Open Access Article
Open Access ArticleUse of whey for a sustainable production of postbiotics with potential bioactive metabolites
Victor E.
Vera-Santander
,
Emma
Mani-López
 ,
Aurelio
López-Malo
,
Aurelio
López-Malo
 and
Maria Teresa
Jiménez-Munguía
and
Maria Teresa
Jiménez-Munguía
 *
*
Chemical, Food and Environmental Engineering Department, Universidad de las Américas, Puebla. Sta. Catarina Mártir S/N, San Andrés Cholula, Puebla, 72810, Mexico. E-mail: mariat.jimenez@udlap.mx
First published on 14th June 2024
Abstract
Postbiotics have gained attention due to their health benefits and potential bioactive metabolites. Short-chain fatty acids (SCFAs) have been identified within these metabolites, which are related to anti-inflammatory properties and antioxidant activity, among others. For the food industry, it is important to consider a suitable culture medium for postbiotic production. Whey, as a by-product from the cheese industry, is rich in nutrients and is proposed to support this purpose. This study is aimed to evaluate the microbial growth of three probiotics, Lactiplantibacillus plantarum 299v, Lacticaseibacillus casei Shirota, and Bifidobacterium animalis subsp. lactis BPL1, using a whey culture medium supplemented with soluble fibres (inulin or chia mucilage) at two concentrations (1% or 2% w/w). Also, analyse the effect of soluble fibres on the production of SCFAs and the antioxidant activity of cell-free supernatant as postbiotics. SCFA production was quantified by HPLC and antioxidant activity was determined by the DPPH+ assay and the KMnO4 agar method. The formulated culture media promoted the growth of probiotics, especially those added with inulin. Lactiplantibacillus plantarum 299v and Lacticaseibacillus casei Shirota produced primary lactic and acetic acid. B. lactis BPL1 had the highest SCFAs production in the culture medium with 2% w/w of inulin. The antioxidant activity from Lactiplantibacillus plantarum 299v postbiotics was significantly improved with soluble fibres (p < 0.05). This study shows postbiotics are produced with a sustainable approach. Moreover, postbiotics based on whey and soluble fibres can be a potential ingredient for the formulation of new food products as sources of SCFAs and antioxidants.
Sustainability spotlightThe sustainable production of postbiotics, rich in antioxidants and short-chain fatty acids (SCFAs), using whey and soluble fibres in culture media is an approach that promotes the principles of the circular economy through the effective use of by-products of the dairy industry such as whey. The use of renewable resources through soluble plant-based fibers, reduces overall environmental impact, offering health benefits to consumers, optimizes nutrient utilization and aligns with market growth demand for sustainable and functional food products. |
1 Introduction
Postbiotic is a relatively new term in industry, food science, and technology research. A wide variety of concepts describe postbiotics; the most current one was given by the consensus panel of the International Scientific Association of Probiotics and Prebiotics (ISAPP). They defined postbiotics as the “preparation of inanimate microorganisms and/or their components that confer a benefit to the health of the host”.1 Postbiotics can provide potential functional properties such as antimicrobial, antioxidant, and strengthen the immune system, which can positively affect microbiota homeostasis and/or consumer metabolic and signaling pathways.2 Incorporating postbiotics into foods has been proposed since they have certain advantages compared to probiotics, such as longer shelf life, storage, handling, and easier transportation.3 The cell-free supernatants (CFS) are considered postbiotics that contain many bioactive metabolites besides the culture media. Short-chain fatty acids (SCFAs) are of great interest among these metabolites for their possible health benefits.4 The main SCFAs produced during bacterial fermentation of soluble fibres are acetate, butyrate, and propionate. SCFAs can confer certain effects such as antitumor, anti-inflammatory in the colon, cardiovascular, obesity control, control of glucose homeostasis, appetite regulation, and protection against the development of immune disorders.5 Also, the consumption of SCFAs as postbiotics through diet has been proposed.6 Several studies focused on food metabolomics have proven that the fermentation of probiotics (Lactobacillus and Bifidobacterium species) with soluble fibres can produce SCFAs.7–10On the other hand, in recent years, postbiotics' antioxidant activity has gained relevance in food science research. Oxidative stress can cause an imbalance of biological processes at the cellular level, such as stem cell depletion, tumorigenesis, autoimmunity (immune response against autoantigens), and accelerated senescence.11 It has been demonstrated that postbiotics (Lactobacillus plantarum AF1 and L. brevis KCCM 12203P) have interesting antioxidant activities.12,13 Some researchers14 have mentioned that conventional techniques (ABTS and DDPH assay) have certain complications in effectively measuring antioxidant activity. For this reason, the method based on potassium permanganate (KMnO4), which is simple, fast to develop, reliable, and economically feasible, has been proposed.
For postbiotics production on an industrial scale, it is essential to have a culture medium suitable for the microorganisms' growth and the production of bioactive metabolites, and preferably, be economically feasible and safe for consumption. Generally, the culture mediums used are laboratory formulations (MRS broth) or milk; however, on a large scale, these can be expensive. Whey has been reported to be suitable for the growth of probiotics,15 since it is rich in sugars (lactose 46–52 g L−1), protein (6.5–6.6 g L−1), and minerals (5.0–5.2 g L−1).16 Besides, whey is a by-product of the cheese industry, is relatively cheap, and is easily accessible. Still, it is sometimes discarded and unused as it should be.17 The cheese industry is known to produce an estimated 115 million tons of whey annually, of which 47% of the by-product is directly disposed of in drains. This is a matter of deep concern, leading to severe environmental pollution issues such as water contamination, dissolved oxygen depletion, and eutrophication.16 Whey has a high biochemical oxygen demand (BOD) ranging from 40–60 g L−1 and chemical oxygen demand (COD) ranging from 50–80 g L−1. These high levels of BOD and COD make whey a significant pollutant by-product worldwide, posing a significant environmental challenge for dairy industries. Furthermore, few studies currently focus on evaluating alternative culture mediums for producing postbiotics that are suitable for the food industry.3,18,19 Recently, Yousefi et al.20 optimised the fermentation conditions of L. plantarum in whey to improve the antibacterial metabolites production whereas Izzo et al.21 analysed the antifungal activity and phenolic compounds from the fermented goat's sweet whey utilized L. plantarum strains. Amiri et al.18 optimised the supplementation of cheese whey to improve the production of conjugated linoleic acid, exopolysaccharides, and bacteriocins by B. lactis BB12 as postbiotics. It is evident the increasing interest to utilise whey as culture medium to produce functional metabolites with food applications. Moreover, it has been reported that several soluble fibers rich in glucose, arabinose, galactose, fructose, fructooligosaccharides (FOS), galactomannans, among others, promotes postbiotic production.22,23
Therefore, this research aims to evaluate the microbial growth of probiotics (Lactiplantibacillus plantarum 299v, Lacticaseibacillus casei Shirota, and Bifidobacterium animalis subsp. lactis BPL1) using a whey culture medium supplemented with soluble fibres (inulin or chia mucilage) at two concentrations (1% or 2% w/w). Also, analyse the production of SCFAs and the antioxidant activity of cell-free supernatant as postbiotics.
2 Material and methods
2.1 Probiotic strains
To obtain postbiotics, it is essential to consider heterofermentative probiotic bacteria to ensure the production of SCFAs. Lactiplantibacillus plantarum 299v, Lacticaseibacillus casei Shirota, and Bifidobacterium animalis subsp. lactis BPL1 are well known probiotics.24–28 Thus, these strains were selected to produce postbiotics. The probiotic strains Lactiplantibacillus plantarum 299v and Bifidobacterium animalis subsp. lactis BPL1 were obtained in the freeze-dried form of Digestive probiotic (Nature Made, West Hills, CA) and Microbiot® Fit (Grupo Columbia, México City, Mexico), and Lacticaseibacillus casei Shirota was obtained from the Food Microbiology Laboratory of the Universidad de las Americas Puebla. Lactiplantibacillus plantarum 299v and L. casei Shirota were reactivated and sub-cultivated in MRS broth (Difco™, BD, Sparks, MD, USA) at 35 °C. For the reactivation and sub-cultivation of B. lactis BPL1, MRS broth was supplemented with 0.5% (w/v) cysteine hydrochloride (Sigma-Aldrich, St. Louis, MO) and grown under anaerobic conditions at 35 °C.2.2 Chia mucilage extraction
The chia seeds (Salvia hispanica L.) were obtained from Verde Limón Trading Company (Mexico City, Mexico). For the extraction of mucilage, chia seeds were hydrated in distilled water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) for 4 h with constant stirring; then, seeds were mechanically separated using a strainer mesh #35 (500 μm), and the mucilage was lyophilized (Triad™ Labconco, USA).29
20 (w/v) for 4 h with constant stirring; then, seeds were mechanically separated using a strainer mesh #35 (500 μm), and the mucilage was lyophilized (Triad™ Labconco, USA).29
2.3 Preparation of culture medium
For the culture medium, the following ingredients were used: whey powder (Food Technologies Trading, Mexico), yeast extract (Difco™, BD, Sparks, MD, USA), magnesium sulfate (MgSO4·7H2O) (Merck, Burlington, MA), manganese sulfate (MnSO4·H2O) (Merck, Burlington, MA), inulin (Agaviotica, Jalisco, Mexico) and chia mucilage. The culture medium used as control (CW) was composed of whey powder 10% w/w, yeast extract 0.3% w/w, MgSO4·7H2O 0.02% w/w, MnSO4·4H2O 0.005% w/w, and water. Based on previous experiments, it was decided to use a range of soluble fibres concentrations of 1% and 2% w/w. Then, 4 culture media were formulated to evaluate inulin and chia mucilage to enrich the CW: CW plus inulin 1% w/w (WIn1%), CW plus inulin 2% w/w (WIn2%), CW plus chia mucilage 1% w/w (WMc1%) and CW plus chia mucilage 2% w/w (WMc2%). The culture medium for B. lactis BPL1 was supplemented with vitamins C (Sigma-Aldrich, St. Louis, MO) and E (Sigma-Aldrich, St. Louis, MO) at 250 ppm and 5.75 ppm, respectively, to provide adequate reducing conditions for bacteria growth. All culture media were sterilized at 120 ± 1.0 °C for 15 min and stored at 25 °C until used. Since MRS broth has been used as a culture medium in most of the studies with postbiotics;30 it was used as reference to compare the culture mediums formulated in this study.2.4 Probiotic culture conditions and bacterial kinetic growth
Fresh cultures of the probiotics (MRS broth) were used to inoculate CW media, adding the necessary amount to obtain an initial population of 106 CFU mL−1. The culture mediums were incubated without stirring at 35 °C for 30 h. For the bacterial growth curve, samples were taken from culture media at 0, 3, 6, 9, 24, and 30 h. For microbial counting, MRS agar (Difco™, BD, Sparks, MD, USA) was used by the surface extension method. After appropriate incubation, colonies were counted. The kinetics of bacterial growth were modelled using the Modified Gompertz equation (eqn (1)). Where A is the maximum net growth, μ is the exponential growth rate (h−1) and λ is the latency time (h). These kinetic parameters were obtained by nonlinear regression. Minitab 20 software (Minitab LLC, State College, PA, USA) was used to calculate the residual analysis (RMSE) and correlation coefficients (R2). The kinetics of bacterial growth were carried out by triplicate. | (1) |
2.5 pH and titratable acidity
Samples were taken from the culture medium to analyse pH and titratable acidity (TA) during fermentation (0, 9, 24, and 30 h). pH was determined by immersion electrode using a pH meter (HI 2210 Hanna Instruments, Woonsocket, RI, USA). The TA was determined following the method 22.061 from AOAC31 and was expressed as a percentage of lactic acid (% w/v). The measurements were performed in triplicate.2.6 Cell-free supernatant preparation as postbiotics
At the end of fermentation, samples were centrifuged at 7000g for 15 min at 5 °C (Sorvall ST 8R, Thermo Fischer Scientific, Schwerte, Germany). The supernatant was then filtered by 0.45 μm cellulose nitrate filter (Advantec, MFS, Dublin, CA, USA). Finally, the cell-free supernatants were frozen at −18 °C until their use.2.7 Characterization of postbiotics
2.8 Antioxidant activity of postbiotics
| TAA = Db − Dc | (2) |
| Scavenging activity % = (Ab − As)/Ab × 100 | (3) |
2.9 Statistical analysis
The data were analysed using Analysis of Variance (ANOVA) and Tukey's mean comparison test, with a confidence level of 95%. Minitab 20 software (Minitab LLC, State College, PA, USA) was utilized for the analysis.3 Results and discussion
3.1 Probiotics growth in the culture media
The metabolites such as SCFAs are produced by different metabolic pathways of fermentation during the microbial growth. MRS broth used as the reference culture medium was optimal for all evaluated probiotics (Fig. 1). Considering that metabolites' production occurs primordialy during the exponential phase of the microorganisms kinetics, we can highlight that the value of the exponential phase growth rate μ for Lactiplantibacillus plantarum 299v was 0.50 h−1 with MRS medium, but with no significant difference (p > 0.05) when using the whey formulations supplemented with chia mucilage or inulin (Table 1). Even though, a clear maximum microbial net growth (A parameter of 3.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU ml−1) was obtained for L. plantarum 299v with MRS broth (Fig. 1, Table 1). For Lacticaseibacillus casei Shirota and Bifidobacterium animalis subsp. lactis BPL1, the highest μ value was attained with the WIn1% formulated medium (0.66 and 0.48 h−1), with no significant difference when using MRS broth (p > 0.05). Burns et al.15 studied the suitability of whey for the growth of probiotics (L. acidophilus A9, 08, and H5; L. paracasei A13 and LS; and L. casei LB) showing that whey medium supplemented with yeast extract (like CW) was optimal for probiotic growth. However, the maximum net growth values (A) reported by Burns et al.15 were lower (1.4–1.6
log CFU ml−1) was obtained for L. plantarum 299v with MRS broth (Fig. 1, Table 1). For Lacticaseibacillus casei Shirota and Bifidobacterium animalis subsp. lactis BPL1, the highest μ value was attained with the WIn1% formulated medium (0.66 and 0.48 h−1), with no significant difference when using MRS broth (p > 0.05). Burns et al.15 studied the suitability of whey for the growth of probiotics (L. acidophilus A9, 08, and H5; L. paracasei A13 and LS; and L. casei LB) showing that whey medium supplemented with yeast extract (like CW) was optimal for probiotic growth. However, the maximum net growth values (A) reported by Burns et al.15 were lower (1.4–1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N/N0) than those obtained in the present study. In another study, Hernandez-Mendoza et al.35 modelled the growth of L. reuteri NRRL 1417, and B. bifidum NCFB2715 in a whey-based food beverage with the modified Gompertz equation, and the A values were also lower (1.76–0.97
N/N0) than those obtained in the present study. In another study, Hernandez-Mendoza et al.35 modelled the growth of L. reuteri NRRL 1417, and B. bifidum NCFB2715 in a whey-based food beverage with the modified Gompertz equation, and the A values were also lower (1.76–0.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N/N0) than our results.
N/N0) than our results.
| Medium |
A (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N/N0, CFU mL−1) N/N0, CFU mL−1) |
μ (h−1) | λ (h) | R 2 | RSME |
|---|---|---|---|---|---|
| a MRS: de Man, Rogosa and Sharpe broth; CW: control; WIn1%: CW plus 1% w/w inulin; WIn2%: CW plus 2% w/w inulin; WMc1%: CW plus 1% w/w chia mucilage; WMc2%: CW plus 2% w/w chia mucilage. Different letters show a significant difference (p < 0.05) between the culture mediums of each probiotic. | |||||
| Lactiplantibacillus plantarum 299v | |||||
| MRS | 3.50 ± 0.06A | 0.50 ± 0.03A | 1.42 ± 0.23B | 0.996 | 0.125 |
| CW | 2.60 ± 0.08BC | 0.21 ± 0.03B | 1.14 ± 0.05C | 0.988 | 0.158 |
| WIn1% | 2.82 ± 0.07BC | 0.36 ± 0.04AB | 1.60 ± 0.39B | 0.993 | 0.133 |
| WIn2% | 2.90 ± 0.07B | 0.38 ± 0.04AB | 1.68 ± 0.36B | 0.990 | 0.166 |
| WMc1% | 2.50 ± 0.05C | 0.35 ± 0.03AB | 1.66 ± 0.30B | 0.995 | 0.104 |
| WMc2% | 2.50 ± 0.04C | 0.34 ± 0.03AB | 3.54 ± 0.30A | 0.996 | 0.098 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Lacticaseibacillus casei Shirota | |||||
| MRS | 4.06 ± 0.04A | 0.50 ± 0.02A | 1.99 ± 0.15BC | 0.999 | 0.078 |
| CW | 2.65 ± 0.04E | 0.43 ± 0.03B | 2.35 ± 0.19B | 0.997 | 0.085 |
| WIn1% | 3.37 ± 0.07B | 0.66 ± 0.06A | 4.91 ± 0.24A | 0.995 | 0.153 |
| WIn2% | 2.87 ± 0.04DE | 0.41 ± 0.02B | 0.75 ± 0.21D | 0.997 | 0.086 |
| WMc1% | 3.05 ± 0.05CD | 0.39 ± 0.03B | 0.83 ± 0.29CD | 0.995 | 0.120 |
| WMc2% | 3.25 ± 0.04BC | 0.41 ± 0.02B | 0.35 ± 0.21D | 0.997 | 0.093 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Bifidobacterium animalis subsp. lactis BPL1 | |||||
| MRS | 3.15 ± 0.04B | 0.42 ± 0.02A | 1.47 ± 0.18A | 0.998 | 0.080 |
| CW | 2.82 ± 0.05BC | 0.37 ± 0.03B | 2.76 ± 0.28A | 0.996 | 0.103 |
| WIn1% | 3.67 ± 0.09A | 0.48 ± 0.05A | 1.70 ± 0.40A | 0.990 | 0.206 |
| WIn2% | 2.84 ± 0.07BC | 0.35 ± 0.03B | 1.79 ± 0.39A | 0.991 | 0.147 |
| WMc1% | 2.80 ± 0.08BC | 0.29 ± 0.04B | 1.61 ± 0.56A | 0.986 | 0.183 |
| WMc2% | 2.55 ± 0.08C | 0.29 ± 0.04B | 1.93 ± 0.58A | 0.983 | 0.183 |
It is well known that inulin is a good prebiotic and enhances the probiotics' growth.36–39 The probiotics studied adapted better to the medium supplemented with inulin than those with chia mucilage. The A values of WIn1% (Lacticaseibacillus casei Shirota and B. lactis BPL1) and WIn2% (Lactiplantibacillus plantarum 299v) were significantly higher (p < 0.05) than the WMc1% and WMc2% medium. In previous investigations with B. lactis BPL1 have reported the stimulation of Bifidobacterium species in culture media containing inulin.37,38 However, the concentration of inulin should be evaluated since high concentrations may cause adverse effects on Bifidobacterium growth. McLaughlin et al.36 reported that several species of Bifidobacterium did not grow adequately at high concentrations (>5% w/v) of inulin. Similar behaviour was observed in the present study with B. lactis BPL1, which showed a reduction in the growth (parameters μ and A) with 2% of inulin. This growth reduction is attributed to a saturation of substrate due to bacteria inability to metabolize all the soluble fibre.36 Likewise, Lacticaseibacillus casei Shirota had the highest μm value at the lowest inulin concentration (1% w/w), showing that this probiotic was better adapted to soluble fibre. This finding was similar to the research of Renye et al.,39 in which several strains of Lacticaseibacillus casei presented greater growth when the medium was supplemented with inulin. In contrast, the chia mucilage had no significant effect (p > 0.05) on the growth rate, probably due to chia mucilage being a very complex carbohydrate, composed primarily of rhamnogalacturonan and arabinoxylans, that probiotics do not metabolize.40 To the best of our knowledge, no previous reports were found about chia mucilage fermentation. The μ value is important since the fermentation time is reduced at higher rates, which could benefit large-scale production costs. Furthermore, a rapid exponential growth rate is key for generating metabolites of interest, such as SCFAs. These components are considered secondary metabolites, presumed to be synthesized at the beginning and during the stationary phase.41
The latency time (λ) represents the bacteria's adaptation time in the medium to grow due to changes in culture conditions. Lactiplantibacillus plantarum 299v required 3.54 h to start growing in the culture medium supplemented with WMc2%, being the longest observed time (p < 0.05). This result can be due to the high viscosity of the culture medium with chia mucilage causing an obstacle to the probiotic adaptation in the culture medium since the nutrients are inaccessible to the microorganisms. B. lactis BPL1 adapted rapidly to all tested culture mediums; showing a λ value very similar (p > 0.05).
3.2 pH and TA of culture media during the growth
Lactiplantibacillus plantarum 299v presented a drastic reduction in pH and an increase in TA from 9 to 16 h of fermentation (Fig. 2); this behaviour was similar for all culture medium and probiotics. For Lacticaseibacillus casei Shirota, the pH values after 30 h of fermentation in CW was 3.55 and did not show significant differences (p > 0.05) when supplementing with chia mucilage or 2% of inulin (Fig. 3). It results important to highlight that B. lactis BPL1 presented the lowest pH (3.3 ± 0.01) values in WIn1% (p < 0.05) among all culture media and compared with the other evaluated lactobacilli. These data were similar to those reported by several studies that used whey as a growth medium.15,42,43 The TA after 30 h of fermentation in the culture medium ranged from 2.04 to 1.05% (see Fig. 3). There were significant differences (p < 0.05) in TA values of the three probiotics in MRS broth compared with the studied culture mediums. Regarding the whey-based mediums supplemented with soluble fibers, Lacticaseibacillus casei Shirota showed the highest values of TA in WIn2%, while for Lactiplantibacillus plantarum 299v was with WMc1% and WMc2%. For Lactiplantibacillus plantarum 299v, TA increased directly proportional to inulin concentration; this effect agrees with previous reports with L. plantarum species.44,45 It is important to emphasize that the pH and TA values of the culture mediums at the end of fermentation were more acidic than those reported in the literature. For instance, a study of the fermentation of a whey-based beverage using L. reuteri NRRL 14171 and B. bifidum NCFB2715 recorded pH values of 4.50–4.86 and TA of 0.315–0.378%.35 The difference in pH and TA between the previous study and this work may be due to the fermentation time. | ||
| Fig. 2 Evolution of pH and titratable acidity in de Man, Rogosa, and Sharpe broth (MRS) (◆) and control medium (CW) (■) for Lactiplantibacillus plantarum 299v. | ||
3.3 Analysis of lactic acid and short-chain fatty acids postbiotics
As is observed in Table 2, postbiotics of Lactiplantibacillus plantarum 299v or B. lactis BPL1 from MRS broth had the highest (p < 0.05) concentration of lactic acid. Lacticaseibacillus casei Shirota yielded the highest amount (p < 0.05) of lactic acid from WIn1% in the tested culture medium. Furthermore, a significant effect (p < 0.05) was observed in all postbiotics from a whey-based medium supplemented with inulin, increasing the concentration of lactic acid. Despite previous reports mentioning that some strains of L. plantarum are not capable of degrading inulin enzymatically, the hydrolysis of inulin in acidic conditions (pH ≤ 4.0) occurs, releasing simple monomers of fructose and glucose, which bacteria could use as carbon source.45–48 Also, Matusek et al.49 observed that oligosaccharide degradation increased with pH reduction. They also mentioned that several strains of Lacticaseibacillus casei can ferment FOS and inulin.| Medium | Lactic acid (mM) | Acetic acid (mM) | Butyric acid (mM) | Propionic acid (mM) |
|---|---|---|---|---|
| a ND: no detectable; MRS: de Man, Rogosa and Sharpe broth; CW: control whey; WIn1%: whey plus 1% w/w inulin; WIn2%: whey plus 2% w/w inulin; WMc1%: whey plus 1% w/w chia mucilage; WMc2%: whey plus 2% w/w chia mucilage. Different letters show a significant difference (P < 0.05) between the culture mediums of each probiotic. | ||||
| Lactiplantibacillus plantarum 299v | ||||
| MRS | 247.53 ± 0.85A | 42.98 ± 1.29A | ND | ND |
| CW | 93.06 ± 0.49E | 18.91 ± 1.39C | ND | ND |
| WIn1% | 142.32 ± 5.22C | 18.53 ± 0.48C | ND | ND |
| WIn2% | 193.08 ± 1.15B | 12.54 ± 0.43D | ND | ND |
| WMc1% | 104.49 ± 0.85D | 28.28 ± 0.11B | ND | ND |
| WMc2% | 94.56 ± 3.41DE | 27.86 ± 0.79B | ND | ND |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Lacticaseibacillus casei Shirota | ||||
| MRS | 160.82 ± 1.16B | 75.09 ± 1.78A | ND | ND |
| CW | 145.63 ± 1.46DE | 14.68 ± 0.28C | ND | ND |
| WIn1% | 170.86 ± 0.43A | 18.42 ± 0.06B | ND | ND |
| WIn2% | 154.42 ± 1.65BC | 17.72 ± 0.09B | ND | ND |
| WMc1% | 151.46 ± 1.14CD | 16.98 ± 0.14BC | ND | ND |
| WMc2% | 138.72 ± 3.48E | 15.63 ± 0.43BC | ND | ND |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Bifidobacterium animalis subsp. lactis BPL1 | ||||
| MRS | 152.93 ± 2.06A | 69.65 ± 1.16A | 9.79 ± 0.58C | 4.71 ± 0.05C |
| CW | 94.37 ± 1.35E | 13.01 ± 0.22E | 14.62 ± 0.21AB | 4.58 ± 0.02C |
| WIn1% | 143.5 ± 1.8B | 28.41 ± 0.36B | 13.5 ± 0.09B | 17.09 ± 0.23B |
| WIn2% | 97.56 ± 2.52E | 17.35 ± 0.23D | 16.07 ± 0.96A | 18.58 ± 0.39A |
| WMc1% | 106.89 ± 0.98D | 25.61 ± 0.54C | ND | ND |
| WMc2% | 117.46 ± 0.77C | 23.43 ± 0.21C | ND | ND |
Concerning the SCFAs, the acetic acid was reported with the highest concentration (p < 0.05) among all postbiotics from MRS broth. In the whey-based media, the supplementation of chia mucilage increased significantly (p < 0.05) the concentration of acetic acid by Lactiplantibacillus plantarum 299v postbiotics. Tamargo et al.50 assessed the effect of chia mucilage on the microbiota using a dynamic gastrointestinal model (Simgi®). The addition of 0.8% chia mucilage stimulated the SCFAs production but did not specified which microorganisms were the producers. Similar to this study, they observed that acetic acid was the primary fermentation product of chia mucilage samples analysed with Simgi®. Probably, acetic acid production was favoured by the major chia mucilage's sugars such as xylose, glucose, arabinose, galactose, glucuronic acid, and galacturonic acid.22 Xylose, arabinose and compounds derived from galactomannan are important precursors in acetic acid production by the glycolytic pathway (Lactobacilli) and pentose phosphate (Bifidobacterium).23 Inulin significantly (p < 0.05) increased the concentration of acetic acid in the postbiotics of Lacticaseibacillus casei Shirota or B. lactis BPL1. The lactic and acetic acid concentrations of culture medium based on whey were similar to those reported by Renye et al.39 (190 and 18 mM, respectively).
As expected, Lactobacilli and Bifidobacterium produced different organic acids. The presence of propionic and butyric acid was only detected in the postbiotics of B. lactis BPL1, in which the WIn2% postbiotics had the highest amounts (p < 0.05). This difference in the production of SCFAs is because Lactobacilli uses the glycolytic pathway (forming pyruvate) and phosphoketolase pathway under heterofermentative conditions. Bifidobacterium species produce organic acids via the pentose phosphate pathway using the enzyme fructose-6-phosphate phosphoketolase (F6PPK).51 In addition, the fermentation of FOS is given by the hydrolytic enzyme and β-fructofuranosidase, which has been related to B. adolescentis, B. breve, and B. animalis subsp. lactis.36 Similarly, Renye et al.39 investigated the production of SCFAs in MRS broth and inulin from various probiotics. B. breve 2141 produced ≈4.5 mM of propionic acid and ≈1.0 mM of butyric acid. In the same way, Ozcan & Eroglu52 employed MRS broth with 1% inulin to grow L. acidophilus and analyse the SFCAs production. Their results showed minimal propionic acid concentrations (1.34 mM) and butyric acid (0.87 mM).
Studies focused on the metabolomics of fermentations with probiotics have identified a great diversity of metabolites, of which SCFAs have stood out.53 A metabolomic profile study from the fermentation of whey with L. plantarum MTCC 5690 only detected the presence of one SCFA (butyric acid).54 Therefore, the supplementation of soluble fibers is necessary to increase these compounds. This was demonstrated with a metabolomic study carried out by Tay et al.,55 who observed that adding soluble fiber (okara) increased the concentration of SCFAs in postbiotics from Bifidobacterium spp. It is important to recognize that Bifidobacterium in lactic fermentations are great probiotic producers of SCFAs.56
As could be noted, B. lactis BPL1 was the largest producer of propionic and butyric acid with WIn2% medium. Propionate has health benefits such as obesity control, glucose homeostasis control, appetite regulation, and potential cardiovascular effects, due to propionate can activate G-Protein Coupled Receptor 41 (GPCR41) which stimulates the satiety hormone leptin via adipocytes.5 Butyrate can be used as an energy source for epithelial cells and has been studied for its antitumor effects. The mechanism of action has not yet been fully understood. In addition, it has been suggested that butyrate inhibits histone deacetylases that dominate the intracellular gene expression of cells, preventing cancer cell proliferation.5,57
In respect to functional concentrations of SCFAs, Nakkarach et al.58 studied the postbiotic anticancer and anti-inflammatory effects from Escherichia coli KUB-36. The SCFAs concentrations tested were low (21.28 mM acetic acid, 0.50 mM propionic acid, and 0.47 mM butyric acid). The SCFAs influenced the inhibition of several cancer cell lines (MCF10-A, MCF7, HT-29 and leukemia cancer cells) and inhibited the expression of proinflammatory cytokines (IL1β, IL6 and TNF-α). Hence, the concentrations reported in the present study with B. lactis BPL1 could have a similar or greater anti-inflammatory and anticancer activity.
3.4 Total phenolic content of postbiotics
Phenolic compounds from LAB have been shown to play an important role in antioxidant capacity34,59,60 The total phenolic content of postbiotics is shown in Fig. 4. All postbiotics obtained from the MRS broth showed the highest phenolic content (p < 0.05) compared to the formulated mediums using whey (449.97–378.90 mg GAE per L). Regarding B. lactis BPL1, CW total phenolic content (396.82 mg GAE per L) from CW did not show significant differences (p > 0.05) compared to MRS. The mechanism of action of phenolic compounds as antioxidants is mainly given by their capability to donate hydrogen to free radicals avoiding oxidative chain reactions such as lipid oxidation.34 In addition, it has been reported that Lactiplantibacillus plantarum species can produce phenolic acids such as DL-3-phenyllactic acid, salicylic acid, and vanillin, and their production varies according to the culture medium used.61 Other study reported gallic acid, protocatechuic acid, chlorogenic acid, syringic acid, vanillin acid, p-coumaric acid, 4-hydroxybenzoic acid, hydrocinnamic acid, sinapic acid, DL-3-phenyllactic acid (PLA), and 1,2 dihydroxybenzene as the main phenolic compounds produced by four L. plantarum strains in goat's sweet whey after 72 h; PLA was the predominant phenolic compound.21 İncili et al.59 have mentioned that phenolic compounds (mainly phenolic acids) are not only antioxidant compounds but also antimicrobials.The importance of phenolic compounds in the human diet focuses on flavonoids which can be used by microbiota in the large intestine as carbon source to produce SCFA providing beneficial physiological benefits to the host (mitigation of obesity, regulates glucose metabolism, inhibitor of lipid synthesis, cancer preventive effects, among others).62 Thus, the formulated postbiotics provide SCFA and their precursors at the same time, which can supply potential health benefits. Also, few studies suggest selected flavonoids suppress the growth of pathogens like Clostridium perfrigens, Clostridium difficile, Bacteroides, Escherichia coli, and Salmonella leading to potential microbiome's modulation.63 Moreover, flavonoids are recognised as antioxidants and to act as a direct radical scavenging with health benefits such as anticancer and anti-inflammatory, among others.63–65
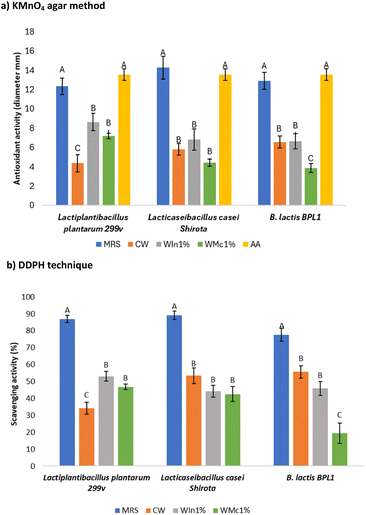
3.5 Antioxidant activity of postbiotics
Only postbiotics from media supplemented with 1% soluble fibres (WIn1% and WMc1%), whey-based (CW), and MRS broth were considered for this determination. Fig. 5a shows the diameters of antioxidant activity of the postbiotics, and Fig. 6 shows the colourless diameters in plates of KMnO4 agar method. The MRS postbiotics had the largest (p < 0.05) diameter of antioxidant activity among the studied postbiotics, which was similar (p > 0.05) to the ascorbic acid solution. The antioxidant activity from Lactiplantibacillus plantarum 299v postbiotics was significantly improved with soluble fibres (p < 0.05). The diameters of antioxidant activity from Lacticaseibacillus casei Shirota postbiotics were not modified by the soluble fibres (p > 0.05). For B. lactis BPL1 postbiotics, the chia mucilage (1%) decreased the antioxidant activity (p < 0.05) compared with inulin or CW. Likewise, the postbiotics from Lactiplantibacillus plantarum 299v and L. rhamnosus GG in MRS presented similar antioxidant activity (diameters of 10 to 15 mm) compared to Lacticaseibacillus casei Shirota of this study.14 On the other hand, the postbiotics' scavenging activity measures using DDPH had a similar tendency (Fig. 5b). For instance, adding soluble fibres such as inulin and chia mucilage in Lactiplantibacillus plantarum 299v growth media significantly increased (p < 0.05) the inhibition of DDPH similar to the KMnO4 agar method. It is reported that probiotics fermentation generates antioxidant compounds such as glutathione (GSH), butyrate, folate, lactate, 3-phenyllactate, indole-3-lactate, β-hydroxybutyrate, γ-aminobutyrate and flavonoids.21,66,67 In the present study, the presence of butyrate in the postbiotics of B. lactis BPL1 was demonstrated, and this compound has been associated with antioxidant activity in previous studies.68 Flavonoids (as phenolic compounds) from postbiotics as mentioned in section 3.4 provide antioxidant and direct radical scavenging activity potentially with anti-cancer and anti-inflammatory action. However, the identification and specific analyses of flavonoids in the postbiotics are necessary. In addition, whey proteins have antioxidative potential as scavenger of free radicals (sulfhydryl containing amino acids) and chelating transition metal ions (lactoferrin) which enhance after whey fermentation.54,69 To show the effect of soluble fibers in the antioxidant activity, the activity from the culture medium with unfermented soluble fibers (control) was subtracted from the obtained data. The diameters in the KMNO4 agar method and inhibition of DDPH were negligible with the unfermented medium. Furthermore, the antioxidant activity of soluble fibers was reviewed in the literature, and at similar concentrations to the tested inulin, the antioxidant activity is minimal.70Even though the DDPH method is the most common to analyse the antioxidant activity, their use in postbiotics is difficult due to sample provides turbidity. The KMnO4 agar method was convenient, and there was no interference in the measurement of colourless diameters, although the results took longer time (24 h) than the DDPH technique. Therefore, the KMnO4 agar method demonstrated to be a viable option for determining the antioxidant capacity of postbiotics as Hanchi et al.14 validated the method of antioxidant activity.
4 Conclusions
The whey-based culture media supplemented with inulin (WIn1%-WIn2%) were optimal for microbial growth of Lactiplantibacillus plantarum 299v, Lacticaseibacillus casei Shirota and B. lactis BPL1. The organic acids in postbiotics, Lactiplantibacillus plantarum 299v and Lacticaseibacillus casei Shirota were primary lactic and acetic acid, while B. lactis BPL1 showed lactic, acetic, propionic, and butyric acid. The supplementation of soluble fibres improved antioxidant activity in postbiotics of Lactiplantibacillus plantarum 299v. In summary, the whey-based culture mediums supplemented with inulin are suitable for microbial growth, SCFAs production, and antioxidant activity sources. Therefore, the postbiotics from these culture mediums could be an economical and sustainable option for human consumption to incorporate postbiotics with SCFAs and antioxidant activity in developing novel food products. The use of whey in the production of postbiotics not only contributes to the effective utilization of byproducts from the cheese industry, avoiding critical environmental issues, but also aligns with sustainability principles, resource efficiency and health promotion. Incorporating these postbiotics into new food products further extends sustainable practices into the consumer market. In future research, it would be interesting to explore the metabolomics of studied probiotics growing in whey and soluble fibres. Finally, investigate the safety, stability, sensory, and bioactive effects of the postbiotics in food products.Author contributions
Conceptualization, V. E. V.-S., M. T. J.-M., E. M.-L. and A. L.-M.; visualization: V. E. V.-S.; validation, V. E. V.-S., M. T. J.-M., E. M.-L. and A. L.-M.; investigation, V. E. V.-S.; resources, M. T. J.-M. and A. L.-M.; writing—original draft preparation, V. E. V.-S.; writing—review and editing, V. E. V.-S., M. T. J.-M., E. M.-L. and A. L.-M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The author Vera-Santander appreciates the financial support for his doctoral studies in Food Science from the National Council of Humanities, Sciences and Technologies (CONAHCyT) and Universidad de las Américas Puebla (UDLAP).References
- S. Salminen, M. C. Collado, A. Endo, C. Hill, S. Lebeer, E. M. M. Quigley, M. E. Sanders, R. Shamir, J. R. Swann, H. Szajewska and G. Vinderola, The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics, Nat. Rev. Gastroenterol. Hepatol., 2021, 18(9), 649–667, DOI:10.1038/s41575-021-00440-6.
- J. E. Aguilar-Toalá, R. Garcia-Varela, H. S. Garcia, V. Mata-Haro, A. F. González-Córdova, B. Vallejo-Cordoba and A. Hernández-Mendoza, Postbiotics: An Evolving Term within the Functional Foods Field, Trends Food Sci. Technol., 2018, 75, 105–114, DOI:10.1016/j.tifs.2018.03.009.
- E. Dunand, P. Burns, A. Binetti, C. Bergamini, G. H. Peralta, L. Forzani, J. Reinheimer and G. Vinderola, Postbiotics Produced at Laboratory and Industrial Level as Potential Functional Food Ingredients with the Capacity to Protect Mice against Salmonella Infection, J. Appl. Microbiol., 2019, 127(1), 219–229, DOI:10.1111/jam.14276.
- C. Klemashevich, C. Wu, D. Howsmon, R. C. Alaniz, K. Lee and A. Jayaraman, Rational Identification of Diet-Derived Postbiotics for Improving Intestinal Microbiota Function, Curr. Opin. Biotechnol., 2014, 26, 85–90, DOI:10.1016/j.copbio.2013.10.006.
- P. A. Gill, M. C. van Zelm, J. G. Muir and P. R. Gibson, Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders, Aliment. Pharmacol. Ther., 2018, 48(1), 15–34, DOI:10.1111/apt.14689.
- P. A. Gill, A. Bogatyrev, M. C. Zelm, P. R. Gibson and J. G. Muir, Delivery of Acetate to the Peripheral Blood after Consumption of Foods High in Short-Chain Fatty Acids, Mol. Nutr. Food Res., 2021, 65(4), 2000953, DOI:10.1002/mnfr.202000953.
- J. G. LeBlanc, F. Chain, R. Martín, L. G. Bermúdez-Humarán, S. Courau and P. Langella, Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria, Microb. Cell Fact., 2017, 16(1), 79, DOI:10.1186/s12934-017-0691-z.
- V. E. Vera-Santander, R. H. Hernández-Figueroa, M. T. Jiménez-Munguía, E. Mani-López and A. López-Malo, Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review, Molecules, 2023, 28(3), 1230, DOI:10.3390/molecules28031230.
- J. Sun, F. Wang, X. Hu, C. Yang, H. Xu, Y. Yao and J. Liu, Clostridium Butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis, J. Agric. Food Chem., 2018, 66(31), 8415–8421, DOI:10.1021/acs.jafc.8b02462.
- H.-J. Chung, J.-H. Sim, T.-S. Min and H.-K. Choi, Metabolomics and Lipidomics Approaches in the Science of Probiotics: A Review, J. Med. Food, 2018, 21(11), 1086–1095, DOI:10.1089/jmf.2017.4175.
- M. Schieber and N. S. Chandel, ROS Function in Redox Signaling and Oxidative Stress, Curr. Biol., 2014, 24(10), R453–R462, DOI:10.1016/j.cub.2014.03.034.
- A. Gholamhosseinpour and S. M. B. Hashemi, Ultrasound Pretreatment of Fermented Milk Containing Probiotic Lactobacillus Plantarum AF1: Carbohydrate Metabolism and Antioxidant Activity, J. Food Process Eng., 2019, 42(1), e12930, DOI:10.1111/jfpe.12930.
- M. W. Song, H. J. Jang, K.-T. Kim and H.-D. Paik, Probiotic and Antioxidant Properties of Novel Lactobacillus Brevis KCCM 12203P Isolated from Kimchi and Evaluation of Immune-Stimulating Activities of Its Heat-Killed Cells in RAW 264.7 Cells, J. Microbiol. Biotechnol., 2019, 29(12), 1894–1903, DOI:10.4014/jmb.1907.07081.
- H. Hanchi, K. Sebei, W. Mottawea, I. Al Kasaa and R. Hammami, An Agar-Based Bioassay for Accurate Screening of the Total Antioxidant Capacity of Lactic Acid Bacteria Cell-Free Supernatants, J. Microbiol. Methods, 2022, 195, 106437, DOI:10.1016/j.mimet.2022.106437.
- P. Burns, G. Vinderola, F. Molinari and J. Reinheimer, Suitability of Whey and Buttermilk for the Growth and Frozen Storage of Probiotic Lactobacilli, Int. J. Dairy Technol., 2008, 61(2), 156–164, DOI:10.1111/j.1471-0307.2008.00393.x.
- P. Papademas and P. Kotsaki, Technological Utilization of Whey towards Sustainable Exploitation, J. Adv. Dairy Res., 2019, 231, DOI:10.35248/2329-888X.19.7.231.
- O. Kareb and M. Aïder, Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review, Probiotics Antimicrob. Proteins, 2019, 11(2), 348–369, DOI:10.1007/s12602-018-9427-6.
- S. Amiri, M. Rezazadeh-Bari, M. Alizadeh-Khaledabad, R. Rezaei-Mokarram and M. Sowti-Khiabani, Fermentation Optimization for Co-Production of Postbiotics by Bifidobacterium Lactis BB12 in Cheese Whey, Waste Biomass Valorization, 2021, 12(11), 5869–5884, DOI:10.1007/s12649-021-01429-7.
- L. Garnier, J. Mounier, S. Lê, A. Pawtowski, N. Pinon, B. Camier, M. Chatel, G. Garric, A. Thierry, E. Coton and F. Valence, Development of Antifungal Ingredients for Dairy Products: From in Vitro Screening to Pilot Scale Application, Food Microbiol., 2019, 81, 97–107, DOI:10.1016/j.fm.2018.11.003.
- H. Yousefi, M. Moosavi-Nasab, S. Soleimanian-Zad, M.-T. Golmakani and M. Majdinasab, Antibacterial Metabolites Production by Lactobacillus Plantarum PTCC 1896 in Fermented Whey and Optimization of Fermentation Conditions for Maximum Production Using RSM, Int. Dairy J., 2024, 152, 105882, DOI:10.1016/j.idairyj.2024.105882.
- L. Izzo, C. Luz, A. Ritieni, J. Quiles Beses, J. Mañes and G. Meca, Inhibitory Effect of Sweet Whey Fermented by Lactobacillus Plantarum Strains against Fungal Growth: A Potential Application as an Antifungal Agent, J. Food Sci., 2020, 85(11), 3920–3926, DOI:10.1111/1750-3841.15487.
- Y. P. Timilsena, R. Adhikari, C. J. Barrow and B. Adhikari, Physicochemical and Functional Properties of Protein Isolate Produced from Australian Chia Seeds, Food Chem., 2016, 212, 648–656, DOI:10.1016/j.foodchem.2016.06.017.
- S. Macfarlane and G. T. Macfarlane, Regulation of Short-Chain Fatty Acid Production, Proc. Nutr. Soc., 2003, 62(1), 67–72, DOI:10.1079/PNS2002207.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Scientific Opinion on the Substantiation of Health Claims Related to Lactobacillus Plantarum 299v (DSM 9843) and “Immune System” (ID 1081), Pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA J., 2010, 8(2), 1488, DOI:10.2903/j.efsa.2010.1488.
- K. Kaźmierczak-Siedlecka, A. Daca, M. Folwarski, J. M. Witkowski, E. Bryl and W. Makarewicz, The Role of Lactobacillus Plantarum 299v in Supporting Treatment of Selected Diseases, Cent. Eur. J. Immunol., 2020, 45(4), 488–493, DOI:10.5114/ceji.2020.101515.
- M. Sharma, A. Wasan and R. K. Sharma, Recent Developments in Probiotics: An Emphasis on Bifidobacterium, Food Biosci., 2021, 41, 100993, DOI:10.1016/j.fbio.2021.100993.
- C. Tarracchini, M. Viglioli, G. A. Lugli, L. Mancabelli, F. Fontana, G. Alessandri, F. Turroni, M. Ventura and C. Milani, The Integrated Probiotic Database: A Genomic Compendium of Bifidobacterial Health-Promoting Strains, Microbiome Res. Rep., 2022, 1(2), 9, DOI:10.20517/mrr.2021.13.
- R. A. Yunes, E. U. Poluektova, T. V. Belkina and V. N. Danilenko, Lactobacilli: Legal Regulation and Prospects for New Generation Drugs, Appl. Biochem. Microbiol., 2022, 58(5), 652–664, DOI:10.1134/S0003683822050179.
- R. Hernández-Nava, A. López-Malo, E. Palou, N. Ramírez-Corona and M. T. Jiménez-Munguía, Encapsulation of Oregano Essential Oil (Origanum Vulgare) by Complex Coacervation between Gelatin and Chia Mucilage and Its Properties after Spray Drying, Food Hydrocolloids, 2020, 109, 106077, DOI:10.1016/j.foodhyd.2020.106077.
- M. Moradi, R. Molaei and J. T. Guimarães, A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria, Enzyme Microb. Technol., 2021, 143, 109722, DOI:10.1016/j.enzmictec.2020.109722.
- Official Methods of Analysis of AOAC International, ed. Latimer, G. W. and AOAC International, AOAC International, Gaithersburg, Md, 21st edn, 2019, vol. 3 Search PubMed.
- R. H. Hernández-Figueroa, E. Mani-López and A. López-Malo, Antifungal Capacity of Poolish-Type Sourdough Supplemented with Lactiplantibacillus Plantarum and Its Aqueous Extracts In Vitro and Bread, Antibiotics, 2022, 11(12), 1813, DOI:10.3390/antibiotics11121813.
- B.-J. Seo, V. K. Bajpai, I. A. Rather and Y.-H. Park, Partially Purified Exopolysaccharide from Lactobacillus Plantarum YML009 with Total Phenolic Content, Antioxidant and Free Radical Scavenging Efficacy, IJPER, 2015, 49(4), 282–292, DOI:10.5530/ijper.49.4.6.
- J. Xing, G. Wang, Q. Zhang, X. Liu, Z. Gu, H. Zhang, Y. Q. Chen and W. Chen, Determining Antioxidant Activities of Lactobacilli Cell-Free Supernatants by Cellular Antioxidant Assay: A Comparison with Traditional Methods, PLoS One, 2015, 10(3), e0119058, DOI:10.1371/journal.pone.0119058.
- A. Hernandez-Mendoza, V. J. Robles, J. O. Angulo, J. De La Cruz and H. S. Garcia, Preparation of a Whey-Based Probiotic Product with Lactobacillus Reuteri and Bifidobacterium Bifidum, Food Technol. Biotechnol., 2007, 45(1), 27–31 Search PubMed.
- H. P. McLaughlin, M. O. Motherway, B. Lakshminarayanan, C. Stanton, R. Paul Ross, J. Brulc, R. Menon, P. W. O'Toole and D. van Sinderen, Carbohydrate Catabolic Diversity of Bifidobacteria and Lactobacilli of Human Origin, Int. J. Food Microbiol., 2015, 203, 109–121, DOI:10.1016/j.ijfoodmicro.2015.03.008.
- D. Meyer and M. Stasse-Wolthuis, The Bifidogenic Effect of Inulin and Oligofructose and Its Consequences for Gut Health, Eur. J. Clin. Nutr., 2009, 63(11), 1277–1289, DOI:10.1038/ejcn.2009.64.
- D. U. Nagy, K. A. Sándor-Bajusz, B. Bódy, T. Decsi, J. Van Harsselaar, S. Theis and S. Lohner, Effect of Chicory-Derived Inulin-Type Fructans on Abundance of Bifidobacterium and on Bowel Function: A Systematic Review with Meta-Analyses, Crit. Rev. Food Sci. Nutr., 2022, 1–18, DOI:10.1080/10408398.2022.2098246.
- J. A. Renye, A. K. White and A. T. Hotchkiss, Identification of Lactobacillus Strains Capable of Fermenting Fructo-Oligosaccharides and Inulin, Microorganisms, 2021, 9(10), 2020, DOI:10.3390/microorganisms9102020.
- J. H. Chiang, D. S. M. Ong, F. S. K. Ng, X. Y. Hua, W. L. W. Tay and C. J. Henry, Application of Chia (Salvia Hispanica) Mucilage as an Ingredient Replacer in Foods, Trends Food Sci. Technol., 2021, 115, 105–116, DOI:10.1016/j.tifs.2021.06.039.
- M. Chávarri, L. Diez-Gutiérrez, I. Marañón and L. Javier R. Barron, Secondary Metabolites From Probiotic Metabolism, in Advances in Probiotics, Elsevier, 2021, pp. 259–276, DOI:10.1016/B978-0-12-822909-5.00017-4.
- A. R. Fornelli, N. S. Bandiera, M. D. R. Costa, C. H. B. de Souza, E. H. W. de Santana, K. Sivieri and L. C. Aragon-Alegro, Effect of Inulin and Oligofructose on the Physicochemical, Microbiological and Sensory Characteristics of Symbiotic Dairy Beverages, Semina: Cienc. Agrar., 2014, 35(6), 3099, DOI:10.5433/1679-0359.2014v35n6p3092.
- C. Pereira, M. Henriques, D. Gomes, A. Gomez-Zavaglia and G. de Antoni, Novel Functional Whey-Based Drinks with Great Potential in the Dairy Industry, Food Technol. Biotechnol., 2015, 53, 307–313, DOI:10.17113/ftb.53.03.15.4043.
- S. da Silva Sabo, A. Converti, S. D. Todorov, J. M. Domínguez and R. P. de Souza Oliveira, Effect of Inulin on Growth and Bacteriocin Production by Lactobacillus Plantarum in Stationary and Shaken Cultures, Int. J. Food Sci. Technol., 2015, 50(4), 864–870, DOI:10.1111/ijfs.12711.
- W. Savedboworn, S. Niyomrat, J. Naknovn and K. Phattayakorn, Impact of Inulin on Viability and Storage Stability of Probiotic Lactobacillus Plantarum TISTR 2075 in Fermented Rice Extract, J. Agric. Nat. Resour., 2017, 51(6), 463–469, DOI:10.1016/j.anres.2018.03.008.
- F. Nazzaro, F. Fratianni, P. Orlando and R. Coppola, Biochemical Traits, Survival and Biological Properties of the Probiotic Lactobacillus Plantarum Grown in the Presence of Prebiotic Inulin and Pectin as Energy Source, Pharmaceuticals, 2012, 5(5), 481–492, DOI:10.3390/ph5050481.
- R. Takagi, Y. Tsujikawa, R. Nomoto and R. Osawa, Comparison of the Growth of Lactobacillus Delbrueckii, L. Paracasei and L. Plantarum on Inulin in Co-Culture Systems, Biosci. Microbiota, Food Health, 2014, 33(4), 139–146, DOI:10.12938/bmfh.33.139.
- S. Young Ng, L. Wee Chia, B. Scott Padam and F. Y. Chye, Effect of Selected Oligosaccharides on the Viability and Fermentation Kinetics of Lactobacillus Acidophilus and Lactobacillus Casei in Cultured Milk, J. Pharm. Nutr. Sci., 2014, 4(2), 92–99, DOI:10.6000/1927-5951.2014.04.02.4.
- A. Matusek, P. Merész, T. K. D. Le and F. Örsi, Effect of Temperature and pH on the Degradation of Fructo-Oligosaccharides, Eur. Food Res. Technol., 2009, 228(3), 355–365, DOI:10.1007/s00217-008-0941-8.
- A. Tamargo, C. Cueva, L. Laguna, M. V. Moreno-Arribas and L. A. Muñoz, Understanding the Impact of Chia Seed Mucilage on Human Gut Microbiota by Using the Dynamic Gastrointestinal Model Simgi®, J. Funct. Foods, 2018, 50, 104–111, DOI:10.1016/j.jff.2018.09.028.
- B. Usta-Gorgun and L. Yilmaz-Ersan, Short-Chain Fatty Acids Production by Bifidobacterium Species in the Presence of Salep, Electron. J. Biotechnol., 2020, 47, 29–35, DOI:10.1016/j.ejbt.2020.06.004.
- T. Ozcan and E. Eroglu, Effect of Stevia and Inulin Interactions on Fermentation Profile and Short-chain Fatty Acid Production of Lactobacillus Acidophilus in Milk and in Vitro Systems, Int. J. Dairy Technol., 2022, 75(1), 171–181, DOI:10.1111/1471-0307.12814.
- Y.-C. Chung, H.-M. Jin, Y. Cui, D. S. Kim, J. M. Jung, J.-I. Park, E.-S. Jung, E.-K. Choi and S.-W. Chae, Fermented Milk of Lactobacillus Helveticus IDCC3801 Improves Cognitive Functioning during Cognitive Fatigue Tests in Healthy Older Adults, J. Funct. Foods, 2014, 10, 465–474, DOI:10.1016/j.jff.2014.07.007.
- S. Kadyan, H. M. Rashmi, D. Pradhan, A. Kumari, A. Chaudhari and G. K. Deshwal, Effect of Lactic Acid Bacteria and Yeast Fermentation on Antimicrobial, Antioxidative and Metabolomic Profile of Naturally Carbonated Probiotic Whey Drink, LWT–Food Sci. Technol., 2021, 142, 111059, DOI:10.1016/j.lwt.2021.111059.
- C. S. Tay, Y. T. Yeo, K. R. Ng, P. K. Yap and W. N. Chen, Metabolomics-Driven Comparison of the Nutritional and Functional Food Characteristics of Postbiotic and Probiotic Okara, ACS Food Sci. Technol., 2023, 3(7), 1165–1174, DOI:10.1021/acsfoodscitech.3c00050.
- Y. Sun, S. Guo, T. Wu, Y. Yang, T. Shen, X. Ma, L.-Y. Kwok, J. Wang, Z. Sun and H. Zhang, Bifidobacterium Adolescentis B8589- and Lacticaseibacillus Paracasei PC-01-Co-Fermented Milk Has More γ-Aminobutyric Acid and Short-Chain Fatty Acids than Lacticaseibacillus Paracasei PC-01-Fermented Milk, LWT–Food Sci. Technol., 2023, 179, 114645, DOI:10.1016/j.lwt.2023.114645.
- M. G. Vander Heiden, L. C. Cantley and C. B. Thompson, Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation, Science, 2009, 324(5930), 1029–1033, DOI:10.1126/science.1160809.
- A. Nakkarach, H. L. Foo, A. A.-L. Song, N. E. A. Mutalib, S. Nitisinprasert and U. Withayagiat, Anti-Cancer and Anti-Inflammatory Effects Elicited by Short Chain Fatty Acids Produced by Escherichia Coli Isolated from Healthy Human Gut Microbiota, Microb. Cell Fact., 2021, 20(1), 36, DOI:10.1186/s12934-020-01477-z.
- G. K. İncili, P. Karatepe, M. Akgöl, A. Güngören, A. Koluman, O. İ. İlhak, H. Kanmaz, B. Kaya and A. A. Hayaloğlu, Characterization of Lactic Acid Bacteria Postbiotics, Evaluation in-Vitro Antibacterial Effect, Microbial and Chemical Quality on Chicken Drumsticks, Food Microbiol., 2022, 104, 104001, DOI:10.1016/j.fm.2022.104001.
- M. S. Darwish, L. Qiu, M. A. Taher, A. A. Zaki, N. A. Abou-Zeid, D. H. Dawood, O. M. A. K. Shalabi, E. Khojah and A. A. Elawady, Health Benefits of Postbiotics Produced by E. Coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis Peruviana L.), Fermentation, 2022, 8(3), 128, DOI:10.3390/fermentation8030128.
- N. Nikmaram, S. Budaraju, F. J. Barba, J. M. Lorenzo, R. B. Cox, K. Mallikarjunan and S. Roohinejad, Application of Plant Extracts to Improve the Shelf-Life, Nutritional and Health-Related Properties of Ready-to-Eat Meat Products, Meat Sci., 2018, 145, 245–255, DOI:10.1016/j.meatsci.2018.06.031.
- M. H. Baky, M. Elshahed, L. Wessjohann and M. A. Farag, Interactions between Dietary Flavonoids and the Gut Microbiome: A Comprehensive Review, Br. J. Nutr., 2022, 128(4), 577–591, DOI:10.1017/S0007114521003627.
- G. Williamson, C. D. Kay and A. Crozier, The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective, Compr. Rev. Food Sci. Food Saf., 2018, 17(5), 1054–1112, DOI:10.1111/1541-4337.12351.
- R. J. Nijveldt, E. Van Nood, D. E. Van Hoorn, P. G. Boelens, K. Van Norren and P. A. Van Leeuwen, Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications, Am. J. Clin. Nutr., 2001, 74(4), 418–425, DOI:10.1093/ajcn/74.4.418.
- M. Yao, J. Xie, H. Du, D. J. McClements, H. Xiao and L. Li, Progress in Microencapsulation of Probiotics: A Review, Compr. Rev. Food Sci. Food Saf., 2020, 19(2), 857–874, DOI:10.1111/1541-4337.12532.
- J. Kim, K.-B. Choi, J. H. Park and K. H. Kim, Metabolite Profile Changes and Increased Antioxidative and Antiinflammatory Activities of Mixed Vegetables after Fermentation by Lactobacillus Plantarum, PLoS One, 2019, 14(5), e0217180, DOI:10.1371/journal.pone.0217180.
- Y. Wang, Y. Wu, Y. Wang, H. Xu, X. Mei, D. Yu, Y. Wang and W. Li, Antioxidant Properties of Probiotic Bacteria, Nutrients, 2017, 9(5), 521, DOI:10.3390/nu9050521.
- H. Endo, M. Niioka, N. Kobayashi, M. Tanaka and T. Watanabe, Butyrate-Producing Probiotics Reduce Nonalcoholic Fatty Liver Disease Progression in Rats: New Insight into the Probiotics for the Gut-Liver Axis, PLoS One, 2013, 8(5), e63388, DOI:10.1371/journal.pone.0063388.
- I. Khan, K. Saeed and I. Khan, Nanoparticles: Properties, Applications and Toxicities, Arabian J. Chem., 2019, 12(7), 908–931, DOI:10.1016/j.arabjc.2017.05.011.
- H.-M. Shang, H.-Z. Zhou, J.-Y. Yang, R. Li, H. Song and H.-X. Wu, In Vitro and in Vivo Antioxidant Activities of Inulin, PLoS One, 2018, 13(2), e0192273, DOI:10.1371/journal.pone.0192273.
| This journal is © The Royal Society of Chemistry 2024 |