Open Access Article
Open Access ArticleRecent advances in the fabrication, characterization and application of starch-based materials for active food packaging: hydrogels and aerogels
Di
Zhao
 a,
Xinyi
Zhang
a,
Yingying
Zhang
a,
Enbo
Xu
b,
Shengkun
Yan
c,
Huaide
Xu
a and
Mei
Li
a,
Xinyi
Zhang
a,
Yingying
Zhang
a,
Enbo
Xu
b,
Shengkun
Yan
c,
Huaide
Xu
a and
Mei
Li
 *a
*a
aCollege of Food Science and Engineering, Northwest A&F University, Yangling 712100, China. E-mail: limei1101@nwafu.edu.cn
bCollege of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou 310058, China
cResearch Institute of Agricultural Machinery, Xinjiang Academy of Agricultural Sciences, Urumqi 830000, China
First published on 8th March 2024
Abstract
With the increasing awareness of the harmful effects of plastics on the environment, the demand for biodegradable packaging materials is growing. Over the last decade, starch-based colloidal materials including hydrogels and aerogels have attracted widespread attention owing to their abundant supply, complete biodegradability and unique properties. This review aimed to provide an overview of the fabrication methods of starch-based hydrogels and aerogels, summarize the effects of internal and external factors on the structural and physicochemical properties of starch-based hydrogels and aerogels, and focus on the evaluation of their application in active food packaging. Hydrogels are sensitive to environmental changes, giving them unique advantages in smart packaging applications. In addition, aerogels have a distinctive place in the delivery and release of active substances based on large surface areas and high porosity. Starch-based hydrogels and aerogels will provide great potential in the application of food packaging.
Sustainability spotlightI would like to declare on behalf of my co-authors that the work described is aligned with the UN's Sustainable Development Goal 13 “Take urgent action to combat climate change and its impacts”. The overwhelming use of plastic related to food packing is a major concern worldwide. Governmental institutions have currently formulated new policies aimed at reducing plastic usage. At the same time, researchers from different countries are making efforts to develop innovative and biodegradable materials for food packaging, which are the topic of this review article. The research and promotion of starch-based food packaging not only contribute to reducing plastic usage and carbon emission, but also increase food byproduct upcycling. |
1 Introduction
Food packaging, served as a storage container, is essential for protecting fresh produce or processed foods from external conditions, such as atmospheric interference, microbial invasion and mechanical collision, and maintaining safety, integrity, freshness and quality during shelf life of packed foods.1,2 Currently, the first-choice of packaging for both fresh food and manufactured products is plastic materials, since they are versatile and cheap and have excellent barrier properties.3 However, due to its non-biodegradability properties, the massive production of plastic is causing serious environmental impacts, such as intensifying pollution and global warming.4 Meanwhile, a large number of studies on the relationship between environmental pollution and human health have confirmed that ingredients in plastics may cause disruptions in the endocrine and immune systems, leading to an increased incidence of chronic diseases.5With emerging global awareness of plastic pollution, as well as growing interest in fresher foods and longer shelf life, demand for environmentally friendly technologies for food packaging is becoming more and more urgent.6 Therefore, agro-based polymers are an excellent source of materials for food packaging.7 Currently, natural biopolymers, including polysaccharides and proteins, have been explored for the development of active food packaging.
Starch, an abundant food-grade resource and renewable material, is approved for use in food products in various forms, including gelling agents, carrier matrices, and emulsifiers.8 In this sense, starch-based materials are regarded as ideal materials for food packaging due to their availability and biocompatibility. More importantly, starch has a high density of surface active hydroxyl, which can promote the generation of intramolecular and/or intermolecular hydrogen bonds with incorporated materials, providing the possibility for the production of antioxidant or antibacterial active packaging materials.9
In the last few decades, fabrication of starch-based hydrogels and aerogels, as well as their application in food packaging, has gained special attention.10,11 Hydrogels, three-dimensional (3D) network structures formed by cross-linking starch chains, are able to hold large amounts of water while maintaining integral structures and have good mechanical resistance, swelling properties and biodegradability.12 Hydrogel packaging is considered an excellent alternative to conventional packaging and has shown potential applications in active packaging systems. Aerogels, porous lightweight solid materials, are derived from hydrogels by replacing the liquid existing in the gel structure with gas.13 In general, any sol–gel derived material that possessed ultra-low density and predominant mesopores could be referred to as an aerogel.14 Due to high porosity, low density and high surface area, aerogels have the potential to become advanced materials in food packaging in various forms, such as internal food packaging layers, wraps and oxygen/humidity sachet scavengers.11,15
Indeed, the use of starch-based hydrogel and aerogels in food packaging has been growing rapidly over the last decade (Fig. 1). Henceforth, the objective of this work is to review the state-of-the-art fabrication techniques of starch-based hydrogels and aerogels and provide a detailed overview of their application in food packaging.
2 Structural and gel characteristics of starch
Starch is one of the most abundant polysaccharides found in nature, which is commonly obtained from plant sources such as cereals (e.g. wheat, maize, rice, etc.), roots and tubers (e.g. cassava, potatoes, etc.), legumes (e.g. beans, peas, etc.), green fruits (e.g. jackfruit, green banana, etc.), and leaves (e.g. arabidopsis, tobacco, etc.). The structure of starch determines its gel characteristics, which in turn affects the structure and physicochemical properties of starch-based hydrogels and aerogels. Here is an overview of the structure and gel characteristics of starch.2.1 Starch structure
Based on the linear or branched structural arrangement, starch has two main categories: amylose and amylopectin. Amylose is a linear polymer with glucose molecules connected in a long, unbranched chain linked by α-D-(1–4) glycosidic bonds and is responsible for the formation of a gel-like texture when starch is heated in the presence of water. Amylose typically makes up approximately 10–35% of the starch content (Fig. 2). In general, legume starches have a higher amylose content (AC), for example, the AC in peas reaches 26–33%, while leafy starches have lower levels, for instance, starch in arabidopsis leaves contains 6–12% amylose, which is lower than the amylose content observed in most storage starches.16 Amylopectin is a highly branched polymer, in which glucose molecules are connected by α-D-(1–4) and α-D-(1–6) glycosidic bonds. It is necessary for the formation of semicrystalline granules and has an impact on the compactness and granule size of the starch.17 Amylopectin constitutes about 65–90% of the starch content (Fig. 2). It is worth noting that high-amylose or high-amylopectin starch has been isolated in various mutants, such as high amylose maize (50–80% amylose) and waxy corn and glutinous rice (<1% amylose).18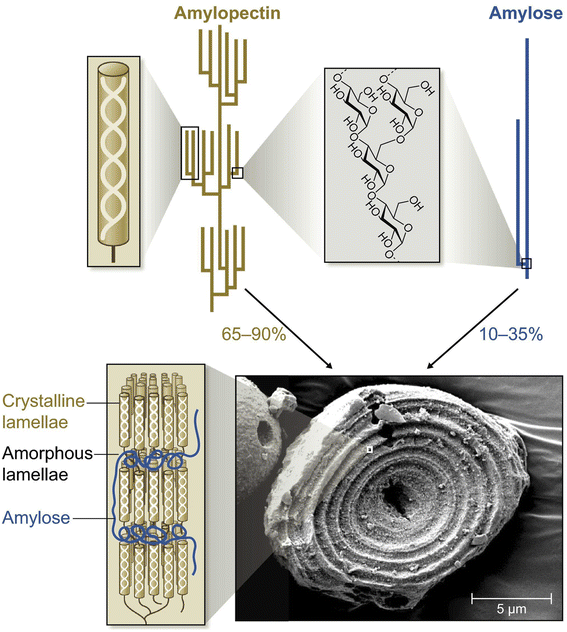 | ||
| Fig. 2 The molecular structure of starch granules.16 | ||
At the morphology level, starch granules from different starch sources present different shaped including spherical, elliptical and irregular, with a size range from 2 μm to 100 μm. Typically, the size and morphology of starch could be manipulated by physical, chemical and/or enzymatic methods. For example, starch could be transformed into starch nanoparticles (referring to particles with dimensions smaller than 1000 nm)19 and porous starch (referring to granules with surfaces presenting abundant voids),20 to obtain innovative starch with enhanced functional properties.
2.2 Starch gel characteristics
The formation of hydrogels and aerogels depends on gel characteristics (gelatinization and retrogradation) of starch. Understanding the processes of colloids is crucial for the food industry, which facilitates exploring novel strategies to utilize starches in innovative ways such as starch-based biomaterials.Gelatinization is described as the transition of starch granules from an ordered state to a disordered state. It involves three processes, including the expansion of starch particles, disruption of ordered structures, and solubilization of starch molecules when exposed to heat and water.21 Retrogradation of starch refers to the process in which gelatinized starch undergoes structural change, forming a semi-crystalline structure. Starch retrogradation is influenced by the type and content of starch as well as the presence of other components in the mixture such as macromolecular polymers22 or small molecular components.23 In addition, external environments such as heating pressure,24 cooling temperature,25 mechanical force26 and nonthermal treatment,27,28 could change gel characteristics by modifying the molecular structure of starch as well as the combination of starch and water, thus affecting the properties of colloids.
3 Starch-based hydrogels
Hydrogels are 3D network hydrophilic polymers that can absorb and retain water. Meanwhile, the 3D network structure of the starch gel could be loaded with antioxidants, antibacterial agents, and a pH indicator, which allows the packaging material to possess functional and responsive intelligent properties. Therefore, we provide a comprehensive review of fabrication, properties and characterization techniques of starch-based hydrogels, as well as the application of various hydrogels on food packaging.3.1 Fabrication of starch-based hydrogels
The 3D network structures of starch-based hydrogels are mainly formed by polymeric association, resulting from interaction forces between the bonds of each polymer chain during different preparation processes. Therefore, the properties of outcomes largely depend on the fabricated method (including the physical and/or chemical method), starch type (such as a native starch source and modified starch), and other additive components in hydrogel formulation.29| Starch type | Additive components | Method | Process | References |
|---|---|---|---|---|
| Oxidized maize starch | — | Physical crosslinking | Hot-extrusion 3D printing | 49 |
| Citrate starch | PVA/PEG | Physical crosslinking | Antimicrobial hydrogels were obtained by physical crosslinking of starch citrate, PVA and PEG via the freeze–thaw technique | 50 |
| Gelatinized or native potato starch | Alginate and gelatin | Physical crosslinking | Emulsion-filled hydrogels were produced by ionic gelation, containing potato starch, alginate and gelatin | 58 |
| Oxidized potato starch | Sodium trimetaphosphate | Chemical crosslinking | The hydrogels were made by chemically cross-linking oxidized potato starch polymers and sodium trimetaphosphate (STMP) | 32 |
| Corn starch | Chitosan and citric acid | Chemical crosslinking | The hydrogels were made by chemically cross-linking starch, chitosan, and citric acid | 33 |
| Native corn starch | Chitosan and tannic acid | Physical and chemical crosslinking | Smart bilayer films were fabricated by a physical and chemical crosslinking reaction with chitosan, tannic acid and corn starch | 10 |
Physically crosslinked hydrogels refer to the 3D network structure formed by the interaction of molecular chains through non-covalent bonds such as hydrophobic, electrostatic, hydrogen, and ionic. These hydrogels exhibit dynamic and reversible physical properties and possess excellent degradability and biocompatibility. Dodda et al.31 designed multicomponent system hydrogels by combining chitosan, starch, PVA, and poly (3,4-ethylenedioxythiophene) polystyrene sulfone (PEDOT:PSS) via one-pot synthesis. The swelling ratio of starch-based hydrogels (from 234% to 280%) was higher than that of cellulose-based hydrogels (from 121% to 156%). Additionally, the cell metabolic activity after 3 days of cell culture in the hydrogel infusion was determined in cytotoxicity experiments, which indicated that there was no release of acute toxic compounds from the hydrogel.
Chemically crosslinked hydrogels are formed through covalent bonding between molecular chains. This process involves the introduction of chemical crosslinking agents into starch solution, which could react with starch chains, leading to the formation of a 3D network structure.32 The covalent bonds formed within the hydrogel provide stability and structural integrity. Citric acid (CA) is widely used as a green crosslinker in starch-based hydrogels because of its non-toxicity and low cost.33 Starch/xanthan gum hydrogels were prepared using different concentrations of CA (0.00, 0.75, 1.50, and 2.25 g/100 g) as crosslinking agents.34 The study found that CA enhanced the mechanical properties of the hydrogel. When the CA concentration was 2.25 g/100 g, the fracture elongation value of the hydrogel increased by 119% compared to the sample without CA. What's more, by the swelling analysis, starch-based hydrogels incorporating CA remained intact, while the sample without CA experienced fragmentation after soaking in water for 2 days. In the fabrication of starch-based hydrogels, chemical crosslinking can occur through the Schiff base reaction, even without a chemical crosslinker. In the study conducted by Liu et al.,35 debranched starch was oxidized to dialdehyde debranched starch (DADBS) and then DADBS was dynamically crosslinked with the amino groups in chitosan to prepare a high-performance chitosan hydrogel. When the molar ratio of amino groups to aldehyde groups is 2.8 in chitosan hydrogels, the hydrogel remains intact under a strain of 70%, indicating its strong compressive performance.
To endow the fabricated hydrogels with excellent water retention capacity, flexibility, and good mechanical properties, physical–chemical or chemical–physical hybrid cross-linked hydrogels have gradually attracted the attention of researchers in this field. Zhao et al.10 developed a chitosan/tannic acid/corn starch (CHT/TA/CS) bilayer hydrogel film, in which tannic acid acted as a bridge connecting the chitosan and corn starch layers by the interaction of the hydroxyl groups on the tannic acid molecular chain and the hydroxyl groups on the corn starch molecular chain. The schematic diagram of the fabrication and formation of CHT/TA/CS bilayer film is shown in Fig. 3. Compared with chitosan and corn starch hydrogel films, the tensile strength of the bilayer hydrogel film was significantly improved, reaching 24.1 MPa, 1.5 times higher than that of the chitosan hydrogel film and 4.6 times higher than that of the starch hydrogel film. This was because a Schiff base reaction occurred between TA and CHT molecules, forming covalent bonds, resulting in the film having strong resistance to tensile forces.
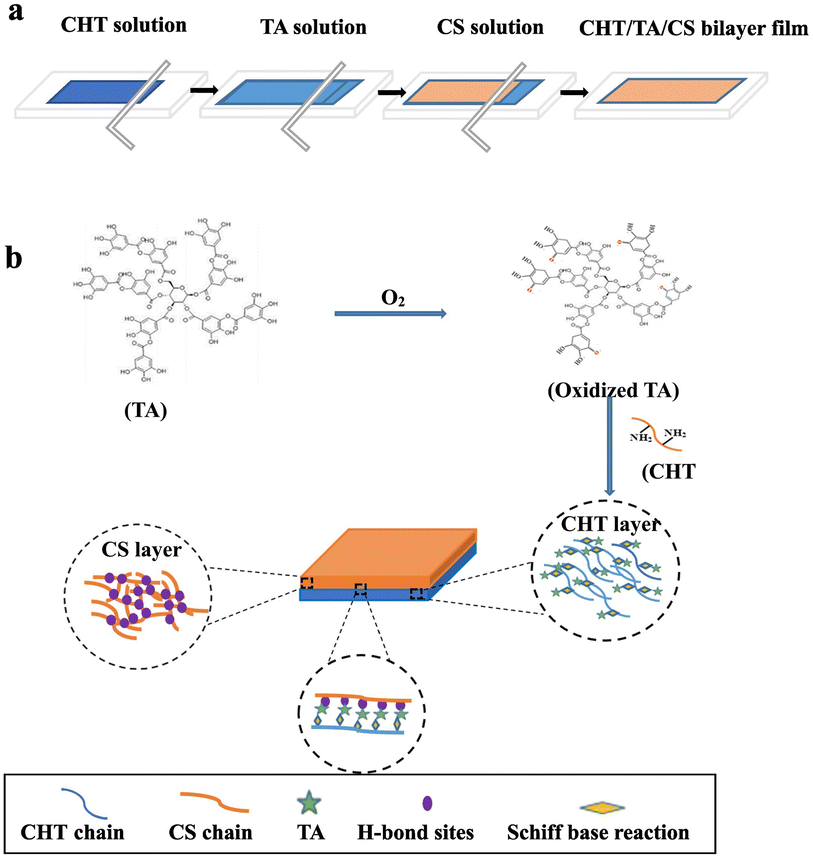 | ||
| Fig. 3 The fabrication (a) and formation (b) of a CHT/TA/CS bilayer film.10 | ||
Dos Santos et al.36 compared the characteristics of starch-based hydrogels prepared from starches derived from various sources with different amylose contents (ACs), including pinhão, guabiju, and uvaia seeds (AC: 34.31% ± 1.24, 43.65 ± 0.70 and 42.68% ± 1.96, respectively). They found that starch with lower AC could improve water absorption capacity of the hydrogel, in which the pinhão, guabiju, and uvaia starch exhibited a water absorption capacity of 722.07%, 457.45% and 604.06%, respectively. It was also concluded that the microstructure of the hydrogels was related to AC of the starch sample. The hydrogel prepared from guabiju starch with higher AC exhibited a uniform structure, with smaller cavities. In contrast, pinhão starch hydrogels (lower AC) showed deeper structural cavities and a spongy appearance. Luo et al.20 found that amylopectin was beneficial for improving the grafting rate of acrylamide (AM). As the amylopectin content increased from 0% to 90%, the grafting rate of AM increased by nearly 1-fold. This was because amylopectin had more branching points, providing more chemical grafting sites. At the same time, the increase in the grafting rate enlarged the pores of the gel network, promoting an increase in water absorption capacity of the hydrogel. When the amylopectin content was 70%, its water absorption capacity was 45.25 times that of the hydrogel prepared with 0% amylopectin content. Therefore, the ratio of amylose and amylopectin should be adjusted to meet the needs of hydrogel applications.
Indeed, a wide range of modification approaches have been explored to tailor the properties of starch materials specifically for active packaging purposes. These modifications mainly include physical, chemical, and enzymatic methods.
High hydrostatic pressure (HHP), shear force, dry heating treatment (DHT), heat moisture treatment (HMT), ultrasonication treatment, pulsed electric field, cold plasma and irradiation (e.g., electron beam, microwave, and gamma) are the main physical modification methods for the preparation of starch-based hydrogels because they are simple to operate, low cost, and harmless.
HHP is a non-thermal technology that can disrupt non-covalent intermolecular interactions, leading to the disordering of biopolymers and inducing pressure-assisted gelatinization. From the previous study, a pressure level of 600 MPa was utilized to prepare a potato starch hydrogel for 15 min, and a conventional thermal treatment hydrogel served as the control sample.37 This indicated that the complete gelatinization of potato starch suspension could be achieved by combining pressure (600 MPa) and moderate heating treatment (50 °C). In the research conducted by Shahbazi et al.,38 high-speed shearing force was used to modify starch and the impact of different shear rates (0/s, NF; 56/s, SF1; 210/s, SF2; 400/s, SF3) on the physicochemical properties of the prepared hydrogel was investigated. The researchers found that increasing the shear rate could cause an increase in the tensile strength and a decrease in water vapor permeability of the hydrogel. This led to an improvement in the texture stiffness and water barrier properties of the hydrogel films. Specifically, the tensile strengths of the SF1, SF2, and SF3 samples were 7.7 MPa, 9.6 MPa, and 12.9 MPa, respectively. The water vapor permeability of NF decreased from 55.2 × 10−1 (g m−1 s−1 Pa−1) down to 0.33 × 10−1 (g m−1 s−1 Pa−1) and 0.1 × 10−1 (g m−1 s−1 Pa−1) regarding the SF2 and SF3 samples, respectively.
DHT usually involves treating starch granules at temperatures ranging from 110 to 150 °C for 1–4 h and a low moisture level (<10%, w/w).39 Researchers have utilized DHT to modify cassava starch, successfully obtaining a water-based gel material that possesses improved elasticity and excellent printing performance. Specifically, compared to the native starch hydrogel, the gel strength and gel hardness of DHT-starch hydrogel decreased from 0.37 N and 0.47 N to 0.06 N and 0.10 N, respectively. They believed that DHT pre-treated starch could be applied in 3D food printing technology.40 Different from DHT, HMT refers to the process of treating starch at higher moisture (10–35%) for a specific period of time in a temperature range of (90–120 °C).41 In the study conducted by Bangar et al.,41 it was found that the light transmittance of starch hydrogels increased from about 15% to 30.5% after HMT modification.
Ultrasonication technology is also a feasible eco-friendly way to improve properties of starch hydrogels for food packaging materials. After subjecting to ultrasonication with a duration of 7.5 minutes and a power output of 360 W, the tensile strength of the PVA film was increased by nearly 29%, while the fracture strain, water vapor permeability and moisture resistance were reduced by 30%, 11%, and nearly 12%, respectively.42 PEF is a non-thermal technique and could be used to modify starch structural and physicochemical properties. In the early work conducted by Singh et al.,43 elephant foot yam starch (EFYS) was subjected to electrical field intensities of 4, 8, 12, and 16 kV cm−1 in pulse width 10 μs and pulse number 1000 for the development of the biodegradable film. It was found that the water absorption capacity of starch increased initially with an increase in PEF intensity from 4 to 12 kV cm−1 and then reduced at 16 kV cm−1 and varied in the range of 1.79 ± 0.01 to 2.08 ± 0.02 g g−1. The variation was explained by the fact that the low intensity PEF treatment caused dissociation of amylose chains in the amorphous region of starch granules which made it easier to absorb water, while high intensity PEF (16 kV) mainly caused destruction of the crystal region which reduced the number of binding sites between starch and water. Cold plasma can change the physical and chemical properties of starch without destroying the integrity of starch granules and is environmentally friendly, which has also been widely used for starch modification. The effects of dielectric barrier discharge (DBD) plasma (a common method for cold plasma generation) treatment (3, 6, and 9 min) on the physicochemical properties of potato starch and its films were studied.44 It has been shown that the permeability and mechanical properties of starch films were improved after DBD plasma treatment, with oxidation, de-polymerization and crosslinking as the main mechanisms. The DBD 9-min film exhibited lower water vapor permeability (4.39 × 10−9 g m m−2 Pa s) and higher tensile strength (more than 21 MPa), compared to the control DBD 0-min film (8.41 × 10−9 g m m−2 Pa s and 16.10 MPa, respectively). This suggested that DBD plasma could enhance the properties of potato starch and its film, making it suitable for use in starch-based packaging.
Radiation technology as an emerging “green technology” has been explored to improve the properties of starch-based hydrogels.45–47 In a previous study,48 waxy maize starch was treated by electron beam irradiation in air with irradiation doses of 2, 4, 6, 8, 10, 15, 20 and 30 kGy. Subsequently, native and irradiated starch films were prepared through a solution casting method. As the radiation dose increased from 0 to 30 kGy, the average molecular weight of starch molecules gradually decreased from 1002.93 × 105 g mol−1 to 2.32 × 105 g mol−1. However, the tensile strength of starch films displayed a trend of initially increasing and then decreasing, reaching a maximum value of 39.94 MPa at 10 kGy. The solubility exhibited a trend of initially increasing and then stabilizing, also reaching 85.56% under 10 kGy irradiation.
Compared with physical modification, chemically modified starch films possess unique advantages, such as enhanced thermal stability and improved mechanical strength.49,50 In essence, chemical modification refers to the reaction where the hydroxyl group of starch is substituted with different functional groups through processes such as esterification, etherification, oxidation, or grafting. Early work showed that modified starch-based hydrogels prepared from maleic anhydride esterified starch had excellent pH sensitivity.51 As the pH increased from 3 to 8, the swelling degree of the starch hydrogel with a maximum degree of substitution (DS, 0.250) increased by nearly 2 times. What's more, the adsorption capacity and encapsulation rate of curcumin increased with an increase in the degree of substitution. When the degree of substitution reached 0.250, the adsorption capacity was 399.23 μg g−1 and the encapsulation rate reached 80%.51 Similarly, Kobryn et al.52 discovered that the properties of starch hydrogels were improved via acetylation with acetic anhydride, resulting in prolongation of the release of β-escin. According to the kinetics and Weibull model, the half-time release of β-escin was determined. The minimal values occurred in the native starch hydrogel, while the maximal values occurred in the hydrogel with 40% modified acetylated potato starch, which were 949.26 min and 4091.24 min, respectively.
Ozone is an emerging and environmentally friendly technology for starch modification. In a previous study,53 when cassava starch samples underwent ozone treatment, the number of carbonyl and carboxyl groups increased while large molecules and branched molecules decreased. Besides, the texture of the hydrogel improved and the strength of the modified starch hydrogel was 280% higher than that of natural starch. According to the study by Maniglia et al.,54 the hydrogel prepared with starch treated with ozone for 30 minutes exhibited a lower peak apparent viscosity of approximately 3000 mPa s compared to native starch (above 4000 mPa s). This provided the optimal rheological properties for food matrices, thereby enabling excellent 3D printing applications. Through evaluating the printability of the gel, the star-shaped pattern produced using ozonated starch exhibits better resolution than native starch, and the result is shown in Fig. 4.
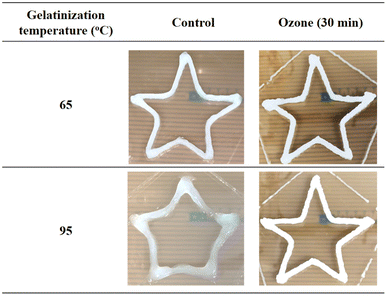 | ||
| Fig. 4 Images of the stars obtained with cassava starch gels and stored for 7 days at 5 °C.54 | ||
Enzymatic modification is often regarded as an efficient avenue to improve hydrogel functionality by altering the starch chain structure and granule microstructure.55 In the study conducted by Rosseto et al.,56 it was found that transglutaminase enzyme (TGase) treatment can increase the tensile strength of hydrogel films by 20%. When exposed to humidity, the solubility of starch treated with TGase was markedly reduced compared to native starch, with a decrease of approximately 25%. As a result, the subsequently prepared packaging materials will be suitable for humid environments.
In general, the hardness of starch hydrogels prepared by physical treatment would increase. However, the viscoelastic properties and thermal stability of starch hydrogels prepared by chemical and enzymatic treatments have improved.
In the early work conducted by Amaral et al.,59 lignin and cellulose were separated from chestnut (Castanea sativa) shells and then the isolated fiber and glycerol were used for the fabrication of the starch-based hydrogel. After formula optimization, the optimal addition ratio of fiber and glycerol was determined to be 10% (w/w) and 50% (w/w), respectively. Compared to the native starch hydrogel, the optimized hydrogel film exhibited approximately 3.5 times higher tensile strength. Furthermore, it was opaque, which possessed the ability to block ultraviolet rays. Therefore, the optimized chestnut shell fiber starch film has the potential for producing biodegradable food packaging. According to Ren et al.,60 the addition of 1% (w/w) Mesona chinensis Benth polysaccharide (MCP) could noticeably improve the tensile strength (about 2 times more than the native sample) of sweet potato starch hydrogel films in the presence of sodium carbonate, resulting from the interaction between the acidic anionic of MCP and the sodium ions of sodium carbonate through electrostatic force. Similarly, kappa carrageenan (κCA) with a high hydrophilicity hydrogel could be cross-linked with other polymers through different chemical and physical mechanisms.61,62 In the previous research, surface modification of hybrid starch/cellulose nanofiber (CNF) with κCA hydrogel coating was conducted by Tavakoli et al.63 The result showed that compared to the hydrogel without κCA, mechanical strength of κCA-coated starch/CNF hydrogels increased by more than two times, depending on the κCA content.
Meanwhile, inorganic materials with high strength characteristics have also been used to improve the overall mechanical properties of the hydrogel, which commonly include biochar, graphene, halloysite, metal oxides, etc.
In the research carried out by Motamedi et al.,64 the effects of natural char (NC), ball-milled natural chars (BMNCs) and chemically modified NC nanoparticles (NCNPs) on the properties of starch-g-poly(acrylic acid-co-acrylamide) composites were determined and compared. The water absorption capacity of NCNPs/hydrogel (389.9 g g−1) was twice as high as that of the neat hydrogel (202.1 g g−1). After storage for 14 days, water absorption capacity of NCNPs/hydrogel (23.1%) was three times that of the neat hydrogel (7.1%). It could be deduced that the presence of NCNPs in the polymer matrix led to the formation of a porous gel structure, which improved the distribution of liquid in the absorbent network during the water absorption process. Clay has the ability to absorb moisture and, when moistened, it exhibits plasticity and high-capacity cation exchange capability, making it a potential choice as an additive material in the packaging industry.65 According to Chaudhuri et al.,66 bentonite clay was incorporated into a starch grafted polyacrylic acid hydrogel. Compared to samples without bentonite clay, the storage modulus of the hydrogel with bentonite clay increased by 3.55 times. The thermal response results indicated that with the incorporation of bentonite clay, the thermal response behavior of the hydrogel transitions from thermophilic behavior to thermophobic behavior. In the study conducted by Su et al.,67 Ca2+ was used to construct a double-network hydrogel, in which the first physical cross-linked network was formed by the hydrogen bond between corn starch and sodium alginate and the second physical ion cross-linked network was formed between Ca2+ and corn starch or sodium alginate. In addition, under a strain of 160%, the composite hydrogel with 0.4% (W/V) Ca2+ had the highest toughness (61.61 kJ m−3) and breaking strength (281.51 kPa). In addition to improving the mechanical properties of hydrogels, metal ions or metal oxides could also endow them with an antimicrobial function.68,69 For example, CuO nanoparticles prepared by the hot injection precipitation method were used to achieve antifungal hydrogel films. Specifically, CuO nanoparticles not only increased the tensile strength of the hydrogel from 0.87 ± 0.40 MPa to 1.92 ± 0.09 MPa, but also gave the gel an inhibitory effect on Alternaria alternata, with a fungistatic rate of 33%.70
The addition of antimicrobials and antioxidants to the hydrogel matrix, such as polyphenols,71,72 essential oil,73,74 and lactic acid bacteria,75 is another efficient approach to improve bio-functional properties of reinforced hydrogel films. The early work indicated that curcumin can improve the antioxidant capacity of hydrogels, and the composite hydrogel film with 5% curcumin had the highest scavenging activity for ABTS and DPPH, reaching 98.09% and 86.77%, respectively.72
3.2 Properties and characterization techniques of starch-based hydrogels
The high water absorption of starch is suitable for food packaging materials, which is beneficial to avoid the water loss of food, so as to properly delay the spoilage of food.4 On the other hand, the swelling properties of starch allows hydrogels to undergo volume or shape transitions in response to physical and chemical stimuli, such as temperature, light, magnetic/electric field, pressure, ionic strength, and pH value.
It has been reported that the rheological strength and structural rigidity of maize starch hydrogels were negatively correlated with the magnitude of inherent mobile fractions within the hydrogels.76 According to a previous study,77 a dual amylopectin (Amy, the first network)/poly(N-hydroxyethyl acrylamide) (PHEAA, the second network) hydrogel was developed through “soft-hard” hydrogen bond (H-bonded) crosslinks. In this hydrogel, the “hard” H-bonded between Amy molecular chains played a key role in maintaining the rigidity, while the “soft” H-bonded between Amy and PHEAA polymer chains provided toughness, thereby synergistically enhancing the mechanical properties of the hydrogel and making it both tough and adhesive.
The permeability or barrier properties of hydrogels to water vapor, oxygen and ultraviolet are of great significance for their application in food packaging. This performance is affected by the microstructure of the hydrogel, which is closely related to the interaction between its components.78 The study conducted by Sifuentes-Nieves et al.79 showed that the roughness of the hydrogel decreased from 612 to 499 nm, the melting temperature increased from 130 to 146 °C, and the Young's modulus increased from 12 to 202 MPa after ultrasonic/plasma dual treatment. This was because the dual treatment of ultrasound/plasma helped form more polar functional groups on the surface of the fibers, further enhancing the bonding between the fibers and starch, resulting in a smoother surface of the starch film and better mechanical properties.
Fully transparent packaging is expected by consumers to see the state of the goods inside; however, the barrier performance of the completely transparent packaging to food destruction factors (such as light, oxygen, and heat) is reduced. Therefore, appropriately reducing the transparency of the packaging is beneficial for the long-term storage of food.80 Zhou et al.73 proved that the light transmittance of a CEO free film was 39.33%, while the transmittance of a film added with 1% CEO decreased to 24.30%, which may be conducive to the packaging of light-sensitive food. Similarly, the water vapor transmission rate increased from 1.04 × 10−12 g cm cm−2 s−1 Pa−1 (CEO free film) to 1.90 × 10−12 g cm cm−2 s−1 Pa−1 (2.5% CEO film), which meant the improvement of the water resistance of the films.
Hardness and tensile strength are the main indicators for evaluating the mechanical strength and flexibility of films, which are important parameters for characterizing hydrogel textural properties. Zhao et al.10 developed a bilayer film composed of chitosan/tannic acid/corn starch (CHT/TA/CS). Through a texture analyzer, it was determined that the tensile strength of the CHT/TA/CS film was 24.1 MPa, which was 1.5 times and 4.6 times higher than that of the CHT film and CS film, respectively. This bilayer hydrogel film holds promising potential for applications in areas such as food packaging materials.
Qin et al. prepared packaging hydrogel films by adding free anthocyanin from wolfberry and nano-complexes into a starch/PVA mixture. From SEM images, it can be observed that anthocyanin-loaded nanocomplexes were uniformly distributed in the membrane, which verified the formation of the starch/PVA film incorporated with anthocyanin-loaded nanocomplexes.71
Previous research81 has reported the preparation of hydrogels by crosslinking lignin sulfonate (KL) with starch. By analyzing the change in the C–O/C–O–C bond mass concentration (KLS, 55.5 ± 2.2%, KL, 37.2 ± 1.8% and starch, 42.3 ± 2.2%) using NMR and XPS, the changes in aromatic and anhydroglucose units of KL and starch were quantified. This confirmed the presence of glyceryl ether crosslinking between starch and KL in KLS, which provided a basis for the mechanism of KL in hydrogels.
According to the research of Lin et al.,82 a hydrogel was successfully prepared by cross-linking N,N′-methylenebisacrylamide (MBA) grafted starch, sorbitol, and eugenol. In order to understand the cross-linking pattern among the three components (MBA, grafted starch and sorbitol), molecular docking technology was employed for evaluation. The results showed that sorbitol formed new hydrogen bonds with starch, providing a cross-linking region through structural reorganization and promoting the formation of a denser network. This offers a new approach for explaining the mechanism of improving hydrogel performance at the molecular level.
3.3 Applications of starch-based hydrogels for food packaging
Due to the properties of high-water content, flexibility and compatibility, hydrogels are widely used in various fields, for example biomedical applications, hygiene products, agriculture, and the food industry. This review focuses on the application of hydrogels in food packaging (Table 2). The basic function of hydrogels as food packaging is to control the humidity inside the packaging, or to capture excess evaporated water inside the packaging caused by physicochemical changes in the packaged food. Novel hydrogel materials can also confer new functions on food packaging, including antibacterial and antioxidant activity, edibility, sensing environmental stimuli and monitoring food quality, thus forming an active packaging system.83| Type of hydrogel | Starch type | Active/intelligent compound | Properties | Potential application | References |
|---|---|---|---|---|---|
| Functional hydrogels | Corn starch | Chitosan and tannic acid | The CHT/TA/CS coating maintained the freshness of banana from 3 days to 7 days | Fruit preservation | 10 |
| Corn starch | CuO nanoparticles | The CuO/starch hydrogel films showed a bacteriostasis rate of 33% for the Alternaria alternata fungus | Antimicrobial packaging | 70 | |
| Cassava starch | Lactobacillus plantarum and Pediococcus pentosaceus | The antioxidant activity of starch/CMC/LAB-2% film was 48.12 ± 1.11%, an increase of 13.18 times compared to the film without LAB | Fruit preservation | 75 | |
| Potato starch | Guar gum, and the extract | The hydrogel can maintain the quality of chicken meat for 12 days at 4 °C | Fresh meat preservation | 86 | |
| Responsive intelligent hydrogels | Soluble starch | Gellan gum and anthocyanin | The pH-indicating smart packaging could display food freshness in real time | A smart tag signaling food freshness | 87 |
| Water chestnuts, maize and potato starches | — | The intensity of luminescence decreased when the packaged meat thawed | An indicator to reveal the state of frozen food | 88 | |
| Edible hydrogel film and carrier system | Hydroxypropyl cornstarch | Xylose, cellulose crystals, and laver | Hydrogels had high tensile properties, ductility and low permeability under dry conditions | Instant noodle preservation | 89 |
| Hydroxypropyl high-amylose corn starch | Glycerol and hawthorn berry (Crataegus pinnatifida) extract | Hydrogels containing 4% glycerol and 6% hawthorn berry extract had inhibitory effects on Pseudomonas aeruginosa | Antibacterial food packaging | 91 | |
| Maize starch | Grape juice, sodium trimetaphosphate and glycerol | The total counts of psychrotrophic bacteria, Enterobacteriaceae and aerobic mesophilic bacteria, in active hydrogel-coated chicken fillets, were reduced by 0.94, 0.85, and 1.58 log, respectively | Coating chicken fillets | 92 | |
| Starch (native and gelatinized) | β-Carotene, alginate, and gelatin | Under accelerated storage conditions (65 °C for 6 days), the hydrogel film improved the stability of beta-carotene | Encapsulation systems for the active substance | 93 |
Furthermore, there are two main important applications focused on functionally active packaging: antioxidant and antimicrobial. The first one is to protect the food from oxidation by incorporating antioxidants into substances, such as natural extracts or essential oils. The second one is generally achieved by the addition of antibacterial agents such as essential oils, probiotics, nanoparticles, and metal ions, resulting in the reduction or inhibition of microorganism growth.3
In the study conducted by De Souza et al.,74 the synergic effect of carvacrol essential oil (CEO) and montmorillonite (MMT) on thermoplastic starch (TPS) films was evaluated. The antimicrobial assay showed that TPS films without CEO or MMT had no antibacterial activity and the inhibition zone was 0. However, the inhibition zone of the hybrid-TPS film incorporating CEO and MMT increased gradually with the increase in the addition of CEO (4.5% and 9%), reaching 1.39 and 1.5 mm2, respectively. When the addition of CEO reached 15%, it exhibited a complete inhibitory effect. The researchers also found that during the preparation or storage of the film, CEO evaporated or CEO in the starch solution did not have antibacterial properties. Thus, it was hypothesized that during the preparation of the hybrid-TPS film, the MMT layers could trap the essential oil, providing protection against its evaporation and volatilization, thereby prolonging and optimizing the bactericidal effect. Nandi et al.86 developed a starch-based functional hydrogel film containing polyphenol extract from betel leaf residue (BLP extract), which effectively extended the shelf life of chicken meat. The hydrogel film with 4% (w/w) BLP extract exhibited the best preservation effect and could extend the shelf life of chicken meat to 12 days at refrigeration temperature. In addition, the biodegradation experiment on the hydrogel film showed that the biodegradation time of the composite hydrogel membrane was noticeably reduced from 28 days (natural film) to 14 days, demonstrating excellent biodegradability. According to Kanatt et al.,47 lime juice (LJ) with a pH of 2.6 and brix value of 8.6° was prepared from lime (Citrus aurantifolia) through extrusion, centrifugation, and filtration. LJ (3%, V/V) was then added to the hydrogel packaging film prepared from irradiated starch (5 g/100 mL), gelatin (5 g/100 mL) and glycerin (0.25 g/100 mL). Compared to a packaging film without LJ, the shelf life of chicken packaged in the film with LJ was extended from 2 days to 12 days during chilled storage.
Li et al.75 prepared starch/CMC/LAB packaging materials by incorporating lactic acid bacteria (LAB) with high exopolysaccharide (EPS) yield at concentrations (0.5%, 1%, 1.5%, and 2%) into the cooled gelatinized solution prepared from cassava starch (4%), glycerol (1.5%), and CMC (0.2%). The antioxidant activity of the starch/CMC/LAB film significantly increased after the addition of probiotics, and it showed concentration dependence (p < 0.05). The highest activity was observed in the starch/CMC/LAB-2% film, which was 48.12 ± 1.11%, an increase of 13.18 times compared to the film without LAB. This proved that the improved antioxidant capacity of the starch/CMC/LAB film is primarily due to the presence of EPSs generated by LAB during the cultivation process. Furthermore, the preservation ability of the film was evaluated through qualitative assessment of the banana's shelf life wrapped in the prepared film. CS/CMC/LAB-2% exhibited better antioxidant effect on bananas. During a 7-day storage period, it resulted in the formation of the smallest black spots, while the bananas wrapped with the free-LAB film showed noticeable black spots on the third day.
The pH smart label is an effective, non-invasive, real-time way to display food freshness. The pH responsiveness of hydrogels is achieved by incorporating pH-sensitive materials, such as anthocyanins (the molecular structure and color of anthocyanins change correspondingly with the variation in pH).71 When acidic or alkaline gases such as NH3, CO2, trimethylamine, and dimethylamine, are released during microbiota growth and food deterioration, significant color shifts may occur. Ma et al.87 developed starch-based hydrogels incorporated with gellan gum and anthocyanin and then attached the hydrogel to the cap of a Petri dish containing milk or shrimp. Under storage conditions at 25 °C, the acidity of the milk reached 18 mg/100 g after 15 hours. According to the Chinese standard GB19645-2010, the standard for fresh milk is also set at 18 mg/100 g. During the process of shrimp decay, intelligent labels exhibited significant color changes from yellow to purple. It is worth noting that the acidity of milk and the color change could be evaluated based on the color parameter measurement of the smart label. Therefore, this smart label fully demonstrates its potential as a pH smart label that can evaluate the freshness level of food.
Frozen food will produce exudates, resulting in decreased food quality and short shelf life in the process of repeated freeze–thaw. The variation in humidity can alter the luminescence intensity of starch by affecting non-bonded interactions between electron-rich heteroatoms in the starch structure. Due to the clusteroluminescence properties of starch, a starch-based hydrogel is sensitive to moisture changes.81 In the study conducted by Lai et al.,88 a responsive intelligent hydrogel packaging bag was developed using maize starch and was applied to the packaging of chicken breast meat. During the experimental process, the weight changes of the meat pieces were measured at regular time intervals under frozen conditions. Simultaneously, the luminescence of the bags containing samples in different states (fresh meat, frozen meat, and thawed frozen meat) was recorded under 365 nm ultraviolet light irradiation, as well as the water content of the meat sample. When the humidity inside the package increased, the packaging film absorbed water and swelled, causing an increase in the intermolecular distance between starch molecules and a decrease in the aggregation degree of non-conjugated electron-rich units. As a result, a decrease in the luminescence intensity of the hydrogel film was observed as shown in Fig. 5.
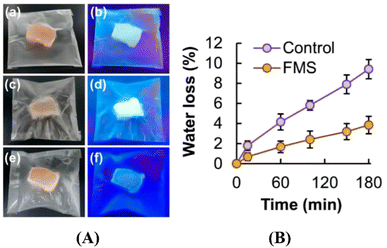 | ||
| Fig. 5 (A) Starch-based packaging under white light (a, c and e) and UV light (b, d and f); fresh chicken meat (a and b); frozen chicken meat (c and d); thawed frozen chicken meat (e and f). (B) The water content changes in the meat stored.88 | ||
In the study by Lai et al.,88 developed edible starch films were generated from water chestnuts, maize and potatoes, and the toxicity of films was examined in cells. The results showed that at all tested concentrations, the loss of 3T3 and HEK293 cell viability was negligible after treatment with starch-based hydrogel films for 5 h and 24 h, indicating that the generated films had neither acute nor chronic cytotoxicity. Besides, edible coatings are transparent films that can also be used in fruit packaging as an alternative to the wax that naturally occurs on the surface of the fruit.90
In addition, several studies have explored the development of starch-based hydrogel edible films as carriers of bioactive compounds, in which the 3D network structure of starch gels could protect the biological activity from oxygen, acidity and free radicals.91,92 Hydrogels have been used for encapsulation of β-carotene, which is sensitive to high temperature, light and other external environmental stimulation, as well as an acidic stomach and other digestive environments.93 In the study conducted by Mala et al.,94 hybrid hydrogel beads were prepared with pectin and resistant starch using the extrusion–gelation method and loaded with bromelain. Through thermal stability experiments, it was found that the highest relative activity of bromelain occurred at a ratio of pectin/resistant starch of 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (w/w) for all heating treatments (at 95 °C for 1, 2, 3, 4, and 5 min), while the activity of free bromelain was only highest at 30 °C. Apart from that, in vitro release experiments were conducted to evaluate the activity of bromelain in the gastrointestinal tract, and it was observed that the presence of pectin and resistant starch-based hydrogel appreciably reduced the release of bromelain, with a reduction in release ranging from 27.41% to 33.85%. Similarly, another study has shown that the starch-coated hydrogel could provide protection for the encapsulated active compound.95
1.5 (w/w) for all heating treatments (at 95 °C for 1, 2, 3, 4, and 5 min), while the activity of free bromelain was only highest at 30 °C. Apart from that, in vitro release experiments were conducted to evaluate the activity of bromelain in the gastrointestinal tract, and it was observed that the presence of pectin and resistant starch-based hydrogel appreciably reduced the release of bromelain, with a reduction in release ranging from 27.41% to 33.85%. Similarly, another study has shown that the starch-coated hydrogel could provide protection for the encapsulated active compound.95
4 Starch-based aerogels
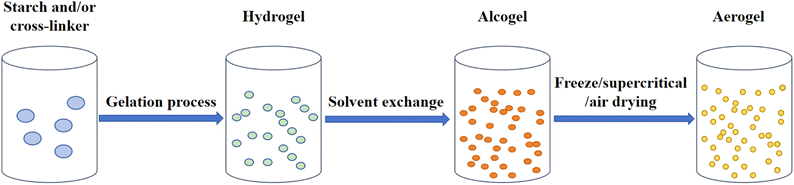
Aerogels are porous materials with a porosity exceeding 90% and an extreme density typically of 0.001 to 0.5 g cm−3.96 The high porosity and lightweight properties endow the aerogel with great potential for application in various fields. The following is some research progress on the preparation, characteristics, and application of starch aerogels in food packaging.4.1 Fabrication of starch-based aerogels
The fabrication of starch-based aerogels involves two steps: the formation of starch-based hydrogels and drying (Fig. 6). Actually, the aerogel complies with the processing methods of starch-based hydrogels, which are reviewed in the above section. This section focuses on the role of gel components (starch type, ratio of amylose to amylopectin, and presence of other additives) and drying methods in the preparation process of aerogels.To improve the stability, homogeneity and open porosity of aerogels, precursor materials are essential, such as polysaccharides,101,102 proteins,103 macromolecular compounds,15 and inorganic molecules,104 which affect the degree of cross-linking between the polymer chains and thus improve the 3D structure of the gel. More importantly, functional aerogels with antibacterial and antioxidant properties can be achieved by incorporating polyphenols,105,106 essential oils,107 and metal ions108 into them.
Air drying is the process of matrix moisture evaporation until reaching a constant weight, which is performed at room temperature under ambient pressure or in a constant oven.110 It is indeed a basic and simple method of drying that can be easily implemented using drying equipment. It should be noted that if air drying is used alone, it is prone to causing the collapse of gel pores, shrinkage of the gel structure, and the formation of cracks, leading to the loss of nanoporous structures in aerogels. Therefore, it is necessary to optimize the parameters of air drying to improve the aerogel characteristics. In the study conducted by Zou et al.,111 a starch-based aerogel with low densities (0.39 g cm−3) and specific surface areas above 100 m2 g−1 (132 m2 g−1, specifically) was obtained by the low-vacuum drying method (using a water aspirator created pressure of around 3 kPa), which were close to those of aerogels obtained by supercritical CO2 drying (density: 0.35 g cm−3 and surface area: 141 m2 g−1). Low-vacuum drying provides a new perspective for the preparation of starch aerogel materials by air drying.
Freeze-drying is another drying method widely used in the preparation of starch aerogels. It involves two steps: a pre-freezing process of a wet material or solution into a solid at −45–−15 °C and a sublimation process of the solvent under vacuum, ultimately resulting in the formation of pores in the aerogel.112 However, it has been found that pre-freezing might damage the structure of the resultant aerogel due to the growth of ice crystals during this stage. Therefore, Ni et al.113 controlled the growth of ice crystals and the formation of aerogel structures by selecting appropriate preparation conditions. The homogeneity of the pore structure in the aerogel depends on the nucleation and growth rate of ice crystals, which could be tuned using pre-freezing temperatures. With the decrease in pre-freezing temperature (from −15 °C to −25 °C and −40 °C), it was observed by SEM that the pore size distribution zones decreased, measuring 18–15 μm, 10–18 μm, and 4–9 μm, respectively. However, the corresponding peak area ratios were 37.0%, 65.1%, and 25.2%, respectively. This indicated that the ice crystal size of the aerogel formed under pre-freezing conditions of −25 °C was most uniform.
It had been reported that gelatin could be used to inhibit the growth of ice crystals and avoid the formation of very large macropores, which was due to the existence of numerous microporous structures and the formation of dense structures in the aerogel.114 The starch concentration has a significant effect on the microstructure of aerogels. From SEM, as the concentration of starch (1–4%, weight/volume) increased, the pore size gradually decreased. When the starch concentration was 4% (w/v), the pore was the smallest, resulting in the most compact structure. Correspondingly, the filtration efficiency of the 4%-starch aerogel was the highest, reaching 92.78%, which was approximately 9 times higher than that of the 1%-starch aerogel.114 Considering the cost, the freeze-drying technique is the most suitable for producing large quantities of porous aerogels at moderate costs.
Supercritical drying is the most efficient way to fabricate starch aerogels with minimal damage to the microstructure. Supercritical carbon dioxide (scCO2) is the most common fluid used for supercritical drying, which possesses the properties of both gas (low viscosity and high diffusion coefficient) and liquid (the density and solvation capacity are similar to those of liquids).115 However, it should be noted that water could not form supercritical mixtures with scCO2 at the critical point. Therefore, solvent exchange is necessary in this dry method, in which ethanol is the most commonly used.116 The previous study found that the starch concentration and drying parameters, such as the CO2 flow rate, temperature and pressure during scCO2 drying, have potential to affect the microstructure of starch aerogels.117 It was indicated that an increase in the starch concentration (from 5 to 10%) promoted an increase in surface area (from 121 to 185 m2 g−1). An increase in CO2 density (from 629 to 839.9 kg m−3) could cause an increase in surface area (from below 40 m2 g−1 to approximately 94 m2 g−1). However, the disadvantages of high energy consumption and solvent exchange steps severely restrict the application of scCO2 drying in the industrial-scale production of aerogels.118
4.2 Properties of starch-based aerogels
| Starch source | Additive components | Drying method | Bulk density (g m−3) | Specific surface area (m2 g−1) | Pore diameter (nm) | Reference |
|---|---|---|---|---|---|---|
| Potato starch, corn starch, and tapioca starch (8–14% w/v) | — | Rotary vacuum evaporator | — | 75.6 | — | 116 |
| Waxy maize starch | — | Freeze-drying | 0.070–0.153 | 3–5 | — | 119 |
| Potato starch (5.0%, w/v) | Konjac glucomannan (0.3%, w/v) | Freeze-drying | — | — | 10–18 | 113 |
| Dialdehyde starch | Nanocellulose and gelatin | Freeze-drying | 0.010–0.020 | 132.28–191.31 | — | 112 |
| Corn starch (27, 55, and 72% amylose content) | Chitosan (0.5 and 0.75 wt%) | SC-CO2 drying | 0.120–0.160 | — | — | 115 |
| Corn starch (70% amylose content) | Agar or microcrystalline cellulose | SC-CO2 drying | 0.145–0.207 | 120–169 | — | 118 |
The pore sizes in aerogels vary from nanometers to micrometers and can be classified into micropores (with a diameter below 1 nm), mesopores (with a diameter ranging from 2 nm to 50 nm), and macropores (with a diameter above 50 nm). The sizes and shapes of these pores in aerogels can be controlled by selecting suitable gel components and drying methods.
Zou et al.119 obtained aerogels with different pore sizes and shapes by changing the concentration of waxy maize starch. From SEM images (Fig. 7), big slit pores, medium irregular honeycomb-like pores, and smaller spherical pores were observed in aerogels with 5 wt%, 8 wt%, and 11 wt% concentrations of starch, respectively.
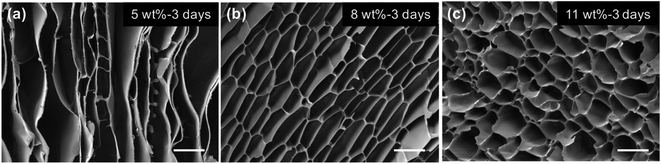 | ||
| Fig. 7 SEM images of porous starch with starch concentrations of 5 wt% (a), 8 wt% (b) and 11 wt% (c). Scale bars are 20 μm.119 | ||
The microstructure of starch aerogels can be modified using additive components such as the cross-linking agent. The effect of trisodium citrate (at three different concentrations of 12.8, 19.3, and 25.7 mg mL−1) on starch-based aerogel porous structures was studied.120 The highest relative density (0.2166 g cm−3) and lowest porosity (72%) were observed in the aerogel with a high concentration trisodium citrate (Aero 25.7), compared to Aero 12.8 (0.1071 g cm−3 and 86%) and Aero 19.3 (0.079 g cm−3 and 90%). The authors explained that this was because the high concentration of trisodium citrate enhanced the cross-linking between molecules and the pore structure was destroyed, forming a thin sheet of interconnected pores.
More importantly, the drying method of gels has a significant impact on the microstructure of starch aerogels. Zou et al.121 compared the effects of scCO2 drying and freeze drying on starch aerogels obtained from different sources (waxy maize, potato and pea). The density of all the aerogels prepared by supercritical drying (0.1–0.6 g cm−3) was higher than that of aerogels produced by freeze drying (0.07–0.16 g cm−3). The specific surface area of aerogels obtained through scCO2 drying was higher, being at least 20–30 times greater than that of freeze-dried aerogels.
To obtain satisfactory mechanical and adsorption properties, starch-based aerogels were prepared by optimizing the proportion of corn starch and trisodium citrate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 1
1.5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) by Camani et al.122 The aerogel with a 1
2) by Camani et al.122 The aerogel with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio exhibited the highest compressive modulus of 123.0 kPa. Correspondingly, it had the lowest specific surface area of 183.5 m2 g−1 and a pore volume of 0.18 cm3 g−1. This indicated that the aerogel (a ratio of 1
2 ratio exhibited the highest compressive modulus of 123.0 kPa. Correspondingly, it had the lowest specific surface area of 183.5 m2 g−1 and a pore volume of 0.18 cm3 g−1. This indicated that the aerogel (a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) possessed excellent mechanical strength and large surface area, thus furnishing a good load-bearing capacity.
2) possessed excellent mechanical strength and large surface area, thus furnishing a good load-bearing capacity.
Water absorption properties of starch-based aerogels, namely affinity to water, are one of the most important features. When an aerogel comes into contact with water, water molecules are easily linked to polar molecules in the aerogel structure and interact with hydrophobic molecules until the water absorption force and retention force reach equilibrium. At this point, the aerogel will reach its maximum water absorption capacity.123 The water transport behavior in aerogels obtained from normal and waxy potato starches (AC: 19.3% and 3.0%) was studied by Le Thanh-Blicharz et al.123 A decrease in equilibrium water activity of the obtained aerogels was observed with an increase in the ratio of amylopectin and amylose starch. The maximum water activity reached by the normal starch aerogel and waxy starch aerogel was 0.904 ± 0.002 and 0.858 ± 0.001, respectively. It could be inferred that waxy potato starch varieties were not preferred for preparing aerogels with stronger water–biopolymer interactions. To further analyze binding ability and mobility of water molecules in the starch-based aerogel at the molecular level, LF NMR was used to determine the spin–lattice relaxation time T1 and the spin–spin relaxation time T2 of water in aerogels. It was found that the T1 value of the normal starch aerogel was 57.5 ± 0.4 ms, which was higher than that of the waxy starch aerogel (30.3 ± 0.1 ms). Principal component analysis was conducted on the starch types and the water properties of aerogels. The results showed that measuring the relaxation rate at the onset of free water in the aerogel was sufficient to characterize the water behavior in starch aerogels.
In terms of oil absorption capacity, it also depends on the microstructure of aerogels.98 In a recent study, microcrystalline cellulose aerogels were synthesized with different amounts of high amylose corn starch (0%, 5%, 10%, 15%, and 20%) and NaOH–urea solution using vacuum freeze-drying technology.124 With an increase in starch content, the density of aerogels showed a trend of initially increasing and then decreasing, as did the adsorption capacity for linseed oil. When the ratio of starch was 15%, the prepared aerogels presented the lowest density (0.125 g cm−3), which endowed the aerogels with the highest absorption ratio of linseed oil (11.44 g g−1).
The excellent heat absorption performance (HAP) of aerogel materials is a key property for food storage and packaging applications.125 In the study conducted by Qian et al.,126 the HAP of konjac glucomannan (KGM)/starch aerogel was investigated, using commercial cellulose acetate (CA) aerogels as the control sample. It was displayed that under continuous heating conditions, the degree of temperature reduction in the air after condensing into a KGM/starch aerogel and CA aerogel was approximately 47.32% and 51.92%, respectively, and the temperature increasing degrees of KGM/starch and CA aerogel were 2.85% and 3.72%, respectively. This meant that the thermal stability of the KGM/starch aerogel was better than that of the CA aerogel. Moreover, the appearance of wrinkle structure in the starch-based aerogel was observed when hot air passed through, so it was assumed that heat absorption performance of aerogels was attributed to the thermal stability of KGM/starch aerogel.126
Three types of starch-based aerogels with different densities and surface areas were prepared and loaded with theophylline, including a potato starch aerogel (density 0.33 g cm−3, surface area 43 m2 g−1), pea starch aerogel (density 0.12 g cm−3, surface area 217 m2 g−1), and waxy maize starch aerogel (density 0.44 g cm−3, surface area 17 m2 g−1).111 The theophylline loading efficiency of the aerogels based on waxy maize starch (240%) was markedly higher than that of aerogels based on potato starch (182%) and pea starch (100%), which was attributed to the noticeably higher density in the waxy maize starch aerogels. What's more, based on the release kinetics experiment, the release profile of theophylline from the starch aerogels varied depending on the formulation. Typically, approximately 60% of the loaded theophylline was released within a timeframe of 30 to 70 minutes. A higher percentage, nearly 98%, was released within a longer period of 3 to 13 hours. The release rate indicated that starch aerogels can effectively control the release of theophylline, allowing for sustained active component delivery.
Starch-based aerogel delivery systems have been widely used to transport various nutrients such as vitamins, as well as bioactive ingredients such as antimicrobials and antioxidants in the food packaging industry. The use of an aerogel as a carrier could improve the bioavailability of these compounds, especially water insoluble bioactives. Due to its crystalline and lipophilic structure, the bioavailability of phytosterol is extremely limited. The bioaccessibility of free phytosterols is only 3%. In the study conducted by Ubeyitogullari et al.,127 phytosterols were incorporated into nanostructured porous starch aerogels using SC-CO2, resulting in a 35% increase in in vitro bioaccessibility.
The mechanical properties of starch-based aerogels have mainly been determined using compression testing with a texture analyzer. The water vapor sorption of aerogels can be evaluated by gravimetry under 97% RH conditions at 25 °C, in which the weight of the sample needs to be measured at intervals until the weight is constant.
The thermal behavior of starch-based aerogels can be investigated using differential scanning calorimetry performed under a nitrogen flow (20 mL min−1) in the temperature range of 25 to 400 °C. The thermal stability can be characterized using a thermogravimetric analyzer. Thermal conductivity is measured by the steady-state plate method through a thermal conductivity tester. The loading properties of starch aerogels are determined by in vitro release tests using simulated gastric fluid (pH 1.2) and simulated intestinal fluid (pH 7.4).
4.3 Applications of starch-based aerogels for food packaging
Different types of food have different characteristics, which consequently impose distinct performance requirements on aerogel packaging materials.128 This section focuses on the applications of aerogels in three types of foods: fruits and vegetables, meat, and fatty foods.In the study carried out by Dhua et al.,131 a starch aerogel was used to package fresh spinach leaves. Under room temperature and refrigerator conditions, the moisture content of fresh spinach leaves wrapped in aerogels increased by 43.12% and 49.57% respectively. The use of aerogel effectively reduced the moisture content within the packaging resulting from the transpiration of fresh spinach leaves. Furthermore, the efficiency of maize starch aerogel in the preservation of fresh spinach leaves has been verified. Without aerogel packaging, fresh spinach leaves spoiled within 2–3 days at room temperature and within 6–7 days at refrigeration temperature. However, aerogel packaging can extend the shelf life to 6 days and 10 days, respectively.
Depending on large surface areas and high porosity, aerogels possess the absorptive capacity of the blood and sap released by fresh meat, so as to prolong its shelf life effectively. Studies have also indicated that aerogels have loading ability on bioactive substances (antimicrobial compounds and antioxidants), giving aerogel packaging materials antibacterial and antioxidant properties.132 Chen et al.133 prepared antibacterial aerogels based on dialdehyde starch and chitosan, incorporated with copper nanoparticles (CuNPs). The result showed that fresh pork packaged with an active aerogel film had a shelf life of 14 days at 4 °C without any signs of spoilage. During this storage period, the amount of CuNPs migrating into the fresh pork was 3.38 mg kg−1, which was within the safe range.133 Such active aerogels loaded with antibacterial agents, such as polyphenols106 and essential oils,107 had the potential for prolonging the shelf life of fresh meat.
In the study conducted by Mirmoeini et al.,107 novel antimicrobial aerogels based on acetylated potato starch (5%, w/w), cellulose (1%, w/w), and Thymus daenensis Celak essential oil (TDEO) were created and used in cheese packaging. And then antimicrobial efficiency of aerogel packaging in Koopeh cheese was evaluated during the storage period. The dosage of TDEO was measured and determined using the minimum inhibitory dose (MID) of TDEO against E. coli O157:H7 in the vapor phase. After 21 days of storage at 7 °C, cheese packaged with the TDEO-aerogel at a high concentration (50 × MID) showed a significant decrease in the count of psychrotrophic bacteria (reduced by 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log) as well as yeast/mold count (reduced by 1
log) as well as yeast/mold count (reduced by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log). Furthermore, in the cheese samples packed with an aerogel incorporating 50 × MID TDEO and 25 × MID TDEO, the quantity of E. coli O157:H7 reduced from 4.2
log). Furthermore, in the cheese samples packed with an aerogel incorporating 50 × MID TDEO and 25 × MID TDEO, the quantity of E. coli O157:H7 reduced from 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU g−1 to undetectable levels after 7 and 14 days of storage, respectively. However, through sensory evaluation, a high concentration TDEO (50 × MID) had a negative influence on sensory characteristics of cheese, resulting in a lower sensory score (<6), while TDEO at a 25 × MID received a higher sensory score (>6). Therefore, in this study, the researchers suggested that new methods such as embedding technology would be adopted in further studies to alleviate the adverse sensory properties associated with high concentrations of essential oils in aerogels.
log CFU g−1 to undetectable levels after 7 and 14 days of storage, respectively. However, through sensory evaluation, a high concentration TDEO (50 × MID) had a negative influence on sensory characteristics of cheese, resulting in a lower sensory score (<6), while TDEO at a 25 × MID received a higher sensory score (>6). Therefore, in this study, the researchers suggested that new methods such as embedding technology would be adopted in further studies to alleviate the adverse sensory properties associated with high concentrations of essential oils in aerogels.
5 Future prospects and challenges
As public awareness of sustainable development increases, the commercialization of biodegradable packaging materials has gained significant attention over the past few years. Based on the global survey by Statista, 61% of consumers are willing to pay extra for eco-friendly products.134 The share of biodegradable and non-biodegradable plastics in 2021 stands at 58.3% and 41.7%, respectively, indicating the shift towards biodegradable products.135 Additionally, the growth of the food packaging industry, which accounts for around 50% of the worldwide biodegradable plastics market, is a major driver of the need for biodegradable packaging materials.136 According to the survey by Market Research Future, the global starch-based packaging market is projected to grow from USD 6.9 Billion in 2022 to USD 12.4 Billion by 2032.137Furthermore, incorporating starch-based plastics into agricultural operations promotes the development of a circular economy by efficiently utilizing resources and reducing dependence on non-renewable materials. Nevertheless, the industrial-scale manufacturing of these starch-based materials encounters numerous challenges, notably concerns related to food supply, land-use conflicts, and agricultural energy consumption. Therefore, utilizing agricultural or food waste for the production of starch-based materials and maintaining the sustainability of large-scale production of agricultural packaging materials is needed.138
It is worth noting that solely focusing on environmental sustainability is not enough to prove that biomaterials can completely replace traditional petroleum-derived plastics across all applications. Certain modification methods, such as the introduction of plasticizers, have been shown to improve the performance of starch-based packaging materials. However, a new problem will arise with the leaching of plasticizers during storage or end-user applications, as well as their harmful effects on consumer health and the environment. Therefore, more research studies are needed to evaluate the toxicity of degradation byproducts to ensure the safety of biomaterials in the environment.138
Overall, to increase the market share in the future, starch-based materials should compete directly with petroleum-based plastics and other biomaterials in areas such as consumer awareness, production costs, toxicity and an acceptable level of biodegradability.
6 Conclusions and future outlook
In general, starch-based hydrogels and aerogels belong to polysaccharide colloids with 3D networks. Both of them show excellent mechanical properties and biodegradability and have potential applications in various fields. When optimizing starch hydrogels and aerogels, it is necessary to consider the type and concentration of starch, modification of starches, the addition of gelling improvers and the selection of preparation methods. In particular, drying methods should also be considered in the preparation process of aerogels.Hydrogel and aerogel packaging materials can prolong the shelf life of perishable foods by absorbing moisture and blocking oxygen and UV radiation. Beyond that, hydrogel and aerogel active packaging incorporated with antioxidants and antimicrobial agents not only provide a physical barrier against external conditions but also exhibit antioxidant and antibacterial effects in food preservation. Starch-based aerogels, as carriers for loading active compounds, exhibit superior encapsulation capability compared to hydrogels. This is primarily due to their high specific surface area and adjustable porosity. On the other hand, starch-based hydrogels possess a unique property known as a stimulus-response system. This characteristic allows hydrogels to undergo changes in response to specific stimuli, making them highly suitable for applications in smart packaging.
Developing a composite material combining hydrogels and aerogels with a double-layer structure of the hydro-coat/aero-core, consisting of a hydrogel shell and an aerogel core, would be an innovative approach in the field of packaging materials. The hydrogel coating facilitates the rapid permeation of the water phase into the aerogel core. The porous structure of the aerogel core provides ample storage space, allowing for the retention of a significant amount of water.139 This makes it an ideal candidate material for applications in various areas such as fried food packaging, where both oil resistance and water absorption properties are desirable characteristics.
To date, starch-based packaging has not yet achieved commercialization on an industrial scale, mainly due to high production costs and poor material properties compared to the production of synthetic plastics. More research should focus on efficient strategies for their bioprocessing and suitable modifications to improve material properties that can be scaled up. Improving the efficiency of processes and the circularity of materials is also needed, aligning with the concept of the circular biomaterial economy, which would ensure the sustainable development of the bio-based packaging market.
Conflicts of interest
The authors declare no conflict of interest. All authors have read and agreed to the published version of the manuscript.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2023YFD2201300), Major Science and Technology Project of Xinjiang Uygur Autonomous Region (2022A02005-5), Key Research and Development Program of Shaanxi (2023-ZDLNY-15), and Qinchuangyuan “Scientists + Engineers” Team Construction Project of Shaanxi (2023KXJ-231).References
- M. E. González-López, S. d. J. Calva-Estrada, M. S. Gradilla-Hernández and P. Barajas-Álvarez, Front. Sustainable Food Syst., 2023, 7, 1225371 CrossRef.
- P. Selvasekaran and R. Chidambaram, Food Hydrocolloids, 2022, 131, 107760 CrossRef CAS.
- M. Taherimehr, H. YousefniaPasha, R. Tabatabaeekoloor and E. Pesaranhajiabbas, Compr. Rev. Food Sci. Food Saf., 2021, 20, 5321–5344 CrossRef CAS PubMed.
- F. J. Leyva-Jiménez, R. Oliver-Simancas, I. Castangia, A. M. Rodríguez-García and M. E. Alañón, Food Hydrocolloids, 2023, 135, 108124 CrossRef.
- P. Kumar, Indian J. Pediatr., 2018, 85, 384–389 CrossRef PubMed.
- T. G. Hoffmann, D. A. Peters, B. L. Angiolett, S. L. Bertoli, L. V. Péres, M. G. R. Reiter and C. K. d. Souza, Chem. Eng. Trans., 2019, 75, 253–258 Search PubMed.
- L. K. Ncube, A. U. Ude, E. N. Ogunmuyiwa, R. Zulkifli and I. N. Beas, Materials, 2020, 13, 13214994 CrossRef PubMed.
- C. Cui, N. Ji, Y. Wang, L. Xiong and Q. Sun, Trends Food Sci. Technol., 2021, 116, 854–869 CrossRef CAS.
- S. K. Mary, R. R. Koshy, R. Arunima, S. Thomas and L. A. Pothen, Carbohydr. Polym. Technol. Appl., 2022, 3, 100190 CAS.
- S. Zhao, R. Jia, J. Yang, L. Dai, N. Ji, L. Xiong and Q. Sun, Int. J. Biol. Macromol., 2022, 205, 419–429 CrossRef CAS PubMed.
- L. M. Fonseca, F. T. d. Silva, G. P. Bruni, C. D. Borges, E. d. R. Zavareze and A. R. G. Dias, Int. J. Biol. Macromol., 2021, 169, 362–370 CrossRef CAS PubMed.
- K. Gul, R.-Y. Gan, C.-X. Sun, G. Jiao, D.-T. Wu, H.-B. Li, A. Kenaan, H. Corke and Y.-P. Fang, Crit. Rev. Food Sci. Nutr., 2022, 62, 3817–3832 CrossRef CAS PubMed.
- Q. Zheng, Y. Tian, F. Ye, Y. Zhou and G. Zhao, Trends Food Sci. Technol., 2020, 99, 608–620 CrossRef CAS.
- S. Zhao, W. J. Malfait, N. Guerrero-Alburquerque, M. M. Koebel and G. Nyström, Angew. Chem., Int. Ed., 2018, 57, 7580–7608 CrossRef CAS PubMed.
- F. T. da Silva, J. P. de Oliveira, L. M. Fonseca, G. P. Bruni, E. da Rosa Zavareze and A. R. G. Dias, Int. J. Biol. Macromol., 2020, 155, 6–13 CrossRef PubMed.
- D. Seung, New Phytol., 2020, 228, 1490–1504 CrossRef CAS PubMed.
- K. Bashir and M. Aggarwal, J. Food Sci. Technol., 2019, 56, 513–523 CrossRef PubMed.
- D. D. Wang, X. Q. Zheng, W. H. Liu, Q. J. Sun, H. H. Chen and H. Y. Mu, Food Chem., 2023, 407, 135141 CrossRef CAS PubMed.
- Q. Liu, L. Gao, Y. Qin, N. Ji, L. Dai, L. Xiong and Q. J. Sun, Food Packag. Shelf Life, 2023, 35, 101032 CrossRef CAS.
- H. Y. Luo, F. P. Dong, Q. Wang, Y. H. Li and Y. Z. Xiong, Molecules, 2021, 26, 3999 CrossRef CAS PubMed.
- W. S. Ratnayake and D. S. Jackson, Adv. Food Nutr. Res., 2008, 55, 221–268 Search PubMed.
- R. Thakur, P. Pristijono, C. J. Scarlett, M. Bowyer, S. P. Singh and Q. V. Vuong, Int. J. Biol. Macromol., 2019, 132, 1079–1089 CrossRef CAS PubMed.
- D. Donmez, L. Pinho, B. Patel, P. Desam and O. H. Campanella, Curr. Opin. Food Sci., 2021, 39, 103–109 CrossRef CAS.
- Z. Chen, Y. Yao, H. Pu, J. Huang and H. Zhong, Int. J. Food Sci. Technol., 2023, 58, 2189–2198 CrossRef CAS.
- P. Owczarz, M. Orczykowska, A. Ryl and P. Ziolkowski, Food Chem., 2019, 271, 94–101 CrossRef CAS PubMed.
- C. Cagnin, B. M. Simoes, F. Yamashita, A. C. Andrello, G. M. de Carvalho and M. V. E. Grossmann, J. Appl. Polym. Sci., 2021, 138, 50194 CrossRef CAS.
- M. L. Zhai, F. Yoshii and T. Kume, Carbohydr. Polym., 2003, 52, 311–317 CrossRef CAS.
- M. M. Ghobashy, H. Abd El-Wahab, M. A. Ismail, A. M. Naser, F. Abdelhai, B. K. El-Damhougy, N. Nady, A. S. Meganid and S. A. Alkhursani, Mater. Sci. Eng., B, 2020, 260, 114645 CrossRef CAS.
- C.-Y. Su, T. Xia, D. Li, L.-J. Wang and Y. Wang, Crit. Rev. Food Sci. Nutr, 2023, 1–19 CrossRef PubMed.
- Y. Ai and J. L. Jane, Starch/Staerke, 2015, 67, 213–224 CrossRef CAS.
- J. M. Dodda, M. G. Azar, P. Belsky, M. Slouf, A. Broz, L. Bacáková, J. Kadlec and T. Remis, Cellulose, 2022, 29, 6697–6717 CrossRef CAS.
- Y. Li, S. Kadam, T. Abee, T. M. Slaghek, J. W. Timmermans, M. A. C. Stuart, W. Norde and M. J. Kleijn, Food Hydrocolloids, 2012, 28, 28–35 CrossRef CAS.
- H. Peidayesh, Z. Ahmadi, H. A. Khonakdar, M. Abdouss and I. Chodák, Polym. Adv. Technol., 2020, 31, 1256–1269 CrossRef CAS.
- B. M. Simoes, C. Cagnin, F. Yamashita, J. B. Olivato, P. S. Garcia, S. M. de Oliveira and M. V. Eiras Grossmann, LWT–Food Sci. Technol., 2020, 125, 108950 CrossRef CAS.
- Q. Liu, N. Ji, L. Xiong and Q. Sun, Carbohydr. Polym., 2020, 246, 116586 CrossRef CAS PubMed.
- J. S. dos Santos, B. Biduski, R. Colussi, V. Z. Pinto and L. R. dos Santos, Food Res. Int., 2023, 173, 113243 CrossRef CAS PubMed.
- D. Larrea-Wachtendorff, G. Tabilo-Munizaga and G. Ferrari, Polymers, 2019, 11, 1673 CrossRef CAS PubMed.
- M. Shahbazi, M. Majzoobi and A. Farahnaky, J. Food Eng., 2018, 223, 10–21 CrossRef CAS.
- W. Liu, W. Pan, J. Li, Y. Chen, Q. Yu, L. Rong, W. Xiao, H. Wen and J. Xie, Curr. Res. Food Sci., 2022, 5, 28–33 CrossRef CAS PubMed.
- B. C. Maniglia, D. C. Lima, M. D. Matta, P. Le-Bail, A. Le-Bail and P. E. D. Augusto, Food Res. Int., 2020, 128, 108803 CrossRef CAS PubMed.
- S. P. Bangar, M. Nehra, A. K. Siroha, M. Petru, R. A. Ilyas, U. Devi and P. Devi, Foods, 2021, 10, 1609 CrossRef PubMed.
- H. Abral, A. Atmajaya, M. Mahardika, F. Hafizulhaq, Kadriadi, D. Handayani, S. M. Sapuan and R. A. Ilyas, J. Mater. Res. Technol., 2020, 9, 2477–2486 CrossRef CAS.
- S. Singh, V. S. Sharanagat, S. Desai, K. Kumar, S. Upadhyay and G. Chakraborty, Starch/Staerke, 2023, 75, 2200229 CrossRef CAS.
- Z. Guo, Q. Gou, L. Yang, Q.-l. Yu and L. Han, Food Chem., 2022, 370, 130992 CrossRef CAS PubMed.
- B. S. Teixeira and N. L. del Mastro, Radiat. Phys. Chem., 2023, 213, 111234 CrossRef CAS.
- K. A. Ciesla and A. Abramowska, Nukleonika, 2021, 66, 3–9 CrossRef CAS.
- S. R. Kanatt, Radiat. Phys. Chem., 2020, 173, 108873 CrossRef CAS.
- X. Zhou, X. Ye, J. He, R. Wang and Z. Jin, Int. J. Biol. Macromol., 2020, 151, 239–246 CrossRef CAS PubMed.
- Z. P. Qiu, B. Zheng, J. C. Xu, J. Chen and L. Chen, Carbohydr. Polym., 2022, 292, 119686 CrossRef CAS PubMed.
- S. F. Chin, A. N. B. Romainor, S. C. Pang and S. Lihan, J. Drug Delivery Sci. Technol., 2019, 54, 101239 CrossRef CAS.
- R. Meng, Z. Z. Wu, H. Q. Xie, G. X. Xu, J. S. Cheng and B. Zhang, Int. J. Biol. Macromol., 2020, 155, 1–5 CrossRef CAS PubMed.
- J. Kobryn, T. Zieba, S. K. Sowa and W. Musial, Pharmaceutics, 2020, 12, 84 CrossRef CAS PubMed.
- D. C. Lima, N. Castanha, B. C. Maniglia, M. D. M. Junior, C. I. A. La Fuente and P. E. D. Augusto, Ozone Sci. Eng., 2021, 43, 60–77 CrossRef CAS.
- B. C. Maniglia, D. C. Lima, M. D. Matta, P. Le-Bail, A. Le-Bail and P. E. D. Augusto, Int. J. Biol. Macromol., 2019, 138, 1087–1097 CrossRef CAS PubMed.
- Y. Benavent-Gil, L. Román, M. Gómez and C. M. Rosell, Starch/Staerke, 2019, 71, 1800171 CrossRef.
- M. Rosseto, C. V. Toniciolli Rigueto, D. D. C. Krein, L. A. Massuda, B. E. Pessini Ostwald, L. M. Colla and A. Dettmer, J. Polym. Environ., 2021, 29, 1063–1075 CrossRef CAS.
- M. C. G. Pellá, A. R. Simao, M. R. Mauricio, R. A. Estrada, G. M. Pereira, R. da Silva and A. F. Rubira, Carbohydr. Polym., 2023, 307, 120614 CrossRef PubMed.
- K. C. G. Silva, A. I. Bourbon, L. Pastrana and A. C. K. Sato, Food Res. Int., 2021, 141, 110059 CrossRef CAS PubMed.
- L. Amaral, F. Rodrigues, A. Silva, P. Costa, C. Delerue-Matos and E. F. Vieira, Food Chem., 2022, 396, 133609 CrossRef CAS PubMed.
- Y. Ren, Z. Wu, M. Shen, L. Rong, W. Liu, W. Xiao and J. Xie, LWT–Food Sci. Technol., 2021, 140, 110679 CrossRef CAS.
- C. X. Jiang, T. T. Liu, S. Y. Wang, Y. F. Zou, J. J. Cao, C. X. Wang, C. Z. Hang and L. F. Jin, Int. J. Biol. Macromol., 2023, 235, 123698 CrossRef CAS PubMed.
- B. S. Bitencourt, J. S. Guedes, A. Saliba, A. G. O. Sartori, L. C. R. Torres, J. Amaral, S. M. Alencar, B. C. Maniglia and P. E. D. Augusto, Food Res. Int., 2023, 170, 113010 CrossRef CAS PubMed.
- S. Tavakoli, M. Kharaziha, S. Nemati and A. Kalateh, Carbohydr. Polym., 2021, 251, 117013 CrossRef CAS PubMed.
- E. Motamedi, B. Motesharezedeh, A. Shirinfekr and S. M. Samar, J. Environ. Chem. Eng., 2020, 8, 103583 CrossRef CAS.
- R. C. Sabadini, M. Fernandes, V. D. Bermudez, A. Pawlicka and M. M. Silva, J. Appl. Polym. Sci., 2022, 139, 52998 CrossRef.
- S. D. Chaudhuri, A. Mandal, A. Dey and D. Chakrabarty, Appl. Clay Sci., 2020, 185, 105405 CrossRef.
- C. Y. Su, D. Li, L. J. Wang and Y. Wang, Chem. Eng. J., 2023, 455, 140653 CrossRef CAS.
- E. Sharmin, M. T. Kafyah, A. A. Alzaydi, A. A. Fatani, F. A. Hazazzi, S. K. Babgi, N. M. Alqarhi, A. A. H. Sindi, D. Akram, M. Alam, J. Alam, L. A. Al-Madboly, N. A. Shoeib, A. M. Alqahtani and M. Mojally, Int. J. Biol. Macromol., 2020, 163, 2236–2247 CrossRef CAS PubMed.
- J. Chen, H. Li, C. Fang, Y. Cheng, T. Tan and H. Han, Polym. Compos., 2020, 41, 838–847 CrossRef CAS.
- J. O. D. Malafatti, M. A. Domingues, M. R. Meirelles, L. G. S. Peres, J. D. Bresolin and E. C. Paris, J. Appl. Polym. Sci., 2023, 140, e54290 CrossRef CAS.
- Y. Qin, D. Yun, F. Xu, D. Chen, J. Kan and J. Liu, Food Hydrocolloids, 2021, 119, 106850 CrossRef CAS.
- Y. Liu, M. Liu, L. Zhang, W. Cao, H. Wang, G. Chen and S. Wang, Food Hydrocolloids, 2022, 130, 107690 CrossRef CAS.
- Y. Zhou, X. Wu, J. Chen and J. He, Int. J. Biol. Macromol., 2021, 184, 574–583 CrossRef CAS PubMed.
- A. G. de Souza, N. M. Agostinho dos Santos, R. F. da Silva Torin and D. d. S. Rosa, Int. J. Biol. Macromol., 2020, 164, 1737–1747 CrossRef CAS PubMed.
- S. Li, Y. Ma, T. Ji, D. E. Sameen, S. Ahmed, W. Qin, J. Dai, S. Li and Y. Liu, Carbohydr. Polym., 2020, 248, 116805 CrossRef CAS PubMed.
- T. T. Koev, J. C. Muñoz-García, D. Iuga, Y. Z. Khimyak and F. J. Warren, Carbohydr. Polym., 2020, 249, 116834 CrossRef CAS PubMed.
- Y. K. Zhang, J. J. Xue, D. P. Li, H. Y. Li, Z. H. Huang, Y. W. Huang, C. J. Gong, S. J. Long and X. F. Li, Polym. Test., 2021, 93, 106976 CrossRef CAS.
- M. Sapper, P. Wilcaso, M. P. Santamarina, J. Rosello and A. Chiralt, Food Control, 2018, 92, 505–515 CrossRef CAS.
- I. Sifuentes-Nieves, R. Yanez-Macias, P. C. Flores-Silva, P. Gonzalez-Morones, C. A. Gallardo-Vega, E. Ramirez-Vargas and E. Hernandez-Hernandez, J. Polym. Environ., 2023, 31, 595–607 CrossRef CAS.
- M. Mohammadi, S. Mirabzadeh, R. Shahvalizadeh and H. Hamishehkar, Int. J. Biol. Macromol., 2020, 149, 11–20 CrossRef CAS PubMed.
- J. A. Diaz-Baca and P. Fatehi, Carbohydr. Polym., 2023, 313, 120846 CrossRef CAS PubMed.
- R. Lin, R. Xu, H. Chen, B. Liu, C. Yuan, L. Guo, B. Cui and Y. Fang, Carbohydr. Polym., 2023, 316, 121044 CrossRef CAS PubMed.
- M. Klein and E. Poverenov, J. Sci. Food Agric., 2020, 100, 2337–2340 CrossRef CAS PubMed.
- R. A. Batista, P. J. P. Espitia, J. d. S. S. Quintans, M. M. Freitas, M. Â. Cerqueira, J. A. Teixeira and J. C. Cardoso, Carbohydr. Polym., 2019, 205, 106–116 CrossRef CAS PubMed.
- C. G. Otoni, P. J. P. Espitia, R. J. Avena-Bustillos and T. H. McHugh, Food Res. Int., 2016, 83, 60–73 CrossRef CAS.
- S. Nandi and P. Guha, Food Chem., 2024, 431, 137103 CrossRef CAS PubMed.
- L. Ma, T. Long, S. Yuan, P. Qi, L. Han and J. Hao, J. Colloid Interface Sci., 2023, 647, 32–42 CrossRef CAS PubMed.
- W.-F. Lai and W.-T. Wong, Membranes, 2022, 12, 437 CrossRef CAS PubMed.
- H. Chen, M. Alee, Y. Chen, Y. Zhou, M. Yang, A. Ali, H. Liu, L. Chen and L. Yu, Foods, 2021, 10, 3105 CrossRef CAS PubMed.
- Q. Xie, G. Liu and Y. Zhang, Crit. Rev. Food Sci. Nutr., 2022, 2153794 Search PubMed.
- Z. E. Leaw, I. Kong and L. P. Pui, J. Food Process. Preserv., 2021, 45, e15752 CAS.
- M. Yildirim-Yalcin, H. Sadikoglu and M. Seker, LWT–Food Sci. Technol., 2021, 142, 111012 CrossRef CAS.
- K. C. G. Silva, G. Feltre, M. D. Hubinger and A. C. K. Sato, J. Food Eng., 2021, 290, 110205 CrossRef.
- T. Mala and A. K. Anal, Front. Bioeng. Biotechnol., 2021, 9, 757176 CrossRef PubMed.
- P. Davoodi-Monfared, S. Akbari-Birgani, S. Mohammadi, F. Kazemi, N. Nikfarjam, M. Nikbakht and S. A. Mousavi, J. Cell. Physiol., 2021, 236, 4066–4075 CrossRef CAS PubMed.
- Y. Wang, Y. Su, W. Wang, Y. Fang, S. B. Riffat and F. Jiang, Carbohydr. Polym., 2019, 226, 115242 CrossRef CAS PubMed.
- I. Ekaette and M. D. A. Saldana, Starch/Staerke, 2021, 73, 2000099 CrossRef CAS.
- J. Le Thanh-Blicharz, J. Lewandowicz, Z. Malyszek, H. M. Baranowska and P. L. Kowalczewski, Gels, 2022, 8, 720 CrossRef CAS PubMed.
- S. Ahmadzadeh and A. Ubeyitogullari, Carbohydr. Polym., 2023, 301, 120296 CrossRef CAS PubMed.
- J. Xie and S. Ding, Int. J. Biol. Macromol., 2023, 226, 102–110 CrossRef CAS PubMed.
- M. Kubicka, M. Bakierska, K. Chudzik and M. Molenda, Nanomaterials, 2020, 10, 1811 CrossRef CAS PubMed.
- K. Wu, Y. Fang, H. Wu, Y. Wan, H. Qian, F. Jiang and S. Chen, Int. J. Biol. Macromol., 2021, 166, 1499–1507 CrossRef CAS PubMed.
- V. Santos-Rosales, I. Ardao, C. Alvarez-Lorenzo, N. Ribeiro, A. L. Oliveira and C. A. Garcia-Gonzalez, Molecules, 2019, 24, 871 CrossRef PubMed.
- Y. Chen, G. Dai and Q. Gao, ACS Sustainable Chem. Eng., 2019, 7, 14064–14073 CrossRef CAS.
- S. Mottola, G. Iannone, M. Giordano, A. Gonzalez-Garcinuno, A. Jimenez, A. Tabernero, E. Martin Del Valle and I. De Marco, Int. J. Biol. Macromol., 2023, 253, 127406 CrossRef CAS PubMed.
- E. J. Santos Araujo, E. Scopel, C. A. Rezende and J. Martinez, J. Food Eng., 2023, 343, 111394 CrossRef.
- S. S. Mirmoeini, S. H. Hosseini, A. L. Javid, M. E. Koutamehr, H. Sharafi, R. Molaei and M. Moradi, Int. J. Biol. Macromol., 2023, 244, 125356 CrossRef CAS PubMed.
- J. Yang, X. Zhang, L. Chen, X. Zhou, X. Fan, Y. Hu, X. Niu, X. Xu, G. Zhou, N. Ullah and X. Feng, Int. J. Biol. Macromol., 2022, 213, 621–630 CrossRef CAS PubMed.
- C. A. Garcia-Gonzalez, J. J. Uy, M. Alnaief and I. Smirnova, Carbohydr. Polym., 2012, 88, 1378–1386 CrossRef CAS.
- A. Ubeyitogullari, S. Brahma, D. J. Rose and O. N. Ciftci, J. Agric. Food Chem., 2018, 66, 9490–9497 CrossRef CAS PubMed.
- F. Zou and T. Budtova, ACS Sustainable Chem. Eng., 2023, 11, 5617–5625 CrossRef CAS.
- J. Li, Y. Wang, L. Zhang, Z. Xu, H. Dai and W. Wu, ACS Sustainable Chem. Eng., 2019, 7, 6381–6389 CrossRef CAS.
- X. Ni, F. Ke, M. Xiao, K. Wu, Y. Kuang, H. Corke and F. Jiang, Int. J. Biol. Macromol., 2016, 92, 1130–1135 CrossRef CAS PubMed.
- W. Wang, Y. Fang, X. Ni, K. Wu, Y. Wang, F. Jiang and S. B. Riffat, Carbohydr. Polym., 2019, 224, 115129 CrossRef CAS PubMed.
- F. Alavi and O. N. Ciftci, Food Hydrocolloids, 2023, 141, 108637 CrossRef CAS.
- M. Soleimanpour, A. M. Tamaddon, M. Kadivar, S. S. Abolmaali and H. Shekarchizadeh, Int. J. Biol. Macromol., 2020, 159, 1031–1047 CrossRef CAS PubMed.
- M. Villegas, A. L. Oliveira, R. C. Bazito and P. Vidinha, J. Supercrit. Fluids, 2019, 154, 104592 CrossRef CAS.
- M. Dogenski, H. J. Navarro-Díaz, J. V. de Oliveira and S. R. S. Ferreira, Food Hydrocolloids, 2020, 108, 106033 CrossRef CAS.
- F. Zou, J.-L. Bouvard, C. Pradille and T. Budtova, Eur. Polym. J., 2022, 176, 111403 CrossRef CAS.
- P. H. Camani, M. G. M. Goncalo, R. F. S. Barbosa and D. S. Rosa, J. Appl. Polym. Sci., 2021, 138, e50863 CrossRef.
- F. Zou and T. Budtova, Carbohydr. Polym., 2021, 255, 117344 CrossRef CAS PubMed.
- P. H. Camani, C. D. M. Dominic, D. F. Parra, H. F. Maltez and D. S. Rosa, Int. J. Biol. Macromol., 2023, 226, 628–645 CrossRef CAS PubMed.
- J. Le Thanh-Blicharz, J. Lewandowicz, Z. Malyszek, P. L. Kowalczewski, K. Walkowiak, L. Masewicz and H. M. Baranowska, Foods, 2021, 10, 2724 CrossRef CAS PubMed.
- Q. Luo, X. Huang, F. Gao, D. Li and M. Wu, Materials, 2019, 12, 1420 CrossRef CAS PubMed.
- F. Zou and T. Budtova, Carbohydr. Polym., 2021, 266, 118130 CrossRef CAS PubMed.
- H. Qian, J. Yang, B. Peng, F. Mi, W. Li, S. Yan, J. Wang and F. Jiang, Int. J. Low-Carbon Technol., 2023, 18, 273–282 CrossRef.
- A. Ubeyitogullari, R. Moreau, D. J. Rose and O. N. Ciftci, J. Food Sci., 2019, 84, 1812–1819 CrossRef CAS PubMed.
- P. Joshi, K. Gupta, P. Uniyal, A. Jana, A. Banerjee, N. Kumar, D. Ghosh, M. Srivastava, A. Ray and O. P. Khatri, Mater. Chem. Phys., 2023, 296, 127282 CrossRef CAS.
- Y. Zhang and W. Jiang, Trends Food Sci. Technol., 2023, 139, 104139 CrossRef CAS.
- P. Ezati, A. Khan, R. Priyadarshi, T. Bhattacharya, S. K. Tammina and J.-W. Rhim, Food Hydrocolloids, 2023, 142, 108771 CrossRef CAS.
- S. Dhua and P. Mishra, Int. J. Biol. Macromol., 2023, 242, 125102 CrossRef CAS PubMed.
- I. Karimi Sani, M. Masoudpour-Behabadi, M. Alizadeh Sani, H. Motalebinejad, A. S. M. Juma, A. Asdagh, H. Eghbaljoo, S. M. Khodaei, J.-W. Rhim and F. Mohammadi, Food Chem., 2023, 405, 134964 CrossRef CAS PubMed.
- L. Chen, X. Niu, X. Fan, Y. Liu, J. Yang, X. Xu, G. Zhou, B. Zhu, N. Ullah and X. Feng, Food Control, 2022, 136, 108644 CrossRef CAS.
- R. Insight, Consumer Readiness to Pay for Sustainable or Environmentally Friendly Products in India as of December 2023, Statista, 2024 Search PubMed.
- B. Market, Bioplastics Market (By Product: Biodegradable, Non-biodegradable; by Application: Packaging, Consumer Goods, Agriculture, Agriculture, Textile, Building & Construction, Others) – Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2023–2032, Precedence Research, 2023 Search PubMed.
- P. Nagrale, Starch-based Plastics Market Research Report Information by End-Use (Automotive and Transportation, Agriculture, Packaging, Consumer Goods and Others), by Type (Thermoplastic Starch and Starch Polymer Blends) and by Region (North America, Europe, Asia-Pacific, and Rest of the World) – Market Forecast till 2032, Market Research Future, 2021 Search PubMed.
- P. Nandi, Starch-based Packaging Market Research Report Information by Type (Starch Blended with PLA, Starch Blended with PHA, and Others), by Technology (Injection Molding, Blow Molding, Extrusion, and Others), by Application (Rigid Packaging, Flexible Packaging, Textile, Consumer Goods, Agriculture, Automotive, Building & Construction, Electronics, and Others), and by Region (North America, Europe, Asia-Pacific, and Rest of the World) – Market Forecast till 2032, Market Research Future, 2024 Search PubMed.
- S. X. Tan, A. Andriyana, H. C. Ong, S. Lim, Y. L. Pang and G. C. Ngoh, Polymers, 2022, 14, 664 CrossRef CAS PubMed.
- E. Zhang, W. Li, Y. Gao, C. Lei, H. Huang, J. Yang, H. Zhang and D. Li, ACS Appl. Bio Mater., 2020, 3, 5872–5879 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |