Open Access Article
Open Access ArticleThe bioavailability of polyphenols following acute consumption of pigmented barley and wheat†
Borkwei
Ed Nignpense
abd,
Nidhish
Francis
acd,
Christopher
Blanchard
ad and
Abishek
Santhakumar
 *ad
*ad
aSchool of Dentistry and Medical Sciences, Faculty of Science and Health, Charles Sturt University, Wagga Wagga, NSW 2650, Australia. E-mail: asanthakumar@csu.edu.au
bFaculty of Health, Southern Cross University, Terminal Drive, Bilinga, Qld 4225, Australia
cSchool of Agricultural, Environment and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW 2650, Australia
dGulbali Institute, Charles Sturt University, Wagga Wagga, NSW 2650, Australia
First published on 23rd August 2024
Abstract
Polyphenols from pigmented cereal grains exert health-promoting effects but data on their bioavailability are limited. This study investigated the acute bioavailability of polyphenols from the consumption of pigmented whole grain cereal porridges, including purple barley (PB), purple wheat (PW), and blue wheat (BW), compared to a non-pigmented regular wheat (RW). A secondary objective was to assess their effects on plasma antioxidant and inflammatory status postprandially. Phenolic characterisation and antioxidant profiling were performed on extracts from the cooked cereals. Three healthy individuals consumed 200 g of a cereal in a 4-way crossover trial with a one-week washout in between meals. Blood samples were collected at fasting baseline, 30 minutes, 1 hour, 2 hours and 4 hours postprandially. Urine samples were collected at fasting baseline and the 4-hour time point. Pigmented grains exhibited significantly higher phenolic content and antioxidant capacity (p < 0.001) compared to RW. This suggests that pigmented grains may be a better source of polyphenols and potentially offer greater health benefits. However, polyphenol bioavailability following pigmented grain consumption was reduced (less than 6%), suggesting that a substantial fraction remained unabsorbed. The bioavailable phenolic compounds detected included phenolic acids (protocatechuic and caffeic acid), hippuric acid and other phenolic metabolites. Interpersonal variability and the type of grain consumed had an impact on the absorption and excretion of phenolic acids. Only PW consumption resulted in significant (p < 0.01) increases in plasma antioxidant status but no short-term impact on the inflammatory status. This study provides valuable insights into the complex dynamics of polyphenol bioavailability from pigmented cereal consumption and warrants further investigation.
1. Introduction
Oxidative stress plays a key role in the pathogenesis of various chronic diseases including cardiovascular diseases, diabetes, and cancer. It is characterised by an imbalance between the generation of unstable free radicals and the antioxidant defence response of the body.1 Excessive production of free radicals, such as reactive oxygen species, counteracts the protective action of endogenous antioxidants such as glutathione, catalase, and superoxide dismutase, resulting in cellular damage and inflammation in the body.2 Dietary polyphenols have been identified in many in vitro studies as exogenous antioxidants that can scavenge free radicals, upregulate endogenous antioxidant systems and attenuate inflammation.3,4 Epidemiological evidence has shown that this health benefit is associated with the consumption of polyphenol-rich foods and a reduced risk of chronic diseases.5 Whole grain cereals are rich in polyphenols, such as flavonoids and phenolic acids, and are linked to a reduced risk of inflammatory diseases.6 However, there is controversy about the role of cereal polyphenols in preventing disease due to their reported low bioavailability.7Several studies have shown that cereal polyphenols are bioactive; however, they must be bioavailable to exert health effects.3,8–10 Bioavailability in the field of pharmacology and nutrition encompasses the study of liberation, absorption, distribution, metabolism, and elimination of drugs or nutrients.7In vitro-simulated gastrointestinal digestion models have been used to mimic the liberation and metabolism of polyphenols from the food matrix in a process termed bioaccessibility.11 However, human studies are still the ideal way to investigate polyphenol bioavailability because of the complex dynamics that occur in vivo. Unfortunately, limited research has been conducted on the in vivo bioavailability of polyphenols from whole grains. Some long-term dietary interventions have studied polyphenol bioavailability and health effects in overweight and obese individuals. An 8-week randomised controlled trial conducted by Vitaglione et al.12 showed that whole grain wheat consumption increased serum dihydroferulic acid and faecal ferulic acid and reduced the inflammatory marker TNF-α compared to refined grains in overweight individuals, which showed no changes. Another 8-week trial compared whole grain purple wheat consumption with regular whole wheat consumption and found that there were no differences in the total plasma phenolic acids, but significant changes in the inflammatory marker IL-6 following purple wheat consumption.13 Both studies focused on the bioavailability of phenolic compounds from whole grain consumption, but whether pigmented grain consumption results in more bioavailable and bioactive phenolic compounds remains unclear.
Acute bioavailability studies have shown conflicting results regarding the plasma phenolic concentration after the consumption of pigmented cereals. Acute intake of red sorghum pasta has been reported to significantly increase total plasma polyphenols and the antioxidant capacity 2 hours postprandially compared with the intake of white sorghum whole grain pasta.14 In contrast, Gamel et al.13 reported rapid absorption and excretion of anthocyanin and phenolic acid metabolites, but no immediate impact on the plasma antioxidant or inflammatory status. The difference in the outcomes of both studies may be explained by the difference in the grain type used. Considering that pigmented whole grains contain more phenolic compounds with antioxidant capacity, more evidence is needed to substantiate health claims over regular whole grain consumption. Ed Nignpense et al.15 demonstrated that pigmented cereal varieties, such as purple barley, purple wheat, and blue wheat, are a source of bioaccessible radical scavenging phenolic compounds following simulated digestion of flour; however, little is known about the bioavailability and antioxidant potential of polyphenols in vivo after consumption in the cooked form. Hence, the current study employed a pilot dietary crossover trial to investigate the bioavailability of polyphenols in healthy human participants following the acute consumption of meals prepared from each of the pigmented grains compared to regular non-pigmented whole grain wheat. Also, this study aimed to assess the postprandial impact of dietary intervention on the plasma antioxidant and inflammatory status.
2. Materials and methods
2.1. Chemicals
The chemicals used included methanol, acetonitrile, formic acid, acetic acid, sulphuric acid, anhydrous sodium acetate, ferric chloride, and sodium bicarbonate obtained from Chem Supply Pty Ltd (Port Adelaide, SA, Australia). The phenolic standards used included, gallic acid, ferulic acid, catechin, caffeic acid, procyanidin B3, prodelphinidin B3, cyanidin 3-O-glucoside, protocatechuic acid and hippuric acid sourced from Sigma-Aldrich (St Louis, MO, USA).2.2. Polyphenol extraction from cooked cereals
The whole grain cereals were grown in Narribrii, New South Wales by the Australian Grain Technologies. The pigmented cereal lines were chosen for this dietary intervention trial based on previous in vitro digestion analysis.15 The varieties included purple barley (Irisaka), purple wheat (Triticum abyssinicum var arraseita) and blue wheat (Sebesta Blue-3) compared with a commercially grown whole grain wheat (Scepter) (Fig. S1†). The cereal grains were ground in a Perten Laboratory Mill 3000 (Hägersten, Sweden) to a particle size of 0.5 mm. Ten grams of flour were cooked in 100 mL of water using a hot plate at 300 °C temperature for 45 min. The cooked samples were lyophilised and concentrated to 1 g mL−1 followed by methanol extraction, as described by Ed Nignpense et al.152.3. Total phenolic content of cooked cereals
The free phenolic content of cooked cereal grains was determined by the Folin–Ciocalteu method as described by Rao et al.16 Phenolic content was expressed as milligrams of gallic acid equivalent (GAE) per 100 g of dry weight (dw) cereal.2.4. ABTS free radical scavenging capacity of cooked cereals
The measurement of total antioxidant capacity of the cooked grains was adopted from a method by Saji et al.17 ABTS free radical solution was used to determine the total radical scavenging capacity of cooked samples. An Agilent 1290 Infinity UHPLC system coupled with an online ABTS system was used for polyphenol profiling and quantifying of compound radical scavenging activity using a method described by Ed Nignpense et al.18 with modifications15 (refer to section 2.8).2.5. Study participants
The human dietary intervention trial was approved by the Charles Sturt University Ethics Committee (Approval No. H222988:) and the Institutional Biosafety Committee of Charles Sturt University (Approval No. B22010HB). The pilot study was performed in compliance with relevant laws and institutional guidelines. All participants provided full informed consent prior to the commencement of the study. Six healthy participants were recruited from the university. The sample size was determined based on previous polyphenol bioavailability pilot studies.19,20 One participant was withdrawn from the trial due to low blood pressure and two participants had only two of the crossover meals. Three (n = 3) healthy participants (2 males, 1 female) completed the crossover trial, and chromatographic analysis was used to evaluate the phenolic compound bioavailability between grain types to evaluate inter- and intra-differences. All recruited participants were age screened by means of questionnaires. A health screening questionnaire was used to determine if the participants were healthy. Participants were included if they were between 21 and 40 years old, non-pregnant, non-smoker who had a healthy body mass index (18.5–24.9 kg m−2), no medical history of chronic metabolic or cardiovascular diseases, no known problems with venipuncture and not currently on anti-inflammatory, anti-dyslipidaemic or anti-clotting medications (Fig. 1). They were excluded if they failed to meet these criteria. The participants were encouraged to avoid phenolic acid-rich diets at least a day before the trial. A food frequency and antioxidant questionnaire was used to screen the participants for their recent consumption of food and medications that were of an anti-inflammatory or antioxidant nature. After initial participant screening, blood pressure and pulse rate were obtained prior to blood collection. | ||
| Fig. 1 Flowchart of dietary intervention trial. PB, purple barley; PW, purple wheat; BW, blue wheat; RW, regular wheat; FBE, full blood examination. | ||
2.6. Study design, dietary intervention and sample collection
The participants were instructed to begin fasting from midnight and to drink two glasses of water in the morning before blood collection. The participants were cannulated in the median cubital arm vein using a 21-gauge Introcan Safety® intravenous (IV) catheter (Braun, Kronberg, Germany) with a ClearLink™ luer activated valve (Baxter, Alabama, USA), using aseptic cannulation procedure. The cannula was fixed into position with Tegaderm™ transparent IV dressing (3M, Minnesota, USA). Post cannulation, fasting blood samples were collected using a vacutainer luer-lok access device (BD Biosciences, California, USA) into 9 mL EDTA and serum-separating (SST) tubes using aseptic blood withdrawal procedures. After baseline blood collection, the participants were randomly fed cooked whole grain porridge, either made from purple barley, purple wheat, blue wheat, or regular wheat (Fig. 1). The porridge was cooked using 200 grams of flour mixed with 500 ml of water, a tablespoon of salt, tablespoon of sugar and honey and 100 ml of milk. A bottle (500 mL) of water was provided to each participant to ensure appropriate hydration. The time at which the final mouthful of cereal was consumed was recorded and subsequent blood samples were collected at thirty minutes, one hour, two hours, and four hours post consumption using the blood withdrawal procedure as previously described. Urine samples were collected at fasting state and after 4 hours to evaluate the phenolic metabolites excreted. Using Microsoft Excel, the participants were randomly assigned to receive each meal type in the consecutive visits and were not informed of the specific type of grain-based meal they were receiving. A one-week wash-out period was applied between supplementation bouts and the study maintained the same experimental routine between each grain variety tested.2.7. Baseline full blood examination and biochemical profile
Baseline haematology and biochemistry profiling was performed on all volunteers to evaluate their health status (Table S1†). Whole blood was used to determine the full blood count using the Cell-Dyn, Emerald 22 haematology analyser (Abbott Diagnostics, IL, USA). EDTA and SST tubes containing whole blood were centrifuged at 3000g for 10 min at 4 °C. SST tubes were used to collect serum for baseline clinical biochemical analysis using the Thermoscientific Indiko Plus Clinical Chemistry analyser (Thermo Fisher Scientific). The resultant plasma from EDTA tubes was aliquoted immediately and stored at −80 °C until further analysis.2.8. UHPLC-ABTS coupled with Q-TOF LC/MS analysis
Before chromatographic analysis, phenolic compounds were extracted by adopting a deproteinisation method described by Vasilakopoulou et al.21 with modifications. Pure methanol was added to plasma or urine samples in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (sample
4 (sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol). The mixture was centrifuged for 15 min at 7000g and the supernatant was collected and evaporated and the residue reconstituted in 100 μL methanol. As part of validating the analytical method, the percentage recovery of the main phenolic compounds was determined using standards (Table S2†).
methanol). The mixture was centrifuged for 15 min at 7000g and the supernatant was collected and evaporated and the residue reconstituted in 100 μL methanol. As part of validating the analytical method, the percentage recovery of the main phenolic compounds was determined using standards (Table S2†).
UHPLC-ABTS mobile phase A consisted of deionized water and 0.1% formic acid. Mobile phase B consisted of acetonitrile with 0.1% formic acid. Samples of 5.7 μL were injected into the system at a flow rate of 0.3 mL min−1 with a gradient elution of 0–1.5 min, 0–5% B; 1.5–2.5 min, 5–15% B; 2.5–5.5 min, 15–60% B; 5.5–11.5 min, 60–90% B; 11.5–15.5 min, 90–100% B; 15.5–20.5 min, 100% B. Scanning using DAD was performed at wavelengths of 280 nm, 520 nm and 414 nm for phenolic compound identification, anthocyanin identification and ABTS˙+ antioxidant activity, respectively. A Trolox standard curve was used to quantify the ABTS˙+ scavenging activity and was expressed as Trolox equivalents (TEs). The peaks were identified by comparing the retention time and mass spectra with the analytical standards.
The UHPLC-ABTS system was coupled with an Agilent 6530 LC/MS Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) to generate mass spectra of molecular features as described by Ed Nignpense et al.18 A complete mass spectral scan was collected in a range of 100–1050 m/z. The peaks were identified in negative mode acquired with capillary and nozzle voltage set at 3500 V and 1000 V, respectively. Mass spectra were extracted using Agilent Mass Hunter Qualitative Analysis software version B.07.00. Peaks were tentatively identified using available reference standards, published literature and databases such as Metlin (Agilent Technologies, Santa Clara, CA, USA) and online tools, including ChemSpider, PubChem, and MassBank.
2.9. Postprandial plasma antioxidant status
2.10. Postprandial plasma inflammatory status
Plasma levels of anti-inflammatory cytokine interleukin-10 (IL-10) were measured using the ProcartaPlex human multiplex kits (Thermo Fisher Scientific, Inc., Waltham, MA, USA) on a bead-based Luminex xMAP technology (Luminex 200 and xPonent/Analyst software) referring to the manufacturer's instructions.2.11. Statistical analysis
Statistical analysis was performed using repeated measures one-way analysis of variance (ANOVA), followed by post-hoc Dunnett's multiple comparisons test using GraphPad Prism 9 software (GraphPad Software Inc. CA, USA). The results are reported as mean ± standard deviation. Statistical significance was determined at a level of p < 0.05.3. Results
3.1. Phenolic content and antioxidant capacity of cooked grains
Cooked purple barley contained 56.4 ± 3.6 mg GAE per 100 g dw free phenolic content, which was significantly (p < 0.0001) greater than the content in purple wheat, 45.6 ± 2.4 mg GAE per 100 g dw, blue wheat, 43.9 ± 1.7 mg GAE per 100 g dw and cooked red wheat, 19.3 ± 0.9 mg GAE per 100 g dw (Fig. 2). Both cooked pigmented wheats contained significantly (p < 0.001) greater phenolic content than the non-pigmented regular wheat, but there was no significant difference (p = 0.724) between purple wheat and blue wheat. The antioxidant capacity for purple barley was 71%, which was more than double that of wheat varieties. Purple wheat had 28.1% antioxidant capacity, which was significantly greater (p < 0.01) than blue wheat 15.2% and RW 16.6%. There was no significant difference in the antioxidant capacity between blue wheat and regular wheat.3.2 Phenolic characterisation and antioxidant activity of cooked grains
The UHPLC-MS-Online ABTS characterisation of the four varieties of cooked cereal grains had notable differences in their phenolic composition and antioxidant profile. A total of 11 antioxidant phenolic compounds were extracted from the cooked cereal grains. The pigmented grains exhibited a greater number of antioxidant peaks compared to the nonpigmented regular wheat (RW). Anthocyanins, C3G and M3G, were only extracted from the cooked purple barley (PB), having relatively the greatest phenolic content of 3.00 ± 0.02 mg GAE per 100 g dw and 2.25 ± 0.02 mg GAE per 100 g dw, respectively.PB showed the highest number of antioxidant peaks that consisted of primarily flavan-3-ols and anthocyanins. From PB, prodelphinidin B3 exhibited the strongest radical scavenging activity at 11.48 ± 0.7 with an antioxidant index of 70.7 (Table 1). This index measures the antioxidant effectiveness of a compound relative to its quantity. Procyanidin B3 and catechin exhibited free radical scavenging activity at 8.91 ± 1.7 mg TE per 100 g dw and 7.74 ± 0.60 mg TE per 100 g dw, respectively. The antioxidant indices of procyanidin B3 and catechin were 17.1 and 13.2, respectively. The free radical scavenging compounds extracted from the cooked wheat cereals included protocatechuic acid, P1, P3 and P4. Cooked purple wheat (PW) showed the greatest amount of protocatechuic acid, P1 and P3 at 0.64 ± 0.01 mg GAE per 100 g dw, 0.64 ± 0.01 mg GAE per 100 g dw and 0.55 ± 0.01 mg GAE per 100 g dw, respectively. Protocatechuic acid exhibited the greatest radical scavenging activity at 4.54 ± 0.86 mg TE per 100 g dw.
| Peak no. | Tentative identification | RT (min) | m/z | Phenolic content (mg GAE per 100 g dw) | Radical scavenging activity (mg TE per 100 g dw) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PB | PW | BW | RW | PB | PW | BW | RW | ||||
| Data presented here are the mean ± SD (n = 3). Values in brackets are the antioxidant index calculated by dividing a compound's radical scavenging activity by its phenolic content. The different letters within the same row represent significant differences between the extract at the 0.05 level. GAE: gallic acid equivalent; TE: Trolox equivalent; DW: dry weight; — not detected; Trace: compound detected in trace amounts; m/z: mass-to-charge ratio; RT: retention time; C3G: Cyanidin 3-O-glucoside; M3G: Malvidin 3-(6′′-acetylglucoside); PB: cooked purple barley porridge; PW: cooked purple wheat porridge; BW: cooked blue wheat porridge; RW: cooked regular wheat porridge. | |||||||||||
| P1 | Unknown1 | 4.0 | 347.0981, 164.0702 | — | — | — | 0.48 ± 0.00c | — | 3.77 ± 1.77a (4.8) | 2.00 ± 1.92 a(3.2) | 0.68 ± 0.97b (1.4) |
| P2 | Protocatechuic acid | 5.1 | 153.0184 | — | — | — | 0.27 ± 0.00b | — | 4.54 ± 0.86a (7.07) | 1.98 ± 0.18b (11.0) | 1.32 ± 0.11c (4.8) |
| P3 | Unknown2 | 6.0 | 671.2025, 625.1972 | — | — | — | 0.25 ± 0.00b | — | 1.85 ± 0.12a (3.3) | 0.88 ± 0.10b (7.0) | — |
| P4 | Unknown3 | 6.6 | 655.2081 | — | — | — | Trace | — | 2.99 ± 0.61a | 0.91 ± 0.27b | — |
| P5 | Prodelphinidin B3 | 9.4 | 593.1287 | — | — | — | — | 11.48 ± 0.66 (70.7) | — | — | — |
| P6 | Catechin | 10.5 | 289.0723 | — | — | — | — | 7.74 ± 0.60 (13.2) | — | — | — |
| P7 | Procyanidin B3 | 11.9 | 577.1336 | — | — | — | — | 8.91 ± 1.66 (17.1) | — | — | — |
| P8 | C3G | 13.3 | 447.0919 | 3.00 ± 0.02 | — | — | — | 5.38 ± 3.09 (1.8) | — | — | — |
| P9 | M3G | 16.6 | 533.0932 | 2.25 ± 0.08 | — | — | — | 3.81 ± 0.00 | — | — | — |
| P10 | Chrysoeriol-7-O-glucoside | 21.9 | 461.0709 | 1.21 ± 0.08 | — | — | — | 4.53 ± 0.00 | — | — | — |
| P11 | Luteolin | 27.9 | 285.0394 | 0.73 ± 0.02 | — | — | — | 4.83 ± 0.00 | — | — | — |
3.3. Phenolic compounds detected in plasma and urine
UHPLC chromatographic analysis detected unidentified major peaks annotated as M1 and M2 in all plasma samples (Table 2). Protocatechuic acid, caffeic acid and hippuric acid were identified in both plasma and urine by comparison with reference standards and mass spectrometry data (Table 2). Other phenolic metabolites were detected in urine but only to a level 4 identification (Table S3†).| Peak annotation | RT (min) | m/z |
|---|---|---|
| M1: metabolite 1; M2: metabolite 2; N/A: not applicable; RT: retention time; m/z: mass-to-charge ratio. | ||
| M1 | 8.4 | N/A |
| M2 | 8.8 | N/A |
| Protocatechuic acid | 6.6 | 153.0278 |
| Caffeic acid | 7.7 | 179.0421 |
| Hippuric acid | 7.6 | 178.0579 |
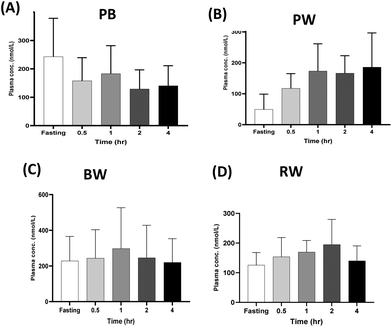
3.4. Plasma kinetics of phenolic compounds over 4 hours post consumption of cereal grains
3.5. Urinary excretion of phenolic compounds
The excretion of phenolic metabolites in urine following the consumption of the different cereal grains revealed distinctive patterns (Fig. 7). Wheat consumption resulted in significant increases in phenolic acids whereas there were no significant changes after consumption of purple barley (PB). Consuming purple wheat (PW), blue wheat (BW), and regular wheat (RW) resulted in significant increases in urinary protocatechuic acid excretion, with concentrations peaking at approximately 200 nmol L−1, 700 nmol L−1, and 400 nmol L−1, respectively, 4 hours postprandially. Furthermore, urinary caffeic acid excretion exhibited increases following the consumption of these grains, reaching concentrations of up to 50 nmol L−1, 180 nmol L−1, and 70 nmol L−1 for PW, BW, and RW, respectively. Interestingly, pigmented grain consumption resulted in a higher postprandial urinary hippuric acid concentration than non-pigmented regular wheat (RW) consumption. Both purple barley (PB) and purple wheat (PW) consumption led to increased urinary hippuric acid excretion, with concentrations peaking at around 10 μmol L−1 and 15 μmol L−1, respectively, after 4 hours.3.6. Postprandial plasma antioxidant capacity and anti-inflammatory activity after consumption of different cereal grains
Table 3 shows that the participants who consumed purple wheat (PW) and purple barley (PB) exhibited an increase in FRAP values with the maximum change detected at the 4-hour ingestion mark. Contrastingly, the participants who consumed regular whole wheat (RW) and barley wheat (BW) showed no significant differences in FRAP after 4 hours. A significant (p < 0.01) increase in FRAP values was observed only 2 hours and 4 hours after PW consumption.| FRAP (mg per 100 g TE) | Time (h) | PB | PW | BW | RW |
|---|---|---|---|---|---|
| Data presented here are mean ± SEM (n = 3). Significant values within the same column are indicated by asterisks: *p < 0.05, **p < 0.01. MFI: mean fluorescence intensity; GPx: glutathione peroxidase; FRAP: ferric iron reducing ability of plasma. | |||||
| Fasting | 45 ± 3 | 50 ± 0.4 | 61 ± 8 | 62 ± 7 | |
| 0.5 | 49 ± 3 | 52 ± 5 | 62 ± 8 | 69 ± 11 | |
| 1 | 50 ± 1 | 56 ± 6 | 55 ± 7 | 60 ± 6 | |
| 2 | 46 ± 3 | 66 ± 7** | 58 ± 7 | 62 ± 6 | |
| 4 | 53 ± 2 | 72 ± 8a** | 61 ± 8 | 63 ± 8 | |
| GPx activity (U L−1) ± SEM | |||||
| Fasting | 596 ± 149 | 486 ± 42 | 514 ± 17 | 503 ± 67 | |
| 0.5 | 521 ± 48 | 596 ± 40 | 549 ± 22 | 626 ± 31 | |
| 1 | 381 ± 135 | 526 ± 52 | 596 ± 22 | 728 ± 116* | |
| 2 | 629 ± 114 | 590 ± 19 | 549 ± 19 | 621 ± 90 | |
| 4 | 549 ± 178 | 727 ± 124* | 578 ± 76 | 558 ± 64 | |
| IL-10 (MFI) | |||||
| Fasting | 34 ± 23 | 50 ± 36 | 30 ± 22 | 25 ± 14 | |
| 0.5 | 51 ± 35 | 35 ± 22 | 24 ± 14 | 35 ± 22 | |
| 1 | 65 ± 52 | 40 ± 31 | 21 ± 9 | 44 ± 31 | |
| 2 | 49 ± 26 | 33 ± 19 | 15 ± 6 | 35 ± 24 | |
| 4 | 63 ± 49 | 30 ± 17 | 16 ± 7 | 35 ± 27 | |
Moreover, postprandial changes in the plasma glutathione peroxidase (GPx) activity were observed. Plasma GPx activity was significantly (p = 0.039) higher than those in the baseline fasting levels at 1 hour and 4 hours after the consumption of RW and PW, respectively. No significant changes in the radical scavenging capacity of plasma were observed after the consumption of purple barley (PB) and blue wheat (BW).
Additionally, the investigation of anti-inflammatory cytokine levels demonstrated an increase in interleukin-10 (IL-10) 2 hours after PB consumption, although this change was not statistically significant (p = 0.12). No significant anti-inflammatory activity was detected following the consumption of wheat grains.
4. Discussion
To our knowledge, the present study is the first to investigate the bioavailability of polyphenols after acute consumption of different whole grain cereal porridges – purple barley (PB), purple wheat (PW), blue wheat (BW), and non-pigmented regular wheat (RW). Polyphenols from pigmented grains have been shown to have disease-preventive properties; however, these compounds need to be stable after cooking the grains and then absorbed in the body to exert any of their health-promoting effects. The nutritional composition of these grains was evaluated using phenolic characterisation and antioxidant profiling (Table 1). Phenolic characterisation revealed substantial differences in the phenolic content and antioxidant capacity of the tested whole grain foods. PB had the highest total phenolic content and antioxidant capacity, followed by the wheat foods PW, BW, and RW (Fig. 2). Analysis of postprandial plasma and urine showed that major bioavailable compounds included phenolic acids, protocatechuic acid, caffeic acid, hippuric acid, and other phenolic metabolites (Table 2). The bioavailability of the polyphenols was dependent on the variety of pigmented grain consumed and the individual (Fig. 4–7 and Table 3). There were significant increases in the plasma antioxidant status, especially after the consumption of purple grains, but no significant acute impact on the plasma inflammatory status.In our previous study, Ed Nignpense et al.,15 we characterised the phenolic composition of raw flour from these pigmented grains. However, to the best of our knowledge, this is the first study to characterise the impact of processing (cooking) on their phenolic composition and antioxidant activity (Fig. 2 and Fig. S2†). Our findings were consistent with our previous study in that PB contained the greatest phenolic content and antioxidant activity, followed by pigmented wheat and regular wheat (Fig. S2†). Although cooking significantly reduced the total phenolic content of PB, flavan-3-ols were still the largest contributors to ABTS radical scavenging activity in both raw and cooked purple barley (Table 1 and Fig. S2†).15 These results suggest that cooking affects the phenolic content, but it may not significantly impact antioxidant capacity and, consequently, its health properties. Overall, the pigmented grains had higher total phenolic content than regular whole wheat (Fig. 2), which is in line with previous studies demonstrating that pigmented grains have better health properties than non-pigmented grains and underscores the potential nutritional benefits of incorporating pigmented grains into a diet.3 Among the cooked wheat varieties, PW had the greatest phenolic content and antioxidant activity, especially with respect to protocatechuic acid concentration, thus highlighting the potential nutritional advantages of this wheat variety. Although pigmented grains are a promising source of anthocyanins, these compounds were only detected in cooked purple barley; this absence may be due to the extraction methodology. Our previous methanol extraction of raw grain polyphenols indicated that the quantifiable antioxidant activity of C3G was lower, but there was little or non-quantifiable amount in the pigmented grain flour.15 Anthocyanins are strongly bound to cereal grain fibre and affected by cooking methods, resulting in less free anthocyanins. Future studies should focus on the extraction of anthocyanins from cereal grain varieties and fortification to increase their anthocyanin content.22
Despite the greater phenolic content in pigmented grains compared to regular whole wheat, the health benefits of these compounds rely on their bioavailability in vivo. Grain polyphenols undergo liberation, absorption, and metabolism during digestion before being distributed in the bloodstream and excreted via urination.7 During gastrointestinal digestion, various compounds such as small phenolic acids and flavones are resistant to alkaline hydrolysis, whereas flavan-3-ols and anthocyanins are highly susceptible.15 Anthocyanins can be absorbed rapidly in the gastric phase, while flavonoid glycosides are absorbed in the intestinal cells following deglycosylation.23 In the liver, flavonoids are conjugated by glucuronidation, methylation, and sulfation or metabolised to small phenolic acids, allowing them to move into systemic circulation and exert bioactivity on target tissues and organs.24 From our study, analysis of the postprandial plasma of the participants revealed the presence of quantifiable amounts of phenolic compound such as protocatechuic acid, caffeic acid, and hippuric acid (Fig. 3–5). The presence of these phenolic compounds is indicative of polyphenol metabolism and absorption after cereal grain consumption. Since the grains contain high flavonoid content, the formation of hippuric acid (conjugation of benzoic acid and glycine) is likely a metabolic pathway that will occur in the liver.24 Also, sulphated metabolites including ferulic acid 4-O-sulphate and dihydrocaffeic acid 3-O-sulphate can be produced during the conjugation of phenolic compounds in the liver (Table 2).25 Low molecular weight hydrophilic compounds including protocatechuic acid and caffeic acid can either be absorbed directly from the small intestine or after extensive colonic metabolism. However, considering that these phenolic acids were detected in plasma within 30 minutes post-consumption, it is likely that the compounds were rapidly absorbed from the upper gastrointestinal tract (Fig. 4–6).
While these phenolic acids, known for preventing cellular oxidative damage and inflammation, contribute to health benefits, their acute bioavailability in this study was relatively low, less than 6% in nanomolar plasma concentrations, suggesting that a considerable amount remains unabsorbed in the gastrointestinal tract after consumption (Fig. 5, 6 and Table S4†).26 The 4-hour timeframe of the trial is relatively short and may have led to this lower bioavailability. However, it allowed for the identification of compounds absorbed in the blood and excreted in urine rapidly. This concurs with previous studies, which have shown that the time for some grain phenolic compounds to reach maximum concentration (Tmax) in plasma falls within 4 hours postprandially.13,27 Like the present study, Bresciani et al.25 showed that the consumption of 94 g of aleurone-rich wheat bread resulted in nanomolar concentrations of plasma ferulic acid. The bioavailability of ferulic acid was 4–8%, which is consistent with the bioavailability calculated for phenolic acids in the present study (Table S4†). In contrast to the present study, which used 200 g of grain porridge, Mateo Anson et al.27 demonstrated that consumption of 300 g of bioprocessed wheat bread resulted in a higher micromolar concentrations of ferulic acid after 1.5 hours. Juxtaposed, these studies illustrate how the dose and the processed forms of the grain can influence the acute bioavailability of the phenolic acids. Other factors including interindividual variability and grain variety, were found to significantly affect the bioavailability of grain polyphenols with respect to their absorption and excretion patterns.
Our current investigation highlighted the interindividual variability in the absorption of phenolic compounds (Fig. 3 and Fig. S3†). These findings align with reports suggesting that the interindividual variation in polyphenol bioavailability can be due to host-related factors such as age, sex, genetic makeup and the gut microbial composition. Age-related factors may contribute to reduced absorption in older individuals due to impaired intestinal permeability, while differences in digestive and metabolic enzymes among sexes can have an impact on polyphenol bioavailability.28,29 Racial differences can result in genetic polymorphisms in transport or metabolising enzymes, which in turn influence polyphenol bioavailability.30 Gut microbiome composition can also influence polyphenol metabolism although this likely happens only if a longer duration in a trial allows for colonic digestion to occur.31 Despite the complexity posed by interindividual variability, a thorough exploration of these underlying factors offers the potential for tailored dietary recommendations.
The type and colour of the grains also seemed to affect the bioavailability of polyphenols. The urinary excretion patterns highlighted the differences in polyphenol bioavailability among the grains consumed. The phenolic acids, protocatechuic acid and caffeic acid, were greatly excreted post-wheat consumption than post-barley consumption (Fig. 7). This may be because cereal genotypes vary in chemical and physical characteristics such as nutrient composition and viscoelastic properties which influence the bioaccessibility of phenolic acids.32 Phenolic acids bound to wheat cell wall structural components may have been released and absorbed in greater amounts during digestion, thus resulting in increased urinary excretion. Interestingly, the levels of hippuric acid excreted after the consumption of pigmented grains were higher than those after the consumption of non-pigmented RW (Fig. 7). This can be explained by hippuric acid excretion being an indicator of anthocyanin metabolism from pigmented grain consumption.23,33 These results concur with findings from previous studies, showing that consumption of anthocyanin-rich foods and beverages leads to relatively high levels of hippuric acid excretion.20,34
The acute intake of pigmented grains had a short-term impact on the postprandial anti-inflammatory and antioxidant status of plasma. The consumption of PW resulted in a significant increase in the antioxidant status of plasma as measured by both FRAP and glutathione peroxidase activity (Table 3). This may indicate the ability of purple wheat polyphenols to positively impact the redox ability and antioxidant enzyme activity. Only a few studies have investigated the intake of pigmented grain consumption on plasma antioxidant status.14,35,36 Khan et al.14 showed that consumption of red wholegrain sorghum increased total polyphenols and the superoxide dismutase activity 2 hours postprandially. Callcott et al.36 showed that purple rice consumption can also significantly increase FRAP values by 70% and decrease the inflammatory status (TNF-α levels) by 25% 4 hours postprandially. Compared with the study by Callcott et al.,36 consumption of PB in this study resulted in nonsignificant decreases of the plasma inflammatory status indicated by increased IL-10 levels postprandially. Surprisingly, although PB was rich in free radical scavenging flavan-3-ols, PB consumption did not show any significant short-term impact on antioxidant status (Table 3). This may be due to factors affecting flavan-3-ol bioavailability including compound instability, the rate of intestinal absorption and gut microbial metabolism.
5. Limitations
This pilot in vivo dietary intervention clinical trial employed a small cohort of participants to investigate potential changes in the polyphenol bioavailability and antioxidant status in healthy adults. While the study provides preliminary novel insights, its findings should be interpreted cautiously due to the reduced statistical power resulting from the small cohort size. Furthermore, many metabolites were not identified in our study including unknown peaks M1 and M2 (Table 2). Other metabolites such as hydroxyhippuric acid, 4-hydroxyhippuric acid sulphate, ferulic acid 4-O sulphate, dihydrocaffeic acid 3-O sulphate and N-feruloylglycine used in this study were identified up to a classification of level 4 identification with low confidence level using only MS data, PubChem and relevant literature23,25 (Table S4†). Though not in the scope of the current study, future studies will benefit from a detailed LC-MS/MS analysis that will provide comprehensive identification and insights into polyphenol metabolic pathways in vivo.6. Conclusion
This pilot crossover dietary intervention study provides novel insights into the bioavailability of polyphenols from the acute consumption of pigmented barley and wheat. Cooked pigmented cereal grains were shown to be composed of a greater amount of radial scavenging phenolic compounds compared to regular whole wheat. Thus, this study presents the potential for the development of functional foods. After consumption of all grains, up to micromolar concentrations of phenolic compounds were detected in plasma and urine. Varied patterns in the absorption and excretion of protocatechuic acid, caffeic acid, and hippuric acid phenolic compounds were observed according to the type of grain consumed and the individual who consumed it. This underscores the complex dynamics involved in the absorption and metabolism of cereal polyphenols and emphasises the need for research on personalised nutrition. Purple wheat consumption showed a significant increase in plasma antioxidant status, which could be attributed to the presence of radical scavenging phenolic compounds in plasma. Future studies should prioritize investigating the large-scale and long-term implications of consuming pigmented grains. Additionally, it is crucial to examine how various processing techniques impact polyphenol bioavailability and associated health benefits.Author contributions
BE wrote the original draft, edited and prepared the final manuscript and conducted the dietary intervention study and analysed results. BE, AS, NF and CB contributed to the conceptualisation of the work. AS and NF were involved in editing the manuscript. All authors reviewed and approved the final manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to acknowledge Australian Grain Technologies for providing the grain samples. The authors would also like to thank all the participants who volunteered to take part in the study. The project was funded by the Australian Government Research Training Program (AGRTP). Borkwei Ed Nignpense is a recepient of an AGRTP scholarship at Charles Sturt University. The authors would also like to acknowledge Charles Sturt University for supporting the open access publication of this article through the 2024 Tri-Faculty Open Access Publishing Scheme.References
- S. Reuter, S. C. Gupta, M. M. Chaturvedi and B. B. Aggarwal, Oxidative stress, inflammation, and cancer: how are they linked?, Free Radicals Biol. Med., 2010, 49, 1603–1616 CrossRef CAS PubMed.
- L. Zuo, E. R. Prather, M. Stetskiv, D. E. Garrison, J. R. Meade, T. I. Peace and T. Zhou, Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments, Int. J. Mol. Sci., 2019, 20, 4472 CrossRef CAS PubMed.
- E. T. Callcott, C. L. Blanchard, P. Oli and A. B. Santhakumar, Pigmented Rice-Derived Phenolic Compounds Reduce Biomarkers of Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells, Mol. Nutr. Food Res., 2018, 62, e1800840 CrossRef PubMed.
- J. L. Song and Y. Gao, Effects of methanolic extract form Fuzhuan brick-tea on hydrogen peroxide-induced oxidative stress in human intestinal epithelial adenocarcinoma Caco-2 cells, Mol. Med. Rep., 2014, 9, 1061–1067 CrossRef CAS PubMed.
- R. D. Mendonça, N. C. Carvalho, J. M. Martin-Moreno, A. M. Pimenta, A. C. S. Lopes, A. Gea, M. A. Martinez-Gonzalez and M. Bes-Rastrollo, Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study, Nutr., Metab. Cardiovasc. Dis., 2019, 29, 69–78 CrossRef PubMed.
- D. Aune, N. Keum, E. Giovannucci, L. T. Fadnes, P. Boffetta, D. C. Greenwood, S. Tonstad, L. J. Vatten, E. Riboli and T. Norat, Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies, Br. Med. J., 2016, 353, i2716 CrossRef PubMed.
- M. D'Archivio, C. Filesi, R. Varì, B. Scazzocchio and R. Masella, Bioavailability of the polyphenols: status and controversies, Int. J. Mol. Sci., 2010, 11, 1321–1342 CrossRef PubMed.
- B. Ed Nignpense, K. A. Chinkwo, C. L. Blanchard and A. B. Santhakumar, Black Sorghum Phenolic Extract Modulates Platelet Activation and Platelet Microparticle Release, Nutrients, 2020, 12, 1760 CrossRef PubMed.
- D. Ferrari, F. Cimino, D. Fratantonio, M. S. Molonia, R. Bashllari, R. Busa, A. Saija and A. Speciale, Cyanidin-3-O-Glucoside Modulates the In Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells, Mediators Inflammation, 2017, 2017, 3454023 Search PubMed.
- S. Rao, K. Chinkwo, A. Santhakumar, S. Johnson and C. Blanchard, Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts, Molecules, 2019, 24, 2465 CrossRef CAS PubMed.
- M. Alminger, A.-M. Aura, T. Bohn, C. Dufour, S. N. El, A. Gomes, S. Karakaya, M. C. Martínez-Cuesta, G. J. McDougall, T. Requena and C. N. Santos, In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility, Compr. Rev. Food Sci. Food Saf., 2014, 13, 413–436 CrossRef CAS PubMed.
- P. Vitaglione, I. Mennella, R. Ferracane, A. A. Rivellese, R. Giacco, D. Ercolini, S. M. Gibbons, A. La Storia, J. A. Gilbert, S. Jonnalagadda, F. Thielecke, M. A. Gallo, L. Scalfi and V. Fogliano, Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber, Am. J. Clin. Nutr., 2015, 101, 251–261 CrossRef CAS PubMed.
- T. H. Gamel, E.-S. M. Abdel-Aal, A. J. Tucker, S. M. Pare, K. Faughnan, C. D. O'Brien, A. Dykun, I. Rabalski, M. Pickard and A. J. Wright, Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity C-reactive protein: a randomised, single-blind parallel-arm study, Br. J. Nutr., 2020, 124, 1179–1189 CrossRef CAS PubMed.
- I. Khan, A. M. Yousif, S. K. Johnson and S. Gamlath, Acute effect of sorghum flour-containing pasta on plasma total polyphenols, antioxidant capacity and oxidative stress markers in healthy subjects: A randomised controlled trial, Clin. Nutr., 2015, 34, 415–421 CrossRef CAS PubMed.
- B. Ed Nignpense, S. Latif, N. Francis, C. Blanchard and A. B. Santhakumar, Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat, Foods, 2022, 11, 3697 CrossRef CAS PubMed.
- S. Rao, E. T. Callcott, A. B. Santhakumar, K. A. Chinkwo, T. Vanniasinkam, J. Luo and C. L. Blanchard, Profiling polyphenol composition and antioxidant activity in Australian-grown rice using UHPLC Online-ABTS system, J. Cereal Sci., 2018, 80, 174–179 CrossRef CAS.
- N. Saji, L. J. Schwarz, A. B. Santhakumar and C. L. Blanchard, Stabilization treatment of rice bran alters phenolic content and antioxidant activity, Cereal Chem., 2020, 97, 281–292 CrossRef CAS.
- B. Ed Nignpense, S. Latif, N. Francis, C. Blanchard and A. B. Santhakumar, The impact of simulated gastrointestinal digestion on the bioaccessibility and antioxidant activity of purple rice phenolic compounds, Food Biosci., 2022, 47, 101706 CrossRef CAS.
- K. Banaszewski, E. Park, I. Edirisinghe, J. C. Cappozzo and B. M. Burton-Freeman, A pilot study to investigate bioavailability of strawberry anthocyanins and characterize postprandial plasma polyphenols absorption patterns by Q-TOF LC/MS in humans, J. Berry Res., 2013, 3, 113–126 CAS.
- M. Netzel, K. Fanning, G. Netzel, D. Zabaras, G. Karagianis, T. Treloar, D. Russell and R. Stanley, Urinary Excretion of Antioxidants In Healthy Humans Following Queen Garnet Plum Juice Ingestion: A New Plum Variety Rich In Antioxidant Compounds, J. Food Biochem., 2012, 36, 159–170 CrossRef CAS.
- P. B. Vasilakopoulou, A.-T. Gousgouni, A. E. Yanni, N. Kostomitsopoulos, V. T. Karathanos and A. Chiou, Polar Phenol Detection in Plasma and Serum: Insights on Sample Pre-Treatment for LC/MS Analysis and Application on the Serum of Corinthian Currant-Fed Rats, Biomolecules, 2022, 12, 1838 CrossRef CAS PubMed.
- S. J. L. Ou, D. Yang, H. P. Pranata, E. S. Tai and M. H. Liu, Postprandial glycemic and lipidemic effects of black rice anthocyanin extract fortification in foods of varying macronutrient compositions and matrices, npj Sci. Food, 2023, 7, 59 CrossRef PubMed.
- T. H. Gamel, A. J. Wright, A. J. Tucker, M. Pickard, I. Rabalski, M. Podgorski, N. Di Ilio, C. O'Brien and E.-S. M. Abdel-Aal, Absorption and metabolites of anthocyanins and phenolic acids after consumption of purple wheat crackers and bars by healthy adults, J. Cereal Sci., 2019, 86, 60–68 CrossRef CAS.
- A. B. Santhakumar, M. Battino and J. M. Alvarez-Suarez, Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis, Food Chem. Toxicol., 2018, 113, 49–65 CrossRef CAS PubMed.
- L. Bresciani, F. Scazzina, R. Leonardi, E. Dall'Aglio, M. Newell, M. Dall'Asta, C. Melegari, S. Ray, F. Brighenti and D. Del Rio, Bioavailability and metabolism of phenolic compounds from wholegrain wheat and aleurone-rich wheat bread, Mol. Nutr. Food Res., 2016, 60, 2343–2354 CrossRef CAS PubMed.
- O. B. Ibitoye and T. O. Ajiboye, Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats, Arch. Physiol. Biochem., 2018, 124, 410–417 CrossRef CAS PubMed.
- N. M. Anson, R. van den Berg, R. Havenaar, A. Bast and G. R. M. M. Haenen, Bioavailability of ferulic acid is determined by its bioaccessibility, J. Cereal Sci., 2009, 49, 296–300 CrossRef.
- N. Hidalgo-Liberona, R. González-Domínguez, E. Vegas, P. Riso, C. Del Bo’, S. Bernardi, G. Peron, S. Guglielmetti, G. Gargari, P. A. Kroon, A. Cherubini and C. Andrés-Lacueva, Increased Intestinal Permeability in Older Subjects Impacts the Beneficial Effects of Dietary Polyphenols by Modulating Their Bioavailability, J. Agric. Food Chem., 2020, 68, 12476–12484 CrossRef CAS PubMed.
- I. Campesi, M. Marino, M. Cipolletti, A. Romani and F. Franconi, Put “gender glasses” on the effects of phenolic compounds on cardiovascular function and diseases, Eur. J. Nutr., 2018, 57, 2677–2691 CrossRef CAS PubMed.
- R. P. Feliciano, C. E. Mills, G. Istas, C. Heiss and A. Rodriguez-Mateos, Absorption, Metabolism and Excretion of Cranberry (Poly)phenols in Humans: A Dose Response Study and Assessment of Inter-Individual Variability, Nutrients, 2017, 9, 268 CrossRef PubMed.
- P. Mena, I. A. Ludwig, V. B. Tomatis, A. Acharjee, L. Calani, A. Rosi, F. Brighenti, S. Ray, J. L. Griffin, L. J. Bluck and D. Del Rio, Inter-individual variability in the production of flavan-3-ol colonic metabolites: preliminary elucidation of urinary metabotypes, Eur. J. Nutr., 2019, 58, 1529–1543 CrossRef CAS PubMed.
- M. Rodehutscord, C. Rückert, H. P. Maurer, H. Schenkel, W. Schipprack, K. E. Bach Knudsen, M. Schollenberger, M. Laux, M. Eklund, W. Siegert and R. Mosenthin, Variation in chemical composition and physical characteristics of cereal grains from different genotypes, Arch. Anim. Nutr., 2016, 70, 87–107 CrossRef CAS PubMed.
- F. Giampieri, D. Cianciosi, J. M. Alvarez-Suarez, J. L. Quiles, T. Y. Forbes-Hernández, M. D. Navarro-Hortal, M. Machì, R. d. J. P. Casanova, J. C. M. Espinosa, X. Chen, D. Zhang, W. Bai, T. Lingmin, B. Mezzetti, M. Battino and Y. A. Diaz, Anthocyanins: What do we know until now?, J. Berry Res., 2023, 13, 1–6 Search PubMed.
- V. Agulló, D. Villaño, C. García-Viguera and R. Domínguez-Perles, Anthocyanin Metabolites in Human Urine after the Intake of New Functional Beverages, Molecules, 2020, 25(2), 371 CrossRef PubMed.
- B. Ed Nignpense, N. Francis, C. Blanchard and A. B. Santhakumar, Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review, Foods, 2021, 10, 1595 CrossRef CAS PubMed.
- E. T. Callcott, C. L. Blanchard, P. Snell and A. B. Santhakumar, The anti-inflammatory and antioxidant effects of acute consumption of pigmented rice in humans, Food Funct., 2019, 10, 8230–8239 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01162g |
| This journal is © The Royal Society of Chemistry 2024 |