Open Access Article
Open Access ArticleLacticaseibacillus paracasei 207-27 alters the microbiota–gut–brain axis to improve wearable device-measured sleep duration in healthy adults: a randomized, double-blind, placebo-controlled trial†
Jinxing
Li‡
 a,
Jincheng
Zhao‡
a,
Xiaolei
Ze
b,
Liang
Li
b,
Yapeng
Li
a,
Zhimo
Zhou
a,
Simou
Wu
a,
Wen
Jia
a,
Meixun
Liu
a,
Yun
Li
a,
Xi
Shen
a,
Fang
He
*a and
Ruyue
Cheng
a,
Jincheng
Zhao‡
a,
Xiaolei
Ze
b,
Liang
Li
b,
Yapeng
Li
a,
Zhimo
Zhou
a,
Simou
Wu
a,
Wen
Jia
a,
Meixun
Liu
a,
Yun
Li
a,
Xi
Shen
a,
Fang
He
*a and
Ruyue
Cheng
 *a
*a
aDepartment of Nutrition and Food Hygiene, West China School of Public Health and West China Fourth Hospital, Sichuan, University, Chengdu 610041, China. E-mail: hf18602880124@163.com; ruyuecheng1993@163.com; Tel: +86-186-0288-0124 Tel: +86-18011462163
bBYHEALTH Institute of Nutrition & Health, Guangzhou 510663, China
First published on 10th October 2024
Abstract
Objective: Probiotics have been reported to exert beneficial effects on sleep through the gut–brain axis. Therefore, this randomized, double-blind, placebo-controlled trial assessed the effects of Lacticaseibacillus paracasei 207-27 supplementation on sleep quality and its safety and potential mechanisms. Method and study design: Healthy adults under mild stress aged 18–35 years consumed low or high doses of L. paracasei 207-27 or a placebo for 28 days. Fecal samples, blood samples, and questionnaires were collected at the baseline and the end of the intervention. Sleep quality was measured using wearable devices and Pittsburgh sleep quality index (PSQI) questionnaire. Serum inflammatory markers, corticotropin-releasing hormone, adrenocorticotropic hormone (ACTH), cortisol (COR), γ-aminobutyric acid, and 5-hydroxytryptamine levels were detected using enzyme-linked immunosorbent assay. The gut microbiota was analyzed using 16S rRNA sequencing and bioinformatics. Short-chain fatty acids levels were detected using gas chromatography–mass spectrometry. Results: Both the low-dose and high-dose groups exhibited significant improvements in wearable device- measured sleep duration compared to the placebo group. The global scores of PSQI in three groups significantly decreased after intervention without statistical difference between groups. At the phylum level, the low-dose group exhibited a higher relative abundance of Bacteroidota and a lower Firmicutes-to-Bacteroidetes (F/B) ratio. At the genus level, two treatment groups had higher relative abundance of Bacteroides and Megamonas, alongside lower levels of Escherichia-Shigella. Furthermore, the low-dose group exhibited significant increases in acetic acid, propionic acid, butyric acid, and valeric acid levels, while two treatment groups exhibited a significant decrease in COR levels. Correlation analysis revealed that the increased levels of acetic acid and butyric acid in the low-dose group may be associated with decreased ACTH. Conclusion: L. paracasei 207-27 administration in healthy adults resulted in improvements in gut microbiota community and sleep duration. The mechanisms might involve modulation of the gut microbiota structure to regulate the function of the gut–brain axis, including increases in SCFA levels and decreases in hypothalamic–pituitary–adrenal axis activity. The Chinese clinical trial registry number is ChiCTR2300069453 (https://www.chictr.org.cn/showproj.html?proj=191193, registered 16 May 2023 - retrospectively registered).
Introduction
The human digestive tract contains trillions of extremely diverse microorganisms.1 The gut microbiota serves as part of the intestinal barrier, and it can modulate the brain.2 The microbiota–gut–brain axis consists of the gut microbiota, metabolites, the enteric nervous system, the sympathetic and parasympathetic branches of the autonomic nervous system, the neural–immune system, the neuroendocrine system, and the central nervous system.3,4 There are five communication pathways between the gut microbiota and the brain, including the gut–brain neural network, the hypothalamic–pituitary–adrenal (HPA) axis, the gut immune system, some neurotransmitters synthesized by the gut microbiota, and barrier pathways including the intestinal mucosal barrier and blood–brain barrier.5,6 Sleep is widely acknowledged as a fundamental physiological process intricately regulated by complex neurobiological mechanisms. Recent scientific investigations have shed light on the potential role of the gut microbiota as a key modulator of the intricate regulatory pathways underlying sleep.7,8The World Health Organization defines probiotics as “live microorganisms that confer a health benefit on the host when administered in adequate quantities”.9 Probiotics have gained increasing attention for their ability to modulate brain health via the microbiota–gut–brain axis.10,11 Indeed, probiotic strains can regulate the gut microbiota and HPA axis hormones in humans, both of which are associated with sleep disorders.12 Numerous investigations have demonstrated that Lactobacillus species can improve sleep quality by influencing the gut–brain axis.13,14 However, data regarding the sleep-improving effect of Lacticaseibacillus paracasei are insufficient and heterogeneous because of the lack of microbiome analyses and the limited sample size and strain-specific effects of probiotics.
L. paracasei 207-27 was originally isolated from healthy infant feces in China and it is now deposited at the Guangdong Microbial Culture Collection Center (GDMCC) under the Budapest Treaty, with deposit code GDMCC 60960. L. paracasei 207-27 is also named Lacticaseibacillus paracasei LPB27 in its commercialized product. In our previous in vitro and in vivo studies, we found that L. paracasei 207-27 has good probiotic properties, and it has been considered a candidate probiotic strain. Furthermore, L. paracasei 207-27 can modulate gut microbiota composition and the levels of its metabolites short-chain fatty acids (SCFAs), and it exhibited an anti-allergy effect in OVA-induced allergic mice.15–17 However, the safety and efficacy of L. paracasei 207-27 in humans remain unstudied. To determine the safety of L. paracasei 207-27 in humans and its effects on the physiological activities of the gut–brain axis such as sleep, we conducted a randomized, double-blind, placebo-controlled trial. In addition, we aimed to elucidate the potential mechanisms of the effects of L. paracasei 207-27 by identifying the alterations and associations among gut microbiota, metabolites, and relevant hormones.
Materials and methods
Study design
We conducted a randomized, double-blind, placebo-controlled trial examining the effects of L. paracasei 207-27 consumption on sleep quality in adults under mild stress aged 18–35. Study researchers who were not involved in the intervention process generated a random sequence using Excel, and the random numbers were stratified by sex with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 allocation. The allocation sequence was concealed from the researchers, and details of the allocated groups were given on number code containing the sequential numbers prepared by the product providers. All participants and researchers were blinded to study allocation throughout the entire study. The study was unblinded after all statistical analyses were completed. Subjects were randomly distributed into three groups (n = 40 per group): placebo group, low-dose L. paracasei group (1 × 1010 colony-forming units [CFU]) and high-dose L. paracasei group (5 × 1010 CFU) group.
3 allocation. The allocation sequence was concealed from the researchers, and details of the allocated groups were given on number code containing the sequential numbers prepared by the product providers. All participants and researchers were blinded to study allocation throughout the entire study. The study was unblinded after all statistical analyses were completed. Subjects were randomly distributed into three groups (n = 40 per group): placebo group, low-dose L. paracasei group (1 × 1010 colony-forming units [CFU]) and high-dose L. paracasei group (5 × 1010 CFU) group.
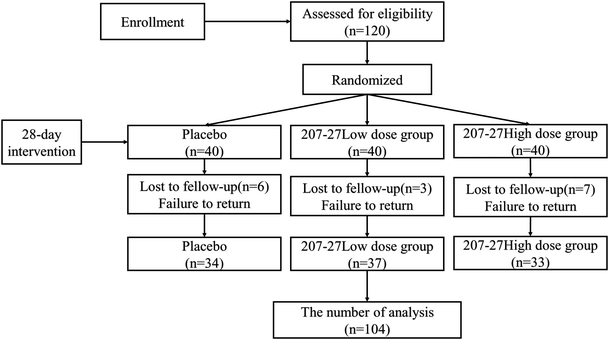
The study consisted of an intervention and a post-observation, as presented in Fig. 1. Participants were asked to wear a wearable device (Huawei B6, Huawei, Guangdong, China) and make no changes to their exercise and eating habits during the study. Participants consumed one sachet of the study product per day after having lunch for 28 consecutive days. The wearable device data, blood samples, fecal samples, and subjects’ questionnaires were collected on days 0 and 28.
Sample size calculation
The sample size was determined using the superiority design method (the sample size was calculated when the outcome measure was a continuous variable).18 We estimated the sample size based on the total PSQI scores of differences after probiotic treatment in published papers (pre: 11.6 ± 3.1, post: 7.75 ± 3.6, P = 0.0007).19 The minimal required sample size was calculated as 96 subjects (n = 32 per group) using the two-sample, continuous outcome, two-tailed, independent t-test approach with a significance level of 5% and power of 90%. Considering a withdrawal rate of 20%, we planned to enroll 120 subjects (n = 40 per group).Subjects
The test subjects were 120 healthy participants (30 men and 90 women). Participants were excluded if they had any known disease or were taking medicine for them, including metabolic arthritis, heart/cardiopulmonary disease, diabetes, hypertension, and constipation. All participants were prohibited from taking probiotics, prebiotics, fermented products (yogurt or foods), antibiotics, and anti-inflammatory medications to avoid unnecessary interference during the experimental period.Study products
L. paracasei 207-27, a human-derived Lactobacillus strain, was cultivated and produced by BY-HEALTH (Guangdong, China). The product of this study consisted of 1 × 1010 or 5 × 1010 CFU of L. paracasei 207-27, and the content was standardized using maltodextrin in sachet packing. The placebo powder contained maltodextrin similarly as the L. paracasei 207-27 powder without the addition of the probiotic. Study products could not be distinguished by package, color, taste, and smell to maintain treatment allocation concealment from participants and study staff. To take the product, participants mixed one sachet with cold water.Data and sample collection
Participants recorded a food diary for 2 days (one workday and one weekend day) each week using the Huawei health application, which automatically accessed energy and macronutrient consumption. The wearable device was used daily to track physical activity. Participants were required to uploaded a week-period energy intake, and energy consumption data to the investigator before the beginning and the end of the intervention.
Outcome assessments
In Deng's investigation, the Huawei smartwatch was employed to assess the sleep quality of the subjects.26 Xie's study performed a comparative examination of several prominent smartwatch models, and the outcomes demonstrated the commendable precision of the Huawei smartwatch in quantifying sleep duration.25 Moreover, the results reported by Liang27 provided additional substantiation regarding the accuracy of the Huawei smartwatch in measuring sleep parameters.
Therefore, the Huawei band 6 was utilized as a non-invasive monitoring tool to obtain objective measurements of sleep attributes, including several key parameters such as the durations of sleep, deep sleep, and rapid eye movement and the number of awakenings. The Huawei band 6 extracted the normal sinus rhythm intervals and respiratory signals from the heart rate signal. It analyzed the coherence and cross-spectral power of these signals using Hilbert-Huang transform (HHT) technology. This process generated a cardiopulmonary coupling (CPC) dynamic spectrum during sleep, which accurately reflected various sleep stages: deep sleep, light sleep, rapid eye movement sleep, and wakefulness.28 The application of this technology in wearable devices enables more convenient, accurate and continuous monitoring of sleep, leading to its increasingly widespread adoption.29–31
Using a wearable device, we collected precise and quantitative sleep data. This objective approach enhanced the accuracy and reliability of our findings, contributing to a more comprehensive understanding of sleep patterns and their potential implications.
After enrollment, all subjects were provided with the Huawei band 6 and instructed to wear it continuously to monitor their sleep from enrollment onwards. The average data collected during the one-week period between enrollment and the start of the intervention was defined as baseline data. Similarly, the average data collected during the week preceding the end of the intervention was defined as outcome data. The sleep duration denoted the total amount of time an individual spent asleep, providing an objective measure of sleep quantity. The number of awakenings indicated the frequency of transitions from sleep to wakefulness throughout the sleep period, reflecting sleep fragmentation and disturbances.
The subsequent data analysis involved a series of rigorous procedures. Initially, the raw sequencing data were demultiplexed according to the barcode sequences and PCR primer sequences, and subsequently, the barcode and primer sequences were trimmed. FLASH software (version 1.2.11)32 was then utilized to merge the reads, resulting in the generation of raw tags. Then, fastp software (version 0.20.0) was employed for quality control to obtain a collection of high-quality clean tags. Subsequently, the clean tags were aligned against a reference database using Vsearch (version 2.9.0) to identify and remove chimeric sequences,33 thereby yielding a set of effective tags representing the valid data. For the obtained effective tags, the DADA2 module within QIIME2 software (Qiime2-202006) was employed for denoising accompanied by the removal of sequences with an abundance smaller than five. Consequently, the amplicon sequence variants (ASVs) and a corresponding feature table were generated. Furthermore, the classify-sklearn module within QIIME2 was utilized to assign species information to each ASV by performing a comparative analysis against a reference database. Furthermore, SVG software (version Perl-5.18.2) was employed to generate a heatmap representing the relative abundance distribution of the species. Alpha diversity indices, including the Chao1, observed_asvs, Shannon, and Simpson indices, were calculated using QIIME2 based on the simplified ASV table. QIIME2 was employed to compute Unifrac distances, and R software (version 2.15.3) was utilized to generate a principal coordinate analysis (PCoA) plot.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 10 min after vortexing for 1 min, and the supernatant was transferred into the vial prior to GC-MS analysis (Agilent 7890A).34
000 rpm and 4 °C for 10 min after vortexing for 1 min, and the supernatant was transferred into the vial prior to GC-MS analysis (Agilent 7890A).34
The categorical variables were presented as absolute numbers and percentages, while continuous variables were expressed as the mean ± standard deviation. Multiple comparisons among the three groups were conducted using one-way analysis of variance or the Kruskal–Wallis H-test, with P values adjusted using the Benjamin–Krieger–Yekutieli method for pairwise comparisons. This adjustment method was also applied to P values in the comparison of multiple taxa in the gut microbiota analysis.
Self-comparison within the groups were conducted using the paired T-test or the Wilcoxon signed-rank test. Spearman correlation analysis was utlized to assess associations between variables.
All statistical analyses were performed using Prism 9.0 (GraphPad, San Diego, CA, USA), and a two-tailed adjusted P-value of less than 0.05 was considered statistically significant.
Results
Participant flow and demographics
As presented in Fig. 2, 120 participants were enrolled in the study, and 104 individuals completed the study (completion rate, 86.67%). No clinically relevant adverse events were reported during the intervention. In all analyses, we included data from participants who completed the intervention. The demographic and clinical characteristics of the participants at baseline and the end of the intervention are summarized in Table 1. The male-to-female ratio did not significantly differ among the groups. The mean ages were 25.02, 25.17, and 24.78 years in the placebo, low-dose, and high-dose groups, respectively, and no significant difference was detected. At baseline and the end of the intervention, no significant differences were observed among the groups in terms of weight, body fat, waistline, hipline, blood pressure, calorie intake, carbohydrates intake, protein intake, fat intake, and energy consumption during exercise. In addition, no significant difference was observed in these variables in the three groups between baseline and the end of the intervention. Therefore, we confirmed that randomization was successful.| Placebo group (n = 34) | Low-dose group (n = 37) | High-dose group (n = 33) | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | 4 weeks | Baseline | 4 weeks | Baseline | 4 weeks | P Baseline | P 4 weeks |
| Sex | ||||||||
| Male | 9(25.7%) | 8(21.6%) | 7(21.2%) | 0.77 | ||||
| Female | 26(74.3%) | 29(78.4%) | 26(78.8%) | 0.86 | ||||
| Age (years) | 25.02 ± 4.32 | 25.17 ± 2.78 | 24.78 ± 3.02 | 0.65 | ||||
| Weight (kg) | 59.96 ± 12.18 | 59.67 ± 12.34 | 57.60 ± 9.10 | 57.24 ± 9.33 | 58.12 ± 11.26 | 57.88 ± 10.93 | 0.63 | 0.62 |
| Body fat (%) | 26.43 ± 6.17 | 25.67 ± 6.2 | 26.41 ± 6.1 | 25.75 ± 6.00 | 27.06 ± 7.50 | 26.41 ± 7.32 | 0.90 | 0.88 |
| Waistline (cm) | 72.91 ± 8.78 | 72.81 ± 9.27 | 71.78 ± 7.67 | 70.85 ± 7.5 | 72.10 ± 9.54 | 71.85 ± 9.25 | 0.86 | 0.62 |
| Hipline (cm) | 94.29 ± 6.97 | 94.03 ± 6.99 | 94.07 ± 5.52 | 93.72 ± 5.70 | 93.76 ± 8.07 | 94.43 ± 7.24 | 0.95 | 0.90 |
| Systolic blood pressure (mmHg) | 114.04 ± 12.06 | 115.50 ± 11.21 | 116.20 ± 10.08 | 115.90 ± 11.73 | 115.50 ± 9.15 | 114.80 ± 8.89 | 0.79 | 0.90 |
| Diastolic blood pressure (mmHg) | 71.21 ± 7.31 | 71.85 ± 8.68 | 73.05 ± 8.16 | 72.05 ± 7.78 | 73.73 ± 6.91 | 72.52 ± 5.73 | 0.36 | 0.93 |
| Calorie intake (kcal) | 1485 ± 446.20 | 1522 ± 514.80 | 1524 ± 386.00 | 1469 ± 418.80 | 1409 ± 392.90 | 1376 ± 489.70 | 0.49 | 0.45 |
| Carbohydrate intake (g) | 194.30 ± 100.40 | 193.30 ± 80.43 | 199.00 ± 65.97 | 179.90 ± 65.76 | 177.80 ± 60.25 | 177.80 ± 85.24 | 0.49 | 0.67 |
| Protein intake (g) | 69.16 ± 29.53 | 75.51 ± 29.31 | 67.85 ± 22.67 | 73.67 ± 35.93 | 65.52 ± 23.62 | 62.19 ± 25.52 | 0.83 | 0.15 |
| Fat intake (g) | 53.94 ± 25.66 | 52.49 ± 22.79 | 54.57 ± 19.24 | 55.31 ± 24.49 | 50.37 ± 17.53 | 48.80 ± 23.81 | 0.68 | 0.52 |
| Energy consumption of exercise (kcal) | 368 ± 190.60 | 366 ± 125.80 | 368.8 ± 130.70 | 359.6 ± 132.20 | 351.1 ± 176.50 | 348.7 ± 109.40 | 0.81 | 0.34 |
Data expressed as mean ± standard deviations. PBaseline were analyzed among three groups at baseline, P4 weeks were analyzed among three groups at the end of intervention.
Safety assessment
Over the entire course of the study, no participant exhibited any untoward gastrointestinal symptoms, such as diarrhea, constipation, vomiting, and regurgitation. Moreover, there were no reported instances of subjective illness perceptions or any form of discomfort. Furthermore, blood lipid profiles, blood glucose levels, serum biochemistry parameters, and complete blood counts were evaluated at baseline and after the intervention to assess any potential changes. The statistical analysis revealed no significant differences in these indices among the three experimental groups (ESI Tables 1 and 2†). The lack of significant differences suggests that the intervention did not significantly affect physiological markers among the study participants.Changes in sleep quality following the probiotic intervention
Significant differences were observed in sleep duration among the three groups. Sleep duration was significantly increased in the low-dose and high-dose groups compared to that in the placebo group (Fig. 3A). Specifically, the mean sleep duration was increased by 1.07 h in the low-dose group and 1.04 h in the high-dose group. However, there were no statistically significant differences observed in the number of awakenings among the three groups (Fig. 3B). Furthermore, although the changes before and after intervention were used for statistical analysis, the original results of pre - and post-intervention comparisons between groups were also accurately presented(ESI Fig. 1†). The PSQI results showed that scores of sleep duration, sleep disturbances in the low-dose group, and global scores in three groups significantly decreased after intervention. However, no significant differences were found in the changes of scores of seven “component” scores and global score between three groups (ESI Table 3†). | ||
| Fig. 3 The changes of sleep duration (A) and awakening times (B) were compared among three groups. **adjusted P < 0.01. | ||
Changes in serum HPA axis hormone levels following the probiotic intervention
The HPA axis, a fundamental component of the gut–brain axis, plays a pivotal role in orchestrating the primary biological response to stressful stimuli. In this study, we quantified the levels of CRH, ACTH and COR to assess the activity of the HPA axis. Significant differences were observed in the changes of CRH levels among the low-dose, high-dose, and placebo groups (Fig. 4A). CRH levels slightly increased in the high-dose group, whereas they decreased in the low-dose and placebo groups. Furthermore, ACTH levels were significantly lower in the low-dose group than in the placebo group (Fig. 4B). In addition, COR levels decreased in the low-dose and high-dose groups but increased in the placebo group (Fig. 4C). | ||
| Fig. 4 The changes of serum levels of CRH (A), ACTH (B) and COR (C) were compared among three groups. *adjusted P < 0.05, **adjusted P < 0.01, ***adjusted P < 0.001. | ||
Changes in serum neurotransmitter levels following the probiotic intervention
The changes in 5-HT and GABA levels during the intervention did not differ among the three groups (Fig. 5A and B).Gut microbiota composition
The relative abundance of specific bacterial strains at both the phylum and genus levels on day 0 and 28 is presented in Fig. 6A and B, as well as in ESI Tables 4 and 5.†At the phylum level, the low-dose group exhibited a significantly higher relative abundance of Bacteroidota than the placebo group (Fig. 6C), whereas the Firmicutes-to-Bacteroidetes (F/B) ratio was significantly lower in the low-dose group than in the placebo group (Fig. 6D).
At the genus level, the relative abundance of Blautia was significantly lower in the low-dose group than in the placebo group (Fig. 6E). Moreover, the relative abundances of Escherichia-Shigella and Megamonas were significantly higher in both the low-dose and high-dose groups than in the placebo group (Fig. 6F and G), whereas Bacteroides was significantly lower (Fig. 6H).
We further analyzed the changes of relative abundance of gut microbiota among groups. Change of relative abundance of Blautia in the low-dose group (−2.951% ± 3.821%) was significantly lower than in the placebo group (1.610% ± 5.297%). The relative abundance of Lactobacillus was increased in the low-dose (0.166% ± 0.784%) and high-dose groups (0.136% ± 0.317%), while decreased in the placebo group (−0.886% ± 1.659%) after intervention. Compared with the placebo group, changes of relative abundance of Lactobacillus in the low-dose and high-dose groups were statistically significant. The relative abundance of Clostridia_UCG_014 decreased in the high-dose groups (−0.110% ± 3.246%), while increased in the placebo (2.146% ± 5.284%) and low-dose groups (0.474% ± 1.444%). The difference between the placebo and high-dose groups was statistically significant. No significant changes were observed in Enterococcus, Enterobacter, etc.
Regarding alpha diversity indices of gut microbiota, no significant differences were observed among the groups (ESI Fig. 2†). Similarly, based on the unweighted Unifrac distance, the PCoA plot revealed that the gut microbiota composition did not exhibit significant dispersion among the groups at the end of the intervention (ESI Fig. 3†).
SCFAs
SCFAs play a crucial role in the bidirectional communication of the gut–brain axis, both directly and indirectly. In this study, we quantified the levels of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid in fecal samples obtained from 104 subjects.Significant differences were observed in the changes of acetic acid, propionic acid, butyric acid, and valeric acid levels among the groups. Specifically, the low-dose group exhibited significant increases in the levels of acetic acid, propionic acid, butyric acid, and valeric acid (Fig. 7A–C and E). However, no significant differences were observed in the changes of isobutyric acid and isovaleric acid levels among the three groups (Fig. 7D and F).
Associations among the gut microbiota, sleep quality, and metabolites
To investigate the potential correlations among sleep quality, HPA axis hormones, SCFAs, and gut microbiota, Spearman correlation analysis was performed.At the phylum level, no significant correlations were found between gut microbiota and other parameters after adjusting P values (Fig. 8A–C).
At the genus level, the relative abundance of Escherichia-Shigella was positively correlated with changes in valeric acid levels (r = 0.619, P < 0.05) in the placebo group (Fig. 8D). Regarding HPA axis hormones, changes in COR levels were negatively correlated with the abundance of Escherichia-Shigella (r = 0.−0.569, P < 0.05) in the low-dose group (Fig. 8E). Changes in ACTH levels were positively correlated with the abundance of Blautia (r = 0.667, P < 0.05) in the high-dose group (Fig. 8F)
Associations among SCFAs, sleep quality, serum hormones, and neurotransmitters
In the placebo group, there were no significant correlations observed among sleep quality, HPA axis hormones, neurotransmitters, and SCFAs (Fig. 9A).In the low-dose group, changes in ACTH levels were negatively correlated with acetic acid (r = −0.562, P < 0.05) and butyric acid (r = −0.491, P < 0.05) levels (Fig. 9B).
In the high-dose group, elevated levels of isobutyric acid and isovaleric acid were positively correlated with GABA (r = 0.502, P < 0.05; r = 0.476, P < 0.05) and CRH (r = 0.501, P < 0.05; r = 0.476, P < 0.05) levels. However, isobutyric acid (r = −0.495, P < 0.05) and isovaleric acid levels ((r = −0.473, P < 0.05)) were negatively correlated with ACTH levels (Fig. 9C).
Discussion
A variety of gut health benefits have been reported for probiotics, and recent evidence suggests that probiotics play a novel role in mental health via the microbiota–gut–brain axis.35 Clinical investigations have demonstrated that probiotic administration can reduce anxiety-like and depressive-like behavior and normalize associated physiological outputs, including corticosterone and neurotransmitter levels and immune function.36,37 However, strain specificity is the key factor. For example, our previous study found that L. paracasei 207-27 and Bifidobacterium breve 207-1, both derived from healthy infant's feces, have similar effects in regulating gut microbiota (for example, they both increase the relative abundance of Alistipes), they show great differences in emotional regulation,16 which actually reflects the specificity of the strains. It reveals that the correlation between probiotics and mental health needs further research. Although studies have demonstrated the health-promoting effects of probiotics on the brain, few studies have examined their effects on mental health, particularly their ameliorative effects on sleep in healthy adults. In the present randomized, double-blind, placebo-controlled trial, we examined the potential effects of different doses of L. paracasei 207-27 on sleep quality, as well as the underlying mechanisms, in young healthy adults.The present study demonstrated that daily consumption of the L. paracasei 207-27 supplement caused no adverse effects or clinical symptoms, such as gastrointestinal symptoms and excessive inflammation. Furthermore, comprehensive analysis of blood lipid profiles, blood glucose, serum biochemistry, and the complete blood count revealed no significant adverse signs. Although self-comparison within the groups showed significant changes in some blood indicators, all values remained within the normal range. Based on self-reported adverse event records from the subjects, there were no significant adverse events attributed to the short-term administration of L. paracasei 207-27. These findings collectively indicated that the regular intake of L. paracasei 207-27 is safe for healthy adults. Furthermore, no statistically significant differences were found in calorie intake, dietary composition (the ratios of carbohydrate, protein, and fat), or exercise expenditure before and after the intervention. These results suggested that the potential effects of probiotic treatment on outcomes were not confounded by variations in these factors (exercise and diet).
Most previous studies employed the PSQI as a measure to assess the sleep quality of participants. However, it should be noted that PSQI relies on subjective responses and only captures a snapshot of sleep quality, failing to provide a comprehensive representation of individuals’ habitual sleep patterns.38 Furthermore, empirical evidence indicates that the PSQI questionnaire does not adequately capture the nuances of sleep–wake behavior disparities between weekdays and non-weekdays.39 Tian et al. found that supplemented Bifidobacterium breve CCFM1025 for four weeks decreased scores of sleep quality, sleep disturbance and PSQI.40 However, there is no significant difference between placebo and treatment groups. Similar results were found in this study, again highlighting the importance of objective measures of sleep quality.
Previous research established the potential of modulating the gut microbiota through live probiotic administration to improve sleep quality via intricate gut–brain axis interactions.10 The findings of Lee's study revealed a statistically significant enhancement in sleep quality following an 8-week intervention of combined L. reuteri NK33 and B. adolescentis NK98 supplementation.41 Furthermore, Ho et al. corroborated these results, demonstrating the effectiveness of L. plantarum PS128 supplementation in prolonging the duration of deep sleep among the study participants.14 Consistently, our findings underscored the remarkable efficacy of different doses of 207-27 in significantly improving sleep quality as indicated by increased sleep duration.
Alterations in the gut microbiota structure might potentially contribute to improvements in sleep quality.42 Several studies observed significant changes in the gut microbiota of patients with sleep disorders.43,44 In this study, the low-dose group had a lower F/B ratio, and they also had lower counts of Blautia compared to the placebo and high-dose groups after the intervention. Compared with the control group, the changes of Lactobacillus in low-dose and high-dose groups were significantly increased, Clostridia_UCG-014 was significantly decreased in high-dose group, while Enterococcus and Enterobacter had no significant differences, indicating the regulatory roles of the tested L. paracasei 207-27 in the gut microbiota. An increased abundance of Firmicutes and decreased abundance of Bacteroidota, resulting in an increased F/B ratio, were detected in the microbiomes of patients with sleep disorders.45 Similarly, a study by Grosicki et al.46 observed a significantly negative association between sleep quality and the F/B ratio in adults. Additionally, Liu et al.47 reported a lower F/B ratio in subjects diagnosed with insomnia. Furthermore, Haimov et al.48 illustrated that a higher relative abundance of Blautia was associated with lower cognitive performance. Consistent with these findings, a previous study detected a higher abundance of Blautia in adults with insomnia.49 Our findings provide evidence supporting the hypothesis that the improvement in sleep quality following L. paracasei 207-27 administration was mediated by targeted alterations in the relative abundance of specific microbial taxa. These results are consistent with prior investigations, which postulated that probiotics, such as Lactobacillus, can exert regulatory effects on the intricate composition of the gut microbiota, thereby potentially enhancing sleep quality.50,51
In addition to differences in the relative abundance of gut microbes, alterations in microbial metabolites play a crucial role in the regulation of sleep quality by probiotics. In our study, the low-dose group exhibited greater changes in acetic acid, propionic acid, butyric acid, and valeric acid levels than the placebo group. The production of SCFAs involves a variety of bacteria and multiple pathways.52–54 Although this study did not identify a relationship between SCFAs and gut microbiota at the top 10 genus level, the increase in SCFAs content may be associated with changes in the abundance of multiple bacteria induced by the low dose of L. paracasei 207-27, particularlly some low-abundance bacteria. However, the specific identification of these key bacteria was not conducted in this study. Some previous studies demonstrated that Bacteroides was a predominant producer of acetic acid, butyric acid, and propionic acid within the gastrointestinal tract.55,56 In animal models, SCFAs produced by the gut microbiota regulate the expression of clock genes in the host.57 Furthermore, in a mouse model of vascular dementia, Clostridium butyricum administration resulted in increased levels of butyrate in both the brain and feces.58 In addition, decreased consumption of dietary fiber, which serves as a substrate to produce SCFAs by the gut microbiota in healthy individuals, has been associated with decreases in the total sleep duration. A study conducted by Magzal et al.59 revealed that individuals with insomnia and shorter sleep durations exhibited decreased SCFA levels. Similarly, Heath et al.60 detected a positive correlation between increased fecal propionic acid levels and a longer sleep duration in infants. These findings consistently suggest a potential association between SCFAs and sleep duration in different populations. The available evidence indicates that L. paracasei 207-27 administration potentially enhances sleep quality through the modulation of SCFA levels.
An additional plausible pathway establishing a connection between the gut microbiota and sleep quality involves the potential inhibition of HPA axis activity. Stress triggers pro-inflammatory cytokines, activating the HPA axis and leading to CRH and ACTH release, resulting in COR secretion.61 Existing evidence has established that excessive activation of the HPA axis can exert adverse effects on sleep, characterized by sleep fragmentation, attenuated deep slow-wave sleep, and a shortened overall sleep duration.62 In this study, the high-dose group exhibited a significant reduction in COR levels accompanied by a relative increase in CRH levels, reflecting the activation of negative feedback regulation. Correlation analysis revealed a negative association between the relative abundance of Escherichia-Shigella and COR levels and a positive correlation between the relative abundance of Blautia and ACTH levels. These findings suggested potential interactions between specific gut microbial taxa and the neuroendocrine regulation of the HPA axis. The results of human studies suggest that probiotics can inhibit HPA axis activation. Rudzki et al.63 found that supplementation with L. plantarum 299v decreased COR levels, thereby improving cognitive function in patients with major depression. Lactobacillus supplementation has displayed promising activity in reducing stress-induced COR levels and improving sleep quality.64 Overall, daily consumption of L. paracasei 207-27 could inhibit HPA axis activation. These findings supported the notion that probiotics can reduce HPA activity consequently enhance sleep quality.
Furthermore, this study identified a significant positive correlation between SCFA levels and GABA content in the high-dose group. GABA has been implicated in the development of various psychiatric disorders, including anxiety, depression, and sleep disorders.65 Recent research by Yu et al.66 demonstrated the potential beneficial effects of GABA-rich fermented milk on sleep quality in mice. Moreover, Strandwitz et al.67 demonstrated that certain probiotic strains, such as Lactobacillus, could produce GABA, thereby improving sleep quality. The present study revealed a non-significant trend toward increased GABA levels in both the low- and high-dose groups, whereas a decrease in GABA levels was observed in the placebo group following the intervention. However, the lack of significant differences among the groups might be attributable to the relatively short duration of the intervention. Further investigations with extended treatment durations are warranted to elucidate the potential interplay between probiotic administration and GABA modulation and its implications for sleep quality. Additionally, no significant differences in inflammatory factor levels were observed among the three groups in this study, likely because of the inclusion of healthy adult participants with initially low levels of inflammation (ESI Table 6†).
This study had several limitations that should be addressed in future research. Firstly, participants were instructed to maintain their usual lifestyle habits, which does not completely rule out the potential influence of factors like caffeinated drinks or sleep aids on the results. Secondly, the study was unable to definitively determine whether participants had severe sleep disorders or other medical conditions, relying solely on self-report for inclusion and exclusion criteria. The subject recruitment and screening were important and irreversible. Researchers need to pay more attention to this period to avoid introducing confounding. Thirdly, although research staff reminded all subjects to take the probiotics every day with text messages, it was difficult to supervise the subjects’ actual adherence to the treatment regimen. Developing a strain-specific primer to determine the relative abundance of the probiotic in the feces and calculate the actual adherence rate could help address this issue. Fourthly, factors that could affect sleep quality, such as women's menstrual cycles, were not adequately considered and adjusted for in the study. Finally, due to operability issues, the study did not use objective sleep tests such as EEG and respiratory events to identify the type of sleep disorders. Although wearable devices were used to obtain sleep parameters indirectly, the accuracy of these measurements warrants further evaluated. Issues such as wearing norms and adaptation periods also need careful considered. Further studies with larger sample sizes and longer follow-up durations are warranted to establish a detailed assessment of the therapeutic effects of L. paracasei 207-27 and unravel the underlying mechanisms. Addressing the issues would provide precise guidance for future probiotic use in managing sleep disorders.
Conclusion
The administration of L. paracasei 207-27 regulated gut microbiota community and improved sleep duration in healthy adults with no obvious side effects. These findings support the health-promoting properties of L. paracasei 207-27 as part of a healthy diet for adults. The mechanisms involve modulation of the gut microbiota structure to regulate the function of the gut–brain axis, including increases in SCFA levels and decreases in HPA axis activity.Author contributions
Conceptualization, R.C., X.S. and F.H.; methodology, R.C., L.L. and X.Z.; formal analysis, Z.Z., S.W. and W.J.; investigation, J.L., J.Z. and P.L.; writing – original draft preparation J.L., and J.Z.; writing – review and editing, R.C. and F.H. All authors have read and agreed to the published version of the manuscript.Ethics approval and consent to participate
The study was performed from May 2022 to June 2022 at Sichuan University (Sichuan, China) in accordance with the guidelines of the Declaration of Helsinki. The protocol was approved by the Medical Ethics Committee of the West China School of Public Health, Sichuan University (Gwll2022003). Information about the clinical trial was registered at the Chinese Clinical Trial Registry (Registration number: ChiCTR2300069453). The purpose and protocol of the study and foreseeable risks were explained to the participating subjects. All subjects have signed written informed consent.Data availability
Data for this article, including types of excel and graph pad prism are available at OSF database at https://osf.io/q93mb/?view_only=c9e558e6bc504a1fbb988286af3b6f3b.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to express their sincere appreciation for the invaluable support extended by the Public Health and Preventive Medicine Provincial Experiment Teaching Center at Sichuan University and the Food Safety Monitoring and Risk Assessment Key Laboratory of Sichuan Province. The substantial contributions and resources provided by these esteemed institutions have played an indispensable role in facilitating the seamless execution and successful completion of this scholarly investigation.References
- A. Adak and M. R. Khan, An insight into gut microbiota and its functionalities, Cell. Mol. Life Sci., 2019, 76(3), 473–493 CrossRef CAS PubMed.
- S. M. Jandhyala, R. Talukdar, C. Subramanyam, H. Vuyyuru, M. Sasikala and D. Nageshwar Reddy, Role of the normal gut microbiota, World J. Gastroenterol., 2015, 21(29), 8787–8803 CrossRef CAS PubMed.
- H. X. Wang and Y. P. Wang, Gut Microbiota-brain Axis, China Med. J., 2016, 129(19), 2373–2380 CrossRef PubMed.
- M. Ding, Y. Lang, H. Shu, J. Shao and L. Cui, Microbiota-Gut-Brain Axis and Epilepsy: A Review on Mechanisms and Potential Therapeutics, Front. Immunol, 2021, 12, 742449 CrossRef CAS.
- L. Dumitrescu, I. Popescu-Olaru, L. Cozma, D. Tulbă, M. E. Hinescu, L. C. Ceafalan, M. Gherghiceanu and B. O. Popescu, Oxidative Stress and the Microbiota-Gut-Brain Axis, Oxid. Med. Cell. Longevity, 2018, 2018, 2406594 CrossRef PubMed.
- P. Strandwitz, Neurotransmitter modulation by the gut microbiota, Brain Res., 2018, 1693(Pt B), 128–133 CrossRef CAS PubMed.
- A. Galbiati, M. Sforza, M. Poletti, L. Verga, M. Zucconi, L. Ferini-Strambi and V. Castronovo, Insomnia Patients With Subjective Short Total Sleep Time Have a Boosted Response to Cognitive Behavioral Therapy for Insomnia Despite Residual Symptoms, Behav. Sleep Med., 2020, 18(1), 58–67 CrossRef PubMed.
- X. Wang, Z. Wang, J. Cao, Y. Dong and Y. Chen, Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation, Microbiome, 2023, 11(1), 17 CrossRef CAS PubMed.
- N. T. Williams, Probiotics, Am. J. Health Syst. Pharm, 2010, 67(6), 449–458 CrossRef CAS PubMed.
- C. Irwin, D. McCartney, B. Desbrow and S. Khalesi, Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: a systematic review and meta-analysis, Eur. J. Clin. Nutr., 2020, 74(11), 1536–1549 CrossRef PubMed.
- M. Shafie, A. Homayouni Rad, S. Mohammad-Alizadeh-Charandabi and M. Mirghafourvand, The effect of probiotics on mood and sleep quality in postmenopausal women: A triple-blind randomized controlled trial, Clin. Nutr. ESPEN, 2022, 50, 15–23 CrossRef PubMed.
- M. L. Jackson, H. Butt, M. Ball, D. P. Lewis and D. Bruck, Sleep quality and the treatment of intestinal microbiota imbalance in Chronic Fatigue Syndrome: A pilot study, Sleep Sci, 2015, 8(3), 124–133 CrossRef PubMed.
- M. Takada, K. Nishida, Y. Gondo, H. Kikuchi-Hayakawa, H. Ishikawa, K. Suda, M. Kawai, R. Hoshi, Y. Kuwano, K. Miyazaki and K. Rokutan, Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: a double-blind, randomised, placebo-controlled trial, Benefic. Microbes, 2017, 8(2), 153–162 CAS.
- Y. T. Ho, Y. C. Tsai, T. B. J. Kuo and C. C. H. Yang, Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial, Nutrients, 2021, 13(8), 2820 CrossRef CAS PubMed.
- H. Liang, Z. Luo, Z. Miao, X. Shen, M. Li, X. Zhang, J. Chen, X. Ze, Q. Chen and F. He, Lactobacilli and bifidobacteria derived from infant intestines may activate macrophages and lead to different IL-10 secretion, Biosci., Biotechnol., Biochem., 2020, 84(12), 2558–2568 CrossRef CAS PubMed.
- H. Liang, X. Ze, S. Wang, Y. Wang, C. Peng, R. Cheng, F. Jiang, S. Wu, R. He, F. He, X. Zhang and X. Shen, Potential Health-Promoting Effects of Two Candidate Probiotics Isolated from Infant Feces Using an Immune-Based Screening Strategy, Nutrients, 2022, 14(17), 3651 CrossRef CAS PubMed.
- H. Liang, Y. Zhang, Z. Miao, R. Cheng, F. Jiang, X. Ze, X. Shen and F. He, Anti-allergic effects of two potential probiotic strains isolated from infant feces in China, J. Funct. Foods, 2022, 92, 105070 CrossRef CAS.
- B. Zhong, How to calculate sample size in randomized controlled trial?, J. Thorac. Dis., 2009, 1(1), 51–54 Search PubMed.
- Y. Lan, J. Lu, G. Qiao, X. Mao, J. Zhao, G. Wang, P. Tian and W. Chen, Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial, Nutrients, 2023, 15(21), 4700 CrossRef CAS PubMed.
- X. Zhang, M. Chen, R. Duan, H. Xue, J. Luo, X. Lv, H. Jia, F. He, L. Zhang and G. Cheng, The Nutrition and Health in Southwest China (NHSC) study: design, implementation, and major findings, Eur. J. Clin. Nutr., 2021, 75(2), 299–306 CrossRef PubMed.
- K. W. Heaton, J. Radvan, H. Cripps, R. A. Mountford, F. E. Braddon and A. O. Hughes, Defecation frequency and timing, and stool form in the general population: a prospective study, Gut, 1992, 33(6), 818–824 CrossRef CAS PubMed.
- L. Wang, L. Wang, P. Tian, B. Wang, S. Cui, J. Zhao, H. Zhang, L. Qian, Q. Wang, W. Chen and G. Wang, A randomised, double-blind, placebo-controlled trial of Bifidobacterium bifidum CCFM16 for manipulation of the gut microbiota and relief from chronic constipation, Food Funct., 2022, 13(3), 1628–1640 RSC.
- S. Miyata, K. Iwamoto and N. Ozaki, Evaluating sleep-wake rhythm using a wearable device in patients with mental disorders, Psychiatry Clin. Neurosci., 2023, 77(8), 419 CrossRef PubMed.
- S. Y. Tobin, P. G. Williams, K. G. Baron, T. M. Halliday and C. M. Depner, Challenges and Opportunities for Applying Wearable Technology to Sleep, Sleep Med. Clin, 2021, 16(4), 607–618 CrossRef PubMed.
- J. Xie, D. Wen, L. Liang, Y. Jia, L. Gao and J. Lei, Evaluating the Validity of Current Mainstream Wearable Devices in Fitness Tracking Under Various Physical Activities: Comparative Study, JMIR Mhealth Uhealth, 2018, 6(4), e94 CrossRef.
- S. Deng, Q. Wang, J. Fan, X. Yang, J. Mei, J. Lu, G. Chen, Y. Yang, W. Liu, R. Wang, Y. Han, R. Sheng, W. Wang, L. Ba and F. Ding, Correlation of Circadian Rhythms of Heart Rate Variability Indices with Stress, Mood, and Sleep Status in Female Medical Workers with Night Shifts, Nat. Sci. Sleep, 2022, 14, 1769–1781 CrossRef PubMed.
- J. Liang, D. Xian, X. Liu, J. Fu, X. Zhang, B. Tang and J. Lei, Usability Study of Mainstream Wearable Fitness Devices: Feature Analysis and System Usability Scale Evaluation, JMIR Mhealth Uhealth, 2018, 6(11), e11066 CrossRef PubMed.
- R. J. Thomas, J. E. Mietus, C. K. Peng, A. L. Goldberger, L. J. Crofford and R. D. Chervin, Impaired sleep quality in fibromyalgia: Detection and quantification with ECG-based cardiopulmonary coupling spectrograms, Sleep Med, 2010, 11(5), 497–498 CrossRef PubMed.
- D. Liu, X. Yang, G. Wang, J. Ma, Y. Liu, C. K. Peng, J. Zhang and J. Fang, HHT based cardiopulmonary coupling analysis for sleep apnea detection, Sleep Med, 2012, 13(5), 503–509 CrossRef PubMed.
- H. S. Al Ashry, Y. Ni and R. J. Thomas, Cardiopulmonary Sleep Spectrograms Open a Novel Window Into Sleep Biology-Implications for Health and Disease, Front. Neurosci., 2021, 15, 755464 CrossRef PubMed.
- R. J. Thomas, Dynamic Tracking of Brain Health via Interrogation of Sleep Networks With Cardiopulmonary Coupling Sleep Spectrograms, Neurology, 2024, 102(10), e209517 CrossRef PubMed.
- T. Magoč and S. L. Salzberg, FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 2011, 27(21), 2957–2963 CrossRef PubMed.
- B. J. Haas, D. Gevers, A. M. Earl, M. Feldgarden, D. V. Ward, G. Giannoukos, D. Ciulla, D. Tabbaa, S. K. Highlander, E. Sodergren, B. Methé, T. Z. DeSantis, J. F. Petrosino, R. Knight and B. W. Birren, Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons, Genome Res., 2011, 21(3), 494–504 CrossRef CAS PubMed.
- X. Han, J. Guo, Y. You, M. Yin, C. Ren, J. Zhan and W. Huang, A fast and accurate way to determine short chain fatty acids in mouse feces based on GC-MS, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1099, 73–82 CrossRef CAS PubMed.
- S. Morkl, M. I. Butler, A. Holl, J. F. Cryan and T. G. Dinan, Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry, Curr. Nutr. Rep, 2020, 9(3), 171–182 CrossRef PubMed.
- E. Navarro-Tapia, L. Almeida-Toledano, G. Sebastiani, M. Serra-Delgado, O. Garcia-Algar and V. Andreu-Fernandez, Effects of Microbiota Imbalance in Anxiety and Eating Disorders: Probiotics as Novel Therapeutic Approaches, Int. J. Mol. Sci., 2021, 22(5), 2351 CrossRef CAS PubMed.
- M. Eslami, A. Bahar, M. Keikha, M. Karbalaei, N. M. Kobyliak and B. Yousefi, Probiotics function and modulation of the immune system in allergic diseases, Allergol. Immunopathol., 2020, 48(6), 771–788 CrossRef CAS PubMed.
- T. Mollayeva, P. Thurairajah, K. Burton, S. Mollayeva, C. M. Shapiro and A. Colantonio, The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis, Sleep Med. Rev., 2016, 25, 52–73 CrossRef PubMed.
- L. K. Pilz, L. K. Keller, D. Lenssen and T. Roenneberg, Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays, Sleep, 2018, 41(5), zsy029 CrossRef PubMed.
- P. Tian, Y. Chen, H. Zhu, L. Wang, X. Qian, R. Zou, J. Zhao, H. Zhang, L. Qian, Q. Wang, G. Wang and W. Chen, Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial, Brain, Behav., Immun., 2022, 100, 233–241 CrossRef CAS PubMed.
- H. J. Lee, J. K. Hong, J. K. Kim, D. H. Kim, S. W. Jang, S. W. Han and I. Y. Yoon, Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial, Nutrients, 2021, 13(8), 2660 CrossRef CAS PubMed.
- B. A. Matenchuk, P. J. Mandhane and A. L. Kozyrskyj, Sleep, circadian rhythm, and gut microbiota, Sleep Med. Rev, 2020, 53, 101340 CrossRef PubMed.
- Z. Wang, Z. Wang, T. Lu, W. Chen, W. Yan, K. Yuan, L. Shi, X. Liu, X. Zhou, J. Shi, M. V. Vitiello, Y. Han and L. Lu, The microbiota-gut-brain axis in sleep disorders, Sleep Med. Rev, 2022, 65, 101691 CrossRef CAS PubMed.
- Z. Wang, W. H. Chen, S. X. Li, Z. M. He, W. L. Zhu, Y. B. Ji, Z. Wang, X. M. Zhu, K. Yuan, Y. P. Bao, L. Shi, S. Q. Meng, Y. X. Xue, W. Xie, J. Shi, W. Yan, H. Wei, L. Lu and Y. Han, Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation, Mol. Psychiatry, 2021, 26(11), 6277–6292 CrossRef CAS PubMed.
- H. Jiang, Z. Ling, Y. Zhang, H. Mao, Z. Ma, Y. Yin, W. Wang, W. Tang, Z. Tan, J. Shi, L. Li and B. Ruan, Altered fecal microbiota composition in patients with major depressive disorder, Brain, Behav., Immun., 2015, 48, 186–194 CrossRef PubMed.
- G. J. Grosicki, B. L. Riemann, A. A. Flatt, T. Valentino and M. S. Lustgarten, Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: a pilot study, Sleep Med, 2020, 73, 76–81 CrossRef PubMed.
- Z. Liu, Z. Y. Wei, J. Chen, K. Chen, X. Mao, Q. Liu, Y. Sun, Z. Zhang, Y. Zhang, Z. Dan, J. Tang, L. Qin, J. H. Chen and X. Liu, Acute Sleep-Wake Cycle Shift Results in Community Alteration of Human Gut Microbiome, mSphere, 2020, 5(1), e00914–19 CrossRef CAS PubMed.
- I. Haimov, F. Magzal, S. Tamir, M. Lalzar, K. Asraf, U. Milman, M. Agmon and T. Shochat, Variation in Gut Microbiota Composition is Associated with Sleep Quality and Cognitive Performance in Older Adults with Insomnia, Nat. Sci. Sleep, 2022, 14, 1753–1767 CrossRef PubMed.
- F. Magzal, T. Shochat, I. Haimov, S. Tamir, K. Asraf, M. Tuchner-Arieli, C. Even and M. Agmon, Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia, Sci. Rep., 2022, 12(1), 2265 CrossRef CAS PubMed.
- T. Teame, A. Wang, M. Xie, Z. Zhang, Y. Yang, Q. Ding, C. Gao, R. E. Olsen, C. Ran and Z. Zhou, Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review, Front. Nutr, 2020, 7, 570344 CrossRef PubMed.
- H. Wang, C. Braun, E. F. Murphy and P. Enck, Bifidobacterium longum 1714 Strain Modulates Brain Activity of Healthy Volunteers During Social Stress, Am. J. Gastroenterol., 2019, 114(7), 1152–1162 CrossRef PubMed.
- H. L. Drake, A. S. Gössner and S. L. Daniel, Old acetogens, new light, Ann. N. Y. Acad. Sci., 2008, 1125, 100–128 CrossRef CAS PubMed.
- M. S. Frolova, I. A. Suvorova, S. N. Iablokov, S. N. Petrov and D. A. Rodionov, Genomic reconstruction of short-chain fatty acid production by the human gut microbiota, Front. Mol. Biosci., 2022, 9, 949563 CrossRef CAS PubMed.
- M. L. Garron and B. Henrissat, The continuing expansion of CAZymes and their families, Curr. Opin. Chem. Biol., 2019, 53, 82–87 CrossRef CAS PubMed.
- S. Macfarlane and G. T. Macfarlane, Regulation of short-chain fatty acid production, Proc. Nutr. Soc., 2003, 62(1), 67–72 CrossRef CAS PubMed.
- N. Reichardt, S. H. Duncan, P. Young, A. Belenguer, C. McWilliam Leitch, K. P. Scott, H. J. Flint and P. Louis, Phylogenetic distribution of three pathways for propionate production within the human gut microbiota, ISME J., 2014, 8(6), 1323–1335 CrossRef CAS PubMed.
- O. Ojo, Q. Q. Feng, O. O. Ojo and X. H. Wang, The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials, Nutrients, 2020, 12(11), 3239 CrossRef CAS PubMed.
- Y. Luo, B. Zeng, L. Zeng, X. Du, B. Li, R. Huo, L. Liu, H. Wang, M. Dong, J. Pan, P. Zheng, C. Zhou, H. Wei and P. Xie, Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus, Transl. Psychiatry, 2018, 8(1), 187 CrossRef PubMed.
- F. Magzal, C. Even, I. Haimov, M. Agmon, K. Asraf, T. Shochat and S. Tamir, Associations between fecal short-chain fatty acids and sleep continuity in older adults with insomnia symptoms, Sci. Rep., 2021, 11(1), 4052 CrossRef CAS PubMed.
- A. M. Heath, J. J. Haszard, B. C. Galland, B. Lawley, N. J. Rehrer, L. N. Drummond, I. M. Sims, R. W. Taylor, A. Otal, B. Taylor and G. W. Tannock, Association between the faecal short-chain fatty acid propionate and infant sleep, Eur. J. Clin. Nutr., 2020, 74(9), 1362–1365 CrossRef CAS PubMed.
- Z. Karaca, A. Grossman and F. Kelestimur, Investigation of the Hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis, Rev. Endocr. Metab. Disord, 2021, 22(2), 179–204 CrossRef PubMed.
- L. D. Asarnow, Depression and sleep: what has the treatment research revealed and could the HPA axis be a potential mechanism?, Curr. Opin. Psychol, 2020, 34, 112–116 CrossRef PubMed.
- L. Rudzki, L. Ostrowska, D. Pawlak, A. Malus, K. Pawlak, N. Waszkiewicz and A. Szulc, Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study, Psychoneuroendocrinology, 2019, 100, 213–222 CrossRef CAS PubMed.
- A. R. Romijn, J. J. Rucklidge, R. G. Kuijer and C. Frampton, A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression, Aust. N. Z. J. Psychiatry, 2017, 51(8), 810–821 CrossRef PubMed.
- S. Kim, K. Jo, K. B. Hong, S. H. Han and H. J. Suh, GABA and l-theanine mixture decreases sleep latency and improves NREM sleep, Pharm. Biol, 2019, 57(1), 65–73 Search PubMed.
- L. Yu, X. Han, S. Cen, H. Duan, S. Feng, Y. Xue, F. Tian, J. Zhao, H. Zhang, Q. Zhai and W. Chen, Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota, Microbiol. Res., 2020, 233, 126409 CrossRef PubMed.
- P. Strandwitz, K. H. Kim, D. Terekhova, J. K. Liu, A. Sharma, J. Levering, D. McDonald, D. Dietrich, T. R. Ramadhar, A. Lekbua, N. Mroue, C. Liston, E. J. Stewart, M. J. Dubin, K. Zengler, R. Knight, J. A. Gilbert, J. Clardy and K. Lewis, GABA-modulating bacteria of the human gut microbiota, Nat. Microbiol, 2019, 4(3), 396–403 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01684j |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |