Open Access Article
Open Access ArticleSilicon-enriched meat positively improves plasma lipidaemia and lipoproteinaemia, LDLr, and insulin capability and the signalling pathway induced by an atherogenic diet in late-stage type 2 diabetes mellitus rats†
Marina
Hernández-Martín‡
a,
Adrián
Macho-González‡
b,
Alba
Garcimartín
*c,
Mª Elvira
López-Oliva
 *a,
Aránzazu
Bocanegra
*a,
Aránzazu
Bocanegra
 c,
Rocío
Redondo-Castillejo
c,
Rocío
Redondo-Castillejo
 c,
Sara
Bastida
b,
Juana
Benedí
c,
Sara
Bastida
b,
Juana
Benedí
 c and
Francisco J.
Sánchez-Muniz
b
c and
Francisco J.
Sánchez-Muniz
b
aDepartmental Section of Physiology, Pharmacy School, Complutense University of Madrid, Plaza Ramón y Cajal s/n, 28040-Madrid, Spain. E-mail: elopez@ucm.es; Tel: (+34) 91 3941700
bNutrition and Food Science Department, Pharmacy School, Complutense University of Madrid, Madrid, Spain
cPharmacology, Pharmacognosy and Botany Department, Pharmacy School, Complutense University of Madrid, Madrid, Spain
First published on 1st January 2024
Abstract
The impact of silicon as a functional ingredient in restructured meat (RM) on lipoprotein composition, metabolism, and oxidation on type 2 diabetes mellitus (T2DM) markers has never been studied. This study aims to evaluate the effect of silicon-enriched-meat consumption on lipidaemia, lipoprotein profile and metabolism, plasma arylesterase, and TBARS and their relationships with glycaemia, insulinaemia, and insulin-signaling markers in late-stage-T2DM rats fed a high-saturated-fat-high-cholesterol (HSFHC) diet. Saturated-fat diets with or without added cholesterol were formulated by mixing a 70% purified diet with 30% freeze-dried RM with or without added silicon. Three groups of seven Wistar rats each were tested. The ED group received the control RM in the framework of a high-saturated-fat diet as early-stage T2DM control. The other two groups received streptozotocin-nicotinamide administration together with the HSFHC diet containing the control RM (LD) or silicon-enriched RM (LD-Si). Scores were created to define the diabetic trend and dyslipidaemia. The ED rats showed hyperglycaemia, hyperinsulinaemia, hypertriglyceridaemia, and triglyceride-rich-VLDLs, suggesting they were in early-stage T2DM. LD rats presented hyperglycaemia, hypoinsulinaemia, and reduced HOMA-beta and insulin signaling markers typical of late-stage T2DM along with hypercholesterolaemia and high amounts of beta-VLDL, IDL, and LDL particles and low arylesterase activity. All these markers were significantly (p < 0.05) improved in LD-Si rats. The diabetic trend and diabetes dyslipidaemia scores showed a high and significant correlation (r = 0.595, p < 0.01). Silicon-enriched-meat consumption counterbalances the negative effects of HSFHC diets, functioning as an active hypolipemic, antioxidant, and antidiabetic dietary ingredient in a T2DM rat model, delaying the onset of late-stage diabetes.
1. Introduction
Type 2 diabetes mellitus (T2DM) is associated with lipid and lipoprotein alterations which may result in the development of severe dyslipidaemia.1–4 An adequate balance between lipoprotein production and clearance minimizes the cardiovascular risk in T2DM and improves the survival of T2DM patients.3,5,6 The scavenger receptor, class B type 1 (SR-B1), is known to participate in maintaining plasma HDL levels,7,8 while low-density lipoprotein receptor (LDLr) is involved in the clearance of Apo E/B100-containing lipoproteins (VLDLs, IDLs, LDLs).9,10 In addition, lipoprotein changes seem to be associated with modifications in the InsRbeta/PI3K/AKT insulin signalling pathway.11 Although plasma lipid and lipoprotein levels play a decisive role in cardiovascular disease (CVD) risk, there is consensus that the oxidation of lipoproteins, such as LDLs and VLDLs, speeds up the atherosclerotic process.9,12 Arylesterase (AE), one of the recognized activities of the paraoxonase enzyme, plays an important role in reducing lipoprotein oxidation12–14 and maintaining high concentrations of other antioxidant enzymes. For this reason, AE is considered a suicide enzyme.15 The excessive consumption of meat and meat products has been associated with prooxidant states and degenerative disease development.16,17 Meat consumption has been associated with the T2DM risk and even used as a clinically useful risk factor for T2DM based on studies evaluating the risks associated with meat consumption as a categorical dietary characteristic (i.e., meat consumption versus no meat consumption), as a scalar variable (i.e., gradations of meat consumption), or as part of a broad dietary pattern.18 Although there is consensus on reducing meat consumption, the possibility to design healthier meat is a valuable tool, which is applicable to people accustomed to eating large amounts of meat with very low adherence to other foods, such as fish and vegetables.19,20 Thus, the design of functional meat products enriched with bioactive ingredients capable of decreasing the T2DM risk and its associated cardiometabolic changes (e.g. glycaemia, lipidaemia, CVD and atherogenic risk associated with metabolic syndrome) is considered a useful dietary tool against T2DM.21–23 Several ingredients have been proposed, with most of them of vegetable origin,22,24 although minerals such as Cr, Si, and Se have been also suggested.25Silicon is an essential mineral that has evidenced important health properties on bone cartilage and cognitive function due to its protective role against aluminium and its implication in Alzheimer's disease.26–29 However, studies associating silicon with CVD protection are relatively scarce. Silicon added to the diet has been found to decrease cholesterol absorption30 and the postprandial lipaemic response in rats.31 On the other hand, supplemental silicon may stimulate endothelial and arterial integrity by mitigating the detrimental effects of cholesterol in rabbits.32 Our group has shown that the consumption of silicon as a supplement by healthy rats31 or as a functional ingredient included in a silicon-enriched restructured meat (RM) in the framework of atherogenic diets induces hypoglycaemic, hypolipaemic, antioxidant, and anti-inflammatory properties and decreases the evolution of non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH) in aged rats.25,33 However, to the best of our knowledge, currently there is no available information regarding the role of silicon and/or silicon-enriched RM in improving hyperglycaemia and dyslipidaemia and plasma oxidation in T2DM murine models.
In this study, we hypothesize that the consumption of a silicon-enriched RM (Si-RM) positively impacts late-stage T2DM by preventing hypoinsulinaemia, excessive levels of plasma glucose, dyslipidaemia, and lipoprotein oxidative changes, and bringing metabolic events closer to early stage T2DM. To this aim, late-stage and early-stage T2DM were induced following validated rat models.32 The objective of this work is to examine the effects of consuming silicon in a RM product in a late-stage T2DM rat model fed with a high-saturated fat high-cholesterol (HSFHC) diet. Specifically, our aim is to evaluate: (a) fat and diet digestibility; (b) glycaemia, insulinaemia, and HOMA-beta; (c) InsRbeta/PI3K/AKT insulin signalling markers; (d) lipidaemia, lipoprotein profile and composition; (e) plasma AE and TBARS; and (f) levels of liver LDLr and SR-B1. In addition, the work seeks to ascertain any existing correlation between glucose homeostasis and lipoprotein metabolism markers and scores and to assess the possible change from late-stage to early-stage in T2DM rats fed silicon.
2. Materials and methods
2.1. Silicon-enriched restructured meat and diet preparations
The preparation of the control RM was carried out as previously described.34 The main ingredient used was lean minced meat (50% pork: 50% veal) after mixing it in a blend with lard for 1 minute using a grinder-homogenizer connected to a cooling bath (2 °C) (Stephan Universal Machine UM5, Stephan u. Söhne GmbH and Co., Hameln, Germany). Si-RM was prepared following the same procedure as the control RM with the addition of silicon. The dose of silicon (G5TM, Glycan Group, Genève, Switzerland) was determined based on differences in silicon consumption between Western and Eastern populations, as indicated by Garcimartín et al.25 Once the RM were prepared, they were lyophilized in a LyoAlfa 10 freeze dryer (Telstar, Terrassa, Spain) for 48 h. The resulting freeze-dried products were then ground into a fine homogeneous powder using a refrigerated mincer (Stephan Universal Machine UM5, Stephan u. Söhne GmbH and Co., Hameln, Germany) for two minutes. The formulation and composition of the diets are detailed in Table 1. Briefly, for each kilogram of diet, 30% of RM and 70% of a purified formulated diet were mixed and subsequently sieved three times until completely homogeneous power was obtained. Three experimental semisynthetic diets were prepared: (a) a high-saturated fat (HSF) diet (50%En and 20.4%En from fat and saturated fatty acids, respectively) containing the control RM; (b) a HSFHC diet (HSF with the addition of 1.4% cholesterol plus 0.2% cholic acid) containing the control RM; (c) a HSFHC diet containing the Si-RM. To reach that supplementation, a specific volume of organic silicon containing 67 mg of elemental silicon was added to 1 kg of meat matrix. The final concentration of silicon in the total diet was 20 mg Si kg−1 diet.| Dietary components | ED diet | LD diet | LD-Si diet |
|---|---|---|---|
| ED: early-stage diabetes control group, diet containing control restructured meat; LD: late-stage diabetes group, diet containing control restructured meat, 1.4% cholesterol, and 0.2% cholic acid; LD-Si: late-stage diabetes silicon-enriched meat group.a En: total energy.b SFA: saturated fatty acids.c MUFA: monounsaturated fatty acids.d PUFA: polyunsaturated fatty acids.e PM 205B SAFE: mineral mix.f PV 200 SAFE: vitamin mix. *Calculated data considered energy equivalents for carbohydrates 16.73 kJ g−1 (4.0 kcal g−1), fat 37.65 kJ g−1 (9.0 kcal g−1), and protein 16.73 kJ g−1 (4.0 kcal g−1). | |||
| Nutrient composition | |||
| Proteins (% En)a | 14.2 | 14.0 | 14.0 |
| Meat Fat (% En) | 49.4 | 49.0 | 49.0 |
| SFAb/MUFAc/PUFAd ratio | 2.7/3.0/1.0 | 2.1/2.3/1.0 | 2.1/2.3/1.0 |
| Cholesterol (%) | 0.02 | 0.93 | 0.93 |
| Energy content (MJ kg−1) * | 20.13 | 20.31 | 20.31 |
| Ingredients (g kg −1 ) | |||
| Sucrose | 68.3 | 68.3 | 68.3 |
| Corn starch | 286.1 | 275.7 | 275.7 |
| Casein | 94.3 | 94.3 | 94.3 |
| Maltodextrin | 94.3 | 94.3 | 94.3 |
| Cellulose | 48.9 | 48.9 | 48.9 |
| PM 205B SAFEe | 50.1 | 50.1 | 50.1 |
| PV 200 SAFEf | 7.2 | 7.2 | 7.2 |
| Soybean oil | 47.9 | 47.9 | 47.9 |
| L-Cysteine | 2.02 | 2.02 | 2.02 |
| Cholesterol | 0 | 9.1 | 9.1 |
| Cholic acid | 0 | 1.3 | 1.3 |
| Silicon | 0 | 0 | 0.002 |
| Lyophilized restructured meat | 301.14 | 301.14 | 301.14 |
2.2. Experimental design
Male Wistar rats aged two-months old were obtained from Harlan Laboratories (Harlan S.L., Barcelona, Spain) and housed in pairs under controlled temperature and lighting conditions (22.3 ± 1.9 °C and 12 h light/dark cycle, respectively) at the Animal Experimentation Centre of Alcalá University of Madrid, Spain (register number ES280050001165). They were randomized into three groups of eight rats each and accorded a 7-day acclimatization period to experimental conditions. Tap water and food were provided ad libitum.To induce early-stage T2DM, eight rats (ED group) were fed the HSF diet formulated with the control RM for eight weeks. For late-stage T2DM, 16 rats were fed the HSFHC diet containing the control RM for three weeks, followed by an intraperitoneal injection of streptozotocin (STZ, 65 mg per kg b.w.) and nicotinamide (NAD, 225 mg per kg b.w.) (both from Sigma Aldrich, Madrid, Spain). The NAD was employed to prevent excessive degeneration of the pancreatic islet cells induced by STZ administration.35 Four days later, fasting hyperglycaemia was confirmed, and the 16 animals were further classified into two groups: LD (control late-stage T2DM rats) and LD-Si. LD rats continued with the HSFHC diet containing the control RM until the end of the experiment, while LD-Si rats received the HSFHC diet containing the Si-RM for 5 weeks. To avoid inter-assay variations, overnight fasted rats were taken, one from each group, anesthetized with isoflurane (5% v/v) and euthanized by extracting blood from the descending aorta with a heparinized syringe. The major lobe of the liver was dissected, weighed, and processed. All experiments were performed in compliance with Directive 2010/63/EU of 22 September 2010 (amended by Regulation (EU) 2019/1010) on the protection of animals used for scientific purposes. The study was approved by the Spanish Science and Technology Advisory Committee (project AGL2014-53207-C2-2-R) and by the Ethics Committee of Complutense University of Madrid, Madrid (Spain).
2.3. Glycaemia and insulin determinations
Blood samples were collected from the tail in fasting conditions in heparinized tubes at the middle of the third week and at the end of the experiment (from the descending aorta). Plasma was isolated by centrifugation for 10 min at 986g, and glycaemia was quantified immediately in a plate reader (SPECTROstar Nano, BMG LABTECH, Offenburg, Germany) at 492 nm using the GOD kit (Spinreact, Barcelona, Spain). Insulin was measured only at the end using an ELISA kit (Rat Insulin Elisa KIT, ELR-Insulin, RayBiotech, Inc, USA) according to the manufacturer's instructions. The colour intensity was evaluated at 450 nm using a microplate reader (SPECTROstar Nano BMG LABTECH, Offenburg, Germany). HOMA-beta was calculated as follows: [20× fasting plasma insulin (μIU mL−1)]/[fasting glucose (mmol L−1) − 3.5].2.4. Lipoprotein isolation
The different lipoprotein fractions were isolated from 2 mL of plasma using saline gradient ultracentrifugation (Beckman L8-70M, IN, USA) with an SW-40.1 rotor following a modification of the Terpstra et al. method,36 as described by Olivero-David et al.37 Briefly, the tubes were centrifuged at 272![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (40
000g (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 4 °C for 21 h 40 min. The isolation of the lipoprotein fractions was performed considering the conventional density range for rats of the different lipoprotein classes (VLDL (ρ20 < 1.0063 g mL−1), IDL (1.006 < ρ20 < 1.019), LDL (1.019 < ρ20 < 1.057), and HDL (1.057 < ρ20 < 1.210)).
000 rpm) at 4 °C for 21 h 40 min. The isolation of the lipoprotein fractions was performed considering the conventional density range for rats of the different lipoprotein classes (VLDL (ρ20 < 1.0063 g mL−1), IDL (1.006 < ρ20 < 1.019), LDL (1.019 < ρ20 < 1.057), and HDL (1.057 < ρ20 < 1.210)).
2.5. Plasma lipid analysis and lipoprotein composition
Triglycerides, total cholesterol, and phospholipids were quantified in plasma and the lipoprotein fractions (VLDL, IDL, LDL, and HDL). The measurements were made in plate readers at 492 nm (SPECTROstar Nano, BMG LABTECH, Offenburg, Germany), using the triglycerides-LQ, cholesterol-LQ and phospholipids kits (Spinreact, Barcelona, Spain) according to the manufacturer's instructions. Total lipids were calculated as the sum of triglycerides, cholesterol, and phospholipids. The protein content of isolated lipoproteins was determined by the Bradford method.38 The total mass of each lipoprotein fraction was calculated as the sum of total lipids plus proteins (both in mg dL−1). The atherogenic index (AI) was determined using the total cholesterol/HDL cholesterol ratio.2.6. Plasma TBARS assay
Plasma oxidation as the TBARS measurement was performed following the Uchiyama and Mihara method.39 Malondialdehyde (MDA), formed after adding thiobarbituric acid at elevated temperature, causes complex colouring directly proportional to the lipid peroxidation. 50 μL of plasma was mixed with a thiobarbituric acid solution and kept at 100 °C for 45 min. The MDA content (mg L−1 for plasma) was quantified using a fluorescence plate reader (FLUOstar Omega, BMG LABTECH, Offenburg, Germany) at λexc = 485/20 nm and λem = 528/20 nm, extrapolating the readings to the standard curve of MDA generated by the acid-catalysed hydrolysis of 1,1,3,3-tetramethoxypropane (Sigma-Aldrich, Madrid, Spain).2.7. Plasma arylesterase activity
AE activity was determined in plasma according to the method of Macho-González et al.40 Simulated body fluid was used as buffer and phenylacetate as substrate. The kinetics of the AE reaction was tested at 270 nm in cuvettes of 10 mm light path quartz thermostatically controlled at 37 °C in a spectrophotometer (SPECTROstar Nano, BMG LABTECH, Offenburg, Germany). Plasma-free blanks were used to correct the spontaneous hydrolysis of phenylacetate that occurs in the buffer. Results were expressed as mmol of phenol formed from phenylacetate per minute, which were normalized per litre of plasma (mmol min−1 L−1).2.8. Immunohistochemical staining; expression of LDL receptor and SR-B1 and insulin signalling markers
Paraffin-embedded liver sections from five rats in each experimental group were deparaffinized and rehydrated in a graded ethanol series. Then, they were heated in 10 mM citrate buffer (pH 6.0) for antigen retrieval, and endogenous peroxidase was quenched with 3% hydrogen peroxide. Subsequently, the sections were incubated overnight at 4 °C with the mouse primary antibodies anti-SR-B1 and anti-LDLr, anti-InsRbeta, anti-PI3K, and anti-pAKTSer473 (Santa Cruz Biotechnology Quimigen, Madrid, Spain). After several washes, the sections were incubated with biotinylated secondary antibody followed by streptavidin–biotin-conjugated horseradish peroxidase (Sigma Aldrich, Madrid, Spain) using 3,3′-diaminobenzidine (DAB) (Sigma Aldrich, Madrid, Spain) as a substrate. The sections were then counterstained with Harris’ haematoxylin. A total of 10 fields per section per rat (20× magnification for image analysis) were selected and analysed. The positive staining intensity was calculated by assigning scores to the stained area relative to the total field assessed.2.9. Plasma markers concurrence
Tertile values were calculated for each plasma variable in the 24 rats studied. Differences in variable distribution among the groups were later tested. Values for insulinaemia, HOMA-beta and AI at the first, second, and third tertiles contributed the values 3, 2, and 1, respectively. In contrast, values of glycaemia, insulin signalling markers, triglycerides, and cholesterol at the first, second, and third tertiles contributed the values of 1, 2, and 3, respectively.Subsequently, several indexes were obtained taking into consideration physiological and/or pathological similarities and associations between variables. This allowed us to obtain multiple single indexes with higher discriminant power than individual markers.41,42 The average score was calculated from the values 1, 2 or 3 assigned to the first, second, and third, respectively, accounting for the number of rats in each tertile. Thus, an average score close to three indicates that most of the animals were located in the third tertile.
Later, a diabetic trend score (DTscore) was obtained by taking into account the association of glucose, insulin and HOMA-beta. The DTscore value was calculated by adding the tertile values of each parameter (DTscore range: 3 to 9). The insulin-signalling score (ISscore) was obtained by considering the association of InsRbeta, PI3K and Akt and the score value was calculated by adding the tertile value of each parameter (ISscore range: 3 to 9). The diabetic-dyslipaemic score (DDscore) was obtained by considering the association of cholesterol, triglyceride and the AI and its value was calculated by adding the tertile value of each parameter (DDscore range: 3 to 9).
2.10. Statistical analysis
The results were expressed as mean ± SD. Continuous variables were compared using one-way ANOVA, followed by the post-hoc Bonferroni or Tamhane T2 method, depending on the assumption of equality or inequality of variances, respectively. The Kruskal–Wallis test followed by the Dunn–Bonferroni approach was used for non-parametric variable comparisons. Chi-square tests were calculated using contingency tables to determine differences for hypercholesterolaemia, hypertriglyceridaemia, hyperphospholipidaemia, and rat distribution among the three experimental groups within the three tertiles of the different indexes. The Pearson product-moment correlations between parameters and the Spearman correlations between scores and parameters were also determined. Statistical significance was considered at p < 0.05. The statistical analysis was performed using SPSS version 28.0 (SPSS Inc., Chicago, Illinois, USA) and graphs were drawn with GraphPad Prism version 9 (GraphPad Software, Inc., La Jolla, California).3. Results
3.1. Feed and cholesterol intakes, faecal excretion, and body weight gain
Feed intake and body weight gain were not significantly affected by the consumption of the experimental diets (p > 0.05) (Table 2). However, significant differences were observed in cholesterol intake, faecal moisture and fat excretion, and dietary and fat digestibilities (p < 0.01). Cholesterol intake was higher in the LD and LD-Si groups compared to the ED group (both p < 0.001). In LD rats, faecal excretion and faecal fat were significantly higher, while dietary and fat digestibilities were lower (all p < 0.05) compared to ED rats. The LD-Si group showed significantly higher faecal excretion, faecal moisture, and faecal fat (p < 0.01), but lower dietary and fat digestibilities (p < 0.01) than the LD group. When compared to the ED group, LD-Si rats exhibited higher faecal excretion, faecal moisture, and faecal fat content together with lower dietary and fat digestibilities (p < 0.01).| ED group | LD group | LD-Si group | ANOVA (p) | |
|---|---|---|---|---|
| Values expressed as mean ± SD. Different letters (a < b < c) indicate significant differences between groups (p < 0.05, calculated using ANOVA, followed by Bonferroni post hoc test). NS: no significant differences between groups.a Fat digestibility: (Fat intake – faecal fat)/fat intake. | ||||
| Feed intake (g day−1) | 17.8 ± 1.2 | 16.5 ± 0.55 | 17.1 ± 0.55 | NS |
| Cholesterol intake (mg day−1) | 3.6 ± 0.36a | 154.0 ± 12.1b | 167.1 ± 8.5b | <0.001 |
| Initial body weight (g) | 253.8 ± 25.3 | 249.1 ± 14.6 | 245.6 ± 15.7 | NS |
| Body weight gain (g) | 141.6 ± 15.5 | 123.0 ± 30.2 | 133.0 ± 33.6 | NS |
| Faecal excretion (g day−1 dry matter) | 1.14 ± 0.08a | 1.60 ± 0.06b | 1.96 ± 0.24c | <0.001 |
| Faecal moisture (%) | 14.35 ± 1.12a | 14.04 ± 1.66a | 16.41 ± 1b | <0.01 |
| Faecal fat (mg g−1 dry matter) | 80.4 ± 7.1a | 168.0 ± 7.4b | 254.9 ± 15.1c | <0.001 |
| Dietary digestibility | 0.92 ± 0.01c | 0.88 ± 0.01b | 0.86 ± 0.01a | <0.001 |
| Fat digestibilitya | 0.99 ± 0.09b | 0.97 ± 0.08b | 0.93 ± 0.08a | <0.01 |
3.2. Glycaemia, insulinaemia and HOMA-beta levels and the diabetic trend score
The dietary treatment had a significant effect on plasma glucose, insulin, and HOMA-beta levels (p < 0.001) and DTscore (p < 0.001) (Table 3). When comparing LD to ED, the LD rats showed significantly higher (p < 0.001) levels of glycaemia and DTscore, but lower levels of insulinaemia and HOMA-beta (p < 0.001). LD-Si rats exhibited intermediate values but significant differences for all parameters (p < 0.01) compared to LD and ED rats (Table 3). The prevalence of severe hyperglycaemia (plasma glucose ≥11.1 mmol L−1 or 200 mg dL−1) was not affected by the dietary treatment (p > 0.05).| ED group | LD group | LD-Si group | ANOVA (p) | |
|---|---|---|---|---|
| Values expressed as mean ± SD. Global dietary effect was measured using one-way ANOVA or Kruskal–Wallis tests, followed by post-hoc Bonferroni test or T2 of Tamhane or the Dunn–Bonferroni approach, respectively. Values bearing different letters (a < b < c) indicate significant differences between groups (p < 0.05). NS: No significant differences between groups. To transform mmol L−1 into mg dL−1 of cholesterol, triglycerides, and phospholipids, multiply data by 38.67, 88.57, and 75, respectively.a HOMA-beta: 20× fasting plasma insulin (μIU mL−1)/(fasting glucose (mmol L−1) – 3.5).b AI: atherogenic index.c AE: arylesterase.d VLDLc: VLDL cholesterol.e VLDLplp: VLDL phospholipids.f HDLc: HDL cholesterol.g HDLplp: HDL phospholipids. For scores, data represent rat distribution per group into tertiles (n = 7 rats per group). The average score was calculated by assigning values 1, 2 and 3 to tertiles 1st, 2nd and 3rd, respectively, based on the number of rats in each tertile. The analysis was made using Kruskal–Wallis test, followed by a non-parametric multiple comparison post hoc test (p < 0.05, a < b < c) or chi-squared test as appropriate (p value).h DTscore, diabetic trend score: glucose score + insulin score + HOMA-beta score.i DDscore, dyslipaemic diabetes score: triglyceride score + cholesterol score + atherogenic index scores.j ISscore, diabetes insulin signalling score: InsRbeta score + PI3K score + pAktser473 score. | ||||
| Diabetic parameters | ||||
| Glucose (mmol L−1) | 13.9 ± 0.99a | 17.74 ± 1.46b | 14.99 ± 2.08a | <0.001 |
| Insulin (μUI mL−1) | 16.05 ± 0.45c | 5.3 ± 1.29a | 7.65 ± 1.32b | <0.001 |
| HOMA-betaa | 31.13 ± 3.33c | 7.24 ± 1.35a | 12.75 ± 3.04b | <0.001 |
| Lipidic parameters | ||||
| Triglycerides (mg dL−1) | 135.8 ± 22.46b | 77.63 ± 19.81a | 52.97 ± 14.95a | <0.001 |
| Cholesterol (mg dL−1) | 79.39 ± 11.52a | 108.2 ± 8.54b | 86.65 ± 5.63a | <0.001 |
| Phospholipids (mg dL−1) | 101.3 ± 24.01 | 95.88 ± 7.63 | 91.9 ± 14.03 | NS |
| Total lipids (mg dL−1) | 316.5 ± 47.1b | 281.7 ± 32.1b | 231.5 ± 27.6a | 0.001 |
| Cholesterol/phospholipids | 0.81 ± 0.16a | 1.13 ± 0.11b | 0.96 ± 0.16ab | <0.01 |
| AIb: total cholesterol/HDLc | 1.43 ± 0.24a | 2.68 ± 0.55c | 2.04 ± 0.16b | <0.001 |
| Peroxidation and antioxidant ratios | ||||
| TBARS (mg L−1) | 2.18 ± 0.24a | 2.58 ± 0.16b | 2.24 ± 0.18a | <0.01 |
| AEc (mmol min−1 L−1) | 184.5 ± 27.5a | 163.4 ± 31.9a | 334.0 ± 47.7b | <0.001 |
| AE/cholesterol (U mg−1) | 0.24 ± 0.04b | 0.15 ± 0.03a | 0.38 ± 0.06c | <0.001 |
| AE/phospholipids (U mg−1) | 0.19 ± 0.05a | 0.17 ± 0.04a | 0.36 ± 0.07b | <0.001 |
| AE/VLDLcd (U mg−1) | 1.29 ± 0.26b | 0.66 ± 0.19a | 2.15 ± 0.51c | <0.001 |
| AE/VLDLplpe (U mg−1) | 0.79 ± 0.12a | 0.71 ± 0.17a | 2.57 ± 0.66b | <0.001 |
| AE/HDLcf (U mg−1) | 0.33 ± 0.06a | 0.36 ± 0.09a | 0.79 ± 0.12b | <0.001 |
| AE/HDLplpg (U mg−1) | 0.25 ± 0.06a | 0.34 ± 0.06a | 0.82 ± 0.12b | <0.001 |
| Scores | Kruskal–Wallis (p) | |||
| DTscoreh (3 to 9) | 3.29 ± 0.49a | 8.57 ± 0.79c | 6.14 ± 0.90b | <0.001 |
| DDscorei (3 to 9) | 5.29 ± 0.95a | 7.71 ± 1.25b | 5.14 ± 0.90a | <0.001 |
| ISscorej (3 to 9) | 8.57 ± 0.54C | 3.57 ± 0.54a | 6.71 ± 0.95b | <0.001 |
3.3. Plasma lipids, atherogenic ratios, and diabetic lipid scores
Plasma triglycerides, cholesterol, total lipids, cholesterol/phospholipid ratio and the AI (total cholesterol/HDL cholesterol) were significantly affected by the dietary treatment (p < 0.01) (Table 3). LD and LD-Si rats displayed lower triglycerides, lower total lipids, and higher AI (p < 0.01) than their ED counterparts. LD vs. ED rats showed significantly higher values for plasma cholesterol, cholesterol/phospholipid ratio and DDscore (p < 0.01). Total lipids were lower (p < 0.001) in LD-Si compared to ED rats. The LD-Si group presented lower values (p < 0.05) for cholesterol (p = 0.001), total lipids (p < 0.05), AI (p < 0.05), and DDscore (p < 0.05) and marginally lower triglyceride levels (p = 0.054) than the LD group.The prevalence of high triglyceride (≥1.69 mmol L−1 or ≥150 mg dL−1) and high cholesterol (≥2.59 mmol L−1 or ≥100 mg dL−1) was significantly affected by the experimental diets (p < 0.001). Hypertriglyceridaemia was present in 62.5% of ED rats but absent in LD and LD-Si rats. Hypercholesterolaemia was prevalent in LD rats (87.5%) but absent in ED and LD-Si rats.
A cholesterol/phospholipid ratio greater than 1 has been suggested as a surrogate index of mild hypercholesterolaemia, while a ratio greater than 2 indicates frank hypercholesterolaemia.43 According to this ratio, 12.5% of ED rats and 37.5% of LD-Si rats presented mild hypercholesterolaemia, indicating that the dietary treatment significantly influenced the prevalence of hypertriglyceridaemia and hypercholesterolaemia.
3.4. Plasma TBARS, arylesterase activity, and arylesterase lipoprotein ratios
Plasma TBARS, AE activity, AE/cholesterol, AE/phospholipids, AE/VLDL cholesterol, AE/VLDL phospholipids, AE/HDL cholesterol, and AE/HDL phospholipids are shown in Table 3. Dietary treatment significantly influenced TBARS (p < 0.01), AE, and AE to any lipid lipoprotein ratios (p < 0.001).LD vs. ED rats showed higher levels of plasma TBARS (p < 0.01) but lower AE/cholesterol and AE/VLDL cholesterol (both p < 0.01). AE and all AE to any lipid lipoprotein ratios were significantly higher (p < 0.05) in LD-Si rats than in ED rats. LD-Si compared to LD rats exhibited lower plasma TBARS (p < 0.05), higher AE activity (p < 0.001) and higher AE to any lipid or lipoprotein ratios (p < 0.001).
3.5. Net and percentage contribution of lipids in lipoproteins to lipoprotein composition
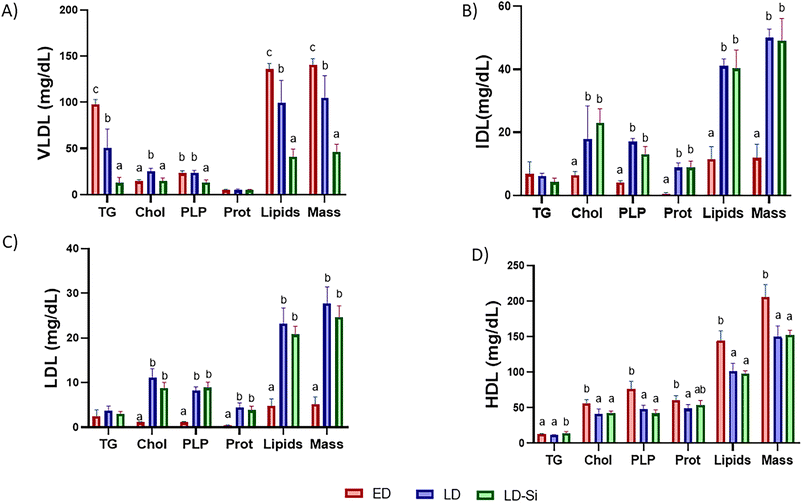
The compositions of the different lipoprotein fractions are shown in Fig. 1. With the exception of VLDL-protein and LDL- and IDL-triglycerides, most lipid components, total lipids, and total lipoprotein mass of the VLDL, IDL, LDL and HDL fractions were significantly affected by the experimental diets (p < 0.001). Regarding the VLDL fraction, the LD group exhibited significantly (p < 0.05) higher cholesterol but lower triglyceride, total lipids and total mass contents compared to the ED group. Finally, LD-Si rats exhibited lower amounts (p < 0.01) of triglycerides, phospholipids, total lipids and total mass than ED rats. LD-Si vs. LD rats had significantly lower levels (p < 0.05) of cholesterol, triglycerides, phospholipids, total lipids, and total mass than LD rats.In relation to IDL, the LD and LD-Si groups showed significantly (p < 0.05) higher levels of cholesterol, phospholipids, protein, total lipids, and total mass (p < 0.001) than the ED group, but lower levels of triglycerides that were not significantly different. The composition of IDL in LD-Si rats did not significantly differ from that of LD rats. These effects were also observed in the LDL fraction, differing in triglyceride contents, which were also higher in LD and LD-Si rats (without significance).
With respect to HDL, the LD group showed significantly (p < 0.05) lower values of cholesterol, phospholipids, proteins, total lipids, and total mass compared to the ED group. LD-Si rats had significantly (p < 0.05) lower levels of cholesterol, phospholipids, total lipids and total mass, but higher triglycerides than the ED group. Lastly, LD-Si rats exhibited significantly higher quantities of triglycerides (p < 0.05) than LD rats.
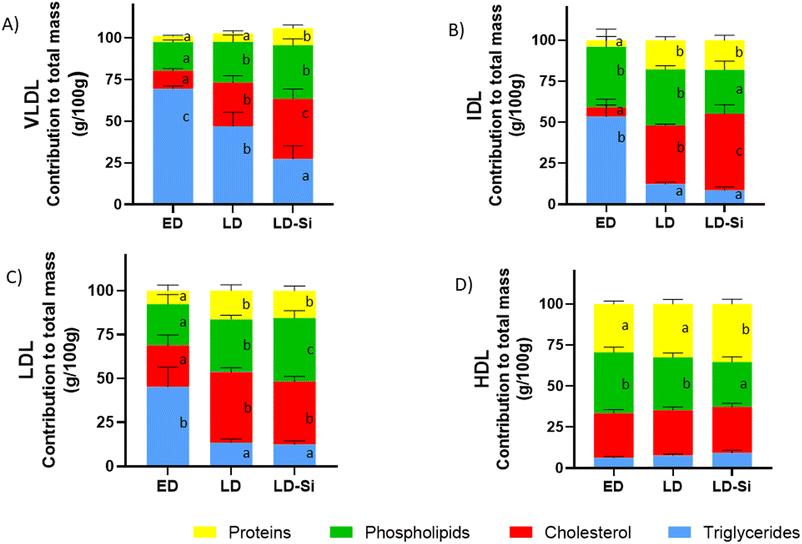
Fig. 2 shows the percentage contributions of the different lipids and proteins to the total mass of plasma VLDL, IDL, LDL, and HDL fractions. The dietary treatment significantly influenced the relative transport of triglycerides, cholesterol, phospholipids, and protein content of VLDL, IDL and LDL (all p < 0.001). For HDL, it only changed triglycerides and proteins (p < 0.05).
Regarding VLDL composition (p < 0.01), all components were significantly affected. ED rats presented VLDL with high triglyceride content (about 2/3 of total mass). Meanwhile, VLDL in LD and LD-Si rats showed higher phospholipids (p < 0.001) and cholesterol (p < 0.001) but lower triglyceride percentages than ED rats (p < 0.001). In comparison, VLDL in LD-Si rats compared to LD rats showed higher cholesterol and protein (p < 0.001) levels, but lower triglycerides (p < 0.001).
LD rats compared to their ED counterparts presented IDL enriched in cholesterol and proteins but scant in triglycerides (p < 0.01). The LD-Si group presented higher cholesterol and protein values but lower triglycerides and phospholipids (p < 0.01). LD-Si rats exhibited higher cholesterol (p < 0.001) but lower phospholipid contribution than LD rats.
The contributions to total LDL mass of cholesterol, proteins and phospholipids in LD and LD-Si rats vs. their ED counterparts were higher (p < 0.0001), while those of triglycerides were lower (p < 0.0001). Furthermore, phospholipids contributed more (p < 0.0001) to the LDL total mass in the LD-Si group than in the LD group.
Regarding HDL mass, in ED rats, cholesterol, phospholipids, and proteins were the major contributors. Regarding HDL fraction, a lower percentage of phospholipids (p < 0.05) and a higher percentage of proteins (p < 0.001) were found in LD-Si rats vs. the ED and LD counterparts.
HDL appeared as the major carrier of cholesterol, phospholipids and proteins, while VLDL was the major carrier of triglycerides, followed distantly by the HDL fraction (Fig. 2).
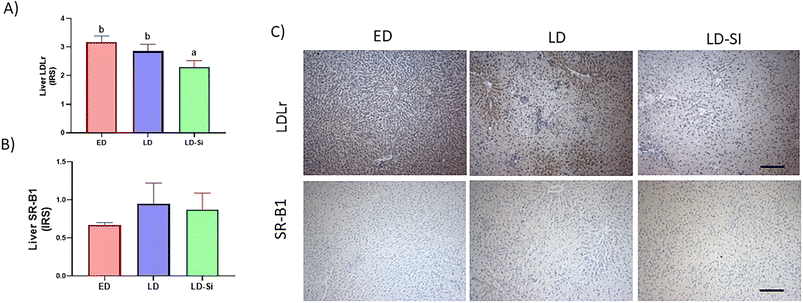
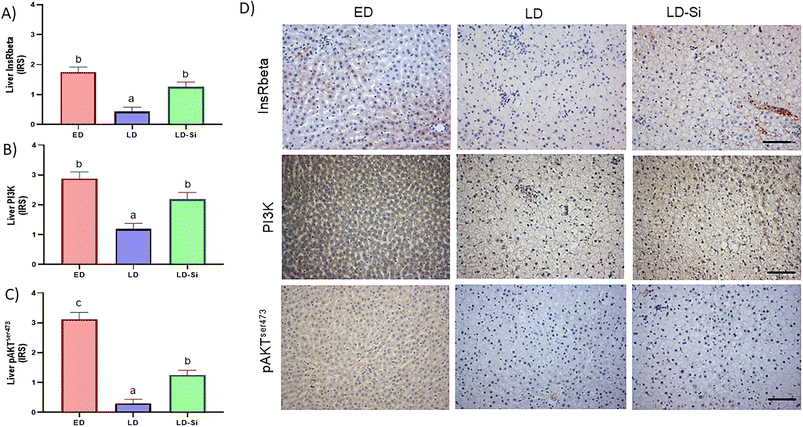
3.6. Liver LDL receptor and SR-B1, InsRbeta, PI3K, pAKTSer473 immunohistochemistry expression; the insulin signalling score
The dietary treatment had a significant influence on all markers except SR-B1 immunohistochemistry expression (p < 0.05) (Fig. 3 and 4).LDLr in LD-Si rats showed lower levels than in LD (p < 0.01) and ED rats (p < 0.0001). LD rats showed lower immunohistochemistry levels than ED for InsRbeta (p < 0.01), PI3K (p < 0.01), and pAKTSer473 (p < 0.01), which are markers of the insulin pathway. LD-Si displayed higher immunohistochemical expression of InsRbeta, PI3K and pAKTSer473 than LD rats (all p < 0.01) but lower pAKTSer473 than ED rats (p < 0.05).
The ISscore was also affected by the dietary treatment (p < 0.01), with LD rats showing significantly lower scores than ED or LD-Si rats (p < 0.05) (Table 3).
3.7. Marker correlation among glycaemic status and dyslipidaemia markers and scores
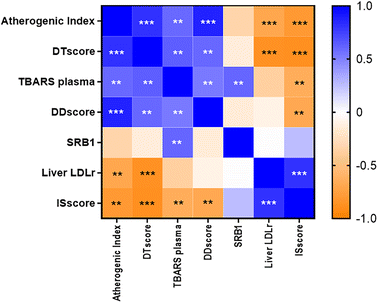
Pearson moment or Spearman correlations between the different scores, AI, TBARS and cholesterol transporters were calculated.Fig. 5 summarizes all significant correlations found, which are highlighted and marked with asterisks. A strong positive association was observed between the DTscore and the AI (r = 0.811, p < 0.001), while ISscore and LDLr (r = −0.867, p < 0.001) showed a negative significant correlation.
DTscore and DDscore were positively and significantly associated (r = 0.595, p < 0.01). ISscore was negatively and significantly correlated with DTscore (r = −0.882, p < 0.001) and DDscore (r = −0.686, p < 0.01).
4. Discussion
Diabetic dyslipidaemia is the primary and most common lipid alteration in T2DM.3 Rat is one of the most used models in cholesterol metabolism studies44 and has been used as NASH and NAFL models.33 However, there is limited evidence available regarding the benefits of incorporating silicon as a dietary functional ingredient for T2DM prevention and treatment. The results presented in this manuscript shed light on the role of silicon in reducing the detrimental effects of HSFHC meat-based diets on late-stage T2DM lipoprotein metabolism, bringing rats near, at least partially, to an earlier stage of T2DM. Silicon contributes to increase insulinaemia and HOMA-beta and reduce glycaemia, plasma oxidation, lipidaemia and the concentration of atherogenic lipoproteins (e.g., VLDL, IDL, and beta-VLDL). Beta-VLDL are highly atherogenic lipoproteins present in animals fed high-cholesterol diets.45 In addition, the consumption of silicon-enriched meat significantly reduced the level of some indices generated by the concurrence of selected markers, clearly indicating improvement in the dyslipidaemia associated with the rat diabetic status.The experimental diets were well accepted by all three groups studied. Feed intake did not differ from previous studies performed in rats fed other functional-meat products.8,25,36,41,46 Feed intake and weight gain were not affected by the dietary treatments, indicating that the differences observed in faecal excretions and dietary digestibility were due to cholesterol and silicon intakes. LD-Si rats, in comparison to LD rats, exhibited higher total faecal and fat excretion, suggesting that the silicon-enriched RM-diet may have an anti-constipation effect. This contributes to lower dietary and fat digestion and absorption, which is particularly valuable for individuals with T2DM who consume HSHC diets. Our findings align with previous research conducted on aged rats with steatosis25 and highlight the capacity of silicon to create molecular crosslinks, allowing the formation of insoluble and non-absorbable esters, similar to the interaction observed between divalent cations and saturated fatty acids.47
Hyperglycaemia and hyperlipidaemia were observed in the ED models, suggesting that feeding a HSF diet induced insulin resistance (IR) and relatively low values of HOMA-beta, which are indicative of early-stage T2DM. These results are consistent with those found in different animal models.48,49 Unpublished data from our research group in healthy control rats suggest that the overall secretion capacity of insulin (given by HOMA-beta) was 3 to 5 times higher than the values observed in ED rats.
The lipid and lipoprotein profile observed in ED rats corresponds to that of diabetic rats, characterized by the presence of a large amount of triglyceride enriched VLDL.3,50 In fact, isolated VLDL in ED rats contained 3 to 4 times more triglycerides compared to that in control rats51 and 2.5 times more than in aged control rats.26 This suggests an imbalance between the production and catabolism of VLDL particles. Almost two-thirds (62.5%) of ED rats exhibited hypertriglyceridaemia, as their plasma triglyceride levels exceeded the cut-off point of 1.7 mmol L−1 for hypertriglyceridaemia.52
The small amount of IDL and LDL particles found in ED rats (only 5% of the total plasma lipoprotein mass) and the high amount and contribution of HDL (53% of total lipoprotein mass, 70% of the total plasma cholesterol) suggest that ED rats were in an early state of diabetes, as normal rats exhibit preponderant lipid transport by HDL. Rats have been considered an HDL animal model.43
Clear differences were observed in the glycaemic status and the amount and composition of lipoproteins in rats belonging to the early and late stages of T2DM in both experimental models. LD rats showed 28% higher glycaemia, 66% less insulinaemia, and 77% lower HOMA-beta values, suggesting clear late-stage T2DM. In contrast, ED rats were in the early stage of T2DM.53 In fact, the DTscore in LD rats was 2.63 times higher than that of their ED counterparts.
The impairment of glycaemic homeostasis had noticeable effects on lipidaemia, lipoproteinaemia, and plasma oxidation status. In fact, the atherogenic index of LD rats was 1.66 to 1.75 times higher than that of ED rats, suggesting an increased CVD risk. LD rats also exhibited higher plasma TBARS levels and lower AE activity or a lower AE/cholesterol ratio than their ED counterparts, confirming the evolution of T2DM. The presence of dyslipidaemia, high oxidation, and hyperglycaemia has a negative effect on pancreatic functionality and T2DM evolution.35,53 Actually, the DTscore and DDscore significantly correlated (Y = −1.104 + 1.186 X, r = 0.595, p < 0.01), with X being the DTscore and Y the DDscore.
Interestingly, the consumption of a HSFHC diet, as opposed to an HSF one, led to a different dyslipaemic pattern: none of the LD rats presented hypertriglyceridaemia, but 87% of them became hypercholesterolaemic (plasma cholesterol ≥2.59 mmol L−1). LD rats exhibited a 40% higher cholesterol/phospholipid ratio than ED rats. It should be noted that a plasma phospholipid/cholesterol ratio (both measurements expressed as mg dL−1) higher than one has been suggested as a potential marker of hypercholesterolaemia for rats.43
Regarding lipoprotein metabolism, LD animals showed reduced levels of HDL-mass and HDL-cholesterol levels compared to ED rats. Conversely, LD vs. ED rats presented in IDL and LDL fractions higher amounts of cholesterol (2.8 and 9.6 times, respectively), proteins (15.7 and 11.6 times, respectively), and total mass (4.2 and 5.3 times, respectively). These findings clearly denote a higher number of remnant and atherogenic particles. On the other hand, the mass of VLDL was 27% lower, with a significant 5-fold increase in the cholesterol/triglyceride ratio of these lipoproteins. This suggests the presence of cholesterol-enriched and triglyceride-depleted VLDL. These results regarding lipoprotein fractions are consistent with previous observations made by our research group on cholesterol-fed rats with the presence of atherogenic cholesterol-enriched VLDL.8,25,37,45,51
Increased levels of IDL and LDL fractions were also observed in previous studies where rats were fed meat-based diets enriched in dietary cholesterol.8,25,37 The results in LD rats suggest a reduction in the catabolism of those lipoproteins due to a decreasing tendency in LDLr levels (26% lower in LD rats compared to ED rats). In situations where LDLr is downregulated, the removal of LDL and VLDL remnants decreases,53 thus contributing to the presence of these atherogenic particles in LD rats. Several significant correlations between lipoprotein markers and glycaemic homeostasis markers were found, adding evidence to the association between the DTscore and DDscore.
With respect to the HDL fraction, a lower amount of the total mass, total protein and lipids was observed. In general terms, it can be accepted that the number of HDL particles was lower in LD rats in comparison with ED rats, as the percentual amount of all components, except of triglycerides, did not significantly change. Lower levels of HDL cholesterol, HDL lipids and HDL mass have been reported in cholesterol-fed animals.3,38,42 This is the result of an increased uptake of HDL by the liver SR-B1 receptor to help eliminate cholesterol through bile.54 Although the dietary treatment did not have a significant effect on SR-B1 abundance, LD rats tended to show 42% higher SR-B1 values than ED rats.
One of the most crucial findings of this study is the significant decrease in glycaemia (16%) and the increase in insulinaemia and HOMA-beta (50%) observed in LD-Si rats when compared to their LD counterparts. This suggests that Si was able to reduce the negative effects observed in LD animals by the combined action of an HSFHC diet and a STZ-NAD injection. In fact, the DTscore was 28% lower in LD-Si rats than in LD animals, indicating that LD-Si rats were in an earlier stage of T2DM than their LD counterparts.35,53 In addition, the glycaemia of LD-Si rats did not significantly differ from that of ED rats, although their insulin (HOMA-beta) levels were higher than those of LD rats, but still lower than those of ED rats. This suggests once more that the evolution of late-stage diabetes was slowed down by the consumption of silicon-enriched meat.
LD-Si rats exhibited lower levels of cholesterol, phospholipid, and triglycerides, as well as lower atherogenic ratios than LD rats, clearly supporting the hypolipemic effects of silicon, which were previously observed in aged rats fed silicon-enriched HSFHC diets.25
In line with previous results, the silicon in the diet induced higher faecal fat content (34%) and total faecal excretion (22%) and reduced dietary and fat digestibilities (6.5% and 4%, respectively). These facts could be explained, at least partially, by the ability of silicon to create molecular crosslinks, enabling the formation of more or less absorbable esters and/or ethers,25 as has been previously discussed.
LD-Si rats showed lower plasma TBARS but higher AE activity in plasma compared to their LD counterparts, suggesting a clear antioxidant effect of dietary silicon. Garcimartín et al.25 reported a significant reduction in VLDL-TBARS in aged rats fed silicon-enriched HSFHC diets, along with increased AE activity, which explains, at least partially, the present results. Silicon has been found to reduce brain oxidation (as indicated by TBARS levels in rats intoxicated with aluminium).27,29 Although the AE/cholesterol and/or total cholesterol/HDL cholesterol ratios55 are normally used as markers of antioxidant capacity, given the more external and pro-oxidative position of phospholipids in lipoproteins, the ratios of AE/phospholipids and AE/VLDL phospholipids could be highly diminished by oxidation. LD-Si rats exhibited increases in AE and the AE/cholesterol, AE/VLDL cholesterol, and AE/VLDL phospholipids ratios compared to their LD counterparts, suggesting that AE activity was higher due to Si ingestion. AE is one of the three activities of the paraoxonase-1 (PON-1) enzyme, which has been proposed to be a suicide enzyme,15 acting prior to other antioxidant enzymes to maintain the antioxidant status. Given the demonstrated antioxidant effects of silicon,25–29 it can be suggested that AE is preserved in ED-Si rats.
The observed changes in lipoproteins in LD-Si rats and LD rats also indicate that the negative effects previously observed in LD rats were partially blocked by silicon-enriched meat compared to control meat.
The ingestion of silicon-enriched meat was found to primarily affect the VLDL fraction, leading to a 3-fold reduction in the total mass of this lipoprotein fraction. These results are once more in agreement with previous findings on aged rats fed silicon-enriched meat.25 A detailed study suggests that the VLDL fraction of LD-Si rats was highly depleted in triglycerides and phospholipids compared to that of LD rats. This hypolipemic effect of silicon could be due to a reduced synthesis of VLDL, probably related to an increased availability of fatty acids for liver cholesterol esterification.45,56 The expression of sterol O-acyltransferase has been found to reduce the cholesterol-free liver pool and increase the cholesterol ester pool as a strategy to enhance LDLr levels.10 In our study, the abundance of LDLr was lower in LD-Si rats compared to LD rats, which is not in agreement with previous results obtained in hypercholesterolemic rats fed Si-enriched meat25 or seaweed-enriched meat.37 This suggests that the late stage of T2DM negatively influenced LDLr expression. However, it should be noted that abundance and gene expression are not always correlated, as post-translational effects can be induced.56 Therefore, other hypotheses should not be disregarded.
Rats show very rapid liver uptake of VLDL after their secretion, which explains the low transfer of apo B 100 from VLDL to IDL and LDL, as well as the low IDL and LDL values in rats.57 Silicon could induce rapid fatty acid beta-oxidation, decreasing its bioavailability for triglyceride synthesis and the formation/secretion of VLDL. It can be suggested that the low LDLr found could be sufficient to assure VLDL and remnant lipoprotein uptake in LD-Si rats. Nonetheless, we are far from knowing the precise mechanism involved.
The InsRbeta/PI3K/AKT pathway participates in the assembly and maturation of VLDL58 and accounts for the lower amount and size of VLDL. Likewise, the improvement in insulin signalling (given by the ISscore) in LD-Si vs. LD rats’ livers also suggests an improvement of glucose uptake in different tissues through AKT phosphorylation, as proposed by Macho-González et al.35
The PI3K (r = −0.539, p < 0.05) and pAktser473 (r = −0.484, p < 0.05) levels, quantified by immunochemistry, significantly and negatively correlated with VLDL cholesterol; the latter also showed a negative correlation with HOMA-beta (r = −0.454, p < 0.05).
Considering the VLDL, IDL and LDL composition of LD-Si rats compared to that of their LD counterparts, it can be suggested that silicon influenced the clearance of remnant lipoproteins. In LD-Si rats, VLDL contributed less to blood cholesterol transport than IDL, while in LD rats, VLDL appeared as a major cholesterol carrier compared to IDL (Fig. 2).
Cholesterol ester transfer protein (CETP) is very poorly expressed in rats,59 which would explain the relatively high contribution of triglycerides by HDL or LDL to total plasma triglycerides in LD-Si rats vs. LD rats. Currently, we do not have a clear explanation for these results; however, the passive transference of lipids between lipoproteins in rats should not be discarded.
The total mass and composition of HDL were not significantly modified in LD rats compared to ED animals, which explains the similar SR-B1 levels in both rat groups. Lecithin cholesterol acyltransferase (LCAT), an enzyme that acts on HDL, is a key component of reverse cholesterol transport.57 In murine lipid metabolism, HDL accepts non-esterified cholesterol from peripheral tissues, and the non-esterified cholesterol is converted into the ester form by the action of LCAT. Subsequently, the cholesteryl esters are delivered to the liver. In rats, a significant hepatic uptake of cholesteryl ester from HDL has been observed.59,60
In line with previous studies in control animals, VLDL was identified as the major carrier of TG, followed by HDL.37,45,46 However, the diet highly influenced the VLDL-triglyceride/HDL-triglyceride ratio (about 8 in ED rats, 4 in LD and about 1 in LD-Si). HDL was the major carrier of cholesterol, phospholipids, and protein, followed by VLDL (the HDL-cholesterol/VLDL cholesterol ratios were 4, 1.66 and 3 in ED, LD and LD-Si, respectively), suggesting that LD-Si rats exhibited an intermediate metabolic state between ED and LD rats.
In order to assess the relationship between modifications in lipid metabolism markers and diabetic status, different parameters were compared and correlated. DDscore and DTscore showed a high correlation coefficient (r = 0.595, p < 0.01). The regression equation found suggests that a one unit increase in the DTscore corresponds to a change of 1.186 units in the DDscore.
Both scores are also positively correlated with the AI and TBARS in plasma but inversely with SR-B1, LDLr and the ISscore (Fig. 5), confirming the validity of this score as a predictive parameter in spite of other isolated markers. Although the relationship found between the diabetic trend and diabetic dyslipidaemia scores is very relevant, some limitations should be acknowledged: (1) the study was conducted solely on young male rats, (2) healthy controls were not included, (3) only one dose of silicon was tested, and (4) the study lasted only 8 weeks. As T2DM is a chronic degenerative disease, future research should analyse the effects of silicon itself and/or silicon-enriched meat in female rats, as well as in animal models following longitudinal studies. In addition, as silicon has been found to be safe in healthy and aged rats,25 it would be desirable to conduct clinical trials in prediabetic and diabetic men and women.
In conclusion, the present results demonstrate, for the first time, that a diet containing meat formulated with silicon improves both dyslipidaemia and HOMA status, leading to a less advanced late stage of T2DM and restoring pancreatic functionality. Fundamentally, silicon reduces plasma triglyceride levels and the presence of VLDL by increasing faecal fat excretion and improving the insulin secretory function (as HOMA-beta). At same time, it favours the clearance of VLDL and IDL while inducing a lower prooxidant status by decreasing TBARS in plasma and preserving high levels of AE and AE/phospholipid activity. Therefore, the results support the idea that silicon-enriched meat could be an adequate functional food for consumption by T2DM patients, as it may slow down disease progression and improve associated dyslipidaemia.
Abbreviations
| pAktser473 | Alpha serine/threonine–protein kinase type 2 |
| CVD | Cardiovascular diseases |
| DDscore | Diabetic dislipidaemia score |
| DTscore | Diabetic trend score |
| HDL | High density lipoproteins |
| HSF | High saturated fat |
| HSFHC | High saturated fat-high cholesterol |
| IDL | Intermediate density lipoproteins |
| InsRbeta | Insulinreceptor beta |
| ISscore | Insulin signalling score |
| LDL | Low density lipoproteins |
| LDLr | Low density receptor gene |
| NAFL | Non-alcoholic fatty liver |
| NASH | Non-alcoholic steatohepatitis |
| PI3K | Phospho-inositol-3 kinase |
| SRB1 | Scavenger receptor type B1 |
| T2DM | Type 2 Diabetes mellitus |
| VLDL | Very low density lipoproteins |
Author contributions
M. H.-M.: Conceptualization; data curation; formal analysis, visualization; writing original draft; and review & editing; A. M.-G.: Conceptualization; data curation; formal analysis, visualization; review & editing. A. G.: Conceptualization, methodology, investigation, supervision, writing – original draft, and review & editing; M. E. L.-O.: Conceptualization; data curation; formal analysis, visualization; writing – original draft; and writing – review & editing. A. B.: Writing-review and editing. R. R.-C.: Formal analysis, review & editing. S. B.: Project administration; funding acquisition, resources; software; supervision. J. B.: Project administration; funding acquisition, resources; supervision. F. J. S.-M.: Project administration; funding acquisition, resources; software; editing and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish Project with references PID2019-103872RB-I00 and /AEI/10.13039/501 100 011 033. Rocío Redondo-Castillejo was supported by grant FPU20/0 2920 from the Spanish Ministry of Universities. Authors acknowledge María José González-Muñoz for her scientific assessment. Special thanks to Rita Shimano for editing the English language manuscript.References
- T. J. Chahil and H. N. Ginsberg, Diabetic dyslipidemia, Endocrinol. Metab. Clin. North Am., 2006, 35, 491–510 CrossRef CAS PubMed.
- M. P. Vaquero, M. Martínez-Suárez, Á. García-Quismondo, F. J. del Cañizo and F. J. Sánchez-Muniz, Diabesity negatively affects transferrin saturation and iron status. The DICARIVA study, Diabetes Res. Clin. Pract., 2021, 172, 108653 CrossRef PubMed.
- U. Galicia-Garcia, A. Benito-Vicente, S. Jebari, A. Larrea-Sebal, H. Siddiqi, K. B. Uribe, H. Ostolaza and C. Martín, Pathophysiology of type 2 diabetes mellitus, Int. J. Mol. Sci., 2020, 21, 6275 CrossRef CAS PubMed.
- T. Hirano, Pathophysiology of diabetic dyslipidemia, J. Atheroscler. Thromb., 2018, 25, 771–782 CrossRef CAS PubMed.
- I. J. Goldberg, Clinical review 124: Diabetic dyslipidemia: causes and consequences, J. Clin. Endocrinol. Metab., 2001, 86, 965–971 CrossRef CAS PubMed.
- International Diabetes Federation, Diabetes Complications, https://idf.org/about-diabetes/diabetes-complications/, (accessed 14 November 2021).
- I. Gracia-Rubio, C. Martín, F. Civeira and A. Cenarro, SR-B1, a key receptor involved in the progression of cardiovascular disease: A perspective from mice and human genetic studies, Biomedicines, 2021, 9, 612 CrossRef PubMed.
- J. A. Santos-López, A. Garcimartín, S. Bastida, M. Bautista-Ávila, M. J. González-Muñoz, J. Benedí and F. J. Sánchez-Muniz, Lipoprotein profile in aged rats fed chia oil- or hydroxytyrosol-enriched pork in high cholesterol/high saturated fat diets, Nutrients, 2018, 26, 1830 CrossRef PubMed.
- R. Ross, Atherosclerosis–an inflammatory disease, N. Engl. J. Med., 1999, 340, 115–126 CrossRef CAS PubMed.
- J. M. Dietschy, Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations, J. Nutr., 1998, 128, 444–448 CrossRef PubMed.
- J. D. Sparks, C. E. Sparks and K. Adeli, Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia, Arterioscler., Thromb., Vasc. Biol., 2012, 32, 2104–2112 CrossRef CAS PubMed.
- R. de la Iglesia, P. Lopez-Legarrea, P. Celada, F. J. Sánchez-Muniz, J. A. Martinez and M. Á. Zulet, Beneficial effects of the RESMENA dietary pattern on oxidative stress in patients suffering from metabolic syndrome with hyperglycemia are associated to dietary TAC and fruit consumption, Int. J. Mol. Sci., 2013, 14, 6903–6919 CrossRef CAS PubMed.
- A. Canales and F. J. Sánchez-Muniz, Paroxonasa, ¿algo más que una enzima? [Paraoxonase, something more than an enzyme?], Med. Clin., 2003, 121, 537–548 CrossRef PubMed.
- P. Celada, F. J. Sánchez-Muniz, G. Delgado-Pando, S. Bastida, M. Espárrago Rodilla, F. Jiménez-Colmenero and B. Olmedilla-Alonso, Effects of improved fat meat products consumption on emergent cardiovascular disease markers of male volunteers at cardiovascular risk, J. Physiol. Biochem., 2016, 72, 669–678 CrossRef CAS PubMed.
- M. Vázquez-Velasco, L. Esperanza Daz, R. Lucas, S. Gómez-Martínez, S. Bastida, A. Marcos and F. J. Sánchez-Muniz, Effects of hydroxytyrosol-enriched sunflower oil consumption on CVD risk factors, Br. J. Nutr., 2011, 105, 1448–1452 CrossRef PubMed.
- A. Macho-González, A. Garcimartín, M. E. López-Oliva, S. Bastida, J. Benedí, G. Ros, G. Nieto and F. J. Sánchez-Muniz, Can meat and meat-products induce oxidative stress?, Antioxidants, 2020, 9, 638 CrossRef PubMed.
- B. C. Johnston, D. Zeraatkar, M. A. Han, R. W. M. Vernooij, C. Valli, R. El Dib, C. Marshall, P. J. Stover, S. Fairweather-Taitt, G. Wójcik, F. Bhatia, R. De Souza, C. Brotons, J. J. Meerpohl, C. J. Patel, B. Djulbegovic, P. Alonso-Coello, M. M. Bala and G. H. Guyatt, Unprocessed red meat and processed meat consumption: Dietary guideline recommendations from the Nutritional Recommendations (NutriRECS) Consortium, Ann. Intern. Med., 2019, 171, 756–764 CrossRef PubMed.
- N. Barnard, S. Levin and C. Trapp, Meat consumption as a risk factor for type 2 diabetes, Nutrients, 2014, 6, 897–910 CrossRef PubMed.
- B. Olmedilla-Alonso, F. Jiménez-Colmenero and F. J. Sánchez-Muniz, Development and assessment of healthy properties of meat and meat products designed as functional foods, Meat Sci., 2013, 95, 919–930 CrossRef PubMed.
- F. Jiménez-Colmenero, F. J. Sánchez-Muniz and B. Olmedilla-Alonso, Design and development of meat-based functional foods with walnut: Technological, nutritional and health impact, Food Chem., 2010, 123, 959–967 CrossRef.
- G. Delgado-Pando, P. Celada, F. J. Sánchez-Muniz, F. Jiménez-Colmenero and B. Olmedilla-Alonso, Effects of improved fat content of frankfurters and pâtés on lipid and lipoprotein profile of volunteers at increased cardiovascular risk: a placebo-controlled study, Eur. J. Nutr., 2014, 53, 83–93 CrossRef CAS PubMed.
- A. Macho-González, S. Bastida, A. Garcimartín, M. E. López-Oliva, P. González, J. Benedí, M. J. González-Muñoz and F. J. Sánchez-Muniz, Functional meat products as oxidative stress modulators: A review, Adv. Nutr., 2021, 12, 1514–1539 CrossRef PubMed.
- F. J. Sánchez-Muniz, A. Macho-González, S. Bastida, A. Garcimartín, A. Bocanegra, A. Canales, M. Nus, M. Vázquez-Velasco, L. González-Torres, R. Redondo Castillejo, M. Hernández Martín, M. E. López-Oliva Muñoz, J. A. Santos, P. Celada, M. J. González-Muñoz, A. R. Schultz Moreira and J. Benedí, Healthy and functional meat products. Scientific evidence of the AFUSAN group, An. R. Acad. Farm., 2022, 88, 603–626 Search PubMed.
- R. Peñalver, L. Martínez-Zamora, J. M. Lorenzo, G. Ros and G. Nieto, Nutritional and antioxidant properties of Moringa oleifera leaves in functional foods, Foods, 2022, 11, 1107 CrossRef PubMed.
- A. Garcimartín, J. A. Santos-López, S. Bastida, J. Benedí, F. J. Sánchez-Muniz, A. Garcimartín, J. A. Santos-López, S. Bastida, J. Benedí and F. J. Sánchez-Muniz, Silicon-enriched restructured pork affects the lipoprotein profile, VLDL oxidation, and LDL receptor gene expression in aged rats fed an atherogenic diet, J. Nutr., 2015, 145, 2039–2045 CrossRef PubMed.
- F. J. Sánchez-Muniz, A. Macho-González, A. Garcimartin, J. A. Santos-López, J. Benedi, S. Bastida and M. J. González-Munoz, The nutritional components of beer and its relationship with neurodegeneration and Alzheimer’s disease, Nutrients, 2019, 11, 1558 CrossRef PubMed.
- M. J. González-Muñoz, A. Garcimartán, I. Meseguer, C. J. Mateos-Vega, J. M. Orellana, A. Peña-Fernández, J. Benedí and F. J. Sánchez-Muniz, Silicic acid and beer consumption reverses the metal imbalance and the prooxidant status Induced by aluminum nitrate in mouse brain, J. Alzheimer's Dis., 2017, 56, 917–927 Search PubMed.
- K. R. Martin, Silicon: the health benefits of a metalloid, Met. Ions Life Sci., 2013, 13, 451–473 Search PubMed.
- P. Merino, J. A. Santos-López, C. J. Mateos, I. Meseguer, A. Garcimartín, S. Bastida, F. J. Sánchez-Muniz, J. Benedí and M. J. González-Muñoz, Can nonalcoholic beer, silicon and hops reduce the brain damage and behavioral changes induced by aluminum nitrate in young male Wistar rats?, Food Chem. Toxicol., 2018, 118, 784–794 CrossRef CAS PubMed.
- M. R. Peluso and B. O. Schneeman, A food-grade silicon dioxide is hypocholesterolemic in the diet of cholesterol-fed rats, J. Nutr., 1994, 124, 853–860 CrossRef CAS PubMed.
- A. Garcimartín, M. E. López-Oliva, A. Macho-González, S. Bastida, J. Benedí and F. J. Sánchez-Muniz, Hypoglycaemic and hypotriglyceridaemic postprandial properties of organic silicon, J. Funct. Foods, 2017, 29, 290–294 CrossRef.
- M. F. McCarty, Reported antiatherosclerotic activity of silicon may reflect increased endothelial synthesis of heparan sulfate proteoglycans, Med. Hypotheses, 1997, 49, 175–176 CrossRef CAS PubMed.
- A. Garcimartín, M. E. López-Oliva, J. A. Sántos-López, R. A. García-Fernández, A. Macho-González, S. Bastida, J. Benedí and F. J. Sánchez-Muniz, Silicon alleviates nonalcoholic steatohepatitis by reducing apoptosis in aged wistar rats fed a high-saturated fat, high-cholesterol diet, J. Nutr., 2017, 147, 1104–1112 CrossRef PubMed.
- M. Hernández-Martín, A. Bocanegra, R. Redondo-Castillejo, A. Macho-González, F. J. Sánchez-Muniz, J. Benedí, S. Bastida, R. A. García-Fernández, A. Garcimartín and M. E. López-Oliva, Could duodenal molecular mechanisms be involved in the hypocholesterolemic effect of silicon used as functional ingredient in late-stage type 2 diabetes mellitus?, Mol. Nutr. Food Res., 2022, 66, 24 CrossRef PubMed.
- A. Macho-González, M. E. López-Oliva, J. J. Merino, R. A. García-Fernández, A. Garcimartín, R. Redondo-Castillejo, S. Bastida, F. J. Sánchez-Muniz and J. Benedí, Carob fruit extract-enriched meat improves pancreatic beta-cell dysfunction, hepatic insulin signaling and lipogenesis in late-stage type 2 diabetes mellitus model, J. Nutr. Biochem., 2020, 84, 108461 CrossRef PubMed.
- A. H. M. Terpstra, C. J. H. Woodward and F. J. Sanchez-Muniz, Improved techniques for the separation of serum lipoproteins by density gradient ultracentrifugation: visualization by prestaining and rapid separation of serum lipoproteins from small volumes of serum, Anal. Biochem., 1981, 111, 149–157 CrossRef CAS PubMed.
- R. Olivero-David, A. Schultz-Moreira, M. Vázquez-Velasco, L. González-Torres, S. Bastida, J. Benedí, M. I. Sánchez-Reus, M. J. González-Muñoz and F. J. Sánchez-Muniz, Effects of Nori- and Wakame-enriched meats with or without supplementary cholesterol on arylesterase activity, lipidaemia and lipoproteinaemia in growing Wistar rats, Br. J. Nutr., 2011, 106, 1476–1486 CrossRef CAS PubMed.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- M. Mihara and M. Uchiyama, Determination of malonaldehyde precursor in tissues by thiobarbituric acid test, Anal. Biochem., 1978, 86, 271–278 CrossRef CAS PubMed.
- A. Macho-González, A. Garcimartín, M. E. López-Oliva, P. Celada, S. Bastida, J. Benedí and F. J. Sánchez-Muniz, Carob-fruit-extract-enriched meat modulates lipoprotein metabolism and insulin signaling in diabetic rats induced by high-saturated-fat diet, J. Funct. Foods, 2020, 64, 103600 CrossRef.
- M. Ruperto, G. Barril and F. J. Sánchez-Muniz, Usefulness of the conicity index together with the conjoint use of adipocytokines and nutritional-inflammatory markers in hemodialysis patients, J. Physiol. Biochem., 2017, 73, 67–75 CrossRef CAS PubMed.
- A. Macho-González, A. Garcimartín, N. Redondo, S. Cofrades, S. Bastida, E. Nova, J. Benedí, F. J. Sánchez-Muniz, A. Marcos and M. E. López-Oliva, Carob fruit extract-enriched meat, as preventive and curative treatments, improves gut microbiota and colonic barrier integrity in a late-stage T2DM model, Food Res. Int., 2021, 141, 110124 CrossRef PubMed.
- F. J. Sánchez-Muniz and S. Bastida, Do not use the Friedewald formula to calculate LDL-cholesterol in hypercholesterolaemic rats, Eur. J. Lipid Sci. Technol., 2008, 110, 295–301 CrossRef.
- M. T. Chiang, Y. C. Chen and A. L. Huang, Plasma lipoprotein cholesterol levels in rats fed a diet enriched in cholesterol and cholic acid, Int. J. Vitam. Nutr. Res., 1998, 68, 328–334 CAS.
- S. Bastida, M. C. García Linares, J. Viejo, M. T. García Arias and F. J. Sánchez-Muniz, Effect of olive oil-fried sardine consumption on liver lipid composition and fatty acid cholesterol esterification in hypercholesterolemic rats, Food Sci. Technol. Int., 2003, 9, 329–338 CrossRef.
- A. Macho-González, A. Garcimartín, M. E. López-Oliva, B. Ruiz-Roso, I. M. de la Torre, S. Bastida, J. Benedí and F. J. Sánchez-Muniz, Can carob-fruit-extract-enriched meat improve the lipoprotein profile, VLDL-oxidation, and LDL receptor levels induced by an atherogenic diet in STZ-NAD-diabetic rats?, Nutrients, 2019, 1, 332 CrossRef PubMed.
- K. Schwarz, Silicon, fibre, and atherosclerosis, Lancet, 1977, 1, 454–457 CrossRef CAS PubMed.
- S. P. Sah, B. Singh, S. Choudhary and A. Kumar, Animal models of insulin resistance: A review, Pharmacol. Rep., 2016, 68, 1165–1177 CrossRef CAS PubMed.
- S. Skovsø, Modeling type 2 diabetes in rats using high fat diet and streptozotocin, J. Diabetes Invest., 2014, 5, 358 Search PubMed.
- M. E. Haas, A. D. Attie and S. B. Biddinger, The regulation of ApoB metabolism by insulin, Trends Endocrinol. Metab., 2013, 24, 391–397 CrossRef CAS PubMed.
- A. R. Schultz Moreira, J. Benedí, L. González-Torres, R. Olivero-David, S. Bastida, M. I. Sánchez-Reus, M. J. González-Muñoz and F. J. Sánchez-Muniz, Effects of diet enriched with restructured meats, containing Himanthalia elongata, on hypercholesterolaemic induction, CYP7A1 expression and antioxidant enzyme activity and expression in growing rats, Food Chem., 2011, 129, 1623–1630 CrossRef CAS.
- L. Berglund, J. D. Brunzell, A. C. Goldberg, I. J. Goldberg, F. Sacks, M. H. Murad and A. F. H. Stalenhoef, Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab., 2012, 97, 2969–2989 CrossRef CAS PubMed.
- V. A. Fonseca, Defining and characterizing the progression of type 2 diabetes, Diabetes Care, 2009, 32, 151–156 CrossRef PubMed.
- W. J. Shen, S. Azhar and F. B. Kraemer, SR-B1: A Unique multifunctional receptor for cholesterol influx and efflux, Annu. Rev. Physiol., 2018, 80, 95–116 CrossRef CAS PubMed.
- L. González-Torres, M. Vázquez-Velasco, R. Olivero-David, S. Bastida, J. Benedí, R. Raposo González, M. J. González-Muñoz and F. J. Sánchez-Muniz, Glucomannan and glucomannan plus spirulina added to pork significantly block dietary cholesterol effects on lipoproteinemia, arylesterase activity, and CYP7A1 expression in Zucker fa/fa rats, J. Physiol. Biochem., 2015, 71, 773–784 CrossRef PubMed.
- A. Schultz Moreira, L. González-Torres, R. Olivero-David, S. Bastida, J. Benedi and F. J. Sánchez-Muniz, Wakame and Nori in restructured meats included in cholesterol-enriched diets affect the antioxidant enzyme gene expressions and activities in Wistar rats, Plant Foods Hum. Nutr., 2010, 65, 290–298 CrossRef PubMed.
- D. W. Bilheimer, S. Eisenberg and R. I. Levy, The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations, Biochim. Biophys. Acta, 1972, 260, 212–221 CrossRef CAS PubMed.
- S. H. Choi and H. N. Ginsberg, Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance, Trends Endocrinol. Metab., 2011, 22, 353–363 CrossRef CAS PubMed.
- D. Steinberg, Drugs Affecting Lipid Metabolism, Springer, Berlin, Heidelberg, 1987, pp. 42–47 Search PubMed.
- C. Glass, R. C. Pittman, D. B. Weinstein and D. Steinberg, Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 5435–5439 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04103d |
| ‡ These two authors should be considered joint first authors of the paper due to their equivalent contribution to the paper. |
| This journal is © The Royal Society of Chemistry 2024 |