Pterostilbene alleviates abdominal aortic aneurysm via inhibiting macrophage pyroptosis by activating the miR-146a-5p/TRAF6 axis†
Huoying
Cai
ab,
Lin
Huang
ab,
Mingshan
Wang
ab,
Ruiming
Liu
c,
Jiacong
Qiu
d,
Yuansen
Qin
ab,
Xi
Yao
 e,
Shenming
Wang
*ab,
Chen
Yao
*ab,
Zuojun
Hu
*ab and
Yu
Zhou
e,
Shenming
Wang
*ab,
Chen
Yao
*ab,
Zuojun
Hu
*ab and
Yu
Zhou
 *ab
*ab
aDepartment of Vascular Surgery, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China. E-mail: zhouy259@mail3.sysu.edu.cn; huozuojun@mail.sysu.edu.cn; yaochen@mail.sysu.edu.cn; shenmingwang@hotmail.com; Fax: +020-28823235; Tel: +020-87755766
bNational-Guangdong Joint Engineering Laboratory for Diagnosis and Treatment of Vascular Diseases, Guangzhou, Guangdong, China
cLaboratory of General Surgery, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
dDepartment of Vascular Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, Jiangxi 330006, China
eDepartment of Biomedical Sciences, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong
First published on 5th December 2023
Abstract
Pterostilbene (PTE), a natural stilbene found in blueberries and several varieties of grapes, has several pharmacological activities, including anti-inflammatory and antioxidative activities. However, its role in abdominal aortic aneurysm (AAA), which is a severe inflammatory vascular disease, remains incompletely understood. In this study, we investigated the protective effects of natural stilbene PTE on AAA formation and the underlying mechanism. Two AAA mouse models (Ang II-induced model and PPE-induced model) were used to examine the effect of PTE on AAA formation. We showed that PTE administration attenuated AAA formation in mice. Furthermore, we found that PTE significantly inhibited inflammatory responses in mouse aortas, as PTE suppressed macrophage pyroptosis and prevented macrophage infiltration in aortas, resulting in reduced expression of pro-inflammatory cytokines in aortas. We also observed similar results in LPS + ATP-treated Raw 264.7 cells (a macrophage cell line) and primary peritoneal macrophages in vitro. We showed that pretreatment with PTE restrained inflammatory responses in macrophages by inhibiting macrophage pyroptosis. Mechanistically, miR-146a-5p and TRAF6 interventions in vivo and in vitro were used to investigate the role of the miR-146a-5p/TRAF6 axis in the beneficial effect of PTE on macrophage pyroptosis and AAA. We found that PTE inhibited macrophage pyroptosis by miR-146a-5p-mediated suppression of downstream TRAF6 expression. Moreover, miR-146a-5p knockout or TRAF6 overexpression abrogated the protective effect of PTE on macrophage pyroptosis and AAA formation. These findings suggest that miR-146a-5p/TRAF6 axis activation by PTE protects against macrophage pyroptosis and AAA formation. PTE might be a promising agent for preventing inflammatory vascular diseases, including AAA.
1. Introduction
Abdominal aortic aneurysm (AAA) is a chronic vascular disease characterized by the dilatation of all layers of the arterial wall as a result of inflammatory response activation, matrix degradation, loss of elastin, smooth muscle cell loss, and compensatory collagen deposition.1,2 With the aging of the population, the incidence of AAA has been increasing significantly, and this condition has become a serious problem threatening human health.3,4 In particular, the mortality associated with ruptured AAA is >90%.5,6 It is currently believed that when the aortic diameter is ≥55 mm in males and 50 mm in females or increases >1 cm per year, surgical treatment is required.7 However, there is currently no pharmacological therapy to alleviate AAA.4 Thus, it is imperative to find pharmacological strategies for treating patients with AAA. Recently, the effects of natural extract on AAA formation have been widely investigated.Given that drugs with anti-inflammatory properties targeting mast cells, neutrophils and cytotoxic T cells failed to limit AAA development in several clinical trials, we focused on inhibiting macrophage inflammation. Macrophage pyroptosis promotes the proinflammatory macrophage phenotype, which has been reported to play an essential role in AAA. Pyroptosis is mainly characterized by inflammasome activation and plays an important role in inflammation-associated diseases.8 Previous studies have shown that the NLRP3 inflammasome is the most well-studied Nod-like receptor, forming a complex composed of adaptor proteins, such as apoptosis-associated speck-like protein (ASC). In addition, the inflammasome recruits pro-caspase-1 and activates it into caspase-1, resulting in the maturation of proinflammatory cytokines, such as IL-1β and IL-18.9–11 In addition, active caspase-1 cleaves Gasdermin-D to form an N-terminal fragment (GSDMD-N), which forms membrane pores to mediate the release of mature IL-1β and IL-18.12–14
Pterostilbene (PTE, trans-3,5-dimethoxy-4-hydroxystilbene), a natural stilbene, is typically found in blueberries and several types of grapes.15 In addition, PTE contains two methoxy groups compared with the three hydroxyl groups of resveratrol, which leads to more lipophilic and better pharmacokinetic profiles than resveratrol.16 PTE also has several pharmacological activities, such as anti-inflammatory, antioxidant and antineoplastic properties.16–18 Moreover, PTE protects vascular endothelial cells against oxidized low-density lipoprotein-induced apoptosis in vitro and in vivo.19 Furthermore, PTE may be an anti-proliferative agent for the treatment of atherosclerosis.20,21 However, the protective effect of PTE on macrophage pyroptosis and AAA formation remains unraveled.
MicroRNAs are endogenous, small noncoding RNAs that can bind the 3′ UTRs of target mRNAs and then inhibit gene expression.22 Numerous studies have revealed that microRNAs play essential roles in AAA, indicating that microRNAs are promising therapeutic targets. To date, several studies have demonstrated that PTE affects the expression of microRNAs. Song et al. demonstrated that PTE exerted a protective effect against liver fibrosis at least partly by inhibiting miR-34a.23 Kumar et al. revealed that PTE significantly downregulated the expression of endogenous miR-17, miR-20a, miR-106a, and miR-106b in DU145 and 22Rv1 prostate cancer cells.24 Moreover, in vivo and in vitro experiments confirmed that miR-146a-5p upregulation inhibited NLRP3 inflammasome and cell pyroptosis in several inflammatory diseases.25,26 Plana et al. also reported that miR-146a-5p was overexpressed in the plasma of AAA patients.27 However, the relationship between PTE and miR-146a-5p in the development of AAA remains to be elucidated.
In this study, we investigated the effect of PTE on macrophage pyroptosis and AAA formation. The mechanism by which PTE affects macrophage pyroptosis and AAA was explored to demonstrate how PTE plays a role in defending against inflammation. Our research provides essential evidence for potential drug development in treating AAA.
2. Materials and methods
2.1 Human sample analysis
Human samples were obtained from 10 AAA patients who underwent open AAA repair surgery, and normal aortas were obtained from 8 patients who underwent donor transplantation at the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong. All procedures for the collection of clinical samples were approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University ([2020]326) and complied with the principles of the Declaration of Helsinki. All clinical parameters of the AAA patients are shown in ESI Table I.† Informed consent was obtained from all study participants or their legal guardians. After dissection, the samples were immediately snap frozen in liquid nitrogen and stored at −80 °C for qPCR or western blot analysis. Some samples were fixed in 10% formalin for embedding, sectioning and staining.2.2 Experimental animals
We obtained 8- to 10-week-old male C57BL/6 mice from the Animal Center of the First Affiliated Hospital of Sun Yat-sen University and 10- to 12-week-old male ApoE−/− mice from GemPharmatech Co., Ltd (Nanjing, China). The miR-146a-5p-knockdown mice were produced by Cyagen Biomodels Co., Ltd (Guangzhou) using CRISPR/Cas9-mediated genome engineering. The miR-146 gene (NCBI reference sequence: NR_029558.1; Ensembl: ENSMUSG00000065601) is located on mouse chromosome 11. As shown in ESI Fig. 11A,† Exon 1 (Transcript miR-146-201: ENSMUST00000083667) was selected as the target site. Finally, Cas9 and gRNA were injected into fertilized eggs to produce knockout mice. The genotypes of the offspring were verified by PCR using the following primers: forwards: 5′- AAGGGAAGGATTGAACATGACACA-3′ and reverse: 5′ TTATTGCCTCTCTACAAGGACCTG-3′. Genotyping of miR-146a-5p- knockdown mice was confirmed by PCR analysis (ESI Fig. 11B and C†). All mice were housed under specific pathogen-free conditions in a controlled environment (20 ± 2 °C with relative humidity ranging from 50% to 60% and a 12-h day/night cycle) and had access to food and water. All animal experiments were approved by the Institutional Review Board for Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat-sen University ([2021]164).2.3 Ang II-infused AAA model
First, ApoE−/− mice were anaesthetized by an intraperitoneal injection of pentobarbital (60 mg kg−1). Then, Ang II (Sigma, A9525, USA) was injected by mini osmotic pumps (Alzet, Model 2004, USA) at a rate of 1000 ng kg−1 min−1. The pumps were placed into the subcutaneous space of the dorsum for 28 days. At the end of the study, the mice were anaesthetized with ketamine/xylazine (100 and 20 mg kg−1 ip, respectively), and the aortas and blood were collected for further analyses.2.4 Porcine pancreatic elastase (PPE)-infused AAA model
Before the operation, all mice were anaesthetized by an intraperitoneal injection of 1% pentobarbital (60 mg kg−1). Using a dissection microscope, the infrarenal aorta was dissected and exposed to a piece of gelfoam soaked in 10 mg mL−1 PPE (A9525-10 MG, Sigma-Aldrich). After 8 min, the gelfoam was removed, the area was rinsed with 0.9% NaCl, and the abdominal incision was closed. After 14 days, the aortas and blood were collected for further analyses.Phenix software (Jiangxi, China) was used to measure the outer aortic diameter under laparotomy on days 0 and 14 after PPE infusion was performed, and images were obtained using a digital camera attached to a Phenix dissection microscope (Jiangxi, China). Measurements were performed at least three times by two individuals. After 14 days, the mice were anaesthetized with ketamine/xylazine (100 and 20 mg kg−1 ip, respectively), and the aortas and blood were collected for further analyses.
2.5 Adeno-associated virus (AAV)-infected mice
The murine TRAF6-overexpression AAV and the corresponding control AAV were provided by GeneChem (Shanghai, China). Before establishing the AAA models, 1 × 1011 viral genome particles of AAV were administered to the abdominal aorta through tail vein injection. The injected mice were subjected to PPE-induced AAA modelling after 14 days.2.6 PTE treatment
PTE and resveratrol (RSV) were dissolved in DMSO to make a stock solution (50 mM) according to the manufacturer's instructions. Several studies have indicated that RSV inhibits the development of experiment AAAs,28,29 so RSV treatment was used as a positive control. C57BL/6 mice were divided into six groups: the sham group mice exposed to normal saline receiving normal drinking water; mice in the PTE groups were divided into three groups, which received three doses of PTE (5 mg kg−1, 10 mg kg−1 and 20 mg kg−1 weights, S26817-25 g, Shanghai Yuanye Bio-Technology Co., Ltd); and RSV group receiving RSV (10 mg kg−1 weight, S30630-5 g, Shanghai Yuanye Bio-Technology Co., Ltd). In comparison, mice in the vehicle group were administered normal drinking water containing the same quantity of DMSO by oral gavage. ApoE−/− mice were divided into three groups: the sham, vehicle and PTE groups (10 mg kg−1 weight).All mice in the PTE and RSV groups received PTE and RSV every other day by oral gavage, beginning on day one after PPE or Ang II infusion, and treatment was continued daily until sacrifice. The concentrations of PTE and RSV were determined based on previous studies.12,30,31
2.7 Ultrasonic imaging
AAA formation and progression were monitored by ultrasonic B-mode imaging at 40 MHz with a Vevo 3100 imaging system (Visual Sonics, Canada). Ultrasonic imaging was used to measure the infrarenal aortic diameter on the day before the mice were harvested on days 14 and 28. AAA was defined as a ≥50% increase in maximal infrarenal aortic diameter compared with the baseline level.2.8 Systolic blood pressure measurement
Systolic blood pressure measurement was performed with a tail-cuff instrument (BP-2010A, Softron, Beijing) on day 28 after Ang II treatment, as described previously.32 In addition, systolic blood pressure measurement was repeated 3 times for each mouse, and the mean value was determined.2.9 Histology
Mouse aorta samples were collected, fixed in 10% formalin and embedded in paraffin. Then, the samples were cut into serial sections (5 μm thickness). Paraffin sections were used for further analysis. Haematoxylin and eosin (H&E), Verhoeff-Van Gieson (Biossci, China) and Masson staining (Biossci, China) were used to evaluate medial elastin and collagen destruction. The destruction of medial elastin and collagen was graded from 1 (no destruction) to 4 (aortic rupture), as previously described.33,342.10 Immunofluorescence analysis
Human and mouse abdominal aorta sections were vertically embedded in an optimal cutting temperature compound (O.C.T, Servicebio, China) and stored at −80 °C. Then, at least 8–10 serial sections (6 μm thickness) were cut with a freezing microtome (Leica, Germany) for immunofluorescence staining. As previously described, aorta sections were stained with antibodies against Caspase 1 p10, NLRP3, GSDMD, α-actin, CD68, CD80 and CD206 (ESI Table IV†). Finally, the tissue sections were visualized using a fluorescence microscope (DMi8, Germany).2.11 Quantitative real-time PCR
Cells and human and mouse aorta samples were extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Then, 1000 ng of RNA was reverse transcribed into cDNA. mRNA synthesis was performed according to the Evo M-MLV RT Mix Kit (ACCURATE BIOLOGY, Hunan, China), and miRNA synthesis was performed according to the 1st Strand cDNA Synthesis Kit by stem-loop (Vazyme, Nanjing, China). RT-qPCR was performed with SYBR Master/Green Mix (Vazyme, Nanjing, China/ACCURATE BIOLOGY, Changsha, China) using a LightCycler 480 II system (Roche Diagnostics, Basel, Switzerland). The primers are shown in ESI Table II.† Finally, we used GAPDH to normalize the relative mRNA expression using the 2−ΔΔCt method. Moreover, the relative miRNA expression was normalized to that of U6.2.12 Cell culture and treatment
The mouse macrophage cell line (Raw 264.7 cells) was purchased from Guangzhou Solarbio Science & Technology Co., Ltd. The cells were cultured in RPMI 1640 (Gibco, USA) supplemented with 10% foetal bovine serum (FBS, Gibco-BRL), penicillin (100 U mL−1), and streptomycin (100 mg mL−1) in a cell culture incubator with 5% CO2 at 37 °C.Primary murine peritoneal macrophages were isolated according to a previously described protocol.35 Briefly, the mice were intraperitoneally injected with 1.5 mL of 3% thioglycolate (TG, Sigma, USA) for 3 days. Then, the mice were sacrificed and sterilized in 75% ethanol for 10 min. An incision was made with scissors to expose the peritoneum, and 8–9 mL of ice-cold PBS was injected into the peritoneal cavity and shaken for 10 seconds. Macrophages were collected with a 10 mL syringe. After the cell suspension was centrifuged, the cell pellet was resuspended in RPMI 1640 (Gibco, USA) supplemented with 10% FBS (Gibco-BRL), penicillin (100 U mL−1), and streptomycin (100 mg mL−1) in a cell culture incubator with 5% CO2 at 37 °C. After 2 h, the cells were washed with PBS to remove nonadherent cells, and the adherent peritoneal macrophages were incubated for further experiments.
2.13 Western blot analysis
Cells and human and mouse aorta samples were lysed in RIPA buffer (Beyotime Biotechnology, Shanghai, China) containing PMSF (Fudebio, Hangzhou, China) for 30 min. Then, the proteins were sonicated and centrifuged to obtain the supernatants. The proteins were separated by SDS-PAGE (8%, 10%, or 15%) and transferred onto polyvinylidene difluoride (PVDF) membranes. Then, the membranes were blocked with 5% fat-free milk in TBST at 37 °C for 1 h and incubated with primary antibodies at 4 °C overnight. After being washed with TBST, the membranes were incubated with secondary antibodies at room temperature for 1 h. Finally, the protein bands were visualized with enhanced chemiluminescence reagents (Millipore, USA). Protein expression was analysed using ImageJ software and normalized to GAPDH expression. The primary antibodies are shown in ESI Table III.†2.14 Cytokine measurements
Blood was collected from the heart after the mice were anaesthetized and then centrifuged to collect the plasma. The levels of proinflammatory cytokines, including TNF-α, IL-6, IL-1β and IL-18, were measured by ELISA kits (Cusabio, Wuhan, China) according to the manufacturer's instructions.2.15 Luciferase assay
293T cells were cotransfected with miR-146a-5p mimic or miR-NC plus WT-TRAF6/Mut-TRAF6. Then, we used Luciferase Assay Reagent II (Luciferase Assay Reagent, Promega) to measure firefly and Renilla luciferase activities after 48 h of transfection.2.16 Measurement of ROS production
Raw 264.7 cells were pretreated with or without PTE (2, 4 or 6 μM) for 24 h, followed by exposure to LPS (1 μg mL−1). After 6 h, ROS production was measured using DCFH-DA (Beyotime Biotechnology, Shanghai, China) according to the manufacturer's protocol. Briefly, the cells were washed three times with PBS and then incubated with the DCFH-DA probe (10 μmol L−1) for 20 min at 37 °C in the dark. Then, the cells were washed three times to rinse away the excess DCFH-DA and visualized using a fluorescence microscope (DMi8, Germany).2.17 YO-PRO-1 dye uptake
YO-PRO-1 (Beyotime Biotechnology, Shanghai, China) is a small molecular dye that is membrane impermeable but pyroptosis-pole permeable. The method used in the dye uptake assay was conducted as previously described.25 Raw 264.7 cells were pretreated with or without PTE (2, 4 or 6 μM) for 24 h, followed by exposure to LPS (1 μg mL−1) for 24 h and then ATP (5 mM) for 2 h. Triton X-100 detergent (0.1%) was used as a positive control. We used the YO-PRO-1 dye (0.2 mM) to observe Raw 264.7 cell pyroptosis. Then, Hoechst 33258 (Beyotime Biotechnology, Shanghai, China) was used to stain the nucleus, indicating the total cells. The observations and images were obtained by applying an inverted microscope (Olympus, Tokyo, Japan).2.18 Calcein AM/propidium iodide (PI) double staining assay
Cell membrane integrity and pyroptosis were detected using another Calcein/PI cell viability/cytotoxicity assay kit (C2015M, Beyotime Biotechnology, Shanghai, China). The method used in the double staining assay was conducted as previously described.36,37 Triton X-100 detergent (0.1%) was used as a positive control. Calcein AM and PI dye were added for 30 min. The observations and images were obtained using a fluorescence microscope (Olympus, Tokyo, Japan).2.19 mRNA sequencing (mRNA-seq) analysis
The mRNA-seq data were analysed as previously described.38 First, the raw data were filtered with SOAPnuke (v1.4.0) before the reliability analysis was performed. Next, we used HISAT (v2.1.0) to align the clean reads to the reference genome. Then, Bowtie2 (v2.2.5) was used to align the clean reads to the reference genes. All samples were examined using the BGISEQ platform (BGI, Shenzhen, China). Within-group differential gene analysis was performed using DESeq under the following conditions: fold change ≥2 and adjusted P value ≤0.001. The pheatmap function was used to examine the differential gene set and draw a heatmap of differential gene clusters. Based on the GO and KEGG annotation results and official classifications, the differentially expressed genes were functionally classified, phyper in R software was used for KEGG enrichment analysis, and the TermFinder package was used for GO enrichment analysis. With a Q value ≤0.05 as the threshold, candidate genes that met this condition were defined as being significantly enriched. The raw data were uploaded to the GEO dataset (GSE221735).2.20 Statistical analysis
The data are presented as the mean ± SEM unless otherwise stated. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). Based on the nature of the data, parametric unpaired t tests, Fisher's exact tests or nonparametric Mann–Whitney tests were used to determine statistical significance between groups. A p value <0.05 was considered statistically significant.3. Results
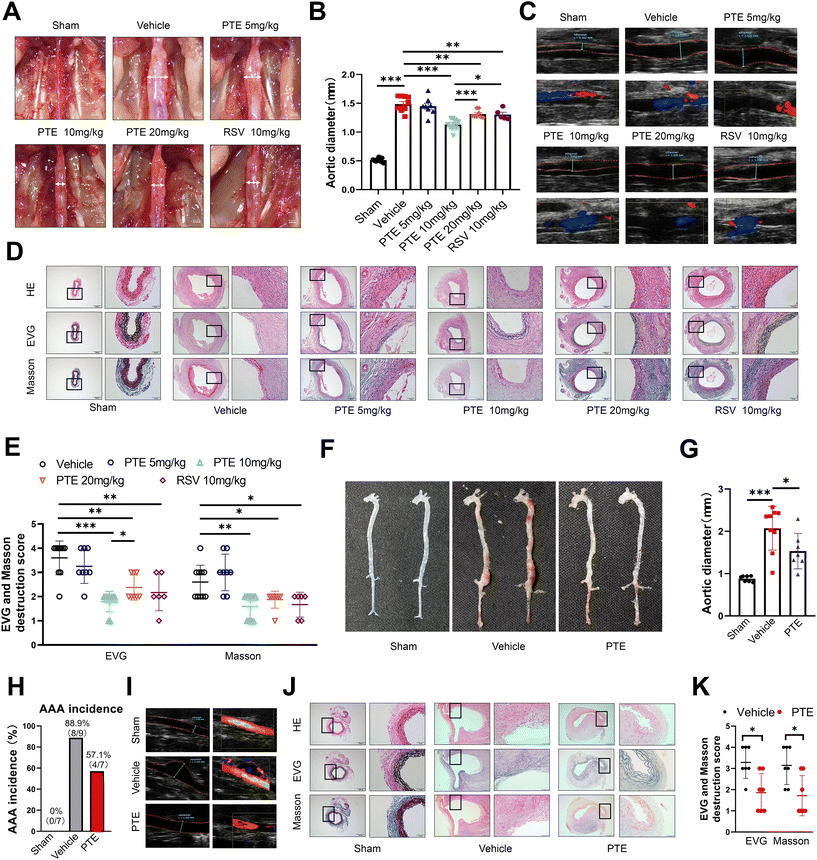
3.1 Oral administration of PTE attenuates AAA formation
To examine the role of PTE in AAA formation, we first investigated the role of PTE in a PPE-induced mouse AAA model. We generated six groups of PPE-infused AAA mice, which were then treated with vehicle, PTE or RSV every other day beginning on day one after PPE infusion for a total of 14 days. Several studies have indicated that RSV inhibits the development of experiment AAAs.28,29 Therefore, RSV treatment was used as a positive control. After 14 days, we successfully created an experimental aneurysm. Maximal outer aortic diameters were no different between vehicle-treated and PTE (5 mg kg−1)-treated mice. However, PTE (10 mg kg−1 and 20 mg kg−1) and RSV (10 mg kg−1)-treated mice were significantly smaller than vehicle-treated mice (p < 0.01, parametric unpaired t test, Fig. 1A and B). Moreover, PTE (10 mg kg−1)-treated mice were the most effective in suppressing the development of experiment AAAs (p < 0.05, parametric unpaired t test, Fig. 1A and B). Therefore, we conducted the following animal experiments at this dose. After PPE exposure, vascular ultrasound imaging showed milder dilation in the abdominal aortas of mice in the PTE (10 mg kg−1 and 20 mg kg−1)- and RSV (10 mg kg−1)-treated groups compared to those in the vehicle-treated group on the 14th day (Fig. 1C). In addition, we assessed elastin levels in vessels by elastic van Gieson (EVG) staining. The results showed that the PTE- and RSV-treated groups had further attenuation of PPE-induced elastin degradation compared with the vehicle-treated group. Masson staining showed that PTE (10 mg kg−1 and 20 mg kg−1) and RSV (10 mg kg−1) treatment promoted the presence of collagen (blue stain) in aneurysmal aortic media (p < 0.05, nonparametric Mann–Whitney test, Fig. 1D and E). These histological results confirmed AAA suppression by PTE treatment. Furthermore, representative immunofluorescence staining of α-actin revealed that PTE treatment significantly enhanced the number of SMCs in the aortic wall (ESI Fig. 1†). We investigated the expression of the proinflammatory cytokines TNF-α, IL-18, IL-6 and IL-1β after 14 days. In addition, qPCR analysis of these proinflammatory cytokines showed that the levels in mouse aortas were decreased after treatment with PTE (p < 0.05 vs. vehicle, parametric unpaired t test, ESI Fig. 2A–D†), suggesting the regulatory effect of PTE on the inflammatory response.To further investigate the role of PTE in AAA, we examined the effects of PTE on an Ang II-infused AAA model, which is a suprarenal AAA model. We generated three groups of Ang II-infused AAA mice: the sham group mice infused with normal saline receiving regular drinking water, and the vehicle and PTE groups infused with Ang II, which were then treated with vehicle or PTE (10 mg kg−1) every other day beginning on day one after Ang II infusion for a total of 28 days. Systolic blood pressure after Ang II infusion did not differ between the two groups (p > 0.05, parametric unpaired t test, ESI Fig. 3A†). Moreover, we observed that body weight was not different between the two groups, indicating that oral administration of PTE had no toxic effects on body weight gain (p > 0.05, parametric unpaired t test, ESI Fig. 3B†). In accordance with previous studies,39,40 the level of blood lipids (low-density lipoprotein, triglyceride, cholesterol) was significantly decreased, but high-density lipoprotein levels were significantly increased in mouse serum after treatment with PTE (p < 0.05, parametric unpaired t test, ESI Fig. 3C–F†). Consistent with the results in the PPE-induced AAA model, the maximal outer aortic diameters of the PTE-treated group were significantly smaller than those in the vehicle-treated group (p < 0.05, parametric unpaired t test, Fig. 1F and G). In the vehicle-treated group, 88.9% (8/9) of the mice exhibited AAA formation, while in the PPE-treated group, only 57.1% (4/7) showed AAA formation (p = 0.2615, Fisher's exact test, Fig. 1H). Ultrasound imaging showed progressively less dilation in the mice in the PTE-treated group compared to the vehicle-treated group (Fig. 1I). H&E, EVG and Masson staining showed that PTE treatment promoted elastin and collagen levels in the aneurysmal aortic media, and elastin destruction scores decreased in mice treated with PTE compared with mice treated with vehicle (p < 0.05, nonparametric Mann–Whitney test, Fig. 1J and K). In addition, the levels of the proinflammatory cytokines TNF-α, IL-6, IL-1β, and IL-18 in mouse aortas and plasma were reduced in the PTE-treated group (p < 0.05, parametric unpaired t test, ESI Fig. 2E–L†). Collectively, these findings suggest that PTE treatment ameliorated AAA formation in mice.
3.2 PTE treatment inhibits macrophage pyroptosis in vivo and in vitro
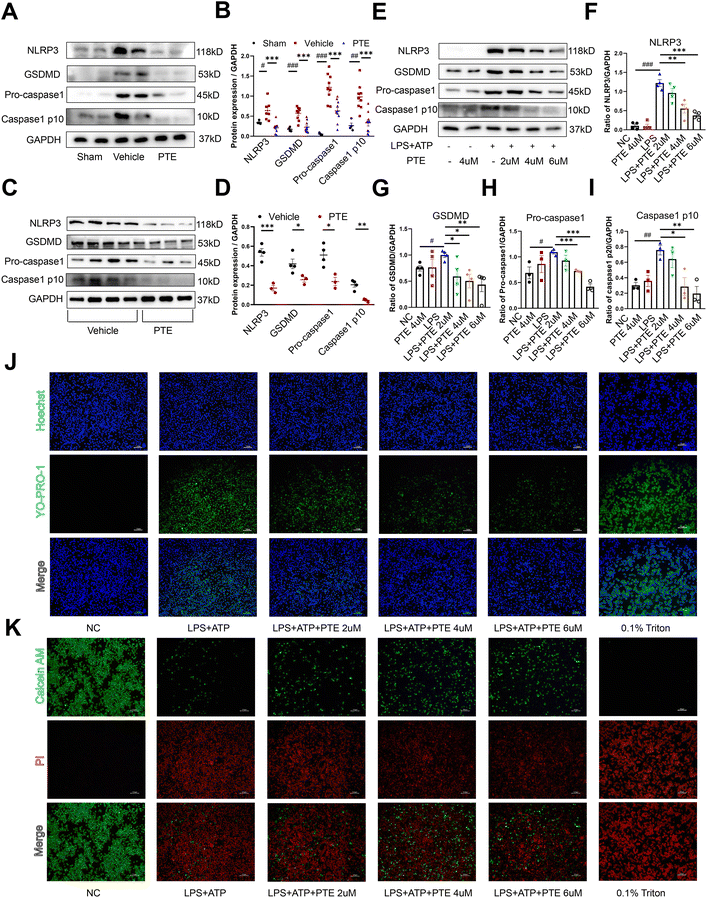
To confirm whether pyroptosis is related to the pathogenesis of AAA, we first measured the expression of pyroptosis-related proteins (NLRP3, GSDMD and Caspase1 p10) in human AAA tissues. Human AAA tissues were obtained from patients undergoing open surgery, and normal aortas were obtained from organ donors. As shown in ESI Fig. 4A and B,† pyroptosis-related protein expression was substantially higher in human AAA tissues than in normal aorta tissues (p < 0.05, parametric unpaired t test). In addition, qPCR revealed that proinflammatory cytokine levels were upregulated in human AAA aortas compared with normal aortas (p < 0.05, parametric unpaired t test, ESI Fig. 4C†).Currently, intraluminal infusion of PPE into the infrarenal aorta to induce AAA formation in mice has become a widely used AAA model. As previously described (24, 25), we established a PPE-induced mouse AAA model. Representative photographs showing the macroscopic features of the aorta at baseline (day 0) and 14 days after PPE infusion in C57BL/6 mice indicate that the PPE-induced AAA model was established successfully (ESI Fig. 4D†). To investigate the localization of pyroptosis in aneurysmal aortas, triple fluorescent staining was performed on mouse and human AAA tissues. Macrophages or vascular SMCs were stained with antibodies conjugated with Alexa Fluor 594 (red colour), pyroptosis-related proteins were stained with antibodies conjugated with Alexa Fluor 488 (green colour), and cell nuclei were stained with DAPI (blue colour). As shown in ESI Fig. 4E and F,† pyroptosis-related proteins were mainly expressed in infiltrating macrophages in the aneurysmal aorta. Collectively, these findings suggest that an increase in macrophage pyroptosis is involved in AAA formation.
We further investigated the role of PTE in pyroptosis in vivo and in vitro. Western blotting showed that the levels of pyroptosis-related proteins in the aortas of PPE-infused C57BL/6 mice were increased in the vehicle group compared with the sham group, and treatment with PTE significantly decreased pyroptosis-related protein expression (Fig. 2A and B). Because macrophage infiltration can also induce inflammation, we examined the effect of PTE on M1 macrophage infiltration. CD68 (macrophage marker) and CD80 (M1 macrophage marker) were stained with antibodies conjugated with Alexa Fluor 594 (red colour), and the nucleus was stained with DAPI (blue colour). As shown in ESI Fig. 5,† PTE decreased the proportions of CD68 and CD80. These results further suggest that PTE treatment attenuated macrophage inflammation. In addition, PTE possesses various pharmacological activities, including anticancer, anti-inflammatory, and antioxidant activities.41–43 We measured intracellular ROS levels in Raw 264.7 macrophages using DCHF as a fluorescent probe. Our results showed that ROS levels were inhibited in the PTE-treated group compared with the LPS-treated group (ESI Fig. 6†).
Thioglycolate (TG)-induced peritoneal macrophages are bone marrow-derived inflammatory cells that produce proinflammatory cytokines, and their behaviours may resemble those of infiltrating macrophages in AAA.22 We used TG to stimulate peritoneal macrophages from PPE-infused C57BL/6 mice (Fig. 2C and D) and Ang II-induced ApoE−/− mice (ESI Fig. 7†), which were then treated with PTE or vehicle and stimulated with LPS and ATP. Western blotting showed that pyroptosis-related protein levels were decreased in the PTE-treated group (p < 0.05, parametric unpaired t test, Fig. 2C and D and ESI Fig. 7†). Moreover, we used Raw 264.7 macrophages to examine the effects of PTE. Our results showed that LPS + ATP significantly increased pyroptosis-related protein levels. However, PTE (4 μM and 6 μM) treatment abolished the expression of these proteins (p < 0.05, parametric unpaired t test, Fig. 2E–I). Pyroptosis is also characterized by the formation of discrete pores in the plasma membrane. Thus, YO-PRO-1, a small membrane impermeable but pyroptosis pole permeable dye, was used to visually observe cell pyroptosis as reported.25,44 Triton 0.1% served as a positive control for dye uptake. Our results showed that PTE (4uM and 6uM) treatment decreased the uptake of YO-PRO-1 dye compared with the LPS + ATP group (Fig. 2J). Moreover, Calcein-AM and PI dyes were used to investigate membrane permeabilization, which could also be used to observe Raw 264.7 macrophage pyroptosis. Calcein-AM is a membrane permeable dye that emits a green, fluorescent signal when the cell membrane remains intact. However, PI dye is a membrane impermeable dye that cannot enter live cells. It can only gain access to the nucleus following membrane permeabilization to emit red fluorescent signals. As shown in Fig. 2K, PTE treatment decreased the uptake of PI dye and increased the uptake of Calcein AM dye. These results further corroborated the protective effect of PTE in macrophage pyroptosis.
To evaluate whether PTE was the most effective in suppressing macrophage pyroptosis, we tested the effects of four polyphenols in the LPS + ATP-induced pyroptosis model in Raw 264.7 cells. Western blot analysis showed that pyroptosis-related proteins were the most significantly decreased after treatment with PTE (4 μM, 24 h) compared with the other three polyphenols (p < 0.05 vs. LPS group, parametric unpaired t test, ESI Fig. 8A–E†). Furthermore, we investigated the expression of the proinflammatory cytokines TNF-α, IL-6 and IL-1β in Raw 264.7 cells. In the PTE-treated group, the levels of TNF-α, IL-6 and IL-1β were the most inhibited among the four groups (p < 0.05 vs. LPS group, parametric unpaired t test, ESI Fig. 8F–H†), suggesting the regulatory effect of PTE on the inflammatory response. Together, the findings suggest that PTE suppresses inflammation induced by macrophage pyroptosis in vitro and in vivo.
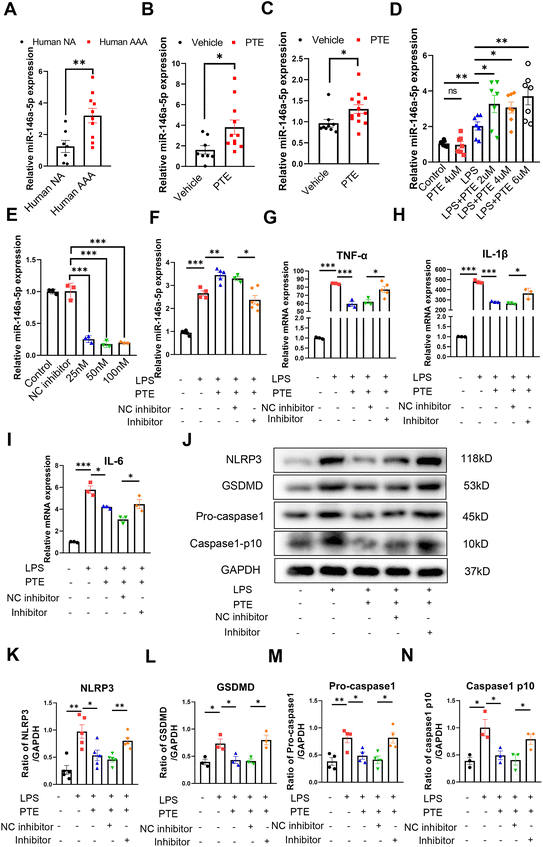
3.3 PTE suppresses macrophage pyroptosis and AAA formation by upregulating miR-146a-5p
MicroRNA-146a-5p (miR-146a-5p) is a well-studied regulator of innate immune responses that has anti-inflammatory functions in multiple cell types, including monocytic THP-1 cells, bone marrow-derived macrophages (BMDMs), BV2 microglial cells and primary astrocytes.45,46 MiR-146a-5p was reported to inhibit cell pyroptosis and was upregulated in the plasma of AAA patients.27 In the present study, we observed that miR-146a-5p expression was increased in human AAA aortas compared with normal aortas (p < 0.01, parametric unpaired t test, Fig. 3A). Furthermore, aortas and peritoneal macrophages from PTE-treated C57BL/6 mice exhibited increased levels of miR-146a-5p expression (p < 0.05, parametric unpaired t test, Fig. 3B and C). To study whether PTE regulated miR-146a-5p expression in Raw 264.7 macrophages, qPCR was performed, revealing that LPS + ATP increased miR-146a-5p levels and that PTE further enhanced miR-146a-5p expression (p < 0.05, parametric unpaired t test, Fig. 3D).Pretreatment with PTE upregulated miR-146a-5p levels in LPS + ATP-induced Raw 264.7 macrophages, and miR-146a-5p exerted anti-inflammatory effects. Therefore, we next examined whether miR-146a-5p was a target of PTE. To reduce the levels of miR-146a-5p in vitro, we transfected Raw 264.7 cells with three concentrations (25 nM, 50 nM and 100 nM) of a miR-146a-5p inhibitor. The interference effects were measured using qPCR (Fig. 3E), and 50 nM induced the most knockdown and was selected for subsequent experiments. As shown in Fig. 3F, the level of miR-146a-5p decreased in the inhibitor group compared with the NC inhibitor group (p < 0.05, parametric unpaired t test, Fig. 3F). The LPS + ATP-induced levels of proinflammatory cytokines TNF-α, IL-1β and IL-6 were also decreased by PTE pretreatment. However, inhibiting miR-146a-5p disrupted this effect, and the levels of these proinflammatory cytokines were higher than those in the NC inhibitor group (p < 0.05, parametric unpaired t test, Fig. 3G–I). Moreover, suppressing miR-146a-5p significantly increased the levels of pyroptosis-related proteins in the inhibitor group compared with the NC inhibitor group, as shown by western blotting (p < 0.05, parametric unpaired t test, Fig. 3J–N). Furthermore, the miR-146a-5p inhibitor weakened the inhibitory effect of PTE on pyroptosis, as measured by YO-PRO-1 staining and Calcein-AM/PI dye staining (ESI Fig. 9 and 10†). These results indicate that the anti-inflammatory and inhibitory effects of PTE on pyroptosis may be partly due to the upregulation of miR-146a-5p levels.
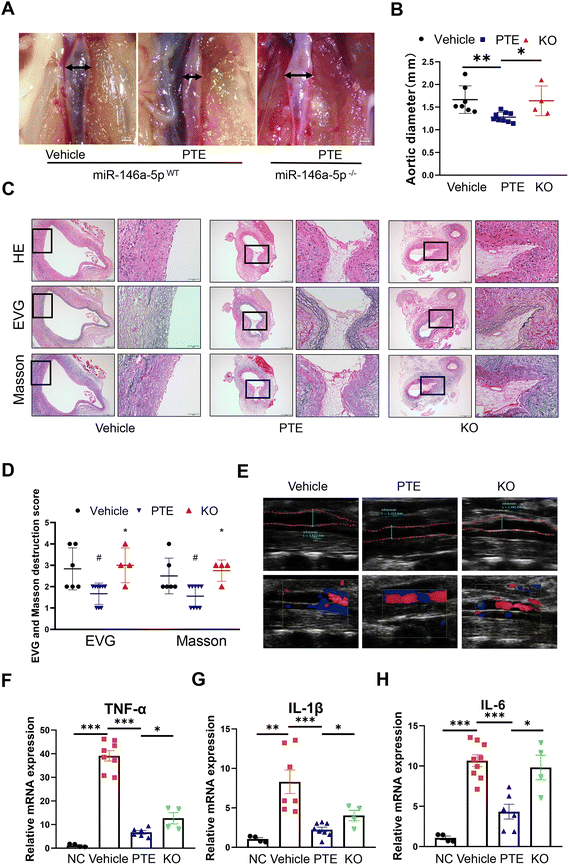
To address the role of miR-146a-5p in PPE-induced AAA, we generated miR-146a-5p−/− mice using CRISPR/Cas9 (ESI Fig. 11A and B†). Moreover, miR-146a-5p knockout in macrophage was confirmed by qPCR analysis (ESI Fig. 11C†). Next, ten- to twelve-week-old male miR-146a-5pWT mice and miR-146a-5p−/− mice were grouped by a random number table according to body weight, exposed to perivascular PPE and then administered water or PTE by gavage. As shown in the macroscopic photographs (Fig. 4A), PTE-treated miR-146a-5pWT mice had smaller aortic diameters compared with those in the vehicle group, while miR-146a-5p knockout significantly reversed this effect (p < 0.05, parametric unpaired t test, Fig. 4B). In addition, vascular ultrasound imaging showed progressively increased dilation in miR-146a-5p−/− mice (Fig. 4E). H&E, EVG and Masson staining showed that elastin and collagen were disrupted and degraded to a greater extent in miR-146a-5p−/− mice than in miR-146a-5pWT mice (p < 0.05, parametric unpaired t test, Fig. 4C and D). On day 14 after exposure to PPE, we extracted TG-induced peritoneal macrophages from all experimental mice and then treated them with LPS (100 ng mL−1) for 24 h. Moreover, qPCR analysis revealed strong upregulation of TNF-α, IL-6 and IL-1β expression in miR-146a-5p-knockdown mice compared with control mice (p < 0.05, parametric unpaired t test, Fig. 4F–H). Overall, we demonstrated that PTE may inhibit macrophage pyroptosis and AAA formation by upregulating miR-146a-5p expression.
3.4 PTE inhibits macrophage pyroptosis and the development of AAA via the miR-146a-5p/TRAF6 axis
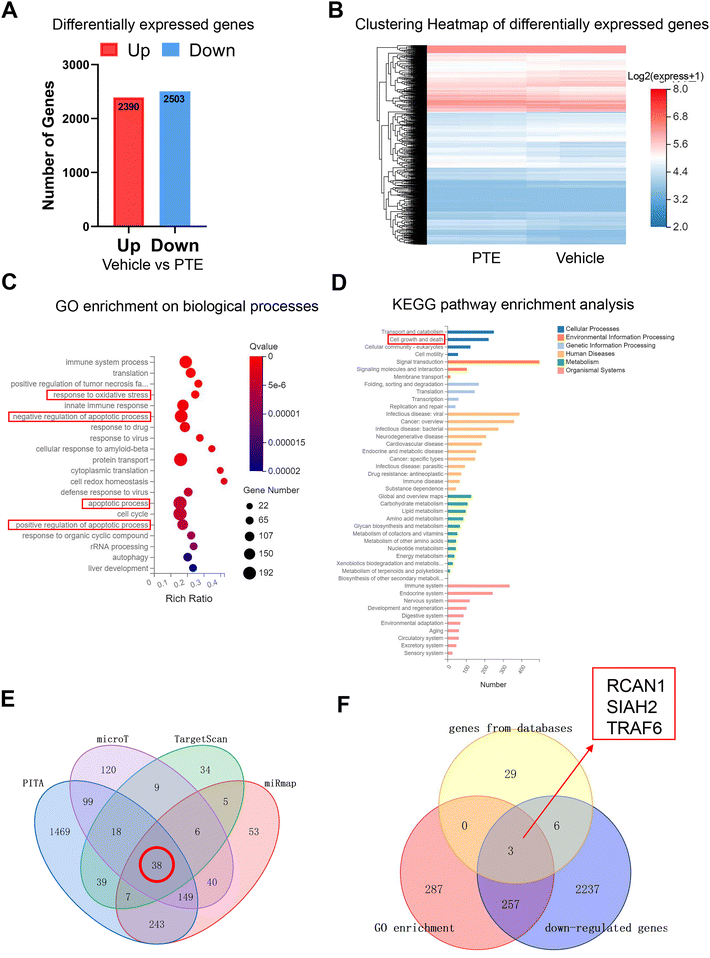
MicroRNAs are endogenous, small noncoding RNAs that can bind the 3′-untranslated regions (3′ UTRs) of target mRNAs and inhibit gene expression (30). To determine the downstream target of miR-146a-5p through which PTE exerts its protective effects, we used mRNA-seq to identify differentially expressed mRNAs in PTE-treated pyroptotic macrophages. As shown in Fig. 5A, there were 2390 genes with upregulated expression and 2503 genes with downregulated expression. The clustering heatmap shows differentially expressed genes between the vehicle and PTE groups (Fig. 5B). GO enrichment analysis of genes in biological processes and KEGG pathway enrichment analysis of genes in different pathways revealed that 547 genes were related to biological processes, such as “response to oxidative stress”, “apoptotic process” and “cell growth and death” (Fig. 5C and D). Then, we predicted the target genes of miR-146a-5p with the PITA, microT, TargetScan and miRm databases (|log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(Fold Change)| > 1 and P < 0.05), and the Venn diagram shows that 38 genes can bind to miR-146a-5p (Fig. 5E). Moreover, the Venn diagram shows that miR-146a-5p can bind to 3 genes (RCAN1, SIAH2 and TRAF6) to exert its effects among 547 genes identified by GO enrichment analysis, 38 genes in the gene databases and 2503 genes with downregulated expression (Fig. 5F).
2(Fold Change)| > 1 and P < 0.05), and the Venn diagram shows that 38 genes can bind to miR-146a-5p (Fig. 5E). Moreover, the Venn diagram shows that miR-146a-5p can bind to 3 genes (RCAN1, SIAH2 and TRAF6) to exert its effects among 547 genes identified by GO enrichment analysis, 38 genes in the gene databases and 2503 genes with downregulated expression (Fig. 5F).
To further investigate the molecules by which PTE suppresses macrophage pyroptosis, we performed validation experiments. After treatment with PTE, the levels of TRAF6, RCAN1 and SIAH2 were decreased in Raw 264.7 macrophages (p < 0.05, parametric unpaired t test, Fig. 6A and ESI Fig. 12A and B†). However, miR-146a-5p inhibitor treatment abolished the effect of PTE by increasing the level of TRAF6 (p < 0.05, parametric unpaired t test, Fig. 6B), while the mRNA levels of RCAN1 and SIAH2 were similar between the inhibitor-NC and inhibitor groups (ESI Fig. 12C and D†). These results indicate that only TRAF6 was the target gene of miR-146a-5p in PTE. Then, we measured the level of TRAF6 in human aortas. Furthermore, qPCR analysis and western blotting showed that the mRNA and protein levels of TRAF6 were significantly upregulated in human AAA samples compared with normal samples (p < 0.05, parametric unpaired t test, Fig. 6C–E). Consistent with the aforementioned results, PTE treatment significantly decreased the mRNA levels of TRAF6 in mouse aortas and peritoneal macrophages, and this effect was abolished in miR-146a-5p-knockout mice (p < 0.05, parametric unpaired t test, Fig. 6F and G). We analysed the protein levels of TRAF6 in Raw 264.7 cells (Fig. 6H and I), mouse aortas (Fig. 6J and K) and peritoneal macrophages from the PPE-induced C57BL/6 mice (Fig. 6L and M) and found similar results (p < 0.05, parametric unpaired t test).
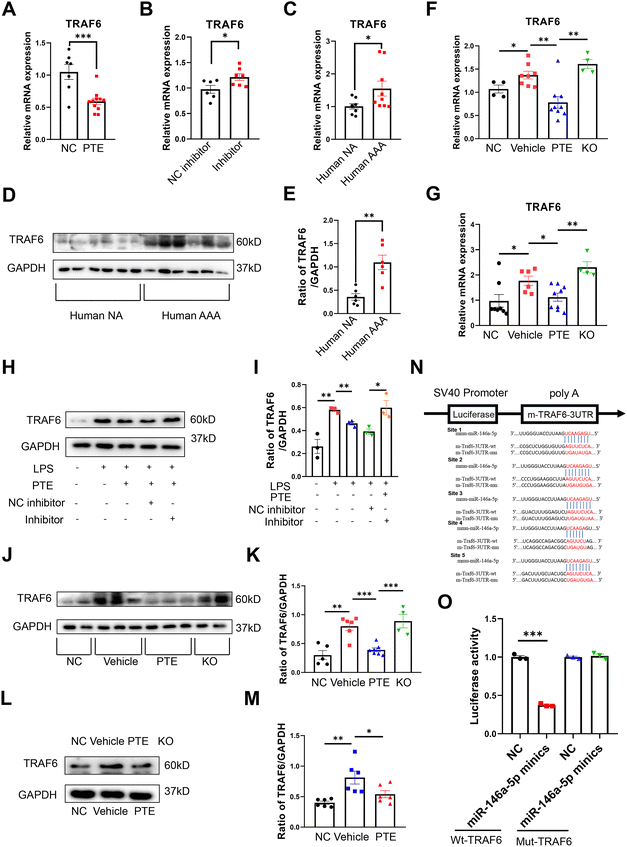 | ||
| Fig. 6 PTE suppresses TRAF6 expression by enabling miR-146a-5p. (A–C) qPCR analysis of TRAF6 expression in Raw 264.7 macrophages (A + B) and human aortas (C). (F–G) qPCR analysis of TRAF6 expression in mouse aortas (F) and peritoneal macrophages (G) from PPE-induced C57BL/6 and miR-146a-5p KO mice. (A) Raw 264.7 macrophages that were pretreated with vehicle or PTE (4 μM, 24 h) and then stimulated with LPS (1 μg mL−1, 24 h) and ATP (5 mM, 2 h), ***p < 0.001; n = 7–11 per group (parametric unpaired t test). (B) Raw 264.7 macrophages that were pretreated with the miR-146a-5p inhibitor (50 nM) or NC inhibitor (50 nM) for 48 h, treated with PTE (4 μM, 24 h) and then stimulated with LPS (1 μg mL−1, 24 h) and ATP (5 mM, 2 h), *p < 0.05; n = 6–7 per group (parametric unpaired t test). (C) Human AAA and normal aorta from organ donor (NA) samples, *p < 0.05; n = 7–8 per group (parametric unpaired t test). (D and E) Western blot analysis of TRAF6 expression in the human aorta as described in (C). (F) qPCR analysis of mouse aortas as described in Fig. 4, ***p < 0.001, **p < 0.01, *p < 0.05; n = 4–9 per group (parametric unpaired t test). (G) qPCR analysis of peritoneal macrophages in four mouse groups, as illustrated in Fig. 4, and then stimulated with LPS (1 μg mL−1, 24 h) and ATP (5 mM, 2 h), **p < 0.01 and *p < 0.05; n = 4–9 per group (parametric unpaired t test). (H–M) Western blot analysis of TRAF6 expression in Raw 264.7 cells (H + I) treated as described in (A + B), mouse aortas (J + K) and peritoneal macrophages (L + M) from the PPE-induced C57BL/6 treated as described in (F + G), ***p < 0.001, **p < 0.01, *p < 0.05; n = 4–7 per group (parametric unpaired t test). (N) Target sequences of miR-146a-5p in the TRAF6 3′-untranslated region (UTR) and mutant sites in the 3′-UTR. (O) Relative luciferase activity of the TRAF6 3′-UTR and mutant in miR-146a-5p mimic-transfected 293T cells, ***p < 0.001; n = 3 pear group (parametric unpaired t test). AAA, abdominal aortic aneurysm; ATP, adenosine triphosphate; KO, miR-146a-5p knockout mice; LPS, lipopolysaccharide; NA, normal aorta; PPE, porcine pancreatic elastase; PTE, pterostilbene. | ||
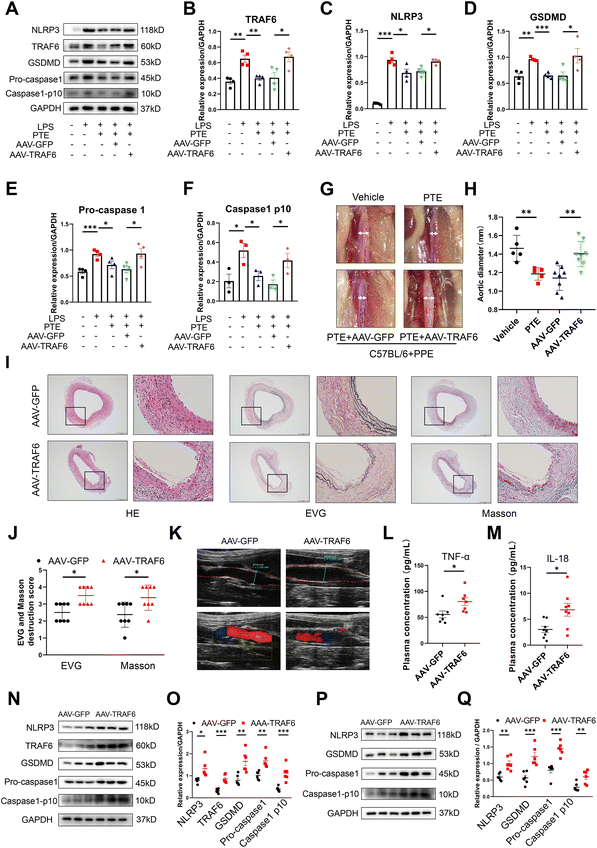
Luciferase reporter assays showed the binding site of miR-146a-5p and TRAF6, and TRAF6 was a direct target of miR-146a-5p (Fig. 6N and O). Because TRAF6 is a target molecule of PTE, we investigated the role of TRAF6 in AAA formation. First, we generated an AAV of TRAF6 that overexpressed TRAF6 and used AAV-GFP for the control group. The viruses were transfected into Raw 264.7 macrophages to identify the efficiency of transfection. Western blot analysis showed that the levels of TRAF6 and pyroptosis were increased in the AAV-TRAF6 group compared with the AAV-GFP group (p < 0.05, parametric unpaired t test, Fig. 7A–F). C57BL/6 mice were injected with these two viruses via the tail vein. After 14 days, the mice were subjected to PPE-induced AAA modelling, as described above. Our results showed that the PTE and PTE + AAV-GFP groups inhibited AAA development compared with the vehicle group. However, TRAF6 overexpression increased the maximal aortic diameter and destruction score of elastin and collagen compared with those in the AAV-GFP group (Fig. 7G–K). The ELISA results showed that plasma levels of the inflammatory cytokines IL-18 and TNF-α were significantly reduced after TRAF6 overexpression compared with those in the AAV-GFP group (p < 0.05, parametric unpaired t test, Fig. 7L and M). Western blotting further confirmed the increase in TRAF6 protein expression and pyroptosis in the aortas and peritoneal macrophages in the AAV-TRAF6 group (p < 0.05, parametric unpaired t test, Fig. 7N–Q). Additionally, qPCR showed the most significant upregulation of TRAF6 and proinflammatory cytokine mRNA levels in mouse aortas and mouse peritoneal macrophages from AAV-TRAF6 mice (p < 0.05, parametric unpaired t test, ESI Fig. 13†). These results indicate that PTE may inhibit macrophage pyroptosis and the development of AAA by activating the miR-146a-5p/TRAF6 axis.
4. Discussion
AAA formation is the result of decreased medial smooth muscle cells, extracellular matrix disruption, and the infiltration of immune cells.47,48 It has been proposed that chronic aortic inflammation induced by immune cell (mast cell, neutrophil, T cell, B cell and macrophage) infiltration destroys the aortic media and causes smooth muscle cell death and dysfunction owing to the release of proteolytic enzymes, which indicates the key role of the inflammatory response in AAA. Thus, suppressing inflammation has become an emerging therapeutic strategy for AAA.49,50 Because anti-inflammatory drugs targeting mast cells, neutrophils and cytotoxic T cells failed to limit AAA growth in several clinical trials, we focused on inhibiting macrophage inflammation in our study. Macrophage pyroptosis is related to the development of many inflammatory diseases, including atherosclerosis and AAA.51–53 The present study revealed the upregulation of pyroptosis indicators (NLRP3, GSDMD and Caspase1 p10) and inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-18) in AAA. Moreover, the upregulation of pyroptosis markers mainly occurred in macrophages but not in vascular smooth muscle cells in human and murine AAA tissue. These data further confirmed that inflammation induced by macrophage pyroptosis plays an essential role in the development of AAA. The modulation of macrophage pyroptosis may be a potential therapeutic approach for AAA.Stilbenes are a class of naturally occurring polyphenolic compounds found in blueberries and various grapes.19 Their antioxidant and anti-inflammatory properties indicate the potential value of stilbenes in the treatment of inflammatory diseases. Recently, a stilbene known as PTE has gained considerable attention owing to its prominent metabolic stability and bioavailability.54,55 Previous studies have demonstrated that PTE protects against gastric and breast cancer.56,57 Moreover, PTE attenuates atherosclerosis by reducing vascular inflammation. However, the role of PTE in AAA remains poorly understood. In this study. We investigated the effect of PTE and three other polyphenolic compounds on macrophage pyroptosis. Our data suggested that PTE had the strongest protective effects against macrophage pyroptosis among the four stilbenes. In vivo, the gavage administration of PTE significantly attenuated macrophage pyroptosis and Ang II- or elastase-induced AAA formation in mice, indicating the protective role of PTE in AAA formation. Our results revealed that PTE (10 mg kg−1 and 20 mg kg−1)-treated mice could prevent the development of experimental AAAs. Interestingly, PTE (10 mg kg−1)-treated mice were the most effective in suppressing the development of experiment AAAs, which was consistent with the dose of aspirin used in the clinic. In previous studies,58–60 a high dose of aspirin had a poorer effect than a low dose of aspirin, which may increase the risk of bleeding. In addition, when the concentration of PTE increases, some toxic side effects may occur in mice.61,62 The underlying mechanism needs to be explored in the future. Moreover, our results showed that oral PTE supplementation significantly suppressed the levels of LDL, triglycerides and cholesterol in mouse serum but had no effect on blood pressure or body weight. We also found that M1 macrophage infiltration and ROS production were inhibited by PTE. These data further support the protective role and safety of PTE in AAA. Taken together, our results indicate that PTE might be a novel and natural therapeutic treatment for AAA.
We further revealed that PTE inhibited macrophage pyroptosis and AAA by mediating miR-146a-5p expression. MicroRNAs are small noncoding RNAs that can bind the 3′ UTRs of target mRNAs and then inhibit gene expression.45 Numerous studies have revealed that microRNAs play essential roles in AAA, which indicates that microRNAs are promising therapeutic targets.24,26,63 MiR-146a-5p has been reported to be upregulated in the plasma of AAA patients and may be a pivotal regulator of AAA. However, the relationship between PTE and miR-146a-5p in the development of AAA remains to be elucidated. Our results showed that miR-146a-5p was significantly increased in macrophages undergoing pyroptosis and in AAA tissues. Moreover, PTE promoted miR-146a-5p expression in vitro and in vivo. Inhibiting miR-146a-5p in vitro and knocking out miR-146a-5p in vivo abrogated the protective effect of PTE on macrophage pyroptosis and AAA formation. In biological or pathological processes, some genes are upregulated in response to the extracellular environment to control deleterious stimulation. This type of regulation is considered a self-protective strategy. Based on previous studies26,63 and our results, miR-146a-5p, which is an inflammation-responsive factor, is significantly upregulated by inflammatory stimuli and exerts a feedback anti-inflammatory effect. However, the endogenous protective effect may not be sufficient to prevent a persistent inflammatory response. Thus, exogenous factors, such as PTE, are needed to enhance the protective effects of miR-146a-5p.
To better understand the crucial role of PTE in AAA formation, we used next-generation sequencing of mRNA to identify potential mechanisms. We found that PTE activated miR-146a-5p and attenuated the development of AAA by suppressing TRAF6 expression. TRAF6, which is a target of miR-146a-5p, is a genetically conserved adapter protein that mediates signalling from the tumour necrosis factor receptor superfamily.46 Exosome-derived miR-146a-5p inhibits IRAK1/TRAF6 signalling pathway-mediated NFκB activation and consequent M1 polarization and the production of potent proinflammatory cytokines.64 In addition, Hua et al. revealed that hc-MSC-derived exosomes attenuated inflammatory pain via miR-146a-5p/TRAF6, which increased the level of autophagy and inhibited microglial pyroptosis.25 However, the role of TRAF6 in macrophage pyroptosis and AAA formation is not well established. Consistent with previous studies, we found that TRAF6 was a direct target of miR-146a-5p in macrophages. Moreover, we were the first to identify a novel role for TRAF6 in AAA. Our data showed that TRAF6 was upregulated in human and murine AAA, and this change could be reversed by PTE treatment. Murine TRAF6-overexpression AAVs were administered to the abdominal aorta, and our results confirmed that PTE significantly inhibited the development of AAA and decreased the expression of pyroptosis-related proteins and proinflammatory cytokines in PPE-induced mice. These effects were abolished by TRAF6 overexpression. Consequently, our findings demonstrate that TRAF6 is essential for the protective effect of PTE against AAA and that the miR-146a-5p/TRAF6 axis plays a critical role in macrophage pyroptosis and AAA formation. Other genes, such as SIAH2, may be the potential downstream molecules of miR-146a-5p binding. The SIAH2 gene belongs to the group of ER-regulated genes. It contains an estrogen response element in the intron separating exons 1 and 2.65 Moreover, SIAH2 functions to control several fundamental cellular processes, including hypoxia, the unfolded protein response (UPR19), cell junction integrity, mitochondrial dynamics, intracellular signalling, cellular metabolism, and cell proliferation.66 Although these activities are associated with immune cell function, direct evidence for SIAH2 regulation of AAA formation is lacking. In the future, several experiments need to be conducted to explore the relationship between SIAH2 and AAA.
However, some limitations should be addressed in this study. First, global miR-146a-5p−/− mice and AAV2-mediated TRAF6 overexpression were used to determine the role of miR-146a-5p and TRAF6 in the protective effect of PTE against macrophage pyroptosis and AAA. In the future, macrophage-specific miR-146a-5p knockout or macrophage-specific TRAF6-knockout mice should be generated to evaluate this effect. Second, our study primarily focused on the PTE/miR-146a-5p/TRAF6 pathway in AAA. However, PTE may modulate multiple signalling pathways, and whether there are other underlying mechanisms of PTE remains to be determined.
5. Conclusions
In summary, we revealed that PTE inhibited the development of experimental AAA by inhibiting macrophage pyroptosis through the miR-146a-5p/TRAF6 pathway. These findings suggest that PTE might be a potential drug for alleviating AAA.Abbreviations
| AAA | Abdominal aortic aneurysm |
| AAV | Adeno-associated virus |
| ASC | Apoptosis-associated speck-like protein |
| ATP | Adenosine triphosphate |
| BMDMs | Bone marrow-derived macrophages |
| CHOL | Cholesterol |
| EVG | Elastic van Gieson |
| FBS | Foetal bovine serum |
| GSDMD-N | Gasdermin-D to form an N-terminal fragment |
| HDL | High density lipoprotein |
| H&E | Haematoxylin and eosin |
| LDL | Low density lipoprotein |
| LPS | Lipopolysaccharide |
| NA | Normal aorta |
| PI | Propidium Iodide |
| PPE | Porcine pancreatic elastase |
| PTE | Pterostilbene |
| PVDF | Polyvinylidene difluoride |
| RSV | Resveratrol |
| TG | Thioglycolate |
| SMCs | Smooth muscle cells |
| 3′ UTRs | 3′-Untranslated regions |
Author contributions
Yu Zhou, Shenming Wang, Chen Yao and Zuojun Hu conceived and designed the study. Huoying Cai and Yu Zhou performed the experiments. Lin Huang and Mingshan Wang collected the data. Ruiming Liu, Yao Xi and Yuansen Qin analyzed the data. Huoying Cai and Yu Zhou wrote the manuscript. Yu Zhou, Shenming Wang, Chen Yao and Zuojun Hu provided substantial revision of manuscript. Zuojun Hu and Yu Zhou obtained funding. All authors have read and approved the final manuscript.Ethical statement
All experiments were performed in compliance with relevant laws or guidelines and followed institutional guidelines. All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Review Board for Clinical Research and Animal Trials of Sun Yat-sen University (Approval Number: 2021-164). The collection of clinical samples was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Approval Number: 2020-326) and complied with the principles of the Declaration of Helsinki. Moreover, informed consent was obtained from all study participants or their legal guardians.Data sharing statement
All data generated or analyzed during this study are included in the main text or ESI.† RNA-seq data collected for the study are available on GEO dataset with the accession number GSE221735.Conflicts of interest
All authors have read the journal's policy on disclosure of potential conflicts of interest and have declared that no conflict of interest exists.Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 82000448) and College Teacher Characteristic Innovation Research Project (2021DZXX11).References
- M. L. LeFevre, Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement, Ann. Intern. Med., 2014, 161, 281–290 CrossRef PubMed.
- H. G. Alcorn, S. K. Wolfson Jr., K. Sutton-Tyrrell, L. H. Kuller and D. O'Leary, Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study, Arterioscler. Thromb. Vasc. Biol., 1996, 16, 963–970 CrossRef CAS.
- T. Huang, S. Liu, J. Huang, B. Xu, Y. Bai and W. Wang, Meta-analysis of the growth rates of abdominal aortic aneurysm in the Chinese population, BMC Cardiovasc. Disord., 2019, 19, 204 CrossRef.
- J. Yu, S. Liu, J. Huang and W. Wang, Current Theories and Clinical Trial Evidence for Limiting Human Abdominal Aortic Aneurysm Growth, Curr. Drug Targets, 2018, 19, 1302–1308 CrossRef CAS PubMed.
- U. K. Sampson, P. E. Norman, F. G. Fowkes, V. Aboyans, Y. Song, F. E. Harrell Jr., M. H. Forouzanfar, M. Naghavi, J. O. Denenberg, M. M. McDermott, M. H. Criqui, G. A. Mensah, M. Ezzati and C. Murray, Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010, Global Heart, 2014, 9, 159–170 CrossRef PubMed.
- J. Huang, G. Li, W. Wang, K. Wu and T. Le, 3D printing guiding stent graft fenestration: A novel technique for fenestration in endovascular aneurysm repair, Vascular, 2017, 25, 442–446 CrossRef.
- E. L. Chaikof, R. L. Dalman, M. K. Eskandari, B. M. Jackson, W. A. Lee, M. A. Mansour, T. M. Mastracci, M. Mell, M. H. Murad, L. L. Nguyen, G. S. Oderich, M. S. Patel, M. L. Schermerhorn and B. W. Starnes, The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm, J. Vasc. Surg., 2018, 67, 2–77 CrossRef PubMed.
- L. Sun, W. Ma, W. Gao, Y. Xing, L. Chen, Z. Xia, Z. Zhang and Z. Dai, Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome, Cell Death Dis., 2019, 10, 542 CrossRef PubMed.
- S. B. Kovacs and E. A. Miao, Gasdermins: Effectors of Pyroptosis, Trends Cell Biol., 2017, 27, 673–684 CrossRef CAS.
- A. Wree, A. Eguchi, M. D. McGeough, C. A. Pena, C. D. Johnson, A. Canbay, H. M. Hoffman and A. E. Feldstein, NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice, Hepatology, 2014, 59, 898–910 CrossRef CAS.
- K. Zhang, Z. Shi, M. Zhang, X. Dong, L. Zheng, G. Li, X. Han, Z. Yao, T. Han and W. Hong, Silencing lncRNA Lfar1 alleviates the classical activation and pyoptosis of macrophage in hepatic fibrosis, Cell Death Dis., 2020, 11, 132 CrossRef CAS PubMed.
- Y. Zhang, Z. Han, A. Jiang, D. Wu, S. Li, Z. Liu, Z. Wei, Z. Yang and C. Guo, Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-κB and Activating Nrf2/HO-1 Signaling Pathways, Front. Pharmacol., 2020, 11, 591836 CrossRef CAS PubMed.
- Y. J. Xu, L. Zheng, Y. W. Hu and Q. Wang, Pyroptosis and its relationship to atherosclerosis, Clin. Chim. Acta, 2018, 476, 28–37 CrossRef CAS.
- Z. Zhaolin, L. Guohua, W. Shiyuan and W. Zuo, Role of pyroptosis in cardiovascular disease, Cell Proliferation, 2019, 52, e12563 CrossRef PubMed.
- W. Wang, Y. R. Wang, J. Chen, Y. J. Chen, Z. X. Wang, M. Geng, D. C. Xu, Z. Y. Wang, J. H. Li, Z. D. Xu, L. L. Pan and J. Sun, Pterostilbene Attenuates Experimental Atherosclerosis through Restoring Catalase-Mediated Redox Balance in Vascular Smooth Muscle Cells, J. Agric. Food Chem., 2019, 67, 12752–12760 CrossRef CAS PubMed.
- E. S. Park, Y. Lim, J. T. Hong, H. S. Yoo, C. K. Lee, M. Y. Pyo and Y. P. Yun, Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway, Vasc. Pharmacol., 2010, 53, 61–67 CrossRef CAS PubMed.
- D. McCormack and D. McFadden, Pterostilbene and cancer: current review, J. Surg. Res., 2012, 173, e53–e61 CrossRef CAS.
- C. M. Remsberg, J. A. Yáñez, Y. Ohgami, K. R. Vega-Villa, A. M. Rimando and N. M. Davies, Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity, Phytother. Res., 2008, 22, 169–179 CrossRef CAS PubMed.
- L. Zhang, G. Zhou, W. Song, X. Tan, Y. Guo, B. Zhou, H. Jing, S. Zhao and L. Chen, Pterostilbene protects vascular endothelial cells against oxidized low-density lipoprotein-induced apoptosis in vitro and in vivo, Apoptosis, 2012, 17, 25–36 CrossRef CAS PubMed.
- H. C. Lin, M. J. Hsieh, C. H. Peng, S. F. Yang and C. N. Huang, Pterostilbene Inhibits Vascular Smooth Muscle Cells Migration and Matrix Metalloproteinase-2 through Modulation of MAPK Pathway, J. Food Sci., 2015, 80, H2331–H2335 CrossRef CAS.
- Y. Zhang and Y. Zhang, Pterostilbene, a novel natural plant conduct, inhibits high fat-induced atherosclerosis inflammation via NF-κB signaling pathway in Toll-like receptor 5 (TLR5) deficient mice, Biomed. Pharmacother., 2016, 81, 345–355 CrossRef CAS.
- K. C. Wang, Y. H. Li, G. Y. Shi, H. W. Tsai, C. Y. Luo, M. H. Cheng, C. Y. Ma, Y. Y. Hsu, T. L. Cheng, B. I. Chang, C. H. Lai and H. L. Wu, Membrane-Bound Thrombomodulin Regulates Macrophage Inflammation in Abdominal Aortic Aneurysm, Arterioscler. Thromb. Vasc. Biol., 2015, 35, 2412–2422 CrossRef CAS PubMed.
- L. Song, T. Y. Chen, X. J. Zhao, Q. Xu, R. Q. Jiao, J. M. Li and L. D. Kong, Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling, Br. J. Pharmacol., 2019, 176, 1619–1634 CrossRef CAS PubMed.
- A. Kumar, A. M. Rimando and A. S. Levenson, Resveratrol and pterostilbene as a microRNA-mediated chemopreventive and therapeutic strategy in prostate cancer, Ann. N. Y. Acad. Sci., 2017, 1403, 15–26 CrossRef CAS.
- T. Hua, M. Yang, H. Song, E. Kong, M. Deng, Y. Li, J. Li, Z. Liu, H. Fu, Y. Wang and H. Yuan, Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis, J. Nanobiotechnol., 2022, 20, 324 CrossRef CAS PubMed.
- F. Olivieri, F. Prattichizzo, A. Giuliani, G. Matacchione, M. R. Rippo, J. Sabbatinelli and M. Bonafè, miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases, Ageing Res. Rev., 2021, 70, 101374 CrossRef CAS PubMed.
- E. Plana, L. Gálvez, P. Medina, S. Navarro, V. Fornés-Ferrer, J. Panadero and M. Miralles, Identification of Novel microRNA Profiles Dysregulated in Plasma and Tissue of Abdominal Aortic Aneurysm Patients, Int. J. Mol. Sci., 2020, 21, 4600 CrossRef CAS.
- H. Kaneko, T. Anzai, M. Morisawa, T. Kohno, T. Nagai, A. Anzai, T. Takahashi, M. Shimoda, A. Sasaki, Y. Maekawa, K. Yoshimura, H. Aoki, K. Tsubota, T. Yoshikawa, Y. Okada, S. Ogawa and K. Fukuda, Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization, Atherosclerosis, 2011, 217, 350–357 CrossRef CAS.
- C. S. Moran, E. Biros, S. M. Krishna, Y. Wang, C. Tikellis, S. K. Morton, J. V. Moxon, M. E. Cooper, P. E. Norman, L. M. Burrell, M. C. Thomas and J. Golledge, Resveratrol Inhibits Growth of Experimental Abdominal Aortic Aneurysm Associated With Upregulation of Angiotensin-Converting Enzyme 2, Arterioscler., Thromb., Vasc. Biol., 2017, 37, 2195–2203 CrossRef CAS PubMed.
- Q. Shi, G. Zhao, S. Wei, C. Guo, X. Wu, R. C. Zhao and G. Di, Pterostilbene alleviates liver ischemia/reperfusion injury via PINK1-mediated mitophagy, J. Pharmacol. Sci., 2022, 148, 19–30 CrossRef CAS.
- D. Palmieri, B. Pane, C. Barisione, G. Spinella, S. Garibaldi, G. Ghigliotti, C. Brunelli, E. Fulcheri and D. Palombo, Resveratrol counteracts systemic and local inflammation involved in early abdominal aortic aneurysm development, J. Surg. Res., 2011, 171, e237–e246 CrossRef CAS.
- J. H. Krege, J. B. Hodgin, J. R. Hagaman and O. Smithies, A noninvasive computerized tail-cuff system for measuring blood pressure in mice, Hypertension, 1995, 25, 1111–1115 CrossRef CAS PubMed.
- H. Song, T. Xu, X. Feng, Y. Lai, Y. Yang, H. Zheng, X. He, G. Wei, W. Liao, Y. Liao, L. Zhong and J. Bin, Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2, EBioMedicine, 2020, 57, 102832 CrossRef.
- W. Xu, Y. Chao, M. Liang, K. Huang and C. Wang, CTRP13 Mitigates Abdominal Aortic Aneurysm Formation via NAMPT1, Mol. Ther., 2021, 29, 324–337 CrossRef CAS PubMed.
- F. J. Rios, R. M. Touyz and A. C. Montezano, Isolation and Differentiation of Murine Macrophages, Methods Mol. Biol., 2017, 1527, 297–309 CrossRef CAS PubMed.
- J. J. Hiu and M. K. K. Yap, The effects of Naja sumatrana venom cytotoxin, sumaCTX on alteration of the secretome in MCF-7 breast cancer cells following membrane permeabilization, Int. J. Biol. Macromol., 2021, 184, 776–786 CrossRef CAS PubMed.
- M. D. Lu, H. Li, J. H. Nie, S. Li, H. S. Ye, T. T. Li, M. L. Wu and J. Liu, Dual Inhibition of BRAF-MAPK and STAT3 Signaling Pathways in Resveratrol-Suppressed Anaplastic Thyroid Cancer Cells with BRAF Mutations, Int. J. Mol. Sci., 2022, 23, 14385 CrossRef CAS.
- X. Zhang, Y. Zhou, Y. Ye, R. Wu, W. Li, C. Yao and S. Wang, Human umbilical cord mesenchymal stem cell-derived exosomal microRNA-148a-3p inhibits neointimal hyperplasia by targeting Serpine1, Arch. Biochem. Biophys., 2022, 719, 109155 CrossRef CAS.
- S. Gómez-Zorita, I. Milton-Laskibar, M. T. Macarulla, L. Biasutto, A. Fernández-Quintela, J. Miranda, A. Lasa, N. Segues, L. Bujanda and M. P. Portillo, Pterostilbene modifies triglyceride metabolism in hepatic steatosis induced by high-fat high-fructose feeding: a comparison with its analog resveratrol, Food Funct., 2021, 12, 3266–3279 RSC.
- L. A. Heikal, A. H. El-Kamel, R. A. Mehanna, H. M. Khalifa and P. S. Hassaan, Improved oral nutraceutical-based intervention for the management of obesity: pterostilbene-loaded chitosan nanoparticles, Nanomedicine, 2022, 17, 1055–1075 CrossRef CAS PubMed.
- A. S. Levenson, Dietary stilbenes as modulators of specific miRNAs in prostate cancer, Front. Pharmacol., 2022, 13, 970280 CrossRef CAS.
- Y. Wang, M. Liu, X. Zhou, H. Zang, R. Zhang, H. Yang, S. Jin, X. Feng and A. Shan, Oxidative stability and gelation properties of myofibrillar protein from chicken breast after post-mortem frozen storage as influenced by phenolic compound-pterostilbene, Int. J. Biol. Macromol., 2022, 221, 1271–1281 CrossRef CAS PubMed.
- Q. Zhang, J. Liu, H. Duan, R. Li, W. Peng and C. Wu, Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress, J. Adv. Res., 2021, 34, 43–63 CrossRef CAS.
- S. E. Adamczak, J. P. de Rivero Vaccari, G. Dale, F. J. Brand 3rd, D. Nonner, M. R. Bullock, G. P. Dahl, W. D. Dietrich and R. W. Keane, Pyroptotic neuronal cell death mediated by the AIM2 inflammasome, J. Cereb. Blood Flow Metab., 2014, 34, 621–629 CrossRef CAS.
- Y. T. Ge, A. Q. Zhong, G. F. Xu and Y. Lu, Resveratrol protects BV2 mouse microglial cells against LPS-induced inflammatory injury by altering the miR-146a-5p/TRAF6/NF-κB axis, Immunopharmacol. Immunotoxicol., 2019, 41, 549–557 CrossRef CAS PubMed.
- Y. Lu, D. L. Cao, B. C. Jiang, T. Yang and Y. J. Gao, MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord, Brain, Behav., Immun., 2015, 49, 119–129 CrossRef CAS PubMed.
- F. M. Davis, D. L. Rateri and A. Daugherty, Abdominal aortic aneurysm: novel mechanisms and therapies, Curr. Opin. Cardiol., 2015, 30, 566–573 CrossRef PubMed.
- M. Vandestienne, Y. Zhang, I. Santos-Zas, R. Al-Rifai, J. Joffre, A. Giraud, L. Laurans, B. Esposito, F. Pinet, P. Bruneval, J. Raffort, F. Lareyre, J. Vilar, A. Boufenzer, L. Guyonnet, C. Guerin, E. Clauser, J. S. Silvestre, S. Lang, L. Soulat-Dufour, A. Tedgui, Z. Mallat, S. Taleb, A. Boissonnas, M. Derive, G. Chinetti and H. Ait-Oufella, TREM-1 orchestrates angiotensin II-induced monocyte trafficking and promotes experimental abdominal aortic aneurysm, J. Clin. Invest., 2021, 131, e142468 CrossRef CAS.
- J. Golledge, Abdominal aortic aneurysm: update on pathogenesis and medical treatments, Nat. Rev. Cardiol., 2019, 16, 225–242 CrossRef PubMed.
- K. Satoh, P. Nigro, T. Matoba, M. R. O'Dell, Z. Cui, X. Shi, A. Mohan, C. Yan, J. Abe, K. A. Illig and B. C. Berk, Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms, Nat. Med., 2009, 15, 649–656 CrossRef CAS PubMed.
- Y. Xin, W. Wang, E. Mao, H. Yang and S. Li, Targeting NLRP3 Inflammasome Alleviates Synovitis by Reducing Pyroptosis in Rats with Experimental Temporomandibular Joint Osteoarthritis, Mediators Inflammation, 2022, 2022, 2581151 Search PubMed.
- H. Guo, M. Xie, C. Zhou and M. Zheng, The relevance of pyroptosis in the pathogenesis of liver diseases, Life Sci., 2019, 223, 69–73 CrossRef CAS.
- X. Zhang, F. Li, W. Wang, L. Ji, B. Sun, X. Xiao, X. Wang, Y. Chen, B. Liu, W. Ye, C. Tian, H. Wang and Y. Zheng, Macrophage pyroptosis is mediated by immunoproteasome subunit β5i (LMP7) in abdominal aortic aneurysm, Biochem. Biophys. Res. Commun., 2020, 533, 1012–1020 CrossRef CAS PubMed.
- J. M. Estrela, A. Ortega, S. Mena, M. L. Rodriguez and M. Asensi, Pterostilbene: Biomedical applications, Crit. Rev. Clin. Lab. Sci., 2013, 50, 65–78 CrossRef CAS PubMed.
- P. Wang and S. Sang, Metabolism and pharmacokinetics of resveratrol and pterostilbene, BioFactors, 2018, 44, 16–25 CrossRef CAS.
- S. Harandi-Zadeh, C. Boycott, M. Beetch, T. Yang, B. J. E. Martin, K. Ren, A. Kwasniak, J. H. Dupuis, K. Lubecka, R. Y. Yada, L. J. Howe and B. Stefanska, Pterostilbene Changes Epigenetic Marks at Enhancer Regions of Oncogenes in Breast Cancer Cells, Antioxidants, 2021, 10, 1232 CrossRef CAS PubMed.
- Y. Hojo, S. Kishi, S. Mori, R. Fujiwara-Tani, T. Sasaki, K. Fujii, Y. Nishiguchi, C. Nakashima, Y. Luo, H. Shinohara and H. Kuniyasu, Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8, Int. J. Mol. Sci., 2022, 23, 4002 CrossRef CAS.
- S. C. Johnston, P. Amarenco, H. Denison, S. R. Evans, A. Himmelmann, S. James, M. Knutsson, P. Ladenvall, C. A. Molina and Y. Wang, Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA, N. Engl. J. Med., 2020, 383, 207–217 CrossRef CAS.
- P. Khatri, D. O. Kleindorfer, T. Devlin, R. N. Sawyer Jr., M. Starr, J. Mejilla, J. Broderick, A. Chatterjee, E. C. Jauch, S. R. Levine, J. G. Romano, J. L. Saver, A. Vagal, B. Purdon, J. Devenport, A. Pavlov and S. D. Yeatts, Effect of Alteplase vs Aspirin on Functional Outcome for Patients With Acute Ischemic Stroke and Minor Nondisabling Neurologic Deficits: The PRISMS Randomized Clinical Trial, J. Am. Med. Assoc., 2018, 320, 156–166 CrossRef CAS PubMed.
- X. Zhang, F. Guo, Q. Wang, W. Bai and A. Zhao, Low-dose aspirin improves blood perfusion of endometrium of unexplained recurrent biochemical pregnancy loss, Int. J. Gynaecol. Obstet., 2022, 157, 418–423 CrossRef CAS.
- Y. Chen, H. Zhang, S. Ji, P. Jia, Y. Chen, Y. Li and T. Wang, Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling, Free Radicals Biol. Med., 2021, 177, 1–14 CrossRef CAS PubMed.
- B. Shen, Y. Wang, J. Cheng, Y. Peng, Q. Zhang, Z. Li, L. Zhao, X. Deng and H. Feng, Pterostilbene alleviated NAFLD via AMPK/mTOR signaling pathways and autophagy by promoting Nrf2, Phytomedicine, 2023, 109, 154561 CrossRef CAS PubMed.
- J. R. Iacona and C. S. Lutz, miR-146a-5p: Expression, regulation, and functions in cancer, Wiley Interdiscip. Rev.: RNA, 2019, 10, e1533 Search PubMed.
- Z. Zhang, X. Zou, R. Zhang, Y. Xie, Z. Feng, F. Li, J. Han, H. Sun, Q. Ouyang, S. Hua, B. Lv, T. Hua, Z. Liu, Y. Cai, Y. Zou, Y. Tang and X. Jiang, Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke, Aging, 2021, 13, 3060–3079 CrossRef CAS PubMed.
- M. P. Jansen, K. Ruigrok-Ritstier, L. C. Dorssers, I. L. van Staveren, M. P. Look, M. E. Meijer-van Gelder, A. M. Sieuwerts, J. Helleman, S. Sleijfer, J. G. Klijn, J. A. Foekens and E. M. Berns, Downregulation of SIAH2, an ubiquitin E3 ligase, is associated with resistance to endocrine therapy in breast cancer, Breast Cancer Res. Treat., 2009, 116, 263–271 CrossRef CAS.
- M. Scortegagna, K. Hockemeyer, I. Dolgalev, J. Poźniak, F. Rambow, Y. Li, Y. Feng, R. Tinoco, D. C. Otero, T. Zhang, K. Brown, M. Bosenberg, L. M. Bradley, J. C. Marine, I. Aifantis and Z. A. Ronai, Siah2 control of T-regulatory cells limits anti-tumor immunity, Nat. Commun., 2020, 11, 99 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo01235b |
| This journal is © The Royal Society of Chemistry 2024 |