Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antimicrobial activity and applications of fermentates from lactic acid bacteria – a review
Ricardo H.
Hernández Figueroa
 ,
Aurelio
López-Malo
,
Aurelio
López-Malo
 and
Emma
Mani-López
and
Emma
Mani-López
 *
*
Departamento de Ingeniería Química, Alimentos y Ambiental, Universidad de Las Américas Puebla, San Andrés Cholula 72810, Mexico. E-mail: emma.mani@udlap.mx
First published on 15th January 2024
Abstract
Fermentates are complex mixtures containing inactivated or viable lactic acid bacteria (LAB) cells, growth metabolites, and culture medium compatible with different food applications, offering a cost-effective and practical alternative to LAB cell-free supernatants (CFS). Contrariwise to CFS, in fermentates, the bacteria in the growth medium are commonly inactivated to be used directly. Despite their commercial availability in the food industry, limited research has explored their antimicrobial potential. This review summarises the current knowledge regarding the antimicrobial activity of fermentates, both in vitro and in food applications. Furthermore, the paper discusses fermentates' physicochemical and sensory effects in foods. Studies indicate that commercial fermentates, applied at low concentrations (<2%), laboratory-produced fermentates at 10%, and sourdoughs at 20–30%, demonstrate effective antimicrobial properties. Notably, the reviewed literature suggests that fermentates minimally impact food products' physicochemical and sensory attributes. The antimicrobial activity of fermentates and their potential to replace chemical preservatives, together with their practical and cost-effective nature, contribute positively to sustainability in food production by reducing dependence on selective media and improving the quality of final products and their applicability in diverse food industries.
Sustainability spotlightThe most common antimicrobial agents used in foods, such as benzoates, sorbates, and propionates, are produced with significant environmental impacts. Additionally, some synthetic antimicrobials are linked to human health problems. This review exposes the use of fermentates from lactic acid bacteria (LAB) as alternative antimicrobials. Fermentates are complex mixtures from the fermentation of LAB containing inactivate cells, metabolites, and culture medium. Fermentates' sustainability comprises free waste, natural production, safe use, “clean label”, and the possibility of using the food's by-products as the substrate for their production. Adequate fermentate development could improve the use of sustainable antimicrobials. |
Introduction
Fermentation is one of the fundamental technologies of modern industrial biotechnology that supports food, water, environment, energy, and medicine, among other applications. Microorganisms are utilised as microbial factories1 to produce food ingredients at high yields with a lower environmental impact. Throughout the centuries, fermented foods and beverages have played a crucial role in the evolution of culinary traditions, offering abundant nutrients and advantages. The fermentation process preserves food, extends its shelf life, and generates valuable bioactive compounds.2 Optimising fermentation processes by carefully choosing suitable microorganisms and fermentation media enables the production of functional ingredients, such as enzymes, vitamins, additives, and antimicrobials, with less environmental impact. The selection of microorganisms and media can also influence the efficiency and yield of production, thus reducing the resources required and the waste generated, resulting in sustainable technologies. When produced by conventional industrial methods, such as mass extraction or organic synthesis, these ingredients are often environmentally unsustainable.3Food processors and consumers often prefer using natural antimicrobials from fermentation processes, as they are considered more natural and label-friendly alternatives to using chemicals such as propionates, sorbates, or benzoates.4–8 Natural food preservation additives using bioprotective cultures could be an alternative to chemical preservatives.7 Cell-free supernatants (CFS) from lactic acid bacteria (LAB) have been widely studied as antimicrobials since they contain the metabolites derived from fermentation and/or secondary microbial products from metabolism.1 CFS from several LAB have demonstrated their efficacy as antimicrobials against bacteria and fungi.9
Depending on the fermentation medium used, CFS from lactic acid bacteria can be included in the food formulation, sprayed on the food's surface, and can also be used to coat the surface of the food10 or incorporated into active packaging. The possible sensory changes in foods depend on the amount added and the type of medium from which they come.6 CFS from LAB are attractive antimicrobials due to their natural origin and safe traditional long-term use of fermented foods. Their supplementation does not involve food fermentation of the product since the microbial cells were eliminated.11 However, sometimes the production of CFS is complex or complicated owing to growth media (which may be rich in solids), the amount of CFS (high volumes require many membrane filters, which may be expensive), and the cost (the growth media could be expensive); therefore, an alternative is the use of whole bacterial cells plus the supernatant of LAB, previously called as fermentate or undefined fermentates.4,7,8,12 For instance, Mathur et al.13 defined “fermentate” as the formulation/composition typically composed of LAB cells (active or inactive) along with the culture supernatants and fermentation media, which contain metabolites and bioactive components. Fermentates are ingredients produced by the fermentation of various raw materials by food-grade microorganisms, typically lactic acid or propionic acid bacteria.8,14 Preservatives made by fermentation processes approved as food additives are divided into nisin preparations, natamycin preparations, undefined cultured milk, and cultured dextrose preparations.4 The latter two can be effective against different microbial groups depending on the culture used for fermentation.
Many lactic acid bacteria are considered probiotics, from which the so-called postbiotics can be generated. These can be defined as metabolic products or by-products produced by some food-grade microorganisms during growth and fermentation in complex microbiological cultures, which have health benefits to the host. Therefore, postbiotics could be considered fermentates since they depend on the LAB type used to produce them.15
Inactivated bacterial cells plus the supernatant are more stable during handling and storage, and the microorganisms will not be able to grow in the foods to which they are added. Besides, fermentates may be incorporated without a previous whole-cell inactivation if used as an ingredient in foods processed at high temperatures during its preparation.
The main advantages of fermentate are its ease of preparation, long shelf life, stability at room temperature, high mass production capability, and a low impact on the sensory properties depending on the amount incorporated and the compatibility of the medium with the food. Therefore, the use of fermentates in certain foods can be advantageous compared to the use of CFS. Additionally, Rajanikar et al.6 reported that using microbiological medium-based cell-free supernatants as ingredients in the food system, rather than employing purified metabolites, remains a topic of debate.
Currently, commercial fermentates are available; several companies market them for different applications, to protect against lactic acid bacteria, bacterial spores, Listeria, Gram-negative bacteria, yeasts, and moulds, in culinary, meat, poultry, seafood, dairy, bakery, beverage, and confectionery applications. The use of fermentates for food preservation occurred in the USA around 1980–1990 with the introduction to the market of the MicroGARD™ product preparations.4,16 They are spray-dried products produced by fermentation with GRAS-status lactic acid bacteria. Since its introduction, several types of fermentates that target specific organisms and are for diverse preparations/applications have been marketed.4
Fermentates or ‘cultured materials’ are marketed as “clean label” or “label-friendly” ingredients due to food-grade bacteria, and safe growth media are utilised during manufacture.8 Despite fermentates' industrial applications, little scientific information is available about their antimicrobial activity, food application, and impact on sensory properties.8,14 Mathur et al.13 recently compiled information about LAB fermentates health benefits, and Fidan et al.17 reported recent developments of LAB and their metabolites' risks and benefits. CFS has been a conventional medium for extracting antimicrobial compounds from LAB. Nevertheless, the associated expenses and complexity in preparation have hindered widespread adoption and limited scalability, leading researchers to seek alternative approaches. Fermentates, characterized by the inactivation of bacterial cells in the growth medium, present an appealing solution due to their simplified and cost-effective production. This review aims to present the main findings of the antimicrobial activity of fermentates in vitro and foods; also, the impacts of fermentates on food physicochemical and sensory properties are revised, exploring the promising possibility of using fermentates as a practical and cost-effective alternative to CFS in LAB-based antimicrobial studies.
Fermentate preparation
For fermentate production, a specific bacterial strain is grown in a suitable culture medium to promote its growth and the production of metabolites.18 The heterogeneous group of LAB with traditional and long-term safe use, recognised as qualified presumption of safety (QPS) or generally recognised as safe (GRAS), should be chosen to fermentate production. Lactic acid bacteria are widespread and are one of the important groups of microorganisms in food fermentation. A wide variety of strains are routinely used as starter cultures in food fermentation processes, contributing to the development of desirable sensory attributes, preservation, and the production of bioactive metabolites.19–21 The selection of specific LAB strains is vital for optimizing these processes. Lactic acid bacteria exhibit remarkable antimicrobial capabilities, making them highly potent among prokaryotes. These bacteria generate multiple antimicrobial substances while metabolizing carbon sources and compete with other species by acidifying their surroundings and the rapid depletion of nutrients. Different LAB strains exhibit varying fermentation capabilities. Selecting strains that efficiently convert sugars into lactic acid helps achieve the desired acidity level for preservation and flavour development. LAB also produce various bioactive compounds during fermentation, such as antimicrobial peptides, vitamins, and bioactive peptides. These compounds may have health-promoting properties, including antimicrobial, antioxidant, and immunomodulatory effects. The current emphasis on biopreservation has renewed interest in exploring antimicrobials compatible with food production, which LAB produces. Selecting LAB strains that can produce specific bioactive metabolites enhances their potential and ensures consistency and reproducibility in the fermentation process. This is crucial for the food industry to maintain product quality and meet expectations.19The other important factor is the growing medium. The culture medium must provide the nutrients for bacterial growth and metabolite production (desirable with antimicrobial activity).
Many reports utilising LAB to produce antimicrobial agents (organic acids, peptides and/or bacteriocins) usually use complex culture media, such as de Man Rogosa and Sharpe (MRS), Brain Heart Infusion (BHI), Tryptone Glucose Extract (TGE), Trypticase Soy Broth (TSB), Trypticase Soy Broth Yeast Extract (TSBYE), among others.22 These media support and stimulate bacterial growth; thus, relatively high levels of bioactive products can be produced, but these media are unsuitable for large-scale production (cost, regulations, possible toxicity). Therefore, other formulations based on more conventional ingredients or industrial by-products, such as cheese whey and molasses, among many others, are being explored as alternative culture substrates. Using these substrates in LAB fermentation can be an attractive alternative to promote the interest in scaling up this process.22,23
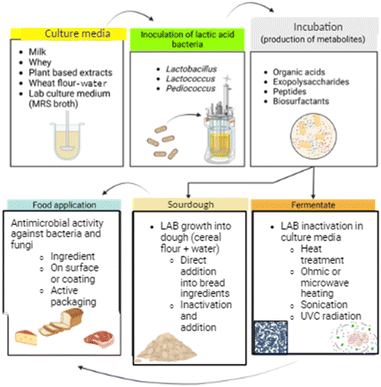
For fermentate production, the culture medium should be food grade and compatible with the foodstuff for food application. Milk, whey, sugar solutions, corn and wheat starches,8 wheat flour/water mixtures, and cereal hydrolysates have been tested in the fermentate formulation. The challenge is formulating a media containing agro-industrial products with appropriate protein and carbohydrate content to promote the simultaneous production of bioactive ingredients (organic acids, peptides, and bacteriocins). Fig. 1 displays an example of a fermentate preparation process. This simplifies the experimental procedure and reduces the overall cost, making fermentates an attractive choice for researchers and industries.
An alternative approach to the commonly employed media to cultivate lactic acid bacteria (MRS broth) involves utilizing whey protein concentrate to produce acid-tolerant LAB biomass7 fermentate, thereby using whey proteins, peptides, and naturally occurring sugars as a sustainable energy source for strain growth, in contrast to relying on MRS broth. The production of this fermentate presents a viable, innovative, natural, and cost-effective means of biomass growth.7 The resulting fermentates from whey protein concentrate lack colour or flavour additives, making them directly integrable to foods.7 Additionally, whey protein concentrate can serve as an economical medium for LAB cultivation, offering an alternative for repurposing acid whey while avoiding the expenses associated with non-food grade LAB growth media like MRS broth and the associated biomass preparation procedures.
LAB fermentation is considered an economical and scalable way to obtain bioactive ingredients once growth conditions that promote their production or release are established.23 The design or selection of culture medium should be considered for the food application to minimise the alteration of physicochemical and sensory properties and to enhance the antimicrobial compound(s) against the target microorganism(s).
After adequate bacterial growth and metabolite production are reached, the viable cells should be inactivated to extend the fermentate shelf life and provide stability for handling and food application. Heat treatment is the most commonly used method; however, sonication, high pressure, or ultraviolet radiation could also be utilised.24 Microwaves, pulsed electric fields, and ohmic heat can reduce the energy consumption to prepare fermentates by heat treatment.
Commercial preparations of bacteriocins are fermentates since bacteriocin is provided with the growth medium commonly pasteurised to inactivate the precursor bacteria. The composition and biological activity could differ with the inactivation process due to changes in the metabolites or cellular fragments. Therefore, each fermentate preparation should be tested as antimicrobial.
Sometimes, all of the metabolites or some of them specifically improve the antimicrobial activity of the fermentate's. Therefore, strategies to promote metabolite production or concentration are implemented during fermentate preparation. For example, the co-culture of Lactococcus lactis (fermentate producer) with Yarrowia lipolytica enhanced the nisin content in the fermentate.25 For the concentration of the fermented product, the elimination of water is required through evaporation, freeze-drying, or spray drying, which reduces the amount of fermentate needed to be added to achieve the antimicrobial action in the food. Consequently, the effects on foods' physicochemical and sensory properties are minimised. For example, a fermentate containing lacticin DPC3147 reached levels of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 AU mL−1 bacteriocin activity after concentration to 40% of solids by evaporation, which was higher than the native medium (10
960 AU mL−1 bacteriocin activity after concentration to 40% of solids by evaporation, which was higher than the native medium (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 AU mL−1).26 Other strategies that can be considered include using LAB mixtures or adding specific substrates to improve the antimicrobial activity of the fermentates.
240 AU mL−1).26 Other strategies that can be considered include using LAB mixtures or adding specific substrates to improve the antimicrobial activity of the fermentates.
When bacteria and their growth medium are incorporated immediately after preparation into a food formulation that receives heat treatment, it also can be considered a fermentate. In this regard, sourdoughs can be formulated using LAB and fermented from 24 to 72 h to incorporate into dough bread.27,28 Afterward, the bread process occurs, including the baking step that inactivates bacteria. Although commercial liquid sourdoughs do not claim antimicrobial properties, it is well known that they improve sensory attributes, bread's stability, and shelf life when incorporated into bread formulations.
Antimicrobial activity of fermentates
Challenges in production and purification of selected metabolites: implications for food applications
Many reviews have been reported about metabolites' antimicrobial effect resulting from fermentation with lactic acid bacteria.1,9,29–32 However, bacteriocins33–35,37 and phenyllactic acid6,36 stand out as antimicrobial agents of interest that could be present in fermented foods and fermentates.27,38,39Several reports isolate and test the antimicrobial activity of LAB and examine their potential as bacteriocins producers. As an example, Haryani et al.2 isolated 55 LAB from homemade fermented foods (20 samples) in Malaysia, identifying Lacticaseibacillus rhamnosus (34.5%), Lactiplantibacillus plantarum (20%), Limosilactobacillus fermentum (20%), Lacticaseibacillus paracasei (12.7%), Lacticaseibacillus casei (3.6%), Lactobacillus sp. (1.8%), and Enterococcus (7.2%). The majority (94%) exhibited broad-spectrum antimicrobial activity (tested with the cell-free supernatant growth in MRS broth) against foodborne pathogens, and four isolates (Lim. fermentum SC1001, Lcb. paracasei K2003, and Lcb. rhamnosus KF1002 and MK2003) could produce bacteriocin-like inhibitory substances.
Bacteriocins constitute various antimicrobial peptides released by bacteria growing in competitive polymicrobial surroundings.40,41 Although Gram-positive and Gram-negative bacteria generate bacteriocins, most documented cases involve Gram-positive bacteria, with a notable emphasis on Lactobacillus, as highlighted by Lee et al.40
Usually, their application includes the use of a bacteriocin-producer strain as inoculation of food, adding purified or semi-purified bacteriocin as a food additive, and using a previously fermented product with a bacteriocin-producing strain during food processing as an ingredient.5,10
Lactic acid bacteria that produce bacteriocins require complex media for growth, leading to heightened production costs and complicating the purification process of these heterogeneous substances.37 This complexity contributes to the limited number of bacteriocins purified to homogeneity, exemplified by nisin.41 The challenges associated with purification add to the overall expense, acting as a significant barrier for applications involving purified bacteriocins.
After screening and identifying new bacteriocins, the most critical and complex stage is purification.37 Currently, the choice of purification method is determined by the molecular weight, charge, and properties of bacteriocin. Bacteriocins synthesized by lactic acid bacteria are currently the only ones used for food preservation and have many advantages over food preservatives obtained by chemical synthesis.37 Three major methods for purifying bacteriocins by LAB to homogeneity have been reported.35
1. Purification can be done by a conventional method based on subsequent steps of ammonium sulphate precipitation, ion exchange, hydrophobic interaction, gel filtration, and reversed-phase high-pressure liquid chromatography (HPLC).
2. Three-step protocol: precipitation (ammonium sulphate), extraction–precipitation (chloroform–methanol), and reversed-phase HPLC.
3. Bacteriocins can be isolated through a unique unit operation (expanded bed adsorption).35
Following the latter two methods, which are more rapid than the first conventional method and yet successful, several bacteriocins with attractive industrial potential have been purified.35
Currently, the cultivation of natural or genetically modified producer organisms remains the primary method for producing bacteriocins, with the production of larger bacteriocins posing particularly tough challenges.41
Lactic acid bacteria antagonism against foodborne microorganisms has been correlated with the production of various antimicrobial substances, such as phenyllactic acid.6 3-Phenyllactic acid, an antimicrobial compound with broad-spectrum activity against bacteria and fungi, is gaining considerable attention as a food additive for controlling microbial contamination and extending the shelf life of food products.36,42 Phenyllactic acid is produced by various microorganisms, including LAB (Lactobacillus, Leuconostoc, and Enterococcus), non-LAB, and fungi. Notably, among Lactobacillus species, Lpb. plantarum, L. acidophilus, and Lcb. paracasei have been reported as significant producers of this compound.42
Producing phenyllactic acid using LAB as a whole-cell catalyst remains a viable and environmentally friendly option.6 As a potential biological preservative, phenyllactic acid can be biosynthesized de novo from glucose by LAB fermentation. During this process, phosphoenolpyruvate and erythrose 4-phosphate, generated from glucose metabolism, enter the shikimate pathway, producing phenylalanine and then converting to phenylpyruvate and phenyllactic acid through a series of reactions.36 Various approaches are implemented in fermentation to enhance phenyllactic acid production, including adding precursors and intermediate compounds and regulating fermentation conditions. As highlighted by Wu et al.,36 these strategies play a crucial role in facilitating the efficient production of phenyllactic acid. Particularly, the concentration of phenylalanine has a significant impact on LAB strains involved in phenyllactic acid production.
There is a lack of large-scale industrial production of phenyllactic acid, primarily due to the low yield and lack of low-cost microbial growth media, indicating the need for optimized agro-based media for industrial applications.6
Fermentates
The antimicrobial activity of fermentates cannot be attributed to a single molecule. Consequently, using analytical assays to determine the concentration of individual components could generate results that do not fully reflect the antimicrobial activity.4 Hence, in vitro inhibition assays are carried out to determine the antimicrobial activity of fermentates, for example, agar diffusion methods.The antimicrobial properties of fermentates can be exhibited primarily in vitro models to define if they inhibit or inactivate the target microorganism(s). Various methods to assay antimicrobial activity include agar diffusion, dilution methods, gradient plates, automated methods, or turbidimetric procedures.43 Then, antimicrobial action should be tested in food even though fermentates' antimicrobial activity can be reduced in foods due to the action of food enzymes (mainly proteolytic), pH, and heat treatment.
In vitro antimicrobial activity of fermentates
Data from in vitro studies are utilised to define the concentration for food tests. Table 1 presents selected studies of in vitro antimicrobial activity from fermentates. The fermentates from Lactococcus and Pediococcus exhibited antimicrobial activity primarily against bacteria (Table 1). Both whey and bacteriological media (MRS) were used to produce the fermentate. Target microorganisms have been Listeria monocytogenes, L. innocua, Escherichia coli, Salmonella, Staphylococcus aureus, Enterococcus, Enterobacter, and Acinetobacter. Antifungal activity was scarcely reported. Samapundo et al.8 evaluated the antifungal activity of 4 commercial fermentates against Penicillium chrysogenum and Penicillium paneum. The time to visible growth (TVG) of P. chrysogenum in malt extract agar at 22 °C was extended up to 26 days at pH 5.5 and >30 days at pH 4.5, whereas for pH 6.5 the TVG ranged from 2.5 to 6.7 days when 3% of fermentates were added. P. paneum was most resistant to fermentates since the TVG was prolonged for 7 to 14 days at pH 4.5, incorporating 3% fermentates, while for pHs 5.5 and 6.5, the mould was inhibited for ≤6 days. Oliveira et al.46 demonstrated the antifungal activity (against Fusarium culmorum) of barley-based malt extract fermented with Lactobacillus amylovorus DSM19280 and Limosilactobacillus reuteri R29 (LAB phenillactic acid producer strains). Kantachote et al.47 used the LAB phenillactic acid-producing strain Lactiplantibacillus plantarum DW3 to produce fermented plant beverages, which inhibited the growth of Rhodotorula mucilaginosa.| Microorganism | Fermentate preparation | Test conditions | Main findings | Reference |
|---|---|---|---|---|
| Lactococcus lactis subsp. lactis DPC3147 | Bacteria was cultivated in reconstituted whey for 24 h, pasteurised at 72 °C for 15 s, concentrated at 40% solids, and spray dried | Different concentrations of fermentate powder were added to target bacteria suspended in phosphate buffer (pH 5.0 or 7.0) supplemented with 10 mM glucose | After 3 h at 30 °C L. monocytogenes lost 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 at pH = 5 and 3.8 log10 CFU mL−1 at pH = 5 and 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 at pH 7, whereas S. aureus reduced by 1.1 log10 CFU mL−1 at pH 7, whereas S. aureus reduced by 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 at pH 5 and 4 log10 CFU mL−1 at pH 5 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 at pH 7 log10 CFU mL−1 at pH 7 |
26 |
| The tested solution of fermentate was 15% of reconstitute powder | Listeria monocytogenes or Staphylococcus aureus were inoculated at ∼108 CFU mL−1 | |||
| Lactococcus lactis DGCC 10042 | Not specify the conditions for preparation | 105 CFU mL−1 of Listeria was placed into wells, and different dilutions of fermentate were tested to determine the minimal inhibitory concentration (MIC) | The MIC was 78 μg mL−1 for L. monocytogenes EGDe, LO28, and Listeria innocua FH1848, 125 μg mL−1 for L. monocytogenes F2365 and 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013, and 62.5 μg mL−1 for L. monocytogenes 33 013, and 62.5 μg mL−1 for L. monocytogenes 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 413 413 |
44 |
| The final fermentate was nisin A | ||||
| Pediococcus acidilactici B-LC-20 | P. acidilactici was cultured in MRS broth at 37 °C for 48 h, then it was sterilised at 121 °C for 15 min | Cultures from indicator bacteria were incubated till absorbances reached ∼0.1 at 600 nm (∼8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1) and adjusted to 7 log10 CFU mL−1) and adjusted to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1. The antibacterial activity was tested by agar disc diffusion using 20 μL of fermentate log10 CFU mL−1. The antibacterial activity was tested by agar disc diffusion using 20 μL of fermentate |
Halos of inhibition against target bacteria ranged from 7.82 to 9.95 mm. The tested bacteria include various strains Escherichia coli, Salmonella Typhimurium, Salmonella Enteritidis, L. monocytogenes, Staphylococcus aureus, Enterococcus faecalis ATCC 29212, Enterobacter aerogenes ATCC 13048, and Acinetobacter haemolyticus ATCC 19002 | 45 |
| Lactobacillus rhamnosus or Propionibacterium jensenii | L. rhamnosus or P. jensenii in two dairy substrates (low-heat milk -LH- and milk permeate -UF) to prepare fermentates | Antifungal activity in a cheese-mimicking model | Mucor racemosus, Penicillium commune, Galactomyces geotrichum, and Yarrowia lipolytica were inhibited | 12 |
Antimicrobial activity of fermentates in foods
LAB fermentates have been investigated for a long time because LAB are recognised as producers of metabolites with antimicrobial activity in fermented foods. The antimicrobial activity should be attributed to the whole action of fermentate compounds; however, sometimes specific compounds, such as bacteriocins, are identified, quantified, and related to the antimicrobial activity. Although fermentates have potential applications in food preservation and there are commercial products, only a few studies have reported the antimicrobial activity of fermentates. Table 2 presents the effect of fermentates from LAB in foods. Lactococcus and Pediococcus have great antibacterial activity against Listeria monocytogenes, Bacillus cereus, Salmonella Typhimurium, and E. coli in dairy products or chicken breast fillets (Table 2). In addition, fermentate antimicrobial action, which is expected to inhibit the target microorganism, has been assessed during food storage. Fermentate from Pediococcus acidilactici in MRS broth inhibited the growth of total viable counts and lactic acid bacteria in chicken breast fillets after 3 or 6 days of storage at 4 °C.45 Better inhibition results against pathogens and spoilage bacteria were reported in chicken breast fillets when fermentate was combined with chitosan and/or thymol.| Microorganism | Fermentate preparation | Test conditions | Main findings | Reference |
|---|---|---|---|---|
| Lactococcus lactis subsp. lactis DPC3147 | Bacteria was cultivated in reconstituted whey for 24 h, pasteurised at 72 °C for 15 s, concentrated at 40% solids, and spray dried | Reconstituted infant milk powder was partially replaced with 1/3 of lacticin powder | After 3 h of incubation at 30 °C, L. monocytogenes Scott A reduced by 97%, whereas the growth in the infant solution without fermentate increased 700% | 26 |
| Listeria monocytogenes was inoculated at 104 CFU mL−1 | ||||
| Lactococcus lactis subsp. lactis DPC3147 | Bacteria was cultivated in reconstituted whey for 24 h, pasteurized at 72 °C for 15 s, concentrated at 40% solids, and spray dried | 1 g of lacticin powder was added to 9 g of natural yoghurt or cottage cheese, mixed, inoculated with 104 CFU g−1L. monocytogenes Scott A, and incubated at 30 °C | In the natural yoghurt, 98.3% of counts were inhibited after 5 min of incubation, and after 60 min, no viable cells remained | 48 |
| Instant vegetable soup (added with boiling water) was added with 1, 5, or 10% of lacticin powder and inoculated with 105 CFU mL−1 of Bacillus cereus and stored at 4 °C | In the cottage cheese, 40% of the cells were inactivated after 5 minutes and 85% after 120 minutes | |||
| 5 or 10% of lacticin inhibited 99.9% of B. cereus within 1 h, whereas 1% of lacticin reduced 80% of the population after 3 h | ||||
| Pediococcus acidilactici B-LC-20 | P. acidilactici was cultured in MRS broth at 37 °C for 48 h, then it was sterilised at 121 °C for 15 min | Chicken breast fillets of 100 g were inoculated with 1 mL (7 log10 CFU mL−1) of pathogenic bacteria by spreading onto the surface. Then, breast fillets were immersed in fermentate solution (50% diluted with sterilised distilled water) for 10 min; after that, fillets were drained for 30 s, packaged, vacuumed, and stored at 4 °C for 15 days | Steady counts of Salmonella Typhimurium cocktail, L. monocytogenes cocktail, and Escherichia coli O157:H7 cocktail were counted during 15 days of storage | 45 |
| Lacticaseibacillus paracasei A11 | Whey protein concentrate | Whey protein concentrate fermentate-based edible coating for cheese | The coating reduced yeast and mould counts by 1.0–1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 during cheese storage (14 days). The resulting fermentates from whey protein concentrate lack colour or flavour additives, making them directly integrable to foods log10 CFU mL−1 during cheese storage (14 days). The resulting fermentates from whey protein concentrate lack colour or flavour additives, making them directly integrable to foods |
7 |
| Lactobacillus rhamnosus or Propionibacterium jensenii | L. rhamnosus or P. jensenii in two dairy substrates (low-heat milk -LH- and milk permeate -UF) to prepare fermentates | Antifungal activity by surface-spraying on semi-hard cheeses | Surface-spraying of each tested fermentate led to increased time to visible growth of fungi (Mucor racemosus, Penicillium commune, Galactomyces geotrichum, and Yarrowia lipolytica) compared to untreated cheese). The most efficient fermentate was that of P. jensenii, which inhibited M. racemosus and P. commune for up to 21 and 14 days, respectively | 12 |
The fermentates' antimicrobial activity is related to their composition, as with CFS antimicrobials. Incili et al.45 analysed the composition of CFS and fermentates from P. acidolactici in MRS broth and reported greater amounts of lactic acid, acetic acid, malic acid, 2-hydroxy-4-methyl-pentanoic acid, 3-phenyl lactic acid, 1,2-butanediol, α-hydroxy-methyl benzyl propanoate, 2-furanmethanol, 1-acetyl-β-carboline, and rutin in the fermentate. Regarding the antibacterial activity from fermentate or CFS had similar action in most tested bacteria (Salmonella, L. monocytogenes, E. coli, Enterobacter, Enterococcus, and Staphylococcus aureus); however, for E. coli O157:H7 ATCC 43895, L. monocytogenes ATCC 13932, and S. aureus ATCC 33591 CFS was most effective (p < 0.05); and for Acinetobacter haemolyticus ATCC 19002 fermentate exhibited best antibacterial action (p < 0.05).45 In another study, fermentates showed higher levels of organic acids such as acetic acid (7.6–23.1%), propionic acid (16.8–27.8%), and lactic acid (3.6%) than the CFS.17 The antimicrobial activity of organic acids is well known, and acetic and propionic acids are especially effective against moulds.
An important property expected from antimicrobials is not affecting or modifying foods' physicochemical and sensory properties. Therefore, it is relevant to assess the effects of fermentate in foods. Incili et al.21 evaluated the impact of fermentate (P. acidolactici in MRS broth) on the physicochemical properties of breast fillets during refrigerated storage. They observed a slight initial reduction in the pH of chicken fillets (∼0.4 units) compared with the control; afterward, pH increased after three days, remained steady for six days, and finally reduced at the end of storage (12–15 days).
Also, thiobarbituric acid analysis was carried out; the results denoted that lipid peroxidation was delayed by fermentate in breast fillets during storage. Fermentate's high total phenolic content, vacuum packaging, and low-fat content of fillets contributed to maintaining low levels of malonaldehyde in meat. The total volatile base-nitrogen (TVB-N) measures the protein oxidation of meat due to enzymatic and microbial activity. The fermentate did not delay the TVB-N formation during the storage of breast fillets, probably because psychrotrophic bacteria growth was observed. The colour of breast fillets treated with fermentate was significantly changed (p < 0.05) due to the brownish colour of the growth medium;45 thus, the fermentate-producing medium could impact the food's physical properties.
Sourdough supplemented as fermentate
Another approach of whole-cell components plus metabolites used as antimicrobials in foods is sourdough incorporation into cereal-baked products. In this case, flour and water combined with LAB are fermented to produce organic acids, peptides, and other antimicrobial compounds. Afterward, the mixture is added to the bread blend and baked. Various studies have shown that whole cells and metabolites preserve the bread during storage due to the antimicrobial activity attributed to organic acids (acetic and lactic acids) and peptides. Axel et al.55 showed that chemical acidification (lactic and acetic acids 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dough did not reach the same antifungal activity as sourdough fermented with three different Lactobacillus (L. amylovorus DSM19280, L. brevis R2Δ, and L. reuteri R29), probably due to fermentate contained other compounds with antimicrobial activity. Hydroxy long-chain fatty acids also have been identified as antifungal compounds in sourdough. Black et al.52 formulated sourdough with Lactobacillus hammesii DSM16381 and linoleic acid to assess the antifungal activity in bread. They identified two compounds (monohydroxy, monounsaturated C18 fatty acid, and coriolic acid) with strong antifungal activity (MIC ≤0.7 g L−1) against Penicillium roqueforti and Aspergillus niger in vitro and bread.
1) dough did not reach the same antifungal activity as sourdough fermented with three different Lactobacillus (L. amylovorus DSM19280, L. brevis R2Δ, and L. reuteri R29), probably due to fermentate contained other compounds with antimicrobial activity. Hydroxy long-chain fatty acids also have been identified as antifungal compounds in sourdough. Black et al.52 formulated sourdough with Lactobacillus hammesii DSM16381 and linoleic acid to assess the antifungal activity in bread. They identified two compounds (monohydroxy, monounsaturated C18 fatty acid, and coriolic acid) with strong antifungal activity (MIC ≤0.7 g L−1) against Penicillium roqueforti and Aspergillus niger in vitro and bread.
Table 3 summarises selected studies of sourdough-like fermentates with antifungal activity. Many species of Lactobacillus have demonstrated their antifungal activity in various types of bread. Lactobacillus plantarum, L. rossiae, L. amylovorus, L. hammessi, L. brevis, L. reuteri, L. delbrueckii subsp. bulgaricus, Lactiplantibacillus plantarum, L. acidophilus or L. casei has been tested. Wheat sourdoughs are mainly studied, but rye, rice, and quinoa are also investigated (Table 3). Regarding fermentation time, 24 to 48 hours were the most frequent conditions to prepare sourdoughs, and ≈20% of sourdough was the amount commonly used to prepare the bread. The antifungal activity extends bread's shelf life between 4 and 28 days, depending on the type of bread and storage conditions (commonly room temperature). Other studies have determined that the antifungal activity from sourdoughs in panettone from Lactobacillus rossiae LD108 prolonged its shelf life for 32 days.59
| Microorganism | Sourdough type and incorporation conditions | Main findings | Reference |
|---|---|---|---|
| Lactobacillus plantarum 1A7 | Cells harvested and suspended from cultured media were incorporated into 200 g of flour, 115 mL of tap water, and 5 mL of cell suspension (108 CFU g−1). The mixture was fermented at 30 °C for 16 h. 30% of sourdough was used to prepare the bread | The fungal growth was inhibited by 14 days of storage of bread slices, whereas control bread presented mould growth after 7 days | 49 |
| L. plantarum LB1 and Lactobacillus rossiae LB5 | Cells from modified MRS broth after ∼10 h were harvested, washed, and re-suspended in tap water. The dough (60% wheat germ and 30% water) was prepared, and the final counts of Lactobacillus were 108 CFU g−1. The dough was fermented for 24 h at 30 °C and freeze-dried. 4% (based on flour) sourdough was added to the bread formulation | Inoculated (102 conidia per mL of Penicillium roqueforti) bread slices displayed fungal growth after 14 days of storage at room temperature. In addition, bread slices without inoculum remained fungal-free after 28 days | 50 |
| Lactobacillus amylovorus DSM 19280 | Cells from 80 mL MRS5 broth were suspended in 40 mL sterile tap water, incorporated into 600 g wheat flour + 560 mL tap water, mixed for 1 min, and fermented at 30 °C for 24 h. 20% of sourdough was supplemented in bread formulation | Bread with sourdough remained mould-free after 14 days, and bread with calcium propionate (0.3%) for 12 days at room temperature | 51 |
| Lactobacillus hammesii DSM16381 | The sourdough was prepared using flour, tap water, and culture (cells from MRS suspended in tap water to 109 CFU mL−1) in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 plus 4 g kg−1 linoleic acid; the dough was fermented at 30 °C for 2 days. Bread was prepared with 20% of sourdough 1 plus 4 g kg−1 linoleic acid; the dough was fermented at 30 °C for 2 days. Bread was prepared with 20% of sourdough |
The environmental moulds were delayed by 13 days and Penicillium roqueforti FUA5005 by 2 days in bread stored (when sourdough was included in the bread formulation). The control bread presented mould growth after 6 days | 52 |
| L. plantarum CRL 778 | 20% of cultured Lactobacillus in MRS broth were inoculated in a mixture (325 g wheat flour, 5 g sucrose, 12 g skimmed milk, and 1 L tap water), adjusted the pH at 5.9, and incubated at 37 °C for 16 h with continuous stirring at 100 rpm. Fermented mixture was stored at 4 °C. For bread, 24% of tap water were replaced with the fermented mixture | Mould growth was inhibited for 20 days in bread formulated with the fermented mixture, whereas in control (0.4 g calcium propionate/100 g of wheat flour) bread, mould growth was detected after 10 days at room temperature (25–30 °C) | 53 |
| L. amylovorus DSM19280, Lactobacillus brevis R2Δ, and Lactobacillus reuteri R29 | Sourdough type II (equal weight of wheat flour and tap water), the inoculum was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 dough stirring after 24 h for total fermentation of 48 h at 30 °C for L. amylovorus and 37 °C for L. reuteri and L. brevis. 20% of sourdough was incorporated into the bread recipe log10 CFU g−1 dough stirring after 24 h for total fermentation of 48 h at 30 °C for L. amylovorus and 37 °C for L. reuteri and L. brevis. 20% of sourdough was incorporated into the bread recipe |
The mould growth was inhibited in bread slices by 6, 7, or 10 days for sourdough fermented with L. brevis, L. reuteri, or L. amylovorus, respectively, whereas the mould growth was evident after 4 days in control bread | 54 |
| L. reuteri R29, L. brevis L1105, or L. brevis R2Δ | For sourdough type II (equal weight of flour (quinoa or rice) and water) preparation, cells from MRS broth were harvested and inoculated at 107 CFU g−1 dough and fermented at 37 or 30 °C for 48 h. Sourdoughs were added at 20% into bread formulation (replace the flour) | Quinoa bread slices had similar mould growth (after 4 days of storage at 20 °C) than acidified (lactic and acetic acids) ones | 55 |
| For rice bread slices, the addition of L. reuteri sourdough delayed 2 days the mould growth more compared with control during the storage at 20 °C | |||
| L. plantarum ATCC 14917 and Saccharomyces cerevisiae ATCC 4126 | 1 g cells from both microorganisms were obtained after 24 h at 30 °C by centrifugation. The sourdough was formulated using 400 g of flour + 2% S. cerevisiae + 1, 2, or 3% of L. plantarum mixed with tap water for 5–10 min and left for 24 h. Balady bread was formulated using 20% of sourdough | The Balady bread shelf life was extended from 3 to 8 days when 2 or 3% of L. plantarum was included in the sourdough formulation | 56 |
| L. reuteri isolated from spontaneous rye dough | ∼10 g wet cells were obtained from MRS broth incubated at 30 °C for 24 h, centrifugated, and washed. The cells were mixed with rye flour and water at a ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) and incubated at 27 °C for 24 h. 20 g sourdough/300 g flour was supplemented in the bread formulation 1.5) and incubated at 27 °C for 24 h. 20 g sourdough/300 g flour was supplemented in the bread formulation |
Breads formulated with sourdough had lower bacteria counts after 7 days of storage at room temperature; in rye bread 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 and in wheat bread 1.5 log10 CFU g−1 and in wheat bread 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 less were observed compared with control breads log10 CFU g−1 less were observed compared with control breads |
57 |
| L. plantarum CECT 749 or Lactobacillus delbrueckii subsp. bulgaricus CECT 4005 | Individual Lactobacillus were cultivated in MRS broth at 37 °C for 10 h, centrifuged, and washed. Then, cells (2 × 108 CFU mL−1) were incorporated into 50 g of wheat flour and 50 mL of sterile tap water and incubated for 48 h at 37 °C. 20% sourdough replaced the total ingredients of bread formulation | The growth of Penicillium expansum CECT 2278 (100 μL of 3 × 105 spores per mL) was delayed for 5 days in bread (slices) formulated with the sourdough of L. bulgaricus and stored at 25 °C. This result was similar to the observed in bread prepared with calcium propionate (0.2%), inoculated, and stored at the same conditions | 58 |
| In addition, the counts of mould were very similar in both types of bread after 7 days | |||
| Lactiplantibacillus plantarum NRRL B-4496 | Cells from MRS broth cultured at 35 °C for 48 h were harvested and incorporated (1 g/100 g) into wheat flour and tap water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture was fermented by 48 h at 37 °C. 28% of poolish-type sourdough was added to the bread formulation 1). The mixture was fermented by 48 h at 37 °C. 28% of poolish-type sourdough was added to the bread formulation |
Environmental mould growth was inhibited in the bread for 9 days, whereas in control bread (without preservatives), the mould growth was observed after 3 days of storage at room temperature | 27 |
| Lactobacillus acidophilus NRRL B-4495 or L. casei 21/1 | Cells cultured at 35 °C for 48 h (MRS broth) were harvested and incorporated (1 g/100 g) into wheat flour and tap water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture was fermented by 48 h at 37 °C. 38% poolish-type sourdough was added to the bread formulation 1). The mixture was fermented by 48 h at 37 °C. 38% poolish-type sourdough was added to the bread formulation |
Bread added with sourdoughs fermented with L acidophilus or L. casei had an increase in shelf life without mould growth (14 days) compared to bread without them (5 days) | 28 |
Incorporating sourdough in bread formulations can modify the bread's physicochemical and sensory properties owing to metabolites from LAB.27,28 Decrements in bread pH and increment of total titratable acidity and specific volume were the main changes reported by Rizzello et al.50 in fresh bread supplemented with 4% (flour-based) of freeze-dried wheat germ sourdough, whereas hardness and fracturability values were reduced. Lower pH was observed in bread formulated with 30% of sourdough (Lactobacillus plantarum 1A7; pH = 4.82) compared with control (pH = 5.45), which also received the highest scores for acid taste and flavour in the sensory evaluation.49
The freshness of bread determined as alkaline water retention capacity (due to staling rate decreased) was enhanced when 20% sourdough (2% Saccharomyces cerevisiae + 3% L. plantarum ATCC 14917) was included in the Balady bread formulation. The sensory properties of the Balady bread did not show differences (p > 0.05) in crust colour, crust characteristics, and crumb colour compared with the control. In contrast, grain, flavour, taste, and texture were improved.56 Jonkuviené et al.57 recorded similar results in odour and taste for rye and wheat bread supplemented with rye sourdough from Lactobacillus reuteri. Fresh and stored (7 days) rye bread with sourdough was softer than control bread, whereas wheat bread was harder than control bread. Colour did not change when rye sourdough was added to rye or wheat bread.57 The acceptance of bread with 24% sourdough from L. brevis SL778 was slightly lower than control bread (0.4% calcium propionate) due to the taste (acidic and spicy) and smell (acidic) attributes.27 Other studies reported that sourdoughs did not modify the moisture, crust colour, and hardness.51
LAB mixtures have also been tested in sourdough formulations to improve their antimicrobial activity. Alkay et al.60 prepared three mixtures of LAB to make sourdoughs and assess the antifungal activity in bread. They evaluated L. brevis 28C1B3 + L. plantarum 59E1B4 + Saccharomyces pastorium HM3 (mixture 1), Lactobacillus crustorum 34 TB6N + L. brevis 34 TB2M + S. pastorium HM3 (mixture 2), and Lactobacillus numerensis 34 TB1M + Lactobacillus paralimentarius 59O1B2 + S. pastorium HM3 (mixture 3) against 3 moulds (Aspergillus flavus, A. niger, and Alternaria alternata). The sourdoughs did not inhibit the growth of Aspergillus, and only mixture 1 or 2 delayed A. alternata growth after seven days of storage. Different amounts of sourdough can be supplemented into bread formulation to enhance the antifungal activity. Denkova et al.61 formulated sourdoughs using mixtures of five LAB and Propionibacterium (Lactobacillus paracasei RN5, L. plantarum X2, L. brevis LBRZ7, L. fermentum LBRH10 plus L. sanfranciscensis LSR or L. buchneri alone or with P. frendenreichii spp. shermanii). They incorporated three levels of sourdough (10, 15, or 20%) into bread dough. The four sourdoughs exhibited mould growth inhibition for five days of storage at 30 °C on bread when 20% of sourdough was added, whereas 10 or 15% delayed growth for 3 or 4 days.
Antimicrobial activity of commercial fermentates
Commercial fermentates are prepared from selected bacteria producers of metabolites that naturally occur in foods. The media to produce the fermentate is commonly a food-grade liquid, such as milk, dextrose and/or starch media, or hydrolysed cereal flours, among many other options. For sourdoughs-like cereal fermentates, wheat and water are the main ingredients.In the United States, for a fermentate to be GRAS by the Food and Drug Administration (FDA), it is expected that the fermentate for food use be approved by a panel of experts who testify that the fermentation ingredients, cultures, and their associated by-products have a history of safe consumption and the naturally occurring antimicrobial compounds have not been selectively purified or concentrated,62 such as cultured skim milk or cultured dextrose that are acceptable in a variety of food products.
Currently, Danisco DuPont offers antimicrobial fermentates with antifungal activity (Natamax®, Microgard® 100, 210, 400, 430, 730, 740, and CM1-50), anti -Listeria or anti-lactic bacteria (Nisaplin®, Microgard® 400, 430, 520, 730, 740, CS1-50, and CSM1-50), and for Gram-negative inhibition (Microgard® 100, 210, 400, 430, 730, and 740) for a wide range of foodstuffs including pasta, sauces, deli salads, dressings (dairy and non-dairy), puddings, liquid eggs, mashed potatoes, dips (dairy based), marinades, cooked meat and poultry, cured meats, raw-marinated- cured-injected- meats, cottage cheese, cultured dairy spreads, flavoured milk, filling confections for bakery products, high protein nutrition bars, and juice-based beverages. The Kerry Group PLC also marketed fermentates containing organic acids and peptides for food protection and preservation under the trademark of DuraFresh™ (dairy, meals, meat, plant-based foods) and UpGrade™ (bakery). BioSafe® and Bactoferm®, from Chr. Hansen, are recommended products with bacteriocins (Nisin, Sakacin, Pediocin) produced by Lactococcus lactis, Latilactobacillus curvatus, Latilactobacillus sakei, or Pediococcus acidilactici for applications in cheese, fermented meats, and meat products mainly against L. monocytogenes.13,68 Other commercial fermentates are Polyfence™35TD (blend of cultured citrus extracts, Martech Research, USA), Defence AM-P (Campus SA, Italy), Nabitor (cultured corn and cultured wheat, AB Mauri Fleischmann's, USA), and Biogard ND concentrate (cultured dextrose, Biorigin, USA). According to the previous list, there are selected fermentates with a broad antimicrobial spectrum.
Puratos Group sold several sourdough-type products for bread quality improvements, such as Fidelio, Traviata, Aroldo, and Durum; however, these products' antimicrobial properties were not described. Table 4 displays selected studies of commercial fermentates with antimicrobial activity. According to Table 4, Microgard products are the most studied fermentates. Commercial fermentates effectively inhibit bacteria in vitro, cottage cheese, and cereal-milk beverages; and delay fungi in bread. Samapundo et al.67 reported that some commercial fermentates (at levels from 0.1% to 2%) have significant inhibitory activity against Zygosaccharomyces bailii, but their potential to replace sorbate in dressings differs greatly from sensorial and microbial stability viewpoints.
| Commercial product | Test conditions | Main findings | Reference |
|---|---|---|---|
| In vitro test | |||
| Microgard™ 100 | Different concentrations of Microgard™ were tested in agar | The growth of Pseudomonas putida, Achromobacter delicatulus 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103, Salmonella Paratyphi 9281, Yersinia enterocolitica 23 103, Salmonella Paratyphi 9281, Yersinia enterocolitica 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 715, and Aeromonas hydrophila 2965 was inhibited with 1%, whereas Salmonella Typhimurium OSU and Pseudomonas aeruginosa 419 required 3% of the fermentate 715, and Aeromonas hydrophila 2965 was inhibited with 1%, whereas Salmonella Typhimurium OSU and Pseudomonas aeruginosa 419 required 3% of the fermentate |
63 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| In foods tests | |||
| Microgard™ 100 | Sorghum malt-based fermented milk beverage was added with 0.1% | Total plate counts and lactic acid bacteria were decreased by 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 after 42 days of storage at 6 °C. Yeast and moulds were inhibited only for 14 days log10 CFU mL−1 after 42 days of storage at 6 °C. Yeast and moulds were inhibited only for 14 days |
64 |
| Microgard™ 400 | Cottage cheese supplemented with 0.20, 0.35, or 0.50% | Fermentate at 0.35 and 0.50% slowed down the psychrotrophs growth by 20 and 24 days of storage at 4 °C, respectively. Coliforms reached levels of ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 after 12 or 16 days when cheese was added with 0.35 or 0.50%, whereas yeast and moulds remained at <1 log10 CFU g−1 after 12 or 16 days when cheese was added with 0.35 or 0.50%, whereas yeast and moulds remained at <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 after 16 days at both tested levels log10 CFU g−1 after 16 days at both tested levels |
65 |
| Four commercial fermentates cultured in wheat solids (FB), dextrose (FC and FD), or corn syrup solids and citric acid (FA) | 1.3 or 2% of the 4 fermentates based on the flour were supplemented in the bread formulation | 2% of fermentates (FA, FC, FD) delayed mould growth (environmental contamination) on bread slices for 13 days of storage at 22 °C whereas FB inhibited it by 20 days | 8 |
| Two commercial fermentates (cultured milk) | 0.5 or 1% of fermentates were directly added to liquid ingredients of cottage cheese (pH 6.00, 56% moisture, and 1.25% salt, made with lactic acid) | After 8 weeks of refrigerated (4 °C) and vacuum-packaged storage, the counts increased by 0.85, 0.83, and 0.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 for aerobic plate count, lactic acid bacteria, and yeast and mould, respectively log10 CFU g−1 for aerobic plate count, lactic acid bacteria, and yeast and mould, respectively |
66 |
| The tests also included cottage cheese inoculated with Listeria monocytogenes at ∼103 CFU g−1 | The time to growth for L. monocytogenes was 2.5 to 3 weeks for fermentates compared with 1 week for control. In addition, reductions of 0.32 and 0.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU g−1 were observed for fermentate 2 at 0.5 or 1.0%, respectively, which contained a bacteriocin log10 CFU g−1 were observed for fermentate 2 at 0.5 or 1.0%, respectively, which contained a bacteriocin |
||
| Six fermentates were evaluated: Polyfence™35TD, MicroGARD® 200, Defence AM-P, Nabitor Nabitor WS, and Biogard ND concentrate | Fermentates as preservatives in dressings and compared with inhibitory activity of potassium sorbate (reference preservative) | The growth of Zygosaccharomyces bailii was inhibited for at least 45 days only by Polyfence 35TD and MicroGARD 200 at 1.5%. The effect of pH on the inhibitory activity of the fermentates towards Z. bailii was expected as their antimicrobial activity is partly attributed to their content of weak organic acids such as propionic, lactic, and acetic acid | 67 |
Information about fermentates' impacts on physicochemical and sensory properties has also been reported. Microgard™ 100 did not modify the flavour and sensory acceptability of sorghum malt-based fermented milk beverage after 21 days at 6 °C,64 and its best antimicrobial activity occurred at pH ≤ 5.6 against Gram-negative bacteria.63 The Microgard™ 400 addition to cottage cheese at 0.35 or 0.50% improved the flavour, colour, appearance, body, and texture scores for 20 days at 4 °C compared with the control, which only remained at acceptable levels for 8 days. The preservative effect was attributed to the strong antimicrobial activity against psychrotrophs, yeast, and moulds.65 Bread supplemented with 1.3 or 2% of 4 commercial fermentates reduced the flavour acceptance (by 1 or 2 points) compared with bread added with calcium propionate and were scored as “neither dislikes no like” and “dislike slightly”.8
On physicochemical properties, incorporating Microgard™ 400 (0.2, 0.35, or 0.50%) in cottage cheese did not modify the moisture content during the refrigerated storage for 24 days. Microgard™ 400 prevented the changes in pH, acidity, free fatty acids, and soluble nitrogen in cottage cheese for 20 days at 4 °C when 0.35 or 0.50% were used.65 Likewise, two commercial fermentates (cultured in milk) at 0.5 or 1.0% in cottage cheese slightly changed the pH up to ±0.1 units.66
Conclusions and future trends
Fermentates come from selected LAB and are complex mixtures of inactivated or active bacteria, growth metabolites, and culture medium with antimicrobial activity. Organic acids, peptides, bacteriocins, and long-chain fatty acids are the main metabolites that exert antimicrobial activity. Fermentates are easy to handle, stable at room temperature, and without impact on food sensory properties if low amounts are used. Fermentates are considered “clean label” ingredients because LAB are safe (traditionally used), and growth media are commonly food grade. Therefore, they are excellent for food with minimal or free synthetic and sustainable preservatives.Sourdoughs can be considered a type of fermentate since they are fermented mixtures of LAB, cereal flour, and water, which are supplemented into bread formulation with subsequent heat treatment (that inactive the viable LAB). Various studies have demonstrated that sourdoughs contributed to prolonging the bread's shelf life.
To expand the understanding of fermentates, future research directions include exploring different LAB genera and species for fermentate production, utilising food-grade and derived by-products as growth media, investigating diverse food applications, and evaluating the impact of fermentates on the physicochemical and sensory properties of food products. Exploring fermentates as substitutes for CFS in LAB-derived antimicrobial research represents a necessary step toward overcoming practical and economic challenges associated with traditional methods. The inherent advantages of fermentates, such as simplified preparation and cost-effectiveness, position them as a compelling option for researchers seeking LAB's full potential in antimicrobial applications. The transition to fermentates may redefine the research on antimicrobial agents for use in foods, offering practical solutions with broad implications.
Author contributions
RHF: conceptualization, writing – original draft, writing – review & editing. ALM: conceptualization, resources, writing – original draft, supervision, writing – review & editing. EML: conceptualization, writing – original draft, writing – review & editing, supervision.Conflicts of interest
We declare that there is no conflict of interest.Acknowledgements
Author Hernández-Figueroa gratefully acknowledges financial support for his PhD studies from Universidad de las Américas Puebla (UDLAP) and the National Council for Humanities, Sciences, and Technologies (CONAHCyT) of Mexico.References
- N. Soto-Reyes, M. Dávila-Rodríguez, A. C. Lorenzo-Leal, F. Reyes-Jurado, E. Mani-López, R. Hernández-Figueroa, J. I. Morales-Camacho and A. López-Malo, Prospects for Food Applications of Products from Microorganisms, in Research and Technological Advances in Food Science, Elsevier, 2022, pp 195–229, DOI:10.1016/B978-0-12-824369-5.00019-1.
- Y. Haryani, N. A. Halid, G. S. Guat, M. A. R. Nor-Khaizura, A. Hatta, S. Sabri, S. Radu and H. Hasan, Characterization, Molecular Identification, and Antimicrobial Activity of Lactic Acid Bacteria Isolated from Selected Fermented Foods and Beverages in Malaysia, FEMS Microbiol. Lett., 2023, 370, DOI:10.1093/femsle/fnad023.
- K. F. Chai, K. R. Ng, M. Samarasiri and W. N. Chen, Precision Fermentation to Advance Fungal Food Fermentations, Curr. Opin. Food Sci., 2022, 47, 100881, DOI:10.1016/j.cofs.2022.100881.
- J. Delves-Broughton and G. N. Weber, Natamycin and Other Commercial Fermentates Used in Food Biopreservation, in Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation, Elsevier, 2011, pp. 63–99, DOI:10.1533/9780857090522.1.63.
- C. Barcenilla, M. Ducic, M. López, M. Prieto and A. Álvarez-Ordóñez, Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review, Meat Sci., 2022, 183, 108661, DOI:10.1016/j.meatsci.2021.108661.
- R. V. Rajanikar, B. H. Nataraj, H. Naithani, S. A. Ali, N. R. Panjagari and P. V. Behare, Phenyllactic Acid: A Green Compound for Food Biopreservation, Food Control, 2021, 128, 108184, DOI:10.1016/j.foodcont.2021.108184.
- A. Vasiliauskaite, J. Mileriene, B. Kasparaviciene, E. Aleksandrovas, E. Songisepp, I. Rud, L. Axelsson, S. Muizniece-Brasava, I. Ciprovica, A. Paskevicius, J. Aksomaitiene, A. Gabinaitiene, D. Uljanovas, V. Baliukoniene, L. Lutter, M. Malakauskas and L. Serniene, Screening for Antifungal Indigenous Lactobacilli Strains Isolated from Local Fermented Milk for Developing Bioprotective Fermentates and Coatings Based on Acid Whey Protein Concentrate for Fresh Cheese Quality Maintenance, Microorganisms, 2023, 11(3), 557, DOI:10.3390/microorganisms11030557.
- S. Samapundo, F. Devlieghere, A. Vroman and M. Eeckhout, Antifungal Activity of Fermentates and Their Potentiel to Replace Propionate in Bread, LWT--Food Sci. Technol., 2017, 76, 101–107, DOI:10.1016/j.lwt.2016.10.043.
- E. Mani-López, D. Arrioja-Bretón and A. López-Malo, The Impacts of Antimicrobial and Antifungal Activity of Cell-Free Supernatants from Lactic Acid Bacteria in Vitro and Foods, Compr. Rev. Food Sci. Food Saf., 2021, 21(1), 604–641, DOI:10.1111/1541-4337.12872.
- A. B. Aljohani, A. M. Al-Hejin and A. B. Shori, Bacteriocins as Promising Antimicrobial Peptides, Definition, Classification, and Their Potential Applications in Cheeses, Food Sci. Technol., 2023, 43, e118021, DOI:10.1590/fst.118021.
- E. L. De Souza, K. Á. De Oliveira and M. E. De Oliveira, Influence of Lactic Acid Bacteria Metabolites on Physical and Chemical Food Properties, Curr. Opin. Food Sci., 2023, 49, 100981, DOI:10.1016/j.cofs.2022.100981.
- L. Garnier, J. Mounier, S. Lê, A. Pawtowski, N. Pinon, B. Camier, M. Chatel, G. Garric, A. Thierry, E. Coton and F. Valence, Development of Antifungal Ingredients for Dairy Products: From in Vitro Screening to Pilot Scale Application, Food Microbiol., 2019, 81, 97–107, DOI:10.1016/j.fm.2018.11.003.
- H. Mathur, T. P. Beresford and P. D. Cotter, Health Benefits of Lactic Acid Bacteria (LAB) Fermentates, Nutrients, 2020, 12(6), 1679, DOI:10.3390/nu12061679.
- D. Elsser-Gravesen and A. Elsser-Gravesen, Biopreservatives, in Biotechnology of Food and Feed Additives, ed. Zorn, H. and Czermak, P., Advances in Biochemical Engineering/Biotechnology, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, vol. 143, pp. 29–49, DOI:10.1007/10_2013_234.
- M. Moradi, S. A. Kousheh, H. Almasi, A. Alizadeh, J. T. Guimarães, N. Yılmaz and A. Lotfi, Postbiotics Produced by Lactic Acid Bacteria: The next Frontier in Food Safety, Compr. Rev. Food Sci. Food Saf., 2020, 19(6), 3390–3415, DOI:10.1111/1541-4337.12613.
- G. Weber and W. Broich, Shelf Life Extension of Cultured Dairy Foods, Cult. Dairy Prod. J., 1986, 21, 19–23 Search PubMed.
- H. Fidan, T. Esatbeyoglu, V. Simat, M. Trif, G. Tabanelli, T. Kostka, C. Montanari, S. A. Ibrahim and F. Özogul, Recent Developments of Lactic Acid Bacteria and Their Metabolites on Foodborne Pathogens and Spoilage Bacteria: Facts and Gaps, Food Biosci., 2022, 47, 101741, DOI:10.1016/j.fbio.2022.101741.
- S. Khubber, F. J. Marti-Quijal, I. Tomasevic, F. Remize and F. J. Barba, Lactic Acid Fermentation as a Useful Strategy to Recover Antimicrobial and Antioxidant Compounds from Food and By-Products, Curr. Opin. Food Sci., 2022, 43, 189–198, DOI:10.1016/j.cofs.2021.11.013.
- J. I. I. Fugaban, E. S. Jung, S. D. Todorov and W. H. Holzapfel, Evaluation of Antifungal Metabolites Produced by Lactic Acid Bacteria, Probiotics Antimicrob. Proteins, 2023, 15(5), 1447–1463, DOI:10.1007/s12602-022-09995-5.
- C. De Muynck, A. I. J. Leroy, S. De Maeseneire, F. Arnaut, W. Soetaert and E. J. Vandamme, Potential of Selected Lactic Acid Bacteria to Produce Food Compatible Antifungal Metabolites, Microbiol. Res., 2004, 159(4), 339–346, DOI:10.1016/j.micres.2004.07.002.
- F. Valerio, P. Lavermicocca, M. Pascale and A. Visconti, Production of Phenyllactic Acid by Lactic Acid Bacteria: An Approach to the Selection of Strains Contributing to Food Quality and Preservation, FEMS Microbiol. Lett., 2004, 233(2), 289–295, DOI:10.1111/j.1574-6968.2004.tb09494.x.
- E. D. L. Chaves De Lima, J. De Moura Fernandes and H. R. Cardarelli, Optimized Fermentation of Goat Cheese Whey with Lactococcus lactis for Production of Antilisterial Bacteriocin-like Substances, LWT, 2017, 84, 710–716, DOI:10.1016/j.lwt.2017.06.040.
- M. G. Venegas-Ortega, A. C. Flores-Gallegos, J. L. Martínez-Hernández, C. N. Aguilar and G. V. Nevárez-Moorillón, Production of Bioactive Peptides from Lactic Acid Bacteria: A Sustainable Approach for Healthier Foods, Compr. Rev. Food Sci. Food Saf., 2019, 18(4), 1039–1051, DOI:10.1111/1541-4337.12455.
- K. Peng, M. Koubaa, O. Bals and E. Vorobiev, Recent Insights in the Impact of Emerging Technologies on Lactic Acid Bacteria: A Review, Food Res. Int., 2020, 137, 109544, DOI:10.1016/j.foodres.2020.109544.
- M. Ariana and J. Hamedi, Enhanced Production of Nisin by Co-Culture of Lactococcus lactis subsp. lactis and Yarrowia lipolytica in Molasses Based Medium, J. Biotechnol., 2017, 256, 21–26, DOI:10.1016/j.jbiotec.2017.07.009.
- S. M. Morgan, M. Galvin, J. Kelly, R. P. Ross and C. Hill, Development of a Lacticin 3147–Enriched Whey Powder with Inhibitory Activity against Foodborne Pathogens, J. Food Prot., 1999, 62(9), 1011–1016, DOI:10.4315/0362-028X-62.9.1011.
- R. H. Hernández-Figueroa, E. Mani-López and A. López-Malo, Antifungal Capacity of Poolish-Type Sourdough Supplemented with Lactiplantibacillus plantarum and Its Aqueous Extracts In Vitro and Bread, Antibiotics, 2022, 11(12), 1813, DOI:10.3390/antibiotics11121813.
- R. H. Hernández-Figueroa, E. Mani-López and A. López-Malo, Antifungal Activity of Wheat-Flour Sourdough (Type II) from Two Different Lactobacillus in Vitro and Bread, Appl. Food Res., 2023, 3(2), 100319, DOI:10.1016/j.afres.2023.100319.
- N. Ahansaz, A. Tarrah, S. Pakroo, V. Corich and A. Giacomini, Lactic Acid Bacteria in Dairy Foods: Prime Sources of Antimicrobial Compounds, Fermentation, 2023, 9(11), 964, DOI:10.3390/fermentation9110964.
- M. K. Yadav and S. K. Tiwari, Methods for Determination of Antimicrobial Activity of Bacteriocins of Lactic Acid Bacteria, Microbiology, 2023, 92(6), 745–765, DOI:10.1134/S0026261723600520.
- J. A. Reis, A. T. Paula, S. N. Casarotti and A. L. B. Penna, Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications, Food Eng. Rev., 2012, 4(2), 124–140, DOI:10.1007/s12393-012-9051-2.
- P. Saranraj, M. A. Naidu and P. Sivasakthivelan, Lactic Acid Bacteria and its Antimicrobial Properties: A Review, 2010, vol. 4 Search PubMed.
- H. W. Tang, P. Phapugrangkul, H. M. Fauzi and J. S. Tan, Lactic Acid Bacteria Bacteriocin, an Antimicrobial Peptide Effective Against Multidrug Resistance: A Comprehensive Review, Int. J. Pept. Res. Ther., 2022, 28(1), 14, DOI:10.1007/s10989-021-10317-6.
- A. H. D. N. Rangel, D. A. F. V. D. A. Bezerra, D. C. Sales, E. D. O. M. Araújo, L. M. D. Lucena, A. L. F. Porto, Í. V. U. M. Véras, A. F. Lacerda, C. V. D. M. Ribeiro and K. Anaya, An Overview of the Occurrence of Bioactive Peptides in Different Types of Cheeses, Foods, 2023, 12(23), 4261, DOI:10.3390/foods12234261.
- L. De Vuyst and F. Leroy, Bacteriocins from Lactic Acid Bacteria: Production, Purification, and Food Applications, Microb. Physiol., 2007, 13(4), 194–199, DOI:10.1159/000104752.
- H. Wu, C. Guang, W. Zhang and W. Mu, Recent Development of Phenyllactic Acid: Physicochemical Properties, Biotechnological Production Strategies and Applications, Crit. Rev. Biotechnol., 2023, 43(2), 293–308, DOI:10.1080/07388551.2021.2010645.
- M. Zimina, O. Babich, A. Prosekov, S. Sukhikh, S. Ivanova, M. Shevchenko and S. Noskova, Overview of Global Trends in Classification, Methods of Preparation and Application of Bacteriocins, Antibiotics, 2020, 9(9), 553, DOI:10.3390/antibiotics9090553.
- D. Arrioja-Bretón, E. Mani-López, E. Palou and A. López-Malo, Antimicrobial Activity and Storage Stability of Cell-Free Supernatants from Lactic Acid Bacteria and Their Applications with Fresh Beef, Food Control, 2020, 115, 107286, DOI:10.1016/j.foodcont.2020.107286.
- D. Arrioja-Bretón, E. Mani-López, H. Bach and A. López-Malo, Antimicrobial Activity of Protein-Containing Fractions Isolated from Lactobacillus plantarum NRRL B-4496 Culture, Braz. J. Microbiol., 2020, 51(3), 1289–1296, DOI:10.1007/s42770-020-00266-5.
- C.-G. Lee, K. H. Cha, G.-C. Kim, S.-H. Im and H.-K. Kwon, Exploring Probiotic Effector Molecules and Their Mode of Action in Gut–Immune Interactions, FEMS Microbiol. Rev., 2023, 47(4), fuad046, DOI:10.1093/femsre/fuad046.
- E. M. Balciunas, F. A. Castillo Martinez, S. D. Todorov, B. D. G. D. M. Franco, A. Converti and R. P. D. S. Oliveira, Novel Biotechnological Applications of Bacteriocins: A Review, Food Control, 2013, 32(1), 134–142, DOI:10.1016/j.foodcont.2012.11.025.
- S. S. Chaudhari and D. V. Gokhale, Phenyllactic Acid: A Potential Antimicrobial Compound in Lactic Acid Bacteria, J. Bacteriol. Mycol., 2016, 2(5), 121–125, DOI:10.15406/jbmoa.2016.02.00037.
- A. López-Malo, E. Mani-López, P. M. Davidson and E. Palou, Methods for Activity Assay and Evaluation of Results, in Antimicrobials in Food, CRC Press, 4th edn, 2021, pp. 13–39 Search PubMed.
- D. Field, K. Daly, P. M. O'Connor, P. D. Cotter, C. Hill and R. P. Ross, Efficacies of Nisin A and Nisin V Semipurified Preparations Alone and in Combination with Plant Essential Oils for Controlling Listeria monocytogenes, Appl. Environ. Microbiol., 2015, 81(8), 2762–2769, DOI:10.1128/AEM.00070-15.
- G. K. İncili, M. Akgöl, P. Karatepe, A. Tekin, H. Kanmaz, B. Kaya and A. A. Hayaloğlu, Whole-Cell Postbiotics: An Innovative Approach for Extending the Shelf Life and Controlling Major Foodborne Pathogens in Chicken Breast Fillets, Food Bioprocess Technol., 2023, 16(7), 1502–1524, DOI:10.1007/s11947-023-03009-0.
- P. M. Oliveira, B. Brosnan, A. Furey, A. Coffey, E. Zannini and E. K. Arendt, Lactic Acid Bacteria Bioprotection Applied to the Malting Process. Part I: Strain Characterization and Identification of Antifungal Compounds, Food Control, 2015, 51, 433–443, DOI:10.1016/j.foodcont.2014.07.004.
- D. Kantachote, P. Prachyakij, W. Charernjiratrakul, M. Ongsakul, Y. Duangjitcharoen, C. Chaiyasut, T. Nitoda and H. Kanzaki, Characterization of the Antiyeast Compound and Probiotic Properties of a Starter Lactobacillus plantarum DW3 for Possible Use in Fermented Plant Beverages, Electron. J. Biotechnol., 2010, 13(5), 2–3, DOI:10.2225/vol13-issue5-fulltext-1.
- S. M. Morgan, M. Galvin, R. P. Ross and C. Hill, Evaluation of a Spray-Dried Lacticin 3147 Powder for the Control of Listeria monocytogenes and Bacillus cereus in a Range of Food Systems, Lett. Appl. Microbiol., 2001, 33(5), 387–391, DOI:10.1046/j.1472-765X.2001.01016.x.
- R. Coda, A. Cassone, C. G. Rizzello, L. Nionelli, G. Cardinali and M. Gobbetti, Antifungal Activity of Wickerhamomyces anomalus and Lactobacillus plantarum during Sourdough Fermentation: Identification of Novel Compounds and Long-Term Effect during Storage of Wheat Bread, Appl. Environ. Microbiol., 2011, 77(10), 3484–3492, DOI:10.1128/AEM.02669-10.
- C. G. Rizzello, A. Cassone, R. Coda and M. Gobbetti, Antifungal Activity of Sourdough Fermented Wheat Germ Used as an Ingredient for Bread Making, Food Chem., 2011, 127(3), 952–959, DOI:10.1016/j.foodchem.2011.01.063.
- L. A. M. Ryan, E. Zannini, F. Dal Bello, A. Pawlowska, P. Koehler and E. K. Arendt, Lactobacillus amylovorus DSM 19280 as a Novel Food-Grade Antifungal Agent for Bakery Products, Int. J. Food Microbiol., 2011, 146(3), 276–283, DOI:10.1016/j.ijfoodmicro.2011.02.036.
- B. A. Black, E. Zannini, J. M. Curtis and M. G. G�nzle, Antifungal Hydroxy Fatty Acids Produced during Sourdough Fermentation: Microbial and Enzymatic Pathways, and Antifungal Activity in Bread, Appl. Environ. Microbiol., 2013, 79(6), 1866–1873, DOI:10.1128/AEM.03784-12.
- C. L. Gerez, M. J. Fornaguera, M. D. Obregozo, G. Font De Valdez and M. I. Torino, Antifungal Starter Culture for Packed Bread: Influence of Two Storage Conditions, Rev. Argent. Microbiol., 2015, 47(2), 118–124, DOI:10.1016/j.ram.2015.02.002.
- C. Axel, B. Brosnan, E. Zannini, L. C. Peyer, A. Furey, A. Coffey and E. K. Arendt, Antifungal Activities of Three Different Lactobacillus Species and Their Production of Antifungal Carboxylic Acids in Wheat Sourdough, Appl. Microbiol. Biotechnol., 2016, 100(4), 1701–1711, DOI:10.1007/s00253-015-7051-x.
- C. Axel, B. Brosnan, E. Zannini, A. Furey, A. Coffey and E. K. Arendt, Antifungal Sourdough Lactic Acid Bacteria as Biopreservation Tool in Quinoa and Rice Bread, Int. J. Food Microbiol., 2016, 239, 86–94, DOI:10.1016/j.ijfoodmicro.2016.05.006.
- S. M. Mohsen, M. H. Aly, A. A. Attia and D. B. Osman, Effect of Sourdough on Shelf Life, Freshness and Sensory Characteristics of Egyptian Balady Bread, Environ. Microbiol., 2016, 4(2), 39–45 Search PubMed.
- D. Jonkuvienė, L. Vaičiulytė-Funk, J. Šalomskienė, G. Alenčikienė and A. Mieželienė, Potential of Lactobacillus reuteri from Spontaneous Sourdough as a Starter Additive for Improving Quality Parameters of Bread, Food Technol. Biotechnol., 2016, 54(3), 342–350, DOI:10.17113/ftb.54.03.16.4143.
- C. Luz, V. D’Opazo, J. Mañes and G. Meca, Antifungal Activity and Shelf Life Extension of Loaf Bread Produced with Sourdough Fermented by Lactobacillus Strains, J. Food Process. Preserv., 2019, 43(10), e14126, DOI:10.1111/jfpp.14126.
- C. Garofalo, E. Zannini, L. Aquilanti, G. Silvestri, O. Fierro, G. Picariello and F. Clementi, Selection of Sourdough Lactobacilli with Antifungal Activity for Use as Biopreservatives in Bakery Products, J. Agric. Food Chem., 2012, 60(31), 7719–7728, DOI:10.1021/jf301173u.
- Z. Alkay, H. Kilmanoğlu and M. Z. Durak, Prevention of Sourdough Bread Mould Spoliage by Antifungal Lactic Acid Bacteria Fermentation, Eur. J. Sci. Technol., 2020, 379–388, DOI:10.31590/ejosat.646043.
- R. Denkova, S. Ilieva, Z. Denkova, L. Georgieva, M. Yordanova, D. Nikolova and Y. Evstatieva, Production of Wheat Bread without Preservatives Using Sourdough Starters, Biotechnol. Biotechnol. Equip., 2014, 28(5), 889–898, DOI:10.1080/13102818.2014.965057.
- Food and Drug Administration, Microorganisms & Microbial-Derived Ingredients Used in Food, 2023, https://www.fda.gov/food/generally-recognized-safe-gras/microorganisms-microbial-derived-ingredients-used-food-partial-list Search PubMed.
- N. Al-Zoreky, J. W. Ayres and W. E. Sandine, Antimicrobial Activity of Microgard™ Against Food Spoilage and Pathogenic Microorganisms, J. Dairy Sci., 1991, 74(3), 758–763, DOI:10.3168/jds.S0022-0302(91)78222-2.
- S. A. Hussain, F. C. Garg and D. Pal, Effect of Different Preservative Treatments on the Shelf-Life of Sorghum Malt Based Fermented Milk Beverage, J. Food Sci. Technol., 2014, 51(8), 1582–1587, DOI:10.1007/s13197-012-0657-4.
- S. Makhal, S. K. Kanawjia and A. Giri, Effect of microGARD on Keeping Quality of Direct Acidified Cottage Cheese, J. Food Sci. Technol., 2015, 52(2), 936–943, DOI:10.1007/s13197-013-1055-2.
- S. K. Engstrom, K. M. Anderson and K. A. Glass, Effect of Commercial Protective Cultures and Bacterial Fermentates on Listeria monocytogenes Growth in a Refrigerated High-Moisture Model Cheese, J. Food Prot., 2021, 84(5), 772–780, DOI:10.4315/JFP-20-247.
- S. Samapundo, I. De Baenst, M. Eeckhout and F. Devlieghere, Inhibitory Activity of Fermentates towards Zygosaccharomyces bailii and Their Potential to Replace Potassium Sorbate in Dressings, LWT--Food Sci. Technol., 2017, 79, 309–315, DOI:10.1016/j.lwt.2017.01.055.
- M. L. Chikindas, R. Weeks, D. Drider, V. A. Chistyakov and L. M. Dicks, Functions and Emerging Applications of Bacteriocins, Curr. Opin. Biotechnol., 2018, 49, 23–28, DOI:10.1016/j.copbio.2017.07.011.
| This journal is © The Royal Society of Chemistry 2024 |