Open Access Article
Open Access ArticleGut microbiota modulation and effects of a diet enriched in apple pomace on inflammation in a DSS-induced colitis mouse model†
Inés
Calvete-Torre
 ab,
Carlos
Sabater
ab,
Carlos
Sabater
 ab,
Begoña
Cantabrana
ab,
Begoña
Cantabrana
 cde,
Abelardo
Margolles
cde,
Abelardo
Margolles
 ab,
Manuel
Sánchez
ab,
Manuel
Sánchez
 cde and
Lorena
Ruiz
cde and
Lorena
Ruiz
 *ab
*ab
aDepartment of Microbiology and Biochemistry of Dairy Products, Instituto de Productos Lácteos de Asturias-Consejo Superior de Investigaciones Científicas (IPLA-CSIC), Paseo Río Linares s/n, 33300, Villaviciosa, Asturias, Spain. E-mail: lorena.ruiz@ipla.csic.es; Tel: +34 985 89 21 31
bFunctionality and Ecology of Beneficial Microbes (MicroHealth) Group, Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Asturias, Spain
cFarmacología, Departamento de Medicina, Universidad de Oviedo, Oviedo, Spain
dInstituto Universitario de Oncología del Principado de Asturias (IUOPA), Oviedo, Spain
ePharmacology of Therapeutic Targets Group, Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Asturias, Spain
First published on 7th February 2024
Abstract
Certain types of soluble dietary fibre, such as pectin and pectic oligosaccharides from different sources, have demonstrated protective effects against inflammation in DSS-induced colitis mouse models. In this work, we have evaluated the impact of a diet enriched in apple pomace (AP-diet), an agricultural by-product with a significant content of pectin and that previously demonstrated prebiotic properties in human fecal batch fermentation models, on the gut microbiota composition, intestinal damage and inflammation markers in a DSS-induced colitis model. We found that the apple pomace enriched diet (AP-diet), providing a significant amount of pectin with demonstrated prebiotic properties, was associated with a slower increase in the disease activity index, translating into better clinical symptomatology of the animals. Histological damage scoring confirmed less severe damage in those animals receiving an AP-diet before and during the DSS administration period. Some serum inflammatory markers, such as TNFα, also demonstrated lower levels in the group receiving the AP-diet, compared to the control diet. AP-diet administration is also associated with the modulation of key taxa in the colonic microbiota of animals, such as some Lachnospiraceae genera and Ruminococcus species, including commensal short chain fatty acid producers that could play a role in attenuating inflammation at the intestinal level.
1. Introduction
Inflammatory bowel disease (IBD) comprises a spectrum of multifactorial disorders, such as ulcerative colitis (UC) and Crohn's disease (CD), characterized by inflammation and ulceration of the gastrointestinal tract. Their incidence is increasing globally, influenced by a multitude of environmental and lifestyle factors associated with westernized lifestyle, including low fibre diets.1 Numerous investigations have described gut microbiome disbalances in CD patients, including the overall reduction of gut microbiota diversity2 and underrepresentation of certain commensals and butyrate producers such as Faecalibacterium, Roseburia, or Akkermansia.3–6 Indeed, some of these microbial groups have been demonstrated to confer protection against intestinal inflammation,5 and there exists interest in developing strategies to promote their representation within the human gut.7,8Diet has been recognized to play a key role in the prevention and management of IBD,1 and high fibre diets have been associated with reduced risk of developing CD.9 However, there are no general recommendations regarding dietary fibre in IBD management, as its consumption has been related to inflammation exacerbation in some patients. Recent research has suggested that the different responses of IBD patients to dietary fibre may relate to basal differences in their gut microbiota composition and, consequently, in their ability to metabolize the fibre that reaches the intestinal environment.10 Furthermore, dietary fibre comprises a vast variety of oligosaccharides and polysaccharides with diverse chemical and structural compositions, influenced by its vegetable source and extraction process, and it ultimately affects its physical–chemical (e.g. solubility) and bioactive properties, including its pattern of fermentation by specific gut microbes. Notably, fibre structures comprise most of the recognized and emerging prebiotics investigated to date, due to their capacity to be selectively used by the microbiota, conferring health benefits.11
Some investigations have pointed to the beneficial effects of particular prebiotic fibres and the metabolites produced during their fermentation by the gut microbiota in IBD patients. Fibres from fruits generally exhibit protective effects against CD, as opposed to those from whole grains and legumes.10 Administration of pectins, which are abundant fibre structures in some fruits, have demonstrated amelioration of inflammation and gut microbiome biomarkers in experimental models of colitis,12,13 enhanced the effects of fecal microbial transplantation in UC patients,14 and demonstrated the capacity to strengthen the gastrointestinal immune barrier through affecting the gut microbiota and intestinal immune cells.15
In previous investigations, we reported a physical–chemical characterization of apple pomaces from monovarietal apple ciders, demonstrating that they are a good source of pectin with appealing properties.16 Furthermore, we demonstrated in vitro the modulatory potential of some of these apple pomaces on the gut microbial populations from healthy subjects and IBD patients.17 Besides, we confirmed through in silico analyses that the gut metagenomes from IBD patients retain the capacity to potentially metabolize prebiotic pectins.18 Hence, in this work, we investigated in vivo the effect of a diet supplemented with apple pomace on the gut microbiome architecture, disease index and inflammatory markers in a mouse model of DSS-induced colitis.
2. Materials and methods
2.1. Diet, animals, experimental designs and samples collected
Following two weeks of administration of the corresponding diets to each cohort, cohorts 2 and 4 received 2.5% DSS (colitis grade, MP Biomedicals, LLC, France) in the drinking water until sacrifice (Fig. 1). Intake and volume of drinking water consumed, weight loss, stool frequency and consistency, and the presence of occult blood in stool were monitored daily (Fig. 1). The food and water ingested were estimated during the entire experiment by the amounts consumed by the five mice in each cage. The three Rs principles were considered at all stages of experimentation. A dose of DSS 2.5% in the drinking water was chosen to minimise pain and suffering and alter the welfare of the animals as little as possible. A pilot study was performed by adding this concentration of DSS to the drinking water (n = 5), confirming the establishment of DSS-induced colitis. A disease activity index (DAI) was estimated following the criteria previously established in the literature: [reduced body weight + stool consistency + presence of bleeding in stool]/3 (ESI Table S3†).19 DAI values ≥1.5 were considered signs of ulcerative colitis. The decision to sacrifice the mice during the experimental period was based on DAI ≥ 3, a weight loss greater than 20%, and/or clinical signs of animal suffering. According to the facility veterinarian, the hunched posture, decreased activity and response to external stimulation, and detachment from the group, which may reflect pain and suffering, lead to the decision to euthanize the mouse or treat it with analgesics (option ruled out).
2.2. 16S rRNA gene sequencing and data analysis
DNA from fecal samples and colonic contents was isolated by using the Power Soil ProKit (Qiagen) and following the modifications previously described.17 Partial 16S rRNA sequencing was performed on 72 fecal samples, corresponding to 3 pools of fecal samples per cage collected at different times (days 1, 15 and 21) from each animal of the groups, and the colon content of each animal (n = 40). The V3–V4 region was sequenced by using the primers 16S-ProV3V4-forward (CCTACGGGNBGCASCAG) and 16S-ProV3V4-reverse (GACTACNVGGGTATCTAATCC) on an Illumina MiSeq instrument in the Sequencing Facilities of Instituto de Parasitología y Biomedicina “López Neyra”. Sequence reads were quality filtered and the resulting ones were processed using a personalized script of QIIME2 v.2021.8 software20 matched by pair-ends. Quality control filtering was performed, keeping sequences with a mean sequence quality score >20 and a length between 140 and 400 bp. Raw sequencing data generated have been deposited in the Short Reads Archive of the NCBI under accession number PRJNA995428.2.3. Short chain fatty acids
The major SCFAs were analyzed in cell free-supernatants from the colon content. The samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in PBS and centrifuged. The supernatants were collected and supplemented with an internal standard solution (2-ethylbutyric acid 1.05 mg mL−1, Sigma, St Louis, USA), acidified with a 20% v/v formic acid solution and extracted with methanol. Then the supernatants were centrifuged and filtered. The samples were used for SCFA separation and quantification by GC in equipment composed of a 6890NGC injection module (Agilent Technologies Inc., Palo Alto, CA, USA) connected to an FID.
10 in PBS and centrifuged. The supernatants were collected and supplemented with an internal standard solution (2-ethylbutyric acid 1.05 mg mL−1, Sigma, St Louis, USA), acidified with a 20% v/v formic acid solution and extracted with methanol. Then the supernatants were centrifuged and filtered. The samples were used for SCFA separation and quantification by GC in equipment composed of a 6890NGC injection module (Agilent Technologies Inc., Palo Alto, CA, USA) connected to an FID.
2.4. Inflammatory marker analysis of serum samples
Blood was extracted after the sacrifice of all mice and left in RT for 2 h; after this time, the samples were centrifuged at 1000g for 15 min and the supernatant was transferred to a new tube and stored at −20 °C until use. A Mouse IL10 (Interleukin10) ELISA Kit (Cat: ELK1143, ELK Biotechnologies) and a Mouse TNFα (Tumor Necrosis Factor Alpha) ELISA Kit (Cat: ELK1287, ELK Biotechnologies) were used for this analysis following the manufacturer's instructions.2.5. Myeloperoxidase (MPO) activity
MPO activity was measured following previously described procedures21 with slight modifications using distal and proximal colon tissue fragments of each mouse. The samples were removed from −80 °C and placed on ice. First, the samples were homogenized with a pestle in HTAB (hexadecyltrimethylammonium bromide) buffer and then, they were transferred to a new tube with silica beads (1 mm) and homogenized in a Fisherbrand™ Homogenizer Bead Mill 24 by employing 4 cycles of alternating 30 s on and 30 s off, maintaining the samples on ice in between the cycles. Then the samples were frozen at −80 °C for 1 h. After this time, the samples were placed on ice and centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400g, for 6 min at 4 °C. The final steps and quantifications were according to the described procedures.21
400g, for 6 min at 4 °C. The final steps and quantifications were according to the described procedures.21
2.6. Histological analysis
Histological analysis was carried out by the Biobank and Microscopy Service from the Health Research Institute of the Principality of Asturias (ISPA). Distal and proximal colonic tissues were processed and fixed in paraffin. Then, 3-micron sections were made using a microtome (Thermo Scientific HM355S) and were stained with hematoxylin and eosin. The microscopic study was carried out with a double observer. Histological lesions were scored considering the depth of lesions, inflammation (based on the length of damaged mucosa and qualitative estimation of the density of cellular infiltrates), and the presence of lymphoid hyperplasia, crypt ectasia, edema, erosion and ulceration. To integrate the results, a score similar to that used for the grading of ulcerative colitis lesions in humans was made (Table S1†).222.7. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics v.28.0.1 (IBM, Armonk, NY, USA). The goodness of fit to normal distribution was analyzed with the Shapiro–Wilk test. When the distribution of variables was skewed, the natural logarithm of each value was used in the statistical test. Box-and-whisker plots were used to represent the data, and the changes between groups were analyzed by the non-parametric Mann–Whitney U test. In the case of normal distribution, the values were represented as the mean ± the standard error of the mean (SEM). One- or two-way analysis of variance (ANOVA) was followed up with a post-hoc test using the Bonferroni adjustment for multiple comparisons among variables. A paired or independent t-test was used to analyze statistical differences between groups. The days at which 50% of the DAI score was elicited (DAI50) were determined by a sigmoid fitting. Two-tailed probability values of p ≤ 0.05 were considered significant in all cases.3. Results
3.1. Food and fluid intake
During the adaptation period, the mice following the control diet (4 cages, n = 20 mice) or AP-diet (4 cages, n = 20 mice) showed an average intake of food of 0.135 (SEM: 0.0035, n = 4 cages) and 0.143 (SEM: 0.0064, n = 4 cages) grams per gram weight of the mouse per day, respectively, and drank an average of 0.182 (SEM: 0.0138, n = 4 cages) and 0.16 (SEM: 0.007, n = 4 cages) ml per gram mouse weight per day of water, respectively. An independent t-test showed no significant differences in food and water intake between both groups of diets. Neither were differences observed by the one-way ANOVA in the food and water intake after the adaptation period when comparing the four groups of mice on both types of diet, treated or not with DSS 2.5% (Fig. 2A and B). | ||
| Fig. 2 Food (A) and water (B) intake of the different groups of animals during the first 2 weeks of the diet adaptation period (adaptation, n = 10 animals per group) and during the DSS administration period from day 15 to sacrifice (DSS 2.5%, n = 10 animals per group). AP-diet: apple pomace enriched diet. (C) Weight evolution in healthy animals receiving either the control or AP-diet and those not receiving any DSS administration along the experimental period (n = 10 animals per group). #p = 0.032, using a t-test for independent samples at day 0, comparing the two groups of mice for the two types of foods. *p = 0.05 and ***p < 0.001, by comparing day 4 vs. day 0 in the control and AP diets, respectively. (D) Normalized weight gain and loss, represented as the percentage of loss referred to the weight of the animals on day 0 (prior to initiating DSS administration) (n = 10 animals per group). (E) Evolution of the disease activity index (DAI) in DSS receiving animals (n = 10 animals per group) determined according to the Cooper scoring system19. **p = 0.007 and ***p < 0.001, by Bonferroni test adjustment for multiple comparisons regarding day 0. | ||
Following the criteria for the sacrifice of the animals, the average number of days in which they were euthanized, after the administration of DSS 2.5%, was significantly lower for those of the control diet, 6.9 (SEM: 0.1) days, compared to those of the AP-diet, 9 (SEM: 0.36) days (p < 0.001, independent t-test).
3.2. Macroscopic indicators of disease
The weights of the animals at day 0 (after the one-week acclimation period) were 22.13 ± 0.24 g in the cohorts receiving the control diet (n = 20) and 22.92 ± 0.25 g (n = 20) in the cohorts that received the AP-diet (p = 0.032, t-test for independent samples). During the housing period, the mice of both groups increased significantly in weight, with respect to the initial value, from day 4 (ANOVA of a factor of repeated measures) (p < 0.001 and p = 0.05, respectively), without any significant differences between the weight of both groups of mice from day 4 (ANOVA of two factors (days of confinement and diet group) of repeated measures) (Fig. 2C).The administration of 2.5% DSS, starting on day 15, limited weight gain in both cohorts, albeit that in the cohort receiving the standard diet, this effect was observed from day 2 of DSS administration, while in the cohort receiving the bagasse-enriched diet, the weight loss was observed from day 6 (Fig. 2D).
The disease activity index (DAI) was calculated during the experimental period to evaluate the disease progression. Overall, among the animals receiving DSS, the evolution of DAI was delayed in the groups receiving the AP-diet. Two-way ANOVA showed that the DAI score significantly increased on day 4 in the AP-diet group (p = 0.007), while this occurred on day 5 (p < 0.001) in the control diet. The curve fitting showed that the DAI50 for the AP-diet was on day 6 (SEM: 0.26) compared to those receiving the standard control diet on day 5.5 (SEM: 0.13) (p < 0.001). No significant differences were observed between diet types regarding the days (Fig. 2E).
3.3. Evaluation of intestinal damage
Colon length has been inversely related to intestinal inflammation in UC models, colon length shortening being an established biomarker of increased intestinal inflammation in models of colitis.23 In this work, a significant colon length shortening was observed in the DSS groups, compared to their respective control groups. Similar results were observed in terms of colon shortening following DSS exposure for the groups fed with the standard diet and those fed with the apple pomace enriched diet (Fig. 3). The administration of the apple pomace enriched diet per se did not induce any colon length shortening in the animals not receiving DSS.Histological examination of distal and proximal tissue sections was performed to compare the severity of the intestinal damage across the four groups of animals in the intervention. Almost all DSS-treated animals showed erosive inflammatory lesions. In all cases, the inflammatory infiltrate was mixed with polymorphonuclear and mononuclear cells, which is indicative of an active and acute lesion (Fig. 4A). In relation to the groups receiving different diets, animals from group 2 (control diet + DSS) were characterized as having exclusively distal pathology, with minimal or absent proximal lesions. The degree of inflammation ranged from moderate to severe, clearly predominating erosion over ulceration. The inflammatory infiltrate was notable in the injured areas, highlighting the acute cellular inflammatory component. This group of animals also showed the maximum degree of reactive lymphoid hyperplasia (Fig. 4B). In contrast, in group 4 (apple pomace enriched diet + DSS), although pathology was apparent in both proximal and distal regions, there was a lower cellular inflammatory component associated with the lesions, ranging from mild to moderate, as well as the absence of edema. There is an increase in the fibroblastic component in the lesion bed and the submucosa affected by inflammation, together with re-epithelialization phenomena (Fig. 4C).
 | ||
| Fig. 4 Representative microscopic images of intestinal tissues analyzed from the DSS-treated animals showing different indications of histological damage: (A) grade 3 mixed inflammation; (B) moderate lymphoid hyperplasia; and (C) re-epithelialization. (D) Histological score of inflammation in the groups receiving DSS treatment (distal region), determined according to Geboes et al. (2000).22 The statistical analysis performed was the non-parametric Mann–Whitney U test (n = 10 animals per group). | ||
In order to facilitate the comparison of the type and severity of the lesions observed at the histological level, these were scored according to previously described procedures, using the scoring levels established in Table 1. No damage was observed in the mice without colitis induction, obtaining a score of 0 in all of them, irrespective of the diet. Comparing the distal sections of the colon of the mice in which 2.5% DSS was added to the drinking water, significant differences (p = 0.023) were observed between cohorts at the level of intestinal tissue damage, with greater scoring in group 2 (standard diet + DSS) as compared to group 4 (AP-diet + DSS) (Fig. 4D). This observation suggests that the severity of the intestinal inflammation is weaker in animals receiving the AP-diet.
| Score | Observation |
|---|---|
| Grade 0 | Minimal changes |
| Grade 1 | Chronic inflammatory infiltrate, 0–3 |
| Grade 2a | Eosinophils presence |
| Grade 2b | Neutrophils presence, 0–3 |
| Grade 3 | Intraepithelial neutrophils, 0–3 |
| Grade 4 | Crypts destruction, 0–3 |
| Grade 5 | Erosion or ulceration: 0 to 3 erosion, 4 ulceration |
3.4. Intestinal and serum inflammatory markers
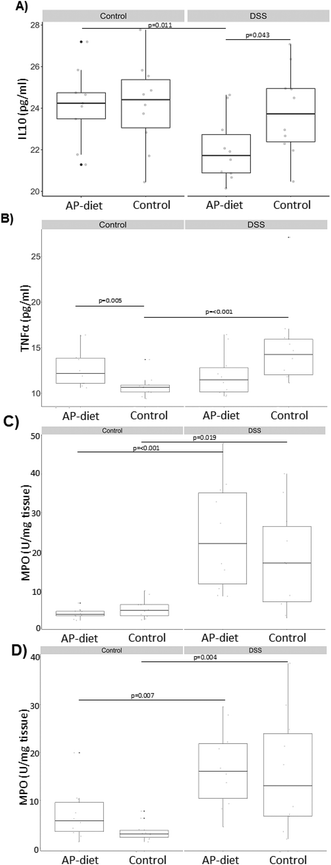
Intestinal MPO activity, determined as an indication of neutrophil accumulation in colonic mucosa,24 did increase significantly during DSS administration in both animals receiving the control diet and AP-diet. No differences in MPO levels were encountered among the DSS-treated animals fed with the control diet and those fed with the AP-diet (Fig. 5).In relation to serum levels of inflammatory markers, serum levels of IL10 and TNFα were determined as an indication of the inflammation level in the animals. The levels of IL10 were reduced in the DSS-treated groups, as compared to their respective control groups, and the same tendency occurred with both diet types. Comparison of the DSS-treated animals from the two diets revealed significant differences (p = 0.043) with higher IL10 levels in group 2 (control diet) as compared to group 4 (AP-diet).
Remarkably, in the healthy control groups, IL10 levels did not show any statistically significant differences between the two types of diet (Fig. 5). IL10 has been recognized as one of the most important anti-inflammatory cytokines regulating mucosal immune responses in the gut25 and has been reported to protect against intestinal damage in models of colitis.26 Several probiotic or prebiotic intervention studies have reported increased expression of IL10 associated with reduced disease severity.27–30 Certain types of pectin, previously proposed as one of the main bioactive components exhibiting prebiotic properties in apple pomace,17 have been reported to be capable of conferring protection against intestinal inflammation in DSS-induced colitis models, generally in association with increased IL10 expression either at the systemic or intestinal level.31 In our work, DSS administration did not significantly affect serum IL10 levels in the animals receiving the standard diet, but animals receiving DSS and the apple pomace enriched diet showed lower IL10 serum levels. While these results appear to contradict those already described, it is not the first time that conflicting results regarding the role of IL10 in inflammation in colitis are presented. Some works have already reported that colitis patients have increased IL10 production, yet the specific physiological consequences of such elevation may be highly influenced by the presence of other cytokines in the local environment.32
TNFα is a proinflammatory cytokine whose levels generally appear increased in both serum and colonic contents in DSS-induced colitis models.33,34 Besides, administration of probiotics or prebiotics demonstrating alleviation of colitis symptoms in such models is generally accompanied by an attenuation of TNFα production in DSS-treated animals.30,33–35 In our experimental groups, no statistically significant differences in TNFα were detected between the cohorts with DSS (p = 0.052). Remarkably, in animals receiving the standard diet, administration of DSS resulted in a significant increase in TNFα (p < 0.001), while in the animals receiving the AP-diet, administration of DSS did not increase the serum levels of TNFα, suggesting that DSS-induced inflammation is reduced in animals fed with the AP-diet (Fig. 5).
These results are consistent with the intestinal tissue damage described above (intestinal and serum inflammatory markers) and the evolution of the DAI in all the experimental groups (evaluation of intestinal damage and Fig. 5) and support the contribution of the AP-diet in ameliorating some inflammatory indicators in the DSS-induced colitis model.
3.5. Gut microbiome
The possible influence of the AP-diet on gut microbiota modulation was analyzed by means of 16S sequencing of fecal samples, collected along the experimental period, and colon contents collected at sacrifice. Concerning colon contents, most alpha diversity coefficients, determined to measure the variability of genera within samples, showed similar trends (ESI Fig. S1†). In the particular case of the Chao1 index, healthy and DSS groups following control diets (31.6 ± 1.4 and 30.5 ± 1.0) and AP-diets (32.9 ± 0.3 and 30.0 ± 0.9) showed similar values. The healthy group following the AP-diet showed alpha diversity estimators significantly (p > 0.05) different from the rest of the groups, including a higher Chao1 index, likely reflecting a higher number of distinct genera in samples from this group.Colonic content diversity among samples and groups was estimated through Bray–Curtis dissimilarity metrics (ESI Fig. S2†). The DSS group following control diets showed significantly higher (p < 0.05) beta-diversity coefficients than the healthy group following AP-diets. These differences might be due to a higher intragroup variability according to alpha diversity analysis (ESI Fig. S1†) and could be associated with the range of differential clinical manifestations of these animals at the end of the experimental period (see sections 3.3. and 3.4). The Bray–Curtis dissimilarity metric was also used to cluster these microbiota samples (ESI Fig. S2B†). Samples corresponding to healthy or DSS animals generally clustered in different branches of the hierarchical tree, yet both sample groups, were not completely discriminated likely due to the high interindividual variability of microbiota profiles. In relation to animals receiving control or AP-diets, no clear clustering between groups was observed, neither in the healthy group nor in the DSS-receiving animals.
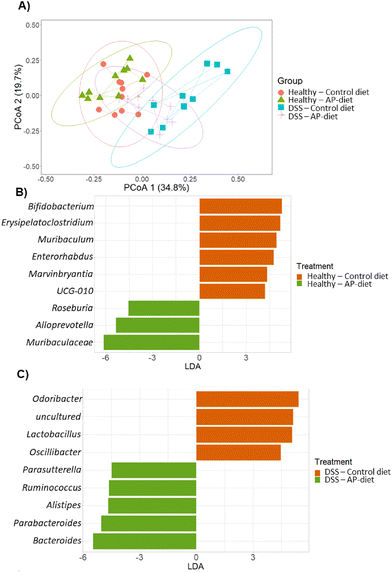
An additional principal coordinates analysis (PCoA) was computed to further characterize these differences in microbial communities among colonic content samples (Fig. 6A). Interestingly, samples from healthy groups following both diets were grouped together and were discriminated from the DSS group following control diets. In contrast, some samples from the DSS group following AP-diets showed similar profiles to those of the healthy groups, revealing the positive influence of apple pomace supplementation and its gut microbiota modulatory properties in the context of DSS-induced colitis.
To further characterize the microbiota modulatory properties of apple pomace, significant differences between groups were determined. Statistical methods designed for microbiome analysis (ANCOM and LEfSe) were performed. To assess the impact of diet on gut microbiota, differences between samples taken at 0 and 15 days of dietary intervention were calculated (ESI Fig. S3†). Along with the dietary intervention, prior to DSS administration, both the control diet and AP-diet led to an increase in Bifidobacterium, Muribaculum, Enterohabdus, and Erysipelatoclostridium and a reduction in Lactobacillus (ESI Fig. S3A and B†).
With regard to the healthy groups, AP-diets resulted in higher abundances of Blautia, Alloprevotella and Muribaculaceae compared to those following control diets after 24 days of intervention (ESI Fig. S3C and D†). An increment in Roseburia was also observed in colonic content samples of mice following AP-diets compared to the control diet group. Other genera that were promoted throughout the administration of AP-diets include Bifidobacterium and the Lachnospiraceae NK4A136 group (Table 2).
| 15 Days | 24 Days | Colonic content | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Healthy group: pomace-enriched diet | ||||||
| Bacteroides | 1.31 | 0.31 | 1.97 | 0.49 | 1.83 | 0.91 |
| Bifidobacterium | 1.21 | 1.39 | 3.41 | 2.30 | 4.75 | 3.90 |
| Candidatus Saccharimonas | 2.65 | 1.12 | 0.67 | 0.21 | 0.57 | 0.41 |
| Colidextribacter | 0.41 | 0.22 | 0.55 | 0.37 | 0.62 | 0.31 |
| Dubosiella | 5.13 | 4.33 | 1.34 | 0.46 | 0.99 | 0.97 |
| Enterorhabdus | 2.30 | 0.23 | 1.44 | 0.34 | 1.30 | 0.42 |
| Erysipelatoclostridium | 1.59 | 1.04 | 0.77 | 0.51 | 0.77 | 0.70 |
| Faecalibaculum | 0.09 | 0.08 | 0.43 | 0.63 | 1.27 | 1.74 |
| Lachnospiraceae NK4A136 group | 3.29 | 0.64 | 5.21 | 4.02 | 8.99 | 5.83 |
| Muribaculaceae | 33.28 | 2.85 | 41.74 | 5.92 | 36.53 | 5.55 |
| Parabacteroides | 0.28 | 0.12 | 0.32 | 0.07 | 0.39 | 0.31 |
| Rikenellaceae RC9 gut group | 0.70 | 0.20 | 0.28 | 0.26 | 0.19 | 0.13 |
| Ruminococcus | 0.07 | 0.06 | 0.17 | 0.11 | 0.27 | 0.27 |
| Uncultured | 0.41 | 0.09 | 0.63 | 0.58 | 1.03 | 1.00 |
| DSS group: pomace-enriched diet | ||||||
| [Eubacterium] coprostanoligenes group | 2.15 | 1.31 | 1.24 | 0.51 | 1.59 | 0.79 |
| Akkermansia | 10.74 | 5.93 | 20.31 | 7.20 | 21.66 | 5.23 |
| Bifidobacterium | 7.83 | 8.60 | 4.68 | 1.84 | 2.01 | 3.01 |
| Blautia | 0.17 | 0.18 | 0.05 | 0.04 | 0.15 | 0.11 |
| Candidatus Saccharimonas | 1.40 | 1.54 | 1.20 | 0.97 | 0.74 | 0.98 |
| Clostridia UCG-014 | 4.10 | 1.85 | 9.36 | 2.66 | 6.04 | 4.10 |
| Colidextribacter | 0.30 | 0.19 | 0.54 | 0.10 | 0.60 | 0.19 |
| Dubosiella | 3.09 | 1.98 | 2.31 | 0.94 | 1.98 | 3.68 |
| Enterorhabdus | 2.45 | 1.79 | 0.68 | 0.24 | 0.44 | 0.09 |
| Erysipelatoclostridium | 1.55 | 1.27 | 0.72 | 0.39 | 2.26 | 0.99 |
| Faecalibaculum | 0.08 | 0.09 | 0.35 | 0.37 | 0.85 | 1.39 |
| Lachnoclostridium | 1.53 | 0.93 | 0.95 | 0.54 | 1.29 | 0.89 |
| Lachnospiraceae NK4A136 group | 3.73 | 0.61 | 6.84 | 1.91 | 9.23 | 4.10 |
| Lachnospiraceae UCG-006 | 0.35 | 0.33 | 1.46 | 0.51 | 3.14 | 3.61 |
| Lactobacillus | 6.11 | 6.34 | 1.80 | 1.13 | 3.27 | 2.69 |
| Marvinbryantia | 0.12 | 0.14 | 0.01 | 0.01 | 0.04 | 0.03 |
| Muribaculaceae | 37.09 | 10.13 | 23.64 | 3.46 | 16.87 | 7.75 |
| Muribaculum | 2.48 | 0.85 | 0.63 | 0.41 | 0.28 | 0.30 |
| Odoribacter | 0.20 | 0.11 | 0.69 | 0.67 | 1.10 | 1.52 |
| Oscillibacter | 0.25 | 0.27 | 0.88 | 0.34 | 1.01 | 0.66 |
| Other | 13.20 | 6.78 | 20.02 | 5.50 | 21.68 | 6.44 |
| Parabacteroides | 0.67 | 0.40 | 1.07 | 0.26 | 2.59 | 2.05 |
| Roseburia | 0.12 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ruminococcus | 0.07 | 0.03 | 0.44 | 0.10 | 1.12 | 1.09 |
| UCG-010 | 0.19 | 0.11 | 0.16 | 0.06 | 0.08 | 0.08 |
In contrast, in the DSS group, the AP-diet led to higher abundances of Alistipes, Parabacteroides and Oscillibacter compared to the control diets (ESI Fig. S3D†). AP-diets also promoted Ruminococcus and Parasutterella in colonic content samples compared to control diets (Fig. 6C). Other genera that were stimulated throughout the administration of AP-diets in the DSS group include Akkermansia, Parabacteroides and novel genera Lachnospiraceae NK4A136 group and Lachnospiraceae UCG-006 (Table 2).
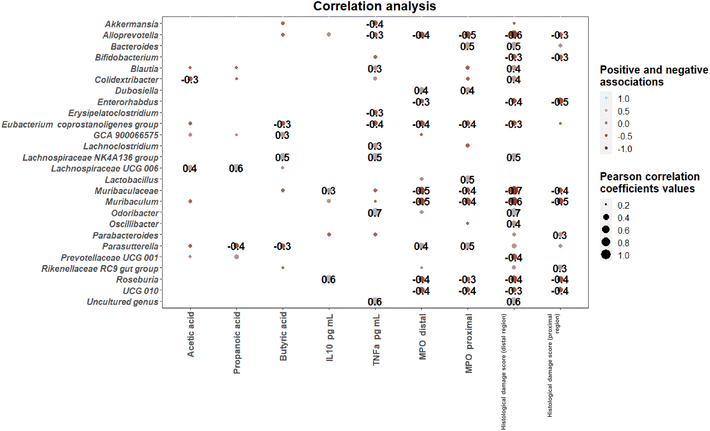
The influence of characteristic taxa on inflammatory markers was investigated through calculation of Pearson correlation coefficients between microbial genera were found showing significant differences among intervention groups’ SCFA levels, inflammatory markers and DAI scores (Fig. 7). Positive correlations between novel Lachnospiraceae genera including the Lachnospiraceae NK4A136 group and Lachnospiraceae UCG 006 and SCFA levels were found. Associations between microbial genera and inflammatory markers included positive correlations between Roseburia and IL10 levels and between Odoribacter and an uncultured genus and TNFα levels. In contrast, the Eubacterium coprostaligenes group, Muribaculum, Roseburia and novel Ruminococcaceae genus UCG 010 correlated negatively with MPO levels in both proximal and distal colon. Finally, histological scores of inflammation in the distal colon were positively correlated with Odoribater, according to previous literature,36 and negatively correlated with Alloprevotella and Muribaculum.
4. Discussion
The present work shows that a diet enriched in apple-pomace, an agricultural by-product with a demonstrated content of complex carbohydrates and polyphenols with potential prebiotic properties,16,17 may exert a protective effect in vivo against intestinal damage induced by DSS in mice. This would open the way to further research aimed at facilitating the development of bioactive and functional ingredients from agricultural by-products, without the need to use highly purified extracts.Prebiotics have been scarcely explored as preventive strategies to protect against intestinal inflammation in IBD patients, mainly due to the fact that diets rich in non-fermentable fibres have been associated with worsening of symptomatology and even with triggering flare-ups in certain IBD patients. Specifically, this effect has been observed with B-glucans (inulin and FOS),37,38 although conflicting results have been presented in preclinical models.39 Regardless of this, with the exception of intestinal obstruction, several authors agree that there is no evidence that fibre intake should be restricted in most IBD patients40,41 and warrant further investigation into the definition of the role of well-defined prebiotic fibres in IBD management.
In this regard, although some preclinical and clinical studies using other prebiotics and/or fibre supplementation reported inconsistent success in ameliorating IBD, certain beneficial effects of defined prebiotic fibres on specific markers of the disease have been reported. For instance, in DSS-induced colitis models, fermented barley and soybean mixtures enhanced intestinal barrier function ameliorating disease progress;42 and pectin from different sources ameliorated inflammation signals12,43 and enhanced barrier function.31 Remarkably, pectin encompasses a wide diverse group of polysaccharides which are recognized as emergent prebiotics. Besides, their specific chemical and structural composition has been linked to variations in their bioactive and functional properties.12,44
In a prior work, we have reported in vitro gut microbiota modulatory properties of apple pomace from selected apple varieties and of their derived pectins,17 supporting a beneficial modulation of beneficial commensal species in human fecal batch cultures. Besides, a prior in silico study based on comparative analyses of predicted carbohydrate utilizing activities in the gut microbiome of healthy vs. IBD patients revealed that despite the important gut microbiota shifts encountered in IBD patients, pectin degraders are still represented in most metagenomes.18 Thus, these results encouraged us to evaluate in vivo the potential beneficial effects of pectin-rich apple pomace as a dietary ingredient. To this end, a basal rodent diet was supplemented with 10% dried apple pomace, an amount that has already been reported to confer metabolic effects in other rodent models45 and which, in our case, is expected to provide sufficient pectin components to exhibit effects on the microbiome46 (ESI Table S1†).
Tolerance of IBD patients to certain prebiotics administration, especially when referring to complex non-digestible carbohydrates, depends on the basal microbiota configuration of the patients and, thus, on their capacity to appropriately metabolize the provided prebiotics.10 Indeed, while some fibre interventions have demonstrated the capacity to ameliorate defined IBD biomarkers, fibre supplementation alone has demonstrated a poor capacity to restore the characteristic IBD gut microbiome disbalances.47 The present work demonstrates the gut modulatory potential of an AP-diet in a DSS-induced colitis mouse model. Apple pomace selectively stimulated bacterial groups that are associated with a healthy gut microbiota composition, including Akkermansia, Parabacteroides and the Lachnospiraceae NK4A136 group and Lachnospiraceae UCG-006. In addition, these novel Lachnospiraceae genera showed positive associations with SCFA levels, known to exert several health benefits in the context of IBD.48 It should be noted that these taxa have been proposed as emerging probiotics associated with specific health benefits.49–51 Specifically, Lachnospiraceae members involve cell wall polysaccharide degraders and butyrate producers52–54 that promote the production of anti-inflammatory cytokines55 and may contribute to the amelioration of intestinal inflammatory conditions.56 In our work, the gut microbiota of mice suffering from colitis following the AP-diet showed profiles more similar to those of healthy mice, in association with amelioration of some inflammatory markers, highlighting the potential of apple pomace fractions to restore the gut ecosystem and ameliorate colitis symptoms. In this regard, previous studies dealing with fecal fermentation experiments of apple pomace and pectin fractions reported similar increases in the abundance of Lachnospira members that could be attributed to the neutral sugar content of polysaccharide chains.17 Similar associations between SCFA levels and the abundance of these taxa were also reported17 in agreement with the present study.
5. Conclusions
Our results demonstrate a significant contribution of AP-diet to reduce intestinal inflammation in a DSS-induced colitis model, reflected by a delayed increase of DAI, reduced promotion of pro-inflammatory TNFα, reduced histological inflammation scores and modification of the overall gut microbiota composition and SCFA production. These results provide an experimental demonstration that pectin containing prebiotic ingredients can be metabolized, to a certain extent, by the disrupted microbiome in colitis models, as previously suggested through in silico18 and in vitro investigations.17 It should be noted that despite its content in prebiotic pectins, the apple pomace, as incorporated in the animals feed, is a complex mixture of ingredients;16 hence, the possibility that other apple pomace components may be affecting the inflammatory response observed in the animals cannot be ruled out. Overall, our results warrant further investigation incorporating well-defined purified substrates for comparison, as well as long-term evaluation of a comprehensive array of health and disease biomarkers associated with consumption of foodstuffs incorporating apple pomace. This will open the avenue towards the formulation of novel and safe prebiotic containing foods from pectin-rich agricultural by-products such as apple pomace.Author contributions
ICT: investigation and formal analyses; CS: formal analyses; BC: investigation; MS: investigation and formal analyses; AM: conceptualization; LR: conceptualization, funding acquisition, and writing – original draft. All authors have read, edited and approved the final version of the manuscript.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
We thank the project RTI2018-095021-J-I00 funded by MCIN/AEI/10.13039/501100011033/, ERDF A way of making Europe and AYUD-2021-50910 from the autonomic Government of Principado de Asturias (FICYT), supported by FEDER. Carlos Sabater was the recipient of a Juan de la Cierva-Formación postdoctoral contract from MICINN, the Spanish Ministry of Science and Innovation (FJC2019-042125-I).References
- J. D. Lewis and M. T. Abreu, Diet as a Trigger or Therapy for Inflammatory Bowel Diseases, Gastroenterology, 2017, 152(2), 398–414 CrossRef CAS.
- A. G. Clooney, J. Eckenberger, E. Laserna-Mendieta, K. A. Sexton, M. T. Bernstein and K. Vagianos, et al., Ranking microbiome variance in inflammatory bowel disease: a large longitudinal intercontinental study, Gut, 2020, 1–12 Search PubMed.
- H. Faden, The Role of Faecalibacterium, Roseburia, and Butyrate in Inflammatory Bowel Disease, Dig. Dis., 2022, 40(6), 793–795 CrossRef PubMed.
- E. Sezgin, G. Terlemez, B. Bozkurt, G. Bengi, H. Akpinar and I. Büyüktorun, Quantitative real-time PCR analysis of bacterial biomarkers enable fast and accurate monitoring in inflammatory bowel disease, PeerJ, 2022, 18(10), e14217 CrossRef PubMed.
- H. Sokol, B. Pigneur, L. Watterlot, O. Lakhdari, L. G. Bermúdez-Humarán and J. J. Gratadoux, et al., Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(43), 16731–16736 CrossRef CAS.
- H. Sokol, P. Seksik, J. P. Furet, O. Firmesse, I. Nion-Larmurier and L. Beaugerie, et al., Low counts of Faecalibacterium prausnitzii in colitis microbiota, Inflammatory Bowel Dis., 2009, 15(8), 1183–1189 CrossRef CAS.
- M. Kumari, P. Singh, B. H. Nataraj, A. Kokkiligadda, H. Naithani and S. A. Ali, et al., Fostering next-generation probiotics in human gut by targeted dietary modulation: an emerging perspective, Food Res. Int., 2021, 150(A), 110716 CrossRef CAS PubMed.
- W. Luo, Z. Shen, M. Deng, X. Li, B. Tan and M. Xiao, et al., Roseburia intestinalis supernatant ameliorates colitis induced in mice by regulating the immune response, Mol. Med. Rep., 2019, 20(2), 1007–1016 CAS.
- X. Liu, Y. Wu, F. Li and D. Zhang, Dietary fiber intake reduces risk of inflammatory bowel disease: result from a meta-analysis, Nutr. Res., 2015, 35(9), 753–758 CrossRef CAS PubMed.
- H. Armstrong, I. Mander, Z. Zhang, D. Armstrong and E. Wine, Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD, Front. Pediatr., 2021, 15(8), 620189 CrossRef.
- G. R. Gibson, R. Hutkins, M. E. Sanders, S. L. Prescott, R. A. Reimer and S. J. Salminen, et al., Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics, Nat. Rev. Gastroenterol. Hepatol., 2017, 14(8), 491–502 CrossRef PubMed.
- K. Ishisono, T. Mano, T. Yabe and K. Kitaguchi, Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner, Front. Immunol., 2019, 102979 Search PubMed.
- V. Singh, B. S. Yeoh and M. Vijay-Kumar, Fermentable Fiber Pectin Improves Intestinal Inflammation by Modulating Gut Microbial Metabolites and Inflammasome Activity, Curr. Dev. Nutr., 2020, 4(Suppl 2), 1535 Search PubMed.
- Y. Wei, J. Gong, W. Zhu, H. Tian, C. Ding, L. Gu and N. Li, Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora, BMC Microbiol., 2016, 16(1), 255 CrossRef PubMed.
- M. Beukema, M. M. Faas and P. de Vos, The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells, Exp. Mol. Med., 2020, 52(9), 1364–1376 CrossRef CAS PubMed.
- I. Calvete-Torre, N. Muñoz-Almagro, M. T. Pacheco, M. J. Antón, E. Dapena and L. Ruiz, et al., Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties, Carbohydr. Polym., 2021, 264, 117980 CrossRef CAS PubMed.
- I. Calvete-Torre, C. Sabater, M. J. Antón, F. J. Moreno, S. Riestra, A. Margolles and L. Ruiz, Prebiotic potential of apple pomace and pectins from different apple varieties: Modulatory effects on key target commensal microbial populations, Food Hydrocolloids, 2022, 133, 107958 CrossRef CAS.
- C. Sabater, I. Calvete-Torre, L. Ruiz and A. Margolles, Arabinoxylan and pectin metabolism in Crohn's disease microbiota: an in silico study, Int. J. Mol. Sci., 2022, 23(13), 7093 CrossRef CAS.
- H. S. Cooper, S. N. S. Murthy, R. S. Shah and D. J. Sedergran, Clinicopathologic study of dextran sulfate sodium experimental murine colitis, Lab. Invest., 1993, 69(2), 238–249 CAS.
- E. Bolyen, J. R. Rideout, M. R. Dillon, N. A. Bokulich, C. C. Abnet and G. A. Al-Ghalith, et al., Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nat. Biotechnol., 2019, 37(8), 852–857 CrossRef CAS.
- J. J. Kim, S. Shajib, M. M. Manocha and W. I. Khan, Investigating intestinal inflammation in DSS-induced model of IBD, J. Vis. Exp., 2012,(60), 3678 Search PubMed.
- K. Geboes, R. Riddell, A. Ost, B. Jensfelt, T. Persson and R. Löfberg, A reproducible grading scale for histological assessment of inflammation in ulcerative colitis, Gut, 2000, 47(3), 404–409 CrossRef CAS PubMed.
- B. Chassaing, J. D. Aitken, M. Malleshappa and M. Vijay-Kumar, Dextran sulfate sodium (DSS)-induced colitis in mice, Curr. Protoc. Immunol., 2014, 104, 15.25.1 Search PubMed.
- J. E. Krawisz, P. Sharon and W. F. Stenson, Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models, Gastroenterology, 1984, 87(6), 1344–1350 CrossRef CAS.
- M. Sasaki, J. M. Mathis, M. H. Jennings, P. Jordan, Y. Wang, T. Ando, T. Joh and J. S. Alexander, Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector, J. Inflammation, 2005, 2(1), 13 CrossRef PubMed.
- A. Son, T. Oshio, Y. I. Kawamura, T. Hagiwara, M. Yamazaki and K. Inagaki-Ohara, et al., TWEAK/Fn14 pathways promotes a T helper 2-type chronic colitis with fibrosis in mice, Mucosal Immunol., 2013, 6(6), 1131–1142 CrossRef CAS PubMed.
- S. G. Jo, E. J. Noh, J. Y. Lee, G. Kim, J. H. Choi and M. E. Lee, et al., Lactobacillus curvatus WiKim38 isolated from kimchi induces IL-10 production in dendritic cells and alleviates DSS-induced colitis in mice, J. Microbiol., 2016, 503–509 CrossRef CAS PubMed.
- M. M. Niu, H. X. Guo, J. W. Cai and X. C. Meng, Bifidobacterium breve Alleviates DSS-Induced Colitis in Mice by Maintaining the Mucosal and Epithelial Barriers and Modulating Gut Microbes, Nutrients, 2022, 14(18), 3671 CrossRef CAS PubMed.
- C. Wang, J. Bai, B. Wang, L. Yu, F. Tian and J. Zhao, et al., Stachyose modulates gut microbiota and alleviates DSS-induced ulcerative colitis in mice, Food Sci. Hum. Wellness, 2023, 12(6), 2211–2220 CrossRef CAS.
- W. Y. Wong, B. Dow Chan, T. W. Leung, M. X. Chen and W. C. S. Tai, Beneficial and anti-inflammatory effects of formulated prebiotics, probiotics, and synbiotics in normal and acute colitis mice, J. Funct. Foods, 2022, 88, 104871 CrossRef CAS.
- C. Sabater, J. A. Molina-Tijeras, T. Vezza, N. Corzo, A. Montilla and P. Utrilla, Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers, Food Funct., 2019, 10(12), 7793–7805 RSC.
- S. Melgar, M. M.-W. Yeung, A. Bas, G. Forsberg, O. Suhr, A. Oberg, S. Hammarstrom, A. Danielsson and M. L. Hammarstron, Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis, Clin. Exp. Immunol., 2003, 134(1), 127–137 CrossRef CAS PubMed.
- Y. Xia, Y. Chen, G. Wang, Y. Yang, X. Song, Z. Xiong, H. Zhang, P. Lai, S. Wang and L. Ai, Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition, J. Funct. Foods, 2020, 67, 103854 CrossRef CAS.
- Y. Zhang, X. Zhao, Y. Zhu, J. Ma, H. Ma and H. Zhang, Probiotic mixture protects dextran sulfate sodium-induced colitis by altering tight junction protein expressions and increasing Tregs, Mediators Inflammation, 2018, 9416391 Search PubMed.
- L. Li, L. Cheng, Z. Li, C. Li, Y. Hong and Z. Gu, Butyrylated starch protects mice from DSS-induced colitis: combined effects of butyrate release and prebiotic supply, Food Funct., 2021, 12, 11290–11302 RSC.
- J. Liu, H. Lin, M. Cao, T. Lin, A. Lin, W. Xu, H. Wang, J. He, Y. Li, H. Tang and B. Zhang, Shifts and importance of viable bacteria in treatment of DSS-induced ulcerative colitis mice with FMT, Front. Cell. Infect. Microbiol., 2023, 13, 1124256 CrossRef CAS PubMed.
- V. Singh, B. San Yeo, R. E. Walker, X. Xiao, P. Saha and R. M. Golonka, Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation, Gut, 2019, 68(10), 1801–1812 CrossRef CAS PubMed.
- H. K. Armstrong, M. Bording-Jorgensen, D. M. Santer, Z. Zhang, R. Valcheva and A. M. Rieger, et al., Unfermented β-fructan fibres fuel inflammation in select inflammatory bowel disease patients, Gastroenterology, 2023, 164(2), 228–240 CrossRef CAS PubMed.
- M. Liao, Y. Zhang, Y. Qiu, Z. Wu, Z. Zhong and X. Zeng, et al., Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice, Food Funct., 2021, 12, 9844–9854 RSC.
- M. C. E. Lomer, B. Wilson and C. L. Wall, British Dietetic Association consensus guidelines on the nutritional assessment and dietary management of patients with inflammatory bowel disease, J. Hum. Nutr. Diet, 2023, 36(1), 336–377 CrossRef PubMed.
- L. Wedlake, N. Salck, J. N. Andreyev and K. Whelan, Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials, Inflammatory Bowel Dis., 2014, 3, 576–586 CrossRef PubMed.
- J. K. Woo, S. Choi, J.-H. Kang, D. E. Kim, B.-S. Hurh and J.-E. Jeon, et al., Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextrain sulfate sodium (DSS)-induced colitis mouse model, BMC Complementary Altern. Med., 2016, 16, 498 CrossRef PubMed.
- Y. Kong, Y. Hu, J. Li, J. Cai, Y. Qiu and C. Dong, Anti-inflammatory effect of a novel pectin polysaccharide from Rubus chingii Hu on colitis mice, Front. Nutr., 2022, 9, 868657 CrossRef PubMed.
- D. Wu, S. Chen, X. Ye, S. Ahmadi, W. Hu and C. Yu, et al., Protective effects of six different pectic polysaccharides on DSS-induced IBD in mice, Food Hydrocolloids, 2022, 127, 1072019 CrossRef.
- R. C. Skinner, D. C. Warren, S. N. Lateef, V. A. Benedito and J. C. Tou, Apple pomace consumption favorably alters hepatic lipid metabolism in young female Sprague-Dawley rats fed a Western diet, Nutrients, 2018, 10, 1882, DOI:10.3390/nu10121882.
- Y. Zhao, J. Bi, J. Yi, J. Peng and Q. Ma, Dose-dependent effects of apple pectin on alleviating high fat-induced obesity modulated by gut microbiota and SCFAs, Food Sci. Hum. Wellness, 2022, 11, 143–154 CrossRef CAS.
- K. Gerasimidis, B. Nichols, M. McGowan, V. Svolos, R. Papadopoulou and M. Kokkorou, et al., The effects of commonly consumed dietary fibres on the gut microbiome and its fibre fermentative capacity in adults with inflammatory bowel disease in remission, Nutrients, 2022, 14(5), 1053 CrossRef CAS PubMed.
- A. Bartoszek, E. V. Moo, A. Binienda, A. Fabisiak, J. B. Krajewska and P. Mosińska, et al., Free fatty acid receptors as new potential therapeutic target in inflammatory bowel disease, Pharmacol. Res., 2020, 152, 104604 CrossRef CAS PubMed.
- T. Lin, C. Shun, W. Lai, C. Tzeng, H. Lai and C. Lu, Investiture of next generation probiotics on amelioration of diseases – Strains do matter, Med. Microecol., 2019, 1, 100002 CrossRef.
- C. Lordan, D. Thapa, R. P. Ross and P. D. Cotter, Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components, Gut Microbes, 2020, 11, 1–20 CrossRef PubMed.
- C. Sabater, I. Calvete-Torre, M. Villamiel, F. Moreno, A. Margolles and L. Ruiz, Vegetable waste and by-products to feed a healthy gut microbiota: Current evidence, machine learning and computational tools to design novel microbiome-targeted foods, Trends Food Sci. Technol., 2021, 118, 399–417 CrossRef CAS.
- W. S. F. Chung, A. W. Walker, D. Bosscher, V. Garcia-Campayo, J. Wagner and J. Parkhill, et al., Relative abundance of the Prevotella, genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides, BMC Microbiol., 2020, 20(1), 1–14 CrossRef PubMed.
- W. Ma, L. H. Nguyen, M. Song, D. D. Wang, E. A. Franzosa and Y. Cao, et al., Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men, Genome Med., 2021, 13(1), 102 CrossRef CAS PubMed.
- D. Rios-Covián, P. Ruas-Madiedo, A. Margolles, M. Gueimonde, C. G. de los Reyes-Gavilán and N. Salazar, Intestinal short chain fatty acids and their link with diet and human health, Front. Microbiol., 2016, 7, 185 Search PubMed.
- W. S. F. Chung, M. Meijerink, B. Zeuner, J. Holck, P. Louis and A. S. Meyer, et al., Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon, FEMS Microbiol. Ecol., 2017, 93(11), fix127 CrossRef PubMed.
- M. Lenoir, R. Martín, E. Torres-Maravilla, S. Chadi, P. González-Dávila and H. Sokol, et al., Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3, Gut Microbes, 2020, 12(1), 1–16 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04277d |
| This journal is © The Royal Society of Chemistry 2024 |