Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nutritional, antioxidant and biological activity characterization of orange peel flour to produce nutraceutical gluten-free muffins†
Giusy Rita
Caponio
 *a,
Alessandro
Annunziato
b,
Mirco
Vacca
b,
Graziana
Difonzo
b,
Giuseppe
Celano
b,
Fabio
Minervini
b,
Marianna
Ranieri
a,
Giovanna
Valenti
a,
Grazia
Tamma‡
a and
Maria
De Angelis‡
b
*a,
Alessandro
Annunziato
b,
Mirco
Vacca
b,
Graziana
Difonzo
b,
Giuseppe
Celano
b,
Fabio
Minervini
b,
Marianna
Ranieri
a,
Giovanna
Valenti
a,
Grazia
Tamma‡
a and
Maria
De Angelis‡
b
aDepartment of Bioscience, Biotechnology and Environment, University of Bari Aldo Moro, Via Orabona 4, 70125 Bari, Italy. E-mail: giusy.caponio@uniba.it
bDepartment of Soil, Plant and Food Sciences, University of Bari Aldo Moro, Via Amendola 165/A, 70126 Bari, Italy
First published on 25th July 2024
Abstract
Celiac disease – a prevalent food intolerance – requires strict adherence to a lifelong gluten-free (GF) diet as the only effective treatment. However, GF products often lack soluble fibre and have a high glycaemic index. Consequently, there is a pressing need in the food industry to develop GF products with improved nutritional profiles. In this context, the impact of incorporating orange peel flour (OPF) into muffins undergoing sourdough fermentation was examined, focusing on their technological, antioxidant, and nutritional characteristics. The functional properties of OPF were investigated using human colon carcinoma HCT8 cells as a model system. Treatment with OPF extract demonstrated a notable reduction in malignant cell viability and intracellular ROS levels, indicating potent antioxidant capabilities. Western blot analysis revealed significant alterations in key signalling pathways, including increased phosphorylation of NF-kB at serine 536 and reduced intracellular levels of caspase-3, alongside increased phosphorylation of RIPK3 and MLKL, suggesting potential involvement in necroptosis. OPF incorporation in muffins with sourdough increased antioxidant activity, reduced glycaemic index, and affected the volatile profile. Furthermore, based on simulated colonic fermentation, muffins with OPF showed a slight prebiotic effect, supported by the significant increase in bacillus-shaped lactic acid bacteria and Clostridia population. Overall, OPF-enriched muffins demonstrated considerable antioxidant effects and impacts on cell viability, underscoring their potential as functional ingredients in GF products. These findings signify the prospect of OPF enhancing the nutritional profiles and conferring health benefits of GF muffins.
1. Introduction
Celiac disease (CD) is a chronic autoimmune disorder that causes an immune reaction of the body to the intake of gluten protein complex found in many grains, including barley, wheat, and rye.1 In Italy, more than 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 patients have a confirmed diagnosis of CD with a prevalence of approximately 1%.2 Typically, CD displays a benign resolution and alleviation of the main symptoms upon the onset of a gluten-free (GF) diet. However, failing to adhere to the GF diet increases the risk of developing gastrointestinal cancer or intestinal lymphoma.3 In this contest, GF baking is very challenging due to differences not only in production formulation but also in production technologies. From a technological point of view, gluten removal poses serious limitations in the management of GF bakery products.4 From a nutritional perspective, GF products have imbalanced nutritional profiles, containing high levels of saturated fatty acids and sugars and lacking several nutrients, such as dietary fibre, iron, zinc, magnesium, calcium, vitamin B12, and folic acid.5 Considering the related issues, various strategies are employed to meet the needs of individuals with CD and improve the quality of GF. Although additives are commonly used, fermentation processes, including natural leavening, are also key to improving product quality and acceptance.6,7 In addition, GF products are often characterized by a high predicted glycaemic index (pGI) due to their starch-based composition.8 It is well known the problem related to the high pGI, for individuals with metabolic disorders, such as diabetes and obesity increases the risk of cardiovascular diseases and the onset of certain diseases including cancer.9
000 patients have a confirmed diagnosis of CD with a prevalence of approximately 1%.2 Typically, CD displays a benign resolution and alleviation of the main symptoms upon the onset of a gluten-free (GF) diet. However, failing to adhere to the GF diet increases the risk of developing gastrointestinal cancer or intestinal lymphoma.3 In this contest, GF baking is very challenging due to differences not only in production formulation but also in production technologies. From a technological point of view, gluten removal poses serious limitations in the management of GF bakery products.4 From a nutritional perspective, GF products have imbalanced nutritional profiles, containing high levels of saturated fatty acids and sugars and lacking several nutrients, such as dietary fibre, iron, zinc, magnesium, calcium, vitamin B12, and folic acid.5 Considering the related issues, various strategies are employed to meet the needs of individuals with CD and improve the quality of GF. Although additives are commonly used, fermentation processes, including natural leavening, are also key to improving product quality and acceptance.6,7 In addition, GF products are often characterized by a high predicted glycaemic index (pGI) due to their starch-based composition.8 It is well known the problem related to the high pGI, for individuals with metabolic disorders, such as diabetes and obesity increases the risk of cardiovascular diseases and the onset of certain diseases including cancer.9
In both CD and non-celiac gluten sensitivity, in which gluten triggers adverse gastrointestinal symptoms, a GF diet remains the only therapy adopted to date. However, several trials have shown how strict adherence to a GF diet leads to an imbalance in microbial composition related to the absence of wheat in GF baked goods, resulting in a critical deficiency of fructans.10 Fructans are a type of fibre with prebiotic properties, promoting the growth of beneficial bacteria and maintaining healthy gut microbiota.11 Oxidative stress, characterized by increased levels of reactive oxygen species (ROS), reduced antioxidant capacity, and a pro-inflammatory state are the main processes potentially involved in gluten toxicity.12 However, antioxidants and polyphenols play a pivotal role in the prevention of oxidative stress-induced human diseases.13 Fruits, known for their high polyphenol content, have been shown to exert anti-inflammatory, antithrombotic, and antiproliferative effects.13,14 Among them, the effects of table grape polyphenolic extracts on the mechanisms of oxidation, cellular inflammation, and metastasis have been studied, which can inhibit cell proliferation and growth by affecting cell morphology and inhibiting their ability to migrate.15 In addition, fruit- and vegetable-derived polyphenols modulated miRNAs involved in various cellular processes, such as inflammation and apoptosis.16–18
Citrus fruits, prominent in the Mediterranean Diet, offer significant nutraceutical benefits. Oranges, for instance, contain flavonoids like hesperidin, naringin, neohesperidin, naringenin, nobiletin, and tangeretin, predominantly found in the juice, which contribute to human health.19 Even orange peel flour (OPF) represents an excellent source of dietary fibre and polyphenols including flavonoids, amino acids, triterpenes, phenolic acids, carotenoids, and contains many nutrients, including vitamins C, A, and B, minerals (calcium, phosphorus, potassium).19,20 It is worth noting that oranges are widely consumed worldwide in both peel and juice form. However, during the production of orange juice, only about half of the weight of the fresh orange is processed into juice, resulting in substantial residues (peel, pulp, seeds) constituting the remaining 50% of the orange weight.21 In the vision of sustainability, one alternative for improving the management of these wastes is the implementation of new recovery processes.22 Orange by-products, due to the high content of bioactive compounds,23 can be of particular importance to the scientific community for their unique and enhanced therapeutic properties against various chronic diseases such as cancer, diabetes, and cardiovascular conditions. These by-products could also be used in the formulation of new GF foods with healthy properties. Numerous studies have explored the antioxidant activity and nutritional value of OPF in various food matrices, such as ice cream and yogurt.24–26
This study examined the functional responses of OPF extracts in HCT8 human colon cancer cells. In addition, research on the incorporation of OPF into GF baked goods is limited, and comprehensive studies assessing the impact of adding OPF to GF muffins undergoing fermentation with sourdough on technological, antioxidant, and nutritional profiles are lacking. Based on these observations, our study aimed to investigate the in vitro effects of OPF extract and OPF-enriched digested muffins on cell viability and intracellular oxidative balance. In addition, we evaluated the impact of OPF muffins on glycaemic index, simulated faecal microbiota, texture, and volatile profile. These analyses provide insights into the potential benefits and effects of incorporating OPF into GF baking.
2. Materials and methods
2.1 Chemical characterization of OPF
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v), vortexed for 10 min, sonicated for 15 min (Elmasonic S 60 H, ELMA, Singen, Germany), and finally centrifugated at 12
10 w/v), vortexed for 10 min, sonicated for 15 min (Elmasonic S 60 H, ELMA, Singen, Germany), and finally centrifugated at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min (SL 16R Centrifuge, Thermo Scientific, Waltham, MA, USA) to recover the extract. Extractions were repeated twice more with 30 mL of water. The three extracts were combined, filtered as above reported, and stored at −20 °C until analysis. All extracts were prepared in triplicate.
000g for 10 min (SL 16R Centrifuge, Thermo Scientific, Waltham, MA, USA) to recover the extract. Extractions were repeated twice more with 30 mL of water. The three extracts were combined, filtered as above reported, and stored at −20 °C until analysis. All extracts were prepared in triplicate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) (solvent A) and acetonitrile–formic acid (99.9
10 v/v) (solvent A) and acetonitrile–formic acid (99.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 v/v) (solvent B). The gradient program of solvent A was as follows: 0–26 min from 94% to 45%; 26–33 from 45% to 30%; 33–35 min isocratic at 30%. Then, equilibration was performed at the initial conditions for 9 min. The PDA detector was set to scan from 220 to 600 nm of wavelength managed by a 3D field. Quantitative analysis was performed according to the external standard method based on calibration curves obtained by injecting different concentrations of standard solutions of naringin and neohesperidin (Sigma Aldrich).
0.1 v/v) (solvent B). The gradient program of solvent A was as follows: 0–26 min from 94% to 45%; 26–33 from 45% to 30%; 33–35 min isocratic at 30%. Then, equilibration was performed at the initial conditions for 9 min. The PDA detector was set to scan from 220 to 600 nm of wavelength managed by a 3D field. Quantitative analysis was performed according to the external standard method based on calibration curves obtained by injecting different concentrations of standard solutions of naringin and neohesperidin (Sigma Aldrich).
2.2 In vitro assays of OPF samples on cell cultures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), pNFkB (1
200), pNFkB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), and RIPK3 (1
200), and RIPK3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) were purchased by Santa Cruz Biotechnology (Santa Cruz, CA). Also, caspase 3 (1
2000) were purchased by Santa Cruz Biotechnology (Santa Cruz, CA). Also, caspase 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) and pRIPK3 (1
300) and pRIPK3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) were obtained from Cell Signalling Technology, (Danvers, MA, USA). MLKL (1
1000) were obtained from Cell Signalling Technology, (Danvers, MA, USA). MLKL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) and pMLKL (1
500) and pMLKL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) were bought from Biorbyt, (Cambridge, UK, 1
500) were bought from Biorbyt, (Cambridge, UK, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500).
500).
HCT8 cells were treated as described above. After treatment, cells were incubated with calcein-AM (1 μM) at 37 °C for 45 min, and then the fluorescence signal was measured and analysed. As an internal positive control, cells were incubated with ethanol (90%) for 1 min.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C and the supernatants were used for ROS detection. As a positive control, cells were treated with tert-butyl hydroperoxide (tBHP, 2 mM for 30 min). The fluorescence emission signal was recorded using a fluorimeter FLUOstar Omega (BMG LABTECH, Offenburg, Germania) at excitation and emission wavelengths of 508 and 529 nm, respectively.
000g for 10 min at 4 °C and the supernatants were used for ROS detection. As a positive control, cells were treated with tert-butyl hydroperoxide (tBHP, 2 mM for 30 min). The fluorescence emission signal was recorded using a fluorimeter FLUOstar Omega (BMG LABTECH, Offenburg, Germania) at excitation and emission wavelengths of 508 and 529 nm, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C. The supernatants were collected and used for the western blotting analyses. Proteins were separated using 10% or 12% stain-free polyacrylamide gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under reducing conditions. Protein bands were electrophoretically transferred onto membrane PVDF Immobilon-P (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and incubated in EveryBlot, blocking solution (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Blots were then incubated with primary antibodies overnight. Immunoreactive bands were detected with secondary goat anti-rabbit and anti-mouse horseradish peroxidase-coupled antibodies obtained from Bio-Rad (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were incubated with Clarity TM Western ECL Substrate (Bio-Rad Laboratories, Hercules, CA, USA), and the signals were visualized with the ChemiDoc System gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Obtained bands were normalized to total protein using stain-free technology gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Densitometry analysis was performed using Image Lab gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
000g for 10 min at 4 °C. The supernatants were collected and used for the western blotting analyses. Proteins were separated using 10% or 12% stain-free polyacrylamide gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under reducing conditions. Protein bands were electrophoretically transferred onto membrane PVDF Immobilon-P (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and incubated in EveryBlot, blocking solution (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Blots were then incubated with primary antibodies overnight. Immunoreactive bands were detected with secondary goat anti-rabbit and anti-mouse horseradish peroxidase-coupled antibodies obtained from Bio-Rad (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were incubated with Clarity TM Western ECL Substrate (Bio-Rad Laboratories, Hercules, CA, USA), and the signals were visualized with the ChemiDoc System gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Obtained bands were normalized to total protein using stain-free technology gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Densitometry analysis was performed using Image Lab gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
2.3 Chemical and nutritional characterization of OPF-enriched muffins
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU per g. To confirm the initial cell density, counting was conducted on plates containing tII-SD samples on Man Rogosa and Sharpe (MRS) agar culture medium. After 24 h of incubation, the pH values and the lactic acid bacteria (LAB) density was collected. The pH was measured using an ultrabasic ub-10 pH meter (Denver Instrument Company, located in Arvada, Colorado, USA), equipped with a food penetration probe. The pH measurement of 3.49 ± 0.01 after 24 h of incubation suggests that the environment or mixture containing tII-SD became slightly acidic during that time. To determine the LAB cell density, 5 g of tII-SD was suspended in 45 mL of a sterile sodium chloride solution (0.9 g L−1) and homogenized in a Bag Mixer 400 P (Interscience, St Nom, France) at room temperature. Serial 10-fold dilutions were then plated with De Man, Rogosa, and Sharpe agar media (MRS agar; Oxoid, Basingstoke, Hampshire, UK) modified with the addition of 1% (w/w) maltose and 5% (w/w) yeast extract and adjusting the pH to 5.6 value. The plated LAB counts were incubated for 48 h at 30 °C. The LAB cell density of tII-SD was of about 8
log CFU per g. To confirm the initial cell density, counting was conducted on plates containing tII-SD samples on Man Rogosa and Sharpe (MRS) agar culture medium. After 24 h of incubation, the pH values and the lactic acid bacteria (LAB) density was collected. The pH was measured using an ultrabasic ub-10 pH meter (Denver Instrument Company, located in Arvada, Colorado, USA), equipped with a food penetration probe. The pH measurement of 3.49 ± 0.01 after 24 h of incubation suggests that the environment or mixture containing tII-SD became slightly acidic during that time. To determine the LAB cell density, 5 g of tII-SD was suspended in 45 mL of a sterile sodium chloride solution (0.9 g L−1) and homogenized in a Bag Mixer 400 P (Interscience, St Nom, France) at room temperature. Serial 10-fold dilutions were then plated with De Man, Rogosa, and Sharpe agar media (MRS agar; Oxoid, Basingstoke, Hampshire, UK) modified with the addition of 1% (w/w) maltose and 5% (w/w) yeast extract and adjusting the pH to 5.6 value. The plated LAB counts were incubated for 48 h at 30 °C. The LAB cell density of tII-SD was of about 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU per g.
log CFU per g.
The decision to incubate at 30 °C was based on the observed pH values. This temperature falls within the mesophilic range, which is typically suitable for the growth of a wide variety of microorganisms.
| Ingredients | SD | YB | YC | SD-OPF | YB-OPF | YC-OPF |
|---|---|---|---|---|---|---|
| Brown rice flour (g) | 200 | 200 | 200 | 186.8 | 186.8 | 186.8 |
| Sugar (g) | 12 | 12 | 12 | 12 | 12 | 12 |
| Stevia (g) | 8 | 8 | 8 | 8 | 8 | 8 |
| Orange peel flour (g) | — | — | — | 13.2 | 13.2 | 13.2 |
| Partially skimmed milk (mL) | 200 | 200 | 200 | 200 | 200 | 200 |
| Sunflower oil (mL) | 90 | 90 | 90 | 90 | 90 | 90 |
| Bakery yeast (g) | 3.3 | 3.3 | — | 3.3 | 3.3 | — |
| Chemical yeast (g) | — | — | 12.1 | — | — | 12.1 |
| Sourdough (g) | 22 | — | — | 22 | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v), sonicated for 20 min (Elmasonic S 60 H, ELMA, Singen, Germany), vortexed for 30 min, and finally centrifugated at 8000g for 10 min (SL 16R Centrifuge, Thermo Scientific, Waltham, MA, USA) to recover the hydroalcoholic extract. Then extracts were stored at −20 °C until analysis. All extracts were prepared in triplicate. Muffin extracts were evaluated for the total phenol content (TPC) and the antioxidant activity (ABTS and DPPH assays) according to methods reported in paragraph 2.1.1. Each sample was analysed in triplicate.
10 w/v), sonicated for 20 min (Elmasonic S 60 H, ELMA, Singen, Germany), vortexed for 30 min, and finally centrifugated at 8000g for 10 min (SL 16R Centrifuge, Thermo Scientific, Waltham, MA, USA) to recover the hydroalcoholic extract. Then extracts were stored at −20 °C until analysis. All extracts were prepared in triplicate. Muffin extracts were evaluated for the total phenol content (TPC) and the antioxidant activity (ABTS and DPPH assays) according to methods reported in paragraph 2.1.1. Each sample was analysed in triplicate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da) for 180 min. Aliquots of dialysate, containing free glucose, and partially hydrolysed starch were sampled every 30 min and further treated with amyloglucosidase. Then, free glucose was determined using the above-mentioned enzyme-based kit and finally converted into hydrolysed (digested) starch in the muffin. Control white wheat bread was used as the control to estimate the hydrolysis index (HI = 100). The predicted glycaemic index (pGI) was calculated using the equation pGI = 0.549 × HI + 39.71 as described by Caponio et al.34 Each sample was analysed in triplicate.
400 Da) for 180 min. Aliquots of dialysate, containing free glucose, and partially hydrolysed starch were sampled every 30 min and further treated with amyloglucosidase. Then, free glucose was determined using the above-mentioned enzyme-based kit and finally converted into hydrolysed (digested) starch in the muffin. Control white wheat bread was used as the control to estimate the hydrolysis index (HI = 100). The predicted glycaemic index (pGI) was calculated using the equation pGI = 0.549 × HI + 39.71 as described by Caponio et al.34 Each sample was analysed in triplicate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CFU per mL) for total aerobes (TAMC), total anaerobes (TANMC), lactic acid bacteria (LAB – bacilli), LAB (cocci), Enterobacteriaceae, and faecal Bifidobacterium, respectively. Except for mBifA, obtained from Becton Dickinson GmbH (Heidelberg, Germany), all other media were procured from Oxoid Ltd (Basingstoke, Hampshire, England). WCAn and mBifA were subjected to anaerobic incubation, while the remaining media were incubated aerobically at 37 °C. The incubation duration adhered to the specifications provided by the respective medium manufacturers. To validate data obtained from MRS and M17 media, cells were randomly observed using optical microscopy.
10 CFU per mL) for total aerobes (TAMC), total anaerobes (TANMC), lactic acid bacteria (LAB – bacilli), LAB (cocci), Enterobacteriaceae, and faecal Bifidobacterium, respectively. Except for mBifA, obtained from Becton Dickinson GmbH (Heidelberg, Germany), all other media were procured from Oxoid Ltd (Basingstoke, Hampshire, England). WCAn and mBifA were subjected to anaerobic incubation, while the remaining media were incubated aerobically at 37 °C. The incubation duration adhered to the specifications provided by the respective medium manufacturers. To validate data obtained from MRS and M17 media, cells were randomly observed using optical microscopy.
2.4 In vitro assays of digested muffins enriched with OPF on cell cultures
HCT8, human ileocecal adenocarcinoma cell line was cultured as mentioned in paragraph 2.2.1. Briefly, cells were incubated with digested muffins obtained from in vitro starch hydrolysis at different concentrations (1 mg mL−1, 0.1 mg mL−1, 0.01 mg mL−1, 0.001 mg mL−1 at 37 °C, 5% CO2, for 24 h). Calcein-AM assay and ROS detection were performed as indicated in paragraphs 2.2.3 and 2.2.4, respectively.2.5 Statistical analysis
The results were expressed as the mean ± standard deviation (SD). Significant differences (p ≤ 0.05) were determined by analysis of variance unidirectional (ANOVA), followed by Tukey test for multiple comparisons. The statistical analysis was carried out using the statistical software Minitab (Minitab Inc., State College, PA, USA).3. Results and discussion
3.1 Chemical characterization of OPF
The OPF extract was first evaluated for its chemical composition. Proximate composition, phenol content of the OPF extract, as well as the antioxidant activity, is detailed in Table 2. The proximate composition of OPF in terms of protein, fibre, and carbohydrates was consistent with those in the literature.23,37 As expected, OPF was low in fat and protein. On the contrary, OPF was high in fibre, containing 34.4 g/100 g fibre. However, results from other authors reported higher results for fibre, about 64.3%, but similar results for protein and lipids, 6.70% and 0.89%, respectively.38 These differences in composition are most likely due to variations in fruit growth and maturity conditions.39| Parameters | OPF |
|---|---|
| Data are represented as means ± SD of three lots of OPF. Abbreviations: ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); DPPH, 2,2-diphenil-1-picrylhydrazyl; OPF, orange peel flour; TPC, total phenol content. | |
| Moisture (g per 100 g) | 8.14 ± 0.05 |
| pH | 4.15 ± 0.01 |
| Protein (g per 100 g) | 5.3 ± 0.03 |
| Lipid (g per 100 g) | 1.1 ± 0.08 |
| Fibre (g per 100 g) | 34.4 ± 0.06 |
| Carbohydrates (g per 100 g) | 45.1 ± 0.29 |
| Salt (g per 100 g) | <0.1 |
| ABTS (μmol TE per g) | 19.71 ± 1.15 |
| DPPH (μmol TE per g) | 18.43 ± 0.94 |
| TPC (mg GAE per g) | 19.08 ± 0.83 |
| Neohesperidin (mg g−1) | 2.02 ± 0.04 |
| Naringin (mg g−1) | 0.87 ± 0.01 |
Remarkably, the OPF extract exhibited high antioxidant activity in both ABTS and DPPH assays, with values of 19.71 ± 1.15 μmol TE per g and 18.43 ± 0.94 μmol TE per g, respectively, as recently published.40,41 Moreover, the TPC evaluated according to the Folin–Ciocalteu method was 19.08 mg GAE per g. OPF is a source of polyphenols that exert antioxidant activity. The main phenolic compounds have been detected and quantified in OPF (Table 2). According to the literature, orange peel waste mainly contains neohesperedin and naringin, according to previous research findings.37,42
3.2 In vitro characterization of OPF samples on cell cultures
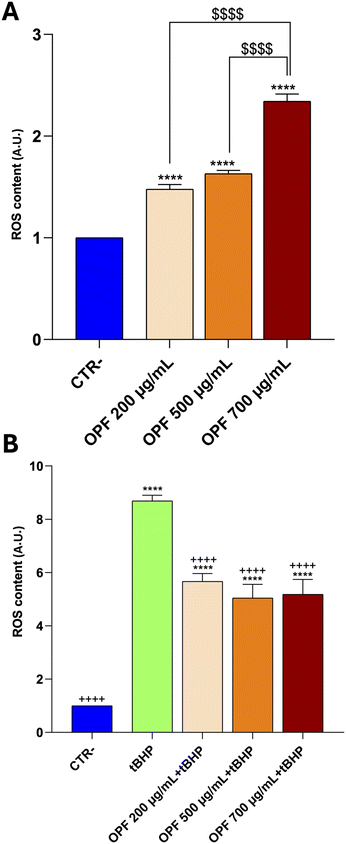
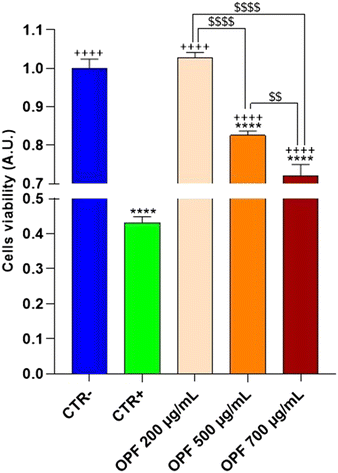
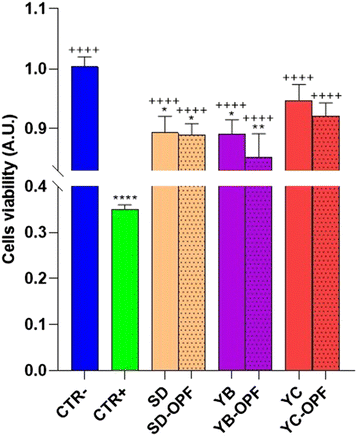
HCT8 cells were left untreated (CTR−) or treated with increasing concentrations (200, 500, 700 μg mL−1) of the OPF extract for 24 h (Fig. 2). As an internal positive control (CTR+) cells were treated with 90% ethanol for 1 min. Compared to the untreated cells (CTR−), treatment with OPF at 500 μg mL−1 and 700 μg mL−1 significantly reduced cell viability. By contrast, treatment with OPF at 200 μg mL−1 does not alter cell viability compared to untreated cells. Altogether, these findings suggest that OPF extract, already at 500 μg mL−1, may promote cell death possibly by inducing oxidative stress. Therefore, 500 μg mL−1 concentration was selected for the subsequent experiments.
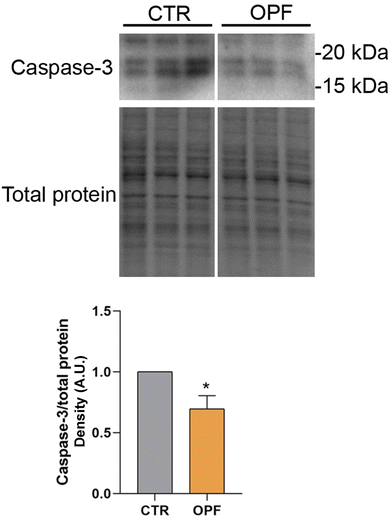
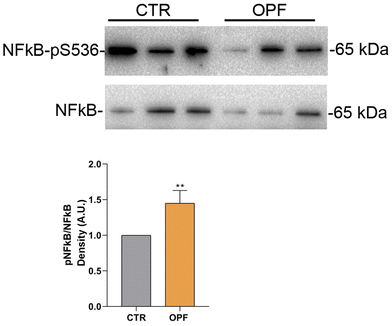
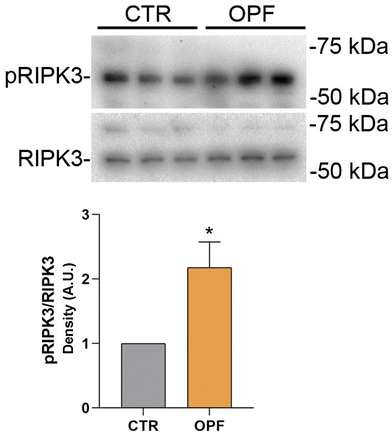
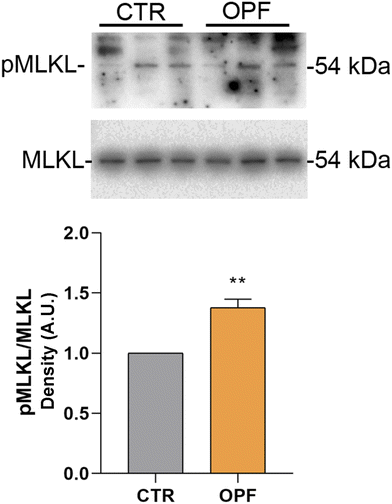
To further investigate the impact of the OPF extract on intracellular signalling modulating cell death, the function of the nuclear factor kB (NFkB) was investigated. Importantly, NFkB is a crucial modulator of inflammation and different forms of programmed cell death.47 Specifically, it has been reported that inhibition of NFkB promotes apoptosis of hepatocytes.48 Conversely, NFkB function counteracts the induction of apoptosis.49 In the present study, immunoblotting analysis showed that incubation with OPF extract caused a slight but significant increase in phosphorylation of NFkB at serine 536 which would explain the possible reduction in cell apoptosis (Fig. 4). On another hand, phosphorylation of NFkB at serine 536 reduces the stability of NFkB,47 likely indicating that the OPF extract would exert an anti-inflammatory function by decreasing the abundance of NFkB. Also, in vivo studies revealed that phosphorylation at serine 536 downregulates NFkB signalling to prevent abnormal and dangerous inflammation50 that, in this case, might be sustained by increased ROS (Fig. 1). Importantly, inhibition of NFkB signalling promotes cell necroptosis in murine keratinocytes.51 In hepatic cells, necroptosis was elicited by ROS-induced NFkB/RIPK1 pathways.52 Therefore, to further investigate the cell fate under OPF extract treatment, cell necroptosis was analysed. Immunoblotting studies (Fig. 5 and 6) revealed that incubation with the OPF extract significantly increased to phosphorylation of RIPK3 and MLKL which are key players in promoting necroptosis.53 Interestingly, the inhibition of certain programmed cell death, such as apoptosis made cells more prone to carcinogenesis.54 In this respect, HCT8 cells displayed a very low level of p53 which is a known oncosuppressor protein that functionally promotes cell apoptosis.55 Impaired apoptosis can be considered a hallmark of cancer. In some cancers, however, induction of necroptosis may represent an alternative type of programmed cell death playing a defensive action against carcinogenesis.56,57 Data showing the phenolic composition of OPF (Table 2) revealed the presence of substantial amounts of naringenin and hesperidin that have been suggested as promising bioactive molecules against cancer progression.58 Our data suggest for the first time that phenols in OPF might stimulate ROS/NFkB-induced cell necroptosis in cells showing possible impaired apoptosis (Fig. 3).
3.3 Chemical and nutritional characterization of OPF-enriched muffins
Notably, the slight significant differences observed between products with and without OPF can also be attributed to the flour used in the muffin's formulation. Brown rice flour is rich in bioactive compounds, particularly phenolic acids, which contribute significantly to antioxidant activity.63
Regarding differences between fermentation types, the TPC value does not seem to be affected, as shown in Table 3 for both SD and YB samples, where only an increasing trend in the value appears for SD samples, but not significantly. A slight difference was observed for YC muffin in terms of ABTS and TPC content, while YC samples achieved the lowest DPPH value compared to SD and YB samples. Several yeast species, including Saccharomyces cerevisiae, are known to possess functional properties (such as potential probiotic effects) as well as improve the bioavailability of phenolic compounds of the products leading to an increased antioxidant capacity.64 The fermentation mixture may also affect the bioavailability of polyphenols. In this regard, scientific evidence exploited the role of Limosilactobacillus reuteri (Ls., basonym Lactobacillus reuteri) and Lactobacillus acidophilus, and their co-culturing, on fermentation performance and the resulting scavenging activity.59,65 The results showed higher values of antioxidant activity in the samples fermented by Ls. reuteri, rather than by the combination. Therefore, the simultaneous use of the two bacteria did not intensify proteolytic and antioxidant activity. Further, fermentative processes, as well as cooking steps, could deeply affect the antioxidant properties of the bakery products.66,67 Also, Saccharomyces cerevisiae and Lacticaseibacillus rhamnosus (basonym Lactobacillus rhamnosus) were able to increase TPC after fermentation of four different flours.68
| Parameters | SD | YB | YC | SD-OPF | YB-OPF | YC-OPF |
|---|---|---|---|---|---|---|
| Data are shown as mean ± SD and analyzed by one-way ANOVA followed by followed by Tukey's multiple comparison test. Different letters (a, b, and c) in the same row mean a significant difference (p ≤ 0.05) among all samples. Comparing the same yeast, “*” indicates a significant difference (P < 0.05) between samples without OFP and samples enriched with OPF. Abbreviations: SD, tII-SD gluten-free muffin; YB, baker's yeast gluten-free muffin; YC, chemical's yeast gluten-free muffin; SD-OPF, tII-SD gluten-free muffin with OPF; YB-OPF, baker's yeast gluten-free muffin with OPF; YC-OPF, chemical's yeast gluten-free muffin with OPF; OPF, orange peel flour. | ||||||
| ABTS (μmol TE per g) | 1.28 ± 0.06c | 1.22 ± 0.05c | 3.14 ± 0.08a | 1.77 ± 0.03b,* | 1.95 ± 0.05b,* | 2.94 ± 0.18a |
| DPPH (μmol TE per g) | 0.89 ± 0.08b | 0.79 ± 0.05b | 0.24 ± 0.04d | 0.98 ± 0.04ab | 1.06 ± 0.04a,* | 0.63 ± 0.05c,* |
| TPC (mg GAE per g) | 2.75 ± 0.43b | 2.69 ± 0.24b | 2.54 ± 0.36b | 2.99 ± 0.28a | 3.32 ± 0.17a,* | 3.32 ± 0.22a,* |
Data from the literature reported how a daily consumption of foods with a high glycaemic index is correlated with the risk of developing cardiovascular disease, insulin resistance, diabetes, and obesity.66,69 GF products often exhibit a higher glycaemic index compared to gluten-containing products. The presence of gluten in foods can inhibit the hydrolysis rates of starch in the small intestine, leading to an increased glycaemic response to carbohydrates upon its elimination from foods.70 Thus, it is important to adopt biotechnological strategies to develop new formulations with improved nutritional value. Bioconversion processes and fermentation strategies for agri-food by-products can both enhance digestibility and nutritional value and reduce levels of anti-nutritional factors in these substrates. Microbial fermentation of agri-food by-products is commonly used to produce ingredients with improved nutritional and health characteristics by promoting the release of bioactive molecules from the plant matrix or through bioconversion of compounds originally present in the by-products.71 Generally, the pGI of foods depends mainly on the amount and type of carbohydrates contained but is also influenced by several other factors, including the presence of organic acids, such as lactic and acetic acid produced by fermentation.72 Fermentation processes are well-known for their role in reducing the glycaemic index of foods.7,34 These studies have also evaluated the influence of the sourdough technique on the glycaemic index, in addition to the health characteristics offered by the enrichment of polyphenols and dietary fibre compounds in OPF.
Furthermore, our results were in line with previous studies, which assessed a decreasing pGI value in muffins enriched with fibre-rich orange bagasse products, achieving a pGI of approximately 70 compared to the control.73 Therefore, the consumption of GF products with a medium/low glycaemic index, such as the SD-OPF sample, could potentially improve the health status of many individuals through significant changes in the glycaemic index.
| Plated bacterial groups | Viable cell density (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CFU per mL) ± SD 10 CFU per mL) ± SD |
|---|---|
| Abbreviations: TAMC, total aerobic microbial count; TANMC, total anaerobic microbial count; LABb, bacillus shaped lactic acid bacteria; LABc, coccus-shaped lactic acid bacteria. SD, standard deviation. | |
| TAMC | 6.3 ± 0.6 |
| TANMC | 7.4 ± 0.7 |
| LABb | 5.4 ± 0.5 |
| LABc | 6.3 ± 0.3 |
| Bifidobacterium | 6.0 ± 0.2 |
| Clostridia | 8.2 ± 0.5 |
| Enterobacteriaceae | 5.1 ± 0.4 |
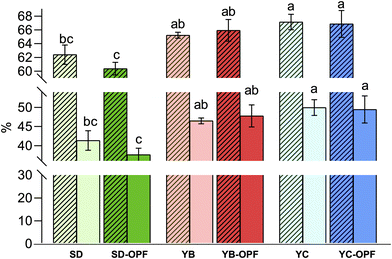
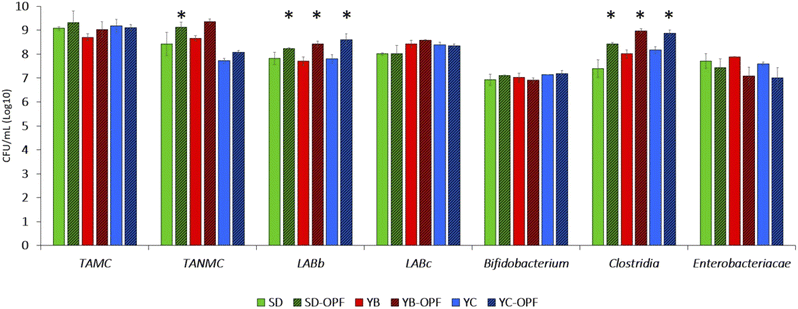
Regarding the results from retentate testing (Fig. 8), variations in OPF influence were observed among different bacterial groups. A 48-hour microbiota fermentation revealed that total aerobic microbial counts (TAMC) were unaffected by the presence or absence of OPF in the medium. However, OPF showed minimal stimulation of the growth of total anaerobes (TANMC), with statistical significance (P < 0.05) achieved only for the SD-OPF compared to itself without OPF. The LAB population was characterized by discerning bacillus-shaped (LABb) from coccus-shaped (LABc) cells, revealing a significant increase of 0.5–1 log in OPF-containing samples for the former group. By contrast, OPF did not stimulate the growth of LABc. Additionally, no prebiotic effect was observed on Bifidobacterium cell density. Therefore, although previous studies demonstrated how polyphenols also enhanced the growth and colonization of bifidobacterial strains, our results confirmed the effective suitability of OPF to stimulate the growth of some specific probiotics.22,75,76
The last two bacterial groups studied through plating were Enterobacteriaceae and cells belonging to the Clostridia class. Once again, OPF led to tendencies in decreasing the viability of Enterobacteriaceae, consistent with its recognized antimicrobial effectiveness against pathogens and pathobionts.77,78 However, it is worth noting how this effectiveness should be considered dose-dependently,78,79 with low concentrations unable to exert significant biological roles on microbes and, therefore, on gut microbiota.80 By contrast, the concentration of OPF used was sufficient to increase the viability of some faecal microbes mostly representative of healthy gut microbiota, including Ruminococcaceae (clostridial cluster IV) and Lachnospiraceae (clostridial cluster XIVa), which are important colonizers of the core microbiota in humans.81,82 In fact, different species within both these families play pivotal roles in the metabolism of undigested food by hosts, producing beneficial compounds such as short-chain fatty acids (SCFAs). These results were aligned with those recently obtained by Núñez-Gómez et al.,83 which observed an increased metabolism of SCFA as a consequence of OPF-fermentation by gut microbiota models in vitro (Núñez-Gómez et al., 2024![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83). In line with this metabolic activity, while not species within Ruminococcaceae and Lachnospiraceae can be claimed as probiotics, they can be considered as health-promoting bacteria of the gut environment.84,85
83). In line with this metabolic activity, while not species within Ruminococcaceae and Lachnospiraceae can be claimed as probiotics, they can be considered as health-promoting bacteria of the gut environment.84,85
| Parameters | SD | YB | YC | SD-OPF | YB-OPF | YC-OPF |
|---|---|---|---|---|---|---|
| Data are shown as mean ± SD and analyzed by one-way ANOVA followed by followed by Tukey's multiple comparison test. Different letters (a, b, and c) in the same row mean a significant difference (p ≤ 0.05) among all samples. Comparing the same yeast, “*” indicates a significant difference (P < 0.05) between samples between OFP and samples enriched with OPF. Abbreviations: SD, tII-SD gluten-free muffin; YB, baker's yeast gluten-free muffin; YC, chemical's yeast gluten-free muffin; SD-OPF, tII-SD gluten-free muffin with OPF; YB-OPF, baker's yeast gluten-free muffin with OPF; YC-OPF, chemical's yeast gluten-free muffin with OPF; OPF, orange peel flour. | ||||||
| Springiness | 0.80 ± 0.02 a | 0.78 ± 0.13a | 0.64 ± 0.02bc | 0.71 ± 0.03abc | 0.77 ± 0.02ab | 0.62 ± 0.01c |
| Chewiness (N) | 6.66 ± 0.52a | 6.44 ± 1.15a | 5.3 ± 0.45ab | 6.2 ± 0.62a | 6.17 ± 1.06a | 3.68 ± 0.49b |
| Cohesiveness | 0.53 ± 0.02a,* | 0.46 ± 0.02b | 0.40 ± 0.02cd | 0.44 ± 0.03bc | 0.44 ± 0.03bc | 0.37 ± 0.02d |
| Hardness (N) | 15.22 ± 1.24c | 17.37 ± 1.12bc | 20.83 ± 1.29a,* | 18.35 ± 1.35abc | 18.55 ± 1.51ab | 16.91 ± 0.66b |
| Volatile compounds | SD | YB | YC | SD-OPF | YB-OPF | YC-OPF |
|---|---|---|---|---|---|---|
| The results are expressed in μg g−1. Data are represented as means ± SD of three lots. Different letters (a, b, and c) in the same row indicate significant differences at P < 0.05 according to two-way ANOVA followed by the Tukey's HSD test. Abbreviations: n.d., not detected. | ||||||
| Aldehydes | ||||||
| Acetaldehyde | 0.26 ± 0.03b | 0.96 ± 0.24a | n.d. | 0.96 ± 0.06a | 1.13 ± 0.26a | n.d. |
| Butanal, 2-methyl- | 1.12 ± 0.03b | 0.86 ± 0.10bc | 0.39 ± 0.02d | 0.79 ± 0.07c | 1.48 ± 0.15a | 0.13 ± 0.00d |
| Butanal, 3-methyl- | 1.49 ± 0.05b | 3.10 ± 0.28a | 0.94 ± 0.08bc | 3.06 ± 0.12a | 1.51 ± 0.27b | 0.36 ± 0.03c |
| Pentanal | 0.55 ± 0.00b | 0.83 ± 0.06a | 0.52 ± 0.02b | 0.86 ± 0.06a | 0.78 ± 0.09a | 0.19 ± 0.01c |
| Hexanal | 20.59 ± 0.62ab | 23.03 ± 1.23a | 21.19 ± 0.94ab | 21.03 ± 0.81ab | 21.35 ± 1.16ab | 19.22 ± 2.56b |
| Heptanal | 2.19 ± 0.56abc | 1.84 ± 0.23bc | 1.55 ± 0.06c | 3.38 ± 0.70ab | 3.94 ± 0.46a | 3.16 ± 0.36abc |
| Octanal | 2.92 ± 0.25b | 2.36 ± 0.33b | 2.05 ± 0.29b | 6.74 ± 0.07a | 6.25 ± 0.28a | 5.98 ± 0.13a |
| 2-Heptenal, (Z)- | 1.91 ± 0.01bc | 1.93 ± 0.10bc | 0.73 ± 0.05c | 3.90 ± 0.58a | 4.83 ± 0.52a | 2.42 ± 0.16b |
| Nonanal | 10.67 ± 1.60b | 9.38 ± 0.66b | 6.69 ± 1.10b | 23.78 ± 4.29a | 23.01 ± 1.03a | 13.38 ± 1.45b |
| Furfural | 0.72 ± 0.01c | 0.33 ± 0.02c | 0.80 ± 0.10c | 3.69 ± 0.27a | 2.96 ± 0.07b | 0.33 ± 0.01c |
| 2-Octenal, (E)- | 2.80 ± 0.30b | 2.40 ± 0.37bc | 0.91 ± 0.01c | 5.81 ± 0.59a | 7.13 ± 0.88a | 2.98 ± 0.01b |
| Benzaldehyde | 1.41 ± 0.08c | 1.74 ± 0.05bc | 1.69 ± 0.02bc | 3.05 ± 0.09a | 3.35 ± 0.39a | 2.29 ± 0.10b |
| Ketones | ||||||
| 2-Butanone | 0.85 ± 0.25a | 0.69 ± 0.07a | 0.59 ± 0.00a | 0.80 ± 0.00a | 0.63 ± 0.02a | 0.77 ± 0.04a |
| 2-Heptanone | 4.91 ± 0.19a | 6.60 ± 1.55a | 4.45 ± 0.34a | 5.39 ± 0.36a | 6.85 ± 0.53a | 5.24 ± 0.01a |
| Acetoin | 1.58 ± 0.15b | 1.92 ± 0.02a | 0.20 ± 0.01d | 1.01 ± 0.11c | 1.56 ± 0.09b | 0.10 ± 0.00d |
| 5-Hepten-2-one, 6-methyl- | 1.43 ± 0.02c | 1.39 ± 0.09c | 1.06 ± 0.01c | 2.70 ± 0.25a | 3.00 ± 0.00a | 2.26 ± 0.05b |
| 2-Nonanone | 1.55 ± 0.13cd | 1.67 ± 0.27cd | 1.35 ± 0.07d | 2.47 ± 0.04bc | 3.54 ± 0.45a | 3.20 ± 0.20ab |
| Ethanone, 1-(2-furanyl)- | n.d. | n.d. | n.d. | 1.49 ± 0.00a | 1.75 ± 0.17a | 1.62 ± 0.22a |
| Alcohols | ||||||
| Ethanol | 185.84 ± 19.13ab | 225.21 ± 25.83a | 6.76 ± 0.08c | 151.97 ± 10.88b | 156.97 ± 6.34b | 7.85 ± 0.81c |
| 1-Propanol, 2-methyl- | 8.35 ± 1.03b | 11.12 ± 0.53a | 0.73 ± 0.04d | 5.74 ± 0.06c | 5.93 ± 0.63c | n.d. |
| 1-Butanol | 0.99 ± 0.04b | 1.62 ± 0.16a | 1.06 ± 0.03b | n.d. | n.d. | n.d. |
| 1-Butanol, 3-methyl- | 155.25 ± 23.3a | 184.16 ± 7.09a | 6.45 ± 0.07c | 79.8 ± 2.22b | 72.01 ± 4.88b | n.d. |
| 1-Pentanol | 6.13 ± 0.73b | 7.72 ± 0.04a | 4.40 ± 0.30c | 5.44 ± 0.08bc | 4.14 ± 0.15c | 2.51 ± 0.22d |
| 2-Buten-1-ol, 3-methyl- | n.d. | n.d. | n.d. | 0.68 ± 0.00ab | 0.84 ± 0.21a | 0.34 ± 0.03bc |
| 1-Hexanol | 33.72 ± 4.49a | 37.52 ± 1.64a | 13.65 ± 0.57b | 31.40 ± 2.53a | 32.52 ± 1.55a | 15.95 ± 1.48b |
| 1-Octen-3-ol | 2.87 ± 0.01a | 3.02 ± 0.21a | 1.52 ± 0.05b | n.d. | n.d. | n.d. |
| 1-Hexanol, 2-ethyl- | 2.37 ± 0.03c | 2.07 ± 0.24c | 3.09 ± 0.08c | 2.97 ± 0.16c | 4.47 ± 0.45b | 5.9 ± 0.41a |
| 1-Octanol | 1.86 ± 0.01c | 1.79 ± 0.04c | 0.83 ± 0.06c | 5.70 ± 0.52ab | 7.16 ± 1.08a | 4.24 ± 0.31b |
| 2-Furanmethanol | 0.77 ± 0.04bc | 0.56 ± 0.04c | 4.76 ± 0.01ab | 2.10 ± 0.19bc | 7.31 ± 0.93a | 7.18 ± 2.26a |
| 2-Methoxy-4-vinylphenol | n.d. | n.d. | n.d. | 1.38 ± 0.13a | 1.31 ± 0.36a | 1.84 ± 0.60a |
| Phenylethyl alcohol | 23.81 ± 0.50ab | 23.96 ± 1.00ab | 2.82 ± 0.28b | 22.85 ± 2.34ab | 38.31 ± 18.16a | 7.36 ± 0.27b |
| Carboxylic acids | ||||||
| Acetic acid | 1.88 ± 0.40b | 1.45 ± 0.01bc | 0.46 ± 0.11c | 6.37 ± 0.44a | 6.45 ± 0.12a | 1.84 ± 0.01b |
| Propanoic acid, 2-methyl- | 0.95 ± 0.20b | 1.28 ± 0.05b | n.d. | 1.83 ± 0.09b | 8.03 ± 1.33a | 0.82 ± 0.05b |
| Butanoic acid, 2-methyl- | 1.76 ± 0.16c | 1.67 ± 0.08c | n.d. | 2.76 ± 0.35b | 4.29 ± 0.09a | 0.67 ± 0.00d |
| Hexanoic acid | 3.00 ± 0.21cd | 1.96 ± 0.22d | n.d. | 5.19 ± 0.35b | 7.36 ± 0.83a | 4.07 ± 0.33bc |
| Octanoic acid | 1.38 ± 0.02ab | 1.18 ± 0.16ab | 0.37 ± 0.05b | 2.73 ± 0.50ab | 4.58 ± 2.46a | 2.66 ± 0.48ab |
| Nonanoic acid | 1.66 ± 0.54bc | 1.45 ± 0.00bc | 0.65 ± 0.01c | 2.69 ± 0.57ab | 3.53 ± 0.09a | 1.57 ± 0.30bc |
| Terpenes | ||||||
| 3-Carene | n.d. | n.d. | n.d. | 25.43 ± 1.52b | 29.67 ± 2.99ab | 34.95 ± 3.23a |
| Limonene | 19.66 ± 0.50b | 23.81 ± 2.01b | 20.19 ± 2.86b | 7158.26 ± 227.09a | 8013.97 ± 417.53a | 8038.43 ± 310.27a |
| Terpinene | n.d. | n.d. | n.d. | 4.61 ± 0.66b | 5.33 ± 0.10ab | 6.35 ± 0.38a |
| o-Cymene | n.d. | n.d. | n.d. | 7.36 ± 0.14b | 8.59 ± 0.42a | 8.38 ± 0.53ab |
| Linalool | n.d. | n.d. | n.d. | 28.57 ± 2.66b | 34.76 ± 2.73ab | 41.02 ± 4.91a |
| Terpineol | 1.09 ± 0.01b | 0.97 ± 0.12b | 0.79 ± 0.01b | 23.15 ± 2.26a | 30.19 ± 4.78a | 35.71 ± 6.79a |
| Ester | ||||||
| Ethyl Acetate | 0.99 ± 0.06b | 1.44 ± 0.11a | 0.4 ± 0.01c | 0.87 ± 0.05b | 1.27 ± 0.24ab | 0.37 ± 0.03c |
| Furan | ||||||
| Furan, 2-penthyl- | 5.45 ± 1.38a | 5.48 ± 1.63a | 1.59 ± 0.03b | 5.09 ± 0.28ab | 6.91 ± 0.04a | 4.48 ± 0.15ab |
| Furfural | 0.72 ± 0.01c | 0.33 ± 0.02c | 0.80 ± 0.10c | 3.69 ± 0.27a | 2.96 ± 0.07b | 0.33 ± 0.01c |
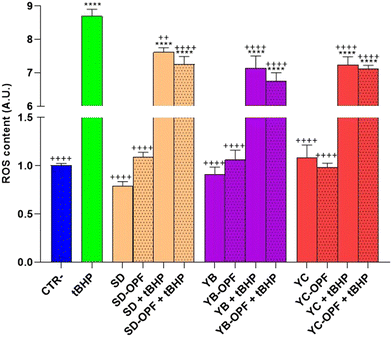
Subsequently, to investigate the effect of the muffin extracts on the intracellular oxidative state, HCT8 cells were left untreated or treated with 1 mg mL−1 of digested muffin extracts and assayed for ROS determination. Fig. 10 clearly shows the antioxidant effects observed in cells treated with the muffin extracts. Specifically, compared to cells treated with the pro-oxidant tBHP, a significant decrease in ROS content was detected in cells incubated with the muffin extracts regardless of the fermentation method. Nevertheless, a slight but not significant decrease in ROS content was found in cells receiving the extracts isolated from OPF muffins, compared to the extracts obtained by untreated muffins.
Conversely, under basal conditions, a slight but not significant increasing tendency in ROS content was detected in cells subjected to the extract isolated from OPF muffins SD and YB fermented. Again, a higher level of OPF in the muffin would have been more effective in promoting ROS release, which as stated above might regulate NFkB function. Nevertheless, together, these findings display a similar tendency in terms of cell viability and ROS content observed in cells treated with OPF extracts.
4. Conclusions
These results underscore the potential of utilizing the polyphenolic compounds and fibres found in OPF to improve the nutritional profile of GF products, particularly when combined with sourdough fermentation. The study conducted a comprehensive evaluation of the nutritional, structural, and volatile aromatic compounds present in these products. Consequently, this research lays the groundwork for exploring the potential advantages of orange by-products as a valuable source of bioactive compounds, which can be incorporated into the development of GF foods. From a nutritional perspective, OPF-enriched muffins fermented with sourdough exhibited increased antioxidant activity, reduced glycaemic index, and affected the volatile profile, influencing the viability of HCT8 cells. Additionally, OPF demonstrated a slight prebiotic effect. Overall, these findings suggest that incorporating OPF enhances the nutritional profiles of GF muffins and offers potential health benefits, highlighting its role as a functional ingredient.Author contributions
Giusy Rita Caponio: formal analysis; data curation; investigation; writing – original draft preparation; Alessandro Annunziato: formal analysis; investigation; Mirco Vacca: formal analysis; data curation; writing – original draft preparation; Graziana Difonzo: formal analysis; data curation; investigation; writing – original draft preparation; Giuseppe Celano: formal analysis; data curation; conceptualization; writing – review & editing; Fabio Minervini: conceptualization; validation; writing – review & editing; Marianna Ranieri: formal analysis; data curation; investigation; Giovanna Valenti: writing – review & editing; Grazia Tamma: resources and funding acquisition; data curation; conceptualization; validation; writing – review & editing; Maria De Angelis: resources and funding acquisition; conceptualization; validation; writing – review & editing. All authors read and approved the final manuscript.Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 – Call for proposals no. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; award number: project code PE00000003, Concession Decree no. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP H93C22000630001, project title “ON Foods – Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods”.References
- M. Gobbetti, G. Rizzello, R. Di Cagno and M. De Angelis, Sourdough lactobacilli and celiac disease, Food Microbiol., 2007, 24, 187–196 CrossRef CAS PubMed.
- J. A. King, J. Jeong, F. E. Underwood, J. Quan, N. Panaccione, J. W. Windsor, S. Coward, J. deBruyn, P. E. Ronksley, A.-A. Shaheen, H. Quan, J. Godley, S. Veldhuyzen Van Zanten, B. Lebwohl, S. C. Ng, J. F. Ludvigsson and G. G. Kaplan, Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis, Am. J. Gastroenterol., 2020, 115, 507–525 CrossRef PubMed.
- I. Marafini, G. Monteleone and C. Stolfi, Association Between Celiac Disease and Cancer, Int. J. Mol. Sci., 2020, 21, 4155 CrossRef CAS PubMed.
- Z. Šmídová and J. Rysová, Gluten-Free Bread and Bakery Products Technology, Foods, 2022, 11, 480 CrossRef PubMed.
- V. Melini and F. Melini, Gluten-Free Diet: Gaps and Needs for a Healthier Diet, Nutrients, 2019, 11, 170 CrossRef CAS PubMed.
- M. Gobbetti and C. G. Rizzello, Biotecnologia dei prodotti lievitati da forno, Casa editrice ambrosiana CEA, Seconda Edizione, 2023 Search PubMed.
- M. Vacca, D. Pinto, A. Annunziato, A. Ressa, M. Calasso, E. Pontonio, G. Celano and M. De Angelis, Gluten-Free Bread Enriched with Artichoke Leaf Extract In Vitro Exerted Antioxidant and Anti-Inflammatory Properties, Antioxidants, 2023, 12, 845 CrossRef CAS PubMed.
- D. El Khoury, S. Balfour-Ducharme and I. J. Joye, A Review on the Gluten-Free Diet: Technological and Nutritional Challenges, Nutrients, 2018, 10, 1410 CrossRef PubMed.
- M. Di Cairano, F. Galgano, R. Tolve, M. C. Caruso and N. Condelli, Focus on gluten free biscuits: Ingredients and issues, Trends Food Sci. Technol., 2018, 81, 203–212 CrossRef CAS.
- D. Paduano, A. Cingolani, E. Tanda and P. Usai, Effect of Three Diets (Low-FODMAP, Gluten-free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life, Nutrients, 2019, 11, 1566 CrossRef CAS PubMed.
- M. De Angelis, G. Garruti, F. Minervini, L. Bonfrate, P. Portincasa and M. Gobbetti, The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome, Curr. Med. Chem., 2019, 26, 3567–3583 CrossRef CAS PubMed.
- M. Rudrapal, S. J. Khairnar, J. Khan, A. B. Dukhyil, M. A. Ansari, M. N. Alomary, F. M. Alshabrmi, S. Palai, P. K. Deb and R. Devi, Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action, Front. Pharmacol., 2022, 13, 806470 CrossRef CAS PubMed.
- G. R. Caponio, T. Lippolis, V. Tutino, I. Gigante, V. De Nunzio, R. Milella, M. Gasparro and M. Notarnicola, Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract, Antioxidants, 2022, 11, 1274 CrossRef CAS PubMed.
- L. Yi, S. Ma and D. Ren, Phytochemistry and bioactivity of Citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities, Phytochem. Rev., 2017, 16, 479–511 CrossRef CAS.
- I. Gigante, R. A. Milella, V. Tutino, G. Debiase, L. Notarangelo, M. A. Giannandrea, V. De Nunzio, A. Orlando, R. D'Alessandro, M. G. Caruso and M. Notarnicola, Autumn Royal and Egnatia Grape Extracts Differently Modulate Cell Proliferation in Human Colorectal Cancer Cells, Endocr. Metab. Immune Disord. Drug Targets, 2020, 20, 1740–1750 CrossRef CAS PubMed.
- T. Ohishi, S. Hayakawa and N. Miyoshi, Involvement of microRNA modifications in anticancer effects of major polyphenols from green tea, coffee, wine, and curry, Crit. Rev. Food Sci. Nutr., 2023, 63, 7148–7179 CrossRef CAS PubMed.
- N. Latruffe, A. Lançon, R. Frazzi, V. Aires, D. Delmas, J. Michaille, F. Djouadi, J. Bastin and M. Cherkaoui-Malki, Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation, Ann. N. Y. Acad. Sci., 2015, 1348, 97–106 CrossRef CAS PubMed.
- T. A. Corrêa and M. M. Rogero, Polyphenols regulating microRNAs and inflammation biomarkers in obesity, Nutrition, 2019, 59, 150–157 CrossRef PubMed.
- L. A. De Castro, J. M. Lizi, E. G. L. Das Chagas, R. A. De Carvalho and F. M. Vanin, From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy, Foods, 2020, 9, 593 CrossRef CAS PubMed.
- N. O'Shea, A. Ktenioudaki, T. P. Smyth, P. McLoughlin, L. Doran, M. A. E. Auty, E. Arendt and E. Gallagher, Physicochemical assessment of two fruit by-products as functional ingredients: Apple and orange pomace, J. Food Eng., 2015, 153, 89–95 CrossRef.
- K. Rezzadori, S. Benedetti and E. R. Amante, Orange waste as raw material for new products, Food Bioprod. Process., 2012, 90, 606–614 CrossRef CAS.
- G. R. Caponio, F. Minervini, G. Tamma, G. Gambacorta and M. De Angelis, Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects, Sustainability, 2023, 15, 9075 CrossRef CAS.
- P. Chavan, A. K. Singh and G. Kaur, Recent progress in the utilization of industrial waste and by–products of citrus fruits: A review, J. Food Process Eng., 2018, 41, e12895 CrossRef.
- T. De Moraes Crizel, A. Jablonski, A. De Oliveira Rios, R. Rech and S. H. Flôres, Dietary fiber from orange byproducts as a potential fat replacer, LWT – Food Sci. Technol., 2013, 53, 9–14 CrossRef CAS.
- P. R. Mary, S. Mutturi and M. Kapoor, Non-enzymatically hydrolyzed guar gum and orange peel fibre together stabilize the low-fat, set-type yogurt: A techno-functional study, Food Hydrocolloids, 2022, 122, 107100 CrossRef CAS.
- T. Erkaya-Kotan, In vitro angiotensin converting enzyme (ACE)-inhibitory and antioxidant activity of probiotic yogurt incorporated with orange fibre during storage, J. Food Sci. Technol., 2020, 57, 2343–2353 CrossRef CAS PubMed.
- G. Caponio, M. Noviello, F. Calabrese, G. Gambacorta, G. Giannelli and M. De Angelis, Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds, Antioxidants, 2022, 11, 567 CrossRef CAS PubMed.
- G. Caponio, M. Cofano, T. Lippolis, I. Gigante, V. De Nunzio, G. Difonzo, M. Noviello, L. Tarricone, G. Gambacorta, G. Giannelli, M. De Angelis and M. Notarnicola, Anti-Proliferative and Pro-Apoptotic Effects of Digested Aglianico Grape Pomace Extract in Human Colorectal Cancer Cells, Molecules, 2022, 27, 6791 CrossRef CAS PubMed.
- M. Troilo, G. Difonzo, V. M. Paradiso, A. Pasqualone and F. Caponio, Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties, Foods, 2022, 11, 1799 CrossRef CAS PubMed.
- A. Torreggiani, C. Demarinis, D. Pinto, A. Papale, G. Difonzo, F. Caponio, E. Pontonio, M. Verni and C. G. Rizzello, Up-Cycling Grape Pomace through Sourdough Fermentation: Characterization of Phenolic Compounds, Antioxidant Activity, and Anti-Inflammatory Potential, Antioxidants, 2023, 12, 1521 CrossRef CAS PubMed.
- Official Methods of Analysis of AOAC International, ed. W. Horwitz, AOAC International, Arlington County, VA, USA, 2000.
- G. Tamma, M. Ranieri, A. Di Mise, M. Centrone, M. Svelto and G. Valenti, Glutathionylation of the Aquaporin-2 Water Channel, J. Biol. Chem., 2014, 289, 27807–27813 CrossRef CAS PubMed.
- G. Difonzo, G. De Gennaro, G. R. Caponio, M. Vacca, G. Dal Poggetto, I. Allegretta, B. Immirzi and A. Pasqualone, Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta, Foods, 2022, 11, 3032 CrossRef CAS PubMed.
- G. R. Caponio, G. Difonzo, G. De Gennaro, M. Calasso, M. De Angelis and A. Pasqualone, Nutritional Improvement of Gluten-Free Breadsticks by Olive Cake Addition and Sourdough Fermentation: How Texture, Sensory, and Aromatic Profile Were Affected?, Front. Nutr., 2022, 9, 830932 CrossRef PubMed.
- S. Pérez-Burillo, S. Molino, B. Navajas-Porras, Á. J. Valverde-Moya, D. Hinojosa-Nogueira, A. López-Maldonado, S. Pastoriza and J. Á. Rufián-Henares, An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality, Nat. Protoc., 2021, 16, 3186–3209 CrossRef PubMed.
- G. R. Caponio, R. Miolla, M. Vacca, G. Difonzo and M. De Angelis, Wine lees as functional ingredient to produce biscuits fortified with polyphenols and dietary fibre, LWT, 2024, 198, 115943 CrossRef CAS.
- L. A. D. Castro, J. M. Lizi, E. G. L. D. Chagas, R. A. D. Carvalho and F. M. Vanin, From Orange Juice By-Product in the Food Industry to a Functional Ingredient: Application in the Circular Economy, Foods, 2020, 9, 593 CrossRef PubMed.
- F. Figuerola, M. L. Hurtado, A. M. Estévez, I. Chiffelle and F. Asenjo, Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment, Food Chem., 2005, 91, 395–401 CrossRef CAS.
- K. Sturm, D. Koron and F. Stampar, The composition of fruit of different strawberry varieties depending on maturity stage, Food Chem., 2003, 83, 417–422 CrossRef CAS.
- E. S. H. Al-Janabi and S. S. Yasen, Determination of chemical composition and antioxidants of wheat flour, orange peel powder and manufactured biscuits, Cape Town, South Africa, 2023, p. 060006 Search PubMed.
- R. O. Obafaye and O. S. Omoba, Orange peel flour: A potential source of antioxidant and dietary fiber in pearl-millet biscuit, J. Food Biochem., 2018, 42, e12523 CrossRef.
- T. Ayora-Talavera, C. Ramos-Chan, A. Covarrubias-Cárdenas, A. Sánchez-Contreras, U. García-Cruz and N. Pacheco L., Evaluation of Pectin Extraction Conditions and Polyphenol Profile from Citrus x lantifolia Waste: Potential Application as Functional Ingredients, Agriculture, 2017, 7, 28 CrossRef.
- S. Toyokuni, K. Okamoto, J. Yodoi and H. Hiai, Persistent oxidative stress in cancer, FEBS Lett., 1995, 358, 1–3 CrossRef CAS PubMed.
- E. G. Russell and T. G. Cotter, New Insight into the Role of Reactive Oxygen Species (ROS) in Cellular Signal-Transduction Processes, in International Review of Cell and Molecular Biology, Elsevier, 2015, vol. 319, pp. 221–254 Search PubMed.
- X. Wen, Z.-Q. Lin, B. Liu and Y.-Q. Wei, Caspase–mediated programmed cell death pathways as potential therapeutic targets in cancer, Cell Proliferation, 2012, 45, 217–224 CrossRef CAS PubMed.
- M. Zhou, X. Liu, Z. Li, Q. Huang, F. Li and C. Li, aspase–3 regulates the migration, invasion and metastasis of colon cancer cells, Int. J. Cancer, 2018, 143, 921–930 CrossRef CAS PubMed.
- J.-P. Pradère, C. Hernandez, C. Koppe, R. A. Friedman, T. Luedde and R. F. Schwabe, Negative regulation of NF-κB p65 activity by serine 536 phosphorylation, Sci. Signaling, 2016, 9, ra85 CrossRef PubMed.
- R. E. Bellas, M. J. FitzGerald, N. Fausto and G. E. Sonenshein, Inhibition of NF-kappa B activity induces apoptosis in murine hepatocytes, Am. J. Pathol., 1997, 151, 891–896 CAS.
- V. R. Baichwal and P. A. Baeuerle, Apoptosis: Activate NF-κB or die?, Curr. Biol., 1997, 7, R94–R96 CrossRef CAS PubMed.
- S. Conejeros-Lillo, F. Aguirre, D. Cabrera, F. Simon, L. Peñailillo and C. Cabello-Verrugio, Role of the ubiquitin-proteasome system in the sarcopenic-like phenotype induced by CCL5/RANTES, Eur. J. Transl. Myol., 2024, 34 DOI:10.4081/ejtm.2024.12249.
- S. Kumari, T.-M. Van, D. Preukschat, H. Schuenke, M. Basic, A. Bleich, U. Klein and M. Pasparakis, NF-κB inhibition in keratinocytes causes RIPK1-mediated necroptosis and skin inflammation, Life Sci. Alliance, 2021, 4, e202000956 CrossRef CAS PubMed.
- S. A. Joosse and K. Pantel, Genetic traits for hematogeneous tumor cell dissemination in cancer patients, Cancer Metastasis Rev., 2016, 35, 41–48 CrossRef CAS PubMed.
- K. Newton, A. Strasser, N. Kayagaki and V. M. Dixit, Cell death, Cell, 2024, 187, 235–256 CrossRef CAS PubMed.
- M. Wang, F. Yu, Y. Zhang and P. Li, Programmed cell death in tumor immunity: mechanistic insights and clinical implications, Front. Immunol., 2024, 14, 1309635 CrossRef PubMed.
- E. E. Gestl and S. A. Böttger, Cytoplasmic sequestration of the tumor suppressor p53 by a heat shock protein 70 family member, mortalin, in human colorectal adenocarcinoma cell lines, Biochem. Biophys. Res. Commun., 2012, 423, 411–416 CrossRef CAS PubMed.
- Y. Gong, Z. Fan, G. Luo, C. Yang, Q. Huang, K. Fan, H. Cheng, K. Jin, Q. Ni, X. Yu and C. Liu, The role of necroptosis in cancer biology and therapy, Mol. Cancer, 2019, 18, 100 CrossRef PubMed.
- G. Pistritto, D. Trisciuoglio, C. Ceci, A. Garufi and G. D'Orazi, Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies, Aging, 2016, 8, 603–619 CrossRef CAS PubMed.
- M. B. Madureira, V. M. Concato, E. M. S. Cruz, J. M. Bitencourt De Morais, F. S. R. Inoue, N. Concimo Santos, M. D. Gonçalves, M. Cremer De Souza, T. Basso Scandolara, M. Fontana Mezoni, M. Galvani, F. Rodrigues Ferreira Seiva, C. Panis, M. M. Miranda-Sapla and W. R. Pavanelli, Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer, Antioxidants, 2023, 12, 586 CrossRef CAS PubMed.
- A. Nikokavoura, D. Christodouleas, E. Yannakopoulou, K. Papadopoulos and A. C. Calokerinos, Evaluation of antioxidant activity of hydrophilic and lipophilic compounds in edible oils by a novel fluorimetric method, Talanta, 2011, 84, 874–880 CrossRef CAS PubMed.
- M. Yaqoob, P. Aggarwal, N. Rasool, W. N. Baba, P. Ahluwalia and R. Abdelrahman, Enhanced functional properties and shelf stability of cookies by fortification of kinnow derived phytochemicals and residues, Food Meas., 2021, 15, 2369–2376 CrossRef.
- V. Laganà, A. M. Giuffrè, A. De Bruno and M. Poiana, Formulation of Biscuits Fortified with a Flour Obtained from Bergamot By-Products (Citrus bergamia, Risso), Foods, 2022, 11, 1137 CrossRef PubMed.
- A. Krajewska and D. Dziki, Enrichment of Cookies with Fruits and Their By-Products: Chemical Composition, Antioxidant Properties, and Sensory Changes, Molecules, 2023, 28, 4005 CrossRef CAS PubMed.
- M. Palla, M. Blandino, A. Grassi, D. Giordano, C. Sgherri, M. F. Quartacci, A. Reyneri, M. Agnolucci and M. Giovannetti, Characterization and selection of functional yeast strains during sourdough fermentation of different cereal wholegrain flours, Sci. Rep., 2020, 10, 12856 CrossRef CAS PubMed.
- L. De Vuyst, H. Harth, S. Van Kerrebroeck and F. Leroy, Yeast diversity of sourdoughs and associated metabolic properties and functionalities, Int. J. Food Microbiol., 2016, 239, 26–34 CrossRef CAS PubMed.
- L. Cui, G. Yang, S. Lu, X. Zeng, J. He, Y. Guo, D. Pan and Z. Wu, Antioxidant peptides derived from hydrolyzed milk proteins by Lactobacillus strains: A BIOPEP-UWM database-based analysis, Food Res. Int., 2022, 156, 111339 CrossRef CAS PubMed.
- D. Restuccia, L. Esposito, U. G. Spizzirri, M. Martuscelli, P. Caputo, C. O. Rossi, M. L. Clodoveo, R. Pujia, E. Mazza, A. Pujia, T. Montalcini and F. Aiello, Formulation of A Gluten-Free Carob-Based Bakery Product: Evaluation of Glycemic Index, Antioxidant Activity, Rheological Properties, and Sensory Features, Fermentation, 2023, 9, 748 CrossRef CAS.
- H. Debelo, M. Li and M. G. Ferruzzi, Processing influences on food polyphenol profiles and biological activity, Curr. Opin. Food Sci., 2020, 32, 90–102 CrossRef.
- Y.-S. Zhao, A. S. Eweys, J.-Y. Zhang, Y. Zhu, J. Bai, O. M. Darwesh, H.-B. Zhang and X. Xiao, Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components, Antioxidants, 2021, 10, 2004 CrossRef CAS PubMed.
- D. J. A. Jenkins, M. Dehghan, A. Mente, S. I. Bangdiwala, S. Rangarajan, K. Srichaikul, V. Mohan, A. Avezum, R. Díaz, A. Rosengren, F. Lanas, P. Lopez-Jaramillo, W. Li, A. Oguz, R. Khatib, P. Poirier, N. Mohammadifard, A. Pepe, K. F. Alhabib, J. Chifamba, A. H. Yusufali, R. Iqbal, K. Yeates, K. Yusoff, N. Ismail, K. Teo, S. Swaminathan, X. Liu, K. Zatońska, R. Yusuf and S. Yusuf, Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality, N. Engl. J. Med., 2021, 384, 1312–1322 CrossRef CAS PubMed.
- G. Zuccotti, V. Fabiano, D. Dilillo, M. Picca, C. Cravidi and P. Brambilla, Intakes of nutrients in I talian children with celiac disease and the role of commercially available gluten–free products, J. Hum. Nutr. Diet., 2013, 26, 436–444 CrossRef CAS PubMed.
- C. Sabater, L. Ruiz, S. Delgado, P. Ruas-Madiedo and A. Margolles, Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes, Front. Microbiol., 2020, 11, 581997 CrossRef PubMed.
- M. De Angelis, C. G. Rizzello, G. Alfonsi, P. Arnault, S. Cappelle, R. Di Cagno and M. Gobbetti, Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread, Br. J. Nutr., 2007, 98, 1196–1205 CrossRef CAS PubMed.
- M. R. Romero-Lopez, P. Osorio-Diaz, L. A. Bello-Perez, J. Tovar and A. Bernardino-Nicanor, Concentrate from Orange (Citrus sinensis L.) Bagase: Characterization and Application as Bakery Product Ingredient, Int. J. Mol. Sci., 2011, 12, 2174–2186 CrossRef CAS PubMed.
- K. Wojtunik-Kulesza, A. Oniszczuk, T. Oniszczuk, M. Combrzyński, D. Nowakowska and A. Matwijczuk, Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review, Nutrients, 2020, 12, 1401 CrossRef CAS PubMed.
- P. Li, X. Yao, Q. Zhou, X. Meng, T. Zhou and Q. Gu, Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in vitro, Front. Nutr., 2022, 9, 888745 CrossRef PubMed.
- A. Manthei, P. Elez-Martínez, R. Soliva-Fortuny and P. Murciano-Martínez, Prebiotic potential of pectin and cello-oligosaccharides from apple bagasse and orange peel produced by high-pressure homogenization and enzymatic hydrolysis, Food Chem., 2024, 435, 137583 CrossRef CAS PubMed.
- S. Saha, T. Do, J. Maycock, S. Wood and C. Boesch, Antibiofilm Efficacies of Flavonoid-Rich Sweet Orange Waste Extract against Dual-Species Biofilms, Pathogens, 2023, 12, 657 CrossRef CAS PubMed.
- T. Anwar, H. Qureshi, A. Fatima, K. Sattar, G. Albasher, A. Kamal, A. Ayaz and W. Zaman, Citrus sinensis Peel Oil Extraction and Evaluation as an Antibacterial and Antifungal Agent, Microorganisms, 2023, 11, 1662 CrossRef CAS PubMed.
- M. H. Baky, M. Elshahed, L. Wessjohann and M. A. Farag, Interactions between dietary flavonoids and the gut microbiome: a comprehensive review, Br. J. Nutr., 2022, 128, 577–591 CrossRef CAS PubMed.
- D. Plamada and D. C. Vodnar, Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics, Nutrients, 2021, 14, 137 CrossRef PubMed.
- C. Milani, S. Duranti, F. Bottacini, E. Casey, F. Turroni, J. Mahony, C. Belzer, S. Delgado Palacio, S. Arboleya Montes, L. Mancabelli, G. A. Lugli, J. M. Rodriguez, L. Bode, W. De Vos, M. Gueimonde, A. Margolles, D. Van Sinderen and M. Ventura, The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota, Microbiol. Mol. Biol. Rev., 2017, 81 CrossRef PubMed , e00036-17.
- M. Vacca, G. Celano, F. M. Calabrese, P. Portincasa, M. Gobbetti and M. De Angelis, The Controversial Role of Human Gut Lachnospiraceae, Microorganisms, 2020, 8, 573 CrossRef CAS PubMed.
- V. Núñez-Gómez, M. Jesús Periago, J. Luis Ordóñez-Díaz, G. Pereira-Caro, J. Manuel Moreno-Rojas and R. González-Barrio, Dietary fibre fractions rich in (poly)phenols from orange by-products and their metabolisation by in vitro digestion and colonic fermentation, Food Res. Int., 2024, 177, 113718 CrossRef PubMed.
- F. P. Douillard and W. M. De Vos, Biotechnology of health-promoting bacteria, Biotechnol. Adv., 2019, 37, 107369 CrossRef CAS PubMed.
- J. Zhang, L. Song, Y. Wang, C. Liu, L. Zhang, S. Zhu, S. Liu and L. Duan, Beneficial effect of butyrate–producing Lachnospiraceae on stress–induced visceral hypersensitivity in rats, J. Gastroenterol. Hepatol., 2019, 34, 1368–1376 CrossRef CAS PubMed.
- A. R. Karnopp, A. M. Figueroa, P. R. Los, J. C. Teles, D. R. S. Simões, A. C. Barana, F. T. Kubiaki, J. G. B. D. Oliveira and D. Granato, Effects of whole-wheat flour and bordeaux grape pomace (Vitis labrusca L.) on the sensory, physicochemical and functional properties of cookies, Food Sci. Technol., 2015, 35, 750–756 CrossRef.
- J. Korus, L. Juszczak, M. Witczak and R. Ziobro, Effect of Citrus Fiber on the Rheological Properties of Dough and Quality of the Gluten-Free Bread, Appl. Sci., 2020, 10, 6633 CrossRef CAS.
- N. O'Shea, L. Doran, M. Auty, E. Arendt and E. Gallagher, The rheology, microstructure and sensory characteristics of a gluten-free bread formulation enhanced with orange pomace, Food Funct., 2013, 4, 1856 RSC.
- E. Campo, L. Del Arco, L. Urtasun, R. Oria and A. Ferrer-Mairal, Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour, J. Cereal Sci., 2016, 67, 75–82 CrossRef.
- C. G. Rizzello, A. Lorusso, M. Montemurro and M. Gobbetti, Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread, Food Microbiol., 2016, 56, 1–13 CrossRef CAS PubMed.
- D. Novotni, N. Čukelj, B. Smerdel and D. Ćurić, Quality attributes and firming kinetics of partially baked frozen wholewheat bread with sourdough, Int. J. Food Sci. Technol., 2013, 48, 2133–2142 CrossRef CAS.
- C. Dingeo, G. Difonzo, V. M. Paradiso, C. G. Rizzello and E. Pontonio, Teff Type-I Sourdough to Produce Gluten-Free Muffin, Microorganisms, 2020, 8, 1149 CrossRef CAS PubMed.
- K. Kaseleht, T. Paalme, A. Mihhalevski and I. Sarand, Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction, Int. J. Food Sci. Technol., 2011, 46, 1940–1946 CrossRef CAS.
- S. Lee, Y. Hwang, M. Kim, M. Chung and Y.-S. Kim, Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria, Molecules, 2019, 24, 1183 CrossRef PubMed.
- L. W. Zheng, H. Chung and Y.-S. Kim, Effects of dicarbonyl trapping agents, antioxidants, and reducing agents on the formation of furan and other volatile components in canned-coffee model systems, Food Res. Int., 2015, 75, 328–336 CrossRef CAS PubMed.
- G. De Gennaro, G. Difonzo, C. Summo, A. Pasqualone and F. Caponio, Olive Cake Powder as Functional Ingredient to Improve the Quality of Gluten-Free Breadsticks, Foods, 2022, 11, 552 CrossRef CAS PubMed.
- Â. Galvan-Lima, S. C. Cunha, Z. E. Martins, A. G. Soares, I. M. P. L. V. O. Ferreira and A. Farah, Headspace volatolome of peel flours from citrus fruits grown in Brazil, Food Res. Int., 2021, 150, 110801 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01395f |
| ‡ Authors sharing equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |