Open Access Article
Open Access ArticleModifications of physicochemical, functional, structural, and nutritional properties of a field bean protein isolate obtained using batch and continuous ultrasound systems
Bhakti Anand
Narale
 ab,
Addanki
Mounika
ab,
Addanki
Mounika
 ab and
Akalya
Shanmugam
ab and
Akalya
Shanmugam
 *ab
*ab
aFood Processing Business Incubation Centre, National Institute of Food Technology, Entrepreneurship and Management, Thanjavur, India. E-mail: akalya@iifpt.edu.in
bCentre of Excellence in Non-Thermal Processing, National Institute of Food Technology, Entrepreneurship and Management, Thanjavur, India
First published on 13th February 2024
Abstract
This study aimed to investigate the effect of ultrasound (US) treatment on improving the yield and the physicochemical, functional, structural, and nutritional properties of a protein isolate from germinated field bean flour at different conditions such as 100 and 200 W at 5, 15, 25, and 35 min. Field bean is an underutilized crop with more protein content and an alternative to animal protein, thus ensuring global food security, responsible consumption, and the well-being of consumers, which is in line with the sustainable development goals (SDGs) 2, 3, 7, 12, and 13. US treatment at 25 min and 200 W gave the best result for all the properties. Upon 25 min of sonication at 200 W, there was an increase in the protein yield, foaming ability, foam stability, emulsion activity index, emulsion stability, solubility, ζ potential, and in vitro protein digestibility from 34.33% to 59.15%, 73.11% to 110.12%, 81.44% to 90.43%, 6.92 to 13.47 m2 g−1, 59.97 to 104.74 min, 56.29% to 73.82%, −9.92 mV to −17.5 mV, and 94.47% to 96.37%, respectively. Moreover, a decrease in the size of particles from 1766 nm to 294.1 nm in comparison to untreated samples using green technology helps in achieving clean energy and decreases extraction time. Water holding capacity and oil holding capacity increased by 52.3% and 51.8%, respectively, after 15 min of US at 200 W. The change in the microstructure of proteins because of the US treatment was analysed using SEM. FTIR analysis confirmed the changes in the secondary structures of proteins. The physical changes caused by acoustic cavitation resulted in the partial denaturation of proteins, which was shown by an increase in their surface hydrophobicity and, thus, functionalities. Outcomes of this work demonstrated that US-assisted protein extraction increased yield and adjusted characteristics to meet the needs of the food sector, indicating a possibility for industrial use and contributed to the accomplishment of SDGs 2, 3, 7, 12, and 13.
Sustainability spotlightThis research compares the advantages of ultrasonic-assisted protein extraction to those of traditional techniques and discusses the significance of plant protein. Field beans are underutilized crops that are used as a source of protein.According to this study, ultrasound improved the functionalities, yield, extraction time and other characteristics of proteins. Germinated field bean flour was ultrasonically treated at 100 and 200 W for 5, 15, 25, and 35 minutes. All the characteristics and yield of protein increased after 25 minutes with an ultrasonic power of 200 W. Owing to their high protein content, beans can help achieve SDG 3. Additionally, they may flourish in areas that are prone to drought, which will help to achieve SDG 2. Impact on environment supporting SDGs 7 and 13 is reduced by ultrasound. |
1. Introduction
The demand for proteins is expected to double by the year 2050 to meet the needs of the increasing population.1 Dietary proteins are important for human health, survival, and reproduction.2 A vast array of plant- and animal-based proteins are used in the food and pharmaceutical industries due to their capacity to improve viscosity, foam, emulsify, gel, and encapsulate qualities.3 Consumers as well as researchers are showing interest in plant protein because of the dietary and religious restrictions on animal protein.4 Plant proteins have several advantages over animal proteins, such as being more affordable, adaptable, productive, nutritious, environmentally sustainable, and highly stress tolerant as well as having a low carbon footprint,5 which helps them meet several sustainable development goals (SDGs). Field bean crops can fix nitrogen content in soil, thus mitigating climate change and helping in achieving SDG 13. All these factors are crucial in achieving SDG 2 to enhance nutritional aspects and support sustainable farming. There are 17 SDGs set by the United Nations (UN) members to eradicate poverty and build a society that is equitable, prosperous, and secure for people and the planet. When compared with the proteins obtained from animal and dairy sources, legume proteins have more technical and bio-functional applications.Field bean, also known as Dolichos bean, hyacinth bean, or Sem or Indian bean, with the scientific name Dolichos lablab, is an important leguminous vegetable. It is cultivated in the tropical regions of Africa, Asia, and, America. The field bean (FB) plant is believed to have its origin in India.6 There are three varieties available in India based on the seed coat, namely, brown, cream, and white with Indian names as Vaal, Pawta, and Rangoon vaal, respectively.7 FB has the potential to grow in saline soil, adapt to acidic conditions, and thrive in drought circumstances. It is a prominent forage and food crop in tropical lowland regions due to its tolerance to warm weather and drought resilience, which supports SDG 3 and achieves SDG 2, allowing it to reach SDG 12, 13. Being a cheap and rich source of protein, it is known as ‘poor man's protein’.8 The National Academy of Science (NAS), India classified lablab beans as a potential source of protein.6 Aside from being a good source of protein, it also offers a lot of complex carbs, minerals, and fibre as well as a low-fat level, which helps to meet SDG 2.9 Also, FB is a promising dietary supplement alternative to prevent infection with COVID-19.10 Despite having these many qualities, it is an underutilized crop.11 Thus, FB poses a good research opportunity, especially for proteins. Very few studies12,13 are available on the yield of proteins and process modifications to the proteins of FB by conventional and few modern methods.14 However, research was absent in the following categories, including a study on Indian seed variations and specific information such as bean type. There is a lack of studies on pretreatment procedures for protein extraction from FBs, including soaking, heating, germination roasting, and process-induced changes.15 During extraction, processing tools can modify the proteins both in positive and negative ways. The process can be tuned to enhance the functional properties, leading to an increase in its application in the development of novel foods. Pretreatments enhance the protein digestibility, nutritional profile, and organoleptic properties of the cereals, e.g., germination.16 Pretreatments applied before the extraction of protein may also alter the constituents along with an improvement in physicochemical parameters of proteins such as an increase in protein yield from rice by soaking in dilute sulphuric acid.17 In a study, various legumes such as mung bean, chickpea, and lentils were germinated under a controlled environment and proteins were isolated; it was found that there was an improvement in the protein yield after gemination.18 In another study, it was mentioned that using pretreatment methods on pulses helps improve protein digestibility and decrease antinutrients.19 The extraction of proteins was enhanced by the pretreatment of hydrothermal cooking accompanied with the action of amylase on rice bran.20 In a study, desi chickpeas were used for the extraction of protein using soaked, germinated, boiled, and raw chickpeas. Among all these trials, germinated chickpea flour showed greater yield for the isolation of protein.21 In a study related to field bean protein isolate, germination was selected as the pre-treatment and the germinated field bean flour was used to extract protein, which improved the protein yield. However, no significant change in the color of the isolate compared to the raw sample was observed, and along with protein increase, there is a decrease in the fat and moisture percentage, which makes it suitable for storage for a longer period.22
Conventional extraction methods for protein include chemical extraction methods, namely, alkali-based protein extraction and organic solvent-based protein extraction. These methods have some constraints like energy-intensive, time-consuming, and are not environment friendly as there is the involvement of chemical solvents, which is against SDG 7.23 have reported the possibilities of undesirable reactions like a decrease in digestibility, lysinoalanine formation, loss of amino acids, and racemization of amino acids. The proteins extracted using conventional extraction technique have inferior quality with low yield.24 Nowadays, the trend is moving towards using environment-friendly, novel non-thermal technologies like microwaves, ultrasound (US), and pulsed electric fields for the extraction of proteins. Researchers are also showing more interest in it because of its advantages over conventional extraction techniques. These techniques not only improve the protein extraction yield but also help in maintaining the protein quality by less degradation. Among other techniques, the US technique remains promising as it has the following advantages over others such as better retention of nutrients in food, providing high purity levels of the final product, low cost, energy, and time.25 Though FB has been studied by,12–14 the ultrasound-assisted extraction (UAE) of germinated FB proteins and the study on its process-induced modification to physicochemical, functional, structural, and quality of protein is not found elsewhere in the literature.
Cavitation is responsible for the generation and implosion of bubbles and produces high shear forces and temperature in the medium.26 Inertial cavitation is formed, which leads to the production of abundant energy because of sonication. The basic principle behind US is ‘Acoustic cavitation’.27 Physical forces like shear forces, microjets, mechanical agitation, hot spots, shockwaves, and microstreaming are found to be efficacious in extraction and emulsification.28 Low-frequency power US has a disruptive effect on the physical, chemical, and biological properties of food. It has applications like activation and inactivation of enzymes, crystallization, defoaming, degassing, dewatering, emulsification, extraction, extrusion, low-temperature pasteurization, particle size reduction, and viscosity alteration, which can be used commercially at a large scale. It is also used in the protein modification and extraction of bioactive compounds.29,30 The cell walls are ruptured by ultrasonic implosion and cavitation, which improves mass transfer from the solid to liquid phase. Microchannels are also produced within the tissues using ultrasonic treatment, which improves solvent penetration into the solid matrix and hence boosts mass transfer. UAE is a viable alternative that improves yields while overcoming the shortcomings of conventional extraction methods and so boosting sustainable energy, hence favoring SDG 7.25 In a study, ultrasound was used for the isolation of protein from germinated chickpea flour, which resulted in increased yield, digestibility, functional, characteristics properties, and a decrease in particle size over conventional methods.21 In another study, the extraction of protein isolates from rice dreg flour was done by ultrasound, which showed an increase in the yield of protein from 44 to 88%. After the extraction process under controlled conditions, it also improved the hydrophobic amino acids content and porosity, and a change in the structure was observed via SEM and FTIR analysis, which indicates that ultrasound can change or alter the microstructure of the protein to improve its quality.31 The UAE of proteins has been studied for different varieties of pulses and legumes such as black bean,32 pea,33 chickpea,34 faba bean,35 kidney bean, and soybean.36 These studies have reported an increase in protein yield as well as an improvement in protein quality. Plant proteins generally have low nutritional value, and poor digestibility; with the help of pre-treatments and ultrasound techniques, these limitations can be overcome.37 These changes help improve the quality of proteins that are suitable in the food to incorporate in sports drinks, ice creams, yogurt, beverages, etc. To prove that proteins plays a major role in improving the quality of food, a study done by (ref. 38) shows that proteins present in the ultrasound-treated chickpea milk helps in attaining the stability of milk with lower sedimentation value. Additionally, in a related study, the mayonnaise prepared from chickpeas and green gram extract had higher viscosity; this is because the proteins in the legumes had stronger emulsifying qualities that improved the quality of the product and also had better sensory characteristics.39,40 However, the UAE of proteins from germinated FB and also FB of Indian variety (pale brown) has not been explored yet. Therefore, this work aims to study the effect of germination on the protein yield of FB and the effect of UAE on the structural and functional properties of germinated field bean protein (FBP).
2. Materials and methods
2.1. Raw materials
Dried FBs were bought from Thanjavur, Tamil Nadu, from a local market. All of the chemicals and reagents utilized were of the analytical grade and purchased from HiMedia Laboratories Pvt. Ltd. and Thermo Fisher Scientific India Pvt. Ltd.2.2. Preparation of field bean flour
FBs were cleaned with water and soaked for 12 h. The soaked beans were kept for germination for 24 h at room temperature and dried in a tray drier at 45–50 °C till a constant moisture content was achieved. The flour was obtained by grinding the beans using a pulverizer and was stored at room temperature. A comparison study was done for the protein isolates obtained from flour of dried beans of raw, soaked, germinated, and boiled FBs. It was observed that the protein isolate from dried germinated FB flour had higher protein content than all others and hence was chosen for further analysis in this whole study.222.3. UAE of field bean protein isolate (FBPI)
The flour was defatted before using it for protein extraction purposes. Hexane was used as a solvent for defatting by the Soxhlet method. To minimize resource use, adopting a more pragmatic way to extract protein, and promote the usage of water, or SDG 6, we used a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio of flour to water. Before protein extraction, 10.0 g of defatted FBPI was dissolved in 100 mL of distilled water and kept in a refrigerator for lowering its temperature to 4 °C. The US probe sonicator with (14 mm probe diameter, 26 kHz frequency 200 W, lab man sonicator) at 200 W input power at 50% and 100% amplitude was used to assist protein extraction. US was applied in pulsation mode with 5 s ON and 5 s OFF. The US treatment times given were 5, 15, 25, and 35 min. The entire US extraction process was performed in a temperature-controlled water bath at 3–4 °C, and the volume of all the samples was kept constant. Distilled water was used as a liquid medium for improving the extraction of protein into the medium, and the solid-solvent ratio used was 1
10 ratio of flour to water. Before protein extraction, 10.0 g of defatted FBPI was dissolved in 100 mL of distilled water and kept in a refrigerator for lowering its temperature to 4 °C. The US probe sonicator with (14 mm probe diameter, 26 kHz frequency 200 W, lab man sonicator) at 200 W input power at 50% and 100% amplitude was used to assist protein extraction. US was applied in pulsation mode with 5 s ON and 5 s OFF. The US treatment times given were 5, 15, 25, and 35 min. The entire US extraction process was performed in a temperature-controlled water bath at 3–4 °C, and the volume of all the samples was kept constant. Distilled water was used as a liquid medium for improving the extraction of protein into the medium, and the solid-solvent ratio used was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. After giving US treatment at different amplitudes and process times, the pH value for the mixture of FB flour and deionized water was adjusted to 11 using 1 N NaOH. This mixture was then stirred using a magnetic stirrer for 2 h and then centrifuged in a refrigerated centrifuge at 4 °C for 20 min at 5000g. For the precipitation of proteins, the pH of the supernatant was adjusted to 4.5 after extraction. Precipitated protein was collected using a refrigerated centrifuge at 5000g for 20 min and washed using distilled water. Finally, FBPs were freeze-dried and stored at 4 °C for further analysis.27,33 FBPI extracted by traditional alkaline extraction process without the usage of US was used as a control. The US-assisted extracted field bean protein isolate is denoted as UFBPI while the untreated one (0 min of sonication time) is the control sample (C).
10. After giving US treatment at different amplitudes and process times, the pH value for the mixture of FB flour and deionized water was adjusted to 11 using 1 N NaOH. This mixture was then stirred using a magnetic stirrer for 2 h and then centrifuged in a refrigerated centrifuge at 4 °C for 20 min at 5000g. For the precipitation of proteins, the pH of the supernatant was adjusted to 4.5 after extraction. Precipitated protein was collected using a refrigerated centrifuge at 5000g for 20 min and washed using distilled water. Finally, FBPs were freeze-dried and stored at 4 °C for further analysis.27,33 FBPI extracted by traditional alkaline extraction process without the usage of US was used as a control. The US-assisted extracted field bean protein isolate is denoted as UFBPI while the untreated one (0 min of sonication time) is the control sample (C).
Similarly, the sample is processed in continuous US with 26 kHz frequency, 200 W input power, 7 mm probe diameter, pulsation mode with 5 s ON and 5 s OFF, and process temperature is maintained at 4 °C.
2.4. Extraction yield
The FBPI yield extraction was calculated using the Kjeldahl technique employing the equation below (1)42 | (1) |
2.5. Physicochemical analysis
2.6. Functional properties
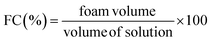 | (2) |
 | (3) |
 | (4) |
 | (5) |
 | (6) |
2.7. Structural properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g to measure the solubility of the supernatant using the biuret reagent, as described in Section 2.6.5 above. Phosphate buffer was then used to dilute the supernatant to 1, 0.5, 0.1, 0.05, and 0.01 mg mL−1, respectively. 8.0 mmol L−1 ANS solution was prepared by the addition of ANS in 0.01 mol L−1 pH−1 7 phosphate buffer. 100 μL of ANS solution was added to 10 mL of protein solution and kept in the dark for 15 min. Later, using a fluorescence spectrophotometer with a 348 nm excitation wavelength and a 511 nm emission wavelength, the absorbance was measured. Through the use of linear regression analysis, the initial slope of the protein concentration and fluorescence intensity was used to determine the Ho index.
000g to measure the solubility of the supernatant using the biuret reagent, as described in Section 2.6.5 above. Phosphate buffer was then used to dilute the supernatant to 1, 0.5, 0.1, 0.05, and 0.01 mg mL−1, respectively. 8.0 mmol L−1 ANS solution was prepared by the addition of ANS in 0.01 mol L−1 pH−1 7 phosphate buffer. 100 μL of ANS solution was added to 10 mL of protein solution and kept in the dark for 15 min. Later, using a fluorescence spectrophotometer with a 348 nm excitation wavelength and a 511 nm emission wavelength, the absorbance was measured. Through the use of linear regression analysis, the initial slope of the protein concentration and fluorescence intensity was used to determine the Ho index.
2.8. Nutritional aspects
2.9. Comparison between batch and continuous process of US system
The protein isolate extracted earlier in this study was obtained using a batch process. Similarly, protein isolate was obtained using the continuous process. The optimized sample, i.e., the UFBPI-25 min-200 W from the batch process, was chosen to compare against the continuous process (26 kHz frequency, 200 W input power, 7 mm probe diameter, pulsation mode with 5 s ON and 5 s OFF, process temperature maintained at 4 °C) using the same US system. The energy density for both the processes was equated, and the processing time required for the continuous process was calculated. Energy density (J mL−1) is a product of power density (W mL−1) and processing time (s) of the protein dispersion (eqn (7)). | (7) |
 ; “m” is the weight of the samples (g), Cp is the specific heat of the medium (4.18 kJ g−1 K−1), and ΔT/Δt is the rate of temperature change with respect to time (°C s−1).49 The efficiency of the system in batch and continuous processes was studied based on the extraction yield and functional properties of the FBPI obtained from both the processes. The FBPI extracted using a continuous process is denoted as CUFBPI.
; “m” is the weight of the samples (g), Cp is the specific heat of the medium (4.18 kJ g−1 K−1), and ΔT/Δt is the rate of temperature change with respect to time (°C s−1).49 The efficiency of the system in batch and continuous processes was studied based on the extraction yield and functional properties of the FBPI obtained from both the processes. The FBPI extracted using a continuous process is denoted as CUFBPI.
2.10. Statistical analysis
All experimental data were collected in triplicate. The findings were presented as mean ± standard deviation. The Minitab 18 program was used to evaluate the statistical data, which was then checked for significance at p < 0.05 using the analysis of variance (ANOVA).3. Results and discussion
3.1. Extraction yield
The US treatment was carried out in duration (0, 5, 15, 25, and 35 minutes) and amplitude (100% and 50%), i.e., with input powers of 200 W and 100 W, respectively (Fig. 1). With the increasing US treatment time, the extraction yield showed an increasing trend. The control sample showed an extraction yield of 33.64%. UAE at 100 W gave a maximum yield of 51.53% in 25 min, which decreased at 35 min of treatment to 50.24%. By increasing the US power from 100 to 200 W, the extraction yield was increased. Furthermore, by increasing the US power to 200 W, the maximum yield of 59.49% in 25 min was observed. The yield showed a decrease to 57.64% at a treatment time of 35 min. The US treatment improved the extraction yield by 76.84%. This trend of increase in the extraction yield, followed by a decrease with the increasing sonication time, was seen with the protein yield of the defatted pumpkin seed at 5 min.50 It was also seen that the extraction done at 100% amplitude was higher than that obtained at 50% amplitude. A similar rise in protein yield was shown in a study of defatted rice bran due to the increase in the sonication power.51 In the extraction process, intense cavitation is caused as a result of the high-power sonication, which is responsible for the increase in the yield.50 The increase in the extraction yield may be attributed to the mechanical vibrations due to US, which increased the contact area between FB flour and alkali solution added during the extraction process. Additionally, US-induced cavitation could disintegrate plant cell walls, sever molecular connections, accelerate mass transfer, and enhance the effectiveness of protein extraction.42 It decreased after a prolonged US treatment, which may be due to the denaturation of proteins that are soluble in the extract as the shear forces generated are also greater. Protein aggregates are formed because of the formation of the intermolecular disulfide bonds.50,52 The maximum yield (59.15%) was found with the combination having a treatment time of 25 min. Based on protein isolate extract time and yield, UAE is capable of extracting the protein effectively. Thus, using UAE instead of traditional extraction reduces the protein extraction time to 25 minutes. To promote SDG 7, the UAE will also be crucial towards the achievement of energy efficiency.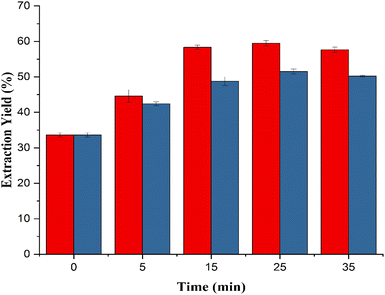 | ||
| Fig. 1 Extraction yield of the FBPI at 200 W and 100 W at sonication times of 0, 5, 15, 25, and 35 min. | ||
3.2. Physicochemical analysis
| Sample | Bulk density (g mL−1) | Tapped density (g mL−1) | L* | a* | b* | In vitro protein digestibility (%) |
|---|---|---|---|---|---|---|
| a C – control sample, A – 200 W input power, B – 100 W input power. | ||||||
| C | 0.28 ± 0.005c | 0.31 ± 0.01a,b | 64.95 ± 0.23a | 2.87 ± 0.12d | 12.44 ± 0.41e | 94.47 ± 0.68b |
| 5A | 0.32 ± 0.015a,b | 0.34 ± 0.01a,b | 61.19 ± 0.57b | 4.50 ± 0.07a,b,c | 15.71 ± 0.03a | — |
| 5B | 0.3 ± 0.01b,c | 0.32 ± 0.01a,b | 62.43 ± 0.29a,b | 4.45 ± 0.72a,b,c | 14.46 ± 0.14c | — |
| 15A | 0.23 ± 0.015d | 0.26 ± 0.015a,b | 56.41 ± 2.04c | 4.88 ± 0.14a,b | 14.66 ± 0.30b,c | — |
| 15B | 0.35 ± 0.01a | 0.32 ± 0.015a,b | 62.34 ± 0.47a,b | 3.96 ± 0.05c | 14.65 ± 0.12b,c | — |
| 25A | 0.32 ± 0.015d | 0.40 ± 0.015a | 55.30 ± 1.83c | 5.14 ± 0.20a | 15.50 ± 0.11a,b | 96.37 ± 0.38a |
| 25B | 0.31 ± 0.01b,c | 0.34 ± 0.020a,b | 54 ± 1.29c,d | 4.31 ± 0.19b,c | 14.01 ± 0.58c,d | — |
| 35A | 0.35 ± 0.015a | 0.23 ± 0.15b | 50.72 ± 1.89d | 4.88 ± 0.20a,b | 13.28 ± 0.36d,e | — |
| 35B | 0.32 ± 0.005a,b | 0.35 ± 0.015a,b | 54.32 ± 0.69c | 5.13 ± 0.07a | 15.68 ± 0.10a | — |
3.3. Functional properties
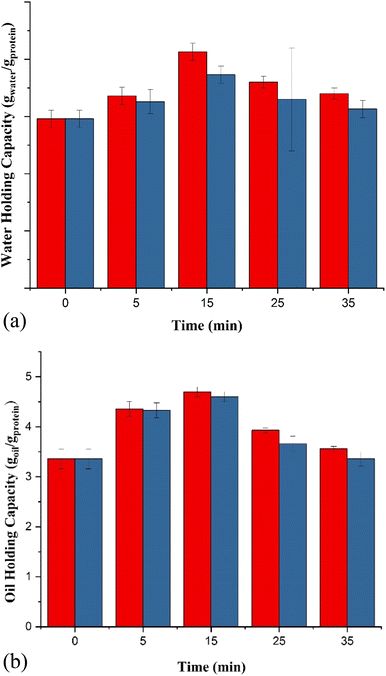
The most significant functional components in a food system are proteins due to their capacity for emulsification, foaming, and nutrition. Plant-based proteins offer a viable alternative to animal-based proteins for human nutritional needs because of their sustainable origin, low cost of production, accessibility, and health benefits (SDG 3).The OHC also showed a similar trend to WHC (Fig. 2b). The OHC for the control sample was 3.36 g g−1, and it increased after giving US treatment up to 15 min at 200 W power input. The OHC of the ultrasonically treated sample increased by 51.8% after treatment for 15 min at 100% amplitude. The OHC decreased on further increasing the treatment time. The increase in OHC may be due to the exposure of hydrophobic groups, which causes the interaction between oil and hydrophobic surface.14,59 The exposure of hydrophobic groups may be seen by the creation of massive protein aggregates in the dry state of the US-treated freeze-dried samples.60
A favorable modification in EAI and ESI was observed in the UFBPI when compared to the control FBPI sample. The EAI and ESI rose initially as the ultrasonic duration was extended, then declined, reaching their peak at 25 minutes. A significant increase (p ≤ 0.05) in EAI was recorded with a value of 16.37 m2 g−1 for a US treatment of 25 minutes at 200 W in comparison to the control (6.92 m2 g−1). The control sample showed an ESI of 60 minutes while the ESI increased up to 104 minutes for UFBPI after US treatment of 25 minutes at 200 W.
It is claimed that the shear forces of US boosted the stability of the protein solution by decreasing the protein particle size and increasing the protein-specific surface area.62 Additionally, the cavitation force brought by ultrasonic waves disrupted the non-covalent interactions that kept the protein spatial structure stable. The protein's hydrophobic group was exposed as a result, and more protein molecules moved to the water–oil interface, improving the protein's ability to emulsify. However, within 25 minutes, the threshold level of the protein's hydrophobic group exposure had been attained. Due to the proteins' molecular mobility and acceleration, hydrophobic interactions were disrupted during extended sonication, which accelerated the process of protein aggregation. Small molecules were quickly replaced by macromolecular proteins when adsorbed at the interface.63 EAI and ESI decreased as a result of flocculation and increased emulsion interfacial tension. The results were consistent with the reported findings.57 A similar observation is noted for UFBPI after 25 min of sonication, irrespective of the applied power. The partial denaturation of protein with an increase in the sonication time for up to 25 min in the 200 W-US processed sample is supported by the result on the increase in the surface hydrophobicity value and aggregation of the protein with prolonged sonication time after 25 min, i.e., the 35 min-200 W US processed sample was supported by the decrease in the surface hydrophobicity value in the later Section 3.3.5 and Fig. 8.
3.3. Structural properties
Table 2 depicts the particle size of FBPI proteins at 200 W US at different processing times. The particle size of the control sample was 1766 nm. The particle size decreases and the particle size distribution narrows as the ultrasonic treatment intensity rises. Up to 25 minutes of treatment time were required to see a decrease in the particle size with 294.1 nm being the lowest value. After prolonging the treatment to 35 minutes, an increase in the particle size was found. The free surface of the material rises when the particle size is decreased. The forces of cavitation in this instance lead the particles to decrease. Aggregates and agglomerates are also destroyed in this process. Aggregates, agglomerates, and even smaller particles may all be broken up by ultrasonic cavitation, which violates the van der Waals forces. Because it creates a strong enough negative pressure to produce bubble implosion and cavitation, sufficiently high-intensity US is responsible for creating friction and turbulence. As a result, it affects the treated material's surface.65 When the ultrasonic power was constant, as the ultrasonic duration increased, protein molecules interacted with one another due to intermolecular interactions, which led to the formation of new aggregates, as noted in the size of the US 200 W 35 min US processed sample.
| Sample at different sonication time at 200 W | Test parameters | Result |
|---|---|---|
| 0 | Particle size | 1766 nm |
| Zeta potential | −9.92 mV | |
| 5 | Particle size | 593.5 nm |
| Zeta potential | −14.5 mV | |
| 15 | Particle size | 471.9 nm |
| Zeta potential | −15.4 mV | |
| 25 | Particle size | 294.1 nm |
| Zeta potential | −17.5 mV | |
| 35 | Particle size | 369.0 nm |
| Zeta potential | −15.9 mV |
The absolute value of the ultrasonic time tends to initially increase and then decrease with increasing ultrasonic time (Table 2). These events demonstrated that the FBPI's degree of ionization was raised by ultrasonic treatment. This was most likely caused by the fact that following ultrasonic treatment, more negatively charged protein groups were exposed. However, as the sonication period and/or ultrasonic power were extended, its absolute value began to trend downward. The protein may reaggregate as a result of the cavitation effect of US, which would result in a drop in the effective charge on the protein surface.61 The expansion of the protein secondary structure occurred during prolonged sonication, exposing the non-polar hydrophobic residues, and the interactions between them to form aggregates may be the cause of the decline in the zeta potential for 35 min 200 W-US for UFBPI, as seen in the study.14 The partial denaturation of protein with an increase in sonication time for up to 25 min in the 200 W-US processed sample is supported by the increase in the surface hydrophobicity value and aggregation of the protein with prolonged sonication time after 25 min, i.e., the 35 min-200 W US processed sample is supported by the decrease in the surface hydrophobicity value in the later Section 3.3.5 and Fig. 8.
The amide I band, amide II band, and amide III band are the three groupings of distinctive absorption bands that make up the protein's FTIR spectrum, and their respective wavenumbers are 1600–1700 cm−1, 1530–1550 cm−1, and 1260–1300 cm−1, respectively. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, which is mostly centered at 1650 cm−1, is the primary cause of the amide I. The hydrogen bond interaction between molecules or within molecules can be reflected in the amide II. When the hydrogen connection between molecules or between molecules is broken, the spectrum band shifts to a high wavenumber.68 Among all amide bands, amide I is sensitive to the type and amount of secondary structures and is not strongly influenced by side chains.69 The amide I zone (1700–1600 cm−1) is mainly used for understanding the secondary structure of proteins because it consists of overlapping component bands that characterize the structures such as α-helix, β-sheet, and β-turn.70
O stretching vibration, which is mostly centered at 1650 cm−1, is the primary cause of the amide I. The hydrogen bond interaction between molecules or within molecules can be reflected in the amide II. When the hydrogen connection between molecules or between molecules is broken, the spectrum band shifts to a high wavenumber.68 Among all amide bands, amide I is sensitive to the type and amount of secondary structures and is not strongly influenced by side chains.69 The amide I zone (1700–1600 cm−1) is mainly used for understanding the secondary structure of proteins because it consists of overlapping component bands that characterize the structures such as α-helix, β-sheet, and β-turn.70
In the FT-IR analysis of FBPI, two sharp peaks were obtained for the control sample for the wavenumbers in the range of 1500–1650 cm−1. One peak was found at 1530 cm−1, which states the presence of N–O stretching. Another peak was found at 1633 cm−1, indicating the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and corresponding to the β-strand and β-sheet structures in the native pulse protein.70 FFBPI showed similar peaks, as seen in the control sample. The predominance of β-structures in the secondary structure also contributes to lowering the digestibility of pulse proteins. The β-sheet content of the control sample was higher than the US-treated samples. The β-sheet content decreased with increasing treatment time. The UFBPI at 25 min had the least β-sheet content, while a rise in the β-sheet content was seen when the treatment time was increased to 35 min.
C stretching and corresponding to the β-strand and β-sheet structures in the native pulse protein.70 FFBPI showed similar peaks, as seen in the control sample. The predominance of β-structures in the secondary structure also contributes to lowering the digestibility of pulse proteins. The β-sheet content of the control sample was higher than the US-treated samples. The β-sheet content decreased with increasing treatment time. The UFBPI at 25 min had the least β-sheet content, while a rise in the β-sheet content was seen when the treatment time was increased to 35 min.
Two small peaks were found in the wavenumber ranging from 1250–950 cm−1, one at 1232 cm−1 and the other at 1065 cm−1 in the control and UFBPI samples. The peak at wavenumber 1232 cm−1 depicts C–N stretching, indicating the presence of an amine group. A broad peak was found at 3280 cm−1 that corresponds to O–H stretching.
3.4. Nutritional aspects
3.5. Comparison between batch and continuous process of US system
Using a batch-type sonicator for 25 minutes at 200 W of 26 kHz input power, the optimal field bean protein isolate was generated based on previous findings. A sample that was jacketed with ice or water at a temperature of 3–4 °C was also taken into consideration for the continuous type. One circulation or a single pass takes 3 min for a volume of 600 mL sample in the continuous system operated at 200 W-26 kHz input US power and processed under controlled temperature using refrigerated circulation at 3–4 °C. Thus, the two systems were compared by equating their energy densities, and the processing time required for the continuous process was mathematically calculated for 150 min or 50 number of passes. The functional properties of field bean protein isolate were analyzed for a continuous system and compared against the optimized batch system for FBPI. Thus, in a continuous system, the extraction yield of the CUFBPI was 43.99%, which was lower than the extraction yield of UFBPI at 25 min-200 W (59.49%). The functional properties of the FBPI were compared to check the efficiency of the systems, i.e., batch and continuous processes. The obtained results are mentioned in Table 3. The WHC, OHC, FC, FS, EAI, ESI, and solubility values obtained for CUFBPI are 3.133 (gwater/gprotein), 3.367 (goil/gprotein), 81.52%, 84%, 10.44 (m2 g−1), 79.68 min, and 60.04%, respectively. It was observed that lower values were obtained compared to the batch-type isolation of UFBPI at 25 min-200 W for all the functional properties analyzed. The WHC and OHC values for CUFBPI were comparable with the values of UFBPI 35 min-100 W (Fig. 2a, b, 3a and b) in magnitude. Also, the result for FC, FS, EAI, and ESI was comparable with the result obtained for UFBPI 15 min-100 W for the same properties (Fig. 4–7). The surface hydrophobicity of CUFBPI was found to be 263.35, which is less than that for UFBPI-25 min (689.32) at the equivalent energy density. This explains the reason for the lower values of functional properties for CUFBPI than UFBPI-25 min, i.e., the less exposure of hydrophobic groups. Hence, it can be concluded that the continuous system at the same power and energy density is less efficient than the batch system. A continuous system with higher input power and intensity operated at the same frequency may provide an equivalent or higher effect in terms of functionality and yield of protein.72| Functional properties | US-25 min-200 W | CUFBPI |
|---|---|---|
| WHC (gwater/gprotein) | 3.6 ± 0.1 | 3.133 ± 0.15 |
| OHC (goil/gprotein) | 3.93 ± 0.05 | 3.367 ± 0.15 |
| Foam capacity (%) | 110.12 ± 0.38 | 81.52 ± 1.68 |
| Foam stability (%) | 90.53 ± 0.38 | 84.00 ± 0.61 |
| Emulsion activity index (m2 g−1) | 16.37 ± 0.45 | 10.44 ± 0.49 |
| Emulsion stability index (min) | 104.74 ± 0.69 | 79.68 ± 0.56 |
| Solubility (%) | 73.82 ± 0.64 | 60.04 ± 0.75 |
One of the most significant indices to assess a protein's conformational shift is its surface hydrophobicity. The surface hydrophobicity of all FBPI is mentioned in Fig. 8. The control sample has a surface hydrophobicity of 183.45. There is not a significant difference (p > 0.05) between the surface hydrophobicity of the control sample and the UFBPI with 5 min treatment time (183.56), indicating that the treatment time is not sufficient enough to make significant changes in the proteins. It can be noted that the surface hydrophobicity of FBPI increased with the expansion of ultrasonication duration. The highest value was seen for the UFBPI at 25 min (689.32). The majority of the hydrophobic groups in the control sample were hidden inside protein molecules. These protein groups or areas were exposed after ultrasonic treatment, which led to an increase in the surface hydrophobicity.73 After 25 min, there is a decrease in the surface hydrophobicity of UFBPI at 35 min (485.08). Along with this, CUFBPI also showed a decrease (263.55) in the protein surface hydrophobicity, which is less than 35 min of UFPBI. These results can justify the changes in the functional properties for both batch and continuous processes, as mentioned in Section 3.2, Fig. 3–5, and Table 3 for CUFBPI. Ultrasonic treatment may induce more hydrophobic groups to re-polymerize into aggregates through hydrophobic interactions, leading to a decrease in the protein's hydrophobicity.74 Thus, from Fig. 8, it is apparent that the protein was partially denatured until the time such as 25 min at 200 W treatment and re-polymerized by hydrophobic binding and other effects as the US time is further increased to 35 min, which might have happened in the continuous ultrasound process, leading to a reduction in the values.62,75
 | ||
| Fig. 8 Surface hydrophobicity (Ho) of FBPI at different sonication treatments at 0, 5, 25, and 35 min of 200 W and in a continuous US system. | ||
4. Conclusions
Plant proteins are gaining more attention in recent times. This study has improved the yield and the physicochemical-functional, structural, and nutritional properties of protein isolate from germinated field bean flour at conditions of 200 W for 25 min. At this level, there was an increase in protein yield, foaming capacity, foam stability, emulsion activity index, emulsion stability, solubility, and zeta potential in vitro protein digestibility. Along with these, there is a decrease in the particle size. The change in the microstructure of the proteins due to US treatment was seen in SEM. FTIR analysis confirmed the changes in the secondary structures of proteins. The physical changes caused by acoustic cavitation resulted in the partial denaturation of proteins shown by an increase in the surface hydrophobicity and thus the functionalities. On prolonged US treatment, i.e., 35 min, the decrease in the values of functional properties was seen due to the protein aggregation, suggesting that 25 min is the optimum treatment time for improving the yield, resulting in the best functional modifications of proteins that help in achieving the SDGs 2, 3, 6, 7, 12, 13. These enhanced qualities demonstrate that US-extracted germinated field bean protein has superior qualities over conventional methods and may be substituted for other proteins in food applications. This study also showed the importance of the pale brown field bean variety with its unique properties that have not received enough attention from researchers as a sustainable alternative protein to use in the food sectors. This study leads to a path to consider the US-treated field bean protein in the development of shakes, yogurt, and other products.Author contributions
Bhakti Anand Narale: data collection, review and research work, analysis, writing – original draft and editing. Addanki Mounika: writing – original draft and editing, reviewing. Shanmugam Akalya: conceptualization, designing of experiments, reviewing, editing and supervision.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to thank the National Institute of Food Technology, Entrepreneurship, and Management – Thanjavur, Ministry of Food Processing Industries, Government of India for providing knowledge and support.References
- United Nations, World Population Projected to Reach 9.6 Billion by 2050 Search PubMed.
- M. López-pedrouso, J. M. Lorenzo, C. Zapata and D. Franco, Proteins and Amino Acids, Elsevier Inc., 2019 Search PubMed.
- J. O'Sullivan, B. Murray, C. Flynn and I. Norton, The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins, Food Hydrocoll., 2016, 53, 141–154 CrossRef.
- S. A. Sofi, J. Singh, K. Muzaffar, D. Majid and B. N. Dar, Physicochemical characteristics of protein isolates from native and germinated chickpea cultivars and their noodle quality, Int. J. Gastron. Food Sci., 2020, 22, 100258 CrossRef.
- F. Boukid, E. Zannini, E. Carini and E. Vittadini, Pulses for bread fortification: a necessity or a choice?, Trends Food Sci. Technol., 2019, 88, 416–428 CrossRef CAS.
- H. M. Habib, S. W. Theuri, E. E. Kheadr and F. E. Mohamed, Functional, bioactive, biochemical, and physicochemical properties of the Dolichos lablab bean, Food Funct., 2017, 8, 872–880 RSC.
- S. A. Marathe, V. Rajalakshmi, S. N. Jamdar and A. Sharma, Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India, Food Chem. Toxicol., 2011, 49, 2005–2012 CrossRef CAS PubMed.
- M. M. Hassan and N. Joshi, Hydrothermal effects on physicochemical, sensory attributes, vitamin C, and antioxidant activity of frozen immature Dolichos lablab, Heliyon, 2020, 6, e03136 CrossRef PubMed.
- T. Longvah, R. Ananthan, K. Bhaskarachary and K. Venkaiah, Indian Food Composition Tables 2017, Pap. Knowl. Towar. a Media Hist. Doc., 2017, 8, pp. 1–578 Search PubMed.
- E. Purwanti, F. E. Hermanto, J. W. Souhaly, W. Prihanta and T. I. Permana, Exploring public health benefits of Dolichos lablab as a dietary supplement during the COVID-19 outbreak: a computational study, J. Appl. Pharm. Sci., 2021, 11, 135–140 CAS.
- P. V. Vaijayanthi, S. Ramesh, M. Byre Gowda, A. Mohan Rao, J. Gowda, H. K. Ramappa, C. M. Keerthi and B. S. Rajendra Prasad, Development and validation of a core set of dolichos bean germplasm, Int. J. Veg. Sci., 2014, 21, 419–428 CrossRef.
- A. Subagio, Characterization of hyacinth bean (Lablab purpureus (L.) sweet) seeds from Indonesia and their protein isolate, Food Chem., 2006, 95, 65–70 CrossRef CAS.
- N. Mohan and J. J. Mellem, Functional properties of the protein isolates of hyacinth bean [Lablab purpureus (L.) Sweet]: an effect of the used procedures, Lwt, 2020, 129, 109572 CrossRef CAS.
- Y. Zhao, C. Wen, Y. Feng, J. Zhang, Y. He, Y. Duan, H. Zhang and H. Ma, Effects of ultrasound-assisted extraction on the structural, functional and antioxidant properties of Dolichos lablab L. Protein, Process Biochem., 2021, 101, 274–284 CrossRef CAS.
- R. D. Myrene, Effect of traditional processing methods on nutritional quality of field bean, Adv. Biores., 2013, 4, 29–33 Search PubMed.
- S. A. Sofi, J. Singh, K. Muzaffar and B. N. Dar, Effect of Germination Time on Physicochemical, Electrophoretic, Rheological, and Functional Performance of Chickpea Protein Isolates, ACS Food Sci. Technol., 2021, 1, 802–812 CrossRef CAS.
- Y. Zhang, B. Wang, W. Zhang, W. Xu and Z. Hu, Effects and mechanism of dilute acid soaking with ultrasound pretreatment on rice bran protein extraction, J. Cereal Sci., 2019, 87, 318–324 CrossRef CAS.
- N. B. Rizvi, S. Aleem, M. R. Khan, S. Ashraf and R. Busquets, Quantitative Estimation of Protein in Sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods, Molecules, 2022, 27(3), 814 CrossRef CAS PubMed.
- S. Gurusamy, C. S. Vidhya, B. Y. Khasherao and A. Shanmugam, Pulses for health and their varied ways of processing and consumption in India – a review, Appl. Food Res., 2019, 2(2), 100171 CrossRef.
- N. Xia, J. Wang, X. Yang, S. Yin, J. Qi, L. Hu and X. Zhou, Preparation and characterization of protein from heat-stabilized rice bran using hydrothermal cooking combined with amylase pretreatment, J. Food Eng., 2012, 110, 95–101 CrossRef CAS.
- G. Sudarsanan, M. Addanki and A. Shanmugam, Improved extraction and modification of plant protein from desi chickpea variety using green technology, Food Chem. Adv., 2023, 3, 100521 CrossRef.
- B. A. Narale, A. Shanmugam and A. Rawson, Effect of pretreatments to field bean for enhancement of protein content, 2022, 11, 550–554 Search PubMed.
- M. Hadnadjev, T. Dapcevic-Hadnadjev, M. Pojic, B. Saric, A. Misan, P. Jovanov and M. Sakac, Progress in vegetable proteins isolation techniques: A review, Food Feed Res., 2017, 44, 11–21 CrossRef.
- M. Kumar, M. Tomar, J. Potkule, R. Verma, S. Punia, A. Mahapatra, T. Belwal, A. Dahuja, S. Joshi, M. K. Berwal, V. Satankar, A. G. Bhoite, R. Amarowicz, C. Kaur and J. F. Kennedy, Advances in the plant protein extraction: Mechanism and recommendations, Food Hydrocoll., 2021, 115, 106595 CrossRef CAS.
- N. Bhargava, R. S. Mor, K. Kumar and V. S. Sharanagat, Advances in application of ultrasound in food processing: A review, Ultrason. Sonochem., 2021, 70, 105293 CrossRef CAS PubMed.
- M. M. Rahman and B. P. Lamsal, Ultrasound-assisted extraction and modification of plant-based proteins: impact on physicochemical, functional, and nutritional properties, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1457–1480 CrossRef CAS PubMed.
- S. Natarajan and V. Ponnusamy, Materials Today: proceedings a review on the applications of ultrasound in food processing, Mater. Today Proc., 2020, 10, 1–4 Search PubMed.
- M. Ashokkumar, Applications of ultrasound in food and bioprocessing, Ultrason. Sonochem., 2015, 25, 17–23 CrossRef CAS PubMed.
- M. Ravikumar, Ultrasonication: An Advanced Technology for Food Preservation, Int. J. Pure Appl. Biosci., 2017, 5, 363–371 CrossRef.
- T. S. Awad, H. A. Moharram, O. E. Shaltout, D. Asker and M. M. Youssef, Applications of ultrasound in analysis, processing and quality control of food: a review, Food Res. Int., 2012, 48, 410–427 CrossRef CAS.
- K. Li, H. Ma, S. Li, C. Zhang and C. Dai, Effect of Ultrasound on Alkali Extraction Protein from Rice Dreg Flour, J. Food Process Eng., 2017, 48, e12377 CrossRef.
- L. Jiang, J. Wang, Y. Li, Z. Wang, J. Liang, R. Wang, Y. Chen, W. Ma, B. Qi and M. Zhang, Effects of ultrasound on the structure and physical properties of black bean protein isolates, Food Res. Int., 2014, 62, 595–601 CrossRef CAS.
- T. Xiong, W. Xiong, M. Ge, J. Xia, B. Li and Y. Chen, Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate, Food Res. Int., 2018, 109, 260–267 CrossRef CAS PubMed.
- Y. Wang, Y. Wang, K. Li, Y. Bai, B. Li and W. Xu, Effect of High Intensity Ultrasound on Physicochemical, Interfacial and Gel Properties of Chickpea Protein Isolate, Lwt, 2020, 129, 109563 CrossRef CAS.
- F. Alavi, L. Chen and Z. Emam-djomeh, Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein, Food Chem., 2021, 354, 129494 CrossRef CAS PubMed.
- B. Byanju, M. M. Rahman, M. P. Hojilla-Evangelista and B. P. Lamsal, Effect of high-power sonication pretreatment on extraction and some physicochemical properties of proteins from chickpea, kidney bean, and soybean, Int. J. Biol. Macromol., 2020, 145, 712–721 CrossRef CAS PubMed.
- A. K. Sarangapany, A. Murugesan, A. S. Annamalai, A. Balasubramanian and A. Shanmugam, An overview on ultrasonically treated plant-based milk and its properties – a review, Appl. Food Res., 2022, 2, 100130 CrossRef CAS.
- A. Vallath and A. Shanmugam, Study on Model Plant Based Functional Beverage Emulsion (Non-Dairy) Using Ultrasound – a Physicochemical and Functional Characterization, SSRN Electron. J., 2022, 8, 106070 Search PubMed.
- K. M. Raghunath, B. Y. Khasherao, A. Shanmugam and Y. Khasherao, Development of medium fat plant-based mayonnaise using chickpea (Cicer arietinum) and green gram (Vigna radiata) and sensory evaluation using fuzzy logic, Pharma Innov. J., 2021, 10, 896–901 CAS.
- D. Betancur-Ancona, R. Martínez-Rosado, A. Corona-Cruz, A. Castellanos-Ruelas, M. E. Jaramillo-Flores and L. Chel-Guerrero, Functional properties of hydrolysates from Phaseolus lunatus seeds, Int. J. Food Sci. Technol., 2009, 44, 128–137 CrossRef CAS.
- J. Fu, Y. Ren, F. Jiang, L. Wang, X. Yu and S. kui Du, Effects of pulsed ultrasonic treatment on the structural and functional properties of cottonseed protein isolate, Lwt, 2022, 172, 114143 CrossRef CAS.
- F. Wang, Y. Zhang, L. Xu and H. Ma, An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities, Lwt, 2020, 127, 109348 CrossRef CAS.
- M. Dabbour, R. He, H. Ma and A. Musa, Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties, J. Food Process Eng., 2018, 41, 12799 CrossRef.
- M. Naik, V. Natarajan, N. Modupalli and S. Thangaraj, Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: kinetics and quality measurements, LWT, 2022, 155, 112997 CrossRef CAS.
- S. M. Zhu, S. L. Lin, H. S. Ramaswamy, Y. Yu and Q. T. Zhang, Enhancement of Functional Properties of Rice Bran Proteins by High Pressure Treatment and Their Correlation with Surface Hydrophobicity, Food Bioprocess Technol., 2017, 10, 317–327 CrossRef CAS.
- A. G. A. Sá, Y. M. F. Moreno and B. A. M. Carciofi, Food processing for the improvement of plant proteins digestibility, Crit. Rev. Food Sci. Nutr., 2019, 1–20 Search PubMed.
- Y. Ding, H. Ma, K. Wang, S. M. R. Azam, Y. Wang, J. Zhou and W. Qu, Ultrasound frequency effect on soybean protein: Acoustic field simulation, extraction rate and structure, Lwt, 2021, 145, 111320 CrossRef CAS.
- C. C. Almeida, M. Lúcia, G. Monteiro, B. Reis, T. S. Alvares and C. A. Conte-junior, In vitro digestibility of commercial whey protein supplements., LWT--Food Sci. Technol., 2015, 61(1), 7–11 CrossRef CAS.
- A. Shanmugam and M. Ashokkumar, Ultrasonic preparation of stable flax seed oil emulsions in dairy systems – physicochemical characterization, Food Hydrocoll., 2014, 39, 151–162 CrossRef CAS.
- G. L. Tu, T. H. N. Bui, T. T. T. Tran, N. M. N. Ton and V. M. Van Le, Comparison of enzymatic and ultrasonic extraction of albumin from defatted pumpkin (cucurbita pepo) seed powder, Food Technol. Biotechnol., 2015, 53, 479–487 CAS.
- T. Chittapalo and A. Noomhorm, Ultrasonic assisted alkali extraction of protein from defatted rice bran and properties of the protein concentrates, Int. J. Food Sci. Technol., 2009, 44, 1843–1849 CrossRef CAS.
- M. Ashokkumar, D. Sunartio, S. Kentish, R. Mawson, L. Simons, K. Vilkhu and C. K. Versteeg, Modification of food ingredients by ultrasound to improve functionality: a preliminary study on a model system, Innov. Food Sci. Emerg. Technol., 2008, 9, 155–160 CrossRef CAS.
- N. A. Mir, C. S. Riar and S. Singh, Food Hydrocolloids physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed, Food Hydrocoll., 2019, 96, 433–441 CrossRef CAS.
- T. Žugčić, R. Abdelkebir, F. J. Barba, A. Rezek-Jambrak, F. Gálvez, S. Zamuz, D. Granato and J. M. Lorenzo, Effects of pulses and microalgal proteins on quality traits of beef patties, J. Food Sci. Technol., 2018, 55, 4544–4553 CrossRef PubMed.
- J. E. Kinsella, C. V. Morr and J. E. Kinsella, Milk proteins : Physicochemical and functional properties, Crit. Rev. Food Sci. Nutr., 1984, 21, 37–41 Search PubMed.
- N. T. Flores-jiménez, J. Armando, J. Esmeralda, U. Silvas, J. Carmen, R. Ramírez, P. Rosas, P. Ulises, B. Rosales, Y. Silva and R. Gutiérrez, Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate, Food Res. Int., 2019, 121, 947–956 CrossRef PubMed.
- H. Cao, R. Sun, J. Shi, M. Li, X. Guan, J. Liu and K. Huang, Ultrasonics Sonochemistry effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates, Ultrason. Sonochem., 2021, 77, 105685 CrossRef CAS PubMed.
- N. A. Mir, C. S. Riar and S. Singh, Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics, Ultrason. Sonochem., 2019, 58, 104700 CrossRef CAS PubMed.
- S. Jiang, J. Ding, J. Andrade, T. M. Rababah, A. Almajwal, M. M. Abulmeaty and H. Feng, Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments, Ultrason. Sonochem., 2017, 38, 835–842 CrossRef CAS PubMed.
- J. A. Resendiz-Vazquez, J. A. Ulloa, J. E. Urías-Silvas, P. U. Bautista-Rosales, J. C. Ramírez-Ramírez, P. Rosas-Ulloa and L. González-Torres, Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate, Ultrason. Sonochem., 2017, 37, 436–444 CrossRef CAS PubMed.
- S. Kang, J. Zhang, X. Guo, Y. Lei and M. Yang, Effects of Ultrasonic Treatment on the Structure, Functional Properties of Chickpea Protein Isolate and Its Digestibility In Vitro, Foods, 2022, 11(6), 880 CrossRef CAS PubMed.
- S. Yan, J. Xu, S. Zhang and Y. Li, Effects of flexibility and surface hydrophobicity on emulsifying properties : Ultrasound-treated soybean protein isolate, LWT, 2021, 142, 110881 CrossRef CAS.
- Q. Cui, A. Zhang, R. Li, X. Wang, L. Sun and L. Jiang, Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates, Food Biosci., 2020, 38, 100747 CrossRef CAS.
- A. R. Jambrak, T. J. Mason, V. Lelas, Z. Herceg and I. L. Herceg, Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions, J. Food Eng., 2008, 86, 281–287 CrossRef CAS.
- A. R. Jambrak, T. J. Mason, V. Lelas, L. Paniwnyk and Z. Herceg, Effect of ultrasound treatment on particle size and molecular weight of whey proteins, J. Food Eng., 2014, 121, 15–23 CrossRef CAS.
- B. Nazari, M. A. Mohammadifar, S. Shojaee-Aliabadi, E. Feizollahi and L. Mirmoghtadaie, Effect of ultrasound treatments on functional properties and structure of millet protein concentrate, Ultrason. Sonochem., 2018, 41, 382–388 CrossRef CAS PubMed.
- S. M. T. Gharibzahedi and B. Smith, The functional modification of legume proteins by ultrasonication: a review, Trends Food Sci. Technol., 2020, 98, 107–116 CrossRef CAS.
- Y. Li, Y. Cheng, Z. Zhang, Y. Wang, B. K. Mintah, M. Dabbour, H. Jiang, R. He and H. Ma, Modification of rapeseed protein by ultrasound-assisted pH shift treatment: ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics, Ultrason. Sonochem., 2020, 69, 105240 CrossRef CAS PubMed.
- Z. Ganim, S. C. Hoi, A. W. Smith, L. P. Deflores, K. C. Jones and A. Tokmakoff, Amide I two-dimensional infrared spectroscopy of proteins, Acc. Chem. Res., 2008, 41, 432–441 CrossRef CAS PubMed.
- K. Shevkani, N. Singh, Y. Chen, A. Kaur and L. Yu, Pulse proteins: secondary structure, functionality and applications, J. Food Sci. Technol., 2019, 56(6), 2787–2798 CAS.
- P. Yu, Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: a new approach, Br. J. Nutr., 2005, 94, 655–665 CrossRef CAS PubMed.
- J. O. Sullivan, B. Murray, C. Flynn and I. Norton, Comparison of batch and continuous ultrasonic emulsification processes, J. Food Eng., 2015, 167, 114–121 CrossRef.
- W. Zhang, G. Zeng, Y. Pan, W. Chen, W. Huang, H. Chen and Y. Li, Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction, Carbohydr. Polym., 2017, 172, 102–112 CrossRef CAS PubMed.
- M. A. Malik, H. K. Sharma and C. S. Saini, High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties, Ultrason. Sonochem., 2017, 39, 511–519 CrossRef CAS PubMed.
- Y. Sun, J. Chen, S. Zhang, H. Li, J. Lu, L. Liu, H. Uluko, Y. Su, W. Cui, W. Ge and L. Jiaping, Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate, J. Food Eng., 2014, 124, 11–18 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |