Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mustard seed major allergen Sin a1 activates intestinal epithelial cells and also dendritic cells that drive type 2 immune responses†
Cristina
Bueno-Díaz
 *a,
Marit
Zuurveld
a,
Verónica
Ayechu-Muruzabal
a,
Sandra G. P. J.
Korsten
ae,
Laura
Martín-Pedraza
b,
Jorge
Parrón-Ballesteros
c,
Frank
Redegeld
a,
Johan
Garssen
ad,
Mayte
Villalba
c and
Linette E. M.
Willemsen
*a,
Marit
Zuurveld
a,
Verónica
Ayechu-Muruzabal
a,
Sandra G. P. J.
Korsten
ae,
Laura
Martín-Pedraza
b,
Jorge
Parrón-Ballesteros
c,
Frank
Redegeld
a,
Johan
Garssen
ad,
Mayte
Villalba
c and
Linette E. M.
Willemsen
 *a
*a
aDivision of Pharmacology, Utrecht Institute for Pharmaceutical Sciences (UIPS), Utrecht University, Utrecht, The Netherlands. E-mail: l.e.m.willemsen@uu.nl
bHospital Ramón y Cajal, Madrid, Spain
cDepartment of Biochemistry and Molecular Biology, Complutense University of Madrid, Madrid, Spain
dDanone Nutricia Research B.V., Utrecht, The Netherlands
eTiofarma B.V., Oud-Beijerland, The Netherlands
First published on 17th May 2024
Abstract
Mustard seeds belong to the food category of mandatory labelling due to the severe reactions they can trigger in allergic patients. However, the mechanisms underlying allergic sensitization to mustard seeds are poorly understood. The aim of this work is to study type 2 immune activation induced by the mustard seed major allergen Sin a1 via the intestinal mucosa, employing an in vitro model mimicking allergen exposure via the intestinal epithelial cells (IECs). Sin a1 was isolated from the total protein extract and exposed to IEC, monocyte derived dendritic cells (DCs) or IEC/DC co-cultures. A system of consecutive co-cultures was employed to study the generic capacity of Sin a1 to induce type 2 activation leading to sensitization: IEC/DC, DC/T-cell, T/B-cell and stem cell derived mast cells (MCs) derived from healthy donors. Immune profiles were determined by ELISA and flow cytometry. Sin a1 activated IEC and induced type-2 cytokine secretion in IEC/DC co-culture or DC alone (IL-15, IL-25 and TSLP), and primed DC induced type 2 T-cell skewing. IgG secretion in the T-cell/B-cell phase was enhanced in the presence of Sin a1 in the first stages of the co-culture. Anti-IgE did not induce degranulation but promoted IL-13 and IL-4 release by MC primed with the supernatant from B-cells co-cultured with Sin a1-IEC/DC or -DC primed T-cells. Sin a1 enhanced the release of type-2 inflammatory mediators by epithelial and dendritic cells; the latter instructed generic type-2 responses in T-cells that resulted in B-cell activation, and finally MC activation upon anti-IgE exposure. This indicates that via activation of IEC and/or DC, mustard seed allergen Sin a1 is capable of driving type 2 immunity which may lead to allergic sensitization.
Introduction
The prevalence of food allergy has dramatically increased over the last few decades, representing a major health problem. It is estimated that nearly 4% of the global population suffers from hypersensitivity reactions against food, especially affecting people from industrialized countries.1 It has been hypothesized that different factors may lead to the increment of food allergy prevalence: from the damaging of the skin barrier upon exposure to pollutants and detergents, to changes in the gut microbiota that may affect the barrier integrity leading to a pro-allergenic profile.2Intestinal epithelial barrier homeostasis is highly regulated by its interplay with both the microbiota and the mucosal immune system, and also with external components like those that come from food.3 The disruption of this homeostasis can lead to the loss of oral tolerance and the development of food allergies.4 The intrinsic properties of major food allergens may contribute to epithelial activation and drive the development of type 2 dendritic cell (DC) activation, the first steps in the immune cascade leading towards allergic sensitization.5,6
Among allergenic sources, plant-derived foods comprise an important percentage of the reported type-I hypersensitivity reactions against food products.7,8 Most allergenic proteins are resistant to digestion allowing crucial epitopes to be recognized by the mucosal immune system.9
Many frequently consumed foods, such as pizza and tomato sauce, contain mustard seeds as a taste enhancer. Thus, despite its relatively low sensitization prevalence,10,11 its ubiquity in meals and the severity of reactions associated with its accidental consumption make mustard one of the foods that must be included on food labels.12 At a clinical level, most patients allergic to mustard seeds are sensitized to Sin a1,13 belonging to the 2S albumin family, along with also major allergens from peanut (Ara h 2 and Ara h 6), hazelnut (Cor a 14) or cashew (Ana o 3). These proteins are characterized by their structural resistance to thermal and enzymatic treatments,14,15 also by their interaction with lipidic components and, consequently, with cell membranes.16,17 Previous studies reported the ability of different 2S albumins to cross intestinal cell monolayers, without affecting permeability, while activating these epithelial cells to produce immune mediators.18,19 However, it is unknown if this would provoke epithelial activation for Sin a1, then contributing to allergic sensitization and food allergy development.20 Therefore, in the present work, human intestinal epithelial cells (IECs) were used to study the sensitizing capacity of Sin a1 through the intestinal mucosa. Moreover, a sequential co-culture system developed by our group21 was employed to further determine if Sin a1 exposure could drive the type 2 phenotype in innate and adaptive immune cells.
Materials and methods
Mustard seed extract and Sin a1 isolation
Sin a1 was isolated from the yellow mustard seed total protein extract (YMSE) as previously described.22 Briefly, the extract was obtained from 5 g of mustard seed powder by saline extraction with 0.15 M sodium borate and 1 M PMSF, pH 8, from mustard powder previously delipidated with cold acetone. Finally, after freeze drying, slurry was reconstituted in 0.15 M ammonium bicarbonate, pH 8.0, in a final concentration of 20 mg ml−1 for further chromatographic steps: 20 mg of the mustard seed extract were loaded in the Sephadex medium matrix (Sigma-Aldrich), in 0.15 M ammonium bicarbonate, pH 8.0, at a flow rate of 0.5 mL min−1.Fractions containing proteins under 30 kDa followed were pooled together, lyophilized and resuspended in 3 mM ammonium pyrophosphate for its application in a SP-Sephadex C-25 (Sigma-Aldrich) ion exchange column, in a 3 to 50 mM sodium pyrophosphate gradient. Protein purification was traced by SDS-PAGE and western blotting using a polyclonal antibody against Sin a1 (ESI Fig. 1†). Fractions containing Sin a1 were pooled together for buffer exchange into ammonium bicarbonate, 50 mM, by centrifugation in 10 kDa size pore Amicon 15 mL tubes (Merck Millipore). After all the purification process, a total of 1 mg of Sin a1 was purified from 20 mg of the total mustard seed extract (5% of total protein).
LPS was removed with Pierce© High-Capacity Endotoxin Removal Spin Columns (Thermo Fisher Scientific). Final endotoxin levels were determined with Endosafe®-PTS Endotoxin test (Charles River Microbial Solutions). Since endotoxin activity was below the limit of detection (0.1 EU mL−1), samples were considered “LPS-free”.
Intestinal epithelial cell lines
Two epithelial cell lines were used as models for IEC. HT-29 (ATCC, HTB-38) was cultured in a McCoy 5A medium (Gibco, Invitrogen), and Caco-2 (ATCC, HTB-37) was cultured in DMEM high glucose medium (Gibco) plus 1% (v/v) L-glutamine (200 mM, 100× stock) (Gibco) and 1% (v/v) non-essential amino acids (100× stock) (Gibco). Both cultures were supplemented with 10% (v/v) fetal calf serum (FCS) and 10% (v/v) penicillin/streptomycin (pen/strep) (100× stock) (Sigma-Aldrich) and grown at 37 °C, 5% CO2, refreshing the medium every 2–3 days. For the experiments, IECs were diluted and seeded based on the surface area in 48-well flat-bottom plates or 12-well transwell inserts (polyester membrane, 0.4 μm pores) (Costar Corning Incorporated). Confluence was determined by optical microscopy for HT-29, and transepithelial electrical resistance (TEER) for Caco-2 (Ω cm2 ≥ 500) with a Millicell® ERS-2 Volt-Ohm meter (Merck Millipore).Immune cell isolation
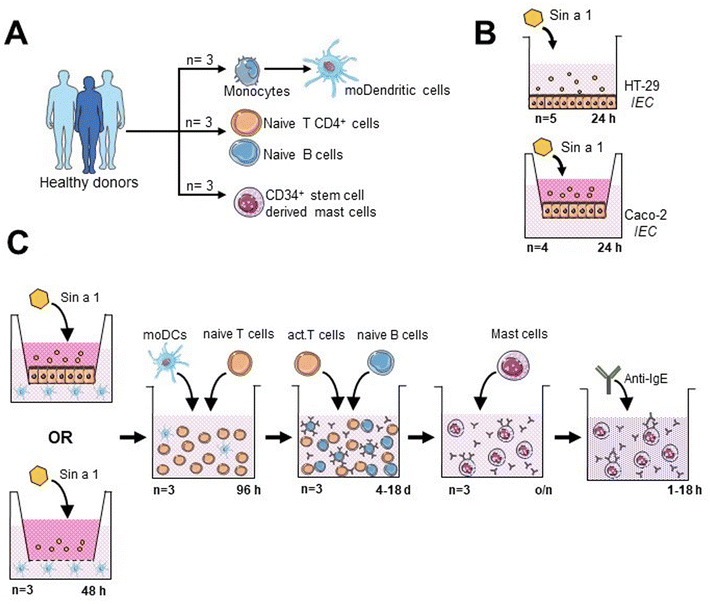
Immune cells were obtained from buffy coats from healthy donors, after informed consent (Sanquin, The Netherlands) (Fig. 1A). Peripheral blood mononuclear cells were isolated using Leucosep tubes (Greiner). Then, monocytes, CD34+ stem cells, naïve T-cells and naïve B-cells were obtained by negative selection with magnetic separation kits, following manufacturer's indications (Miltenyi Biotec). Monocytes were differentiated into dendritic cells (DC) by culturing them for 6 days in RPMI 1640 (Lonza) containing 10% (v/v) FCS, 1% (v/v) pen/strep (100× stock), 100 ng mL−1 IL-4 and 60 ng mL−1 GM-CSF (Prospec). Naïve T- and naïve B-cells were maintained in IMDM (Sigma-Aldrich), 5% (v/v) FCS, 1% (v/v) pen/strep (100× stock), 20 μg mL−1 apo-transferrin (Sigma-Aldrich) and 50 μM β-mercaptoethanol (Sigma-Aldrich). Monocytes, T-cells and B-cells were obtained from three different donors. DC/T-cell cultures were from allogenic donors and T/B-cell co-cultures were from autologous donors. Primary human mast cells (MC) were differentiated from CD34+ stem cells as described.23 Purity of the isolated immune cells was assayed immediately after purification.21Exposure of IECs to Sin a1 or the yellow mustard seed extract
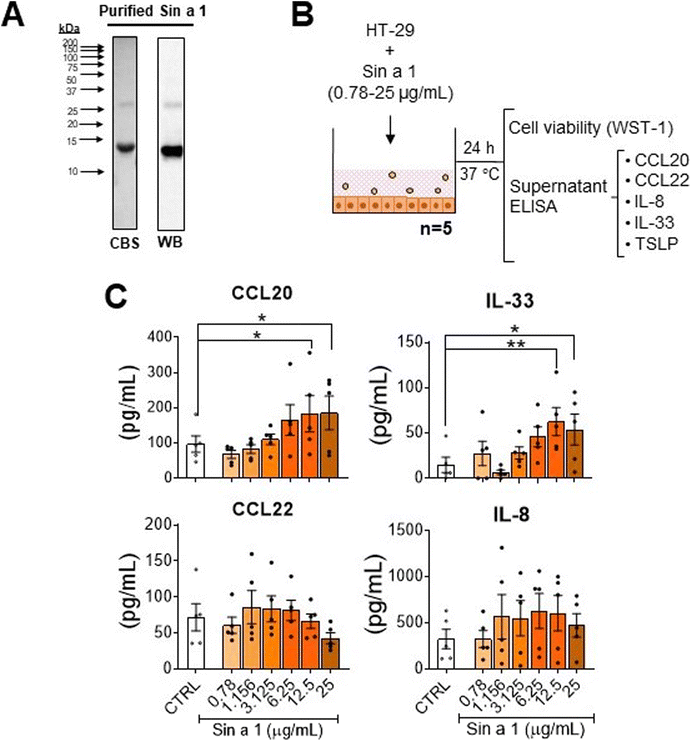
HT-29 cells in 48-well flat-bottom plates were exposed for 24 h to different concentrations of “LPS-free” Sin a1 (0.75–25 μg mL−1), the yellow mustard seed extract (YMSE) (15.65–250 μg mL−1), or LPS (1 × 10−4 to 1 μg mL−1), plus only cell medium condition as the control. Supernatants were collected for cytokine analysis, and cell viability was determined using a WST-1 assay kit (Roche). In addition, prior to co-culture models, HT-29 were seeded in 12-well transwell inserts (Fig. 1B) and exposed apically to different concentrations of Sin a1 (5–50 μg mL−1) for up to 72 h. The basolateral supernatant was collected every 24 h and replaced with a new medium to determine the cytokine profile.Caco-2 cells were seeded in 12-well transwell inserts. Once the cells were differentiated (day 21 after confluence, TEER ≥ 500 Ω cm2), Sin a1 was added (0–25 μg mL−1) in the apical compartment (Fig. 1B), after 24 h basolateral supernatants were collected for cytokine analysis by ELISA. Barrier integrity was studied by measuring TEER and FITC-dextran (3–5 kDa; Sigma-Aldrich) permeability one hour after apical application.
Co-culture models
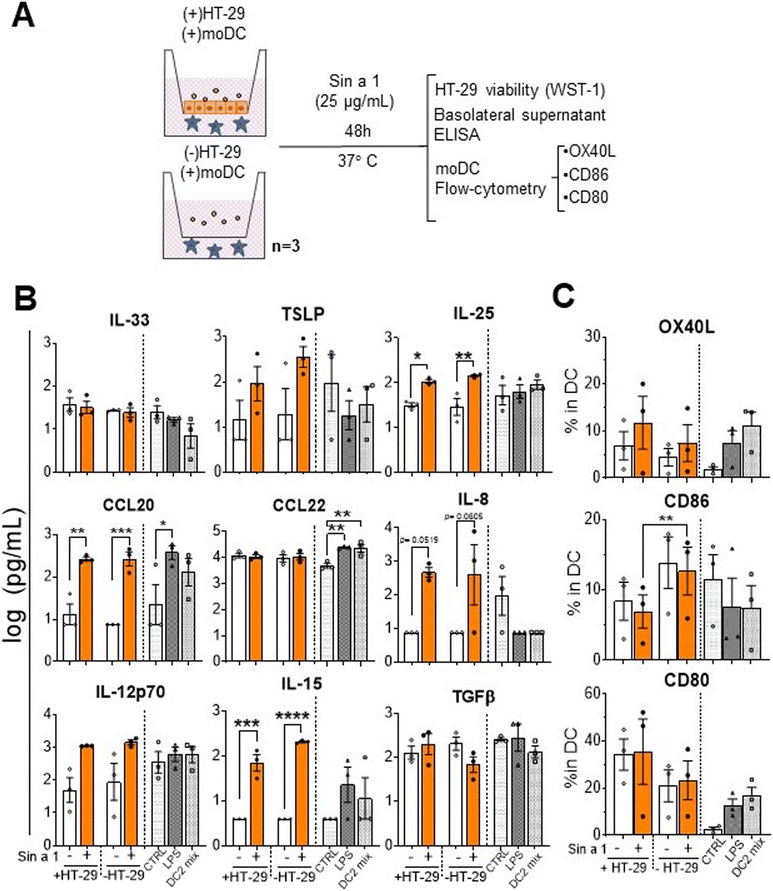
In order to mimic the oral sensitization process, we established an in vitro model based on sequential co-cultures (Fig. 1C): IEC/DC or DC alone, followed by primed DC/T-cells and T/B-cells, and finally, primary stem cell-derived human MC exposed to supernatants from T/B-cell cocultures.21 After each step, supernatants were collected for cytokine analysis, and part of the cells was employed for phenotyping by flow cytometry.For the IEC/DC co-culture, HT-29 cells were seeded in 6-wells of a 12-well transwell insert plate (Costar) until they reached confluence. Then, 5 × 105 DCs were added to each well in the basolateral compartment; so two conditions were analysed: IEC-DCs or DCs in the absence of epithelial cells. Apically, 25 μg mL−1 Sin a1 was added to the cultures for 48 h. DC control conditions were placed in a separate 12-well plate, using 100 ng mL−1 LPS (type 1 DC profile) or DC2-mix (50 ng mL−1 TNFα, 25 ng mL−1 IL-1β, 10 ng mL−1 IL-6 and 1 μg mL−1 PGE2; type 2 DC profile) as previously described.24
Next step was the DC/T-cell co-culture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, T
1 ratio, T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DC) for 96 h in the presence of 5 ng mL−1 IL-2 (Prospec) and 150 ng mL−1 anti-CD3 (BD Biosciences), washing the DCs prior to incubation with T cells, to avoid the presence of Sin a1 in the supernatant. Appropriate T-cell control conditions included: anti-CD3 + IL-2 stimulated, and non-stimulated cells cultured in the absence of DC.
DC) for 96 h in the presence of 5 ng mL−1 IL-2 (Prospec) and 150 ng mL−1 anti-CD3 (BD Biosciences), washing the DCs prior to incubation with T cells, to avoid the presence of Sin a1 in the supernatant. Appropriate T-cell control conditions included: anti-CD3 + IL-2 stimulated, and non-stimulated cells cultured in the absence of DC.
Next, T-cells were co-cultured with B-cells (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in the presence of 5 μg mL−1 anti-IgM (Sigma-Aldrich), employing appropriate B-cell controls as described previously.21 Finally, B-cells were collected for flow cytometry analysis, while supernatants were employed for cytokine determination and MC degranulation and activation assays. For this purpose, MCs were incubated overnight with B cell supernatants. Afterwards, cell medium was replaced and anti-IgE was added, measuring MC degranulation after 1 hour incubation (β-hexosaminidase release), and type 2 cytokine (IL-4 and IL-13) secretion 18 hours after exposure to anti-IgE.
1 ratio) in the presence of 5 μg mL−1 anti-IgM (Sigma-Aldrich), employing appropriate B-cell controls as described previously.21 Finally, B-cells were collected for flow cytometry analysis, while supernatants were employed for cytokine determination and MC degranulation and activation assays. For this purpose, MCs were incubated overnight with B cell supernatants. Afterwards, cell medium was replaced and anti-IgE was added, measuring MC degranulation after 1 hour incubation (β-hexosaminidase release), and type 2 cytokine (IL-4 and IL-13) secretion 18 hours after exposure to anti-IgE.
β-Hexosaminidase assays
After overnight incubation with the B-cell supernatant, cell medium was replaced, and primary human MCs were activated with anti-human IgE Mab (eBioscience) for 1 h. The supernatant was collected and incubated with 158 μM 4-methylumbelliferyl-β-d-glucopyranoside (4-MUG) for 1 h. The enzymatic reaction was stopped with 0.11 M glycine buffer. β-Hexosaminidase content was quantified by measuring fluorescence at ex350 nm/em460 nm and represented as a percentage compared to a negative (0%) and positive control (100%) samples.Cytokine detection
Supernatants collected from IEC, IEC-DC, DC, DC/T-cell, T/B-cell or MC cultures were analysed for cytokine, chemokine and immunoglobulin secretion (ESI Table 1†): IFNγ, IL-4, IL-8, IL-10, IL-12p70, IL-17, IL-13, TGFβ, TNFα, TSLP, IgE and IgG (Invitrogen), IL-15 (Biolegend), CCL20, CCL22, IL-25, IL-33 (R&D systems). In brief, 96-well ELISA plates (Corning™ Costar™ 9018) were coated overnight with a capture antibody solution diluted in PBS. Afterward, the plates were washed using 0.05% Tween 20 in PBS, and blocked with reagent diluent (1% BSA in PBS) for 1 h at room temperature (RT). Subsequently, samples and standards were added, following another wash step, and they were appropriately diluted in the reagent diluent, and incubated for 2 h at RT and washed. The detection antibody, also diluted in the reagent diluent, was added and incubated at RT for up to 2 h and washed. Following this, diluted streptavidin-HRP was added and incubated for up to 30 min and washed. For the final step, a substrate solution (TMB) was applied and left to incubate for up to 20 min. The reaction was terminated by adding 2 N H2SO4. The optical density was then measured at 450 nm, with wavelength correction at 570 nm with a GloMax microplate reader (Promega). Variations on the protocol are indicated by the manufacturer.Flow cytometry of immune cells
The phenotype of DCs, T-cells and B-cells after co-culture was analysed by flow cytometry. Nonspecific binding sites were blocked with human Fc block (BD Biosciences) in PBS containing 1% BSA (Roche). Antibodies for extracellular staining are indicated in Table 2 ESI.† T-cells were permeabilized with Intracellular Fixation & Permeabilization Buffer Set (eBioscience) to allow staining of intracellular IL-13 (BioLegends). Flow cytometric measurements were performed using BD FACS Canto II (Becton Dickinson) and acquired data were analysed using FlowLogic software (Inivai Technologies).For DC analyses, CD11c + HLA-DR + population was gated from the life cells. Subsequently, the expression of activation markers CD80, CD86, and OX40L was assessed and reported as percentages in DCs (see ESI Fig. 6† for gating strategy including FMO controls).
For T cells, CD4+ population was gated within the life cell population. Then, the expression of CRTH2, CXCR3 and intracellular IL-13 markers was assessed in the CD4+ population and expressed as median MFI or percentage of cells (ESI Fig. 4 and 7† for gating strategy including FMO controls). Finally, B cells were selected over T cells according to CD19 expression. For those CD19+ cells, CD25 and CD27 markers were measured and expressed as percentage of cells.
Statistical analysis
Data were analysed using Prism software 8.0 (GraphPad software), using one-way repeated measures ANOVA followed by Bonferroni's multiple comparison on selected pairs, or by a paired t-test for CTRL vs. DC2 condition. Data are represented as mean ± SEM; p values below 0.05 were considered statistically significant. When data were not normally distributed (IL-8, TGFβ, IL-13 from DC/T cell co-culture, IgG from T/B cell co-culture, IL-13 from MC incubated with the supernatant from day 18 of T/B cell co-culture), logarithm transformation (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) was applied prior to ANOVA analysis. All data regarding cytokine or immunoglobulin levels from the experiments using donor cells are represented in the logarithmic scale to show them in a consistent manner.
10) was applied prior to ANOVA analysis. All data regarding cytokine or immunoglobulin levels from the experiments using donor cells are represented in the logarithmic scale to show them in a consistent manner.
Results
Sin a1 activates intestinal epithelial cells and promotes type-2 cytokine secretion
The interaction of food allergens with the intestinal epithelial barrier is considered the first step in oral sensitization. Epithelial activation and barrier integrity upon exposure to Sin a1, purified from YMSE, was studied in HT-29 and/or Caco-2 cells. To determine epithelial activation, we measured the presence cytokines (IL-33 and TSLP) and chemokines (CCL20 and CCL22) that are involved in the recruitment of immune cells including DC and/or priming the DC to instruct Th2 development, driving a type-2 immune response. On the other hand, IL-8 was included as a marker of the general inflammatory response.First, HT-29 cells were grown confluent in 48-well flat bottom plates and exposed to increasing concentrations of purified Sin a1 for 24 h (Fig. 2B). In ESI Fig. 1† the purification of Sin a1 from the YMSE is shown (ESI Fig. 1†). Sin a1 increased CCL20 and IL-33 concentrations in a dose-dependent manner, while CCL22 and IL-8 remained unaffected, and TSLP was not detected (Fig. 2C). Moreover, Sin a1 did not affect cell viability and neither YMSE nor LPS was capable of inducing epithelial activation into type-2 profile like Sin a1. The 62.5 μg ml−1 dose of YMSE induced IL-8 secretion by HT-29 cells (ESI Fig. 2A–C†).
To study the effects of Sin a1 on epithelial barrier properties, Caco-2 cells were cultured in 12-well transwell inserts, and 21 days after reaching confluence, exposed to different concentrations of Sin a1 for 24 h (ESI Fig. 3A†). The presence of the allergen did not affect cell viability according to the WST-1 test, nor TEER or 3–5 kDa FITC dextran permeability (ESI Fig. 3B and C†). The highest dose of Sin a1 enhanced CCL20 (p < 0.01) release by IEC, while IL-33 levels increased (p < 0.05) using 5 μg mL−1 Sin a1 (ESI Fig. 3D†). No significant changes were observed in CCL22, and neither TSLP nor IL-8 were detectable.
These results suggest an ability of Sin a1 to interact and activate epithelial cells to initiate a pro-allergenic immune response. Since in HT-29 Sin a1 dose-dependently increased both IL-33 and CCL20, these cells were chosen to further study the effects of epithelial activation by Sin a1 on DC maturation and downstream type 2 polarization using a dose of 25 μg mL−1.
Epithelial activation by Sin a1 primes DC and its interaction with T-cells in a type-2 context
The next step in sensitization via the gastro-intestinal tract is the recruitment of antigen presenting cells like DCs. Epithelial mediators may modify the DC function into a type 2 driving phenotype; upon allergen capture, they migrate to the lymph nodes and present it to naïve T-cells, driving type 2 polarization of the immune response. In order to mimic this process, we established a sequential co-culture model.21The first step of this system consisted of two initial conditions established in a transwell plate: HT-29 co-culture with DC (IEC-DC) or DC alone. In both cases, cells were exposed to a single dose of Sin a1 in the apical compartment (Fig. 3A). After 48 h, analysis of target cytokines from the basolateral compartment revealed immune activation having both a type-2 and type-1 phenotype (Fig. 3B), either in the presence or absence of HT-29 in the apical compartment. Sin a1 increased IL-25 (p < 0.05; p < 0.01), CCL20 (p < 0.01; p < 0.005), and IL-15 levels (p < 0.005; p < 0.001). The presence of IEC reduced CD86 expression in DC in the presence or absence of Sin a1, while CD80 tended to increase (Fig. 3C). Sin a1 did not affect OX40L, CD86 nor CD80 expression of DC compared to medium controls in the presence or absence of IEC. Maturation controls using LPS (DC1) or cytokine mix (DC2) showed a tendency towards the increase of OX40L and CD80 and increased CCL20 and/or CCL22 secretion of matured DC compared to medium controls.
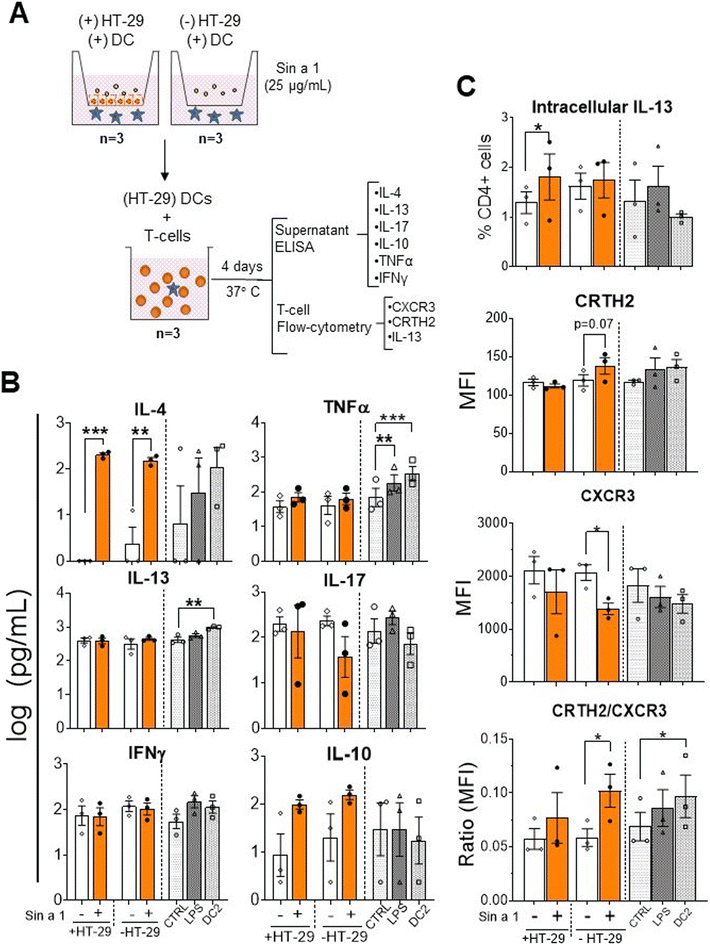
Next step in the sensitization phase is the interaction of DC with T-cells. Therefore, the primed DCs were cultured in the presence of allogenic naïve T-cells for four days (Fig. 4A) and cytokine profiles and T-cell phenotypes were determined. Cytokine analysis revealed a significant increment in IL-4 in both IEC-DC/T-cells (p < 0.001) and DC/T-cells (p < 0.01) when either IEC-DC or DC had been exposed to Sin a1 in the previous step (Fig. 4B). IL-10 followed a similar tendency. IL-17 concentrations remained unaffected and showed a declining pattern in the Sin a1 DC/T-cell culture. Flow cytometry showed a significant increment in intracellular IL-13 expression (p < 0.05) in those T-cells from the IEC-DC/T-cell when IEC had been exposed to Sin a1. Furthermore, DCs that were directly exposed to Sin a1 tended to increase the expression of CRTH2 (Th2 marker) on T cells, while the expression of CXCR3 (Th1 marker) and the frequency of CXCR3 expressing T-cells was reduced (Fig. 4C and ESI Fig. 4B†). Moreover, the ratio MFI CRTH2/MFI CXCR3 significantly increased in this condition, and also in the DC2/T-cell control, indicating a shift in the balance towards the Th2 profile (Fig. 4C). Control DC/T-cell cultures of T-cells exposed to matured DC1 (LPS) or DC2 showed increased TNF secretion, and in DC2/T-cell cultures also IL-13 secretion increased, while IL-4 showed a similar inclining pattern.
T/B-cell supernatants of Sin a1 exposed IEC-DC-T/B or DC-T/B provoke mast cell activation upon anti-IgE stimulation
To study if Sin a1 also initiates a humoral response, instructed T-cells from the previous step were co-cultured with B-cells from autologous donors for four up to 18 days. Then, IgE and IgG levels were determined, as well as the B-cell phenotype (Fig. 5A). After co-culture, no significant changes in IgE levels were observed either in Sin a1-IEC-DC-T/B-cell or Sin a1-DC-T/B-cell supernatants (Fig. 5B). Nevertheless, IgG levels significantly increased in the B-cell supernatants of day 4 and 18 from both conditions when IEC-DC or DC had been exposed to Sin a1 (p < 0.05) (Fig. 5B). Moreover, while IL-13 could be measured in T/B cell coculture supernatants at day 4 and day 18, IL-4 was only detected in supernatants after 18 days coculture, and levels of both cytokines were unaffected in the Sin a1 conditions (ESI Fig. 5†), thus the same in Sin a1-(IEC-) DC-T/B-cell supernatants compared to (IEC-)DC-T/B-cell supernatants (ESI Fig. 5B†). The B-cell phenotype was not affected (CD25, CD27 and CD38 expression) among the different groups (Fig. 5C). When B-cells were co-cultured with DC2 primed T-cells, this did not affect IgE or IgG secretion, nor B-cell maturation (Fig. 5A–C). Control B-cells exposed to anti-IgM or IgE mix (anti-IgM + anti-CD40 + IL-4) tended to increase IgE release at day 4 and showed enhanced CD25 or CD27 expression.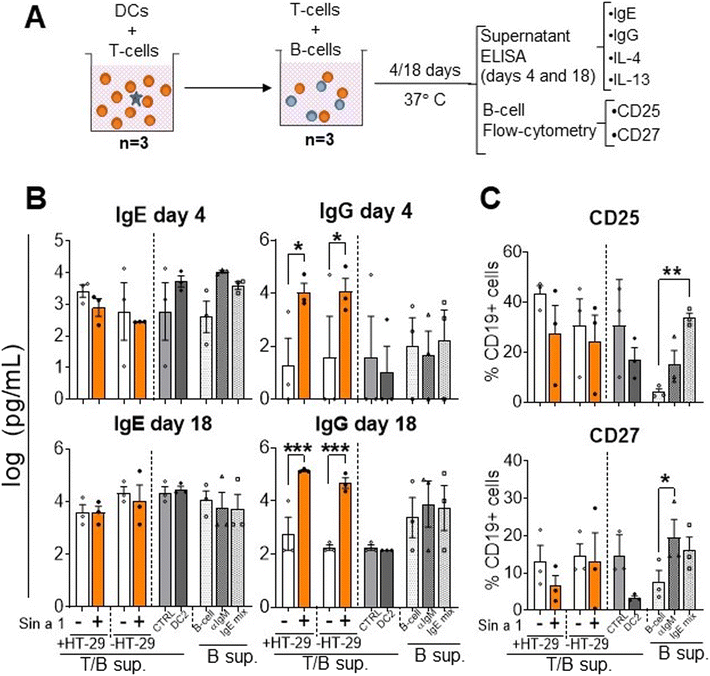 | ||
| Fig. 5 Co-culture of Sin a1 exposed IEC-DC or DC-primed T-cells with naïve B-cells. (A) T-cells primed with DCs that were previously exposed to Sin a1 exposed IEC or DC directly exposed to Sin a1 were co-cultured with autologous naïve B-cells for four up to 18 days (n = 3 independent donors). T/B-cell supernatants were collected to measure IgE and IgG antibody levels (B), while the B cell phenotype was determined by flow cytometry (C). Controls are B-cells alone, medium-exposed (B-cell), anti-IgM exposed (aIgM), or anti-IgM + antiCD40 + IL-4 (IgE mix). Data were analysed by one-way ANOVA, or by paired t-test for CTRL vs. DC2, mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). IL-4 and IL-13 data are shown in ESI Fig. 5.† | ||
Finally, supernatants obtained from both IEC-DC-T/B-cell and DC-T/B-cell co-cultures were exposed over night to primary human MC to determine effector cell activation mediated by antibodies produced by B-cells in the previous step (Fig. 6A). In the conditions with the T/B-cell supernatants of the Sin a1 exposed IEC/DC or DC primed T-cells anti-IgE induced MC degranulation tended to increase when using the day 4 supernatants, while this effect was lost using the day 18 supernatants (Fig. 6B). However, both the day 4 and day 18 supernatant from the Sin a1-IEC-DC-T/B-cell co-culture induced IL-13 secretion by exposed MC (p < 0.05), while this was also the case for the Sin a1-DC-T/B-cell supernatant of day 18 (Fig. 6C). IL-4 was significantly increased in the supernatants of MC exposed to 18 days Sin a1-DC-T/B-cell supernatant (ESI Fig. 5B†). These results reveal Sin a1 exposed IEC/DC or DC to drive an immune cascade, resulting in MC activation as indicated by type 2 cytokine secretion. 18 days T/B-cell supernatant of DC2 primed T-cells also facilitated anti-IgE provoked MC degranulation, while day 4 supernatant enhanced IL-13 secretion by MC and a similar pattern was observed with the day 18 supernatant (Fig. 6A–C). The background in MC degranulation upon anti-IgE exposure was negligible, while IgE exposed MC showed a percentage of degranulation of more than 60%, while tending to increase IL-13 secretion (n = 1, data not shown).
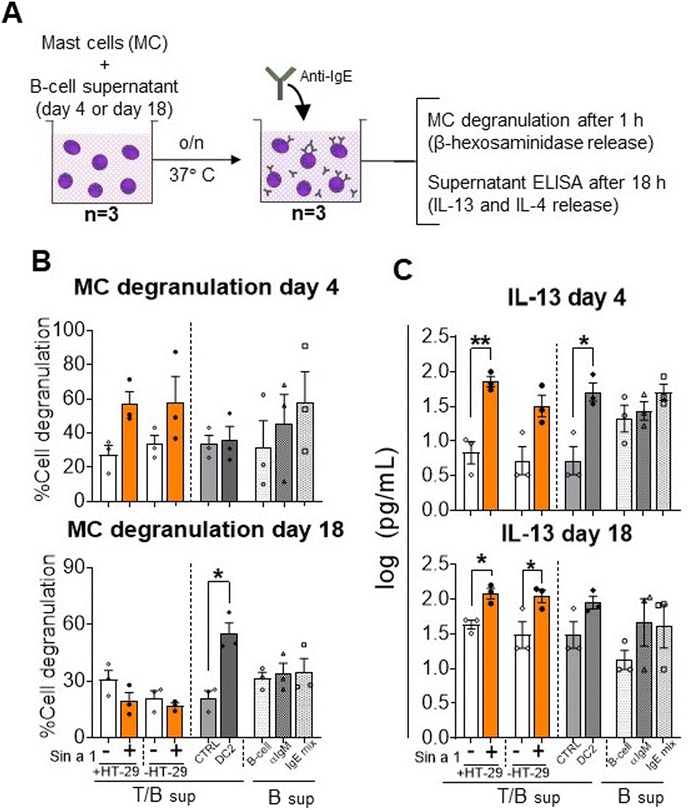 | ||
| Fig. 6 Mast cell degranulation and IL-13 release. (A) Mast cells were cultured overnight in the supernatant from T-cell/B-cell co-culture (n = 3 independent T/B cell donors) of T-cells that were previously exposed to primed DC from Sin a1 exposed IECs or DCs directly exposed to Sin a1. Mast cells were washed and incubated with anti-IgE. Mast cell degranulation and activation were determined by β-hexosaminidase release (B), and IL-13 levels (C), respectively. Data were analysed by one-way ANOVA, or by paired t-test for CTRL vs. DC2, mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001. IL-4 data are shown in ESI Fig. 5.† | ||
Discussion and conclusions
The prevalence of food allergy is rising, especially among children;25 however, the sensitization process is still not completely understood. Reasons of loss of oral tolerance remain unknown; however, it is generally accepted that it starts with epithelial barrier impairment2 and epithelial activation. This allows DC activation, while allergenic proteins interact with the mucosal immune system, which may result in a type-2 pro-inflammatory effect leading to allergic sensitization.Allergen interaction with IEC has been addressed in the past, and mainly focused on the transport of proteins across the epithelial barrier.19,26,27 However, little information can be found regarding epithelial activation and further type-2 immune polarization.28,29 Murine models of peanut and egg allergy are already available,30,31 and a similar model could be developed for Sin a1; however, results may be affected by mouse strains and the sensitization route.32,33 Moreover, the actual politics towards animal experimentation makes necessary the development of suitable in vitro tools that, if not replace murine models, reduce their use.34,35 In the present study, a novel developed sequential human in vitro co-culture model was used to study sensitizing allergenicity.21 Similar to the reported results for egg allergen ovalbumin, Sin a1 was capable of independently activating IECs and DCs, first step in allergy development. Sin a1 was washed after incubation with IEC and/or DC. Both IECs and DCs were however activated by the previous exposure to Sin a1 to promote secretion of type 2 mediators. Moreover, this activation leads to generic type-2 T-cells that prime B-cells to produce more IgG. Even though no increase in IgE was observed, the mast cell supernatant did facilitate anti-IgE-induced MC activation, indicating a functional IgE response could be triggered by upstream Sin a1 exposure of IEC/DC or DC. These findings show the intrinsic capacity of Sin a1 to promote type 2 immunity, either via IEC (structural cells) or directly via DC (innate immune cells) in a similar manner.
Direct exposure of IEC to Sin a1 induced epithelial activation and the release of CCL20 and IL-33, key mediators in starting a type-2 immune response. These results were in line with those reported for Pru p 3,28 a major allergen from peach; however, cytokine profiles were determined at the mRNA level. Only isolated Sin a1 and not YMSE, containing the same amount of target allergen, induced epithelial activation into a type-2 profile. YMSE contains not only allergenic proteins but also other components, such as polyphenols and pigments, that may interfere in the epithelial response to allergens.36 Even though within its matrix, Sin a1 did not activate IEC, in vivo Sin a1 may be released from the protective food matrix as it may escape from digestion,37 allowing its allergenic properties.38,39
HT-29 and Caco-2 have been generally used to test allergen transport and epithelial activation.40 In the current study, we used both cell lines to study the effects of Sin a1. As Caco-2 cells are known to polarize in culture and the gold standard in performing intestinal barrier studies, we studied effects of Sin a1 on TEER and functional permeability (4 kDa FITC dextran) in these cells. Sin a1 was not consistently found to affect epithelial barrier properties (ESI Fig. 3†). However, other 2S albumins have been reported to cross epithelial lining via transepithelial transport,18,19 hence also without affecting tight junction permeability. Beyond studying barrier effects, the purpose of our studies was to study the capability of Sin a1 to activate epithelial cells. For this purpose, we used HT29 and Caco-2 cells. In both cell lines, Sin a1 was able to enhance CCL20 and IL33 release; albeit these effects were most strong in HT-29 which showed greater levels in the release of these type 2 mediators. Therefore, the HT-29 cells were used for the follow up studies, to study the effect of epithelial activation on DC phenotype and function and consequent type 2 immune development. This novel mucosal immune model represents a first step in studying the possible contribution of IEC in sensitizing allergenicity of food allergens. In this model, cell lines were used, which are of carcinogenic origin, and therefore may not be representative of physiological conditions regarding type-2 response development due to their inflammatory background.41,42 Future studies may therefore make use of primary human IEC cultures to further validate the use of these generally used model cell lines to study sensitizing allergenicity of proteins to predict risk on food allergy.43 Nonetheless, primary intestinal epithelial cell cultures have constraints in terms of availability, since obtaining these cultures usually requires special permits for biopsies from donors. Another alternative is intestinal organoids; however, these are of foetal origin, which may not be representative in the case of mustard seed allergy since this is not an early-life allergy. Furthermore, organoids require complex high-priced treatment to expose their apical side and need 2D culture conditions.44 For these reasons, the epithelial models employed in the present study may represent an easy-to-use and economy-wise first approximation in identifying type 2 driving capacity of food proteins.
Regarding the immune response driven by Sin a1, the allergen induced the release of type-2 mediators by DC, such as CCL20, IL-15 and IL-25, independent of IEC and a similar pattern was observed for IL-8 and TSLP.45–47 Moreover, a similar response was observed upon Sin a1 exposure via IEC in the IEC/DC coculture; however, here the contribution of IECs in DC priming remains unclear. Nevertheless, previous data from our group demonstrated that allergen pre-exposed IECs were capable of priming DCs, inducing a shift in the immune response towards the type-2 profile.48 Furthermore, the outcome of the T-cell and mast cell response did differ in some aspects, indicating that Sin a1 exposure to IEC/DC may have modified the function of the primed DCs when compared to direct exposure of DCs. Although IL-33, TSLP and IL-25 release is canonically attributed to IECs, recent studies have described that DCs can produce these cytokines in a type-2 environment.49 On the other hand, control DCs exposed to LPS or DC2 cytokine mix showed increased secretion of CCL20 and/or CCL22. Sin a1 therefore differentially affected DC properties compared to these controls. In contrast to studies using OVA,21 Sin a1 did enhance DC maturation markers. Beyond the difference in dose, and the OVA source containing some LPS contamination, also differences in allergen structural characteristics between Sin a1 and OVA may explain these discrepancies.9,50 Moreover, Sin a1-primed IEC-DCs and DCs were functionally affected since they were capable of inducing IL-4 release by T-cells. Furthermore, intracellular IL-13 expression in T-cells was significantly enhanced after their co-culture with Sin a1-IEC-DC, while the Sin a1-DC/T-cell condition decreased the frequency of Th1 (CXCR3) cells and increased Th2 (CRTH2) over Th1 balance. These findings reinforced the idea of Sin a1 induced epithelial activation, shifting the immune balance towards the type-2 profile.51 However, also in the absence of IECs, Sin a1 was able to affect DC properties leading to type 2 T-cell characteristics, indicating that Sin a1 activation of IECs is not an absolute requirement to enable mucosal sensitization to Sin a1.
When addressing the interaction of T-cells with B-cells, no significant changes were observed in the expression of activation markers, nor in the IgE levels. However, the DC2-T/B-cell controls nor B-cell controls that were found to enhance B-cell maturation (CD27 expression) and/or activation (CD25 expression) also did not show enhanced IgE or IgG secretion. In contrast, in previous studies, OVA exposed IEC-DC-T/B-cells were found to secrete IgE after 18 days.21 Sin a1 exposed IEC-DC-T/B-cells or Sin a1 DC-T/B-cells did show increased total IgG in the supernatants, already after day 4 of co-culture. Class-switching from IgM to IgE is a sequential process, obtaining IgG as intermediate necessary for affinity maturation of the antibody.52 However, after 18 days of co-culture, still only increase in IgG was detected.
However, even though Sin a1 exposure to IECs/DCs or DCs did not result in the instruction of B-cells to produce humoral factors facilitating full mast cell degranulation; similar to hen's egg allergen ovalbumin,21 mast cells were activated since IL-13 and IL-4 was released by the mast cells. Indeed IgE receptor crosslinking can lead to mast cell degranulation and/or cytokine secretion. It is known that anti-IgE dependent MC activation could be provoked as shown by IL-13 release.53 This may indicate that IgE present in the Sin a1 condition is functionally different from that in the control condition, leading to higher sensitivity for anti-IgE crosslinking and thus mast cell activation.54 Hence, we hypothesize that in the current set up the Sin a1 signal on IECs/DCs or DCs was not strong enough to fully give rise to increased IgE levels. Therefore, anti-IgE may not be capable of activating the mast cell degranulation pathway, but could induce type 2 cytokine (IL-4 and IL-13) secretion by mast cells which could further drive the pathway of allergic sensitization. Alternatively, other humoral factors present in the B-cell supernatant could influence anti-IgE mediated mast cell degranulation and type 2 activation as well. IgE mediated mast cell degranulation can be affected by regulatory mediators such as IL-10 and TGFβ, or other immunoglobulins such as inhibitory IgG4.55 In addition, future studies could aim to quantify the amount of IgE bound to the mast cells, which may differ between conditions, as indication of sensitization. Thus by including the primary human mast cells in this sequential model to study the sensitizing capacities of Sin a1, it was aimed to gain insight into the full phenotype of B-cell activation and its capacity to provoke allergic symptoms.
In this model, however, generic and not allergen specific mast cell degranulation was studied. Generation of allergen specific IgE would require both T- and B-cells capable of recognizing Sin a1 via either the T-cell or B-cell receptor. Chances of having present naïve T-cells and B-cells that can recognize Sin a1 within a healthy donor population is very small. Hence, in the current assay it was not aimed to study allergen specific responses, but the generic capacity of Sin a1 to drive type 2 responses via activating IECs and/or DCs. Studying allergen specific responses would be possible when making use of PBMC derived from allergic patients which already have more T and B cells in their repertoire to recognize the allergen.56
On the other hand, beyond IgE there are also other factors, such as IL-4 secretion by other immune cells, that can cause mast cell activation.57 IL-4 was detected in supernatants from T/B-cell cultures at day 18, but these were not affected by the Sin a1 conditions, and at day 4, IL-4 was not detected. Still, in MC exposed to these B-cell supernatants, IL-13 release was observed under Sin a1 exposed conditions. However, these were not associated with the presence of IL-4 in the B-cell supernatants, thus IL-4 was not responsible for the mast cell activation upon exposure to the T/B-cell supernatants (ESI Fig. 5†). Alternatively suboptimal activation of mast cells through FcεRI has been described as another possible explanation to cause mast cell activation without degranulation,58,59 in accordance with our results.
The supernatants from DC2-T/B-cell controls were found to facilitate IL-13 production (day 4 and 18) and degranulation (day 18) of MCs upon anti-IgE exposure also implying sensitization of MCs. This suggests that exposure to Sin a1 via IEC-DC or DCs induced sequential immune polarization and immunoglobulin class-switching, resulting in MC priming.
In conclusion, mustard seed allergen Sin a1 was shown to enhance type 2 activation of IECs as well as DCs, leading to type 2 skewing of the T-cells and humoral response in B-cells, facilitating MC activation resulting in IL-4 and IL-13 release. The present model may be further explored to identify the intrinsic activity of food proteins to induce type 2 activation which could lead to sensitization for these proteins. Furthermore, it could help to better understand the sensitization process while enabling exploration of therapeutical approaches for food allergy prevention and treatment.
Author contributions
This study was designed by CBD and LW. Data collection was performed by CBD and MZ. CBD, MZ and LW analysed and interpreted the data. The manuscript was drafted by CBD and critically reviewed by MZ, VAM, SK, LMP, JPB, FR, JG, MV and LW. All listed authors have given approval for publication.Conflicts of interest
The author JG is partly employed by Danone Nutricia Research B. V. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interestAcknowledgements
The authors would like to thank Bart Blokhuis for his kind contribution in providing the primary human mast cell culture and technical support for the β-hexosaminidase assays. The FEBS Short term grant and an EMBO Short term grant made these studies possible. In addition, the studies were financed within the framework of the Dutch government TKI-Health Holland public-private project with the acronym HMOS4ALL, project number LSHM18037.References
- S. H. Sicherer and H. A. Sampson, Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management, J. Allergy Clin. Immunol., 2018, 141(1), 41–58, DOI:10.1016/j.jaci.2017.11.003
.
- C. A. Akdis, H. Renz, K. J. Allen, S. H. Sicherer, H. A. Sampson and G. Lack,
et al., Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions?, Nat. Rev. Immunol., 2021, 21(4), 739–751, DOI:10.1038/s41577-021-00538-7
.
- N. Cerf-Bensussan and V. Gaboriau-Routhiau, The immune system and the gut microbiota: Friends or foes?, Nat. Rev. Immunol., 2010, 10(10), 735–744, DOI:10.1038/nri2850
.
- R. S. Chinthrajah, J. D. Hernandez, S. D. Boyd, S. J. Galli and K. C. Nadeau, Molecular and cellular mechanisms of food allergy and food tolerance, J. Allergy Clin. Immunol., 2016, 137(4), 984–997, DOI:10.1016/j.jaci.2016.02.004
.
- W. Yu, D. M. H. Freeland and K. C. Nadeau, Food allergy: Immune mechanisms, diagnosis and immunotherapy, Nat. Rev. Immunol., 2016, 16(12), 751–765, DOI:10.1038/nri.2016.111
.
- J. H. M. Van Bilsen, E. Sienkiewicz-Szłapka, D. Lozano-Ojalvo, L. E. M. Willemsen, C. M. Antunes, E. Molina, J. J. Smit, B. Wróblewska, H. J. Wichers, E. F. Knol, G. S. Ladics, R. H. H. Pieters, S. Denery-Papini, Y. M. Vissers, S. L. Bavaro, C. Larré, K. C. M. Verhoeckx and E. L. Roggen, Application of the adverse outcome pathway (AOP) concept to structure the available in vivo and in vitro mechanistic data for allergic sensitization to food proteins, Clin. Transl. Allergy, 2017, 7, 13, DOI:10.1186/s13601-017-0152-0
.
- B. I. Nwaru, L. Hickstein, S. S. Panesar, G. Roberts, A. Muraro and A. Sheikh, Prevalence of common food allergies in Europe: A systematic review and meta-analysis, Allergy Eur. J. Allergy Clin. Immunol., 2014, 69(8), 992–1007, DOI:10.1111/all.12423
.
- L. Zuidmeer, K. Goldhahn, R. J. Rona, D. Gislason, C. Madsen, C. Summers, E. Sodergren, J. Dahlstrom, T. Lindner, S. T. Sigurdardottir, D. McBride and T. Keil, The prevalence of plant food allergies: A systematic review, J. Allergy Clin. Immunol., 2008, 121(5), 1210–1218.e4, DOI:10.1016/j.jaci.2008.02.019
.
- J. Costa, S. L. Bavaro, S. Benedé, A. Diaz-Perales, C. Bueno-Diaz and E. Gelencser,
et al., Are Physicochemical Properties Shaping the Allergenic Potency of Plant Allergens?, Clin. Rev. Allergy Immunol., 2020, 62(1), 37–63, DOI:10.1007/s12016-020-08810-9
.
- M. Morisset, D. A. Moneret-Vautrin, F. Maadi, S. Frémont, L. Guénard and A. Croizier,
et al., Prospective study of mustard allergy: First study with double-blind placebo-controlled food challenge trials (24 cases), Allergy Eur. J. Allergy Clin. Immunol., 2003, 58(4), 295–299, DOI:10.1034/j.1398-9995.2003.00074.x
.
- A. Sharma, A. K. Verma, R. K. Gupta, Neelabh and P. D. Dwivedi, A Comprehensive Review on Mustard-Induced Allergy and Implications for Human Health, Clin. Rev. Allergy Immunol., 2019, 57(1), 39–54, DOI:10.1007/s12016-017-8651-2
.
- M. Gamella, C. Bueno-Díaz, V. Ruiz-Valdepeñas Montiel, E. Povedano, A. J. Reviejo and M. Villalba,
et al., First electrochemical immunosensor for the rapid detection of mustard seeds in plant food extracts, Talanta, 2020, 219, 121247, DOI:10.1016/j.talanta.2020.121247
.
- A. Vereda, S. Sirvent, M. Villalba, R. Rodríguez, J. Cuesta-Herranz and O. Palomares, Improvement of mustard (Sinapis alba) allergy diagnosis and management by linking clinical features and component-resolved approaches, J. Allergy Clin. Immunol., 2011, 127(5), 1304–1307, DOI:10.1016/j.jaci.2011.01.020
.
- B. Cabanillas and N. Novak, Effects of daily food processing on allergenicity, Crit. Rev. Food Sci. Nutr., 2019, 59(1), 31–42, DOI:10.1080/10408398.2017.1356264
.
-
C. Bueno-Díaz, L. Martín-Pedraza, J. Parrón, J. Cuesta-Herranz, B. Cabanillas and C. Pastor-Vargas, et al., Characterization of Relevant Biomarkers for the Diagnosis of Food Allergies: An Overview of the 2S Albumin Family, 2021, DOI:10.3390/foods10061235
.
- M. Oñaderra, R. I. Monsalve, J. M. Mancheño, M. Villalba, A. M. Del Pozo and J. G. Gavilanes,
et al., Food mustard allergen interaction with phospholipid vesicles, Eur. J. Biochem., 1994, 225(2), 609–615, DOI:10.1111/j.1432-1033.1994.00609.x
.
- L. Mirotti, E. Florsheim, L. Rundqvist, G. Larsson, F. Spinozzi and M. Leite-De-Moraes,
et al., Lipids are required for the development of Brazil nut allergy: The role of mouse and human iNKT cells, Allergy Eur. J. Allergy Clin. Immunol., 2013, 68(1), 74–83, DOI:10.1111/all.12057
.
- D. Price, L. Ackland and C. Suphioglu, Nuts “n” guts: transport of food allergens across the intestinal epithelium, Asian Pac. Allergy, 2013, 3(4), 257–265, DOI:10.5415/apallergy.2013.3.4.257
.
- F. J. Moreno, L. A. Rubio, A. Olano and A. Clemente, Uptake of 2S albumin allergens, Ber e 1 and Ses i 1, across human intestinal epithelial Caco-2 cell monolayers, J. Agric. Food Chem., 2006, 54(22), 8631–8639, DOI:10.1021/jf061760h
.
- D. Lozano-Ojalvo, C. Berin and L. Tordesillas, Immune basis of allergic reactions to food, J. Invest. Allergol. Clin. Immunol., 2019, 29(1), 1–14, DOI:10.18176/jiaci.0355
.
- M. Zuurveld, C. B. Díaz, F. Redegeld, G. Folkerts, J. Garssen and B. van’t Land,
et al., An advanced in vitro human mucosal immune model to predict food sensitizing allergenicity risk: A proof of concept using ovalbumin as model allergen, Front. Immunol., 2023, 13, 1073034, DOI:10.3389/fimmu.2022.1073034
.
- M. González de la Peña, L. Menéndez-Arias, R. I. Monsalve and R. Rodríguez, Isolation and characterization of a major allergen from oriental mustard seeds, Braj I, Int. Arch. Allergy Appl. Immunol., 1991, 96(3), 263–270, DOI:10.1159/000235505
.
- J. Folkerts, F. Redegeld, G. Folkerts, B. Blokhuis, M. P. M. van den Berg and M. J. W. de Bruijn,
et al., Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling, Allergy Eur. J. Allergy Clin. Immunol., 2020, 75(8), 1962–1974, DOI:10.1111/all.14254
.
- T. Hoppenbrouwers, J. H. Cvejić Hogervorst, J. Garssen, H. J. Wichers and L. E. M. Willemsen, Long chain polyunsaturated fatty acids (LCPUFAs) in the prevention of food allergy, Front. Immunol., 2019, 10, 1118, DOI:10.3389/fimmu.2019.01118
.
- W. Loh and M. L. K. Tang, The epidemiology of food allergy in the global context, Int. J. Environ. Res. Public Health, 2018, 15(9) DOI:10.3390/ijerph15092043
.
- S. E. Howe, D. J. Lickteig, K. N. Plunkett, J. S. Ryerse and V. Konjufca, The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine, PLoS One, 2014, 9(1), 1–11, DOI:10.1371/journal.pone.0086656
.
- D. B. Price, M. L. Ackland, W. Burks, M. I. Knight and C. Suphioglu, Peanut allergens alter intestinal barrier permeability and tight junction localisation in Caco-2 cell cultures, Cell. Physiol. Biochem., 2014, 33(6), 1758–1777, DOI:10.1159/000362956
.
- L. Tordesillas, C. Gómez-Casado, M. Garrido-Arandia, A. Murua-García, A. Palacín and J. Varela,
et al., Transport of Pru p 3 across gastrointestinal epithelium - an essential step towards the induction of food allergy?, Clin. Exp. Allergy, 2013, 43(12), 1374–1383, DOI:10.1111/cea.12202
.
- L. Tordesillas, N. Cubells-Baeza, C. Gómez-Casado, C. Berin, V. Esteban and W. Barcik,
et al., Mechanisms underlying induction of allergic sensitization by Pru p 3, Clin. Exp. Allergy, 2017, 47(11), 1398–1408, DOI:10.1111/cea.12962
.
- Y. Jin, K. E. Guzmán, A. P. Boss, V. Gangur and C. E. Rockwell, The protective effect of butylated hydroxytoluene and 3-hydroxytyrosol on food allergy in mice, Immunopharmacol. Immunotoxicol., 2023, 45(4), 426–432, DOI:10.1080/08923973.2022.2160732
.
- J. M. Sobczak, P. S. Krenger, F. Storni, M. O. Mohsen, I. Balke and G. Reseviča,
et al., The next generation virus–like particle platform for the treatment of peanut allergy, Allergy, 2023, 78(7), 1980–1996, DOI:10.1111/all.15704
.
- M. Paolucci, V. Homère, Y. Waeckerle-Men, N. Wuillemin, D. Bieli and N. Pengo,
et al., Strain matters in mouse models of peanut-allergic anaphylaxis: Systemic IgE-dependent and Ara h 2-dominant sensitization in C3H mice, Clin. Exp. Allergy, 2023, 53(5), 550–560, DOI:10.1111/cea.14279
.
- M. Briard, M. Guinot, M. Grauso, B. Guillon, S. Hazebrouck and H. Bernard,
et al., Route of Sensitization to Peanut Influences Immune Cell Recruitment at Various Mucosal Sites in Mouse: An Integrative Analysis, Nutrients, 2022, 14(4), 790, DOI:10.3390/nu14040790
.
- N. Shanks, R. Greek and J. Greek, Are animal models predictive for humans?, Philos. Ethics Humanit. Med., 2009, 15(4) DOI:10.1186/1747-5341-4-2
.
- B. Garthoff, Alternatives to animal experimentation: The regulatory background, Toxicol. Appl. Pharmacol., 2005, 207(2), 388–392, DOI:10.1016/j.taap.2005.02.024
.
- S. Kobayashi, J. Watanabe, E. Fukushi, J. Kawabata, M. Nakajima and M. Watanabe, Polyphenols from some foodstuffs as inhibitors of ovalbumin permeation through Caco-2 cell monolayers, Biosci., Biotechnol., Biochem., 2003, 67(6), 1250–1257, DOI:10.1271/bbb.67.1250
.
- S. Sirvent, O. Palomares, J. Cuesta-Herranz, M. Villalba and R. Rodríguez, Analysis of the structural and immunological stability of 2S albumin, nonspecific lipid transfer protein, and profilin allergens from mustard seeds, J. Agric. Food Chem., 2012, 60(23), 6011–6018, DOI:10.1021/jf300555h
.
- I. Prodic, D. Stanic-Vucinic, D. Apostolovic, J. Mihailovic, M. Radibratovic and J. Radosavljevic,
et al., Influence of peanut matrix on stability of allergens in gastric-simulated digesta: 2S albumins are main contributors to the IgE reactivity of short digestion-resistant peptides, Clin. Exp. Allergy, 2018, 48(6), 731–740, DOI:10.1111/cea.13113
.
- J. M. Aguilera, The food matrix: implications in processing, nutrition and health, Crit. Rev. Food Sci. Nutr., 2019, 59(22), 3612–3629, DOI:10.1080/10408398.2018.1502743
.
- M. Gavrovic-Jankulovic and L. E. M. Willemsen, Epithelial models to study food allergen-induced barrier disruption and immune activation, Drug Discovery Today: Dis. Models, 2015, 17–18, 29–36, DOI:10.1016/j.ddmod.2016.09.002
.
-
D. Martínez-Maqueda, B. Miralles and I. Recio. HT29 cell line, in The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models. 2015. DOI:10.1007/978-3-319-16104-4_11
.
- Y. Sambuy, I. De Angelis, G. Ranaldi, M. L. Scarino, A. Stammati and F. Zucco, The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics, Cell Biol. Toxicol., 2005, 21(1), 1–26, DOI:10.1007/s10565-005-0085-6
.
- A. Castellanos-Gonzalez, M. M. Cabada, J. Nichols, G. Gomez and A. C. White, Human primary intestinal epithelial cells as an improved in vitro model for cryptosporidium parvum infection, Infect. Immun., 2013, 81(6), 1996–2001, DOI:10.1128/iai.01131-12
.
- J. Costa and A. Ahluwalia, Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest, Front. Bioeng. Biotechnol., 2019, 7, 456869, DOI:10.3389/fbioe.2019.00144
.
- G. Varricchi, A. Pecoraro, G. Marone, G. Criscuolo, G. Spadaro and A. Genovese,
et al., Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer, Front. Immunol., 2018, 9(JUL), 400340, DOI:10.3389/fimmu.2018.01595
.
- C. Afferni, C. Buccione, S. Andreone, M. R. Galdiero, G. Varricchi, G. Marone, F. Mattei and G. Schiavoni, The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity, Front. Immunol., 2018, 9(9) DOI:10.3389/fimmu.2018.02601
.
- J. B. Lee, C. Y. Chen, B. Liu, L. Mugge, P. Angkasekwinai and V. Facchinetti,
et al., IL-25 and CD4+ TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy, J. Allergy Clin. Immunol., 2016, 137(4), 1216–1225.e5, DOI:10.1016/j.jaci.2015.09.019
.
- M. Zuurveld, P. C. J. Kiliaan, S. E. L. Van Grinsven, G. Folkerts, J. Garssen, T. Van, B. Land and L. E. M. Willemsen, Ovalbumin-Induced Epithelial Activation Directs Monocyte-Derived Dendritic Cells to Instruct Type 2 Inflammation in T Cells Which Is Differentially Modulated by 2′-Fucosyllactose and 3-Fucosyllactose, J. Innate Immun., 2022, 15(1), 222–239, DOI:10.1159/000526528
.
- R. Ebina-Shibuya and W. J. Leonard, Role of thymic stromal lymphopoietin in allergy and beyond, Nat. Rev. Immunol., 2022, 23(1), 24–37, DOI:10.1038/s41577-022-00735-y
.
- J. Costa, C. Villa, K. Verhoeckx, T. Cirkovic and D. Schrama, Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens?, Clin. Rev. Allergy Immunol., 2021, 62(1), 1–36, DOI:10.1007/s12016-020-08826-1
.
- A. B. Blázquez and M. C. Berin, Gastrointestinal Dendritic Cells Promote Th2 Skewing via OX40L, J. Immunol., 2008, 180(7), 4441–4450, DOI:10.4049/jimmunol.180.7.4441
.
- H. Xiong, J. Dolpady, M. Wabl, M. A. C. de Lafaille, J. J. Lafaille and A. Muraro,
et al., Sequential class switching is required for the generation of high affinity IgE antibodies, J. Exp. Med., 2012, 209(2), 353–364, DOI:10.1084/jem.20111941
.
- J. J. A. McLeod, B. Baker and J. J. Ryan, Mast cell production and response to IL-4 and IL-13, Cytokine, 2015, 75(1), 57–61, DOI:10.1016/j.cyto.2015.05.019
.
- K. Plattner, M. F. Bachmann and M. Vogel, On the complexity of IgE: The role of structural flexibility and glycosylation for binding its receptors, Front. Allergy, 2023, 4, 32, DOI:10.3389/falgy.2023.1117611
.
- L. Tordesillas and M. C. Berin, Mechanisms of Oral Tolerance, Clin. Rev. Allergy Immunol., 2018, 55(2), 107–117, DOI:10.1007/s12016-018-8680-5
.
- S. M. Hayen, H. G. Otten, S. A. Overbeek, A. C. Knulst, J. Garssen and L. E. M. Willemsen, Exposure of intestinal epithelial cells to short- and long-chain fructo-oligosaccharides and CpG oligodeoxynucleotides enhances peanut-specific T Helper 1 polarization, Front. Immunol., 2018, 9, 923, DOI:10.3389/fimmu.2018.00923
.
- H. C. Oettgen, Mast cells in food allergy: Inducing immediate reactions and shaping long-term immunity, J. Allergy Clin. Immunol., 2023, 151(1), 21–25, DOI:10.1016/j.jaci.2022.10.003
.
- O. T. Burton, A. R. Darling, J. S. Zhou, M. Noval-Rivas, T. G. Jones and M. F. Gurish,
et al., Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy, Mucosal Immunol., 2013, 6(4), 740–750, DOI:10.1038/mi.2012.112
.
- M. Huber, Activation/Inhibition of mast cells by supra-optimal antigen concentrations, Cell Commun. Signaling, 2013, 11(1), 1–11, DOI:10.1186/1478-811x-11-7
.
Footnote |
| † Electronic supplementary information (ESI) available: Details. See DOI: https://doi.org/10.1039/d4fo01980f |
| This journal is © The Royal Society of Chemistry 2024 |