Open Access Article
Open Access ArticleMetabolic profiling of (poly)phenolic compounds in mouse urine following consumption of hull-less and purple-grain barley†
María-Engracia
Cortijo-Alfonso
a,
Silvia
Yuste
 a,
Iván
Friero
a,
Mariona
Martínez-Subirà
a,
Marian
Moralejo
a,
Carme
Piñol-Felis
bc,
Laura
Rubió-Piqué
a,
Iván
Friero
a,
Mariona
Martínez-Subirà
a,
Marian
Moralejo
a,
Carme
Piñol-Felis
bc,
Laura
Rubió-Piqué
 *a and
Alba
Macià
a
*a and
Alba
Macià
a
aUniversity of Lleida-Agrotecnio CERCA Center, Av. Alcalde Rovira Roure 191, 25198 Lleida, Spain. E-mail: laura.rubio@udl.cat
bDepartment of Medicine and Surgery, University of Lleida, Lleida, Spain
cInstitut de Recerca Biomèdica de Lleida, Fundació Dr Pifarré IRBLleida, Lleida, Spain
First published on 18th July 2024
Abstract
The present study attempted for the first time to investigate the metabolic fate of (poly)phenolic compounds provided by a hull-less and purple grain barley genotype biofortified in anthocyanins. Balb/c mice were supplemented either with standard purified diet (SD) or whole-grain barley supplemented diet (WGB) for six weeks. Subsequently, (poly)phenolic metabolites were determined in urine samples by UPLC-MS/MS, and the principal metabolic pathways were elucidated. Thirty-nine (poly)phenolics compounds were identified in WGB which were distributed between the free (58%) and bound (42%) fractions, encompassing anthocyanins, phenolic acids, flavan-3-ols and flavones. Upon WGB intake, forty-two (poly)phenolic metabolites were identified, predominantly comprising phase-II sulphate, glucuronide, and/or methylated conjugates, along with colonic catabolites. Noteworthy metabolites included peonidin-3-O-glucuronide, peonidin-3-O-6′′-O-malonylglucoside, and peonidin-3-O-glucoside among anthocyanins; hydroxyphenylpropanoic acid-O-sulphate among phenolic acids; and 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone-O-sulphate among flavan-3-ols. Metabolites like phenylpropionic, phenylacetic, hydroxybenzoic, and hippuric acids were found in both WGB and SD groups, with higher levels after barley consumption, indicating both endogenous and polyphenolic metabolism origins. Overall, this study offers valuable insights into the metabolism of (poly)phenols in purple barley, setting the stage for future investigations into the health benefits linked to the consumption of purple grain barley.
1. Introduction
Barley (Hordeum vulgare L.) ranks among the world's most widely cultivated cereals, following maize, wheat, and rice. This highly adaptable crop thrives in diverse climates, making it a staple cereal crop across various regions. Global barley production reached approximately 145.9 million metric tons in the 2021/2022 crop year.1 Traditionally utilized as animal feed and malt, barley has garnered increasing attention as an ingredient in the production of nutritious food products. This interest stems from its exceptional nutritional profile and the functional properties of its bioactive compounds.1,2 These include dietary fiber (β-glucans and arabinoxylans), as well as various phytochemicals such as phenolic acids, flavonoids, tocols, lignans, phytosterols, and folates. In terms of health, bioactive compounds in barley have demonstrated positive effects on cardiovascular diseases, diabetes, obesity, cancer or the immune system, which are in great part attributed to the (poly)phenolic content.2(Poly)phenols from barley are categorized into several major classes, including flavonoids and phenolic acids which can be free or ester-bound to the fiber. The major flavonoids contained in barley are flavan-3-ols and flavones, especially catechins, procyanidins, and prodelphinidins, which are the main (poly)phenols in the free (poly)phenolic fraction. Conversely, phenolic acids, such as 4′-hydroxy-3′-methoxycinnamic acid (ferulic acid), are major components of the bound (poly)phenolic fraction.3
Cereals displaying black, purple, blue or red pigments are gaining attention due to their rich (poly)phenolic content. Colored grain barley, enriched with anthocyanins (ACNs), stands out as a promising candidate, presenting significantly higher levels of antioxidant capacity compared to common barley varieties.4 Studies investigating the consumption of purple and red rice or purple wheat have revealed a decrease in plasma inflammatory markers among obese individuals.5,6 These findings underscore the potential health benefits of whole-grain cereal (poly)phenols, particularly those from colored varieties, and highlight the importance of further research in this area, especially concerning the metabolic fate of (poly)phenols, which is crucial for unraveling their full therapeutic potential.
To date, scientific evidence regarding the metabolism of barley (poly)phenols remains limited, primarily relying on in vitro studies,7–11 along with a few in vivo investigations focusing on the fate of (poly)phenols from hull-less barley in rat intestinal contents12 or in fecal and plasma samples of growing pigs.13 In addition to ACNs present in purple barley, a variety of (poly)phenols have been identified, but there is a notable gap in understanding the metabolism of these compounds in the context of whole-grain barley consumption. During the digestion of cereal grains, free (poly)phenols are swiftly liberated and absorbed within the digestive tract. Conversely, fiber-bound (poly)phenols, act as co-passengers gradually releasing along the digestive tract and undergoing enzymatic transformations by the gut microbial community.14 Ultimately, these metabolites enter circulation and target tissues, potentially mediating the health effects associated with the consumption of barley-based food products.14
The primary aim of this study was to elucidate the metabolic fate and pathways of (poly)phenols from a hull-less, purple-grain barley genotype by analyzing mouse urine samples. This study is the first to characterize the metabolic fate of (poly)phenolic compounds following sustained consumption of colored whole-grain barley in vivo. This paper uses the metabolite nomenclature recommended by several authors,15 and all cited literature has been adjusted as necessary.
2. Materials and methods
2.1. Chemicals and reagents
Cyanidin-3-O-glucoside chloride, and luteolin-7-O-glucoside were purchased from Extrasynthese (Genay, France). 4-Hydroxybenzoic acid, 3,5-dimethoxy-4-hydroxybenzoic acid (aka syringic acid), 3,5-dimethoxy-4-hydroxycinnamic acid (aka sinapic acid), and catechin were from Sigma-Aldrich (St Louis, MO, USA); 4′-hydroxy-3′-methoxycinnamic acid (aka ferulic acid) was from Fluka (Buchs, Switzerland). Methanol (HPLC grade), acetonitrile (HPLC grade), acetic acid, formic acid and hydrochloric acid (HCl) were purchased from Scharlab Chemie (Sentmenat, Catalonia, Spain). Sodium hydroxide (NaOH) was from Fluka (Buchs, Switzerland). The water used was Milli-Q quality (Millipore Corp, Bedford, MA, USA).Stock solutions of standard compounds were prepared by dissolving each standard compound in methanol at a concentration of 1000 mg L−1, and stored in a dark flask at −30 °C.
2.2. Plant material
The double haploid barley line 151340, a hull-less and purple-grain barley, was used in this study. This genotype was provided by Semillas Batlle SA (Bell-lloc d'Urgell, Lleida, Spain). Barley grains were milled to a particle size smaller than 0.5 mm before inclusion in the diets (Foss Cyclotec 1093™ mill, Foss Iberia, Barcelona, Spain).2.3. Animals and experimental procedure
Twenty Balb/c mice BALB/cAnNCrl between forty-five and fifty-two days of life were purchased from Charles River Laboratories (Barcelona, Spain). The animals were separated into two experimental groups, each consisting of five males and five females. One group received a purified standard diet (SD) and the other a diet supplemented with purple whole grain barley (WGB). They were fed these diets for 6 weeks.The purified standard diet (Envigo Teklad Diets; TD.94045; AIN-93G Purified Diet) included casein as protein source, L-cystine, corn starch, maltodextrin, soybean oil, sucrose, cellulose, vitamins, choline bitartrate and minerals (see ESI Table 1†).
To prepare the WGB and SD diets, purified diet pellets and barley grains were crushed in a mill (MC300132, Moulinex, Alençon, France) until a homogeneous powder was obtained. SD diet consisted of 100% purified diet, while the WGB diet was a mix of 75% purified diet and 25% barley. Distilled water was added, the mixture was homogenized, and pellets were prepared and lyophilized using a Lyobeta 15 TELSTAR Lyophilizer (Terrassa, Spain).
The supplementation of ACNs through WGB was based on the human equivalent dose of 140 mg day−1 of ACNs, according to Reagan-Shaw et al.16 Therefore, the quantity of WGB administered was adjusted to a dose of 616 ± 26.5 μg per day per mouse of ACNs.
During the 6 weeks of the experiment, mice were housed in cages on a 12 h light–12 h dark schedule at a controlled temperature (21 ± 1 °C), and humidity (55 ± 10%). Food and water were available ad libitum. Body weight, food intake and water intake were recorded every 3 days.
At the end of the 6-week experimental period, urine samples were collected from all animals and stored at −80 °C for subsequent chromatographic analysis of (poly)phenolic metabolites. Following sample collection, the animals were anesthetized with isoflurane (IsoFlo, Veterinarian Esteve, Bologna, Italy) and sacrificed by intracardiac puncture.
Animal procedures were conducted in accordance with the guidelines of the European Communities Directive 2010/63/EU for regulating animal research. The protocols were approved by the Animal Ethical Committee of the University of Lleida (CEEA of UdL 03-03/20), and were performed under the Generalitat de Catalunya Project License (Generalitat 11498). The study complies with the ARRIVE guidelines developed by the NC3Rs.17
2.4. Whole-grain barley and urine analysis of (poly)phenolic compounds by ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS)
To extract the bound (poly)phenols, the methodology used was reported by Martínez et al.,18 with some modifications. Briefly, the residue remaining after the extraction of free (poly)phenols underwent alkaline hydrolysis by adding 4 mL of 2 M NaOH. The samples were allowed to stand for 24 hours at room temperature to ensure complete hydrolysis. Subsequently, samples were vortexed and centrifuged at 8784g for 10 minutes; the supernatant was transferred to clean tubes and acidified with 400 μL HCl 37% to achieve a pH of 2. 350 μL of the supernatant was mixed with 350 μL phosphoric acid 4%, vortexed, centrifuged again (8784g for 10 minutes), and subjected to micro-Elution solid-phase extraction (μSPE) (OASIS HLB (2 mg), Waters, Milford, MA, USA). Previously, the micro-cartridges were sequentially preconditioned with 250 μL of methanol and 250 μL of acidified water (pH 2). Once the sample was loaded into the micro-cartridge, it was sequentially cleaned-up with 100 μL of Milli-Q-water and 100 μL of Milli-Q water/methanol solution (95/5, v/v). Finally, the retained (poly)phenolic compounds were eluted with methanol (2 × 50 μL), and 2.5 μL of the eluate was directly injected and analyzed by UPLC-MS/MS.
ESI Table 2† shows the selected reaction monitoring (SRM) conditions as well as the cone voltage and collision energy used for the quantification of (poly)phenolic compounds. For barley (poly)phenolics quantification, some compounds were tentatively quantified using the calibration curve of their precursor or another (poly)phenolic compound with a similar structure, due to the lack of commercial (poly)phenolic standards. All the ACNs compounds were tentatively quantified by using the calibration curve of cyanidin-3-O-glucoside; the benzoic acids by the calibration curve of p-hydroxybenzoic acid; 4′-hydroxy-3′-methoxycinnamic acid derivatives by the calibration curve of 4′-hydroxy-3′-methoxycinnamic acid; (epi)catechin derivatives with the calibration curve of catechin; and flavone glycosides with the calibration curve of luteolin-7-O-glucoside.
3. Results and discussion
3.1. Daily dose of (poly)phenolics administered
Complete (poly)phenolic characterization and quantification of WGB and SD diets was performed as a preliminary step to study their metabolic fate after supplementation in mice. Table 1 shows a comprehensive characterization of the daily doses of the main (poly)phenolics. The daily dosage of total (poly)phenols administered with WGB averaged 1467 ± 49.0 μg per day per mouse, equivalent to a human dosage of 333 mg day−1, calculated according to Reagan-Shaw et al.16 The control diet contained a minimal amount of (poly)phenols, approximately 8.91 μg per day per mouse.| (Poly)phenolic compounds | SD | WGB |
|---|---|---|
| n.d.: not detected. | ||
| Free (poly)phenols | ||
| Cyanidin-3-O-arabinoside | n.d. | 0.07 ± 0.01 |
| Cyanidin-3-O-glucoside | n.d. | 48.8 ± 2.96 |
| Cyanidin-3-O-malonylglucoside | n.d. | 221 ± 12.4 |
| Cyanidin-3-O-dimalonylglucoside | n.d. | 295 ± 18.5 |
| Pelargonidin-3-O-glucoside | n.d. | 1.52 ± 0.28 |
| Pelargonidin-3-O-malonylglucoside | n.d. | 18.8 ± 0.83 |
| Peonidin-3-O-glucoside | n.d. | 2.32 ± 0.08 |
| Peonidin-3-O-malonylglucoside | n.d. | 19.0 ± 1.52 |
| Peonidin-3-O-dimalonylglucoside | n.d. | 8.51 ± 0.38 |
| Delphinidin-3-O-glucoside | n.d. | 0.27 ± 0.03 |
| Total anthocyanins | n.d. | 616 ± 26.5 |
| 4-Hydroxybenzoic acid | n.d. | 1.86 ± 0.22 |
| 3,4-Dihydroxybenzoic acid | n.d. | 1.51 ± 0.38 |
| 4-Hydroxy-3-methoxybenzoic acid | n.d. | 1.27 ± 0.09 |
| 3,5-Dimethoxy-4-hydroxybenzoic acid | n.d. | 1.21 ± 0.24 |
| 4′-Hydroxycinnamic acid | 1.77 ± 0.44 | 0.73 ± 0.07 |
| 3′,4′-Dihydroxycinnamic acid | n.d. | 0.53 ± 0.03 |
| 4′-Hydroxy-3′-methoxycinnamic acid | n.d. | 4.72 ± 0.33 |
| 3′-Hydroxy-4′-methoxycinnamic acid | n.d. | 0.63 ± 0.06 |
| Total phenolic acids | 1.77 ± 0.44 | 12.4 ± 0.02 |
| Catechin | n.d. | 11.4 ± 2.99 |
| Catechin glucoside | n.d. | 20.1 ± 0.88 |
| Procyanidin B3 | n.d. | 73.8 ± 1.23 |
| GC-C/prodelphinidin B4 | n.d. | 75.9 ± 11.1 |
| Procyanidin diglucoside | n.d. | 3.94 ± 0.53 |
| Total flavan-3-ols | n.d. | 185 ± 6.74 |
| Apigenin-O-glucoside | n.d. | 1.39 ± 0.08 |
| Apigenin-6-C-arabinoside-8-C-glucoside | n.d. | 6.35 ± 0.46 |
| Isovitexin-C-glucoside | n.d. | 6.61 ± 0.23 |
| Isovitexin-C-rutinoside | n.d. | 1.60 ± 0.19 |
| Luteolin-O-glucoside | n.d. | 7.81 ± 0.58 |
| Isoorientin | n.d. | 2.66 ± 0.08 |
| Isoscoparin-C-glucoside | n.d. | 10.8 ± 0.88 |
| Isoscoparin-C-rutinoside | n.d. | 2.68 ± 0.07 |
| Total flavones | n.d. | 39.8 ± 1.16 |
| Total free (poly)phenols | 1.77 ± 0.44 | 853 ± 31.0 |
| Bound (poly)phenols | ||
| Cyanidin-3-O-glucoside | n.d. | 0.56 ± 0.15 |
| Pelargonidin-3-O-glucoside | n.d. | 0.18 ± 0.04 |
| Peonidin-3-O-glucoside | n.d. | 0.15 ± 0.03 |
| Delphinidin-3-O-glucoside | n.d. | 0.01 ± 0.00 |
| Malvidin-3-O-glucoside | n.d. | 0.01 ± 0.00 |
| Total anthocyanins | n.d. | 0.90 ± 0.22 |
| 4-Hydroxybenzoic acid | 0.86 ± 0.07 | 7.51 ± 0.84 |
| Hydroxybenzoic acid | 1.54 ± 0.34 | 1.44 ± 0.14 |
| 3,4-Dihydroxybenzoic acid | n.d. | 0.53 ± 0.03 |
| 4-Hydroxy-3-methoxybenzoic acid | 0.21 ± 0.05 | 4.05 ± 0.42 |
| 3,5-Dimethoxy-4-hydroxybenzoic acid | n.d. | 1.63 ± 0.13 |
| Cinnamic acid | n.d. | 0.99 ± 0.11 |
| 4′-Hydroxycinnamic acid | 0.56 ± 0.05 | 11.0 ± 0.85 |
| 3′,4′-Dihydroxycinnamic acid | n.d. | 0.23 ± 0.02 |
| 3′,4′-Dihydroxycinnamic acid-O-glucoside | n.d. | 0.14 ± 0.05 |
| 4′-Hydroxy-3′-methoxycinnamic acid | 2.82 ± 0.19 | 340 ± 23.5 |
| 3′-Hydroxy-4′-methoxycinnamic acid | 1.16 ± 0.38 | 56.9 ± 4.48 |
| 3,5-Dimethoxy-4-hydroxycinnamic acid | n.d. | 26.1 ± 3.96 |
| 3,5-Dimethoxy-4-hydroxycinnamic acid-O-glucoside | n.d. | 9.01 ± 2.16 |
| 8/5-5′-Diferulic acid | n.d. | 113 ± 4.31 |
| Diferulic acid decarboxylated | n.d. | 23.1 ± 1.72 |
| Triferulic acid | n.d. | 15.1 ± 2.51 |
| Total phenolic acids | 7.14 ± 0.52 | 611 ± 39.0 |
| Apigenin-O-glucoside | n.d. | 0.40 ± 0.16 |
| Apigenin-6-C-arabinoside-8-C-glucoside | n.d. | 0.56 ± 0.06 |
| Isovitexin-O-glucoside | n.d. | 0.45 ± 0.06 |
| Isovitexin-O-rutinoside | n.d. | 0.39 ± 0.07 |
| Luteolin-O-glucoside | n.d. | 0.25 ± 0.05 |
| Isoscoparin-C-glucoside | n.d. | 0.36 ± 0.16 |
| Total flavones | n.d. | 2.41 ± 0.19 |
| Total bound (poly)phenols | 7.14 ± 0.52 | 614 ± 38.9 |
| Total (poly)phenols (free + bound) | 8.91 ± 0.63 | 1467 ± 49.0 |
The free (poly)phenol fraction of WGB represented 58% of the total (poly)phenols ingested per day, with ACNs accounting for the majority of this fraction (72.2% of the total free (poly)phenols), followed by flavan-3-ols, flavones and phenolic acids (21.7%, 4.68% and 1.45%, respectively). ACNs, responsible for the purple grain color, represented 42% (616 ± 26.5 μg per day per mouse) of the total (poly)phenols ingested per day, the main ones being cyanidin-3-O-(3′′,6′′)-dimalonylglucoside, cyanidin-3-O-6′′-O-malonyl-glucoside and cyanidin-3-O-glucoside. These findings align with a previous study14 which investigated the food potential of biscuits made with a purple hull-less barley genotype. The major flavan-3-ols detected in the free (poly)phenol fraction were procyanidin B3 and GC-C/prodelphinidin B4; the major flavones were isoscoparin-C-glucoside and luteolin-O-glucoside; and the major phenolic acid was 4′-hydroxy-3′-methoxycinnamic acid (ferulic acid).
The remaining (poly)phenols detected were found in a bound form, constituting 42% of the total (poly)phenols ingested per day. Notably, phenolic acids accounted for 99.5% of this fraction, with a daily intake of 611 ± 39.0 μg per day per mouse. Among phenolic acids, 4′-hydroxy-3′-methoxycinnamic acid was the most abundant, representing 23.5% of the total daily (poly)phenolic content administered (340 ± 23.5 μg per day per mouse). Additionally, diferulic and triferulic acids, esters linked to arabinoxylans, were quantified at 136 ± 6.03, and 15.1 ± 2.51 μg per day per mouse in the bound fraction, respectively. Flavones and ACNs were also detected but at lower concentrations, accounting for less than 0.60% of the total bound (poly)phenols. The amount, type, and distribution of free and bound (poly)phenols detected in WGB were in accordance with previous findings.7,14,18,22
3.2. Metabolic pathways and (poly)phenolic profile in mice urine samples
Our findings, illustrated in Table 2, ESI Table 3,† and Fig. 1 and 2, provide insights into the diverse array of urinary (poly)phenolic metabolites upon WGB intake. Table 2 and ESI Table 3† highlight the exclusive detection and identification of twenty-five (poly)phenolic compounds derived from WGB compared to SD, where they were undetectable. Among these compounds eight were ACNs, one 1,2-benzene-diol, seven phenolic acids, and ten flavan-3-ols. In Table 2, these (poly)phenolic metabolites are indicated with a “+” symbol, signifying their presence in WGB, while in SD, they are absent (denoted with a “−” signal).| (Poly)phenolic metabolites | SD | WGB |
|---|---|---|
| ++ indicates heavily presence respect to the standard diet (SD), + indicates presence, and − indicates the absence. | ||
| Anthocyanins | ||
| Cyanidin-3-O-glucoside | − | + |
| Cyanidin-3-O-6′′-O-malonylglucoside | − | + |
| Cyanidin-3-O-(3′′,6′′)-dimalonylglucoside | − | + |
| Peonidin-3-O-glucoside | − | + |
| Peonidin-3-O-6′′-O-malonylglucoside | − | + |
| Peonidin-3-O-(3′′,6′′)-dimalonylglucoside | − | + |
| Peonidin-3-O-glucuronide | − | + |
| Pelargonidin-3-O-6′′-O-malonylglucoside | − | + |
| Benzene-1,2-diols | ||
| Benzene-1,2-diol-O-sulphate | + | ++ |
| Methyl benzene-1,2-diol-O-sulphate | + | + |
| Phenolic acids | ||
| Hydroxybenzoic acid-O-sulphate | + | + |
| 3,4-Dihydroxybenzoic acid-O-sulphate | + | ++ |
| 3,5-Dimethoxy-4-hydroxybenzoic | + | + |
| 4-Hydroxy-3-methoxybenzoic acid-O-sulphate | + | ++ |
| Hippuric acid | + | ++ |
| 4′-Hydroxycinnamic-O-sulphate | + | + |
| 3′,4′-Dihydroxycinnamic acid (CA) | + | + |
| 3′,4′-Dihydroxycinnamic acid-O-sulphate | − | + |
| 4′-Hydroxy-3′-methoxycinnamic acid | + | + |
| 4′-Hydroxy-3′-methoxycinnamic acid-O-sulphate | + | ++ |
| 3′-Hydroxy-4′-methoxycinnamic acid-O-sulphate | + | + |
| 4′-Hydroxy-3′-methoxycinnamic acid-O-glucuronide | + | ++ |
| 3-(4′-Hydroxy-3′-methoxyphenyl)propanoic acid | − | + |
| 3-(4′-Hdroxy-3′-methoxyphenyl)propanoic acid-O-sulphate | + | ++ |
| 3-(4′-Hydroxy-3′-methoxyphenyl)propanoic acid-O-glucuronide | − | + |
| 4′-Hydroxy-3′-methoxycinnamic acid glycine | − | + |
| 3,5-Dimethoxy-4-hydroxycinnamic acid | − | + |
| Hydroxyphenylacetic acid-O-glucuronide | − | + |
| Dihydroxyphenylacetic acid-O-sulphate | + | ++ |
| Hydroxyphenylpropanoic acid-O-sulphate | + | ++ |
| Hydroxyphenylpropanoic acid-O-glucuronide | − | + |
| Dihydroxyphenylpropanoic acid-O-sulphate | + | ++ |
| Flavan-3-ols | ||
| Catechin-O-sulphate | − | + |
| Epicatechin-O-sulphate | − | + |
| Methyl catechin-O-sulphate | − | + |
| Methyl epicatechin-O-sulphate | − | + |
| Methyl catechin-O-glucuronide | − | + |
| Methyl epicatechin-O-glucuronide | − | + |
| 5-(4′-Hydroxyphenyl)-γ-valerolactone-O-sulphate | − | + |
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone-O-sulphate | − | + |
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone-O-glucuronide | − | + |
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone-O-sulphate-O-glucuronide | − | + |
Conversely, certain metabolites (17 in total), such as phenylpropionic, phenylacetic, hydroxybenzoic, and hippuric acids, were detected in both the barley-supplemented and control groups. However, their instrumental responses were significantly higher after WGB consumption compared to SD alone. When the instrumental response of these metabolites after WGB consumption exceeded 80% compared to SD (as detailed in ESI Table 3†), they were designated with a “++” signal in Table 2.
Given the minimal (poly)phenols in the control diet, these results suggest that phenolic acids can arise from both barley (poly)phenols and endogenous sources. Recent studies indicate that many (poly)phenolic catabolites in plasma and urine originate not only from dietary (poly)phenols but also from aromatic amino acids like L-phenylalanine and L-tyrosine, and to a lesser extent, from catecholamines such as dopamine.23 Thus, phenylalanine and tyrosine from both endogenous protein turnover and dietary sources significantly contribute to these low molecular weight phenolic catabolites, explaining their presence even after the SD diet.
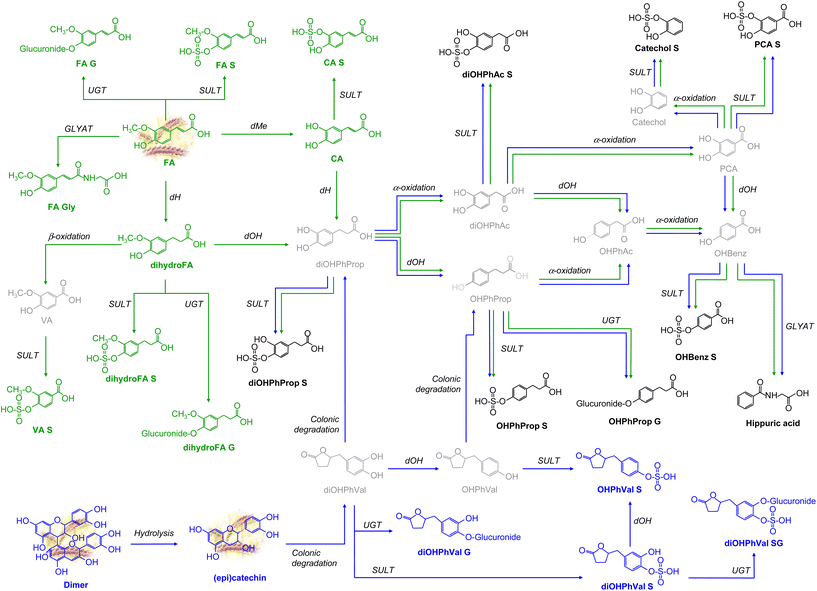
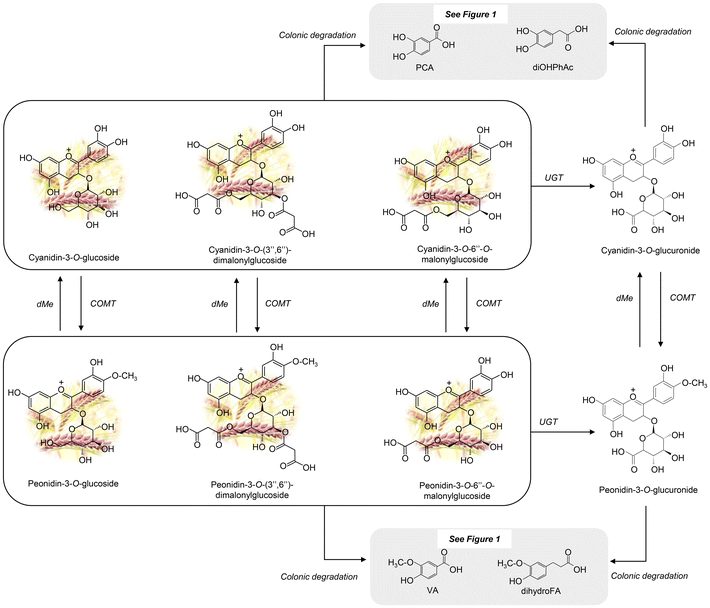
Most of the detected metabolites primarily exist as phase-II sulphate, glucuronide, and/or methylated conjugates, as well as microbial catabolites resulting from colonic degradation. In Fig. 1 and 2, we propose the main metabolic pathways to elucidate how each (poly)phenolic metabolite is generated from its precursor present in WGB. Results from each specific (poly)phenolic family and their corresponding metabolic pathways are described in the following sections.
It is important to note that this study was limited by its focus on qualitative assessments and descriptive interpretations of metabolite presence and composition, rather than quantitative measurements of absolute metabolite quantities. Pooling urine samples hindered the accurate measurement of individual urine volumes, thus limiting the ability to report metabolite concentrations in urine. This qualitative framework allows valuable insights into metabolite profiles, contributing to a comprehensive understanding of the metabolic processes under investigation.
Peonidin-3-O-glucoside, peonidin-3-O-6′′-O-malonylglucoside and peonidin-3-O-(3′′,6′′)-dimalonylglycoside were found both in the barley matrix and in urine samples. This intriguing observation suggests a potential pathway wherein these compounds originate from the methylation of precursor ACNs such as cyanidin-3-O-glucoside, cyanidin-3-O-(3′′,6′′)-dimalonyl-glucoside, and cyanidin-3-O-6′′-O-malonylglucoside, catalyzed by the enzyme catechol-O-methyltransferase (COMT), as illustrated in Fig. 2. This metabolic conversion, occurring within the biological sample, sheds light on the dynamic interplay between dietary ACNs and their metabolic fate within the human body. Methylation is one of the first metabolic reactions of cyanidin glucosides and has also been reported by other authors in human plasma and urine samples after acute intake of red-fleshed apple snack21 or aronia berry extract.24
Cyanidin-3-O-6′′-O-malonylglucoside and peonidin-3-O-6′′-O-malonyl-glucoside can also be conjugated in other phase II reactions with glucuronic acid by the action of the UGT enzyme, resulting in cyanidin-3-O-glucuronide and peonidin-3-O-glucuronide, respectively. This reaction, which is also common to cyanidin glycosides, has been reported in human urine samples after the acute intake of hot air-dried red-fleshed apple and pasteurized red-fleshed apple purée,25 and in rat urine samples after a sustained intake of an infusion from aronia fruit.19 In the present study cyanidin-3-O-glucuronide was not detected. This fact could suggest the rapid methylation of cyanidin glycoside derivatives by COMT action, leading to the formation of peonidin-3-O-glucuronide.
Other cyanidin metabolites based on B-ring fission and cleavage of the C-ring by the action of enzymes of colonic microbiota were also detected (Fig. 2). As a result of this colonic degradation, 3,4-dihydroxybenzoic acid and dihydroxyphenylacetic acid are generated.26 This degradation was previously reported in human plasma and urine samples after acute intake of a red-fleshed apple snack.21 It must be noted that dihydroxyphenylacetic acid and 3,4-dihydroxybenzoic acid can also be generated by colonic degradation via valerolactones of flavan-3-ols (dimer and (epi)catechin) or via 4′-hydroxy-3′-methoxycinnamic acid after dehydrogenation, demethylation, and dehydroxylation reactions (Fig. 2). Finally, 4-hydroxy-3-methoxybenzoic acid and 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid can also be formed from the different forms of peonidin via the gut microbiota after fission of the B-ring of peonidin glucosides.26–28
After the sustained consumption of WGB, a total of twenty-two phenolic acid compounds were detected in the urine samples. Among these, four parent compounds were directly sourced from barley, while the remaining eighteen metabolites were products of various metabolic phases, including phase I, phase II, and colonic metabolism pathways. From 4′-hydroxy-3′-methoxycinnamic acid, which is the predominant (poly)phenol found in WGB (Table 1), various conjugated forms can be directly generated through processes such as sulphation, glucuronidation, and glycine conjugation. Additionally, a dehydrogenation reaction can lead to the formation of 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid, which, upon β-oxidation (undergoing two consecutive decarboxylations), produces 4-hydroxy-3-methoxybenzoic acid. This compound can then undergo sulphation to yield 4-hydroxy-3-methoxybenzoic acid-O-sulphate. The conversion of 4′-hydroxy-3′-methoxycinnamic acid into 4-hydroxy-3-methoxybenzoic acid via 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid is a well-established metabolic pathway.30
3-(4′-Hydroxy-3′-methoxyphenyl)propanoic acid, previously identified as a colonic metabolite following oryzanol intake,31 is susceptible to further metabolic transformations. It can undergo dehydroxylation to yield 3-(3,4-dihydroxyphenyl)propanoic acid or can be conjugated with a sulphate or glucuronide group to form 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid-O-sulphate or 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid-O-glucuronide, respectively (Fig. 1). 3′,4′-Dihydroxycinnamic acid can also be directly formed from 4′-hydroxy-3′-methoxycinnamic acid by a demethylation reaction. From this compound (3′,4′-dihydroxycinnamic acid), 3-(3,4-dihydroxyphenyl)propanoic acid can also be formed following phase I dehydrogenation. 3-(3,4-Dihydroxyphenyl)propanoic acid can be degraded to 3,4-dihydroxyphenylacetic acid and generate 3,4-dihydroxybenzoic acid after α-oxidation (one decarboxylation).19,32 Then, 3,4-Dihydroxybenzoic acid can be further degraded by microbial activity to generate benzene-1,2-diol metabolites.21
On the other hand, if 3-(3,4-dihydroxyphenyl)propanoic acid undergoes a dehydroxylation reaction, it generates 3-(4-hydroxyphenyl)propanoic acid, which can be sulphated and glucuronidated, forming the phase II metabolites 3-(4-hydroxyphenyl)propanoic acid-O-sulphate and 3-(4-hydroxyphenyl)propanoic acid-O-glucuronide, respectively. 3-(4-Hydroxyphenyl)propanoic acid can also be decarboxylated to form 4-hydroxyphenylacetic acid, and this metabolite after the microbiota action can generate hydroxybenzoic acid after α-oxidation which in turn after a glycine conjugation reaction by the action of the GLYAT enzyme can form hippuric acid. Hydroxybenzoic acid can also be formed from 3,4-dihydroxybenzoic acid if it undergoes dehydroxylation and, thus, hippuric acid.33
Regarding phenylpropionic, phenylacetic, hydroxybenzoic and hippuric acids, their presence was also observed following consumption of the SD diet, but their instrumental responses were notably higher after WGB diet than those observed after SD consumption (see Table 2 and ESI Table 3†). Although these phenolic metabolites are known to be colonic metabolites derived from various (poly)phenolic compound families, these phenolic acids are considered endogenous, as they can also be originated from the colonic fermentation of aromatic amino acids.29
The metabolites observed in the present study derived principally from 4′-hydroxy-3′-methoxycinnamic acid and all the phenolic acids present in WGB were in agreement with previous in vitro studies reported in the literature describing the metabolic fate of these compounds in barley products.7,11,34 Additionally, the results aligned with both in vitro35 and in vivo6,21,28 studies conducted on other foods rich in these phenolics, such as coffee,35 red-fleshed apples21 and berry mixtures28 or in other coloured cereals such as purple wheat.6
The first metabolic step that suffers flavan-3-ols is the hydrolysis of the proanthocyanidin dimer to catechin and epicatechin. These monomers were detected in urine as glucuronided ((epi)catechin glucuronide), sulphated ((epi)catechin sulphate), and further methylated (methyl (epi)catechin sulphate) conjugates. Moreover, these monomers can be metabolized by the intestinal microbiota to 5-(3′,4′-dihydroxyphenyl)-γ-valerolactones. Similarly, other studies have reported the γ-valerolactones as specific flavan-3-ol metabolites.36–39 These colonic metabolites are known to exhibit antioxidant and anti-inflammatory activities, which are associated with various health benefits such as cardiovascular protection and cancer prevention.37
5-(3′,4′-Dihydroxyphenyl)-γ-valerolactones undergo degradation by the colonic microbiota, resulting in the formation of 3-(3,4-dihydroxyphenyl)-propanoic acids. Additionally, they can undergo dehydroxylation to yield hydroxyphenyl-γ-valerolactones, which are further metabolized by the intestinal microbiota to hydroxyphenylpropanoic acids (Fig. 2), as documented in previous studies.26,36 The metabolic pathways originating from these two metabolites, 3-(3,4-dihydroxyphenyl)propanoic acid and 3-(4-hydroxyphenyl)-propanoic acid, coincide with those elucidated in section 3.2.3.
Although excellent qualitative outcomes were yielded in this study, the absence of quantitative data represents a noteworthy limitation. This challenge arises from the difficulty in obtaining sufficient volumes of rat urine, preventing accurate measurement of individual urine volumes and necessitating the pooling of samples for (poly)phenolic extraction (μSPE). Furthermore, pooling urine samples also precluded the study of inter-individual variability.
4. Conclusions
This study represents the first comprehensive analysis of the metabolic fate of (poly)phenolic compounds following sustained consumption of whole grain barley, focusing on purple-grain barley. The characterization and quantification of (poly)phenols in a hull-less and purple-grain barley genotype paved the way for investigating their metabolic fate in mice upon supplementation. Detected compounds in urine were mainly phase-II conjugates and microbial catabolites resulting from colonic degradation. Twenty-five metabolites exclusively generated from WGB consumption were identified, indicating its distinct metabolic impact compared to a control diet. Although certain metabolites, like phenylpropionic, phenylacetic, hydroxybenzoic, and hippuric acids, were detected in both groups, their levels significantly increased after WGB consumption. This suggests their origin from colonic metabolism of barley (poly)phenolics but also from aromatic amino acid fermentation, indicating a broader dietary origin. ACNs, prominent in purple WGB, underwent extensive metabolism, with various metabolites detected in urine. Notably, conversion of cyanidin-3-O-glucoside to peonidin-3-O-glucoside highlighted the interplay between dietary ACNs and their metabolic fate. Phenolic acids constituted a significant portion of WGB (poly)phenolic content, evident in urine samples, which exhibited elevated levels of derived metabolites from colonic catabolism. Flavan-3-ols in barley were metabolized into valerolactones by colonic microbiota, renowned for their potent antioxidant and anti-inflammatory effects. Overall, this research sheds light on (poly)phenol metabolism in purple barley and sets the stage for exploring its health benefits through dietary interventions.Author contributions
Conceptualization: M.-E. C.-A., A. M. and L. R.-P.; methodology: M.-E. C.-A., S. Y., M. Ma. and I. F.; validation: M.-E. C.-A., C. P.-F. and L. R.-P.; investigation: M.-E. C.-A.; resources: C. P.-F. and M. M.; data curation: M.-E. C.-A., S. Y. and A. M.; writing – original draft preparation: M.-E. C.-A.; writing – review and editing: S. Y., A. M., and L. R.-P.; visualization: L. R.-P., and A. M.; supervision: C. P.-F. and L. R.-P.; project administration: L. R. P. and M. Mo.; and funding acquisition: M. Mo. All authors have read and agreed to the published version of the manuscript.Data availability
The raw data files used to generate the experimental results presented in our manuscript have been made available in the CORA repository. You can access the data via the following https://doi.org/10.34810/data1387.Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.Acknowledgements
This study was supported by the Spanish Ministry of Industry, Economy and Competitiveness, the Agencia Estatal de Investigación (AEI) and the European Regional Development Fund (ERDF) through the PID2020-113009RB-100 INNOBAR project and by the Generalitat de Catalunya through the María Engracia Cortijo predoctoral FI SDUR grant. Silvia Yuste has a Margarita Salas postdoctoral grant funded by the European Union – NextGenerationEU through the Ministerio de Universidades and the Universitat de Lleida. Iván Friero was supported by a pre-doctoral fellowship (PRE2021-100591). The authors are grateful to SCT-Estabulari of the Universitat de Lleida where the animal experiment was carried out. We would like to acknowledge Dr María-Paz Romero for her valuable contribution and supervision throughout this work.References
- Faostat, Food and Agriculture Data. https://faostat.fao.org (accessed September 14, 2023).
- E. Idehen, Y. Tang and S. Sang, Bioactive phytochemicals in barley, J. Food Drug Anal., 2017, 25, 148–161 CrossRef CAS PubMed.
- M. S. Izydorczyk and J. E. Dexter, Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products–a Review, Food Res. Int., 2008, 41, 850–868 CrossRef CAS.
- M. Martínez-Subirà, M. P. Romero, E. Puig, A. Macià, I. Romagosa and M. Moralejo, Purple, high β-glucan, hulless barley as valuable ingredient for functional food, LWT–Food Sci. Technol., 2020, 131, 109582 CrossRef.
- E. T. Callcott, C. L. Blanchard, P. Snell and A. B. Santhakumar, The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort, Food Funct., 2019, 10, 8016–8025 RSC.
- T. H. Gamel, E. S. M. Abdel-Aal, A. J. Tucker, S. M. Pare, K. Faughnan, C. D. O'Brien, A. Dykun, I. Rabalski, M. Pickard and A. J. Wright, Consumption of whole purple and regular wheat modestly improves metabolic markers in adults with elevated high-sensitivity C-reactive protein: A randomised, single-blind parallel-arm study, Br. J. Nutr., 2020, 124, 1179–1189 CrossRef CAS PubMed.
- J. I. Mosele, M. J. Motilva and I. A. Ludwig, Beta-Glucan and Phenolic Compounds: Their Concentration and Behavior during in Vitro Gastrointestinal Digestion and Colonic Fermentation of Different Barley-Based Food Products, J. Agric. Food Chem., 2018, 66, 8966–8975 CrossRef CAS PubMed.
- B. Ed Nignpense, S. Latif, N. Francis, C. Blanchard and A. B. Santhakumar, Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat, Foods, 2022, 11, 3697 CrossRef CAS PubMed.
- Z. Xiang, J. Deng, K. Yang, Y. Zhu, C. Xia, J. Chen and T. Liu, Effect of processing on the release of phenolic compounds and antioxidant activity during in vitro digestion of hulless barley, Arabian J. Chem., 2021, 14, 103447 CrossRef CAS.
- Y. Zhu, S. Yang, Y. Huang, J. Huang and Y. Li, Effect of in vitro gastrointestinal digestion on phenolic compounds and antioxidant properties of soluble and insoluble dietary fibers derived from hulless barley, J. Food Sci., 2021, 86, 628–634 CrossRef CAS PubMed.
- P. C. Drawbridge, F. Apea-Bah and T. Beta, Bioaccessibility of ferulic acid in hulless barley varieties at stages of simulated in vitro digestion, Cereal Chem., 2023, 100, 954–965 CrossRef CAS.
- Y. Xie, T. Gong, H. Liu, Z. Fan, C. Zhaojun and X. Liu, In vitro and in vivo digestive fate and antioxidant activities of polyphenols from hulless barley: impact of various thermal processing methods and β-glucan, J. Agric. Food Chem., 2022, 70, 7683–7694 CrossRef CAS PubMed.
- A. S. Hole, N. P. Kjos, S. Grimmer, A. Kohler, P. Lea, B. Rasmussen, R. L. Lima, J. Narvhus and S. Sahlstrøm, Extrusion of barley and oat improves the bioaccessibility of dietary phenolic acids in growing pigs, J. Agric. Food Chem., 2013, 61, 2739–2747 CrossRef CAS PubMed.
- B. Ed Nignpense, N. Francis, C. Blanchard and A. B. Santhakumar, Bioaccessibility and bioactivity of cereal polyphenols: A review, Foods, 2021, 10, 1595 CrossRef CAS PubMed.
- C. D. Kay, M. N. Clifford, P. Mena, G. J. McDougall, C. Andres-Lacueva, A. Cassidy, D. Del Rio, N. Kuhnert, C. Manach, G. Pereira-Caro, A. Rodriguez-Mateos, A. Scalbert, F. Tomás-Barberán, G. Williamson, D. S. Wishart and A. Crozier, Recommendations for standardizing nomenclature for dietary (poly)phenol catabolites, Am. J. Clin. Nutr., 2020, 112, 1051–1068 CrossRef.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, Dose translation from animal to human studies revisited, FASEB J., 2008, 22, 659–661 CrossRef CAS PubMed.
- C. Kilkenny, W. J. Browne, I. C. Cuthill, M. Emerson and D. G. Altman, Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research, PLoS Biol., 2010, 8, e1000412 CrossRef PubMed.
- M. Martínez, M. J. Motilva, M. C. López de Las Hazas, M. P. Romero, K. Vaculova and I. A. Ludwig, Phytochemical composition and β-glucan content of barley genotypes from two different geographic origins for human health food production, Food Chem., 2018, 245, 61–70 CrossRef PubMed.
- S. Yuste, I. A. Ludwig, M. P. Romero, C. Piñol-Felis, Ú. Catalán, A. Pedret, R. M. Valls, S. Fernández-Castillejo, M. J. Motilva, A. Macià and L. Rubió, Metabolic fate and cardiometabolic effects of phenolic compounds from red-fleshed apple in hypercholesterolemic rats: A comparative study with common white-fleshed apple. The AppleCOR study, Mol. Nutr. Food Res., 2021, 65, 2001225 CrossRef CAS PubMed.
- D. Bars-Cortina, A. Macià, I. Iglesias, M. P. Romero and M. J. Motilva, Phytochemical profiles of new red-fleshed apple varieties compared with traditional and new white fleshed varieties, J. Agric. Food Chem., 2017, 65, 1685–1696 CrossRef.
- S. Yuste, I. A. Ludwig, L. Rubió, M. P. Romero, A. Pedret, R. M. Valls, R. Solà, M. J. Motilva and A. Macià, In vivo transformation of (poly)phenols and anthocyanins of red-fleshed apple and identification of intake biomarkers, J. Funct. Foods, 2019, 55, 146–155 CrossRef CAS.
- N. Gangopadhyay, D. K. Rai, N. P. Brunton, E. Gallagher and M. B. Hossain, Antioxidant-guided isolation and mass spectrometric identification of the major polyphenols in barley (Hordeum vulgare) grain, Food Chem., 2016, 210, 212–220 CrossRef CAS PubMed.
- M. N. Clifford, I. A. Ludwig, G. Pereira-Caro, L. Zeraik, G. Borges, T. M. Almutairi, S. Dobani, L. Bresciani, P. Mena, C. I. R. Gill and A. Crozier, Exploring and disentangling the production of potentially bioactive phenolic catabolites from dietary (poly)phenols, phenylalanine, tyrosine and catecholamines, Redox Biol., 2024, 103068 CrossRef CAS PubMed.
- L. Xie, S. G. Lee, T. M. Vance, Y. Wang, B. Kim, J. Y. Lee, O. K. Chun and B. W. Bolling, Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of aronia berry extract, Food Chem., 2016, 211, 860–868 CrossRef CAS PubMed.
- S. Yuste, A. Macià, M. J. Motilva, N. Prieto-Diez, M. P. Romero, A. Pedret, R. Solà, I. A. Ludwig and L. Rubió, Thermal and non-thermal processing of red-fleshed apple: how are (poly) phenol composition and bioavailability affected?, Food Funct., 2020, 11, 10436–10447 RSC.
- J. I. Mosele, A. Macià and M. J. Motilva, Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review, Molecules, 2015, 20, 17429–17468 CrossRef CAS PubMed.
- T. Nurmi, J. Mursu, M. Heinonen, A. Nurmi, R. Hiltunen and S. Voutilainen, Metabolism of berry anthocyanins to phenolic acids in humans, J. Agric. Food Chem., 2009, 57, 2274–2281 CrossRef CAS PubMed.
- A. Gomes, C. Oudot, A. Macià, A. Foito, D. Carregosa, D. Stewart, T. Van de Wiele, T. Berry, M. J. Motilva, C. Brenner and C. Nunes dos Santos, Berry-enriched diet in salt-sensitive hypertensive rats: metabolic fate of (poly)phenols and the role of gut microbiota, Nutrients, 2019, 11, 2634 CrossRef CAS PubMed.
- J. J. Rodríguez-Romero, A. C. Duran-Castañeda, A. P. Cárdenas-Castro, J. A. Sánchez-Burgos, V. M. Zamora-Gasga and S. G. Sáyago-Ayerdi, What we know about protein gut metabolites: Implications and insights for human health and diseases, Food Chem.: X, 2022, 13, 100195 Search PubMed.
- Z. Huang, L. Dostal and J. P. Rosazza, Mechanisms of ferulic acid conversions to vanillic acid and guaiacol by Rhodotorula rubra, J. Biol. Chem., 1993, 268, 23954–23958 CrossRef CAS PubMed.
- C. Perez-Ternero, A. Macià, M. A. de Sotomayor, J. Parrado, M. J. Motilva and M. D. Herrera, Bioavailability of the ferulic acid-derived phenolic compounds of a rice bran enzymatic extract and their activity against superoxide production, Food Funct., 2017, 8, 2165–2174 RSC.
- S. G. Sáyago-Ayerdi, K. Venema, M. Tabernero, B. Sarriá, L. Bravo and R. Mateos, Bioconversion of polyphenols and organic acids by gut microbiota of predigested Hibiscus sabdariffa L. calyces and Agave (A. tequilana, Weber) fructans assessed in a dynamic in vitro model (TIM-2) of the human colon, Food Res. Int., 2021, 143, 110301 CrossRef PubMed.
- C. Khoo and H. Liu, Effect of Cranberry Polyphenols and Metabolites on Microbial Activity and Impact on Urinary Tract Health, in Polyphenols: Prevention and Treatment of Human Disease, Academic Press, 2018, pp. 89–105 Search PubMed.
- X. Orozco-Angelino, J. Espinosa-Ramírez and S. O. Serna-Saldívar, Extrusion as a tool to enhance the nutritional and bioactive potential of cereal and legume by-products, Food Res. Int., 2023, 169, 112889 CrossRef CAS PubMed.
- I. A. Ludwig, M. P. de Peña, C. Cid and A. Crozier, Catabolism of coffee chlorogenic acids by human colonic microbiota, BioFactors, 2013, 39, 623–632 CrossRef CAS PubMed.
- A. Serra, A. Macià, M. P. Romero, N. Anglés, J. R. Morelló and M. J. Motilva, Metabolic pathways of the colonic metabolism of procyanidins (monomers and dimers) and alkaloids, Food Chem., 2011, 126, 1127–1137 CrossRef CAS.
- P. Mena, L. Bresciani, N. Brindani, I. A. Ludwig, G. Pereira-Caro, D. Angelino, R. Llorach, L. Calani, F. Brighenti, M. N. Clifford, C. I. R. Gill, A. Crozier, C. Curti and D. Del Rio, Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity, Nat. Prod. Rep., 2019, 36, 714–752 RSC.
- P. Mena, I. A. Ludwig, V. B. Tomatis, A. Acharjee, L. Calani, A. Rosi, F. Brighenti, S. Ray, J. L. Griffin, L. J. Bluck and D. Del Rio, Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes, Eur. J. Nutr., 2019, 58, 1529–1543 CrossRef CAS PubMed.
- D. Angelino, A. Caffrey, H. McNulty, C. I. Gill, P. Mena, A. Rosi, K. Moore, L. Hoey, M. Clements, E. Laird, K. Boyd, B. Mullen, B. Pucci, H. Jarrett, C. Cunningham, M. Ward, J. J. Strain, K. McCarroll, A. J. Moore, A. M. Molloy and D. Del Rio, Association of dietary flavan-3-ol intakes with plasma phenyl-γ-valerolactones: analysis from the TUDA cohort of healthy older adults, Am. J. Clin. Nutr., 2023, 118, 476–484 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01275e |
| This journal is © The Royal Society of Chemistry 2024 |