Open Access Article
Open Access ArticleAn assessment of spent coffee grounds as a replacement for peat in the production of whisky: chemical and sensory analysis of new make spirits†
Kacper P.
Krakowiak
 a,
Irene
Baxter
b,
Barry
Harrison
b,
Nicholas
Pitts
b,
Sam
Fergusson
c,
Nicholle G. A.
Bell
a,
Irene
Baxter
b,
Barry
Harrison
b,
Nicholas
Pitts
b,
Sam
Fergusson
c,
Nicholle G. A.
Bell
 c,
David
Ellis
*a and
Ruaraidh D.
McIntosh
c,
David
Ellis
*a and
Ruaraidh D.
McIntosh
 *a
*a
aInstitute of Chemical Sciences, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK. E-mail: d.ellis@hw.ac.uk; r.mcintosh@hw.ac.uk
bScotch Whisky Research Institute, Riccarton, Edinburgh, EH14 4AP, UK
cEaStCHEM School of Chemistry, University of Edinburgh, David Brewster Rd, Edinburgh, EH9 3FJ, UK
First published on 15th October 2024
Abstract
The chemical composition of whisky spirits produced using malt smoked with spent coffee grounds (SCG) or traditionally peated were established using high resolution 1H NMR spectroscopy and Fourier Transform-Ion Cyclotron Resonance-Mass Spectrometry. Extracts of malts used for the process were also analysed using Gas Chromatography-Mass Spectrometry. Analytical findings were augmented by sensory analysis to establish whether differences and similarities observed between samples translate to the human sensory experience. Our studies revealed notable matches between new make spirits produced using different sources of smoke, including the presence of several phenolic species related to smoky aroma, such as phenol, and ortho- and para-cresol. The greatest differences were observed in pyridine and furan species concentrations, which were notably higher in SCG spirits, compared to those produced traditionally. These findings were reflected by the sensory analysis, which showed no statistically significant differences in terms of smoky and medicinal scores but a higher burnt score for SCG samples. These findings suggest the potential for creating an alternative to peated whisky that retains some of the desirable sensory characteristics, yet utilises a more sustainable raw material.
Sustainability spotlightIn this work we demonstrate the potential for using a sustainable alternative to peat in the production of Scotch whisky. Peat is a finite natural resource that is capable of both absorbing and storing carbon dioxide. However, ongoing commercial extraction has led to degradation of peatlands, which has had a significant deleterious impact on its capacity to act as a carbon sink. Peat harvesting must be restricted in order for the restoration of peatlands to be successful; therefore current users, including the Scotch Whisky industry, must look towards minimizing peat use and exploring the use of more sustainable alternatives. This work contributes towards the UN's Sustainable Development Goals 12 (responsible production and consumption), 13 (climate action), and 15 (life on land) are all aided by this study. |
1 Introduction
Scotch Whisky is Scotland's largest food and drink export, generating £6.2 billion of export sales in 2022.1 The production process and ingredients used are strictly regulated in order to preserve the heritage and characteristics of the drink, with water, cereals, yeast and caramel colouring being the only permissible raw materials.2 Nevertheless, variation in flavour between spirits has been noted.3,4 For example, many whiskies produced on the island of Islay have distinctive flavours and aromas, due to smoky notes imparted during grain treatment.5,6 Variations in the flavour profile can also be influenced by factors such as the yeast used and the type of barrel used to age the whisky. One of the most distinctive factors impacting flavour is whether peat is used during grain treatment. Traditionally used as fuel for the drying process during malting, the products of the thermal decomposition of peat are transferred onto the grain (referred to as malt).7 Details of the ensuing processes have been published. 8,9 Mashing, fermentation and distillation then follow, the latter involving the wash being raised to boiling with the resulting vapours passing through a copper still, before condensing into a separate vessel.10 The resulting distillates, called low wines, are then redistilled. The resulting liquid is referred to as new make spirit (NMS) and is transferred into casks for maturation. Foreshots, along with the last runnings of the final distillation, called feints, are usually recycled back into subsequent distillations.The importance of reducing peat usage is a critical element of peatland preservation and restoration efforts.11 Previous studies have shown that a delicate carbon balance exists within peatlands.12–14 Approximately 80% of UK peatlands have been damaged by human exploitation and climate change, details of the threats to peat are published elsewhere15,16 They function as natural habitats for animals and plants unique to their environment and play a key role in several other areas, such as water filtration, flood prevention and historical archiving.17–19 While the volume of peat used by the Scotch Whisky industry is not as large as some other sectors, the industry, led by the Scotch Whisky Association has recognised the need to use peat responsibly.20 As a result, distilleries have begun to look for ways to reduce the usage of peat through improving the efficiency of kilning.21
A new approach would be to replace peat with a more sustainable alternative, while preserving the smoky flavour.22 Such a replacement would need to be composed primarily of cellulose, hemicellulose and lignins, which are major constituents of most peat.23 It has been previously found that the thermal decomposition products of this biomass are responsible, in large part, for the smoky characteristics of peated whisky.5,24–27 Of particular interest are phenolic compounds, specifically guaiacol, cresol, phenol, and their derivatives and isomers. Spent coffee grounds (SCG) are an abundant waste product known to produce smoke rich in these key congeners.22 The question remains as to whether these compounds could be transferred to the spirit, and can a methodology be developed which will allow for thorough analysis of this, and other potential peat substitutes.
Whisky is a complex mixture which is challenging to analyse through a single technique. Therefore, we have chosen to combine several analytical methods to investigate the chemical composition of whisky spirits, including nuclear magnetic resonance (NMR) spectroscopy, gas chromatography coupled with mass spectrometry (GC-MS), Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) and sensory analysis. The principal advantages of NMR are short analysis time, simple sample preparation and the ability to profile the major species present in a non-selective way. While NMR is most commonly used by chemists to determine the structures of small molecules it has also been successfully applied to analysis of complex mixtures, including beers,28,29 wines,30,31 whisky32,33 and other spirits.34,35 In our work it was used to establish chemical profiles of the distillates and identify variations. One of the main disadvantages of NMR for analysis of natural mixtures is the need to suppress strong signals of solvent-type matrices. This can be achieved by utilisation of solvent suppression pulse sequences, some of which have been prepared specifically to work with ethanol/water mixtures.36,37 A second factor for NMR is relatively low sensitivity, which is particularly detrimental given that taste and aroma active congeners can have a noticeable effect at concentrations of the order of μg L−1, which is beyond the detection capability of conventional NMR experiments.
GC-MS and FT-ICR-MS were utilised to provide greater sensitivity. Various chromatographic methods have been applied over the years to complex mixture analysis and are the most commonly used techniques for whisky analysis.38–40 Gas chromatography combined with mass spectrometry allows for separation of compounds in the mixture and their identification even at concentrations in the order of μg L−1. The main disadvantage of the technique is time-consuming sample preparation and difficulty in detecting divergent chemical species in one experiment. FT-ICR-MS is a powerful, nontargeted technique which has been used previously to unveil the full complexity of matured whisky, identifying thousands of compounds across a large number of samples.41,42 The main downside of high resolution mass spectrometry, by itself, is that, while it can detect an astonishing number of molecules and their formulas, the molecular formulae could exist as many potential structures and therefore structural information can only be inferred.
The combined range of techniques presented above allows for thorough exploration of the chemical makeup of the distillates but provides no information on how the complex chemistry is reflected in the sensory profile of the samples. This is particularly important in this case as whisky will ultimately be subject to human consumption, however, the connection between congeners and sensory perception remains poorly understood so any information inferred through chemical analysis would be speculative.43 Therefore we have chosen to use sensory analysis as a starting point for our study; using this data, we are then in a position to predict whether a spirit would have a similar sensory profile to a traditionally peated whisky based on its chemical identity. With this holistic approach, we aim to investigate whether the smoky whisky experience can be replicated or approximated without using peat.
2 Experimental
2.1 Samples
Eighteen samples were analysed, including two of each of the following: industrially peated new make spirit (IPNMS1, IPNMS2), industrially peated low wines (IPLW1, IPLW2), industrially peated feints (IPF1, IPF2), laboratory peated new make spirit (LPNMS1, LPNMS2), laboratory peated low wines (LPLW1, LPLW2), laboratory peated feints (LPF1, LPF2), SCG smoked new make spirit (CSNMS1, CSNMS2), SCG smoked low wines (CSLW1, CSLW2), SCG smoked feints (CSF1, CSF2). Details on how the new make spirits and other distillates were produced can be found in the ESI.†2.2 Sensory analysis
All sensory evaluations were carried out by the expert sensory panel at The Scotch Whisky Research Institute (SWRI). For this study, sixteen panellists (SWRI employees, over 18 years of age, mixed gender) were involved, who all have training and expertise in the evaluation of Scotch whisky. Duplicates of each type of NMS and feints samples were nosed by the SWRI sensory scientist to ensure similarity before compositing to provide adequate volume for analysis. Low wines were not assessed because the available volume was insufficient. Samples were diluted to 20% ABV using distilled water on the day of testing.Quantitative Descriptive Profiling (QDP) conducted in accordance with ISO 13299:2016 was used to assess the peaty characteristics (burnt, smoky, medicinal and overall peat intensity) of the samples. The intensity of each of these attributes was scored using a 0–3 continuous line scale marked at 0.5 intervals.
Mean scores for each attribute were calculated, and analysis of variance (ANOVA) used to determine statistically significant (p < 0.05) differences between the samples followed by Tukey's post hoc test to determine sample groupings for any significant result. Unistat® 10.0 for Excel (Unistat Ltd, London, UK) was used for statistical analysis of the sensory data. Additional detail on sensory analysis can be found in the ESI.†
2.3 NMR spectroscopy
NMR samples were prepared by adding acetic acid/sodium acetate buffer solution containing sodium trimethylsilylpropanesulfonate (DSS) (0.1 mL) to the sample (0.5 mL) to give a total volume of approximately 0.6 mL. The buffer was prepared following methodology of Kew et al. by mixing sodium acetate-d3 (0.0277 g, 0.204 mmol), acetic acid-d4 (0.0820 g, 1.28 mmol), DSS (0.0131 g, 0.0600 mmol) and filling up to mark with D2O in a 10 mL volumetric flask.37 The final concentration of the DSS standard was 1.00 mM in a standard 5 mm NMR tube and the pH of the solution was 3.95. Some of the samples were diluted with deionised water prior to buffer addition in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample
1 sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water proportions in order to standardise the ethanol concentration at 40% which was necessary for the correct functioning of the employed solvent suppression sequence. Selected congeners were quantified through comparison of integration values of NMR signals, compared to the signal of the DSS standard of known concentration. ABV (alcohol by volume, measured as mL of ethanol in 100 mL of beverage) values of low wines and feints were determined using NMR methodology previously used to measure ABVs of other alcoholic beverages.44
water proportions in order to standardise the ethanol concentration at 40% which was necessary for the correct functioning of the employed solvent suppression sequence. Selected congeners were quantified through comparison of integration values of NMR signals, compared to the signal of the DSS standard of known concentration. ABV (alcohol by volume, measured as mL of ethanol in 100 mL of beverage) values of low wines and feints were determined using NMR methodology previously used to measure ABVs of other alcoholic beverages.44
All NMR experiments were performed on a 600 MHz Bruker AVIII spectrometer equipped with a 5 mm TCI cryoprobe with z gradients at 300 K at the School of Chemistry, University of Edinburgh. NMR data obtained was processed using TopSpin and Microsoft Excel software. Spectra were acquired using a multiple solvent suppression sequence optimised for whisky analysis used previously and described in detail by Kew et al.37 The assignment of signals was done by comparing with online databases, literature and spectra of pure compounds.32,45–49
2.4 Peated malt headspace SPME-GC-MS analysis
Malt sample (5.00 g ± 0.05 g) was weighed directly into a 100 mL reagent bottle followed by addition of 40% EtOH/UHQ water extraction solution (25 mL). The internal standard (deuterated phenol) was added (concentration 4 mg kg−1). Samples were incubated in a shaking incubator at 30 °C overnight whilst being constantly agitated at 100 rpm. A sample of the extraction solution (1 mL) was then diluted to 20% EtOH with 1 mL of UHQ water solution in a 10 mL headspace vial.The SPME ARROW fibre used was 1.1 mm ∅ 120 μm phase thickness, DVB/Carbon WR/PDMS. Sample pre-incubation time was 20 min at 50 °C and extraction time was 15 min. Analyses were performed using a 60 m × 0.32 mm DB-WaxUI capillary column with a film thickness of 0.5 μm. The carrier gas was He, at a flow-rate of 1.4 mL min−1. The initial oven temperature was 35 °C, held for 2 min, increasing to 250 °C at 6 °C min−1 with a final hold time of 10 min. The SPME fibre was thermally desorbed in the multimode injector held at a temperature of 270 °C. The split valve was closed for 4 min. The transfer line temperature was maintained at 250 °C. The mass spectrometer was operated in the electron ionisation (EI) mode and ions from 35 to 350 amu were scanned at a rate of 4 scans/s.
2.5 FT-ICR-MS
MS samples were prepared by adding 300 μL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of LC-MS grade water/methanol (Fisher) to 30 μL of sample. Triplicate samples were prepared for each NMS. Each sample was prepared immediately prior to the introduction into the electrospray ionisation source (ESI). Mass spectra were acquired on a Bruker SolariX 12 Tesla FT-ICR mass spectrometer with an ESI source in negative mode. This has been widely reported as the preferred ionisation method for whisky samples.50 The flow rate was set to 120 μL h−1; nebuliser gas flow was set to 1.8 bar; drying gas flow was 6 L min−1 at 180 °C. Ions were accumulated over 0.3 s with a time of flight between the quadrupole and the ICR cell set to 0.6 ms. 4 MW FIDs were acquired for 200 total scans sampling between 98.29 m/z and 1000 m/z.
1 mixture of LC-MS grade water/methanol (Fisher) to 30 μL of sample. Triplicate samples were prepared for each NMS. Each sample was prepared immediately prior to the introduction into the electrospray ionisation source (ESI). Mass spectra were acquired on a Bruker SolariX 12 Tesla FT-ICR mass spectrometer with an ESI source in negative mode. This has been widely reported as the preferred ionisation method for whisky samples.50 The flow rate was set to 120 μL h−1; nebuliser gas flow was set to 1.8 bar; drying gas flow was 6 L min−1 at 180 °C. Ions were accumulated over 0.3 s with a time of flight between the quadrupole and the ICR cell set to 0.6 ms. 4 MW FIDs were acquired for 200 total scans sampling between 98.29 m/z and 1000 m/z.
Spectra were acquired in a randomised order and the ion source was flushed with water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol between samples. Blank solvent spectra were acquired using the same water/methanol mixture at the start and end of the session as well as after every third sample. Details on spectral processing can be found in the ESI.†
methanol between samples. Blank solvent spectra were acquired using the same water/methanol mixture at the start and end of the session as well as after every third sample. Details on spectral processing can be found in the ESI.†
3 Results and discussion
3.1 Sensory analysis
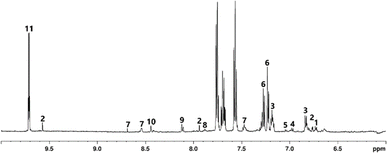
Average panel scores for each sample and the results of ANOVA are shown in Fig. 1 for the NMS and in Fig. S4† for the feints. Considering the scores, there were no statistically significant differences between any of the NMS samples with regard to three of the four peat-related characteristics (Fig. 1) using the standardised descriptors from the Scotch Whisky Flavour Wheel. There was a statistically significant difference in the ‘burnt’ aroma of the NMS samples and a similar trend was noted in the feints. Seven of the sixteen panellists commented that the coffee smoked sample had a very different aroma compared to the industrial peated and lab peated samples: chlorine-like (panellist 5); burnt sugar (panellist 6); burnt plastic/chemical (panellist 7); odd, almost minty (panellist 8); damp lawn clippings that have not composted (panellist 9); very perfumed (panellist 11); very different, gassy, scored for medicinal as the closest descriptor but was very alien (panellist 13). In contrast there was only one additional comment for the other samples, with the lab peated NMS described by one panellist as being like old lawn clippings, seaweed-like. The comments show that the current peaty vocabulary on the middle ring of the Scotch Whisky Flavour Wheel (i.e. burnt, smoky, medicinal) was not adequate to describe the sensory perception of the aroma from the smoked coffee grounds sample. This suggests that, moving forwards, additional descriptors may be required to describe newly created spirits and the Flavour Wheel may need to be modified accordingly. It should be noted however, that these findings reflect the character of the unmatured spirit, and that aging may significantly modify the sensory response.There were no statistically significant differences between the feints samples with regard to peat-related aromas (Fig. S4†). Generally, the coffee smoked feints sample was more burnt in aroma. The overall peat intensity was higher in the feints samples than in the corresponding NMS samples. Phenols that contribute to peaty flavour have low volatility and therefore distil at the end of the spirit run, so the higher scores for peaty attributes in the feints is to be expected. Comparing the sensory results for the two fractions, the burnt aroma is higher in the SCG samples than the lab peated or industrially peated samples. There were no panellist comments on any of the feints samples, suggesting that the compounds responsible for the burnt aromas are collected in the NMS rather than the feints and that the sensory vocabulary was suitable for describing these samples.
3.2 NMR analysis of smoked new make spirits
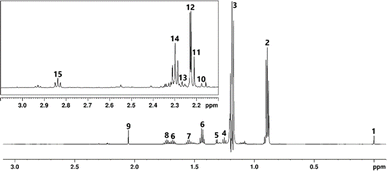
In order to ease analysis, 1H NMR spectra were divided into three regions, shown as Fig. 2–4 in an example spectrum of a NMS produced using traditionally peated malt. The low frequency region (0–3 ppm, see Fig. 2) in this case contains the most intense signals, many of which correspond to alcohols. The singlet at 0 ppm in this and every other spectrum arises from the DSS standard, which was used as both a reference for quantitation and to calibrate the spectra. A group of overlapping triplets and doublets arising from higher alcohols (often referred to as fusel alcohols), the byproducts of alcoholic fermentation, are observed at 2.2–2.9 ppm. While it is difficult to separate and assign these signals accurately it may be deduced, from the presence of other multiplets in the spectrum, that isobutanol, isoamyl alcohol, among others, present.The distorted residual signal at 1.18 ppm (3) is the suppressed CH3 group of ethanol. Of more interest is the triplet at 1.25 ppm corresponding to a CH3 group of ethyl acetate, The doublet at 1.31 ppm relates to the CH3 group of 1,1-diethoxyethane, a known flavour compound in malt whisky.51 Multiplets between 1.36 ppm and 1.78 ppm arise from fusel alcohol CH2 units. For this low frequency part of the spectra, the profiles observed for the NMS produced using malt smoked with spent coffee grounds showed minimal differences compared to those peated industrially or in the laboratory (see Fig. S5–S7†). Moving to slightly higher frequency, the singlet at 2.05 ppm is assigned to either/both acetic acid or ethyl acetate CH3 groups the intensities of the signal differing significantly between the samples. It should be noted that some proton–deuterium exchange may occur involving components of the buffer so caution must be applied in interpretation. Several signals in this region originate from alkyl side groups of aromatics. Examples of these are singlets at 2.18 ppm and 2.27 ppm, which correspond to p-cresol and o-cresol, respectively. The observed concentrations of these in the industrially peated NMS are on average 4.6 mg L−1, which aligns well with concentrations previously found in whisky. Such compounds are of particular interest, being linked to smoky characteristics in peated whisky.5,52 The apparent concentration of both is higher in the SCG sample, at variance with the results obtained using py-GC-MS previously to compare peat and spent coffee grounds. 20 A possible explanation is that the pyrolysis process does not perfectly reflect combustion. Alternatively, cresols found in SCG smoke may be transferred more easily into the malt and consequently the spirit, possibly through distillation. The central part of the spectrum (see Fig. 3) is dominated by a group of signals, a doublet at 3.33 ppm, singlet at 3.35 ppm and triplets at 3.52 ppm and 3.59 ppm from fusel alcohols. As previously, the concentrations of alcohols are nearly identical in all samples. The cluster of signals at 3.7–3.8 ppm contain overlapping multiplets, making assignment particularly difficult. Based on chemical shifts we assign these signals to CHx groups bound directly to strongly electronegative atoms (most likely oxygen), in compounds such as guaiacol, syringol or phenylethanol and their isomers or derivatives. Based on comparison with the spectrum of guaiacol, the singlet at 3.81 ppm is assigned to the methoxy group of guaiacol, with measured concentration of 7.86 and 8.04 mg L−1 in the two industrially peated samples. For comparison, samples peated in the laboratory showed concentrations of 19.63 and 9.36 mg L−1, while the samples smoked using spent coffee grounds showed 12.38 and 11.11 mg L−1. This aligns with the previous observations of phenolics originating from smoke being higher in concentration in the SCG new make spirits, compared to those peated traditionally. Notably, the variation in concentrations of the two samples prepared using peat in the laboratory may suggest that the peating method used was not as consistent as the industrial method. The quartet at 4.12 ppm originates from ethyl acetate and appears to be overlapping with another, more intense, quartet from 2-propanol. The second split signal arises from lactic acid, which has been previously found in some whiskies, arising through fermentation.53
Superimposed on the residual water signal are quartets originating from two hemiacetals of acetaldehyde (ethyl hemiacetal at 4.86 ppm and hydrate hemiacetal at 5.20 ppm). From 6 ppm is the region of the spectrum where species containing double bonds, including aromatics, are observed (see Fig. 4). This is also where the majority of differences between the spectra can be found (exemplified in Fig. 5). One significant variation is the presence of two doublets in the SCG smoked and lab peated NMS spectra, arising from the aromatic protons of 2-methylfuran. This compound was identified in coffee and has been described as one of the congeners responsible for its unique aroma and taste, along with other furan derivatives. The difference in concentration of the species measured from the spectra is large with an average of 18.5 mg L−1 in the SCG samples, compared to 2.15 mg L−1 in the samples peated in the laboratory. This relatively high concentration, combined with presence of other furan derivatives (such as 5-hydroxymethylfurfural) in the coffee grounds' NMS may contribute to the increased ‘burnt’ characteristic identified in the sensory tests, as furan species have been correlated with this type of aroma when present at higher concentrations. Unsurprisingly, the concentrations of 2-phenylethanol (multiplets at 7.22 ppm and 7.27 ppm) in all of the samples are similar, as it originates from fermentation and not the smoking process. Less expected is the similarity in concentrations of phenol (6.83 ppm, 7.18 ppm) and its derivatives, o-cresol (doublet at 7.04 ppm) and p-cresol (doublet at 6.96 ppm). These compounds are important markers responsible in large part for the ‘medicinal’ or ‘phenolic’ characteristics in whisky spirit. This similarity in concentration correlates well with the analogous ‘peaty’ scores given by the sensory panel for the different samples. It should be noted that the concentrations calculated from the aromatic signals are notably lower than those from methyl group intensities. These discrepancies may be caused by proximity of the methyl resonances to residual ethanol signals and other signal overlap. Another major difference between the spirit samples derived from SCG and peat are the presence in the former of relatively high concentrations of nitrogen containing heteroaromatic compounds, which had been previously observed in SCG smoke. Signals of pyridine present at 7.44 ppm, 7.88 ppm, 8.54 ppm and 2-methylpyridine at 7.32 ppm and 8.41 ppm range from being five to ten times more intense in the SCG smoked sample. While the difference in concentration is significant, the sensory panel has not detected consequent strong off notes in the spirit aroma, which could potentially be explained by previously reported protonation of pyridines in acidic media and their consequent absence from the headspace.54 This effect is magnified as the pH reduces over the maturation period, so the mature spirit may not be as strongly affected by the presence of undesirable heterocyclic nitrogen species as initially expected. The NMR analysis of NMS produced using spent coffee grounds as a source of smoke for malt drying and its comparison to traditionally peated spirit suggests that the composition of the spirits is largely the same with the major difference being the presence of furan species typically associated with coffee notes, and pyridines. These variations in chemistry create different sensory responses reported by the sensory panel.
3.3 NMR analysis of feints and low wines
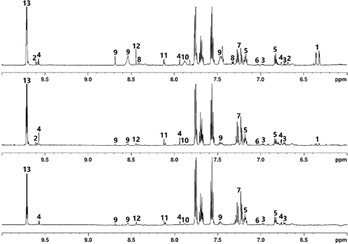
Various distillation fractions were analysed to trace the changes in chemical makeup of the spirit. The following discussion is based primarily on comparison between feints, low wines and NMS peated industrially, unless specified otherwise, as most of the observed changes were applicable to all samples and the same general trends were observed in most cases. The spectra appear broadly similar, however the position of signals in low wines and feints samples are shifted compared to their equivalents in the NMS, this being particularly noticeable in the aromatic region of the spectrum (see Fig. 6). This is believed to stem from the wide variation in ethanol content of the samples – NMS has ABV of 60–70%, while low wines exhibit 18.9–26.8% ABV and the feints showed values in a broad range of 31.2–54.6% ABV.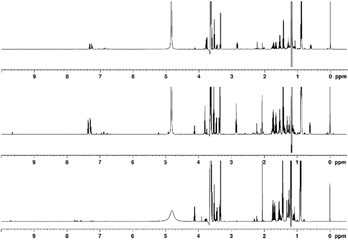 | ||
| Fig. 6 1H NMR spectra of industrially peated (from top to bottom): feints, low wines, new make spirit, recorded using a 600 MHz instrument. | ||
The cluster of CH3 signals centred around 0.88 ppm in the spectra indicates that the low wines sample contains a greater variety of fusel alcohols, compared to the NMS, though in both low wines and feints, these are less abundant than in the new-make. E.g., 3-methylbutanol is present at 2.6 g L−1 in new-make but in low wines, is measured to be 0.36 g L−1 and in feints at 0.44 g L−1, representing a nearly tenfold decrease in relative concentration. Similar comparisons may be made for 2-methylbutanol, n-propanol and isobutanol. Methanol also follows this pattern, though to a lesser degree, showing an approximately threefold concentration decrease in low wines and feints, compared to NMS. It appears that the fraction containing most ethanol (the NMS) is also rich in other alcohols, while the low wines and feints exhibit significantly lower concentrations of these. The signals for fusel alcohols, present above 3 ppm, follow the same trends observed and discussed above. Contrary trends can be observed for phenylethanol, despite its similar origin. The phenylethanol concentration in NMS varies between 24.4 mg L−1 and 35.6 mg L−1, between 124 mg L−1 and 182 mg L−1 in low wines, and 125–145 mg L−1 in feints. This is believed to be a consequence of the large discrepancy between the boiling point of ethanol (78.4 °C) and phenylethanol (204 °C).
The signals for the isolated CH3 groups of acetic acid and ethyl acetate are notably different between samples, with two distinct signals observed at 2.06 and 2.07 ppm in low wines and only one signal at 2.07 ppm in feints. As discussed previously, only one signal is observed in the NMS, at 2.05 ppm. This suggests that while both acetic acid and its ethanol ester are present initially following fermentation, the distillation process significantly reduces the concentration of ethyl acetate, which is recycled with the foreshots, while acetic acid accumulates in the feints. It is worth noting that the SCG samples show the same pattern of signals, though at lower intensity, which, as before, suggests that source of smoke has an impact on concentration of acetic acid and its esters. The doublet from acetaldehyde shows a large decrease in intensity between the NMS and feints (calculated concentrations 43.0 mg L−1 to 2.14 mg L−1 respectively), as expected given the aldehyde's very low boiling point (20.2 °C). In the feints, a group of signals at a frequency higher than that of the suppressed ethanol CH2 quartet contain a triplet of increased intensity (compared to the NMS), correlating to the phenylethanol CH2 group adjacent to OH. Other signals in the region, however, are notably lower in intensity in low wines, while remaining comparatively strong in the feints. This suggests the presence of compounds with high boiling points, which would be more abundant primarily in the later fractions of the distillation, such as guaiacol or methylpyridines. The spectrum of the NMS shows two overlapping quartets at 4.11 ppm and 4.12 ppm (unassigned), while the other samples only contain one quartet in this region (though the other may be too low in intensity to be observable).
The aromatic region displays the largest differences in terms of chemical shifts observed between the samples (up to 0.08 ppm difference observed for the 2-phenylethanol signals between the NMS and low wines). In terms of concentrations, evidence of cresols is comparable between the NMS and feints, with the latter's signals showing higher signal intensity. Low wines, on the other hand, show lower cresol concentration. Phenol concentration remains similar between samples, though a slight concentration increase in the feints can be noted. These observations suggest that significant volumes of phenolic species are concentrated in the last runnings of the distillation, which are subsequently recycled. Modification of the distillation cut may therefore result in higher concentration of compounds responsible for ‘smoky’ characteristics, though other undesirable compounds may also transfer into the spirit as a result. Notably pyridine signals appear only in NMS, and those from 2-methylfuran appear only in the feints and low wines. Both compounds show notably higher concentrations in the SCG spirit with 2-methylfuran showing an increase in concentration for low wines and feints compared to NMS, while the signals of pyridine disappears. Even though spirits smoked with alternative materials to peat may contain higher concentrations of undesirable compounds, adjustments in the distillation process could lower their concentration sufficiently to reduce their impact on the flavour and aroma. In terms of phenolics, both the laboratory and SCG samples follow the same trends discussed above.
3.4 GC-MS
Twenty seven potential smoke aroma congeners were analysed in the malt produced using either peat or spent coffee grounds (Fig. 7).Comparing the levels of these congeners in spent coffee ground smoked malt against peated malt, nitrogen containing heterocycles were relatively abundant in the coffee ground smoked malt. This suggests an increased level of nitrogen containing precursors in the spent coffee grounds. These nitrogen containing heterocycles, notably pyrazines, are associated with earthy aroma in coffee.55
Comparing different species of phenolic compounds, methoxyphenols were found to be present at relatively low levels compared to other phenols in spent coffee grounds malt. A lower level of methoxyphenols could suggest a different lignin or other polyphenolic structure in the spent coffee grounds compared to peat. It may also suggest an influence of the coffee grounds production process where roasting may preferentially decrease methoxyphenols or their precursors.
3.5 FT-ICR-MS
Triplicate analysis of the three NMS samples resulted in complex mass spectra containing thousands of peaks between 150 m/z and 800 m/z. As shown previously, these peaks arise from singly charged monoisotopic species, including isotopologues such as 13C1, 13C2 etc.41Taking only species present in at least two replicates, the number of assigned species was 3795, 2700 and 3225 for SCG, IPM and LPM, respectively. These assignments account for ∼70% of the peaks picked above S/N = 4. The higher number of compounds in SCG NMS is surprising, however, this pattern was also observed in the py-GC-MS studies of peat and SCG previously reported.22 The lower number of assigned species found in industrial NMS vs. lab NMS likely reflects the differing production processes, for example the peating level or distillation cuts. From the assigned species, the vast majority are CHO compounds (75–76%) followed by CHOS (13–14%) with mean mass of 399 (SCG), 369 (IPM) and 382 (LPM) Da. Other metrics such as aromaticity index56 or nominal oxidation state57 commonly examined in high resolution MS data showed insignificant differences between distillates (see ESI†).
The chemical composition and diversity of assigned species can be visualised using van Krevelen diagrams, which plot the assigned molecular formulae as points based on H/C and O/C ratios. The van Krevelen of the SCG NMS is shown in Fig. 8, colour coded by Kernel density. Similar plots for LPM and IPM can be found in the ESI (Fig. S2).†
Superimposed on the van Krevelen plot are boxes labelled by compound class. It should be noted that molecules within these regions should only be described as lipid-like, carbohydrate-like etc, as further MS/MS data would be required to provide evidence of their true classification. Very low numbers of compounds in the carbohydrate-like region are expected due to the poor ionisation efficiency of carbohydrates compared to other compound types.
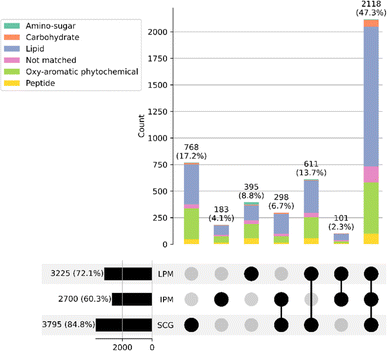
Inspecting the van Krevelen obtained from SCG NMS, the plot is typical of NMS41,50 with the vast majority of compounds located in the lipid-like region which correspond to the products of fermentation that survive distillation and lower numbers in the tannin or lignin-like areas reflecting, in part, the smoked malt. Comparing SCG with peated NMS visually in this way shows very little difference (Fig. S2†). The differences between the samples are much clearer from UpSet analysis (Fig. 9).
The UpSet plots shows that 2118 of the molecular formulae assigned are found in all three samples. If compared with non-peated NMS (Fig. S1†) this number drops to 989 species common to all three samples and 1129 found in all distillates. This demonstrates the common influence of the fermentation and distillation stages on the composition of NMS, dominated by lipid-like (fatty acid) molecules.
From UpSet analysis there are 768, 161 and 359 compounds unique to SCG NMS, industrially peated NMS and laboratory peated NMS, respectively. The compound class breakdown of these compounds is very similar showing a dominance of lipid-like and oxy-aromatic phytochemicals. The elemental composition varied slightly with a ∼10% higher abundance of CHO compounds found in SCG NMS compared to the peated NMS samples, while the % of CHON compounds unique to laboratory peated NMS was almost double that found in SCG NMS (Table S1†). These observations indicate that within the FT-ICR-MS observation window the compounds unique to SCG NMS are not nitrogen or sulphur compounds and perhaps the differences are due to the fermentation of the SCG smoked malt. It should be noted however, that FT-ICR-MS is not quantitative and so would not reflect variations in concentrations of these compound groups in the spirits.
4 Conclusions
The chemical composition and sensory profiles of whisky spirits produced from peat and spent coffee grounds as a source of smoke were investigated using a variety of methods. NMR analysis of the spirits revealed that altering the source of smoke has minimal impact on many of the compounds that are responsible for flavour and aroma making up the mixture, with the notable exception of nitrogen-aromatics and furans. In the case of former, differences in concentrations were noted, showing slightly higher levels of cresols and phenol in SCG new make spirit, compared to samples peated traditionally. Additional furan species, most notably 2-methylfuran as well as noticeably higher levels of pyridine species were detected in the coffee smoked samples.These observations correlated well with results obtained from sensory analysis, where the scores revealed no statistically significant differences between NMS or feints samples produced using different smoke sources, for the smoky and medicinal aromas. The increase in burnt aroma was statistically significant and the narrative data suggested that current whisky flavour wheel descriptors did not fully encompass the atypical ‘burnt’ characteristic which showed largest deviation between samples, likely due to presence of the aforementioned furan species, which have been correlated with such aroma at high concentrations.
The ‘burnt’ aroma, judged by panellists to be too intense could be addressed by modifying the smoking conditions (i.e. temperature, air flow) or by mixing SCG with peat to produce NMS with the desired peaty flavour whilst using less peat. Another solution would be to alter the distillation process, as analysis of low wines and feints revealed differences in concentrations of some congeners between different distillates. While the levels of most phenolic species showed only slightly elevated levels in feints, the concentration of pyridines decreased in NMS compared to other distillates and 2-methylfuran increased. However, any alterations in the distillation process must be made carefully, as our results have also revealed large changes in concentration of other compounds depending on which fraction was analysed. For fusel alcohols, a more than tenfold increase was observed from low wines to NMS, the figure is threefold for methanol, while 2-phenylethanol showed a significant decrease in concentration.
Use of GC-MS and FT-ICR-MS techniques allowed us to overcome the limited sensitivity of NMR. The first of the two methods allowed for measurement of aromatic species in malt extracts, revealing lower levels of methoxyphenols in SCG smoked malt, compared to malt peated traditionally, elevated levels of pyridine species and similarity in terms of cresols and other phenolic species, confirming and expanding on results obtained using NMR. FT-ICR-MS revealed a staggering number of compounds in the analysed spirits, with nearly 4000 chemicals in SCG NMS and around 3000 in laboratory and industrially peated samples. More than 2000 of these compounds were common to all three samples, suggesting a degree of chemical similarity. The species unique to the SCG spirit were not nitrogen or sulphur compounds, which may suggest that the observed differences in composition were due to fermentation of the malt, rather than differences in smoke composition.
Overall, spent coffee grounds show promise as an alternative to peat for smoky whisky production. Similar levels of phenolic species and peaty characteristics between analysed NMS are reassuring, while potential difficulties in using a replacement material could be remedied through alterations of the smoking process and distillation. It is recognised that any process innovation applied specifically to Scotch Whisky, such as use of SCG, would need to comply with the Scotch Whisky Definition. In this context this research is equally as important as a validation of an analytical methodology, which can be extended to production and analysis of whisky spirits using other alternative materials in the future.
Data availability
A data availability statement (DAS) is required to be submitted alongside all articles. Please read our full guidance on data availability statements for more details and examples of suitable statements you can use. The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Lorna Murray and Juraj Bella for maintaining the NMR facility at The University of Edinburgh.Notes and references
- Scotch Whisky Association, Scotch Whisky Exports Over £6bn for First Time, https://www.scotch-whisky.org.uk/newsroom/scotch-whisky-exports-2022/, accessed 08.03.2023.
- UK Parliament, The Scotch Whisky Regulations 2009, 2009, 2890 Search PubMed.
- K. Y. M. Lee, PhD Thesis, University of Strathclyde, 2000.
- C. Roullier-Gall, J. Signoret, C. Coelho, D. Hemmler, M. Kajdan, M. Lucio, B. Schäfer, R. D. Gougeon and P. Schmitt-Kopplin, Food Chem., 2020, 323, 126748 CrossRef CAS.
- B. Guthrie, J. Beauchamp, A. Buettner, S. Toth and M. Qian, Sex, Smoke, and Spirits the Role of Chemistry, Washington, DC: American Chemical Society, Washington, DC, 2019 Search PubMed.
- Islay.com, Islay Whisky Making, Distilling on Islay, Peat, https://islay.com/islay-whisky-making/peat/, accessed 04.02.2022.
- T. A. Bringhurst and J. Brosnan, in Whisky, Elsevier Ltd, 2nd edn, 2015, ch. 6, pp. 49–122 Search PubMed.
- I. Russell and G. Stewart, in Whisky, Elsevier Ltd, 2nd edn, 2015, ch. 7, pp. 123–146, DOI:10.1016/B978-0-12-401735-1.00007-6.
- H. Suomalainen, J. Inst. Brew., 1971, 77, 164–177 CrossRef CAS.
- D. A. Nicol, in Whisky, Elsevier Ltd, 2nd edn, 2015, ch. 9, pp. 155–178, DOI:10.1016/B978-0-12-401735-1.00009-X.
- H. Rydin and J. K. Jeglum, in The Biology of Peatlands, Oxford: Oxford University Press, Oxford, 2nd edn, 2013, ch. 13, pp. 274–295, DOI:10.1093/acprof:osobl/9780199602995.003.0013.
- S. Yamulki, R. Anderson, A. Peace and J. I. L. Morison, Biogeosciences, 2013, 10, 1051–1065 CrossRef.
- H. Vasander and A. Kettunen, in Boreal Peatland Ecosystems, ed. R. K. Wieder and D. H. Vitt, Springer Berlin Heidelberg, Berlin, Heidelberg, 2006, pp. 165–194, DOI:10.1007/978-3-540-31913-9_9.
- E. Gorham, Ecol. Appl., 1991, 1, 182–195 CrossRef.
- T. Moore and N. Basiliko, in Boreal Peatland Ecosystems, ed. R. K. Wieder and D. H. Vitt, Springer Berlin Heidelberg, Berlin, Heidelberg, 2006, pp. 125–143, DOI:10.1007/978-3-540-31913-9_7.
- A. L. Hinwood and C. M. Rodriguez, J. R. Soc. West. Aust., 2005, 88, 133–138 Search PubMed.
- H. Rydin and J. K. Jeglum, in The Biology of Peatlands, Oxford: Oxford University Press, Oxford, 2nd edn, 2013, ch. 1, pp. 1–20, DOI:10.1093/acprof:osobl/9780199602995.003.0001.
- H. Rydin and J. K. Jeglum, in The Biology of Peatlands, Oxford University Press, 2nd edn, 2013, ch. 2, pp. 21–47, DOI:10.1093/acprof:osobl/9780199602995.003.0002.
- IUCN UK, Peatland Programme, https://www.iucn-uk-peatlandprogramme.org/, accessed 18.10.2022.
- The Scottish Government, Ending the Sale of Peat in Scotland: Analysis of Consultation Responses, 2023 Search PubMed.
- Scotch Whisky Association, Caring for the Land, https://www.scotch-whisky.org.uk/insights/sustainability/caring-for-the-land/, accessed 23.11.2022.
- K. Krakowiak, R. D. McIntosh and D. Ellis, Sustainable Food Technol., 2024, 2, 92–103 RSC.
- H. Biester, K. H. Knorr, J. Schellekens, A. Basler and Y. M. Hermanns, Biogeosciences, 2014, 11, 2691–2707 CrossRef CAS.
- H. H. Jeleń, M. Majcher and A. Szwengiel, Food Sci. Technol., 2019, 107, 56–63 Search PubMed.
- D. M. Goldberg, B. Hoffman, J. Yang and G. J. Soleas, J. Agric. Food Chem., 1999, 47, 3978–3985 CrossRef CAS.
- C. Macfarlane, J. B. Lee and M. B. Evans, J. Inst. Brew., 1973, 79, 203–209 CrossRef.
- R. T. Toledo, in International Smoked Seafood Conference Proceedings, ed. D. E. Kramer, L. Brown, Alaska Sea Grant College Program, Anchorage, 2008, pp. 55–62 Search PubMed.
- I. Duarte, A. Barros, P. S. Belton, R. Righelato, M. Spraul, E. Humpfer and A. M. Gil, J. Agric. Food Chem., 2002, 50, 2475–2481 CrossRef CAS.
- D. W. Lachenmeier, W. Frank, E. Humpfer, H. Schafer, S. Keller, M. Mortter and M. Spraul, Eur. Food Res. Technol., 2005, 220, 215–221 CrossRef CAS.
- L. Viggiani and M. A. C. Morelli, J. Agric. Food Chem., 2008, 56, 8273–8279 CrossRef CAS.
- M. A. Brescia, V. Caldarola, A. De Giglio, D. Benedetti, F. P. Fanizzi and A. Sacco, Anal. Chim. Acta, 2002, 458, 177–186 CrossRef CAS.
- W. Kew, I. Goodall and D. Uhrin, Food Chem., 2019, 298, 8 CrossRef.
- M. Stockwell, I. Goodall and D. Uhrín, Anal. Sci. Adv., 2020, 1, 132–140 CrossRef.
- J. R. Belmonte-Sanchez, R. Romero-Gonzalez, J. L. M. Vidal, F. J. Arrebola and A. G. Frenich, Food Chem., 2020, 317, 8 CrossRef.
- C. Fotakis, D. Christodouleas, K. Kokkotou, M. Zervou, P. Zoumpoulakis, P. Moulos, M. Liouni and A. Calokerinos, Food Chem., 2013, 138, 1837–1846 CrossRef CAS.
- Y. B. Monakhova, H. Schafer, E. Humpfer, M. Spraul, T. Kuballa and D. W. Lachenmeier, Magn. Reson. Chem., 2011, 49, 734–739 CrossRef CAS.
- W. Kew, N. G. A. Bell, I. Goodall and D. Uhrín, Magn. Reson. Chem., 2017, 55, 785–796 CrossRef CAS.
- G. Fitzgerald, K. J. James, K. MacNamara and M. A. Stack, J. Chromatogr. A, 2000, 896, 351–359 CrossRef CAS.
- L. Poisson and P. Schieberle, J. Agric. Food Chem., 2008, 56, 5813–5819 CrossRef CAS.
- P. Wiśniewska, T. Dymerski, W. Wardencki and J. Namieśnik, J. Sci. Food Agric., 2015, 95, 2159–2166 CrossRef.
- W. Kew, I. Goodall, D. Clarke and D. Uhrín, J. Am. Soc. Mass Spectrom., 2017, 28, 200–213 CrossRef CAS.
- J. S. Garcia, B. G. Vaz, Y. E. Corilo, C. F. Ramires, S. A. Saraiva, G. B. Sanvido, E. M. Schmidt, D. R. J. Maia, R. G. Cosso, J. J. Zacca and M. N. Eberlin, Food Res. Int., 2013, 51, 98–106 CrossRef CAS.
- F. Jack, in Whisky, Elsevier Ltd, 2nd edn, 2015, ch. 13, pp. 229–242, DOI:10.1016/B978-0-12-401735-1.00013-1.
- K. Krakowiak, R. D. McIntosh and D. Ellis, Measurement of Beverage ABV by Benchtop NMR Spectroscopy, Oxford Instruments, 2023 Search PubMed.
- ChemicalBook, Chemical Book, https://www.chemicalbook.com/, accessed 09.12.2022.
- I. John Wiley & Sons, SpectraBase, https://spectrabase.com/, accessed 09.12.2022.
- T. Y. T. Saito, K. Hayamizu, M. Yanagisawa, O. Yamamoto, Spectral Database for Organic Compounds SDBS, https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=hnmr%26sdbsno=1898, accessed 16.02.2022.
- D. S. Wishart, A. Guo, E. Oler, F. Wang, A. Anjum, H. Peters, R. Dizon, Z. Sayeeda, S. Tian, B. L. Lee, M. Berjanskii, R. Mah, M. Yamamoto, J. Jovel, C. Torres-Calzada, M. Hiebert-Giesbrecht, V. W. Lui, D. Varshavi, D. Varshavi, D. Allen, D. Arndt, N. Khetarpal, A. Sivakumaran, K. Harford, S. Sanford, K. Yee, X. Cao, Z. Budinski, J. Liigand, L. Zhang, J. Zheng, R. Mandal, N. Karu, M. Dambrova, H. B. Schiöth, R. Greiner and V. Gautam, Nucleic Acids Res., 2022, 50, D622–D631 CrossRef CAS.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512–7515 CrossRef CAS.
- C. Roullier-Gall, J. Signoret, D. Hemmler, M. A. Witting, B. Kanawati, B. Schäfer, R. D. Gougeon and P. Schmitt-Kopplin, Front. Chem., 2018, 6, 29 CrossRef.
- L. Nykänen and I. Nykänen, in Volatile Compounds in Foods and Beverages, ed. H. Maarse, Marcel Dekker, New York, 1st edn, ch. 15, pp. 547–580, 1991, DOI:10.1201/9780203734285.
- K. Y. M. Lee, A. Paterson, J. R. Piggott and G. D. Richardson, J. Inst. Brew., 2001, 107, 287–313 CrossRef.
- N. R. Wilson, PhD Thesis, Heriot-Watt University, 2010.
- C. M. Delahunty, J. M. Conner, J. R. Piggott and A. Paterson, J. Inst. Brew., 1993, 99, 479–482 CrossRef CAS.
- W. Grosch, H.-D. Belitz and P. Schieberle, in Food Chemistry, Germany: Springer Berlin/Heidelberg, Germany, 2009, ch. 21, pp. 938–970 Search PubMed.
- J. J. Melendez-Perez, M. J. Martinez-Mejia and M. N. Eberlin, Org. Geochem., 2016, 95, 29–33 CrossRef CAS.
- D. E. LaRowe and P. Van Cappellen, Geochim. Cosmochim. Acta, 2011, 75, 2030–2042 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00251b |
| This journal is © The Royal Society of Chemistry 2024 |