Open Access Article
Open Access ArticleTamarind seed polysaccharides, proteins, and mucilage: extraction, modification of properties, and their application in food
M.
Geethalaxmi
,
C. K.
Sunil
 * and
N.
Venkatachalapathy
* and
N.
Venkatachalapathy

Department of Food Engineering, National Institute of Food Technology and Management-Thanjavur (NIFTEM-T), Thanjavur, Tamil Nadu 613005, India. E-mail: sunil.ck@iifpt.edu.in
First published on 20th September 2024
Abstract
Tamarind seeds, a by-product of the tamarind processing industry, are an excellent source of vital fats and amino acids and they also contain a good amount of carbohydrates and proteins. Apart from their nutritional importance, tamarind seeds are frequently utilized as hydrocolloids due to their capacity to interact with water to form networks and change the rheological properties of food systems. Polysaccharides, proteins, and mucilage are extracted from tamarind seeds using conventional and non-thermal processing techniques (high-pressure processing, sub-critical water extraction, ultrasound, electron beam, gamma irradiation, microwave, and enzyme-assisted extraction). Process conditions significantly contribute to the structural and techno-functional alteration of extracted polysaccharides, proteins, and mucilage. In a variety of food items, including bakery, dairy, confectionery, frozen desserts, beverages, meat, seafood, and so forth, the proteins, mucilage, and polysaccharides derived from tamarind seeds are used as hydrocolloids for stabilizing, thickening, emulsifying, foaming, gelling, and other purposes. The primary focus of this review is on the various extraction methods of tamarind seed polysaccharides, mucilage, and proteins as well as their influence on structural, physicochemical, and techno-functional properties and their application as hydrocolloids in different food products.
Sustainability spotlightTamarind seeds, a by-product of the tamarind processing industry, are an excellent source of vital fats and amino acids and they also contain a good amount of carbohydrates and proteins. Apart from their nutritional importance, tamarind seeds are frequently utilized as hydrocolloids due to their capacity to interact with water to form networks and change the rheological properties of food systems. The proteins, mucilage, and polysaccharides derived from tamarind seeds are used as hydrocolloids and have various applications in food. As a food by-product and a source of fats, proteins and carbohydrates, their valorisation can be explored for the development of different products and application as an ingredient in food. This aligns with the UN SDGs of promoting good health and well-being as well as responsible consumption and production. |
1. Introduction
The word hydrocolloid originates from Greek, where ‘hydro’ implies water, and ‘kola’ implies glue. An alternative name for hydrocolloids is “food gums”. Hydrocolloids belong to the broad family of long-chain polymers, such as proteins and polysaccharides, characterized by their capacity to form gels and thick, sticky dispersions in water. The hydroxyl groups present increase the affinity of hydrocolloids for hydrogen bonding, allowing them to confine water molecules and transfer them into hydrophilic or water-soluble substances. The resulting dispersion between the true solution (particle size less than 1 nm) and suspension (particle size greater than 100 nm) exhibits colloidal quality. These two qualities give rise to the terms “hydrocolloids” or “hydrophilic colloids”.1 Hydrocolloids were developed to be utilized in food systems as stabilizers, thickeners, gelling agents, emulsifiers, and other applications. The main reason for the extensive application of hydrocolloids is their potential to form networks with water and modify rheological properties in food systems. In food systems, this comprises two primary properties, i.e., viscosity or flow behavior and texture. Hydrocolloids are used as food additives with specialized functions since modifying these two aspects of the food system helps to modify the sensory qualities.Hydrocolloids are categorized into three groups based on the source of origin: natural, synthetic, and semi-synthetic. Natural hydrocolloids are water-soluble polymers derived from naturally occurring sources, such as animals (caseinate, gelatin, protein obtained from egg white, and whey), plants (plant starch, pectin, seeds, endosperm gum, trees, tuber, and tree gum exudates), seaweeds (carrageenans, brown and red seaweeds) and microbes (gellan gum, xanthan gum, curdlan, dextran, and cellulose). By modifying natural hydrocolloids, we obtain semi-synthetic hydrocolloids, which are further classified into starch derivatives (phosphorylated starch, hydroxypropylated starch, and acetylated starch) and cellulose derivatives (carboxy methyl cellulose, methylcellulose, microcrystalline cellulose, and hydroxypropyl methyl cellulose). Petroleum-derived materials are used in the chemical synthesis of hydrocolloids to create a product that closely resembles naturally occurring polysaccharides.2
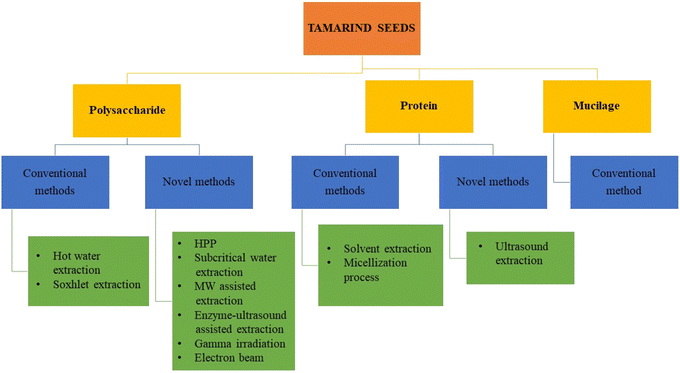
In recent years, compared to hydrocolloids derived from semi-synthetic and synthetic sources, hydrocolloids from natural sources have been commonly used in food industries due to various reasons like safety, health and nutritional benefits, quality attributes, functional properties and stability, easy availability, cost-effectiveness, environmental safety, and suitability for chemical and physical modifications.2 Hydrocolloids can be produced from various non-traditional sources, such as fruit, barks, leaves, mucilage, and seed extracts or exudates.3–5 The effective use of biodegradable waste materials or byproducts from food processing industries helps in ensuring the maintenance of a clean environment, provides a substitute to non-biodegradable materials, increases their economic value by turning them into value-added products, and produces commercially viable biomolecules that serve as an additional means of disposing of and treating waste.2 One such example is the extraction of mucilage, protein, and polysaccharides from tamarind seeds and their subsequent use as a source of hydrocolloids. The conventional and various novel extraction techniques for the utilization of tamarind seeds as hydrocolloids in food are shown in Fig. 1.
Tamarindus indica is the taxonomic name for tamarind, which is categorized under the Leguminosae or Fabaceae family.6 It is claimed that Madagascar is the origin of tamarind. The tamarind tree is more prevalent in Asia, South America, and Africa, especially in India and Thailand.7 It is also grown in countries like Malaysia, Myanmar, and Bangladesh. India stands first in the world in the production and export of tamarind. The tamarind fruit, also known as the tamarind pod, is the mature outer light brown layer. It covers soft pulp that is deep brown or reddish to purple and contains two to ten dark brown seeds per pod. The attachment of seeds and pulp is facilitated by a large fiber network.7 The ripe tamarind fruit or pod comprises 30 to 50% of pulp, 11–30% shell fiber, and 25 to 40% of seed. The pulp is the most economical and frequently used portion of the tamarind fruit. The primary composition of pulp is pectin, reducing sugars, tartaric acid, tannin, cellulose, and fiber. The fresh, ripe tamarind seeds have the following chemical composition: moisture content (20.15–24.50%), total solids (65–77%), tartaric acid (15.84–20.16%), and ascorbic acid (0.68–2%).6 Tamarind seeds are hard and glossy, with shapes varying from oblique to rhomboidal, slightly flattened, and dicotyledonous with thick cotyledons. Physiochemical properties of tamarind seeds are shown in Table 1. Tamarind seeds comprise testa, outer seed coat, which accounts for 20 to 23% and the kernel, also known as the endosperm, which constitutes 70–75%. The seeds also contain a good amount of proteins (13 to 20%), carbohydrates (50 to 57%), and fats (4.5 to 16.2%). The fiber is mainly found in the seed testa, which constitutes about 20%, and it also contains 20% of tannins.9
| Composition | References | |
|---|---|---|
| Physical composition | ||
| Length (mm) | 10.59 | 8 |
| Breadth (mm) | 8.84 | 9 |
| Seed coat (%) | 20–23 | |
| Seed kernel (%) | 70–75 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Proximate composition | ||
| Moisture (%) | 10.75 | 10 |
| Carbohydrate (%) | 53.66 | 11 |
| Protein (%) | 23.06 | |
| Fat (%) | 4.30 | |
| Fibre (%) | 7 | |
| Ash (%) | 2.6 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Amino acid content (g/100 g of protein) | ||
| Aspartic acid | 12.3 | 12 |
| Glutamine | 16.9 | |
| Serine | 5.6 | |
| Glycine | 5.3 | |
| Histidine | 2.3 | |
| Arginine | 7.2 | |
| Threonine | 3.2 | |
| Alanine | 5.0 | |
| Proline | 4.6 | |
| Tyrosine | 4.6 | |
| Valine | 4.9 | |
| Methionine | 1.8 | |
| Isoleucine | 5.0 | |
| Leucine | 8.5 | |
| Phenylalanine | 5.1 | |
| Lysine | 7.6 | |
| Cysteine | 1.7 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Fatty acid content (mg per dry weight) | ||
| Palmitic acid | 0.54 | 13 |
| Stearic acid | 0.17 | 10 |
| Oleic acid | 1.07 | |
| Linoleic acid | 1.65 | |
| Gamma-linolenic acid | 0.014 | |
| Arachidic acid | 0.064 | |
| Eicosadienoic acid | 2.12 | |
| Eicosenoic acid | 3.13 | |
| Behenic acid | 0.39 | |
| Lignoceric acid | 0.51 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Mineral content (mg/100 g) | ||
| Calcium | 285.66 | 11 |
| Magnesium | 86.43 | 14 |
| Potassium | 30.53 | |
| Sodium | 12.63 | |
| Phosphorous | 165 | |
| Copper | 18.97 | |
| Zinc | 3 | |
The composition of fatty acids obtained from tamarind seed kernel oil indicates the presence of fatty acids like oleic acid, palmitic acid, behenic acid, eicosanoic acid, linoleic acid, and lignoceric acid. The fatty acids help to prevent and control various health problems like hypertension and heart and circulatory system-related diseases; they also act as a remedy for constipation and dysentery and exhibit antioxidant effects.6 The quantity of essential amino acids, or necessary amino acids, is appropriately balanced in tamarind seed protein. Excluding a deficit in a few amino acids, seeds have a good amount of lysine, leucine, isoleucine, valine, phenylalanine, and methionine.12,13 Tamarind seeds meet the requirement for three of the eight amino acids which are essential compared to the World Health Organization's protein content requirements. Additionally, it has been discovered that decorticated tamarind seeds have a significant amount of pectin. Rutin, catechin, gallic and ferulic acid are some of the major phenols and flavonoids present in tamarind seeds, which possess various properties like antiviral, antimalarial, anticarcinogenic, and antioxidant properties.15 The current review focuses on analysing various extraction methods for tamarind seed polysaccharides, mucilage, and proteins as well as their influence on structural, physicochemical, techno-functional properties; it also explores their application as hydrocolloids in different food products.
2. Tamarind seed polysaccharides
Plant seed polysaccharides can be broadly categorized into three groups: non-starch endosperm components (like galactomannans); mucilaginous seed coat constituents; and endosperm cell wall material (like xyloglucans).16 Tamarind seed polysaccharides (TSPs) are obtained from the seed endosperm, where xyloglucan is the fundamental constituent of polysaccharides found in the seeds.17 Xyloglucan is the chief cell wall component in higher or vascular plants. In tamarind seeds, it acts as a repository polysaccharide.18 TSP is a high molecular weight galactoxyloglucan that contains glucose, xylose, and galactose in the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. TSP consists of a β-(1-4)-D-glucan backbone with side chains of β-D-xylopyranose and β-D-galactopyranosyl.19 The unique physiochemical characteristics of polysaccharides derived from tamarind seeds in liquid solutions include Newtonian flow behavior under practical conditions; gelling and thickening properties aided by alcohol, polyphenol, and sugar; stability or tolerance against acid, salt, heat, and mechanical shock; and good water retention capacity.17 TSP is used more widely in the food industry as hydrocolloids in various food products and other applications because of its enhanced stability.19
1 ratio. TSP consists of a β-(1-4)-D-glucan backbone with side chains of β-D-xylopyranose and β-D-galactopyranosyl.19 The unique physiochemical characteristics of polysaccharides derived from tamarind seeds in liquid solutions include Newtonian flow behavior under practical conditions; gelling and thickening properties aided by alcohol, polyphenol, and sugar; stability or tolerance against acid, salt, heat, and mechanical shock; and good water retention capacity.17 TSP is used more widely in the food industry as hydrocolloids in various food products and other applications because of its enhanced stability.19
2.1 Tamarind seed mucilage
Glycosidic linkages, glycoproteins, and bioactives mainly connect monosaccharides and uronic acids to form a complex, water-soluble polysaccharide known as mucilage.20 Mucilage, which constitutes around 72% of the seed content of tamarind, is primarily made up of polysaccharides but also includes chemical compounds that have positive effects on health.21 Glucose, galactose, and xylose are the primary polysaccharides that make up tamarind seed mucilage (TSM), which has a molecular weight ranging from 700–880 kDa.22–24In TSM, the dry basis contents of protein, carbohydrates, fat, and ash are 14.78%, 79.76%, 4.76%, and 0.70%, respectively.24 TSM is an inexpensive food ingredient since it has high concentrations of several important amino acids, including valine, phenylalanine, leucine, lysine, methionine, and isoleucine.24 According to Chiang et al. (2021)25 the lipid fraction of mucilage can hold oil, whereas the protein and dietary fiber fractions exhibit their water affinity and gel-forming capabilities.20 It has physicochemical, thermal, rheological, and functional properties, which make it suitable for use as an emulsifying agent and wall material in the process of encapsulating bioactive lipophilic compounds. The mucilage extracted from tamarind seeds has drawn a lot of interest and attention from the scientific community in recent years.22
The extraction techniques are classified into two groups: conventional and novel methods. The impact of different extraction methods on the rheological, functional, and structural characteristics of seeds was investigated. Several procedures, such as dehulling, pulverizing, fat and protein separation, dehydration, etc., are required for the TSP or xyloglucan separation from tamarind seeds. Table 2 summarizes the effect of different extraction methods on the structural and functional properties of TSP, while Table 3 outlines their impact on color properties.
| Method | Processing conditions | Structural & functional modifications | References |
|---|---|---|---|
| Conventional method | |||
| • Hot water extraction | Heating at 100 °C/20 min; 5000 rpm for 20 & 5 min; tray drying at 50 °C/4 h | Reduced viscosity and surface tension | 18 |
| • Soxhlet extraction | Temperature 70 °C for 6 h; hot air oven drying at 35–40 °C | Enhanced WHC, swelling index, free flowability and thermal stability | 26 and 27 |
| High pressure processing | Pressure – 0, 125, 250, 500 MPa; time 5 to 15 min | Enhanced viscosity and WAI | 28 |
| Reduced WSI | |||
| Subcritical water extraction | Temperature 100, 125, 150, 175, 200 °C | Reduced viscosity, pasting property, molecular weight, and WAI | 29 |
| Enhanced WSI | |||
| Microwave assisted extraction | (1) Power – 850 W; time 120, 150, 180 & 210 s | Stiffer, tightly packed gel structure | 30 |
| (2) Power – 850 W; time 120 s; soaking for 2, 3, 4 & 8 h; hot air oven drying at 80 °C/8 h, 90 °C/2, 4, 6, 8 h and 100 °C/2, 4, 6, 8 h | Reduced apparent viscosity | ||
| Enzyme – ultrasound assisted extraction | Protease concentration 0.48 U mL−1; amplitude 25%, 50% (0.5, 1 watt cm−3); time 15 or 30 min | Reduced molecular weight and flow behavior index | 31 |
| Enhanced elasticity and consistency coefficient | |||
| Gamma irradiation method | Source – cobalt-60 gamma irradiator; dosage – 0, 5, 10 kGy; dose strength – 11.1 PBq; dose rate – 10 kGy h−1 | Reduced molecular weight, interlinkage between fiber bunches, radical scavenging and antioxidant capacity | 32 |
| Enhanced flat sheet like structures | |||
| Electron beam method | Beam current – 1 mA; beam power – 570 kW; distance – 50 cm (from source); thickness – 3 mm | Reduced molecular weight | 32 |
| Enhanced radical scavenging ability, antioxidant capacity |
| Methods | Changes in color | Reason | References |
|---|---|---|---|
| Conventional method | • Light brown or cream color | Polyphenol oxidase enzyme deactivation during the boiling step | 28, 29 and 33 |
| High pressure processing | • Light color | Deactivation of polyphenol oxidase enzyme at a higher pressure level (>200 MPa) | 28 and 34 |
| • Enhanced L*, a*, and b* values | |||
| Subcritical water extraction | • Dark color | Due to Maillard reaction and caramelization which occur during high-temperature extraction (>100 °C) | 29 and 35 |
| • Reduced L*, enhanced a* and b* values | |||
| • Light color | Due to less exposure to heat (<100 °C) | ||
| • Enhanced L*, reduced a* and b* values | |||
| Microwave-assisted extraction | • Dark brown color | Maillard reaction due to elevated temperature and time | 30 |
| • Reduced L*, enhanced a*and b* values |
2.2 Conventional methods of TSP extraction
The polysaccharide extraction involves mixing TKP with distilled water and then boiling it at 100 °C for a set time (20 min). The mixture is centrifuged (5000 rpm for 20 min) after being left overnight. The obtained supernatant is transferred to methanol/ethanol (95%) and continuously stirred. The final solution's pH (5) is adjusted, and the resulting mixture is centrifuged (5000 rpm for 5 min) before extracting the precipitated xyloglucan. The precipitated xyloglucan is dried (50 °C for 4 h) using a tray dryer and then ground using a simple grinder.18 This approach is limited to extracting polysaccharides outside cells since it cannot break the material's cell wall. This extraction method is the most widely utilized because of its simple procedure, although it requires a significant amount of time and energy.26,38
2.2.1.1 Purification. Precipitation is a key factor in polysaccharide purification since it helps to remove impurities and improve bulk stability. Polysaccharide precipitation involves the intermolecular interaction of alcohols and organic solvents. The drop in the polysaccharide dielectric constant leads to alterations in the molecular organization, causing molecules to cluster and precipitate.26 Crude polysaccharide purification also involves removing released protein with the Sevage reagent, which consists of chloroform and n-butanol in a ratio of 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; this reagent helps to break down protein. At a specific temperature (25 °C), the polysaccharide is combined with the Sevage reagent and deionized water, and then the mixture is centrifuged (7000 rpm for 15 min) at ambient temperature. The supernatant is removed, and the process is repeated until the protein layer disappears. Following centrifugation, the polysaccharide is found at the top, followed by the protein, and finally, after repeating the process, the protein layer diminishes. Ethanol is used to precipitate the gathered polysaccharides, which are dissolved in an aqueous solution, i.e., water and dialyzed (MW 3500 dalton) with tap water flow for two days and deionized water for twelve hours. Then, the lyophilization procedure is used for TSP production.39
1; this reagent helps to break down protein. At a specific temperature (25 °C), the polysaccharide is combined with the Sevage reagent and deionized water, and then the mixture is centrifuged (7000 rpm for 15 min) at ambient temperature. The supernatant is removed, and the process is repeated until the protein layer disappears. Following centrifugation, the polysaccharide is found at the top, followed by the protein, and finally, after repeating the process, the protein layer diminishes. Ethanol is used to precipitate the gathered polysaccharides, which are dissolved in an aqueous solution, i.e., water and dialyzed (MW 3500 dalton) with tap water flow for two days and deionized water for twelve hours. Then, the lyophilization procedure is used for TSP production.39
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) and leaving it undisturbed (2 h) for finer precipitation of the polysaccharide, followed by clarification, ethanol washing, and pressing. The obtained TSP in the pressed form is dehydrated utilizing a hot air oven (35 to 40 °C) and then pulverized.26,27 In certain instances, tamarind seed powder is first rendered fat-free, and the resulting defatted tamarind seed powder (DTKP) is then used to extract polysaccharides using a hot water extraction process followed by purification, resulting in the production of water-soluble TSP.37
2 ratio) and leaving it undisturbed (2 h) for finer precipitation of the polysaccharide, followed by clarification, ethanol washing, and pressing. The obtained TSP in the pressed form is dehydrated utilizing a hot air oven (35 to 40 °C) and then pulverized.26,27 In certain instances, tamarind seed powder is first rendered fat-free, and the resulting defatted tamarind seed powder (DTKP) is then used to extract polysaccharides using a hot water extraction process followed by purification, resulting in the production of water-soluble TSP.37
2.3 Novel methods of TSP extraction
Conventional extraction techniques have several shortcomings, such as applying heat to the product, which alters its properties, prolonged extraction time, more solvent absorption, and low extraction efficiency. These shortcomings prompted the development of novel extraction techniques that produce better results and more desirable product attributes. The merits and demerits of conventional and novel extraction methods of TSP are shown in Table 4.| Extraction methods | Merits | Demerits | References |
|---|---|---|---|
| Conventional methods | • Simple procedure | • Low extraction efficiency | 26, 38, 28 and 43 |
| • Low cost | • More energy and time requirements | ||
| • Simple equipment | • Low purity | ||
| • Absorption of solvent | |||
| • Heat-induced changes in the properties of TSP | |||
| • Environmental hazards and toxicological impacts | |||
| Novel methods | • High extraction efficiency | • High cost | 26, 29, 28, 30 and 43 |
| • Less energy and time requirements | • Advanced equipment | ||
| • Low temperature | • Dark color of extracted TSP (subcritical water and MAE techniques) | ||
| • Ecologically sound | |||
| • Eliminates the requirement for hazardous solvents | |||
| • Enhanced purity | |||
| • Non-toxic |
2.3.1.1 Effect of high-pressure processing on TSP properties. The tamarind polysaccharide powder that was extracted under high pressure had a light color that showed improvements in lightness (L*), yellow (b*), and red (a*) color values. This is because the polyphenol oxidase enzyme was deactivated during extraction at a higher pressure of more than 200 MPa, which in turn helped to prevent enzymatic browning.28,34 The viscosity increases with a rise in pressure; this is because the damaged plasma membrane leads to the release of xyloglucan along extended molecular chains from the cell wall of the tamarind kernel. This increases the solvent's capacity to enter cells, accelerating the mass transfer rate.28,44,45 The high-pressure extracted TSP exhibited less viscosity when compared with conventionally extracted TSP. This could result from xyloglucan's molecular mass reduction after high-pressure treatment.28 Compared to high-pressure extraction, the conventional method involves high temperatures, which cause damage to the tamarind seed kernel's cell membrane, thus releasing a longer chain of xyloglucan molecules. The other reason is that under high pressure, the disintegration of the hydrogen bonds between molecules causes a reduction in viscosity through xyloglucan chain disintegration.28
The TSP's water absorption index (WAI) increased as the pressure increased. The increase in WAI is associated with xyloglucan molecular weight; that is, higher molecular weights have a greater capacity to absorb water, which causes an increase in viscosity when dissolved in water. However, compared with the conventional method, high-pressure extraction had low WAI. This is because, in the conventional method, high temperature is used for extraction.28 The water solubility index (WSI) of high-pressure extracted polysaccharides was found to be lower than that of polysaccharides obtained by the conventional method carried under atmospheric pressure. This could be caused by a reduction in the surface area available for binding water due to the denser structure of xyloglucan resulting from high pressure.28,29
The DTKP is blended using distilled water, and the solution is poured into a vessel that is resistant to high pressure, which is further heated using a thermostat block heater made of aluminum to attain certain temperatures (100, 125, 150, 175, and 200 °C). The vessel is detached from the heater and immediately allowed to cool using ice water to stop further reaction. The variation in pressure level is decided based on the correlation between the saturation pressure of water vapor and treatment temperature. Protease is added to the subcritical water-treated extract, and the conventional precipitation procedure is carried out to obtain TSP.29,50
2.3.2.1 Effect of subcritical water extraction on TSP properties. Subcritical water extraction produced a higher yield than conventional extraction because the TKP's inner prime cell wall degenerated due to extreme conditions. The yield of xyloglucan (TSP) production was greater at 175 °C.29 The subcritical water extracted from xyloglucan showed reduced lightness (L*) and increased yellowness (b*), and redness (a*) values. This is caused by the Maillard reaction, which happens during high-temperature extraction and causes xyloglucan to break down into reducing sugars and their combination with amino acids. The other reason for the enhanced dark color of extracted xyloglucan is caramelization. At 160 °C, the subcritical extraction process of xyloglucan breaks down the sugar molecules in it, causing them to become fragmented and caramelized. At low temperatures (100 °C), an increase in the L* value along with a decrease in a* and b* values was observed; this is due to less exposure to heat.29,35 Light-colored xyloglucan components are more appealing and are used in the food industry. The extraction method notably altered the pasting properties of the xyloglucan component. The disintegration of the xyloglucan structure and reduced molecular weight under extreme extraction conditions is the reason behind the decreased holding strength of subcritical hot water extracted xyloglucan compared to that obtained from the conventional method. Similarly, the viscosity of TSP also decreased.29
When compared to the conventional approach, the molecular mass of xyloglucan decreased because of a significant hydrolysis process caused by an increase in extraction process temperature.29,51,52 The WAI of extracted xyloglucan was lower than that obtained by the conventional method due to the degradation of the structure of xyloglucan, resulting in weaker water absorption, even though the increase in WAI with increasing temperature was noted when subcritical extraction was tested with various process temperatures. In contrast to WAI, the WSI of the extracted xyloglucan component was increased compared to the conventional method; this is also due to the degeneration of the structure of xyloglucan under severe process conditions, which enhanced the solubility of xyloglucan powder in water.29
Tamarind seeds are pretreated with microwave energy in two ways. The first approach involves placing the tamarind seed in a ceramic dish and inside a microwave oven on a glass turntable plate. Operating parameters such as power (850 W) and time (120, 150, 180, and 200 s) are predetermined. After microwave heating, the seeds are allowed to cool to room temperature for a while before decortication. Like the previous procedure, the second method's operating conditions include power (850 W) and varying times (60, 90, 120, and 150 s). Following microwave pretreatment, the tamarind seeds are steeped immediately in tap water (2, 3, 4, and 8 h) and then dried using a hot air oven (80 °C/8 h, 90 °C/2, 4, 6, 8 h, and 100 °C/2, 4, 6, 8 h), followed by decortication. The microwave-pretreated tamarind seeds are then ground into powder and subjected to defatting. Then, using the same protocol as the conventional approach, DTKP is utilized to extract xyloglucan, followed by purification.30
2.3.3.1 Effect of microwave-assisted extraction on TSP properties. The first approach (at 180 s) of microwave pretreatment removed the tamarind seed coat, but the result was an unacceptable dark color. Dark-colored TKP was formed due to the prolonged microwave pretreatment period, which increased the a*, and b* values and reduced the L* value. The Maillard reaction in tamarind seeds, which results from elevated temperature, is the process that causes the production of a dark brown powder.30 Similarly, in the second approach, longer heating times (>120 s) ensured higher decortication percentages, and led to the production of too-dark powder. The higher rate of decortication is caused by a gap that was created between the tamarind seed's outer testa and cotyledons when microwave heating was applied. This gap allowed easy removal of the seed coat.30,55 The best results, with a safe moisture level, creamy white powder, and a high decortication rate, were obtained by applying microwave pretreatment (120 s), steeping (3 h), and hot air oven drying (100 °C for 4 h). This is because after soaking for 3 h, the kernel could imbibe water to reach equilibrium moisture, or while drying, the necessary gap between the kernel and testa was established, making decortication simple. Soaking for more than 3 h did not show any increase in decortication percentage. Because of the influence of the moisture content, there is no variation in the percentage of decortication when drying time and temperature are increased beyond 100 °C and 4 h.30
Increased ethanol addition during the precipitation phase of xyloglucan purification causes a strong interaction among the xyloglucan and alcohol molecules, and as a consequence a stiffer, tightly packed gel structure forms with less trapped protein and water.30,56,57 With an increase in the shear rate, the apparent viscosity dropped, and the xyloglucan demonstrated pseudoplastic or shear thinning properties.30 Thivya et al. (2021)58 observed a xyloglucan yield of 21.75% from tamarind seeds treated with MAE with process parameters of 400 W power level and 99.43 mL g−1 liquid to solid ratio at 83.33 °C for 14.29 min.
The ultrasound-assisted technique is extensively used in applications like extraction, emulsification, and sterilization processes in food industries.31,61,62 The fundamental mechanism of ultrasonic extraction is acoustic cavitation, in which bubbles develop under pressure conditions and collide. In ultrasound-assisted extraction, dispersion or diffusion is the first phase, followed by cell wall breakdown and washing of the constituents of the cell after cell disruption. By breaking down the cell wall, the mass transfer rate is accelerated, dispersion of solid materials is enhanced, and contents inside the cell are released into the solvent. To minimize the size of particles contained in the compounds, this method uses less energy and solvent.26,53 By decreasing the molecular mass of proteins, high-power ultrasonic treatment increases the extraction of proteins and thus increases their solubility.31,62,63 The protease enzyme breakdown in combination with ultrasound extraction resulted in the separation of protein from TKP and enhanced the effectiveness of polysaccharide extraction and purity of the obtained extract.31,63
Protease enzyme digestion is the initial step of TSP extraction. To decrease cluster formation and improve DTKP dispersion, ethanol (95%) is mixed with DTKP and then distilled water. A magnetic stirrer-equipped water bath maintains the temperature (37 °C). The addition of the protease enzyme (0.16, 0.48, and 0.80 units per mL) to the slurry for a certain reaction period (1, 3, and 5 h) is followed by enzyme digestion; the obtained suspension is centrifuged (1500×g for 10 min), and the supernatant is collected, and then the precipitate is mixed with ethanol (95% for 5 min), filtered under vacuum conditions and then dried.31
The second method of TSP extraction combined high-power ultrasound with protease digestion, using an ultrasonic standard probe (½-inch) and administering ultrasonography in three separate sequences: before, during, and following protease enzyme digestion. As the enzyme digestion procedure specified, the DTKP slurry temperature (37 °C) and protease concentration (0.48 units per mL) are maintained. The sonicator probe amplitude [25%, 50% (0.5, 1 watt cm−3)] is set for a certain duration (15 or 30 min), with every 5 seconds of “on” and “off” periods. Subsequently, the suspension separation is followed by drying and sifting.31
2.3.4.1 Effect of enzyme and ultrasound-assisted extraction on TSP properties. The increase in protease enzyme concentration and digestion time were linked to increased extracted polysaccharide purity and yield, and decreased protein content. A small amount of protein is still present in the extracted TSP which is due to protease's incapacity to hydrolyze amino acids and protein enclosed inside the polysaccharide granules, hence inaccessible to the protease. The prolonged exposure of the polysaccharide in solution caused swelling and dissolution of the polysaccharide, which decreased the yield of the polysaccharide.31 The polysaccharide purity is enhanced in the second approach, which combines enzyme digestion and ultrasound treatment, by prolonging the ultrasound treatment's duration (15 to 30 min) and raising its amplitude (25 to 50%). The combination treatment resulted in an increased polysaccharide yield (95.86%) with a purity >90% and a decrease in protein content (<2.06%) when the sonication amplitude level was maintained at 50% with a protease enzyme concentration of 0.48 U mL−1 for a duration of 15 min. This results from cavitation caused by ultrasonic treatment, which weakens the bond between the protein matrix and polysaccharide granules.31 When sonication treatment was combined with enzyme digestion, the extracted TSP's molecular weight was lower compared to when enzyme digestion was used alone. This is because high-intensity ultrasonic treatment decreases the molecular size and breaks down the D-glucan backbone's β-(1-4) linkage. This is caused by increasing the time and amplitude level of the sonication treatment.31,64 An increased intertwining of the polymer mixture in the extracted TSP solution demonstrated viscoelastic behavior. The point of storage shear modulus (G′) and loss shear modulus (G′′) cross-over below 0.3 Hz was lowered by the increased protease concentration (0–0.80 U mL−1) and treatment duration (up to 5 h), indicating an elevated intertwining of the polymer solution caused by improved protein elimination. The solution's elasticity improved as a result of the reduction in protein concentration. The consistency coefficient (K) increased along with an increase in protease enzyme concentration, power, sonication, and enzyme digestion time. Conversely, a drop in the flow behavior index was observed. This is due to the enhanced purity of the extracted polysaccharides.31
2.3.5.1 Effect of electron beam and gamma irradiation on TSP properties. In comparison with traditional approaches, the extraction yield was greater. There was little variation in the extraction yield when utilizing gamma and electron beam irradiation treatments at absorbed doses (5 and 10 kGy). The degeneration of TSP with high molecular weight is responsible for the enhanced dry weight or mass of the extracted polysaccharide and the conversion of polysaccharides from an insoluble to a soluble form.32,65 Gamma-irradiation reduced the molecular mass of tamarind seeds at doses of 5 and 10 kGy compared to the non-irradiated control sample. Compared to gamma irradiation, the molecular mass of the sample treated with an electron beam decreased significantly at absorbed doses (5 kGy and 10 kGy), respectively. This is due to the impact of radiolysis on starch, β-glucan, where depolymerization of the polysaccharides takes place. The sample that was exposed to gamma radiation had the morphology of enhanced structures, such as flat sheets, and decreased interlinkage between fiber bunches. Extremely tiny particles are produced in samples treated with electron beams due to complete fibril structural breakdown. Because electron beam irradiation has a higher radical concentration than gamma irradiation, the degeneration patterns of the samples exposed to both types of radiation exhibited a minor variation at similar absorption doses.32 Compared to high molecular weight TSP, low molecular weight TSP exhibited stronger superoxide radical scavenging efficacy. This is caused by the impact of a greater or more intensified intramolecular hydrogen bond found in high molecular mass TSP with a compact structure, which diminishes the ability to scavenge radicals by limiting hydroxyl group exposure and weakening hydroxyl group activities.32,66 Hence, TSP exposed to electron beam radiation has a greater capacity for radical scavenging than sample exposed to gamma radiation. Because of its lower molecular weight, enhanced hydrogen intermolecular bonding, and superoxide radical scavenging ability, the TSP treated with an electron beam exhibited a greater antioxidant capacity than the gamma-irradiated sample.32,67,68
2.4 Extraction of TSM
Distilled water is combined with powdered tamarind seeds in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Distilled water is added again at a weight ratio (of :
10. Distilled water is added again at a weight ratio (of :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 while the solution mixture is continuously stirred for a specified duration of time (10 min) using a hotplate stirrer. The solution is heated at a temperature of 80 °C for a duration of 60 min. The mixture is centrifuged (524×g for 8 min) after being left undisturbed (20 °C for 24 h) to release the mucilage. The supernatant obtained after centrifugation is the fraction of mucilage, which is then carefully decanted and stored (at 4 °C) for further processing steps and analysis. The TSM extracted is then subjected to spray drying with operating conditions like an inlet temperature of 135 ± 5 °C, a feed rate of 40 mL min−1, and an outlet temperature of 80 ± 5 °C, with an injection of pressurized air at 4 bar. The high water activity and carbohydrate content of mucilage cause it to be exceedingly unstable; at room temperature. Therefore, preserving the active components and drying the mucilage is imperative to turn it into a powder. Spray drying is preferred since it preserves the sensory qualities of mucilage, such as flavor, color, and nutrients.24
40 while the solution mixture is continuously stirred for a specified duration of time (10 min) using a hotplate stirrer. The solution is heated at a temperature of 80 °C for a duration of 60 min. The mixture is centrifuged (524×g for 8 min) after being left undisturbed (20 °C for 24 h) to release the mucilage. The supernatant obtained after centrifugation is the fraction of mucilage, which is then carefully decanted and stored (at 4 °C) for further processing steps and analysis. The TSM extracted is then subjected to spray drying with operating conditions like an inlet temperature of 135 ± 5 °C, a feed rate of 40 mL min−1, and an outlet temperature of 80 ± 5 °C, with an injection of pressurized air at 4 bar. The high water activity and carbohydrate content of mucilage cause it to be exceedingly unstable; at room temperature. Therefore, preserving the active components and drying the mucilage is imperative to turn it into a powder. Spray drying is preferred since it preserves the sensory qualities of mucilage, such as flavor, color, and nutrients.24
The swelling index of TSM increased as temperature and pH increased. The granules with weak binding forces have a higher capability for swelling. Temperature increases cause the mucilage molecules' connectivity to weaken, which in turn causes the mucilage chains to expand and trap more water molecules. The electrostatic repulsion caused by the functional groups resulted in an ample space accessible for the reservation of the water molecules which resulted in an increase in the swelling index in response to a pH increase.23,70 An increased TSM spray dried powder to oil volume ratio led to improved emulsifying ability (EA) and reduced emulsion stability (ES). This is caused by the rise in mucilage content and then a subsequent rise in the number of chains in a branching arrangement in the area where oil molecules are absorbed, which lowers surface tension.23,71 Mucilage's structure was flexible, which reduced surface tension and improved foaming ability. The weight-to-volume dispersion ratio was increased, which improved foaming capacity (FC) as well as foam stability (FS). This is because more mucilage was present, which caused the mucilage to travel to the interface and create viscoelastic films, which enhanced foam development.23,72
Thermogravimetric (TGA) and differential scanning calorimetry (DSC) measurements of TSM's thermal properties, such as thermal stability and transitions during the heating process, revealed that the extracted mucilage is exceptionally thermostable, serving as a helpful component in applications of food such as suspension stabilization, emulsions, and micro encapsulations.23 Shear thinning non-Newtonian flow behavior with a flow behavior index (n) n < 1 was demonstrated by the TSM dispersions. Pseudoplasticity and dispersion viscosity are directly correlated with solution concentration and inversely correlated with solution temperature. Increased pseudoplasticity, with higher viscosity at lower shear rates that decreases with increasing shear, and a decreased n value resulted from increased TSM content.24,73 Because of the decrease in intermolecular and molecular mobility resulting from energy dissipation, an increase in temperature corresponds to a reduction in pseudoplasticity and an increase in the n value. Consequently, less energy is needed for flow and hydrodynamic domain intervention.24 As TSM concentration increased, its coefficient of consistency (k) also increased. This is a result of TSM's superior ability to increase intermolecular interactions and bind water. The temperature rise caused the value of k to fall, indicating a decrease in viscosity.24,74 The effect of the mucilage extraction method on structural and functional properties is summarised in Table 5.
| Method | Processing conditions | Structural & functional modifications | References |
|---|---|---|---|
| Conventional method | Temperature – 80 °C; time – 60 min; centrifugation – 524 g for 8 min; spray drying: Inlet temperature – 135 ± 5 °C, outlet temperature – 80 ± 5 °C, feed rate – 40 mL min−1, air pressure – 4 bar | Enhanced solubility, WHC, OHC, swelling index, EA, FA, FC, thermostability, coefficient of consistency | 24 |
| Reduced ES, surface tension, viscosity |
3. Tamarind seed protein
The amount of protein in tamarind seed kernels varies from 18.4% to 26.9%. Additionally, they are an abundant source of critical amino acids, including lysine, isoleucine, leucine, phenylalanine, valine, aspartic acid, and glutamic acid. The protein-digestibility index of tamarind seed kernel protein, as determined in vitro, was 71.3.60 The effect of different protein isolation methods on structural and functional properties is summarised in Table 6.| Method | Processing conditions | Structural & functional modifications | References |
|---|---|---|---|
| Conventional method | (1) Solvent extraction: vacuum drying (70 cm vacuum) under room temperature (29 ± 2 °C) | Enhanced FC, EC (raw/defatted TKP) | 60 |
| (2) Micellization process: pH – 10; centrifugation – 6000 rpm for 15 min; precipitation (ammonium sulfate); centrifugation – 8000 rpm for 15 min | Reduced FC, EC (roasted TKP) | 60 and 75 | |
| Reduced WAC (defatted & roasted TKP meal) | |||
| Ultrasound modification | Frequency – 25 kHz; probe diameter – 6 mm; power −100 & 200 W; time – 15 & 30 min | Enhanced solubility, WHC, OHC, FC, FS, ES, EC | 76 |
| Reduced particle density |
3.1 Conventional extraction method of protein concentrates and meal from TKP
The first method involves defatting TKP with hexane as a solvent to produce kernel meal, after or before roasting. The resulting tamarind kernel meal is ground to produce protein concentrates in powder form after being vacuum-dried (70 cm vacuum) for solvent removal at room temperature (29 ± 2 °C). The disadvantage of this extraction technique is that protein precipitation occurs at the isoelectric pH point (pH 4–5), which leads to the development of a gel and impedes the filtration process.60 The second extraction technique uses sodium chloride to initiate a micellization process. In this process, DTKP was combined with sodium chloride (1 M), the pH was adjusted (10), the mixture was stirred (30 min), then centrifuged (6000 rpm for 15 min), and then extracted twice. The supernatant's pH is adjusted using HCl (1 N) (4–6). After adding ammonium sulfate in solid form till the saturation point, the suspension is centrifuged (8000 rpm for 20 min) until the production of protein in precipitated form. This precipitation process is repeated to obtain the appropriate amount of TKP protein. Cellophane bags are used as the dialysis membrane against deionized water (for 48 h) at a temperature of 2 to 4 °C during the dialysis step of the precipitated protein purification process. The deionized water (9 L) used for the dialysis process, is replaced every 8 h. Protein concentrates are obtained by freeze-drying the contents of the cellophane bag and storing it in the refrigerator.60,753.2 Novel method of extraction
Most proteins lack the necessary techno-functional properties in their native state to be utilized in various food applications. The functional qualities can be improved by modifying them physically or chemically or treating them with enzymes. The disadvantages of these approaches include the high expense of enzymatic treatment, allergic reactions, nutritional imbalances, and numerous other adverse effects on health from using chemicals for modification. Additionally, the protein's functionality can be changed by performing various physical operations like freezing, extrusion, heating, and others.76,78 Novel isolation technique's merits include being time- and energy-efficient, non-toxic, and environmentally safe; as a result, they might be utilized as an alternative to traditional protein modification techniques. The food industry utilizes ultrasonic technology, one of the many innovative technologies, for modification and has several uses.76The protein was initially extracted from TKP using the standard extraction technique, which involves isoelectric point precipitation followed by centrifugation, precipitation, drying, and grinding of the precipitate. The isolated protein was modified using an ultrasound probe with a diameter of 6 mm at a frequency a 25 kHz. After mixing the protein isolate powder with the deionized water, various power level (100, 200 W) and time (15 and 30 min) combinations were applied during the sonication process. After sonicating the protein isolate, it is separated from the solution using centrifugation (7800×g for 15 min), followed by precipitation. The isolated protein is denatured by drying the precipitate (40 °C for 24 h).76
3.2.1.1 Effect of ultrasonication on the properties of tamarind seed protein. The increase in ultrasonic treatment strength and duration improved the solubility of the protein isolate from tamarind seeds. The increase is caused by a greater number of bubbles cavitating during sonication, which aids in protein unfolding due to a significant rise in temperature and pressure locally close to the bursting bubbles and subsequent peptide bond breaking during hydrolysis.76,79 To unravel the protein and expose its carboxylic or amino groups, prolonging the sonication period is more crucial than increasing power. Proteins with weaker hydrogen bonds are broken down by sonication, modifying the protein's structure and increasing its solubility. Furthermore, reducing the size of protein aggregates with ultrasonic treatment results in greater water–protein interaction and increased solubility.76,80 Because of sonication-induced protein unfolding and subsequent protein structural rearrangement, the tamarind seed protein isolate particle density was slightly lower for the treated sample.76 When treatment duration and intensity were increased, the WHC was raised for the ultrasound-modified sample. This is because ultrasound treatment causes the hydrophilic groups in the polypeptide chain to unfold, exposing them. The balance of hydrophobic–hydrophilic groups in amino acid composition of protein molecules, in addition to, the interaction of lipids, carbohydrates, tannins–protein association, and numerous other criteria such as shape, size, steric factors, and conformational properties, also have a substantial impact on the WHC of protein isolates.76,81
Ultrasound treatment increased the OHC because it caused structural changes in the polypeptide chains due to rupturing of cavitation bubbles near protein molecules during sonication. This exposed hydrophobic chains, which are non-polar and present on the side of amino acids, increasing the attachment of oil molecules to protein.76,82 The FC and FS both improved with ultrasound treatment. The unwinding of the polypeptide chain and native protein denaturation are the causes of the increase in FC. The isolate's hydrophobicity and protein solubility were related to its FC and FS.76,83 As the intensity and duration of ultrasonic treatment increased, both EC and ES also increased correspondingly. This is caused by several factors, including changes in the protein's second-order structure and the combined effects of chemical, mechanical, and thermal shock caused by sonication treatment.76,84,85 The primary cause is the abruptly high temperature and pressure produced by sonication, which altered the basic structure of the protein molecules. Additionally, the ability to emulsify is attributed to the protein's solubility and hydrophobicity. The ultrasonic treatment unaffected the main structure and molecular weight of proteins.76
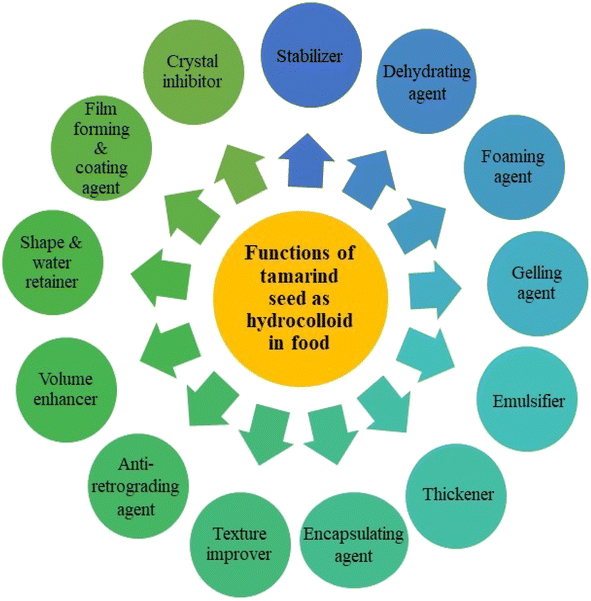
4. Tamarind seed application as hydrocolloids in food
The use of TSP, mucilage, and protein as hydrocolloids in food is associated with their ability to function as stabilizers, thickening agents, gelling agents, emulsifiers, water retainers, film-forming agents, and starch modifiers. Fig. 2 shows different applications of tamarind seeds as hydrocolloids in food. The various applications of tamarind seed as a hydrocolloid are summarized in Table 7. The TSM as a hydrocolloid, is used in applications like inhibition of crystallization and formation of edible films, flavor retention, stabilizing emulsions, and in encapsulation techniques as a coating material for flavors and nutrients.23,99 The sesame seed oil is microencapsulated by utilizing mucilage from tamarind seeds as a coating material.87 The modified tamarind seed protein improves functional qualities like foaming, viscosity, and the previously mentioned qualities like gelation and emulsification.76 Bread, jelly, and fortified biscuits are made with protein isolates from raw or roasted TKP.55,60| Tamarind seed fractions | Function | Application | References |
|---|---|---|---|
| Tamarind seed mucilage | Flavor retention, enhance mouthfeel, emulsion stabilizer | Fat replacers, dairy products | 23 |
| Gel former, thickener | Jams, jellies, marmalade, mayonnaise, sauces | 86 | |
| Encapsulating agent (coating material) | Flavors, nutrients (sesame oil) | 87 | |
| Tamarind seed protein | Foaming, gelling, emulsifying agents, fortification | Jelly, biscuit, bread | 55, 60 and 76 |
| Tamarind seeds polysaccharide (TSP) | Thickening agents | Sauces for meat products (cutlets) | 17 |
| Texture improver | Milk, cocoa and fruit beverages, starch, gluten-free bread | ||
| Viscosity stabilizer | Batter mixes: jam, jelly, puddings | ||
| Gelling agent | Japanese desserts | ||
| Emulsion stabilizer | Ice-cream, salad dressings, mayonnaise, low fat dressings | ||
| Starch substitute | Bakery products, flour paste, noodles, stew | ||
| Anti-retrograding agent, dough improver, volume enhancer, stabilizer | Bread | 17, 88, 89, 90 and 91 | |
| Retard starch gelatinization, improve volume | Bread | 19 and 91 | |
| Reduce gluten deterioration (during freezing), uneven porosity | Bread dough, steamed bread | 91 and 92 | |
| Hardness reducer and volume enhancer | Sponge cakes | 17 and 93 | |
| Water retainer | Seaweed preserve products (in Japan) | 17 | |
| Retard ice recrystallization | Ice cream mixes, frozen desserts | 94 | |
| Cryoprotectant | Frozen surimi gels | 95 | |
| TSP + xanthan gum | Crystallization inhibitor (sugar, ice), provides overrun, stabilizer | Frozen desserts | 17 |
| Texture adjustment, shape retention, gelling property | Dressing applications | 96 | |
| TSP + κ-carrageenan | Emulsion stabilizer | Instant coffee, butter, skim milk, glucose and fructose syrup | 55 |
| TSP + locust bean gum | Shape retainer, helps in meltdown | Ice cream | |
| TSP + gelatin | Oleogels | Confectionery products, ground meat products, shortenings | 97 |
| Tamarind seed powder | Film forming agent | Fish, pineapple | 55 |
| Coating material | Casings of sausage, fried food products | ||
| Dehydrating agent | Powdered foods | ||
| Stabilizer | Mango sauce | 98 and 86 |
Thickening is accomplished by TSP, or xyloglucan, which decreases stickiness, flows easily, and gives the product a body after tasting. When strong stability and viscosity are required in sauces for meat items like cutlets, it is utilized as a thickening ingredient. In low-fat milk, chocolate, and fruit-based beverages, TSP contributes to the improved texture and body of the product. It stabilizes the tiny particles that are suspended in fruit pulp-based drinks. In batter mixtures, the TSP is employed to stabilize viscosity. Over a wide pH range, the concentrated TSP and sugar solution combine to produce an elastic gel. This gel is free from water release, heat-stable, and unaffected by freezing and thawing, making it an excellent substitute for pectin in fruit products like jam and jelly.17 Traditional Japanese delicacies like kudzu mochi and yokan require gelling agents, which are largely produced using TSP.
Furthermore, TSP is commonly utilized in Japanese puddings, jellies, and preserves known as tsukudani, which are formed from seaweed and help in quenching water release.17 By providing mechanical and thermal stability, the TSP strengthens the starch's texture. The stabilizing properties of polysaccharides are primarily caused by changes in viscosity or gelation within the continuous aqueous phase.100 In products like ice cream, salad dressings, and mayonnaise, the TSP is used as a stabilizer of emulsions. TSP is used in place of or in addition to starch in various baked goods, flour paste, noodles, custard cream, and stews.17
In bakery products like bread, it is used to prevent starch retrogradation and enhance the performance of dough and its preservation.17,101 It also acts as a bread volume improver and enhances storage properties. The water-soluble TSP fractions and their hydrolysates improve the stability, and quality of bread's dough. The smaller gas bubble distribution also improves the appearance of bread.17,88,89 By inhibiting the aging of gelatinized starch, hydrolyzed TSP preserves gelatinization and contributes to the reduction of hardness and increase in volume in sponge cakes. TSP enhances rice-based gluten-free bread's volume, appearance, texture, and storage qualities.17,93 TSP also helps in the inhibition of recrystallization of ice cream mixes94 and also acts as a cryoprotectant in frozen surimi gels.95
When used in dressing applications, TSP and xanthan gum work together to modify texture, maintain shape, and exhibit high viscosity due to their gel-like, thermoreversible properties.96 The TSP is an emulsion stabilizer in no-fat or low-fat dressings and mayonnaise.17 In frozen desserts, the TSP serves as a stabilizing agent and, over extended storage time, helps to prevent the crystallization of sugar, inhibit the growth of ice crystals, and also provide overrun to the product. The WHC of TSP, which in freezing mixtures retains extra unbound water surrounding ice crystals, is the cause of the prevention of ice crystal formation. The sugar-TSP gel's freezing and thawing operations create a stronger, more elastic, and more rigid gel-like compound.17 After a freeze–thaw process, the TSP in gels reduces syneresis. The food items including glucose, fructose syrup, butter, skim milk, and instant coffee use TSP and κ-carrageenan as emulsion stabilizers. Locust bean gum and TSP work together to assist ice cream in maintaining its shape and melting more quickly when stored at room temperature.55
Tamarind seed powder can be utilized as an essential oil emulsifying agent and as a dehydrating agent when producing powdered goods. Low-calorie candies, dry cakes, gum, frozen dairy desserts, cookie mixes, energy bars, baked products, gel-filled desserts, as well as spoonable sauces can all be made with the oligosaccharide derived from tamarind seeds. The TKP is also used to coat and create films for products like sausage casings, fried food, and pineapple, which helps in preserving these products.55 In mango sauce, tamarind seed powder acts as a stabilizer and also improves the viscosity of the product.86,98
5. Conclusion
The findings from the studies above suggest that tamarind seeds have potential as hydrocolloids for utilization in various foods. Hydrocolloids are employed in food items to enhance texture, mouthfeel, gelling, thickening, stabilizing, foaming, emulsifying, and other properties. The hydrocolloid derived from tamarind seeds, a by-product of the tamarind processing industry, is inexpensive, comes from a natural source, and reduces waste by being employed in food products. To achieve the desired effects on the product, it is essential to comprehend the rheological, structural, functional, and nutritional benefits of hydrocolloids along with specific parameters of the product. Eco-friendly, non-thermal approaches and conventional processes extract the polysaccharide, protein, and mucilage from tamarind seeds. Based on the outcomes of these extraction methods on the physicochemical, functional, and structural characteristics, tamarind seeds are utilized as hydrocolloids in various food products, including dairy, confectionery, bakery, frozen desserts, beverages, meat, and seafood. Apart from the non-thermal techniques discussed in this review, other techniques like radiofrequency wave (RF) assisted extraction, supercritical carbon dioxide extraction, and pulsed electric field (PEF) extraction can also be investigated, and their consequences on structural and techno-functional alterations can be scrutinized.Data availability
No additional data are available related to the article.Conflicts of interest
The authors declare no conflict of interest in the decision to publish this manuscript.Acknowledgements
The work was funded (project no. IMP-02/PMFME/02) by the National Institute of Food Technology, Entrepreneurship and Management – Thanjavur (NIFTEM-T), Thanjavur, India.References
- D. Saha and S. Bhattacharya, J. Food Sci. Technol., 2010, 47, 587–597 CrossRef CAS PubMed.
- B. Bisht, U. C. Lohani, V. Kumar, P. Gururani and R. Sinhmar, Crit. Rev. Food Sci. Nutr., 2022, 62, 693–725 CrossRef CAS PubMed.
- S. Khushbu, C. K. Sunil, D. V. Chidanand and R. Jaganmohan, J. Food Process Eng., 2019, 43, e13237 CrossRef.
- P. Himashree, A. S. Sengar and C. K. Sunil, Int. J. Gastron. Food Sci., 2022, 27, 100468 CrossRef.
- S. V. Medina-López, C. M. Zuluaga-Domínguez, J. P. Fernández-Trujillo and M. S. Hernández-Gómez, Foods, 2022, 11, 401 CrossRef PubMed.
- S. A. Idris, N. R. Rosli and R. M. A. R. Aris, Mater. Today Proc., 2022, 63, S462–S466 CrossRef CAS.
- A. S. Rao, A. A. Kumar and M. V. Ramana, Agric. Eng. Int.: CIGR J., 2015, 17, 200–204 Search PubMed.
- K. Mahajani, J. AgriSearch, 2020, 7, 51–53 Search PubMed.
- N. B. Shankaracharya, J. Food Sci. Technol., 1998, 35, 193–208 CAS.
- I. A. Ajayi, R. A. Oderinde, D. O. Kajogbola and J. I. Uponi, Food Chem., 2006, 99, 115–120 CrossRef CAS.
- M. Richa, R. Prasad and A. Verma, J. Pharmacogn. Phytochem., 2018, 7, 1078–1080 CAS.
- B. O. De Lumen, R. Becker and P. S. Reyes, J. Agric. Food Chem., 1986, 34, 361–364 CrossRef CAS.
- R. H. Glew, D. J. Vanderjagt, C. Lockett, L. E. Grivetti, G. C. Smith, A. Pastuszyn and M. Millson, J. Food Compos. Anal., 1997, 10, 205–217 CrossRef CAS.
- M. M. Ishola, E. B. Agbaji and A. S. Agbaji, J. Sci. Food Agric., 1990, 51, 141–143 CrossRef CAS.
- C. M. Martins, J. A. C. Guedes, E. S. de Brito and S. R. S. Ferreira, J. Supercrit. Fluids, 2022, 183, 105556 CrossRef CAS.
- C. Soukoulis, C. Gaiani and L. Hoffmann, Curr. Opin. Food Sci., 2018, 22, 28–42 CrossRef.
- K. Yamatoya, A. Tabuchi, Y. Suzuki and H. Yamada, in Biopolymer-Based Formulations: Biomedical and Food Applications, Elsevier Inc., 2020, pp. 445–461 Search PubMed.
- S. S. Hettiarachchi, W. M. I. Thakshila Kumari and R. Amarakoon, Eur. J. Adv. Chem. Res., 2023, 4, 15–21 CrossRef.
- F. Xie, H. Zhang, Y. Xia and L. Ai, Food Hydrocolloids, 2020, 105, 105854 CrossRef.
- H. Cakmak, H. Ilyasoglu-Buyukkestelli, E. Sogut, V. H. Ozyurt, C. E. Gumus-Bonacina and S. Simsek, Food Hydrocolloids Health, 2023, 3, 100131 CrossRef CAS.
- C. M. Martins, D. M. Ferro, E. S. de Brito and S. R. S. Ferreira, Innovative Food Sci. Emerging Technol., 2020, 66, 102518 CrossRef CAS.
- S. Cortés-Camargo, A. Román-Guerrero, J. Alvarez-Ramirez, E. Alpizar-Reyes, S. K. Velázquez-Gutiérrez and C. Pérez-Alonso, Carbohydr. Polym. Technol. Appl., 2023, 5, 100302 Search PubMed.
- E. Alpizar-Reyes, H. Carrillo-Navas, R. Gallardo-Rivera, V. Varela-Guerrero, J. Alvarez-Ramirez and C. Pérez-Alonso, J. Food Eng., 2017, 209, 68–75 CrossRef CAS.
- E. Alpizar-Reyes, A. Román-Guerrero, R. Gallardo-Rivera, V. Varela-Guerrero, J. Cruz-Olivares and C. Pérez-Alonso, Int. J. Biol. Macromol., 2018, 107, 817–824 CrossRef CAS PubMed.
- J. H. Chiang, D. S. M. Ong, F. S. K. Ng, X. Y. Hua, W. L. W. Tay and C. J. Henry, Trends Food Sci. Technol., 2021, 115, 105–116 CrossRef CAS.
- S. Muthusamy, G. Prabu and V. Ramireddy, Bioact. Carbohydr. Diet. Fibre, 2021, 26, 100276 CrossRef CAS.
- J. Bansal, K. Nitin, R. Malviya and P. K. Sharma, Am.-Eurasian J. Sci. Res., 2014, 9, 01–05 CAS.
- N. Limsangouan, C. Charunuch, S. K. Sastry, W. Srichamnong and W. Jittanit, LWT–Food Sci. Technol., 2020, 134, 110112 CrossRef CAS.
- N. Limsangouan, N. Milasing, M. Thongngam, P. Khuwijitjaru and W. Jittanit, J. Food Process. Preserv., 2019, 43, 1–10 Search PubMed.
- T. T. Nguyen, W. Jittanit and W. Srichamnong, J. Food Process. Preserv., 2019, 43, 1–14 Search PubMed.
- S. Poommarinvarakul, J. Tattiyakul and C. Muangnapoh, J. Food Sci., 2010, 75, E253–E260 CrossRef CAS PubMed.
- J. Choi, J. Kim, P. Srinivasan, J. Kim, H. Park, M. Byun and J. Lee, Radiat. Phys. Chem., 2009, 78, 605–609 CrossRef CAS.
- R. Malviya, S. Sundram, S. Fuloria, V. Subramaniyan, A. K. Azad, M. Sekar, D. H. Kumar, S. Chakravarthi, O. Porwal, D. U. Meenakshi and N. K. Fuloria, Polymers, 2021, 13, 3023 CrossRef CAS PubMed.
- A. Sulaiman and F. V. M. Silva, Food Control, 2013, 33, 424–428 CrossRef CAS.
- K. Watchararuji, M. Goto, M. Sasaki and A. Shotipruk, Bioresour. Technol., 2008, 99, 6207–6213 CrossRef CAS PubMed.
- M. A. Cerqueira, A. C. Pinheiro, B. W. S. Souza, Á. M. P. Lima, C. Ribeiro, C. Miranda, J. A. Teixeira, R. A. Moreira, M. A. Coimbra, M. P. Gonçalves and A. A. Vicente, Carbohydr. Polym., 2009, 75, 408–414 CrossRef CAS.
- N. Sivakumar and K. Karuppaiyan, J. Food Process Eng., 2020, 43, 1–17 Search PubMed.
- N. Addoun, Z. Boual, C. Delattre, A. V Ursu, J. Desbrières, D. Le Cerf, C. Gardarin, M. D. O. El-hadj, P. Michaud and G. Pierre, Int. J. Biol. Macromol., 2020, 155, 1333–1341 CrossRef CAS PubMed.
- L. Ren, Y. Yang, X. Bian, X. Li, B. Wang, D. Wang, D. Su, L. Liu, D. Yu, X. Guo, X. Zhang and N. Zhang, J. Food Qual., 2022, 2022, 9788248 CrossRef.
- U. Farooq, P. K. Sharma and R. Malviya, Am.-Eurasian J. Agric. Environ. Sci., 2014, 14, 269–274 Search PubMed.
- A. P. Gorle and S. S. Chopade, J. Drug Delivery Ther., 2020, 10, 295–307 CrossRef CAS.
- H. N. Lavudi, S. Kottapalli and F. M. Goycoolea, Eurobiotech J., 2017, 1, 303–309 CrossRef PubMed.
- M. Puri, D. Sharma and C. J. Barrow, Trends Biotechnol., 2012, 30, 37–44 CrossRef CAS PubMed.
- K. N. Prasad, B. Yang, M. Zhao, X. Wei, Y. Jiang and F. Chen, Sep. Purif. Technol., 2009, 70, 41–45 CrossRef CAS.
- X. Guo, D. Han, H. Xi, L. Rao, X. Liao, X. Hu and J. Wu, Carbohydr. Polym., 2012, 88, 441–448 CrossRef CAS.
- O. Pourali, F. S. Asghari and H. Yoshida, Food Chem., 2009, 115, 1–7 CrossRef CAS.
- J. Zhang, C. Wen, M. Chen, J. Gu, J. Zhou, Y. Duan, H. Zhang and H. Ma, Int. J. Biol. Macromol., 2019, 134, 172–179 CrossRef CAS PubMed.
- P. Khuwijitjaru, Jpn. J. Food Eng., 2016, 17, 33–39 CrossRef.
- L. R. Adetunji, A. Adekunle, V. Orsat and V. Raghavan, Food Hydrocolloids, 2017, 62, 239–250 CrossRef CAS.
- K. Klinchongkon, P. Khuwijitjaru, J. Wiboonsirikul and S. Adachi, J. Food Process Eng., 2017, 40, e12269 CrossRef.
- X. Wang, Q. Chen and X. Lü, Food Hydrocolloids, 2014, 38, 129–137 CrossRef CAS.
- K. Klinchongkon, N. Chanthong, K. Ruchain, P. Khuwijitjaru and S. Adachi, J. Food Process. Preserv., 2016, 41, e13138 CrossRef.
- Z. Zhu, J. He, G. Liu, F. J. Barba, M. Koubaa, L. Ding, O. Bals, N. Grimi and E. Vorobiev, Innovative Food Sci. Emerging Technol., 2016, 33, 1–9 CrossRef CAS.
- J. Zhao, M. Zhang and H. Zhou, Molecules, 2019, 24, 1605 CrossRef CAS PubMed.
- C. S. Kumar and S. Bhattacharya, Crit. Rev. Food Sci. Nutr., 2008, 48, 1–20 CrossRef CAS PubMed.
- S. Yamanaka, Y. Yuguchi, H. Urakawa, K. Kajiwara, M. Shirakawa and K. Yamatoya, Food Hydrocolloids, 2000, 14, 125–128 CrossRef CAS.
- Y. Yuguchi, T. Kumagai, M. Wu, T. Hirotsu and J. Hosokawa, Cellulose, 2004, 11, 203–208 CrossRef.
- P. Thivya, Y. Bhosale, S. Anandakumar, V. Hema and V. Sinija, Waste Biomass Valorization, 2021, 12, 5779–5794 CrossRef CAS.
- S. S. Nadar, P. Rao and V. K. Rathod, Food Res. Int., 2018, 108, 309–330 CrossRef CAS PubMed.
- S. Bhattacharya, S. Bal, R. K. Mukherjee and S. Bhattacharya, Food Chem., 1994, 49, 1–9 CrossRef CAS.
- N. Kardos and J. Luche, Carbohydr. Res., 2001, 332, 115–131 CrossRef CAS PubMed.
- F. Priego-Capote and L. M. D. de Castro, J. Biochem. Biophys. Methods, 2007, 70, 299–310 CrossRef CAS PubMed.
- L. Wang and Y. Wang, J. Cereal Sci., 2004, 39, 291–296 CrossRef CAS.
- M. Vodeničarová, G. Dřímalová, Z. Hromádková, A. Malovíková and A. Ebringerová, Ultrason. Sonochem., 2006, 13, 157–164 CrossRef PubMed.
- S.-J. Huang and J.-L. Mau, Food Chem., 2007, 105, 1702–1710 CrossRef CAS.
- R. Xing, S. Liu, Z. Guo, H. Yu, P. Wang, L. Cuiping, Z. Li and P. Li, Bioorg. Med. Chem., 2005, 13, 1573–1577 CrossRef CAS PubMed.
- J. K. Kim, P. Srinivasan, J.-H. Kim, J. Choi, H. J. Park, M. W. Byun and J. W. Lee, Food Chem., 2008, 109, 763–770 CrossRef CAS PubMed.
- E. Byun, J. Kim, N. Sung, J. Choi, S. Lim, K. Kim, H. Yook, M. Byun and J. Lee, Radiat. Phys. Chem., 2008, 77, 781–786 CrossRef CAS.
- B. T. Amid and H. Mirhosseini, Food Chem., 2012, 132, 1258–1268 CrossRef CAS PubMed.
- M. R. P. Rao, D. U. Warrier, S. R. Gaikwad and P. M. Shevate, Int. J. Biol. Macromol., 2016, 85, 317–326 CrossRef CAS PubMed.
- M. Jindal, V. Kumar, V. Rana and A. K. Tiwary, Ind. Crops Prod., 2013, 45, 312–318 CrossRef CAS.
- J. E. Kinsella, Food Chem., 1981, 7, 273–288 CrossRef CAS.
- M. I. Capitani, L. J. Corzo-Rios, L. A. Chel-Guerrero, D. A. Betancur-Ancona, S. M. Nolasco and M. C. Tomás, J. Food Eng., 2015, 149, 70–77 CrossRef.
- A. Koocheki, A. Reza and A. Bostan, Food Res. Int., 2013, 50, 446–456 CrossRef CAS.
- O. P. López and C. Ordorica-Falomir, J. Sci. Food Agric., 1986, 37, 1097–1103 CrossRef.
- B. Biswas and N. Sit, J. Food Sci. Technol., 2020, 57, 2070–2078 CrossRef CAS.
- H. B. Bull and K. Breese, Arch. Biochem. Biophys., 1968, 1128, 488–496 CrossRef PubMed.
- L. Mirmoghtadaie, S. S. Aliabadi and S. M. Hosseini, Food Chem., 2015, 199, 619–627 CrossRef PubMed.
- K. J. Moulton and L. C. Wang, J. Food Sci., 1982, 47, 1127–1129 CrossRef.
- C. Arzeni, K. Martínez, P. Zema, A. Arias, O. E. Pérez and A. M. R. Pilosof, J. Food Eng., 2012, 108, 463–472 CrossRef CAS.
- H. Hu, E. C. Y. Li-chan, L. Wan, M. Tian and S. Pan, Food Hydrocolloids, 2013, 32, 303–311 CrossRef CAS.
- S. Jitngarmkusol, J. Hongsuwankul and K. Tananuwong, Food Chem., 2008, 110, 23–30 CrossRef CAS PubMed.
- M. Barac, S. Cabrilo, M. Pesic, S. Stanojevic and S. Zilic, Int. J. Mol. Sci., 2010, 11, 4973–4990 CrossRef CAS PubMed.
- G. Krešić, V. Lelas, A. R. Jambrak, Z. Herceg and S. R. Brnčić, J. Food Eng., 2008, 87, 64–73 CrossRef.
- H. Hu, J. Wu, E. C. Y. Li-chan, L. Zhu, F. Zhang, X. Xu, G. Fan, L. Wang, X. Huang and S. Pan, Food Hydrocolloids, 2013, 30, 647–655 CrossRef CAS.
- C. K. Nagar, S. K. Dash and K. Rayaguru, J. Food Process. Preserv., 2022, 46, e16872 CAS.
- E. Alpizar-Reyes, V. Varela-Guerrero, J. Cruz-Olivares, H. Carrillo-Navas, J. Alvarez-Ramirez and C. Pérez-Alonso, Int. J. Biol. Macromol., 2020, 145, 207–215 CrossRef CAS PubMed.
- T. Maeda, H. Yamashita and N. Morita, Carbohydr. Polym., 2007, 68, 658–664 CrossRef CAS.
- N. Morimoto, A. Tabara and M. Seguchi, Food Sci. Technol. Res., 2015, 21, 309–316 CrossRef.
- C. Ferrero, Food Hydrocolloids, 2017, 68, 15–22 CrossRef CAS.
- H. Zhang, H. Cui, F. Xie, Z. Song and L. Ai, Food Hydrocolloids, 2024, 155, 110222 CrossRef CAS.
- X. Ren, W. Zheng, L. Li, S. Feng, H. Zhang, Z. Xiong, Y. Wu, Z. Song, L. Ai and F. Xie, J. Sci. Food Agric., 2023, 103, 6574–6583 CrossRef CAS PubMed.
- K. Jang, Y. E. Hong, Y. Moon, S. Jeon, S. Angalet and K. Meera, Food Sci. Biotechnol., 2018, 27, 1639–1648 CrossRef CAS PubMed.
- X. Sun, R. Guo, T. Zhan, Y. Kou, X. Ma, H. Song, W. Zhou, L. Song, H. Zhang, F. Xie, C. Yuan, Z. Song and Y. Wu, Food Hydrocolloids, 2024, 149, 109579 CrossRef CAS.
- F. Xie, W. Zheng, T. Fu, K. Zhu, H. Zhang, Z. Song and L. Ai, Food Hydrocolloids, 2024, 153, 110022 CrossRef CAS.
- B. Kim, M. Takemasa and K. Nishinari, Biomacromolecules, 2006, 7, 1223–1230 CrossRef CAS PubMed.
- F. Xie, X. Ren, Z. Zhu, J. Luo, H. Zhang, Z. Xiong, Y. Wu, Z. Song and L. Ai, Food Hydrocolloids, 2023, 142, 108761 CrossRef CAS.
- W. koosamart, N. Veerasugwanit and W. Jittanit, SHS Web Conf., 2016, 23, 03003 CrossRef.
- C. Viebke, S. Al-assaf and G. O. Phillips, Bioact. Carbohydr. Diet. Fibre, 2014, 4, 101–114 CrossRef CAS.
- E. Dickinson, Food Hydrocolloids, 2003, 17, 25–39 CrossRef CAS.
- C. Ferrero, Food Hydrocolloids, 2016, 68, 15–22 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |