 Open Access Article
Open Access ArticleEffect of heat-treated flaxseed lignan macromolecules on the interfacial properties and physicochemical stability of α-linolenic acid-enriched O/W emulsions†
Chen
Cheng
ab,
Xiao
Yu
bc,
Fenghong
Huang
b,
Lei
Wang
b,
Zhenzhou
Zhu
*a,
Jing
Yang
b,
Peng
Chen
b and
Qianchun
Deng
 *b
*b
aNational R&D Center for Se-rich Agricultural Products Processing, Hubei Engineering Research Center for Deep Processing of Green Se-rich Agricultural Products, School of Modern Industry for Selenium Science and Engineering, Wuhan Polytechnic University, Wuhan 430023, China. E-mail: zhenzhou.zhu@whpu.edu.cn; Tel: +86-27-86827874
bOil Crops Research Institute of the Chinese Academy of Agricultural Sciences, Hubei Key Laboratory of Lipid Chemistry and Nutrition and Key Laboratory of Oilseeds Processing, Oil Crops and Lipids Process Technology National & Local Joint Engineering Laboratory, Wuhan 430062, China. E-mail: dengqianchun@caas.cn
cCollege of Food and Bioengineering, Henan Key Laboratory of Cold Chain Food Quality and Safety Control, Zhengzhou University of Light Industry, Zhengzhou 450001, China
First published on 19th August 2024
Abstract
Flaxseed lignan macromolecules (FLMs) are important polyphenols present in flaxseeds with interfacial adsorption behavior. However, FLMs are easily degraded during thermal treatment in emulsions, which further influences their interfacial properties and application. In this work, the interfacial properties of FLMs between oil and water were evaluated using compression isotherms and interfacial tension to investigate the regulation mechanism of FLMs and their heat-treated products on the stability of O/W emulsions. Furthermore, the improvement mechanism of FLM heat-treated products on the physicochemical stability of flaxseed oil emulsions was clarified. Studies showed that thermal degradation occurred on terminal phenolic acids in FLMs when treated under 100 and 150 °C (FLM-100 and FLM-150) without any decrease in antioxidant activity. FLM-100 and FLM-150 improved the physicochemical stability of sunflower lecithin (S90)-stabilized flaxseed oil emulsions and reduced the concentration of hydroperoxides and TBARS by 26.7% and 80% (p < 0.05), respectively, during storage. This was due to the high interfacial anchoring of FLM-100 and FLM-150, which further strengthened the interface of oil droplets and improved the interfacial antioxidant effect of FLMs. This implies that FLM-100 and FLM-150 could act as new efficient antioxidants for application in food emulsions.
1. Introduction
Flaxseed oil (FO) is one of the richest sources of α-linolenic acid (ALA)—an essential fatty acid. Epidemiological surveys indicated that ALA plays a crucial role in human health throughout the entire lifecycle, including brain development in babies, the fertility of men and women, and the prevention of neurodegenerative diseases in the elderly.6 ALA can be metabolized into higher activity long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs), such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and other n-3 derivatives in mammals.6 However, due to ALA's low water solubility, high oxidation susceptibility, and limited in vivo conversion into DHA and EPA, the effective intervention dose of ALA was calculated to exceed the normal intake. More importantly, reports proved that enterolactone, the metabolite of secoisolariciresinol (SECO), could regulate ALA metabolism by promoting the extracellular efflux of free fatty acids (FFAs) and triglycerides (TAGs) and decreasing the expression of fatty acid β-oxidation-related protein (CPT1).9 Therefore, it also could be a wonderful antioxidant for the design of FO-enriched food.7,8Lignans refer to a series of plant secondary metabolites that are formed via the polymerization of two phenylpropanoid (C6–C3) derivatives in various configurations, existing as dimers, trimers, and polymers in plants. Approximately 769 lignans have been isolated and identified over the past few years; among them, flaxseed lignan has been found to have the highest content in nature.1 Flaxseed lignan exists in flaxseed hull as an SDG copolymer structure, which is named the flaxseed lignan macromolecule (FLM). The FLM is surrounded by flaxseed kernel and can protect FO from oxidation and other external environment attack. Considering the natural structure of the FLM, it occurs as a linear copolymer ester-linked by 3-hydroxy-3-methylglutaric acid (HMGA), and its skeleton comprises SDG and a few flavonoid glycosides (e.g., pinoresinol diglucosides (PDGs) and herbacetin diglucoside (HDG)), among which HDG/PDG predominantly existed with a FerAG moiety in longer chain FLMs, which may further influence the biosynthesis of these longer chain variants of FLMs. Besides, ferulic acid glucoside (FeAG), p-coumaric acid glucoside (CouAG) or caffeic acid glucoside (CafAG) was grafted at terminals for the formation of complete FLMs.5 The critical role of flaxseed lignans in preventing multiple chronic diseases has been well confirmed, including reducing the risk of cardiovascular diseases, type 2 diabetes, and different types of cancers.2 Pharmacokinetic parameters for SDG and FLM have been previously evaluated in female Wistar rats after a single oral dose.14 The study indicated that the total enterolactone and enterodiol (final metabolite of FLM and SDG) reached similar maximum concentrations between 11 and 12 h, and the total body exposures were similar for both enriched SDG and FLM. Considering the economy and safety of the application of flaxseed lignans, FLMs can serve as cost-effective alternatives to the SDG due to their pharmacokinetic characteristics.5
To achieve the co-delivery of ALA and flaxseed lignans, an emulsion system can be designed due to its high tolerance for both lipophilic and hydrophilic bioactive substances.2,7 Compared with other emulsifiers, phospholipids could motivate the activity of lipolytic enzymes and participate in the micellization process of bile salts, free fatty acids, monoglycerides, etc., which promoted the absorption of ALA.10 When co-absorbed with ALA, phospholipids were reported to improve transportation and metabolism by promoting the expression of the protein that is related to the formation and exocytosis of chylomicrons.8 Therefore, co-intake of flaxseed lignans and ALA by phospholipid-stabilized FO emulsions could improve the bioaccessibility of ALA via decreasing consumption by inhibiting ALA oxidation, enhance the absorption of ALA by promoting the formation of ALA micelles, and improve the metabolism of ALA (to EPA/DHA) through decreasing β-oxidation.
FLM has also been recently promoted as a nutraceutical with known safety effects and has generally been employed in commercial products such as plant milk, bread, coarse grain biscuits, and mooncakes.2,3 However, the release of lignans was seriously limited during digestion due to the remaining integral cell structure for flaxseeds or sesame powder. It has been proved that the daily intake of more than 500 mg per day lignans is likely to play their biological activity.4 However, the daily intake of lignans from flaxseed products ranged from 300 to 500 mg per day for secoisolariciresinol diglucoside (SDG) for most populations, resulting in the insufficient intake dose to develop a significant dose-effect relationship for adults.4,5 Therefore, with the wide concern for a healthy life, the delivery efficiency of FLM in FO emulsion is also an important research field. Besides, thermal processing is a common and necessary process in the manufacture of lignan-enriched foods such as pasteurization, boiling, cooking and roasting. From modest heat treatments, the input of thermal energy can exert significant effects on the content, structure of food polyphenols due to their degradation, solubilization and mutual effects with other food components.11 For example, for sesamin and sesamol-enriched sesame meal, thermal processing (240 °C for 20 min) increased the release of lignans (sesamin and sesamol) to 19.6% after in vitro digestion due to the degradation of aglycones and glycosides.12 The batch pasteurization (85 °C for 20 min) for SDG-enriched whey drinks does not affect the content of SDG.13 However, the concentration of SDG degraded by 25% after 6 months of storage under 8 °C.13 However, there is no in-depth study on the structural and functional changes of FLMs under different heat treatments, particularly for the structure–function (antioxidant activity) relationship and the microscopic behavior changes in emulsions. Therefore, it was urgent to investigate the structural and functional changes of FLMs under different thermal processes and strive to achieve the optimal use of lignans in the food industry.
Accordingly, low-temperature drying (37 and 55 °C), pasteurization (70 °C), boiling (100 °C) and baking (150 °C) temperatures are set to simulate different process temperatures of FLMs. The antioxidant activity of heat-treated/untreated FLMs is detected and further analyzed based on their structural and composition changes. Furthermore, a previous study has proved that using phospholipids as emulsifiers to prepare an emulsion delivery system could improve the release and absorption of ALA compared with proteins and polysaccharides.8 Therefore, sunflower lecithin-stabilized FO emulsion was selected to evaluate the effect of heat-treated/untreated FLM on the physicochemical stability, focusing on the interfacial antioxidant theory. This study aims to develop effective plant-based antioxidants, which can be further applied in functional foods and beverages.
2. Materials and methods
2.1 Chemicals and reagents
FO was obtained from Hongjingyuan Oil Co. Ltd (Xilingol, China). Sunflower lecithin Sunlipon™ 90 (S90) was provided by a commercial supplier (Perimondo, New York, NY, USA) Secoisolariciresinol diglycoside (SDG, 97%), secoisolariciresinol (SECO, 95%), thiobarbituric acid (TBA), 1,1,3,3-tetraethoxypropane, sodium azide, cumene hydroperoxide, 2,2-azobis-2-methylpropanimidamide dihydrochloride (AAPH), lipase (from porcine pancreas), and bile extract (porcine) were offered by Sigma-Aldrich (Saint Louis, MS, USA). Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China).2.2 Extraction of FLM
In brief, flaxseed hull powder (FHP, 20 g) was defatted by incubating with 100 mL n-hexane at 25 °C for 24 hours. Then, defatted FHP (20 g) was subjected to three extractions using 100 mL of 70% ethanol (v/v) for 24 hours. After each extraction, the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 40 minutes. The FLM-enriched supernatant obtained from each extraction was obtained using a C18 solid-phase extraction column (Waters, C18, 5 cm) to purify polar small-molecule polyphenols. The purified FLM was concentrated using a rotary evaporator (IKARV10D, Germany), followed by drying using a vacuum freeze dryer, and then stored at −18 °C for in-depth analyses.
000g for 40 minutes. The FLM-enriched supernatant obtained from each extraction was obtained using a C18 solid-phase extraction column (Waters, C18, 5 cm) to purify polar small-molecule polyphenols. The purified FLM was concentrated using a rotary evaporator (IKARV10D, Germany), followed by drying using a vacuum freeze dryer, and then stored at −18 °C for in-depth analyses.
2.3 Preparation of heat-treated FLMs
FLMs were re-dissolved in 0.5 mL 70% ethanol water (v/v) and then diluted to 10 mL with distilled water (5 mg mL−1, w/v). Subsequently, FLM solutions were heated in a water bath at 37, 55, 70, and 100 °C for 30 min to simulate different heating processes, low-temperature drying (55 °C), pasteurization (70 °C), and boiling (100 °C), respectively. Moreover, the temperature of 150 °C in the oil bath for 30 min was set to simulate the roasting process of FLMs. FLM solutions were immediately cooled in ice water to stop heat processing and then freeze-dried for subsequent analyses. FLMs prepared at different heating temperatures were termed FLM-37, FLM-55, FLM-70, FLM-100, and FLM-150, respectively. The FLM without heat treatment served as a control.The retention rate of FLMs after simulating the heat-treated process was examined using a UPLC (Waters, USA) equipment ACQUITY UPLC ® BEH Shield RP 18 column (2.1 × 100 mm, 1.7 μm) and calculated as follows:
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 (x: μg mL−1, r2 = 0.996). The selected gradient was consistent with the parameters described previously.7,17
758 (x: μg mL−1, r2 = 0.996). The selected gradient was consistent with the parameters described previously.7,17
2.4 Composition analysis of heat-treated FLM
According to our previous study,7 the untreated and heat-treated FLM (2 mg mL−1) were fully saponified in a 75 mM NaOH solution at 55 °C for 24 h. The reaction was terminated by adjusting the pH to 6.5–7.0 with 1 M HCl. The compositions of different FLM samples were examined by UPLC (Waters, USA). The standards employed for flaxseed lignan quantification include secoisolariciresinol diglucoside (SDG), herbacetin diglucoside (HDG), pinoresinol diglucoside (PDG), p-coumaric acid glycoside (CouAG), and ferulic acid glycoside (FerAG).The freeze-dried FLM samples were employed for spectroscopy analysis using a TENSOR 27 Fourier transform infrared (FT-IR) spectrometer (Bruker) to analyze its structural properties. The spectra were recorded at a resolution of 4 cm−1 and a scan number of 32 in the range of 4000–700 cm−1 with air as the background at ambient temperature (25 °C).
2.5 Interfacial property of heat-treated/untreated FLMs
To simulate the realistic interfacial adsorption process of FLMs and S90 between flaxseed oil and water, the dynamic interfacial tension (γ) at the interface of FO and heat-treated/untreated FLM (0.03% w/w)-enriched aqueous phase was examined using a drop profile tensiometer (Tracker, Teclis Technologies, France). The droplet area was set to 15 mm2 and the respective experiment was monitored at 25 °C for 3600 s. The drop profiles of γ were continuously monitored and then recorded as a function of time. The surface pressure was calculated using the following mathematical conversion relation (eqn (2)):16| π = γs − γp | (2) |
The mica flakes were immersed in a trough with an ultrapure water aqueous phase and then pulled upwards (1 mm min−1) at a certain surface pressure (20 mN m−1). A freshly cut mica sheet (Highest Grade V1 Mica, Ted Pella, INC, USA) was used. The mica sheet with the film was reproduced and then dried in a desiccator for 2–3 days before observing through AFM (Bruker Dimension ICON, USA). Topographic measurements were performed using a ScanAsyst-Air model non-conducting tapered silicon nitride probe. The AFM images were analyzed and processed using Nanoscope Analysis software v1.5.
2.6 Antioxidant activity of heat-treated/untreated FLM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 proportion, and the absorbance of this mixture was recorded using a UV-vis spectrophotometer (Beckman Coulter Inc., USA) at 729 nm after 10 minutes of reaction.17 The ABTS˙+ scavenging capacity was determined by the percentage of inhibition as follows:
10 proportion, and the absorbance of this mixture was recorded using a UV-vis spectrophotometer (Beckman Coulter Inc., USA) at 729 nm after 10 minutes of reaction.17 The ABTS˙+ scavenging capacity was determined by the percentage of inhibition as follows: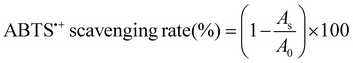 | (3) |
To maintain the same free radical concentration with ABTS˙+, the DPPH working solution was diluted with methanol to 0.05 μmol mL−1 with the absorbance between 0.5 and 0.6 at 517 nm. Twenty microliters of FLMs (1 mg mL−1) were mixed with 200 μL DPPH. After 30 min incubation, the absorbance of samples was recorded using a UV-vis spectrophotometer (Beckman Coulter Inc., USA) at 517 nm. The DPPH radical scavenging capacity was determined from the percentage of inhibition as follows:
 | (4) |
2.7 Emulsion preparation
Emulsions were produced by high-speed homogenization followed by high-pressure microfluidics. The continuous phase was prepared by solubilizing 3% S90 and 0.5 mg mL−1 FLMs. Coarse FO emulsions were prepared by blending 20% FO (w/w) and 80% (w/w) aqueous phase using a high-speed blender (IKA, T25, Germany) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. Fine FO emulsions were prepared using a microfluidizer (model M-110L, Microfluidics, Newton, MA) at 12
000 rpm for 5 min. Fine FO emulsions were prepared using a microfluidizer (model M-110L, Microfluidics, Newton, MA) at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi for four cycles.
000 psi for four cycles.
2.8 Morphology of emulsions
The interfacial morphology of heat-treated/untreated FLM-enriched emulsion droplets was captured by cryo-scanning electron microscopy (Cryo-SEM, SU8010, Hitachi, Tokyo, Japan). Briefly, the emulsion (5.0–10.0 μL) was frozen in liquid nitrogen, transferred into a cryo-preparation chamber, cut into the cross-section at −90 °C and 1.3 × 10−6 mbar and then sputter-coated at 10 mA for 30 s. Finally, the samples were observed using a Cryo-SEM at 3 kV.2.9 Analysis of physical and chemical stability during storage
FO emulsions with 0.02% (w/w) of sodium azide were packed into 10 mL glass tubes, sealed with a silicone plug and parafilm and then stored in the darkness at 37 °C for 3 weeks. The particle size and ζ-potential of FO emulsions were determined using a Mastersizer 3000 and a Nano-ZS, respectively. The microscopic morphology of FO emulsions was identified by confocal laser scanning microscopy (CLSM) (Nikon D-Eclipse C1 80i, Nikon, Melville, NY), and the CLSM images were processed using the Zen 2.6 software. The interface topography of FO emulsion droplets was observed by cryo-scanning electron microscopy (cryo-SEM) (SU8010, Hitachi, Japan).The generation of primary oxidative products and secondary oxidative products of emulsions during storage was determined by a spectrophotometric method.7 The values of hydroperoxides were expressed as mmol cumene hydroperoxide equivalents per kg of oil. The secondary oxidative products, malonaldehyde, were expressed as mmol 1,1,3,3-tetraethoxypropane (TEP) equivalents per kg of oil.
2.10 Statistical analysis
All assays were performed in triplicate, and the data are expressed as mean ± SD (n = 3). One-way ANOVA was performed to analyze the significant differences between data (p < 0.05) using SPSS 24 (SPSS Inc., Chicago, IL, USA). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca).3. Results and discussion
3.1 Thermostability of FLM under different heating stresses
As shown in Fig. S1,† the purity of FLMs after extraction and purification was detected by UPLC at 280 nm, which reached 98%. Different heating processes were simulated for FLMs as digestion temperatures (37 °C, FLM-37), low-temperature sterilization temperatures (55 °C, FLM-55), pasteurization temperatures (70 °C, FLM-70), boiling temperatures (100 °C, FLM-100), and baking temperatures (150 °C, FLM-150). As shown in Fig. 1a, after treatment at 37 and 55 °C, there was no difference between the heat-treated FLMs and untreated FLMs. However, about 31%, 38%, and 52% of the FLM were degraded under higher temperature treatments (FLM-70, FLM-100, and FLM-150), respectively. As depicted in Fig. 1b, the heat-treated/untreated FLMs displayed typical absorption peaks of phenolic compounds, i.e., the O–H and C–O stretching vibration of phenolic hydroxyl (Ar-OH), at 3369.36 and 1218 cm−1. The C–H bending vibration of the methyl (–CH3) groups was at 1458 cm−1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the FLM was identified at the wavenumber of 1900–1650 cm−1. The Calkyl–O stretching vibration of the methoxy (Ar-OCH3) group was at 1120 cm−1, and the C–H vibration of aromatic units was at 1600 cm−1 and 1510 cm−1.18–20 However, after high-temperature heat treatment, the O–H and C–O stretching vibration of FLM-70, FLM-100, and FLM-150 maximally shifted from 3369.36 cm−1 to 3421.44 cm−1, and the C
O stretching vibration of the FLM was identified at the wavenumber of 1900–1650 cm−1. The Calkyl–O stretching vibration of the methoxy (Ar-OCH3) group was at 1120 cm−1, and the C–H vibration of aromatic units was at 1600 cm−1 and 1510 cm−1.18–20 However, after high-temperature heat treatment, the O–H and C–O stretching vibration of FLM-70, FLM-100, and FLM-150 maximally shifted from 3369.36 cm−1 to 3421.44 cm−1, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration maximally shifted from 1726 cm−1 to 1718.43 cm−1 compared with the FLM. This indicated that O–H was degraded, and formed quinone compounds after heating under 70 °C, 100 °C and 150 °C.
O stretching vibration maximally shifted from 1726 cm−1 to 1718.43 cm−1 compared with the FLM. This indicated that O–H was degraded, and formed quinone compounds after heating under 70 °C, 100 °C and 150 °C.
As shown in Fig. 1c–f, after complete hydrolysis, thirteen phenolic compounds were identified in the FLM, which comprised five lignans (+, −) SDG, PDG, and their monoglucoside (PMG) and aglycones, and four phenolic acids and their glucosides, namely, caffeic acid glucoside (CarfG), p-coumaric acid glucoside (CouAG), ferulic acid glucoside and its isomers ((E)-form/(Z)-form FerAG), and dihydroferulic acid dimers. Compared with FLM, the concentration of the composition in heat-treated FLM decreased under different thermal treatments. Besides, lignans and flavonoids in FLM-100 and FLM-150, such as SDG, PDG, and HDG, serving as the skeleton of FLMs, were slightly degraded (<1%). However, the concentration of phenolic acids at the end of the chain of FLM was decreased by 14% (CouAG), 10% (FerAG and its isomers), and 21% (dihydroferulic acid dimer), respectively (p < 0.05), which was consistent with the phenomena and conclusions reported in previous studies.15
The partial least squares discriminant analysis (PLS-DA) of the composition for heat-treated/untreated FLMs represented the disjoint union for FLM, FLM-100 and FLM-150, indicating the significant composition changes of FLMs under 100 and 150 °C heat treatment (Fig. 2). PLS-DA combined with the variable importance in projection (VIP) scores was adopted to select the phenolic compounds that significantly changed during heat treatment according to the criterion of VIP > 1. Dihydroferulic acid dimers such as FerA, (+, −) SDG, PDG, CouAG and (+, −) FerAG were regarded as characteristic compositions during heating with a higher VIP value (Fig. 2a). As shown in Fig. 2b, the cluster analysis had the same results as PLS-DA, which indicated that the phenolic compounds in FLM-100 and FLM-150 were significantly decreased compared with FLMs. Besides, phenolic acid glucuronides and flavonols such as FerAG, CouAG, and (+, −) SDG and PDG showed a significant positive correlation with heat treatment temperature.
FT-IR spectra indicated that the stretching vibration of compound hydroxyl appeared in the infrared wavenumber ranging from 3650 to 3500 cm−1, which can be adapted to determine whether there exist phenols, acids, and alcohols in the compounds.19 The FLM displayed characteristic peaks at wave numbers of 3369 cm−1, 1726 cm−1 and 1458 cm−1, suggesting the existence of hydroxyl, carbonyl, and benzene ring characteristic structures, respectively.21 As revealed by the shift of O–H and C–O stretching vibrations for heat-treated FLMs, the phenolics were slightly decreased to aldehyde compounds. Besides, the ArO-H vibration signals of aromatic units were normalized to evaluate the relative contents of Ar-OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (ESI, Fig. S1†). As the treatment temperature of FLMs increases, the signal intensity of the O–H stretching vibration tended to be reduced, whereas the signal intensity of C
O (ESI, Fig. S1†). As the treatment temperature of FLMs increases, the signal intensity of the O–H stretching vibration tended to be reduced, whereas the signal intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O increased, confirming the transformation of Ar-OH group into the C
O increased, confirming the transformation of Ar-OH group into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. Therefore, it also indicated that the molecular structure of FLMs can be effectively reconstructed by heat-treatment, in particular phenolic-OH groups.22 Those results indicated the good thermal stability of FLMs at lower temperatures such as the digestion temperature (37 °C) and sterilization temperature (55 °C) for 30 min. However, under higher temperature heat treatment (100 °C and 150 °C), the phenolic acids on the terminal of FLMs were easily degraded without the release of polyphenols, suggesting that the degradation of FLMs was limited and only existed in the oxidation of internal groups.5
O group. Therefore, it also indicated that the molecular structure of FLMs can be effectively reconstructed by heat-treatment, in particular phenolic-OH groups.22 Those results indicated the good thermal stability of FLMs at lower temperatures such as the digestion temperature (37 °C) and sterilization temperature (55 °C) for 30 min. However, under higher temperature heat treatment (100 °C and 150 °C), the phenolic acids on the terminal of FLMs were easily degraded without the release of polyphenols, suggesting that the degradation of FLMs was limited and only existed in the oxidation of internal groups.5
At higher temperatures, the serious degradation of phenolic acids compared to lignans and flavonoids suggested that the degradation mainly occurred at the terminal of the chain of FLMs. However, the concentration of FerAG in FLM-150 was slightly higher than that of FLM-100 but without significance (p > 0.05), and this might be due to the isomerization between FerAG and its isomers or undifferentiated degradation of FerAG in FLMs under 100 °C and 150 °C.23 As postulated in Fig. 3, the depolymerization, isomerization, and oxidation of FLMs may occur simultaneously when exposed to heat treatment.24 Then, the oxidation of phenolic hydroxyl to form reactive semiquinone and quinone radicals can lead to the further formation of stabilized quinone structures.25,26 Moreover, the isomerization of FerAG during thermal treatment has been demonstrated as a vital change, especially at higher processing temperatures.11 Besides, the depolymerization of FLM may exist during heat processing and the release is relatively complex with a small molecular weight.24,27
3.2 Effect of heat treatment on the antioxidant activity of FLM
The total phenolic contents and FRAP of FLMs were measured to indicate the antioxidant activities of different mechanisms, i.e., the transfer of electrons of phenolics.28 SDG (0.1 mg mL−1) and SECO (0.025 mg mL−1) possessed the same number of hydroxyl groups with 1 mg mL−1 FLM (Mw ≈ 4641, c(–OH) = 275 μmol L−1) and were set as the control group. As indicated in Table 1, the total phenolic contents of SDG and SECO were 29.48 and 14.01 μg SA per mL, and the values of FRAP were 10.59 and 3.18 μg SA per mL, respectively. Besides, the ABTS˙+ and DPPH˙ free radical scavenging ability of SDG reached 76.17% and 63.07%, respectively, which was 66.7% and 50% higher than those of SECO (p < 0.05). For heat-treated/untreated FLMs, there were no obvious differences in the reducing power (FRAP), which was 40% lower than that of SDG but 50% higher than that of SECO (p < 0.05), respectively. Moreover, FLM-55 exhibited the highest total phenolic contents compared to other samples (34.51 ± 0.73 μg SA per mL, p < 0.05), which were 16.9% and 146% higher than those of SDG and SECO, respectively (p < 0.05). Moreover, for the ability to scavenge DPPH˙ free radicals, there was no obvious difference between heat-treated/untreated FLMs (∼85%), but 11.8% and 112% higher than those of SDG and SECO, respectively. Besides, FLM-150 exhibited the highest ABTS free radical scavenging ability (84.48 ± 2.87%, p < 0.05) compared to other samples, which was 34.1% and 310% higher than those of SDG and SECO (p < 0.05), respectively.| Total phenolic (μg SA per mL) | FRAP (μg SA per mL) | DPPH free radicals scavenge (%) | ABTS free radicals scavenge (%) | |
|---|---|---|---|---|
| FLM | 31.44 ± 1.06 bc | 6.00 ± 0.22 b | 85.25 ± 0.35 a | 81.37 ± 0.54 c |
| FLM-37 | 33.82 ± 0.09 b | 5.47 ± 0.50 b | 85.25 ± 0.25 a | 81.47 ± 2.11 c |
| FLM-55 | 34.51 ± 0.73 ab | 6.36 ± 0.37 b | 83.97 ± 0.33 a | 82.09 ± 0.65 bc |
| FLM-70 | 29.80 ± 0.84 bc | 5.14 ± 0.29 b | 84.54 ± 1.37 a | 80.22 ± 2.34 c |
| FLM-100 | 30.28 ± 1.19 bc | 6.23 ± 0.52 b | 84.45 ± 0.21 a | 79.79 ± 2.23 c |
| FLM-150 | 30.44 ± 2.18 bc | 6.00 ± 0.45 b | 84.89 ± 0.77 a | 84.48 ± 2.87 a |
| SDG | 29.48 ± 0.45 c | 10.59 ± 0.35 a | 76.17 ± 0.77 b | 63.07 ± 2.31 d |
| SECO | 14.01 ± 0.34 d | 3.18 ± 0.15 c | 40.14 ± 5.30 c | 20.1 ± 0.34 e |
In theory, the total phenolic content is positively correlated with the antioxidant capacity of plant polyphenols, which also reflects the number of phenolic hydroxyl groups to a certain extent.29,30 As revealed by the above-mentioned results, the total phenolic contents of SDG were nearly two times higher than those of SECO at the identical concentration of hydroxyl groups, which was also in accordance with the number of hydroxyl groups in SDG and SECO. Therefore, FLMs had the same amount of total phenolic content with SDG, indicating no significant difference in the amount of phenolic hydroxyl groups among all samples. However, the DPPH and ABTS free radical scavenging capacity of heat-treated/untreated FLMs was slightly better than that of SDG at the identical concentration of hydroxyl groups. It was due to the higher antioxidant activity of flavonoids in the terminal of FLM chains such as HDG and PDG.2 In general, at the same concentration of hydroxyl groups, HDG had a higher free radical scavenging capacity than that of SDG due to the meta-position dihydroxy moiety, which was an essential feature to evaluate the antioxidant activity.31 Besides, due to the inconsistent antioxidant mechanisms, there was no significant difference between the results of FRAP with DPPH and ABTS-free radical scavenging activity for FLMs and SDG and SECO.32 However, SDG had a higher value of FRAP than that of FLM due to the good water solubility, thus contributing to the transfer of a single electron in a polar reaction environment.28 This suggested that the heat-treated/untreated FLM possessed a higher antioxidant activity than those of SDG and SECO at the identical concentration of hydroxyl groups. These results were in accordance with Kim et al.,33 who confirmed that a simple heating process can be used as a tool to increase the antioxidant activity of grape seed extracts. Therefore, the FLM can serve as a more economic and efficient antioxidant than SDG and SECO.
As depicted in Fig. 1c–f, CouAG was seriously degraded by 12% after heat treatment under 150 °C. However, due to the weak antioxidant activity of CouAG, this change did not influence the antioxidant activity of FLMs.34 Besides, after low-temperature heat treatment (37 °C and 55 °C), the total phenolic content of FLM was increased, suggesting the increased exposure of hydroxyl groups. It might be the depolymerization of FLMs under heat treatment due to the broken glycosidic bonds. Besides, after high-temperature heat treatment (70, 100, and 150 °C), the total phenolic content of FLMs declined due to the oxidation of phenolic hydroxyl groups.35
3.3 Effect of heat treatment on the interfacial adsorption behavior of FLMs
An in-depth study was conducted to illustrate the effect of heat-treated/untreated FLMs on the compression isotherm curves of interfacial films formed by S90 during the interfacial adsorption. As depicted in Fig. 4b, the unit molecular area was nearly 41 Å when the surface pressure of S90 film reached the maximum (27.5 mN m−1), suggesting “condensed phase” formation of S90 under that condition.40 Besides, three stages for the behavior of heat-treated/untreated FLM-enriched S90 solutions are elucidated as follows: stage I: the zero-pressure stage is extremely low interfacial coverage, which was termed “gaseous phase”; stage II: with the interface compressing, the interfacial pressure of S90 is subjected to an initial increase, which was termed “fluid phase”; stage III, the tiled FLMs come closer with each other and then form the “condensed phase”.38 The emulsifier S90 began to form the “fluid phase” when the molecular area took up nearly 100 Å (Fig. 4b). After the addition of FLMs, the molecular area for “fluid phase” advanced to 125 Å, which was due to the higher molecular area of FLMs (MW > 4000) than S90 (MW ∼768). Moreover, the surface pressure of the FLM-enriched S90 solution increased compared with that of S90, suggesting that competitive adsorption might exist between FLMs and S90 during co-absorption onto the interface. However, the potential noncovalent interactions between FLMs and S90 might lead to an undefined variation tendency of surface pressure of FLM-enriched S90 solutions.
To further investigate the microstructure of heat-treated/untreated FLM-enriched S90 films, the LB films were prepared on the mica flakes when the surface pressure was under 20 mN m−1. Furthermore, the mica flakes were observed by atomic force microscopy (AFM). As presented in Fig. 4e, the films formed by S90 were continuous and without any defect due to the excellent interfacial properties of phospholipids. Under the scale of 3 μm, the images indicated that FLM, FLM-37, and FLM-55 did not affect the dispersibility and compactness of S90 films, while FLM-70, FLM-100, and FLM-150 led to the depression formation on S90 films. Under the scale of 0.5 μm, the images showed obvious defects for all FLM-enriched S90 films, especially for FLM-37, FLM-55, and FLM-70. Besides, FLM-enriched S90 (except for FLM-55) films showed obvious depression under the surface pressure at 20 mN m−1. However, FLM-55-enriched S90 collapsed since the pressure approached the critical pressure (22 mN m−1). FLM-150-enriched S90 formed relatively intact films for their prominent interfacial property. Therefore, for heat-treated/untreated FLMs, FLM-150 exhibited a higher increased rate of interfacial pressure than other samples, suggesting that the dense film may be formed.
3.4 Effect of heat-treated FLM on the stability of FO emulsions
As revealed by the macroscopic evaluation of emulsions, all emulsions were kept stable during the storage with no leakages of either oil or water phases or aggregation of droplets. The microspheres with an average diameter of nearly 400 nm for S90-stabilized emulsion were observed from the SEM images (Fig. 6b). In contrast, the average diameter of FLM-enriched emulsions was larger than that of S90-stabilized emulsions, and their surface was micro-concave with densely distributed shallow craquelure. Moreover, the observed results of particle size by SEM were consistent with the D(4,3). Notably, FLM-150 enriched emulsions exhibited a much smoother surface, and their particle size was evenly distributed (Fig. 6b). Furthermore, as depicted in Fig. 5d, all CLSM images showed very fine lipid droplets (red) evenly distributed in the aqueous phase (black) at the initial stage. Similar to the results of D(4,3), the size of the lipid particles increased after storage.
Moreover, D(3,2) has been extensively employed to evaluate the droplet size of freshly prepared emulsions.46 Besides, D(4,3) has been often adopted to monitor the droplet size changes of the emulsion during storage.47 The results of D(3,2) suggested that the addition of FLMs led to 32%–79% increase in the particle size of S90-stabilized emulsions due to the bridged effect between FLMs and S90.48,49 The change in CLSM images and D(4,3) during storage indicated a positive effect on the physical stability of S90-stabilized emulsions by all types of FLMs, especially for FLM-150. The compression isotherm curves further illustrated that the thicker and higher viscoelastic interfacial film formed in FLM-150-enriched S90. Besides, Fig. 4c further revealed the decreased emulsion stability of S90 by FLMs due to the interactions between them. For heat-treated/untreated FLMs, the value of interfacial tension between FLM-150-enriched S90 solution and FO was maximum, which showed the largest particle size after emulsification to form an emulsion. Furthermore, FLMs could also interact with phospholipids via hydrogen bond and hydrophobic interactions, which were mainly determined by the polyphenol's polarity. Due to the weak polarity of FLMs, they were expected to interact with the nonpolar acyl chain region deep of phospholipids and this depth decreased with the increase in polarity.50 Therefore, the different polarity of heat-treated FLMs further influenced their interface positioning and stability of emulsions.
 | ||
| Fig. 7 Hydroperoxide (a and b) and malonaldehyde (c and d) formation in FO emulsions containing different types of lignans (FLM, FLM-37, FLM-55, FLM-75, FLM-100 and FLM-150) during storage. | ||
According to previous study, the antioxidant activity of plant polyphenols generally reduced after high-temperature heat treatments (e.g., tea polyphenols and grape seed extract).24,35 However, the antioxidant activity of FLMs was improved after heat treatment, which might be due to the prominent stability of FLMs against heat treatment attributed to its relatively highly stable ester-bonded oligomer structure compared with those plant polyphenolics. Accordingly, when treated at high temperatures (100 and 150 °C), the FLM depolymerized and released monomers and other lower oligomers, which increased its antioxidant activity.51 Notably, all heat-treated/untreated FLMs were proved to possess interfacial properties, especially for FLM-100 and FLM-150, suggesting the possible higher adsorption contents of FLM-100 and FLM-150 than FLM, FLM-37, FLM-55 and FLM-70. The better interfacial anchoring of FLM-100 and FLM-150 further increased their interfacial antioxidant effect. Besides, FLM-100 and FLM-150-enriched S90 formed a thick and dense interfacial film, which can inhibit the attack of prooxidative factors for lipids. However, there was no significant difference in the antioxidant capability between low-temperature-treated FLMs in emulsions, which might be because (i) no significant changes in the FLM structure at low temperatures, (ii) no depolymerization of FLM occurred at low temperatures, which further kept higher lipophilicity and were likely to be embedded deeply into the phospholipid molecule, hindering their interfacial antioxidant reactions and even reducing interfacial density, (iii) the un-depolymerized FLMs exhibited higher steric hindrance on the interface of emulsion droplets, which were likely to interfere with the radical scavenging capacity exhibited by the phenol hydroxyl group.
4. Conclusion
In this study, the chemical composition and antioxidant activity of FLMs under different thermal processes were examined. The results indicated that all heat-treated and untreated FLMs had superior antioxidant activity to their structural units SDG and SECO at the same hydroxyl concentration. This was attributed to the higher antioxidant activity of flavonoids located at the terminal of the FLM chain, such as HDG and PDG. Although the phenolic acids at the end of the FLM chain were significantly reduced, this did not affect their antioxidant activity. Furthermore, we systematically explored the relationship between the structural changes of FLMs under different heat processes and their effects on the physicochemical stability of emulsions. The results indicated that the FLM and its low-temperature-treated products (FLM-37, FLM-55, and FLM-70) exhibited limited antioxidant activity in S90-stabilized FO emulsions, due to the limited interfacial properties of their intact and large molecular structures. In contrast, FLM-100 and FLM-150 demonstrated higher antioxidant activity and interfacial properties, further enhancing the physicochemical stability of the S90-stabilized FO emulsion system. Therefore, the interfacial anchoring of FLMs primarily contributed to their higher interfacial antioxidant activity in emulsions. In conclusion, FLM-100 and FLM-150 were identified as excellent antioxidants for PUFA-enriched emulsions, owing to their outstanding interfacial properties and antioxidant activity. Our findings are also expected to offer a novel and environmentally friendly strategy for promoting the widespread use of natural polymerized plant polyphenols as antioxidants in the food industry.Abbreviations
| FLM | Flaxseed lignan macromolecules |
| DFHP | Defatted flaxseed hull powder |
| S90 | Sunflower lecithin Sunlipon™ 90 |
| SDG | Secoisolariciresinol diglucoside |
| SECO | Secoisolariciresinol |
| HDG | Herbacetin diglucoside |
| PDG | Pinoresinol diglucoside |
| CouAG | p-Coumaric acid glycoside |
| FerAG | Ferulic acid glycoside |
| FO | Flaxseed oil |
| ALA | α-Linolenic acid |
| EPA | Eicosapentaenoic |
| DHA | Docosahexaenoic |
| TBA | 2-Thiobarbituric acid |
| CLSM | Confocal laser scanning microscopy |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| TCA | Trichloroacetic acid |
| TEP | 1,1,3,3-Tetraethoxypropane |
| AAPH | 2,2-Azobis-2-methylpropanimidamide dihydrochloride |
| FRAP | Ferric reducing antioxidant power |
| SAE | Sinapic acid equivalents |
Author contributions
Chen Cheng: investigation, formal analysis, writing – original draft preparation and review & editing; Xiao Yu: development or design of methodology, writing – review & editing preparation; Fenghong Huang: data curation and supervision; Lei Wang: investigation and formal analysis; Zhenzhou Zhu: methodology, development or design of methodology and creation of models; Jing Yang: writing – critical review, commentary and revision; Peng Chen: methodology; Qianchun Deng: resources; conceptualization, ideas; formulation or evolution of overarching research goals and aims; funding acquisition, acquisition of the financial support for the project leading to this publication.Data availability
The data for this article are available at Web of Science at https://doi.org/10.1039/d4fo02663b. Besides, the data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (32072267) and supported by the Earmarked Fund for China Agriculture Research System (CRAS-14).References
- L. X. Wang, H. L. Wang, J. Huang, T. Z. Chu, C. Peng, H. Zhang, H. L. Chen, Y. A. Xiong and Y. Z. Tan, Review of lignans from 2019 to 2021: Newly reported compounds, diverse activities, structure-activity relationships and clinical applications, Phytochemistry, 2022, 202, 113326 CrossRef.
- T. J. Tse, Y. Guo, Y. Y. Shim, S. K. Purdy, J. H. Kim, J. Y. Cho, J. Alcorn and M. J. T. Reaney, Availability of bioactive flax lignan from foods and supplements, Crit. Rev. Food Sci. Nutr., 2022, 1–16 Search PubMed.
- Health Canada 2014. Summary of health Canada's assessment of a health claim about ground whole flaxseed and blood cholesterol lowering. Accessed September 17, 2021. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assess-evalu/flaxseed-graines-de-lin-eng.pdf.
- J. L. Adolphe, S. J. Whiting, B. H. J. Juurlink, L. U. Thorpe and J. Alcorn, Health effects with consumption of the flax lignan secoisolariciresinol diglucoside, Br. J. Nutr., 2010, 103(07), 929–938 CrossRef PubMed.
- A. Mueed, M. Ibrahim, S. Shibli, P. Madjirebaye, Z. Deng and M. Jahangir, The fate of flaxseed-lignans after oral administration: a comprehensive review on its bioavailability, pharmacokinetics, and food design strategies for optimal application, Crit. Rev. Food Sci. Nutr., 2022, 1–19 Search PubMed.
- S. Rahmawaty and B. J. Meyer, Stunting is a recognized problem: Evidence for the potential benefits of omega-3 long-chain polyunsaturated fatty acids, Nutrition, 2020, 73, 110564 CrossRef CAS PubMed.
- C. Cheng, X. Yu, F. Huang, D. Peng, H. Chen, Y. Chen, Q. Huang and Q. Deng, Effect of different structural flaxseed lignans on the stability of flaxseed oil-in-water emulsion: An interfacial perspective, Food Chem., 2021, 357, 129522 CrossRef.
- L. Couedelo, S. Amara, M. Lecomte, E. Meugnier, J. Monteil and L. Fonseca, et al., Impact of various emulsifiers on ALA bioavailability and chylomicron synthesis through changes in gastrointestinal lipolysis, Food Funct., 2015, 6(5), 1726–1735 RSC.
- S. Trattner, B. Ruyter, T. K. Østbye, T. Gjøen, V. Zlabek and A. Kamal-Eldin, et al., Sesamin increases alpha-linolenic acid conversion to docosahexaenoic acid in atlantic salmon (salmo salar l.) hepatocytes: role of altered gene expression, Lipids, 2008, 43(11), 999–1008 CrossRef PubMed.
- O. Pabois, C. D. Lorenz, R. D. Harvey, I. Grillo, M. M. Grundy and P. J. Wilde, et al., Molecular insights into the behaviour of bile salts at interfaces: A key to their role in lipid digestion, J. Colloid Interface Sci., 2019, 556, 266–277 CrossRef PubMed.
- H. Debelo, M. Li and M. G. Ferruzzi, Processing influences on food polyphenol profiles and biological activity, Curr. Opin. Food Sci., 2020, 32, 90–102 CrossRef.
- Y. Chen, H. Lin, M. Lin, P. Lin and J. Chen, Effects of thermal preparation and in vitro digestion on lignan profiles and antioxidant activity in defatted-sesame meal, Food Chem. Toxicol., 2019, 128, 89–96 CrossRef CAS PubMed.
- H. K. Hyvärinen, J. M. Pihlava, J. A. Hiidenhovi, V. Hietaniemi, H. J. T. Korhonen and E. L. Ryhänen, Effect of processing and storage on the stability of flaxseed lignan added to bakery products, J. Agric. Food Chem., 2006, 54(1), 48–53 CrossRef PubMed.
- X. Yang, Y. Guo, T. J. Tse, S. K. Purdy, R. Mustafa, J. Shen, J. Alcorn and M. J. T. Reaney, Oral pharmacokinetics of enriched secoisolariciresinol diglucoside and its polymer in rats, J. Nat. Prod., 2021, 84(6), 1816–1822 CrossRef CAS PubMed.
- E. Gerstenmeyer, S. Reimer, E. Berghofer, H. Schwartz and G. Sontag, Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye, Food Chem., 2013, 138(2), 1847–1855 CrossRef CAS PubMed.
- J. Yang, Y. Duan, H. Zhang, F. Huang, C. Wan, C. Cheng, L. Wang, D. Peng and Q. Deng, Ultrasound coupled with weak alkali cycling-induced exchange of free sulfhydryl-disulfide bond for remodeling interfacial flexibility of flaxseed protein isolates, Food Hydrocolloids, 2023, 140 Search PubMed.
- C. Cheng, K. Yu, X. Yu, F. Geng, F. Huang, L. Wang, Q. Huang, S. Quan and Q. Deng, Optimized endogenous lipid concomitants in flaxseed oil by different oil extraction technologies: Their positive roles in emulsions, LWT – Food Sci. Technol., 2022, 155 Search PubMed.
- J. S. Rodrigues, A. d. S. M. de Freitas, C. C. Maciel, S. F. Mendes, D. Diment, M. Balakshin and V. R. Botaro, Selection of kraft lignin fractions as a partial substitute for phenol in synthesis of phenolic resins: Structure-property correlation, Ind. Crops Prod., 2023, 191 Search PubMed.
- C. Wen, D. Song, L. Zhuang, G. Liu, L. Liang, J. Zhang, X. Liu, Y. Li and X. Xu, Isolation and identification of polyphenol monomers from celery leaves and their structure-antioxidant activity relationship, Process Biochem., 2022, 121, 69–77 CrossRef CAS.
- G. Zhu, D. Ye, X. Chen, Y. Wu, Z. Yang, Y. Mai, B. Liao and J. Chen, Lignin-derived polyphenols with enhanced antioxidant activities by chemical demethylation and their structure-activity relationship, Int. J. Biol. Macromol., 2023, 237, 124030 CrossRef PubMed.
- M. A. Hossain and S. M. Mizanur Rahman, Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus, Arabian J. Chem., 2015, 8(2), 218–221 CrossRef.
- A. Majira, B. Godon, L. Foulon, J. C. van der Putten, L. Cezard, M. Thierry, F. Pion, A. Bado-Nilles, P. Pandard, T. Jayabalan, V. Aguie-Beghin, P. H. Ducrot, C. Lapierre, G. Marlair, R. J. A. Gosselink, S. Baumberger and B. Cottyn, Enhancing the Antioxidant Activity of Technical Lignins by Combining Solvent Fractionation and Ionic-Liquid Treatment, ChemSusChem, 2019, 12(21), 4799–4809 CrossRef PubMed.
- C. Arruda, V. Pena Ribeiro, M. Oliveira Almeida, J. A. Aldana Mejia, R. Casoti and J. Kenupp Bastos, Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis, J. Pharm. Biomed. Anal., 2020, 178, 112922 CrossRef PubMed.
- S. Chamorro, I. Goñi, A. Viveros, D. Hervert-Hernández and A. Brenes, Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace, Eur. Food Res. Technol., 2011, 234(1), 147–155 CrossRef.
- M. Y. Coseteng and C. Y. Lee, Changes in Apple Polyphenoloxidase and Polyphenol Concentrations in Relation to Degree of Browning, J. Food Sci., 2010, 52(4), 985–989 CrossRef.
- L. Pourcel, J. M. Routaboul, V. Cheynier, L. Lepiniec and I. Debeaujon, Flavonoid oxidation in plants: From biochemical properties to physiological functions, Trends Plant Sci., 2007, 12(1), 29–36 CrossRef CAS.
- M. J. Gonzalez, J. L. Torres and I. Medina, Impact of thermal processing on the activity of gallotannins and condensed tannins from hamamelis virginiana used as functional ingredients in seafood, J. Agric. Food Chem., 2010, 58(7), 4274–4283 CrossRef CAS PubMed.
- J. Rumpf, R. Burger and M. Schulze, Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins, Int. J. Biol. Macromol., 2023, 233, 123470 CrossRef CAS.
- Y. Guo, S. Shi, N. Yang, M. X. Tang, Z. J. Duan, X. R. Guo and Z. H. Tang, Comparative assessment of nutritional composition, polyphenol profile and antioxidative properties of wild edible ferns from northeastern China, Food Res. Int., 2023, 163, 112237 CrossRef CAS.
- C. Y. Woumbo, D. Kuate and H. M. Womeni, Cooking methods affect phytochemical composition and anti-obesity potential of soybean (Glycine max) seeds in Wistar rats, Heliyon, 2017, 3(8), e00382 CrossRef.
- R. Praveena, K. Sadasivam, V. Deepha and R. Sivakumar, Antioxidant potential of orientin: A combined experimental and DFT approach, J. Mol. Struct., 2014, 1061, 114–123 CrossRef CAS.
- R. L. Prior, X. Wu and K. Schaich, Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements, J. Agric. Food Chem., 2005, 53(10), 4290–4302 CrossRef CAS PubMed.
- S. Y. Kim, S. M. Jeong, W. P. Park, K. C. Nam, D. U. Ahn and S. C. Lee, Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts, Food Chem., 2006, 97(3), 472–479 CrossRef.
- C. Cheng, X. Yu, D. J. Mcclements, Q. D. Huang, H. Tang, K. Yu, X. Xiang, P. Chen, X. T. Wang and Q. C. Deng, Effect of flaxseed polyphenols on physical stability and oxidative stability of flaxseed oil-in-water nanoemulsions, Food Chem., 2019, 301, 125207 CrossRef.
- Z. Jiang, Z. Han, M. Zhu, X. Wan and L. Zhang, Effects of thermal processing on transformation of polyphenols and flavor quality, Curr. Opin. Food Sci., 2023, 51 Search PubMed.
- V. Aguie-BeGhin, P. Sausse, E. Meudec, V. Cheynier and R. Douillard, Polyphenol β-Casein Complexes at the Air/Water Interface and in Solution: Effects of Polyphenol Structure, J. Agric. Food Chem., 2008, 56(20), 9600–9611 CrossRef PubMed.
- G. Fabre, I. Bayach, K. Berka, M. Paloncyova, M. Starok, C. Rossi, J. L. Duroux, M. Otyepka and P. Trouillas, Synergism of antioxidant action of vitamins E, C and quercetin is related to formation of molecular associations in biomembranes, Chem. Commun., 2015, 51(36), 7713–7716 RSC.
- F. Sun, Q. Wang, C. Gao, H. Xiao and N. Yang, Effect of extraction pH and post-extraction heat treatment on the composition and interfacial properties of peanut oil bodies, Colloids Surf., A, 2023, 656 Search PubMed.
- A. Mohammadi, P. A. Kashi, M. Kashiri, A. Bagheri, J. Chen, R. Ettelaie, H. Jäger and M. Shahbaz, Self-assembly of plant polyphenols-grafted soy proteins to manufacture a highly stable antioxidative pickering emulsion gel for direct-ink-write 3d printing, Food Hydrocolloids, 2023, 142, 108851 CrossRef CAS.
- A. S. Girard-Egrot, S. P. Godoy and L. C. Blum, Enzyme association with lipidic Langmuir–Blodgett films: Interests and applications in nanobioscience, Adv. Colloid Interface Sci., 2005, 116, 205–225 CrossRef CAS PubMed.
- X. Feng, H. Dai, L. Ma, Y. Fu, Y. Yu, H. Zhou, T. Guo, H. Zhu, H. Wang and Y. Zhang, Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: influence of the nanoparticles concentration, Colloids Surf., B, 2020, 196, 111294 CrossRef CAS PubMed.
- W. Xiong, C. Ren, M. Tian, X. Yang, J. Li and B. Li, Emulsion stability and dilatational viscoelasticity of ovalbumin/chitosan complexes at the oil-in-water interface, Food Chem., 2018, 252, 181–188 CrossRef CAS PubMed.
- A. F. H. Ward and L. Tordai, Time–Dependence of Boundary Tensions of Solutions I. The Role of Diffusion in Time–Effects, J. Chem. Phys., 1946, 14(7), 453–461 CrossRef CAS.
- S. Mad-Ali, S. Benjakul, T. Prodpran and S. Maqsood, Interfacial properties of gelatin from goat skin as influenced by drying methods, LWT – Food Sci. Technol., 2016, 73, 102–107 CrossRef.
- C. Cheng, X. Yu, F. Geng, L. Wang, J. Yang, F. Huang and Q. Deng, Review on the Regulation of Plant Polyphenols on the Stability of Polyunsaturated-Fatty-Acid-Enriched Emulsions: Partitioning Kinetic and Interfacial Engineering, J. Agric. Food Chem., 2022, 70(12), 3569–3584 CrossRef PubMed.
- H. Niu, W. Chen, W. Chen, Y. Yun, Q. Zhong, X. Fu, H. Chen and G. Liu, Preparation and Characterization of a Modified-beta-Cyclodextrin/beta-Carotene Inclusion Complex and Its Application in Pickering Emulsions, J. Agric. Food Chem., 2019, 67(46), 12875–12884 CrossRef PubMed.
- H. Niu, W. Wang, Z. Dou, X. Chen, X. Chen, H. Chen and X. Fu, Multiscale combined techniques for evaluating emulsion stability: A critical review, Adv. Colloid Interface Sci., 2023, 311, 102813 CrossRef PubMed.
- C. Le Bourvellec and C. M. Renard, Interactions between polyphenols and macromolecules: quantification methods and mechanisms, Crit. Rev. Food Sci. Nutr., 2012, 52(3), 213–248 CrossRef CAS PubMed.
- X. Pan, Y. Fang, L. Wang, Y. Shi, M. Xie, J. Xia, F. Pei, P. Li, W. Xiong, X. Shen and Q. Hu, Covalent Interaction between Rice Protein Hydrolysates and Chlorogenic Acid: Improving the Stability of Oil-in-Water Emulsions, J. Agric. Food Chem., 2019, 67(14), 4023–4030 CrossRef CAS PubMed.
- X. Yu, S. Chu, A. E. Hagerman and G. A. Lorigan, Probing the interaction of polyphenols with lipid bilayers by solid-state NMR spectroscopy, J. Agric. Food Chem., 2011, 59(12), 6783–6789 CrossRef CAS PubMed.
- J. Yu, M. Ahmedna, I. Goktepe and J. Dai, Peanut skin procyanidins: Composition and antioxidant activities as affected by processing, J. Food Compos. Anal., 2006, 19(4), 364–371 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02663b |
| This journal is © The Royal Society of Chemistry 2024 |






