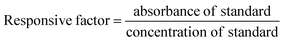Open Access Article
Open Access ArticleA two-stage extraction model for simultaneous extraction of essential oil and phenolics from tulsi leaves: implementing a blended mode microwave hydrodiffusion and gravity (MHG) model
Souvik
Mukherjee
a,
Monika
Chandrakar
a,
Pragya
Gupta
a,
Altamash
Khan
a,
Riya
Pal
a,
Apoorva
Dwivedi
a,
Kavi Bhushan Singh
Chouhan
ab,
Sinchan
Das
a,
Arjun
Patra
a and
Vivekananda
Mandal
 *a
*a
aDepartment of Pharmacy, Division of Pharmacognosy (Green Extraction of Botanicals Research Group), Guru Ghasidas Central University, Bilaspur, Chhattisgarh 495009, India. E-mail: v.mandal@ggu.ac.in
bDepartment of Pharmacognosy, Chhatrapati Shivaji Institute of Pharmacy, Durg, Chhattisgarh 491001, India
First published on 29th August 2024
Abstract
The work is based on implementing a blend of high, medium, and sustained low power microwave heating for the extraction of essential oil from tulsi leaves using the principle of MHG. The blended mode was implemented to target the simultaneous extraction of essential oil and non-volatile principles (phenolics) from the same biomass through a two-stage process. The first stage dealt with the extraction of essential oil using an optimized MHG protocol comprising of a blend of high- (510 W) and medium-power (340 W) microwave surges of 5 min each, followed by the completion of the experiment with low power microwave (170 W). The yield of essential oil obtained from the optimized MHG protocol (50 min) was found to be 5% w/w. On the other hand, MHG with single-power microwaving at 170 W (60 min), 340 W (40 min) and 510 W (25 min) produced yields of 1.9%, 2.9% and 1.0% w/w, respectively. Hydrodistillation (240 min) could achieve a yield of 1.9% w/w only. As per gas chromatography results, the % area of eugenol content was found to be 16.64%, slightly higher than the 15.45% obtained from hydrodistillation. The second stage was about retention capabilities of the biomass with reference to the non-volatile components. The total phenolic content of the leftover biomass after the MHG blended mode protocol was found to be 6.1 mg GAE per g of dried extract, which was more than the control (untreated) sample that retained a phenolic content of 5.4 mg GAE per g of dried extract. However, biomass obtained after hydrodistillation showed a severe depletion of phenolic content (1.9 mg GAE per g of dried extract). Thus, MHG (blended mode) allows the extraction of essential oil in the first stage, followed by the extraction of non-volatile compounds from the same biomass in the second stage, ensuring judicious and exhaustive use of plant biomass.
Sustainability spotlightTechnology can be made sustainable only when it is aligned with the environment; otherwise, it is likely to perish over time. To be sustainable, a technology should ideally be clean, green and eco-friendly, with reduced carbon emissions. Today, with the world increasingly concerned about carbon emissions and many countries are targeting zero carbon emissions, it is crucial that our innovations align with this objective. Drastic and unpredictable climate changes result from excessive carbon load, which threatens the existence of coastal countries. The extraction of botanicals is the starting point of the herbal production line and medicinal plant research. It is important to use biomass judiciously and ensure waste recycling to avoid excessive strain on our biodiversity. This extraction model perfectly meets these goals, ensuring the simultaneous extraction of two different classes of bioactive components (volatile and non-volatile compounds) from the same biomass, which otherwise would not have been possible. The task is accomplished in less time with improved yield compared to traditional hydrodistillation. The work is aligned with SDG 7: Affordable and clean energy. |
Introduction
Nowadays, consumers prefer using natural resources for promoting health and facilitating healing, which has significantly resulted in increased usage of natural products or plant-based products, with special emphasis on nutraceuticals, antioxidants and essential oils. This inclination is an outcome of growing trust resulting in reliance on various natural products. As interesting natural substitutes for synthetic chemicals, essential oils are becoming increasingly popular in the food, beverage, cosmetics, aromatherapy, and pharmaceutical industries.1–4 Multiple studies have demonstrated that essential oils possess a diverse range of biological activities, such as antioxidant, antibacterial, antiviral, antiaging, anti-inflammatory, anticancer, and anti-tyrosinase effects.1,4 No herbal drug industry is devoid of an extraction line. But when it comes to extraction, manufacturers prefer to traditional methods even if there is lack of automation, low yield and increased operational time. As far as the extraction of essential oils is concerned, hydrodistillation and steam distillation are two globally respected traditional methods. However, in this era of artificial intelligence and digital awakening, working with methods that do not support automation and involve long operational time along with excessive carbon load and thermal threat is considered inefficient.5 To overcome these limitations, a novel technique called solvent-free microwave-based extraction (SFME) has been implemented widely and has gained the research interest of researchers globally. This methodology is regarded as a cutting-edge and very promising method for extracting essential oils. At atmospheric pressure conditions, SFMAE utilizes the synergistic effects of microwave heating and distillation without the need for water or organic solvents.6 This method has significant benefits, including enhanced heating efficiency, rapid energy transfer, time efficiency, and cost-effectiveness, and is regarded as an environmentally sustainable green approach for extracting essential oils from plants.7 There are basically two variants available for SFME. In the first variant, a traditional distillation setup is used in which fresh plant material is loaded without adding any solvent.8 The natural moisture content of the plant material acts as a medium for the absorption of microwaves and thus generates heat inside the biomass. In the other method, which is popularly known as microwave hydrodiffusion and gravity (MHG), fresh biomass is kept inside an extraction vessel that is placed in an inverted position. When subjected to microwaves, heat is generated inside the biomass due to the absorption of microwaves and the contents (oil and water vapours) move downward. A vivid illustrative description of these methods is given by the authors in their previously published research articles.9 With a focus on sustainability, this work specifically aims to address the above-mentioned issues by implementing a unique heating strategy involving the MHG model for the extraction of essential oil from tulsi leaves (Ocimum gratissimum). Notably, according to the prominent Indian business journal Economic Times, the worldwide market for tulsi oil was US$ 724.4 million in 2023.10 Experts forecast the market to reach a value of US$ 1107.4 million by 2030. Therefore, the significant involvement of commerce and trade in this product demands innovative interventions and the development of a robust green method for the extraction of essential oil. The main objective of this study is to strategize MHG through a blended mode microwave heating approach so that both essential oil and phenolic compounds are extracted from the same biomass simultaneously. Furthermore, the Paris Convention on Climate Change, which has adopted strategies addressing carbon emissions, may be sustained only when environment-friendly technologies are developed, encouraged and adopted. The work also aligns with the resolutions passed at the United Nations Paris Convention on Climate Change and the Sustainable Development Goals proposed by the UN.Materials and methods
Plant material
Fresh fully grown leaves of Ocimum gratissimum were collected locally and used for extraction. Leaves were identified by a taxonomist from the host university, and a voucher specimen number of BOT/GGV/2022/48 was assigned. The leaves were washed with distilled water to get rid of any surface dirt and subjected to extraction after physically draining excess water. Based on the loss-on-drying method, the moisture content in the fresh sample was found to be 78.0%.Chemicals
The Folin reagent used for the determination of total phenolic content and methanol (analytical grade) were purchased from Central Drug House Private Limited, Mumbai. 2,2-Diphenylpicrylhydrazyl (DPPH) was purchased from Sigma Chemicals (St. Louis, MO, USA). High-Performance Thin Layer Chromatography (HPTLC) plates from Merck were used for chromatographic studies.Apparatus
A commercial microwave extractor (CATA R-invert) manufactured by catalyst systems was used to execute the MHG protocol. The extractor was equipped with a magnetron operating at a frequency of 2450 MHz and 850 W nominal (maximum) power. The extraction chamber comprised a 1 L glass extraction vessel placed in an inverted position and connected to a condenser operated below the extraction chamber. The condenser was fed with cold water from a recirculating chiller operated at a temperature of 10 °C. The condenser, in turn, led to a florentine flask in which the essential oil and water were collected and eventually separated. As the oil oozed out of the ruptured oil glands, gravity pulled it through the condenser, and it was collected in a container attached to the bottom.An HPTLC system from Camag (Switzerland) equipped with a Linomat 5 injector and thin layer chromatography (TLC) scanner 3 (WinCATS version 1.4.4) was used for analytical quantification.
Extraction protocols
MHG protocol for the implementation of blended mode microwave heating
Fresh tulsi leaves were washed as stated above, weighed accurately to 180 g and loaded into the extraction vessel without the addition of any solvent. The MHG strategy was carried out in a blended mode; a mix of high-, medium- and low power microwaving was carried out in three controlled microwave firing phases. In the first phase, a high power microwave surge of 510 W was applied for 5 min followed by a 2 min cooling period. The second phase consisted of microwave firing at 340 W microwave power for 5 min followed by 2 min of cooling. In the third phase, low power microwave firing at 170 W was continued till the completion of the extraction in short cycles of 5 min each with intermittent cooling periods of 2 min each. The biomass was not changed during the stages of blended mode operation. The rationale behind the blended mode operation is to facilitate the quick and immediate rupture of oil glands due to the development of sudden thermal stress under controlled high-and medium-power microwaving, followed by smooth extraction at 170 W microwave power. The extraction was considered complete when no visible drops of oil were observed. The oil was collected over a 15% brine solution kept in a florentine flask prior to the beginning of the extraction process to facilitate quick separation of the oil and water layers and provide a soft landing to the condensing oil droplets in the florentine flask. The collected oil was allowed to stand overnight and then dried over anhydrous sodium sulphate (Fig. 1). The oil was also kept in the refrigerator at 0 °C overnight to ensure that the oil was totally moisture-free. The extraction yield was calculated as per the following equation. | (1) |
Conventional hydrodistillation (HD)
Hydrodistillation from 180 g of fresh leaves was carried out for 4 h using a classical Clevenger apparatus as the collection device. The condenser of the Clevenger apparatus was connected to a recirculating chiller operated at 10 °C. The oil collected at the end of the extraction process was allowed to stand overnight, then dehydrated by passing through anhydrous sodium sulphate, and stored at 4 °C in amber-coloured bottles.6Post-MHG biomass treatment
The biomass obtained after MHG and steam distillation was dried (2–3 days) and then subjected to maceration for the extraction of non-volatile principles with special reference to phenolics and flavonoids. A control was also prepared using dried untreated leaves (180 g) for comparison. Maceration was performed for 24 h with occasional stirring for the first 6 hours using 150 mL methanol as the solvent. At the end of the maceration step, the extract thus obtained was partitioned thrice with petroleum ether (60–80 °C grade) to remove any colouring or waxy material, which could serve as potential interfering substances during the analysis. The petroleum ether layer was discarded, and the semi-purified methanol layer was dried and weighed.Gas chromatography-flame ionization detection (GC-FID) analysis
The GC-FID analysis was carried out using a Shimadzu Nexis GC-2030 gas chromatograph equipped with a DB-5ms column (30.0 m × 0.25 mm ID, film thickness 0.25 μm) and an FID detector operated at 300 °C. Helium (99.99%) was used as the carrier gas at a flow rate of 1.0 mL min−1. The analyses were performed by injecting 1.0 μL of the sample at a split ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The oven temperature was programmed as follows: 40 °C for 3 min, 4 °C min−1 up to 220 °C and then 240 °C for 5 min.9,11
1. The oven temperature was programmed as follows: 40 °C for 3 min, 4 °C min−1 up to 220 °C and then 240 °C for 5 min.9,11
Thermogravimetric analysis (TGA)
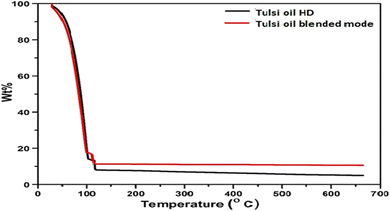
Using the PerkinElmer TGA 4000 instrument, the thermogravimetric analysis of tulsi oil obtained from MHG and HD was performed in a nitrogen environment by heating from 30 °C to 600 °C at a rate of 5 °C min−1. The general data were used to measure weight loss.12Dot blot analysis (TLC bioautography) of the oil sample
Through TLC bioautography, the antioxidant potential of the extracted essential oil was tested on a real-time basis on the HPTLC plates. Silica-precoated HPTLC plates measuring 10 cm × 5 cm were utilized for this experiment. Tulsi oils (undiluted) obtained from HD and MHG were spotted on the precoated HPTLC plates using a capillary tube. The plates were developed using toluene–ethyl acetate–formic acid (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the mobile phase. The developed plate was then dipped in a purple DPPH solution (0.1 mM). While the entire plate was stained purple, yellow spots were visualized in some areas where the purple colour was bleached by the antioxidant compounds. Thus, the appearance of yellow spots directly indicated the presence of antioxidant compounds in those areas, and the intensity of the yellow colour is directly proportional to the concentration of the antioxidant compounds.9,13
1 v/v) as the mobile phase. The developed plate was then dipped in a purple DPPH solution (0.1 mM). While the entire plate was stained purple, yellow spots were visualized in some areas where the purple colour was bleached by the antioxidant compounds. Thus, the appearance of yellow spots directly indicated the presence of antioxidant compounds in those areas, and the intensity of the yellow colour is directly proportional to the concentration of the antioxidant compounds.9,13
Field-emission scanning electron microscopy (SEM)
The SEM images of the following dried and powdered tulsi leaf samples were obtained: (a) control sample: dried untreated tulsi leaves, (b) MHG: leftover biomass after MHG, and (c) HD: leftover biomass after HD.Every specimen was analyzed by a JEOL JSM-7610F (Tokyo, Japan) scanning electron microscope under a high-vacuum condition at an accelerating voltage of 5.0 kV using a Schottky-type field-emission (T-FE) electron gun.14
Total phenolic content (TPC) determination
The total phenolic content in the extract obtained from the post-MHG biomass was determined by using the Folin–Ciocalteu reagent method, as described by the authors in their previous publications.9 TPC was expressed as gallic acid equivalent (GAE) mg per g of dried extract. For gallic acid, the calibration curve (y = 0.036x − 0.012, r2 = 0.995) was established by preparing standard solutions of gallic acid (10, 20, 40, 60 and 80 μg mL−1 in methanol).HPTLC analysis
The tulsi extracts obtained from the leftover biomass after SFME and HD were subjected to HPTLC for the identification and quantification of phenolic principles (biomarkers), namely gallic acid, chlorogenic acid, quercetin, and p-coumaric acid, as per the method described by the authors in their previous publications.9,14 The Rf values of the peaks extracted from the samples were compared to those of external standards (biomarkers). Methanolic solutions of the tulsi extracts at a concentration of 10 mg mL−1 were applied onto precoated silica gel 60 F254 aluminium plates (10 cm × 10 cm), positioned 10 mm from the bottom and 15 mm from the side of the plate, with an 8 mm bandwidth, using a Camag Linomat 5 automated TLC applicator under a nitrogen flow providing a delivery speed of 150 nL s−1 from the syringe. The mobile phase used was toluene–ethyl acetate–formic acid (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the plate was developed till 8 cm at room temperature (25 ± 2 °C). Quantification was carried out by measuring absorbance at 254 nm, with a slit width of 6 × 0.45 μm at a data resolution of 100 μm per step and a scanning speed of 10 mm s−1 using a computerized TLC scanner-3. The results were recorded and analyzed using WinCATS software version 1.4.4.
1), and the plate was developed till 8 cm at room temperature (25 ± 2 °C). Quantification was carried out by measuring absorbance at 254 nm, with a slit width of 6 × 0.45 μm at a data resolution of 100 μm per step and a scanning speed of 10 mm s−1 using a computerized TLC scanner-3. The results were recorded and analyzed using WinCATS software version 1.4.4.
Antioxidant activity assay
The antioxidant activity of the extract was measured using the DPPH (2,2-diphenylpicrylhydrazyl) method. A 0.1 mM solution of DPPH and the sample solution (100 μg mL−1) were mixed at a fixed concentration. The mixture was incubated for 30 min at room temperature in the dark. The absorbance was recorded at 517 nm. The % scavenging activity was calculated as mentioned in the below equation.9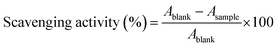 | (2) |
Total protein and carbohydrate content determination
Leaf samples (1 g of marc after oil extraction) from each treatment (MHG & HD) were homogenized in 2 mL of 50 mM Tris HCL buffer at pH 7.2. To this, 0.1 mM ethylenediamine-tetra acetic acid (EDTA) was added along with 1% polyvinylpyrrolidone at 4 °C. For protein estimation, the crude supernatant was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. Protein concentration was determined by the Bradford method. The absorbance at 595 nm was measured using a spectrophotometer. Bovine serum albumin was used for the calibration curve. According to eqn (3), the protein concentration was calculated as
000 rpm for 15 min. Protein concentration was determined by the Bradford method. The absorbance at 595 nm was measured using a spectrophotometer. Bovine serum albumin was used for the calibration curve. According to eqn (3), the protein concentration was calculated as | (3) |
The estimation of carbohydrates was done using the anthrone reagent as per the method described by Chouhan et al., 2019 with glucose as the external standard.6 In a test tube, 500 μL of the sample solution, 500 μL of distilled water, and 4 mL of anthrone reagent were pipetted out; then, the solution was mixed on a vortex mixer for 2–3 min. The standards were prepared in the same manner as the test samples. Their absorbance at 620 nm was measured using a UV spectrophotometer. The carbohydrate content was calculated using eqn (4).
 | (4) |
Statistical analysis
All experiments were carried out in triplicate, and the means were compared using the Student's t-test and Duncan multiple range test. P values < 0.05 are considered significant. All statistical analyzes were performed using the free online statistical software Graph Pad Prism version 7.0.Results and discussion
Impact of blended mode MHG on the extraction yield and extraction time
The results (in terms of % yield) indicated in Fig. 2 clearly demonstrate that the maximum yield was obtained under blended mode MHG operation using a strategic mix of high-, medium- and low power microwaves compared to fixed-power microwave treatment and HD. The yield obtained with HD was found to be 10 times less than that obtained using the blended mode MHG protocol. As far as single-power microwaving was concerned, all tested microwave powers proved less effective than the blended mode microwaving strategy, with the 170 W microwave power (lowest power level used) producing less than half the yield obtained using the blended mode. The yield obtained at the highest microwave power level (510 W) was found to be the minimum for all microwave-based protocols. The above findings indicate that operation at a low power level may not be enough to recover the essential oil in totality, which is evident from the low yield of the essential oil obtained using the 170 W fixed microwave power.15 Low microwave power may not be able to generate sufficient heat to cause the rupture of oil glands and the subsequent release of oil. Moreover, less steam (due to the evaporation of in situ moisture content) is formed at low microwave power, resulting in insufficient force to drive the diffusion of the essential oil from the inside of the matrix to the outside environment.12 All these issues lead to low yields. Notably, the situation did improve in terms of yield while 340 W microwave power was used but it did not prove to be the optimal operating condition.Operation at a fixed high microwave power (510 W) generates intense heat due to localized thermal stress. The intense heat generated within the plant matrix causes the decomposition and combustion of the essential oil.14,15 A burning smell could be felt and the burning of biomass also occurred at high microwave powers. It must be noted that burning of biomass will render it unsuitable for any further use and hence is not a desirable situation with regard to the objective of this research work.
Blended mode microwave operation presents a perfect combination of high, medium, and low microwave power levels. The initial high power creates a sudden rise in temperature, leading to localized thermal stress, which is sufficient to impact the oil glands and release the essential oil. The medium microwave power 340 W facilitates the diffusion of the oil from the inner matrix along with sufficient steam generation to drive the oil towards the condenser. The subsequent low power microwave prevents any further degradation of the oil and facilitates the extraction process to its completion state. On the other hand, if continuous high power is applied, degradation and combustion of the oil are certain, which is evident from the reduced yield at 510 W microwave power.16,17
As for extraction time, Fig. 3 clearly indicates that operation at a low microwave power would require more time for extraction than at a high microwave power level. It can be clearly observed that when the microwave power was increased to 340 W, the operation time was reduced by 18%. A further increase in microwave power to 510 W reduced the operation time by more than 35%. In this case, operation time was considered as the time period till visible collection of oil/water drops occurs. Extraction was deemed complete when no more visible drops were seen. At this juncture, the operation time was considered as the viable extraction time (visible appearance of oil/water drops), and no conclusion was obtained at this stage regarding the quality of the oil obtained. Any questions/concerns regarding the quality of oil were dealt with in the subsequent experiments.
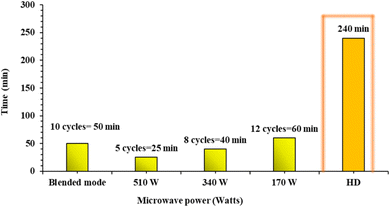 | ||
| Fig. 3 Number of extraction cycles required for tulsi oil extraction by different methods (1 cycle = 5 min). | ||
Determination of oil quality
Increased yield and reduced operation time alone do not dictate the success of blended mode MHG heating. Only the quality of the oil can provide conclusive evidence. In order to adjudge the quality of the extracted essential oil, the identification of volatile biomarkers, thermal analysis and antioxidant activity check were used as the basic parameters, and the findings are explained below.| Compound identified | % area | ||||
|---|---|---|---|---|---|
| HD | MHG (blended mode) | 170 W | 340 W | 510 W | |
| α-Pinene | — | 25.2 | — | — | — |
| β-Myrcene | 24.2 | 33.0 | 32.5 | 17.2 | — |
| Sabinene | — | 21.4 | 21.8 | — | — |
| Limonene | — | 25.3 | 25.4 | — | — |
| Eugenol | 15.5 | 18.6 | — | 16.6 | — |
| Geraniol | 21.2 | 24.4 | — | 19.9 | — |
Furthermore, no peak of any volatile principles was identified at 510 W microwave power. This clearly indicates that when extracted at a high microwave power, such as 510 W, the essential oil can undergo combustion and degradation.21 Oxygenated compounds are highly interactive with microwaves and hence undergo tremendous heating at high microwave powers due to their polar nature.22 Operation at higher microwave powers also produced a burned odour due to the charring of the biomass. The oil obtained by HD showed the presence of the following volatile principles: 24.21% β-myrcene, 15.45% eugenol, and 21.16% geraniol%. The content of the major constituent eugenol was found to be significantly high in the essential oil obtained by the blended mode of microwave operation, which clearly indicates the superiority of this extraction method in terms of the quality of the oil obtained compared with the oil obtained by HD.
The oil obtained by the blended mode MHG method showed the presence of oxygenated terpenes, such as eugenol and geraniol, which are likely to contribute to its biological activity. It is a well-established fact that oxygenated compounds are predominantly responsible for the flavour/fragrance and biological activity of any essential oil. The dot blot analysis (Fig. 5) revealed yellow antioxidant zones on the TLC plate for the oil sample obtained by MHG (blended mode). Meanwhile, the oil obtained by HD showed only faint yellow zones on the TLC plate. When dipped in a 0.4 mM DPPH solution, the entire plate turned purple, and the spots with antioxidant activity were bleached and appeared yellow, reflecting positive antioxidant activity. The intensity of the yellow colour was directly proportional to the antioxidant activity of the compound.24,25 In this experiment, the antioxidant activity was considered to indicate its biological activity, assuming that, if the oil can retain its biological activity, then it is likely to retain its other activity as well. These findings clearly indicate that the oil extracted by the MHG (blended mode) method is not only rich in terms of volatile principles but also possesses improved biological activity (evaluated in terms of antioxidant potential) compared with the oil obtained by HD.
Elucidating the mechanism of action through SEM analysis
Fig. 6 shows the physical structural changes in the oil glands of tulsi leaves after microwave extraction and HD. The images clearly demonstrate total rupture of the oil glands during SFME, allowing gravity to drain the oil from the ruptured glands. During HD, the oil glands were squeezed, wrinkled, and reduced in size but no conclusive evidence of rupture was visible compared with the intensity of the massive bursting of oil glands observed during the microwave treatment. As a result of the internal heating of the in situ water, pressure builds up in the oil glands, causing a massive bursting. The in situ water absorbs the microwaves and heats up tremendously, and the vapours formed increase the pressure on the inside, and when this pressure exceeds the expanding ability of the oil glands, massive ruptures are caused. As a result of volumetric heating of the internal organic molecules present in the glands and vascular systems, thermal stress is also produced.26,27 However, the rupture of oil glands was not observed in the case of HD since it operates on the principle of heat transfer governed by the convection and conduction theories. Several researchers have reported similar disruptions of oil glands due to intense microwave heating.28 During MHG, heat and mass transfers (transfer of oil) take place from the inside of the oil gland to the outer surface of the matrix, while in HD, both transfers occur in the opposite direction. As heat and mass transfer are synergistic in MHG, the acceleration is magnified. The two phenomena antagonize each other and slow down the extraction process during HD.22Determination of biomass integrity
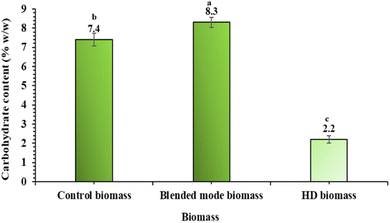
The quality of the biomass obtained after oil extraction was assessed to ascertain its reusability factor. MHG is a gentle and exhaustive process of oil extraction, hence the biomass obtained after oil extraction is likely to retain its non-volatile principles and can be further used for the extraction of phenolics and flavonoids. In order to determine the quality of the biomass, the following parameters were evaluated, and the findings are presented below.The biomass obtained after MHG (blended mode), on the other hand, showed remarkable results in terms of TPC. The TPC of the biomass was 13% more than that of the control (Fig. 7). The biomass exposed to microwave during oil extraction undergoes cellular disruption, as evident from the SEM studies. Such cellular disruption serves as a direct exit route for the phenolics and flavonoids present inside, thus allowing oil extraction with an improved quantity of phenolics and flavonoids compared with the control. During the process of MHG, the biomass does not lose its morphological integrity, and only the volatile principles are extracted in the process. Since no solvent is used in the process of extraction, the phenolic and flavonoid principles and other solid phytoconstituents do not undergo any leaching and remain safe in the biomass. These findings strongly indicate that MHG is a targeted extraction process for essential oil and does not compromise the non-volatile principles present in the biomass. Even after the extraction of essential oils, the biomass can be made available for the extraction of other non-volatile principles, such as phenolics and flavonoids. The findings of this work are similar to a study on the simultaneous extraction of volatile and non-volatile antioxidants from rosemary using MHG.29 This extraction strategy ensures the simultaneous extraction of different classes of compounds, leading to judicious utilization of biomass.
| S. no. | Sample name | Identified phenolics | AUC | Cumulative area |
|---|---|---|---|---|
| 1 | Biomass leftover after MHG (blended mode) | Chlorogenic acid | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 998.9 998.9 |
5284.9 |
| p-Coumaric acid | 2071.1 | |||
| 110.7 | ||||
| 460.0 | ||||
| 1783.7 | ||||
| 2 | Control sample (untreated) | Chlorogenic acid | 7954.0 | 3618.7 |
| p-Coumaric acid | 255.1 | |||
| 2801.7 | ||||
| 3464.0 | ||||
| 3 | Biomass leftover after HD | Chlorogenic acid | 555.8 | 923.5 |
| p-Coumaric acid | 1872.7 | |||
| 1133.3 | ||||
| 132.2 |
Conclusion
A strategically designed blended mode MHG protocol for two-stage extraction is proposed for the simultaneous extraction of volatile and non-volatile components from the same biomass. In the first stage, the essential oil (volatile component) was extracted using a strategically crafted blend of high (510 W) microwave power for 5 min, followed by medium (340 W) microwave power for 5 min and finally a prolonged low power (170 W) microwave treatment in cycles of 5 min each till the completion of extraction until no visible appearance of oil drops. In the second stage, the same biomass was dried and used for the extraction of phenolic principles (non-volatile components), and the results were comparable to that of the control sample (untreated sample which did not undergo any extraction process). On the other hand, the biomass subjected to HD reflected a massive depletion of phenolic principles, which may have degraded during the HD process or leached out into the boiling water. Therefore, the blended mode MHG protocol clearly reflects the extraction possibility of two widely varying classes of bioactive principles from the same biomass. This method ensures judicious use of biomass and can reduce unnecessary load on the biodiversity of a particular region. A comparative table depicting the performance of MHG (blended mode) and HD is presented in Table 3. In light of growing awareness towards environmental issues, it is very important to understand that every technological innovation must have a green edge so that it can be sustainable in the long run. Anything that does not align with the environment is likely to perish with time.30 In this regard, the MHG variant of SFME stands out as a promising technique for essential oil extraction from aromatic plants and has all the traits to replace traditional distillation. However, it should be borne in mind that the composition of essential oil varies from plant to plant, and hence, the optimized MHG condition for one plant may not be applicable for another plant and there is no universal operating condition for MHG. Henceforth, it is of prime importance that optimal conditions for every essential-oil-bearing plant. Currently, the technology is in its infancy and may take a few years to mature based on growing research evidence. Solvent-free extraction is no more a jargon in the field of essential oil extraction and is likely to gain prominence in the coming years. The market of essential oil is equally promising due to its wide scope of applicability. Therefore, such technological interventions as MHG can make us future-ready in a greener way to meet the increasing demand for essential oils.| Performance parameters | Extraction methods | |
|---|---|---|
| MHG (blended mode) | HD | |
| a Data marked by different alphabets row-wise are significantly different at p < 0.05. | ||
| Performance comparison | ||
| Extraction time (min) | 50.0 | 240.0 |
| Number of extraction cycle | 10.0 | NA |
| Yield of oil (wt%) | 5.0 ± 0.2a | 0.5 ± 0.2b |
| Antioxidant zone intensity | 546.1a | 353.1b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Profile of leftover biomass | ||
| TPC of biomass (mg GAE per gm of dried extract) | 6.1 ± 0.2a | 1.9 ± 0.2b |
| % scavenging activity | 86.8 ± 2.6a | 10.0 ± 1.7b |
| Carbohydrate content (% w/w) | 8.3 ± 0.3a | 2.2 ± 0.2b |
| Protein content (mg gm−1 of fresh weight) | 27.4 ± 1.1a | 13.0 ± 0.7b |
| SEM | Ruptured | Squeeze |
Data availability
The datasets used and/or analysed during the present study are included in this article.Author contributions
Souvik Mukherjee: conceptualisation: equal; data curation: equal; formal analysis: equal; investigation: lead; methodology: equal; software: lead; validation: equal; visualization: equal; writing—review and editing: supporting. Monika Chandrakar: conceptualisation: supporting; formal analysis: supporting; methodology: supporting; writing—review & editing: supporting. Pragya Gupta: conceptualisation: supporting; formal analysis: supporting; methodology: supporting; writing—review & editing: supporting. Kavi Bhusan Singh Chouhan: data curation: supporting; formal analysis: supporting; investigation: supporting; software: supporting. Altamash Khan: conceptualisation: supporting; methodology: supporting; writing—original draft: equal. Riya Pal: conceptualisation: supporting; data curation: supporting; methodology: supporting; writing—original draft: supporting. Sinchan Das: methodology: supporting; software: supporting. Arjun Patra: conceptualisation: supporting; methodology: supporting; resources: supporting; supervision: supporting; writing—review and editing: supporting. Vivekananda Mandal: conceptualisation: lead; data curation: lead; formal analysis: lead; funding acquisition: lead; investigation: equal; methodology: lead; project administration: lead; resources: lead; software: supporting; supervision: lead; validation: equal; writing—original draft: equal; writing—review and editing: lead.Conflicts of interest
The authors declare that there is no conflict of interest associated with this article.Acknowledgements
The authors wish to thank the host Guru Ghasidas Central University (accredited by NAAC with the highest grade of A++) for providing infrastructural and research support. The authors are equally thankful to the Internal Quality Assurance Cell (IQAC) of the host university for removing various research hurdles and ensuring a quality research ecosystem within the University. Support from Birbal Sahni Institute of Palaeosciences, Lucknow, for SEM studies (Dr Subodh Kumar, SEM In-charge) and Central University of Punjab, Bhatinda, for GC-FID studies (Mr Ajit Paul Singh) are deeply acknowledged.References
- F. Bakkali, S. Averbeck, D. Averbeck and M. Idaomar, Food Chem. Toxicol., 2008, 46, 446–475 CrossRef CAS PubMed.
- M. Chenni, D. El Abed, N. Rakotomanomana, X. Fernandez and F. Chemat, Molecules, 2016, 21, 113 CrossRef PubMed.
- N. Nasim, A. Ray, S. Singh, S. Jena, A. Sahoo, B. Kar, I. S. Sandeep, S. Mohanty and S. Nayak, Nat. Prod. Res., 2017, 31, 853–856 CrossRef CAS PubMed.
- N. S. Dosoky and W. N. Setzer, Nutrients, 2018, 10, 10–17 CrossRef PubMed.
- S. Mukherjee, K. B. S. Chouhan, M. Chandrakar, P. Gupta, K. Lal and V. Mandal, Crit. Rev. Food Sci. Nutr., 2022, 63, 1–23 Search PubMed.
- K. B. Singh Chouhan, R. Tandey, K. K. Sen, R. Mehta and V. Mandal, J. Cleaner Prod., 2019, 225, 587–598 CrossRef CAS.
- F. Chemat, A. S. Fabiano-Tixier, M. A. Vian, T. Allaf and E. Vorobiev, TrAC, Trends Anal. Chem., 2015, 71, 157–168 CrossRef CAS.
- M. E. Lucchesi, F. Chemat and J. Smadja, J. Chromatogr. A, 2004, 1043, 323–327 CrossRef CAS PubMed.
- S. Mukherjee, K. B. S. Chouhan and V. Mandal, J. Chem. Technol. Biotechnol., 2024, 99, 931–945 CrossRef CAS.
- Economic Times Markets, Global Demand Lifts Basil Tulsi Oil Prices; Outlook Bullish, 2023, https://www.24chemicalresearch.com/download-sample/252681/global-basil-oil-forecast-market-2024-2030-663 Search PubMed.
- M. Z. Hou, L. L. Chen, C. Chang, J. F. Zan and S. M. Du, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2021, 1181, 122904 CrossRef CAS PubMed.
- S. Mukherjee, K. B. S. Chouhan, A. Khan, S. Meshram, S. C. Srivastava, S. Das, V. Yadav, A. Patra and V. Mandal, Biomass Convers. Biorefin., 2024, 24, 1–19 Search PubMed.
- K. B. S. Chouhan, S. Mukherjee and V. Mandal, Sustainable Chem. Pharm., 2023, 33, 101113 CrossRef CAS.
- K. B. S. Chouhan, S. Mukherjee and V. Mandal, Sustainable Chem. Pharm., 2022, 29, 100805 CrossRef CAS.
- N. Bousbia, M. Abert Vian, M. A. Ferhat, E. Petitcolas, B. Y. Meklati and F. Chemat, Food Chem., 2009, 114, 355–362 CrossRef CAS.
- K. B. Singh Chouhan, R. Tandey, K. K. Sen, R. Mehta and V. Mandal, Trends Food Sci. Technol., 2019, 92, 12–21 CrossRef CAS.
- J. Song, D. Li, C. Liu and Y. Zhang, Innovative Food Sci. Emerging Technol., 2011, 12, 282–287 CrossRef CAS.
- K. Bhushan, S. Chouhan, S. Mukherjee, K. Mahato, A. Sinha and V. Mandal, Trends Anal. Chem., 2023, 165, 117131 CrossRef.
- M. E. Lucchesi, J. Smadja, S. Bradshaw, W. Louw and F. Chemat, J. Food Eng., 2007, 79, 1079–1086 CrossRef CAS.
- B. Berka-Zougali, M. A. Ferhat, A. Hassani, F. Chemat and K. S. Allaf, Int. J. Mol. Sci., 2012, 13, 4673–4695 CrossRef CAS PubMed.
- B. Y. Meklati, F. Chemat and M. A. Ferhat, Flavour Fragrance J., 2007, 22, 494–504 CrossRef.
- Z. Liu, B. Deng, S. Li and Z. Zou, Ind. Crops Prod., 2018, 124, 353–362 CrossRef CAS.
- C. H. Ma, L. Yang, Y. G. Zu and T. T. Liu, Food Chem., 2012, 134, 2532–2539 CrossRef CAS PubMed.
- W. Jesionek, B. Majer-Dziedzic and I. M. Choma, J. Liq. Chromatogr. Relat. Technol., 2017, 40, 292–296 CrossRef CAS.
- N. Tabanca, J. Niogret, P. E. Kendra and N. D. Epsky, Biomolecules, 2020, 10, 683 CrossRef CAS PubMed.
- S. Hilali, A. S. Fabiano-Tixier, M. Elmaataoui, E. Petitcolas, A. Hejjaj, F. Aitnouh, A. Idlimam, M. Jacotet-Navarro, A. Bily, L. Mandi and F. Chemat, ACS Sustain. Chem. Eng., 2018, 6, 10969–10979 CrossRef CAS.
- H. Benmoussa, W. Elfalleh, S. He, M. Romdhane, A. Benhamou and R. Chawech, Ind. Crops Prod., 2018, 124, 633–642 CrossRef CAS.
- A. Farhat, C. Ginies, M. Romdhane and F. Chemat, J. Chromatogr. A, 2009, 1216, 5077–5085 CrossRef CAS PubMed.
- D. F. Ferreira, B. N. Lucas, M. Voss, D. Santos, P. A. Mello, R. Wagner, G. Cravotto and J. S. Barin, Ind. Crops Prod., 2020, 145, 112094 CrossRef CAS.
- H. K. Kala, R. Mehta, K. K. Sen, R. Tandey and V. Mandal, TrAC, Trends Anal. Chem., 2016, 85, 140–152 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |