Open Access Article
Open Access ArticleDiet enriched with high-phenolic cocoa potentiates hippocampal brain-derived neurotrophic factor expression and neurogenesis in healthy adult micewith subtle effects on memory†
Sonia
Melgar-Locatelli
 abd,
M. Carmen
Mañas-Padilla
abh,
Adriana
Castro-Zavala
ab,
Patricia
Rivera
ac,
María
del Carmen Razola-Díaz
de,
Francisco J.
Monje
f,
Celia
Rodríguez-Pérez‡
abd,
M. Carmen
Mañas-Padilla
abh,
Adriana
Castro-Zavala
ab,
Patricia
Rivera
ac,
María
del Carmen Razola-Díaz
de,
Francisco J.
Monje
f,
Celia
Rodríguez-Pérez‡
 *deg and
Estela
Castilla-Ortega‡
*ab
*deg and
Estela
Castilla-Ortega‡
*ab
aInstituto de Investigación Biomédica de Málaga y Plataforma en Nanomedicina-IBIMA Plataforma BIONAND, Spain
bDepartamento de Psicobiología y Metodología de las Ciencias del Comportamiento, Universidad de Málaga, Spain
cUnidad de Gestión Clínica de Salud Mental, Hospital Regional Universitario de Málaga, Spain
dDepartamento de Nutrición y Bromatología, Universidad de Granada, Campus Universitario de Cartuja, Spain
eInstituto de Nutrición y Tecnología de los Alimentos ‘José Mataix’ (INYTA), Universidad de Granada, Granada, Spain
fCenter for Physiology and Pharmacology, Department of Neurophysiology and Neuropharma-cology, Medical University of Vienna, 1090 Vienna, Austria
gInstituto de Investigación Biosanitaria de Granada (ibs.GRANADA), 18012, Granada, Spain
hUniversidad Internacional de la Rioja (UNIR), Spain
First published on 18th July 2024
Abstract
Cocoa is widely known for its health benefits, but its neurocognitive impact remains underexplored. This preclinical study aimed to investigate the effects of cocoa and cocoa polyphenols on hippocampal neuroplasticity, cognitive function and emotional behavior. Seventy young-adult C57BL/6JRj male and female mice were fed either a standard diet (CTR) or a diet enriched with 10% high-phenolic content cocoa (HPC) or low-phenolic content cocoa (LPC) for at least four weeks. In a first experiment, behavioral tests assessing exploratory behavior, emotional responses and hippocampal-dependent memory were conducted four weeks into the diet, followed by animal sacrifice a week later. Adult hippocampal neurogenesis and brain-derived neurotrophic factor (BDNF) expression in the hippocampus and prefrontal cortex were evaluated using immunohistochemistry and western blot. In a different experiment, hippocampal synaptic response, long-term potentiation and presynaptic-dependent short-term plasticity were studied by electrophysiology. Cocoa-enriched diets had minimal effects on exploratory activity and anxiety-like behavior, except for reduced locomotion in the LPC group. Only the HPC diet enhanced object recognition memory, while place recognition memory and spatial navigation remained unaffected. The HPC diet also increased adult hippocampal neurogenesis, boosting the proliferation, survival and number of young adult-born neurons. However, both cocoa-enriched diets increased immobility in the forced swimming test and hippocampal BDNF expression. Hippocampal electrophysiology revealed no alterations in neuroplasticity among diets. The results were mostly unaffected by sex. Overall, the HPC diet demonstrated greater potential regarding cognitive and neuroplastic benefits, suggesting a key role of cocoa flavanols in dietary interventions aimed at enhancing brain health.
Introduction
The consumption of cocoa, obtained from the Theobroma cacao tree whose beans are used to produce various food products, including chocolate, has significantly increased since its introduction to Europe in the 16th century.1,2Cocoa contains more than 300 different constituents, including minerals (magnesium, potassium, iron and zinc), fiber, methylxanthines (caffeine and – most abundantly – theobromine, a caffeine derivative) and polyphenols.3,4 Phenolic compounds are a large group of compounds present in plants and plant-based foods.5 Among them, natural cocoa powder is a major source of polyphenols, which are found in cocoa at a higher concentration (up to 50 mg per gram) than in many other natural foods.4,6 The main polyphenols found in cocoa are flavanols; specifically, monomeric (−)-epicatechin and (+)-catechin and polymerics procyanidins are the most abundant.4 Nevertheless, it is important to note that the phenolic composition of commercial cocoa-derived products varies widely, with pure cocoa powder typically containing higher levels.7–9 Factors including cocoa plant genotype, region and method of cultivation and manufacturing processes (especially alkalinization, which reduces polyphenol content) contribute significantly to this variability.10–12
Polyphenols may explain cocoa's long recognized health benefits, as they have been shown to have preventive and/or therapeutic effects on a number of diseases such as cancer, diabetes, cardiovascular and even neurodegenerative disorders.13 Among other actions, cocoa flavanols directly neutralize free radicals and reduce levels of reactive oxygen species, contribute to arterial elasticity and nitric oxide-mediated vasodilation, have anti-inflammatory properties, reduce insulin resistance – by lowering the circulating levels of glucose – and enhance the growth of beneficial gut microbiota.2,11 However, in addition to flavanols, other cocoa components such as methylxanthines may influence health outcomes. For example, recent studies have highlighted the potential of theobromine as a protective agent against cancer, inflammation and cardiovascular disease.7,14 For caffeine, both healthy and harmful effects on several physiological systems have been described.7,15 In any case, an interesting possibility is that some cocoa components may act synergistically or in interaction, so the combination of caffeine and theobromine in cocoa may have the expected methylxanthine-derived benefits without the side effects reported for caffeine.14,16 Therefore, it is not only relevant to study the properties of each of the cocoa components separately, but also important to study the effects of the whole natural product, which is highly present in the everyday diet of millions around the world.
Lately, cocoa and dark chocolate have been proposed as cognitive enhancers. Acute intake of cocoa has been consistently associated with a rapid improvement in cognitive measures.17 For example, ingestion of 35 g of a 70% dark chocolate bar is enough to potentiate hippocampal-dependent episodic-like memory, probably through flavanols’ rapid actions on brain irrigation18 which increase cerebral blood flow in memory-related brain regions including the hippocampus.19 Regarding chronic consumption, a meta-analysis of studies that administered chocolate or cocoa for 1–6 months to healthy adults revealed an improvement in executive function and language-related measures.20 However, a recent randomized clinical trial with 2262 elderly participants that consumed cocoa extract for 3 years did not find an effect of cocoa on global cognition, in contrast to the cognitive benefits found after multivitamin–mineral supplementation.21 Another review focused on cocoa flavanols17 supports that flavanols improve general cognition, attention, memory or processing speed. Nevertheless, flavanols’ cognitive effects are not unequivocal and consistently replicated compared to their action on other physiological responses, and findings may depend on methodological differences; for example, consumption of cocoa flavanols may be more beneficial for populations at risk of neurocognitive impairment.17
Preclinical research could provide a valuable tool to unveil the cognitive functions of cocoa and cocoa flavanols, as well as their brain mechanisms. In addition to their cerebrovascular, anti-inflammatory and antioxidant properties, some in vivo and in vitro studies point out that cocoa flavanols stimulate the cAMP-response element-binding protein (CREB) pathway in neurons, leading to long-term potentiation as well as the production of survival factors such as the brain-derived-neurotrophic factor (BDNF).22,23 Cocoa methylxanthines may also exert neuroprotective actions; for example, theobromine in cocoa has demonstrated anti-amyloidogenic activity in cellular models of Alzheimer's disease.24 In rodents, adult hippocampal neurogenesis (AHN) is a key neuroplastic phenomenon that seems to be involved in maintaining normal cognitive and emotional function, and dysregulation of this process has been associated with behavioral impairments in animal models of aging, dementia, stress and many other pathologies.25 Adult-born hippocampal neurons are highly sensitive to both extrinsic and intrinsic factors, including neurotrophins such as BDNF and inflammatory cytokines, which can be influenced by diet.26 However, the influence of non-alkalized (natural) cocoa on AHN has not yet been properly evaluated. Flavanols are usually considered as AHN enhancers,27 but mixed results have been reported for caffeine.28,29 Other studies have revealed a potentiated AHN – cell proliferation and differentiation – and a pro-cognitive effect in both normal mice and Alzheimer's disease mice that consumed a diet enriched with high-phenolic cocoa. Unfortunately, the diet was also enriched with other nutrients such as polyunsaturated fatty acids from nuts, which prevented the determination of the specific contribution of cocoa.30 Other experiments that fed rats with a high-phenolic cocoa powder extract have described behavioral outcomes typically associated with AHN – i.e. antidepressant-like effects and improved cognitive performance in spatial navigation tasks –, but no brain correlates were analyzed.31–33
In this study, we aimed to study the effect of chronic administration of a cocoa-enriched diet on emotional and cognitive behavior in healthy adult male and female mice, assessing AHN and BDNF expression (in the hippocampus and prefrontal cortex) as measures of brain neuroplasticity. Additionally, we conducted electrophysiological experiments to further investigate the impact of the cocoa-enriched diet on the hippocampal response, specifically focusing on the CA3-Schaffer collateral to CA1 pathway synapses. Considering the potential contribution of cocoa polyphenols to both behavioral and brain outcomes, we used two different cocoa powders that differed in their phenolic composition.
Materials and methods
The present manuscript is grounded in the findings of two distinct experiments. The first experiment (Experiment 1) encompassed behavioral assessment, western blot analysis and immunohistochemistry. Conversely, the second experiment (Experiment 2) was specifically focused on electrophysiology.Animals and ethics statement
Seventy young adult male and female C57BL/6JRj mice, divided into two experiments, were sourced from Janvier Labs (Le Genest-St-Isle, France) and arrived at the animal facility at 10 weeks of age. After one week of acclimation, mice were individually housed in standard laboratory cages with nesting material under standard conditions, which consisted of a controlled temperature of 22 ± 2 °C and a 12-hour light/dark cycle (lights on at 8:00 a.m.). Additionally, they were provided with ad libitum access to water and food.Procedures were performed according to the European and Spanish regulations for animal research (Directive 2010/63/UE, Real Decreto 53/20130 and Ley 32/2007) and were approved by the research ethics committees of the University of Málaga (code: 104-2021-A) and Junta de Andalucía (code: 3/11/2021/170). Experimenters held the appropriate training certification for the use of laboratory animals in experimental sciences.
Diets
High-phenolic content cocoa (HPC) and low-phenolic content cocoa (LPC) were selected considering their nutritional and phenolic composition as we previously published.10 First, more than 20 cocoa powders commercially available in the Spanish market were evaluated considering the information from their nutrition fact labels (data not shown). After that, seven cocoa powders with similar composition in macronutrients and energy from different origins (5 alkalized and 2 non-alkalized) were finally selected for determining their total phenolic content (TPC), procyanidin content and methylxanthine content and for assessing their antioxidant activity as described elsewhere.10 Briefly, the TPC was evaluated using the Folin–Ciocalteu spectrophotometric method while the procyanidin content (flavan-3-ols) was measured by high performance liquid chromatography coupled to a fluorescence detector (HPLC-FLD). Furthermore, the total methylxanthine content (expressed as the sum of caffeine and theobromine concentration) was determined by HPLC coupled to a photodiode array detector (DAD). For that purpose, an Agilent 1200 Series (Agilent Technologies, Palo Alto, CA, USA), equipped with a quaternary pump delivery system, a degasser, an autosampler and a photodiode array detector (DAD) set up at 264 nm, was employed for the analysis using the procedure described by Alañón et al.34 In addition, three different methods were employed for determining the antioxidant activity of the cocoa samples, i.e., 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and Ferric Reducing Antioxidant Power (FRAP) assays.The cocoa-supplemented diets were provided by the Scientific Animal Food & Engineering (SAFE, Bourgogne, France) company, by incorporating 10% concentration of the respective high and low phenolic content cocoa powder into the standard diet pellets (SAFE® D40 pellets). Additionally, a green or a blue dye was added to facilitate accurate identification of either dietary profile. All diets were stored at −80 °C upon their arrival and unfrozen two days before their use.
Statistical analysis
To assess the normality of the data distributions, a Shapiro–Wilk test was conducted. To explore the differences among dietary treatments, one-way analysis of variance (ANOVA) or repeated measures ANOVAs were performed, followed by post-hoc Fisher's least significant difference (LSD) or Tukey's test when required. Tukey's test was employed to assess variations in bioactive compounds between the two different cocoas, with two samples analyzed per cocoa powder. Furthermore, additional factorial and repeated measures ANOVA analyses were subsequently carried out to explore sex differences and potential interactions between sex and diet, which are reported in the ESI† in the case of significant effects. Comparisons were considered significant at p ≤ 0.05. Non-statistically significant differences are shown in ESI Table 1.†Experiment 1
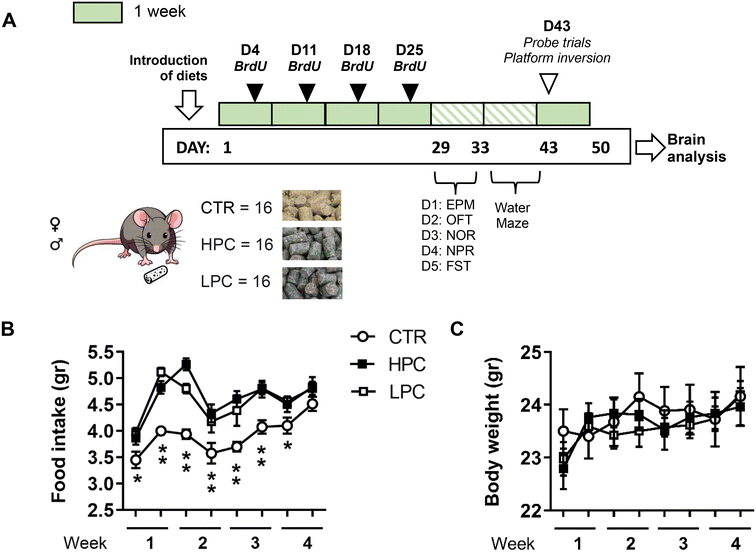
Forty-eight young-adult C57BL/6JRj mice – 24 mice of each sex – were divided into three groups based on comparable average body weight. They were then randomly assigned to receive either the standard (‘control’; CTR), HPC or LPC diet (n = 16 mice; 8 mice per sex). The cocoa-enriched diets were introduced on Day 1 (Fig. 1A), coinciding with the animals reaching 12.5 weeks of age. Throughout the first four weeks, food intake and body weight measurements were recorded twice a week. Mice consumed their assigned diets until the end of the experiment (i.e. on Day 50).Bromodeoxyuridine administration
To study AHN, bromodeoxyuridine (BrdU, Sigma-Aldrich, Madrid, Spain) was administered over the initial four weeks of dietary administration, specifically on days 4, 11, 18, and 25 (Fig. 1A), in order to tag the newly generated cells. Mice were subjected to two daily intraperitoneal administrations of BrdU at a dosage of 75 mg kg−1, diluted in physiological saline, with a 4-hour interval between each administration.35 This BrdU administration protocol aims to investigate whether the diets modulated AHN, without specifying the precise timing at which this effect occurs.Behavioral testing
The evaluation of exploration, emotional responses, and cognitive performance was conducted in accordance with our previously published protocols.36,37 The behavioral assessment started five weeks after the separation of mice in individual cages. Mice were carried to a noise-isolated room (illuminated at 75 lux) at 8:30 a.m. and were habituated for at least 20 minutes before starting behavioral testing. To eliminate odor cues, a 30% ethanol solution was utilized to clean the dry maze arena. The experimental sessions were recorded, and spatiotemporal parameters were analyzed with the software Ethovision XT.17. (Noldus, Wageningen, The Netherlands). Observational scorings were carried out by a proficient observer who was blind to the mice's sex and treatment conditions and possessed no preconceived notions regarding the study's outcome.The behavioral schedule was structured as shown in Fig. 1A:
• Elevated plus maze (EPM) (Day 29). The plus-shaped (+) apparatus was positioned at a height of 47 cm from the floor and comprised two unprotected open arms and two enclosed arms (each measuring 29.5 × 5 cm) connected by a central platform (5 × 5 cm). The mouse was introduced onto the central platform and allowed to freely explore the apparatus for a duration of 6 min. Total locomotion (cm), time spent (s) in the open arms and the latency to enter an open arm (s) were analyzed.
• Open field test (OFT) (Day 30). On the second day of behavioral assessment, mice were placed at one corner of an empty open field (40 × 40, 40 cm high) and allowed to freely explore for 5 min (habituation session). Total locomotion (cm) and time spent (s) in the center zone (comprising an imaginary central square of 20 × 20 cm) were evaluated.
• Novel object recognition (NOR) and novel place recognition (NPR) memory (Days 30–32). Sixty minutes following the OFT, mice were re-exposed to the apparatus. It included two identical copies of an object, referred to as the ‘familiar’ object, positioned near two adjacent corners (sample session; Fig. 2C). On Day 31, mice were left to explore an identical copy of the familiar object and a ‘novel’ unknown object, located in the previous positions (NOR test). Finally, on Day 32, the open field was equipped with two identical copies of the familiar object. One of them remained in its habitual position (i.e. ‘stationary’), while the other was ‘displaced’ to the opposite corner of the arena (NPR test). The mouse was given 10 min to freely explore the apparatus in each session. The total duration of object exploration (s), defined as nose or paw contact with the object or pointing its nose towards the object at a distance of 1–2 cm away, was analyzed observationally as per prior research.35 Furthermore, the ‘object memory ratio’ [(time spent exploring the novel object − time spent exploring the familiar object)/total time spent exploring both objects] and the ‘place memory ratio’ [(time spent exploring the displaced object − time spent exploring the stationary object)/total time spent exploring both objects] were calculated to gauge object and place memory, respectively.
• Forced swimming test (FST) (Day 33). Mice were introduced into a cylindrical container made of a transparent material (10 cm diameter, 27 cm height) filled with water (22 ± 1 °C) reaching a depth of 15 cm for a duration of 6 min. The total time (s) of two distinct behaviors, which included ‘immobility’ (i.e. minimal movements to keep the head above water) and ‘struggling’ (i.e. vigorous attempts to climb the cylinder walls while breaking the water surface using the forelimbs), as well as latency to immobility (s) were observationally recorded and analyzed.
• Water maze (Days 36–48). A circular pool (1.2 m diameter) was filled with opaque water (containing non-toxic white paint) at a temperature of 24 °C ± 1 °C. The pool was partitioned into four equal quadrants, which were conceptual divisions rather than physically separated compartments. Mice were released from one of the four possible starting positions in each quadrant's peripheral region (north, N; west, W; east, E; and south, S). The experimental room was furnished with distal extra-maze cues, including visible black cardboard panels with distinct geometric shapes placed on each wall, as well as various furniture elements to facilitate spatial orientation. During the training session, latency (s) to reach the platform and total path length (cm) were evaluated as indicators of learning.
The water maze training consisted of the following stages:
– Habituation (Day 36). Mice swam freely in the pool for 1 min, starting from the S position. Various parameters were analyzed, including the total distance swum (cm) and time spent (s) in the peripheral area (defined as an outer zone located 24 cm inward from the walls).
– Visible platform training (Days 36–37). Forty-five min after the habituation session, a white goal platform (11 cm diameter) was placed in the center of one quadrant. The platform was slightly above the water level and featured a vertically standing black polystyrene tube. Each day, the mice underwent four training sessions (with an inter-trial interval – ITI – of 30 min), during which the platform's location was moved across all quadrants, and the starting positions were alternated. If a mouse successfully reached the visible platform, it was promptly removed from the pool. However, if a mouse failed to reach the platform within a duration of 1 min, the experimenter guided the animal towards it. This training was repeated on Day 37. Path length (cm), velocity (cm s−1) and latency (s) to reach the platform were analyzed.
– Reference memory training (Days 38–41). The platform was permanently positioned within the target quadrant, submerged under 1 cm of water. Spatial memory training took place over four consecutive days (Days 10–13), with a total of six training sessions per day (IT: 30 min). During each session, mice were released into the pool from one of the four starting positions (N, W, E, S). Upon discovering the hidden platform, mice were allowed to remain on it for 5 s. If a mouse failed to locate the platform within 1 min, the experimenter guided the mouse to it, permitting the mouse to stay on the platform for 10 s. Total locomotion (cm), velocity (cm s−1) and latency (s) to reach the platform were evaluated.
– Probe trials (Day 42). Following the removal of the platform from the maze, mice were released from the S position and given a 1 min swimming period. Performance evaluation encompassed measurements of the total time (s) spent swimming within the target and opposite quadrants, the latency (s) to reach the circular region where the platform was previously positioned, and the number of crossings made towards the imaginary platform. Two probe trials were carried out on Day 42. The first probe trial occurred early in the day, 72 h after the last spatial training session, to evaluate the long-term retention of spatial memory. The second probe trial took place 30 min after the last training session with platform inversion, focusing on cognitive flexibility and the acquisition of short-term memory regarding the new platform location.
– Platform inversion (Day 43). Forty-five minutes after the 72 h probe trial, the platform was hidden in the center of quadrant E, which was positioned opposite to the target quadrant utilized during spatial training. Six training sessions were then conducted according to the previously described procedure.
Brain sample collection
Mice were deeply anesthetized with intraperitoneal sodium pentobarbital (50 mg per kg body weight) and sacrificed on Day 50, one week after the platform inversion test. Five minutes after administering the anesthetic, mice were intracardially perfused with 0.1 M phosphate buffered saline pH 7.4 (PBS) and subsequently sacrificed by decapitation. Brain samples were immediately extracted; the right hemisphere was post-fixed in paraformaldehyde (PFA) for 48 h at 4 °C and cut into coronal (45 μm) vibratome sections for immunohistochemistry, while the left hemisphere was directly frozen at −80 °C for protein analysis by western blot.Western blot
The left hemisphere of the hippocampus, frozen at −80 °C, was dissected from frozen brain samples using the Paxinos and Watson's mouse brain atlas38 as a reference. Since three hippocampal samples were missed during tissue extraction (two CTR males and one LPC female), a total of 45 animals were studied for western blot analysis. The hippocampus samples (17–20 mg per sample) and prefrontal cortex samples (18–21 mg per sample) of forty-five animals were individually homogenized in 1 ml of cold radioimmunoprecipitation assay lysis buffer (RIPA). The RIPA buffer consisted of 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton, 0.1% SDS, 1 mM Na3VO4, and 1 mM NaF. Additionally, the homogenization buffer was supplemented with a phosphatase (Phosphatase Inhibitor Cocktail Set III, 524527, Millipore, Darmstadt, Germany) and a protease (cOmplete™ Protease Inhibitor Cocktail, 11836145001, Roche, Basel, Switzerland) inhibitor cocktail. Following a 2 h incubation at 4 °C, the suspension was centrifuged at 12,000 rpm for 15 min at 4 °C. The resulting protein extracts (obtained from the supernatant) were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in loading buffer (dithiothreitol [DTT] 2×) and heated for 5 min at 99 °C.
1 in loading buffer (dithiothreitol [DTT] 2×) and heated for 5 min at 99 °C.
Protein expression levels of BDNF were assessed using western blot analysis following methods from our laboratory.39 Tissue protein samples (10–15 μg) were subjected to electrophoresis on 4–12% Criterion XT Precast Bis-Tris gels (3450125, Bio-Rad, California, USA) for 30 min at 80 V, followed by 2 h at 150 V. The separated proteins were then transferred onto a 0.2 μm nitrocellulose membrane (Bio-Rad, USA) using wet transfer equipment (Bio-Rad, USA) for 1 h at 80 V. Ponceau Red staining (10× diluted to 1× in H2O) was utilized for protein visualization. The membrane was subsequently washed with TBST 1× Tween 20 (150 mM NaCl, 10 mM Tris-HCl, 0.1% Tween 20, pH 7.6) until it became clean and clear. The membrane was blocked with 2% bovine serum albumin-Tris buffered saline Tween 20 (BSA-TBST 1×) on a shaker platform at room temperature for 1 h. Next, the membrane was incubated overnight at 4 °C with the primary antibodies [rabbit anti-BDNF antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250, AB1534SP, Sigma-Aldrich, Massachusetts, USA)], diluted in 2% BSA-TBST 1×. The following day, the membrane was washed three times for 10 min with TBST 1× and then incubated with appropriate horseradish peroxidase conjugated secondary antibodies (goat anti-rabbit IgG, W4011, Promega, Wisconsin, USA) diluted 1
250, AB1534SP, Sigma-Aldrich, Massachusetts, USA)], diluted in 2% BSA-TBST 1×. The following day, the membrane was washed three times for 10 min with TBST 1× and then incubated with appropriate horseradish peroxidase conjugated secondary antibodies (goat anti-rabbit IgG, W4011, Promega, Wisconsin, USA) diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in 2% BSA-TBST 1× for 1 h at room temperature on a shaker. After washing the membrane, it was exposed to a chemiluminescent reagent (Santa Cruz Biotechnology) for 5 min. If required, stripping/reproving steps were performed. The protein bands on the membrane were visualized using chemiluminescence (ChemiDoc Imaging System, Bio-Rad, California, USA) and quantified using ImageJ software (densitometric analysis https://imagej.nih.gov/ij). Normalization was accomplished by using a reference protein, γ-adaptin (1
000 in 2% BSA-TBST 1× for 1 h at room temperature on a shaker. After washing the membrane, it was exposed to a chemiluminescent reagent (Santa Cruz Biotechnology) for 5 min. If required, stripping/reproving steps were performed. The protein bands on the membrane were visualized using chemiluminescence (ChemiDoc Imaging System, Bio-Rad, California, USA) and quantified using ImageJ software (densitometric analysis https://imagej.nih.gov/ij). Normalization was accomplished by using a reference protein, γ-adaptin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000, 610385, BD Biosciences, New Jersey, USA), which was present on the same membrane. The results were expressed as the ratio between the total protein expression and γ-adaptin as described.40 Data were normalized to the CTR mean group.
2000, 610385, BD Biosciences, New Jersey, USA), which was present on the same membrane. The results were expressed as the ratio between the total protein expression and γ-adaptin as described.40 Data were normalized to the CTR mean group.
Immunohistochemistry and cell quantification
Following a 48-hour post-fixation period, the hippocampus of the right hemisphere of 24 mice (four males and four females of each treatment, selected at random) was sectioned into 45 μm coronal sections, resulting in six equivalent tissue series, using a Leica VT1000S vibratome. For free-floating immunohistochemistry, the following steps were undertaken: first, sections were subjected to an antigen retrieval method using EnVision Flex high pH solution (Dako, Glostrup, Denmark) for 1 min in a microwave instrument. Subsequently, an endogenous-peroxidase blocking solution consisting of 80% PBS, 10% methanol, and 10% hydrogen peroxide was applied in darkness for 30 min. After PBS rinses, the sections were incubated overnight with primary antibodies, which were diluted in a solution of PBS, 0.5% Triton X-100, and donkey serum. The primary antibodies used were as follows: rabbit anti-doublecortin (DCX; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000, ab18723, Abcam, Cambridge, UK), rat anti-BrdU (1
2000, ab18723, Abcam, Cambridge, UK), rat anti-BrdU (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, ab6326, Abcam), and mouse anti-proliferating cell nuclear antigen (PCNA; 1
500, ab6326, Abcam), and mouse anti-proliferating cell nuclear antigen (PCNA; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, P8825, Sigma). On the following day, appropriate biotin-conjugated secondary antibodies were incubated for 90 min, including rabbit anti-rat (1
1500, P8825, Sigma). On the following day, appropriate biotin-conjugated secondary antibodies were incubated for 90 min, including rabbit anti-rat (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, 31834, Invitrogen, Carlsbad, USA), goat anti-mouse (1
200, 31834, Invitrogen, Carlsbad, USA), goat anti-mouse (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, B7264, Sigma-Aldrich), and donkey anti-rabbit (1
800, B7264, Sigma-Aldrich), and donkey anti-rabbit (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, RPN1004 V, Thermo Fisher Scientific, Massachusetts, USA). The staining process was carried out using the biotin and peroxidase-conjugated extravidin method, employing diaminobenzidine (DAB) and hydrogen peroxide as the chromogen/substrate. PBS rinses followed each step of the protocol.
500, RPN1004 V, Thermo Fisher Scientific, Massachusetts, USA). The staining process was carried out using the biotin and peroxidase-conjugated extravidin method, employing diaminobenzidine (DAB) and hydrogen peroxide as the chromogen/substrate. PBS rinses followed each step of the protocol.
Co-labeling of BrdU with a mature neuron marker was confirmed by immunofluorescence in four animals per treatment. Sections were first incubated overnight with a combination of rat anti-BrdU antibodies (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) and rabbit anti-neuronal nuclei antibodies (NeuN; 1
500) and rabbit anti-neuronal nuclei antibodies (NeuN; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, ABN78, Merck Millipore, Madrid, Spain), as a marker for mature neurons. Subsequently, sections were incubated for 90 min in a mixture of fluorescent secondary antibodies. This included goat anti-rat Alexafluor-594 (AB150160, Abcam, Cambridge, UK) and goat anti-rabbit Alexafluor-594 (A11012, Invitrogen Thermo Fisher, Carlsbad, USA), both diluted 1
500, ABN78, Merck Millipore, Madrid, Spain), as a marker for mature neurons. Subsequently, sections were incubated for 90 min in a mixture of fluorescent secondary antibodies. This included goat anti-rat Alexafluor-594 (AB150160, Abcam, Cambridge, UK) and goat anti-rabbit Alexafluor-594 (A11012, Invitrogen Thermo Fisher, Carlsbad, USA), both diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in PBS.
500 in PBS.
Cell quantification
The dentate gyrus (DG) within the dorsal hippocampus (bregma −1.06 mm to −3.08 mm)38 was examined for immunohistochemical expression to determine the presence of the aforementioned specific markers related to AHN. To quantify the cells stained with DAB, detailed photographs of every sixth hippocampal section were captured using an Olympus BX41TF-5 microscope equipped with an Olympus DP70 digital camera (Olympus, Glostrup, Denmark). The software ImageJ (National Institutes of Health, Maryland, USA) was used to measure and analyze the drawn regions of interest. The number of positive cells within each region was counted and expressed as the number of cells per mm2. For the DCX + neurons, we distinguished two categories according to their morphological features: type 1: with absent or short dendritic processes (i.e., immature-like morphology) and type 2: with at least one prominent apical dendrite penetrating the granular cell layer.41 Confocal microscopy (Leica SP8; Leica Microsystems, Solms, Germany) was used to detect the co-expression of BrdU and NeuN by immunofluorescence in 1 in every 12 dorsal hippocampal sections.Experiment 2
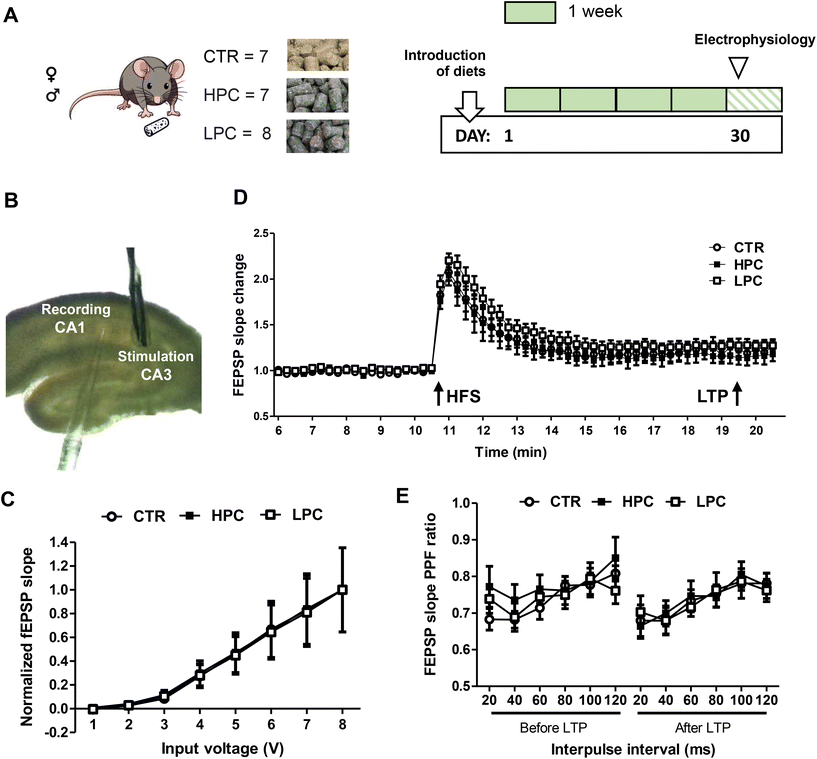
Twenty-two young-adult C57BL/6JRj mice were divided into three groups based on comparable body weight. They were then randomly assigned to receive either the standard CTR, HPC or LPC diet (n = 7–8 animals per experimental group, comprising 3–4 males and 4 females each). The cocoa-enriched diets were introduced on Day 1, coinciding with the animals reaching 12.5 weeks of age (Fig. 6A). Throughout the first four weeks, food intake and body weight measurements were recorded once a week. Mice consumed their assigned diets until the end of the experiment (i.e. on Day 30).Hippocampal slice preparation
Animals were deeply anesthetized using a solution containing 110 mg kg−1 ketamine and 15 mg kg−1 Rompun, diluted in saline. Subsequently, they were quickly sacrificed by decapitation. The hippocampal slices were prepared in accordance with previous reports.42,43 The right hemisphere of the brain was immediately extracted and placed in an ice-cold artificial cerebrospinal fluid (aCSF) solution containing (in mM) 125 NaCl, 2.5 CaCl2, 2.5 KCl, 1 MgCl2, 20 NaHCO3, 25 D-glucose and 1 NaH2PO4 (all compounds from Sigma-Aldrich, Wien, Austria). Sagittal hippocampal slices were then cut using a McIlwain tissue chopper (TC752, Campden Instruments Ltd, Loughborough, UK) into 400 μm-thick sections and transferred to an aCSF-filled recovery chamber submerged in a water bath at 32 °C, where they recovered for 1 h before electrophysiology measurements. The aCSF solution was consistently gassed with 95% O2 and 5% CO2 to maintain a pH of 7.4.Electrophysiology
Slice recordings (3–4 slices per animal) were carried out in a submerged chamber that received a continuous supply of pre-carbogenated aCSF, pre-warmed at 30 ± 2 °C, at a rate of 3–4 mL min−1. Field excitatory postsynaptic potentials (fEPSPs) were recorded in the CA1 stratum–radiatum layer using glass micropipettes filled with aCSF (2–4 MΩ). The recordings were made after electrically stimulating the collateral projections of Schaffer originating from the CA3 region (Fig. 6B). To induce the fEPSPs, biphasic-square voltage pulses were delivered through bipolar electrodes made of tungsten wire, which were insulated with a Teflon coating up to the electrode tips. The voltage pulses were generated using an ISO-STIM 01D stimulator (NPI Electronics, Tamm, Germany). All recordings were made within the dorsal hippocampus, following previously described protocols.43–45The input/output (I/O) curves were established by applying progressively increasing voltage discharges (0–7 V with Δ1 V, 200 μs per pulse; 15 s inter-pulse interval). The fEPSP slopes of each field potential were normalized to the maximum inducible value, and the average was used as an indicator of the synaptic response. For the induction of long-term potentiation (LTP), a high-frequency stimulation (HFS) protocol was delivered. Six separate series of electrical stimulations were applied at 200 ms intervals. Each series consisted of a total of 10 biphasic voltage pulses (100 μs per phase) delivered at a frequency of 100 Hz. A 10 min baseline of field recordings was established, followed by the delivery of the LTP-inducing protocol. Subsequently, field recordings for an additional 20 min were conducted. LTP was identified by observing the temporal changes in fEPSP slope values (decaying phase) subsequent to LTP induction and normalizing them to the average slope values recorded in the baseline. Finally, the properties of presynaptic-dependent short-term plasticity were studied by implementing electrophysiological protocols of Paired-pulse-induced Facilitation (PPF). Two successive pulses of electrical stimulation, delivering voltages that evoke approximately 50% of the maximum inducible field amplitude, were administered with “delta” of increments of 20 ms, increasing up to 120 ms interpulse interval. Ratios for the values of the initial decay slope of the field potential response (EPSP2/EPSP1) were employed to quantify the power of paired-pulse induced facilitation. A second PPF protocol was applied after the LTP induction in order to examine possible changes in metaplasticity properties.
Recordings were obtained using an AxoClamp-2B amplifier, digitalized using the Digidata-1440 interface, and acquired and analyzed using the pCLAMP-11 (version 11.3) software (all from Axon Instruments, Molecular Devices, 660–665 Eskdale Rd, Winnersh, Triangle, Wokingham RG41 5TS, UK).
Results
Composition of different cocoa powders
Significant differences among the studied cocoa powders in terms of bioactive compounds and antioxidant activity were found.10 The nutritional composition based on labelling of the selected cocoas is shown in ESI Table 2.† Concerning bioactive compound composition, the cocoa powder with the highest values presented a TPC of 57.4 mg gallic acid equivalents (GAE) per g d.w., flavanol content of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575.06 μg catechin equivalents (CE) per g d.w. and antioxidant activity of 97.94, 267.43 and 98.74 mg Trolox equivalents (TE) per g d.w. for DPPH, ABTS and FRAP, respectively, while the one with the lowest values had a TPC of 9.2 mg GAE per g d.w., flavanol content of 3,298.89 μg CE per g d.w., and 30.77, 73.97 and 28.88 mg TE per g d.w. of antioxidant activity measured by DPPH, ABTS and FRAP assays, respectively. Those two cocoa powders were finally selected for further experiments, one with the highest TPC, procyanidins and antioxidant activity (HPC) and other with the lowest values (LPC). Considering an average pellet intake per mouse of 5 g per day (enriched with 10% HPC or LPC), the calculated intake of phenolics, flavanols, and methylxanthines was higher in mice on the HPC diet than in those on the LPC diet (Table 1).
575.06 μg catechin equivalents (CE) per g d.w. and antioxidant activity of 97.94, 267.43 and 98.74 mg Trolox equivalents (TE) per g d.w. for DPPH, ABTS and FRAP, respectively, while the one with the lowest values had a TPC of 9.2 mg GAE per g d.w., flavanol content of 3,298.89 μg CE per g d.w., and 30.77, 73.97 and 28.88 mg TE per g d.w. of antioxidant activity measured by DPPH, ABTS and FRAP assays, respectively. Those two cocoa powders were finally selected for further experiments, one with the highest TPC, procyanidins and antioxidant activity (HPC) and other with the lowest values (LPC). Considering an average pellet intake per mouse of 5 g per day (enriched with 10% HPC or LPC), the calculated intake of phenolics, flavanols, and methylxanthines was higher in mice on the HPC diet than in those on the LPC diet (Table 1).
| Bioactive compounds | HPC | LPC |
|---|---|---|
| Different letters (a,b) in the same line indicate significant differences. | ||
| Total phenolic content (TPC) (mg GAE per kg) | 1195.83 ± 27.5a | 191.67 ± 30.42b |
| Flavanols (mg CE per kg) | 595.42 ± 1.25a | 68.75 ± 0.83b |
| Methylxanthines (mg kg−1) | 815.83 ± 17.92a | 573.75 ± 60.83b |
| – Caffeine (mg caffeine per kg) | 563.75 ± 1.25a | 296.67 ± 59.36b |
| – Theobromine (mg theobromine per kg) | 251.88 ± 42.92 | 276.88 ± 1.67 |
Supplementing cocoa in diets increased food consumption
Both groups of cocoa-fed mice freely increased food consumption when assessed during the four following weeks compared to control animals [repeated measures ANOVA ‘diet x day’: effect for ‘diet’: F(2, 45) = 17.731, p < 0.001; ‘day’: F(7, 315) = 22.613, p < 0.001; ‘diet x day’: F(14, 315) = 2.405, p = 0.003; LSD post hoc analysis is shown in Fig. 1B]. However, such increased food consumption did not yield notable changes in body weight gain which was similar to that in controls. Moreover, LSD post hoc analysis did not reveal significant differences between the CTR group and any of the cocoa-treated groups (Fig. 1C). During the analysis of sex differences, food consumption was not influenced by the sex of the animals. Nevertheless, male mice exhibited a higher body weight than females (ESI Fig. 1†).Locomotor activity was reduced by LPC-enriched diet, with no differences in anxiety-like behavior
In the elevated plus maze, there were no significant differences among treatments in total locomotion, total time spent in open arms or latency to enter an open arm (Fig. 2A). However, exploration of a novel open field revealed reduced locomotor activity in the LPC group [F(2, 45) = 5.790, p = 0.006; LSD post hoc analysis is shown in Fig. 2B], while the time spent in the center of the apparatus was not altered (Fig. 2B). There were no differences related to sex in these tasks.Only HPC-enriched diet promoted object recognition memory
Results from the sample, object and place recognition memory sessions are reported at a 7 min trial duration, as we found that this cut-off time maximized between-group differences. HPC-fed mice showed increased object memory [one-way ANOVA: F(2, 45) = 3.729, p = 0.032; LSD post hoc analysis is shown in Fig. 2C], while all groups performed similarly in the place memory test (Fig. 2C). Mice fed either of the cocoa-enriched diets spent less time exploring objects than CTR mice [repeated measures ANOVA ‘diet x session’: effect for ‘diet’: F(2, 45) = 3.193, p = 0.050; ‘session’: F(2, 45) = 4.387, p = 0.015; ‘diet x session’: F(4, 90) = 1,343, p = 0.260; LSD post hoc analysis is shown in Fig. 2C], although locomotion was similar in all groups. During the analysis of sex differences, the effects of diet on recognition-related measures and locomotion were not influenced by the sex of the animals. Nevertheless, male mice spent more time exploring objects than females (ESI Fig. 2†).Both cocoa-enriched diets increased immobility behavior during forced swimming
In the forced swimming test, mice fed with any of the cocoa-enriched diets showed more immobility and less struggling than their CTR counterparts [one-way ANOVA: struggling: F(2, 45) = 8.206, p < 0.001; immobility: F(2, 45) = 8.578, p < 0.001; latency to immobility: F(2, 45) = 9.736, p < 0.001; LSD post hoc analyses are shown in Fig. 2D]. There were no differences related to sex in this task.Both cocoa-enriched diets initially reduced swimming velocity but did not modulate spatial memory
In the 5 min habituation trial with no platform, the treatment did not affect the preference for different maze zones, such as the periphery (thigmotaxis) (Fig. 3A) or each of the four different quadrants (data not shown).However, the cocoa-treated groups initially showed a reduced swimming velocity compared to controls. This was evident during habituation [F(2, 45) = 4.479, p = 0.017; LSD post hoc analysis is shown in Fig. 3A] and also during visible platform training, mostly evident during the first sessions [repeated measures ANOVA ‘diet x trial’: effect for ‘diet’: F(2, 45) = 8.703, p < 0.001; ‘trial’: F(7, 315) = 2.886, p = 0.006; ‘diet x trial’: F(14, 315) = 1.118, p = 0.340; LSD post hoc analysis is shown in Fig. 3B]. Interestingly, the CTR mice progressively reduced their higher swimming velocity as they were trained to reach the visible platform (i.e. sessions 1–3 on day 1 were different from sessions 3 and 4 on day 2; LSD post hoc analysis; p < 0.05). This reduced swimming velocity may affect the outcome of a classic learning-related measure in the water maze such as the escape latency. In fact, both cocoa-fed groups initially took more time to reach the visible platform than controls [effect for ‘diet’: F(2, 45) = 8.195, p < 0.001; ‘trial’: F(7, 315) = 12.663, p < 0.001; ‘diet x trial’: F(14, 315) = 0.656, p = 0.817; LSD post hoc analysis is shown in Fig. 3B]. Nevertheless, the path length (i.e. total distance swam), which is based on the trajectory followed from the starting position to the platform, independently of velocity, was similar among groups (Fig. 3B).
During the reference memory training (Fig. 3C), there were no differences among diets, either for the path length, the latency to find the hidden platform or the swimming velocity.
A probe trial was performed 72 hours after the last reference memory session to study long-term memory retention (Fig. 3D). All groups spent more time in the pool quadrant that previously contained the hidden platform than in the opposite quadrant and showed similar frequency of crossings over the previous platform location (‘platform crossings’). A second probe trial was performed 30 minutes after six training sessions with the hidden platform displaced to the opposite quadrant (‘inversion’ training) to study short-term acquisition and cognitive flexibility (Fig. 3E). All groups of mice performed the same number of platform crossings and were unable to discriminate the new target quadrant from the opposite. This outcome suggests that the short ‘inversion’ training was insufficient for mice to develop a significant preference for the new platform-containing quadrant, but they were able to extinguish the previously acquired preference.
When sex differences were investigated in the water maze task, the sex of the animals did not modulate the effects of the diet on the learning-related measures. However, when considering the overall data across all groups, male mice generally outperformed females in the visual platform training (escape latency; ESI Fig. 3†) and during reference memory training (path length, escape latency; ESI Fig. 4 and 5†).
HPC-enriched diet potentiated hippocampal adult neurogenesis and BDNF expression
BDNF expression was analyzed by western blot in both the hippocampus and the prefrontal cortex (Fig. 4A). In the hippocampus, BDNF expression augmented in both cocoa-fed groups compared to the CTR group [one-way ANOVA: F(2, 42) = 4.937, p = 0.012; LSD post hoc analysis is shown in Fig. 4B]. Contrary to the hippocampus, western blot analysis of BDNF in the PFC did not reveal differences per diet [F(2, 42) = 0.111, p = 0.895; Fig. 4B].Regarding AHN markers, diets did not influence the number of DCX+ neurons with an immature-like (Type 1) morphology (Fig. 5B). However, the HPC diet increased the population of proliferating PCNA+ cells [F(2, 19) = 4.279, p = 0.029; Fig. 5A], the DCX+ neurons with a mature-like morphology (Type 2) [F(2, 21) = 13.651, p < 0.001; LSD post hoc analysis is shown in Fig. 5B] and the number of BrdU+ cells that survived until the end of the experiment [F(2, 21) = 14.019, p < 0.001; LSD post hoc analysis is shown in Fig. 5C]. While the sex of the animals did not modulate the impact of diet, female mice showed less DCX+ neurons than males (ESI Fig. 6†). There were no differences in all treatments in their differentiation into mature neurons, as shown in the double staining of BrdU/NeuN (Fig. 5D).
Cocoa intake did not influence synaptic plasticity at the CA3–CA1 region
Input–output curves, obtained in order to analyze the impact of cocoa on basal synaptic transmission, revealed no statistically significant differences between experiments (Fig. 6C). Recordings from slices obtained from animals treated with cocoa exhibited no significant differences compared to those from the CTR group in terms of LTP (Fig. 6D). Regarding PPF, analysis of the changes in field slope ratios measured across all interpulse intervals examined, including both the PPF before and after LTP, indicated no significant differences between groups (Fig. 6E), suggesting that cocoa did not impact the properties of short- and long-term synaptic plasticity in the dorsal hippocampus.Discussion
This work was aimed to describe the effects of chronic consumption of two cocoa-enriched diets on brain plasticity and behavior in healthy young adult mice of both sexes. HPC diet surpassed the LPC diet not only in the total phenolic component (approximately 6.2 times higher in the HPC), but also in its total levels of caffeine (approximately 1.9 times higher in the HPC). Conversely, cocoas were similar in nutritional components such as fat, proteins, and sugars. Despite the fact that caffeine doses consumed by mice fed both cocoa-enriched diets were elevated compared to those in other mouse studies (roughly 2.8 times higher in the HPC diet and 1.5 higher in the LPC diet),46,47 polyphenol doses in the HPC diet were also markedly elevated (∼6 times higher) compared to those in the previous literature.48,49 Importantly, while considering the potential adverse effects of caffeine on health, the action of polyphenols is known to mitigate methylxanthine's effects.16 Thus, while the varying effects of cocoa-enriched diets in this study are likely attributable to the action of polyphenols, we cannot discount other alternative explanations, such as potential interactions among other constituents. Hence, it is valuable to study the effects of whole cocoa products, which are commonly consumed in everyday diets, rather than administering the components separately.Despite the similar caloric content of the three diets, mice fed either of the cocoa-enriched diets showed increased (approximately by +18%) total food consumption during the first four weeks compared to mice fed the CTR diet. A possible explanation is that cocoa supplementation may result in rewarding for mice, hence it could stimulate appetite and consumption (i.e. ‘hedonic hunger’) in the absence of homeostatic hunger [reviewed in ref. 50]. Chocolate is regarded as highly palatable for rodents.50 However, reward studies should be performed specifically with natural cocoa, since chocolate reward may not only be attributed to components present in cocoa – such as methylxanthines – but to chocolate's particular attributes, such as its sweetness.51 In any case, consumption of the cocoa-enriched diets did not increase body weight, in accordance with previous reports. In fact, cocoa is regarded as a natural anti-obesity product in animal models acting through different mechanisms such as reducing adipogenesis or increasing antioxidant response.52,53 Accordingly, a meta-analysis of randomized clinical trials concluded that diet supplementation with cocoa or dark chocolate did not affect body weight and other anthropometric measures, and body weight could even be reduced by certain consumption schedules.54
Regarding emotional measures, it is a popular belief that cocoa-containing foods are positive regulators of mood. Clinical literature in healthy adults with no psychiatric disorders overall supports that cocoa-rich products reduce depressive and anxiety symptoms; however, caution has been drawn on the short administration schedule used in the available studies (usually less than 1 week) as well as on the reduced number of participants involved.55 On the other hand, experimental research in rodents using cocoa, cocoa flavanol extract or cocoa-rich products is scarce and inconclusive. Regarding anxiety-like responses, cocoa mass had an anxiolytic effect in adult male rats when administered acutely but not when administered for 2 weeks.56In contrast, chronic administration (i.e. for 14 or 24 weeks) of the flavanol (−)epicatechin may reduce anxiety-like measures in healthy male mice;57 however, another study concluded that only pathologically anxious obese male mice, and not healthy controls, were benefited by (−)epicatechin treatment.58 For depression-like responses, adult male rats administered a cocoa polyphenolic extract for 14 days showed reduced immobility in the FST with no changes in exploratory activity. But this effect was not evident in the initial exposure to the FST but in a second retention trial, in which the animals were already familiarized with the test.32 Notably, the second FST trial involves non-emotional components, for example, memory for the previous exposure to the task.59 In a different research study, mice's diet was supplemented with 10% cocoa-based dark chocolate for 3 weeks, revealing increased locomotor activity and motor coordination but no effects on anxiety or depression-related variables (examined with the EPM and FST tests, among others).42 In conclusion, preclinical data do not yet support a strong effect of cocoa-derived products on emotional variables. Methodological differences among studies regarding the properties of the cocoa-derived product administered, the animal model used, or the administration schedule are likely responsible for some of the discrepancies. Furthermore, it is noteworthy that few studies used natural cocoa or female rodents, making direct comparisons between the present study and previous data challenging.
In the present work, cocoa – regardless of its phenolic content – had no clear effects on modulating anxiety-like behaviors (i.e. in the OFT and EPM) when chronically administered to healthy young male and female mice. Nevertheless, both groups of cocoa-fed mice showed reduced highly active attempts to escape from water (struggling) while increasing immobility behavior in the FST. While immobility has been classically considered as a depression or despair-like response,60,61 several factors should be taken into account to interpret this outcome. First, mice fed either of the cocoa-enriched diets also showed a decreased swimming velocity in the first water maze sessions (i.e. the habituation session and the initial sessions with a visible platform). Since the CTR mice showed gradually decreased swimming velocity as they became habituated to the water maze task, the cocoa-treated mice may have displayed increased resilience to an acute water stressor. In this regard, there has been a shift towards the interpretation of the FST immobility behavior as an adaptative mechanism for mice to cope with the inescapable water stressor60,61 whereas animals vulnerable to stress would expend higher energy attempting to escape fruitlessly.62 Given these considerations, further investigation is warranted to examine the role of cocoa-enriched diets in regulating the stress response and its habituation.
Second, FST behavior may be strongly modulated by changes in motor activity,63 so it is advisable to combine the FST with motor activity tests to rule out this confounding factor.32 For example, environmentally-enriched male mice that travelled less distance in novel environments would struggle less in water.35 Reduced motor and exploratory activation was induced by cocoa (specifically the LPC) in the OFT when this environment was novel, and both cocoa-enriched diets reduced total object exploration. Locomotion, exploration and novelty-seeking are reward-related responses modulated by the mesolimbic system, which in turn interacts with food reward, hunger and satiety signals.64 As we have discussed previously, cocoa-enriched diets could have been more rewarding for mice than the standard diet, considering increased food intake measures.
We also researched whether cocoa could modulate declarative memory, measured using object and place recognition memory and spatial navigation tasks. Episodic-like memory is the memory for everyday life experiences, and allows to encode several components of an event such as what, when and where. The object recognition paradigm mainly assesses the ‘what’ component, while the place recognition memory paradigm would assess the ‘where’.65 Although both cocoa-enriched diets reduced total object exploration, such diminished motivation for objects did not prevent novelty preference. In fact, the HPC-enriched diet, but not the LPC-enriched diet, potentiated long-term object recognition memory compared to the control diet. Previous research converge with our results as administering the flavanol (−)epicatechin for 4 weeks improved object recognition memory at 1 h and 24 h retention intervals in both young and aged male mice, supporting a role of cocoa polyphenols in object recognition memory.66 In our case, monomers (catechin + epicatechin) accounted for > 27% of the total flavanols present in the HPC, which means 74.4% higher content than in the LPC diet. On the other hand, neither cocoa-enriched diet influenced novel place recognition memory, nor spatial memory acquisition and consolidation tested in the water maze.
All of these declarative memory tasks require the integrity of the hippocampus and the medial temporal lobe as well as its associated structures such as the medial prefrontal cortex.66–68 However, their neurobiological substrate does not completely overlap. For example, while the hippocampal participation in the novel place memory is consistently recognized, the hippocampal involvement in novel object memory seems to be conditioned to a number of methodological factors,65 and it usually requires a long delay imposed between the sample and the test trials such as the one used here (24 h).67 Object memory is also frequently dissociated from spatial navigation memory, as it is not unusual for different enhancing or deleterious treatments to modulate one type of memory but not the other [e.g. ref. 35, 69 and 70]. While previous finding has reported that the flavanol (−)epicatechin would improve spatial reference memory, the effect was most evident when this treatment was combined with physical exercise in female mice.71 In another report, a diet containing cocoa prevented spatial memory impairment in aged rats.31 Thus, our study does not rule out a potential beneficial effect of cocoa or its phenolic compounds on spatial memory, which may require certain adjunct treatments or be more evident in individuals with cognitive impairment. On a different note, we did not find a sex-dependent effect on the modulation of behavior by the cocoa-enriched diets. Independently of the diet, here there was a slightly delayed reference memory learning in female mice. While sex differences are not the focus of this manuscript, this effect is extensively discussed in our previous report37 and in the ESI.†
At the neurobiological level, we evaluated BDNF expression in the prefrontal cortex and the hippocampus, only finding changes in the latter. Increases in either circulating BDNF concentration in humans72 or hippocampal BDNF expression in rodents73 have been associated with diets rich in polyphenols. However, we did find that hippocampal BDNF was increased by both cocoa-enriched diets, independently of their phenolic content. It should be noted that other cocoa components present in both diets might account for this effect. For instance, theobromine, which was equally present in our cocoa-enriched diets, could have contributed to the increase in hippocampal BDNF levels.74–76
In addition, a key result of this study is that only the HPC-enriched diet upregulated AHN, compared to the CTR and LPC diets. This upregulation in the HPC group appeared to be driven both by enhanced cell proliferation, as indicated by the increased number of PCNA+ cells, as well as by improved cell survival. Specifically, there was a marked increase in the number of BrdU+ cells that differentiated into neurons and survived until the end of the experiment in the HPC-fed mice. This was accompanied by a higher number of young DCX+ neurons that were postmitotic and exhibited a mature-like morphology.41 Previous studies have linked dietary polyphenols to stimulation of AHN, acting through several mechanisms that may involve anti-inflammatory and antioxidant activity, as well as modulation of neurotransmitters or neurotrophins,27,77 but a pro-neurogenic effect was not studied after specific administration of cocoa-derived polyphenols or whole natural cocoa. Considering that the adult-born hippocampal neurons may enter the hippocampal circuitry at a young age, while still immature – the first output synapses may occur at 2–3 weeks of age –,78 it is possible for the increased AHN to contribute to the behavioral phenotype of the HPC mice. However, the functional role of AHN remains ambiguous. In humans, the existence of AHN is currently under debate.79,80 In rodents, AHN is interpreted as a mood regulator that provides resilience to stress, being also involved in hippocampal-dependent memory consolidation, updating and forgetting.81 Nevertheless, most literature studies, such as the present report, only show a correlational relationship between increased AHN and a healthier behavioral response after unspecific manipulations of AHN (i.e. by exercise, learning, novelty, dietary changes,…). Compared to loss-of-function studies, there are very few gain-of-function studies that selectively increase AHN to causally demonstrate an effect of an augmented number of these neurons on behavior.82 In any case, AHN is a well-established marker of hippocampal neuroplasticity in preclinical literature which may be stimulated by natural cocoa, but only when sufficient amounts of phenolic compounds are present in its composition.
Finally, we evaluated the influence of cocoa on hippocampal synaptic plasticity, finding no differences among groups. It was previously shown that mice fed a diet supplemented with 10% cocoa-based dark chocolate – with unknown phenolic content – displayed hippocampal hyperexcitability and reduced GABA-α1 receptor levels in the dentate gyrus, when using a combined low Mg2+ and pharmacological GABAergic neurotransmitter-inhibition as a strategy to unmask potential seizure-like responses resembling features of epilepsy.42 This raised concerns regarding a deleterious epileptogenic effect of cocoa, which may be explained by its methylxanthine content.83 In contrast to Cicvaric et al. (2018), here we purposely avoided using seizure-like models of epilepsy in order to experimentally recreate physiological conditions more closely resembling in vivo synaptic activity and plasticity-related functions. Under our experimental conditions, none of the cocoa-enriched diet groups displayed hippocampal seizure-like hyperexcitability or an impairment or enhancement of the properties of basal hippocampal synaptic transmission or short- or long-term forms of synaptic plasticity as examined in CA3–CA1 synapses. These observations dismiss possible detrimental effects of the different cocoa sources on the examined synaptic functions and align with the absence of effects on hippocampal memory evaluated in the water maze, as it has been widely established that CA3–CA1 synapses play key roles in spatial memory acquisition and maintenance.84 Nevertheless, there are several other potential target areas of the brain not examined here by electrophysiological methods (including the hippocampal dentate gyrus), which are known to play key roles in memory-related functions and that could be influenced by polyphenols and other bioactive molecules found in the cocoa varieties used here. Given that we report specific effects on the dentate gyrus region (significantly enhanced AHN) in response to HPC, future electrophysiological studies examining the effects of HPC on the electrophysiological synaptic properties of dentate gyrus synapses are therefore encouraged. This exploration may shed light on potential effects of HPC on neurogenesis-dependent long-term forms of synaptic potentiation.85,86
In conclusion, our study shows that chronic consumption of two different cocoa-enriched diets increased hippocampal neuroplasticity (BDNF expression) and modulated behavior in both male and female mice. However, only cocoa with a high phenolic content was able to potentiate object memory and increase AHN, a neuroplastic process in rodents that usually correlates with healthy cognition and emotional regulation. This result may have important implications when using cocoa as a nutraceutical tool, considering that the available cocoa products in the market may strongly differ in their phenolic composition. However, several limitations in this study should be noted. First, the findings are based on animal models, which may limit direct translation to human physiology. In human clinical trials, the effects of dietary interventions involving cocoa on brain health have been inconsistently reported. Second, although the study focused on hippocampal neuroplasticity and behavior, as well as the actions of cocoa polyphenols, further investigation into other neurobiological mechanisms or the influence of other cocoa components is warranted. These limitations underscore the importance of future research to thoroughly explore the potential benefits and mechanisms of cocoa-derived compounds for enhancing cognitive function – in both normal and pathological conditions – and mitigating age-related cognitive decline, across both animal and human models.
Author contributions
Sonia Melgar-Locatelli: conceptualization, methodology, formal analysis, investigation, writing – original draft preparation, visualization. M. Carmen Mañas-Padilla: methodology, investigation writing – original draft preparation, visualization. Adriana Castro-Zavala: methodology, investigation, visualization. María del Carmen Razola-Díaz: methodology, investigation writing – original draft preparation, visualization. Francisco J. Monje: methodology, investigation, visualization, supervision. Patricia Rivera: investigation, supervision. Celia Rodríguez-Pérez: funding acquisition, conceptualization, methodology, formal analysis investigation, writing – original draft preparation, visualization, project administration, supervision. Estela Castilla-Ortega: funding acquisition, conceptualization, methodology, formal analysis, investigation, writing – original draft preparation, visualization, project administration, supervision.Data availability
Dataset for this article is available at Digibug at https://hdl.handle.net/10481/92723.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was funded by Grant PID2020-114374RB-I00 funded by MCIN/AEI/10.13039/501100011033 (to C. R.-P. and E. C.-O.), Junta de Andalucía-Consejería de Universidad, Investigación e Innovación – Proyect P21_00777 (to C. R.-P. and E. C-O.) and Universidad de Málaga (C.2. II Plan Propio de Investigación, Transferencia y Divulgación Científica).Author P. R. holds a “Miguel Servet I” research contract from the National System of Health, EU-ERDF-ISCIII (CP19/00068). Author M. C. M.-P. holds a predoctoral grant from the Spanish Ministry of Science, Innovation and Universities (FPU17/00276). Author A. C. Z. holds a postdoctoral research contract from Secretaría General de Universidades, Investigación y Tecnología– Junta de Andalucía (POSTDOC21_00365). Author M. C. R.-D. holds a predoctoral grant from the Spanish Ministry of Science, Innovation and Universities (FPU19/02009).
The authors acknowledge the IBIMA's common research support structure—ECAI—of animal experimentation and behavior (“Centro de Experimentación y Conducta Animal”, University of Malaga) for maintenance of the mice.
References
- ICCO, International Cocoa Organization. Available online: https://www.icco.org/statistics/#tab-id-6. Accessed on 7 February 2024, 2022.
- T. Magrone, M. A. Russo and E. Jirillo, Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications, Front. Immunol., 2017, 8, 677 CrossRef PubMed.
- Q. R. De Araujo, J. N. Gattward, S. Almoosawi, M. Silva, P. A. Dantas and Q. R. De Araujo Júnior, Cocoa and Human Health: From Head to Foot–A Review, Crit. Rev. Food Sci. Nutr., 2016, 56, 1–12 CrossRef PubMed.
- D. L. Katz, K. Doughty and A. Ali, Cocoa and chocolate in human health and disease, Antioxid. Redox Signaling, 2011, 15, 2779–2811 CrossRef CAS PubMed.
- T. K. Siroma, D. J. Machate, V. A. Zorgetto-Pinheiro, P. S. Figueiredo, G. Marcelino, P. A. Hiane, D. Bogo, A. Pott, E. R. J. Cury, R. C. A. Guimarães, M. L. B. Vilela, R. D. S. Ferreira and V. A. do Nascimento, Polyphenols and ω-3 PUFAs: Beneficial Outcomes to Obesity and Its Related Metabolic Diseases, Front. Nutr., 2021, 8, 781622 CrossRef PubMed.
- S. J. Crozier, A. G. Preston, J. W. Hurst, M. J. Payne, J. Mann, L. Hainly and D. L. Miller, Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products, Chem. Cent. J., 2011, 5, 5 CrossRef CAS PubMed.
- R. Franco, A. Oñatibia-Astibia and E. Martínez-Pinilla, Health benefits of methylxanthines in cacao and chocolate, Nutrients, 2013, 5, 4159–4173 CrossRef CAS PubMed.
- C. C. Meng, A. M. Jalil and A. Ismail, Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market, Molecules, 2009, 14, 200–209 CrossRef CAS PubMed.
- K. B. Miller, W. J. Hurst, N. Flannigan, B. Ou, C. Y. Lee, N. Smith and D. A. Stuart, Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao, J. Agric. Food Chem., 2009, 57, 9169–9180 CrossRef CAS PubMed.
- M. D. C. Razola-Díaz, M. J. Aznar-Ramos, V. Verardo, S. Melgar-Locatelli, E. Castilla-Ortega and C. Rodríguez-Pérez, Exploring the Nutritional Composition and Bioactive Compounds in Different Cocoa Powders, Antioxidants, 2023, 12, 716 CrossRef PubMed.
- V. Sorrenti, S. Fortinguerra, G. Caudullo and A. Buriani, Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics, Nutrients, 2020, 12, 1265 CrossRef CAS PubMed.
- B. Urbańska and J. Kowalska, Comparison of the Total Polyphenol Content and Antioxidant Activity of Chocolate Obtained from Roasted and Unroasted Cocoa Beans from Different Regions of the World, Antioxidants, 2019, 8, 283 CrossRef PubMed.
- H. Cory, S. Passarelli, J. Szeto, M. Tamez and J. Mattei, The Role of Polyphenols in Human Health and Food Systems: A Mini-Review, Front. Nutr., 2018, 5, 87 CrossRef PubMed.
- E. Martínez-Pinilla, A. Oñatibia-Astibia and R. Franco, The relevance of theobromine for the beneficial effects of cocoa consumption, Front. Pharmacol., 2015, 6, 30 Search PubMed.
- R. M. van Dam, F. B. Hu and W. C. Willett, Coffee, Caffeine, and Health, N. Engl. J. Med., 2020, 383, 369–378 CrossRef CAS PubMed.
- J. Schuster and E. S. Mitchell, More than just caffeine: psychopharmacology of methylxanthine interactions with plant-derived phytochemicals, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2019, 89, 263–274 CrossRef CAS PubMed.
- V. Socci, D. Tempesta, G. Desideri, L. De Gennaro and M. Ferrara, Enhancing Human Cognition with Cocoa Flavonoids, Front. Nutr., 2017, 4, 19 CrossRef PubMed.
- D. J. Lamport, E. Christodoulou and C. Achilleos, Beneficial Effects of Dark Chocolate for Episodic Memory in Healthy Young Adults: A Parallel-Groups Acute Intervention with a White Chocolate Control, Nutrients, 2020, 12, 483 CrossRef CAS PubMed.
- A. M. Brickman, U. A. Khan, F. A. Provenzano, L. K. Yeung, W. Suzuki, H. Schroeter, M. Wall, R. P. Sloan and S. A. Small, Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults, Nat. Neurosci., 2014, 17, 1798–1803 CrossRef CAS PubMed.
- Z. Shateri, A. Kooshki, R. Hormoznejad, S. A. Hosseini, R. Mousavi and E. Foroumandi, Effects of chocolate on cognitive function in healthy adults: A systematic review and meta-analysis on clinical trials, Phytother. Res., 2023, 37, 3688–3697 CrossRef PubMed.
- L. D. Baker, J. E. Manson, S. R. Rapp, H. D. Sesso, S. A. Gaussoin, S. A. Shumaker and M. A. Espeland, Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial, Alzheimers Dement., 2023, 19, 1308–1319 CrossRef CAS PubMed.
- A. Cimini, R. Gentile, B. D'Angelo, E. Benedetti, L. Cristiano, M. L. Avantaggiati, A. Giordano, C. Ferri and G. Desideri, Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer's disease model by modulating BDNF signaling pathway, J. Cell. Biochem., 2013, 114, 2209–2220 CrossRef CAS PubMed.
- J. P. Spencer, Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance, Proc. Nutr. Soc., 2008, 67, 238–252 CrossRef CAS PubMed.
- C. Ciaramelli, A. Palmioli, A. De Luigi, L. Colombo, G. Sala, M. Salmona and C. Airoldi, NMR-based Lavado cocoa chemical characterization and comparison with fermented cocoa varieties: Insights on cocoa's anti-amyloidogenic activity, Food Chem., 2021, 341, 128249 CrossRef CAS PubMed.
- T. Toda, S. L. Parylak, S. B. Linker and F. H. Gage, The role of adult hippocampal neurogenesis in brain health and disease, Mol. Psychiatry, 2019, 24, 67–87 CrossRef CAS PubMed.
- S. Melgar-Locatelli, M. de Ceglia, M. C. Mañas-Padilla, C. Rodriguez-Pérez, E. Castilla-Ortega, A. Castro-Zavala and P. Rivera, Nutrition and adult neurogenesis in the hippocampus: Does what you eat help you remember?, Front. Neurosci., 2023, 17, 1147269 CrossRef PubMed.
- F. Sarubbo, D. Moranta and G. Pani, Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition, Neurosci. Biobehav. Rev., 2018, 90, 456–470 CrossRef CAS PubMed.
- M. E. Han, K. H. Park, S. Y. Baek, B. S. Kim, J. B. Kim, H. J. Kim and S. O. Oh, Inhibitory effects of caffeine on hippocampal neurogenesis and function, Biochem. Biophys. Res. Commun., 2007, 356, 976–980 CrossRef CAS PubMed.
- Z. F. Mao, S. H. Ouyang, Q. Y. Zhang, Y. P. Wu, G. E. Wang, L. F. Tu, Z. Luo, W. X. Li, H. Kurihara, Y. F. Li and R. R. He, New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation, FASEB J, 2020, 34, 10998–11014 CrossRef CAS PubMed.
- T. Valente, J. Hidalgo, I. Bolea, B. Ramirez, N. Anglés, J. Reguant, J. R. Morelló, C. Gutiérrez, M. Boada and M. Unzeta, A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain, J. Alzheimer's Dis., 2009, 18, 849–865 CAS.
- J. F. Bisson, A. Nejdi, P. Rozan, S. Hidalgo, R. Lalonde and M. Messaoudi, Effects of long-term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats, Br. J. Nutr., 2008, 100, 94–101 CrossRef CAS PubMed.
- M. Messaoudi, J. F. Bisson, A. Nejdi, P. Rozan and H. Javelot, Antidepressant-like effects of a cocoa polyphenolic extract in Wistar-Unilever rats, Nutr. Neurosci., 2008, 11, 269–276 CrossRef PubMed.
- P. Rozan, S. Hidalgo, A. Nejdi, J. F. Bisson, R. Lalonde and M. Messaoudi, Preventive antioxidant effects of cocoa polyphenolic extract on free radical production and cognitive performances after heat exposure in Wistar rats, J. Food Sci., 2007, 72, S203–S206 CrossRef CAS PubMed.
- M. E. Alañón, S. M. Castle, P. J. Siswanto, T. Cifuentes-Gómez and J. P. Spencer, Assessment of flavanol stereoisomers and caffeine and theobromine content in commercial chocolates, Food Chem., 2016, 208, 177–184 CrossRef PubMed.
- M. C. Mañas-Padilla, P. Tezanos, E. Cintado, L. Vicente, L. Sánchez-Salido, S. Gil-Rodríguez, J. L. Trejo, L. J. Santín and E. Castilla-Ortega, Environmental enrichment alleviates cognitive and psychomotor alterations and increases adult hippocampal neurogenesis in cocaine withdrawn mice, Addict. Biol., 2023, 28, e13244 CrossRef PubMed.
- M. C. Mañas-Padilla, F. Ávila-Gámiz, S. Gil-Rodríguez, D. Ladrón de Guevara-Miranda, F. Rodríguez de Fonseca, L. J. Santín and E. Castilla-Ortega, Persistent changes in exploration and hyperactivity coexist with cognitive impairment in mice withdrawn from chronic cocaine, Physiol. Behav., 2021, 240, 113542 CrossRef PubMed.
- S. Melgar-Locatelli, M. C. Mañas-Padilla, A. L. Gavito, P. Rivera, C. Rodríguez-Pérez, E. Castilla-Ortega and A. Castro-Zavala, Sex-specific variations in spatial reference memory acquisition: Insights from a comprehensive behavioral test battery in C57BL/6JRj mice, Behav. Brain Res., 2024, 459, 114806 CrossRef PubMed.
- G. Paxinos and K. B. L. Franklin, The mouse brain in stereotaxic coordinates, Academic Press, San Diego, 4th edn, 2012 Search PubMed.
- D. Medina-Vera, J. A. Navarro, P. Rivera, C. Rosell-Valle, A. Gutiérrez-Adán, C. Sanjuan, A. J. López-Gambero, R. Tovar, J. Suárez, F. J. Pavón, E. Baixeras, J. Decara and F. Rodríguez de Fonseca, d-Pinitol promotes tau dephosphorylation through a cyclin-dependent kinase 5 regulation mechanism: A new potential approach for tauopathies?, Br. J. Pharmacol., 2022, 179, 4655–4672 CrossRef CAS PubMed.
- J. J. Bass, D. J. Wilkinson, D. Rankin, B. E. Phillips, N. J. Szewczyk, K. Smith and P. J. Atherton, An overview of technical considerations for Western blotting applications to physiological research, Scand J. Med. Sci. Sports, 2017, 27, 4–25 CrossRef CAS PubMed.
- T. Plümpe, D. Ehninger, B. Steiner, F. Klempin, S. Jessberger, M. Brandt, B. Römer, G. R. Rodriguez, G. Kronenberg and G. Kempermann, Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation, BMC Neurosci., 2006, 7, 77 CrossRef PubMed.
- A. Cicvaric, T. Bulat, D. Bormann, J. Yang, B. Auer, I. Milenkovic, M. Cabatic, R. Milicevic and F. J. Monje, Sustained consumption of cocoa-based dark chocolate enhances seizure-like events in the mouse hippocampus, Food Funct., 2018, 9, 1532–1544 RSC.
- S. Kouhnavardi, M. Cabatic, M. C. Mañas-Padilla, M. A. Malabanan, T. Smani, A. Cicvaric, E. A. Muñoz Aranzalez, X. Koenig, E. Urban, G. Lubec, E. Castilla-Ortega and F. J. Monje, miRNA-132/212 Deficiency Disrupts Selective Corticosterone Modulation of Dorsal vs. Ventral Hippocampal Metaplasticity, Int. J. Mol. Sci., 2023, 24, 9565 CrossRef CAS PubMed.
- S. Kouhnavardi, A. Ecevitoglu, V. Dragačević, F. Sanna, E. Arias-Sandoval, P. Kalaba, M. Kirchhofer, J. Lubec, M. Niello, M. Holy, M. Zehl, M. Pillwein, J. Wackerlig, R. Murau, A. Mohrmann, K. R. Beard, H. H. Sitte, E. Urban, C. Sagheddu, M. Pistis, R. Plasenzotti, J. D. Salamone, T. Langer, G. Lubec and F. J. Monje, A Novel and Selective Dopamine Transporter Inhibitor, (S)-MK-26, Promotes Hippocampal Synaptic Plasticity and Restores Effort-Related Motivational Dysfunctions, Biomolecules, 2022, 12, 881 CrossRef CAS PubMed.
- T. Stojanovic, H. Benes, A. Awad, D. Bormann and F. J. Monje, Nicotine abolishes memory-related synaptic strengthening and promotes synaptic depression in the neurogenic dentate gyrus of miR-132/212 knockout mice, Addict. Biol., 2021, 26, e12905 CrossRef CAS PubMed.
- G. B. Kaplan, D. J. Greenblatt, M. A. Kent and M. M. Cotreau-Bibbo, Caffeine treatment and withdrawal in mice: relationships between dosage, concentrations, locomotor activity and A1 adenosine receptor binding, J. Pharmacol. Exp. Ther., 1993, 266, 1563–1572 CAS.
- T. Paula, L. C. Cardoso, F. Felicioni, A. L. Caldeira-Brant, T. G. Santos, H. Castro-Oliveira, G. B. Menezes, E. Bloise, H. Chiarini-Garcia and F. de Almeida, Maternal chronic caffeine intake impairs fertility, placental vascularization and fetal development in mice, Reprod. Toxicol., 2023, 121, 108471 CrossRef CAS PubMed.
- D. Wang, M. Zhang, T. Wang, T. Liu, Y. Guo and D. Granato, Green tea polyphenols mitigate the plant lectins-induced liver inflammation and immunological reaction in C57BL/6 mice via NLRP3 and Nrf2 signaling pathways, Food and chemical toxicology : an international journal published for the British, Ind. Biol. Res. Assoc., 2020, 144, 111576 CAS.
- P. R. Yeganeh, J. Leahy, S. Spahis, N. Patey, Y. Desjardins, D. Roy, E. Delvin, C. Garofalo, J. P. Leduc-Gaudet, D. St-Pierre, J. F. Beaulieu, A. Marette, G. Gouspillou and E. Levy, Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis, J. Nutr. Biochem., 2018, 57, 56–66 CrossRef CAS PubMed.
- N. Samerphob, D. Cheaha, A. Issuriya, S. Chatpun, W. Lertwittayanon, O. Jensen and E. Kumarnsit, Changes in neural network connectivity in mice brain following exposures to palatable food, Neurosci. Lett., 2020, 714, 134542 CrossRef CAS PubMed.
- D. Hosová and L. P. Spear, Voluntary Binge Consumption of Ethanol in a Sweetened, Chocolate-Flavored Solution by Male and Female Adolescent Sprague Dawley Rats, Alcohol: Clin. Exp. Res., 2017, 41, 541–550 CrossRef PubMed.
- S. Y. Min, H. Yang, S. G. Seo, S. H. Shin, M. Y. Chung, J. Kim, S. J. Lee, H. J. Lee and K. W. Lee, Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor, Int. J. Obes. (2005), 2013, 37, 584–592 CrossRef CAS PubMed.
- M. Sun, Y. Gu, S. L. Glisan and J. D. Lambert, Dietary cocoa ameliorates non-alcoholic fatty liver disease and increases markers of antioxidant response and mitochondrial biogenesis in high fat-fed mice, J. Nutr. Biochem., 2021, 92, 108618 CrossRef CAS PubMed.
- H. Kord-Varkaneh, E. Ghaedi, A. Nazary-Vanani, H. Mohammadi and S. Shab-Bidar, Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials, Crit. Rev. Food Sci. Nutr., 2019, 59, 2349–2362 CrossRef CAS PubMed.
- L. Fusar-Poli, A. Gabbiadini, A. Ciancio, L. Vozza, M. S. Signorelli and E. Aguglia, The effect of cocoa-rich products on depression, anxiety, and mood: A systematic review and meta-analysis, Crit. Rev. Food Sci. Nutr., 2022, 62, 7905–7916 CrossRef PubMed.
- T. Yamada, Y. Yamada, Y. Okano, T. Terashima and H. Yokogoshi, Anxiolytic effects of short- and long-term administration of cacao mass on rat elevated T-maze test, J. Nutr. Biochem., 2009, 20, 948–955 CrossRef CAS PubMed.
- T. P. Stringer, D. Guerrieri, C. Vivar and H. van Praag, Plant-derived flavanol (-)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice, Transl. Psychiatry, 2015, 5, e493 CrossRef CAS PubMed.
- J. Kang, Z. Wang, E. Cremonini, G. Le Gall, M. G. Pontifex, M. Muller, D. Vauzour and P. I. Oteiza, (-)-Epicatechin mitigates anxiety-related behavior in a mouse model of high fat diet-induced obesity, J. Nutr. Biochem., 2022, 110, 109158 CrossRef CAS PubMed.
- A. P. West, Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 1990, 14, 863–877 CrossRef CAS PubMed.
- E. R. de Kloet and M. L. Molendijk, Floating Rodents and Stress-Coping Neurobiology, Biol. Psychiatry, 2021, 90, e19–e21 CrossRef PubMed.
- M. L. Molendijk and E. R. de Kloet, Coping with the forced swim stressor: Current state-of-the-art, Behav. Brain Res., 2019, 364, 1–10 CrossRef PubMed.
- R. D. Moreno-Fernández, M. Pérez-Martín, E. Castilla-Ortega, C. Rosell Del Valle, M. I. García-Fernández, J. Chun, G. Estivill-Torrús, F. Rodríguez de Fonseca, L. J. Santín and C. Pedraza, maLPA1-null mice as an endophenotype of anxious depression, Transl. Psychiatry, 2017, 7, e1077 CrossRef PubMed.
- C. Vierira, R. C. De Lima, P. Carobrez Ade and C. Lino-de-Oliveira, Frequency of climbing behavior as a predictor of altered motor activity in rat forced swimming test, Neurosci. Lett., 2008, 445, 170–173 CrossRef PubMed.
- C. Hansson, R. H. Shirazi, J. Näslund, H. Vogel, C. Neuber, G. Holm, H. Ancharsäter, S. L. Dickson, E. Eriksson and K. P. Skibicka, Ghrelin influences novelty seeking behavior in rodents and men, PLoS One, 2012, 7, e50409 CrossRef CAS PubMed.
- O. Y. Chao, S. Nikolaus, Y. M. Yang and J. P. Huston, Neuronal circuitry for recognition memory of object and place in rodent models, Neurosci. Biobehav. Rev., 2022, 141, 104855 CrossRef PubMed.
- V. Navarrete-Yañez, A. Garate-Carrillo, A. Rodriguez, P. Mendoza-Lorenzo, G. Ceballos, C. Calzada-Mendoza, M. C. Hogan, F. Villarreal and I. Ramirez-Sanchez, Effects of (-)-epicatechin on neuroinflammation and hyperphosphorylation of tau in the hippocampus of aged mice, Food Funct., 2020, 11, 10351–10361 RSC.
- S. J. Cohen and R. W. Stackman Jr., Assessing rodent hippocampal involvement in the novel object recognition task, Behav. Brain Res., 2015, 285, 105–117 CrossRef PubMed.
- L. J. Lissner, K. M. Wartchow, A. P. Toniazzo, C. A. Gonçalves and L. Rodrigues, Object recognition and Morris water maze to detect cognitive impairment from mild hippocampal damage in rats: A reflection based on the literature and experience, Pharmacol., Biochem. Behav., 2021, 210, 173273 CrossRef CAS PubMed.
- M. Milić, T. Timić, S. Joksimović, P. Biawat, S. Rallapalli, J. Divljaković, T. Radulović, J. M. Cook and M. M. Savić, PWZ-029, an inverse agonist selective for α5 GABAA receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats, Behav. Brain Res., 2013, 241, 206–213 CrossRef PubMed.
- D. H. Kim and J. H. Ryu, Differential Effects of Scopolamine on Memory Processes in the Object Recognition Test and the Morris Water Maze Test in Mice, Biomol. Ther., 2008, 16, 173–178 CrossRef CAS.
- H. van Praag, M. J. Lucero, G. W. Yeo, K. Stecker, N. Heivand, C. Zhao, E. Yip, M. Afanador, H. Schroeter, J. Hammerstone and F. H. Gage, Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice, J. Neurosci., 2007, 27, 5869–5878 CrossRef CAS PubMed.
- E. Gravesteijn, R. P. Mensink and J. Plat, Effects of nutritional interventions on BDNF concentrations in humans: a systematic review, Nutr. Neurosci., 2022, 25, 1425–1436 CrossRef CAS PubMed.
- C. Rendeiro, D. Vauzour, M. Rattray, P. Waffo-Téguo, J. M. Mérillon, L. T. Butler, C. M. Williams and J. P. Spencer, Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor, PLoS One, 2013, 8, e63535 CrossRef CAS PubMed.
- C. Lao-Peregrín, J. J. Ballesteros, M. Fernández, A. Zamora-Moratalla, A. Saavedra, M. Gómez Lázaro, E. Pérez-Navarro, D. Burks and E. D. Martín, Caffeine-mediated BDNF release regulates long-term synaptic plasticity through activation of IRS2 signaling, Addict. Biol., 2017, 22, 1706–1718 CrossRef PubMed.
- C. Sallaberry, F. Nunes, M. S. Costa, G. T. Fioreze, A. P. Ardais, P. H. Botton, B. Klaudat, T. Forte, D. O. Souza, E. Elisabetsky and L. O. Porciúncula, Chronic caffeine prevents changes in inhibitory avoidance memory and hippocampal BDNF immunocontent in middle-aged rats, Neuropharmacology, 2013, 64, 153–159 CrossRef CAS PubMed.
- M. Yoneda, N. Sugimoto, M. Katakura, K. Matsuzaki, H. Tanigami, A. Yachie, T. Ohno-Shosaku and O. Shido, Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice, J. Nutr. Biochem., 2017, 39, 110–116 CrossRef CAS PubMed.
- C. Angeloni, L. Pirola, D. Vauzour and T. Maraldi, Dietary polyphenols and their effects on cell biochemistry and pathophysiology, Oxid. Med. Cell. Longevity, 2012, 2012, 583901 Search PubMed.
- N. Toni and A. F. Schinder, Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus, Cold Spring Harbor Perspect. Biol., 2015, 8, a018903 CrossRef PubMed.
- A. Duque, J. I. Arellano and P. Rakic, An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases, Mol. Psychiatry, 2022, 27, 377–382 CrossRef PubMed.
- E. P. Moreno-Jiménez, J. Terreros-Roncal, M. Flor-García, A. Rábano and M. Llorens-Martín, Evidences for Adult Hippocampal Neurogenesis in Humans, J. Neurosci., 2021, 41, 2541–2553 CrossRef PubMed.
- G. Kempermann, What Is Adult Hippocampal Neurogenesis Good for?, Front. Neurosci., 2022, 16, 852680 CrossRef PubMed.
- K. L. Jones, M. Zhou and D. J. Jhaveri, Dissecting the role of adult hippocampal neurogenesis towards resilience versus susceptibility to stress-related mood disorders, NPJ Sci. Learn., 2022, 7, 16 CrossRef PubMed.
- G. Defer, [Central adverse effects of methylxanthines], Therapie, 1992, 47, 67–73 CAS.
- A. Pavlowsky, E. Wallace, A. A. Fenton and J. M. Alarcon, Persistent modifications of hippocampal synaptic function during remote spatial memory, Neurobiol. Learn. Mem., 2017, 138, 182–197 CrossRef PubMed.
- B. Cuccurazzu, E. Zamberletti, C. Nazzaro, P. Prini, M. Trusel, M. Grilli, D. Parolaro, R. Tonini and T. Rubino, Adult Cellular Neuroadaptations Induced by Adolescent THC Exposure in Female Rats Are Rescued by Enhancing Anandamide Signaling, Int. J. Neuropsychopharmacol., 2018, 21, 1014–1024 CrossRef CAS PubMed.
- J. S. Snyder, N. Kee and J. M. Wojtowicz, Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus, J. Neurophysiol., 2001, 85, 2423–2431 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01201a |
| ‡ Joint senior authors. |
| This journal is © The Royal Society of Chemistry 2024 |