Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of highly effective growth strategies aiming at improving the content of carotenoids in Dunaliella salina IFDSAL-JY215†
Vítor
Sousa
a,
Filipe
Maciel
ab,
António A.
Vicente
 ab,
Óscar
Dias
ab and
Pedro
Geada
ab,
Óscar
Dias
ab and
Pedro
Geada
 *ab
*ab
aCentre of Biological Engineering, Universidade do Minho, 4710-057 Braga, Portugal. E-mail: pedrogeada@ceb.uminho.pt
bLABBELS – Associated Laboratory, Braga, Guimarães, Portugal
First published on 18th September 2024
Abstract
Dunaliella salina is the most promising natural source of β-carotene, presenting itself as a valid alternative to traditional chemically synthesized carotenoids. Microalgal pigments present several advantages compared to their synthetically produced counterparts, revealing, for instance, higher bioaccessibility. In the present study, a central composite rotatable design and a central composite design were employed to maximize β-carotene production through the optimization of 4 cultivation variables (salinity, airflow, and the nitrogen and phosphorus concentration in the growth medium). The optimal conditions found for β-carotene production were 64 PSU of salinity, an airflow of 500 mL min−1, and a nitrate and phosphate concentration of 6 mmol L−1 and 0.4 mmol L−1, respectively. When compared to the standard conditions, optimized cultures resulted in an improvement in the β-carotene concentration of around 88%. Concomitantly, a biomass concentration increase of 132% was observed for D. salina, from 0.93 g L−1 – under standard conditions – to 2.16 g L−1, under the optimal conditions. The microalga's carotenoid profile was also found to be positively influenced by the optimization process.
Sustainability spotlightThe food industry has been looking for more sustainable processes. However, some products are still resulting from non-environmentally friendly practices. For example, most of the β-carotene – a widely used pigment in the food industry – supplied to the market is obtained by chemical synthesis, though it presents several disadvantages – lower bioaccessibility, etc. – compared to its natural counterparts, such as that produced by the microalga Dunaliella salina. By assessing the synergistic effect of 4 growth parameters, we were able to significantly improve microalgal biomass and carotenoid production, which might contribute to their widespread use in the food industry helping the successful adoption of the UN SDGs 2 (end hunger, achieve food security and improved nutrition and promote sustainable agriculture) and 12 (ensure sustainable consumption and production patterns). |
1. Introduction
Modern society faces exponential population growth and foresees a global food1 crisis by 2050, with this being just an example of the biggest problems of the 21st century. This context turns even more critical in the search for more sustainable and efficient ways to meet human nutritional needs.2 A potential bio-based solution to overcome this problem is the use of microalgal biomass as a source of biocompounds, namely as an emergent alternative to non-animal protein for human consumption, contributing to sustainable food production.2 Microalgae are a potential source of high-value biocompounds – as in the case of carotenoids or proteins – which makes them excellent food additives.1 Moreover, proper growing conditions and bulk biomass production are easy to achieve in a wide range of environments, even under extreme conditions (i.e., temperature, pH, light intensity, and salinity), avoiding the need for arable land.1Dunaliella salina is a unicellular halophile green microalga whose cells are egg-shaped, spherical, and spindly or elliptical, with two flagella.3–5 It is known as salt-tolerant due to its capacity to live in a wide range of salt concentrations, from 0.5 to 4.5 mmol L−1 of NaCl.3,5–7 In this regard, these microalgae do not present a rigid cell wall, which makes them easier to disrupt under high shear stress conditions.8,9D. salina is a novel source of compounds – such as proteins or carotenoids (e.g., lutein, neoxanthin, violaxanthin, zeaxanthin, and β-carotene) – for industrial applications, namely in the pharmaceutical, cosmetics, health, or food sectors.10–12 The main interest in these microalgae is related to their capacity to accumulate high amounts of β-carotene, which can reach up to 10% of their dry weight.4,6,13 Indeed, D. salina is known as the most interesting source of natural β-carotene. This carotenoid is widely used in cosmetics – for protection against UV radiation and oxidative stress, food – as a food colorant or source of pro-vitamin A, pharmaceuticals – as an antioxidant or in the formulation of anticancer drugs –, and the nutraceutical industry – as a preventative supplement against heart disease.1,4,6,13,14 The traditional source of carotenoids for industrial applications is chemical synthesis. In the case of β-carotene, chemically synthesized compounds represent approximately 90% of the products in the market.8,15 However, microalgal pigments present several advantages when compared to those from traditional sources, revealing, for instance, higher bioaccessibility and better antioxidant properties. This occurs because all synthetic compounds exclusively contain the trans-isomer, while the cis-isomer, found in some strains of D. salina, is responsible for these interesting properties.4,8,15 Carotenoids play an important role in microalgal cells. They are involved in several vital functions, such as capturing light energy, helping to form structural apparatus for photosystem assembly, regulating the non-photochemical quenching, or scavenging reactive oxygen species (ROS) from algal cells and preventing oxidative stress.5,15,16 Usually, carotenoids are produced in higher amounts under metabolic stress conditions. Despite all the benefits presented by natural β-carotene, its cost (250–2000 USD/kg) is significantly greater when compared to its chemically synthesized counterparts – 350–750 USD per kg,5 evidencing the need to optimize the cultivation step in order to increase β-carotene productivity and, consequently, decrease the overall cost of the production process. In this regard, several studies were conducted aiming to understand which conditions favour the accumulation of β-carotene in D. salina cells. Most of the studies link the application of high light intensities, high salinity, and nitrogen deprivation to higher accumulation of β-carotene.6,10,11,17 In addition, other factors, such as phosphorus and sulfur content or the aeration rate, have also revealed relevant impacts on β-carotene accumulation.5,12,18
To the best of our knowledge, there is limited information regarding the synergetic effect of different parameters involved in microalgal cultivation, with these mechanisms being poorly understood. Therefore, the present study aimed at improving the productivity of carotenoids from D. salina biomass and assessing the combined effect of the variables under study. For that purpose, an experimental design was applied to evaluate the impact of 4 cultivation variables (salinity, airflow, and nitrogen and phosphorus content) on microalgal biomass production and accumulation of carotenoids.
2. Materials and methods
2.1 Microalga strain and cultures' maintenance
The microalga Dunaliella salina IFDSAL-JY215 was acquired from the Roscoff Culture Collection, France (https://www.roscoff-culture-collection.org/). Microalgae were grown in 2 L flat bottom flasks under autotrophic conditions with a constant light supply of 100 μmolphotons m−2 s−1 and airflow of 0.6 vvm in f/2 medium. The chemical composition of f/2 medium was as follows: nutrient solution (NaNO3: 10 mmol L−1; NaH2PO4·H2O: 0.1 mmol L−1), trace metal solution (Na2EDTA·2H2O: 11.7 μmol L−1; FeCl3·6H2O: 11.7 μmol L−1; MnCl2·4H2O: 0.91 μmol L−1; ZnSO4·7H2O: 0.0765 μmol L−1; CoCl2·6H2O: 0.042 μmol L−1; CuSO4·5H2O: 0.0393 μmol L−1, and Na2MoO4·2H2O: 0.026 μmol L−1), and vitamin solution (thiamine HCl: 0.296 μmol L−1; cyanocobalamin: 3.69 × 10−4 μmol L−1; and biotin: 2.05 × 10−4 μmol L−1). Salinity was set at 35 PSU.19 The nutrient concentrations used in the maintenance and inoculum preparation correspond to those used as standard conditions – central points (CPs) of Table 1.| Trial | Independent variables | X max (g L−1) | β-Carotene concentration (μg mg−1) | β-Carotene productivity (mg per L per day) | |||
|---|---|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | X 4 | ||||
| N content (mmol L−1) | P content (mmol L−1) | Salinity (PSU) | Aeration rate (mL min−1) | ||||
| 1 | −1 (7.5) | −1 (0.05) | −1 (35) | −1 (600) | 0.66 | 0.51 | 0.02 |
| 2 | 1 (12.5) | −1 (0.05) | −1 (35) | −1 (600) | 0.58 | 0.39 | 0.02 |
| 3 | −1 (7.5) | 1 (0.15) | −1 (35) | −1 (600) | 1.75 | 0.59 | 0.05 |
| 4 | 1 (12.5) | 1 (0.15) | −1 (35) | −1 (600) | 1.17 | 0.55 | 0.04 |
| 5 | −1 (7.5) | −1 (0.05) | 1 (89) | −1 (600) | 0.60 | 0.39 | 0.01 |
| 6 | 1 (12.5) | −1 (0.05) | 1 (89) | −1 (600) | 0.66 | 0.31 | 0.01 |
| 7 | −1 (7.5) | 1 (0.15) | 1 (89) | −1 (600) | 1.70 | 0.70 | 0.06 |
| 8 | 1 (12.5) | 1 (0.15) | 1 (89) | −1 (600) | 1.68 | 0.49 | 0.04 |
| 9 | −1 (7.5) | −1 (0.05) | −1 (35) | 1 (800) | 0.62 | 0.12 | 0.01 |
| 10 | 1 (12.5) | −1 (0.05) | −1 (35) | 1 (800) | 0.59 | 0.12 | 0.01 |
| 11 | −1 (7.5) | 1 (0.15) | −1 (35) | 1 (800) | 1.27 | 0.43 | 0.03 |
| 12 | 1 (12.5) | 1 (0.15) | −1 (35) | 1 (800) | 1.15 | 0.26 | 0.02 |
| 13 | −1 (7.5) | −1 (0.05) | 1 (89) | 1 (800) | 0.49 | 0.11 | 0.01 |
| 14 | 1 (12.5) | −1 (0.05) | 1 (89) | 1 (800) | 0.49 | 0.14 | 0.01 |
| 15 | −1 (7.5) | 1 (0.15) | 1 (89) | 1 (800) | 1.48 | 0.33 | 0.02 |
| 16 | 1 (12.5) | 1 (0.15) | 1 (89) | 1 (800) | 1.46 | 0.35 | 0.02 |
| 17 | −2 (5) | 0 (0.10) | 0 (62) | 0 (700) | 0.82 | 0.38 | 0.02 |
| 18 | 2 (15) | 0 (0.10) | 0 (62) | 0 (700) | 0.84 | 0.25 | 0.01 |
| 19 | 0 (10) | −2 (0) | 0 (62) | 0 (700) | 0.18 | 0.16 | 0.01 |
| 20 | 0 (10) | 2 (0.20) | 0 (62) | 0 (700) | 1.67 | 0.56 | 0.04 |
| 21 | 0 (10) | 0 (0.10) | −2 (6) | 0 (700) | 0.10 | 0 | 0.00 |
| 22 | 0 (10) | 0 (0.10) | 2 (116) | 0 (700) | 0.87 | 0.16 | 0.01 |
| 23 | 0 (10) | 0 (0.10) | 0 (62) | −2 (500) | 0.93 | 0.53 | 0.03 |
| 24 | 0 (10) | 0 (0.10) | 0 (62) | 2 (900) | 0.72 | 0.34 | 0.02 |
| CP1 | 0 (10) | 0 (0.10) | 0 (62) | 0 (700) | 0.99 | 0.41 | 0.04 |
| CP2 | 0 (10) | 0 (0.10) | 0 (62) | 0 (700) | 0.85 | 0.40 | 0.04 |
| CP3 | 0 (10) | 0 (0.10) | 0 (62) | 0 (700) | 0.99 | 0.40 | 0.04 |
| CP4 | 0 (10) | 0 (0.10) | 0 (62) | 0 (700) | 1.00 | 0.42 | 0.04 |
2.2 Experimental design
The optimization process to achieve maximum microalgal biomass and β-carotene production was carried out based on design of experiments methodologies, particularly using the Protimiza Experimental Design software (http://experimental-design.protimiza.com.br). To investigate the main, interaction, and quadratic effects of four independent variables (nitrogen content (X1), phosphorus content (X2), salinity (X3), and aeration rate (X4)) on algal biomass and β-carotene production, the central composite rotational design (CCRD) and central composite design (CCD) were applied. The experimental design was performed in 1 L bubble column reactors (6.5 cm diameter and 43 cm high) under a constant light supply of 150 μmolphotons m−2 s−1 and a CO2 stream of 6 mL min−1. Reactors were continuously aerated with filtered air (0.2 μm) through an internal glass tube located at the center of the reactor. At the end of each growth, cultures were centrifuged for 20 min at 3000g (Centurion, model CR7000, 2/3 L, India) and the pellet was frozen (−20 °C) for later lyophilization.The CCRD screening design investigated the impact of the 4 independent variables in the response variables: maximum biomass concentration (Xmax) and β-carotene production. These independent variables were studied at 5 different levels (−α, −1, 0, 1, and α), where the values of (−α) and (α) were −2 and 2, respectively – Table S1.† Regarding the range of aeration rates tested, it is important to note that 500 mL min−1 was selected as a minimum since preliminary tests using the 1 L bubble column reactors proved that aeration rates below this value would be insufficient to enable the homogeneous mixing of the growing culture, resulting in cell sedimentation. Based on such considerations, an experimental matrix of 24 assays and 4 central points (CPs) was formulated. As mentioned in Section 2.1 the culture conditions of the CPs correspond to the intermediate values (level 0) of each independent variable, representing the standard growth conditions. All the independent variables selected to proceed with the optimization process have shown a significant effect on β-carotene accumulation at a confidence level higher than 90% and were used to determine the model equation of the coded variables.
The concentrations of nitrogen (X1) and phosphorus (X2) were further investigated in more detail in a CCD. These independent variables were studied at low (−1) and high (1) levels, resulting in a total of 4 assays combining the 2 variables under study simultaneously and 3 additional CPs (Table S2†). All the independent variables – as well as the respective interactions – that have shown a significant effect on β-carotene accumulation at a confidence level higher than 95% were selected to determine the model equation of the coded variables.
2.3 Determination of growth kinetics
Microalgal growth was followed by optical density (OD) at 750 nm and used for dry weight estimation (X, g L−1) according to the following calibration curve, eqn (1):| X (g L−1) = 1.3027OD − 0.0872 | (1) |
Dry cell weight was measured by gravimetric determination, where 10 mL of culture was filtered through glass filters (VWR, USA), washed twice with 20 mL of ammonium formate (0.5 mol L−1), and dried at 105 °C for 24 h.
Biomass productivity (P, g per L per day) was obtained through eqn (2):
| P = (Xt − X0)/(t − t0) | (2) |
β-Carotene productivity (Pβcar, mgβcar per L per day) was obtained from eqn (3):
| Pβcar = [βcar] × P | (3) |
2.4 Metabolite characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) – was added to 10 mg of lyophilized biomass. This mixture was vortexed for 2 min and then centrifuged for 10 min at 225g (Hettich Mikro 120, Tuttlingen, Germany). The organic phase was pipetted to a glass tube and the biomass residue was re-extracted one more time. After this, the resulting organic phase was dried under a stream of nitrogen gas. To remove the non-lipid contaminants, the initial extract was re-dissolved in a mixture of dichloromethane, methanol, and water using 2 mL, 1 mL, and 0.75 mL, respectively. The mixture was centrifuged again for 10 min at 225g. The organic phase was pipetted into dark vials previously dried and weighed. To finish the procedure, the organic phase was dried under a nitrogen stream and weighed.
1 v/v) – was added to 10 mg of lyophilized biomass. This mixture was vortexed for 2 min and then centrifuged for 10 min at 225g (Hettich Mikro 120, Tuttlingen, Germany). The organic phase was pipetted to a glass tube and the biomass residue was re-extracted one more time. After this, the resulting organic phase was dried under a stream of nitrogen gas. To remove the non-lipid contaminants, the initial extract was re-dissolved in a mixture of dichloromethane, methanol, and water using 2 mL, 1 mL, and 0.75 mL, respectively. The mixture was centrifuged again for 10 min at 225g. The organic phase was pipetted into dark vials previously dried and weighed. To finish the procedure, the organic phase was dried under a nitrogen stream and weighed.
| Carbohydrates (%) = 100 − (ash + crude protein + crude lipid) | (4) |
2.5 Nitrate concentration
To evaluate the amount of nitrate consumption in the growth medium at the end of the assay, the nitrate concentration was attained using the ultraviolet spectrophotometric screening method described by the American Public Health Association.25 For this method, samples were analyzed using an ultraviolet-visible spectrophotometer (Jasco V-560, Japan) in the ultraviolet (UV) range at the wavelengths 220 nm and 275 nm. The nitrate concentration was calculated according to eqn (5). Then, the corrected absorbance was compared to a calibration curve developed using potassium nitrate standards with known nitrate concentrations ranging between 0.009 and 0.286 m L−1 (R2 = 0.995).| Nitrate concentration = Abs220 − 2Abs275 | (5) |
2.6 Statistical analysis
Second-order models of the CCRD and CCD were obtained using a 90% (p < 0.10) and 95% (p < 0.05) confidence level, respectively, and the quality of the fitted model was statistically evaluated by analysis of variance (ANOVA) and using the coefficient of determination (R2). The statistical analysis of the validation assays (Section 2.2.1) was carried out using GraphPad Prism 9 (GraphPad Software, La Jolla, USA). Tests were used as indicated in figure legends: two-tailed unpaired t-test. Data are represented as mean ± standard error of the mean (SD). Statistical significance was set for p < 0.05 and it is indicated by different superscript letters according to their significance.3. Results
3.1 Assessment of the synergetic impact of 4 variables on β-carotene production
Biomass productivity, as well as the accumulation of biocompounds, is directly influenced by several factors (e.g., abiotic factors). The determination of optimal growth conditions could improve cost-effectiveness of the microalgal cultivation process. In this regard, the present work employed a CCRD to evaluate the impact of four independent variables – nitrogen content, phosphorus content, salinity, and aeration rate – on the accumulation of β-carotene, one of the most important carotenoids produced by D. salina. Table 1 contains the data resulting from the CCRD assays.Based on the results from CCRD (Table 1), it was possible to identify the most suitable conditions for β-carotene production. β-Carotene content on D. salina varied from 0 to 0.70 μg mg−1 within the CCRD. Additionally, considering a confidence level of 90% (p-value < 0.1), one was also able to determine the significant variables of the process, as well as synergistic interactions between them, and, consequently, set the models. According to the R2 and calculated F values in Table 2, it is possible to say that these are adequate to obtain a second-order model (eqn Y1) for β-carotene production, within the range of values studied. Also, all the variables in the study showed a significant effect. The model presents a high value of the coefficient of determination (R2 = 84%) indicating a good correlation between the experimental and predicted values. The highest β-carotene content was achieved in trial 7 (0.70 μg mg−1), which represents an increase of 72.5% when compared to the standard conditions (CPs) – that reached 0.41 μg mg−1. Concomitantly, this increase in β-carotene content also represented an improvement in β-carotene productivity, from 0.04 to 0.06 mg per L per day. These results reveal a substantial improvement in β-carotene accumulation and highlight the importance of optimizing the variables under study. On the other hand, the minimal content was achieved in trial 21, where no β-carotene was detected.
| Response | R 2 (%) | F calc | F tab | Equation | |
|---|---|---|---|---|---|
| CCRD | β-Carotene concentration (μg mg−1) | 84 | 30.2 | 2.21 | Y 1 = 0.41 − 0.03X1 + 0.10X2 − 0.07X32 − 0.10X4 |
| X max (g L−1) | 85 | 32.3 | 2.21 | Y 2 = 1.01 + 0.41X2 + 0.10X3 − 0.09X32 − 0.07X4 | |
| β-Carotene productivity (mg per L per day) | 81 | 25.1 | 2.21 | Y 3 = 0.030 − 0.003X12 + 0.011X2 − 0.006X32 − 0.007X4 | |
| CCD | β-Carotene concentration (μg mg−1) | 95 | 98.3 | 6.61 | Y 4 = 0.95 + 0.19X1 |
| X max (g L−1) | 97 | 170.9 | 6.61 | Y 5 = 1.90 + 0.28X1 | |
| β-Carotene productivity (mg per L per day) | 99 | 688.9 | 6.61 | Y 6 = 0.17 + 0.04X1 |
The model (Fig. 1) suggests that decreasing the nitrogen content of the medium until 5 mmol L−1 significantly increases β-carotene production (linear effect). For this reason, nitrogen content was further evaluated on the CCD and the range of values was changed from 5–15 mmol L−1 to 2–6 mmol L−1.
Regarding the model for phosphorus (Fig. 1), it suggests that higher β-carotene production was promoted with the increment of phosphorus content (linear effect). Therefore, phosphorus content was also further evaluated on the CCD, with the new range under study being 0.2–0.4 mmol L−1, instead of 0–0.2 mmol L−1.
In terms of salinity, the model indicates that enhancing the salinity from 8 to 64 PSU significantly increases β-carotene production. However, salinity above 64 PSU would lead to a sharp decrease (quadratic effect) (Fig. 1). Therefore, CCRD trials resulted in the optimal conditions when a salinity of 64 PSU was applied.
Additionally, the model demonstrates that decreasing the aeration rate of the medium until 500 mL min−1 significantly increases β-carotene production (linear effect) (Fig. 1). Considering that sufficient mixing is crucial for cultivation of microalgae, the aeration rate applied in further CCD experiments was 500 mL min−1, in order to prevent cell sedimentation and maintain a homogeneous culture suspension, ensuring that each cell has access to light, nutrients, and CO2.
3.2 Assessment of the synergetic impact of the 4 variables on D. salina growth performance
Similar to that observed for β-carotene content, biomass production was also significantly affected by the growth conditions tested in the CCRD (Table 1). The biomass concentration varied from 0.10 to 1.75 g L−1, representing a decrease of 86% and an increase of 82%, respectively, compared to the CPs. According to the model presented in Table 2 (eqn Y2), the differences observed were promoted by three out of the four variables under study: phosphorus content, salinity, and aeration rate. In contrast, nitrogen content did not affect Xmax when ranging from 5 to 15 mmol L−1.According to the model, biomass production observed an enhancement at a higher phosphorus concentration in the culture medium, up to 0.2 mmol L−1 (linear effect), and lower aeration rates (linear effect). In the case of salinity, the model indicates that the increase in salinity, up to 70 PSU, favors the growth performance of D. salina (linear effect). Above this salinity, it exponentially decreases (quadratic effect) (Fig. 2).
3.3 CCD for nitrogen and phosphorus content
Based on the indications from CCRD, a CCD was applied to assess the impact of new ranges of nitrogen and phosphate content on β-carotene production. Considering the new ranges under study, only nitrogen content showed a significant effect, with a confidence level of 95% (p-value < 0.05). Analysing Table 3, both R2 and calculated F values were shown to be adequate to obtain a second-order model (eqn Y4) for β-carotene production (Table 2). The model presents a high value for the coefficient of determination (R2 = 95%) and reveals that the enhancement of nitrogen content, in the range of values studied in the CCD, contributes to significant increases in β-carotene production (linear effect).| Trial | Independent variables | X max (g L−1) | β-Carotene concentration (μg mg−1) | β-Carotene productivity (mg per L per day) | |||
|---|---|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | X 4 | ||||
| N content (mmol L−1) | P content (mmol L−1) | Salinity (PSU) | Aeration rate (mL min−1) | ||||
| 1 | −1 (2) | −1 (0.2) | 64 | 500 | 1.61 | 0.74 | 0.13 |
| 2 | 1 (6) | −1 (0.2) | 2.14 | 1.14 | 0.21 | ||
| 3 | −1 (2) | 1 (0.4) | 1.57 | 0.76 | 0.12 | ||
| 4 | 1 (6) | 1 (0.4) | 2.16 | 1.12 | 0.21 | ||
| CP1 | 0 (4) | 0 (0.3) | 1.94 | 1.02 | 0.17 | ||
| CP2 | 0 (4) | 0 (0.3) | 1.95 | 0.94 | 0.17 | ||
| CP3 | 0 (4) | 0 (0.3) | 1.92 | 0.91 | 0.17 | ||
As mentioned before, phosphorus content was not statistically significant for β-carotene production within the range of values of the CCD (0.2 to 0.4 mmol L−1). Consequently, for further model validation, the phosphorus concentration was kept at 0.2 mmol L−1.
Considering the effect of the variables under study on growth performance, it is important to mention that the model obtained (eqn Y5) showed similar tendencies to those found for β-carotene production.
Regarding the results obtained in the CCD, when compared to the CCRD (Sections 3.1 and 3.2), the increase reported in both dependent variables under study is notable. Taking into account the trials that presented the best performances in each of the experimental designs, there is an increase of 23.43%, 62.86% and 23.53% for biomass, β-carotene production, and β-carotene productivity, respectively. These results are in line with the expected outcomes – with a more pronounced increase in β-carotene – since the CCD was designed to enhance the accumulation of β-carotene in D. salina IFDSAL-JY21 biomass.
3.4 Validation of the model
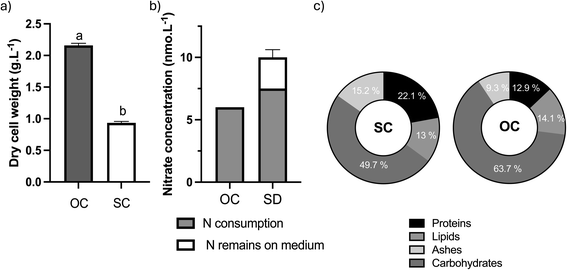
Regarding the growth performance (Fig. 4a), and compared to standard conditions, optimal conditions led to an increase of 132% in Xmax, yielding 0.93 g L−1. Based on nitrate variation throughout the growth process (Fig. 4b), it was possible to realize that the nitrates supplied to the culture (6 mmol) were completely consumed under optimal conditions. In contrast, cultures grown under standard conditions consumed 7.5 out of the 10 mmol L−1 of nitrates provided. The biochemical composition of D. salina biomass grown under both conditions was analyzed in terms of proteins, lipids, and carbohydrates. As demonstrated in Fig. 4c, protein content in D. salina cells decreased under optimal conditions whereas carbohydrate content increased. No significant differences were observed in lipid content.
4. Discussion
This study was carried out to understand the combined effect of nitrogen, phosphorus, salinity, and aeration rate both on β-carotene production and growth performance of D. salina. The whole study was divided into two experimental phases. The first experimental phase (CCRD) allowed an initial screening of the four variables under study, enabling the determination the optimal values for salinity and the aeration rate as well as trends for nitrogen and phosphorus content. Subsequently, these trends were the starting point of the second experimental phase (CDD), meant to determine the optimal conditions regarding nitrogen and phosphate content.The nitrogen content in the culture medium has direct interference with β-carotene accumulation. Based on the results from DCCR, presented in Table 1, higher amounts of β-carotene were achieved at lower concentrations of nitrogen. Indeed, these results are in agreement with the information reported in previous studies. A low nitrogen concentration is considered one of the most stimulant conditions to promote β-carotene synthesis.11,13,17,18 As happened in the present study, when testing a wide range of nitrogen concentrations (from 1.4 mgN L−1 to 716 mgN L−1), Sui et al. (2019) reached maximum carotenoid accumulation (16 pg per cell) using the lowest amount of nitrogen.10 Despite the carotenoid increase reported by the authors, cell growth was almost inexistent under this condition. In contrast, the present study shows an increase in β-carotene production combined with significant biomass yields, which makes this biotechnological process profitable in industrial terms. In the absence of a suitable nitrogen concentration, cells promote the synthesis of non-nitrogen-induced pigments – as in the case of β-carotene – in order to maintain their normal metabolic activity.13 However, the results of DCC presented in Table 3 demonstrate that, below a certain threshold, lower availability of nitrogen does not contribute to a positive effect on β-carotene production. Since the accumulation of β-carotene occurs mainly in the β-carotene globules,3,11,17 it is possible to hypothesize that, below a certain nitrogen level, the impact of nitrogen starvation on β-carotene production becomes negative. This might be explained by the decreasing production of carotenoids' globule proteins under unsuitable conditions of nitrogen, which is responsible for β-carotene globule stability.11,26 Consequently, without a proper amount of such proteins, a decrease can possibly occur in β-carotene content, as observed in the present work.
Concerning the impact of phosphorus content on β-carotene accumulation, higher values were achieved at higher concentrations of this element in the growth medium (Table 1). Therefore, the metabolic response of D. salina to the phosphorus concentration was more deeply investigated on the DCC (Table 3), which pointed out its direct effect on β-carotene accumulation up to 0.2 mmol L−1. Phosphorus is an essential macronutrient for all organisms. Previous studies about the induction of β-carotene synthesis by nutrient deprivation were mainly focused on nitrogen. The few studies dealing with the impact of phosphorus on β-carotene accumulation report different trends.12,18,27 Some authors concluded that phosphorus limitation favour β-carotene accumulation, as demonstrated by Lv et al. (2016). In this case, β-carotene increased – from 2 to 8 pg per cell – in cells subjected to phosphorus deprivation.12 In contrast, other authors reported results similar to those observed in the present study, where elevating the phosphate concentration contributes to increasing the amount of β-carotene, until a certain level.18 The inconsistency between the results reported in other studies and the results of the present study may be related to the different physiological pathways presented by different D. salina strains. It is reported that some D. salina strains present intracellular pools of phosphorus,12,28 which gives them the capacity to store phosphorus and better react to stressful conditions. Dunaliella salina IFDSAL-JY215 may not be able to accumulate phosphorus in phosphorus pools, consequently, hindering the production of β-carotene under limited phosphorus growth conditions.
The results obtained regarding the aeration rate showed that the D. salina species under study has a greater tendency to accumulate β-carotene at low aeration rates. Nevertheless, at higher surface gas velocities, a faster alternation of cells would be expected between dark and light zones of the reactor and, consequently, a greater accumulation of β-carotene.9 This would happen since, with greater exposure to light, higher generation of ROS is expected inside the cells, thus triggering the production of pigments with antioxidant capacity – such as β-carotene – as a response.3,5,16 As shear stress forces have also been shown to influence the growth rate of D. salina (Table 1), with a clear negative impact on its cellular metabolism, it might explain the reported results.6,14
D. salina is a microalgal species well-known for its capacity to live in a wide range of salt concentrations. Different strains of D. salina present different optimal salinity ranges for β-carotene accumulation. However, all of them present the same tendency: higher salinities contribute to a higher accumulation of β-carotene.6,14 Our results are in agreement with these findings. High salinities result in stressful conditions for cells due to the generation of ROS. The high accumulation of ROS on the photosynthetic machinery of cells can damage their normal metabolic functions.14,26 Therefore, the overexpression of carotenoids, as in the case of β-carotene, occurs to protect the cells.16,17 As evidenced by the present study, salinity was positively correlated with β-carotene production until a certain level. Similar results were reported by Lou et al. (2020),6 obtaining an increase in β-carotene content from 44.90 mg g−1, under control conditions (0.5 mol L−1 of salinity and 50 μmolphotons m−2 s−1), to 305.63 mg g−1, under high light and salinity treatment – 2.5 mol L−1 and 400 μmolphotons m−2 s−1.
Nitrogen and phosphorus play an important role in microalgal cells, being essential components of biological macromolecules such as proteins, DNA, RNA, ATP, and phospholipids, constituents of microalgal biomembranes. Therefore, below a certain amount of these elements, D. salina growth performance is compromised.12 On the other hand, as previously observed for β-carotene production, lower aeration rates also contribute to higher biomass accumulation. Similar findings were found in the literature, where increasing superficial gas velocity – from 0.029 to 0.052 m s−1 – enhanced the death rate constant of D. salina by approximately 4.76-fold.9 The fact that D. salina does not have a cell wall makes this microalga particularly sensitive to shear forces.8 The lack of a cell wall also plays an important role in the ability of this microalga to adapt to high salinities. This halophilic microalga requires high salinities to enhance its growth.3,14 Consistent with these facts, the growth performance of D. salina at higher salinities increases almost concomitantly with β-carotene production. The results obtained in the presented work indicate that the optimal salinity for β-carotene accumulation and biomass production was 64 and 70 PSU, respectively. Salinity directly interferes with biomass productivity, contributing to the inhibition of photosynthetic activity. This is compensated for by the increase of β-carotene, protecting cells against the negative impact induced by salt stress and allowing them to enhance growth efficiency.6,14 This protective mechanism works while photoprotection promoted by β-carotene and other carotenoids outcompetes the impact of salt stress. With the optimization process developed in this study, one was capable of significantly increasing biomass production of D. salina, showing clear increases when compared with similar studies in the literature. Morowvat et al. (2016) also used a response surface methodology for the optimization of medium composition aiming for better biomass production of D. salina. The authors reported a maximum biomass concentration of 0.997 g L−1 under optimal conditions,18 which is quite below the value reached in the present work: 2.16 g L−1.
The Dunaliella genus can be divided into two groups, depending on the protection mechanism they adopt when subjected to stress conditions.5,17 Carotenogenic Dunaliella species are characterized by producing large amounts of β-carotene under stress conditions5,17 (Fig. 5). On the other hand, non-carotenegenic Dunaliella, instead of producing large amounts of β-carotene, carries out other protection mechanisms in similar environments, such as the xanthophyll cycle5,17 (Fig. 5). The vast majority of Dunaliella salina are carotenogenic; however, some strains may be non-carotenogenic.5,17 Contrary to our expectations, the carotenoid with the highest amounts in D. salina IFDSAL-JY215 is lutein, suggesting that this is a non-carotenogenic strain. Lutein is a xanthophyll that acts as protection against photodamage, as happens with β-carotene.7,15,29 Its most important function is believed to be in quenching triplet chlorophyll (3Chl*), to prevent energy transfer to molecular oxygen and consequent formation of singlet oxygen  . Lutein also quenches excited 1Chl* (NPQ) to prevent the formation of ROS under high-light conditions.15 This carotenoid has been previously reported as having a strong correlation with photosynthesis and respiration.15,29 This can explain the concomitant increase of carotenoid content and biomass production, observed in our data. Non-carotenogenic Dunaliella species demonstrate a resourceful and flexible mechanism to deal with stress conditions, not depending on massive β-carotene accumulation but on a different energy dissipation mechanism reflected in a clear increase of non-photochemical quenching, where the xanthophyll cycle carotenoids play a major role.17 Lv et al. (2016) showed that, under nutrient deprivation conditions, D. salina increases its transcript levels of β-carotene hydroxylase.12 This is a key regulatory enzyme in the beta–beta-branch of carotenoid biosynthesis, which catalyzes the hydroxylation of β-carotene for the xanthophyll cycle.12,30 This metabolic pathway may be responsible for a potential decrease in β-carotene content (Fig. 5).
. Lutein also quenches excited 1Chl* (NPQ) to prevent the formation of ROS under high-light conditions.15 This carotenoid has been previously reported as having a strong correlation with photosynthesis and respiration.15,29 This can explain the concomitant increase of carotenoid content and biomass production, observed in our data. Non-carotenogenic Dunaliella species demonstrate a resourceful and flexible mechanism to deal with stress conditions, not depending on massive β-carotene accumulation but on a different energy dissipation mechanism reflected in a clear increase of non-photochemical quenching, where the xanthophyll cycle carotenoids play a major role.17 Lv et al. (2016) showed that, under nutrient deprivation conditions, D. salina increases its transcript levels of β-carotene hydroxylase.12 This is a key regulatory enzyme in the beta–beta-branch of carotenoid biosynthesis, which catalyzes the hydroxylation of β-carotene for the xanthophyll cycle.12,30 This metabolic pathway may be responsible for a potential decrease in β-carotene content (Fig. 5).
Based on these assumptions and considering our results, D. salina IFDSAL-JY215 appears to adopt mechanisms of protection and accumulation of carotenoids similar to those reported for non-carotenogenic Dunaliella. Therefore, phylogenetically, D. salina IFDSAL-JY21 may be closer to non-carotenogenic Dunaliella. There is a huge diversity of D. salina strains, each one with a specific response to unfavourable conditions. The effectiveness of these mechanisms varies greatly between strains, as does the potential for β-carotene production.
In addition to the increase in β-carotene content that resulted from this optimisation process, there was also an increase in other carotenoids (Fig. 3). Among these carotenoids, lutein stands out because it is the carotenoid that is produced in the greatest quantity, as well as for its industrial relevance. As a result of this optimization process for β-carotene, a 48% increase in lutein content was observed under the optimized conditions, compared to the standard conditions. These results can be explained by the fact that certain key parameters in the production optimization of these carotenoids show the same trend, as in the case of salinity. In the current study, as well as in the literature, it is clear that higher salinities favour the production of β-carotene and similar trends were observed in the case of lutein. According to Bermejo et al. (2018), a concentration increase in NaCl, from 0 to 500 mmol L−1, enhanced C. onubensis lutein content (from 5.3 to 7.8 mg g−1).31 However, other factors display opposite tendencies for both carotenoids, such as the N content. In the present work (and in the literature), it is shown that lower N content contributes to a higher β-carotene content. In contrast, studies devoted to lutein indicated that a higher N content leads to a higher lutein content, as demonstrated by Xie et al. (2013). The mentioned work demonstrated an increase in the lutein content of Desmodesmus sp. – from 3.76 mg g−1 to 4.97 mg g−1 – by increasing the nitrate concentration from 4.4 mmol L−1 to 13.2 mmol.32 These indications regarding lutein content show promising signs, opening the possibility of improving the lutein value. In the present study, a lutein yield of 1.63 mg g−1 was reported, which makes D. salina IFDSAL-JY21 a promising alternative to the traditional sources of this carotenoid, as in the case of marigold flowers (which have a lutein yield between 0.17 and 5.7 mg g−1).33
5. Conclusion
β-Carotene content, as well as the content of the remaining carotenoids of D. salina IFDSAL-JY21, could be significantly enhanced using a response surface methodology. This showed that all the independent variables under study (nitrogen, phosphorus, salinity, and aeration rate) have a significant effect on β-carotene and biomass production, contributing to an increase of 88% and 132%, respectively, after the optimization process. Concomitantly, an increase in all carotenoids was also observed as a result of this optimisation process targeting β-carotene, especially lutein which increased by 48%. The lutein content presented makes D. salina IFDSAL-JY21 a promising alternative to traditional sources of this carotenoid (marigold flowers). Although the strain under study in the present study is considered a D. salina, it showed mechanisms of protection and accumulation of carotenoids similar to those reported to non-carotenogenic Dunaliella.Data availability
The data supporting this article have been included either in the main document or as part of the ESI.†Author contributions
Vitor Sousa: conceptualization, methodology, validation, investigation, formal analysis, visualization, writing – original draft, writing – review and editing; Filipe Maciel: methodology, writing – review and editing; António A. Vicente: resources, writing – review and editing, supervision, funding acquisition; Oscar Dias: writing – review and editing, supervision; Pedro Geada: conceptualization, resources, writing – review and editing, supervision.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of the UIDB/04469/2020 unit, with DOI http://dx.doi.org/10.54499/UIDB/04469/2020, and by LABBELS – Associate Laboratory in Biotechnology, Bioengineering and Microelectromechanical Systems, LA/P/0029/2020. Vitor Sousa acknowledges the Foundation for Science and Technology (FCT) for their fellowship UI/BD/151238/2021 (https://doi.org/10.54499/UI/BD/151238/2021). This work was financially supported by “Pacto da Bioeconomia Azul” (Project No. C644915664-00000026) within the WP5 Algae Vertical, funded by the Next Generation EU European Fund and the Portuguese Recovery and Resilience Plan (PRR). Pedro Geada acknowledges FCT for the Junior Research contract obtained under the scope of the Scientific Stimulus Employment with the ref. 2022.00930.CEECIND (https://doi.org/10.54499/2022.00930.CEECIND/CP1718/CT0023). Oscar Dias acknowledges FCT for the Assistant Research contract obtained under CEEC Individual 2018 (https://doi.org/10.54499/CEECIND/03425/2018/CP1581/CT0020).References
- P. Geada, R. Rodrigues, L. Loureiro, R. Pereira, B. Fernandes and J. A. Teixeira,
et al., Electrotechnologies applied to microalgal biotechnology – Applications, techniques and future trends, Renewable Sustainable Energy Rev., 2018, 94(November 2017), 656–668, DOI:10.1016/j.rser.2018.06.059
.
- P. Geada, C. Moreira, M. Silva, R. Nunes, L. Madureira and C. M. R. Rocha,
et al., Algal proteins: Production strategies and nutritional and functional properties, Bioresour. Technol., 2021, 332(April), 125125, DOI:10.1016/j.biortech.2021.125125
.
- J. E. W. Polle, R. Roth, A. Ben-Amotz and U. Goodenough, Ultrastructure of the green alga Dunaliella salina strain CCAP19/18 (Chlorophyta) as investigated by quick-freeze deep-etch electron microscopy, Algal Res., 2020, 49(May), 101953, DOI:10.1016/j.algal.2020.101953
.
- S. Pourkarimi, A. Hallajisani, A. Nouralishahi, A. Alizadehdakhel and A. Golzary, Factors affecting production of beta-carotene from Dunaliella salina microalgae, Biocatal. Agric. Biotechnol., 2020, 29(August), 101771, DOI:10.1016/j.bcab.2020.101771
.
- R. K. Goswami, K. Agrawal and P. Verma, Microalgae Dunaliella as biofuel feedstock and β-carotene production: An influential step towards environmental sustainability, Energy Convers. Manage.: X, 2022, 13, 100154, DOI:10.1016/j.ecmx.2021.100154
.
- S. Lou, X. Zhu, Z. Zeng, H. Wang, B. Jia and H. Li,
et al., Identification of microRNAs response to high light and salinity that involved in beta-carotene accumulation in microalga Dunaliella salina, Algal Res., 2020, 48(October 2019), 101925, DOI:10.1016/j.algal.2020.101925
.
- H. Bonnefond, N. Moelants, A. Talec, O. Bernard and A. Sciandra, Concomitant effects of light and temperature diel variations on the growth rate and lipid production of Dunaliella salina, Algal Res., 2016, 14, 72–78, DOI:10.1016/j.algal.2015.12.018
.
- J. Monte, J. Bernardo, M. Sá, C. Parreira, C. F. Galinha and L. Costa,
et al., Development of an integrated process of membrane filtration for harvesting carotenoid-rich Dunaliella salina at laboratory and pilot scales, Sep. Purif. Technol., 2020, 233(August 2019), 116021, DOI:10.1016/j.seppur.2019.116021
.
- M. J. Barbosa and W. R. H. Hadiyanto, Overcoming Shear Stress of Microalgae Cultures in Sparged Photobioreactors, Biotechnol. Bioeng., 2004, 85(1), 78–85 CrossRef CAS PubMed
.
- Y. Sui, M. Muys, D. B. Van de Waal, S. D'Adamo, P. Vermeir and T. V. Fernandes,
et al., Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity, Bioresour. Technol., 2019, 287(April 2019), 121398, DOI:10.1016/j.biortech.2019.121398
.
- H. Bonnefond, N. Moelants, A. Talec, P. Mayzaud, O. Bernard and A. Sciandra, Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation, Biotechnol. Biofuels, 2017, 10(1), 1–10 CrossRef PubMed
.
- H. Lv, X. Cui, F. Wahid, F. Xia, C. Zhong and S. Jia, Analysis of the physiological and molecular responses of Dunaliella salina to macronutrient deprivation, PLoS One, 2016, 11(3), e0152226, DOI:10.1371/journal.pone.0152226
.
- Z. Wu, P. Duangmanee, P. Zhao and C. Ma, The effects of light, temperature, and nutrition on growth and pigment accumulation of three dunaliella salina strains isolated from saline soil, Jundishapur J. Microbiol., 2016, 9(1), 1–9 CrossRef CAS PubMed
.
- R. Reshma, K. Chitra Devi, S. Dinesh Kumar, P. Santhanam, P. Perumal and N. Krishnaveni,
et al., Enhancement of pigments production in the green microalga Dunaliella salina (PSBDU05) under optimized culture condition, Bioresour. Technol. Rep., 2021, 14(March), 100672, DOI:10.1016/j.biteb.2021.100672
.
- Y. Xu, I. M. Ibrahim, C. I. Wosu, A. Ben-Amotz and P. J. Harvey, Potential of new isolates of dunaliella salina for natural β-carotene production, Biology, 2018, 7(1), 14, DOI:10.3390/biology7010014
.
- Y. Xi, J. Bian, G. Luo, F. Kong and Z. Chi, Enhanced β-carotene production in Dunaliella salina under relative high flashing light, Algal Res., 2022, 67, 102857, DOI:10.1016/j.algal.2022.102857
.
- S. Pereira and A. Otero, Effect of light quality on carotenogenic and non-carotenogenic species of the genus Dunaliella under nitrogen deficiency, Algal Res., 2019, 44(November), 101725, DOI:10.1016/j.algal.2019.101725
.
- M. H. Morowvat and Y. Ghasemi, Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture, Biocatal. Agric. Biotechnol., 2016, 7, 217–223, DOI:10.1016/j.bcab.2016.06.008
.
-
R. R. L. Guillard, Culture of phytoplankton for feeding marine invertebrates, Culture of Marine Invertebrate Animals, 1975, pp. 29–60, DOI:10.1007/978-1-4615-8714-9_3
.
- V. Sousa, L. Loureiro, G. Carvalho and R. N. Pereira, Extraction of biomolecules from Coelastrella sp. LRF1 biomass using Ohmic Heating technology, Innovative Food Sci. Emerg. Technol., 2022, 80(December 2021), 103059, DOI:10.1016/j.ifset.2022.103059
.
- E. G. Bligh and W. J. Dyer, A rapid method of total lipd extraction and purification, Can. J. Biochem. Physiol., 1959, 37(8), 911–917, DOI:10.1139/o59-099
.
- P. Geada, D. Francisco, F. Pereira, F. Maciel, L. Madureira and A. Barros,
et al., Multivariable optimization process of heterotrophic growth of Chlorella vulgaris, Food Bioprod. Process., 2023, 138, 1–13 CrossRef CAS
.
- N. Sanz, A. García-Blanco, A. Gavalás-Olea, P. Loures and J. L. Garrido, Phytoplankton pigment biomarkers: HPLC separation using a pentafluorophenyloctadecyl silica column, Methods Ecol. Evol., 2015, 6(10), 1199–1209 CrossRef
.
- F. Maciel, P. Berni, P. Geada, J. Teixeira, J. Silva and A. Vicente, Identification and optimization of the key growth parameters involved in carotenoids production of the marine microalgae Pavlova gyrans, Sci. Rep., 2024, 17224, DOI:10.1038/s41598-024-66986-y
.
-
A. E. Greenberg, L. S. Clesceri and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 18th edn, 1992, vol. 552 Search PubMed
.
- F. Elleuch, H. B. Hlima, M. Barkallah, P. Baril, S. Abdelkafi and C. Pichon,
et al., Carotenoids overproduction in Dunaliella sp.: Transcriptional changes and new insights through lycopene cyclase regulation, Appl. Sci., 2019, 9(24), 5389, DOI:10.3390/app9245389
.
- P. Sanpapao, W. Tawong, P. Pongpadung, P. Ponza and S. Ponza, Effect of phosphorus and sodium chloride levels on growth performance, carotenoid accumulation and isomerization to 9-cis β-carotene in Thai Dunaliella salina NUAC09, Journal of Biological Research - Bollettino Della Società Italiana Di Biologia Sperimentale, 2023, 15, 27–38, DOI:10.22034/IAR.2023.1968808.1336
.
- H. Lv, X. Cui, Z. Tan and S. Jia, Analysis of metabolic responses of Dunaliella salina to phosphorus deprivation, J. Appl. Phycol., 2017, 29(3), 1251–1260 CrossRef CAS
.
- W. Fu, G. Paglia, M. Magnúsdóttir, E. A. Steinarsdóttir, S. Gudmundsson and B. Ø. Palsson, Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina, Microb. Cell Fact., 2014, 13, 3, DOI:10.1186/1475-2859-13-3
.
- W. Fu, Ó. Guomundsson, G. Paglia, G. Herjólfsson, Ó. S. Andrésson and B. O. Palsson,
et al., Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution, Appl. Microbiol. Biotechnol., 2013, 97(6), 2395–2403 CrossRef CAS PubMed
.
- E. Bermejo, M. C. Ruiz-Domínguez, M. Cuaresma, I. Vaquero, A. Ramos-Merchante and J. M. Vega,
et al., Production of lutein, and polyunsaturated fatty acids by the acidophilic eukaryotic microalga Coccomyxa onubensis under abiotic stress by salt or ultraviolet light, J. Biosci. Bioeng., 2018, 125(6), 669–675 CrossRef CAS PubMed
.
- Y. Xie, S. H. Ho, C. N. N. Chen, C. Y. Chen, I. S. Ng and K. J. Jing,
et al., Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation, Bioresour. Technol., 2013, 144, 435–444 CrossRef CAS PubMed
.
- M. S. Kadri, R. R. Singhania, G. S. Anisha, N. Gohil, V. Singh and A. K. Patel,
et al., Microalgal lutein: Advancements in production, extraction, market potential, and applications, Bioresour. Technol., 2023, 389, 129808, DOI:10.1016/j.biortech.2023.129808
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00229f |
| This journal is © The Royal Society of Chemistry 2024 |