 Open Access Article
Open Access ArticleInvestigation of ultrasound processing for homogenisation of blackberry dairy-based beverages†
Gontorn
Putsakum
ab,
Dilip K.
Rai
 *a,
Brijesh K.
Tiwari
a and
Colm P.
O'Donnell
b
*a,
Brijesh K.
Tiwari
a and
Colm P.
O'Donnell
b
aTeagasc Food Research Centre Ashtown, Dublin D15 DY05, Ireland. E-mail: dilip.rai@teagasc.ie
bSchool of Biosystems and Food Engineering, University College Dublin, Dublin D04 V1W8, Ireland
First published on 9th July 2024
Abstract
Ultrasound (US) processing is a novel technology that has many potential applications in food processing. Pilot-scale batch US homogenisation of a blackberry-enriched dairy beverage was investigated in this study. Particle size, apparent viscosity, colour, phenolic content and antioxidant activities of US homogenised beverages were compared to those of conventionally homogenised products. Blackberry powder was mixed with preheated whole milk (37 °C) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v). The mixture was treated at an US intensity of 10.37 ± 0.58 W cm−2 for selected treatment times of 1, 3 and 5 min, while the control sample was homogenised using a conventional homogeniser for 1 min at 10
20 (w/v). The mixture was treated at an US intensity of 10.37 ± 0.58 W cm−2 for selected treatment times of 1, 3 and 5 min, while the control sample was homogenised using a conventional homogeniser for 1 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. US treatment resulted in smaller particle size compared to the control, and longer US treatment time increased the number of smaller particles (p < 0.05). A higher viscosity value was measured in all US-treated samples when compared to the untreated blackberry–milk beverage (p < 0.05). Minor significant changes in colour parameters of all US-treated samples were observed compared to the control (p < 0.05). The application of US treatment to blackberry–milk beverages resulted in comparable retention of phenolic contents and antioxidant activities compared to conventional homogenisation.
000 rpm. US treatment resulted in smaller particle size compared to the control, and longer US treatment time increased the number of smaller particles (p < 0.05). A higher viscosity value was measured in all US-treated samples when compared to the untreated blackberry–milk beverage (p < 0.05). Minor significant changes in colour parameters of all US-treated samples were observed compared to the control (p < 0.05). The application of US treatment to blackberry–milk beverages resulted in comparable retention of phenolic contents and antioxidant activities compared to conventional homogenisation.
Sustainability spotlightUltrasound processing is an eco-friendly and sustainable technology that is gaining applications in food and beverage production. The ultrasound technology is considered a ‘green’ technology as it requires low volumes and/or no organic solvents. Optimized ultrasound technology in food processing aids in the reduction of energy consumption through enhanced efficiency. This study focused on the utilization of ultrasound as a sustainable alternative processing for functional beverages and investigated its effects on the rheological properties, colour changes, phenolic contents and antioxidant activities. This research aligns with the United Nations' (UN) sustainable development goals (SDGs), which are Goal 12 – Responsible Consumption and Production and Goal 13 – Climate Action. |
Introduction
Increased focus on environmental sustainability has led to an increase in sustainable research in all industry fields aiming to reduce the negative impact of processing and manufacturing technologies on the environment.1–3 In the food industry, much research has focused on investigating new alternative sustainable novel technologies that can be adopted into food manufacture, for instance, high-pressure processing, pulsed-electric field, ultrasound/sonication, microwave heating, infra-red heating, UV and pulsed light.4 These novel technologies were explored extensively to replace or efficiently assist the existing conventional thermal approaches (i.e. pasteurisation and sterilisation) in order to reduce the intensive energy usage during the heat treatment of products, which is the main drawback that leads to more CO2 emission.1 For instance, a life cycle assessment (LCA) study was reported by Cacace et al. where high pressure processing showed lower index values for overall environmental impact categories when compared to pasteurisation.5 Furthermore, novel technologies can be used to improve other commercial practices commonly utilised within the food industry, such as the extraction of high value compounds or freezing processes of food materials. As mentioned in the LCA study reported by Garcia-Garcia et al., the microwave-assisted extraction of pectin resulted in lower environmental impact indices when compared to commercial acid extraction.6 In another LCA study, the application of ultrasound during the atmospheric freezing of apples, carrots and eggplants could reduce energy consumption by up to 70% as well as reduce the overall impact indices when compared to a normal freezing process.7 Through some of these examples, it can be implied that proper applications of novel technologies can lead to more sustainable and eco-friendly food production and processing.Ultrasound (US) processing, which can be employed to apply sound waves at selected frequencies to food matrices, is a non-thermal processing technology which is increasingly being considered for industrial adoption in food manufacture. The technology has ‘green’ characteristics as the technique can be employed using low volumes of clean or ‘green’ solvents (e.g. water, butanol, ethyl lactate, limonene, liquid salts/ionic liquids, etc.).8 US has been commonly utilised in liquid food applications such as juice, puree and milk, rather than solid foods. Sono-physical and sono-chemical effects caused by the cavitation phenomenon can lead to desirable changes in foods in terms of physical,9,10 rheological,11,12 chemical,13,14 physiochemical15,16 and microbiological properties,17,18 as well as increased shelf-life stability.19,20 Thus, the application of US has also been widely explored for many types of products as well as in various unit operations, such as emulsification/homogenisation, sterilisation/pasteurisation and extraction.21
There is an increased demand from consumers for foods and beverages with enhanced functional and health benefits.22,23 The food industry has responded to this market demand through research and development of products incorporating plant extracts or concentrated forms of plants rich in health-beneficial properties. In the recently completed ‘RubusElite’ research project funded through the Irish Department of Agriculture, Food and Marine, the use of blackberry polyphenols and proteins in functional beverages to enhance sport and exercise performance showed promising results.24 It was reported that ingestion of flavonoids (polyphenols) can improve the recovery of muscle strength and reduce muscle soreness due to their high antioxidant activity.25 Blackberries contain high amounts of polyphenols, dominantly anthocyanin and specifically cyanidin-3-O-glucoside (C3G) which contributes to the high level of antioxidant activity of the fruit.26,27 Thus, blackberries were reported to provide better long-term insulin resistance, cognitive function, bone density, and cardiovascular function when consumed.27 Additionally, it was reported that the consumption of blackberry beverages could significantly decrease levels of triglycerides, total cholesterol, and glucose in plasma.28 The anti-inflammatory effect of polyphenol extracts, rich in anthocyanin and proanthocyanidin, from blackberry fruits was evident in a study by Van de Velde et al. where the extract could induce fibroblast migration up to 50%, implying its wound healing effect.29 Therefore, the fortification of blackberry and its extract into food and beverages as functional products can be potentially commercialised for consumers.
One of the practical challenges in developing a milk beverage fortified with blackberry polyphenols was the non-homogeneous product due to the formation of powder clumps when whole milk and blackberry powder extract were mixed. This could affect consumers' sensorial acceptance in terms of product commercialisation. The application of homogenisation is a key solution to create a homogeneous and stabilised functional beverage incorporated with plant extracts or ingredients. Wu et al. reported that there was an increase in colloidal stability of acidified milk drinks incorporated with carboxymethylcellulose when homogenisation was applied (p < 0.05).30 Alongi, Calligaris and Anese reported that the high-pressure homogenisation of milk-based coffee beverages reduced the particle size and ζ-potential as well as increased the bioaccessibility of chlorogenic acid by more than 50% (p < 0.05).31 Research studies relating to the homogenisation of polyphenol-enriched dairy-based beverages using US processing are limited. However, there are reports of potential uses of US as homogenisation with promising results when compared to conventional and other types of homogenisation: Zhou et al. found that an US-assisted emulsification resulted in lower emulsion droplet size and higher stability indices of a myofibrillar protein–soybean oil emulsion than high-speed homogenisation (p < 0.05).32 Another study showed that US homogenisation in milk could significantly reduce the size of fat globules in milk when compared to both single-stage and two-stage homogenisation (p < 0.05).33 Thus, US can be potentially employed as a homogenisation process for blackberry–milk beverages.
In this study, pilot-scale batch US homogenisation of a novel blackberry–milk beverage was investigated. Changes in physical, physiochemical, rheological and chemical properties of a blackberry–milk mixture were investigated to assess the effects of US homogenisation compared to traditional homogenisation.
Experimental
Preparation of blackberry–milk samples
Batches of freeze-dried blackberry powder prepared from fresh berries (Wild Orchard, Limerick, Ireland) were provided by RubusElite (University College Cork, Cork, Ireland) and stored at −30 °C prior to trials. The fresh berries were pureed as described in the previous literature.34 The blackberry purees were then freeze-dried using an FD80 Cuddon freeze dryer (Cuddon Freeze Dryer, South Island, New Zealand) for 36 hours, and the lyophilized blackberries were ground into fine powders using a Microtron MB800 laboratory mixer (Kinematica AG, Switzerland). Total phenolic content in the blackberry powder was ∼3% (w/w). Pasteurised whole milk (composed of 12.2% total solids, 3.5% fat, 4.6% carbohydrate, 3.4% protein and 0.57% ash) was purchased from a local supermarket and was used for beverage preparation on the same day. Whole milk was preheated in a water bath to a temperature of 35 ± 2 °C. Blackberry powder was then added to pre-heated milk samples at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (20 g powder
20 (20 g powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 mL milk). The mixture was lightly mixed with a stirring rod and then transferred to a stainless steel beaker prior to homogenisation.
400 mL milk). The mixture was lightly mixed with a stirring rod and then transferred to a stainless steel beaker prior to homogenisation.
For the UPLC-mass spectrometry analysis of polyphenols, 10 mL of US-treated beverage sample was diluted with 40 mL of methanol solution containing 1% formic acid and vortexed at 2000 rpm for 1 h to facilitate the precipitation of milk protein as described previously by Tzima, Brunton and Rai.35 The samples were left for 30 min to allow precipitation. The mixture was then centrifuged at 2173 × g (model 2 – 16KL, SIGMA, Germany) at 4 °C for 10 min and the supernatant was collected for further chemical analyses.
Chemical reagents
Folin–Ciocalteu's phenol reagent, gallic acid, sodium acetate (anhydrous), ferric chloride hexahydrate (FeCl3·6H2O), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), 2,2-diphenyl-1-picrylhydrazyl (DPPH), formic acid and acetic acid were obtained from Sigma-Aldrich (Merck, Wicklow, Ireland). Hydrochloric acid (HCl, 37%) was purchased from Honeywell Fluka (Fisher Scientific, Carrigaline, Ireland). Acetonitrile (ACN) and MeOH were purchased from Romil (Lennox Laboratory Supplies LTD, Dublin, Ireland). Sodium carbonate (Na2CO3) was obtained from Merck (Merck, Darmstadt, Germany). The different standards of phenolic compounds, namely protocatechuic acid, chlorogenic acid, cyanidin-3-O-rutinoside, quercetin-3-O-glucoside, kaempferol-3-O-rutinoside and rutin were obtained from Extrasynthese (Extrasynthese Co., Genay Cedex, France). The standard of cyanidin-3-O-glucoside was obtained from PhytoLab (PhytoLab GmbH & Co. KG, Vestenbergsgreuth, Germany). Milli-Q® (18 mΩ) (Merck Millipore, Molsheim, France) water (H2O) was used for all experiments.US homogenisation of blackberry–milk samples
A UIP1000hdT (Hielscher Ultrasonics GmbH, Teltow, Germany) pilot-scale ultrasonic transducer (1000 W) with an operational frequency of 20 kHz was utilized for US homogenisation of blackberry–milk samples. A titanium sonotrode with a diameter of 40 mm (model BS4d40) equipped with a B4-1.4 amplitude booster (max. amplitude, 24 μm) was submerged in samples at a depth of 40 mm from the surface and operated in continuous mode. A magnetic stirrer was also employed at 450 rpm along with the US treatment. Temperature was monitored throughout the whole treatment period using thermocouples. Samples were treated at an US intensity of 10.37 ± 0.58 W cm−2 for selected treatment times of 1, 3 and 5 min. Control samples were homogenised using a homogeniser (model T25 digital ULTRA-TURRAX®, IKA-Werke GmbH & Co. KG, Staufen, Germany) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. Smoothie-like beverages were obtained. All samples were stored at 4 °C after the treatment for 24 hours prior to analyses.
000 rpm for 1 min. Smoothie-like beverages were obtained. All samples were stored at 4 °C after the treatment for 24 hours prior to analyses.
An estimated calculation of energy was adapted from the method proposed by Putsakum et al. to determine the US power output (W) within the blackberry–milk samples13 using the following equation:
 | (1) |
Estimation of US intensity (UI) was calculated based on the equation stated by Tiwari and Mason:36
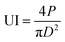 | (2) |
Determination of colour spaces
Colour spaces of blackberry–milk samples were determined using a portable chroma meter (model CR-400, Konica Minolta Ltd., Japan) with a D65 illuminant and 2° observer angle. The colorimeter was calibrated against a CR-A43 calibration plate prior to the sample measurement. A portion of each blackberry–milk sample was poured into CR-A502 optical glass tube cells (60 mm diameter and 40 mm depth) which were filled to an approximate depth of 10 mm from the bottom. The chroma meter was positioned at the top opening of the cell. Colour parameters, L* (+ lightness/− darkness), a* (+ redness/− greenness), b* (+ yellowness/− blueness), C* (chroma) and h (hue angle), of the samples were then measured under a white background. The total colour change (ΔE) was calculated using the equation below: | (3) |
Analysis of particle size distribution (PSD)
PSD of blackberry–milk samples was analysed using a Mastersizer 3000 (Malvern Instruments Ltd, Malvern, UK) as described by van de Langerijt et al. with slight modifications.37 The analysis mode was set for ‘general purpose’ with particle and dispersant refractive indices of 1.46 and 1.33, respectively. Samples were pipetted into a Hydro SM dispersion unit until the laser obscuration was within the range of 12%. All tested samples had weighted residuals below 1% indicating a good fit curve. The particle size distribution curve was expressed as particle size (μm) against volume density (%). Standard distribution percentiles of 10 (Dv10), 50 (Dv50) and 90% (Dv90) were also expressed, including volume weighted mean (D [4, 3]) and specific surface area.Analysis of rheological properties
Apparent viscosity of blackberry–milk samples was measured using a Physica MCR-301 rheometer (Anton Paar GmbH, Graz, Austria) as described by Keenan et al. with slight modifications.38 The instrument was equipped with smooth parallel plates (diameter, 50 mm) with a built-in Peltier device (model H-PTD200) to control the temperature during the analysis. Approximately 2 mL of samples were placed onto the base plate prior to lowering the top rotating plate to set the gap at 1 mm. The excess sample along the edge of the plates was removed. Samples were then rested between the plates for 5 min to achieve a measurement temperature of 25 °C and prevent residual stresses. The shear rate was increased from 0.01 to 100 s−1 through 40 data points.Amplitude strain sweep was also conducted using the same setup as previously mentioned to observe the linear viscoelastic region (LVE) and the overall rheological nature of the blackberry–milk samples. Strain was set to increase from 0.01 to 100% through 25 data points. The curves were expressed as shear strain against the storage modulus (G′) and loss modulus (G′′). Data are presented in the ESI data sheet.†
Determination of pH
Whole milk and blackberry–milk samples' pH was measured using a probe (model FiveGo F2, Mettler-Toledo AG, Schwerzenbach, Switzerland) at ambient temperature.Determination of total phenolic contents (TPCs), DPPH radical scavenging activity and ferric reducing antioxidant power (FRAP)
Assays for TPC, DPPH and FRAP of blackberry samples were performed as outlined by Singleton et al.,39 Goupy et al.40 and Stratil et al.41 respectively with slight modifications as reported by Putsakum et al.13 For TPCs, an aliquot of 100 μL of diluted sample was mixed with 100 μL of MeOH, 100 μL of Folin–Ciocalteu reagent and 700 μL of 20% (w/v) Na2CO3. The solution was vortexed and incubated in the dark for 20 min. Incubated mixtures were centrifuged at 7200 × g (model Galaxy 7D Digital Micro-centrifuge, VWR International, Germany) for 3 min at ambient temperature. The supernatant (200 μL) was collected and pipetted into a polystyrene 96-well microplate. Absorbance was measured at 735 nm using an EPOCH 2 microplate reader (BioTek Instruments, Inc., Dublin, Ireland). Gallic acid was used as a standard for calculation; absorbance of each sample was corrected after subtracting the reagent blank, MeOH. TPC values were expressed as mg of gallic acid equivalents (GAE)/100 mL of sample.For DPPH radical scavenging activity, the reagent stock solution was freshly prepared by dissolution of DPPH in MeOH (0.238 mg mL−1) prior to sonication for 20 min. Subsequently, 100 μL of diluted DPPH solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dilution with MeOH) was mixed with an aliquot of diluted sample (100 μL) in a polystyrene 96-well microplate. The mixture was incubated in the dark for 30 min at ambient temperature. Trolox was used as a standard and the absorbance of the samples was determined with an EPOCH 2 microplate reader (BioTek Instruments, Inc., Dublin, Ireland) at 515 nm. The results were expressed as mg of Trolox equivalents (TE)/100 mL of blackberry juice.
5 dilution with MeOH) was mixed with an aliquot of diluted sample (100 μL) in a polystyrene 96-well microplate. The mixture was incubated in the dark for 30 min at ambient temperature. Trolox was used as a standard and the absorbance of the samples was determined with an EPOCH 2 microplate reader (BioTek Instruments, Inc., Dublin, Ireland) at 515 nm. The results were expressed as mg of Trolox equivalents (TE)/100 mL of blackberry juice.
For FRAP assay, the working reagent was prepared by combining 100 mL of anhydrous sodium acetate buffer (38 mM, pH 3.6), 10 mL of FeCl3·6H2O in Milli-Q H2O (20 mM) and 10 mL of TPTZ in 40 mM HCl (10 mM). The reagent was incubated at 37 °C for a minimum of 5 min and maintained at this temperature prior to use. In a polystyrene 96-well microplate, 180 μL of FRAP reagent was mixed with 20 μL of diluted sample and incubated at 37 °C for 40 min. The absorbance was then measured using an EPOCH 2 microplate reader (BioTek Instruments, Inc., Dublin, Ireland) at 593 nm. Trolox was used as a standard and MeOH was used for blanks. The obtained values were expressed as mg of TE/100 mL of sample.
Quantification of specific phenolic compounds via UPLC-ESI-MS/MS
The quantification of the major phenolic compounds in blackberry juice samples was conducted using the modified method proposed by Putsakum et al.13 The analysis was performed on a Waters Acquity UPLC (Waters Corporation, Milford, MA, USA) system coupled to a tandem quadrupole (TQD) mass spectrometer. An Acquity UPLC HSS T3 (2.1 mm × 100 mm; 1.8 μm particle size) column was used and the mobile phases were composed of 0.1% formic acid in H2O (solvent A) and 0.1% formic acid in ACN (solvent B). The flow rate was set at 0.5 mL min−1 for 10 min. A multiple reaction monitoring (MRM) method was used for the quantification, while the MRM transitions of each compound were obtained through the Waters Intellistart™ software (Waters Corp., Milford, MA, USA). The UPLC-ESI-MS/MS data were obtained in negative ESI mode for all the phenolic compounds, except for cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, for which positive ion mode [M + H]+ was used. The quantification of phenolic compounds in the samples and standards was processed using the TargetLynx™ software (Waters Corporation, Milford, MA, USA).Statistical analysis
Three independent samples for each different treatment were evaluated (n = 3). For the colour spaces, pH, selected PSD parameters and antioxidant indices, three technical replicates were analysed and the results were reported as mean ± standard deviation (SD). A coefficient of variation (CV) below 15% was considered acceptable among the replicates for the spectrophotometric measurements. To assess the concentration of phenolic compounds via UPLC-ESI-MS/MS, the concentration of each compound was measured in triplicate (n = 3) and was reported as mean ± SD. Data normality was assessed through a combination of the Shapiro–Wilk test and Z-scores of skewness and kurtosis viaeqn (4) and (5):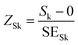 | (4) |
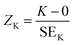 | (5) |
Results & discussion
Particle size distribution
The PSD curves of blackberry–milk samples and related parameters are shown in Fig. 1 and Table 1, respectively. The results show that the application of sonication significantly reduced the particle size of blackberry–milk beverages (p < 0.05). The PSD curves of US-treated samples (Fig. 1) shifted to the left compared to homogenised samples. Peak area of larger size particles decreased and the peak areas of smaller size particles increased as US exposure time was prolonged. This shows that longer treatment time resulted in a higher number of smaller particles in the US-treated samples. The ‘Dv’ value represents the maximum diameter of particles below a certain percentage volume of samples. In this study, analysis at 10, 50 and 90% of the tested sample volume is reported (Table 1). The data show that the application of sonication decreased the particle size significantly compared to the control (p < 0.05). For example, the Dv50 of samples treated with US for 1 min was 12.50 ± 0.30 μm and was further reduced to 10.03 ± 0.12 μm for 5 min US treatment. In contrast, a control sample had significantly larger particle sizes with a Dv50 of 42.70 ± 8.31 μm. A similar trend was observed for D [4, 3], which represents the mean particle size based on the whole volume (and/or mass) of tested samples. The decrease in particle size observed can be explained through the US mechanism. When a liquid matrix is sonicated, acoustic cavitation – the formation and collapse of micro-bubbles – occurs within the matrix, which in turn causes localised high heat and pressure. The mechanical forces generated through this phenomenon at the micro-scale can disrupt and cause breakage of particles within the matrix,42,43 consequently reducing the particle sizes of large compounds. In a study by Kumar et al. (2023), a significant decrease in particle size compared to the control was observed for smoothies processed with US-assisted heat treatment.44 In a reported study45 investigating US treatment of avocado puree, US was also shown to reduce sample particle size and increase specific surface area as the samples were treated with US (p < 0.05). The specific surface area of US-treated samples increased from 513.63 ± 9.13 m2 kg−1 (US, 1 min) to 623.50 ± 4.94 m2 kg−1 (US, 5 min). The surface area of particles within the food matrix increased due to an increase in the number of smaller particles. | ||
| Fig. 1 Particle size distribution curves of conventional and ultrasound (US)-homogenised blackberry–milk beverages. | ||
| Treatment | Dv10 (μm) | Dv50 (μm) | Dv90 (μm) | D [4, 3] (μm) | Specific surface area (m2 kg−1) |
|---|---|---|---|---|---|
| a Different superscript alphabetical letters (a and b) represent significant differences in means in the same column (p < 0.05). | |||||
| Conventional homogenisation | 11.85 ± 1.74a | 42.70 ± 8.31a | 126.00 ± 7.00a | 61.23 ± 5.84a | 225.60 ± 33.88b |
| US 1 min | 5.58 ± 0.11ab | 12.50 ± 0.30ab | 112.33 ± 7.77ab | 39.63 ± 3.44ab | 513.63 ± 9.13ab |
| US 3 min | 5.66 ± 0.25ab | 11.97 ± 0.91ab | 94.77 ± 7.13b | 36.60 ± 3.33ab | 534.23 ± 32.46ab |
| US 5 min | 5.07 ± 0.10b | 10.03 ± 0.12b | 80.37 ± 12.01b | 30.30 ± 2.41b | 623.50 ± 4.94a |
Temperature and pH changes
Changes in the temperature of US-treated samples are shown in Fig. 2a. During sonication, temperature continues to increase significantly with time (p < 0.05). From an initial temperature of samples at 36.67 ± 0.58 °C, the final temperatures were 41.67 ± 0.58 °C, 50.33 ± 1.15 °C and 58.67 ± 0.58 °C after US treatment for 1, 3 and 5 min, respectively. While US is considered a ‘non-thermal’ technology, the soundwaves generated from US transducers transfer energy to the matrix during processing, thus increasing the temperature of the treated matrix. Changes in pH were observed in the milk samples before addition of blackberry powder, in the blackberry–milk mixtures prior to homogenisation and in the blackberry–milk samples after homogenisation (Fig. 2b). The pH decreased after addition of the blackberry powder to the milk samples (from 6.69 ± 0.01 to 5.47 ± 0.02) due to the presence of organic acids within the blackberry powder (p < 0.05). The predominant organic acids within blackberries are malic acids and citric acids depending on the cultivar, regions and seasons.46 Lumps of non-homogeneous mixtures were observed when blackberry powder was added to the milk samples prior to homogenisation due to the aggregation of milk proteins under acidic conditions. This made the dissolution of blackberry powder more difficult in milk. After homogenisation, all samples showed a further reduction in pH (p < 0.05). This indicates that both conventional and US homogenisation assist with the dissolution of blackberry powder allowing the organic acids and other compounds within the powder to dissolve more easily in the milk matrix. However, samples treated with US for 5 min resulted in a slightly significantly lower pH than the conventionally homogenised samples (p < 0.05). It is possible that the presence of smaller particles and the increased surface area of particles further enhanced the dissolution of organic acids in the blackberry powder into the milk matrix. Mild heat generated by US treatment may also have assisted the rate of dissolution. Previous studies have shown that US treatment improves the solubilisation of milk protein concentrate powder by more than 90% under mild heating at 50 °C47 and that US-assisted processing can induce complete dissolution of maize starch in dimethyl sulfoxide.48Apparent viscosity
Apparent viscosity curves for US treated and untreated beverage samples are shown in Fig. 3. All samples had smoothie-like consistencies after homogenisation. The curves showed that US-treated beverages had a higher apparent viscosity compared to the control (p < 0.05) at low shear rates. A 1 minute treatment time resulted in a lower viscosity (p < 0.05) compared to 3 and 5 minute treatment times. As the shear rate increased, the viscosity of all samples decreased. The blackberry–milk samples exhibited pseudoplastic flow behaviour. The significant increase in viscosity observed is due to interactions between various compounds within the blackberry–milk matrix including: (1) gelation of milk proteins, (2) gelation of blackberry polysaccharides and (3) interaction between different types of macromolecules. As reported in the literature, the gelation of casein micelles occurs at pH < 5.5 as the acidified conditions decrease the net charge of the protein and induce colloidal calcium phosphate within the micelles to solubilize into the matrix.49,50 Destabilization within the micelles then occurs leading to the clustering of casein micelles and the formation of a gel-like structure within the matrix. As milk was the largest portion of the whole mixture, it is highly likely that this phenomenon was the main contributor to the observed increase in viscosity. Gelation of blackberry polysaccharides, for instance, pectin, can also occur due to low pH and the presence of Ca2+ ions within the milk matrix.51 However, due to the low proportion of blackberries in the whole mixture, it is likely that weak gel-like structures were formed within the matrix. Interactions between proteins and polysaccharides are other possibilities that may contribute to increased viscosity of blackberry–milk beverages. Many types of complex interactions can occur within the matrix as proteins, polysaccharides and fats are present within the sample matrix. Electrostatic interactions, van der Waals forces, hydrophobic interactions and hydrogen bonding between proteins and polysaccharides can lead to biopolymer complexes.52 The contribution effect on viscosity of the emulsion should also be noted as a colloidal dispersion was formed within the matrix. When polysaccharides are present in the matrix with an emulsion, a phenomenon called ‘bridging flocculation’ can occur, which induces a gel-forming structure,52,53 thus stabilizing the matrix and, in this case, possibly increasing the viscosity of the samples. Additionally, the effect of reducing the particle size and increasing particle surface area through US homogenisation (Table 1) promotes these interactions, promoting the weak binding between macromolecules to occur easily within the blackberry–milk matrix. | ||
| Fig. 3 Apparent viscosity of blackberry–milk beverages treated with conventional and ultrasound (US) homogenisation. | ||
Though the investigation of possible interactions between macromolecules was not the focus of the current study, the determination of the elastic (G′) and viscous (G′′) modulus can indirectly indicate the presence of gel formation within the blackberry–milk matrix (ESI data Fig. S1†). The G′ curve represents the elastic behaviour showing a solid-like nature of the samples. In contrast, the G′′ curve represents the viscous behaviour showing a liquid-like nature of the samples. Based on the obtained viscoelastic curves, it was found that G′ was initially higher than G′′ in all samples. This implies that the blackberry–milk mixture contains more elastic than viscous components, indicating that the matrix shows gel-like behaviour. This phenomenon was also reported in other related rheological studies for US-treated strawberry pulp,54 US-treated whey protein solution,55 and other dairy products treated with US.56 Importantly, it was observed that samples treated with US had longer LVE regions than traditionally homogenised samples, and as the US exposure time increased, the LVE regions extended (p < 0.05). In conventionally homogenised samples, the LVE region started to reduce as the G′ curve gradually decreased before the shear strain reached 1%. For samples treated with US for 1 min the G′ curve reduced to close to 1% shear strain. For 3 and 5 min US treatments, the G′ curves declined when the shear strain reached 1%, showing that sample matrixes treated at these parameters were slightly more resistant to deformation as shear strain was applied. US homogenisation also resulted in higher values for LVE regions. The LVE regions of US-treated samples (1, 3 and 5 min) were 11.00–17.60 Pa, 28.70–31.50 Pa and 26.50–28.60 Pa, respectively. Furthermore, the differences between the LVE regions of G′ and G′′ were significantly higher for US-treated samples and increased for longer US exposure times (p < 0.05). US treatment can induce stronger elastic behaviour resulting in a stronger gel-like structure forming between compounds within the blackberry–milk matrix.
Colour spaces and total colour changes
The colour values (L*, a*, b*, C* and h) and total colour changes (ΔE) observed are shown in Table 2. Changes in L* and a* values were not significant (p > 0.05). For b* values, a small significant increase was observed in samples treated with US for 3 and 5 min, which were 11.27 ± 0.27 and 11.15 ± 0.09, respectively (p < 0.05). C* or chroma represents the saturation or the intensity of colour and h or the hue angle indicates the quality of colour (red hue, 0°; yellow hue, 90°; green hue, 180°; and blue hue, 270°). C* values ranged from 20.96 ± 0.33 to 21.86 ± 0.29 and h values ranged from 28.19 ± 0.21 to 31.68 ± 0.43 (p < 0.05). These two parameters indicate that blackberry–milk samples had a reddish hue and that the colour was not highly saturated. The colour of the blackberry–milk samples was light pinkish red. Though there were slight significant differences in b*, C* and h, the overall colour of homogenised and US-treated samples were closely identical. From Table 2, the ΔE of US-treated samples were 22.01 ± 0.23, 23.05 ± 0.49 and 23.50 ± 0.62 for US 1, 3 and 5 min, respectively while the ΔE of the control was 23.04 ± 0.21. The ΔE between homogenised and US-treated samples showed non-significant differences (p > 0.05), with the exception of US 1 and 5 min, which were significantly different (p < 0.05). This implied that ΔE increased as the US treatment time progressed, which was possibly caused by the increase in temperature and longer exposure to cavitation. With some minor changes, it can be indicated that US treatment within 5 min along with continuous stirring does not significantly affect the overall colour of blackberry–milk samples. Comparable effects were reported in another study where carrot-based smoothies treated with US showed non-significant colour changes compared to the control.57 Another investigation of short-term US treatment for complex matrixes in avocado puree also reported non-significant effects on colour parameters.45 They reported that the colour of diluted avocado puree (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio) treated with US (for 1–10 min) was not statistically different compared to homogenised puree (p > 0.05).
9 ratio) treated with US (for 1–10 min) was not statistically different compared to homogenised puree (p > 0.05).
| Treatment | Colour parameters | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h | ΔE | |
| a Different superscript alphabetical letters (a, b, c and d) represent the significant difference in means in the same column (p < 0.05). | ||||||
| Homogenisation | 75.11 ± 0.17a | 18.81 ± 0.21a | 10.01 ± 0.29b | 21.22 ± 0.43ab | 28.19 ± 0.21d | 23.04 ± 0.21ab |
| US 1 min | 77.44 ± 0.45a | 18.30 ± 0.27a | 10.24 ± 0.20b | 20.96 ± 0.33b | 29.63 ± 0.26c | 22.01 ± 0.23b |
| US 3 min | 74.78 ± 2.31a | 18.27 ± 0.18a | 11.27 ± 0.27a | 21.47 ± 0.28ab | 31.68 ± 0.43a | 23.05 ± 0.49ab |
| US 5 min | 75.19 ± 1.11a | 18.80 ± 0.32a | 11.15 ± 0.09a | 21.86 ± 0.29a | 30.67 ± 0.41b | 23.50 ± 0.62a |
Total phenolic content and antioxidant indices
TPC, DPPH radical scavenging activities and FRAP activities of blackberry–milk samples are shown in Table 3. The results showed that samples treated with US for 3 min had the highest TPC, 561.65 ± 12.22 mg GAE/100 mL, among US-treated samples (p < 0.05), but the value was not statistically different from that of the control, 534.31 ± 17.41 mg GAE/100 mL (p > 0.05). This trend was also observed for DPPH radical scavenging and FRAP activities. However, there was a slight significant reduction in TPC, DPPH and FRAP values for samples treated with US for 1 min (p < 0.05). This slight decrease was possibly due to the formation of hydroxyl radicals (˙OH) induced by ultrasonic waves.58 The rate of hydroxyl radical formation may have outweighed the rate of polyphenol release from blackberry powder for a treatment time of 1 min. At 3 min, the significant increase observed in these three parameters could imply that more polyphenols were released into the matrix because of the cell disruption by sono-mechanical effects and increases in temperature (from 36.67 ± 0.58 to 50.33 ± 1.15 °C) of the blackberry–milk samples.59 Recent studies also reported increases in phenolic contents and antioxidant activities in fruit juice samples where both US and temperature treatment were applied.13,60 Overall, US treatment resulted in minor changes in phenolic contents and antioxidant indices of blackberry–milk beverages when compared to homogenised samples.| Treatment | TPC (mg GAE/100 mL) | DPPH (mg TE/100 mL) | FRAP (mg TE/100 mL) |
|---|---|---|---|
| a Different superscript alphabetical letters (a and b) represent the significant difference in means in the same column (p < 0.05). | |||
| Conventional homogenisation | 534.31 ± 17.41ab | 574.40 ± 9.16a | 1294.91 ± 16.69a |
| US 1 min | 512.53 ± 7.28b | 547.22 ± 9.60b | 1210.80 ± 3.54b |
| US 3 min | 561.65 ± 12.22a | 570.47 ± 8.30a | 1289.51 ± 5.83a |
| US 5 min | 540.04 ± 6.74ab | 547.83 ± 3.14b | 1232.41 ± 4.63b |
Quantification of polyphenols in blackberry–milk samples
The polyphenol contents of blackberry–milk samples are shown in Table 4. Similar trends in concentrations of polyphenols and TPCs were observed, except for rutin and caffeic acid. Slight decreases in concentrations of cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, quercetin-3-O-glucoside, kaempferol-3-O-rutinoside and protocatechuic acid were observed in US-treated samples for 1 min (p < 0.05). This is indicative of polyphenol degradation by ˙OH radicals formed by ultrasonic waves as previously mentioned.58 The slight significant increase in overall polyphenol a content also occurred in US-treated samples for 3 and 5 min (p < 0.05) due to an increase in polyphenol release from cell disruption; however, no statistical differences were observed compared to the control (p > 0.05). A gradual increase observed in the concentration of cyanidin-3-O-glucoside as the processing time increased may result from an interaction between the anthocyanin and milk proteins. A study on the interaction between cyanidin-3-O-glucoside and β-lactoglobulin with US treatment showed an enhancement of the binding ability of both compounds forming anthocyanin–protein complexes (through van der Waals forces, hydrogen bonds and hydrophobic interactions) which increased the stability of cyanidin-3-O-glucoside.61 This was also evident in another study where the interaction of cyanidin-3-O-glucoside and β-lactoglobulin was shown to provide a protective effect on colour degradation and enhance bioaccessibility after heat treatment.62 An interaction between cyanidin-3-O-glucoside and caseins in cow's milk was also reported as anthocyanin–casein complexes were mainly formed through hydrogen and hydrophobic interactions, inducing higher stability and bioavailability.63 Though studies on the interaction between cyanidin-3-O-glucoside and casein under US treatment were not the focus of this study, it is possible that the enhancement of anthocyanin–casein binding occurred similar to β-lactoglobulin. Only rutin and caffeic acid were stable throughout the US treatment as no significant differences were found for all samples. It was reported in another study that caffeic acid has a relatively high response threshold to ˙OH radicals as well as thermal degradation compared to cyanidin-3-O-glucoside and rutin.58 In contrast to the cited literature, the concentration of rutin in the blackberry–milk mixture appeared to be stable throughout the US process. This is possibly due to the release of more rutin from the dissolution of blackberry powder as the US homogenisation progresses. Overall, this study shows that US homogenisation has minor degradation effects on the concentration of major polyphenols in blackberry–milk beverages.| Treatment | Concentration of polyphenols (mg per 100 mL sample) | ||||||
|---|---|---|---|---|---|---|---|
| C3G | C3R | Q3G | K3R | Rut | PcA | CafA | |
| a Different superscript alphabetical letters (a and b) represent the significant difference in means in the same column (p < 0.05). C3G, cyanidin-3-O-glucoside; C3R, cyanidin-3-O-rutinoside; Q3G, quercetin-3-O-glucoside; K3R, kaempferol-3-O-rutinoside; Rut, rutin; PcA, protocatechuic acid; and CafA, caffeic acid. | |||||||
| Conventional homogenisation | 5.127 ± 0.512ab | 0.148 ± 0.007a | 0.120 ± 0.008ab | 0.013 ± 0.002a | 0.065 ± 0.005a | 0.133 ± 0.014ab | 0.026 ± 0.002a |
| US 1 min | 4.022 ± 0.250b | 0.124 ± 0.004b | 0.114 ± 0.002b | 0.009 ± 0.001b | 0.059 ± 0.006a | 0.106 ± 0.003b | 0.029 ± 0.004a |
| US 3 min | 4.272 ± 0.483b | 0.148 ± 0.006a | 0.131 ± 0.005a | 0.012 ± 0.001ab | 0.066 ± 0.007a | 0.169 ± 0.024a | 0.035 ± 0.007a |
| US 5 min | 5.677 ± 0.457a | 0.146 ± 0.004a | 0.122 ± 0.003ab | 0.013 ± 0.001a | 0.065 ± 0.002a | 0.166 ± 0.025a | 0.032 ± 0.005a |
Conclusions
This study investigated the effects of pilot-scaled batch US homogenisation compared to conventional homogenisation for blackberry–milk beverages. US homogenisation enhanced the overall apparent viscosity of blackberry–milk beverages through the effects of particle size reduction and the consequent increase in specific surface area of the particles that allowed efficient interaction with milk. These effects were more pronounced with an increase in temperature (from 35 °C to 60 °C after 5 min of US treatment). Rheology data indicated the formation of weak gel-like structures that occurred due to US treatment, which further contributed to the enhancement of apparent viscosity. A drop in pH after US homogenisation, from pH 5.47 to pH 4.86–4.80, indicates that acidic contents within the blackberry powder were dissolved within the milk matrix. US homogenisation for 5 min was more effective for dissolution of blackberry powder compared to conventional homogenisation, without compromising visual colour and health-beneficial constituents. Ideally, US homogenisation for 3 min was preferable for retention of antioxidant phenolic compounds in blackberry–milk samples. Further work investigating the application of US homogenisation coupled with conventional heat treatment as pilot-scale unit processing for blackberry–milk functional beverages is recommended. The investigation on that aspect can provide an improved understanding of the effect of process combinations on the final product quality and sensory-related attributes.Data availability
Data for this article, including quantitative and qualitative data, are available from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial and consumable support for the execution of this work from FIRM funded RubusElite (FIRM 17/F/277) from the Department of Agriculture, Food and Marine is acknowledged. Additionally, Royal Thai Government support, specifically from Ministry of Higher Education, Science, Research and Innovation (ST G5729), is also acknowledged for providing partial funding and contribution to the study.References
- L. Wang, Energy Effic., 2014, 7, 791–810 CrossRef.
- A. Shamoon, A. Haleem, S. Bahl, M. Javaid, S. Bala Garg, R. Chandmal Sharma and J. Garg, Mater. Today: Proc., 2022, 57, 936–941 CAS.
- J. Oláh, N. Aburumman, J. Popp, M. A. Khan, H. Haddad and N. Kitukutha, Sustainability, 2020, 12, 4674 CrossRef.
- A. Priyadarshini, G. Rajauria, C. P. O'Donnell and B. K. Tiwari, Crit. Rev. Food Sci. Nutr., 2019, 59, 3082–3101 CrossRef PubMed.
- F. Cacace, E. Bottani, A. Rizzi and G. Vignali, Innovative Food Sci. Emerging Technol., 2020, 60, 102281 CrossRef.
- G. Garcia-Garcia, S. Rahimifard, A. S. Matharu and T. I. J. Dugmore, ACS Sustain. Chem. Eng., 2019, 7, 5167–5175 CrossRef CAS.
- D. Merone, D. Colucci, D. Fissore, N. Sanjuan and J. A. Carcel, J. Food Eng., 2020, 283, 110031 CrossRef CAS.
- N. Pallarés, H. Berrada, E. Ferrer, J. Zhou, M. Wang, F. J. Barba and M. Brnčić, in Sustainable Production Technology in Food, ed. J. M. Lorenzo, P. E. S. Munekata and F. J. Barba, Academic Press, 2021, pp. 155–164 Search PubMed.
- M. L. Rojas, T. S. Leite, M. Cristianini, I. D. Alvim and P. E. Augusto, Food Res. Int., 2016, 82, 22–33 CrossRef CAS.
- G. Yildiz and G. Izli, J. Food Process Eng., 2019, 42, e13223 CrossRef.
- J. Wang, J. Wang, S. K. Vanga and V. Raghavan, Food Chem., 2020, 313, 126121 CrossRef PubMed.
- A. J. Vela, M. Villanueva, Á. G. Solaesa and F. Ronda, Food Hydrocolloids, 2021, 113, 106480 CrossRef CAS.
- G. Putsakum, K. Tzima, B. K. Tiwari, C. P. O'Donnell and D. K. Rai, Int. J. Food Sci. Technol., 2023, 58, 2304–2311 CrossRef CAS.
- A. Starek, Z. Kobus, A. Sagan, B. Chudzik, J. Pawłat, M. Kwiatkowski, P. Terebun and D. Andrejko, Sci. Rep., 2021, 11, 3488 CrossRef CAS PubMed.
- L. Chen, X. Bi, X. Cao, L. Liu and Z. Che, J. Sci. Food Agric., 2018, 98, 5378–5385 CrossRef CAS PubMed.
- B. Xu, M. Feng, B. Chitrakar, J. Cheng, B. Wei, B. Wang, C. Zhou and H. Ma, Innovative Food Sci. Emerging Technol., 2023, 103295 CrossRef CAS.
- B. Basumatary, P. K. Nayak, C. M. Chandrasekar, A. Nath, M. Nayak and R. K. Kesavan, Int. J. Fruit Sci., 2020, 20, S2056–S2073 CrossRef.
- R. Sasikumar, D. Pradhan and S. C. Deka, J. Food Process. Preserv., 2019, 43, e14220 CAS.
- H. Wahia, C. Zhou, A. T. Mustapha, R. Amanor-Atiemoh, L. Mo, O. A. Fakayode and H. Ma, Ultrason. Sonochem., 2020, 64, 104982 CrossRef CAS PubMed.
- L. Cassani, B. Tomadoni and M. del Rosario Moreira, J. Sci. Food Agric., 2020, 100, 5518–5526 CrossRef CAS PubMed.
- N. Bhargava, R. S. Mor, K. Kumar and V. S. Sharanagat, Ultrason. Sonochem., 2021, 70, 105293 CrossRef CAS PubMed.
- E. Sloan, IFT - Food Technology Magazine, 2021, vol. 75, pp. 23–35 Search PubMed.
- V. M. Ivanov, O. Shevchenko, A. Marynin, V. Stabnikov, E. Stabnikova, O. Gubenia, A. Shevchenko, O. M. Gavva and A. Salyuk, Ukr. Food J., 2021, 10, 7–36 CrossRef CAS.
- Department of Agriculture Food and the Marine, Development of a novel high protein, polyphenol enriched dairy beverage for athletes and physically active individuals (RubusElite), Report 17F277, Department of Agriculture, Food and the Marine, Dublin, 2024 Search PubMed.
- C. C. Carey, A. Lucey and L. Doyle, Sports Med., 2021, 51, 1293–1316 CrossRef PubMed.
- K. Tzima, G. Putsakum and D. K. Rai, Molecules, 2023, 28, 1933 CrossRef CAS PubMed.
- J. A. Robinson, J. E. Bierwirth, P. Greenspan and R. B. Pegg, J. Food Bioact., 2020, 9, 40–51 Search PubMed.
- M. S. Quesada-Morúa, O. Hidalgo, J. Morera, G. Rojas, A. M. Pérez, F. Vaillant and L. Fonseca, J. Berry Res., 2020, 10, 459–474 Search PubMed.
- F. Van de Velde, D. Esposito, M. H. Grace, M. E. Pirovani and M. A. Lila, Food Res. Int., 2019, 121, 453–462 CrossRef CAS PubMed.
- J. Wu, B. Du, J. Li and H. Zhang, LWT–Food Sci. Technol., 2014, 56, 370–376 CrossRef CAS.
- M. Alongi, S. Calligaris and M. Anese, J. Funct. Foods, 2019, 58, 130–137 CrossRef CAS.
- L. Zhou, W. Zhang, J. Wang, R. Zhang and J. Zhang, Ultrason. Sonochem., 2022, 82, 105885 CrossRef CAS PubMed.
- V. Akdeniz and A. S. Akalın, Turkish Journal of Agriculture - Food Science and Technology, 2020, 8, 252–259 CrossRef.
- T. M. van de Langerijt, T. R. Murphy, J. A. O'Mahony and S. V. Crowley, Int. Dairy J., 2024, 150, 105843 CrossRef CAS.
- K. Tzima, N. P. Brunton and D. K. Rai, J. Food Biochem., 2020, 44, e13148 CrossRef PubMed.
- B. K. Tiwari and T. J. Mason, in Novel Thermal and Non-Thermal Technologies for Fluid Foods, ed. P. J. Cullen, B. K. Tiwari and V. P. Valdramidis, Academic Press, San Diego, 2012, pp. 135–165 Search PubMed.
- T. M. van de Langerijt, Y. C. O'Callaghan, K. Tzima, A. Lucey, N. M. O'Brien, J. A. O'Mahony, D. K. Rai and S. V. Crowley, Int. J. Dairy Technol., 2023, 76, 828–843 CrossRef CAS.
- D. F. Keenan, B. K. Tiwari, A. Patras, R. Gormley, F. Butler and N. P. Brunton, Int. J. Food Sci. Technol., 2012, 47, 827–836 CrossRef CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, in Methods in Enzymology, Academic Press, 1999, vol. 299, pp. 152–178 Search PubMed.
- P. Goupy, M. Hugues, P. Boivin and M. J. Amiot, J. Sci. Food Agric., 1999, 79, 1625–1634 CrossRef CAS.
- P. Stratil, B. Klejdus and V. Kubáň, J. Agric. Food Chem., 2006, 54, 607–616 CrossRef CAS PubMed.
- F. J. Fuchs and J. D. Kramlick, in Power Ultrasonics, ed. J. A. Gallego-Juárez, K. F. Graff and M. Lucas, Woodhead Publishing, 2nd edn, 2023, pp. 455–471 Search PubMed.
- H. Delmas, L. Barthe and R. Cleary, in Power Ultrasonics, ed. J. A. Gallego-Juárez, K. F. Graff and M. Lucas, Woodhead Publishing, 2nd edn, 2023, pp. 665–685 Search PubMed.
- S. Kumar, R. Chawla, S. Sivakumar, S. K. Khatkar, N. Kumar and N. Goel, Int. J. Food Sci. Technol., 2023, 58, 3420–3429 CrossRef CAS.
- X. Bi, Y. Hemar, M. O. Balaban and X. Liao, Ultrason. Sonochem., 2015, 27, 567–575 CrossRef CAS PubMed.
- M. Mikulic-Petkovsek, R. Veberic, M. Hudina, Z. Zorenc, D. Koron and M. Senica, Foods, 2021, 10, 1581 CrossRef CAS PubMed.
- N. A. McCarthy, P. M. Kelly, P. G. Maher and M. A. Fenelon, J. Food Eng., 2014, 126, 142–148 CrossRef CAS.
- P. Guo, W. Wang, S. Dai, S. Shen, W. Zhang, Y. Lian and H. Dou, Microchem. J., 2019, 150, 104092 CrossRef.
- H. Sinaga, N. Bansal and B. Bhandari, Int. J. Food Prop., 2017, 20, 179–197 CrossRef CAS.
- M. Alexander and D. G. Dalgleish, Colloids Surf., B, 2004, 38, 83–90 CrossRef CAS PubMed.
- B. R. Thakur, R. K. Singh, A. K. Handa and M. A. Rao, Crit. Rev. Food Sci. Nutr., 1997, 37, 47–73 CrossRef CAS PubMed.
- K. K. T. Goh, A. Teo, A. Sarkar and H. Singh, in Milk Proteins, ed. M. Boland and H. Singh, Academic Press, 3rd edn, 2020, pp. 499–535, DOI:10.1016/B978-0-12-815251-5.00013-X.
- E. Dickinson, Trends Food Sci. Technol., 1998, 9, 347–354 CrossRef CAS.
- L. Chen, L. Chen, K. Zhu, X. Bi, Y. Xing and Z. Che, Ultrason. Sonochem., 2020, 67, 105144 CrossRef CAS PubMed.
- A. B. Khatkar, A. Kaur, S. K. Khatkar and N. Mehta, Ultrason. Sonochem., 2018, 49, 333–342 CrossRef CAS PubMed.
- L. M. Carrillo-Lopez, I. A. Garcia-Galicia, J. M. Tirado-Gallegos, R. Sanchez-Vega, M. Huerta-Jimenez, M. Ashokkumar and A. D. Alarcon-Rojo, Ultrason. Sonochem., 2021, 73, 105467 CrossRef CAS PubMed.
- M. Buniowska, E. Arrigoni, A. Znamirowska, J. Blesa, A. Frígola and M. J. Esteve, Foods, 2019, 8, 492 CrossRef CAS PubMed.
- P. Wang, C. Cheng, Y. Ma and M. Jia, Sep. Purif. Technol., 2020, 247, 116967 CrossRef CAS.
- J. Živković, K. Šavikin, T. Janković, N. Ćujić and N. Menković, Sep. Purif. Technol., 2018, 194, 40–47 CrossRef.
- Y. Wu, L. Xu, X. Liu, K. M. F. Hasan, H. Li, S. Zhou, Q. Zhang and Y. Zhou, LWT, 2021, 150, 112021 CrossRef CAS.
- L. Wang, X. Wang, F. Luo and Y. Li, Int. J. Food Sci. Technol., 2022, 57, 7057–7065 CrossRef CAS.
- X. Qie, W. Chen, R. Wu, Z. Wang, M. Zeng, J. Chen, H. Douglas Goff and Z. He, Food Res. Int., 2022, 157, 111494 CrossRef CAS PubMed.
- F. Pan, J. Li, L. Zhao, T. Tuersuntuoheti, A. Mehmood, N. Zhou, S. Hao, C. Wang, Y. Guo and W. Lin, J. Food Biochem., 2021, 45, e13570 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fb00065j |
| This journal is © The Royal Society of Chemistry 2024 |

