Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Allamanda cathartica (Linn.) leaf extract-encapsulated phytoemulsions: formulation, characterization and in vitro–in vivo biological evaluations
Ritu
Tomar†
a,
Sabya Sachi
Das†
 *a,
Vivek
Sahu
a,
Neha
Kapoor
b,
Divya
Chaudhary
c,
Jagannath
Sahoo
d,
Santosh Kumar
Rath
a and
Kavindra Kumar
Kesari
*a,
Vivek
Sahu
a,
Neha
Kapoor
b,
Divya
Chaudhary
c,
Jagannath
Sahoo
d,
Santosh Kumar
Rath
a and
Kavindra Kumar
Kesari
 *ef
*ef
aSchool of Pharmaceutical and Population Health Informatics, DIT University, Dehradun, Uttarakhand 248009, India. E-mail: ss.das@dituniversity.edu.in
bSchool of Applied Science, Suresh Gyan Vihar University, Jaipur, Rajasthan 302017, India
cDepartment of Biotechnology and Microbiology, Meerut Institute of Engineering and Technology, Meerut, Uttar Pradesh 250005, India
dShobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM'S NMIMS, V. L. Mehta Road, Vile Parle (W), Mumbai, Maharashtra 400056, India
eDepartment of Applied Physics, School of Science, Aalto University, Espoo 00076, Finland. E-mail: kavindra.kesari@aalto.fi
fCentre of Research Impact and Outcome, Chitkara University, Rajpura 140417, Punjab, India
First published on 22nd April 2024
Abstract
Allamanda cathartica Linn. (A. cathartica L.). belongs to the Apocynaceae family and is reported to have various pharmacological activities. The present study aims to extract and formulate A. cathartica leaf extract-based emulsions, termed phytoemulsions, for improved therapeutic efficacy. Based on the results of solubility and FTIR studies, OA, T80 and P400 were selected as the oil, surfactant, and co-surfactant/stabilizer. Four different phytoemulsions (F1–F4) were formulated based on extracts obtained from different extraction solvents, F1 (organic methanolic), F2 (aqueous), F3 (ethyl acetate), and F4 (hydroalcoholic). In vitro antioxidant (DPPH and ABTS) assay results showed that all phytoemulsions exhibited potential antioxidant activity, with F3 showing the highest activity. In vitro antibacterial (zone of inhibition) assay results showed that F2 and F3 exhibited antibacterial effects against Streptococcus pyogenes (6.3 ± 0.58 mm) and Klebsiella pneumoniae (7.68 ± 0.58 mm), respectively. In vitro MTT assay findings demonstrated that all the phytoemulsions exhibited potential chemotherapeutic effects against both A431 (human epidermoid cancer) and MCF-7 (breast cancer) cells in a dose-dependent manner (24 h), with F3 showing the highest effect. Also, F3-treated cells exhibited the highest intracellular ROS and nitric oxide release. In vivo skin irritation studies revealed that phytoemulsion-treated Wistar rats showed no signs of inflammation, redness, and ablation with a primary irritation index of zero, confirming that the phytoemulsions were safe for topical application. In conclusion, A. cathartica leaf extract-based phytoemulsions showed potential antioxidant, anticancer (A431 and MCF-7) and antibacterial (F3 and F2 only) effects with high biodegradability and stability.
Sustainability spotlightEmulsion-based systems are a potential platform for encapsulating herbal or medicinal plant extracts for improving their therapeutic efficacy. In the present study, Allamanda cathartica Linn. dried leaf extract-based phytoemulsions were formulated and evaluated. The results led to a sustainable strategy for effective delivery of phytocompounds of herbal/medicinal plants like A. cathartica that could be utilized further as a substitute for conventional therapeutics and also evolving functional food products. This work aligns with the sustainable development goal (SDG) 3 stating “healthy lives and promoting well-being for all at all ages”. In addition, the works also aligns with the SDG 12, precisely goal target 12.2 which states “by 2030, achieve the sustainable management and efficient use of natural resources”. |
1. Introduction
In the last few decades, medicinal plants have been the mainstay of traditional medicine, thanks to extensive pharmacological research. They are thought to be potential sources of lead compounds for drug development as well as novel compounds with medicinal promise. It is estimated that 80% of people in underdeveloped nations receive their primary treatment from traditional medicine. To provide a foundation for future pharmacological research, it becomes necessary to screen medicinal plants for bioactive compounds.1 Secondary metabolites with intriguing biological activity can be found in abundance in plants. These secondary metabolites, which have a diversity of structural configurations and characteristics, are generally important sources.2Numerous medicinal plants with a diverse array of biological functions and intriguing phytochemical components belong to the Apocynaceae family. Allamanda cathartica L. (A. cathartica) is often called the Golden Trumpet, Buttercup Flower, or Yellow Bell, and this genus of tropical shrubs and vines is a part of the Apocynaceae family.3 This genus, which is native to tropical America—Brazil specifically—was named by the Swiss botanist Dr Frederic Allamand, who brought seeds of the species to the Swedish botanist Linnaeus in the eighteenth century. The definition of “cathartica” is purgative.3 The genus Allamanda is incredibly common across the globe. It belongs to the family Apocynaceae and, according to “The Plant List,” contains approximately 15 species (A. cathartica, A. blanchetti, A. augustifolia, A. caccicola, A. laevis, A. doniana, A nobilis, A. martii, A. oenotherifolia, A. polyantha, A. puberula, A. thevetifolia, A. schottii, A. weberbaueri, and A. setulosa).4 Around the world, A. cathartica is used in traditional medicine for a variety of conditions. It exhibits anticancer, antioxidant, antibacterial, antifungal, antiviral, antimalarial, anti-inflammatory, anti-diabetic, and cathartic properties.4 Apart from these pharmacological activities, species of A. cathartica are also reported to be used as anti-ascites, anti-hypertensive, diuretic and emetic, antipyretic, and laxative and for treating jaundice. The most used plant parts are the leaves, stem bark, flowers, roots, stem, sap, seeds, and branches, in decreasing order.4 Since 1954, a great deal of research has been done on the chemical components of A. cathartica.5 Initial chemical analyses revealed the presence of phenolic compounds, quinones, anthraquinones, anthocyanins, carbohydrates, carotenoids, coumarin, flavonoids, glycosides, lignin, lipids, phenolic compounds, quinones, tannins, and terpenes from a variety of extracts, primarily leaves, flowers, stems, stem bark, roots, and shoots.6–10
Traditional medicinal applications of A. cathartica has been described, and the first biological and pharmacological investigations were recorded in 1943.11 In diabetic rats treated with streptozotocin, aqueous extracts from the aerial portions of A. cathartica (400 mg kg−1 for 28 days) lowered blood glucose levels when compared to glibenclamide (5 mg kg−1) as the standard.12 In Sprague-Dawley rats, aqueous leaf extracts of A. cathartica (150 mg kg−1 day−1 for 14 days) enhanced the activity of wound healing. The treated rats exhibited increased rates of wound contraction, reduced epithelialization periods, increased skin breaking strength, considerably higher granulation tissue weight, and increased hydroxyproline concentration in comparison to the controls. Less inflammatory cells and more collagen were formed in the granulation tissue of treated rats, according to histological investigations.13 Allotides with α-amylase inhibitory activity and proline-rich nature were found in leaves extracted with 50% (v/v) ethanol.14 In a different investigation using methanol extracts from leaves, P388 leukemia cells showed an IC50 of 85 μg mL−1.3 At doses of 0.5, 1, 2, and 5 mg mL−1, the ethanol extracts from the leaves exhibited dose-dependent antioxidant activity (based on the DPPH technique).15 On BHK-21 cells, leaf extracts in methanol and aqueous form at doses of 10, 5, 2.5, 1.25, and 0.6 mg mL−1 did not exhibit any cytotoxic activity.16 By using the DPPH technique, the flower methanolic extracts demonstrated antioxidant activity at a concentration of 0.6 mg mL−1.17
The site-specific local effect, less first-pass metabolism, and decreased systematic exposure of topical administration make it appealing.18 Nonetheless, medications must be able to pass through the stratum corneum, the skin's primary barrier, to reach the site of action.19–21 Drug penetration is influenced by the formulation and physical and chemical characteristics of the drug as well as the characteristics of the skin. Researchers have studied and reported the usage of various enhanced drug delivery techniques composed of surfactants and new penetration enhancers, in both transdermal and oral drug administration systems.22–24 Studies have indicated that the application of this technology can improve drug stability and increase drug absorption via the skin, leading to improved treatment outcomes.25,26
Plant extracts have been demonstrated to have a variety of biological functions, but not enough research has been done on creating formulations for their application as natural plant protectants.4,12 Emulsions are classified into three categories: (1) W/O emulsion, in which oil is the continuous phase and water is the dispersed phase; (2) O/W emulsion, in which water is the continuous phase and oil is the dispersed phase; and (3) multiphase emulsion, which combines both O/W and W/O emulsions at the same time.27 According to Bancroft's rule, water-soluble emulsifiers typically forms oil-in-water (O/W) emulsions, and oil-soluble emulsifiers typically form water-in-oil (W/O) emulsions, which can be used to forecast the types of microemulsions.28 Emulsions are thermodynamically stable, isotopically transparent dispersions of two immiscible liquid phases including oil and water, stabilized by the interfacial coating of any surfactant and/or co-surfactant. It is well known that they increase the bioactive chemicals' solubility, stability, and effectiveness.29,30
Moreover, the choice of a suitable extraction method depends on the behavior of the plant material, solvent used, solvent to sample ratio, solvent pH, and temperature.31 Thus to explore these properties, we collected the extracts of A. cathartica leaves, further formulated them as emulsions, termed phytoemulsions, and evaluated them for compatibility studies (FTIR), rheology, in vitro antioxidant (DPPH and ABTS assays) and antibacterial (agar well diffusion assay) abilities and in vivo skin irritation effects. These findings will assist researchers in exploring the therapeutic potential of A. cathartica through appropriate delivery systems.
2. Materials and methods
2.1. Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT), phosphate buffered saline (PBS), trypsin–EDTA solution, Dulbecco's modified Eagle medium (DMEM), penicillin-streptomycin (Pen-Strep), fetal bovine serum (FBS), Muller Hinton Broth media dimethyl sulfoxide (DMSO), Tween 80 (T80) and olive oil (OO) were purchased from HiMedia Laboratories Pvt. Ltd, India. Span 80 (S80), polyethylene glycol 200 (P200), corn oil (CO), oleic acid (OA) and potassium persulfate (K2S2O8) were procured from Central Drug House (P) Ltd, India. Solutol HS 15 (SHS15) and polyethylene glycol 400 (P400) were purchased from Merck, India. Miglyol 829 (M829) was purchased from Oleochemicals, Germany. Coconut oil (CCO) of Parachute brand was procured from a local market in India. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) were purchased from Sigma-Aldrich chemicals, USA. All other chemicals and reagents were of analytical grade and were used as received.2.2. Antibacterial assay microorganisms
In the present study, eight test organisms were used viz. Bacillus subtilis (B. subtilis; MTCC 121), Staphylococcus aureus (S. aureus; MTCC 96), Escherichia coli (E. coli; MTCC 730), Streptococcus agalactiae (S. agalactiae; ATCC 13813), Streptococcus pyogenes (S. pyogenes; MTCC 1924), Klebsiella pneumoniae (K. pneumoniae; MTCC 432), Proteus vulgaris (P. vulgaris; MTCC 426), and Candida albicans (C. albicans; MTCC 7315). The test organisms were activated by inoculating onto Mueller Hinton Broth media. The number of bacterial cells in the broth was adjusted by using 0.5 McFarland solution as the turbidity standard.2.3. Preparation and extraction of the plant material
The plant material was isolated and collected from the local regions of villages in Dehradun, India and was authenticated by Dr S. K. Singh, Scientist E, Forest Research Institute (FRI), Dehradun, India. The leaves of A. cathartica (AC) were collected, dried (natural sun drying), and crushed into fine powder followed by mixing in precise amounts of selected solvent media for the extraction process. For maceration and extraction, four different solvent media (organic mixture, ethyl acetate, hydroalcoholic, and aqueous) were used individually. An equal amount of AC (∼250 mg) was added in solvent media, and the mixture was macerated (described in Table 1) and filtered (0.45 micron Whatman filter) followed by the collection of filtrate. The residue of each sample was re-macerated 2–3 times, and all filtrates were combined as a whole volume and evaporated to dryness using a rotary evaporator (40–50 °C, 55–70 mbar and 100–150 rpm). The dried residues were kept under vacuum until further analysis.| Plant extract batch codes | Solvent media composition | Rotary evaporator conditions | ||
|---|---|---|---|---|
| Temperature (°C) | Pressure (mbar) | Rotation (rpm) | ||
| OME | Organic mixture (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (1 methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) 1)) |
40 | 55 | 100 |
| EAE | Ethyl acetate | 45 | 55 | 120 |
| AE | Aqueous | 55 | 70 | 150 |
| HAE | Hydroalcoholic (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3 water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)) 7)) |
45 | 60 | 130 |
2.4. Solubility assessment of A. cathartica extracts
Solubility studies of all the A. cathartica plant extracts (OME, EAE, AE and HAE) in various oils and surfactants were performed using the conventional shaking water bath method.32 Initially, excess amount of each sample batch was added to separate vials containing oil (3.0 g) and surfactants (3 g) followed by vortexing followed by the addition of samples to each vial until precipitation was noticed. Further, the vials were left to shake overnight in the water bath (37 ± 2 °C) to enable proper mixing of extracts. The studies were performed in triplicate (n = 3).2.5. Formulation of A. cathartica dried leaf extract-encapsulated phytoemulsions
It is essential to appropriately select the ratio of oil, surfactant, and water to formulate and develop stable and effective phytoemulsions. Initially, the excipients (oil and surfactant) showing maximum solubility and stability of the plant extract were selected for the formulation approach. Later, the plant extract samples were added to the oil phase (oil + surfactant) and mixed with the aqueous phase (D.I. water + solubilizer/co-surfactant). The ratio of the oil phase and aqueous phase was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was kept stirring (400 rpm, 37 ± 2 °C) overnight for proper mixing. After 24 h of mixing the mixture, the batches were observed for any phase separation or precipitation.32
1. The mixture was kept stirring (400 rpm, 37 ± 2 °C) overnight for proper mixing. After 24 h of mixing the mixture, the batches were observed for any phase separation or precipitation.32
2.6. Characterization studies
2.7. Antioxidant assay
The antioxidant effects of A. cathartica-encapsulated phytoemulsions were evaluated using two major in vitro antioxidant assays, DPPH and ABTS, and details are mentioned below.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) stock solution of ABTS·+ (7 mM) with K2S2O8 (2.45 mM). Then, the working solution was kept overnight (12–16 h) in the dark at room temperature (25 ± 2 °C) and on the day of study it was diluted with phosphate buffered saline (PBS; pH 7.4) to maintain the absorbance (700 nm). Further, stock solutions of test samples (10, 50 and 100 μg mL−1) and Trolox (standard/control) were prepared by diluting with PBS (pH 7.4). Finally, the ABTS·+ stock solution (100 μL) was added into a 96-well plate followed by the addition of each test sample (50 μL), incubated for 30 min in the dark at room temperature and the absorbance was determined by using a 96-well-microtiter plate reader (Thermo Scientific, Multiskan FC).
1) stock solution of ABTS·+ (7 mM) with K2S2O8 (2.45 mM). Then, the working solution was kept overnight (12–16 h) in the dark at room temperature (25 ± 2 °C) and on the day of study it was diluted with phosphate buffered saline (PBS; pH 7.4) to maintain the absorbance (700 nm). Further, stock solutions of test samples (10, 50 and 100 μg mL−1) and Trolox (standard/control) were prepared by diluting with PBS (pH 7.4). Finally, the ABTS·+ stock solution (100 μL) was added into a 96-well plate followed by the addition of each test sample (50 μL), incubated for 30 min in the dark at room temperature and the absorbance was determined by using a 96-well-microtiter plate reader (Thermo Scientific, Multiskan FC).
The percent radical scavenging activity (% RSA) for both assays (DPPH and ABTS) was estimated using eqn (1):
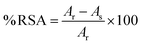 | (1) |
2.8. In vitro cell viability study
The in vitro cell viability or anticancer activity of all the test samples (blank emulsion and phytoemulsions) was analyzed against A431 (human epidermoid cancer) and MCF-7 (breast cancer) cell lines using MTT assay methods, as reported earlier.33 Initially, the cells were seeded in specific culture media overnight in a CO2 incubator (5% CO2; 37 °C) for pertinent adherence. After this, homogeneous and healthy cells (∼1 × 104 cells per well) were seeded in a 96 well-microplate (Tarson) and treated with samples (10, 50 and 100 μL mL−1). The samples were then treated with MTT solution (10 μL) and diluted with DMSO (as required), followed by incubation (5% CO2; 37 °C). The cancer cells untreated with any samples were considered as control samples. Later, the absorbance for each sample was determined at regular intervals using a 96-well-microtiter plate reader (Thermo Scientific, Multiskan FC) and percent cell viability (% CV) was calculated using eqn (2): | (2) |
2.9. In vitro ROS assay
The in vitro reactive oxygen species (ROS) assay for all the test samples was performed using the 2,7-dichlorofluorescein-diacetate (DCF-DA) based spectrofluorometric method, as reported earlier.34 The study was based on quantification of the intracellular ROS levels which is measured as DCF fluorescence intensity. In brief, 100 μL of A431 cells (∼1 × 104 cells per well) were seeded in 96-well plates, followed by addition of 50 μL of test sample (100 μg mL−1) and then incubated overnight in a CO2 incubator (5% CO2; 37 °C). Post-treatment, the supernatant from each well was relocated to new microplates, was treated with the DCF-DA solution and was incubated for the next 1 h. The supernatant from each treated well was withdrawn and the cells were washed (2–3 times) with PBS (pH 7.4). Finally, the cells were treated with H2O2 (100 μL), kept undisturbed for 30 min and then the fluorescence of each sample was estimated at two wavelengths (λex. = 485 nm and λem. = 535 nm) using a 96-well-microtiter plate reader (Thermo Scientific, Multiskan FC).34 The results were plotted by estimating the relative DCF fluorescence intensity by calculating the ratio of mean fluorescence of test samples and control.2.10. In vitro NOS assay
The in vitro nitric oxide synthase (NOS) assay was based on the production of nitric oxide (NO) by estimating the nitrite (metabolite of NO) accumulation in the supernatant of test samples treated against A431 carcinogenic cells.35 Initially, the cells (1 × 104 cells per well) were taken in sterile Eppendorf tubes, treated with 100 μL of the test sample (100 μg mL−1) and incubated in BOD at 24 °C. Afterward, the supernatants (50 μL) were added to a 96-well microplate, treated with the Griess reagent solution (50 μL), and incubated at room temperature (25 ± 2 °C) for 15 min. Finally, the optical density of each test sample was determined at 570 nm using a 96-well-microtiter plate reader (Thermo Scientific, Multiskan FC). The findings were compared with the pre-determined values of nitrite concentration (standard/control).2.11. Anti-bacterial studies
The agar well diffusion assay was carried out to assess the anti-bacterial activity of all test samples using agar well diffusion assay, as described earlier.36 Briefly, 5 mm wells were punched out in sterile Mueller Hinton Agar (MHA) plates, pre-swabbed with test 0.5 McFarland adjusted organism, using a sterile cork borer. Thereafter, 50 μL of each purified compound (stock concentration-1 mg mL−1) under study was dispended into the wells and allowed to diffuse for 15 min followed by incubation at 37 ± 2 °C for 12–24 hours. DMSO served as negative control and streptomycin (1 mg mL−1) was used as positive control. After the incubation was over, the diameter of the inhibition zone around the well was recorded to infer antibacterial activity.2.12. Stability testing
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min using a cold ultracentrifuge (CPR-24 I Plus 365, Remi). The physical appearance of each sample was evaluated and observed for changes.
000 rpm for 30 min using a cold ultracentrifuge (CPR-24 I Plus 365, Remi). The physical appearance of each sample was evaluated and observed for changes.
2.13. In vivo studies (skin irritation)
The animals (Wistar rats) were selected for this study according to the protocol approved by the Institutional Animal Ethical Committee (IAEC) under reference C57BL/6J, registered CPCSEA (Reg No. 1156/ac/07/CPCSEA), DIT University, Uttarakhand, India. The Draize patch test was used to determine if the formulations caused primary cutaneous irritation.37,38 Wistar rats (150–180 g), five in each group, were used in this investigation. The rats' sides and backs were made hair-free by using a razor/trimmer a few hours before the formulations were applied. After a few hours, the animals' skin was treated with selective groups: group I (normal control), group II (F1-organic methanolic extract); group III (F2-aqueous extract); group IV (F3-ethyl acetate); group V (F4-hydroalcoholic extract). A homogeneous 4 cm2 area was disseminated with each 0.5 g formulation application on the hairless skin of the rats. Every discernible alteration, such as erythema (redness) or edema (swelling), was noted on the skin.39 The scoring scale for the Draize test typically ranges from 0 to 4 for skin irritation assessments. Each parameter, such as erythema (redness), edema (swelling), or other relevant observations, is assigned a score based on the severity of the observed effect (Table 2).37| Range value | Observation |
|---|---|
| 0 | No irritation |
| 1 | Marginal reaction |
| 2 | Slight perceptible erythema |
| 3 | A greater than slight reaction which is not sufficient to be classed as distinct |
| 4 | Distinct erythema |
| 5 | A greater than distinct reaction that is insufficient to be classed as well-developed |
| 6 | Possibly spreading erythema |
| 7 | A reaction which is not sufficient to be classed as strong |
| 8 | Deep erythema which may extend beyond the treatment site |
| 9 | A more intense reaction than the above |
2.14. Statistical analysis
All experiments were performed in triplicate (n = 3) and the findings were represented as mean ± standard deviation (s.d.). The statistical analysis of the studies was performed using ordinary one-way analysis of variance (ANOVA) followed by Sidak's multiple comparison test using GraphPad Prism 8 (Ver. 8.0.2) software. The difference in population mean was considered significantly different with test difference at the 0.05 level (p < 0.05). In addition, statistical analysis for MTT and ROS assay was performed using one-way ANOVA followed by Tukey's multiple comparison test through GraphPad Prism 8 (Ver. 8.0.2) software. Furthermore, p-values less than 0.05 were considered as significant and represented as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, whereas p-value more than 0.05 was considered as non-significant (ns).3. Results and discussion
3.1. Extraction and solubility assessment of A. cathartica (dried leaves)
Extraction of A. cathartica plant dried leaves was performed using a solvent-extraction process followed by rotary evaporation (40–50 °C, 55–70 mbar and 100–150 rpm) with details of processing conditions previously mentioned in Table 1. Furthermore, the plant extracts were tested for the presence of phytocompounds using specific chemical tests (Table 3).| Phytocompounds screening | Chemical tests | Observation |
|---|---|---|
| a Presence of phytoconstituent level: ‘+’ good; ‘+++’ abundantly found. | ||
| Carbohydrates | Molisch's | +++ |
| Amino acid | Ninhydrin | +++ |
| Fats & fixed oils | Acrolein (potassium bisulfite reagent) | +++ |
| Alkaloids | Millon's | +++ |
| Glycosides | Keller–Kiliani | +++ |
| Terpenoids | Salkowski's | +++ |
| Tannins | Ferric chloride; lead acetate; potassium dichromate | + |
| Steroids | Salkowski's | +++ |
| Flavonoid | Ferric chloride; alkaline reagent; lead acetate | +++ |
| Protein (absent) | — | — |
The extraction of A. cathartica (dried leaves) was carried out using four different solvent media, organic mixture, aqueous, ethyl acetate, and hydroalcoholic, and the extracts were coded as OME, AE, EAE, and HAE, respectively. Furthermore, these extracts were individually analyzed for solubility in selective excipients. In our study, the solubility studies were performed in various oils and surfactants. The results (Fig. 1) show that A. cathartica (dried leaves) (shown for EAE) has maximum solubility in OA (8.23 ± 0.861 mg g−1; oil), T80 (54.25 ± 0.581 mg g−1; surfactant), and P400 (48.79 ± 0.347 mg g−1; co-surfactant). In this study, we have considered P400 as the co-surfactant/stabilizer for improving solubility and stability. It becomes difficult to achieve the therapeutic levels of plant extracts due to poor solubility leading to trouble in permeating via skin barriers. The solubility of the phytocompounds can be improved by encapsulating them in specific carrier medium (oil/surfactants). This helps in maintaining the content consistency of dosage form that plays a crucial role in topical delivery that may suffer from inconsistent and erratic drug absorption.40,41 Thus, OA, T80 and P400 were selected for developing the extract of A. cathartica dried leaf-encapsulated emulsions (phytoemulsions).
 | ||
| Fig. 1 Solubility of A. cathartica dried leaf extracts (EAE) in various (a) surfactants and (b) oils. All the studies performed in triplicate (n = 3) and data represented as mean ± s.d. | ||
3.2. Formulation of A. cathartica dried leaf extract-encapsulated phytoemulsions
It is essential to appropriately select the ratio of oil, surfactant, and water to formulate and develop stable and effective phytoemulsions. Based on the solubility studies, OA, T80 and P400 were selected as the oil, surfactant, and co-surfactant/solubilizing agent, respectively, for the development of A. cathartica (dried leaves) extract phytoemulsions. The phytoemulsions were prepared as per the earlier reported phase mixing method.32 Initially the extracts (10 mg; OME, AE, EAE and HAE) were mixed in the oil phase (10 mL; OA). Once the extracts were properly mixed, they were mixed into the aqueous phase comprising D.I. water (10 mL), T80 (3 mL) and P400 (2 mL). The overall formulation (25 mL) was then kept under magnetic stirring (300 rpm, 40 °C, 24 h) and was observed for any physical changes. After 24 h, the phytoemulsions [F1 (OME-based), F2 (AE-based), F3 (EAE-based) and F4 (HAE-based)] were found to be stable and were stored at 10 °C until further analysis.3.3. Characterization studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
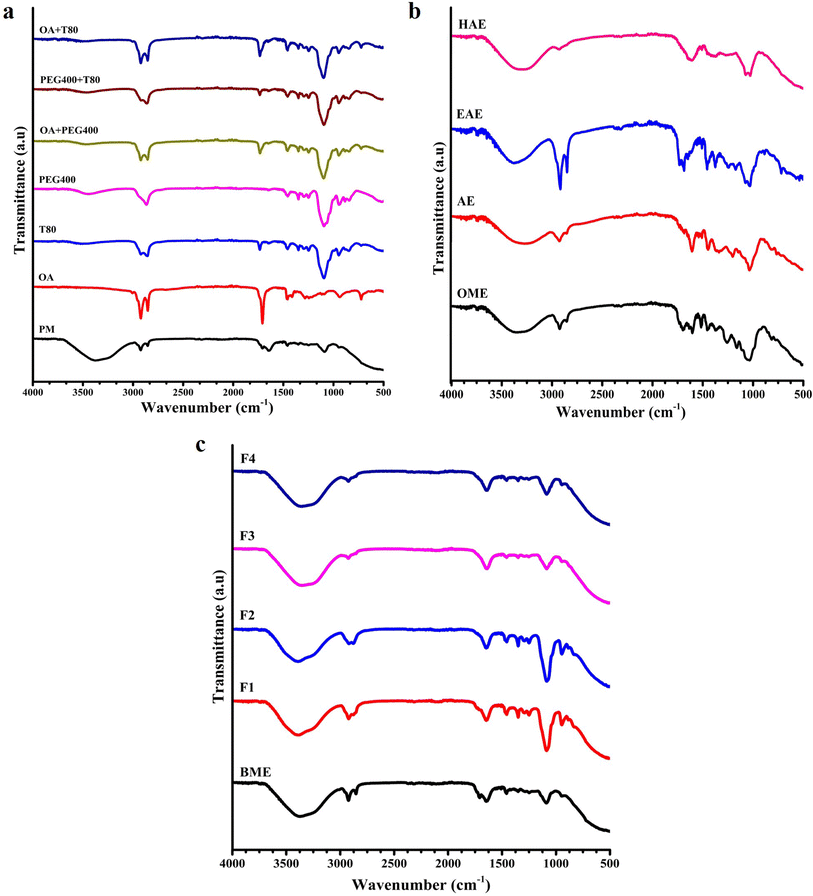
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), all plant (dried leaves) extracts, blank phytoemulsion (BPE) composed of OA + T80 + PEG400, and plant dried leaf extract-encapsulated phytoemulsions (F1, F2, F3 and F4). Furthermore, overall results demonstrated that the extract has been embedded correctly in the microemulsion mixture system deprived of any substantial interaction or formation of new conjugates. These results and findings were obtained for Allamanda cathartica (A. cathartica), and the identified wavenumbers of the bonds have been reported in Table 4.
1), all plant (dried leaves) extracts, blank phytoemulsion (BPE) composed of OA + T80 + PEG400, and plant dried leaf extract-encapsulated phytoemulsions (F1, F2, F3 and F4). Furthermore, overall results demonstrated that the extract has been embedded correctly in the microemulsion mixture system deprived of any substantial interaction or formation of new conjugates. These results and findings were obtained for Allamanda cathartica (A. cathartica), and the identified wavenumbers of the bonds have been reported in Table 4.
| Sample name | Observed peak wavelength (cm−1) |
|---|---|
| OME | 3359.11, 2919.92, 2854.59, 1693.11, 1602.88, 1512.66, 1452.51, 1368.51, 1260.14, 1045.48, 815.25 |
| AE | 3276.66, 2930.81, 2849.4, 1609.11, 1451.48, 1334.81, 1197.4, 1034.59, 816.29 |
| EAE | 3373.11, 2915.77, 2839.55, 1684.29, 1455.62, 1373.18, 1252.37, 1169.92, 1023.71, 884.74 |
| HAE | 3296.88, 2925.62, 1614.29, 1507.48, 1370.07, 1044.96 |
| BPE | 3271.48, 2922.51, 1588.87, 1506.44, 1385.62, 1258.59, 1049.11, 821.22 |
| F1 | 3391.25, 2926.14, 2842.14, 1644.37, 1458.74, 1344.66, 1260.14, 1092.14, 947.48 |
| F2 | 3395.4, 2925.62, 2871.7, 1644.88, 1461.85, 1349.85, 1293.85, 1248.22, 1080.74, 948.51, 887.33 |
| F3 | 3328.51, 2922.51, 1639.71, 1455.62, 1347.77, 1094.74, 947.48 |
| F4 | 3327.48, 2920.96, 1639.69, 1461.85, 1355.03, 1075.55, 932.96 |
3.3. Contact angle analysis
While performing the contact angle studies, it is very essential to fulfill two general conditions: (1) the analyzed surface should not be reactive towards the analyzing solvent, and (2) the drop of the sample must be stable (unchanged shape) while coming in contact with the surface.42 Comparatively, at room temperature (25 ± 2 °C), F2 (Fig. 4c) exhibited the maximum contact angle value (27.3–34.7°) followed by F4 > F1 > F3, similar to the results for rheological findings. This shows that formulation F2 exhibited maximum stability with good viscoelastic behaviour as compared to other formulations. Overall (Fig. 4), all the formulations showed a value of contact angle (θ): (0° < θ < 90°), which ensures that the formulations have larger adhesive forces than the cohesive forces, which confirms the tendency of the formulation to wet the surface with improved surface endurance. Thus, these formulations are established as oil-in-water emulsions (θ < 90°), can be used for delivering the API transdermally/topically, and are also applicable for performing in vivo biological studies.3.4. In vitro antioxidant assay
In the present study, we have evaluated the antioxidant potential of the plant dried leaf extract-encapsulated phytoemulsions, F1 (OME-based), F2 (AE-based), F3 (EAE-based) and F4 (HAE-based) (Fig. 5). Furthermore, the effects of sample concentration (10 μg mL−1, 50 μg mL−1, and 100 μg mL−1) with respect to DPPH solution and ABTS+ solution have been shown in Fig. 5. Comparatively, at room temperature (25 ± 2 °C), F3 exhibited maximum % RSA for both DPPH and ABTS followed by F2 > F1 > F4. Also, the % RSA of all the formulations (F1, F2, F3 and F4) and (standards) for both DPPH (Fig. 5a) and ABTS (Fig. 5b) assay increased with the increase in sample concentration and further became stable over time.Similar findings associated with the antioxidant effects of A. cathartica extracts were reported earlier and confirmed using various in vitro assays. In this study, the results of DPPH assay showed that the ethanolic extract of A. cathartica leaves exhibited superior antioxidant activity in a dose-dependent manner.15 In another study, researchers reported that the carbon tetrachloride (CCl4) fractions achieved from the methanolic extracts of A. cathartica leaves exhibited potential antioxidant activity, confirmed using DPPH assay, with an IC50 value of 47.5 ± 0.11 μg mL−1.43 Hameed et al.44 applied ABTS radical-scavenging assay to determine the total antioxidant activity (5.43 ± 0.29 mM g−1) of crude methanolic extracts of A. cathartica leaves. The assay demonstrated chain infringement and hydrogen contributing ability of the A. cathartica leaf extracts towards the free radicals. In addition, the A. cathartica root extracts showed high levels of various enzymatic antioxidants including peroxidase, catalase, and superoxide dismutase and also higher levels of total phenolic content.44
3.5. In vitro cell viability study
A. cathartica L. belonging to the family Apocynaceae is well-known for its antibacterial and anticancer effects.45 In the present study, the anticancer effects of A. cathartica dried leaf extract-encapsulated phytoemulsions (F1, F2, F3 and F4) were analyzed against A431 (human epidermoid cancer) and MCF-7 (breast cancer) cell lines using the MTT assay.The results (Fig. 6) demonstrated that all the phytoemulsions (F1, F2, F3 and F4) exhibited anticancer activity against both A431 (Fig. 6a) and MCF-7 (Fig. 6b) cell lines in a dose-dependent manner. As compared to all the phytoemulsions, F3 exhibited the highest potential anticancer effects both against A431 and MCF-7 cells, followed by F2 > F1 > F4 phytoemulsions, in a dose-dependent manner. Thus, it can be concluded that the phytoemulsions F2 (AE-based) and F3 (EAE-based) can be potentially used as chemotherapeutic agents in treating skin as well as breast cancer. Furthermore, the anticancer potential and mechanism of phytoemulsions, specifically against A431 (human epidermoid cancer) cells, were established using ROS and NOS assay.
3.6. In vitro ROS assay
The cellular metabolism leads to the intracellular production of three reactive oxygen species (ROS) i.e. superoxide anion (O2−), hydroxyl radical (OH−), and hydrogen peroxide (H2O2), having physiological significance.46 Oxidative stress is a vital cellular event associated with physiological, biological and pathological conditions.46The purpose of our study was to determine total cellular ROS levels in the A. cathartica dried leaf extract-encapsulated phytoemulsion-treated A431 carcinogenic cells using DCFH-DA staining. The quantification of the intracellular ROS levels was measured as DCF fluorescence intensity, identified through the treated A431 carcinogenic cells. ROS assay results (Fig. 7) demonstrated that the F3 (EAE-based) formulation exhibited maximum DCF relative fluorescence intensity (relative to −ve control fluorescence intensity) followed by F2 (AE-based) > F1 (OME-based) > F4 (HAE-based). These results were similar to the findings of MTT assay where F3 exhibited superior anticancer effects as compared to other phytoemulsions. In addition, oxidative stress can be detected as an unevenness between antioxidants and prooxidants.47 Thus, the presence of more antioxidants in samples can trigger ROS levels leading to inducing of oxidative stress within the cells leading to potential anticancer behaviour.
3.7. In vitro nitric oxide (NO·) assay
Nitric oxide (NO·) exhibits both pro- and antitumorigenic effects as reported in the literature leading to its bimodal effects in carcinogenesis and tumor progression.48 It is reported that higher levels of NOS expression may be cytotoxic or cytostatic against carcinogenic or tumor cells. Paradoxically thus, NO· may exhibit both angiogenic and genotoxic activity.49 This study focused on determining the NO· production using nitric oxide assay that was based on the estimation of the nitrite (metabolite of NO·) accumulation in the supernatant of test samples treated against A431 human epidermoid carcinogenic cells.The results showed that all the phytoemulsions (F1–F4) (Fig. 8) upregulated the NO· levels (demonstrated in terms of relative optical density i.e. the higher the relative optical density the higher the amount of NO· in the sample supernatant). The F3 (EAE-based) phytoemulsion exhibited the maximum relative optical density followed by F2 (AE-based) > F1 (OME-based) > F4 (HAE-based), thus being the most potential formulation for upregulating NO· levels. In another study, rape seed proanthocyanidins inhibited expressions of cyclooxygenase (COX)-2, inducible nitric oxide synthase (iNOS), proliferation of cell nuclear antigen, cyclin D1 and matrix metalloproteinase (MMP)-9, in the treated A431 carcinoma cells.50 Earlier researchers demonstrated the potential anticancer effects of Rumex obtusifolius combined with arginase/nitric oxide synthase inhibitors against HT29 colorectal adenocarcinoma and MCF-7 breast cancer cells.51 Overall, the ROS and NO· assay results show correlation with MTT assay results where, F3 exhibited superior anticancer effects as compared to the other phytoemulsions. Thus, it can be concluded that the F3 (EAE-based) phytoemulsion exhibits potential anticancer (A431) effects due to inducing ROS and NO· production at intracellular levels.
3.8. Antimicrobial activity
To determine the antimicrobial activity, we performed antibacterial and antifungal studies for all four formulations (F1, F2, F3 and F4) against Gram-positive (S. pyogenes, B. subtilis and S. agalactiae) and Gram-negative (E. coli, K. pneumoniae and P. vulgaris) test microorganisms. The results of antibacterial studies showed that only two formulations specifically exhibited antimicrobial effects. The zone of inhibition (Table 5) was found to be 7.68 ± 0.58 mm for the F3 formulation specifically against K. pneumoniae (MTCC 432) and 6.3 ± 0.45 mm for the F2 formulation specifically against S. pyogenes (MTCC 1924), similar to that of respective control values (Fig. 9). However, it was noticed that both F1 and F4 formulations exhibited no significant antibacterial activity against any of the tested microorganisms. Our results also exhibit similar findings as reported earlier in the study where researchers demonstrated the antimicrobial activity of various plant part extracts (leaf and flower) of A. cathartica when evaluated against some multidrug resistant pathogenic test microorganisms including B. subtilis, Agrobacterium tumifaciens, S. aureus, and K. pneumoniae. It was noticed that the leaf extracts showed the best activity against K. pneumoniae as compared to other pathogens.52| Batch code | Zone of inhibition (in mm) | ||||||
|---|---|---|---|---|---|---|---|
| Bacterial strains | Fungal strain | ||||||
| Gram positive | Gram negative | Gram positive | |||||
| S. pyogenes (MTCC 1924) | B. subtilis (MTCC 121) | S. agalactiae (ATCC 13813) | E. coli (MTCC 730) | K. pneumoniae (MTCC 432) | P. vulgaris (MTCC 426) | C. albicans (MTCC 7315) | |
| a Data presented are mean ± s.d. of three replications (n = 3); (—) = no significant antimicrobial activity. | |||||||
| F1 | — | — | — | — | — | — | — |
| F2 | 6.3 ± 0.58 | — | — | — | — | — | — |
| F3 | — | — | — | — | 7.68 ± 0.58 | — | — |
| F4 | — | — | — | — | — | — | — |
 | ||
| Fig. 9 Images for zone of inhibition assay of the tested samples: (a) F2 phytoemulsion effects on S. pyogenes strains and (b) F3 phytoemulsion effects on K. pneumoniae strains. | ||
In another study, researchers have reported the antibacterial effects of flavonoids (Quercitrin), isolated from A. cathartica plant, against Staphylococcus aureus (S. aureus; Gram-positive) and E. coli (Gram-negative) bacterial strains. It was noticed that the isolated compound specifically exhibited better antibacterial effects (higher zone of inhibition) against the Gram-negative strain as compared to the Gram-positive strain.53
The results seem to be very unpredictive, but this could be due to the composition of the formulation of F2 and F3 that allowed them to exhibit specific antibacterial effects (Fig. 9). Also, the blank emulsions exhibited no significant activity against any of the tested microorganisms (results not shown). In addition to the antibacterial studies, all the formulations were also tested for antifungal activity against the Gram-positive fungal strain (C. albicans). The results showed that none of the formulations exhibited any activity against the fungal strain, leading to the conclusion that the formulations have no substantial antifungal activity. Overall, it can be concluded that the F2 formulation can be used for treating S. pyogenes causing skin infections such as impetigo, erysipelas, cellulitis, necrotizing fasciitis, and myositis and myonecrosis.54 On the other hand, the F3 formulation can be used for treating K. pneumoniae causing pneumonic infections, particularly hospital-acquired pneumonia.55
3.9. Stability testing
The stability studies play an important role in determining the apt formulation approach and storage abilities of the lipid- and oil-based formulations. We performed the stability studies using two methods: centrifugation and heating-cooling methods. As per the method, the blank phytoemulsion and all the formulations (F1, F2, F3 and F4) were studied under physical stress conditions including centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 30 min) and temperature stress conditions (4 °C → 40 °C → 4 °C), similarly repeated for three cycles. The physical appearance of each sample was evaluated for both studies. The results showed that all the formulations showed superior stability under both centrifugation (Fig. 10a) and temperature (Fig. 10b) stress conditions.
000 rpm, 30 min) and temperature stress conditions (4 °C → 40 °C → 4 °C), similarly repeated for three cycles. The physical appearance of each sample was evaluated for both studies. The results showed that all the formulations showed superior stability under both centrifugation (Fig. 10a) and temperature (Fig. 10b) stress conditions.
3.10. In vivo skin irritation studies
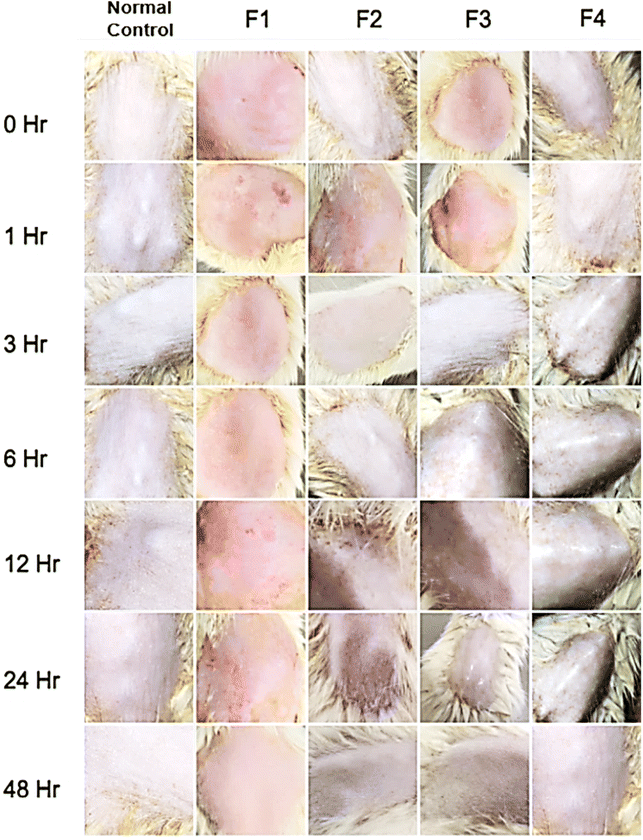
Skin irritation happens due to unspecified harm to the specimen upon contact with the external stimuli, growing skin sensitivity and producing inflammatory reactions. The prime pathogenic mechanisms associated with skin irritation include damage to skin barriers, the release of cytokine cascade, and oxidative stress, causing perceptible and subclinical inflammatory reactions.56,57 Plant-based active phytocomponents and their extracts have been explored extensively for topical applications. However, their frequent usage is limited due to poor permeation and absorption of components into the skin. Consequently, emulsions have led to improvement in the absorption as well as permeation of the phytocompounds leading to enhanced therapeutic efficacy. Emulsions (micro- or nanoemulsions) are composed of high concentrations of surfactant which helps in the absorption of therapeutic through skin barriers, though they may cause skin irritation.58,59 Thus, in our study, we evaluated the skin irritation effects of all the A. cathartica dried leaf extract-encapsulated phytoemulsions, F1 (OME-based), F2 (AE-based), F3 (EAE-based) and F4 (HAE-based), over the tested Wistar rats using the open Draize patch test. The results of the primary tests showed that none of the formulations showed any skin irritation, confirmed through micro images (Fig. 11) of the treated skin of Wistar rats with no signs of inflammation redness and ablation. Also, the primary irritation index (Table 6) for all the phytoemulsions, at the end of 24 and 48 h, was found to be zero, confirming that the phytoemulsions were safe for topical application.| Formulation code | Irritation index | |
|---|---|---|
| 24 h | 48 h | |
| Normal control | 0 | 0 |
| F1 | 0 | 0 |
| F2 | 0 | 0 |
| F3 | 0 | 0 |
| F4 | 0 | 0 |
4. Conclusion
In the present study, we have collected and extracted the leaves of Allamanda cathartica L. (A. cathartica) plant using various solvent media including aqueous, organic mixture, ethyl acetate, and hydroalcoholic. These extracts were initially checked for their solubility and compatibility (FTIR) and based on these findings oleic acid (oil), tween 80 (surfactant) and polyethylene glycol 400 (co-surfactant/stabilizer) were selected for formulating A. cathartica dried leaf extract-based phytoemulsions (F1–F4). All the phytoemulsions showed good rheological behavior with high stability. Furthermore, as compared to all formulations, the F3-based phytoemulsion exhibited superior antioxidant (DPPH and ABTS) and anticancer (A431 and MCF-7) activity. Moreover, the in vitro antibacterial studies showed that F2 and F3 exhibited antibacterial effect against S. pyogenes (6.3 ± 0.58 mm) and K. pneumoniae (7.68 ± 0.58 mm), respectively. Also, F3-treated cells exhibited the highest intracellular ROS and nitric oxide release. In vivo skin irritation studies revealed that phytoemulsion-treated Wistar rats showed no signs of inflammation, redness, and ablation with a primary irritation index of zero, confirming that the phytoemulsions were safe for topical application. It can be concluded that emulsion-based systems can be a potential platform for encapsulating herbal or medicinal plant extracts to achieve improved therapeutic efficacy.Author contributions
Conceptualization, S. S. D. and S. K. R.; methodology, R. T., S. K. R. and S. S. D.; formal analysis, S. S. D., D. C., N. K. and V. S.; investigation, R. T., D. C., N. K. and S. S. D.; data curation, S. S. D., J. S. and K. K. K.; writing – original draft preparation, S. S. D. and V. S.; writing – review and editing, S. S. D., J. S. and K. K. K.; supervision, S. S. D., J. S. and S. K. R. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.References
- P. Hasani, N. Yasa, S. Vosough-Ghanbari, A. Mohammadirad, G. Dehghan and M. Abdollahi, Acta Pharm., 2007, 57, 123–129 CAS.
- A. de Fatima, L. V. Modolo, L. S. Conegero, R. A. Pilli, C. V. Ferreira, L. K. Kohn and J. E. de Carvalho, Curr. Med. Chem., 2006, 13, 3371–3384 CrossRef CAS PubMed.
- T. N. Tiwari, V. B. Pandey and N. K. Dubey, Phytother. Res., 2002, 16, 393–394 CrossRef CAS PubMed.
- V. L. Petricevich and R. Abarca-Vargas, Molecules, 2019, 24, 1238 CrossRef PubMed.
- H. R. Arthur and W. H. Hui, J. Chem. Soc., 1954, 2782–2784 RSC.
- P. A. Akah and V. N. Offiah, Int. J. Pharmacogn., 2008, 30, 213–217 Search PubMed.
- U. A. Essiett and E. Udo, Int. J. Sci. Technol., 2015, 4, 248–253 Search PubMed.
- J. Joselin, T. S. S. Brintha, A. R. Florence and S. Jeeva, Asian Pac. J. Trop. Dis., 2012, 2, S260–S264 CrossRef CAS.
- K. Mukherjee and L. N. Ray, Int. J. Crude Drug Res., 2008, 24, 187–205 Search PubMed.
- N. Savithramma, R. M. Linga and D. Suhrulatha, Int. J., 2013, 1, 821–825 Search PubMed.
- E. M. Osborn, Br. J. Exp. Pathol., 1943, 24, 227–231 Search PubMed.
- A. B. Chaithra, S. Satish, N. Abhishek and K. K. Ajay, Int. J. Pharm. Chem. Res., 2017, 3, 242–247 Search PubMed.
- S. Nayak, P. Nalabothu, S. Sandiford, V. Bhogadi and A. Adogwa, BMC Complementary Altern. Med., 2006, 6, 12 CrossRef PubMed.
- P. Q. Nguyen, T. T. Luu, Y. Bai, G. K. Nguyen, K. Pervushin and J. P. Tam, J. Nat. Prod., 2015, 78, 695–704 CrossRef CAS PubMed.
- A. C. Omonhinmin, I. P. Dike and S. O. Rotimi, Res. J. Med. Plant, 2015, 9, 81–89 CrossRef CAS.
- S. Mehta, S. Roy and A. Chowdhary, Virusdisease, 2017, 28, 127–132 CrossRef PubMed.
- O. Victor, A. Emeka, A. Chukwuka, A. Victor, E. Simeon, A. Victor and O. Patience, Annu. Res. Rev. Biol., 2015, 5, 357–365 CrossRef.
- R. T. Gerhardt, J. M. Matthews and S. G. Sullivan, Prehospital Emerg. Care, 2009, 13, 500–504 CrossRef PubMed.
- D. Ramadon, M. T. C. McCrudden, A. J. Courtenay and R. F. Donnelly, Drug Delivery Transl. Res., 2022, 12, 758–791 CrossRef PubMed.
- C. Gorzelanny, C. Mess, S. W. Schneider, V. Huck and J. M. Brandner, Pharmaceutics, 2020, 12, 684 CrossRef PubMed.
- Y. Q. Yu, X. Yang, X. F. Wu and Y. B. Fan, Front. Bioeng. Biotechnol., 2021, 9, 646554 CrossRef PubMed.
- S. S. Das, D. Sharma, V. K. R. Balaga, M. K. Arora, J. Ruokolainen, M. Dhanka, H. Singh and K. K. Kesari, Mater. Adv., 2023, 4, 6064–6091 RSC.
- V. S. Sivasankarapillai, S. S. Das, F. Sabir, M. A. Sundaramahalingam, J. C. Colmenares, S. Prasannakumar, M. Rajan, A. Rahdar and G. Z. Kyzas, Mater. Today Chem., 2021, 19, 100382 CrossRef CAS.
- A. Sood, S. S. Das, A. Dev, D. Bhardwaj, A. Kumar, G. Agrawal and S. S. Han, Eur. Polym. J., 2023, 196, 112323 CrossRef CAS.
- V. Hmingthansanga, N. Singh, S. Banerjee, S. Manickam, R. Velayutham and S. Natesan, Pharmaceutics, 2022, 14, 2818 CrossRef CAS PubMed.
- A. Otto, J. du Plessis and J. W. Wiechers, Int. J. Cosmet. Sci., 2009, 31, 1–19 CrossRef CAS PubMed.
- A. B. Yaakob and A. A. Sulaimon, J. Jpn. Pet. Inst., 2017, 60, 186–193 CrossRef CAS.
- W. D. Bancroft, J. Phys. Chem., 1913, 17, 501–519 CrossRef CAS.
- A. K. Anal, N. Boonlao and U. R. Ruktanonchai, Curr. Opin. Food Sci., 2023, 50, 101001 CrossRef CAS.
- J. Teixe-Roig, G. Oms-Oliu, I. Odriozola-Serrano and O. Martin-Belloso, Foods, 2023, 12, 1502 CrossRef CAS PubMed.
- A. R. Abubakar and M. Haque, J. Pharm. BioAllied Sci., 2020, 12, 1–10 CrossRef CAS PubMed.
- S. S. Das, P. R. P. Verma and S. K. Singh, LWT, 2020, 124, 109141 CrossRef CAS.
- S. S. Das, A. Sarkar, S. C. Chabattula, P. R. P. Verma, A. Nazir, P. K. Gupta, J. Ruokolainen, K. K. Kesari and S. K. Singh, Antioxidants, 2022, 11, 1378 CrossRef CAS PubMed.
- M. Grajzer, B. Wiatrak, T. Gebarowski, A. Matkowski, H. Grajeta, E. Roj, A. Kulma and A. Prescha, Food Chem., 2021, 335, 127649 CrossRef CAS PubMed.
- S. S. Das, A. K. Dubey, P. R. P. Verma, S. K. Singh and S. K. Singh, Mol. Pharm., 2022, 19, 3367–3384 CrossRef CAS PubMed.
- M. Balouiri, M. Sadiki and S. K. Ibnsouda, J. Pharm. Anal., 2016, 6, 71–79 CrossRef PubMed.
- D. Basketter, F. Reynolds, M. Rowson, C. Talbot and E. Whittle, Contact Dermatitis, 1997, 37, 218–220 CrossRef CAS PubMed.
- W. Rattanatayarom and S. Wattanasirichaigoon, J. Med. Assoc. Thailand, 2007, 90, 724–729 Search PubMed.
- P. A. Patel, S. C. Patil, D. R. Kalaria, Y. N. Kalia and V. B. Patravale, Int. J. Pharm., 2013, 446, 16–23 CrossRef CAS PubMed.
- P. Batheja, L. Sheihet, J. Kohn, A. J. Singer and B. Michniak-Kohn, J. Controlled Release, 2011, 149, 159–167 CrossRef CAS PubMed.
- K. Frederiksen, R. H. Guy and K. Petersson, Eur. J. Pharm. Biopharm., 2015, 91, 9–15 CrossRef CAS PubMed.
- K. G. Kabza, J. E. Gestwicki and J. L. McGrath, J. Chem. Educ., 2000, 77, 63–65 CrossRef CAS.
- R. Sarker, T. Sharmin, F. Islam and S. R. Chowdhury, J. Med. Plants Res., 2014, 8, 63–67 CrossRef.
- A. Hameed, G. Nawaz and T. Gulzar, Nat. Prod. Res., 2014, 28, 2066–2071 CrossRef CAS PubMed.
- I. Prakash Sharma, A. Balkrishna, A. Saini, R. Bhandari and V. Arya, Current Indian Science, 2023, 1, e211222212074 CrossRef.
- H. Kim and X. Xue, J. Visualized Exp., 2020, 160, e60682 Search PubMed.
- A. Rahal, A. Kumar, V. Singh, B. Yadav, R. Tiwari, S. Chakraborty and K. Dhama, BioMed Res. Int., 2014, 2014, 761264 Search PubMed.
- J. Mintz, A. Vedenko, O. Rosete, K. Shah, G. Goldstein, J. M. Hare, R. Ramasamy and H. Arora, Vaccines, 2021, 9, 94 CrossRef CAS PubMed.
- W. Xu, L. Z. Liu, M. Loizidou, M. Ahmed and I. G. Charles, Cell Res., 2002, 12, 311–320 CrossRef PubMed.
- S. M. Meeran and S. K. Katiyar, Front. Biosci., 2008, 13, 887–897 CrossRef CAS PubMed.
- M. Ginovyan, H. Javrushyan, G. Petrosyan, B. Kusznierewicz, I. Koss-Mikolajczyk, Z. Koziara, M. Kuczynska, P. Jakubek, A. Karapetyan, N. Sahakyan, A. Maloyan, A. Bartoszek and N. Avtandilyan, Int. J. Biochem. Cell Biol., 2023, 158, 106396 CrossRef CAS PubMed.
- M. Fartyal and P. Kumar, Int. J. Adv. Pharm., Biol. Chem., 2016, 5, 303–313 CAS.
- K. Hema and R. Krishnaveni, Int. J. Pharma Bio Sci., 2014, 5, 588–593 Search PubMed.
- D. L. Stevens and A. E. Bryant, Impetigo, Erysipelas and Cellulitis, https://www.ncbi.nlm.nih.gov/books/NBK333408/, accessed 15 February, 2024 Search PubMed.
- J. V. Ashurst and A. Dawson, Klebsiella Pneumonia, https://www.ncbi.nlm.nih.gov/books/NBK519004/, accessed 15 February, 2024 Search PubMed.
- D. Basketter, D. Jirova and H. Kandarova, Interdiscip. Toxicol., 2012, 5, 98–104 Search PubMed.
- G. Novak-Bilic, M. Vucic, I. Japundzic, J. Mestrovic-Stefekov, S. Stanic-Duktaj and L. Lugovic-Mihic, Acta Clin. Croat., 2018, 57, 713–720 Search PubMed.
- J. W. Guo, Y. P. Cheng, C. Y. Liu, H. Y. Thong, C. J. Huang, Y. Lo, C. Y. Wu and S. H. Jee, Pharmaceutics, 2020, 12, 457 CrossRef CAS PubMed.
- S. Vibhute, V. Kasture, S. Kasture, P. Kendre, S. Rupnar and P. Vishal, Der Pharm. Lett., 2015, 7, 7–16 Search PubMed.
Footnote |
| † These authors contributed equally to the manuscript. |
| This journal is © The Royal Society of Chemistry 2024 |