Open Access Article
Open Access ArticleAssessing the impact of insect protein sources on intestinal health and disease: insights from human ex vivo and rat in vivo models
Helena
Segú
 a,
Florijan
Jalševac
a,
Florijan
Jalševac
 a,
Marta
Sierra-Cruz
a,
Francesc
Feliu
b,
Jamileh
Movassat
c,
Esther
Rodríguez-Gallego
a,
Ximena
Terra
a,
Marta
Sierra-Cruz
a,
Francesc
Feliu
b,
Jamileh
Movassat
c,
Esther
Rodríguez-Gallego
a,
Ximena
Terra
 a,
Montserrat
Pinent
a,
Montserrat
Pinent
 a,
Anna
Ardévol
a,
Anna
Ardévol
 *a and
M. Teresa
Blay
*a and
M. Teresa
Blay
 a
a
aMoBioFood Research Group, Departament de Bioquímica i Biotecnologia, Universitat Rovira i Virgili, C/Marcel.lí Domingo 1, 43007 Tarragona, Spain. E-mail: anna.ardevol@urv.cat
bServei de Gastroenterologia, Institut Sanitari Pere Virgili, Tarragona, Spain
cUniversité Paris Cité, CNRS, Unité de Biologie Fonctionnelle et Adaptative, F-75013, Paris, France
First published on 2nd April 2024
Abstract
The exploration of edible insects, specifically Alphitobius diaperinus and Tenebrio molitor, as sustainable sources of protein for human consumption is an emerging field. However, research into their effects on intestinal health, especially in relation to inflammation and permeability, remains limited. Using ex vivo and in vivo models of intestinal health and disease, in this study we assess the impact of the above insects on intestinal function by focusing on inflammation, barrier dysfunction and morphological changes. Initially, human intestinal explants were exposed to in vitro-digested extracts of these insects, almond and beef. Immune secretome analysis showed that the inflammatory response to insect-treated samples was comparatively lower than it was for samples exposed to almond and beef. Animal studies using yellow mealworm (Tenebrio molitor) and buffalo (Alphitobius diaperinus) flours were then used to evaluate their safety in healthy rats and LPS-induced intestinal dysfunction rats. Chronic administration of these insect-derived flours showed no adverse effects on behavior, metabolism, intestinal morphology or immune response (such as inflammation or allergy markers) in healthy Wistar rats. Notably, in rats subjected to proinflammatory LPS-induced intestinal dysfunction, T. molitor consumption did not exacerbate symptoms, nor did it increase allergic responses. These findings validate the safety of these edible insects under healthy conditions, demonstrate their innocuity in a model of intestinal dysfunction, and underscore their promise as sustainable and nutritionally valuable dietary protein sources.
Introduction
There is an urgent need nowadays to feed an exponentially growing human population. As a result, the demand for protein is expected to increase by 70% in the next 30 years.1 Current food research therefore focuses on incorporating foods derived from novel and sustainable protein sources such as insects.2,3 Although insects have long been a common component of the human diet, in Western countries entomophagy is still in its early stages.4 However, several reasons support incorporating insects into our diets as a valuable source of protein.Firstly, insects contain a large amount of protein, accounting for over 50 percent of their crude weight. This abundant protein has high biological quality thanks to the presence of all essential amino acids.5–7 Insects also contain large amounts of unsaturated fatty acids, minerals, and vitamins.8 In addition, insects have been identified as a source of bioactive compounds with anti-hypertensive, antidiabetic, antioxidant, and anti-inflammatory activity.9
Also in support of insect consumption is the fact that insect production is more environmentally sustainable than the production of conventional livestock.8 Indeed, insect-based protein is emerging as a cost-effective alternative to meat protein since its production employs minimal resources, requires less water and less land, and is responsible for a much lower carbon footprint than the production of meat protein.4,10 Moreover, since insects can feed on bio-waste (such as mushroom waste and fruit peels), they can mitigate the environmental impact by turning waste into compost.11
For all these reasons, in the not-too-distant future insect protein will probably become a component of the total protein consumed by humans.5 To enhance social acceptance, insect protein can also be processed into food ingredients such as flour, which can then be incorporated into food products.12
The yellow mealworm Tenebrio molitor, and the lesser mealworm Alphitobius diaperinus (Coleoptera: family Tenebrionidae), are both larvae of the beetles that grow in flour and grain cereals. These edible larvae possess significant nutritional value as they encompass a full spectrum of amino acids that are rich in essential fatty acids and vitamins as well as a higher mineral content (including calcium, copper, magnesium, iron and zinc) than conventional meats and eggs.10,13–15Tenebrio molitor commonly breeds in Europe and, together with Alphitobius diaperinus, is the main species used for farm-animal feed and considered for human consumption.10
Recent reports from the European Food Safety Authority (EFSA) positively evaluated both these mealworms as novel foods suitable for human consumption.16,17 The safety profiles of T. molitor and A. diaperinus larvae as novel foods are based on nutritional studies that assessed the microbiological risk of zoonosis, heavy metal contamination and allergy. However, the EFSA recommends that further research should be conducted in this area.14,17
Recent studies have suggested beneficial effects of edible insects in terms of inflammation and intestinal health. For instance, a study with Tenebrio molitor larvae powder described its ability to attenuate pathologic changes in colon tissue and down-regulate the expression of inflammatory cytokine genes in mice (DSS)-induced colitis.18 Moreover, an in silico analysis indicated that Tenebrio molitor could be a source of peptides with anti-inflammatory activity.19 Further, in vitro-digested proteins from three different insects have been proposed to exhibit antioxidant and anti-inflammatory effects.20 Additionally, in a human intervention study, cricket consumption was found to improve gut health and reduce systemic TNF-α levels;21 while other studies also indicated improvements in microbiota and metabolic benefits in animals after the consumption of mealworms.22,23 Collectively, this evidence suggests potential health benefits associated with insect consumption, particularly in the context of intestinal tract and inflammatory response.24
However, a current concern about insect consumption is the potential allergic effects on sensitized individuals or those allergic to other inhalant or food allergen sources such as house dust mites or crustaceans.24,25 The yellow mealworm and lesser mealworm belong to the Insecta class, one of the four subphyla of Arthropoda. In relation to arthropods, several allergens have been reported, including tropomyosin,26 arginine kinase,27 chitinases28 and glutathione S-transferase,29 that affect humans through cross-reactivity. However, studies in this context suggest that the allergenicity of edible insects is species-specific and that thermal processing may partially reduce cross-allergenicity.30 In the context of insect mealworm protein, individuals allergic to shrimps exhibited cross-reactivity due to high protein homology across different arthropod subphyla,31 while one case report that documented food-induced anaphylaxis to T. molitor in a patient allergic to dust mites attributed the allergic response to α-amylase, tubulin, and larval cuticle proteins.32
The origins of allergic diseases have traditionally been explained by immunoglobulin E-mediated immune responses to account for asthma, atopic dermatitis, atopic rhinitis, and food allergy. Research insights into disease origins support a broader array of factors that predispose, initiate, or exacerbate altered immunity in allergic diseases, such as (1) inherent epithelial barrier dysfunction; (2) loss of immune tolerance; (3) disturbances in the gut; and (4) organ-specific microbiomes, diet, and age.33 Here, we focus on the gut barrier function as a key factor in preventing or facilitating food allergy. In fact, Galli et al. previously reported that epithelial barrier deficiencies often lead to a state of constant inflammation that makes tissue repair difficult. Deficient barrier integrity facilitates allergen entry and lowers the threshold for sensitization to innocuous substances because of the inflammatory environment, and therefore likely precipitates allergic sensitization at distal organs.34
Although the consumption of Alphitobius diaperinus and Tenebrio molitor has raised concerns about their potential to induce food allergic reactions in sensitized or allergic individuals, research into the specific health impacts of these insects, including both their detrimental and beneficial effects, is still limited and further studies are needed to understand their role in inflammation and intestinal dysfunction.
Thus, this paper analyses how insect flours interacted with the gastrointestinal tract in the human intestine ex vivo and how this interaction might influence the response of the whole organism in rats, focussing on the inmunologic response. From a more holistic view of allergy development, we evaluated how the effects of low-dose insect supplementation depend on disease status by comparing the effects on a healthy animal vs. a model of LPS-induced inflammation and intestinal barrier dysfunction.
Experimental methods
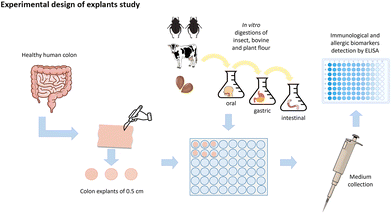
Ex vivo study with human colonic explants exposed to in vitro digested insect, beef and almond
Buffalo (A. diaperinus) powder and insect protein concentrate powder (IPC) from A. diaperinus (Protifarm, Belgium), a lean portion of beef (Bos primogenitors) from the Central Market in Tarragona, Spain, and almond (Prunus dulcis) flour from Borges Agricultural & Industrial Nuts (BAIN) were used for human colon treatments. Samples were stored in the dark at −20 °C until use.Buffalo, IPC, beef and almond food powders were then in vitro digested according to the INFOGEST harmonized protocol.35 This digestion involved simulating in vivo digestion in oral, gastric and intestinal stages in vitro using commercial enzymes such as amylase, pancreatin and trypsin and inactivating the enzymes. The detailed procedures and characterization of the digestion products have been described previously.36
To check the effectiveness of enzymatic digestion, SDS-PAGE was performed with the samples obtained.37
For the explant culture, healthy viable colon tissues were obtained from 10 patients (average age 65 years) who had undergone colon surgery. All donor patients met the study criteria and gave their informed consent. The exclusion criteria were anti-inflammatory drug use, alcohol abuse, and intestinal disease. The study was approved by the Clinical Research Ethics Committee (CEIC) of the Hospital Universitari Joan XXIII in Tarragona (CEIm 101/2017).
Tissues obtained from the proximal and distal colon were transferred to the culture laboratory within 30 min in cold oxygenated Krebs–Ringer buffer (pH = 7.4) with D-mannitol 10 mM.38
The excised samples were cut up to generate six explants from each human donor. The 5 mm diameter explants were placed in a 48-well plate pre-filled with 400 μL of KRB buffer with D-mannitol, and divided into five treatment groups: control, beef, almond, insect, and IPC. The control group was treated with the same KRB buffer with glucose. The digested samples were adjusted to a dose of 5 mg protein per mL. The medium was collected after 30 minutes of incubation and stored at −80 C.
The secretion of human inflammatory cytokines and intestinal immunoglobulins TNF-α, IL-10, IL-8, IgE and sIgA was quantified using Enzyme-linked Immunosorbent Assay (ELISA) with colorimetric detection. The overall experimental procedure is shown in Fig. 1. For immunosecretome analysis in human colon explants, intestinal secretion of IL-10, IL-1β, IgE and sIgA was measured with the Elabscience ELISA kit (Texas, United States). The human TNF-α ultrasensitive ELISA kit was obtained from Thermofisher (Invitrogen, Barcelona) (Cat. no: KHC3014). Colon IL-8 secretion was measured with Millipore (Sigma Aldrich, Madrid) (Cat. no: RAB0319). All immunomarkers were measured according to manufacturers’ instructions.
In vivo study of the consumption of A. diaperinus and T. molitor flour by Wistar rats
Buffalo (A. diaperinus) flour was obtained from Protifarm NV (Ermelo, Gelderland, The Netherlands) with a caloric content of 6.1 kcal g−1 and a macronutrient composition of 56.31% protein, 18.82% fat, 7.44% fibre, and 6.3% carbohydrates (1.30% starch).T. molitor flour from larvae was prepared from insects purchased from a local supplier (Iberinsect, S.L; Reus, Spain). Insect flour was prepared and processed by the FoodIE Research Group and the Mobiofood Research Group at Universitat Rovira i Virgili (URV), Spain. The composition of T. molitor flour was 56.10% protein, 26.31% lipids and 7.78% carbohydrates (3.34% starch), as measured by AGROLAB, S.A. The caloric content was determined to be 6.23 kcal g−1 by the Institute of Agrifood Research and Technology (IRTA, Catalonia, Spain).
The microbial content of A. diaperinus flour was determined at Protifarm (Ermelo, Gelderland, The Netherlands). The microorganism content of T. molitor flour was analysed at AGROLAB S.A (Tarragona, Spain). Both microbiological analyses showed levels of bacteria, moulds and yeasts absent or below the toxicity levels for both insects.
Forty, six-week-old, Wistar female rats (Janvier, Castellar del Vallès, Spain) were included in the in vivo study. The animals spent an adaptation period of 14 days at the Universitat Rovira Virgili animal facility under standard conditions. They were caged in pairs at 22 °C with a standard 12-hour light–dark cycle, ventilation, ad libitum access to tap water, and a standard Teklad diet (Cat no: Teklad 2014, Envigo++, Barcelona, Spain) consisting of 20% protein, 13% fat and 63% carbohydrates. The rats’ standard diet was a plant-based protein maintenance diet with 4.073 kcal g−1, as measured by IRTA, Catalonia, Spain.
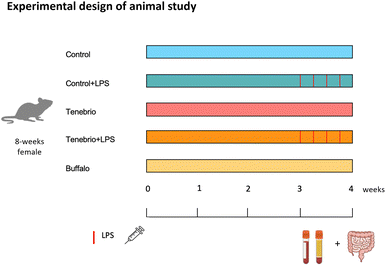
After this adaptation period, the animals were individualized and randomly divided into five experimental groups, with identical initial body weight, each of which was given a different treatment. Lipopolysaccharide (LPS) treatment was given, based on the body weight (BW) of the rats, to induce intestinal immune disruption. LPS was obtained from E. coli serotype O111.B5 (Merck Lifesciences, Madrid, Spain; Cat no: 4357765). As detailed in Miguéns-Gómez et al.,39 the treatment lasted 26 days (also shown in Fig. 2) and involved five groups as follows:
Group 1 comprised animals that received a standard (STD) diet ad libitum (C group);
Group 2 comprised animals that received an STD diet plus five intraperitoneal (i.p.) doses of LPS at 0.5 mg kg−1 of BW on the last five days (C + LPS group);
Group 3 comprised animals that received an STD diet plus a daily oral dose of T. molitor flour (300 mg of protein per kg BW per day) (T group);
Group 4 comprised animals that received STD diet plus a daily oral dose of T. molitor flour (300 mg of protein per kg BW per day) and an i.p. dose of LPS 0.5 mg per kg of BW on the last five days (T + LPS group); and
Group 5 comprised animals that received STD diet plus a daily oral dose with A. diaperinus flour (300 mg of protein per kg BW per day) (Buffalo group).
The insect flours were administered by controlled voluntary oral intake with a syringe, at a dose of 300 mg of protein per kilogram of body weight dissolved in water, at 6 pm. The treatment thus consisted only of raw insect meal mixed with tap water, while the control groups received the equivalent volume of tap water.
During the experiment, the health status of the animals was regularly monitored for cleanliness, general physical appearance, faecal consistency, and stress symptoms (hair loss, lack of appetite, etc.).
After 21 days, the animals were euthanized via exsanguination under anaesthesia, administered at a dose of 100 mg per kg BW of pentobarbital. Some of the rats’ main organs (the duodenum, jejunum and ileum from the small intestine; the distal and proximal large intestine; and the thymus, spleen, kidney and liver) were excised, weighed and frozen immediately in liquid nitrogen for future analysis. White abdominal adipose tissue (WAT) from mesenteric, retroperitoneal and epididymal locations was excised and weighed to calculate the percentage of adiposity. Blood was collected with ethylenediaminetetraacetic acid (Deltalab, Barcelona, Spain) as anticoagulant. Plasma was obtained by centrifugation at 1500g for 15 min at 4 °C and frozen immediately at −80 °C for future parameter quantification. All procedures were approved by the GENCAT Animal Experimentation Committee (number 11701).
Biochemical analysis
Colorimetric kits from QCA, (Tarragona, Spain), Materlab (Madrid, Spain) and Wako (Kyoto, Japan) were used to determine the following plasma biochemical parameters: cholesterol (QCA, Ref. 995282), glucose (QCA, Ref. 998282), triacylglycerols (QCA, Ref. 992330), urea (QCA, Ref. 993648), creatinine (QCA, Ref. 990310) and ketone bodies (β-hydroxybutyrate), (Materlab, Ref. HB8855).Intestinal barrier, immunological and allergenic analyses
Two days before euthanasia, the ovalbumin (OVA) test was run to determine intestinal permeability.40 These analyses were carried out using Ovalbumin ELISA kits (Cat. no. MBS2000240) from MyBioSource (San Diego, CA, USA).Plasma levels of total IgE and histamine were measured as biomarkers of allergy at the end of the experimental period. IgE (Cat. no. CN: E-EL-R0517) and histamine (Cat. no. CN: E-EL-0032) kits were purchased from Elabscience (Texas, United States) and performed according to the manufacturer's instructions.
Plasma inflammatory cytokine markers (IL-1β, IL-10, and TNF-α) were determined by ELISA. The ELISA kit for IL-10 (Cat. no. CN: 88-50629) was purchased from Thermofisher (Invitrogen) (Life Technologies, Madrid, Spain). ELISA for TNF- α (Cat. no. E-EL-R2856) and IL-1β (Cat. no. E-ELL-R0012) were purchased from Elabscience.
After the intestine was removed and before the intestinal parts were excised, the intestinal lumen contents were subjected to two lavages with 4 mL of PBS. The intestinal lumen contents of the small and large intestine were obtained separately and frozen immediately at −80 °C for future analysis. Secretory IgA (sIgA) levels were measured in small and large intestinal lavage fluids using the ELISA kit from MyBioSource (Cat. no. MBS9711882).
Total RNA and cDNA were obtained as previously reported.41 Quantitative PCR amplification was performed using specific TaqMan® probes for the sIgA inducing protein (Rn01406210_s1) and IL-1β (Rn00580432_m1) genes, and PPIA (cyclophilin) (Rn00690933_m1) as reference gene. The relative expression of each gene was compared with the control group using the 2-ΔΔCt method and with the cyclophilin gene as reference.
Histological analysis of the intestinal sections
One centimetre of each part of the intestine was fixed for 24 h in 4% formaldehyde solution and transferred to 70% ethanol solution for preservation until it was embedded in paraffin blocks. The samples analysed (at the Laboratory of Biology and Pathology of the Endocrine Pancreas of the Unité de Biologie Fonctionnelle et Adaptative, CNRS at Université Paris Cité, F-75013 Paris, France) were the duodenum, jejunum, ileum and ascending colon from five animals from each experimental group.Samples were cut at 5 mm and placed on glass slides. Haematoxylin and eosin (H&E) staining was then performed following standard procedures.
Using an OLYMPUS BX60 microscope equipped with Histolab 10.5.1 (Microvision Instruments, Evry, France) software for histology and morphometrics counting, the intestinal epithelium was analysed by measuring the villus height, villus width, crypt depth, epithelium height and villus-to-crypt ratio, as described in our previous study.42 The percentage of goblet cells was also analysed by counting the number of goblet cells and the number of epithelial cells.
Statistical analysis
Results are presented as means ± SEM. Data analysis was conducted with the XLSTAT 2023 statistical software (Addinsoft, USA). In the ex vivo human experiment, group differences were assessed through one-way ANOVA, followed by Tukey HSD multiple comparisons test.For the rat experiment, statistical differences were analysed using Student's t test, comparing each experimental group with the corresponding control group. Specifically, Tenebrio, Buffalo and Control + LPS were compared with Control, while Tenebrio + LPS was compared to Control + LPS.
Statistical significance was considered for mean differences if p < 0.05.
Results
Insect-derived flours produced a healthier inflammatory secretome in human colonic explants
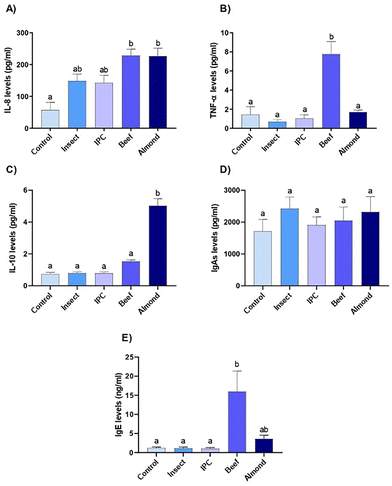
To assess how the human colonic immune system responds to various protein sources, we exposed healthy human colon explants to digested flours for 30 minutes at a concentration protein that stimulated enterohormone secretions in paired colonic samples.37The levels of proinflammatory protein IL-8 and TNF-α were determined in the media of colonic explants after insect, almond and beef exposure (Fig. 3). IL-8 levels from human explants treated with insect and IPC showed lower inflammatory profiles than those treated with beef or almond (Fig. 3A). Beef also had a proinflammatory effect by inducing an increase in TNF-α levels, whereas neither insect, IPC nor almond treatment led to elevated TNF- α levels (Fig. 3B).
Also assessed was the anti-inflammatory cytokine IL-10, which was significantly higher in explants treated with almond flour but remained unchanged after insect, IPC or beef treatment (Fig. 3C).
The levels of sIgA showed the extent of protection from intestinal pathogens in the intestinal lumen. No treatment showed changes in sIgA levels with respect to the control (Fig. 3D).
Also determined was the level of allergy-related IgE immunoglobulin (Fig. 3E). Neither whole insect nor IPC from A. diaperinus showed a similar allergenic profile to that of almond extract, whereas beef induced a significant increase in IgE levels.
Whole-health status of insect-fed rats
To analyse the effects on an in vivo system, we worked with two animal models, one of which was a healthy rat and the other was a model of LPS-induced mild inflammation and intestinal dysfunction.43 This LPS-induced disease model wants to mimic all pathologies that presented an altered physical barrier and a proinflammatory intestinal environment in the small intestine which would be the best candidates to be sensitive to this atypical food component.44,45 Using these animal models, we tested two species of insects to detect potential species-specific effects.In the healthy rat model, after 21 days of insect administration no animal showed any physical sign of stress (gastrointestinal, respiratory, or skin alteration) during the nutritional intervention. Treatment with buffalo flour did not change the weight of any organs in comparison with those of the control. Supplementation with T. molitor insect flour produced a trend towards an increase in % body weight gain but not in adiposity and did not change the weight of the thymus, liver, stomach, spleen, or kidney. There were no differences in total intestinal length between the groups (Table 1). In healthy rats, the ingestion of insect flour did not modify glucose, triglycerides (TAGs) or total cholesterol (Table 2). The levels of ketone bodies were undetectable and urea and creatinine in plasma were unchanged by insect consumption.
| Organ (g) | Control | Tenebrio | Buffalo | LPS | LPS + Tenebrio |
|---|---|---|---|---|---|
| n = 7–8 animals per group. Data are mean ± SEM. Student's t test was performed. * indicates p < 0.05 vs. control group; # indicates p < 0.1 vs. control group; $p < 0.05 vs. LPS. | |||||
| Liver | 7.46 ± 0.35 | 7.86 ± 0.19 | 8.06 ± 0.25 | 8.91 ± 0.37* | 9.41 ± 0.28 |
| Stomach | 1.38 ± 0.07 | 1.42 ± 0.04 | 1.41 ± 0.03 | 1.33 ± 0.03 | 1.37 ± 0.05 |
| Kidney | 0.34 ± 0.01 | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 |
| Thymus | 0.49 ± 0.04 | 0.57 ± 0.05 | 0.52 ± 0.10 | 0.33 ± 0.03* | 0.49 ± 0.06$ |
| Small Intestine length (cm) | 97.6 ± 0.57 | 100.1 ± 0.17 | 98.0 ± 0.44 | 96.2 ± 0.68 | 100.6 ± 0.52 |
| Spleen | 0.84 ± 0.05 | 0.84 ± 0.03 | 0.86 ± 0.04 | 1.53 ± 0.10* | 1.49 ± 0.13 |
| % BW gain | 9.42 ± 1.03 | 13.98 ± 2.83# | 9.80 ± 1.04 | 7.01 ± 1.40 | 6.64 ± 1.26 |
| % Adiposity | 4.38 ± 0.25 | 5.12 ± 0.59 | 4.26 ± 0.26 | 4.12 ± 0.21 | 4.37 ± 0.49 |
| Plasma | Control | Tenebrio | Buffalo | LPS | LPS + Tenebrio |
|---|---|---|---|---|---|
| Plasma levels of glucose, triglycerides, cholesterol, urea and creatinine at the end of the study. n = 7–8 animals per group. Data are mean ± SEM. Student's t test was performed. * indicates p < 0.05 vs. control group; $ 0.05 > p < 0.1 vs. LPS. | |||||
| Glucose (mM) | 7.18 ± 0.15 | 7.21 ± 0.38 | 7.35 ± 0.14 | 6.44 ± 0.24* | 7.09 ± 0.16$ |
| TAGs (mM) | 0.18 ± 0.02 | 0.22 ± 0.05 | 0.17 ± 0.02 | 0.18 ± 0.03 | 0.20 ± 0.01 |
| Cholesterol(mM) | 0.45 ± 0.01 | 0.46 ± 0.03 | 0.48 ± 0.03 | 0.35 ± 0.02* | 0.37 ± 0.02 |
| Urea (mM) | 3.20 ± 0.21 | 2.93 ± 0.13 | 3.28 ± 0.13 | 1.70 ± 0.18* | 2.11 ± 0.13$ |
| Creatinine (μM) | 2.68 ± 0.49 | 2.59 ± 0.28 | 3.18 ± 0.24 | 2.64 ± 0.56 | 3.43 ± 0.34 |
In line with the results in humans, in this healthy model the administration of insect protein (Buffalo or Tenebrio flour) did not alter the levels of the proinflammatory cytokines IL-1β and TNF-α. Similarly, no changes were observed in IL-10.
In the intestinal dysfunction model, the weights of the liver and spleen in the LPS group clearly increased while that of the thymus decreased (Table 1). Tenebrio molitor ingestion in LPS-treated animals slightly prevented the decrease in thymus weight compared to the LPS group. The disease model presented mild alterations in some biochemical parameters (Table 2). LPS treatment reduced the plasma levels of total cholesterol, glucose and the urea. However, TAGs and creatinine remained unchanged. Interestingly, ingestion of T. molitor seemed to prevent the decrease in blood glycemia and uraemia.
Effects of insect consumption on intestinal permeability, immune barrier and allergenicity
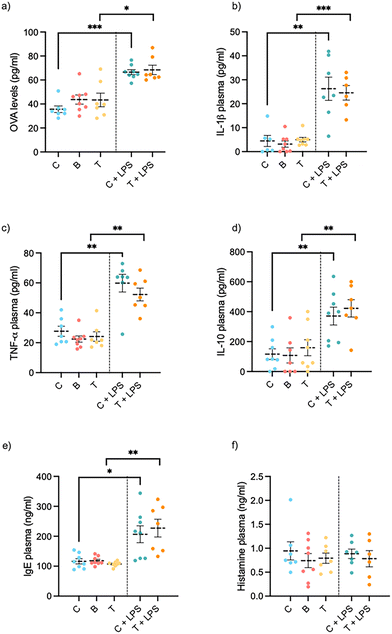
Both LPS-treated groups showed an increase in intestinal permeability compared to controls, though insect supplementation did not exert any significant effect with this time and dose (Fig. 4a). In healthy rats, insect supplementation showed no change in intestinal permeability compared to controls (Fig. 4a).To evaluate the effect of insect protein consumption in a pro-inflammatory environment, we measured the serum levels of TNF-α, IL-1β and IL-10. Fig. 4c shows that treatment with LPS significantly increased the levels of TNF-α and increased Il-10 and IL-1β almost three-fold (Fig. 4b and d) in comparison with the control group. Treatment with T. molitor, on the other hand, did not modify the secretion of these cytokines.
To assess the health and functionality of the mucosal immune system, we quantified the secretion of secretory immunoglobulin A (sIgA) at the intestinal level. In the healthy model, the results showed that more sIgA was secreted in the small intestine than in the colon (Table 3). Ingestion of the Buffalo and Tenebrio flours alone had no effect on sIgA levels in either the small intestine or the colon.
| Control | Tenebrio | Buffalo | LPS | LPS + Tenebrio | |
|---|---|---|---|---|---|
| n = 7–8 animals per group. Data are mean ± SEM. Student's t test was performed. # indicates 0.05 > p < 0.1 vs. control. | |||||
| Small intestine sIgA (μg ml−1) | 9.94 ± 1.25 | 8.00 ± 0.63 | 8.65 ± 1.37 | 17.2 ± 3.49# | 13.6 ± 1.95 |
| Ileal IgA i.p. gene expression | 0.91 ± 0.14 | 1.19 ± 0.30 | 0.87 ± 0.07 | 1.04 ± 0.19 | 0.76 ± 0.20 |
| Colonic sIgA (μg ml−1) | 1.27 ± 0.09 | 1.19 ± 0.08 | 1.17 ± 0.05 | 1.13 ± 0.05 | 1.23 ± 0.08 |
In the disease model, when comparing the LPS group with the Control group, the statistical analysis indicates a tendency to increase. However, animals that received both LPS injection and consumed Tenebrio did not show significant differences or a trend, neither with the Tenebrio group nor the LPS group. Intraperitoneal LPS administration had local immunology effects in the small intestine but not in the colon. We also analyzed relative expression in the ileum of slgA activation protein (Table 3) but observed no significant changes in gene expression in either the healthy or the LPS-induced inflammatory group.
To evaluate potential in vivo sensitization to the consumption of A. diaperinus or T. molitor insect flour for 21 days, IgE and histamine plasma biomarkers were tested. In the healthy model, neither the histamine nor the IgE levels showed any significant change between groups when the animals received insect flour (see Fig. 4f). In the disease model, IgE plasmatic levels increased significantly compared to controls, while histamine levels remained unchanged after insect consumption.
Intestinal morphometry in healthy and LPS-Wistar rat models under insect supplementation
To evaluate any putative micro-gut morphological alterations caused by the consumption of insect flour from mealworm and buffalo, we histochemically analysed the morphometric size of the villus and crypt and determined the total epithelium and surface amplification ratio of all intestinal sections (duodenum, jejunum, ileum and proximal colon) (see Table 4) as well as their Goblet cell percentage. Scheme 1 shows examples of the images used for the analysis.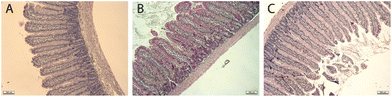 | ||
| Scheme 1 Examples of images obtained of jejunal sections of (A) Control group, (B) Tenebrio group and (C) LPS group. Magnification 10×. | ||
| Small intestine | Control | Tenebrio | Buffalo | LPS | LPS + Tenebrio |
|---|---|---|---|---|---|
| Duodenum (μm) | |||||
| Villus length | 548.13 ± 31.24 | 616.33 ± 29.62 | 597.29 ± 23.19 | 558.27 ± 25.37 | 549.71 ± 34.04 |
| Villus width | 78.48 ± 3.27 | 79.57 ± 2.43 | 76.21 ± 3.03 | 83.32 ± 2.88 | 79.80 ± 2.43 |
| Crypt depth | 168.94 ± 8.63 | 196.92 ± 17.41 | 187.20 ± 10.81 | 191.89 ± 17.18 | 181.79 ± 9.71 |
| Crypt width | 71.25 ± 4.42 | 71.05 ± 2.77 | 74.86 ± 4.77 | 78.86 ± 5.83 | 73.85 ± 2.31 |
| Epithelium w | 777.34 ± 22.76 | 848.24 ± 54.28 | 854.31 ± 48.23 | 803.30 ± 40.45 | 791.76 ± 45.40 |
| Villus/crypt ratio | 3.34 ± 0.45 | 3.50 ± 0.67 | 3.20 ± 0.08 | 3.01 ± 0.32 | 3.06 ± 0.24 |
| M (surface ratio) | 8.4 ± 0.12 | 9.4 ± 0.52 | 8.79 ± 0.46 | 7.93 ± 0.60 | 8.17 ± 0.52 |
| Jejunum (μm) | |||||
| Villus length | 404.72 ± 14.96 | 407.33 ± 14.02 | 414.78 ± 12.81 | 393.50 ± 26.82 | 366.32 ± 17.30 |
| Villus width | 55.28 ± 2.74 | 55.86 ± 5.69 | 53.77 ± 5.20 | 58.14 ± 2.68 | 52.73 ± 5.39 |
| Crypt depth | 168.94 ± 8.63 | 196.92 ± 17.41 | 187.20 ± 10.81 | 191.89 ± 17.18 | 181.79 ± 9.71 |
| Crypt width | 58.34 ± 4.42 | 58.44 ± 5.54 | 51.92 ± 3.79 | 56.76 ± 3.00 | 54.76 ± 2.49 |
| Epithelium w. | 586.74 ± 24.38 | 576.25 ± 20.85 | 588.07 ± 18.35 | 576.25 ± 20.85 | 533.05 ± 22.47 |
| Villus/crypt ratio | 2.90 ± 0.03 | 3.37 ± 0.12* | 3.22 ± 0.15& | 3.28 ± 0.33 | 3.00 ± 0.05 |
| M (surface ratio) | 7.8 ± 0.43 | 7.96 ± 0.67 | 8.8 ± 0.51 | 7.7 ± 0.57 | 7.45 ± 0.39 |
| Ileum (μm) | |||||
| Villus length | 269.50 ± 21.89 | 282.90 ± 5.14 | 248.96 ± 16.71 | 244.04 ± 10.28 | 261.89 ± 11.79 |
| Villus width | 55.92 ± 6.38 | 46.60 ± 2.03 | 47.93 ± 7.06 | 53.98 ± 5.08 | 47.16 ± 1.33 |
| Crypt depth | 102.55 ± 7.06 | 100.05 ± 5.51 | 103.73 ± 3.39 | 105.62 ± 3.13 | 104.95 ± 3.64 |
| Crypt width | 58.14 ± 1.98 | 54.08 ± 2.60 | 55.74 ± 1.43 | 53.10 ± 2.79 | 51.49 ± 1.67 |
| Epithelium w | 408.46 ± 24.50 | 429.35 ± 12.06 | 381.82 ± 20.50 | 374.73 ± 16.02 | 397.30 ± 15.42 |
| Villus/crypt ratio | 2.61 ± 0.15 | 2.85 ± 0.13 | 2.41 ± 0.20 | 2.21 ± 0.01* | 2.49 ± 0.05$ |
| M (surface ratio) | 5.41 ± 0.52 | 6.02 ± 0.29 | 5.20 ± 0.20 | 5.28 ± 0.05 | 5.87 ± 1.21 |
| Large intestine | Control | Tenebrio | Buffalo | LPS | LPS + Tenebrio |
|---|---|---|---|---|---|
| n = 7–8 animals per group. Data are mean ± SEM. Student's t test was performed. * indicates p < 0.05 vs. control group; $p < 0.05 vs. LPS; & 0.05 > p < 0.1 vs. control. | |||||
| Colon (μm) | |||||
| Crypt depth | 113.76 ± 15.40 | 103.56 ± 8.61 | 106.84 ± 7.06 | 106.13 ± 6.58 | 95.22 ± 6.27 |
| Crypt width | 40.95 ± 1.35 | 40.75 ± 1.50 | 38.55 ± 2.27 | 39.46 ± 1.53 | 39.47 ± 1.69 |
In healthy animals, our results showed that insect flour consumption did not modify villus width, villus height, crypt depth, villus-to-crypt ratio, epithelium width or the M-surface amplification ratio from any intestinal location. This is an indicator of the absence of a deleterious effect. Only Tenebrio increased the villus-to-crypt ration in the jejunum. No changes in Goblet cell number were observed in any intestinal section.
The same parameters were also measured in the inflammatory rat model with and without insect flour supplementation (Table 4). In the inflammatory model, a slight proinflammatory state influenced some parameters, including the ileum villus-to-crypt ratio, which decreased, thus indicating a morphological deleterious effect. Interestingly, T. molitor supplementation prevented this effect. No changes in Goblet cell number were observed.
Discussion
We have investigated the effect of a sustainable source of protein on human explants and Wistar rats, focusing on intestinal immunological effects. In a previous complementary work39 we analysed their effects in food intake and enterohomone modulation. Specifically, two edible insect species approved for human consumption by EFSA were analysed: Alphitobius diaperinus and Tenebrio molitor.47 Here our results showed healthy responses with regard to systemic and intestinal inflammation, allergenic response, and intestinal morphology in rats after chronic insect supplementation in both healthy and intestinal disfunction models. This is the first description of the non-allergenic effect of insect ingestion in a disrupted permeability and intestinal inflammation animal model.The gastrointestinal tract – the primary barrier against food components – plays a crucial role in host defence and immune response.46 Some studies have reported that increased permeability of the intestinal epithelium can facilitate the entry of potential food allergens, thus heightening sensitization and allergy risks.33,47 Notably, the intraperitoneal injection of lipopolysaccharides (LPS) leads to systematic and local alterations, including increased intestinal permeability and exacerbated inflammation.43,48
In the context of insect consumption, the potential allergenic response is a significant concern. While most existing research focuses on pre-sensitized individuals or those with allergies to crustaceans and inhalants,49 our study aimed to advance this field by assessing allergenic responses in the context of disrupted intestinal permeability and inflammation. Our results from the LPS model rats revealed an impaired inflammatory and allergic response, as has previously been described.43 However, in the group that received insect supplementation, no aggravation of this situation was observed, which suggests that the insect did not exert any allergenic response in this rat model of intestinal dysfunction.
Moreover, by evaluating the response to insect consumption in both healthy and non-sensitized human and rat models, our study offers a comprehensive overview of the allergenic potential of low-dose insect consumption. In this sense, the healthy rats that were chronically supplemented by yellow mealworm or lesser mealworm had a normal profile of secreted inflammatory and anti-inflammatory cytokines, while histamine and total IgE were not altered. Previous studies conducted with Sprague-Dawley rats, with longer oral exposures to insects and maximum doses (300–3000 mg kg−1 day−1) of mealworm, also found no statistically significant increases in serum histamine or IgE concentrations.50 Our data with doses of 300 mg kg−1 day−1 for 21 days in Wistar females corroborate those results.
We also directly compared the human colonic immune response to insect extracts to the response to almond and beef protein sources. Our results indicated that exposure to insect extracts led to a lower secretion of inflammatory cytokines and allergenic immunoglobulin compared to almond or beef extracts. Beef especially appeared to be more proinflammatory, as was evidenced by higher cytokines and IgE levels. On the other hand, the response to almond appeared to be counterbalanced by an anti-inflammatory effect. These findings align with previous in vivo studies that suggest that beef consumption can alter gut microbiota and activate inflammatory pathways,51,52 while almond consumption is known to exert a protective effect against the development of gastrointestinal inflammation.53 Moreover, while explants treated with beef exhibited higher IgE secretion, these levels are still within the low and normal range for intestinal tissue,54 which suggests that this is not indicative of an allergic response. All these results support the notion of a healthier inflammatory secretome for insect-derived flours compared to those from almond and beef.
An important effector function of gut-associated lymphoid tissue involves the production and secretion of immunoglobulin A (IgA). This immunoglobulin plays a crucial role in protecting the intestine from pathogens, mainly by limiting their interactions with the epithelial cell monolayer.55 The transcytosis of dimeric IgA antibodies through epithelial cells is mediated by the polymeric Ig receptor, which results in the release of secretory IgA (sIgA).56 In this context, local antigen exposure could lead to increased sIgA levels in both the exposed area and the enteric mucosa. Measuring IgA in intestinal lavage fluids is therefore a common method for assessing mucosal immune responses.55 Our animal study showed a tendency only for increased sIgA secretion in rats administered with LPS. In contrast, neither human explants nor rats treated solely with protein sources showed significant alterations in this intestinal immune marker. This suggests that the consumption of these protein sources, including insects, does not cause major immune disturbances, thereby aligning with findings reported by Stull et al.21
To continue with the intestinal analysis, our findings also showed that insect supplementation did not affect normal intestinal morphology. The typical gradient of morphometric indices from the duodenum to the ileum was maintained, which corroborates the preservation of physiological gut development and absorption processes.57 Similar to these results obtained by Biasato et al. in chickens, chronic insect consumption did not alter the intestinal morphology. Main modification was in the jejunum, where animals supplemented with insects exhibited a higher villus-height to crypt-depth ratio. This effect has been suggested as adaptative response to expand the surface area for nutrient absorption.58 Our study does not allow support this hypothesis, because there was not a clear effect on food intake, we saw an increased food intake at the first week, but it was lost afterwards.39 And the other challenging aspect regarding insects-derived products is their digestibility. In this respect, we have previously shown a similar digestibility of A. diaperinus to beef samples.59 Moreover, a lower ileal villus-to-crypt ratio was observed in rats administered LPS, which confirms the finding from studies on mice that the intraperitoneal injection of LPS affects the integrity of the small intestine.60 Interestingly, the consumption of T. molitor appeared to counteract this reduction in the ileal ratio.
Moreover, our findings reveal a reduction in thymus weight following LPS injections, consistent with other studies that have previously reported the potential for LPS-induced thymic atrophy.61,62 The thymus is a vital organ of the immune system, and its atrophy could compromise its ability to effectively respond to other infections.63 Interestingly, our observations suggest that the consumption of T. molitor may confer protection to the thymus against changes induced by LPS. Despite not observing other improvements in terms of inflammatory status, this result aligns with the potential anti-inflammatory effects associated with insect consumption, as indicated by existing literature.24 Furthermore, some authors have proposed that thymus gland dysfunction can be ameliorated through nutritional intervention, involving a balance of macronutrients, micronutrients, and the incorporation of probiotics, indicating thymus-gut connections.64,65 Thus, the potential of insect supplementation to modulate gut microbiota with prebiotic effects,66 may be linked to thymus healthy. Further research exploring both the inflammatory protection and the prebiotic effects of insect consumption could contribute to a comprehensive understanding of its impact on inflammation.
Although this study primarily focused on the immune system's response, we also considered whole-health parameters. With regard to the plasma parameters, our study indicates good metabolic tolerance to insect administration, with T. molitor intake notably ameliorating LPS-altered glucose and urea levels. Our study does not allow to explain these effects, but we can clearly state that they cannot relate it with improvement of TNF-α or IL-1β, which have been demonstrated to induce hypoglycaemia.45 We cannot discard results from other studies that have reported improved circulating triglycerides, glucose and cholesterol levels in humans and animals following insect consumption.1 Our initial screening of plasmatic cytokine levels, known to participate in systemic inflammation and exhibiting increased levels in the LPS situation,67 revealed that LPS administration alone increased the immunological parameters, but the ingestion of insect flour did not exacerbate this effect. Notably, the LPS-induced increase in IL-10 plasma levels is a typical counter-regulatory response to intraperitoneal insult mediated by T-regulatory cells to resolve inflammation.68
Note that the EFSA recently approved the insects used in this study as novel food for human consumption and encouraged further research in this area.16,17 There is growing consensus on the need for further studies to demonstrate the health benefits of insect consumption and enhance its social acceptance, especially in Western diets, which urgently need to change from a conventional to a more sustainable source of protein.69 The present study describes, for the first time, the chronic effects of consuming two species of insects on both general and intestinal health in rat and human samples and provides novel insights into the immune response in a healthy and a disrupted intestinal model.
In view of all the evidence in support of adding insects to diets and the encouraging results of this study, to validate these findings further research should involve human participants without known allergies. Long-term studies are needed to fully understand the impact of edible insects on human health. As the food industry explores alternative sources of protein, edible insects present a unique opportunity to improve environmental sustainability while also providing substantial nutritional advantages.
Conclusions
The human ex vivo studies have shown that A. diaperinus digested in vitro induced a less inflammatory profile than either almond or beef extract in colon samples. Moreover, neither A. diaperinus nor T. molitor administered chronically in moderate doses to healthy Wistar rats compromised gut morphology, plasma biochemistry profile or immune response. In the LPS-induced intestinal dysfunction model, insect consumption ameliorated glycaemia, uraemia and gut villus-to-crypt ratio. These data reinforce the healthy immunological profile of both species of insect assayed. More insights are needed on the bioactive effects of insect flour, especially with regard to the less studied Alphitobius diaperinus.Author contributions
HS, FJ and MSC: data curation, formal analysis, investigation; FF: investigation; ERG, JM and XT: methodology; AA, MTB, XT and MP: conceptualization, supervision. AA, MTB, HS: project administration, original draft; MP, XT, JM and AA: resources. All the authors participated in funding acquisition and revised and edited the final document before submission.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to thank Niurka Llopiz and Rosa M. Pastor for respective technical support.This research was funded by Diputació de Tarragona and Universitat Rovira i Virgili (URV) grant number DIPTA-007 2020/26 (ESSARPI), MCIN/AEI PID2021-122636OB-I00 by ERDF “A way of making Europe”, TED2021-131783B-100 funded by MCIN/AEI/10.13039/501100011033 and by the “European Union NextGenerationEU/PRTR”. M. Sierra and H. Segú were funded by the M. Franquès programme of the URV. F. Jalševac was funded by Marie Skłodowska Curie grant agreement no. 945413 and Universitat Rovira i Virgili (URV). Montserrat Pinent and Ximena Terra are Serra Húnter fellows.
References
- M. Ros-Baró, P. Casas-Agustench, D. A. Díaz-Rizzolo, L. Batlle-Bayer, F. Adrià-Acosta, A. Aguilar-Martínez, F. X. Medina, M. Pujolà and A. Bach-Faig, Edible Insect Consumption for Human and Planetary Health: A Systematic Review, Int. J. Environ. Res. Public Health, 2022, 19, 11653 CrossRef PubMed.
- IPIFF, The European insect sector today: Challenges, opportunities and regulatory landscape. IPIFF vision paper on the future of the insect sector towards 2030, https://ipiff.org/wp-content/uploads/2019/12/2019IPIFF_VisionPaper_updated.pdf, (accessed 20 April 2022).
- IPIFF, An overview of the European market of insects as feed, https://ipiff.org/wp-content/uploads/2021/04/Apr-27-2021-IPIFF_The-European-market-of-insects-as-feed.pdf, (accessed 15 August 2023).
- N. M. de Carvalho, A. R. Madureira and M. E. Pintado, The potential of insects as food sources–a review, Crit. Rev. Food Sci. Nutr., 2020, 60, 3642–3652 CrossRef PubMed.
- A. Jantzen da Silva Lucas, L. Menegon de Oliveira, M. da Rocha and C. Prentice, Edible insects: An alternative of nutritional, functional and bioactive compounds, Food Chem., 2020, 311, 126022 CrossRef CAS PubMed.
- B. A. Rumpold and O. K. Schlüter, Nutritional composition and safety aspects of edible insects, Mol. Nutr. Food Res., 2013, 57, 802–823 CrossRef CAS PubMed.
- D. A. T. Sosa and V. Fogliano, Potential of Insect-Derived Ingredients for Food Applications, Insect Physiol. Ecol., 2017 DOI:10.5772/67318.
- C. L. R. Payne, P. Scarborough, M. Rayner and K. Nonaka, Are edible insects more or less ‘healthy’ than commonly consumed meats? A comparison using two nutrient profiling models developed to combat over- and undernutrition’, Eur. J. Clin. Nutr., 2016, 70, 285 CrossRef CAS PubMed.
- Z. Ma, M. Mondor, F. Goycoolea Valencia and A. J. Hernández-Álvarez, Current state of insect proteins: extraction technologies, bioactive peptides and allergenicity of edible insect proteins, Food Funct., 2023, 14, 8129–8156 RSC.
- L. Selaledi, C. A. Mbajiorgu and M. Mabelebele, The use of yellow mealworm (T. molitor) as alternative source of protein in poultry diets: a review, Trop. Anim. Health Prod., 2020, 52, 7–16 CrossRef CAS PubMed.
- A. Nikkhah, S. Van Haute, V. Jovanovic, H. Jung, J. Dewulf, T. Cirkovic Velickovic and S. Ghnimi, Life cycle assessment of edible insects (Protaetia brevitarsis seulensis larvae) as a future protein and fat source, Sci. Rep., 2021, 11, 14030 CrossRef CAS PubMed.
- S. Mancini, G. Sogari, S. E. Diaz, D. Menozzi, G. Paci and R. Moruzzo, Exploring the Future of Edible Insects in Europe, Foods, 2022, 11, 455 CrossRef CAS PubMed.
- L. Soetemans, N. Gianotten and L. Bastiaens, Agri-Food Side-Stream Inclusion in The Diet of Alphitobius Diaperinus. Part 2: Impact on Larvae Composition, Insects, 2020 DOI:10.3390/INSECTS11030190.
- C. Garino, H. Mielke, S. Knüppel, T. Selhorst, H. Broll and A. Braeuning, Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor), Food Chem. Toxicol., 2020, 142, 111460 CrossRef CAS PubMed.
- E. Siemianowska, A. Kosewska, M. Aljewicz, K. A. Skibniewska, L. Polak-Juszczak, A. Jarocki and M. Jędras, Larvae of mealworm (Tenebrio molitor L.) as European novel food, Agric. Sci., 2013, 04, 287–291 Search PubMed.
- D. Turck, T. Bohn, J. Castenmiller, S. De Henauw, K. Ildico Hirsch-Ernst, A. Maciuk, I. Mangelsdorf, H. J. McArdle, A. Naska, C. Pelaez, K. Pentieva, A. Siani, F. Thies, S. Tsabouri, M. Vinceti, F. Cubadda, T. Frenzel, M. Heinonen, R. Marchelli, M. Neuh, M. Poulsen, M. Prieto Maradona, J. Rudolf Schlatter, H. van Loveren, E. Ververis and H. Katrine Knutsen, Safety of frozen and freeze-dried formulations of the lesser mealworm (Alphitobius diaperinus larva) as a Novel food pursuant to Regulation (EU) 2015/2283 EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), EFSA J., 2022, 20, 7325 Search PubMed.
- D. Turck, J. Castenmiller, S. De Henauw, K. I. Hirsch-Ernst, J. Kearney, A. Maciuk, I. Mangelsdorf, H. J. McArdle, A. Naska, C. Pelaez, K. Pentieva, A. Siani, F. Thies, S. Tsabouri, M. Vinceti, F. Cubadda, T. Frenzel, M. Heinonen, R. Marchelli, M. Neuhäuser-Berthold, M. Poulsen, M. Prieto Maradona, J. R. Schlatter, H. van Loveren, E. Ververis and H. K. Knutsen, Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283, EFSA J., 2021 DOI:10.2903/J.EFSA.2021.6343.
- B. M. Park, B. G. Jung, J. A. Lee and B. J. Lee, Mitigating Effects of Tenebrio molitor Larvae Powder Administration in Mice with Dextran Sodium Sulfate (DSS)-Induced Colitis, Asian Pac. J. Cancer Prev., 2023, 24, 1751 CrossRef CAS PubMed.
- J. Rivera-Jiménez, C. Berraquero-García, R. Pérez-Gálvez, P. J. García-Moreno, F. J. Espejo-Carpio, A. Guadix and E. M. Guadix, Peptides and protein hydrolysates exhibiting anti-inflammatory activity: sources, structural features and modulation mechanisms, Food Funct., 2022, 13, 12510–12540 RSC.
- E. Zielińska, B. Baraniak and M. Karaś, Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria), Int. J. Food Sci. Technol., 2018, 53, 2542–2551 CrossRef.
- V. J. Stull, E. Finer, R. S. Bergmans, H. P. Febvre, C. Longhurst, D. K. Manter, J. A. Patz and T. L. Weir, Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial, Sci. Rep., 2018 DOI:10.1038/S41598-018-29032-2.
- Y. Kang, P. M. Oba, C. A. Gaulke, L. Sánchez-Sánchez and K. S. Swanson, Dietary Inclusion of Yellow Mealworms (T. molitor) and Lesser Mealworms (A. diaperinus) Modifies Intestinal Microbiota Populations of Diet-Induced Obesity Mice, J. Nutr., 2023, 153, 3220–3236 CrossRef CAS PubMed.
- E. Colombino, I. Biasato, I. Ferrocino, S. B. Oddon, C. Caimi, M. Gariglio, S. Dabbou, M. Caramori, E. Battisti, S. Zanet, E. Ferroglio, L. Cocolin, L. Gasco, A. Schiavone and M. T. Capucchio, Effect of insect live larvae as environmental enrichment on poultry gut health: Gut mucin composition, microbiota and local immune response evaluation, Animals, 2021, 11, 2819 CrossRef PubMed.
- N. Cunha, V. Andrade, P. Ruivo and P. Pinto, Effects of Insect Consumption on Human Health: A Systematic Review of Human Studies, Nutrients, 2023 DOI:10.3390/NU15143076/S1.
- K. C. M. Verhoeckx, S. van Broekhoven, C. F. den Hartog-Jager, M. Gaspari, G. A. H. de Jong, H. J. Wichers, E. van Hoffen, G. F. Houben and A. C. Knulst, House dust mite (Der p 10) and crustacean allergic patients may react to food containing Yellow mealworm proteins, Food Chem. Toxicol., 2014, 65, 364–373 CrossRef CAS PubMed.
- G. Reese, R. Ayuso and S. B. Lehrer, Tropomyosin: An Invertebrate Pan–Allergen, Int. Arch. Allergy Immunol., 1999, 119, 247–258 CrossRef CAS PubMed.
- M. Binder, V. Mahler, B. Hayek, W. R. Sperr, M. Schöller, S. Prozell, G. Wiedermann, P. Valent, R. Valenta and M. Duchêne, Molecular and immunological characterization of arginine kinase from the Indianmeal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen, J. Immunol., 2001, 167, 5470–5477 CrossRef CAS PubMed.
- X. Zhao, L. Li, Z. Kuang, G. Luo and B. Li, Proteomic and immunological identification of two new allergens from silkworm (Bombyx mori L.) pupae, Cent. Eur. J. Immunol., 2015, 40, 30–34 CrossRef PubMed.
- P. A. Galindo, M. Lombardero, J. Borja, E. Gómez, F. Feo, D. Barber and R. García, A new arthropod panallergen?, Allergy, 2001, 56, 195–197 CrossRef CAS PubMed.
- C. Lamberti, S. Nebbia, S. Cirrincione, L. Brussino, V. Giorgis, A. Romito, C. Marchese, M. Manfredi, E. Marengo, M. G. Giuffrida, G. Rolla and L. Cavallarin, Thermal processing of insect allergens and IgE cross-recognition in Italian patients allergic to shrimp, house dust mite and mealworm, Food Res. Int., 2021, 148, 110567 CrossRef CAS PubMed.
- H. C. H. P. Broekman, A. C. Knulst, G. de Jong, M. Gaspari, C. F. den Hartog Jager, G. F. Houben and K. C. M. Verhoeckx, Is mealworm or shrimp allergy indicative for food allergy to insects?, Mol. Nutr. Food Res., 2017 DOI:10.1002/MNFR.201601061.
- P. Beaumont, J. Courtois, X. Van der Brempt and S. Tollenaere, Food-induced anaphylaxis to Tenebrio molitor and allergens implicated, Rev. Fr. Allergol., 2019, 59, 389–393 CrossRef.
- J. W. Krempski, C. Dant and K. C. Nadeau, The origins of allergy from a systems approach, Ann. Allergy, Asthma, Immunol., 2020, 125, 507–516 CrossRef CAS PubMed.
- S. J. Galli, M. Tsai and A. M. Piliponsky, The development of allergic inflammation, Nature, 2008, 454, 445–454 CrossRef CAS PubMed.
- L. Egger, O. Ménard, C. Delgado-Andrade, P. Alvito, R. Assunção, S. Balance, R. Barberá, A. Brodkorb, T. Cattenoz, A. Clemente, I. Comi, D. Dupont, G. Garcia-Llatas, M. J. Lagarda, S. Le Feunteun, L. JanssenDuijghuijsen, S. Karakaya, U. Lesmes, A. R. Mackie, C. Martins, A. Meynier, B. Miralles, B. S. Murray, A. Pihlanto, G. Picariello, C. N. Santos, S. Simsek, I. Recio, N. Rigby, L. E. Rioux, H. Stoffers, A. Tavares, L. Tavares, S. Turgeon, E. K. Ulleberg, G. E. Vegarud, G. Vergères and R. Portmann, The harmonized INFOGEST in vitro digestion method: From knowledge to action, Food Res. Int., 2016, 88, 217–225 CrossRef CAS.
- A. Miguéns-Gómez, C. Grau-Bové, M. Sierra-Cruz, R. Jorba-Martín, A. Caro, E. Rodríguez-Gallego, R. Beltrán-Debón, M. T. Blay, X. Terra, A. Ardévol and M. Pinent, Gastrointestinally digested protein from the insect alphitobius diaperinus stimulates a different intestinal secretome than beef or almond, producing a differential response in food intake in rats, Nutrients, 2020, 12, 1–15 CrossRef PubMed.
- A. Miguéns-Gómez, C. Grau-Bové, M. Sierra-Cruz, R. Jorba-Martín, A. Caro, E. Rodríguez-Gallego, R. Beltrán-Debón, T. Blay, X. Terra, A. Ardévol and M. Pinent, Gastrointestinally Digested Protein from the Insect Alphitobius diaperinus Stimulates a Different Intestinal Secretome than Beef or Almond, Producing a Differential Response in Food Intake in Rats, Nutrients, 2020, 12, 2366 CrossRef PubMed.
- C. Grau-bové, C. González-quilen, X. Terra, M. Teresa Blay, R. Beltrán-Debón, R. Jorba-martín, B. Espina, M. Pinent and A. Ardévol, Effects of Flavanols on Enteroendocrine Secretion, Biomolecules, 2020, 10, 1–14 CrossRef PubMed.
- A. Miguéns-Gómez, M. Sierra-Cruz, H. Segú, R. Beltrán-Debón, E. Rodríguez-Gallego, X. Terra, M. T. Blay, A. M. Pérez-Vendrell, M. Pinent and A. Ardévol, Administration of Alphitobius diaperinus or Tenebrio molitor before meals transiently increases food intake through enterohormone regulation in female rats, J. Sci. Food Agric., 2023, 103, 1660–1667 CrossRef PubMed.
- K. Gil-Cardoso, I. Ginés, M. Pinent, A. Ardévol, M. Blay and X. Terra, The co-administration of proanthocyanidins and an obesogenic diet prevents the increase in intestinal permeability and metabolic endotoxemia derived to the diet, J. Nutr. Biochem., 2018, 62, 35–42 CrossRef CAS PubMed.
- C. González-Quilen, K. Gil-Cardoso, I. Ginés, R. Beltrán-Debón, M. Pinent, A. Ardévol, X. Terra and M. T. Blay, Grape-Seed Proanthocyanidins are Able to Reverse Intestinal Dysfunction and Metabolic Endotoxemia Induced by a Cafeteria Diet in Wistar Rats, Nutrients, 2019 DOI:10.3390/nu11050979.
- H. Segú, F. Jalševac, M. Pinent, A. Ardévol, X. Terra and M. T. Blay, Intestinal Morphometric Changes Induced by a Western-Style Diet in Wistar Rats and GSPE Counter-Regulatory Effect, Nutrients, 2022 DOI:10.3390/NU14132608.
- K. Gil-Cardoso, R. Comitato, I. Ginés, A. Ardévol, M. Pinent, F. Virgili, X. Terra and M. Blay, Protective Effect of Proanthocyanidins in a Rat Model of Mild Intestinal Inflammation and Impaired Intestinal Permeability Induced by LPS, Mol. Nutr. Food Res., 2019 DOI:10.1002/MNFR.201800720.
- M. J. Rodriguez, A. Aranda, T. D. Fernandez, N. Cubells-Baeza, M. J. Torres, F. Gomez, F. Palomares, J. R. Perkins, J. Rojo, A. Diaz-Perales and C. Mayorga, LPS promotes Th2 dependent sensitisation leading to anaphylaxis in a Pru p 3 mouse model, Sci. Rep., 2017 DOI:10.1038/SREP40449.
- S. Seemann, F. Zohles and A. Lupp, Comprehensive comparison of three different animal models for systemic inflammation, J. Biomed. Sci., 2017 DOI:10.1186/s12929-017-0370-8.
- T. Takiishi, C. I. M. Fenero and N. O. S. Câmara, Intestinal barrier and gut microbiota: Shaping our immune responses throughout life, Tissue Barriers, 2017 DOI:10.1080/21688370.2017.1373208.
- R. P. Schleimer, A. Kato, R. Kern, D. Kuperman and P. C. Avila, Epithelium: At the interface of innate and adaptive immune responses, J. Allergy Clin. Immunol., 2007, 120, 1279 CrossRef CAS PubMed.
- K. Ramírez, D. Quesada-Yamasaki and J. Fornaguera-Trías, A protocol to perform systemic lipopolysacharide (LPS) challenge in rats, Odovtos – Int. J. Dent. Sci., 2019, 21, 53–66 CrossRef.
- L. De Marchi, A. Wangorsch and G. Zoccatelli, Allergens from Edible Insects: Cross-reactivity and Effects of Processing, Curr. Allergy Asthma Rep., 2021 DOI:10.1007/S11882-021-01012-Z.
- S. R. Han, B. S. Lee, K. J. Jung, H. J. Yu, E. Y. Yun, J. S. Hwang and K. S. Moon, Safety assessment of freeze-dried powdered Tenebrio molitor larvae (yellow mealworm) as novel food source: Evaluation of 90-day toxicity in Sprague-Dawley rats, Regul. Toxicol. Pharmacol., 2016, 77, 206–212 CrossRef CAS PubMed.
- D. P. Li, M. Cui, F. Tan, X. Y. Liu and P. Yao, High Red Meat Intake Exacerbates Dextran Sulfate-Induced Colitis by Altering Gut Microbiota in Mice, Front. Nutr., 2021, 8, 646819 CrossRef PubMed.
- Y. Yin, J. Cai, L. Zhou, L. Xing and W. Zhang, Dietary oxidized beef protein alters gut microbiota and induces colonic inflammatory damage in C57BL/6 mice, Front. Nutr., 2022, 9, 980204 CrossRef PubMed.
- G. Mandalari, T. Gervasi, D. W. Rosenberg, K. G. Lapsley and D. J. Baer, Effect of Nuts on Gastrointestinal Health, Nutrients, 2023, 15, 1733 CrossRef CAS PubMed.
- W. R. Brown, B. K. Borthistle and S.-T. Chen, Immunoglobulin E (IgE) and IgE-containing cells in human gastrointestinal fluids and tissues, Clin. Exp. Immunol., 1975, 20, 227–237 CAS.
- S. S. Ghosh, J. Wang, P. J. Yannie and S. Ghosh, Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development, J. Endocr. Soc., 2020 DOI:10.1210/JENDSO/BVZ039.
- J. L. Gommerman, O. L. Rojas and J. H. Fritz, Re-thinking the functions of IgA+ plasma cells, Gut Microbes, 2014, 5, 652 CrossRef PubMed.
- I. Biasato, I. Ferrocino, E. Biasibetti, E. Grego, S. Dabbou, A. Sereno, F. Gai, L. Gasco, A. Schiavone, L. Cocolin and M. T. Capucchio, Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens, BMC Vet. Res., 2018 DOI:10.1186/S12917-018-1690-Y.
- S. R. Taylor, S. Ramsamooj, R. J. Liang, A. Katti, R. Pozovskiy, N. Vasan, S. K. Hwang, N. Nahiyaan, N. J. Francoeur, E. M. Schatoff, J. L. Johnson, M. A. Shah, A. J. Dannenberg, R. P. Sebra, L. E. Dow, L. C. Cantley, K. Y. Rhee and M. D. Goncalves, Dietary fructose improves intestinal cell survival and nutrient absorption, Nature, 2021, 597, 263–267 CrossRef CAS PubMed.
- F. Accardo, A. Miguéns-Gómez, V. Lolli, A. Faccini, A. Ardévol, X. Terra, A. Caligiani, M. Pinent and S. Sforza, Molecular composition of lipid and protein fraction of almond, beef and lesser mealworm after in vitro simulated gastrointestinal digestion and correlation with the hormone-stimulating properties of the digesta, Food Res. Int., 2022 DOI:10.1016/j.foodres.2022.111499.
- E. Im, F. M. Riegler, C. Pothoulakis and S. H. Rhee, Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice, Am. J. Physiol.: Gastrointest. Liver Physiol., 2012, 303, 490–497 CrossRef PubMed.
- Y. Ogikubo, M. Norimatsu, Y. Sasaki, A. Yasuda, J. Saegusa and Y. Tamura, Effect of Lipopolysaccharide (LPS) Injection on the Immune Responses of LPS-Sensitive Mice, J. Vet. Med. Sci., 2004, 66, 1189–1193 CrossRef CAS PubMed.
- M. P. Ullewar and S. N. Umathe, A possible role of endogenous central corticotrophin releasing factor in lipopolysaccharide induced thymic involution and cell apoptosis: Effect of peripheral injection of corticotrophin releasing factor, J. Neuroimmunol., 2015, 280, 58–65 CrossRef CAS PubMed.
- M. Luo, L. Xu, Z. Qian and X. Sun, Infection-Associated Thymic Atrophy, Front. Immunol., 2021, 12, 652538 CrossRef CAS PubMed.
- W. Savino, J. Durães, C. Maldonado-Galdeano, G. Perdigon, D. A. Mendes-da-Cruz and P. Cuervo, Thymus, undernutrition, and infection: Approaching cellular and molecular interactions, Front. Nutr., 2022, 9, 948488 CrossRef PubMed.
- F. Balcells, M. J. Martínez Monteros, A. L. Gómez, S. I. Cazorla, G. Perdigón and C. Maldonado-Galdeano, Probiotic Consumption Boosts Thymus in Obesity and Senescence Mouse Models, Nutrients, 2022 DOI:10.3390/NU14030616/S1.
- V. J. Stull, Impacts of insect consumption on human health, Wageningen Academic Publishers, 2021, 7, 695–713, DOI:10.3920/JIFF2020.0115.
- M. Aries, M. Cook and T. Hensley-McBain, Peripheral Low Level Chronic LPS Injection as a Model of Neutrophil Activation in the Periphery and Brain in Mice, Res. Sq., 2023 DOI:10.21203/RS.3.RS-3443401/V1.
- Y. P. Rubtsov, J. P. Rasmussen, E. Y. Chi, J. Fontenot, L. Castelli, X. Ye, P. Treuting, L. Siewe, A. Roers, W. R. Henderson, W. Muller and A. Y. Rudensky, Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces, Immunity, 2008, 28, 546–558 CrossRef CAS PubMed.
- M. Molfetta, E. G. Morais, L. Barreira, G. L. Bruno, F. Porcelli, E. Dugat-Bony, P. Bonnarme and F. Minervini, Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation, Foods, 2022, 11, 2065 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |