 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New findings in the metabolism of the saffron apocarotenoids, crocins and crocetin, by the human gut microbiota†
Carlos Javier
García
,
David
Beltrán
,
Maria Dolores
Frutos-Lisón
,
Maria Teresa
García-Conesa
,
Francisco A.
Tomás-Barberán
 * and
Rocío
García-Villalba
* and
Rocío
García-Villalba
 *
*
Research Group on Quality, Safety and Bioactivity of Plant-Derived Foods, CEBAS-CSIC, 30100 Murcia, Spain. E-mail: fatomas@cebas.csic.es; rgvillalba@cebas.csic.es
First published on 15th August 2024
Abstract
The main constituents of saffron are the apocarotenoids crocins and crocetin, present in the stigmas. Numerous healthy properties, especially those related to the effects on the central nervous system, have been attributed to these compounds but the metabolites responsible for these effects are still unknown. Previous evidences in animal models suggest a role for the gut microbiota in the pharmacokinetics and the neuroprotective effects of these compounds. However, the interaction between these apocarotenoids and the gut microbiota has been poorly studied. In this article, we have thoroughly investigated the batch fermentation of crocin-1 and crocetin (10 μM) with human fecal samples of two donors at different incubation times (0–240 h) using a metabolomic approach. We corroborated a rapid transformation of crocin-1 which looses the glucose molecules through de-glycosylation reactions until its complete transformation into crocetin in 6 hours. A group of intermediate crocins with different degrees of glycosylation were detected in a very short time. Crocetin was further metabolized and new microbial metabolites produced by double-bond reduction and demethylation reactions were identified for the first time: dihydro and tetrahydro crocetins and di-demethyl crocetin. In addition, we detected changes in the levels of the short chain fatty acids valeric acid and hexanoic acid suggesting further structural modifications of crocetin or changes in the catabolic production of these compounds. This research is a pioneering study of the action of the human gut microbiota on the saffron apocarotenoids and goes one step further towards the discovery of metabolites potentially involved in the benefits of saffron.
1. Introduction
Crocins and crocetin are natural carotenoid-type compounds found in the stigmas of the saffron (Crocus sativus L.) flowers that give them their characteristic red color and are of great interest in the agrifood, phytopharmaceutical and cosmetic industries.1,2 There are numerous studies that highlight the biomedical and pharmacological properties of saffron in the prevention and development of various chronic diseases.3 Specifically, crocins and crocetin are attributed cardioprotective, hepatoprotective, antiviral, anticancer, antiatherosclerotic, and antidiabetic activities.4 More recently, saffron and its apocarotenoids have been associated with pharmacological effects on the central nervous system with biological action on memory and learning, neurodegenerative diseases, depression and anxiety.5,6 Despite all the biological and neuroprotective activities, neither the specific molecules or metabolites responsible for these effects nor the mechanisms of action involved in them have been sufficiently studied.Pharmacokinetic studies mainly in animal models7,8 and, to a lesser extent, in healthy human volunteers9 have revealed a low absorption for crocins. In these studies, when crocins were administered as pure compounds or in extracts, they were rapidly hydrolyzed by β-glucosidase enzymes of the intestinal epithelium to crocetin which is then easily absorbed and detected in plasma in the free form and as glucuronide derivatives (1 hour after crocin administration).5,9–11 Rapid absorption of crocetin was also observed after its single oral administration in humans.12 It is possible that part of the non-hydrolyzed crocins and (or) non-absorbed crocetin reach the colon. Indeed, different authors have identified the presence of these compounds in animal feces after crocin ingestion.13–15 Xi et al.,13 reported than 80% of the crocin administered to rats (40 mg kg−1) was excreted in the feces or remained in the intestinal content. This means that approximately 6.4 mg of crocin (6.5 μmol) reached the colon. Once they reach the large intestine, they can interact with the microbiota and be subjected to further metabolic transformation. The potential metabolism of crocins to crocetin by the intestinal microbiota was already proposed in previous studies where the pharmacokinetics of these compounds in pseudo-germ free rats14 and rats subjected to antibiotic pretreatment15 were compared to that of normal control rats. In both cases the amount of crocetin circulating in plasma was higher in the control animals suggesting that the gut microbiota contributes to the transformation of crocin to crocetin.
Regarding the neuroprotective effects attributed to the saffron apocarotenoids, there is little in vivo evidence of crocetin reaching the brain after oral administration16 and only some in vitro studies have demonstrated that trans-crocetin was able to permeate through the blood–brain barrier (BBB) in a quite slow process.11 Therefore, a direct interaction of these molecules with the brain tissues may not be responsible for the potential benefits in this organ. Of note, Zhang et al., showed that oral administration of crocin and crocetin to a rat model of cerebral ischemia-reperfusion injury, was associated to a higher cerebral protection effect than an intravenous administration.14 For oral administration, crocin (60 mg kg−1) and crocetin (20 mg kg−1)were administered once daily for 4 days. Further, orally administered crocin showed less cerebral-protective effect in pseudo germ-free rats that in normal rats.14 In this study the neuroprotective effects were evaluated with: neurological deficit score, measurement of infarct volume in perfused brains, measurement of MDA level and total antioxidant capacity in damaged brain tissues and metabolomics study of brain tissue. In other study the consumption of saffron extracts improved anxiety related behavior (analyzed with behavioral tests) in a mouse model of low-grade chronic inflammation and this improvement coincided with changes in gut microbial community (increase of Akkermansia, Muribaculaceae, Christensenellacae and Alloprevotella) and gut derived metabolites (reduction of dimethylamine).17 All these studies have clearly indicated a key role of the microbiota in the metabolism and neurological effects of crocins and crocetin.
Since there is increasing evidence of the existence of a bidirectional signaling between the gut microbiota and the brain (the gut–brain axis),18 it is plausible that yet unknown microbial metabolites derived from crocins and crocetin, or changes in the fecal metabolome derived from the interaction between these compounds and the intestinal bacteria may be involved in the neurocognitive effects of these compounds. In this sense, analysis of fatty acids is of particular interest because they are key microbial metabolites to know bacteria activity and their production, especially of short-chain fatty acids (SCFAs) have been related to many health benefits, including neuroprotective activities.19 Besides, the similarity of fatty acids with the structure of the crocetin molecule suggests that they could be produced from crocetin after decarboxylation reactions. However, the knowledge about all important metabolic changes produced by the interaction of saffron carotenoids with the gut microbiota is still scarce, especially in humans.
Overall, the interaction of carotenoids with gut microbiota in the colon is an important gap in carotenoids research. The potential interaction of carotenoids with the gut microbiota has been generally overlooked but may be of relevance, as carotenoids largely bypass absorption in the small intestine and are passed on to the colon.20 Some studies have indicated that the intestinal microbiota may be a major factor underlying the effectiveness of carotenoids’ beneficial effects.21
The main aim of this work was to advance in the investigation of the role of the human gut microbiota in the metabolism of crocins and crocetin using in vitro human fecal fermentation and different analytical methodologies. The specific objectives were: (1) further identify new metabolites potentially derived from these compounds; (2) detect changes in the composition and levels of fecal fatty acids as a mean to detect changes in the bacterial catabolic activity.
2. Materials and methods
2.1. Materials
Crocin-1 (crocetin digentiobioside ester, trans-4-GG) (>95% purity) (PHL80391) and trans-crocetin (>98% purity) (SML3255) were purchased from Sigma-Aldrich (St Louis, MO, USA). Standard stock solutions 1.7 mM of crocin-1 were prepared in water/DMSO (60/40, v/v) and 3 mM of crocetin in DMSO. Chrysin also from Sigma-Aldrich (St Louis, MO, USA) was used as internal standard and the standard stock solution was prepared in methanol. Standards of short chain fatty acids (SCFAs) (acetic, propionic, butyric, isobutyric, valeric, isovaleric and 2-ethyl butyric acid (IS)) and FAME (Fatty Acids Methyl Ester) Mix 37 components were purchased from Sigma-Aldrich (St Louis, MO, USA). Fermentation medium to grow anaerobes (anaerobe basal broth, ABB) and Nutrient Broth were from Oxoid (Basingstoke, Hampshire, UK) and L-cysteine hydrochloride from Panreac Química (Barcelona, Spain). DMSO, methanol, 0.1% formic acid in water, acetonitrile, hexane and ethyl acetate were supplied from Scharlab (Barcelona, Spain) methyl tert-butyl ether from Sigma-Aldrich and formic acid from Honeywell (Barcelona, Spain). Water was deionized using a Milli-Q-system (Millipore, Bedford, MA, USA).2.2. Gut microbiota incubations
Colonic fermentations were carried out using human fecal samples from two healthy volunteers, a 42-year-old woman with BMI 22 kg m−2 and a 45-year-old man with BMI 24.6 kg m−2. They followed a normal diet avoiding the consumption of saffron at least three days before donating the samples and declared not having ingested antibiotics for at least 3 months before sample collection. In previous works these two volunteers had showed differences in the gut microbiota composition related to differences in the metabolism of other bioactive compounds.22 All experiments were performed in accordance with the ethical guidelines outlined in the Declaration of Helsinki and approved by the CSIC ethics committee. Informed consents were obtained from human participants of this study. Preparation of fecal suspension and subsequent culturing experiments were conducted under anoxic conditions in an anaerobic chamber (Don Whitley Scientific Limited, Shipley, UK) with an atmosphere consisting of N2/H2/CO2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 37 °C. Fecal samples (10 g) were diluted 1/10 (w/v) in Nutrient Broth supplemented with 0.06% L-cysteine hydrochloride and homogenized by stomacher in filter bags.
10) at 37 °C. Fecal samples (10 g) were diluted 1/10 (w/v) in Nutrient Broth supplemented with 0.06% L-cysteine hydrochloride and homogenized by stomacher in filter bags.
In a first experiment (Fig. 1), to know more about the kinetics of transformation of both apocarotenoids, fecal inocula from two volunteers (1%, 800 μL) were independently incubated in a fermentation medium to grow anaerobes (80 mL) with 10 μM of crocin-1 or 10 μM of crocetin. The amount of fecal inoculum used (1% of a stool solution previously diluted 1/10) with around 109 cfu g−1 had already been shown to provide optimal conditions for studying the human microbial metabolim of bioactive compounds.22 The concentration of apocarotenoids (10 μM) was chosen based on the information available in literature,13,14 taking into account the extraction protocol and the sensitivity of the analytical method used and not exceeding 0.5% DMSO in the medium. Two jars only with crocetin or crocin-1 and medium were incubated as control. The jars were incubated in anaerobic chambers at 37 °C to mimic colon conditions and 2.5 mL of samples were collected at 0, 1, 2, 4, 6, 24, 48 and 120 h. Three replicates were taken at each time point.
In a second experiment, to study the transformation kinetics of crocetin derived metabolites and other gut microbial metabolites, a fecal inoculum from one of the volunteers (1%, 2.25 mL) was inoculated into the fermentation medium to grow anaerobes (225 mL) and incubated with 10 μM of crocetin (750 μL of standard stock 3 mM) for a longer period of time, i.e. 0, 6, 24, 30, 48, 54, 72, 78, 120 and 240 h. Two jars were prepared as controls: fermentation medium with fecal inoculum but without crocetin and fermentation medium with crocetin but in the absence of bacteria. Three replicates were again taken at each time point.
2.3. Quantitation of crocins and crocetin by HPLC-DAD-ESI IT MS
In the first experiment, 2.5 mL of medium obtained after incubation were extracted with 5 mL of ethyl acetate in a thermoblock with agitation for 10 min at room temperature. Samples were centrifuged at 3500g and 4 °C for 10 min (Thermo Scientific™ Sorvall™ ST 16, Germany.) The supernatants (4 mL) were evaporated in a speed vacuum concentrator (Savant SPD121P, ThermoScientific, Alcobendas, Spain), re-dissolved in 200 μL of methanol with 0.1 μM of IS (chrysin) and filtered through a 0.22 μm 13 mm PVDF filter (Sharlab, Barcelona, Spain) before injection. This fraction mainly contained crocetin (recovery percentage 89 ± 3%). In the samples incubated with crocin-1 due to the more polar character of this molecule, it was necessary to apply a different extraction protocol. The remaining aqueous fraction after removing the ethyl acetate layer was passed through a Sep-Pak C18 cartridge (Chromafix C18) size L (Machery-Nagel, Düren, Germany) previously conditioned with 10 mL of methanol and then with 10 mL of water. Crocin-1 and other crocins were eluted with 2 mL of methanol that was filtered through a 0.22 μm filter before injection.Samples were injected in an Agilent 1100 HPLC system equipped with a photodiode array detector (G1315D) (Agilent Technologies, Waldbronn, Germany) and coupled in series to a HCT ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany) through an electrospray ionization (ESI) interface (HPLC-DAD-ESI-MS/MS (IT)). The chromatographic separation was achieved using a reversed-phase C18 Poroshell column, 100 mm × 3 mm, and 2.7 μm particle size (Agilent Technologies). The method used was a binary gradient, A (water/formic acid, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)) and B (acetonitrile), settled in the following gradients: 0 min, 3% B; 20 min, 50% B; 29 min, 90% B; 31 min, 90% B; 32 min, 3% B; 35 min, 3% B. The flow rate was 0.4 mL min−1, the injection volume was 10 μL, and the column temperature was settled at 25 °C. The UV-Vis spectra were acquired in the range of 200 to 600 nm and the chromatograms were registered at 430 nm. In the mass spectrometer, nitrogen was used as drying, nebulizing and collision gas. The ESI parameters were: nebulizer pressure 65 psi, dry gas flow 11 L min−1 and dry gas temperature 350 °C. The capillary voltage was set at 4 kV and spectra were acquired in negative ionization mode in the range of m/z 100–1200, and target mass 327. Automatic MS/MS mode was applied with fragmentation amplitude 1 V and number of parents, 3. Carotenoids (crocins and crocetin) were identified by their UV spectra, retention time, molecular weight and MS/MS fragmentation pattern and were quantified in UV at 430 nm using calibration curve of the authentic standards.
1 (v/v)) and B (acetonitrile), settled in the following gradients: 0 min, 3% B; 20 min, 50% B; 29 min, 90% B; 31 min, 90% B; 32 min, 3% B; 35 min, 3% B. The flow rate was 0.4 mL min−1, the injection volume was 10 μL, and the column temperature was settled at 25 °C. The UV-Vis spectra were acquired in the range of 200 to 600 nm and the chromatograms were registered at 430 nm. In the mass spectrometer, nitrogen was used as drying, nebulizing and collision gas. The ESI parameters were: nebulizer pressure 65 psi, dry gas flow 11 L min−1 and dry gas temperature 350 °C. The capillary voltage was set at 4 kV and spectra were acquired in negative ionization mode in the range of m/z 100–1200, and target mass 327. Automatic MS/MS mode was applied with fragmentation amplitude 1 V and number of parents, 3. Carotenoids (crocins and crocetin) were identified by their UV spectra, retention time, molecular weight and MS/MS fragmentation pattern and were quantified in UV at 430 nm using calibration curve of the authentic standards.
2.4. UPLC-ESI-QTOF analysis and untargeted metabolomics approach
Samples from the first experiment incubated with crocetin at times 0, 24 and 120 h and extracted with ethyl acetate as described in the section 2.3 were also analyzed using an Agilent 1290 Infinity LC system coupled to the 6550 Accurate-Mass Quadrupole time-of-flight (QTOF) (Agilent Technologies, Waldbronn, Germany) using an electrospray interface (Jet Stream Technology). The chromatographic conditions were the same described previously but using water with 0.1% formic acid as phase A and acetonitrile with 0.1% formic acid as phase B and 5 μL of injection. The optimal conditions of the electrospray interface were as follows: gas temperature 280 °C, drying gas 11 L min−1, nebulizer 45 psi, sheath gas temperature 400 °C and sheath gas flow 12 L min−1. Spectra were acquired in the m/z range 100–1100 in negative mode, and fragmentor voltage was 100 V. MS/MS product ion spectra were collected at a m/z range of 50–800 using a retention time window of 1 min, a collision energy of 10, 20 and 40 V and an acquisition rate of 4 spectra per s.Data were acquired in both centroid and profile mode. Raw data acquired was converted to .abf data file and then was processed by MS-DIAL 5.1.2 (prime.psc.riken.jp/compms) for creating the data matrices. The features extraction parameters were set in order to cover the complete metabolome of the samples. Data collection was set at 0.01 Da of MS1 tolerance and the peak detection parameters included a minimum peak height of abundance 1000 and a mass slide with 0.1 Da. Regarding to the smoothing method the linear Weighted moving average with a smoothing level of 3 scans. Then the data matrices were exported to Metaboanalyst platform (metaboanalayst.ca, Xia Lab) for evaluation and creation the multivariate models by partial least square discriminant analysis (PLS-DAD). Both missing values and abundance filters and log transformation and autoscaling were applied prior to creating the multivariate models. Additionally, and after the evaluation of the multivariate models, a featured extraction parameters based on the in-house database built for crocins and crocetin were applied. This step allowed the tentative identification of new molecules derived from crocetin. After data processing, the crocetin derivatives identified were analyzed by Mass Hunter Qualitative 10.0 qualitative (Version B.10.0, Agilent software metabolomics, Agilent Technologies, Waldbronn, Germany).
2.5. Analysis of crocetin-derived metabolites
Samples from the second experiment were extracted with ethyl acetate as described in section 2.3 and were analyzed with UPLC-ESI-QTOF as described in section 2.4. A target screening strategy was applied to identify the metabolites previously identified and to search for the potential biotransformation products. The screening was based on mass filtering at the exact mass of the compound investigated using narrow mass extraction windows (0.01 m/z). The identification was based on accurate mass, isotopic pattern, MS/MS fragmentation patterns, elution order.2.6. Analysis of fatty acids
Samples from the second experiment incubated at time 0, 6, 24, 48 and 120 h were chosen for the analysis of fatty acids: long, medium and short-chain fatty acids.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C. Organic phase was collected and 50 μL was transferred to an insert in a vial for the injection in the GC-MS. For the analysis of SCFAs we used a GC-MS consisted of an Agilent 7890A (Agilent Technologies, Santa Clara, CA, USA) equipped with an automatic liquid sampler (MPS2; Gerstel, Mülheim, Germany) and coupled with an Agilent 5975C mass selective detector using a previously published method.23
000g at 4 °C. Organic phase was collected and 50 μL was transferred to an insert in a vial for the injection in the GC-MS. For the analysis of SCFAs we used a GC-MS consisted of an Agilent 7890A (Agilent Technologies, Santa Clara, CA, USA) equipped with an automatic liquid sampler (MPS2; Gerstel, Mülheim, Germany) and coupled with an Agilent 5975C mass selective detector using a previously published method.23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). After 10 min in a thermoblock at 4 °C with agitation, samples were centrifuged at 3500g and 4 °C for 10 min, 3 mL were evaporated and the residue was subjected to a methyl esterification. For this, 3 mL of methanolic HCl (10%) were added to the lipid extract and were heated at 90 °C for 2 h after vigorous shaking. After cooling to room temperature 1 mL of n-hexane was added. The mix was vortexed and centrifuged at 3500g and 4 °C for 10 min. The organic phase was transferred to an amber vial with 10 mg of sodium sulfate to eliminate water residues and after centrifugation the recovered hexane was put into an insert vial for the injection. For the analysis of FAME gas chromatograph was fitted with a high-polarity, polyethylene glycol, fused silica capillary column DB-WAXetr (30 m, 0.25 mm id, 0.25 mm film thickness; Agilent Technologies), and helium was used as the carrier gas at 1 mL min−1. The injection volume was 1 μL and the injection temperature 250 °C. The column temperature was initially 90 °C, then increased to 150 °C at 15 °C min−1, to 170 °C at 5 °C min−1, and finally to 250 °C at 20 °C min−1 and kept at this temperature for 2 min (total time 14 min). Solvent delay was 3.5 min. The detector was operated in electron impact ionization mode (electron energy 70 eV), scanning the m/z 30–250 range. The temperatures of the ion source, quadrupole, and interface were 230, 150, and 280 °C, respectively.
v). After 10 min in a thermoblock at 4 °C with agitation, samples were centrifuged at 3500g and 4 °C for 10 min, 3 mL were evaporated and the residue was subjected to a methyl esterification. For this, 3 mL of methanolic HCl (10%) were added to the lipid extract and were heated at 90 °C for 2 h after vigorous shaking. After cooling to room temperature 1 mL of n-hexane was added. The mix was vortexed and centrifuged at 3500g and 4 °C for 10 min. The organic phase was transferred to an amber vial with 10 mg of sodium sulfate to eliminate water residues and after centrifugation the recovered hexane was put into an insert vial for the injection. For the analysis of FAME gas chromatograph was fitted with a high-polarity, polyethylene glycol, fused silica capillary column DB-WAXetr (30 m, 0.25 mm id, 0.25 mm film thickness; Agilent Technologies), and helium was used as the carrier gas at 1 mL min−1. The injection volume was 1 μL and the injection temperature 250 °C. The column temperature was initially 90 °C, then increased to 150 °C at 15 °C min−1, to 170 °C at 5 °C min−1, and finally to 250 °C at 20 °C min−1 and kept at this temperature for 2 min (total time 14 min). Solvent delay was 3.5 min. The detector was operated in electron impact ionization mode (electron energy 70 eV), scanning the m/z 30–250 range. The temperatures of the ion source, quadrupole, and interface were 230, 150, and 280 °C, respectively.
3. Results
3.1. Transformation kinetics of crocin-1 and crocetin
The transformation kinetics of crocin-1 in the control incubation and in both volunteers is shown in Fig. 2. It can be already observed in Fig. 2A (control) that the total amount of crocin-1 added to the medium (10 μM) was not completely recovered at time 0. Indeed, the HPLC-UV chromatogram obtained at time 0 (Fig. 3A) shows crocin-1 (trans-4-GG) as the main compound (peak 1, tr 13.9 min) but also the presence of other peak at longer retention time (18.1 min) that correspond to the cis isomer (cis-4-GG) and smaller peaks (peaks 2 and 3) corresponding to: crocetin gentiobiosylglucosyl ester (trans-3-Gg) and crocetin diglucosyl ester (trans-2-gg) (structures information in Fig. 4A and Table 1). We checked that the original standard of crocin-1 was pure so, this cis–trans isomerization and slight hydrolysis probably occurred during the extraction protocol. It has been reported that the cis–trans isomerization can be induced by light and temperature.24 Total crocins (sum of all crocins) and crocin-1 were quite stable in the absence of bacteria throughout the incubation period and the ratio crocin-1/total crocins remained the same, which indicates that there was no transformation between them during the incubation period without bacteria (Fig. 2A). Only a decrease in the total crocins and specially in crocin-1 was observed at the end of the study (5 days of incubation), showing the instability of these compounds at this incubation time in the absence of bacteria. | ||
| Fig. 3 HPLC-UV chromatograms at 430 nm of fecal fermentations (volunteer 1) of crocin-1 at different times (A) time 0, SPE extraction (B) time 4 h, SPE extraction (C) time 6 h, SPE extraction (D) time 6 h, ethyl acetate extraction. Numbers correspond to compounds in Table 1 and Fig. 4. 1: trans-4-GG, 2: trans-3-Gg, 3: trans-2-gg; 4:cis-4-GG; 5:trans-2-G; 6: trans-1-g; 7: cis-2-gg, 8: cis-2-G; 9: cis-2-G; 10:cis-1-g, 11:cis-1-g; 12: trans-crocetin; 13: cis-crocetin. | ||
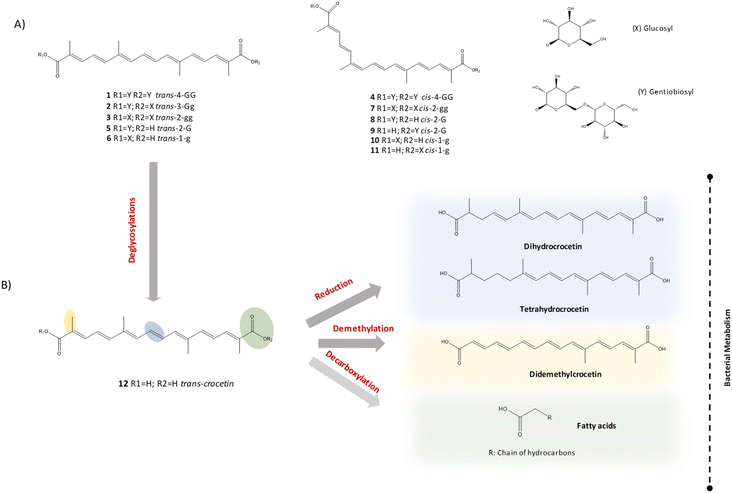 | ||
| Fig. 4 Structures of the different types of crocins identified in the study (A), possible transformation route of crocins and crocetin (B). Nomenclature of crocins was based on the proposal of Carmona et al.,25 the first part describes the cis/trans form of the aglycon part, followed by the total number of sugar moieties, and finally, the type of sugar in each part of the crocin structure. Namely, G refers to gentiobiose, g, to glucose and H to hydrogen. The dashed arrow indicated proposed but not totally confirmed metabolic pathways. | ||
| Number | Compounda | Retention time (min) | [M − H]− | MS/MS |
|---|---|---|---|---|
| a Nomenclature of crocins was based on the proposal of Carmona et al.,25 the first part describes the cis/trans form of the aglycon part, followed by the total number of sugar moieties, and finally, the type of sugar in each part of the crocin structure. Namely, G refers to gentiobioside, and g to glucose. | ||||
| 1 | trans-4-GG | 13.97 | 533 (z = 2) | 975, 697, 651, 510, 323 |
| 2 | trans-3-Gg | 15.06 | 452 (z = 2) | 813, 651, 535, 489, 323 |
| 3 | trans-2-gg | 16.34 | 697 (651 + 46) | 651, 489, 327 |
| 4 | cis-4-GG | 18.11 | 976 | 651, 489, 327 |
| 5 | trans-2-G | 18.27 | 651 | 327, 283 |
| 6 | trans-1-g | 20.05 | 489 | 446, 327, 283, 239 |
| 7 | cis-2-gg | 20.77 | 651 | 489, 327 |
| 8 | cis-2-G | 21.70 | 651 | 327, 283, 239 |
| 9 | cis-2-G | 22.03 | 651 | 327, 283, 239 |
| 10 | cis-1-g | 23.82 | 489 | 446, 327, 283, 239 |
| 11 | cis-1-g | 24.06 | 489 | 446, 327, 283, 239 |
| 12 | trans-Crocetin | 24.71 | 327 | 283, 239 |
| 13 | cis-Crocetin | 27.32 | 327 | 283, 239 |
In the presence of bacteria, both crocin-1 and total crocins rapidly disappeared from the medium within the first 6 hours of incubation. The behavior during these first hours was a bit different between the two volunteers. The decrease was faster in volunteer 2 that showed lower disappearance half-life time of total crocins (3.2 h) than volunteer 1 (4.78 h) and also lower concentrations of total crocins after 4 hours (2.03 μM in volunteer 2 vs. 8.12 μM in volunteer 1) (Fig. 2B and C). In volunteer 1, the ratio crocin-1/total crocins decreased with the incubation time reaching very low values at 4 hours which indicates the transformation of crocin-1 into other intermediate crocins by the gut microbiota. Fig. 3B shows the chromatogram of this volunteer after 4 h of incubation and we can observe many different crocins (Table 1) with different degrees of glycoslylation obtained from crocin-1 (structures in Fig. 4A). Identification of crocins was performed comparing the UV spectra and the retention time of the peaks with literature and MS data.25,26 The main compounds in the chromatogram (peaks 2, 3, 5 and 6) were the trans-isomers obtained by the gradual loss of different glucose molecules from the crocin-1 (trans-4-GG). Smaller peaks with longer retention times (peaks 8, 9, 10, 11) were the corresponding cis-isomers probably produced from the previous ones during the extraction protocol.
At the same time that the crocins begin to disappear (around 4 hours), crocetin starts to appear reaching a maximum concentration in both volunteers around 6 hours, where a complete transformation of crocins into crocetin was observed. HPLC-UV chromatograms at 6 h of incubation (Fig. 3C and D) shows the complete disappearance of crocins and the appearance of crocetin. As occurred with crocins, the main peak corresponds to trans-crocetin, the natural form of the carotenoids, but a small percentage (around 10%) was transform to the cis-isomer. After this time, crocetin decreased with the incubation time indicating further metabolization. This transformation was further confirmed in the incubations with crocetin (Fig. 5). Crocetin was very stable throughout the incubation time in the absence of bacteria but a rapid decrease was observed between 6 and 24 hours in the fecal samples incubation. After 120 h less than 25% of crocetin was detected in the medium, clearly indicating its transformation by the gut microbiota. Considering these results, we studied the formation of new microbial metabolites derived from crocetin.
 | ||
Fig. 5 Kinetic of transformation of crocetin by the human gut microbiota in vitro.  Control (medium with crocetin), Control (medium with crocetin),  volunteer 1, volunteer 1,  volunteer 2. Each value represents the mean ± SD of 3 replicates. volunteer 2. Each value represents the mean ± SD of 3 replicates. | ||
3.2. Untargeted metabolomics approach after fecal fermentation with crocetin
Samples from the first experiment incubated with crocetin at three times (0, 24 and 120 h), were chosen to be analyzed by UPLC-ESI-QTOF and an untargeted approach was applied.From the full data set based on 36 samples (3 times × (2 volunteers with crocetin + 2 volunteers without crocetin) × 3 replicates), a total of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 097 ions were found and used for creating the data matrix. After filtering by abundance and RSD to remove uninformative features, 25
097 ions were found and used for creating the data matrix. After filtering by abundance and RSD to remove uninformative features, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 ions were selected for modelling by PLS-DA. The multivariate model created based on 5 components presented a fit of the model of 99.7% (R2 = 0.997) and a prediction value of 75.1% (Q2 = 0.751). The score plot of the PLS-DA model based on two components (Fig. 6) explained the 37.2% of the total variability of the data.
419 ions were selected for modelling by PLS-DA. The multivariate model created based on 5 components presented a fit of the model of 99.7% (R2 = 0.997) and a prediction value of 75.1% (Q2 = 0.751). The score plot of the PLS-DA model based on two components (Fig. 6) explained the 37.2% of the total variability of the data.
The major source of variation was the incubation time explained by component 1 and showed in Fig. 6 with different colors. The highest differences were observed between time 0 (negative values, on the left) and times 24 and 120 h (positive values, on the right). In addition, we found differences in component 2 scores between control samples and samples incubated with crocetin at both times 24 and 120 h. The impact in the metabolome of the samples incubated with crocetin was explained by the component 2: control samples appear in the upper part (positive values) and samples incubated with crocetin in the bottom (negative values). So, the incubation with crocetin seems to affect the fecal metabolome of both volunteers. In view of these results, an untargeted strategy was applied by searching for new metabolites that only appeared after incubation of crocetin with fecal bacteria and that could be derived from the crocetin molecule. We applied different filters to consider only those molecules that did not appear at time 0 and were found at time 24 and 120 h. We only considered metabolites that were absent in the control samples to eliminate those molecules that could come from the degradation of crocetin in the absence of bacteria or from the bacteria activity in the absence of substrate. Using these filters and applying featured extraction parameters based on the in-house database built for crocins and crocetins with more than 180 possible derived metabolites, more than 400 compounds were tentatively identified as originated in the medium because of the presence of crocetin. An exhaustive evaluation of the data was done using exact mass, isotopic distribution and MS/MS fragmentation patterns. The final list of candidates was filtered and classified by intensity at any time sampling point, and those up to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 counts were passed to further MS/MS analysis. The experimental MS/MS data set and the in-house database SMILES of the candidates were used to compare the fragmentation with the Competitive Fragmentation Modelling for Metabolite identification (CFM-ID 4.0). With all this valuable information we could identify new metabolites derived from crocetin (Table 2). The main reactions detected were reduction of the double bonds and demethylations (Fig. 4B). Six new compounds derived from the reduction of the double bonds of crocetin were identified and confirmed by MS/MS, including three isomers of dihydrocrocetin and other three of tetrahydrocrocetin. In reverse phase chromatography these compounds were separated by chain length and the degree of unsaturation. Dihydrocrocetin and tetrahydrocrocetin showed less polarity and longer retention time than crocetin because, in general, the double bonds reduce the retention time in reverse phase chromatography. The MS/MS profile of dihydrocrocetin was characterized by two fragments that corresponded to the loss of one carboxylic acid (−44) or both (−88) (Fig. 7). The same behavior was observed for crocetin (the original molecule). Besides, this family of dicarboxylic acids suffer spontaneous in-source fragmentation so, their characteristic fragments could be identified in the MS scan without applying MS/MS. In the case of tetrahydrocrocetin isomers, only one of the fragments was identified due to a low signal intensity. The same happened with another compound tentatively identified as di-demethyl crocetin obtained in samples incubated with bacteria after demethylation reactions. This metabolite showed a shorter retention time than crocetin due to the loss of the two non-polar methyl groups. Using the in-house database we searched for other dicarboxylic acids considering all possible combinations of double bound reductions and demethylations of the crocetin molecule but the ions obtained did not reach the minimum score value in the assignment of the correct molecular formula or in the isotopic distribution or did not have enough intensity to be confirmed by MS/MS.
000 counts were passed to further MS/MS analysis. The experimental MS/MS data set and the in-house database SMILES of the candidates were used to compare the fragmentation with the Competitive Fragmentation Modelling for Metabolite identification (CFM-ID 4.0). With all this valuable information we could identify new metabolites derived from crocetin (Table 2). The main reactions detected were reduction of the double bonds and demethylations (Fig. 4B). Six new compounds derived from the reduction of the double bonds of crocetin were identified and confirmed by MS/MS, including three isomers of dihydrocrocetin and other three of tetrahydrocrocetin. In reverse phase chromatography these compounds were separated by chain length and the degree of unsaturation. Dihydrocrocetin and tetrahydrocrocetin showed less polarity and longer retention time than crocetin because, in general, the double bonds reduce the retention time in reverse phase chromatography. The MS/MS profile of dihydrocrocetin was characterized by two fragments that corresponded to the loss of one carboxylic acid (−44) or both (−88) (Fig. 7). The same behavior was observed for crocetin (the original molecule). Besides, this family of dicarboxylic acids suffer spontaneous in-source fragmentation so, their characteristic fragments could be identified in the MS scan without applying MS/MS. In the case of tetrahydrocrocetin isomers, only one of the fragments was identified due to a low signal intensity. The same happened with another compound tentatively identified as di-demethyl crocetin obtained in samples incubated with bacteria after demethylation reactions. This metabolite showed a shorter retention time than crocetin due to the loss of the two non-polar methyl groups. Using the in-house database we searched for other dicarboxylic acids considering all possible combinations of double bound reductions and demethylations of the crocetin molecule but the ions obtained did not reach the minimum score value in the assignment of the correct molecular formula or in the isotopic distribution or did not have enough intensity to be confirmed by MS/MS.
| Compound | Formula | m/z | ppm | Rt | MS/MS fragments (neg polarity) | Collision energy (eV) |
|---|---|---|---|---|---|---|
| Dihydrocrocetin 1 | C20H26O4 | 329.1751 | −2.08 | 22.8 | 285.1867;241.1962 | 10 |
| Dihydrocrocetin 2 | C20H26O4 | 329.1750 | −1.02 | 23.47 | 285.1863;241.1962 | 10 |
| Dihydrocrocetin 3 | C20H26O4 | 329.1750 | −1.63 | 23.87 | 285.1864;241.1965 | 10 |
| Tetrahydrocrocetin 1 | C20H28O4 | 331.1911 | −2.73 | 23.7 | 287.2024 | 10 |
| Tetrahydrocrocetin 2 | C20H28O4 | 331.1907 | 0.75 | 24.08 | 287.2020 | 10 |
| Tetrahydrocrocetin 3 | C20H28O4 | 331.1913 | −0.72 | 24.37 | 287.2021 | 10 |
| Dedimethylcrocetin | C18H20O4 | 299.1288 | −3.55 | 13.69 | — | — |
3.3. Transformation kinetics of crocetin derivatives with a dicarboxylic structure
The transformation kinetics of the new identified metabolites derived from crocetin were studied in a second experiment incubating crocetin with a larger number of sampling points at longer times (Fig. 8). The reduced metabolites (dihydro and tetrahydro derivatives) began to appear at 24 h coinciding with the decrease of crocetin in the medium. Their values increased over time reaching the maximum between 54 and 78 h depending on the compound and remaining constant after 78 hours. A different behavior was observed for the demethylated compound that appeared later, sometime between 120 and 240 hours of incubation.3.4. Changes in fatty acids
We also studied the possibility of finding decarboxylated metabolites from crocetin. The decarboxylation and chain shortening of crocetin molecule could produce fatty acids with different chain lengths (Fig. 4B). To study changes in fatty acids, samples at time 0, 6, 24, 48 and 120 h from the second experiment (incubation with crocetin) were subjected to different analytical methodologies. Medium and long chain fatty acids were analyzed by GC-MS after extraction and methyl esterification of the samples and four compounds were identified: tridecanoic acid 12-methyl-methyl ester, hexadecanoic acid methyl ester, octadecanoic acid methyl ester, and 13-docosenoic acid methyl ester (ESI Fig. 1†). These compounds were already present in the medium at time 0 and their concentration was modified with the incubation time in the presence of fecal bacteria. However, no significant differences were found when crocetin was added to the medium (data not shown).SCFAs were also analyzed by GC-MS. These SCFAs were analyzed for two reasons: (1) they could be directly derived from the crocetin molecule after reactions of decarboxylation, reduction and degradation that include aliphatic-chain shortening reactions and (2) these SCFAs are one of the main microbial metabolites produced by gut bacteria, so changes in these SCFAs could indicate changes in the microbial populations due to the interaction with crocin and crocetin. No significant changes were found in the smallest fatty acids (acetic, propionic, butyric and isobutyric) between the samples incubated with and without crocetin but a clear statistically significant increase was observed in fatty acids with 5 and 6 carbon atoms (valeric acid and hexanoic acid) (ESI Fig. 2†). After 48 and 120 h a higher concentration of these metabolites was observed after the incubation with crocetin.
4. Discussion
In this paper we have described for the first time the transformation kinetics of the saffron apocarotenoids, crocin and crocetin, by human gut microbiota. Only two in vitro studies with animal fecal samples have been previously reported.11,14 We herein first time report and confirm that crocin-1 (a digentiobiosyl ester of crocetin) (Fig. 2B and C) was totally transformed by the human gut microbiota in less than 6 hours into crocetin (the aglycone form containing an apocarotenoid dicarboxylic acid). This fast conversion of crocin-1 into crocetin was previously reported in in vitro incubations with gut content of rats adding 2 μM of crocin or crocetin and incubating in anaerobic conditions for 2 hours,14 but this is the first time that these results are observed with humans. In another work, an 80% reduction of a mixture of crocins was observed in the incubation for 5 hours of mice fecal homogenate with saffron extracts but no presence of crocetin was detected.11 They attributed the decline of crocins to fecal metabolism towards backbone-cleaved products with smaller alkyl units that are not detectable at 440 nm. They did not indicate the formation of any specific degradation products. The deglycosylation is a common reaction of the intestinal bacteria which show extensive metabolic capacity, primarily by β-glucosidase enzymes for the hydrolysis of glycosidic bonds. This reaction has been extensively reported in the family of polyphenols because most of them exist in foods as glycosylated forms. They cannot be absorbed in these native forms and are hydrolyzed by the gut microbiota.27,28 A gradual deglycosylation of crocin-1 (trans-4-GG) was observed, especially in volunteer 1. After 4 hours of incubation a series of intermediate crocins (trans-3-Gg, trans-2-gg, trans-2-G and trans-1-g) resulting from the sequential loss of different glucose molecules was identified (Table 1). However, this transformation was fast and the metabolites did not remain in the medium for a long time. This means that different crocin patterns could be present in the colon at some time after ingestion where they could exert local effects and affect the microbiota-gut–brain crosstalk. These intermediate metabolites were identified for the first time in this study. In the paper reported by Lautenschläger et al., the incubation of saffron extracts with mouse feces did hardly change the crocin pattern, but in this case the incubation conditions were different.11 Once generated in the colon, crocetin could be bioavailable. The presence of higher concentration of crocetin in urine and plasma of control rats consuming crocin compared with germ free rats and rats treated with antibiotics indicate that crocetin is produced and absorbed in the colon.14,15 Besides, other studies have shown in vitro permeation of crocetin through intestinal barrier by passive transcellular diffusion.11After the complete deglycosylation of crocin-1 to trans-crocetin, the catabolic activity of bacteria continued metabolizing the crocetin molecule but at longer times, between 6 and 24 hours. After 24 hours of incubation less than 30% of crocetin was detected in the medium. This decrease of crocetin in the medium with bacteria was also previously reported in rats14 but a lower transformation rate (around 50%) was observed probably because of the shorter incubation times used (2 hours). However, they did not investigate the presence of other metabolites.
The use of untargeted metabolomics, applying multivariate analysis and considering the complete metabolome, allowed to discriminate between the samples incubated with and without crocetin, although the incubation time was the most discriminant parameter. These results indicate that the incubation with crocetin seems to affect the fecal metabolome produced by bacteria in both volunteers. Fecal metabolomics provided important information of gut microbial metabolism and its interaction with environmental factors, such as dietary intake, although there is a considerable unexplored potential.29 These results open the possibility of further studies to identify possible pathways related to neuroprotective effects affected by the presence of crocetin.
In this work we focused on the identification of crocetin-derived metabolites. After an exhaustive analysis of the untargeted metabolomics data, different dicarboxylic acids derived from crocetin were identified: three isomers of dihydrocrocetin, three isomers of tetrahydrocrocetin and di-demethylcrocetin. There is still no evidence of its possible absorption in the colon and presence in plasma and urine. Future human intervention studies will be needed to shed light to these questions. The reduction of the double bonds and demethylations were the main metabolic reactions identified on the crocetin molecule. Particular fragmentation profiles of these dicarboxylic acids were observed, sequentially losing both carboxylic groups. These molecules were fragmented under the conditions of the ionization source. This may help to find biomarkers derived from crocetin in further untargeted metabolomics studies only using MS data. Reductions of double bonds and demethylation are common reactions of the intestinal bacteria together with decarboxylation, dehydroxylation or chain shortening.30 Other bioactive compounds are subjected to reduction reactions by the gut microbiota: curcumin that is reduced to dihydrocurcumin and tetrahydrocurcumin by the highly expressed NADPH-dependent curcumin/dihydrocurcumin reductase (CurA) of E. coli DH10B,31 daidzein that is reduced to dihydrodaidzein by Lactobacilluss p. Niu-016 with daidzein reductase activity,32 or resveratrol that is reduced to dihydroresveratrol by Slackia equolifaciens and Adlercreutzia equolifaciens.33 Double bond hydrogenation was also the main catabolic reaction observed in the microbial metabolism of sesquiterpene lactones, terpenoid phytochemicals present in lettuce, endive and escarole.34 Demethylation is also a common gut microbial reaction and compounds such as hesperetin, xanthohumol, dimethoxyflavone and methoxylated isoflavonoids can undergo demethylation reaction under the action of gut microbes.30 As an example, formononetin and biochanin A udergo demethylation to produce daidzein and genistein under the action of E. limosum ATCC 8486.35 The specific microorganisms and enzymes involved in crocetin metabolism should be investigated in the future to fully understand the apocarotenoids metabolism and their impact in human health. We could expect the presence of other metabolites resulting from the reduction of the different double bonds (up to 7) or demethylation of the different methyl groups (up to 4) present in the crocetin molecule. However, although masses corresponding to these compounds were detected we were unable to definitely confirm the identity of these metabolites with the available data. Future studies with higher concentrations and with larger number of volunteers could be proposed to explore the presence of other metabolites of this family. These results are pioneers in the study of carotenoids colonic fermentation, a topic that still lacks information and clear evidence.21,36 Recently, in vitro fermentation of β-carotene with human fecal samples showed its transformation into retinol and a further hydrolyzation into retinoic acid by human gut microbiota.37
Apart from the dicarboxylic acid derivatives we also studied the possibility of finding decarboxylated metabolites from crocetin. Decarboxylation reactions represent another of the biotransformation capabilities of the gut microbiota. A well-known example of such reaction involves the decarboxylation of L-dopa to produce dopamine or the decarboxylation of aromatic amino acids to produce aromatic amines.38 The decarboxylation and chain shortening of crocetin molecule could produce fatty acids with different chain lengths. Using different GC-MS methodologies we analyzed short, medium and long chain fatty acids after the incubation with crocetin. In this case, we did not expect to find unique metabolites derived from crocetin because fatty acids are metabolites commonly present in fecal fermentations. No differences were observed in medium and long chain fatty acids (tridecanoic acid 12-methyl, hexadecanoic acid, octadecanoic acid and 13-docosenoic acid) as a response to the incubation with crocetin. Regarding short chain fatty acids, only larger amounts were observed in valeric acid and hexanoic acid (caproic acid) in the presence of crocetin. However, it is not possible to conclude that these compounds derived from crocetin molecule after decarboxylation and chain shortening reactions because they are also the main metabolites produced in the colon by bacterial fermentation of undigested carbohydrates. In any case the presence of crocetin seems to modulate the presence of this SCFAs. Although less studied than the more abundant acetic, propionic and butyric acids, they have also been shown to exert important physiological effects (regulation of metabolic, endocrine and immune functions, prevention of inflammation, maintenance of gut integrity),39,40 including their key role in central nervous sytem.41 In particular, valeric and caproic acid have demonstrated anti-inflammatory properties,42 activity as a potent histone deacetylase inhibitor (HDI)43 and modulation of brain functions.44 Knowing whether these metabolites derive from the crocetin molecule or are simply modulated by it through changes in gut microbial populations requires further research. Gut microbiota composition is associated to health effects, so studying these potential changes in gut microbiota abundance and diversity as a consequence of the interaction with these apocarotenoids and their metabolites is also an important and little explored issue17 to be considered in future studies.
5. Conclusions
This work has given further and strong evidence of the role of human gut microbiota in the metabolism of the saffron carotenoids, crocin and crocetin. We report for the first time, the rapid and total transformation of crocin-1 into crocetin through a series of intermediate crocins with different degree of glycosylation and the further transformation of crocetin. We have identified several crocetin-derived microbial metabolites, mainly dicarboxylic acids, produced after reduction and demethylation reactions. These metabolites constitute potential molecules candidates to be responsible for the biological effects attributed to the saffron apocarotenoids and highlighted the possibility to expand the family of microbial metabolites of crocetin. Besides, possible changes in fatty acids as a consequence of the metabolization and/or interaction of crocetin with the gut bacteria were detected. Identifying all these changes at the metabolome level will be very important to understand the mechanisms by which the protective effects of apocarotenoids are exerted. This new and future insights in the bacterial metabolism of crocetin constitute a relevant advance in understanding the relationship between crocetin and human health.Author contributions
R.G.V and F.A.T.B conceptualized and designed the experiments and analytical approaches; D.B.R performed the in vitro fecal fermentations; M.D.F.L extracted and analyzed all the samples; R.G.V and C.J.G. performed the untargeted and targeted metabolomics analyses; R.G.V, C.J.G and M.T.G.C wrote the manuscript with contributions from all the co-authors. All authors have read and agreed to the published version of the manuscript.Data availability
Raw metabolomics data generated in the untargeted metabolomics study are available at ZENODO at https://doi.org/10.5281/zenodo.13124702. Other data supporting this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work has been funded by the Project 22050/PI/22 from the Program for the Promotion of Scientific and Technical Research (Action Plan 2022) of the Seneca Foundation-Science and Technology Agency of the Region of Murcia (Spain) and it is part of the AGROALNEXT programme, supported by Spanish Ministry of Science, Innovation and Universities (MCIN) with funding from European Union NextGenerationEU (PRTR-C17.I1).References
- M. J. Bagur, G. L. Alonso-Salinas, A. M. Jiménez-Monreal, S. Chaouqui, S. Llorens, M. Martínez-Tomé and G. L. Alonso, Saffron: an old medicinal plant and a potential novel functional food, Molecules, 2018, 23, 30 CrossRef PubMed.
- D. Kothari, R. Thakur and R. Kumar, Saffrom (Crocus sativus L.): gold of the spices-a comprehensive review, Hortic., Environ. Biotechnol., 2021, 62, 661 CrossRef.
- S. F. Omidkhoda and H. Hosseinzadeh, Saffron and its active ingredients against human disorders: a literature review on existing clinical evidence, Iran. J. Basic Med. Sci., 2022, 25, 913 Search PubMed.
- T. Abu-Izneid, A. Rauf, A. A. Khalil, A. Olatunde, A. Khalid, F. A. Alhumaydhi, A. S. M. Aljohani, M. S. Uddin, M. Heydari, M. Khayrullin, M. A. Shariati, A. O. Aremu, A. Alafnan and K. R. R. Rengasamy, Nutritional and health beneficial properties of saffron (Crocus sativus L): a comprehensive review, Crit. Rev. Food Sci. Nutr., 2022, 62, 2683 CrossRef CAS PubMed.
- D. Cerdá-Bernad, L. Costa, A. T. Serra, M. R. Bronze, E. Valero-Cases, F. Pérez-Llamas, M. E. Candela, M. B. Arnao, F. Tomás-Barberán, R. García-Villalba, M. T. García-Conesa and M. J. Frutos, Saffron against neuro-cognitive disorders: an overview of its main bioactive compounds, their metabolic fate and potential mechanisms of neurological protection, Nutrients, 2022, 14, 5368 CrossRef PubMed.
- E. Bej, A. R. Volpe, P. Cesare, A. Cimini, M. D'Angelo and V. Castelli, Therapeutic potential of saffron in brain disorders: from bench to bedside, Phytother. Res., 2024, 38, 2482 CrossRef CAS PubMed.
- Y. Zhang, F. Fei, L. Zhen, X. Zhu, J. Wang, S. Li, J. Geng, R. Sun, X. Yu, T. Chen, S. Feng, P. Wang, N. Yang, Y. Zhu, J. Huang, Y. Zhao, J. Aa and G. Wang, Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1044, 1 Search PubMed.
- E. Christodoulou, M. E. Grafakou, E. Skaltsa, N. Kadoglou, N. Kostomitsopoulos and G. Valsami, Preparation, chemical characterization and determination of crocetin's pharmacokinetics after oral and intravenous administration of saffron (Crocus sativus L.) aqueous extract to C57/BL6J mice, J. Pharm. Pharmacol., 2019, 71, 753–764 CrossRef CAS PubMed.
- P. Almodovar, D. Briskey, A. Rao, M. Prodanov and A. M. Inarejos-García, Bioaccesibility and pharmacokinetics of a commercial saffron (Crocus sativus L.) extract, J. Evidence-Based Complementary Altern. Med., 2020, 11, 1 Search PubMed.
- A. Asai, T. Nakano, M. Takahashi and A. Nagao, Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice, J. Agric. Food Chem., 2005, 53, 7302 CrossRef CAS PubMed.
- M. Lautenschläger, J. Sendker, S. Hüwel, H. J. Galla, S. Brandt, M. Düfer, K. Riehemann and A. Hensel, Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier, Phytomedicine, 2015, 22, 36 CrossRef PubMed.
- N. Umagi, K. Murakami, M. V. Ulit, L. S. Antonio, M. Shirotori, H. Morikawa and T. Nakano, The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration, Phytomedicine, 2011, 18, 575 CrossRef PubMed.
- L. Xi, Z. Qian, P. Du and J. Fu, Pharmacokinetic properties of crocin (crocetin digentibioside ester) following oral administration in rats, Phytomedicine, 2007, 14, 633 CrossRef CAS PubMed.
- Y. Zhang, J. Geng, Y. Hong, L. Jiao, S. Li, R. Sun, Y. Xie, C. Yan, J. Aa and G. Wang, Orally administered crocin protects against cerebral ischemia/reperfusion injury through the metabolic transformation of crocetin by gut microbiota, Front. Pharmacol., 2019, 10, 440 CrossRef CAS PubMed.
- R. Shakya, M. R. Nepal, M. J. Kang and T. C. Jeong, Effects of intestinal microbiota on pharmacokinetics of crocin and crocetin in male Sprague-Dawley rats, Metabolites, 2020, 10, 424 CrossRef CAS PubMed.
- F. Yoshino, A. Yoshida, N. Umigai, K. Kubo and M. C. Lee, Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain, J. Clin. Biochem. Nutr., 2011, 49, 182 CrossRef CAS PubMed.
- M. G. Pontifex, E. Connell, G. Le Gall, L. Pourtau, D. Gaudout, C. Angeloni, L. Zallocco, M. Ronci, L. Giusti, M. Müller and D. Vauzour, Saffron extract (Safr'InsideTM) improves anxiety related behavior in a mouse model of low-grade inflammation through the modulation of the microbiota and gut derived metabolites, Food Funct., 2022, 13, 12219 RSC.
- S. A. L. Queiroz, A. M. M. Ton, T. M. C. Pereira, B. P. Campagnaro, L. Martinelli, A. Picos, M. Campos-Toimil and E. C. Vasquez, The gut microbiota-brain axis: a new frontier on neuropsychiatric disorders, Front. Psychiatry, 2022, 13, 872594 CrossRef PubMed.
- Y. P. Silva, A. Bernardi and R. L. Frozza, The role of short-chain fatty acids from gut microbiota in gut-brain communication, Front. Endocrinol., 2020, 11, 25 CrossRef PubMed.
- A. Eroglu, I. S. Al'Abri, R. E. Kopec, N. Crook and T. Bohn, Carotenoids and their health benefits as derived via their interactions with gut microbiota, Adv. Nutr., 2023, 14, 238 CrossRef CAS PubMed.
- H. R. Rocha, M. C. Coelho, A. M. Gomes and M. E. Pintado, Carotenoids diet: digestion, gut microbiota modulation and inflammatory diseases, Nutrients, 2023, 15, 2265 CrossRef CAS PubMed.
- R. García-Villalba, D. Beltrán, J. C. Espín, M. V. Selma and F. A. Tomás-Barberán, Time course production of urolithins from ellagic acid by human gut microbiota, J. Agric. Food Chem., 2013, 61, 8797 CrossRef PubMed.
- R. García-Villalba, J. A. Giménez-Bastida, M. T. García-Conesa, F. A. Tomás-Barberán, J. C. Espín and M. Larrosa, Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples, J. Sep. Sci., 2012, 36, 1906 CrossRef PubMed.
- F. Fusi, G. Romano, G. Speranza and G. Agati, Photon-and singlet-oxygen-induced cis-trans isomerization of the water-soluble carotenoid crocin, Int. J. Mol. Sci., 2023, 24, 10783 CrossRef CAS PubMed.
- M. Carmona, A. Zalacain, A. M. Sánchez, J. L. Novella and G. L. Alonso, Crocetin esters, pirocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS, J. Agric. Food Chem., 2006, 54, 973 CrossRef CAS PubMed.
- A. Mena-García, D. Herreo-Gutiérrez, M. L. Sanz, M. Díez-Municio and A. I. Ruiz-Matute, Fingerprint of characteristic saffron compounds as novel standardization of comercial Crocus sativus, extracts, Foods, 2023, 12, 1634 CrossRef PubMed.
- G. Catalkaya, K. Venema, L. Lucini, G. Rocchetti, D. Delmas, M. Daglia, A. De Filippis, H. Xiao, J. L. Quiles, J. Xiao and E. Capanoglu, Interaction of dietary polyphenols and gut microbiota: microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health, Food Front., 2020, 1, 109 CrossRef.
- M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, Interaction between phenolics and gut microbiota: Role in human health, J. Agric. Food Chem., 2009, 57, 6485 CrossRef CAS PubMed.
- S. Han, W. V. Treuren, C. R. Fischer, B. D. Merrill, B. C. DeFelice, J. M. Sanchez, S. K. Higginbottom, L. Guthrie, L. A. Fall, D. Dodd, M. A. Fischbach and J. L. Sonnenburg, A metabolomics pipeline for the mechanistic interrogation of the gut microbiome, Nature, 2021, 595, 415 CrossRef CAS PubMed.
- Y. Zhao, X. Zhong, J. Yan, C. Sun, X. Zhao and X. Wang, Potential roles of gut microbes in biotransformation of natural products: an overview, Front. Microbiol., 2022, 13, 956378 CrossRef PubMed.
- S. Tan, T. W. Rupasinghe, D. L. Boughton, C. Oliver, C. McSweeny, S. L. Gras and M. A. Augustin, Degradation of curcuminoids by in vitro pure culture fermentation, J. Agric. Food Chem., 2014, 62, 11005 CrossRef CAS PubMed.
- Y. Heng, M. J. Kim, H. J. Yang, S. Kang and S. Park, Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybean, Arch. Microbiol., 2019, 201, 1009 CrossRef CAS PubMed.
- L. M. Bode, D. Bunzel, M. Huch, G. S. Cho, D. Ruhland, M. Bunzel, A. Bub, C. Franz and S. E. Kulling, In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota, Am. J. Clin. Nutr., 2013, 2, 295–309 CrossRef PubMed.
- C. J. García, D. Beltrán and F. A. Tomás-Barberán, Human gut microbiota metabolism of dietary sesquiterpene lactones: Untargeted metabolomics study of lactucopicrin and lactucin conversion in vitro and in vivo, Mol. Nutr. Food Res., 2020, 64, 2000619 CrossRef PubMed.
- H. Hur and F. Rafii, Biotransformation of the isoflavonoids biochanin A, formononetin and glycitein by Eubacterium limosum, FEMS Microbiol. Lett., 2000, 192, 21 CrossRef CAS PubMed.
- P. S. Hedge, M. B. Agni, P. Rai, M. Kumar and D. Gowda, Impact of carotenoids on gut microbiome: implications in human health and disease, J. Appl. Nat. Sci., 2022, 14, 1085 Search PubMed.
- Z. Li, Z. Dai, E. Shi, P. Wan, G. Chen, Z. Zhang, Y. Xu, R. Gao, X. Zeng and D. Li, Study of the interaction between β-carotene and gut microflora using an in vitro fermentation mode, Food Sci. Hum. Wellness, 2023, 12, 1369 CrossRef CAS.
- I. D. Wilson and J. K. Nicholson, Gut microbiome interactions with drug metabolism, efficacy and toxicity, Transl. Res., 2017, 179, 204 CrossRef CAS PubMed.
- J. Tan, C. McKenzie, M. Potamitis, A. N. Thorburn, C. R. Mackay and L. Macia, The role of short-chain fatty acids in health and disease, Adv. Immunol, 2014, 121, 91 CAS.
- P. G. Roopashree, S. S. Shetty and N. S. Kumari, Effect of mdium chain fatty acid in human health and disease, J. Funct. Food, 2021, 87, 104724 CrossRef CAS.
- Y. P. Silva, A. Bernardi and R. L. Frozza, The role of short-chain fatty acids from gut microbiota in gut-brain communication, Front. Endocrinol., 2020, 11, 25 CrossRef PubMed.
- M. Luu, S. Pautz, V. Kohl, R. Singh, R. Romero and S. Lucas, The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes, Nat. Commun., 2019, 10, 760 CrossRef CAS PubMed.
- S. Yuille, N. Reichardt, S. Panda, H. Dunbar and I. E. Mulder, Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid, PLoS One, 2018, 13, e0201073 CrossRef PubMed.
- N. Shekhar, S. Tyagi, S. Rani and A. K. Thakur, Potential of capric acid in neurological disorders: an overview, Neurochem Res., 2023, 48, 697 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02233e |
| This journal is © The Royal Society of Chemistry 2024 |











