 Open Access Article
Open Access ArticleEffect of lactic acid fermentation on the physico-chemical, functional, and antioxidant properties, and in vitro protein digestibility of malted ragi (Eleusine coracana L.)
Rahul
Dev
,
Shriya
Bhatt
and
Mahesh
Gupta
 *
*
Food and Nutraceutical Laboratory, Dietetics and Nutrition Technology Division, CSIR-Institute of Himalayan Bioresource Technology, Palampur, 176061, Himachal Pradesh, India. E-mail: mgupta@ihbt.res.in
First published on 14th June 2024
Abstract
Ragi is a widely recognized “Shree Anna” that should be included in diets to augment food diversity and security amid climate change challenges. The aim of the study was to evaluate the effect of lactic acid fermentation on the physico-chemical, functional, antinutritional, and antioxidant properties, and in vitro protein digestibility of raw and malted ragi flour at intervals of 24 and 48 h. RRF and MRF were inoculated with Lactiplantibacillus plantarum, oven-dried and milled into flour samples at each fermentation time. The process of optimizing malted ragi involves germinating soaked grains at 28 °C for 24 h, followed by open-pan roasting at 70 °C. The results showed a significant increase (p < 0.05) in carbohydrate content, water absorption index, and in vitro protein digestibility (63.66 to 79.98% and 85.77 to 90.27%) with increased fermentation time. However, the antinutrient content of phytic acid was significantly reduced with increasing fermentation time. During the 48 hour fermentation period, the crude protein content of both raw ragi flour and malted ragi flour varies from 7.01% to 7.75% and 8.18% to 8.64%, respectively. The 48 h fermented malted flour contains a significant amount of bioactive compounds, including catechin and protocatechuic acid. There was a significant increase (p < 0.05) in the total phenolic content and total flavonoid content. Thus, fermenting malted ragi flour for 48 h is an effective approach for enhancing protein digestibility and bioactive components, with a significant reduction in antinutrient content.
Sustainability spotlightTraditional food processing techniques, such as malting and fermentation, have the potential to significantly enhance the nutritional quality and functional properties of ragi flour. Incorporating ragi into diets can enhance their diversity, increase resilience against climate change, and address food insecurity and malnutrition in vulnerable regions. The study highlights fermented malted ragi flour as a sustainable solution, utilizing local, resilient crops and employing sustainable processing techniques that align with various United Nations Sustainable Development Goals (SDGs). This supports SDG 2 (Zero Hunger) by promoting nutritious food options, SDG 3 (Good Health and Well-being) through enhanced bioactive compounds, and SDG 12 (Responsible Consumption and Production) by fostering sustainable food systems. |
1 Introduction
Finger millet, locally known as mandua or ragi (Eleusine coracana L.), is an important cereal (minor millet) cultivated in diverse regions of the North Western Himalayas under rainfed organic conditions.1 It can be grown alone or as an intercrop with pulses and oilseeds to provide food, fodder, fuel, and nutritional security.2 Climate change threatens crop productivity through various stresses. Prioritizing staple crops overlooks resilient, nutritious millets like finger millet, vital for food and nutritional security in harsh climates.3 Being a climate-smart crop, it contributes significantly to sustainable agriculture because it requires minimal carbon and water inputs for growth and can thrive on marginal land.2 According to data from the World Summit on Food Security, food production needs to increase by at least 70% by 2050 to meet the demands of the growing population.4 Because of this scenario, finger millet has emerged as a crucial point of scientific research due to its distinctive capacity to flourish in conditions of low moisture and high temperature.3 Compared to other cereals, millets are more nutritious and are now referred to as “nutri-cereals”.5India is the world's leading producer of millet crops, contributing 18 million tons (mt), which accounts for 60% of the world's production, followed by China (2.7 mt) and Niger (2.15 mt).6 In the hilly and sub-mountainous regions of the Indian Himalayas, ragi is the most significant minor millet cultivated, serving as a staple food for poor communities in these areas. Ragi is a traditional crop with significant health benefits and high nutritional value.7 On average, it contains 1.5% fat, 7.7% protein, 2.7% minerals, 18% dietary fiber, 72.6% carbohydrates, 0.61% tannins, and 0.48% phytates.8 It is an excellent source of micronutrients like calcium, phosphorus, iron, and magnesium, with a higher concentration of calcium (344 mg per 100 g) than rice and wheat, as well as essential amino acids such as lysine, cysteine, and methionine. It provides B-group vitamins such as niacin, thiamine, riboflavin, and folic acid.9 Additionally, it has nutraceutical properties that have antimicrobial, antidiabetic, anti-inflammatory, and anti-tumorigenic effects.10 Therefore, finger millet is not only a vital crop, but it also offers substantial nutritional value and various health benefits, making it a valuable component of the local diet and economy. Numerous products can be derived from finger millet, such as malted and fermented flour and beverages.11 Traditionally, it is consumed in the form of flour-based foods like puttu (sweet pudding), mudde (stiff porridge/dumpling), roti (unleavened pancake), jandh (a type of beer), and ambli (thin porridge), each with their own distinct characteristics.12
To improve the nutritional qualities and extend the shelf life of food, various techniques can be used, such as soaking, germination, malting, and fermentation.13 Malted and fermented cereals are an important part of our diet, providing a substantial source of nutrients.14 As reported by Desai et al.15 and Kalpana et al.,16 malting enhances the activity of hydrolytic enzymes, which boosts amino acid content, total sugars, and B-group vitamins while reducing starch and dry matter. Furthermore, fermentation enhances the texture, shelf life, taste, aroma, digestibility, and nutritional value of cereals while notably reducing antinutrients.17 Jan et al.18 found that the lactic acid fermentation of finger millet is an effective method for enhancing the bioavailability of micronutrients and increasing their phenolic content and antioxidant activities. Fermentation and malting were found to enhance pearl millet flour's physicochemical and nutritional properties.19 Onyango et al.20 found that combining malting and fermentation improves protein digestibility, reduces anti-nutrients, and extends the shelf life of sorghum and pearl millet. Overall, Adebiyi et al.21 also suggest that integrating these techniques enhances nutritional value, protein digestibility, mineral availability, and functional properties while reducing phytic acid, thereby extending the shelf life of finger millet flour. This improvement over conventional processing methods, such as grinding and sieving, also supports the diversification of finger millet-based products.
Hence, the combination of malting and fermentation can have a synergistic effect on the nutritional profile of ragi. There is, however, limited information on the physicochemical changes induced by the fermentation and malting of ragi flour. Therefore, the present study aimed to evaluate the effect of malting and fermentation treatments on an underutilized local ragi cultivar on the physicochemical, functional, and antioxidant properties, and in vitro protein digestibility of ragi flour, thereby including it in diets to improve food diversity and strengthen food security.
2 Materials and methods
2.1 Material
The ragi variety (VL-M-380) was purchased from the Bandal Ghati Swayat Shkarita, Dehradun (India). The bacterial strains used in this study, namely Lactiplantibacillus plantarum strains, were previously isolated in the lab. The total dietary fiber kit (K-TDFR-100A) was obtained from Megazyme (Wicklow, Ireland). Analytical-grade reagents and chemicals of the highest purity were used for the analysis.2.2 Preparation of ragi malt
The seeds of ragi (Eleusine coracana) millet (VL-M-380) were manually cleaned and washed to remove the adhering dust, dirt, or foreign particles. After draining excess water, the grains were dried in a hot air oven at 60 °C for 1 h before being milled to make raw ragi flour (RRF). Additionally, the washed seeds were optimized for soaking in (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/v) water for about 24 h and then drained. The seeds were then spread in a thin layer within a tray covered with moist muslin cloth. The germination process was optimized to allow the seeds to germinate in the dark at 28 °C for 24 h, using the acrospire length as a basis for optimization. Later, the seeds were oven-dried at 45 °C for 6 h and roasted in an open pan at 70 °C until the color of the acrospires turned from white to brownish-yellow. The grains obtained were then ground using a cutting mill (SM-100, Retsch, Germany) and then subjected to sieving through a 42-mesh (0.354 mm) sieve to produce malted ragi flour (MRF) and stored in polyethylene bags to facilitate further analysis (Fig. 1).
4 w/v) water for about 24 h and then drained. The seeds were then spread in a thin layer within a tray covered with moist muslin cloth. The germination process was optimized to allow the seeds to germinate in the dark at 28 °C for 24 h, using the acrospire length as a basis for optimization. Later, the seeds were oven-dried at 45 °C for 6 h and roasted in an open pan at 70 °C until the color of the acrospires turned from white to brownish-yellow. The grains obtained were then ground using a cutting mill (SM-100, Retsch, Germany) and then subjected to sieving through a 42-mesh (0.354 mm) sieve to produce malted ragi flour (MRF) and stored in polyethylene bags to facilitate further analysis (Fig. 1).
2.3 Physical and functional properties of ragi grain/flour
The physical and functional properties of the RRF and MRF were analyzed using standard procedures, specifically examining their thousand kernel weight, length, and thickness. Bulk density was determined using eqn (1), which is the ratio of the mass of the sample to its volume. The dispersibility index is a metric used to measure the ability of flour to rehydrate with water. The percent dispersibility was calculated using eqn (2), whereby 1 g of the flour sample was weighed into a 15 mL measuring Falcon tube, and 10 mL of distilled water was added, vigorously stirred, and allowed to stand for 3 h. The water activity meter model 4TE by Aqua Lab with a dew point sensor was used to measure water activity (aw) from the ground sample. 2 g of sample was loaded into the sample chamber and allowed to equilibrate before measuring the aw values. | (1) |
| Dispersibility (%) = 100 − volume of settled particles | (2) |
The oil absorption index and water absorption index (OAI and WAI) were calculated by using eqn (3) and (4) as outlined by Dev and Gupta.22 Briefly, 2.5 g of samples was blended with 30 mL of distilled water for WAI and vegetable oil (sunflower) for OAI, vortexed for a minute, and allowed to stand at room temperature for 30 min. The samples were then centrifuged, and the residues were weighed after decanting the excess oil and water.
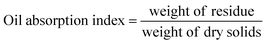 | (3) |
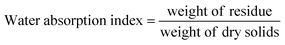 | (4) |
2.4 Physicochemical properties of ragi flours
The compositional analysis of RRF and MRF with other fermentation treatments was carried out using an AOAC method23 for measuring moisture, protein, fat, ash, and crude fiber contents. The moisture content of the sample flours was determined by drying the samples in a hot air oven at 105 ± 5 °C for 1 h until a stable weight was obtained. The weight reduction indicated the moisture content of the sample. The crude protein and fat content were analyzed using a KjelTRON KDIGB 20M nitrogen analyzer and SoxTRON Sox-6 from Tulin Equipments. To calculate the protein content, the determined nitrogen content was calculated using a Kjeldahl apparatus and then multiplied by a factor of 6.25. For fat content, the samples were weighed and placed in thimbles, then extracted using a Soxhlet extractor with petroleum ether. The weight of the fat was noted after the complete evaporation of the solvent. Ash content was analyzed using an electric muffle furnace by incinerating the samples at 580 ± 5 °C for 6 h. The acid–alkali digestion method was used to estimate the crude fibre content, while the percentage difference method of the other proximate indexes was used to determine the total carbohydrate content. Furthermore, the energy value (kcal per 100 g) was calculated by summing the energy conversion factors described by FAO24 for carbohydrate, crude fat, and protein, namely 4, 9, and 4 kcal g−1, respectively. Analysis of total dietary fiber, including soluble and insoluble dietary fiber, was performed using an enzymatic-gravimetric method, AOAC method 985.29, and a K-TDFR 100A kit (Megazyme). Analysis of phytic acid was performed using a colorimetric molybdenum-blue assay (K-PHYT 50A kit, Megazyme) by the method outlined by McKie and McCleary.25 The pH of the ragi flour samples was estimated by adding 100 mL of distilled water to 10 g of each flour sample and thoroughly stirring until homogeneous. The pH was subsequently measured using a pre-calibrated Eutech digital pH meter (Eutech India Pvt. Ltd). For total titratable acidity (TTA), 10 g of fermented raw and malted ragi flour samples were dissolved in 10 mL of distilled water with 2 drops of phenolphthalein and titrated using a base solution of 0.1 N NaOH until a pale pink color appeared. TA was expressed as citric acid equivalents.2.5 Fermentation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 dilution into the previously autoclaved RRF & MRF slurry. After the inoculation, samples were incubated at 37 °C for intervals of 24 h (RRF 24 h and MRF 24 h) and 48 h (RRF 48 h and MRF 48 h), respectively. Subsequently, the samples were subjected to oven drying at 50 °C for 12 h (Fig. 1).
CFU mL−1 dilution into the previously autoclaved RRF & MRF slurry. After the inoculation, samples were incubated at 37 °C for intervals of 24 h (RRF 24 h and MRF 24 h) and 48 h (RRF 48 h and MRF 48 h), respectively. Subsequently, the samples were subjected to oven drying at 50 °C for 12 h (Fig. 1).
2.6 Microbiological analysis
The LAB count in each fermenting sample blend (RR and MR) was determined by homogenizing 1 g of each sample with 9 mL of PBS. Subsequently, 100 μL of the sample was mixed with 900 μL of phosphate-buffered saline (PBS) solution for a ten-fold serial dilution at a 10−1 level. The resulting dilutions were cultured onto MRS agar plates using the spread plate technique. Pure cultures of isolated colonies of fermenting organisms (LAB) were obtained on MRS medium by incubating inoculated plates at 37 °C for 48 h under anaerobic conditions.26 For each sample, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 was estimated, and plate counts were determined at 24 h and 48 h.
CFU mL−1 was estimated, and plate counts were determined at 24 h and 48 h.
2.7 In vitro protein digestibility of ragi flours
A three-stage in vitro digestion model simulating the oral, gastric, and intestinal phases of human digestion was constructed, with some minor modifications as described by Menekus et al.27 In the oral phase, 5 g of RRF and MRF was mixed with 3.5 mL of SSF (simulated salivary fluid) and left to activate for 10 min. Afterwards, 0.5 mL of porcine pancreas α amylase (75 U mL−1), 25 μL of 0.3 M CaCl2, and 975 μL of distilled water were added, and the mixture was incubated at 37 °C for 2 min. Thereafter, in the gastric phase, 10 mL of oral bolus was taken, and to that 7.5 mL of simulated gastric fluid (SGF), 1.6 mL of porcine pepsin solution (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1), 5 μL of 0.3 M CaCl2, and 0.695 μL of distilled water were added, and the pH was adjusted to 3.0 with the help of 0.2 mL of 1 M HCl. The mixture was then incubated for 2 h at 37 °C in a shaking incubator. In the intestinal phase, 20 mL of gastric chyme was combined with 11 mL of SGF, 750 μL of pancreatin from porcine pancreas, 2.5 mL of bile, 40 μL of 0.3 M CaCl2, and 1.31 mL of distilled water, and the pH was adjusted to 7.0 with the help of 1 M NaOH and incubated for 2 h at 37 °C. After incubation, the samples were preserved at 4 °C to cease enzyme activity and later used for further analysis. The total and residual protein contents of all the samples were quantified using the Kjeldahl method. Protein digestibility is expressed as a percentage and calculated using eqn (5).
000 U mL−1), 5 μL of 0.3 M CaCl2, and 0.695 μL of distilled water were added, and the pH was adjusted to 3.0 with the help of 0.2 mL of 1 M HCl. The mixture was then incubated for 2 h at 37 °C in a shaking incubator. In the intestinal phase, 20 mL of gastric chyme was combined with 11 mL of SGF, 750 μL of pancreatin from porcine pancreas, 2.5 mL of bile, 40 μL of 0.3 M CaCl2, and 1.31 mL of distilled water, and the pH was adjusted to 7.0 with the help of 1 M NaOH and incubated for 2 h at 37 °C. After incubation, the samples were preserved at 4 °C to cease enzyme activity and later used for further analysis. The total and residual protein contents of all the samples were quantified using the Kjeldahl method. Protein digestibility is expressed as a percentage and calculated using eqn (5).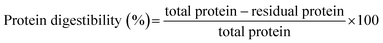 | (5) |
2.8 Total phenolic content (TPC) and total flavonoid content (TFC)
The phenolic and flavonoid content of the RRF and MRF 48 h samples was determined by preparing an extract using 70% ethanol in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (sample
10 (sample![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent) ratio using a digital magnetic stirrer, and keeping it at 28 °C for 2 h. Following centrifugation, the supernatant was collected for quantification of TPC and TFC using the methodology outlined by Dev and Gupta.22 Gallic acid and catechin were used as reference standards for TPC and TFC, respectively.
solvent) ratio using a digital magnetic stirrer, and keeping it at 28 °C for 2 h. Following centrifugation, the supernatant was collected for quantification of TPC and TFC using the methodology outlined by Dev and Gupta.22 Gallic acid and catechin were used as reference standards for TPC and TFC, respectively.
2.9 High-performance liquid chromatography (HPLC) quantification
For quantification of phenolics, a gradient method developed by Dadwal et al.28 was performed on an HPLC system (Agilent 1260 Infinity II) equipped with a diode array detector and separation column (LIChrospher®100 RP-18, 5 μm) at a temperature of 35 °C. The mobile phase used consisted of water and acetonitrile, containing 0.1% (v/v) and 0.05% (v/v) formic acid, respectively. Several gradient combinations were optimized to effectively separate the target phenolics using an injection volume of 10 μL at a flow rate of 1 mL min−1. Chromatograms were analyzed at 280 nm using reference standards including gallic acid, protocatechuic acid, caffeic acid, chlorogenic acid, ferulic acid, syringic acid, catechin, rutin, and quercetin.2.10 Color measurements
A reflectance colorimeter from KONICA MINOLTA, Chroma-Meter model CR-400, was used to record the colorimetric analysis (L*, a*, and b*) of RRF and MRF, with L* representing the lightness–darkness, a* indicating red–green, and b* indicating yellow–blue values of the samples.2.11 Statistical analysis
Analytical tests were performed in triplicate (n = 3), with the standard deviation (±) recorded as errors from the mean, and statistical analysis was performed using GraphPad Prism 9 software by Dotmatics employing two-way analysis of variance (ANOVA) with Tukey's multiple comparisons test at a significance level of p < 0.05.3 Results and discussion
3.1 Physical and functional properties of raw and malted ragi grains/flours
The physical properties of RRF and MRF exhibited changing characteristics, as described in Table 1. The dimensions of the raw and malted ragi grains ranged from 1.47 to 1.55 mm in length, 1.41 to 1.57 mm in width, and 1.04 to 1.1 mm in thickness. Another important physical property includes the weight of the kernel, which ranges from 3.22 g to 3.39 g per thousand samples.| Parameters | Raw ragi | Malted ragi |
|---|---|---|
| a Results are expressed as mean ± std. dev.; values in the same row which are not significantly different (p < 0.05) are indicated by identical superscript letters. | ||
| Length (mm) | 1.47 ± 0.01a | 1.55 ± 0.01a |
| Width (mm) | 1.41 ± 0.01a | 1.57 ± 0.01a |
| Thickness (mm) | 1.04 ± 0.02a | 1.1 ± 0.01a |
| 1000 sample wt (g) | 3.22 ± 0.08a | 3.39 ± 0.12a |
| Bulk density (kg m−3) | 771.28 ± 0.97a | 678.48 ± 0.79b |
| WAI (g g−1) | 1.88 ± 0.07a | 1.97 ± 0.04a |
| OAI (g g−1) | 2.04 ± 0.05a | 2.26 ± 0.08a |
| Water activity (in aw) | 0.51 ± 0.01a | 0.53 ± 0.01a |
| Dispersibility (%) | 83.59 ± 0.24a | 84.72 ± 0.39b |
The functional characteristics, which include bulk density, oil absorption capacity, water absorption capacity, water activity and dispersibility, also revealed changes in characteristic properties. The bulk density exhibited a significant decrease (p < 0.05) from 771.28 ± 0.97 kg m−3 to 678.48 ± 0.79 kg m−3 in raw and malted ragi grain. Yenasew and Urga29 observed that the malting process results in a decrease in bulk density, leading to the breakdown of complex compounds of starch into smaller units. This is due to the action of enzymes, which dextrinify the starch into smaller subunits, resulting in flours with a lower bulk density and a higher nutrient density.30 Ramappa et al.31 also reported comparable findings, showing similar ranges in bulk density and thousand sample weight. In contrast, the water absorption index and oil absorption index showed no significant difference between raw and malted ragi grains. A similar observation has also been reported by Kumar et al.32 The water activity of RRF and MRF was 0.51 ± 0.01 and 0.53 ± 0.01 aw, respectively. However, dispersibility exhibited a significant difference between RRF and MRF, indicating that the germination process enhanced the dispersibility of the MRF. Dispersibility represents the ease of reconstitution of flour samples, and a higher dispersibility value indicates better reconstitution in water with less ability of lump formation during preparation.33
3.2 Composition of raw and malted ragi flour
The changes in composition of RRF and MRF are presented in Table 2. The non-significant difference in moisture content observed in malted ragi as compared to raw ragi could be attributed to water absorption by germinated seeds.34 Interestingly, the protein content and crude fiber exhibited a significant increase (p < 0.05) in MRF as compared to RRF. This increase could be accredited to the process of germination, resulting in the synthesis of new protein–fiber complexes that support the growth of seedlings, including roots and shootlets.19 Owheruo et al.35 and Hiremath and Geetha36 also observed a similar increase in the protein and crude fiber content in the malted finger millet flour samples. In contrast, a non-significant difference (p < 0.05) in fat and ash content was found between RRF and MRF. However, Hiremath and Geetha36 also reported a significant reduction in fat and ash content in malted ragi when compared to raw ragi.| Parameters | Raw ragi flour | Malted ragi flour |
|---|---|---|
| a Results are expressed as mean ± std. dev.; values in the same row which are not significantly different (p < 0.05) are indicated by identical superscript letters. | ||
| Moisture (%) | 9.92 ± 0.35a | 10.73 ± 0.33a |
| Fat (%) | 1.44 ± 0.12a | 1.19 ± 0.17a |
| Protein (%) | 7.01 ± 0.45a | 8.18 ± 0.57b |
| Crude fibre (%) | 4.78 ± 0.41a | 5.93 ± 0.35b |
| Ash (%) | 2.41 ± 0.12a | 2.18 ± 0.11a |
| Carbohydrates (%) | 74.44 ± 1.03a | 71.79 ± 0.77b |
| Energy (kcal) | 338.75 ± 1.9a | 330.62 ± 3.36b |
| Phytic acid (mg per 100 g) | 252.14 ± 3.04a | 220.96 ± 1.99b |
| Dietary fibre (%) | 12.66 ± 0.47a | 16.31 ± 0.33b |
In addition, ragi is a rich source of dietary fibre. Its high fibre content slows down the digestion process, allowing individuals to sustain their energy levels for extended periods of time on a single meal.37 Malting increases the dietary fiber content of ragi flour from 12.66% to 16.31%. This significant rise (p ≤ 0.05) resulted from changes to the cell walls, tissue integrity, and protein–carbohydrate interactions, leading to new fiber biosynthesis.38 Similar effects were observed in kodo millet and amaranth flour.38,39 In the present study, a significant (p < 0.05) reduction was observed in the phytic acid content from RRF to MRF due to an increase in the hydrolytic activity of the enzyme phytase as a result of germination.40 Sharma et al.39,41 also observed a decrease in phytic acid content in foxtail and kodo millets as a result of germination. The carbohydrate content in RRF was observed to be 74.68 ± 1.07%, while in MRF it was 70.73 ± 0.90%. According to Murungweni et al.,42 malting enhanced the enzymatic degradation of carbohydrates in ragi flour by encouraging the production of enzymes like α-amylase, which breaks down starch into simple sugars and provides energy for seed growth, thus lowering its energy value. The energy content of RRF and MRF was 339.72 ± 1.75 kcal and 326.37 ± 3.17 kcal, respectively. Devi and Modgil34 have also observed a similar decrease in carbohydrate and energy content in MRF, which is positively correlated with the present study.
3.3 Changes in pH and titratable acidity during fermentation of RRF and MRF
A statistically significant (p < 0.05) decrease in pH was observed with a simultaneous rise in titratable acidity during the fermentation of RRF and MRF (Fig. 2). The pH of the MRF and RRF decreased from 6.23 to 4.38 and 5.69 to 4.25, respectively, during the 48 hour fermentation period, whereas the total titratable acidity (TTA) of MRF and RRF increased during the fermentation process from 0.03 to 0.10% and 0.05 to 0.11%, respectively. Mutshinyani et al.43 reported a similar finding on TTA and a decrease in pH during the fermentation of ragi flour. The reduction in pH may be attributed to the soluble organic acids released from ragi flour during fermentation by lactic acid bacteria. A decrease in pH during fermentation prolongs the shelf life of the flours, as bacteria cannot survive close to a pH of 4.44 Additionally, the rise in TTA in ragi flours is also due to the production of organic acids through the bacterial conversion of glucose and carbon dioxide into an equal mix of carbon dioxide, ethanol, and lactic acid.35 | ||
| Fig. 2 Changes in pH and TTA of fermented raw and malted ragi flour. All values are expressed as mean ± std. dev. (n = 3), while significant differences (p < 0.05) are indicated by letters. | ||
3.4 Microbial analysis of fermented flours
The lactobacilli count in fermented RRF and MRF samples with fermentation time is shown in Fig. 3. At the end of 24 h of fermentation, the cell count of RR was 2.2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1, which increased to 9.2 log
CFU mL−1, which increased to 9.2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 at the end of 48 h. While the cell count of MR after 24 h of fermentation was observed to be 4.2 log
CFU mL−1 at the end of 48 h. While the cell count of MR after 24 h of fermentation was observed to be 4.2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1, it increased to 5.8 log
CFU mL−1, it increased to 5.8 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 at the end of 48 h. The initial increase in lactobacilli count observed in MRF might be due to the hydrolysis of germinated flours, which produces desirable acids, flavors, and peptides that inhibit the growth of undesirable organisms.11 The results of this combination of RRF and MRF fermented treatments at 48 h revealed a significant (p < 0.05) decrease in values of 9.2 log
CFU mL−1 at the end of 48 h. The initial increase in lactobacilli count observed in MRF might be due to the hydrolysis of germinated flours, which produces desirable acids, flavors, and peptides that inhibit the growth of undesirable organisms.11 The results of this combination of RRF and MRF fermented treatments at 48 h revealed a significant (p < 0.05) decrease in values of 9.2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 and 5.2 log
CFU mL−1 and 5.2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1, respectively. The findings were consistent with those of Ilango and Antony,45 who demonstrated that pathogens were inhibited during the fermentation of finger millet beverages, apparently due to the acid production by LAB.
CFU mL−1, respectively. The findings were consistent with those of Ilango and Antony,45 who demonstrated that pathogens were inhibited during the fermentation of finger millet beverages, apparently due to the acid production by LAB.
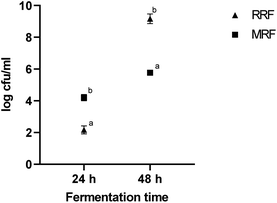 | ||
| Fig. 3 Cell counts of fermented RRF and MRF. All values are expressed as mean ± std. dev. (n = 3), while significant differences (p < 0.05) are indicated by letters. | ||
3.5 Fermentation treatments with LAB
Table 3 presents the variations in the nutritional and functional components of fermented RRF and MRF with LAB. The moisture content ranged from 9.92% to 7.75% in RRF and 10.73% to 7.24% in MRF, exhibiting decreased moisture content with increasing fermentation time, which is likely attributable to the drying process of fermented flours. The LAB treated RRF and MRF reported reduced moisture content compared to unfermented flours, which can be attributed to their related dry matter content. Mutshinyani et al.43 observed a similar decline in the moisture content of fermented ragi flours with the increase of fermentation time. A non-significant difference in the ash content was observed during the fermentation of RRF and MRF, from 2.41 to 2.54% and 2.18 to 2.24%, respectively. The crude fat content varied from 1.44 to 1.2% in RRF and 1.19 to 0.9% in MRF. The present study observed comparable fat content in the fermented RRF to that reported by Jan et al.18 and Mutshinyani et al.43| Sample | RRF | Fermentation | MRF | Fermentation | ||
|---|---|---|---|---|---|---|
| RRF 24 h | RRF 48 h | MRF 24 h | MRF 48 h | |||
| a Results are expressed as mean ± std. dev.; values in the same row which are not significantly different (p < 0.05) are indicated by identical superscript letters. RRF = raw ragi flour, RRF 24 h = raw ragi flour after 24 h of fermentation, RRF 48 h = raw ragi flour after 48 h of fermentation, MRF = malted ragi flour, MRF 24 h = malted ragi flour after 24 h of fermentation, MRF 48 h = malted ragi flour after 48 h of fermentation. | ||||||
| Moisture (%) | 9.92 ± 0.35a | 8.85 ± 0.24b | 7.75 ± 0.43c | 10.73 ± 0.33a | 8.44 ± 0.39b | 7.24 ± 0.32c |
| Crude fat (%) | 1.44 ± 0.12a | 1.32 ± 0.30a | 1.21 ± 0.31a | 1.19 ± 0.17a | 1.06 ± 0.21a | 0.9 ± 0.18a |
| Crude protein (%) | 7.01 ± 0.45a | 7.18 ± 0.20a | 7.75 ± 0.16a | 8.18 ± 0.57a | 8.56 ± 0.37a | 8.64 ± 0.35a |
| Ash (%) | 2.41 ± 0.12a | 2.49 ± 0.07a | 2.54 ± 0.09a | 2.18 ± 0.11a | 2.23 ± 0.10a | 2.24 ± 0.08a |
| Crude fiber (%) | 4.78 ± 0.41a | 3.7 ± 0.50a | 2.94 ± 0.31b | 5.93 ± 0.35a | 4.42 ± 0.21b | 2.78 ± 0.26c |
| Carbohydrate (%) | 74.44 ± 1.03a | 76.46 ± 0.30b | 77.81 ± 0.76c | 71.79 ± 0.07a | 75.28 ± 0.23b | 78.20 ± 0.97c |
| Energy (kcal) | 338.75 ± 1.99a | 346.44 ± 2.84b | 353.07 ± 5.08c | 330.62 ± 3.36a | 344.91 ± 1.25b | 355.45 ± 3.84c |
| Water absorption index (g g−1) | 1.88 ± 0.07a | 3.02 ± 0.07b | 3.77 ± 0.09b | 1.97 ± 0.04a | 3.22 ± 0.10b | 4.19 ± 0.08c |
| Oil absorption index (g g−1) | 2.04 ± 0.05a | 2.30 ± 0.06a | 2.43 ± 0.08a | 2.26 ± 0.08a | 2.54 ± 0.11a | 2.57 ± 0.04a |
| Water activity (aw) | 0.51 ± 0.01a | 0.44 ± 0.00a | 0.42 ± 0.00a | 0.53 ± 0.01a | 0.43 ± 0.01a | 0.40 ± 0.00a |
| Dispersibility (%) | 84.72 ± 0.39a | 82.11 ± 0.81b | 81.14 ± 0.36b | 83.59 ± 0.24a | 78.31 ± 0.66b | 77.51 ± 0.57b |
| Phytic acid (mg per 100 g) | 252.14 ± 3.04a | 232.24 ± 1.49b | 212.33 ± 2.29c | 220.96 ± 1.99a | 208.35 ± 2.29b | 180.48 ± 1.14c |
| In vitro protein digestibility IVPD (%) | 63.66 ± 1.78a | 76.77 ± 1.12b | 79.98 ± 0.69c | 85.77 ± 1.03a | 89.31 ± 1.05b | 90.27 ± 0.35c |
The non-significant difference in crude protein content during fermentation was observed from 7.01 to 7.75% in RRF and 8.81 to 8.64% in MRF, respectively. The fermented flours of both RRF (7.75%) and MRF (8.64%) samples after 48 h of fermentation contain the highest protein value. The synthesis of amino acids may have contributed to elevating protein levels in the fermentation process of ragi flours, thereby enhancing the overall protein content of the fermented flours.11 The crude protein values of ragi from fermented and malted samples were a consequence of the accumulation of proteins and the creation of additional amino acids as a result of an increase in fermentation and malting duration.19 The protein content of RR and MR flours during fermentation in this study is comparable to the values reported by Oshodi et al.46 and Inyang and Zakari47 for pearl, quinoa, and fermented ragi flour.
There was a significant decrease (p < 0.05) in the crude fiber content with an increase in fermentation time, ranging from 2.94 to 4.78% in RRF and 2.78 to 5.93% in MRF, respectively. The decrease in the crude fiber content of fermented flours could be ascribed to the degradation of fiber by fermenting microbes.18 This fermentation might result in the enzymatic degradation of fiber by LAB during fermentation, using fiber as a source of carbon. The values of carbohydrate increased significantly (p < 0.05) from 74.44 to 77.81% in RRF and 71.79 to 78.20% in MRF, respectively. Ragi is a carbohydrate rich grain containing both free sugars and starch.48 This significant increase in carbohydrate content could be attributed to the reduced moisture content resulting from the increased fermentation time. Furthermore, Sharma et al.49 reported that LAB fermentation may have resulted in an enhanced carbohydrate content due to the degradation of complex polysaccharides into smaller molecular weight components, which also resulted in an increased caloric value of the food product. The results could be positively correlated with the crude fibre content values of fermented flour, where a significant reduction in fiber content was observed during fermentation. Additionally, LAB fermentation processes can produce secondary metabolites like organic acids and alcohols, which also contribute to the overall energy content of the fermented product.49 The energy values of the fermented RRF and MRF also increased significantly (p < 0.05) from 338.75 kcal per 100 g (RRF) to 353.07 kcal per 100 g (RRF 48 h) and 330.62 kcal per 100 g (MRF) to 355.45 kcal per 100 g (MRF 48 h), respectively.
The variations in the functional properties of fermented RRF and MRF samples with increasing fermentation time are shown in Table 3. The dispersibility of the fermented flours of RRF and MRF significantly (p < 0.05) decreased with the increase in fermentation. The reduction in the percentage dispersibility of fermented flours can be attributed to the formation of lumps with lower reconstitution ability.50 Oloyede et al.51 also observed a significant decrease in dispersibility of moringa seed flour with an increase in fermentation time. The WAI of fermented flours significantly (p < 0.05) increased from 1.88 to 3.77% in RRF and 1.97 to 4.19% in MRF with increasing fermentation time. This increase in WAI could be attributed to the increased damaged starch and surface area. Damaged starch is more hygroscopic than native starch and hence absorbs more water.44 The results are consistent with the previously reported literature by Oloyede et al.51 and Azeez et al.52 for moringa seed flour and brown ragi flour with increasing fermentation time. The OAI of fermented flour samples observed a non-significant difference ranging from 2.26 to 2.57% in MRF and 2.04 to 2.43% in RRF, respectively, with increased fermentation time. A similar trend was observed with a non-significant difference in the water activity of MRF and RRF with increased fermentation time. Oil absorption capacity and water absorption capacity are useful indicators of the protein's ability to prevent fluid loss during food storage.53
The anti-nutrient content of phytic acid was significantly reduced (p < 0.05) with an increase in fermentation time from 252.14 mg per 100 g (RRF) to 212.33 mg per 100 g (RRF 48 h), 220.96 mg per 100 g (MRF), and 180.48 mg per 100 g (MRF 48 h), with a maximum reduction of 15.79% in RRF 48 h, 12.37% in MRF, and 28.42% in MRF 48 h, respectively. The reduction in phytic acid may be attributed to the elevated activity of phytase, which significantly reduces the phytic acid content by hydrolyzing phytate into phosphate and myoinositol phosphate during fermentation.54,55 Furthermore, the reduction in phytic acid that occurs during fermentation improves mineral bioavailability, enhances digestibility, and reduces antinutritional factors.56 Therefore, the fermentation process with MRF proved to be the most effective in reducing the phytic acid content.
3.6 In Vitro protein digestibility (IVPD)
Table 3 presents the in vitro protein digestibility (IVPD) values of RRF and MRF with increasing fermentation time. The observation revealed that increased fermentation times of RRF and MRF resulted in a significant (p < 0.05) increase in the IVPD. Highest values of 79.98% in RRF and 90.27% in MRF were observed following 48 h of fermenting the flours, respectively. The enhanced IVPD could be attributed to the breakdown of proteins into smaller biopeptides, potentially adding biological value through increased proteolytic enzyme activity in fermenting bacteria, which is triggered by a reduction in pH.57 Moreover, lowering the levels of anti-nutritional factors promotes protein digestibility, while their increase leads to bonding with amino acids, thereby obstructing the proteolytic process.58 A similar trend indicating increased IVPD due to fermentation was reported by Azeez et al.,52 Sharma and Sharma,57 and Arshad et al.59 for finger millet, foxtail millet, and grass pea, respectively. Meanwhile, the increase in IVPD% from RRF to MRF during germination could be ascribed to the enhanced proteolysis carried out through intrinsic hydrolytic enzyme activity (proteases), resulting in increased protein digestibility and amino acid availability.60 The IVPD results of the present study showed that malting with fermentation can effectively enhance protein digestibility in ragi.3.7 Antioxidant profile
Table 4 presents the TPC, TFC, and antioxidant profile of the RRF and fermented MRF 48 h flour samples. It was observed that the TPC and TFC of RRF and the corresponding fermented MRF 48 h increased significantly (p < 0.05) with the fermentation. The increase in TPC observed during fermentation may be due to the synthesis of enzymes by the microorganisms involved in the process.18 These enzymes are likely to facilitate the release of phenolic compounds, converting bound phenols into free phenols.56 Furthermore, the observed increase in the TFC may be attributed to the metabolic alterations that occurred in the flour and produced secondary metabolites or flavonoids.61 Mutshinyani et al.43 and Jan et al.18 both observed a similar increase in TPC and TFC during the fermentation of ragi flour.| Sample | RRF | MRF 48 h |
|---|---|---|
| a Results are expressed as mean ± std. dev.; values in the same row which are not significantly different (p < 0.05) are indicated by identical superscript letters. RRF = raw ragi flour, MRF 48 h = malted ragi flour after 48 h of fermentation, NQ = not quantified. | ||
| TPC (mg GAE per 100 g) | 169.84 ± 3.37a | 241.85 ± 2.77b |
| TFC (mg CE per 100 g) | 76.41 ± 13.77a | 100.12 ± 9.25b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Polyphenols (mg per 100 g) | ||
| Gallic acid | 1.12 ± 0.00a | 0.15 ± 0.02a |
| Protocatechuic acid | 0.06 ± 0.07a | 2.16 ± 0.08b |
| Chlorogenic acid | 0.12 ± 0.05a | 0.10 ± 0.01a |
| Ferulic acid | 0.31 ± 0.05 | NQ |
| Caffeic acid | 0.12 ± 0.07 | NQ |
| Syringic acid | 0.12 ± 0.06a | 0.18 ± 0.05a |
| Catechin | 48.36 ± 0.27a | 85.71 ± 0.11b |
| Rutin | 0.26 ± 0.16 | NQ |
| Quercetin | 0.05 ± 0.00a | 0.07 ± 0.00a |
The polyphenolic profiles of the samples were analyzed through an HPLC analytical method. Previous studies have demonstrated that lactobacillus fermentation enhances the bioactive compounds and reduces the antinutritional factors in the millets.62 Consequently, the findings of the present study also demonstrate that fermenting MRF for 48 h resulted in a non-significant difference in the polyphenolic profile of some of the compounds in comparison to the RRF. The HPLC analysis successfully detected and validated the nine polyphenolic compounds in RRF, consistent with Xiang et al.63 Gallic acid, protocatechuic acid, chlorogenic acid, t-ferulic acid, caffeic acid, and syringic acid were the phenols, while three flavanols, namely catechin, rutin, and quercetin, were also identified in the RRF (Table 4). Catechin was identified as the predominant flavanol in the samples, with a significant increase of 77% observed between the RRF and MRF samples after 48 hours of fermentation. Furthermore, Rocchetti et al.64 also reported an increase in phenols in cooked pseudocereals fermented with lactic acid bacteria, which improves their nutritional value and supports health-promoting properties.
3.8 Color profile
The color values L*a*b* of fermented RRF and MRF are shown in Fig. 4. The L* (lightness) of MRF increased significantly (p < 0.05) compared to RRF, but the a* (redness) decreased. This increase in the L* of MRF compared to RRF could be ascribed to the starch content, while the decrease in a* and increase in b* values can be attributed to the soaking and drying process, as previously reported by Shingare and Thorat.65 Murungweni et al.42 observed that the color of malted ragi flour is often lighter than that of native ragi flour due to the enzymatic activities that occur during the malting process, which break down complex molecules, including the color pigments of the grain. Moreover, Devi et al.66 reported that production of malted FM flours involves heating and drying, which affects the pigmentation of the grains, and the presence of phenolic compounds, in the testa and pericarp of the grain, which was reduced by leaching, may be responsible for the color shift observed during malting, particularly in a* values. Furthermore, the results show a similar trend reported by Hejazi and Orsat67 and Nefale and Mashau68 for germinated and raw ragi flour. The lightness (L*) of both the RRF and MRF showed a significant decrease (p < 0.05) ranging from 61.53 to 56.75 and 62.84 to 59.29, respectively, with an increase in the fermentation time. The bright color of the ragi flour changed as the fermentation progressed. During fermentation, the a* (redness) decreased steadily from 3.43 to 2.45 and 3.63 to 3.13 for RR & MR, respectively. Conversely, the b* (yellowness) increased with prolonged fermentation, ranging from 8.06 to 8.64 and 8.28 to 8.79 for RR & MR, respectively. The reduction in redness could be due to the disintegration of the anthocyanins on the surface of ragi flour, while the increase in yellowness could be due to enzymatic oxidation of starch and polyphenols present in the flour during fermentation.434 Conclusions
Fermentation treatments were found to be effective in enhancing the nutritional composition and bioactive compounds of the MRF compared with the RRF. Fermentation time significantly increases the protein digestibility and water absorption index. However, dispersibility percentage and phytic acid content exhibited a decline with fermentation time. The lactobacilli count increased with the fermentation time, resulting in a significant (p < 0.05) decrease in pH and an increase in titratable acidity. A statistically significant increase in L* (lightness) and b* (redness) values was observed with a decrease in a* (redness) with increased fermentation time. Furthermore, the TPC, TFC, and polyphenolic profile of fermented MRF were enhanced. Thus, the traditional processing technique of fermentation enhances the utilization of underutilized native ragi by incorporating it into the development of functional products with high protein digestibility, lower phytic acid content, and better bioactive compounds.Author contributions
Rahul Dev: experimental work execution, data curation, methodology, software, manuscript writing – original draft; Shriya Bhatt: experimental work execution, software; Mahesh Gupta: conceptualization, supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors express sincere gratitude to the Director, CSIR-Institute of Himalayan Bioresource Technology for their valuable suggestions and encouragement. The institute manuscript number is 5537.References
- H. O. UdeH, K. G. Duodu and A. I. O. Jideani, Finger millet bioactive compounds, bio accessibility, and potential health effects – a review, Czech J. Food Sci., 2017, 35(1), 7–17, DOI:10.17221/206/2016-CJFS.
- S. Maitra and T. Shankar, Agronomic Management in Little Millet (Panicum sumatrense L.) for Enhancement of Productivity and Sustainability, Int. J. Bioresour. Sci., 2019, 6(2), 91–96, DOI:10.30954/2347-9655.02.2019.9.
- S. Purohit and J. B. Palai, Finger millet a boon to food security under changing climatic condition, Int. J. Chem. Stud., 2020, 8(3), 1385–1388, DOI:10.22271/chemi.2020.v8.i3s.9390.
- M. Tester and P. Langridge, Breeding technologies to increase crop production in a changing world, Science, 2010, 327(5967), 818–822 CrossRef CAS PubMed.
- B. V. Bhat, B. D. Rao and V. A. Tonapi, The Story of Millets, Karnataka State Department of Agriculture, Bengaluru and ICAR-Indian Institute of Millets Research, Hyderabad, India, 2018 Search PubMed.
- FAOSTAT, Crops Production, Food and Agriculture Organization of the United Nations, 2023 Search PubMed.
- B. V. Bhat, A. Arunachalam, D. Kumar, V. A. Tonapi and T. Mohapatra, Millets in the Indian Himalaya, Indian Council of Agricultural Research, New Delhi, 2019, p. 84 Search PubMed.
- Food and Agriculture Organization (FAO), 2014, http://faostat.fao.org/default.aspx.
- Y. Babita, A review of the nutritional properties of ragi (Eleusine coracana L.), Int. J. Plant Sci., 2023, 18(1), 73–78, DOI:10.15740/HAS/IJPS/14.1/19-24.
- D. Chandra, S. Chandra and A. Sharma, Review of finger millet (Eleusine coracana (L.) Gaertn): A power house of health benefiting nutrients, Food Sci. Hum. Wellness, 2016, 5, 149–155 CrossRef.
- M. R. Kambabazi, E. Hitayezu, Y. Mukandahiro and H. Vasanthakaalam, Assessment of microbiological changes during production of malted and fermented finger millet flour, RWA J. Agric. Sci., 2019, 1, 31–35 Search PubMed.
- J. R. N. Taylor and K. G. Duodu, Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes, AACC International Press, 2019, pp. 259–292, DOI:10.1016/B978-0-12-811527-5.00009-5.
- S. N. Hejazi, V. Orsat, B. Azadi and S. Kubow, Improvement of the in vitro protein digestibility of amaranth grain through optimization of the malting process, J. Cereal Sci., 2016, 68, 59–65, DOI:10.1016/j.jcs.2015.11.007.
- I. Amadou, M. E. Gounga and G. W. Le, Millets: nutritional composition, some health benefits and processing e a review, Emir. J. Food Agric., 2013, 25, 501e508 Search PubMed.
- A. D. Desai, S. S. Kulkarni, A. K. Sahoo, R. C. Ranveer and P. B. Dandge, Effect of Supplementation of Malted Ragi Flour on the Nutritional and Sensorial Quality Characteristics of Cake, Adv. J. Food Sci. Technol., 2010, 2(1), 67–71 CAS.
- P. Kalpana, W. Sushima and S. Krishnapura, Bio accessible Mineral Content of Malted Finger Millet (Eleusine coracana), Wheat (Triticum aestivum), and Barley (Hordeum vulgare), J. Agric. Food Chem., 2010, 58, 8100–8103 CrossRef PubMed.
- Z. Kohajdova and J. Karovicova, Fermentation of cereals for specific purpose, J. Food Nutr. Res., 2007, 46, 51e57 Search PubMed.
- S. Jan, K. Kumar, A. N. Yadav, N. Ahmed, P. Thakur, D. Chauhan, Q. H. Rizvi and H. S. Dhaliwal, Effect of diverse fermentation treatments on nutritional composition, bioactive components, and anti-nutritional factors of finger millet (Eleusine coracana L.), J. Appl. Biol. Biotechnol., 2022, 10(2), 46–52, DOI:10.7324/JABB.2022.10s107.
- J. A. Adebiyi, A. O. Obadina, A. F. Mulaba-Bafubiandi, O. A. Adebo and E. Kayitesi, Effect of fermentation and malting on the microstructure and selected physicochemical properties of pearl millet (Pennisetum glaucum) flour and biscuit, J. Cereal Sci., 2016, 70, 132–139, DOI:10.1016/j.jcs.2016.05.026.
- C. A. Onyango, S. O. Ochanda, M. A. Mwasaru, J. K. Ochieng, F. M. Mathooko and J. N. Kinyuru, Effects of malting and fermentation on anti-nutrient reduction and protein digestibility of red sorghum, white sorghum and pearl millet, J. Food Res., 2013, 2, 41, DOI:10.5539/jfr.v2n1p41.
- J. A. Adebiyi, A. O. Obadina, O. A. Adebo and E. Kayitesi, Fermented and malted millet products in Africa: Expedition from traditional/ethnic foods to industrial value-added products, Crit. Rev. Food Sci. Nutr., 2018, 58(3), 463–474, DOI:10.1080/10408398.2016.1188056.
- R. Dev and M. Gupta, Elucidating the physical, chemical, functional and antioxidant properties of protein-rich quinoa (Chenopodium quinoa) crisp, Food and Humanity, 2024, 2, 100205, DOI:10.1016/j.foohum.2023.100205.
- Association of Official Analytical Chemists (AOAC), Official Methods of Analysis, Washington DC, AOAC International, 2010 Search PubMed.
- FAO, Food energy - methods of analysis and conversion factors, FAO Food and Nutrition Paper, 2003, p. 77, https://www.fao.org/3/Y5022E/y5022e00.htm Search PubMed.
- V. A. McKie and B. V. McCleary, A Novel and Rapid Colorimetric Method for Measuring Total Phosphorus and Phytic Acid in Foods and Animal Feeds, J. AOAC Int., 2016, 99(3), 738–743, DOI:10.5740/jaoacint.16-0029.
- FSSA, Microbiological Testing, Manual of Methods of Analysis of Foods, Ministry of Health and Family welfare/Government of India, New Delhi, 2012 Search PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. L. Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Ménard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food–an international consensus, Food Funct., 2014, 5, 1113–1124, 10.1039/C3FO60702J.
- V. Dadwal, R. Joshi and M. Gupta, A Multidimensional UHPLC-DAD-QTOF-IMS Gradient Approach for Qualitative and Quantitative Investigation of Citrus and Malus Fruit Phenolic Extracts and Edibles, ACS Food Sci. Technol., 2021, 1(10), 2006–2018, DOI:10.1021/acsfoodscitech.1c00323.
- A. Yenasew and K. Urga, Effect of the germination period on functional properties of finger millet flour and sensorial quality of porridge, Food Sci. Nutr., 2023, 11(5), 2336–2343, DOI:10.1002/fsn3.3240.
- D. I. Gernah, C. C. Ariahu and E. K. Ingbian, Effects of malting and lactic fermentation on some chemical properties of maize (Zea mays), Am. J. Food Technol., 2011, 6, 404–412 CrossRef CAS.
- K. T. Ramappa, S. B. Batagurki, A. V. Karegoudar and H. Shranakumar, Study on physical properties of finger millet (Eleusine coracana), Int. J. Agric. Eng., 2011, 4(1), 13–15 Search PubMed.
- A. Kumar, A. Kaur, K. Gupta, Y. Gat and V. Kumar, Assessment of germination time of finger millet for value addition in functional foods, Curr. Sci., 2021, 120(2), 406–413, DOI:10.18520/cs/v120/i2/406-413.
- M. Banti, T. Atlaw, B. Agza, G. Kanchora, R. Jiso, M. Tsegaye, A. Bisrat, S. Fenta, A. Mengst and A. Kassa, Injera making quality evaluation of tef and cassava composite flour, Am. J. Biosci. Bioeng., 2020, 8(6), 99–104 Search PubMed.
- S. Devi and R. Modgil, Physico-chemical Characteristics, Functional Properties and Antinutritional Factors of Domestically Processed Sub Himalayan Non- conventional Millet koda (Eleusine coracana), Int. J. Curr. Microbiol. Appl. Sci., 2020, 9(11), 2808–2817, DOI:10.20546/ijcmas.2020.911.340.
- J. O. Owheruo, B. O. T. Ifesan and A. O. Kolawole, Physicochemical properties of malted finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum), Food Sci. Nutr., 2019, 7, 476–482, DOI:10.1002/fsn3.816.
- S. P. Hiremath and K. Geetha, Nutritional Composition of Raw, Malted and Popped Finger Millet (Eleusine coracana) Varieties, Int. J. Curr. Microbiol. Appl. Sci., 2019, 8(2), 966–974, DOI:10.20546/ijcmas.2019.802.112.
- I. Patel, K. Patel, S. V. Pinto and S. M. Patel, Ragi: A Powerhouse of Nutrients. Research & Reviews, J. Dairy Sci. Technol., 2018, 5(3), 36–47, DOI:10.37591/rrjodst.v5i3.461.
- J. X. K. Perales-Sanchez, C. Reyes-Moreno, M. A. Gomez-Favela, J. Milan-Carrillo, E. O. Cuevas-Rodriguez, A. Valdez-Ortiz and R. Gutierrez-Dorado, Increasing the antioxidant activity, total phenolic and flavonoid contents by optimizing the germination conditions of amaranth seeds, Plant Foods Hum. Nutr., 2014, 69, 196–202 CrossRef CAS PubMed.
- S. Sharma, D. C. Saxena and C. S. Riar, Using combined optimization, GC-MS and analytical technique to analyze the germination effect on phenolics, dietary fibers, minerals and GABA contents of Kodo millet (Paspalum scrobiculatum), Food Chem., 2017, 233, 20–28, DOI:10.1016/j.foodchem.2017.04.099.
- R. Sinha and A. Kawatra, Effect of processing on phytic acid and polyphenol contents of cowpeas (Vigna unguiculata (L.) Walp), Plant Foods Hum. Nutr., 2003, 58, 1–8 CrossRef.
- S. Sharma, D. C. Saxena and C. S. Riar, Antioxidant activity, total phenolics, flavonoids and antinutritional characteristics of germinated foxtail millet (Setaria italica), Cogent Food Agric., 2015, 1, 108172 Search PubMed.
- K. T. Murungweni, S. E. Ramashia and M. E. Mashau, Effect of malting on physicochemical, antioxidant, and microstructural properties of finger millet (Eleusine coracana) flours, Food Sci. Nutr., 2023, 12(1), 547–563 CrossRef PubMed.
- M. Mutshinyani, M. E. Mashau and A. I. O. Jideani, Bioactive compounds, antioxidant activity and consumer acceptability of porridges of finger millet (Eleusine coracana) flours: Effects of spontaneous fermentation, Int. J. Food Prop., 2020, 23(1), 1692–1710, DOI:10.1080/10942912.2020.1825485.
- A. Usha, G. Sripriya and T. S. Chandra, Effect of Fermentation on Primary Nutrients in Finger Millet (Eleusine Coracana), J. Agric. Food Chem., 1999, 44(9), 2616–2818, DOI:10.1021/jf950787q.
- S. Ilango and U. Antony, Assessment of the microbiological quality of koozh, a fermented millet beverage, Afr. J. Microbiol. Res., 2014, 8(3), 308–312 CrossRef.
- H. N. Oshodi, M. O. Ogungbenle and A. A. Oladimeji, Chemical Composition, Nutritionally Valuable Minerals and Functional Properties of Benniseed (Sesamum Radiatum), Pearl Millet (Pennisetum Typhoides) and Quinoa (Chenopodium Quinoa) Flours, Int. J. Food Sci. Nutr., 1999, 50(5), 25–331, DOI:10.1080/096374899101058.
- C. U. Inyang and U. M. Zakari, Effect of Germination and Fermentation of Pearl Millet on Proximate, Chemical and Sensory Properties of Instant ‘Fura’– A Nigerian Cereal Food, Pak. J. Nutr., 2008, 7(1), 9–12, DOI:10.3923/pjn.2008.9.12.
- C. Gopalan, B. Ramshashtri and S. Balasubramanian, in Nutritive Value of Indian Foods, ed. Narasinga R. B. S., Deosthale Y. G. and Pant K. C., NIN, Hyderabad, 1999, p. 156 Search PubMed.
- R. Sharma, P. Garg, P. Kumar, S. K. Bhatia and S. Kulshrestha, Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods, Fermentation, 2020, 6(4), 106, DOI:10.3390/fermentation6040106.
- M. Feyera, Effects of fermentation time and blending ratio on functional properties and organoleptic acceptability of complementary food, Food Sci. Quality Management, 2021, 104, 2224–6088, DOI:10.7176/FSQM/104-03.
- O. O. Oloyede, S. James, O. B. Ocheme, C. E. Chinma and V. E. Akpa, Effects of fermentation time on the functional and pasting properties of defatted Moringa oleifera seed flour, Food Sci. Nutr., 2016, 4(1), 89–95, DOI:10.1002/fsn3.262.
- S. O. Azeez, C. E. Chinma, S. O. Bassey, U. K. Eze, A. F. Makinde, A. A. Sakariyah, S. S. Okubanjo, N. Danbaba and O. A. Adebo, Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours, LWT, 2022, 154, 112734, DOI:10.1016/j.lwt.2021.112734.
- A. L. Iswarya and A. Narayanan, Effect of Germination on Biofortified Pearl Millet Cultivars' Nutrient Content, Int. J. Innov. Res. Educ. Sci., 2016, 3(6), 391–396 Search PubMed.
- S. M. Abd El Rahaman, H. B. Elmaki, W. H. Idris, A. B. Hassan, E. E. Babiker and A. H. ElTinay, Antinutritional factor content and hydrochloric acid extractability of minerals in pearl millet cultivars as affected by germination, Int. J. Food Sci. Nutr., 2007, 58, 6–17, DOI:10.1080/09637480601093236.
- D. Fardiaz and P. Markakis, Degradation of phytic acid in Oncom (fermented peanut press cake), J. Food Sci., 1981, 46, 523–526 CrossRef CAS.
- S. G. Nkhata, E. Ayua, E. H. Kamau and J. B. Shingiro, Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes, Food Sci. Nutr., 2018, 6(8), 2446–2458 CrossRef CAS PubMed.
- R. Sharma and S. Sharma, Anti-nutrient & bioactive profile, in vitro nutrient digestibility, techno-functionality, molecular and structural interactions of foxtail millet (Setaria italica L.) as influenced by biological processing techniques, Food Chem., 2022, 368, 130815, DOI:10.1016/j.foodchem.2021.130815.
- Z. Ma, J. I. Boye and X. Hu, Nutritional quality and techno-functional changes in raw, germinated and fermented yellow field pea (Pisum sativum L.) upon pasteurization, LWT--Food Sci. Technol., 2018, 92, 147–154, DOI:10.1016/j.lwt.2018.02.018.
- N. Arshad, S. Akhtar, T. Ismail, W. Saeed, M. Qamar, F. Özogul, E. Bartkiene and J. M. Rocha, The Comparative Effect of Lactic Acid Fermentation and Germination on the Levels of Neurotoxin, Anti-Nutrients, and Nutritional Attributes of Sweet Blue Pea (Lathyrus sativus L.), Foods, 2023, 12, 2851, DOI:10.3390/foods12152851.
- A. K. Singh, J. Rehal, A. Kaur and G. Jyot, Enhancement of attributes of cereals by germination and fermentation: A review, Crit. Rev. Food Sci. Nutr., 2015, 55(11), 1575–1589, DOI:10.1080/10408398.2012.706661.
- H. Kaur and B. S. Gill, Changes in physicochemical, nutritional characteristics and ATR–FTIR molecular interactions of cereal grains during germination, J. Food Sci. Technol., 2021, 58(6), 2313–2324 CrossRef CAS PubMed.
- H. Wang, Y. Fu, Q. Zhao, D. Hou, X. Yang, S. Bai, X. Diao, Y. Xue and Q. Shen, Effect of Different Processing Methods on the Millet Polyphenols and Their Anti-diabetic Potential, Front. Nutr., 2022, 9, 780499, DOI:10.3389/fnut.2022.780499.
- J. Xiang, F. B. Apea-Bah, V. U. Ndolo, M. C. Katundu and T. Beta, Profile of phenolic compounds and antioxidant activity of finger millet varieties, Food Chem., 2019, 275, 361–368, DOI:10.1016/j.foodchem.2018.09.120.
- G. Rocchetti, F. Miragoli, C. Zacconi, L. Lucini and A. Rebecchi, Impact of cooking and fermentation by lactic acid bacteria on phenolic profile of quinoa and buckwheat seeds, Food Res. Int., 2019, 119, 886–894, DOI:10.1016/j.foodres.2018.10.073.
- S. P. Shingare and B. N. Thorat, Effect of Drying Temperature and Pretreatment on Protein Content and Color Changes during Fluidized Bed Drying of Finger Millets (Ragi, Eleusine coracana) Sprouts, Drying Technol., 2013, 31(5), 507–518, DOI:10.1080/07373937.2012.744033.
- P. B. Devi, R. Vijayabharathi, S. Sathyabama, N. G. Malleshi and V. B. Priyadarisini, Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fibre: A review, J. Food Sci. Technol., 2014, 51, 1021–1040 CrossRef CAS PubMed.
- S. N. Hejazi and V. Orsat, Malting process optimization for protein digestibility enhancement in finger millet grain, J. Food Sci. Technol., 2016, 53(4), 1929–1938, DOI:10.1007/s13197-016-2188-x.
- F. E. Nefale and M. E. Mashau, Effect of germination period on the physicochemical, functional and sensory properties of finger millet flour and porridge, Asian J. Appl. Sci., 2018, 6(5), 360–367, DOI:10.24203/ajas.v6i5.5466.
| This journal is © The Royal Society of Chemistry 2024 |


