 Open Access Article
Open Access ArticleProcess characterization for tisane development using pomegranate waste: an herbal drink optimization strategy
Aastha
Dewan
a,
Sanya
Dawra
a,
Nita
Kaushik
a,
Ajay
Singh
 b,
Sheetal
Thakur
b,
Sheetal
Thakur
 *c,
Sandeep
Kaur
d and
Janifer Raj
Xavier
e
*c,
Sandeep
Kaur
d and
Janifer Raj
Xavier
e
aDepartment of Food Technology, Guru Jambheshwar University of Science and Technology, Hisar, Haryana, India
bDepartment of Food Technology, Mata Gujri College, Fatehgarh Sahib, Punjab, India
cUniversity Centre for Research and Development, Department of Biotechnology, Chandigarh University, Gharuan-Mohali, Punjab 140413, India. E-mail: sheetalt2402@gmail.com
dDepartment of Microbiology, Punjab Agricultural University, Ludhiana, Punjab, India
eDefence Food Research Laboratory (DFRL), Defence Research and Development Organisation (DRDO), Ministry of Defence, Mysore, Karnataka 570 011, India
First published on 22nd April 2024
Abstract
The present study aimed to utilize pomegranate waste in the form of its peel and seed for the development of an herbal extract called “tisane”. A series of preliminary evaluations were performed where the dried pomegranate peel powder (PPP) and pomegranate seed powder (PSP) were studied for proximate analysis viz. moisture, crude protein, crude fat, ash, crude fiber and carbohydrate content. Notably, higher macromolecule percentages were recorded in both peel and seed powder, with peel containing 79.05% carbohydrate content and seed displaying 34% crude fiber content. The study then explored various blends of PPP and PSP viz. 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 6
3, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and 5
4, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, resulting in an optimized blend, B-73 (7PPP:3PSP), exhibiting the highest antioxidant potential (98.7%), total phenolic content (46.926 mg GAE/100 g), and total flavonoid content (91 mg CE/100 g) along with a higher diffusivity rate (0.678 g min−1). The combination of PPP and PSP showed better results with respect to the antioxidant behavior when compared with the individual contribution of PPP and PSP. Furthermore, analysis of total solubility solids (TSS), pH and color of tisane prepared from different blends was carried out. In sensory evaluation, blend D-55 showed the highest overall acceptability score (8.7) surpassing other blends as well as market samples. Despite this, B-73 emerged as the best-formulated blend, boasting the highest concentration of bioactive constituents, suggesting its potential for promoting well-being when consumed as an herbal drink. This research underscores the innovative use of pomegranate by-products to develop a healthy beverage rich in bioactive components, offering a sustainable approach to utilizing pomegranate waste.
5, resulting in an optimized blend, B-73 (7PPP:3PSP), exhibiting the highest antioxidant potential (98.7%), total phenolic content (46.926 mg GAE/100 g), and total flavonoid content (91 mg CE/100 g) along with a higher diffusivity rate (0.678 g min−1). The combination of PPP and PSP showed better results with respect to the antioxidant behavior when compared with the individual contribution of PPP and PSP. Furthermore, analysis of total solubility solids (TSS), pH and color of tisane prepared from different blends was carried out. In sensory evaluation, blend D-55 showed the highest overall acceptability score (8.7) surpassing other blends as well as market samples. Despite this, B-73 emerged as the best-formulated blend, boasting the highest concentration of bioactive constituents, suggesting its potential for promoting well-being when consumed as an herbal drink. This research underscores the innovative use of pomegranate by-products to develop a healthy beverage rich in bioactive components, offering a sustainable approach to utilizing pomegranate waste.
Sustainability spotlightFood product development is considered as a cost-effective and scientifically proven intervention to address the issues of deficiencies related to micronutrients and in promoting the health aspects of mankind. It becomes a more sustainable option, environmentally as well as economically, when the peels and seeds, which are generally considered as food waste, can be reutilized for the development of healthy products. In the present study, tisane, a herbal drink has been developed by fortifying with pomegranate peel and seed waste. The proximate, chemical and sensory analysis showed that pomegranate waste is a vital source of phenols, flavonoids and antioxidants along with sensory acceptability for developed herbal tea. Therefore, pomegranate waste left after the extraction of juice could deliver nutritionally beneficial characteristics that can promote well-being. |
1. Introduction
Pomegranate, scientifically named Punica granatum L., is an ancient mystical fruit that grows across the temperate zone, including the northern Himalayas and certain areas in the United States.1 The medicinal benefits of pomegranate make it a widely acceptable fruit among the majority of people. The processing of pomegranate into jams, juices, wine and other products results in a significant volume of waste. Every year, pomegranate production and processing reach approximately 3 million tons, leading to a global production of around 1.62 million tons of byproducts.2 However, the nutrient rich profile of pomegranate necessitates appropriate handling techniques as the leftovers in the form of seed and peel are either discarded or used for manure conversion.Pomegranate waste (seeds and peels) contains several valuable constituents including minerals, proteins, dietary fiber, etc. and is considered to have anti-inflammatory, anti-cancer and anti-microbial properties. Previous studies have asserted that pomegranate peel contains several minerals (calcium, magnesium, phosphorus, potassium and sodium), sugars (fructose, glucose, sucrose and maltose), fatty acids, crude fibers, proteins, organic acids and alkaloids.3 92% of the total antioxidant compounds found in pomegranates are attributed to water-soluble tannins present in the peel membrane.4 It has been reported that pomegranate peel rich in ellagic acid is used in derma care to protect against UV rays and other related disorders viz. de-pigmentation, skin ageing and skin cancer issues.5 Gallotannins, ellagitannins, anthocyanins, hydroxycinnamic acids, and hydroxybenzoic acids have also been studied to be present in the pomegranate peel.6 Encapsulated pomegranate peel phenolics were utilized as bio-functional ingredients by Kaderides et al. in the formulation of cookies.7 In a similar way, researchers have claimed that the pomegranate seed matrix contains lignins,8 fusion products of cell wall components and hydroxybenzoic/cinnamic acids, isoflavones, and potently antioxidant lignin derivatives.9 Also, pomegranate seed oil is considered as an enriched source of steroidal as well as non-steroidal estrogens.10 The pomegranate fruit, its peel and seed oil demonstrate anti-cancer properties and are considered to have the potential to effectively counteract the invasion of cancer cells.11
Taking into account the different challenges in the area of food-based industries, efforts must be made to optimize and standardize the processing technologies along with the alternative ways to utilize and hence, reduce the generated food leftovers. In this context, developing a healthy formulation by using food waste could offer solutions to various challenges concerning human health and the environment. Researchers have started focusing on developing herbal beverages and value-added functional drinks like tisane by utilizing fruit waste. For instance, Andika et al.12 utilized red dragon fruit peel, and Trimedona et al.13 and Al-Zughbi and Krayem14 used ginger peel and quince fruit peel, respectively for the development of herbal drinks. Hence, in order to transform pomegranate leftovers into valuable products, the present work was conceptualized and designed with an aim to utilize the bioactive constituents of pomegranate seed and peel for the development of wellness drinks.15 Tisane, often termed herbal tea, is a beverage created from the infusion or decoction of different spices, herbs, or any other plant material in hot water, usually without caffeine and is consumed for therapeutic purposes.16
Pomegranate fruit, along with its peel and seeds, is a rich source of various bioactive compounds viz. antioxidants and total polyphenolics.17 Therefore, exploring such a material to be utilized by the food and pharma industries for propagation of novel products will somehow help to minimize the waste and also potentially beneficial for the well-being of mankind. The major aim of this research was to develop “tisane” by using pomegranate waste in the form of seed and peel through a series of standardization phases. The prepared blends of peel and seed powder were subjected to proximate and phytochemical analysis and the prepared tisane compositions were further analyzed and compared to that of the market samples.
2. Materials and methods
2.1. Raw material procurement and sample preparation
The pomegranate waste was collected from the regional local market of Hisar, Haryana, India. The variety “Phule Arakta” was selected based on the high yield of seeds and peel.11,18 Empty filter tea bags were purchased from an online store (Flipkart). Manually, arils were separated from peels, further juice was extracted from seeds using a manual screw juicer to obtain the seeds without juice. The waste (peels and seeds) was thoroughly washed and dried in a hot air oven (60 ± 5 °C for 42 h) (Fig. 1a), packed in suitable metallized airtight polyester pouches (Fig. 1b) and were stored in a deep freezer. The stored peels and seeds were brought to room temperature and then ground to a fine powder using a Sujata mixer-grinder with a pause of 5 seconds in between grinding cycles to avoid heat production and further 400 mesh size was used to sieve the powder in order to get the uniform particle size. The prepared powdered samples of pomegranate peels and seeds were stored in metallized pouches separately and stored in the dark for further analysis.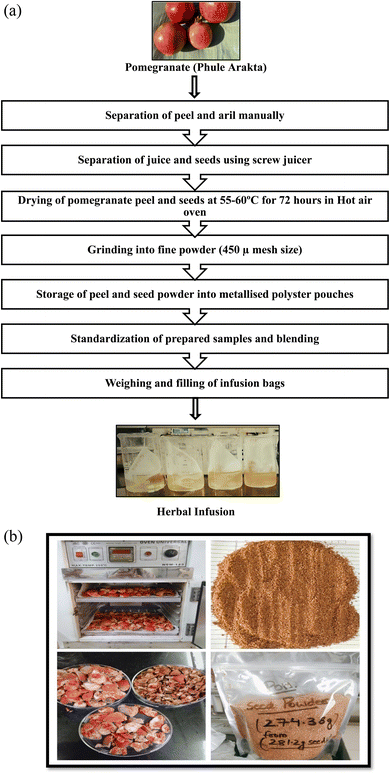 | ||
| Fig. 1 (a) Schematic illustration to develop herb infusion formulation. (b) Pictorial view for the preparation of pomegranate peel and seed powder. | ||
2.2. Proximate analysis of dried pomegranate peel and seed powder
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 copper sulphate and potassium sulphate) and 15 mL of nitrogen-free concentrated sulphuric acid (conc. H2SO2) were added. The tubes were then moved to the digestion unit where the content was digested for 90 min at 420 °C. Then, the digested content was allowed to cool to room temperature, and each tube was loaded into Kjeldahl distillation apparatus, and 45 mL of 40% NaOH was automatically added for neutralization. The ammonia that had been freed was concentrated and collected in a 50 mL saturated boric acid solution containing a mixed indicator. A reagent blank was immediately run after the distillate was titrated against 0.02 N H2SO4.
4 copper sulphate and potassium sulphate) and 15 mL of nitrogen-free concentrated sulphuric acid (conc. H2SO2) were added. The tubes were then moved to the digestion unit where the content was digested for 90 min at 420 °C. Then, the digested content was allowed to cool to room temperature, and each tube was loaded into Kjeldahl distillation apparatus, and 45 mL of 40% NaOH was automatically added for neutralization. The ammonia that had been freed was concentrated and collected in a 50 mL saturated boric acid solution containing a mixed indicator. A reagent blank was immediately run after the distillate was titrated against 0.02 N H2SO4.
Crude protein content was calculated as % nitrogen × 6.25.
| Total carbohydrate (%) = 100 − (moisture% + protein% + fat% + ash% + crude fibre%) | (1) |
2.3. Chemical analysis
2.4. Standardization of “tisane”
Pomegranate peel and seed powder were blended into four different proportions as mentioned in Table 1.| Sample code | Pomegranate peel powder (PPP) (%) | Pomegranate seed powder (PSP) (%) |
|---|---|---|
| A-91 | 90 | 10 |
| B-73 | 70 | 30 |
| C-64 | 60 | 40 |
| D-55 | 50 | 50 |
2.5. Preparation of blends in tea bags
Dried and powdered samples (PPP and PSP) were blended using a Sujata mixer grinder as per the prepared proportions (Table 1), and packed in tea bags with a total weight of 3 g per bag.2.6. “Tisane” preparation and analysis
The formulation was standardized using a teacup in which the volume was measured and fixed as 125 mL. Based on this, 125 mL of distilled water was taken and heated using a pan till it reached the boiling point, then the prepared teabag was added to it and allowed to boil for 3 min. After boiling, the infusion was allowed to steep for 2 min for the extraction of the essence and was then transferred to a cup. All samples were prepared in a similar manner. The prepared tisane samples along with market samples have been shown in Fig. 2. | ||
| Fig. 2 Formulated herbal infusions along with market samples (sample sequence from left starts with A-91, B-73, C-64, D-55, Brand-A, Brand-B, Brand-C). | ||
| W0 − W1 = DR | (2) |
2.7. Comparative evaluation of formulated tisane with market available herbal teas
In order to understand the sensory aspects and acceptability of pomegranate waste formulated tea, different parameters were tested and compared with market available herbal teas (labelled as Brand A, B and C). The selection criteria for off the shelf herbal teas were their composition i.e., the teas composed of fruit and flower extracts were randomly selected for study and compared with formulated tisane. These brands were formulated with synthetic colors and additives to provide fruity flavor. Parameters like pH, TSS, diffusion rate, color and sensory evaluation were determined for comparison.2.8. Statistical analysis
The data reported are average of triplicate readings and otherwise stated. The data given in tables were subjected to one-way analysis of variance (ANOVA) to identify the significant differences among the samples and means were compared by the least significant difference (LSD) test at P < 0.05. All statistical analyses were done using Statistical Package for the Social Sciences (SPSS) 19.0.3. Results and discussion
3.1. Proximate composition of pomegranate peel powder (PPP) and pomegranate seed powder (PSP)
The proximate composition of PPP and PSP has been given in Table 2. The moisture, protein, ash and fat content of PPP were observed to be as 12.26%, 3.08%, 3.56%, and 1.52%, respectively (Table 2) which was in proximity with the results obtained by Akhtar et al.6 The carbohydrate content of PPP was 79.05% which was consistent with the results of Chaudhary et al.26 claiming the total carbohydrate content of 79.6% in pomegranate peel. Carbohydrates are naturally occurring organic substances essential for the sustenance and nourishment of both plant and animal life, and they serve as fundamental raw materials in numerous industries.27 Pomegranate peel constituting 43% w/w of the fruit is a rich source of bioactive compounds, including complex carbohydrates, minerals and dietary fiber.2 The moisture, fat, protein, and ash content of PSP were 6.87%, 32.87%, 13.67%, and 1.49%, respectively which were consistent with the results reported by Jalal et al.28 The pomegranate seeds from different cultivars were found to be a rich source of calcium, magnesium and sodium, while iron, manganese and zinc were present in trace amounts.29 A previous study has claimed that the fat in PSP comprises 45 identified different fatty acids where the major type is punicic acid followed by linoleic and oleic acids.2,30 Several studies have revealed the health benefits of punicic acid in reducing the risk of skin cancer, type-II diabetes and, anti-obesity, anti-inflammatory and anti-oxidant properties.2 A significant amount of crude fiber content was recorded in PPP and PSP where crude fiber in PSP (34%) was observed to be higher than that of PPP (10.25%). PPP is considered a good source of ash, crude fiber and carbohydrates and PSP is considered a good source of crude proteins crude fat and crude fiber.28| Parameters | Pomegranate peel powder (PPP) (%) | Pomegranate seed powder (PSP) (%) |
|---|---|---|
| a Data represented as mean value ± standard deviation. Values with different superscripts in a column differ significantly (P ≤ 0.05). | ||
| Moisture content (%) | 12.26b ± 0.02 | 6.87a ± 0.01 |
| Ash content (%) | 3.56b ± 0.02 | 1.49a ± 0.02 |
| Crude fat content (%) | 1.52a ± 0.04 | 32.87b ± 0.06 |
| Crude protein content (%) | 3.08a ± 0.02 | 13.67b ± 0.05 |
| Crude fiber content (%) | 10.25a ± 0.03 | 34.00b ± 0.07 |
| Total carbohydrate (%) | 79.05b ± 0.01 | 12.12a ± 0.02 |
3.2. Phytochemical analysis comprising total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity
The results of the phytochemical analysis of raw materials, i.e., pomegranate peel and seed powder along with prepared blends for standardization of tisane are given in Table 3. The plant material used in the present study was evaluated for its phytochemical properties and the results showed the presence of secondary metabolites. The findings indicated that PPP had much greater TPC and TFC values than that of PSP. Pomegranate peel was observed to be rich in tannins, such as gallic and ellagic acid.31 Furthermore, blends had greater TPC and TFC levels than native crude extracts of peel and seeds. Phenols encompass a broad spectrum of complex organic chemical compounds, the quantity and quality of which vary based on growth phases, environmental conditions, extraction conditions, extraction solutions employed, and other variables.32 The phenolic compounds present in pomegranate seeds and pericarp have been documented to display potent antioxidant activity.33 The results revealed that the TPC of blend B-73 was the highest, followed by the TPC of blends D-55, C-64, and A-91, respectively. Similarly, blend B-73 had the highest TFC (91.14 mg CE/100 g) followed by C-64, A-91 and D-55. The TPC and TFC results showed that standardization with 70% peel powder and 30% seed powder had the best results in the composition of herbal tisane.| Samples | TPC (mg GAE/100 g) | TFC (mg CE/100 g) | AOA (% inhibition) |
|---|---|---|---|
| a Data represented as mean value ± standard deviation. Values with different superscripts in a column differ significantly (P ≤ 0.05). | |||
| PPP (crude) | 34.11b ± 0.02 | 56.22b ± 0.03 | 40.12a ± 0.02 |
| PSP (crude) | 15.05a ± 0.04 | 47.21a ± 0.01 | 87.33c ± 0.04 |
| A-91 | 45.17c ± 0.03 | 72.11d ± 0.06 | 80.41b ± 0.01 |
| B-73 | 46.93e ± 0.01 | 91.14f ± 0.05 | 98.72f ± 0.02 |
| C-64 | 45.92d ± 0.02 | 75.05e ± 0.04 | 89.84d ± 0.03 |
| D-55 | 46.07e ± 0.02 | 68.55c ± 0.05 | 97.71e ± 0.06 |
Due to the presence of colored pigments and other bioactive compounds, fruits and vegetables are considered a principal source of natural antioxidant compounds.34 PSP showed a higher antioxidant activity than that of PPP (Table 3). Punica granatum fruit waste extracts, i.e., peel and seed powder exhibited enhanced phenolic content and antioxidant capabilities, indicating that they can be employed as a natural source of antioxidants.35 Pomegranate pericarp exhibits antioxidant behavior due to its polyphenolic elements (majorly, punicalagin and ellagic acid) which helps in the inhibition of the oxidation process, even in minute quantities and hence, display a positive influence on the human body.36 Blend B-73 was found to have the highest antioxidant activity, followed by D-55, C-64 and A-91. PSP and PPP extracts were observed to be potent free radical scavengers and antioxidants that bind with free radicals. A similar study of antioxidant extraction from pomegranate peel and seed by using water, acetone and methanol has been reported previously.37,38
3.3. Water holding capacity (WHC) of PSP and PPP
WHC refers to a moist object's retaining water under the influence of an external centrifugal/gravitational pull or tension.39 Water is retained in dietary fiber via absorption and adsorption mechanisms, with some water remaining outside the fiber matrix (free water).40 As per the observations, PPP and PSP showed water holding capacities of 4.24 and 4.47 times their own weight, respectively. These findings were in accordance with the results obtained by Jalal et al.28 who reported that PPP and PSP exhibited a water holding capacity of 4.84 and 4.45 times its own weight. For other fibrous residues like, sugarcane,41 pear,42 similar results were reported by researchers in previous studies. Hence, our results indicate that WHC is a critical parameter in terms of water retention and formulation affecting hydration with dried PPP and PSP blends, implying good water absorption during the infusion.3.4. Comparative analysis of pomegranate peel-seed-based herbal tisane with commercially available herbal teas
| Samples | Rate of diffusionb (g min−1) | pH | TSS (0brix) | Color analysis | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| a Data represented as mean value ± standard deviation. Values with different superscripts in a column differ significantly (P ≤ 0.05). b Except for rate of diffusion. | ||||||
| A-91 | 0.667b | 3.76c ± 0.15 | 1.97a ± 0.15 | 41.45b ± 1.12 | 0.42a ± 0.12 | 10.74b ± 1.48 |
| B-73 | 0.678b | 3.30ab ± 0.10 | 2.30bc ± 0.15 | 46.98d ± 1.04 | 0.53a ± 0.01 | 8.76a ± 0.02 |
| C-64 | 0.603a | 4.06d ± 0.05 | 3.00d ± 0.20 | 38.71a ± 0.13 | 0.71a ± 0.03 | 12.75c ± 0.51 |
| D-55 | 0.593a | 3.90b ± 0.10 | 2.16ab ± 0.15 | 37.48a ± 0.91 | 1.65b ± 0.07 | 20.23d ± 0.50 |
| Brand-A | 0.668b | 3.34b ± 0.05 | 2.45c ± 0.12 | 44.92c ± 1.05 | 3.87c ± 0.03 | 23.08e ± 0.67 |
| Brand-B | 0.692b | 3.39b ± 0.10 | 2.20b ± 1.10 | 40.63b ± 0.32 | 3.99c ± 0.21 | 25.37f ± 1.23 |
| Brand-C | 0.687b | 3.23a ± 0.05 | 2.95d ± 0.16 | 50.66e ± 0.96 | 1.5b ± 0.43 | 11.33bc ± 1.58 |
Blend C-64 indicated the maximum TSS, while blend A-91 was observed to have a minimum brix. Increasing the amount of PSP increased the brix value till sample C-64 which might be due to the high concentration of compounds like crude fat and crude protein in pomegranate seed. However, blend D-55 did not show a further increase in brix with an increasing proportion of PSP which indicated the addition of an optimized amount of seed powder for a high brix value. Brix values of B-73 and D-55 blends were close to that of most commercially available herbal teas (Table 4).
The L* values varied greatly among different formulated infusions from blends (Table 4). Blend D-55 had the lowest L* value, indicating the lighter color of the formulation, followed by blends C-64 and A-91. Blend B-73 showed the highest L* value, indicating the dark colored formulation. The pale color of blend A-91 might be due to the high peel content and low seed percentage. In all samples, there was a significant lightening in the color of the formulation with increasing PPP content. A positive a* (indicating redness) was recorded in all samples (Table 4). Among the blends, D-55 had the highest and A-91 had the lowest a* values (Table 4). The high a* for D-55 indicated that the infusion was bright and red which also reflects the results of sensory evaluation.49 This variation in red hue can be attributed to different pomegranate peel contents in different blends. The highest b* value (indicating yellowness) was observed in blend D-55, while the lowest b* was recorded for blend B-73. The possible reason behind this variation might be the infusion of carotenoids and lipophilic chlorophylls present in seeds and peels of pomegranate.50
Green and yellow color in fruits is due to carotenoids and lipophilic chlorophylls present in plastids, whereas red coloration is due to hydrophilic anthocyanins and carotenoids present in the vacuole.51 On ripening, pomegranate (Punica granatum L.) cultivars yield fruit that ranges in color from green to scarlet. Anthocyanins found in peels contribute to the nutritional value of pomegranate juice. Various research studies reported a great number of polyphenols including hydrolysable tannins, flavonoids, and anthocyanins in peels.38,52 While present in lower quantities compared to hydrolysable tannins, anthocyanins are the main molecules accountable for the bioactivity observed in pomegranate peel extract.53 The seeds are considered unsuitable for anthocyanin study due to the lack of enticing red colors and it has been reported previously that no anthocyanins were discovered in seeds.54 The significantly higher color values (L*, a* and b*) for infusions prepared from market samples might be due to the inclusion of synthetic color and additives in order to make final formulation appealing to the consumers.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) was required to achieve the acceptable sensory characteristics of developed tisane.
5) was required to achieve the acceptable sensory characteristics of developed tisane.
| Sample | Appearance | Flavor | Taste | Overall acceptability |
|---|---|---|---|---|
| a Data represented as mean value ± standard deviation. Values with different superscripts in a column differ significantly (P ≤ 0.05). | ||||
| A-91 | 7.1a ± 0.43 | 6.0a ± 0.37 | 7.02b ± 0.27 | 6.7ab ± 0.35 |
| B-73 | 7.0a ± 0.24 | 7.02b ± 0.25 | 6.99b ± 0.27 | 7.0b ± 0.25 |
| C-64 | 8.04b ± 0.30 | 6.1a ± 0.23 | 7.0b ± 0.19 | 7.04bc±0.24 |
| D-55 | 8.98c ± 0.60 | 9.05c ± 0.23 | 7.99c ± 0.23 | 8.67d ± 0.35 |
| Brand-A | 8.04b ± 0.36 | 7.0b ± 0.19 | 7.0b ± 0.19 | 7.35c ± 0.24 |
| Brand-B | 8.02b ± 0.26 | 7.02b ± 0.24 | 7.06b ± 0.19 | 7.36c ± 0.23 |
| Brand-C | 6.99a ± 0.26 | 6.03a ± 0.13 | 6.06a ± 0.24 | 6.33a ± 0.21 |
4. Conclusion
Pomegranate waste (peel and seed) was utilized to formulate herbal infusion “tisane” providing a rich blend of antioxidants and phenolics. Pomegranate peel powder was observed with high carbohydrate and low-fat content, while pomegranate seed powder had high protein, crude fat and fiber content. Antioxidant activity was exceptionally high in both peel and seed extracts along with high TPC and TFC values. The comparison between PPP and PSP indicated that PPP had more TPC and TFC than PSP. Blending of PSP and PPP in the right proportion boosted their antioxidant behavior by more than two-fold. Among all blends, blend B-73 showed the highest diffusion rate and antioxidant activity as well as the maximum TPC and TFC, thus, making it the best blend with respect to health benefits. However, in terms of sensory evaluation, the highest scores for appearance, flavor and taste were recorded for blend D-55 among all the samples. Despite the fact that the market available brands were formulated with synthetic flavor and color, these secured low sensory scores in comparison with blend D-55. Thus, pomegranate waste when utilized wisely in the form of herbal extract may not only provide beneficial phytonutrients and bioactive compounds but also protect consumer health from products developed using synthetic additives.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Aastha Dewan: conceptualization, supervision, statistical analysis; Sanya Dawra: writing draft, experimentation, statistical analysis; Nita Kaushik: editing, reviewing; Ajay Singh: data verification and correction; Sheetal Thakur: reviewing, plagiarism detection, manuscript preparation and revision; Sandeep Kaur: reviewing and correction; Janifer Raj Xavier: revision and correction.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors are grateful for lab facilities and the chemicals provided by the Department of Food Technology, Guru Jambheshwar University of Science and Technology, Hisar.References
- J. Chen, C. Liao, X. Ouyang, I. Kahramanoǧlu, Y. Gan and M. Li, Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation, J. Food Qual., 2020, 1–8 Search PubMed.
- K. Ko, Y. Dadmohammadi and A. Abbaspourrad, Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review, Foods, 2021, 10(3), 1–17 CrossRef PubMed.
- A. H. Rahmani, A. A. Mohamed and A. A. Saleh, Active constituents of pomegranates (Punica granatum) as potential candidates in the management of health through modulation of biological activities, Pharmacogn. J., 2017, 9(5), 689–695 CrossRef CAS.
- D. A. Van Elswijk, U. P. Schobel, E. P. Lansky, H. Irth and J. van der Greef, Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry, Phytochemistry, 2004, 65(2), 233–241 CrossRef CAS PubMed.
- C. B. Stowe, The effects of pomegranate juice consumption on blood pressure and cardiovascular health, Compl. Ther. Clin. Pract., 2011, 17(2), 113–115 CrossRef PubMed.
- S. Akhtar, S. Ismail, D. Fraternale and P. Sestili, Pomegranate peel and peel extracts: chemistry and food features, Food Chem., 2015, 174, 417–425 CrossRef CAS PubMed.
- K. Kaderides, I. Mourtzinos and A. M. Goula, Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies, Food Chem., 2020, 310, 125849 CrossRef CAS PubMed.
- D. N. Dalimov, G. N. Dalimova and M. Bhatt, Chemical composition and lignins of tomato and pomegranate seeds, Chem. Nat. Compd., 2003, 39, 37–40 CrossRef CAS.
- R. F. Wang, W. D. Xie, Z. Zhang, D. M. Xing, Y. Ding, W. Wang, C. Ma and L. J. Du, Bioactive compounds from the seeds of Punica granatum (Pomegranate), J. Nat. Prod., 2004, 67(12), 2096–2098 CrossRef CAS PubMed.
- B. Khalili, M. Rafieian, S. H. Hejazi, H. A. Yusefi, N. Yektaian and L. Shirani-Bidabadi, Effect of Achillea millefolium, Artemisia absinthium & Juglans regia leaves extracts on Trichomonas vaginalis in vitro, J. Shahrekord Univ. Med. Sci., 2011, 12(4), 62–69 Search PubMed.
- M. Dikmen, O. Nilgün and O. Yusuf, The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer, J. Med. Food, 2011, 14(12), 1638–1646 CrossRef CAS PubMed.
- V. K. Andika, A. R. Hasana and S. D. Sawu, Empowerment of PKK Members in Training for the Production of Red Dragon Fruit Peel Tisane in the Kauman Subdistrict of Malang City, J. Community Pract. Soc. Welfare, 2023, 3(2), 26–36 CrossRef.
- N. Trimedona, R. Rahzarni, S. Syahrul, Y. Muchrida and I. Roza, Antioxidant properties of herbal tea prepared from red dragon fruit Peel with the addition of ginger, J. Appl. Agri. Sci. Technol., 2020, 4(2), 181–188 Search PubMed.
- I. Al-Zughbi and M. Krayem, Quince fruit Cydonia oblonga Mill nutritional composition, antioxidative properties, health benefits and consumers preferences towards some industrial quince products: A review, Food Chem., 2022, 393, 133362 CrossRef CAS PubMed.
- A. Gorguc, E. Gencdag and F. M. Yilmaz, Industrial pomegranate wastes and their functional benefits in novel food formulations, in Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications, Springer International, Cham, 2022, pp. 721–738 Search PubMed.
- F. S. Poswal, G. Russell, M. Mackonochie, E. MacLennan, E. C. Adukwu and V. Rolfe, Herbal Teas and their Health Benefits: A Scoping Review, Plant Foods Hum. Nutr., 2019, 4(3), 266–276 CrossRef PubMed.
- S. C. Kushwaha, M. B. Bera and P. Kumar, Nutritional composition of detanninated and fresh pomegranate peel powder, IOSR J. Environ. Sci., Toxicol. Food Technol., 2013, 7(1), 38–42 CrossRef.
- N. Perveen, S. S. Cholin, K. Hipparagi, B. N. S. Murthy and D. Peerjade, Genetic variability studies in pomegranate, Indian J. Hortic., 2018, 75(3), 355–361 CrossRef.
- AOAC, Official Method of Analysis, AOAC Association of Official Analytical Chemists International, Gaithersburg Maryland, USA, 2, 19th edn, 2012, pp. 20877–22417 Search PubMed.
- AOAC, Official Method of Analysis, Association of Officiating Analytical Chemists, Washington DC, 18th edn, 2005 Search PubMed.
- AOAC, Methods of Analysis - Official Methods 923.03, 923.05, 925.09, 962.09, and 979.09, Association of Official Analytical Chemists International, Washington, DC, USA, 17th edn, 2006, vol. II Search PubMed.
- J. Zhishen, M. Tang and J. Wu, The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food Chem., 1999, 64(4), 555–559 CrossRef CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent, Methods Enzymol., 1999, 299, 152–178 CAS.
- W. Brand-Williams, M. E. Cuvelier and C. L. W. T. Berset, Use of a freeradical method to evaluate antioxidant activity, LWT--Food Sci. Technol., 1995, 28, 25–30 CrossRef CAS.
- T. M. Lu, C. C. Lee, J. L. Mau and S. D. Lin, Quality and antioxidant property of green tea sponge cake, Food Chem., 2010, 119(3), 1090–1095 CrossRef CAS.
- A. Chaudhary, Z. Hussain, A. Aihetasham, M. El-Sharnouby, R. A. Rehman and M. A. U. Khan, et al., Pomegranate peels waste hydrolyzate optimization by Response Surface Methodology for Bioethanol production, Saudi J. Biol. Sci., 2021, 28(9), 4867–4875 CrossRef CAS PubMed.
- E. O. Oladele and A. Oshodi, Nutritional Potential of Berlandier Nettle Spurge (Jatropha cathartica) Seed, Pak. J. Nutr., 2007, 6(4), 345–348 CrossRef.
- H. Jalal, M. Ashraf Pal, S. Rafeh Ahmad, M. Rather, M. Andrabi and S. Hamdan, Physico-chemical and functional properties of pomegranate peel and seed powder, Pharma Innov. Int. J., 2018, 7(4), 1127–1131 Search PubMed.
- Y. Peng, Comparative analysis of the biological components of pomegranate seed from different cultivars, Int. J. Food Prop., 2019, 22(1), 784–794 CrossRef CAS.
- E. Hornung, C. Pernstich and I. Feussner, Formation of conjugated Δ11Δ13-double bonds by Δ12-linoleic acid (1, 4) -acyl-lipid-desaturase in pomegranate seeds, Eur. J. Biochem., 2002, 269(19), 4852–4859 CrossRef CAS PubMed.
- Y. Amakura, M. Okada, S. Tsuji and Y. Tonogai, High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits, J. Chromatogr. A, 2000, 896(1–2), 87–93 CrossRef CAS PubMed.
- P. Garcia-Salas, A. Morales-Soto, A. Segura-Carretero and A. Fernández-Gutiérrez, Phenolic-compound-extraction systems for fruit and vegetable samples, Molecules, 2010, 15(12), 8813–8826 CrossRef CAS PubMed.
- M. I. Gil, F. A. Tomás-Barberán, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing, J. Agric. Food Chem., 2000, 48(10), 4581–4589 CrossRef CAS PubMed.
- A. I. Jideani, H. Silungwe, T. Takalani, A. O. Omolola, H. O. Udeh and T. A. Anyasi, Antioxidant-rich natural fruit and vegetable products and human health, Int. J. Food Prop., 2021, 24(1), 41–67 CrossRef CAS.
- F. Licciardello, S. Kharchoufi, G. Muratore and C. Restuccia, Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage, Food Packag. Shelf Life, 2018, 17, 114–119 CrossRef.
- S. Jalili, A. Tabatabee Naini, M. Ashrafi and M. Aminlari, Antioxidant Activity of Pericarp Extract from Different Varieties of Pomegranate Fruit, J. Agric. Sci. Technol., 2020, 22, 95–107 Search PubMed.
- R. P. Singh, K. N. C. Murthy and G. K. Jayaprakasha, Studies on the antioxidant activity of pomegranate peel and seed extracts using in vitro models, J. Agric. Food Chem., 2002, 50, 81–86 CrossRef CAS PubMed.
- W. Elfalleh, H. Hannachi, N. Tlili, Y. Yahia, N. Nasri and A. Ferchichi, Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower, J. Med. Plants Res., 2012, 6(32), 4724–4730 CAS.
- C. G. Awuchi, V. S. Igwe and C. K. Echeta, The functional properties of foods and flours, Int. J. Adv. Acad. Res., 2019, 5(11), 139–160 Search PubMed.
- E. Sánchez-Zapata, E. Fuentes-Zaragoza, J. Fernández-López, E. S. Esther Sendra, C. Navarro and J. A. Pérez-Álvarez, Preparation of Dietary Fiber Powder from Tiger Nut (Cyperus esculentus) Milk (“Horchata”) Byproducts and Its Physicochemical Properties, J. Agric. Food Chem., 2009, 57(17), 7719–7725 CrossRef PubMed.
- L. H. Mckee and T. A. Latner, Underutilized sources of dietary fiber: A review, Plant Foods Hum. Nutr., 2000, 55, 285–304 CrossRef CAS PubMed.
- A. Sangnark and A. Noomhorm, Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse, Food Chem., 2003, 80(2), 221–229 CrossRef CAS.
- A. Büyükbalci and S. N. El, Determination of In vitro Antidiabetic Effects, Antioxidant Activities and Phenol Contents of Some Herbal Teas, Plant Foods Hum. Nutr., 2008, 63(1), 27–33 CrossRef PubMed.
- P. Bassi, V. Kumar, S. Kumar, S. Kaur, Y. Gat and I. Majid, Importance and prior considerations for development and utilization of tea bags: A critical review, J. Food Process Eng., 2020, 43(1), e13069 CrossRef.
- A. Polat, Z. Kalcıoğlu and N. Müezzinoğlu, Effect of infusion time on black tea quality, mineral content and sensory properties prepared using traditional Turkish infusion method, Int. J. Gastron. Food Sci., 2022, 29, 100559 CrossRef.
- M. Viuda-Martos, Y. Ruiz-Navajas, A. Martin-Sánchez, E. Sánchez-Zapata, J. Fernández-López and E. Sendra, et al., Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product, J. Food Eng., 2012, 110(2), 220–224 CrossRef CAS.
- S. Kaur, S. Kumar, Z. F. Bhat and A. Kumar, Effect of pomegranate seed powder, grape seed extract and tomato powder on the quality characteristics of chicken nuggets, Nutr. Food Sci., 2015, 45(4), 583–594 CrossRef.
- A. Monsalve-Gonzalez, G. V. Barbosa-Cánovas, R. P. Cavalieri, A. J. McEvily and R. Iyengar, Control of Browning During Storage of Apple Slices Preserved by Combined Methods. 4-Hexylresorcinol as Anti-Browning Agent, J. Food Sci., 1993, 58(4), 797–800 CrossRef CAS.
- Q. Wu, Z. Ziwei, Z. Yining, H. Huiqing, O. Xiaoxi and S. Yun, Identification of Key Components Responsible for the Aromatic Quality of Jinmudan Black Tea by Means of Molecular Sensory Science, Foods, 2023, 12(9), 1794 CrossRef CAS PubMed.
- A. Loranty, E. Rembiałkowska, E. A. Rosa and R. N. Bennett, Identification, quantification and availability of carotenoids and chlorophylls in fruit, herb and medicinal teas, J. Food Compos. Anal., 2010, 23(5), 432–441 CrossRef CAS.
- L. Kapoor, A. J. Simkin, C. George Priya Doss and R. Siva, Fruit ripening: dynamics and integrated analysis of carotenoids and anthocyanins, BMC Plant Biol., 2022, 22(1), 1–22 CrossRef PubMed.
- I. Bar-Ya'akov, L. Tian, R. Amir and D. Holland, Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit, Front. Plant Sci., 2019, 10, 620 CrossRef PubMed.
- O. A. Fawole, N. P. Makunga and U. L. Opara, Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract, BMC Complementary Altern. Med., 2012, 12(1), 1–11 Search PubMed.
- C. Arlotta, G. D. Puglia, C. Genovese, V. Toscano, R. Karlova, J. Beekwilder, R. C. H. de Vos and S. A. Raccuia, MYB5-like and bHLH influence flavonoid composition in pomegranate, Plant Sci., 2020, 298, 110563 CrossRef CAS PubMed.
- D. Wu and D. W. Sun, Colour measurements by computer vision for food quality control – A review, Trends Food Sci. Technol., 2013, 29(1), 5–20 CrossRef.
- R. Bennet, S. Vijayalakshmi, R. Dinesh and J. Yuvaraj, Formulation and sensory evaluation of tisanes, Int. J. Pharma Bio Sci., 2016, 7(4), 115–120 CAS.
- H. Wasila, X. Li, L. Liu, I. Ahmad and S. Ahmad, Peel effects on phenolic composition, antioxidant activity, and making of pomegranate juice and wine, J. Food Sci., 2013, 78(8), C1166–C1172 CrossRef CAS PubMed.
- A. Ayoubi, M. Balvardi, H. R. Akhavan and R. Hajimohammadi-Farimani, Fortified cake with pomegranate seed powder as a functional product, J. Food Sci. Technol., 2022, 1–9 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
