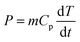Open Access Article
Open Access ArticleConverting potato peel waste into bioactive extracts: reduction of pesticides by traditional and novel pretreatment technologies
Thanh-Tri
Nguyen
 *a,
Carmen
Rosselló
b,
Sergey
Mikhaylin
*a,
Carmen
Rosselló
b,
Sergey
Mikhaylin
 c and
Cristina
Ratti
a
c and
Cristina
Ratti
a
aDepartment of Soils Science and Agri-Food Engineering, Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec City, QC G1V 0A6, Canada. E-mail: thanh-tri.nguyen.1@ulaval.ca
bDepartment of Chemistry, University of the Balearic Islands, Palma, 07122 Mallorca, Spain
cEcoFoodLab, Food Science Department, Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec City, QC G1V 0A6, Canada
First published on 26th December 2023
Abstract
Potato peel, a primary component of potato processing waste, is rich in bioactive phenolic compounds. Nevertheless, it often contains elevated levels of pesticide residues that require reduction before further processing. This study aimed to diminish pesticide content in potato peel using water immersion (WI), ultrasound (US), liquid nitrogen immersion (LNI), and pulsed electric field (PEF) pretreatment processes while preserving its bioactive value. Specific pesticide compounds, including Chlorpropham, Spirotetramat, Azoxystrobin, Propiconazole, and Captan, were diluted in water and spiked onto potato peel samples. The spiked samples underwent WI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 sample-to-water ratio), US (acoustic energy density: 592.46 ± 3.59 W L−1, 1 to 5 min duration, 1
4 sample-to-water ratio), US (acoustic energy density: 592.46 ± 3.59 W L−1, 1 to 5 min duration, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 sample-to-water ratio), PEF (3 kV cm−1, 12 to 50 pulses, 1
4 sample-to-water ratio), PEF (3 kV cm−1, 12 to 50 pulses, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 sample-to-water ratio), and LNI (2 min-immersion-thawing cycles: 1 to 4). Changes in total phenolic content, chlorogenic acid, hardness, color, and water electrical conductivity, along with light microscopy images, were evaluated before and after pretreatments to assess their impact on potato peel. Ultrasound treatment proved to be the most effective in reducing pesticide content, achieving a 100% reduction for Captan, followed by PEF (up to 80%) and LNI (20%). Removal of pesticides from potato peel using WI, with or without intensification processes, correlated well with the octanol–water partition coefficient of individual pesticide compounds. Furthermore, the retention of total phenolic content exceeded 90% for LNI, while for the US, it surpassed PEF (88% and 54%, respectively). Results of potato peel hardness, color, water electrical conductivity, and microscopic tissue images led to a plausible explanation of the differing polyphenol content. Overall, ultrasound pretreatment exhibited excellent potential for reducing hydrophilic pesticides in potato peel while preserving a significant amount of phenolic compounds.
4 sample-to-water ratio), and LNI (2 min-immersion-thawing cycles: 1 to 4). Changes in total phenolic content, chlorogenic acid, hardness, color, and water electrical conductivity, along with light microscopy images, were evaluated before and after pretreatments to assess their impact on potato peel. Ultrasound treatment proved to be the most effective in reducing pesticide content, achieving a 100% reduction for Captan, followed by PEF (up to 80%) and LNI (20%). Removal of pesticides from potato peel using WI, with or without intensification processes, correlated well with the octanol–water partition coefficient of individual pesticide compounds. Furthermore, the retention of total phenolic content exceeded 90% for LNI, while for the US, it surpassed PEF (88% and 54%, respectively). Results of potato peel hardness, color, water electrical conductivity, and microscopic tissue images led to a plausible explanation of the differing polyphenol content. Overall, ultrasound pretreatment exhibited excellent potential for reducing hydrophilic pesticides in potato peel while preserving a significant amount of phenolic compounds.
Sustainability spotlightThis study addresses the challenge of potato peel waste, focusing on its impact on environmental sustainability and resource utilization. The innovative use of pre-treatment techniques such as water immersion, ultrasound, pulsed electric field, and liquid nitrogen immersion aims to enhance the quality and safety of potato peel waste. By reducing pesticide residues and preserving bioactive compounds, these methods align with the principles of responsible consumption and production (Goal 12) and industry innovation (Goal 9) set by the United Nations' Sustainable Development Goals. This work contributes to sustainable practices in the agri-food sector, promoting efficient resource use and minimizing environmental impacts. |
1. Introduction
Canadian exports of potatoes and potato products reached an estimated value of $2.6 billion in 2021–2022, with frozen and processed potatoes accounting for 73% of the total.1 Potato peel waste represents a significant proportion of the processing operations, ranging from 15 to 40% of the potato weight depending on the peeling process.2 The enormous magnitude of potato waste poses economic challenges and environmental concerns. Thus, developing an environmentally friendly solution for potato peel waste is imperative, particularly within the context of organic waste decomposition and environmental pollution.3The conversion of fruit and vegetable waste from agri-food by-products into valuable products has lately gained significant attention, particularly in extracting polyphenols and other antioxidants (e.g., fiber, vitamin E, lycopene, etc.) done by converting these extracts into stable powders.4,5 In the case of potato waste, dried peel extracts have been reported to contain phenolic compounds with antioxidant and antiviral activities.3,6 The predominant phenolic compound in potato peel is chlorogenic acid, constituting approximately 80% of the total phenolic acids and contributing to its potent antioxidant activity.7
Potato waste tends to contain high amounts of pesticide residues predominantly concentrated in the outer parts of plants such as the peel.8 Since potato waste primarily consists of peel, which is the focus of this study, it is crucial to address pesticide residue issues and their elimination through pretreatment methods before further waste transformation. Previous studies have demonstrated that washing and peeling can reduce pesticide residues to different extents. For example, peeling resulted in a reported 98% reduction in Captan residues in apples.9 Conventional washing methods could be moderately effective in removing residual pesticides from fruits and vegetables, especially in the case of pesticides with higher water solubility.10 Wu et al. (2019) reported removal rates of less than 35% and 32% for pesticides applied on cucumber and spinach, respectively, when washed with tap water.11 Heshmati et al. (2020) found that washing grapes with tap water for 15 minutes resulted in removal rates of only 20.3%, 18.5%, 37.5%, 15.2%, and 16.6% for Penconazole, Hexaconazole, Diazinon, Ethion, and Phosalone, respectively.12 Similarly, washing cabbage leaves with tap water showed low effectiveness, with only 17.6%, 17.1%, 19.1%, and 15.2% reduction in Chlorpyrifos, p,p-DDT, Cypermethrin, and Chlorothalonil residues, respectively.13 Water immersion is especially effective in eliminating hydrophilic pesticide compounds with lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (octanol–water partition coefficient).14 For instance, washing kumquat fruits with tap water, removal rates of Dimethoate, Chlorpyrifos, Malathion, Methidathion, and Triazophos were 23.0%, 8.0%, 19.0%, 16.0%, and 10.0%, respectively, depending on their log
P values (octanol–water partition coefficient).14 For instance, washing kumquat fruits with tap water, removal rates of Dimethoate, Chlorpyrifos, Malathion, Methidathion, and Triazophos were 23.0%, 8.0%, 19.0%, 16.0%, and 10.0%, respectively, depending on their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, where lower log
P values, where lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values correlated with greater removal of residues.15 In addition, in the case of potatoes, organochlorine residues exhibited less reduction compared to organophosphorus residues after washing with tap water.16
P values correlated with greater removal of residues.15 In addition, in the case of potatoes, organochlorine residues exhibited less reduction compared to organophosphorus residues after washing with tap water.16
Ultrasound has been used to intensify the reduction of pesticide residues during washing. For instance, Zhang et al. (2010) demonstrated the effectiveness of ultrasonic treatment at T = 15 ± 2 °C and 25 kHz in degrading diazinon in apple juice, with the degradation percentage depending on the initial concentration and ultrasonic power. Higher initial pesticide concentrations resulted in decreased degradation percentages, and the treatment at 500 W was 2.7 times more effective than at 100 W.17 Similarly, Chen et al. (2009) observed that the degradation of Methamidophos and Chlorpyrifos in apple juice after pulsed electric field pretreatment increased as the electric field strength was augmented from 8 kV cm−1 to 20 kV cm−1. This increase in voltage induced the vibration and rotation of polar molecules, facilitating the degradation of these pesticides.18 However, no studies have explored the application of ultrasound and PEF for reducing pesticides on potato peel waste.
Cyclic liquid nitrogen immersion has been used to accelerate drying processes by utilizing the freeze-cracking effects on the waxy layer of fruits such as blueberries.19 Additionally, studies have shown that this technique can effectively extract waxes from the epidermis of plant materials such as grains20 and straw.21 Given that a significant portion of pesticides tends to reside in the waxy layer of the peel, it could be possible to reduce pesticide compounds through the application of liquid nitrogen immersion, since this technique targets the specific location where these compounds are predominantly found.
Although these previously described pretreatments present an interesting potential to diminish residual pesticides in foods, they may as well cause a negative impact on the retention of valuable bioactive compounds. For instance, US and PEF intensification processes have been found to decrease the retention of total antioxidant compounds (20.7%), polyphenolic content (63%), and vitamin C when used during the processing of blackberry (US pretreatment), orange peel (PEF-600 μs), and red bell pepper (US and PEF), respectively.22–24
Considering the above-mentioned issues related to pesticides and the final quality in potato waste, the objective of this study is to reduce pesticide residue content in potato peel waste using ultrasound (US), liquid nitrogen immersion (LNI), and pulsed electric field (PEF) pretreatment processes, with diverse operating variables such as time, immersion cycles, and pulse numbers, respectively. The effect of these pretreatment methods on phenolic compounds and quality characteristics was assessed as well.
2. Materials and methods
2.1. Raw materials
Pre-washed potatoes (Russet) purchased from a local grocery store were manually peeled using a knife. Samples were obtained by cutting rectangular slabs from the peel, measuring 10 mm in length, 20 mm in width, and 1 ± 0.1 mm in thickness.2.2. Pesticides and spiking method
In this study, specific pesticide compounds, commercial-grade, including Chlorpropham (98A, AG-Services Inc., Canada), Spirotetramat (Movento 240 SC, Bayer, Canada), Azoxystrobin (Quadris, Syngenta, Canada), Propiconazole (Tilt fungicide, Syngenta, Canada), and Captan (Captan fungicide, Southern Ag, USA), were selected based on their applicability to potato growing and storage protection, and on their varying affinities with water and other physicochemical properties. These commercial-grade pesticides were dissolved in water to create solutions following concentrations recommended by the manufacturer. Initial pesticide concentrations in the solutions were 1.25 g L−1 for Azoxystrobin, Propiconazole, and Spirotetramat, and 1.5 g L−1 for Chlorpropharm, and Captan.Spiking of pesticides was carried out following the method presented by Wu et al.11 with modifications. Potato peel samples were immersed in the pesticide solutions at T = 20 ± 2 °C for 1 hour. After immersion, the spiked samples were drained using a sieve, and any excess solution was blotted with absorbent paper to remove surface moisture. The samples were then placed in a closed container at T = 20 ± 2 °C for 1 hour to facilitate pesticide penetration. Two types of spiking solutions were employed: individual compound solutions, where single pesticide solutions were used to spike each sample, and mixed compound solutions, where a mixture of all five pesticides was used.
2.3. Pesticide standards and chemical reagents
All chemical reagents used for GC determinations were of analytical reagent grade, and solvents were of HPLC grade. Standards for Chlorpropham, Spirotetramat, Azoxystrobin, Propiconazole, and Captan were purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions (100 mg kg−1) of each pesticide were prepared in acetonitrile (MeCN) and stored at −20 °C. Methanol (MeOH) and MeCN, magnesium sulfate (MgSO4), and sodium chloride (NaCl) were purchased from Sigma-Aldrich (St. Louis, MO, USA).2.4. Pretreatment experiments
The pretreatment experiment protocol is illustrated in Fig. 1. Pretreatment conditions were selected from preliminary experiments. Initially, potato peel samples (prepared as described in the previous section) were spiked with pesticides following the spiking procedure explained earlier. Subsequently, the potato samples were subjected to different pretreatment methods: liquid nitrogen immersion (LNI), water immersion (WI, used as a control), ultrasound (US), and pulsed electric field (PEF).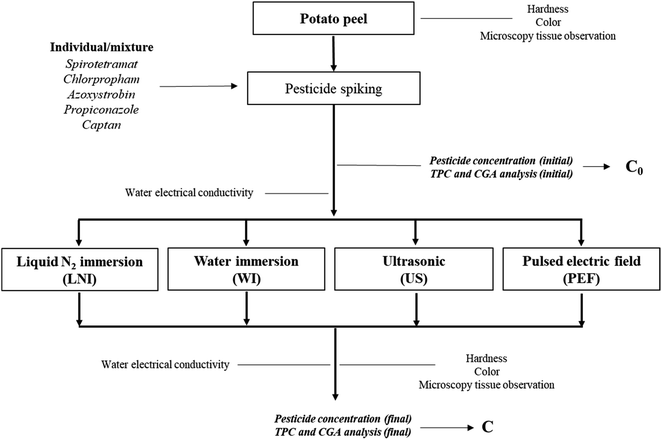 | ||
| Fig. 1 Experimental protocol of the pretreatment studies for potato peel pesticide reduction and quality impact. | ||
Pesticide concentration and various physicochemical evaluations, including total phenolics (TPC) and chlorogenic acid (CGA) contents, microscopy tissue observation, hardness and color of potato peel, and water electrical conductivity, were carried out as a function of pretreatment conditions. These evaluations were performed after the pretreatment procedures and compared to the initial values. Detailed information regarding all the operations involved and the technical methods employed in this protocol will be provided in the following sections. All experiments were carried out in triplicates.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w) without agitation, for different durations (1, 2, 3, 4, and 5 minutes) at 20 ± 2 °C.
4 w/w) without agitation, for different durations (1, 2, 3, 4, and 5 minutes) at 20 ± 2 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/w). The samples were processed at a constant frequency of 24 kHz for different durations: 1, 2, 3, 4, and 5 minutes. Ultrasound treatments were performed under a continuous mode. Temperature (25 ± 2 °C) during the treatment was maintained through a cooling circulation system connected to the jacketed beaker.
4 (w/w). The samples were processed at a constant frequency of 24 kHz for different durations: 1, 2, 3, 4, and 5 minutes. Ultrasound treatments were performed under a continuous mode. Temperature (25 ± 2 °C) during the treatment was maintained through a cooling circulation system connected to the jacketed beaker.
The actual power dissipated was determined using the calorimetric method.25 For this purpose, temperature increase in 200 mL water, being sonicated without sample nor cooling circulation, was recorded using a thermocouple. From the temperature versus time data, the initial temperature increase rate dT/dt was obtained through linear regression. Ultrasound power was then calculated using the following equation:
Experiments were done in triplicate. The acoustic energy density (AED) was then calculated by dividing the measured power by the volume of water. The AED value for the applied amplitude levels was 592.46 ± 3.59 W L−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/w).
4 (w/w).
The samples were subjected to different pulse numbers, namely 12, 25, and 50, to evaluate the effects of varying pulse numbers on pesticide reduction. Water temperature after PEF treatment was also measured (24 ± 1 °C).
2.5. Physicochemical analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 3 minutes (in an ice bath). Then, 10 g of the homogenized sample was transferred into a 50 mL Falcon centrifuge tube. Acetonitrile (10 mL) was added to the tube, and the mixture was vigorously shaken for 1 minute using a Vortex Vortex (Scientific Industries G560 Vortex-Genie® 2 Shaker) at maximum speed (3200 rpm). Anhydrous MgSO4 (4 g) and NaCl (1 g) were added to the tube, followed by another 1 min mixing on the Vortex mixer. The resulting mixture was then centrifuged for 6 min at 6000 rpm. The supernatant was filtered through a 0.2 μm PTFE filter and transferred to a 2 mL vial for GC-FID analysis.
500 rpm for 3 minutes (in an ice bath). Then, 10 g of the homogenized sample was transferred into a 50 mL Falcon centrifuge tube. Acetonitrile (10 mL) was added to the tube, and the mixture was vigorously shaken for 1 minute using a Vortex Vortex (Scientific Industries G560 Vortex-Genie® 2 Shaker) at maximum speed (3200 rpm). Anhydrous MgSO4 (4 g) and NaCl (1 g) were added to the tube, followed by another 1 min mixing on the Vortex mixer. The resulting mixture was then centrifuged for 6 min at 6000 rpm. The supernatant was filtered through a 0.2 μm PTFE filter and transferred to a 2 mL vial for GC-FID analysis.
Pesticide residue analysis was performed using a gas chromatograph (HP 6890 series) equipped with a flame ionization detector (FID). An RTX®-65TG (Restek, Bellefonte, PA, USA) column (30 m × 0.25 mm, 0.10 μm film thickness) was used for compound separation. Ultra-high purity hydrogen was used as the carrier gas at a flow rate of 1.1 mL min−1. The column temperature was initially set at 80 °C with a hold time of 0.6 minutes, then ramped to 200 °C at a rate of 10 °C min−1. It was further increased to 280 °C at a rate of 20 °C min−1 and held for 5 minutes. The injector and detector temperatures were maintained at 250 °C and 280 °C, respectively. The injector was operated in split mode, and 1 μL of the sample was injected using an autosampler.
Calibration curves (i.e. pic area as a function of concentration) were conducted for each pesticide standard. Thus, initial pesticide concentration in potato peel (Co) was estimated by comparing, for each pesticide compound, the GC elution areas of spiked potato peel samples with individual pesticide standard areas from calibration curves.
Pesticide retention values were reported as the percentage ratio between final (after pretreatment) concentration (C) and initial concentration:
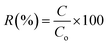 | (1) |
The total phenolic content was determined using the Folin–Ciocalteu method as described by Al-Weshahy et Venket Rao2 with slight modifications. Freeze-dried samples (500 mg) were mixed with 5 mL of methanol, followed by centrifugation (6000 rpm, 10 min). Then, a 200 μL aliquot of the phenolic extract was mixed with 100 μL of Folin–Ciocalteu reagent, and 700 μL of saturated Na2CO3 solution was added. After incubation in the dark at room temperature (T = 20 ± 2 °C) for 2 h, the absorbance was measured at 765 nm using a microplate spectrophotometer (xMark, Bio-Rad Laboratories, Hercules, CA, USA). Gallic acid (Sigma-Aldrich, Oakville, Ontario, Canada) was used as the standard, and the total phenolic content was expressed as mg of gallic acid equivalent per g dry matter (mg GAE/g matter). TPC retention values were reported using eqn (1).
The total color difference (ΔE) was then determined using the following equation:
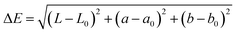 | (2) |
In addition, hue angle (h), and chroma (C*) were calculated using the following equations:
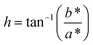 | (3) |
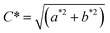 | (4) |
ImageJ 1.53k software program28 was used to estimate inner cell surface area, epidermal cell surface area, and epidermal cell thickness from image data. The measurements were done in triplicate.
2.6. Statistical analysis
The data were expressed as the mean ± SD of triplicate experiments (n = 3) and analyzed using one-way analysis of variance (ANOVA) with Minitab 16.0 software (Minitab Inc., USA). The significant difference between means was evaluated using the Tukey test for means comparison. A p-value of less than 0.05 was considered statistically significant.3. Results and discussion
3.1. Pesticide chromatographic determination
Fig. 2 shows the GC chromatogram for Chlorpropharm (a), Captan (b), Propiconazole (c), Spirotetramat (d), and Azoxystrobin (e) chemical standards together with that for potato peel sample after spiking with pesticides of commercial grade (f), which provides the initial pesticide concentrations. From Fig. 2, it can be observed that elution times for different pesticides were 7.7 min (Chlorpropharm), 12.8 min (Captan), 13.9 min (Propiconazole), 16.1 min (Spirotetramat), and 18.8 min (Azoxystrobin) (Fig. 2-a–e, respectively). As well, Fig. 2-f shows the presence of the corresponding different spiked commercial pesticides in the potato peel sample at similar times, together with other compounds coming from additives of commercial pesticides and natural wax compounds from the potato peel.21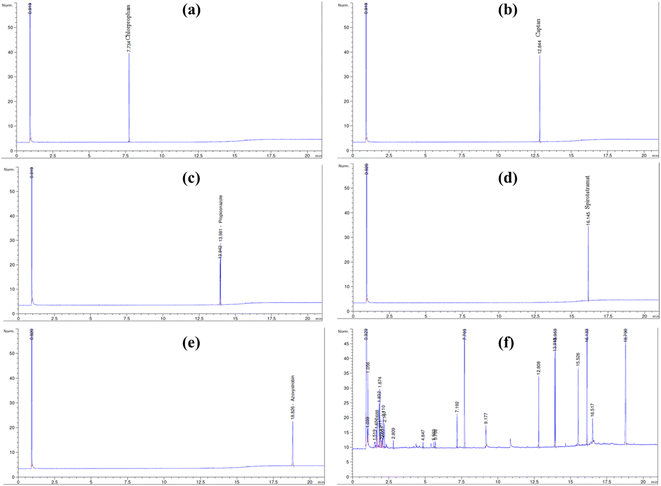 | ||
| Fig. 2 An example of GC chromatograms of pesticide chemical standards (a–e) and untreated potato peel, spiked with a mixture of 5 pesticides (f). | ||
Initial pesticide concentrations (Co) of potato peel were found to be 476 ± 7 mg L−1, 866 ± 36 mg L−1, 786 ± 47 mg L−1, 1016 ± 100 mg L−1, and 1197 ± 71 mg L−1 for Spirotetramat, Chlorpropham, Azoxystrobin, Propiconazole, and Captan, respectively.
3.2. Effect of pretreatments on pesticide residues
The retention of spiked pesticides in potato peel after diverse pretreatments is presented in Fig. 3 for individually spiked samples. All the pretreatments had a positive impact in decreasing pesticides but to different extents. The effect of each pretreatment will be described separately in the following paragraphs.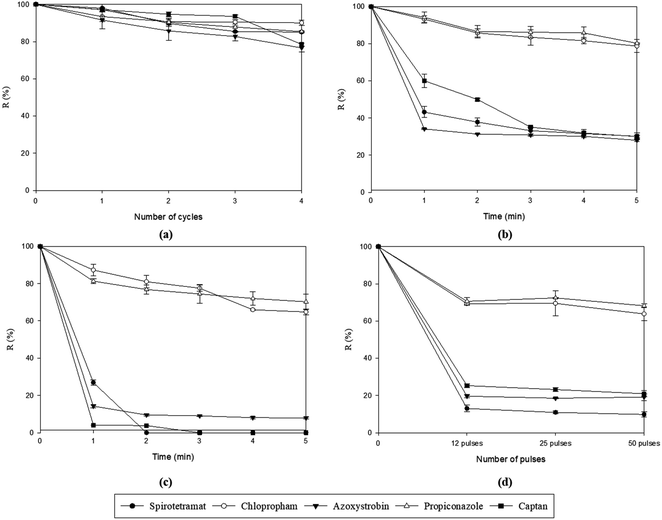 | ||
| Fig. 3 Pesticides retention after different pretreatments: LNI (a), WI (b), US (c), and PEF (d) for potato peel samples. | ||
As shown in Fig. 3-a, pesticide residues on potato peel after LNI pretreatment were just slightly reduced, decreasing linearly along with the number of immersion-thawing cycles. Results showed a significant decrease (p < 0.05) of pesticide residues (Azoxystrobin, Captan, Spirotetramat, and Propiconazole) on potato peel between 1 and 4 cycles of LNI pretreatment. No clear differential behavior of individual pesticide retention values was observed (Fig. 3-a). After four (4) cycles of pretreatment, two pesticides exhibited similar reductions (approximately 23.0% and 21.5% for Azoxystrobin and Captan, respectively). In contrast, Spirotetramat and Propiconazole showed slightly lower reductions of 14.8% and 14.6% while Chlorpropham exhibited the smallest reduction at only 10%, and no significant difference (p < 0.05) between the number of cycles pretreatment. These results could be explained by the impact of liquid nitrogen immersion on the modification of epidermal waxes from plant-based materials19 and by the distribution of compounds in intracuticular and extracuticular waxes.29 Ketata et al. found that after three liquid nitrogen immersion-thawing cycles, the cuticle thickness of highbush blueberries was reduced by up to 80%, while for lowbush blueberry species, it decreased by up to 55%.19 Other authors also reported that vegetal waxes from rice, sorghum, wheat,20 wheat, and flax straw21 were effectively extracted by immersion cycles in liquid nitrogen. As indicated by Angioni et al.,30 pesticides are mainly present in the plant epidermis, although they could be distributed in epicuticular or intra-cuticular waxes depending on their affinity to different compounds. Therefore, this could explain the slight impact of LNI pretreatment on decreasing pesticide residues from potato peel (around 20%) merely accompanying the wax disappearance due to freeze-cracking of superficial waxes by liquid nitrogen immersions, and additionally, indicating that Chlorpropham could be located internally (intracuticular waxes) rather than at the surface of the potato peel (epicuticular waxes) making its removal more difficult by LNI.
The results of water immersion (WI) pretreatment on pesticide residues indicate a significant reduction (p < 0.05) of applied pesticides between 1 to 5 minutes of pretreatment time (Fig. 3-b). These curves show an initial rapid decrease in pesticide retention followed by a “plateau”, from which the equilibrium retention value could be determined. Also, from Fig. 3-c, pesticide residues are significantly reduced by intensification of water immersion with US (p < 0.05), with reduction increasing with pretreatment duration. During the ultrasonic process, alternative pressure changes cause the creation, expansion, and implosive collapse of microbubbles in ultrasonically irradiated liquids, which is known as “acoustic cavitation”, striking the surface of sample.31 This phenomenon accelerates external mass transfer happening during WI and eases the removal of pesticide compounds from the potato peel matrix. For instance, the removal of pesticide residues from Bok choy (pakchoi cabbage) leaves using ultrasonic treatment was more efficient compared to deionized water soaking, which was attributed to the powerful cavitation effect on the surface of the leaves.32 Similarly, Lozowicka et al. observed that ultrasonic treatment resulted in a greater reduction of 16 pesticide residues from strawberries compared to tap water soaking alone, explained by the formation of cavitation bubbles, which generate mechanical energy in the form of shockwaves and cause agitation within the small pores on the uneven surfaces of strawberries.33 To end, PEF pretreatment (Fig. 3-d) showed a positive impact in decreasing final pesticide retention compared to WI, however, this reduction is less important than the one observed for US pretreatment. The reduction of pesticide residues was no significant difference (p < 0.05) between 25 and 50 pulses of PEF pretreatment, except for Propiconazole residue. Thus, the number of PEF pulses further than 25 does not seem to increase further the reduction of residual pesticides, which could be a good consideration for reducing energy consumption associated with this pretreatment method. To improve PEF performance, a higher electric field strength could be a good venue to test.
As expected, Fig. 3-b–d show that the reduction of pesticides exhibited two different behaviors for WI, US, and PEF pretreatments depending on the type of pesticide: Chlorpropham and Propiconazole presented significantly smaller reductions compared to the group consisting of Spirotetramat, Azoxystrobin, and Captan (Fig. 3-b–d). For instance, the retention amount of Chlorpropham and Propiconazole after 5 minutes of US pretreatment reached 64.8%, and 70.2%, respectively (Fig. 3-b), but Spirotetramat, Captan, and Azoxystrobin residues decreased to 0%, 0% and 7.9%, respectively. For PEF pretreatment (Fig. 3-d), the retention of Chlorpropham and Propiconazole at 50 pulses was reduced to 63.8% and 68.2%, respectively, while the retention of Spirotetramat, Captan, and Azoxystrobin was 9.8%, 20.9%, and 19.1%, respectively. These results demonstrate an effective reduction of pesticide retention of potato peel waste through PEF and US pretreatments, particularly for the US regarding the latter group of pesticides. This difference in reduction behavior depending on the type of pesticides can be attributed to variations in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, which aligns with the results of previous authors.11,15,33 Discussion about the relationship between pesticide retention and their physicochemical properties will be presented later.
P values, which aligns with the results of previous authors.11,15,33 Discussion about the relationship between pesticide retention and their physicochemical properties will be presented later.
The behavior of pesticides, both individually spiked (Fig. 3-b–d) and mixture spiked (not shown), exhibited similar trends. The differential pesticide retention behavior observed previously was also found to happen for potato peel spiked with a mixture of pesticides. For instance, after 5 minutes of US pretreatment, the retention percentages of Chlopropham and Propiconazole in potato peel spiked with pesticide mixture were 45.2% and 46.9%, respectively, which were higher than those of Spirotetramat, Azoxystrobin, and Captan (12.4%, 11.8%, and 0.0%, respectively). Retentions of Chlopropham and Propiconazole residues in a mixture of spiked potato peel for US and PEF pretreatments were found to be somewhat lower than those for individually spiked ones, while the retention of Spirotetramat, and Azoxystrobin residues were slightly higher. This could be explained by the interaction occurring of the pesticides in the mixture.34–36
Fig. 3-a shows that LNI pretreatment is the least effective in reducing pesticide compounds compared to WI, US, or PEF pretreatments (Fig. 3-b–d).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) P.
To understand better the difference in retention behavior of pesticide compounds after pretreatments, equilibrium retention values for each pesticide are presented in Table 1 together with the octanol–water partition coefficient (log
P.
To understand better the difference in retention behavior of pesticide compounds after pretreatments, equilibrium retention values for each pesticide are presented in Table 1 together with the octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) representing the lipophilicity of a compound.10 This property affects various aspects of pesticide behavior, including absorption, transport, persistence, and bioaccumulation.10 Pesticides with higher log
P) representing the lipophilicity of a compound.10 This property affects various aspects of pesticide behavior, including absorption, transport, persistence, and bioaccumulation.10 Pesticides with higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values are generally more hydrophobic, indicating a greater affinity with nonpolar solvents like lipids and organic matter. From Table 1, it can be observed that Chlorpropham and Propiconazole have higher log
P values are generally more hydrophobic, indicating a greater affinity with nonpolar solvents like lipids and organic matter. From Table 1, it can be observed that Chlorpropham and Propiconazole have higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (3.76 and 3.72, respectively) compared to Spirotetramat, Azoxystrobin, and Captan (2.51, 2.50, and 2.50, respectively). Additionally, retention values at equilibrium also presented in Table 1 show that the reduction of these pesticides during pretreatment correlates positively with log
P values (3.76 and 3.72, respectively) compared to Spirotetramat, Azoxystrobin, and Captan (2.51, 2.50, and 2.50, respectively). Additionally, retention values at equilibrium also presented in Table 1 show that the reduction of these pesticides during pretreatment correlates positively with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, as found previously by other authors.11,15,33 For instance, in a study by Wu et al., Chlorpyrifos and Bifenthrin pesticides applied on cucumber, exhibited retention rates of 76% and 85%, respectively, after a 5 minutes washing with tap water. These retention rates presented a positive correlation with their respective log
P values, as found previously by other authors.11,15,33 For instance, in a study by Wu et al., Chlorpyrifos and Bifenthrin pesticides applied on cucumber, exhibited retention rates of 76% and 85%, respectively, after a 5 minutes washing with tap water. These retention rates presented a positive correlation with their respective log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 4.7 and 6.6.11 Another study focused on kumquat fruits, found that Dimethoate and Triazophos residues showed removal rates (inverse of retention rate) of 23% and 10%, respectively, after a 5 minutes washing with tap water. These rates also depended on their log
P values of 4.7 and 6.6.11 Another study focused on kumquat fruits, found that Dimethoate and Triazophos residues showed removal rates (inverse of retention rate) of 23% and 10%, respectively, after a 5 minutes washing with tap water. These rates also depended on their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 0.75 and 3.55.15 Furthermore, Lozowicka et al. (2016) reported that Acetamiprid (log
P values of 0.75 and 3.55.15 Furthermore, Lozowicka et al. (2016) reported that Acetamiprid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.8) and Deltamethrin (log
P = 0.8) and Deltamethrin (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 4.6) spiked on strawberries exhibited removal rates of 56% and 27%, respectively, following a 5 minutes washing with tap water.33 In the case of potatoes, where the applied pesticides are located on the surface of the potato peel containing epicuticular waxes, those pesticides with higher log
P = 4.6) spiked on strawberries exhibited removal rates of 56% and 27%, respectively, following a 5 minutes washing with tap water.33 In the case of potatoes, where the applied pesticides are located on the surface of the potato peel containing epicuticular waxes, those pesticides with higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values tend to remain attached to the waxes and epidermis and are more difficult to remove, which correlates adequately with our results. This can be explained by the different bonding behaviors of the spiked pesticide molecules on the waxy layer (lipid) of the potato peel.10,37
P values tend to remain attached to the waxes and epidermis and are more difficult to remove, which correlates adequately with our results. This can be explained by the different bonding behaviors of the spiked pesticide molecules on the waxy layer (lipid) of the potato peel.10,37
| Pesticides | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P57 (at pH 7, 20 °C) P57 (at pH 7, 20 °C) |
Equilibrium pesticide retention Req (%) | |||
|---|---|---|---|---|---|
| LNI | WI | US | PEF | ||
| a Values are mean ± SD of triplicates (n = 3). Means in the same row (pesticide) with different lowercase superscripts are significantly different (p < 0.05). | |||||
| Spirotetramat | 2.51 | 85.13 ± 0.46a | 30.04 ± 1.33b | Not detected | 9.79 ± 1.50c |
| Chlorpropham | 3.76 | 90.10 ± 1.37a | 90.01 ± 0.74a | 64.78 ± 1.60b | 61.78 ± 0.26c |
| Azoxystrobin | 2.50 | 77.09 ± 2.82a | 28.99 ± 0.78b | 7.89 ± 0.47d | 19.09 ± 1.78c |
| Propiconazole | 3.72 | 85.41 ± 0.34a | 79.93 ± 2.07b | 70.23 ± 4.25c | 68.20 ± 1.08c |
| Captan | 2.50 | 78.52 ± 0.98a | 24.96 ± 0.57b | Not detected | 20.93 ± 1.59c |
Comparing all the pretreatment methods used in this study, the US was found to be the most effective in decreasing the retention of pesticides, especially for hydrophilic compounds having a weak bonding with epidermal waxes. As discussed previously, pesticide compounds are attached to different extents to the waxy epidermis and US intensification acts mainly on the surface of potato peel easing the removal of hydrophilic compounds.
3.3. Effect of pretreatments on quality parameters
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), which is higher than most ratios used in the literature,38–42 hampering the polyphenol removal by quickly saturating the solvent phase. In the literature, the extraction yield of phenolic compounds is influenced by the material-to-solvent ratio used during the extraction process, a higher ratio leads to a reduced mixture density, which affects the efficiency of extraction.43 For example, previous studies used various lower material-to-solvent ratios, such as 1
4), which is higher than most ratios used in the literature,38–42 hampering the polyphenol removal by quickly saturating the solvent phase. In the literature, the extraction yield of phenolic compounds is influenced by the material-to-solvent ratio used during the extraction process, a higher ratio leads to a reduced mixture density, which affects the efficiency of extraction.43 For example, previous studies used various lower material-to-solvent ratios, such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v, g mL−1) for grape pomace,38 1
20 (w/v, g mL−1) for grape pomace,38 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 (w/v, g mL−1) for fresh blackberries,22 1
10.5 (w/v, g mL−1) for fresh blackberries,22 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.7 (w/v, g mL−1) for mulberry leaves,39 and 1
16.7 (w/v, g mL−1) for mulberry leaves,39 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (w/v, g mL−1) for freeze-dried apples40 treatments. Razola-Díaz et al. (2021)41 also reported the successful extraction of phenolic compounds from orange by-products using a solid-to-solvent ratio of 1
24 (w/v, g mL−1) for freeze-dried apples40 treatments. Razola-Díaz et al. (2021)41 also reported the successful extraction of phenolic compounds from orange by-products using a solid-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 (w/v, g mL−1).
24 (w/v, g mL−1).
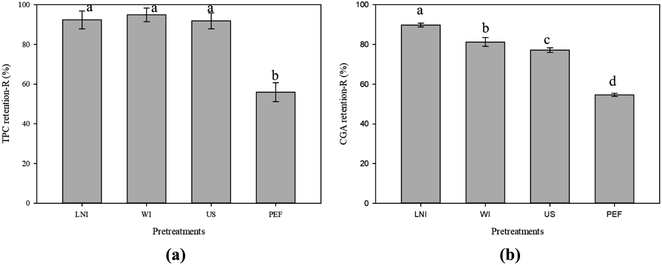 | ||
| Fig. 4 Average values of total phenolics (a) and chlorogenic acid (b) content retention (R%) of potato peel waste after pretreatments. | ||
From Fig. 4-a, a slight decrease in polyphenol content (less than 9%) from fresh potato peel was observed in samples pretreated with LNI, WI, and US (not significantly different between pretreatments, p < 0.05). In contrast, the retention of TPC in samples pretreated with PEF was significantly lower compared to the other pretreatments, with a retention rate of only 55.9%. Similarly, a 10% decrease in CGA content was observed in samples pretreated with LNI, while decreases of 19% and 23% were observed in samples pretreated with WI and US, respectively. On the other hand, the retention of CGA in samples pretreated with PEF was significantly lower compared to the other pretreatments, with a retention rate of only 54.6%.
In the research conducted by Peiro et al., it was found that the total phenolic content (TPC) extracted from lemon peels significantly increased by 1.6 times when the peels were pretreated with PEF at 7 kV cm−1 for 30 pulses, compared to untreated samples.42 Similarly, Luengo, Álvarez, & and Raso reported a remarkable 2.3-fold increase in TPC yield from orange peel extracts when treated with PEF at 3 kV cm−1 and 20 pulses. This increase was attributed to the permeabilization effect of PEF on the cell membranes of the orange peel, which facilitated the release of polyphenols from inside the cells.44 However, in the study conducted by Roselló-Soto et al., no significant differences (p < 0.05) were observed in the amount of total phenolic compounds extracted from olive kernels with water extraction assisted by PEF and US45 operated at similar specific powers from 18 to 109 kJ kg−1. This different result could be due to the olive kernel matrix structure and composition (different from plant cellular structures), equivalent to a hardwood with cellulose and lignin predominating, or because the solid/liquid ratio used for extraction (1/10) was smaller than in our study (1/4).
Results in Fig. 4 suggest that while there is a slight reduction in both TPC and CGA after certain pretreatments, the differences in TPC were not significant for LNI, WI, and US. However, it was found that PEF pretreatment has a significant negative impact on the retention of both TPC and CGA in potato peel samples, which could be explained by the different mechanisms of US and PEF pretreatments on the potato peel samples. In the case of US, cavitation bubbles near the material surface during a compression cycle collapsed, leading to the formation of microjets directed towards the epidermal layer of the peel.22 This phenomenon facilitates the removal of pesticide residues and other contaminants present on the surface of the peel, while causing minimal or negligible changes in the internal cellular structure where polyphenols are located, slowing down their removal, consistent with the findings of Wiktor et al. (2021).46 On the other hand, when PEF was applied to potato peel samples, short intense electric pulses were employed causing electroporation of the internal cell membranes.47 This electroporation phenomena could lead to the formation of temporary pores in the cell walls, allowing for the release of intracellular compounds, including phenolic compounds and other bioactive substances. It is worth noting that phenolic compounds are mainly located in the vacuoles of plant cells,48 and the electroporation process during PEF treatment could ease their release.
| Samples | Fresh | US | WI | PEF | |
|---|---|---|---|---|---|
| a Values are mean ± SD. Means in the same row (color parameter) with different lowercase superscripts are significantly different (p < 0.05). | |||||
| Peel part | L* | 49.66 ± 7.33a | 47.87 ± 10.27a | 48.93 ± 7.25a | 42.53 ± 2.68a |
| a* | 2.86 ± 2.18a | 8.05 ± 5.85a | 6.37 ± 5.71a | 6.37 ± 5.76a | |
| b* | 28.90 ± 0.81a | 16.38 ± 7.92b | 30.16 ± 9.64ab | 26.19 ± 7.62ab | |
| h | 84.43 ± 4.12a | 65.50 ± 6.39b | 76.19 ± 14.44ab | 74.59 ± 15.91ab | |
| C* | 29.07 ± 1.02a | 18.30 ± 9.67b | 31.30 ± 8.13ab | 27.47 ± 5.93ab | |
| Flesh part | L* | 64.07 ± 5.81a | 61.78 ± 5.25a | 65.18 ± 2.48a | 46.93 ± 3.05b |
| a* | 0.03 ± 1.33b | −1.09 ± 0.08b | −1.23 ± 0.49b | 7.38 ± 4.93a | |
| b* | 19.86 ± 0.74a | 21.02 ± 0.65a | 19.46 ± 0.99a | 23.34 ± 5.45a | |
| h | 89.84 ± 3.84a | 92.97 ± 0.11a | 93.65 ± 1.61a | 71.36 ± 14.83b | |
| C* | 19.88 ± 0.73a | 21.04 ± 0.65ab | 19.51 ± 0.96a | 24.89 ± 3.65b | |
Consequently, Fig. 5 presents the color changes (ΔE) in both the peel and flesh sides of potato peel, respectively, following various pretreatments (WI, US, and PEF) at end values of operational variables (time and pulse numbers, respectively). Regarding the peel side (Fig. 5-c), remarkable color changes were observed from the control sample to the pretreated samples, with ΔE values of 9.1, 10.9, and 16.1 for WI, PEF, and US pretreated samples, respectively. There was no significant difference in color change between WI and PEF pretreated samples. However, a distinct difference was observed for the US-pretreated sample, which became lighter in color after pretreatment. This can be attributed to the intensity of the US waves on the surface of the sample leading to a more rapid superficial compound dissolution (such as residual dust or dirt left after pre-washing), subsequently causing lightening of the peel surface and an increase in ΔE value. These findings are consistent with previously published data regarding the impact of washing methods on the residual presence of these compounds.51
Slight color changes were observed on the flesh side of the potato peel (Fig. 5-d) for WI and US pretreated samples, with no significant differences between them (p < 0.05). However, in the case of PEF pretreatment, the ΔE value for the flesh side was 19.0, which was significantly higher than for WI and US pretreatments. After PEF pretreatment, the flesh side of the samples was darker, which was indicated by lower L* values, and higher chroma (C*) values.
Polyphenol oxidase (PPO) activity plays a crucial role in the browning reaction that occurs in minimally processed potatoes. It oxidizes phenolic compounds present in potatoes, converting them into quinones, which further polymerize to form melanin pigments.52 In addition, chlorogenic acid, constituting 80% of total phenolic acids,7 significantly contributes to the browning of fresh-cut potatoes. These oxidation processes lead to the development of undesirable colors and texture in the potatoes, ultimately reducing the nutritional and economic value of the food. This points out that PEF-treated potato peel samples may have experienced a higher deterioration in the intracellular space of the flesh side, resulting in browning and corroborating previous results on TPC and CGA lower retention values, while US pretreatment under the conditions used in this study is more promising in terms of final product visual quality.
3.3.4.1 Water electrical conductivity. Water electrical conductivity measurement of the immersion media, before and after the pretreatment processes that use water immersion (WI, US, PEF), allowed for the monitoring of electrolytes or small molecule leakage from the cell tissue and intracellular compartments, which can provide valuable insights of the cellular integrity and permeability of the potato peel samples. The impact of pretreatments on changes in water electrical conductivity is shown in Fig. 5-a at maximum end values of operational variables. The water used for WI and US pretreatments on potato peel exhibited moderate increases of 2.8-fold and 3.7-fold in water electrical conductivity, respectively. In contrast, PEF pretreatment led to a significant 10-fold surge in water electrical conductivity. Similarly, the results obtained by Wiktor et al. (2015) showed that PEF application (5 kV cm−1, 50 pulses) on apple and carrot samples resulted in water electrical conductivity increases of 6-fold and 8-fold, respectively.53 This substantial increase in electrical conductivity with PEF can be attributed to the phenomenon of electroporation, resulting in the release of intracellular compounds, whereas, the impact of US and WI seems to affect less the internal tissue, as previously discussed. Results of water electrical conductivity corroborate previous results regarding TPC and CGA retention rates, hardness, and color of pretreated potato peel samples.
3.3.4.2 Light microscopy images. Fig. 6 shows light microscopic images of the cross-section of potato peel for a fresh sample (Fig. 6-a) along with those after WI, US, PEF, and LNI pretreatments (Fig. 6-b–e) at their respective maximum operational variable values (time, pulse numbers, and cycles). Fig. 6-a offers a clear observation of the distinct layers of potato peel: a thin layer of epicuticular waxes on the surface, a layer of epidermal cells, and another layer of inner tissue cells. The fresh sample (Fig. 6-a) exhibited a microstructure characterized by an intact, compact, and uniform waxy layer and uniform distribution (in cell size and shape) of epidermal and inner tissue layers. Cells in the epidermal layer are distributed densely compared to the inner tissue layer, with a smaller size. Their shape is rectangular. For inner tissue cells, the size is superior, and their shape is oval. For WI pretreated samples (Fig. 6-b), the waxy later seems stretched compared to fresh peel (Fig. 6-a), which could be because of water flux exchange between water and the sample. The inner tissue cells are round, likely due to water sorption during immersion pretreatment. Conversely, the US pretreated sample (Fig. 6-c) displayed surface damage and loss of the epicuticular waxy layer. The inner tissue cells showed shrinkage, attributable to water flux to the surface where cavitation and accelerated mass transfer occur.54 The microscopic image of the PEF-treated sample (Fig. 6-d) exhibited as well, a destroyed and absent waxy layer, but significantly smaller inner tissue cells compared to the fresh sample (Fig. 6-a), accompanied by signs of high cellular damage. This phenomenon can be attributed to PEF-caused disintegration of cellular membranes, followed by the release of water and vacuole compounds,55 resulting in a reduction in vacuole size and shape.56 As expected, LNI pretreated samples revealed the disappearance of some epicuticular waxes of potato peel surfaces (Fig. 6-e). Although similar in shape, smaller inner tissue cell size was observed compared to initial samples (Fig. 6-a), which could be attributed to the freeze-cracking impact caused by LNI immersion freeze-thawing cycles.21
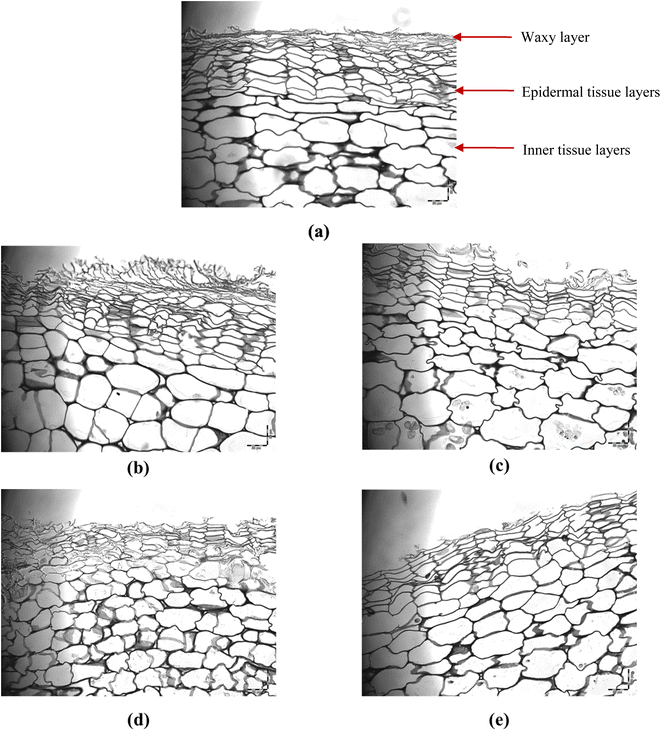 | ||
| Fig. 6 Microscopy observation of the cross-section through the potato peel under different pretreatments. (a) Fresh potato peel (untreated), (b) WI, (c) US, (d) PEF, and (e) LNI. | ||
Table 3 provides estimated values for inner cell surface area, epidermal cell surface area, and epidermal cell layer thickness, further highlighting significant reductions in these parameters for PEF and LNI pretreated samples (p < 0.05) as found previously from Fig. 6. In the case of PEF pretreatment, the average inner cell surface area was reduced by 56.3%, while epidermal cell surface area and thickness together decreased by 42.7%, and 42.5% compared to untreated samples, respectively. Additionally, LNI pretreatment resulted in a 24.4% reduction in the average inner cell surface area and a 23.8% reduction in epidermal cell layer thickness. These results corroborate the visual image observations described previously.
| Sample | Inner cell surface area (μm2) | Epidermal cell surface area (μm2) | Epidermal cell layer thickness (μm) |
|---|---|---|---|
| a Values are mean ± SD. Means in the same column with different lowercase superscripts are significantly different (p < 0.05). | |||
| Fresh | 4614 ± 2236a | 805 ± 484a | 160 ± 15a |
| WI | 5173 ± 1977a | 834 ± 318a | 154 ± 36ab |
| US | 5099 ± 2505a | 881 ± 396a | 130 ± 8bc |
| PEF | 2015 ± 793c | 461 ± 187b | 92 ± 28d |
| LNI | 3490 ± 1467b | 905 ± 323a | 122 ± 26cd |
Microscopic image results support previous findings of increased water electrical conductivity post-PEF pretreatment, along with lower retention values for TPC and CGA, lower hardness, and darker color of potato peel samples.
4. Conclusions
This study focused on innovative pretreatment techniques (WI, US, PEF, and LNI) to enhance the safety of potato peel, a by-product of potato production, by removing residual pesticides while maintaining an interesting bioactive quality before further processing. US and PEF pretreatments emerged as effective strategies for reducing pesticide residues. The elimination of pesticides from potato peel, whether through water immersion independently or with US/PEF intensification processes, was influenced by the compounds' affinity to lipids/water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P). A higher solid/solvent ratio used in most of the pretreatments together with the impact of ultrasounds mainly on potato peel surface, led to superior retention of phenolic compounds of US pretreated samples, compared to PEF samples for which cell electroporation was predominant. The observed changes in hardness, water electrical conductivity, color, and microstructure after pretreatments corroborated the mechanisms by which these intensification processes worked on pesticide reduction and polyphenol preservation. Thus, using a higher material-to-solvent ratio (1/4) combined with ultrasound pretreatment presented an interesting advantage for preserving bioactive compounds while effectively reducing pesticide residues on potato peel, enhancing the value of such waste towards the application of circular economy in potato transformation industries.
P). A higher solid/solvent ratio used in most of the pretreatments together with the impact of ultrasounds mainly on potato peel surface, led to superior retention of phenolic compounds of US pretreated samples, compared to PEF samples for which cell electroporation was predominant. The observed changes in hardness, water electrical conductivity, color, and microstructure after pretreatments corroborated the mechanisms by which these intensification processes worked on pesticide reduction and polyphenol preservation. Thus, using a higher material-to-solvent ratio (1/4) combined with ultrasound pretreatment presented an interesting advantage for preserving bioactive compounds while effectively reducing pesticide residues on potato peel, enhancing the value of such waste towards the application of circular economy in potato transformation industries.
The implementation of these results at the industrial level requires the scale-up of innovative pretreatment techniques, which may pose challenges and considerations. While ultrasound (US) and pulsed electric field (PEF) pretreatments show efficacy in reducing pesticide residues in potato peel on a lab scale, transitioning these methods to large-scale production requires careful evaluation. Scaling up these novel technologies, in addition to the cost and skills required by the industry, necessitates addressing factors such as the need for consistent and reliable power sources, optimization of treatment parameters, and the integration of these technologies into existing production processes. Furthermore, addressing the environmental impact of pesticide residues in wastewater is another important consideration.
List of abbreviations
| WI | Water immersion |
| US | Ultrasound |
| PEF | Pulse electric field |
| LNI | Liquid nitrogen immersion |
| TPC | Total phenolic content |
| CGA | Chlorogenic acid |
| R | Pesticide retention |
| C 0 | Initial concentration |
| C | Final concentration |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | Octanol-water partition coefficient |
| P | Power |
| m | Mass of water |
| C p | Heat capacity of water |
| dT/dt | The initial temperature increase rate |
| AED | Acoustic energy density |
| GC | Gas chromatography |
| FID | Flame ionization detector |
| κ e | Electrical conductivity |
| H | Hardness |
| L* | Lightness to darkness |
| a* | Redness to greenness |
| b* | Yellowness to blueness |
| ΔE | Total color difference |
| h | Hue angle |
| C* | Chroma |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Dr Paul Angers, Françoise Nadeau, and Diane Gagnon for technical support regarding GC and microscopic determinations. As well, authors would like to acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (RGPIN - 2017-04774) (NSERC - operating grant), from the Spanish research agency (AEI) MCIN/AEI/10.13039/501100011033 (projects PID2019-106148RR-C43 and PID2022-136889OB-C21) and FEDER A way of making Europe.References
- Government of Canada, https://agriculture.canada.ca/en/sector/horticulture/reports/potato-market-information-review-2021-22 (visit date: 2023-11-25).
- A. Al-Weshahy and A. Venket Rao, Food Res. Int., 2009, 42, 1062–1066 CrossRef CAS.
- H. Y. Gebrechristos and W. Chen, Food Sci. Nutr., 2018, 6, 1352–1356 CrossRef PubMed.
- M. P. Martins, E. J. Cortés, V. Eim, A. Mulet and J. A. Cárcel, Drying Technol., 2019, 37, 559–568 CrossRef CAS.
- B. Chitrakar, M. Zhang, X. Zhang and S. Devahastin, Powder Technol., 2020, 366, 275–282 CrossRef CAS.
- D. Rodriguez de Sotillo, M. Hadley and E. T. Holm, J. Food Sci., 1994, 59, 649–651 CrossRef CAS.
- C. R. Brown, Am. J. Potato Res., 2005, 82, 163–172 CrossRef CAS.
- T. T. Nguyen, C. Rosselló, R. Bélanger and C. Ratti, Foods, 2020, 9, 38 CrossRef PubMed.
- D. F. K. Rawn, S. C. Quade, W. F. Sun, A. Fouguet, A. Bélanger and M. Smith, Food Chem., 2008, 109, 790–796 CrossRef CAS PubMed.
- N. Yigit and Y. S. Velioglu, Crit. Rev. Food Sci. Nutr., 2019, 0, 1–20 Search PubMed.
- Y. Wu, Q. An, D. Li, J. Wu and C. Pan, Int. J. Environ. Res. Public Health, 2019, 16, 1–20 Search PubMed.
- A. Heshmati, A. Nili-Ahmadabadi, A. Rahimi, A. Vahidinia and M. Taheri, J. Cleaner Prod., 2020, 270, 122287 CrossRef CAS.
- Z. Y. Zhang, X. J. Liu and X. Y. Hong, Food Control, 2007, 18, 1484–1487 CrossRef CAS.
- J. Yang, L. Song, C. Pan, Y. Han and L. Kang, Int. J. Environ. Anal. Chem., 2020, 1–14 Search PubMed.
- W. Chen, Y. Liu and B. Jiao, Food Control, 2016, 66, 87–92 CrossRef CAS.
- A. Zohair, Food Chem. Toxicol., 2001, 39, 751–755 CrossRef CAS PubMed.
- Y. Zhang, W. Zhang, X. Liao, J. Zhang, Y. Hou, Z. Xiao, F. Chen and X. Hu, Ultrason. Sonochem., 2010, 17, 662–668 CrossRef CAS PubMed.
- F. Chen, L. Zeng, Y. Zhang, X. Liao, Y. Ge, X. Hu and L. Jiang, Food Chem., 2009, 112, 956–961 CrossRef CAS.
- M. Ketata, Y. Desjadins and C. Ratti, J. Food Eng., 2013, 116, 202–212 CrossRef CAS.
- T. C. T. Pham, P. Angers and C. Ratti, Can. J. Chem. Eng., 2018, 96, 2273–2281 CrossRef CAS.
- D. Canizares, P. Angers and C. Ratti, Ind. Crops Prod., 2019, 141, 111700 CrossRef CAS.
- C. A. Romero J. and B. D. Yépez V., Ultrason. Sonochem., 2015, 22, 205–210 CrossRef CAS PubMed.
- R. E. Mello, A. Fontana, A. Mulet, J. L. G. Corrêa and J. A. Cárcel, Innovative Food Sci. Emerging Technol., 2021, 72, 1–8 CrossRef.
- K. Rybak, A. Wiktor, M. Kaveh, M. Dadan, D. Witrowa-Rajchert and M. Nowacka, Molecules, 2022, 27, 1–22 Search PubMed.
- T. J. Mason, J. P. Lorimer and D. M. Bates, Ultrasonics, 1992, 30, 40–42 CrossRef CAS.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F. J. Schenck, J. AOAC Int., 2003, 86, 412–431 CrossRef CAS PubMed.
- D. W. Griffiths, H. Bain and M. F. B. Dale, J. Sci. Food Agric., 1992, 58, 41–48 CrossRef CAS.
- W. S. Rasband, ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, 2012 Search PubMed.
- C. Buschhaus and R. Jetter, J. Exp. Bot., 2011, 62, 841–853 CrossRef CAS PubMed.
- A. Angioni, M. Schirra, V. L. Garau, M. Melis, C. I. G. Tuberoso and P. Cabras, Food Addit. Contam., 2004, 21, 1065–1070 CrossRef CAS PubMed.
- R. Pandiselvam, A. Y. Aydar, N. Kutlu, R. Aslam, P. Sahni, S. Mitharwal, M. Gavahian, M. Kumar, A. Raposo, S. Yoo, H. Han and A. Kothakota, Ultrason. Sonochem., 2023, 92, 1–20, DOI:10.1016/j.ultsonch.2022.106261.
- Y. Zhu, T. Zhang, D. Xu, S. Wang, Y. Yuan, S. He and Y. Cao, Food Control, 2019, 95, 176–180 CrossRef CAS.
- B. Lozowicka, M. Jankowska, I. Hrynko and P. Kaczynski, Environ. Monit. Assess., 2016, 188, 1–19 CrossRef CAS PubMed.
- R. Pan, H. P. Chen, M. L. Zhang, Q. H. Wang, Y. Jiang and X. Liu, PLoS One, 2015, 10, 1–12 Search PubMed.
- J. E. Casida, J. Agric. Food Chem., 2017, 65, 4553–4561 CrossRef CAS PubMed.
- A. F. Hernández, F. Gil and M. Lacasaña, Arch. Toxicol., 2017, 91, 3211–3223 CrossRef PubMed.
- U. Bajwa and K. S. Sandhu, J. Food Sci. Technol., 2014, 51, 201–220 CrossRef CAS PubMed.
- M. R. González-Centeno, F. Comas-Serra, A. Femenia, C. Rosselló and S. Simal, Ultrason. Sonochem., 2015, 22, 506–514 CrossRef PubMed.
- Y. Tao, P. Wang, Y. Wang, S. U. Kadam, Y. Han, J. Wang and J. Zhou, Ultrason. Sonochem., 2016, 31, 310–318 CrossRef CAS PubMed.
- A. Lammerskitten, A. Wiktor, C. Siemer, S. Toepfl, V. Mykhailyk, E. Gondek, K. Rybak, D. Witrowa-Rajchert and O. Parniakov, J. Food Eng., 2019, 252, 36–43 CrossRef CAS.
- M. D. C. Razola-Díaz, E. J. Guerra-Hernández, C. Rodríguez-Pérez, A. M. Gómez-Caravaca, B. García-Villanova and V. Verardo, Foods, 2021, 10, 1–24 CrossRef PubMed.
- S. Peiro, E. Luengo, F. Segovia, J. Raso and M. Pilar Almajano, Waste Biomass Valorization, 2019, 10, 889–897 CrossRef CAS.
- C. S. Dzah, Y. Duan, H. Zhang, C. Wen, J. Zhang, G. Chen and H. Ma, Food Biosci., 2020, 35, 1–9 Search PubMed.
- E. Luengo, I. Álvarez and J. Raso, Innovative Food Sci. Emerging Technol., 2013, 17, 79–84 CrossRef CAS.
- E. Roselló-Soto, F. J. Barba, O. Parniakov, C. M. Galanakis, N. Lebovka, N. Grimi and E. Vorobiev, Food Bioprocess Technol., 2015, 8, 885–894 CrossRef.
- A. Wiktor, A. Landfeld, A. Matys, P. Novotná, M. Dadan, E. Kováříková, M. Nowacka, M. Mulenko, D. Witrowa-Rajchert, J. Strohalm and M. Houška, Foods, 2021, 10, 1–15 CrossRef PubMed.
- N. I. Lebovka, M. V. Shynkaryk and E. Vorobiev, Drying Technol., 2006, 24, 601–608 CrossRef.
- P. Gramazio, J. Prohens, M. Plazas, I. Andjar, F. J. Herraiz, E. Castillo, S. Knapp, R. S. Meyer and S. Vilanova, BMC Plant Biol., 2014, 14, 1–15 CrossRef PubMed.
- P. A. Zongo, S. Khalloufi, S. Mikhaylin and C. Ratti, Foods, 2022, 11, 2551 CrossRef CAS PubMed.
- A. Wiktor, M. Schulz, E. Voigt, D. Witrowa-Rajchert and D. Knorr, J. Food Eng., 2015, 146, 8–16 CrossRef.
- C. Alister, M. Araya, K. Becerra, C. Volosky, J. Saavedra and M. Kogan, Food Chem., 2018, 268, 264–270 CrossRef CAS PubMed.
- C. Bøjer Rasmussen, J. J. Enghild and C. Scavenius, Food Chem., 2021, 365, 1–8 CrossRef PubMed.
- A. Wiktor, M. Sledz, M. Nowacka, K. Rybak, T. Chudoba, W. Lojkowski and D. Witrowa-Rajchert, Innovative Food Sci. Emerging Technol., 2015, 30, 69–78 CrossRef CAS.
- I. Lavilla and C. Bendicho, in Water Extraction of Bioactive Compounds, Elsevier Inc., 2017, pp. 291–316 Search PubMed.
- J. Blahovec and P. Kouřím, J. Food Eng., 2019, 240, 183–190 CrossRef CAS.
- M. Botero-Uribe, M. Fitzgerald, R. G. Gilbert and J. Midgley, Trends Food Sci. Technol., 2017, 67, 1–11 CrossRef CAS.
- University of Hertfordshire, The PPDB-Pesticide properties database, http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |