Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
(R,S)-Equol 7-β-D-glucuronide, but not other circulating isoflavone metabolites, modulates migration and tubulogenesis in human aortic endothelial cells targeting the VEGF pathway†
Juan Antonio
Giménez-Bastida
*a,
María Ángeles
Ávila-Gálvez
ab,
Alicia
Martínez-López
cd,
Diana
García-Moreno
cd,
Juan Carlos
Espín
 a and
Antonio
González-Sarrías
a and
Antonio
González-Sarrías
 *a
*a
aLaboratory of Food & Health, Research Group on Quality, Safety and Bioactivity of Plant Foods, CEBAS-CSIC, Murcia, Spain. E-mail: jgbastida@cebas.csic.es; agsarrias@cebas.csic.es
bNOVA Medical School|Faculdade de Ciências Médicas, NMS|FCM, Universidade NOVA de Lisboa, Lisboa, Portugal
cCenter for Biomedical Research in Rare Diseases Network (CIBERER), Carlos III Health Institute, 28029, Madrid, Spain
dBiomedical Research Institute of Murcia (IMIB)-Pascual Parrilla, 30120, Murcia, Spain
First published on 28th November 2023
Abstract
Current knowledge indicates that the consumption of isoflavone-rich foodstuffs can have a beneficial impact on cardiovascular health. To what extent these isoflavones act as the main actors of that benefit is less clear. Genistein (GEN), daidzein (DAZ), and the DAZ-derived microbial metabolite equol (Eq) exhibit antiangiogenic effects in vitro, but their low bloodstream concentrations make it difficult to rationalize the in vivo effects. Their derived phase-II metabolites (glucuronides and sulfates) are major metabolites found in plasma, but their role as antiangiogenic molecules remains unexplored. We aimed here to first assess the anti-angiogenic activities of the main circulating isoflavone metabolites (glucuronides and sulfates) and compare them with their corresponding free forms at physiological concentrations (0.1–10 μM). The effects of the conjugated vs. free forms on tubulogenesis, cell migration, and VEGF-induced signalling were investigated in primary human aortic endothelial cells (HAECs). While (R,S)-equol 7-β-D-glucuronide (Eq 7-glur) exerted dose-dependent inhibition of tubulogenesis and endothelial migration comparable to that exerted by the free forms (GEN, DAZ, and Eq), the rest of the phase-II conjugates exhibited no significant effects. The underlying molecular mechanisms were independent of the bFGF but related to the modulation of the VEGF pathway. Besides, the observed dissimilar cellular metabolism (conjugation/deconjugation) places the phase-II metabolites as precursors of the free forms; however, the question of whether this metabolism impacts their biological activity requires additional studies. These new insights suggest that isoflavones and their circulating metabolites, including Eq 7-glur, may be involved in cardiovascular health (e.g., targeting angiogenesis).
Introduction
Targeting angiogenesis is a pivotal strategy against chronic diseases such as atherosclerosis and (or) cancer. Tumour progression and metastasis are angiogenesis-dependent processes that require sustained cell division, migration, and endothelial cell assembly to form capillary-like structures from preexisting vessels providing nutrients and oxygen to cancerous cells.1–4 Currently, approved anti-angiogenic drugs have numerous associated side effects and are not effective for all patients. Even those who initially respond well may develop resistance over time. This situation highlights the need to search for alternative therapies, such as using new compounds (e.g., natural products), which could overcome these drawbacks and limitations.5 The interest in using non-toxic phytochemicals as a broad-spectrum alternative includes dietary (poly)phenols as antiangiogenic agents. Dietary (poly)phenols exhibit pleiotropic and multi-target activities, and therefore, their use represents a cost-effective and easily accessible method with reduced adverse side effects compared to conventional therapies.5,6 In this regard, an attractive approach is to consider angiogenesis as a target of (poly)phenols resulting in beneficial effects against chronic diseases such as cancer.6,7 Curcumin, resveratrol, quercetin, genistein (GEN), and epigallocatechin gallate exemplify the role of (poly)phenols as effective antiangiogenic agents.8 However, several issues should be considered:9 (i) current evidence mainly comes from in vitro studies conducted with free forms, overlooking phase-II metabolism; (ii) (poly)phenols-gut microbiota interaction is not considered; and (iii) the concentrations tested are much higher than those detected in vivo. Therefore, the circulating phenolic-derived metabolites (at plausible in vivo concentrations) deserve attention as potential candidates to exert the anti-angiogenic effects attributed to the (poly)phenolic dietary precursors.Isoflavones are a subclass of flavonoids found almost exclusively in legumes (Fabaceae), with the highest amounts found in soybeans (Glycine max (L.)) and soy products such as soy flour, soy milk, miso, tofu, among others, while lower levels are detected in the seeds and roots of other legumes such as fava beans, lentils, or chickpeas.10,11 Regarding the anti-angiogenic properties of isoflavones, GEN is recognized for its anti-angiogenic efficacy via modulation of a wide range of molecular mechanisms, including the reduced proliferation of endothelial and cancer cells, down-regulation of proangiogenic factors such as protein tyrosine kinase activity (PTKs), matrix metalloproteinases (MMPs), mitogen-activated protein kinases (MAPKs), epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor (VEGF) and its receptors (VEGFR).12–15 Further, endothelial cellular mechanisms include inhibition of cell migration and tube formation against MH7A and human umbilical vein endothelial cells (HUVECs) stimulated with VEGF.13,16,17 Much less information regarding the effects (proliferation and migration) of DAZ and equol (Eq) on endothelial cells is available,18,19 while studies on phase-II metabolites and angiogenesis effects are scarce.
Isoflavone glycosides (i.e., daidzin, genistin, and glycitin) are poorly absorbed in the gut. Instead, they are hydrolyzed to their aglycones and reach the colon. These molecules readily absorb or undergo microbial metabolism to form Eq, O-desmethylangolensin (ODMA), or dihydrogenistein. Further phase-II metabolism in the enterocyte and liver will biosynthesize glucuronides, sulfates and sulfo-glucuronides as the main metabolites (i.e., Eq 7-glucuronide, DAZ 4′-sulfate, and GEN 7-glucuronide), which will reach the circulatory system.10,20 GEN and DAZ 4′-sulfo-7-glucuronides have been identified as major metabolites in human plasma, reaching concentrations between 0.5 and 0.8 μM.21 Eq 7-glucuronide is the main metabolite derived from Eq, which can reach concentrations between 30 and 100 nM in plasma and systemic tissues, respectively.22 Previous clinical trials have reported that these circulating conjugated metabolites achieve concentrations in serum ranging from high nM to low μM, being detected in target tissues such as the human mammary gland,22,23 where they may exert biological effects, including their anti-angiogenic properties.24,25 However, to the best of our knowledge, the effects and molecular mechanisms of circulating isoflavone metabolites on angiogenesis in primary human aortic endothelial cells (HAECs), which may resemble physiological conditions in adult tissues, remain elusive.
The current study aims at determining, for the first time, the anti-angiogenic effects and the underlying mechanisms of physiologically relevant phase-II conjugates (glucuronides and sulfates) of GEN, DAZ, and the microbiota-derived metabolite Eq on HAECs. Specifically, we investigated the effect of these metabolites on the capacity of the endothelial cells to form capillary-like structures, migrate, and modulate the basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) pathways. For comparative purposes, we also tested the effects of the free forms and their formation via deconjugation by cell metabolism.
Materials and methods
Materials
(R,S)-Equol 7-β-D-glucuronide sodium salt (Eq 7-glur), genistein (GEN), GEN 4′-β-D-glucuronide (GEN 4′-glur), GEN 7-β-D-glurcuronide (GEN 7-glur), daidzein (DAZ), DAZ 4′-β-D-glucuronide (DAZ 4′-glur), DAZ 7-β-D-glucuronide glur potassium salt (DAZ 7-glur), and DAZ 4′-sulfate disodium salt (DAZ 4′-sulf) were obtained from LGC Standards (Barcelona, Spain) and Toronto Research Chemicals (North York, ON, Canada). Endothelial cell growth medium (ECGM) enriched with growth supplements (complete medium) was purchased from Tebu-Bio (Barcelona, Spain). Corning® Matrigel® Growth Factor Reduced was purchased from Cultek (Madrid, Spain). Recombinant human vascular endothelial growth factor 165 protein (VEGF165) and recombinant human basic fibroblast growth factor (bFGF) protein were supplied by Bio-techne (Minneapolis, MN, USA). (S)-Equol, Hanks’ balanced salt solution (HBSS), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Trypan Blue Solution, and dimethyl sulfoxide (DMSO) were provided by Sigma-Aldrich (St Louis, MO, USA). The antibodies used to study protein expression/activation (see below) were obtained from cell signalling (MA, USA), Werfen (Barcelona, Spain), and Bio-techne (Minneapolis, MN, USA).Cell culture conditions and treatments
Human aortic endothelial cells (HAECs) were purchased from the European Collection of Cell Cultures (Salisbury, UK). HAECs were cultured in endothelial cell basal medium (ECBM) enriched with growth supplements (GS) and seeded at 5000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 in 25 cm2 flasks. Cells were maintained at 37 °C, 95% relative humidity, and 5% CO2. The culture medium was replaced every other day until ∼80% confluence. Then, following the manufacturer's instructions, HAECs were subcultured using the reagent kit (Salisbury, UK) and treated as described below. Passages 2–6 were used for all the experiments.
000 cells per cm2 in 25 cm2 flasks. Cells were maintained at 37 °C, 95% relative humidity, and 5% CO2. The culture medium was replaced every other day until ∼80% confluence. Then, following the manufacturer's instructions, HAECs were subcultured using the reagent kit (Salisbury, UK) and treated as described below. Passages 2–6 were used for all the experiments.
Dose information
We used DMSO as a vehicle to dilute the standards of the isoflavones. The presence of sodium or potassium salts in the structure of the molecules tested was considered in the preparation of the standards (stock solutions were prepared at 1 mM). To test whether the concentrations selected in this study exerted cytotoxic effects, the cells were seeded at 5000 cells per cm2 in a 96-well plate and incubated at 37 °C for 24 h. Once attached, we treated the cells with 1% (v/v) DMSO as the control or 10 μM of each isoflavone tested (Fig. 1). After 24 h, the culture medium with the treatments was removed, the cells washed with HBSS (twice) and the cell viability and proliferation measured using the MTT reduction assay. The assays were performed in triplicate (n = 3), while each treatment was performed 6 times (6 wells per treatment).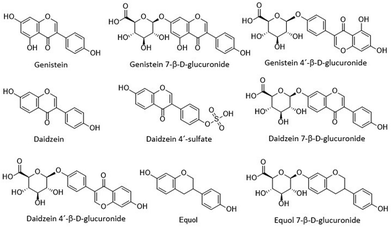 | ||
| Fig. 1 The chemical structure of relevant free forms and their conjugated metabolites of genistein and daidzein. | ||
Tubulogenesis assay
The effect of the compounds investigated on tube formation was determined as described elsewhere.26,27 Briefly, 96-well plates coated with cold reduced growth factor Matrigel (50 μL) were incubated at 37 °C for 30 min to allow its solidification. Confluent HAEC cells (≥80%) were incubated in ECBM (without GS) at 37 °C for 3 hours and then trypsinised. Next, 50 μL cell suspension (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cells) was added to each Matrigel-coated well before the addition of each treatment (10 μM) in basal ECBM and incubated at 37 °C for 4 hours. Immediately after, the cells were stimulated with GS (0.65% v/v), and this time point was set as time 0 hours (h). A picture at 5x magnification of each treatment was taken at four different time points (time 0, 7, 11, and 24 h) using a microscope (Zeiss Fluorescence Microscope). According to prior studies,28,29 we used the Angiogenesis Analyzer plug-in (ImageJ 1.54d, National Institute of Health, NIH) to quantify the effect of the isoflavones on tube network formation. We repeated (at least n = 3) the dose-dependent effect (10, 1, and 0.1 μM) of the compounds studied, choosing those isoflavones that significantly inhibited tubulogenesis.
500 cells) was added to each Matrigel-coated well before the addition of each treatment (10 μM) in basal ECBM and incubated at 37 °C for 4 hours. Immediately after, the cells were stimulated with GS (0.65% v/v), and this time point was set as time 0 hours (h). A picture at 5x magnification of each treatment was taken at four different time points (time 0, 7, 11, and 24 h) using a microscope (Zeiss Fluorescence Microscope). According to prior studies,28,29 we used the Angiogenesis Analyzer plug-in (ImageJ 1.54d, National Institute of Health, NIH) to quantify the effect of the isoflavones on tube network formation. We repeated (at least n = 3) the dose-dependent effect (10, 1, and 0.1 μM) of the compounds studied, choosing those isoflavones that significantly inhibited tubulogenesis.
Migration assay
The capacity of the endothelial cells to migrate was investigated as previously described elsewhere.30,31 Briefly, HAECs were seeded at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2 in 48-well plates and grown to reach a confluence ≥90%. Next, the endothelial monolayer was wounded by scratching the cellular surface with a sterile tip, and the medium containing debris and dislodged cells was removed. The attached cells were washed with HBSS before adding fresh ECBM (GF-deprived) containing DMSO (1% v/v) as the control or 10 μM isoflavones. The cells were incubated for 4 h, stimulated by the addition of 1.5% (v/v) GF (set as time 0 h) and incubated for 20 h (set as time 24/final point). Random pictures (2 or 3 of each treatment) taken along the scratched area using a microscope (Zeiss Fluorescence Microscope) were quantified following the reported protocols.31 The treatments (10 μM), resulting in significant migration inhibition, were tested at lower concentrations (10, 1, and 0.1 μM) to determine a dose-dependent effect. The quantification of the scratched area at the different time points (4 and 24 h after each treatment) was repeated at least three times (n = 3).
000 cells per cm2 in 48-well plates and grown to reach a confluence ≥90%. Next, the endothelial monolayer was wounded by scratching the cellular surface with a sterile tip, and the medium containing debris and dislodged cells was removed. The attached cells were washed with HBSS before adding fresh ECBM (GF-deprived) containing DMSO (1% v/v) as the control or 10 μM isoflavones. The cells were incubated for 4 h, stimulated by the addition of 1.5% (v/v) GF (set as time 0 h) and incubated for 20 h (set as time 24/final point). Random pictures (2 or 3 of each treatment) taken along the scratched area using a microscope (Zeiss Fluorescence Microscope) were quantified following the reported protocols.31 The treatments (10 μM), resulting in significant migration inhibition, were tested at lower concentrations (10, 1, and 0.1 μM) to determine a dose-dependent effect. The quantification of the scratched area at the different time points (4 and 24 h after each treatment) was repeated at least three times (n = 3).
Western blot analysis
Cells at ∼80% confluence were incubated in ECBM (GS deprived) for 3 h prior to treatment with 10 μM DAZ, GEN, Eq, or Eq 7-glur for 4 h. Immediately after, the cells were stimulated for 5 min with (i) 100 ng mL−1 bFGF (optimization is described in ESI†) or (ii) 100 ng mL−1 VEGF165 (as determined elsewhere).32 Next, the cells were washed with HBSS, and the protein extracted using RIPA buffer. The cellular protein lysates (20 μg protein loaded in each well) separated into 10%–12% acrylamide gels were transferred to nitrocellulose membranes, incubated with monoclonal primary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution) against total (t)-FGF receptor 1 (D8E4, #9740), p-VEGFR2 (Tyr1175; 19A10, #2478), t-VEGFR2 (55B11, #2479), p-VEGFR1 (Y1213; AF4170), t-VEGFR1 (E7T9H, #64094), p-ERK (Thr202/Tyr204, 20G11; #4377), t-ERK (137F5; #4695), p-Akt (Ser473, 193H12; #4058), t-Akt (C67E7; #4691), p-p38 (Thr180/Tyr182; D3F9, #4511), and t-p38 (D13E1, #8690). GAPDH (D4C6R, #97166) at a dilution of 1
1000 dilution) against total (t)-FGF receptor 1 (D8E4, #9740), p-VEGFR2 (Tyr1175; 19A10, #2478), t-VEGFR2 (55B11, #2479), p-VEGFR1 (Y1213; AF4170), t-VEGFR1 (E7T9H, #64094), p-ERK (Thr202/Tyr204, 20G11; #4377), t-ERK (137F5; #4695), p-Akt (Ser473, 193H12; #4058), t-Akt (C67E7; #4691), p-p38 (Thr180/Tyr182; D3F9, #4511), and t-p38 (D13E1, #8690). GAPDH (D4C6R, #97166) at a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 was the loading protein control selected. The anti-rabbit and anti-mouse secondary antibodies were used at 1
2500 was the loading protein control selected. The anti-rabbit and anti-mouse secondary antibodies were used at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000. The membranes developed with SuperSignal West Pico PLUS Chemiluminescent Substrate detection system (ThermoFisher, Barcelona, Spain) were scanned by Amersham Imager 600 (Chicago, IL, USA), and the protein level determined using the ImageJ software v 1.54d (NIH, USA).
5000. The membranes developed with SuperSignal West Pico PLUS Chemiluminescent Substrate detection system (ThermoFisher, Barcelona, Spain) were scanned by Amersham Imager 600 (Chicago, IL, USA), and the protein level determined using the ImageJ software v 1.54d (NIH, USA).
Immunofluorescence analysis
HAEC cells (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per 24-well plate foci) were seeded in Poly-L-Lys Cellware 12 mm cover (Corning) in 250 μl ECBM media and allowed to adhere at 37 °C overnight. The cells were treated as described for western blot analysis (incubation in deprived medium for 3 h, treatment with 10 μM isoflavones for 4 h, and stimulation with 100 ng mL−1 VEGF165 for 5 min). Next, the cells were washed with PBS, fixed with 4% paraformaldehyde in PBS (v/v) for 10 min, incubated at room temperature with 20 mM glycine for 20 min, permeabilised with 0.5% NP40 and blocked for 1 h with 2% BSA. Cells were then labeled with the corresponding primary antibody dilution 1
000 per 24-well plate foci) were seeded in Poly-L-Lys Cellware 12 mm cover (Corning) in 250 μl ECBM media and allowed to adhere at 37 °C overnight. The cells were treated as described for western blot analysis (incubation in deprived medium for 3 h, treatment with 10 μM isoflavones for 4 h, and stimulation with 100 ng mL−1 VEGF165 for 5 min). Next, the cells were washed with PBS, fixed with 4% paraformaldehyde in PBS (v/v) for 10 min, incubated at room temperature with 20 mM glycine for 20 min, permeabilised with 0.5% NP40 and blocked for 1 h with 2% BSA. Cells were then labeled with the corresponding primary antibody dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, followed by Alexa 594-conjugated secondary antibody dilution 1
200, followed by Alexa 594-conjugated secondary antibody dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (ThermoFisher Scientific). Samples were mounted using a Dako mounting medium and examined with a Leica laser scanning confocal microscope AOBS and software (Leica Microsystems). The objective used was HCX PL APO CS × 63 glycerol.
400 (ThermoFisher Scientific). Samples were mounted using a Dako mounting medium and examined with a Leica laser scanning confocal microscope AOBS and software (Leica Microsystems). The objective used was HCX PL APO CS × 63 glycerol.
Docking interaction analysis
To perform the in silico study of the isoflavones-VEGFR2 interaction, we selected the MCULE software (MCULE, Inc. Palo Alto, CA, USA). This platform offers a wide range of online compounds and discovery tools with comprehensive chemical databases. Once obtained, the “InChIKey” of GEN and Eq (compounds that inhibited VEGFR2 phosphorylation) together with the specific inhibitor AZD322933 as well as the structural model of VEGFR2 (coded as 6GQO within the Protein Data Bank; https://www.rcsb.org/structure/6GQO) we uploaded the information to MCULE. This program provides information about the critical interactions between the molecules tested with the receptor studied based on how the 3D structure of the ligands fit into the 3D binding site of the target. This interaction is measured as docking scores, which are used to predict the affinity of a molecule to a target (the more negative, the higher the affinity). We next downloaded the files of the docking simulations with the best scores as “pbd” files to generate images of the docking sites and the molecular interactions using the Biovia Discovery Studio 2021.Assessment of stability and metabolism by HAEC cells
To determine the stability and metabolism of isoflavones (free and conjugated forms) by HAEC cells, cell culture supernatants (from tubulogenesis and migration assays) were collected at the initial (0 h) and final points (24 h), processed, and analysed using an Agilent 1290 Infinity UPLC-ESI system coupled to a 6550 Accurate-Mass quadrupole-time-of-flight (QTOF) (mass spectrometer (Agilent Technologies, Waldbronn, Germany)) as previously described.31,32Statistical analysis
Data were expressed as the average ± standard deviation (SD) of at least three independent experiments (n ≥ 3). Normally distributed data were analysed by the ANOVA test and Dunnet's post-hoc analysis. The software used for statistical analysis was Prism 9.0 (GraphPad, La Jolla, CA, USA). Graphics and figures were prepared using Prism 9.0 (GraphPad) and ChemDraw Professional v. 16.0.1.4 (PerkinElmer Informatics Inc., Cambridge, MA, USA). Statistically significant differences were considered at *p < 0.05, **p < 0.01, and ***p < 0.001.Results and discussion
Isoflavones at 10 μM lack cytotoxicity on HAECs
The results of the MTT assay showed an absence of cytotoxic effects in the cells treated with the vehicle (1% DMSO v/v) and 10 μM isoflavones (maximum concentration used) for 24 h (results not shown).Isoflavones inhibit the tubulogenic capacity of HAECs time- and dose-dependently
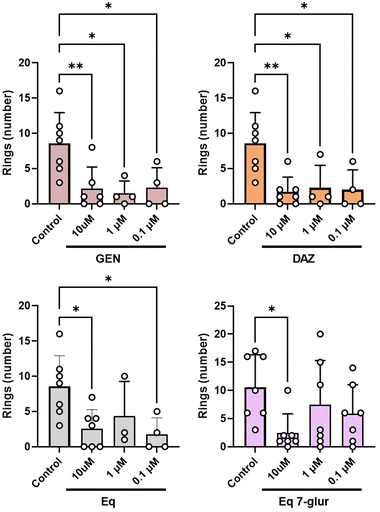
The in vitro tubulogenesis assay is a widely used methodology to investigate the capacity of molecules of interest (i.e., dietary phenolics) in modulating angiogenesis.34 Regarding isoflavones, information about their role in in vitro tubulogenesis encompasses a small number of studies focused on GEN-treated endothelial cells,18,35,36 thus justifying the investigation of other relevant dietary isoflavones such as DAZ as well as their derived-circulating metabolites.In this study, we used HAECs as a relevant cellular line for two reasons: (i) they are primary cells isolated from adult tissues (i.e., aorta) and show a phenotype closer to in vivo (patho)physiological processes compared to other endothelial cell lines;37,38 (ii) modulation of in vitro angiogenic mechanisms in this cell line by isoflavones remains unexplored. According to the guidance reported elsewhere,9 in the first group of experiments, we compared the capacity of a set of circulating phase-II metabolites and their free forms (as shown in Fig. 1) to modulate HAECs tubulogenesis, in a time-dependent manner, using the Matrigel-based experiment. The phase-II metabolites (glucuronides and sulfates) of DAZ and GEN at 10 μM were unable to promote or inhibit the formation of tubulogenesis-related structures in stimulated cells (Fig. S1†). In contrast, at the same concentration, DAZ and GEN exerted a substantial inhibition (p < 0.05 at 24 h) of the characteristic features of in vitro tubulogenesis (Fig. 2). The weaker biological activity of phase-II metabolites compared to their free forms is a recurrent result described in prior studies that assessed phase-II (poly)phenolic conjugates, including isoflavones ones, which exerted less or no biological activity compared to their free forms.39–41 Our results show the inhibitory effects of GEN and DAZ on HAECs for the first time. However, the systemic nature of this cell line and the negligible level of GEN and DAZ detected in plasma makes it difficult to rationalize their direct effect at the vascular level. An alternative explanation to this resides in the role of these molecules as precursors of gut microbial metabolites,25 which might be more bioactive molecules. For example, the DAZ-derived microbial Eq can bind to estrogen receptors (ERs) with higher affinity than its precursor42 or inhibit endothelial migration at lower concentrations.18 Based on this, we next tested whether Eq and its main circulating phase-II metabolite Eq 7-glur were active molecules against tubulogenesis. Under the same conditions described above, Eq and Eq 7-glur hampered the tubulogenic capacity of HAECs at 10 μM (Fig. 2). Notably, 10 μM Eq 7-glur showed similar potency to the free forms, exerting a significant reduction (p < 0.05 at 14 and 24 h) of the number of rings formed compared to the stimulated cells (Fig. 2). To the best of our knowledge, the anti-angiogenic properties of Eq and its glucuronide are unprecedented, and it is in accordance with the effect exerted by other circulating metabolites, such as urolithin A-glucuronide,30 6-methoxyequol,43 and(or) quercetin 3-glur44 at the vascular level.
Considering the concentrations achieved in vivo45 and the results of the time-course assay, we next treated the cells with DAZ, GEN, Eq, and Eq 7-glur at three different concentrations (10, 1, and 0.1 μM) for 24 h (significant effects observed at this time point). The potential of the free forms in inhibiting tubulogenesis in HAECs was similar within the range of concentrations tested (Fig. 3 and Fig. S2†). The similar potency exhibited by GEN and DAZ (even at the lowest concentration) was in agreement with the results obtained using the chorioallantoic membrane assays (CAM),46 but in contrast with the lower (or no) activity showed by DAZ compared to GEN in vivo.19,47,48 This discrepancy might be related to the different conditions of the assays (such as models used, concentrations tested, and duration of the experiments) described in these studies. The circulating metabolite Eq 7-glur also effectively reduced the tubulogenic capacity of the endothelial cells. Although less potent than its free form, Eq 7-glur (only effective at 10 μM) was recognized, for the first time, as an active phase-II metabolite with the ability to inhibit tubulogenesis in vitro.
Equol and equol 7-glur inhibit endothelial cell migration in a dose-dependent manner
Endothelial migration is another critical step in angiogenesis. We used the wound healing assay as an easy and reliable technique to test how isoflavones affect endothelial migration. The glucuronides and sulfates of GEN and DAZ, at 10 μM, were ineffective in reducing the capacity of the stimulated HACEs to migrate (Fig. S3†). The inability of the conjugated metabolites was in agreement with our tubulogenesis results (no studies have been published that support or challenge this effect). At the same concentration, GEN and DAZ could not inhibit HAEC cell migration (Fig. S3†). This result agrees with previous studies reporting that these molecules showed no effect on HUVEC36 and HEMC-119 migration and is at odds with the inhibitory effect observed on EA.hy92617 and rat aortic-isolated endothelial cells.49 Otherwise, the lack of effect of GEN and DAZ against migration contrasts with their notably inhibitory effect observed on tubulogenesis. This disparity seems to be related to cell density. For example, Berndt et al. described a greater inhibitory effect of GEN (25–100 μM) on tubulogenesis compared to endothelial migration in HUVECs,36 while Fotsis et al. showed a density-dependent effect of GEN on bovine brain capillaries-derived endothelial cells.18There is little information regarding the role of Eq and Eq 7-glur on cellular migration. Eq acts as an inhibitor of cell migration against a range of cancer cells50 (the effect is untested in endothelial cells), whereas nothing is known about the biological activity of its glucuronide. Our results showed that both molecules reduced cell migration dose-dependently, albeit only Eq 7-glur was significantly effective at 10 and 1 μM (Fig. 4 and Fig. S3†). These results are relevant as the transformation of dietary isoflavones by gut microbiota provides Eq and, subsequently, Eq 7-glur as biologically active metabolites potentially capable of modulating a wider range of processes (under the conditions of our study) related to angiogenesis. The effect of Eq 7-glur is relevant because it is a circulating metabolite detected in the bloodstream and systemic tissues,22,51,52 where it can exert its antiangiogenic effects.
Isoflavones target the VEGF pathway through different mechanisms
Angiogenesis is a complex system regulated by a plethora of key molecules, including proteases, transcription factors, and (or) growth factors.53 We studied how DAZ, GEN, Eq, and Eq 7-glur (at 10 μM) affected the proangiogenic bFGF and VEGF pathways54 activation in stimulated endothelial cells to evaluate the inhibition targets.In HAECs, the treatment of the cells with 100 ng mL−1 bFGF exerted proangiogenic effects, inducing receptor FGFR155 phosphorylation, which in turn triggers the activation of essential signalling pathways (i.e., ERK, p38, and JNK, among others) modulating angiogenesis.56 In an initial test, we optimized the stimulation conditions with bFGF, testing the activation of downstream pathways such as ERK. A time-course assay of HAECs treated with 100 ng mL−1 bFGF for 2–30 min showed the highest phosphorylation of ERK at 5 min (Fig. S4A†). Using these experimental conditions, we investigated whether our compounds could target the FGF pathway via targeting the ERK pathway (phosphorylation inhibition) or down-regulating FGFR1 expression. The treatment with DAZ, GEN, Eq, and Eq 7-glur for 4 h before the stimulation with bFGF for 5 min did not affect the level of phosphorylated ERK (Fig. S4B†) or t-FGFR1 (Fig. S4C†) compared to the stimulated cells, indicating that the inhibitory effects exerted on tubulogenesis and(or) migration are independent of this pathway.
The VEGF pathway is another key target in (patho)physiological angiogenesis that could help to understand how the isoflavones exert their antiangiogenic effects. VEGF165 is one of the most relevant isoforms in this pathway, exerting its effects via interaction with its tyrosine kinase receptors VEGFR1 and VEGFR2 and activation of multiple downstream pathways.57 Current knowledge places the effect of isoflavones (e.g., GEN and 6-methyl-Eq) via inhibition of the downstream pathways (i.e., JNK, p38, ERK) in VEGF-treated HUVECs.13,43 Still, it overlooks whether this effect involves receptor modulation. Hence, we first studied whether our compounds modulated VEGFR1 since its blockage is a known mechanism associated with the suppression of angiogenesis.58 However, our data show that VEGFR1 is an unlikely target of GEN, DAZ, Eq, and Eq 7-glur since its receptor level, as well as the VEGF165-induced activation (measured as Y1213 residue phosphorylation), is similar between the different treatments (Fig. S5A†).
A second mechanism approached was the interaction of the isoflavones with VEGFR2. Specifically, we determined their effects on key factors on angiogenesis regulation, such as Y1175 residue phosphorylation59 and ERK, Akt, and p38 activation.60 VEGFR2 was identified as a target of GEN and Eq in VEGF165-treated cells, given the lower phosphorylation level at the Y1175 residue and the related sequential inactivation of ERK and Akt. DAZ and Eq 7-glur did not affect VEGFR2 activation but prevented the VEGF165-induced activation of Akt and ERK, respectively (Fig. 5). The inhibitory effects seemed independent of the p38 pathway as none of these compounds modulated its activation (Fig. S5B†).
Structure-based docking of GEN and Eq to VEGFR2
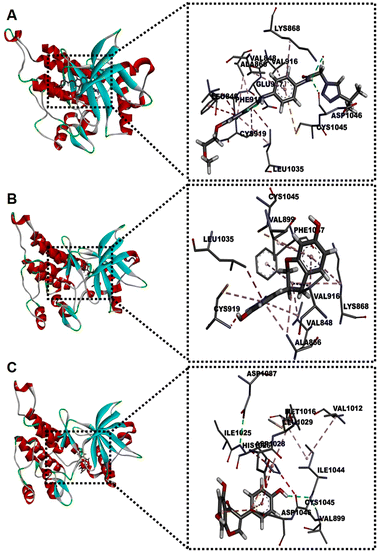
Next, we conducted an in silico approach to explore whether GEN and (or) Eq can bind to VEGFR2 (Fig. 6). We excluded DAZ and Eq 7-glur since they did not affect VEGFR2 activation. Taking as a reference the docking score of the VEGFR2 inhibitor AZD3229 (−9.7 kcal mol−1), the predicted binding affinity to the receptor was higher for Eq (−8.7 kcal mol−1) compared to GEN (−7.8 kcal mol−1). Also, the hotspot predicted for Eq (Fig. 6B) has numerous residues in common with the inhibitor AZD3229, whereas for GEN, the similarities are much more limited. The amino acid CYS1045 formed a Pi-sulfur interaction in AZD3229 and Eq, both with the phenyl ring, whereas this amino acid bound GEN with VEGFR2 through a hydrogen bond. Likewise, AZD3229 and Eq shared more similarities.As shown in Fig. 6A and B, both compounds could interact with the VEFGR2 surface pocket by forming Pi-Sigma and Pi-alkyl interactions with LEU1035 and ALA866, respectively. However, these interactions were not observed with GEN (Fig. S6†). This suggests that Eq could bind to VEGFR2 and block its activation VEGF165, whereas the effect exerted by GEN might imply alternative mechanisms such as intracellular inhibition of protein tyrosine kinase activity.13 These in silico results require validation through additional experiments, including competitive binding assays.
VEGFR2 location is unaffected by isoflavones
VEGFR2 is a membrane receptor detected intracellularly due to the recycling/degradation cycle. The receptor location is an important factor that regulates the access of the ligands and, therefore, its activation.61 Thus, cytoplasmic accumulation of VEGFR2 could reduce its exposure to VEGF165, altering its activation. The analysis of the images (Fig. 7) included in Fig. S7† shows a similar distribution of the VEGFR2 in the cytoplasm of the VEGF165-stimulated endothelial cells in the presence or absence of GEN and Eq. These results indicate that the reduced activation of the VEGFR2 in GEN- and Eq-treated HAECs described in Fig. 5 is not due to a cytoplasmic accumulation of the receptor induced by the isoflavones. | ||
| Fig. 7 Immunofluorescence detection of VEGFR2 in VEGF165-treated HAEC cells in the presence or absence of 10 μM GEN or Eq. DAPI staining allowed the visualization of the cellular nuclei. | ||
Isoflavones metabolism in endothelial migration and tubulogenesis assays
We next analysed the culture medium of the tubulogenesis and migration assays to study the metabolic transformations of the isoflavones tested under different experimental conditions (Fig. S8–S10†). Table 1 summarizes the concentrations of the compounds used in the cell assays (time 0) and the metabolites produced after 24 h incubation.| Migration | Tubulogenesis | |||
|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | |
| a GEN-sulf was tentatively identified and expressed as area values of the integrated extracted ion chromatograms (EICs) due to the lack of authentic standards. | ||||
| Treatment DAZ | ||||
| DAZ | 10.49 ± 1.40 | 9.92 ± 0.65 | 10.90 ± 0.81 | 8.94 ± 0.57 |
| DAZ 4′-sulf | n.d. | 0.16 ± 0.14 | n.d. | 0.39 ± 0.31 |
| Treatment DAZ 4′-glur | ||||
| DAZ | 0.25 ± 0.11 | 5.21 ± 0.57 | 0.18 ± 0.16 | 10.49 ± 2.93 |
| DAZ 4′-glur | 10.07 ± 1.94 | 5.96 ± 0.62 | 11.58 ± 0.49 | 1.93 ± 0.72 |
| DAZ 4′-sulf | n.d. | 0.10 ± 0.14 | n.d. | 0.10 ± 0.004 |
| Treatment DAZ 7-glur | ||||
| DAZ | 0.06 ± 0.07 | 4.91 ± 2.90 | n.d. | 9.36 ± 3.88 |
| DAZ 7-glur | 10.49 ± 0.81 | 5.44 ± 3.86 | 9.48 ± 0.39 | 1.58 ± 2.19 |
| DAZ 4′-sulf | n.d. | 0.07 ± 0.09 | n.d. | 0.03 ± 0.03 |
| Treatment DAZ 4′-sulf | ||||
| DAZ | 0.06 ± 0.07 | 2.56 ± 2.10 | 0.30 ± 0.01 | 2.10 ± 2.10 |
| DAZ 4′-sulf | 9.38 ± 2.13 | 6.86 ± 0.13 | 10.02 ± 1.73 | 8.35 ± 0.14 |
| Treatment GEN | ||||
| GEN | 9.92 ± 1.89 | 9.18 ± 1.11 | 9.90 ± 0.01 | 7.32 ± 2.39 |
| GEN-sulfa | n.d. | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 ± 9051a 291 ± 9051a |
n.d. | 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132 ± 110 132 ± 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940a 940a |
| Treatment GEN 4′-glur | ||||
| GEN | 0.02 ± 0.01 | 2.31 ± 2.14 | n.d. | 3.09 ± 1.03 |
| GEN 4′-glur | 11.97 ± 2.43 | 6.72 ± 1.86 | 10.26 ± 2.74 | 5.44 ± 1.62 |
| GEN-sulfa | n.d. | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335 ± 13 335 ± 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 637a 637a |
n.d. | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 776 ± 5361a 776 ± 5361a |
| Treatment GEN 7-glur | ||||
| GEN | 0.03 ± 0.02 | 0.11 ± 0.14 | 0.06 ± 0.02 | 3.77 ± 0.71 |
| GEN 7-glur | 11.41 ± 0.97 | 9.61 ± 0.93 | 10.43 ± 0.53 | 5.22 ± 1.14 |
| GEN-sulfa | n.d. | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 613 ± 25 613 ± 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 873a 873a |
n.d. | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 369 ± 25 369 ± 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906a 906a |
| Treatment Equol | ||||
| Equol | 10.20 ± 1.18 | 10.62 ± 0.20 | 10.75 ± 1.24 | 9.57 ± 0.35 |
| Treatment Equol 7-glur | ||||
| Equol | n.d. | n.d. | n.d. | 4.09 ± 0.57 |
| Equol 7-glur | 11.27 ± 2.03 | 11.75 ± 1.99 | 10.53 ± 1.42 | 4.02 ± 0.21 |
In line with the metabolic transformations described for curcumin, urolithins, resveratrol, and (or) flavanones,31,32,62,63 the incubation of HAEC cells with DAZ resulted in the formation of sulfate metabolites (i.e., 0.1–0.4 μM DAZ-sulf). These cells were also able to form sulfates from GEN (Fig. S9†), although the lack of standards made it difficult to determine the amount of phase-II metabolite generated. No glucuronides were detected, thus supporting the reported prevalence of sulfation over glucuronidation in these cells.62 Similar to the results described by Toro-Funes et al.,64 Eq was less prone to the conversion to its conjugated form, as revealed by the absence of derived metabolites at 24 h.
Interestingly, we have observed deconjugation with different isoflavones under our experimental conditions. There are notable differences in the metabolism of conjugated metabolites: (i) sulfate deconjugation (only tested in DAZ 4′-sulf) was similar in migration and tubulogenesis assays, (ii) tubulogenesis conditions promoted deconjugation of DAZ conjugates (the glucuronide position influence was negligible) and Eq 7-glur compared to migration, and (iii) the glucuronide position determined the release of GEN from its conjugate only in the migration assay. The in vitro metabolism of the isoflavones may be understood as a dynamic process characterized by cycles of conjugation/deconjugation influenced by several assay- and compound-dependent factors. The presence of the Matrigel promotes deconjugation of glucuronides, albeit its ill-defined and complex composition65 makes it difficult to determine what component(s) is(are) responsible and the underlying mechanism(s). Cell density (confluent vs. sub-confluent) is another factor to be considered based on its influence on phase-II metabolism.66 Another element to contemplate is the structure of (poly)phenols, which influences their susceptibility to undergo enzymatic conjugation/deconjugation.67–69 Spontaneous deconjugation appears very unlikely as the isoflavones are stable in the culture medium under the conditions of our assays (without cells) for 24 h (results not shown). While the correlation between isoflavones metabolism and migration effects was trivial, deconjugation of the phase-II of GEN and DAZ metabolites resulted in the release of their free forms, reaching concentrations high enough to inhibit tubulogenesis (Fig. 2 and 3). Nonetheless, the treatment with the conjugated metabolites was inefficient. By mechanisms which remain unclear, the coexistence of free and conjugated forms of isoflavones resulted in an inactive mixture against tubulogenesis. Studies with RSV described a comparable circumstance that involved senescence induction in breast cancer cells treated with individual compounds, RSV, or their derived metabolites, but no effects in the presence of a physiologically relevant mixture with different RSV metabolites. The differences between individual compounds and their mixture were related to the potential competition of the individual molecules for ABC transporters.70 The well-known expression of influx/efflux transporters in endothelial cells71,72 and their reported interaction with isoflavones73–76 align with the free/conjugated isoflavones competition hypothesis. This could be an avenue to explain why the conjugated metabolites hydrolysed to free forms that act intracellularly are inactive (e.g., DAZ targets the Akt pathway; Fig. 6B), yet additional studies are requested to support this idea. Interestingly, Eq 7-glur seemed to inhibit migration by itself (no release of Eq in this assay) and kept its ability to inhibit angiogenesis despite being hydrolysed to Eq, indicating no competition in the mixture Eq 7-glur/Eq.
Conclusions
This study documents important findings on the role of isoflavones in targeting angiogenesis. First, the description of Eq 7-glur as an active molecule at the vascular level. The relevance of this lies in (i) the variable capacity of humans to produce equol (25–50% are equol producers)25 and, in turn, the circulating glucuronide; (ii) it is an exception to the reduced biological activity exhibited by conjugated metabolites compared to their free forms. Second, the previously identified cell metabolism of HAECs could also impact the biological activity of isoflavones. This discovery sets phase-II metabolites of isoflavones as precursors of their free forms in endothelial cells but underscores the complexity of the mixtures of conjugated metabolites/free forms. Properly comprehending isoflavone deconjugation and its relation to biological activity requires further studies. Third, the use of a relevant cell line, the description of the underlying molecular mechanisms (i.e., VEGF and downstream pathway), and the integration of metabolism/bioavailability of isoflavones in the design of the in vitro assays will be the basis of future in vivo studies.The design of future studies should also consider the main limitations of our study. Intestinal microbiota exclusively produce the (S)-equol enantiomer77 (used in our study), which undergoes phase-II metabolism. The (R,S)-equol 7-β-D-glucuronide used in our study is a racemic mixture that differs from the (S)-equol-derived glucuronide formed in vivo. The different effects observed between (S)-equol and (R)-equol78 highlight the importance of testing the enantiomer of the glucuronide formed in vivo.
Further in vivo investigations are warranted to explore the possible role of isoflavone metabolites as potential antiangiogenic compounds contributing to the prevention/treatment of angiogenesis-related chronic diseases such as cancer and(or) atherosclerosis upon consumption of isoflavone foodstuffs.
Author contributions
Conceptualization: J.A.G.-B., A.G.-S. and J.C.E.; methodology: J.A.G.-B., A.G.-S., M.A.A-G., D.G.-M, and A. M.-L.; resources: J.A.G.-B., A.G.-S. and J.C.E.; writing original draft: J.A.G.-B. and A.G.-S.; writing – review & editing: all authors. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. A. G.-B. was supported by a Standard European Marie Curie Individual Fellowship from the European Commission. D. G.-M. was supported by a Miguel Servet contract (AES 2021; CP21/00028) funded by the Institute Carlos III and by “ERDF a way of making Europe”. This work has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie Grant Agreement No. 838991. This work was supported by the Ramón y Cajal grant (RyC2021-032111-I) and by the grants CNS2022-135253 and TED2021-130962B-C22 funded by the MCIN/AEI/10.13039/501100011033 and by the “European Union NextGenerationEU/PRTR” program, grants PID2022-136915NA-I00 and PID2022-136419OB-I00 funded by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe” by the European Union, and the grant 22030/PI/22 funded by the Programa Regional de Fomento de la Investigación Científica y Técnica (Plan de Actuación 2022) de la Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia, Spain.References
- A. S. Chung, J. Lee and N. Ferrara, Targeting the Tumour Vasculature: Insights from Physiological Angiogenesis, Nat. Rev. Cancer, 2010, 10, 505–514 CrossRef PubMed.
- P. Carmeliet and R. K. Jain, Molecular Mechanisms and Clinical Applications of Angiogenesis, Nature, 2011, 473, 298–307 CrossRef PubMed.
- M. Rajabi and S. A. Mousa, The Role of Angiogenesis in Cancer Treatment, Biomedicines, 2017, 5, 34 CrossRef PubMed.
- R. Lugano, M. Ramachandran and A. Dimberg, Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities, Cell. Mol. Life Sci., 2020, 77, 1745–1770 CrossRef PubMed.
- Z. Wang, C. Dabrosin, X. Yin, M. M. Fuster, A. Arreola, W. K. Rathmell, D. Generali, G. P. Nagaraju, B. El-Rayes, D. Ribatti, Y. C. Chen, K. Honoki, H. Fujii, A. G. Georgakilas, S. Nowsheen, A. Amedei, E. Niccolai, A. Amin, S. S. Ashraf, B. Helferich, X. Yang, G. Guha, D. Bhakta, M. R. Ciriolo, K. Aquilano, S. Chen, D. Halicka, S. I. Mohammed, A. S. Azmi, A. Bilsland, W. N. Keith and L. D. Jensen, Broad Targeting of Angiogenesis for Cancer Prevention and Therapy, Semin. Cancer Biol., 2015, 35 Suppl, S224–S243 CrossRef PubMed.
- A. D. Marrero, A. R. Quesada, B. Martínez-Poveda and M. Á. Medina, Antiangiogenic Phytochemicals Constituent of Diet as Promising Candidates for Chemoprevention of Cancer, Antioxidants, 2022, 11, 302 CrossRef CAS PubMed.
- H. Abbaszadeh, B. Keikhaei and S. Mottaghi, Review of Molecular Mechanisms Involved in Anticancer and Antiangiogenic Effects of Natural Polyphenolic Compounds, Phytother. Res., 2019, 33, 2002–2014 CrossRef PubMed.
- M. Caban and U. Lewandowska, Inhibiting Effects of Polyphenols on Angiogenesis and Epithelial-Mesenchymal Transition in Anterior Segment Eye Diseases, J. Funct. Foods, 2021, 87, 104761 CrossRef CAS.
- M. Á. Ávila-Gálvez, A. González-Sarrías and J. C. Espín, In Vitro Research on Dietary Polyphenols and Health: A Call of Caution and a Guide on How To Proceed, J. Agric. Food Chem., 2018, 66, 7857–7858 CrossRef PubMed.
- K. Zaheer and M. Humayoun Akhtar, An Updated Review of Dietary Isoflavones: Nutrition, Processing, Bioavailability and Impacts on Human Health, Crit. Rev. Food Sci. Nutr., 2017, 57, 1280–1293 CrossRef CAS PubMed.
- C. Danciu, S. Avram, I. Z. Pavel, R. Ghiulai, C. A. Dehelean, A. Ersilia, D. Minda, C. Petrescu, E.-A. Moaca and C. Soica, Main Isoflavones Found in Dietary Sources as Natural Anti-Inflammatory Agents, Curr. Drug Targets, 2018, 19, 841–853 CrossRef CAS PubMed.
- R. Ambra, G. Rimbach, S. de Pascular Teresa, D. Fuchs, U. Wenzel, H. Daniel and F. Virgili, Genistein Affects the Expression of Genes Involved in Blood Pressure Regulation and Angiogenesis in Primary Human Endothelial Cells, Nutr., Metab. Cardiovasc. Dis., 2006, 16, 35–43 CrossRef CAS PubMed.
- X. Yu, J. Zhu, M. Mi, W. Chen, Q. Pan and M. Wei, Anti-Angiogenic Genistein Inhibits VEGF-Induced Endothelial Cell Activation by Decreasing PTK Activity and MAPK Activation, Med. Oncol., 2012, 29, 349–357 CrossRef CAS PubMed.
- L. Varinska, P. Gal, G. Mojzisova, L. Mirossay and J. Mojzis, Soy and Breast Cancer: Focus on Angiogenesis, Int. J. Mol. Sci., 2015, 16, 11728–11749 CrossRef CAS PubMed.
- C. Spagnuolo, G. L. Russo, I. E. Orhan, S. Habtemariam, M. Daglia, A. Sureda, S. F. Nabavi, K. P. Devi, M. R. Loizzo, R. Tundis and S. M. Nabavi, Genistein and Cancer: Current Status, Challenges, and Future Directions, Adv. Nutr., 2015, 6, 408–419 CrossRef CAS.
- Y. Guo, S. Wang, D. R. Hoot and S. K. Clinton, Suppression of VEGF-Mediated Autocrine and Paracrine Interactions between Prostate Cancer Cells and Vascular Endothelial Cells by Soy Isoflavones, J. Nutr. Biochem., 2007, 18, 408–417 CrossRef CAS PubMed.
- W.-X. Cheng, H. Huang, J.-H. Chen, T.-T. Zhang, G.-Y. Zhu, Z.-T. Zheng, J.-T. Lin, Y.-P. Hu, Y. Zhang, X.-L. Bai, Y. Wang, Z.-W. Xu, B. Song, Y.-Y. Mao, F. Yang and P. Zhang, Genistein Inhibits Angiogenesis Developed during Rheumatoid Arthritis through the IL-6/JAK2/STAT3/VEGF Signalling Pathway, J. Orthop. Translat., 2020, 22, 92–100 CrossRef PubMed.
- T. Fotsis, M. Pepper, H. Adlercreutz, G. Fleischmann, T. Hase, R. Montesano and L. Schweigerer, Genistein, a Dietary-Derived Inhibitor of in Vitro Angiogenesis, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 2690–2694 CrossRef CAS.
- S.-J. Su, T.-M. Yeh, W.-J. Chuang, C.-L. Ho, K.-L. Chang, H.-L. Cheng, H.-S. Liu, H.-L. Cheng, P.-Y. Hsu and N.-H. Chow, The Novel Targets for Anti-Angiogenesis of Genistein on Human Cancer Cells, Biochem. Pharmacol., 2005, 69, 307–318 CrossRef CAS PubMed.
- S.-M. Heinonen, K. Wähälä and H. Adlercreutz, Metabolism of Isoflavones in Human Subjects, Phytochem. Rev., 2002, 1, 175–182 CrossRef CAS.
- S. T. Soukup, J. Helppi, D. R. Müller, O. Zierau, B. Watzl, G. Vollmer, P. Diel, A. Bub and S. E. Kulling, Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: a cross-species and sex comparison, Arch. Toxicol., 2016, 90, 1335–1347 CrossRef CAS PubMed.
- M. Á. Ávila-Gálvez, A. González-Sarrías, F. Martínez-Díaz, B. Abellán, A. J. Martínez-Torrano, A. J. Fernández-López, J. A. Giménez-Bastida and J. C. Espín, Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin, Mol. Nutr. Food Res., 2021, 65, e2100163 CrossRef PubMed.
- S. Bolca, M. Urpi-Sarda, P. Blondeel, N. Roche, L. Vanhaecke, S. Possemiers, N. Al-Maharik, N. Botting, D. De Keukeleire, M. Bracke, A. Heyerick, C. Manach and H. Depypere, Disposition of Soy Isoflavones in Normal Human Breast Tissue, Am. J. Clin. Nutr., 2010, 91, 976–984 CrossRef CAS PubMed.
- A. A. Franke, J. F. Lai and B. M. Halm, Absorption, Distribution, Metabolism, and Excretion of Isoflavonoids after Soy Intake, Arch. Biochem. Biophys., 2014, 559, 24–28 CrossRef CAS.
- A. Cortés-Martín, M. V. Selma, F. A. Tomás-Barberán, A. González-Sarrías and J. C. Espín, Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes, Mol. Nutr. Food Res., 2020, 64, e1900952 CrossRef.
- I. Arnaoutova and H. K. Kleinman, In Vitro Angiogenesis: Endothelial Cell Tube Formation on Gelled Basement Membrane Extract, Nat. Protoc., 2010, 5, 628–635 CrossRef PubMed.
- B. M. Huuskes, R. J. DeBuque, P. G. Kerr, C. S. Samuel and S. D. Ricardo, The Use of Live Cell Imaging and Automated Image Analysis to Assist With Determining Optimal Parameters for Angiogenic Assay in Vitro, Front. Cell Dev. Biol., 2019, 7, 45 CrossRef PubMed.
- G. Carpentier, S. Berndt, S. Ferratge, W. Rasband, M. Cuendet, G. Uzan and P. Albanese, Angiogenesis Analyzer for ImageJ—A Comparative Morphometric Analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”, Sci. Rep., 2020, 10, 11568 CrossRef.
- K. L. DeCicco-Skinner, G. H. Henry, C. Cataisson, T. Tabib, J. C. Gwilliam, N. J. Watson, E. M. Bullwinkle, L. Falkenburg, R. C. O'Neill, A. Morin and J. S. Wiest, Endothelial Cell Tube Formation Assay for the In Vitro Study of Angiogenesis, J. Visualized Exp., 2014, e51312 Search PubMed.
- J. A. Giménez-Bastida, A. González-Sarrías, M. Larrosa, F. Tomás-Barberán, J. C. Espín and M.-T. García-Conesa, Ellagitannin Metabolites, Urolithin A Glucuronide and Its Aglycone Urolithin A, Ameliorate TNF-α-Induced Inflammation and Associated Molecular Markers in Human Aortic Endothelial Cells, Mol. Nutr. Food Res., 2012, 56, 784–796 CrossRef PubMed.
- J. A. Giménez-Bastida, A. González-Sarrías, F. Vallejo, J. C. Espín and F. A. Tomás-Barberán, Hesperetin and Its Sulfate and Glucuronide Metabolites Inhibit TNF-α Induced Human Aortic Endothelial Cell Migration and Decrease Plasminogen Activator Inhibitor-1 (PAI-1) Levels, Food Funct., 2016, 7, 118–126 RSC.
- J. A. Giménez-Bastida, M. Á. Ávila-Gálvez, M. Carmena-Bargueño, H. Pérez-Sánchez, J. C. Espín and A. González-Sarrías, Physiologically Relevant Curcuminoids Inhibit Angiogenesis via VEGFR2 in Human Aortic Endothelial Cells, Food Chem. Toxicol., 2022, 166, 113254 CrossRef PubMed.
- J. G. Kettle, R. Anjum, E. Barry, D. Bhavsar, C. Brown, S. Boyd, A. Campbell, K. Goldberg, M. Grondine, S. Guichard, C. J. Hardy, T. Hunt, R. D. O. Jones, X. Li, O. Moleva, D. Ogg, R. C. Overman, M. J. Packer, S. Pearson, M. Schimpl, W. Shao, A. Smith, J. M. Smith, D. Stead, S. Stokes, M. Tucker and Y. Ye, Discovery of N-(4-{[5-Fluoro-7-(2-Methoxyethoxy)Quinazolin-4-Yl]Amino}phenyl)-2-[4-(Propan-2-Yl)-1H-1,2,3-Triazol-1-Yl]Acetamide (AZD3229), a Potent Pan-KIT Mutant Inhibitor for the Treatment of Gastrointestinal Stromal Tumors, J. Med. Chem., 2018, 61, 8797–8810 CrossRef PubMed.
- M. W. Irvin, A. Zijlstra, J. P. Wikswo and A. Pozzi, Techniques and Assays for the Study of Angiogenesis, Exp. Biol. Med., 2014, 239, 1476–1488 CrossRef PubMed.
- M. Piao, D. Mori, T. Satoh, Y. Sugita and O. Tokunaga, Inhibition of Endothelial Cell Proliferation, in Vitro Angiogenesis, and the down-Regulation of Cell Adhesion-Related Genes by Genistein. Combined with a CDNA Microarray Analysis, Endothelium, 2006, 13, 249–266 CrossRef.
- S. Berndt, M. E. Issa, G. Carpentier and M. Cuendet, Bivalent Role of Genistein in Sprouting Angiogenesis, Planta Med., 2018, 84, 653–661 CrossRef CAS.
- D. Wang, Z. Chen, A. Wai Kan Yeung and A. G. Atanasov, Differences between Common Endothelial Cell Models (Primary Human Aortic Endothelial Cells and EA.Hy926 Cells) Revealed through Transcriptomics, Bioinformatics, and Functional Analysis, Curr. Res. Biotechnol., 2021, 3, 135–145 CrossRef CAS.
- P. H. Tan, C. Chan, S. A. Xue, R. Dong, B. Ananthesayanan, M. Manunta, C. Kerouedan, N. J. W. Cheshire, J. H. Wolfe, D. O. Haskard, K. M. Taylor and A. J. T. George, Phenotypic and Functional Differences between Human Saphenous Vein (HSVEC) and Umbilical Vein (HUVEC) Endothelial Cells, Atherosclerosis, 2004, 173, 171–183 CrossRef CAS.
- Y. Zhang, T. T. Song, J. E. Cunnick, P. A. Murphy and S. Hendrich, Daidzein and Genistein Glucuronides in Vitro Are Weakly Estrogenic and Activate Human Natural Killer Cells at Nutritionally Relevant Concentrations, J. Nutr., 1999, 129, 399–405 CrossRef CAS.
- G. Rimbach, P. D. Weinberg, S. de Pascual-Teresa, M. G. Alonso, B. A. Ewins, R. Turner, A. M. Minihane, N. Botting, B. Fairley, S. Matsugo, Y. Uchida and A. Cassidy, Sulfation of Genistein Alters Its Antioxidant Properties and Its Effect on Platelet Aggregation and Monocyte and Endothelial Function, Biochim. Biophys. Acta, 2004, 1670, 229–237 CrossRef CAS.
- L. E. Pritchett, K. M. Atherton, E. Mutch and D. Ford, Glucuronidation of the Soyabean Isoflavones Genistein and Daidzein by Human Liver Is Related to Levels of UGT1A1 and UGT1A9 Activity and Alters Isoflavone Response in the MCF-7 Human Breast Cancer Cell Line, J. Nutr. Biochem., 2008, 19, 739–745 CrossRef CAS PubMed.
- K. D. R. Setchell, N. M. Brown and E. Lydeking-Olsen, The Clinical Importance of the Metabolite Equol-a Clue to the Effectiveness of Soy and Its Isoflavones, J. Nutr., 2002, 132, 3577–3584 CrossRef CAS PubMed.
- S. Bellou, E. Karali, E. Bagli, N. Al-Maharik, L. Morbidelli, M. Ziche, H. Adlercreutz, C. Murphy and T. Fotsis, The Isoflavone Metabolite 6-Methoxyequol Inhibits Angiogenesis and Suppresses Tumor Growth, Mol. Cancer, 2012, 11, 35 CrossRef PubMed.
- X.-D. Guo, D.-Y. Zhang, X.-J. Gao, J. Parry, K. Liu, B.-L. Liu and M. Wang, Quercetin and Quercetin-3-O-Glucuronide Are Equally Effective in Ameliorating Endothelial Insulin Resistance through Inhibition of Reactive Oxygen Species-Associated Inflammation, Mol. Nutr. Food Res., 2013, 57, 1037–1045 CrossRef CAS PubMed.
- V. van der Velpen, P. C. Hollman, M. van Nielen, E. G. Schouten, M. Mensink, P. Van't Veer and A. Geelen, Large Inter-Individual Variation in Isoflavone Plasma Concentration Limits Use of Isoflavone Intake Data for Risk Assessment, Eur. J. Clin. Nutr., 2014, 68, 1141–1147 CrossRef CAS.
- L. Krenn and D. H. Paper, Inhibition of Angiogenesis and Inflammation by an Extract of Red Clover (Trifolium Pratense L.), Phytomedicine, 2009, 16, 1083–1088 CrossRef CAS PubMed.
- X. Kang, S. Jin and Q. Zhang, Antitumor and Antiangiogenic Activity of Soy Phytoestrogen on 7,12-Dimethylbenz[Alpha]Anthracene-Induced Mammary Tumors Following Ovariectomy in Sprague-Dawley Rats, J. Food Sci., 2009, 74, H237–H242 CrossRef CAS.
- S. Kiriakidis, O. Högemeier, S. Starcke, F. Dombrowski, J. C. Hahne, M. Pepper, H. C. Jha and N. Wernert, Novel Tempeh (Fermented Soyabean) Isoflavones Inhibit in Vivo Angiogenesis in the Chicken Chorioallantoic Membrane Assay, Br. J. Nutr., 2005, 93, 317–323 CrossRef CAS PubMed.
- M. J. Sandoval, P. H. Cutini, M. B. Rauschemberger and V. L. Massheimer, The Soyabean Isoflavone Genistein Modulates Endothelial Cell Behaviour, Br. J. Nutr., 2010, 104, 171–179 CrossRef CAS PubMed.
- H. S. Tuli, M. J. Tuorkey, F. Thakral, K. Sak, M. Kumar, A. K. Sharma, U. Sharma, A. Jain, V. Aggarwal and A. Bishayee, Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances, Front. Pharmacol., 2019, 10, 1336 CrossRef CAS.
- C. Gardana and P. Simonetti, Long-Term Kinetics of Daidzein and Its Main Metabolites in Human Equol-Producers after Soymilk Intake: Identification of Equol-Conjugates by UPLC-Orbitrap-MS and Influence of the Number of Transforming Bacteria on Plasma Kinetics, Int. J. Food Sci. Nutr., 2017, 68, 496–506 CrossRef CAS.
- A. Obara, M. Kinoshita, K. Hosoda, A. Yokokawa, H. Shibasaki and K. Ishii, Identification of Equol-7-Glucuronide-4′-Sulfate, Monoglucuronides and Monosulfates in Human Plasma of 2 Equol Producers after Administration of Kinako by LC-ESI-MS, Pharmacol. Res. Perspect., 2019, 7, e00478 CrossRef PubMed.
- Z.-L. Liu, H.-H. Chen, L.-L. Zheng, L.-P. Sun and L. Shi, Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer, Signal Transduction Targeted Ther., 2023, 8, 198 CrossRef CAS PubMed.
- M. Przybylski, Review of the Current Research on the Role of BFGF and VEGF in Angiogenesis, J. Wound Care, 2009, 18, 516–519 CrossRef PubMed.
- H.-R. Seo, H. E. Jeong, H. J. Joo, S.-C. Choi, C.-Y. Park, J.-H. Kim, J.-H. Choi, L.-H. Cui, S. J. Hong, S. Chung and D.-S. Lim, Intrinsic FGF2 and FGF5 Promotes Angiogenesis of Human Aortic Endothelial Cells in 3D Microfluidic Angiogenesis System, Sci. Rep., 2016, 6, 28832 CrossRef PubMed.
- Y. Xie, N. Su, J. Yang, Q. Tan, S. Huang, M. Jin, Z. Ni, B. Zhang, D. Zhang, F. Luo, H. Chen, X. Sun, J. Q. Feng, H. Qi and L. Chen, FGF/FGFR Signaling in Health and Disease, Signal Transduction Targeted Ther., 2020, 5, 181 CrossRef.
- L. Pérez-Gutiérrez and N. Ferrara, Biology and Therapeutic Targeting of Vascular Endothelial Growth Factor A, Nat. Rev. Mol. Cell Biol., 2023, 24, 816–834 CrossRef.
- H. Huang, J. Shen and S. A. Vinores, Blockade of VEGFR1 and 2 Suppresses Pathological Angiogenesis and Vascular Leakage in the Eye, PLoS One, 2011, 6, e21411 CrossRef PubMed.
- X. Wang, A. M. Bove, G. Simone and B. Ma, Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role, Front. Cell Dev. Biol., 2020, 8, 599281 CrossRef.
- A. Endo, S. Fukuhara, M. Masuda, T. Ohmori and N. Mochizuki, Selective Inhibition of Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2) Identifies a Central Role for VEGFR-2 in Human Aortic Endothelial Cell Responses to VEGF, J. Recept. Signal Transduction Res., 2003, 23, 239–254 CrossRef.
- J. A. F. Silva, X. Qi, M. B. Grant and M. E. Boulton, Spatial and Temporal VEGF Receptor Intracellular Trafficking in Microvascular and Macrovascular Endothelial Cells, Sci. Rep., 2021, 11, 17400 CrossRef.
- S. Fernández-Castillejo, A. Macià, M.-J. Motilva, Ú. Catalán and R. Solà, Endothelial Cells Deconjugate Resveratrol Metabolites to Free Resveratrol: A Possible Role in Tissue Factor Modulation, Mol. Nutr. Food Res., 2019, 63, e1800715 CrossRef.
- V. Spigoni, P. Mena, M. Cito, F. Fantuzzi, R. C. Bonadonna, F. Brighenti, A. Dei Cas and D. Del Rio, Effects on Nitric Oxide Production of Urolithins, Gut-Derived Ellagitannin Metabolites, in Human Aortic Endothelial Cells, Molecules, 2016, 21, 1009 CrossRef PubMed.
- N. Toro-Funes, F. J. Morales-Gutiérrez, M. T. Veciana-Nogués, M. C. Vidal-Carou, J. P. E. Spencer and A. Rodriguez-Mateos, The Intracellular Metabolism of Isoflavones in Endothelial Cells, Food Funct., 2015, 6, 98–108 RSC.
- E. A. Aisenbrey and W. L. Murphy, Synthetic Alternatives to Matrigel, Nat. Rev. Mater., 2020, 5, 539–551 CrossRef.
- K. Lněničková, M. Šadibolová, P. Matoušková, B. Szotáková, L. Skálová and I. Boušová, The Modulation of Phase II Drug-Metabolizing Enzymes in Proliferating and Differentiated CaCo-2 Cells by Hop-Derived Prenylflavonoids, Nutrients, 2020, 12, 2138 CrossRef.
- K. A. O'Leary, A. J. Day, P. W. Needs, W. S. Sly, N. M. O'Brien and G. Williamson, Flavonoid Glucuronides Are Substrates for Human Liver Beta-Glucuronidase, FEBS Lett., 2001, 503, 103–106 CrossRef PubMed.
- J. Chen, S. Wang, X. Jia, S. Bajimaya, H. Lin, V. H. Tam and M. Hu, Disposition of Flavonoids via Recycling: Comparison of Intestinal versus Hepatic Disposition, Drug Metab. Dispos., 2005, 33, 1777–1784 Search PubMed.
- Z.-M. Weng, P. Wang, G.-B. Ge, Z.-R. Dai, D.-C. Wu, L.-W. Zou, T.-Y. Dou, T.-Y. Zhang, L. Yang and J. Hou, Structure-Activity Relationships of Flavonoids as Natural Inhibitors against E. Coli β-Glucuronidase, Food Chem. Toxicol., 2017, 109, 975–983 CrossRef PubMed.
- J. A. Giménez-Bastida, M. Á. Ávila-Gálvez, J. C. Espín and A. González-Sarrías, Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of P53/P21 and P16/Rb Pathways, and ABC Transporters, Mol. Nutr. Food Res., 2019, 63, e1900629 CrossRef.
- M. Eilers, U. Roy and D. Mondal, MRP (ABCC) Transporters-Mediated Efflux of Anti-HIV Drugs, Saquinavir and Zidovudine, from Human Endothelial Cells, Exp. Biol. Med., 2008, 233, 1149–1160 CrossRef.
- P. T. Ronaldson, H. Brzica, W. Abdullahi, B. G. Reilly and T. P. Davis, Transport Properties of Statins by Organic Anion Transporting Polypeptide 1A2 and Regulation by Transforming Growth Factor-β Signaling in Human Endothelial Cells, J. Pharmacol. Exp. Ther., 2021, 376, 148–160 CrossRef.
- A. I. Álvarez, F. Vallejo, B. Barrera, G. Merino, J. G. Prieto, F. Tomás-Barberán and J. C. Espín, Bioavailability of the Glucuronide and Sulfate Conjugates of Genistein and Daidzein in Breast Cancer Resistance Protein 1 Knockout Mice, Drug Metab. Dispos., 2011, 39, 2008–2012 CrossRef.
- M. K. Bircsak and M. L. Aleksunes, Interaction of Isoflavones with the BCRP/ABCG2 Drug Transporter, Curr. Drug Metab., 2015, 16, 124–140 CrossRef.
- G. Grosser, B. Döring, B. Ugele, J. Geyer, S. E. Kulling and S. T. Soukup, Transport of the Soy Isoflavone Daidzein and Its Conjugative Metabolites by the Carriers SOAT, NTCP, OAT4, and OATP2B1, Arch. Toxicol., 2015, 89, 2253–2263 CrossRef.
- A. Boronat, J. Rodriguez-Morató, G. Serreli, M. Fitó, R. F. Tyndale, M. Deiana and R. de la Torre, Contribution of Biotransformations Carried Out by the Microbiota, Drug-Metabolizing Enzymes, and Transport Proteins to the Biological Activities of Phytochemicals Found in the Diet, Adv. Nutr., 2021, 12, 2172–2189 CrossRef.
- K. D. R. Setchell and C. Clerici, Equol: History, Chemistry, and Formation, J. Nutr., 2010, 140, 1355S–1362S CrossRef PubMed.
- M. Tanaka, S. Fujii, H. Inoue, N. Takahashi, Y. Ishimi and M. Uehara, (S)-Equol Is More Effective than (R)-Equol in Inhibiting Osteoclast Formation and Enhancing Osteoclast Apoptosis, and Reduces Estrogen Deficiency-Induced Bone Loss in Mice, J. Nutr., 2022, 152, 1831–1842 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo03946c |
| This journal is © The Royal Society of Chemistry 2024 |