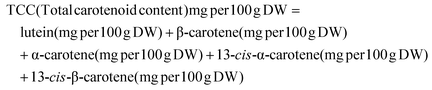Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Genotype and ripening method affect carotenoid content and bio-accessibility in banana†
Bryan
Munoz
 ac,
Micaela
Hayes
ab,
Penelope
Perkins-Veazie
ac,
Micaela
Hayes
ab,
Penelope
Perkins-Veazie
 ac,
Nicholas
Gillitt
d,
Miguel
Munoz
ac,
Nicholas
Gillitt
d,
Miguel
Munoz
 e,
Colin D.
Kay
e,
Colin D.
Kay
 abf,
Mary Ann
Lila
abf,
Mary Ann
Lila
 ab,
Mario G.
Ferruzzi
ab,
Mario G.
Ferruzzi
 *abf and
Massimo
Iorizzo
*ac
*abf and
Massimo
Iorizzo
*ac
aPlants for Human Health Institute, North Carolina State University, 600 Laureate Way, Kannapolis, NC 28081, USA. E-mail: miorizz@ncsu.edu; MFerruzzi@uams.edu
bDepartment of Food, Bioprocessing and Nutrition Sciences, North Carolina State University, 600 Laureate Way, Kannapolis, NC 28081, USA
cDepartment of Horticultural Science, North Carolina State University, 600 Laureate Way, Kannapolis, NC 9 28081, USA
dThe Osmolality Lab, 600 Laureate Way, Kannapolis, NC 28081, USA
eResearch & Development Department, Dole, Standard Fruit Company de Costa Rica, San José, Costa Rica
fArkansas Children's Nutrition Center (ACNC), University of Arkansas for Medical Sciences, 4301 West Markham Street, Little Rock, AR 72202, USA
First published on 4th March 2024
Abstract
Bananas (Musa spp.) are a target crop for provitamin A carotenoids (pVACs) biofortification programs aiming at reducing the negative impact on health caused by vitamin A deficiency in vulnerable populations. However, studies to understand the effect of ripening methods and stages and the genotype on carotenoid content and bioaccessibility in the banana germplasm are scarce. This study evaluated carotenoid content and bioaccessibility in 27 different banana accessions at three maturation stages and two ripening methods (natural ripening and ethylene ripening). Across most accessions, total carotenoid content (TCC) increased from unripe to ripe fruit; only two accessions showed a marginal decrease. The ripening method affected carotenoid accumulation; 18 accessions had lower TCC when naturally ripened compared with the ethylene ripening group, while nine accessions showed higher TCC when ripened with exogenous ethylene, suggesting that treating bananas with exogenous ethylene might directly affect TCC accumulation, but the response is accession dependent. Additionally, carotenoid bioaccessibility varied across genotypes and was correlated with the amount of soluble starch and resistant starch. These findings highlight the importance of ripening methods and genotypes in maximizing banana carotenoid content and bioaccessibility, which could contribute to improving pVACs delivery in biofortification programs.
1 Introduction
Carotenoids are pigments synthesized by plants and microorganisms, commonly found in green, orange, and yellow plant tissues. These pigments have important functions in human health, and since humans cannot synthesize carotenoids, they must ingest them from food or via supplementation. While there are around 700 carotenoid forms in nature, only 40 of them can be metabolized by humans, and six of those can be detected in blood plasma: α-carotene, β-carotene, lutein, lycopene, β-cryptoxanthin, and zeaxanthin.In humans, some carotenoids exert an important function as provitamin A precursors; others may exert antioxidant effects in cells, while others, like lutein and zeaxanthin, have been linked to ocular development and health as they accumulate in macular pigments in the eye.1
In addition, some carotenoids can influence cellular signal pathways and induce detoxifying enzymes and gene expression.2 Of the many species of carotenoids present in the diet, only a small subset, including α- and β -cryptoxanthin as well as α- and β-carotene can be metabolized by beta-carotene oxygenase (BCO1) to retinal, the active form of vitamin A3,4. These pigments are called provitamin A carotenoids (pVAC) and are considered the most important for the human diet.5,6
Vitamin A is essential for human health since it supports systemic biological functions such as vision, immunity response, growth, and development.7 Vitamin A has also been associated with a reduced risk of cardiovascular disease, diabetes, and several forms of cancer.8,9 Individuals with vitamin A deficiency (VAD) are typically more susceptible to infections than those with adequate vitamin A levels.10 VAD is the leading cause of blindness in children worldwide;11 it may also cause fatal diseases from severe infections and has been linked to HIV transmission from mother to baby.11 VAD has become a public health problem in more than half of all countries, especially in Africa and South-East Asia.12 To reduce VAD in developing countries, breeders, farmers, and plant and food scientists have been and are still working on vitamin A biofortification in staple crops.10,13
Among staple crops in developing countries, banana has been explored as a target crop for carotenoid biofortification as some banana accessions are a rich source of carotenoids.14 Many cultures from Latin America, the Caribbean, Southeast Asia, Polynesia, and Africa have selected banana landraces and local varieties best adapted to their needs, making it a staple in their diets.15 Indeed, eighty-seven percent of the bananas grown worldwide are local varieties and landraces adapted in the communities where they are produced.16 Through the research done on carotenoid biofortification programs in bananas, carotenoid content, profile, and genes involved in their biosynthesis have been extensively studied.8,17 The most abundant carotenoids in bananas are α-carotene, β-carotene, lutein, β-cryptoxanthin, and zeaxanthin. Also, previous studies have demonstrated that carotenoid accumulation in banana varieties is genotype dependent18,19 and varies across banana germplasm.8
Besides the effect of the genotype on carotenoid content and profile, other factors should be considered when breeding for vitamin A biofortification in bananas. For instance, postharvest treatment plays an important role in the banana supply chain. Indeed, bananas are climacteric fruits, and to avoid spoilage and increase marketability, commercial bananas are harvested unripe. They remain as such until they reach their distribution center, where they are treated with exogenous ethylene to promote ripening.20 Several studies have covered the effect of this controlled ripening system on sugars, starch, soluble solids, chlorophylls, and some market quality traits (texture, flavor, color), but little is known about the effect of the controlled ripening treatment on pVAC content, profile, when compared to fruit ripening on the plant.21–24
In addition to the post-harvest treatment, it is essential to understand what fraction of the carotenoids accumulated in the fruit is, in fact, bioavailable after ingestion. The potential for carotenoids from plant-based foods to have an effect on the human body is contingent on their release from the matrix into the digestive tract and their availability for absorption. This characteristic is commonly referred to as bioaccessibility.25 In contrast, bioavailability is defined as the amount of bioaccessible nutrient (or bioactive) that reaches the tissues and participates in metabolic.26 The amount of bioaccessible carotenoids from a particular food product can be correlated to the amount of carotenoid that becomes bioavailable;27 however, the actual accessibility may be a trait that is independent of content and related more to the digestibility of the food matrix. These factors are crucial in determining the amount of pVAC intake from a particular food product like bananas.28
As previously found in other crops, the genotype and, in some cases, specific chemical components (e.g., fiber content) can directly affect bioaccessibility and/or bioavailability.13,29–31 However, studies on pVAC bioaccessibility in bananas have focused only on the effect of cooking processes, and yet, no study has evaluated the variation in bioaccessibility across multiple genotypes of banana.21,32 Based on this information and as reported in the literature, there is a knowledge gap in understanding bioaccessibility and bioavailability in the context of biofortification programs.33–35
Considerations about the bioavailability and bioaccessibility of pVAC in relation to genotypes and post-harvest treatments are essential to establish goals for banana vitamin A biofortification programs.8 Working toward these research goals, this study aimed to characterize 27 edible banana accessions to evaluate the impact of the genotype, ripening stages, and treatment (using natural and controlled ripening treatments) on carotenoid content and bioaccessibility, establishing their potential nutritional value.
2 Materials and methods
2.1 Plant materials and sample collection
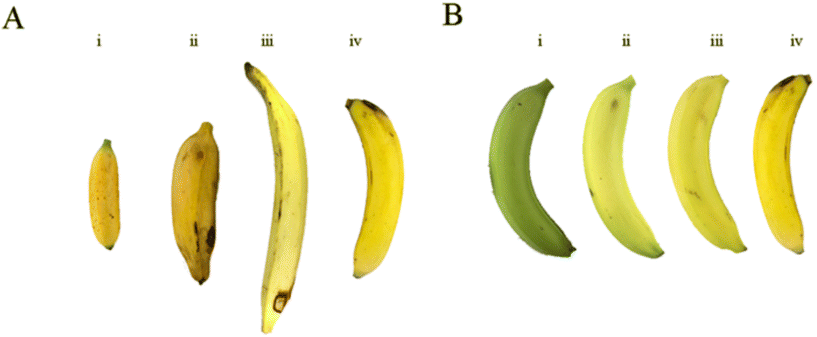
Twenty-seven banana accessions or genotypes, which included cultivars and landraces, were collected from the Dole Food Company's banana collection in Rio Frio, Costa Rica (10°18′29.9′′ North and 83°52′59, 6′′) (Table A.1†). The 27 accessions were all edible banana types. They represented the three major banana market types, including the dessert banana, cooking banana, and plantain banana. Besides the market category, accessions were also selected to represent different pulp colors (white, yellow, and orange), flavors and tastes, ploidy levels (2×, 3×, 4×), and other plant characteristics (e.g., resistance to diseases).Banana samples were collected at multiple ripening stages (from unripe to fully ripe) and ripened under natural or controlled conditions. The ripening scale developed by Soltani (2011) was modified to identify the appropriate stages for sampling (Fig. 1B) and to account for the difference in color and texture across all accessions included in this study. The Soltani scale that ranges from 1–7 ripening stages was simplified into three stages, A = unripe (deep to light green, Soltani scale 1–2), B = semi-ripe (break in color and texture, Soltani scale 3–5), and C = fully ripe (no green color visible, Soltani scale 6–7). Samples at stage B were only collected from fruit ripened in controlled conditions. In contrast, samples at stage C were collected from bananas ripened in controlled conditions (Ccc) and fully ripe naturally on the plant (Cnc). Fruits were collected from three biological replicates for each accession, totaling 324 samples.
For ripening under controlled conditions, unripe green banana fruits (A) were harvested and stored in a ripening chamber at 14.5 °C at 85–90% relative humidity. The temperature was raised to 17 °C for 24 h to initiate the ripening process. Then ethylene was applied by employing a catalytic generator with 1 L of technical grade ethylene at 95% for 24 h. Following the ethylene treatment, the room was ventilated for 20–30 minutes, and the fruit was held at 17 °C for two days, at 15.5 °C for one day, and finally at 14.5 °C for one day or two days, depending on the ripening stage. Semi-ripe bananas (B) were sampled during this treatment by visually inspecting the ripening stage. Fully ripe bananas (C) were collected at the end of the ripening treatment when all fruits were visually inspected to ensure they were meeting the classification criteria for stage C. The banana fruits were evaluated visually and collected from the plants at the fully ripened stage (Ccn) for natural ripening.
2.2 Sample preparation
Banana fruits from each biological replicate were peeled, and the pulp was sliced and immediately frozen in liquid nitrogen to avoid oxidation. Frozen samples were stored at −80 °C for approximately 12 h and then lyophilized for five days (Labconco Freezone 2.5L Freeze Dry System). The lyophilizer was light-sealed to avoid phytochemical oxidation and degradation, and each sample was placed into Mylar bags. Dried samples were stored in light-protected Falcon tubes at −80 °C until processed. The complete set of accessions (N = 27) collected from all the conditions was used for carotenoid content analysis, and a subset of accessions (N = 11) collected at the ripening stage (Ccn and Ccc) was used for bioaccessibility and starch content analysis, as described below.2.3 Carotenoid extraction
All samples were processed under yellow lights to minimize the potential for photodegradation of carotenoids. An aliquot of 50 mg of lyophilized banana powder was dispersed in 1 ml of chilled methanol to create a slurry, and 100 μL of β-apo-8′-carotenal (10 μg mL−1) was spiked into the slurry as an internal standard. Samples were sonicated for 5 minutes, then placed on ice for 10 minutes, before extraction with 5 ml of acetone: petroleum ether (0.1% BHT) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). A total of 3 sequential extracts were combined and dried under nitrogen gas, re-solubilized in 300 μL of ethyl acetate: methanol (1
3). A total of 3 sequential extracts were combined and dried under nitrogen gas, re-solubilized in 300 μL of ethyl acetate: methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), filtered using a 0.45 μm cellulose acetate filter in preparation for analysis by liquid chromatography (LC) with photodiode array detection (LC-PDA).31
4), filtered using a 0.45 μm cellulose acetate filter in preparation for analysis by liquid chromatography (LC) with photodiode array detection (LC-PDA).31
2.4 Carotenoid analysis
Carotenoid analysis was performed by LC-PDA using a Waters Alliance 2695 Liquid Chromatography system (Waters, Milford, MA, USA) equipped with a model 2998 Photo Diode Array Detector (Milford, MA, USA). Carotenoids separation was performed using a YMC C30 column (3 μm 150 mm × 2 mm) thermostated at 35 °C and a gradient elution method, 17-minute run consisting of: (1) 95% solvent A (methanol: ammonium acetate, adjusted to pH 4.6, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 5% solvent B (ethyl acetate) for three minutes; (2) 85% solvent A and 15% solvent B for five minutes; (3) 20% solvent A and 80% solvent B for one minute; (4) 100% solvent B for four minutes; and (5) 95% solvent A and 5% solvent B. The flow rate was 0.37 mL min−1, with an injection volume of 10 μl. External standards of reference materials were run daily, which ensured inter-day consistency of the LC measurements. All compounds of interest, including the main banana carotenoids lutein, β-carotene, α-carotene, 13-cis-α-carotene, and 13-cis-β-carotene, were quantified at 450 nm. All carotenoids were quantified using calibration curves developed for each standard in the concentration range of 0.01–7.5 μM.31
2) and 5% solvent B (ethyl acetate) for three minutes; (2) 85% solvent A and 15% solvent B for five minutes; (3) 20% solvent A and 80% solvent B for one minute; (4) 100% solvent B for four minutes; and (5) 95% solvent A and 5% solvent B. The flow rate was 0.37 mL min−1, with an injection volume of 10 μl. External standards of reference materials were run daily, which ensured inter-day consistency of the LC measurements. All compounds of interest, including the main banana carotenoids lutein, β-carotene, α-carotene, 13-cis-α-carotene, and 13-cis-β-carotene, were quantified at 450 nm. All carotenoids were quantified using calibration curves developed for each standard in the concentration range of 0.01–7.5 μM.31
2.5 Carotenoid bioaccessibility
Ten banana accessions with a total carotenoid content >1mg per 100 g of dry weight representing the fully ripened fruit samples (natural and controlled conditions), plus Cavendish (Landrace Ecuadorian Dwarf – Super Green), were selected to evaluate carotenoid bioaccessibility. This analysis was performed using a three-phase, high-throughput digestion method adapted from Hayes et al. (2020)31 that uses a Tecan Freedom EVO liquid handling robot (Tecan Group Ltd, Männedorf, Switzerland) to automate all the liquid handling steps during the protocol.Briefly, 150 mg of lyophilized banana fruits were dispersed in 5 mL of boiling water, and 5% (w/w) canola oil was added to facilitate micellization of carotenoids and the digestion process.
The digestion consisted of three phases: (1) the oral phase, where the banana solution was incubated for 10 min at 37 °C, 120 rpm with α-amylase (10 units per mg); (2) the gastric phase where the oral phase solution was incubated 60 min at 37 °C, 120 rpm with of 0.6 mL of pepsin (final concentration of 0.4 g L−1) and adjusted to pH 2.5 ± 0.1; (3) the intestinal phase, where the gastric phase solution was incubated for 120 min at 37 °C, 120 rpm with 0.6 mL of pancreatin-lipase solution (final concentration of 0.8 g L−1 for both pancreatin and lipase) and 0.9 mL of bile solution (final concentration of 1.8 g L−1) and adjusted to pH 7.0 ± 0.1.
After digestion, an aliquot of the final digesta was transferred and stored at −80 °C, and a second aliquot of digesta was centrifuged (Beckman Coulter, Allegra X-30R Centrifuge, Indianapolis, IN, USA) at 4255g for 60 min. The supernatant was collected and filtered through a 0.2 μm cellulose acetate filter to isolate the fraction containing the micellarized carotenoids (aqueous fraction). As described above, 1 mL of aqueous and digesta fraction was used for carotenoid extraction and analysis (section 2.3–2.4). Extraction recovery of aqueous and digesta was 97% ± 3.05%, as determined by using trans-β-apo-8′-carotenal spiked into samples.
2.6 Starch analyses
Starch content was evaluated on the 11 accessions used for carotenoid bioaccessibility to assess the possible impact of starch on carotenoid bioaccessibility analysis. Megazyme's K-RSTAR 05/19 kit was used for measuring starch (McClearly et al., 2019; Englyst et al., 1992). Approximately 100 mg of lyophilized banana powder was dispersed in a 4 mL solution of sodium maleate (100 mM) with pancreatic α-amylase (10 mg mL−1) and amyloglucosidase (AMG) (3U mL−1) and incubated for 16 h at 37 °C with continuous lateral shaking. The reaction was stopped by adding an equal volume of 100% ethanol, and the resistant starch was recovered by centrifugation at 1800g for 10 minutes. The pellet was washed twice by adding 8 mL of 50% ethanol. All the supernatants from this step were collected and diluted to 100 mL with sodium maleate buffer (100 mM) to measure soluble starch.The pellet was dissolved in 2 mL of KOH solution (2M) by continuously stirring in a cold room at 3 °C for 20 min. Afterward, it was neutralized with 8 mL of NaOAc buffer (1.2 M) at room temperature. The resistant starch was quantitatively hydrolyzed to D-glucose with 0.1 mL of AMG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3300 U mL−1) at 50 °C for 30 min. Both resistant and soluble starch were measured as D-glucose by the reaction of glucose oxidase/peroxidase reagent (GOPOD) in a microplate reader at 510 nM.36
3300 U mL−1) at 50 °C for 30 min. Both resistant and soluble starch were measured as D-glucose by the reaction of glucose oxidase/peroxidase reagent (GOPOD) in a microplate reader at 510 nM.36
2.7 Data analyses
The data used for statistical analysis included three biological replicates for each accession, ripening stage, and ripening treatment. The results are presented as the mean ± standard error.Total carotenoid content (TCC) was calculated as the sum of individual lutein, β-carotene, α-carotene, 13-cis-α-carotene, and 13-cis-β-carotene as determined by LC analysis. Relative bioaccessibility was calculated as the ratio of a particular carotenoid or total carotenoids from the aqueous phase to their concentration in the crude digesta. Absolute bioaccessibility was calculated as the relative bioaccessibility expressed as a percentage multiplied by total carotenoid content and expressed as mg per 100 g. All carotenoid concentration data were calculated per 100 g of banana (dry weight, DW.)
Formula:
The recommended dietary allowances for Vitamin A were estimated as Retinol Equivalence (RAE). For the purposes of this study, we utilized a conversion of one mg of RAE, which is equivalent to 12 mg of β-carotene and 24 mg for all other dietary pVAC (α-carotene and β-cryptoxanthin) (FNB, 2001).
All statistical analyses were performed using the software R Studio,37,38 and results were plotted using the package ggplot2.39 Means were compared using a one-way analysis of variance (ANOVA) to determine the significant difference (α < 0.05) among accessions and ripening stages. In contrast, a paired t-test was used to determine any significant difference between ripening methods (α < 0.05).
3 Results
3.1 Carotenoid content
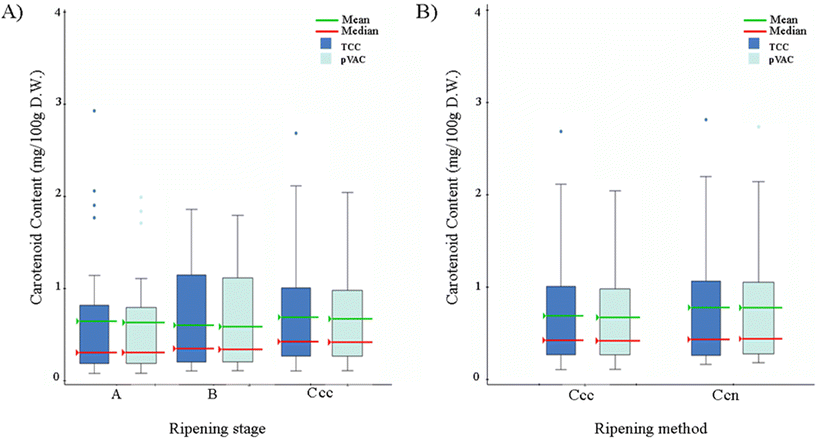
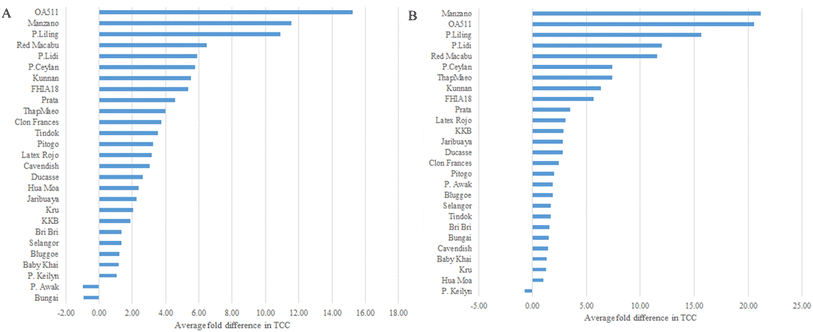
Individual carotenoid species, lutein, β-carotene, α-carotene, 13-cis-α-carotene, and 13-cis-β-carotene were measured and detected in all 27 banana accessions, three ripening stages (A, B, C), and two ripening methods, fully ripened under controlled conditions (Ccc) and fully ripened naturally on the plant (Ccn) (Fig. 1).Total carotenoid content (TCC), measured as the sum of the individual carotenoids detected here, ranged from 0.018–1.497 mg per 100 g in unripe banana (Stage A) (Table A.2 and Fig. A.1†) and 0.042–2.68 mg per 100 g in semi-ripe banana (stage B) (Table A.3 and Fig. A.1†). TCC varied from 0.201 to 5.29 mg per 100 g in samples ripened under controlled conditions (Stage Ccc) (Table A.4 and Fig. A.1†), and from 0.241 to 2.542 mg per 100 g in samples ripened on the plant (Stage Ccn) (Table A.5 and Fig. A.1†). The accessions with the highest TCC were Tindok and Kru, collected at stage Ccc. These samples had 2 to 7-fold higher carotenoid content than Cavendish (Fig. A.1†). Based on the TCC and pVAC aggregate average; there were no significant differences among the ripening methods or stages, where carotenoid content was accession-dependent (ANOVA p < 0.05) (Fig. 2).
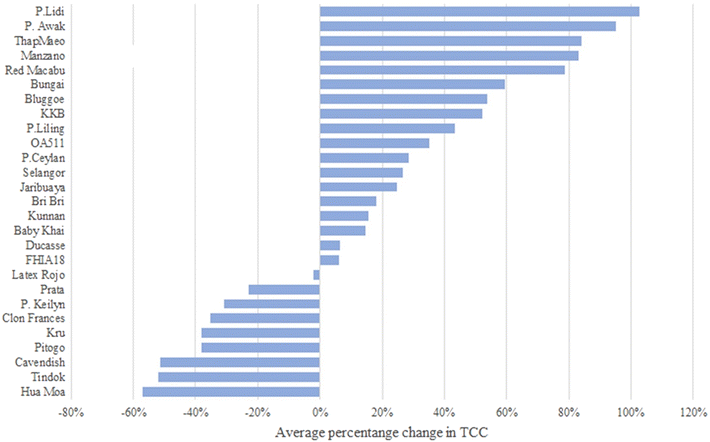
In most of the accessions (N = 26), TCC from unripe (stage A) to fully ripe fruits (stage Ccc) increased (Fig. 3). Tindok, the accession with the highest TCC in all conditions, had a 3.5-fold (3.79 mg per 100 g) increase in TCC from stage A to Ccc. In accessions such as Bungai, P. Awak, P. Keilyn, Selangor, and Bluggoe, TCC showed low variance during the maturation process. A similar trend was observed for the comparison with stage Ccn where P. Keilyn showed a slight decrease, and the remaining 26 accessions showed an increase in TCC (Fig. 3 and Table A.6†). Comparison of naturally ripened vs. ripened under controlled conditions indicated that TCC was lower in controlled conditions for 18 accessions and higher for nine accessions. The differences in TCC content between the Ccc and Ccn stages ranged from a decrease in Tindok (2.75 ± 0.24 mg per 100 g) to an increase in KKB (0.7 ± 0.15 mg per 100 g) (Fig. 4 and Table A.6†).
Across all conditions, carotenoid profiles were significantly different among the 27 accessions (ANOVA p < 0.005). The average carotenoid profile was 22% lutein, 30% α-carotene, and 48% β-carotene, which indicates that about 78% of carotenoids are pVAC (Tables A.2–A.5 and Fig. A.1†). Accessions with a high content of β-carotene had lower lutein content and vice versa (Fig. A.1†). However, no significant correlation was observed between TCC and carotenoid composition. When comparing accessions at all ripening stages and methods, the average α-carotenoid and lutein content were not significantly different (ANOVA, p < 0.05). Similar results were obtained for the average β-carotenoid content between stages A, B, and Ccc. In contrast, the average β-carotene content was significantly higher in the samples fully ripened under controlled conditions (Stage Ccc) than in the samples fully ripened on the plant (Stage Ccn) (paired t-test, p < 0.05) (Fig. A.1†).
The 27 accessions were further divided by market type (cooking, dessert, and plantains), and the effects of natural or controlled ripening over carotenoid accumulation were examined. For fully ripe bananas (Ccc and Ccn), the plantain market type had the highest average TCC (2.44 ± 0.40 mg per 100 g at stage Ccc), followed by dessert bananas (0.75 ± 0.09 mg per 100 g at stage Ccn); the cooking market type bananas had the lowest average amount of TCC (0.45 ± 0.07 mg per 100 g at stage Ccn). Overall, plantains at stage C (Ccc and Ccn) samples had a significantly higher TCC (ANOVA p < 0.05) than the rest of the accessions (Fig. A.2†).
3.2 Carotenoid bioaccessibility
| Ripening Stage | Accession Name | Relative bioaccessibility (%) | Bioaccessible content (mg per 100g D.W.) | Bioaccessible content (RAE mg per 100g D.W.) | %DV Standard portiona | %DV Single portionb | ||
|---|---|---|---|---|---|---|---|---|
| *Letters next to the numbers indicate Tukey's HSD (Honest Significant Difference) grouping.a Percent daily values (%DV) were based on FDA recommended DV for Vitamin A (0.9 mg RAE) on a standard portion of a medium banana (Cavendish, 126 g) for a 2000 calories diet, assuming that all the bioaccessible carotenoid content is bioavailable.40.b Percent daily values (%DV) were based on FDA-recommended DV for Vitamin A (0.9 mg RAE) on a single portion (1 banana) for a 2000-calorie diet, assuming that all the bioaccessible carotenoid content is bioavailable.40. | ||||||||
| Ccc | Baby Khai | 18.98 ± 4.50 | bc | 0.27 ± 0.10 | abc | 0.082 ± 0.010 | 4 | 2 |
| Bri Bri | 34.68 ± 0.45 | a | 0.57 ± 0.17 | ab | 0.110 ± 0.028 | 4 | 2 | |
| Cavendish | 29.79 ± 3.91 | ab | 0.04 ± 0.02 | bc | 0.034 ± 0.004 | 1 | 1 | |
| Hua Moa | 15.89 ± 1.63 | c | 0.56 ± 0.09 | abc | 0.167 ± 0.004 | 7 | 18 | |
| KKB | 25.35 ± 3.61 | abc | 0.22 ± 0.02 | abc | 0.087 ± 0.002 | 3 | 3 | |
| Kru | 20.75 ± 3.14 | bc | 0.77 ± 0.05 | a | 0.191 ± 0.022 | 6 | 5 | |
| Latex Rojo | 21.44 ± 1.37 | abc | 0.27 ± 0.01 | abc | 0.074 ± 0.007 | 3 | 2 | |
| Pitogo | 18.37 ± 1.26 | bc | 0.46 ± 0.06 | abc | 0.172 ± 0.025 | 8 | 3 | |
| Prata | 18.04 ± 1.47 | bc | 0.24 ± 0.02 | bc | 0.065 ± 0.001 | 2 | 1 | |
| Red Macabu | 13.14 ± 2.60 | c | 0.14 ± 0.03 | c | 0.045 ± 0.005 | 2 | 2 | |
| Tindok | 25.53 ± 9.33 | c | 0.56 ± 0.11 | a | 0.347 ± 0.013 | 17 | 54 | |
| Ccn | Baby Khai | 17.57 ± 2.77 | a | 0.25 ± 0.01 | bc | 0.099 ± 0.015 | 4 | 5 |
| Bri Bri | 32.96 ± 6.76 | ab | 0.62 ± 0.08 | a | 0.129 ± 0.008 | 5 | 4 | |
| Cavendish | 32.69 ± 7.21 | a | 0.03 ± 0.01 | c | 0.018 ± 0.001 | 1 | 1 | |
| Hua Moa | 9.89 ± 6.06 | b | 0.32 ± 0.09 | c | 0.071 ± 0.002 | 3 | 11 | |
| KKB | 13.07 ± 0.65 | ab | 0.22 ± 0.13 | bc | 0.138 ± 0.004 | 4 | 9 | |
| Kru | 16.21 ± 1.65 | ab | 0.27 ± 0.01 | b | 0.119 ± 0.007 | 3 | 4 | |
| Latex Rojo | 7.82 ± 1.26 | b | 0.21 ± 0.04 | c | 0.072 ± 0.009 | 3 | 3 | |
| Pitogo | 16.96 ± 1.56 | ab | 0.11 ± 0.02 | bc | 0.103 ± 0.007 | 5 | 2 | |
| Prata | 18.04 ± 1.47 | ab | 0.16 ± 0.04 | bc | 0.048 ± 0.005 | 2 | 1 | |
| Red Macabu | 10.53 ± 2.13 | b | 0.29 ± 0.18 | bc | 0.089 ± 0.022 | 3 | 6 | |
| Tindok | 43.02 ± 1.59 | b | 0.23 ± 0.03 | bc | 0.163 ± 0.013 | 8 | 29 | |
Lutein had the highest average relative bioaccessibility (23.50%± 1.55), followed by α-carotene (18.60%± 1.63) and β-carotene (16.40%± 1.48). Analysis of variance showed significant differences between accessions and a significant interaction between accessions and ripening stages (p < 0.05).
The ripening method had a significant effect on relative bioaccessibility (paired t-test, p < 0.05). Banana samples at stage Ccn had a slightly lower average relative bioaccessibility (18% ± 1.80) than the corresponding samples ripened with exogenous ethylene (stage Ccc, 21% ± 1.32) (Table 1).
When accessions were divided by carotenoid content, banana accessions with the highest content (Tindok, Pitogo, Hua moa, Bri Bri, and Kru) at stage Ccc had a significantly higher relative bioaccessibility than the corresponding samples at stage Ccn (paired T-test p < 0.05). In contrast, for the accessions with the lowest TCC (KBB, Prata, Latex Rojo, Baby Khay, and Red Macabu), ripening conditions did not have a significant effect on relative bioaccessibility (paired T-test p = 0.14) (Table 1).
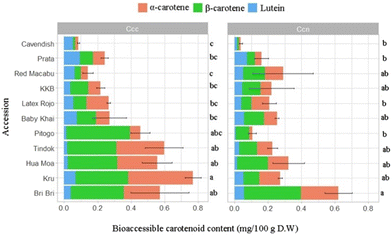
Bioaccessible content measured as RAE ranged from 0.018 ± 0.01 mg per 100 g for Cavendish at stage Ccn to 0.347 ± 0.0.013 mg per 100 g for Tindok at stage Ccc. Total bioaccessible carotenoid content differed among accessions (ANOVA, p < 0.05), and the ripening treatment and the accession interaction were significant (ANOVA, p < 0.005). β-carotene bioaccessible content (ANOVA, P < 0.05) also differed, ranging from 0.043 ± 0.0.028 mg per 100 g in Cavendish (stage Ccc) to 0.248 ± 0.0.013 mg per 100 g in Bri Bri (stage Ccc) (Table 1).
When individual carotenoids were compared across ripening conditions (stages Ccc and Ccn), accessions showed significant differences in bioaccessible content for α-carotene and β-carotene (paired t-test, p < 0.05) but not for lutein (Fig. 5).
The ripening method significantly affected the carotenoid bioaccessible content. Accessions ripened with exogenous ethylene (stage Ccc) had a significantly higher carotenoid bioaccessible content than accessions ripened on the plant (stage Ccn) (paired t-test p < 0.05) (Table 1). As observed for the relative bioaccessibility data, there were differences in bioaccessible content when accessions with the highest and the lowest total carotenoid content were compared by the ripening method (stages Ccc and Ccn). Indeed, considering the five accessions with the highest carotenoid content (Tindok, Pitogo, Hua moa, Bri Bri, and Kru), the Ccn samples had a significantly lower carotenoid bioaccessible content than the Ccc samples (paired T-test p < 0.05). For the accessions with the lowest carotenoid content (KBB, Prata, Latex Rojo, Baby Khai, Red Macabu, and Cavendish), the carotenoid bioaccessible content of the samples ripened in the two conditions (Ccc vs. Ccn) was not significantly different (paired T-test p = 0.23) (Table 1).
3.3 Starch content
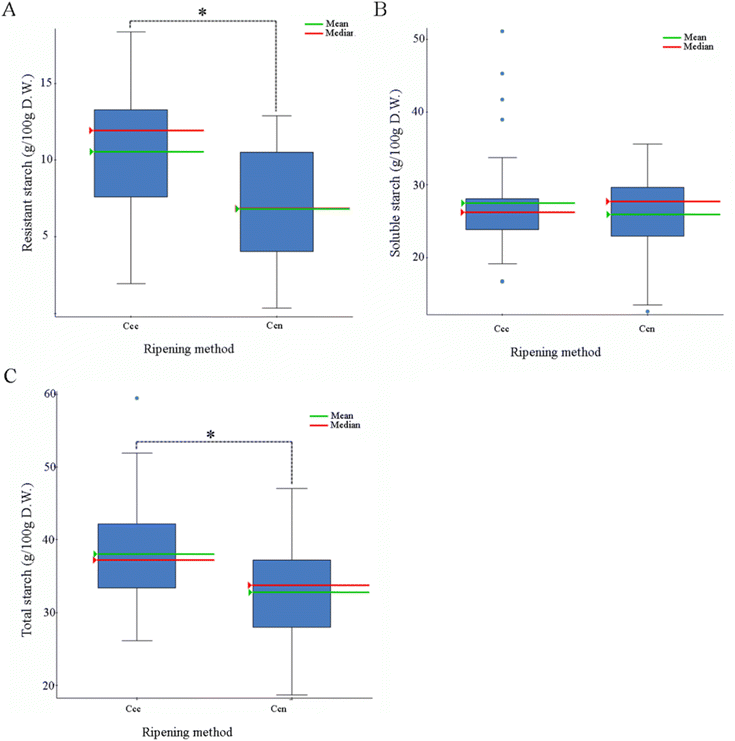
Dietary fiber and starch play crucial roles in the bioaccessibility and bioavailability of nutrients within the human body. Both components contribute to the overall digestion and absorption processes, affecting the release and utilization of various nutrients (Palafox-Carlos & Gonzalez-Aguilar, 2011).Total starch, resistant starch, and soluble starch content were evaluated to assess their effect on carotenoid bioaccessibility. Red Macabu had the lowest total starch content (Ccc = 28.24 ± 1.8 gper 100 g; Ccn = 19.53 ± 0.81 g per 100 g), and Baby Khai had the highest starch content (Ccc = 49.51 ± 6.54 g per 100 g; Ccn = 39.18 ± 2.34 g per 100 g). In Cavendish, the total starch content was 38.11 ± 0.78 g per 100 g at stage Ccc and 31.63 ± 2.16 g per 100 g at stage Ccn (Fig. A.3†).
Resistant starch, a main fiber component in banana, represented about 25% of the starch content in all banana samples evaluated; Its value ranged from 0.49 g per 100 g (2% of total starch) at stage Ccn in Latex Rojo to 14.09 g per 100 g (44% of total starch) at stage Ccc in Pitogo. The resistant starch content in Cavendish was 13.56 ± 0.36 g per 100 g at stage Ccc and 5.49 ± 0.79 g per 100 g at stage Ccn (25% of total starch) (Fig. 6 and Fig. A.3†).
When analyzed by starch type (total, soluble, and resistant starch), accessions showed significant differences across ripening conditions (stages Ccc and Ccn) for total and resistant starch (ANOVA, p < 0.05), with an average decrease of 3.73 g per 100 g of resistant starch, and 4.96 g per 100 g of total starch in natural ripening (Ccn). No significant differences were observed for soluble starch (Fig. 6).
Resistant starch content has a significantly positive correlation with total bioaccessible carotenoid content (R2 = 0.20, p < 0.05) and bioaccessible β-carotene content (R2 = 0.25, p < 0.05). No other significant correlations were found between starch content and relative bioaccessibility.
4 Discussion
4.1 Carotenoid content is affected by ripening stages and ripening method
Fruit ripening is defined by four significant physiological changes: color (due to the loss of chlorophyll and the gain of carotenoids and anthocyanins); texture (softening of the cell wall); flavor (an increase in sugars and/or accompanied by decreased organic acids); and smell (production of volatile compounds).41 These physiological changes and the conditions under this process can directly or indirectly affect carotenoid content and profile.20Across all comparisons between green fruit (stage A) vs. ripened fruit (Ccn and Ccc) for most of the accessions, an increase of TCC from unripe (stage A) to fully ripe fruits (stage Ccc and Ccn) was observed, and in only two accessions, TCC marginally decreased. With few exceptions, genotypes with lower TCC also had the slightest TCC increase from the unripe to the ripe stage. These results agree with previous banana studies,42 as is the case with other fruit that does not stay green when ripe. The reduction of chlorophyll and the increase in carotenoids during ripening is an important physiological process coordinated by a network of genes that activate carotenoid accumulation.43
When comparing ripening methods (Ccc and Ccn), 18 accessions had lower TCC in the Ccn than in the Ccc samples. In contrast, TCC was higher in the Ccn samples than in the Ccc samples in nine accessions, suggesting that treating bananas with exogenous ethylene might directly affect TCC accumulation. Previous studies have indicated that the application of exogenous ethylene directly affects metabolite accumulation in bananas including sugars, vitamin C, organic acids, volatile compounds, and minerals.44–46 Two studies evaluated the effect of exogenous ethylene on carotenoids. Hakim (2012)47 concluded that bananas, tomatoes, and pineapple treated with ethephon (exogenous ethylene) had lowered carotenoid content when compared with the non-treated group. Similarly, Maduwanthi (2021)46 found bananas treated with ethephon and acetylene had significantly lower levels of total carotenoids in peel and flesh when compared to the non-treated groups. The two studies used analogous maturation conditions to the ones used in this study; however, those studies used only one type of banana, and neither study reported which cultivar or accession was used for the experiments.
In contrast with these observations in banana, previous studies in tomatoes have found that postharvest ripened fruits accumulated more carotenoids than those ripened on the plant.48 One of the keyways ethylene influences carotenoid accumulation in climacteric fruits is by upregulating the expression of genes encoding enzymes involved in carotenoid biosynthesis, such as phytoene synthase, ζ-carotene desaturase, and β-carotene hydroxylase, which are responsible for the initial steps in carotenoid synthesis.49 This upregulation of gene expression can enhance the production of carotenoids in plant tissues. Thus, the application of exogenous ethylene has the potential to accelerate the carotenoid biosynthesis naturally occurring during maturation.50
At the same time, Rodrigo & Zacarias (2007)50 also observed that fruit protected against UVB radiation on the field showed a higher β-carotene accumulation, suggesting a particular sensitivity of lycopene β-cyclase to UV-B radiation. Becatti et al. (2009)51 reported that TCC and carotenoid profiles in tomatoes were controlled by ethylene concentration (endogenous and exogenous) and UV-B radiation. The latter influenced carotenoid metabolism either in an ethylene-dependent or -independent way, acting antagonistically.
Overall, compared with previous studies on banana, our study evaluated a relatively large sample of banana accessions and indicated that while ethylene treatment has a significant effect on TCC accumulation, this effect is genotype-dependent, which has been previously suggested as a plausible mechanism to explain the influence of ethylene in the carotenoid pathway in other climacteric crops like apricots,49 and tomatoes.52
4.2 Carotenoid bioaccessibility varies across genotypes and ripening conditions
Bioaccessibility, which measures the amount of carotenoids released from the food matrix into the gut lumen by normal digestion, directly affects the amount of those components available for absorption and, by extension, their bioavailability and potential health benefits. Carotenoid bioaccessibility is a predictor of bioavailability in humans,53 and as an in vitro technique, by virtue, has a lower cost and higher throughput to measure, it is a valuable model for studying broad variation in bioavailable nutrients and phytochemicals in plant matrices. Bioaccessibility and bioavailability of pVAC in biofortified food have been extensively reviewed for many crops.33,54–56 However, despite the multiple efforts on carotenoid biofortification in banana, only one study evaluated carotenoid bioaccessibility in banana and focused on the effect of cooking recipes21 and not on the effect of the genotypes.35 Most of the carotenoid studies in banana focused on characterizing carotenoid content, profile, and genetics, but no study evaluated the variation of carotenoid bioaccessibility in fresh fruits from different genotypes and the effect of the ripening method. Recent studies in other crops, like spinach31 and cassava,57 demonstrated that variation in carotenoid bioaccessibility exists across genotypes.Here, we measured the bioaccessibility of 11 banana accessions at the fully ripened stage and two ripening methods (Ccc and Ccn), in which its carotenoid content was higher than 1 mg per 100 g DW. The relative bioaccessibility of TCC varied among accessions. Interestingly, no correlation between TCC and relative bioaccessibility was found, suggesting that the release of carotenoids from the food matrix is genotype-dependent and unrelated to the carotenoid content. Overall, the range of relative carotenoid bioaccessibility ranged from 6% to 34%, which is within previously reported ranges in banana (boiled banana, 10–41%)21 and in other crops like spinach.31 Lutein had the highest relative bioaccessibility, which might be due to its high concentration relative to other carotenoids58 and that its physical deposition within the food matrix is different from other carotenoids, which have been previously assumed to exert a significant influence on the bioavailability.59
The ripening method was found to have a significant effect on relative bioaccessibility and bioaccessible content. Bananas ripened on the plant (stage Ccn) had a lower average relative bioaccessibility and bioaccessible TCC than the corresponding samples ripened with exogenous ethylene. Note that the four accessions (Tindok, Pitogo, Hua Mao, and Kru) with the highest carotenoid bioaccessibility were the same, with the highest increase in TCC in the controlled condition. The higher accumulation of TCC in these accessions under controlled conditions likely explains the higher absolute carotenoid bioaccessibility.
According to the FDA (2017)40 and their guidelines for a healthy diet, an average adult must consume at least 0.9 mg RAE of Vitamin A per day (Daily Value, DV). In their calculations, one portion of banana (based on a Cavendish banana with an average weight of 126 g) contributes 2% of the DV for this vitamin. However, it is not specified if such an amount is calculated using bioaccessible content. Based on bioaccessible TCC, our study found that one portion of Cavendish banana, one of the most consumed worldwide, has 1% of DV at stage C, regardless of the ripening method. In contrast, pVACs in the same size portion of Tindok has 17% DV at stage Ccc and 8% DV at stage Ccn. Leafy greens are considered one of the main dietary sources of β-carotene with a content that ranges from 0.46 to 6.4 mg per 100 g of fresh weight, while in fruit like orange, cherry, peach, apple, and pear, β-carotene ranges from 0.010–0.17 mg per 100 g fresh weight.57 In comparison, in Tindok, β-carotene content ranges from 0.42 to 1 mg per 100 g of fresh weight, and in Cavendish, it ranges from 0.07 to 0.09 mg per 100 g of fresh weight (Tables A.4 and A.5†). These results highlight that depending on the genotype, β-carotene content can reach value that approach those on the lower end of leafy green. Considering the high intake of banana and frequency of consumption these levels could represent an important path to enhance provitamin A intake from fruit and vegetables.60,61
At the same time, one whole, Tindok banana (average weight 326 g) could provide up to 54% of vitamin A DV, which could make a difference in countries where banana is a staple fruit and vegetables and fruits with high pVAC content are hard to find or expensive to buy.
For Tindok, Pitogo, Kru, and Hua Moa, which have the highest TCC at stage Ccc, the Percent Daily Value (%DV) for a standard portion was lower in the samples ripened on the tree (Ccn) when compared to those ripened with exogenous ethylene (Ccc). For the remaining accessions, the %DV for a standard portion was the same or slightly different (>1% difference) for both maturation methods. The data suggest that this trait is also accession-dependent.
Relative bioaccessibility has been inversely correlated with the fiber content of food.62 High pectin concentration and other dietary fibers such as cellulose, guar, and alginate significantly reduce carotenoid micellization by increasing intestinal content viscosity.32 Furthermore, dietary fiber and resistant starch can influence the bioaccessibility of nutrients by forming complexes with them during digestion.63 These complexes can protect nutrients from degradation by digestive enzymes and promote their release for absorption in the small intestine.64 This mechanism has been observed with lipophilic compounds such as carotenoids, where dietary fiber and resistant starch may improve their solubility and transport across the intestinal membrane.65
For this reason, our study measured the amount of resistant starch and soluble starch content and their effect on carotenoid bioaccessibility. On average, resistant starch represented about 25% of the starch content in all banana accessions. While resistant starch showed significant differences according to maturation stage (Ccc and Ccn), soluble starch did not; both types of starch are accession dependent. Resistant starch content was significantly correlated with total bioaccessible carotenoid content (R2 = 0.20, p < 0.05) (Fig. A.4†) and bioaccessible β-carotene content (R2 = 0.25, p < 0.05). Resistant starch acts as a substrate for colonic fermentation, where it undergoes microbial breakdown, producing short-chain fatty acids (SCFAs).66 These SCFAs create an environment in the colon that promotes the absorption and utilization of carotenoids. SCFAs increase the solubility of carotenoids and enhance their transport across the intestinal epithelium, facilitating their uptake into the bloodstream.67,68 Our findings align with several studies, suggesting an intrinsic correlation between resistant starch and bioaccessible carotenoid content.69–71
Studies on starch content and composition in bananas have found that the starch content, on average, is approximately 65% of the total dry weight, while resistant starch may vary from 37.2% to 79.2%. These studies suggest that starch content and composition are accession-dependent.72–74 A study by Vatanasuchart et al. (2012)73 on 11 banana cultivars grown in Thailand showed that the RS content observed in the common cultivars ranges between 52.2–61.4%, and values for indigenous cultivars are between 50.7–68.1%, most of them being resistant starch type II which has less impact on carotenoids bioaccessibility in comparison to soluble fiber. Our sample's composition and type of resistant starch might explain our results.
5 Conclusions
Our study is the first study to compare the carotenoid and starch content with carotenoid bioaccessibility at multiple ripeness stages and ripening methods in a relatively large set of banana accessions. Our data shows that carotenoid, starch content, and carotenoid bioaccessibility vary across accessions. Our study revealed that the application of exogenous ethylene during the ripening process influences carotenoid and starch accumulation in banana. This, in turn, has profound implications for bioaccessibility across various banana genotypes, thereby changing the Vitamin A intake. This is essential knowledge for successfully translating any banana biofortification effort to the public. This study concludes that the genotype, ripening methods (natural vs. exogenous ethylene application), and carotenoid bioaccessible content should be variables that need to be considered when setting a nutrition goal for vitamin A biofortification in banana breeding programs.Author contributions
M. Iorizzo, M. Ferruzzi, N. Gillit: conceptualization, funding acquisition. B. Munoz, M. Ferruzzi, M. Iorizzo: data curation, formal analysis, methodology, drafting the Paper. B. Munoz, M. Hayes: data curation, investigation. M. Munoz: plant Material. P. Perkins-Veazie, C. Kay, M.A. Lila, N. Gillitt, M. Munoz: editing.Conflicts of interest
Dr. M. Munoz, representing this project's funder (Dole Plc.), was involved in collecting the plant material. Still, his contribution did not influence the experimental design, data analysis, results, interpretation, and conclusion of the work presented here. All other authors declare no competing financial interests or personal relationships that could have appeared to influence the outcomes reported in this paper.Acknowledgements
This research was supported by the Dole Nutrition Institute at Dole Food Company Inc., and M. I. was also supported by the United States Department of Agriculture National Institute of Food and Agriculture, Hatch project 1008691. MF was in part supported by USDA-ARS Project 6026-51000-012-000-D funding at the Arkansas Children's Nutrition Center.References
- M. Eggersdorfer and A. Wyss, Carotenoids in human nutrition and health, Arch. Biochem. Biophys., 2018, 652, 18–26 CrossRef CAS PubMed.
- R. Rühl, et al., Carotenoids and their metabolites are naturally occurring activators of gene expression via the pregnane X receptor, Eur. J. Nutr., 2004, 43, 336–343 CrossRef PubMed.
- A. Nagao, Oxidative conversion of carotenoids to retinoids and other products, J. Nutr., 2004, 134(1), 237S–240S CrossRef CAS PubMed.
- B. J. Burri, M. R. La Frano and C. Zhu, Absorption, metabolism, and functions of β-cryptoxanthin, Nutr. Rev., 2016, 74(2), 69–82 CrossRef PubMed.
- M. W. Davey, et al., Genetic variability in Musa fruit provitamin A carotenoids, lutein and mineral micronutrient contents, Food Chem., 2009, 115(3), 806–813 CrossRef CAS.
- G. F. Combs Jr. and J. P. McClung, The vitamins: fundamental aspects in nutrition and health, Academic press, 2016 Search PubMed.
- Z. Huang, et al., Role of vitamin A in the immune system, J. Clin. Med., 2018, 7(9), 258 CrossRef CAS PubMed.
- D. Amah, et al., Recent advances in banana (Musa spp.) biofortification to alleviate vitamin A deficiency, Crit. Rev. Food Sci. Nutr., 2019, 59(21), 3498–3510 CrossRef CAS PubMed.
- A. G. Murillo and M. L. Fernandez, Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications, Adv. Nutr., 2016, 7(1), 14–24 CrossRef CAS PubMed.
- G. A. Stevens, et al., Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys, Lancet Glob. Health, 2015, 3(9), e528–e536 CrossRef PubMed.
- UNICEF, Vitamin A deficiency in children, 2022 Search PubMed.
- FAO, Micronutrient deficiencies Vitamin A deficiency, 2018 Search PubMed.
- D. Amah, Genetic variability of carotenoids and polypoid induction towards vitamin A biofortification in plantain (Musa spp.), University of the Free State, 2018 Search PubMed.
- A. Amini Khoozani, J. Birch and A. E.-D. A. Bekhit, Production, application and health effects of banana pulp and peel flour in the food industry, J. Food Sci. Technol., 2019, 56, 548–559 CrossRef CAS.
- B. Singh, et al., Bioactive compounds in banana and their associated health benefits–A review, Food Chem., 2016, 206, 1–11 CrossRef CAS.
- FAO, Banana facts and figures, 2020 Search PubMed.
- R. Fungo and M. Pillay, β-Carotene content of selected banana genotypes from Uganda, Afr. J. Biotechnol., 2011, 10(28), 5423–5430 CAS.
- B. Mlalazi, et al., Isolation and functional characterisation of banana phytoene synthase genes as potential cisgenes, Planta, 2012, 236, 1585–1598 CrossRef CAS PubMed.
- S. Buah, et al., The quest for golden bananas: investigating carotenoid regulation in a Fe'i group Musa cultivar, J. Agric. Food Chem., 2016, 64(16), 3176–3185 CrossRef CAS PubMed.
- N. Kaur, et al., CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit, Metab. Eng., 2020, 59, 76–86 CrossRef CAS PubMed.
- B. Ekesa, et al., Bioaccessibility of provitamin A carotenoids in bananas (Musa spp.) and derived dishes in African countries, Food Chem., 2012, 133(4), 1471–1477 CrossRef CAS.
- M. Hailu, T. S. Workneh and D. Belew, Review on postharvest technology of banana fruit, Afr. J. Biotechnol., 2013, 12(7), 635–647 Search PubMed.
- H. Akter, et al., Effects of variety and postharvest treatments on shelf life and quality of banana, Int. J. Environ. Sci. Nat. Resour., 2013, 6(2), 163–175 Search PubMed.
- M. S. Hosseini, et al., Effects of postharvest treatments with chitosan and putrescine to maintain quality and extend shelf–life of two banana cultivars, Food Sci. Nutr., 2018, 6(5), 1328–1337 CrossRef CAS PubMed.
- F. Shahidi and H. Peng, Bioaccessibility and bioavailability of phenolic compounds, J. Food Bioact., 2018, 4, 11–68 Search PubMed.
- D. B. Rodrigues, et al., Trust your gut: Bioavailability and bioaccessibility of dietary compounds, Curr. Res. Food Sci., 2022, 5, 228–233 CrossRef CAS PubMed.
- C. Galanakis, What is the difference between bioavailability bioaccessibility and bioactivity of food components, SciTech Connect, 2017, Available online: https://scitechconnect.elsevier.com/bioavailabilitybioaccessibility-bioactivity-food-components/(accessed on 27 April 2017) Search PubMed.
- C. Dima, et al., Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods, Compr. Rev. Food Sci. Food Saf., 2020, 19(6), 2862–2884 CrossRef PubMed.
- C. Agostoni, et al., Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre, EFSA J., 2010, 8(3), 1462 Search PubMed.
- J. Díaz-Gómez, et al., Biofortification of crops with nutrients: factors affecting utilization and storage, Curr. Opin. Biotechnol., 2017, 44, 115–123 CrossRef PubMed.
- M. Hayes, et al., In vitro bioaccessibility of carotenoids and chlorophylls in a diverse collection of spinach accessions and commercial cultivars, J. Agric. Food Chem., 2020, 68(11), 3495–3505 CrossRef CAS PubMed.
- R. M. Schweiggert, et al., Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato, Food Chem., 2012, 135(4), 2736–2742 CrossRef CAS PubMed.
- R. E. Kopec and M. L. Failla, Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles, J. Food Compos. Anal., 2018, 68, 16–30 CrossRef CAS.
- S. L. Huey, et al., Bioaccessibility and bioavailability of biofortified food and food products: Current evidence, Crit. Rev. Food Sci. Nutr., 2022, 1–23 CrossRef PubMed.
- J. Low, et al., Scaling readiness of biofortified root, tuber, and banana crops for Africa, in Root, tuber and banana food system innovations, 2022 Search PubMed.
- B. V. McCleary, et al., Measurement of available carbohydrates, digestible, and resistant starch in food ingredients and products, Cereal Chem., 2020, 97(1), 114–137 CrossRef CAS.
- R. Team, RStudio: Integrated Development for R, RStudio, Boston, 2020 Search PubMed.
- R. C. Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, 2021 Search PubMed.
- W. Hadley, ggplot2: Elegant Graphics for Data Analysis. 2016 Search PubMed.
- FDA, Fruits Nutrition Facts Raw, Edible Weight Portion Percent Daily Values (%DV). 2017 Search PubMed.
- S. Osorio, F. Scossa and A. R. Fernie, Molecular regulation of fruit ripening, Front. Plant Sci., 2013, 4, 198 Search PubMed.
- B. Ekesa, et al., Provitamin A carotenoid content of unripe and ripe banana cultivars for potential adoption in eastern Africa, J. Food Compos. Anal., 2015, 43, 1–6 CrossRef CAS.
- L.-s. Zhu, et al., Banana MaSPL16 modulates carotenoid biosynthesis during fruit ripening through activating the transcription of lycopene β-cyclase genes, J. Agric. Food Chem., 2019, 68(5), 1286–1296 CrossRef PubMed.
- A. S. Sonmezdag, H. Kelebek and S. Selli, Comparison of the Aroma and Some Physicochemical Properties of G rand Naine (M usa acuminata) Banana as Influenced by Natural and Ethylene–Treated Ripening, J. Food Process. Preserv., 2014, 38(5), 2137–2145 CrossRef CAS.
- S. Maduwanthi and R. Marapana, Comparative study on aroma volatiles, organic acids, and sugars of Ambul banana (Musa acuminata, AAB) treated with induced ripening agents, J. Food Qual., 2019, 2019, 1–9 CrossRef.
- S. Maduwanthi and R. Marapana, Comparison of pigments and some physicochemical properties of banana as affected by ethephon and acetylene induced ripening, Biocatal. Agric. Biotechnol., 2021, 33, 101997 CrossRef CAS.
- M. Hakim, et al., Role of health hazardous ethephone in nutritive values of selected pineapple, banana and tomato, J. Food, Agric. Environ., 2012, 10(2), 247–251 CAS.
- G. Giovanelli, et al., Variation in antioxidant components of tomato during vine and post–harvest ripening, J. Sci. Food Agric., 1999, 79(12), 1583–1588 CrossRef CAS.
- I. Marty, et al., Ethylene regulation of carotenoid accumulation and carotenogenic gene expression in colour-contrasted apricot varieties (Prunus armeniaca), J. Exp. Bot., 2005, 56(417), 1877–1886 CrossRef CAS PubMed.
- M. J. Rodrigo and L. Zacarias, Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit, Postharvest Biol. Technol., 2007, 43(1), 14–22 CrossRef CAS.
- E. Becatti, et al., Solar UV− B radiation influences carotenoid accumulation of tomato fruit through both ethylene-dependent and-independent mechanisms, J. Agric. Food Chem., 2009, 57(22), 10979–10989 CrossRef CAS PubMed.
- L. Su, et al., Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance, BMC Plant Biol., 2015, 15(1), 1–12 CrossRef PubMed.
- E. Reboul, et al., Bioaccessibility of carotenoids and vitamin E from their main dietary sources, J. Agric. Food Chem., 2006, 54(23), 8749–8755 CrossRef CAS PubMed.
- R. Estévez-Santiago, B. Olmedilla-Alonso and I. Fernández-Jalao, Bioaccessibility of provitamin A carotenoids from fruits: application of a standardised static in vitro digestion method, Food Funct., 2016, 7(3), 1354–1366 RSC.
- G. Giuliano, Provitamin A biofortification of crop plants: a gold rush with many miners, Curr. Opin. Biotechnol., 2017, 44, 169–180 CrossRef CAS PubMed.
- E. Fernández-García, et al., Carotenoids bioavailability from foods: From plant pigments to efficient biological activities, Food Res. Int., 2012, 46(2), 438–450 CrossRef.
- I. J. Aragón, et al., Pro-vitamin A carotenoids stability and bioaccessibility from elite selection of biofortified cassava roots (Manihot esculenta, Crantz) processed to traditional flours and porridges, Food Funct., 2018, 9(9), 4822–4835 RSC.
- G. Britton and F. Khachik, Carotenoids in food, in Carotenoids, Nutrition and Health, Springer, 2009, vol. 5, pp. 45–66 Search PubMed.
- J. Hempel, et al., Ultrastructural deposition forms and bioaccessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.), Food Chem., 2017, 218, 525–533 CrossRef CAS PubMed.
- B. S. Gebregziabher, et al., Carotenoids: Dietary sources, health functions, biofortification, marketing trend and affecting factors–A review, J. Agric. Food Res., 2023, 14, 100834 CAS.
- OECD, Food and A. O. o. t. U. Nations, OECD-FAO Agricultural Outlook 2023–2032, 2023 Search PubMed.
- H. Palafox-Carlos, J. F. Ayala-Zavala and G. A. González-Aguilar, The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants, J. Food Sci., 2011, 76(1), R6–R15 CrossRef CAS PubMed.
- M. M.-L. Grundy, et al., Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism, Br. J. Nutr., 2016, 116(5), 816–833 CrossRef CAS PubMed.
- R. Khorasaniha, et al., Diversity of fibers in common foods: Key to advancing dietary research, Food Hydrocolloids, 2023, 108495 CrossRef CAS.
- A. Y. Hart, Effects of resistant starch and soluble fiber on the bioaccessibility of dietary carotenoids from spinach and carrot using simulated in vitro digestion, The Ohio State University, 2012 Search PubMed.
- G. den Besten, et al., The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism, J. Lipid Res., 2013, 54(9), 2325–2340 CrossRef CAS PubMed.
- Z. Li, et al., Study on the interaction between β-carotene and gut microflora using an in vitro fermentation model, Food Sci. Hum. Wellness, 2023, 12(4), 1369–1378 CrossRef CAS.
- A. Nogal, A. M. Valdes and C. Menni, The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health, Gut Microbes, 2021, 13(1), 1897212 CrossRef PubMed.
- D. Mbogo, T. Muzhingi and S. Janaswamy, Starch digestibility and β–carotene bioaccessibility in the orange–fleshed sweet potato puree–wheat bread, J. Food Sci., 2021, 86(3), 901–906 CrossRef CAS PubMed.
- C. Molteni, C. La Motta and F. Valoppi, Improving the bioaccessibility and bioavailability of carotenoids by means of nanostructured delivery systems: A comprehensive review, Antioxidants, 2022, 11(10), 1931 CrossRef CAS PubMed.
- D. Kim, et al., Improved stability and in vitro bioavailability of β-carotene in filled hydrogel prepared from starch blends with different granule sizes, Food Hydrocolloids, 2023, 139, 108546 CrossRef CAS.
- N. Faisant, et al., Digestion of raw banana starch in the small intestine of healthy humans: structural features of resistant starch, Br. J. Nutr., 1995, 73(1), 111–123 CrossRef CAS PubMed.
- N. Vatanasuchart, B. Niyomwit and K. Wongkrajang, Resistant starch content, in vitro starch digestibility and physico-chemical properties of flour and starch from Thai bananas, Maejo Int. J. Sci. Technol., 2012, 6(2), 259 CAS.
- A. Chávez-Salazar, et al., Isolation and partial characterization of starch from banana cultivars grown in Colombia, Int. J. Biol. Macromol., 2017, 98, 240–246 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04632j |
| This journal is © The Royal Society of Chemistry 2024 |