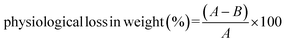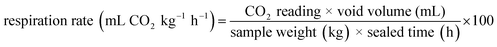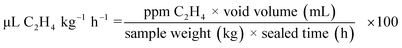Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Storage stability assessment of guava fruit (Psidium guajava L.) cv. ‘Gola’ in response to different packaging materials
Ali Asad
Yousaf
 *,
Kashif Sarfraz
Abbasi
,
Muhammad Suhail
Ibrahim
,
Asma
Sohail
,
Mamoona
Faiz
and
Mehwish
Khadim
*,
Kashif Sarfraz
Abbasi
,
Muhammad Suhail
Ibrahim
,
Asma
Sohail
,
Mamoona
Faiz
and
Mehwish
Khadim
Institute of Food & Nutritional Sciences, PMAS-Arid Agriculture University, Rawalpindi, 46000, Pakistan. E-mail: aliasad.yousaf@gmail.com; yousafm1@msu.edu
First published on 28th November 2023
Abstract
Guava (Psidium guajava L.) is a nutritious fruit of sub-tropical regions that displays climacteric characteristics and short postharvest life. The quality of guava fruit deteriorates within two to three days at ambient temperatures; therefore, specific practices are needed to preserve the quality and increase the shelf life of guavas. In the present work, we aimed to assess how various packing materials affected the shelf life and quality of guava fruit (cv. Gola) stored at room temperature. Guava fruits were stored for a period of 20 days in different packing materials such as biodegradable packaging (BDP), corrugated fiber box (CFB), polypropylene (PP), high density polyethylene (HDPE), low density polyethylene (LDPE) and polystyrene (PS). During the storage period, transitions in multiple quality metrics were observed at predetermined intervals of four (4) days. According to the findings, each packing material significantly affected the fruit's quality in comparison to control samples. During the storage period, there was a natural decline in firmness (93.4%), ascorbic acid (55.6%), and TPC (48.6%); however, the packaged fruit samples showed a considerably slower rate of reduction (p < 0.05) than the unpackaged control samples. Moreover, respiratory gases were effectively suppressed under packaging viz. HDPE (ethylene; 8.76 μL kg−1 h−1, CO2; 19.76 mL kg−1 h−1) and BDP (ethylene; 10.16 μL kg−1 h−1, CO2; 21.37 μL kg−1 h−1), respectively. In terms of enzyme dynamics, un-packed fruit samples had relatively low CAT activity (69.45 U mg−1 protein), while guava fruits that were packed in BDP exhibited much increased CAT activity (82.28 U mg−1 protein). Likewise, PPO activity was significantly inhibited in packaged fruit samples. Among the different packaging employed, biodegradable packaging, PP, and HDPE exhibited better overall retention of quality attributes during 20 days of storage under ambient conditions. The study's outcome should open up new opportunities for the fruit and vegetable sectors while also offering a cost-effective approach to preserving fresh guavas and increasing their economic potential.
Sustainability spotlightThe current investigation is expected to address the first SDG, that is, poverty alleviation, through farmers' income generation by reduction in their post-harvest losses which are estimated to be more than 30% in fruits in particular. Reducing postharvest losses in nutritionally important fruits will also have a meaningful impact towards sustainable agriculture, which is another important SDG. It will also promote the access of the masses to nutrient-dense food (indigenous guava fruit cultivars in the case of the current study) and would ensure food as well as nutritional security. Reduction in postharvest losses would also address waste pollution thus promoting a clean and healthy environment. |
1. Introduction
Guava (Psidium guajava L.) is a major fruit crop that is widely cultivated in sub-tropical and tropical parts of the world. It is a gregarious member of the Myrtaceae family, which has 121 genera and about 5800 species of perennial trees with edible fruits.1 According to the Food and Agriculture Organization's database, the world’s annual production of guava fruit is 55 million tons approximately,2 with India and Pakistan collectively contributing around 50 percent of the total production.3 With 0.57 million tons of yearly production, guava takes the 4th place among Pakistan's major fruit crops, just behind citrus, mango, and dates; however, Pakistan merely exported 0.2% of guava fruits in the fiscal year of 2021–22, due to the lack of proper postharvest management practices.4Guava is aptly called the “poor man's apple of the tropics” and the “winter national fruit of Pakistan”.5 Nutritionists often classify it under “super-fruits” due to its varied bioactive compounds and significant antioxidant activity.6 In addition, it can offer four times more vitamin-C than an average sized orange fruit.7 Pharmacological studies also demonstrate its antimicrobial, antidiarrheal, antidiabetic, anti-allergic, anti-plasmodia, anti-spasmodic and anti-inflammatory activities.5,8,9 Indigenous guava fruits carry 83–84% moisture content, 8–10% total carbohydrates, 1.9–2.2% protein, 2.96–3.46% crude fiber, 0.6–0.85% fat and 0.6–0.7% ash.7 It is also a generous source of micronutrients and nutraceuticals such as calcium (23 mg/100 g), phosphorous (42 mg/100 g), iron (0.09 mg/100 g), vit. C (250–300 mg/100 g), vit. A (200–400 IU/100 g), phenolic compounds (94–190 mg GAE/100 g) and flavonoids (81–154 mg QE/100 g).7,10,11
Guava fruit is highly perishable and placed under the climacteric category of fruits as it ripens speedily after harvest under ambient conditions.12 In climacteric commodities, such as guava, high respiration rates are regulated in various biological systems, governed by a natural growth hormone, known as ethylene which is produced in a complex signal transduction pathway through L-methionine via the 1-aminocyclopropane-1-carboxylicacid (ACC) synthase enzyme.13 Resultantly, guava fruit attains its senescence peaks in three to four post-harvest days depending on the variety, harvest time and storage conditions.12,14 The critical changes that occur during fruit's senescence include weight loss, chlorophyll degradation, turgidity loss, reduced nutritional value, and finally a decline in marketability.15,16 In addition to these physiological losses, it is estimated that approximately 30–50% of guava fruit is wasted annually due to inadequate postharvest handling.17 To improve storage life, different approaches are being instigated for perishable commodities including controlled atmospheric (CA) storage;18,19 modified atmospheric packaging;20–23 hypobaric storage;24 edible coatings;25–28 storage at low temperatures16,29 and irradiation.30,31 However, guava has received less attention, regardless of its nutraceutical potential and commercial importance.
Amongst multiple postharvest interventions, packaging is one of the cheapest and easy to adopt approaches to extending the storage life of perishable commodities. Packaging can offer a barrier to gaseous exchange and restrict the rate of respiration at varying levels, thus conserving the freshness of the product for much longer periods.32 By keeping perishable goods isolated from the outside world and shielding them from pollutants and microbes, packaging aids in maintaining sterile conditions.33 In addition, packaging improves aesthetic value, fulfills industry requirements and consumer desires and also minimizes food wastage during the supply chain.34–37 For perishable goods, several packaging materials, such as corrugated fibres, polypropylene (PP), low density polyethylene (LDPE), and high density polyethylene (HDPE), have been testified to decrease postharvest losses.38–40 The majority of prior research, nonetheless, has concentrated on the combined effects of packing and low temperatures. In contrast, the current research emphasized how various packing materials influenced the postharvest quality of guava fruit (cv: “Gola”) in ambient circumstances. [The present study is an extension of our previous work7 from which we selected an indigenous guava variety ‘Gola’ on the basis of its physio-chemical and nutraceutical characterization].
2. Materials and methods
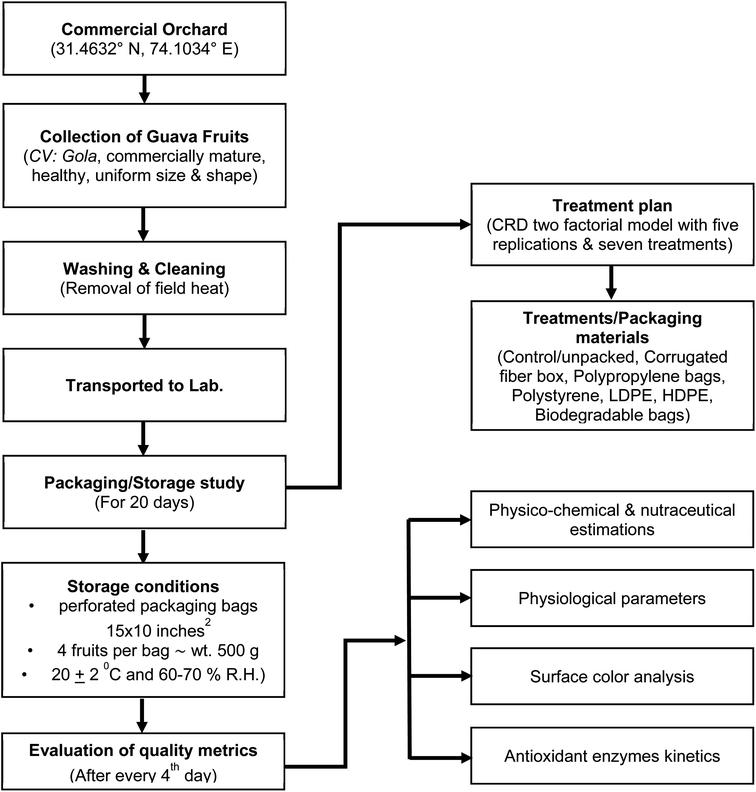
The present investigation was carried out at the Institute of Food and Nutritional Sciences (IFNS), PMAS-Arid Agriculture University, Rawalpindi, Pakistan. Fig. 1 shows the flow diagram of the whole investigation.2.1. Materials
Based on the findings of our prior investigation,7 the indigenous guava variety “Gola” was chosen for the current study. Commercially mature, healthy fruits consistent in size and shape were harvested from a guava orchard in District Sheikhupura, Punjab, Pakistan (31.4632° N, 74.1034° E). The fruit samples were carefully transferred to the IFNS for further analysis after being graded, sorted, and precooled to avoid field heat. The packaging materials (corrugated fiber, polypropylene, polystyrene, LDPE, HDPE, and biodegradable), in the form of bags, having 15 × 10 inches2 area and provided perforations with 5 holes of 0.5 mm diameter on each side, were obtained from the local wholesale market, while Sapphire Textiles Mills, Pvt. Ltd. Lahore, Pakistan, provided the biodegradable packaging bags made from compressed chrysanthemum seeds.2.2. Treatments and experimental design
The experiment was laid out in a two factorial model of completely randomized design with five replications and seven treatment combinations which were as follows: T0 (control/unpacked), T1 (corrugated fiber box), T2 (polypropylene bags), T3 (polystyrene), T4 (LDPE), T5 (HDPE), and T6 (biodegradable bags). Correspondingly, a plastic tray containing un-packed fruits served as a control. Each package contained 4 fruits in each replication with 500 g approximate weight. Both the control and the packaged fruits were stored at room temperature (20 ± 2 °C and 60–70% R.H.) in accordance with the aforementioned treatment plan after being visually screened and having their surfaces dried with muslin cloth. The below mentioned methodologies were employed to assess transitions in various quality metrics during the course of storage (20 days) at a predetermined interval of 4 days.2.3. Physico-chemical and nutraceutical estimations
The firmness of the guava fruits under storage was measured by using fruit penetrometer FT10 (Wagner, Italy) equipped with an 8 mm size plunger tip as described by Abbasi et al.37 The ripening index (RI) was calculated as the ratio of total soluble solids to titratable acidity, whereas nutraceutical properties such as total phenolic content (TPC) and vitamin C content (ascorbic acid) of guava fruits were estimated by following the procedures as described in our earlier study.72.4. Physiological parameters
Physiological loss in weight (PLW) of guava fruits under storage was calculated at every 4 days of sampling interval and expressed in percentage as:where A is the fruit weight just before storage and B is the fruit weight after a fixed storage period.41
Ethylene and the CO2 exchange rate were measured according to the method described by Nair, Saxena & Kaur12 with some modifications. Three (3) pre-weighed guava fruits from each replication were placed in an airtight plastic container (3.0 L volume) enclosed with a rubber septum on the lid for one hour. For readings, a syringe of a handheld three gas analyzer (Felix: F-950, USA) was inserted into the plastic container through the rubber septum. After noting the readings (CO2 and ethylene, as appeared on the analyzer screen), the respiration rate (CO2) was calculated as:
while the release of ethylene gas was calculated using the following formula:
2.5. Surface color analysis
Surface color of the fruit was measured with a handheld colorimeter (Konica Minolta CR300, Japan) in terms of L*, a*, and b* coordinates. The chroma value was calculated according to Mendoza, Dejmek & Aguilera.422.6. Antioxidant enzyme kinetics
The activities of antioxidant enzymes such as catalase (CAT) and polyphenol oxidase (PPO) were assessed adopting the methods similar to those used by Ali, Khan, Malik, and Shahid43 with a few minor adjustments. For this purpose, frozen fruit pulp (1 g) was homogenized in 2 mL phosphate buffer (pH 7.2) using a mortar and pestle. After thorough homogenization, samples were centrifuged using refrigerated centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 minutes. The obtained supernatant was collected and further used to measure enzymatic activity. The method by Liu et al.44 was used with slight modifications to assess the CAT activity in guava fruit. In order to initiate the enzyme reaction, 100 μL of enzyme extract was mixed with freshly prepared 100 μL solution of 5.9 mM H2O2 (Sigma-Aldrich, USA). Using a UV-vis spectrophotometer (Jenway 6850 D, Cole-Parmer, Germany), absorbance at 240 nm was recorded and the activity of the CAT enzyme was expressed as U mg−1 protein, where one unit (U) of enzyme activity was defined as absorbance change in 0.1 unit min−1. Likewise, PPO activity of stored guava fruits was measured according to the method adopted by Lo'ay & Taher.45 For this purpose, 1 g of fruit pulp was combined with 20 mM, pH 7 Tris–HCl buffer (VWR International, Canada) and centrifuged at 10
000 × g for 10 minutes. The obtained supernatant was collected and further used to measure enzymatic activity. The method by Liu et al.44 was used with slight modifications to assess the CAT activity in guava fruit. In order to initiate the enzyme reaction, 100 μL of enzyme extract was mixed with freshly prepared 100 μL solution of 5.9 mM H2O2 (Sigma-Aldrich, USA). Using a UV-vis spectrophotometer (Jenway 6850 D, Cole-Parmer, Germany), absorbance at 240 nm was recorded and the activity of the CAT enzyme was expressed as U mg−1 protein, where one unit (U) of enzyme activity was defined as absorbance change in 0.1 unit min−1. Likewise, PPO activity of stored guava fruits was measured according to the method adopted by Lo'ay & Taher.45 For this purpose, 1 g of fruit pulp was combined with 20 mM, pH 7 Tris–HCl buffer (VWR International, Canada) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes. The supernatant was collected at −20 °C to which 200 μL of fruit extract was instantly mixed along with 3 mL of 20 mM methyl catechol (Sigma-Aldrich, USA). Spectrophotometric measurements at 400 nm wavelength over the course of 4 minutes exposed the change in activity, whereas an increase or decrease in absorbance of 0.1 units (U) per minute was used to measure enzyme activity. However, bovine serum albumin (Thermo Fisher Scientific, USA) was used as a standard for the determination of protein content.46
000 rpm for 5 minutes. The supernatant was collected at −20 °C to which 200 μL of fruit extract was instantly mixed along with 3 mL of 20 mM methyl catechol (Sigma-Aldrich, USA). Spectrophotometric measurements at 400 nm wavelength over the course of 4 minutes exposed the change in activity, whereas an increase or decrease in absorbance of 0.1 units (U) per minute was used to measure enzyme activity. However, bovine serum albumin (Thermo Fisher Scientific, USA) was used as a standard for the determination of protein content.46
2.7. Statistical analyses
All the experiments were executed in triplicate according to completely randomized design (CRD) with a factorial layout. The factors were different packaging and storage times. Analysis of variance (ANOVA) was used to analyze the data and the LSD test was applied to compare means at P < 0.05 using MINITAB 18.1 software.3. Results
3.1. Fruit firmness
The data regarding firmness of stored guava samples under the influence of different packing materials are presented in Fig. 2A. It was observed that the firmness showed a decreasing tendency with a storage period of 20 days. The highest decline in firmness (93%) was perceived in the fruit samples which were stored without any packing; however, the guava fruit placed in various packaging materials exhibited retaining behavior. While analyzing the results, it was noted that T6 had significantly the lowest (77%) firmness drop in contrast to control samples followed by T5 (79.23%) and T2 (79.87%), respectively. Meanwhile, the treatments T1 (corrugated fiber box) and T4 (LDPE) were found to be statistically at par (p < 0.05) in terms of retaining fruit firmness.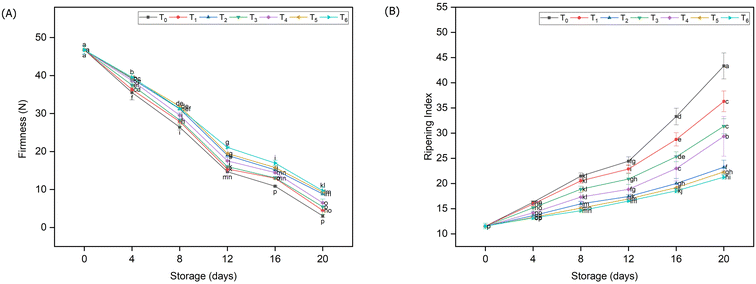 | ||
| Fig. 2 Transitions in firmness (A) and ripening index (B) of guava fruits stored under different packaging materials. | ||
3.2. Ripening index
The ripening index refers to the ratio of soluble solids to acidity. The finding of the current investigation showed that most of the treatments were significantly at par (p < 0.05) with each other; however, considerably dissimilar from control samples (Fig. 2B). A significantly high ripening index was calculated in control samples (23.54), demonstrating that unpacked fruits ripened far more quickly than those receiving other treatments. Compared to alternative packaging materials, the treatments, particularly biodegradable, HDPE and PP, exhibited substantially more encouraging outcomes.3.3. Vitamin C content and total phenolic compounds
In the current study, vitamin C values were preserved in treated samples but drastically decreased in unpackaged fruits (Fig. 3A). The results demonstrated that only 44% of ascorbic acid was preserved in T0 compared with packed guava fruits (for instance in T6), where 62% of ascorbic acid was retained through the whole span of storage. Similarly, the distribution of total phenolic compounds (TPCs) in guava fruits during storage (Fig. 3B) showed that TPCs gradually decreased across all treatments; however, those tended to be retained in packed fruit samples compared to control samples. Significantly higher mean values were calculated in T6 (153.14 mgGAE/100 g) followed by T5 (150.28 mgGAE/100 g) & T2 (144.39 mgGAE/100 g). The findings clearly showed that throughout the 20 days of storage, 48.61% of TPCs was lost in unpackaged guava fruit samples in comparison to the packed guava fruit samples where TPCs were lost up to 39%.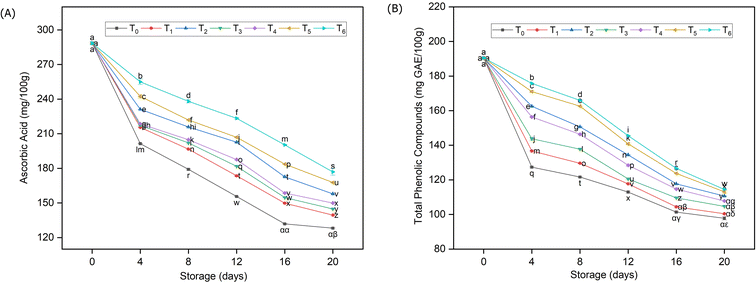 | ||
| Fig. 3 Transitions in ascorbic acid (A) and total phenolic compounds (B) of guava fruits stored under different packaging materials. | ||
3.4. Physiological parameters (weight loss, respiration rate, and ethylene rate)
It was evident from the data presented in Fig. 4A that the significantly (p < 0.05) highest loss in weight was noted in control samples i.e. 27%, while under biodegradable packaging and HDPE packaging, only 11% & 13% of weight loss were recorded at the end of storage (20 days), respectively. Fig. 4B exhibits the effect of different packaging materials on the respiration rate of guava fruits during storage. It was observed that all the treatments were considerably different from each other. However, a significantly higher amount of CO2 was released from control samples, while the treatments T5 (19.76 mL kg−1 h−1) & T6 (21.37 mL kg−1 h−1) significantly retarded the release of respiratory gases followed by T2 (22.93 mL kg−1 h−1). Similarly, the lowest mean values of ethylene (Fig. 4C) were recorded in T5 (8.75 μL kg−1 h−1) as compared to other treatments. It is worth mentioning that the climacteric peak was significantly delayed by packaging treatments such as T2, T5 & T6 where the climacteric peak was delayed to the 8th day of storage, while it appeared on the 4th day in other treatments.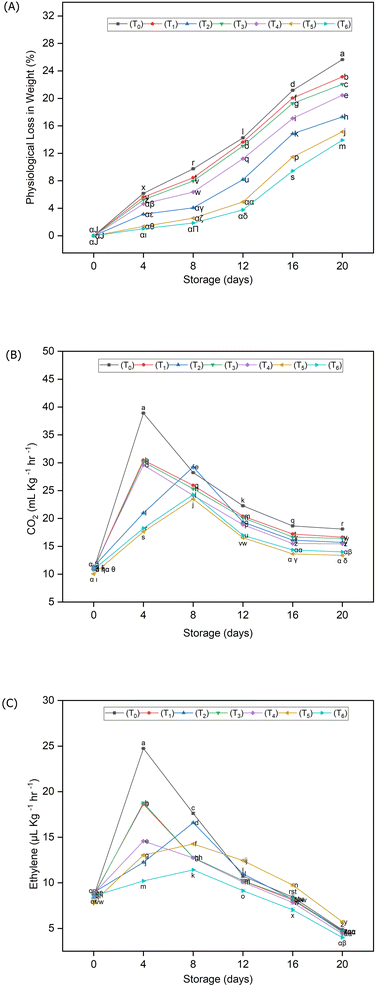 | ||
| Fig. 4 Transitions in weight loss (A), respiration rate (B) and ethylene rate (C) of guava fruits stored under different packaging materials. | ||
3.5. Surface color of fruit
The findings of the current study regarding the surface color of guava fruits stored at room temperature under different packaging materials showed that the luminosity (L* coordinates) dramatically decreased in the un-packed fruits, but it tended to retain its values in the packaged samples (Fig. 5A). Similarly, the coordinates (a* & b*) indicating greenness and yellowness, respectively, increased with the lapse of storage days; however, the pace of that increase was significantly slower in guava fruits that were experiencing different packaging materials compared to those of non-packaged guava fruits (Fig. 5B–D). The results of the current study further demonstrated that biodegradable packaging and HDPE were statistically at par (p < 0.05) and exhibited the lowest values of color coordinates (a*, b*, & chroma values), which suggests that packaging maintained the greenish color of guava fruits up to the 12th day of storage, while the control samples turned yellow at a much faster rate.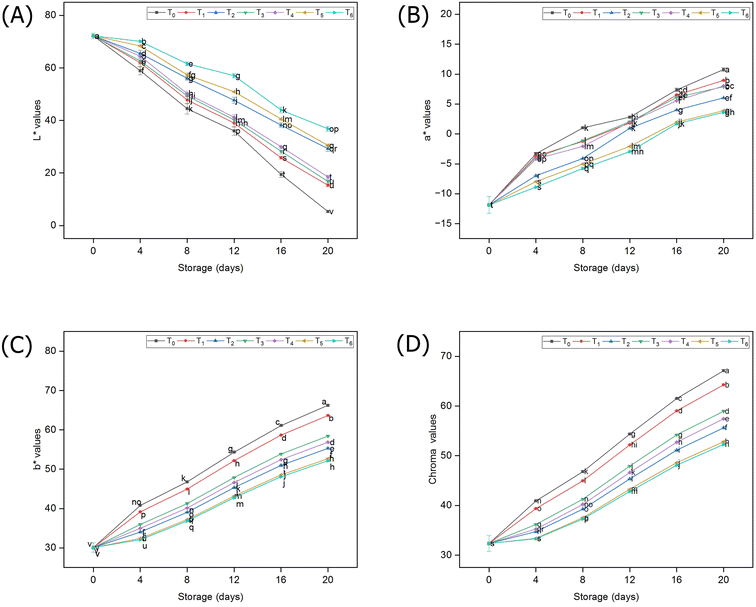 | ||
| Fig. 5 Surface color evaluation of guava fruits under storage; L* values (A), a* values (B), b* values (C) and chroma (D). | ||
3.6. Antioxidant enzyme kinetics
The influence of different packaging materials on the kinetics of the antioxidant enzymes in guava fruits during storage is shown in Fig. 6. According to the revealed data, the packaging materials had a highly significant (p < 0.05) impact on polyphenol oxidase (PPO) and catalase (CAT) activities as compared to un-packed guava fruit samples. It was observed that biodegradable, HPPE and PP packing significantly (p < 0.05) inhibited the activity of PPO (0.258, 0.264, and 0.266 U mg−1 protein), correspondingly as compared to fruit samples (T0) stored without any packing material (0.328 U mg−1 protein) (Fig. 6A). Likewise, the packaging materials also significantly affected the catalase activity as compared to untreated samples. The activity of catalase enzyme (CAT) was dropped in all treatments throughout the storage time, while the rate of decline was noticeably reduced under the influence of different packaging materials (Fig. 6B). Unpackaged fruit samples had very little CAT activity (69.45 U mg−1 protein), whereas guava fruits that had been stored in biodegradable packaging had a much higher CAT activity (82.28 U mg−1 protein).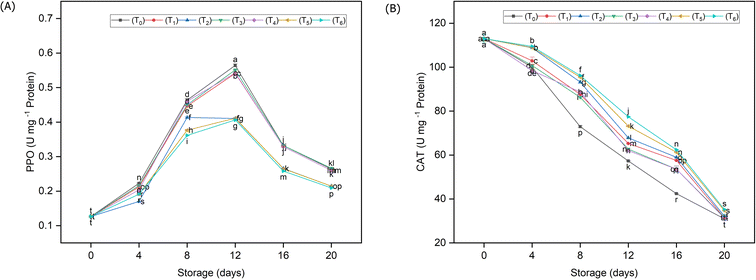 | ||
| Fig. 6 Influence of different packaging materials on the enzyme kinetics of guava fruits during storage; activity of the PPO enzyme (A) and catalase activity (B). | ||
4. Discussion
Perishable horticultural produce needs to be managed at all times particularly after harvest. To reduce post-harvest losses, these commodities are preserved by a variety of methods. The simplest and the most cost-effective way of reducing postharvest losses while maintaining the intact quality characteristics of fruits and vegetables is packing technology. Packaging is often a viable option for increasing fruit's shelf life, showing a considerable advantage in reducing physical damage through direct treatments.47 The current investigation revealed the effect of different packaging materials on the storage stability of guava fruits specifically the ‘Gola’ cultivar. The results regarding the influence of packaging on the firmness of guava fruit samples demonstrated that the guava fruit samples lost their firmness gradually during storage at room temperatures. However, the firmness tended to be retained in packed guava fruits. Significantly higher firmness may be due to the low rate of respiration and transpiration under the influence of multiple packaging materials leading to retaining the firmness of the guava fruits during storage. Owing to the limited supply of available oxygen, particularly in the cases of HDPE, PP and biodegradable packing, the activities of polygalacturonase and pectin methylesterase enzymes, which are responsible for pectin hydrolysis, may be reduced, thus resulting in the retention of firmness during storage.48 The findings of the current study are also in conformity with those of Gaspar et al.49 and Sahoo et al.,21 who examined the storage stability of guava in different packing systems. Rana, Siddiqui & Goyal50 also produced similar results from wrapped guava fruits in which firmness remained significantly higher for control fruits (8.6 kg cm−2) and declined thereafter. Comparable results were also outlined by Sahoo, Bal, Pal & Sahoo51 that bell pepper could be stored for 4 days under ambient conditions using ventilated PP and LDPE based packing materials.One of the most important quality indicators to be assessed during postharvest storage is the ripening index (RI), which is the ratio of total soluble solids to titratable acidity. Horticulturists and even advanced growers can better comprehend the appropriate harvesting time and the actual state of fruit ripeness by calculating the ripening index. Furthermore, instead of measuring soluble solids and titratable acidity separately, the ratio provides a more accurate indication of fruit's flavor.52 In the current investigation, the RI of guava fruits steadily increased in all treatments with each subsequent interval during storage; however, packaging had an advantageous effect on slowing the pace of RI escalation. This might be due to the retaining tendency of total soluble solids in the packed guava fruit samples owing to the reduced respiration rate and as a consequence, the metabolic activity was also compromised, resulting in slow conversion of carbohydrates into simple sugars.53 On the other hand, it could be the reason for significantly lower values of titratable acidity as well. Rodriguez-Nunez et al.54 also provided a similar explanation for the pattern of RI in which it gradually increased during the course of storage. The observations that were noted from the current study are also in line with the findings of Yamashita & Benassi,55 Miano, Jokhio & Miano,56 and Rana, Siddiqui & Goyal.50
When performing a storage study of horticultural goods, surface color is one of the essential matrices that may be used to measure quality and characterize the product.57 The color value “L” reflects luminosity or the degree of lightness, whereas the corresponding coordinates “a” and “b” denote the degree of redness or greenness and the degree of yellowness or blueness, respectively. A lower value of ‘a’ indicates a more greenish color of the fruit, which can be attributed to the richness of chlorophyll pigments, whereas a positive value of ‘b’ signifies yellowing of a particular fruit.47 Referring to the results of the current study, it was revealed that the non-packaged guava fruits lost their luminosity at much faster rates as compared to the packaged fruit samples. Similarly, the values of a* coordinates gradually increased from a negative value (−ve) to positive values (+ve) at the end of just the 4th day of storage in the case of un-packed, corrugated fiber box packaging and LDPE packaging; however, positive (+ve) a* values were recorded at the 12th day of storage in the fruit samples which were under PP, HDPE and biodegradable packaging. This was further supported by visual inspection of guava fruit samples and the color shift from green to reddish brown, which may have resulted from enzyme activity.21 In terms of the b* coordinates, biodegradable packaging and HDPE packaging were statistically at par (p < 0.05) which showed that the guava fruit samples packed under the specified packing maintained their greenish color to some extent, whereas unpackaged guavas quickly went yellow. This might be due to the loss of chlorophyll pigments or enzymatic synthesis of Maillard products.58 In addition, a low respiration rate may have contributed to the retention of green color in packed guava fruit samples which resulted in maintaining fruit quality.59 The findings of the current investigation regarding the influence of different packaging materials upon guava storage were in close conformity with those of Jacomino, Kluge, Sarantópoulos & Sigrist.39 Sahoo et al.21 and Afrin et al.23 inferred that the packing improved the color retention and marketability of guavas. They also suggested perforated PP and PE packaging for storage of climacteric fruits under ambient conditions.
As previously stated, guava fruits are frequently classified as super fruits due to the presence of several health boosting phytochemicals that go beyond basic nutritional value. Furthermore, it contains a high concentration of vitamin C, making guava fruit an excellent source of antioxidants. In general, during storage studies, nutraceutical compounds (phenolics, flavonoids, and vitamins) reduce with the passage of time; so while selecting any postharvest intervention, the preservation of this dynamic trait of fruits should be considered. In the current investigation, the total phenolic compounds and values of vitamin C concentration declined in all the packaging treatments with the passage of the storage period; however, biodegradable, HDPE and polypropylene packaging showed promising results in retaining TPC and vitamin C values in comparison to un-packaged guava fruit samples. The drop in TPC resulted in a decrease in astringency of guava fruits during storage, which might be attributed to polymerization of leuco-anthocyanidins and hydrolysis of the astringent arabinose ester of hexa-hydrodiphenic acid.14 The retention of nutraceutical substances may also be ascribed to the packaging system's endurance to oxidative stress. As a result, less reactive oxygen species (ROS) would be produced, perhaps preserving bioactive molecules.60 On the other hand, packaging delayed the enzymatic conversion of ascorbic acid to L-dehydro ascorbic acid due to reduced oxygen supply, resulting in vitamin C retention in packed fruits.61 Comparable results have also been reported by Wu62 and Nath et al.32 Partial reduction in ascorbic values was also reported by Pal et al.63 during the storage of individually wrapped guava fruits.
Physiological weight loss is considered a crucial determining factor for the limited storage life of guava fruits and their visual quality. Weight loss is mainly caused by enzymatically catalyzed natural catabolism of horticultural products and is directly connected to transpiration and the vapor pressure differential.21 With respect to the current investigation, the weight loss was gradually increased during the storage period; however, the loss in weight was significantly higher than that of packing treatments. The packaging materials especially biodegradable packaging, PP and HDPE restricted the release of breathing gases responsible for weight loss. This fact proved the delayed ripening under the influence of packing materials, thus enhancing the shelf life of guava fruits with intact quality attributes. The observance of decreased weight loss under the influence of packing treatments might be attributed to controlled respiration and senescence related metabolic changes during storage, whereas, a higher water vapor transmission rate may be responsible for higher physiological loss in weight as in the case of un-packaged guava fruit samples. Identical justification was also given by Tirkey et al.,64 while studying the shelf-life of fresh-cut unripe papaya packed in low density polyethylene (LDPE) and metallized polyester poly (MPP) pouches. Our study's findings are also consistent with those of Hailu, Seyoum, Workneh & Belew,65 who determined that the weight loss was significantly reduced (24.8% to 2.1%) from bananas stored at ambient temperatures using LDPE and HDPE packing. Similar results were also produced by Rodov et al.,66 who concluded that the fruit weight loss in biodegradable packaging was 4–5 times lower than that in un-packed fruits.
Primarily, guava is a climacteric fruit; hence, it continues to respire after being harvested and attains its respiratory/ethylene peak within 2–3 days at ambient temperatures. An early inception of the climacteric peak involves an increase in the respiration rate as well as other biochemical modifications, such as changes in skin color, increased TSS and firmness decline.14 In the present study, the guava fruit samples showed a typical climacteric pattern of respiration, reaching a climacteric peak on the 4th day of storage at room temperature with significantly higher amounts of CO2 released in the control samples. However, the packaging treatments significantly postponed the onset of respiratory peaks on the 8th day with lower values of CO2 and ethylene production rates. Due to the selective gaseous permeability offered by the packaging materials, the packaging innovations attempt to change the gaseous environment surrounding the guava fruit in a way that lowers the respiration rate and delays the start of the respiratory peak.47 During storage, the respiration rate is reduced, which further limits the ripening-related changes in guava fruit.67 In addition, due to the limited availability of respiration gases, the activity of enzymes such as acetyl-CoA carboxylase (ACC) synthase and ACC oxidase, involved in ethylene biosynthesis, lowers, which in turn causes ethylene production to slow down and delays the climacteric peak in packaged fruits.68,69 The findings of the current investigation are in line with Sahoo et al.21 and Rana, Siddiqui & Goyal50 who also produced similar results while studying the effect of different packaging materials on the shelf life of guava fruits. According to Islam et al.,70 modified atmospheric packing effectively controls the respiration and transpiration of climacteric jujube fruit during storage.
Compared to the unpacked fruits, all the packing materials in the current investigation more efficiently retained the modified atmospheric conditions surrounding the guava fruits. As a result, there was less moisture loss and less oxygen available for oxidation of the polyphenols. In the present study, the activity of PPO enzyme initially increased in all treatments owing to the availability of substrates; however, it declined with senescence during storage. When compared to the control, the PPO activity was dramatically reduced by packaging treatments especially in the case of biodegradable packaging and HDPE. The change in PPO activity may be due to the difference in oxygen permeability between various packing materials which was also demonstrated by Abbasi et al.37 Low PPO activity is also favorable in non-browning of fruits because it is involved in the oxidation of phenolics and the degradation of anthocyanins24,71 as we have observed in the present study also. On the other hand, the activity of the catalase enzyme was steadily decreased due to the typical climacteric behavior of guava fruits; however, the magnitude of that decrease was significantly slower under the influence of packaging. CAT is the primary enzyme of the plant's antioxidant system, which can reduce the accumulation of superoxide anions and H2O2, hence reducing cyto-dermal damage.72 Higher CAT activity also indicates that the cells have better capacity to scavenge reactive oxygen species and H2O2.73 The findings of the current study are also in conformity with those of Ali et al.74 and Sun et al.75
5. Conclusion
It has been revealed that packaging materials, in general, significantly influence the physiological weight loss, ripening index, firmness, phenolic compounds and vitamin C content. Most importantly, the climacteric peak was substantially (p ≤ 0.05) delayed in packaged fruits as opposed to un-packaged fruits during storage as optimized ethylene and CO2 concentrations were attained with packaging materials such as HDPE, PP and biodegradable packaging. Additionally, the current study showed that the activity of PPO enzymes was significantly lowered in packed guava fruits, which in turn minimized fruit browning. As a conclusion to this study, perforated PP, HDPE and biodegradable packaging may be a preferable choice for guava fruit packing. The study's findings would also boost fresh guavas' economic potential and present new prospects for the fruit and vegetable industries in addition to providing an affordable method of preserving them. Future studies may be carried out by employing the current packing system in combination with other postharvest interventions for the longer storage life of horticultural produce.Conflicts of interest
The authors have no conflict of interests regarding the current research work.References
- M. É. Stefanello, A. C. Pascoal and M. J. Salvador, Essential oils from neotropical Myrtaceae: chemical diversity and biological properties, Chem. Biodiversity, 2011, 8(1), 73–94 CrossRef PubMed.
- Food and Agricultural Organization of the United Nations, Major Tropical Fruits: Preliminary results 2021, 2021, available from: https://www.fao.org/3/cb9412en/cb9412en.pdf, accessed on 25th September 2023 Search PubMed.
- E. M. Yahia, Fruits and vegetable phytochemicals: chemistry and human health, Wiley, Hoboken. 2018, pp. 1067–1076 Search PubMed.
- Government of Pakistan, Ministry of National Food Science and Research, Agricultural Statistics of Pakistan - Economic Wing, Ministry of National Food Science and Research, Islamabad, Pakistan, 2022 Search PubMed.
- S. Z. Hussain, B. Naseer, T. Qadri, T. Fatima and T. A. Bhat. Guava (Psidium guajava)-Morphology, Taxonomy, Composition and Health Benefits, in Fruits Grown in Highland Regions of the Himalayas: Nutritional and Health Benefits, Springer International Publishing, Cham, Switzerland, 2021, pp. 257–267 Search PubMed.
- B. Joseph and M. Priya, Review on nutritional, medicinal and pharmacological properties of guava (Psidium guajava Linn.), Int. J. Pharma Bio Sci., 2011, 2(1), 53–69 Search PubMed.
- A. A. Yousaf, K. S. Abbasi, A. Ahmad, I. Hassan, A. Sohail, A. Qayyum and M. A. Akram, Physico-chemical and nutraceutical characterization of selected indigenous Guava (Psidium guajava L.) cultivars, Food Sci. Technol., 2020, 41, 47–58 CrossRef.
- M. Gupta, A. Wali, S. Gupta and S. K. Annepu, Nutraceutical potential of Guava, Bioact. Mol. Food, 2018, 1–27 Search PubMed.
- R. Upadhyay, J. F. Dass, A. K. Chauhan, P. Yadav, M. Singh and R. B. Singh. Guava enriched functional foods: therapeutic potentials and technological challenges, The Role of Functional Food Security in Global Health, 2019, pp. 365–378 Search PubMed.
- G. Flores, S. B. Wu, A. Negrin and E. J. Kennelly, Chemical composition and antioxidant activity of seven cultivars of guava (Psidium guajava) fruits, Food Chem., 2015, 170, 327–335 CrossRef PubMed.
- D. M. Kadam, P. Kaushik and R. Kumar, Evaluation of guava products quality, Int. J. Food Sci. Nutr. Eng., 2012, 2(1), 7–11 CrossRef.
- M. S. Nair, A. Saxena and C. Kaur, Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.), Food Chem., 2018, 240, 245–252 CrossRef PubMed.
- F. D. Rueda, Guava (Psidium guajava L.) fruit phytochemicals, antioxidant properties and overall quality as influenced by postharvest treatments, Doctoral dissertation, University of Florida, 2005 Search PubMed.
- H. A. Bashir and A. B. Abu-Goukh, Compositional changes during guava fruit ripening, Food Chem., 2003, 80(4), 557–563 CrossRef.
- M. Goutam, H. S. Dhaliwal and B. Mahajan, Effect of pre-harvest calcium sprays on post-harvest life of winter guava (Psidium guajava L.), J. Food Sci. Technol., 2010, 47, 501–506 CrossRef PubMed.
- M. A. Rehman, M. R. Asi, A. Hameed and L. D. Bourquin, Effect of Postharvest application of aloe vera gel on shelf life, activities of anti-oxidative enzymes, and quality of ‘Gola’guava fruit, Foods, 2020, 9(10), 1361 CrossRef CAS PubMed.
- S. Sultana, S. Khanam, M. T. Islam, T. Anannya Sharif and M. R. Haque, Assessment of Post-Harvest Storage Stability and Physico-Chemical Properties of Guava (Psidium guajava) at Different Maturity Stages, Asian J. Biochem. Genet. Mol. Biol., 2021, 11–24 CrossRef.
- S. Mangaraj, T. K. Goswami, S. K. Giri and C. G. Joshy, Design and development of modified atmosphere packaging system for guava (cv. Baruipur), J. Food Sci. Technol., 2014, 51, 2925–2946 CrossRef CAS PubMed.
- G. H. Teixeira, L. C. Júnior, A. S. Ferraudo and J. F. Durigan, Quality of guava (Psidium guajava L. cv. Pedro Sato) fruit stored in low-O2 controlled atmospheres is negatively affected by increasing levels of CO2, Postharvest Biol. Technol., 2016, 111, 62–68 CrossRef CAS.
- D. K. Antala, A. K. Varshney, P. R. Davara and V. P. Sangani, Modified atmosphere packaging of guava fruit, Packag. Technol. Sci., 2015, 28(6), 557–564 CrossRef CAS.
- N. R. Sahoo, M. K. Panda, L. M. Bal, U. S. Pal and D. Sahoo, Comparative study of MAP and shrink wrap packaging techniques for shelf life extension of fresh guava, Sci. Hortic., 2015, 182, 1–7 CrossRef CAS.
- S. K. Pandey, Physicochemical Changes under Bulk Perforated Modified Atmospheric Storage of Guava, Agricultural Engineering Today, 2018, 42(4), 42–47 Search PubMed.
- T. Afrin, N. A. Husna, M. M. Hossain, A. Iqbal and P. Karmoker, Efficacy of Packaging on the Quality and Shelf Life of Guava (Psidium guajava), European J. Agric. Food Sci., 2022, 4(5), 7–12 Search PubMed.
- X. Zhang, L. Xin, C. Wang, S. Sun and Y. Lyu, Short-term hypobaric treatment enhances chilling tolerance in peaches, J. Food Process. Preserv., 2021, 45(9), e15362 CAS.
- S. F. El-Gioushy, M. F. Abdelkader, M. H. Mahmoud, H. M. Abou El Ghit, M. Fikry, A. M. Bahloul, A. R. Morsy, A. A. Lo’ay, A. M. R. A. Abdelaziz, A. S. A. Haifa, D. M. Hikal, M. A. Abdein, K. H. A. Hassan and M. S. Gawish, The effects of a gum arabic-based edible coating on guava fruit characteristics during storage, Coatings, 2022, 12(1), 90 CrossRef CAS.
- A. S. Formiga, E. M. Pereira, J. S. Junior, F. B. Costa and B. H. Mattiuz, Effects of edible coatings on the quality and storage of early harvested guava, Food Chem. Adv., 2022, 1, 100124 CrossRef.
- A. Rani, J. Tokas, P. Gupta, H. Punia and S. Baloda, Efficacy of herbal extracts-based nano-formulations in extending guava fruit shelf-life, Appl. Sci., 2022, 12(17), 8630 CrossRef CAS.
- M. Zaidi, A. Akbar, S. Ali, H. Akram, S. Ercisli, G. Ilhan, E. Sakar, R. A. Marc, D. A. Sonmez, R. Ullah and A. Bari, Application of Plant-Based Edible Coatings and Extracts Influences the Postharvest Quality and Shelf Life Potential of “Surahi” Guava Fruits, ACS Omega, 2023, 8(22), 19523–19531 CrossRef.
- M. BVC, S. R. Sharma and R. K. Dhall, Optimization of storage temperature for maintaining quality of guava, J. Food Sci. Technol., 2009, 46(6), 604–605 Search PubMed.
- I. Ihsanullah and A. Rashid, Current activities in food irradiation as a sanitary and phytosanitary treatment in the Asia and the Pacific Region and a comparison with advanced countries, Food Control, 2017, 72, 345–359 CrossRef.
- N. Munir, M. Tahir and S. Naz, Effect of Gamma Radiation to Cope with Post-harvest Losses and Nutritional Components in Guava (Psidium guajava L.), Curr. Nutr. Food Sci., 2020, 16(6), 934–944 CrossRef.
- A. Nath, B. C. Deka, A. Singh, R. K. Patel, D. Paul, L. K. Misra and H. Ojha, Extension of shelf life of pear fruits using different packaging materials, J. Food Sci. Technol., 2012, 49, 556–563 CrossRef PubMed.
- S. Lu, Effects of bactericides and modified atmosphere packaging on shelf-life of Chinese shrimp (Fenneropenaeus chinensis), LWT--Food Sci. Technol., 2009, 42(1), 286–291 CrossRef.
- R. Coles, D. McDowell and M. J. Kirwan, Plastic in Food Packaging: Food Packaging Technology, CRC press, 2003 Search PubMed.
- K. Marsh and B. Bugusu, Food packaging—roles, materials, and environmental issues, J. Food Sci., 2007, 72(3), 39–55 CrossRef.
- S. Quintavalla and L. Vicini, Antimicrobial food packaging in meat industry, Meat Sci., 2002, 62(3), 373–380 CrossRef PubMed.
- K. S. Abbasi, T. Masud, A. Qayyum, S. U. Khan, A. Ahmad, A. Mehmood, A. Farid and M. A. Jenks, Transition in tuber quality attributes of potato (Solanum tuberosum L.) under different packaging systems during storage, J. Appl. Bot. Food Qual., 2016, 89, 142–149 Search PubMed.
- M. A. Del Nobile, F. Licciardello, C. Scrocco, G. Muratore and M. Zappa, Design of plastic packages for minimally processed fruits, J. Food Eng., 2007, 79(1), 217–224 CrossRef.
- A. P. Jacomino, R. A. Kluge, C. I. Sarantópoulos and J. M. Sigrist, Evaluation of plastic packages for guava refrigerated preservation, Packag. Technol. Sci., 2001, 14(1), 11–19 CrossRef.
- L. M. Pereira, A. C. Rodrigues, C. I. Sarantópoulos, V. C. Junqueira, R. L. Cunha and M. D. Hubinger, Influence of modified atmosphere packaging and osmotic dehydration on the quality maintenance of minimally processed guavas, J. Food Sci., 2004, 69(4), 172–177 CrossRef.
- K. Hong, J. Xie, L. Zhang, D. Sun and D. Gong, Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage, Sci. Hortic., 2012, 144, 172–178 CrossRef CAS.
- F. Mendoza, P. Dejmek and J. M. Aguilera, Calibrated color measurements of agricultural foods using image analysis, Postharvest Biol. Technol., 2006, 41(3), 285–295 CrossRef.
- S. Ali, A. S. Khan, A. U. Malik and M. Shahid, Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits, Food Chem., 2016, 206, 18–29 CrossRef CAS PubMed.
- D. Liu, J. Zou, Q. Meng, J. Zou and W. Jiang, Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity, Ecotoxicology, 2009, 18, 134–143 CrossRef CAS PubMed.
- A. A. Lo’ay and M. A. Taher, Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv.‘Banati’, Sci. Hortic., 2018, 235, 424–436 CrossRef.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72(1–2), 248–254 CrossRef CAS.
- A. Yadav, N. Kumar, A. Upadhyay, O. A. Fawole, M. K. Mahawar, K. Jalgaonkar, D. Chandran, S. Rajalingam, G. Zengin, M. Kumar and M. Mekhemar, Recent advances in novel packaging technologies for shelf-life extension of guava fruits for retaining health benefits for longer duration, Plants, 2022, 11(4), 547 CrossRef CAS.
- L. Jat, S. S. Lakhawat, V. Singh, R. Meena, J. L. Choudhary and S. Gathala, Postharvest γ-irradiation treatment enhance nutritional and antioxidant potential of Indian jujube (Ziziphus mauritiana Lamk) fruit, Sci. Hortic., 2022, 301, 111127 CrossRef CAS.
- J. Gaspar, F. Couto, L. C. Salomão, F. L. Finger and A. Cardoso, Effect of low temperature and plastic films on post-harvest life of guava (Psidium guajava L.), International Symposium on Myrtaceae, 1996, vol. 452, pp. 107–114 Search PubMed.
- S. Rana, S. Siddiqui and A. Goyal, Extension of the shelf life of guava by individual packaging with cling and shrink films, J. Food Sci. Technol., 2015, 52, 8148–8155 CrossRef CAS PubMed.
- N. R. Sahoo, L. M. Bal, U. S. Pal and D. Sahoo, A comparative study on the effect of packaging material and storage environment on shelf life of fresh bell-pepper, J. Food Meas. Charact., 2014, 8, 164–170 CrossRef.
- A. A. Kader, Flavor quality of fruits and vegetables, J. Sci. Food Agric., 2008, 88(11), 1863–1868 CrossRef CAS.
- Z. Razali, D. S. George and N. A. Ibrahim, Improvements in quality and antioxidant capacity of guava (Psidium guajava L.) exposed to UV-C irradiation, in Proc. 3rd International Conf. on Chemical, Agricultural and Medical Sciences, CAMS-2015, 2015, pp. 10–11 Search PubMed.
- J. R. Rodríguez-Núñez, A. Rodríguez-Félix, P. I. Campa-Siqueiros, L. Val-Félix and T. J. Madera-Santana, Ionizing Technology Effects on Bioactive Compounds from Food Products, in Ionizing Radiation Technologies: Managing and Extracting Value from Wastes, ed. S. Shima and D. Suresh, Wiley, UK, 2022, pp. 104–119 Search PubMed.
- F. Yamashita and M. D. Benassi, Influence of modified atmosphere packaging and calcium treatment on kinetic of ascorbic acid degradation and weight loss in guavas (Psidium guajava L.), Food Sci. Technol., 2000, 20, 27–31 CrossRef CAS.
- T. F. Miano, J. A. Jokhio and T. F. Miano, Effect of different packaging materials and storage conditions on physicochemical characteristics of guava var Allahabadi, J. Agrofor. Environ., 2010, 4(2), 33–36 Search PubMed.
- A. D. Siqueira, J. M. da Costa, M. R. Afonso and E. Clemente, Pigments of guava paluma cultivar stored under environmental conditions, Afr. J. Food Sci., 2011, 5(6), 320–323 CAS.
- I. Aguiló-Aguayo, G. Oms-Oliu, R. Soliva-Fortuny and O. Martín-Belloso, Flavour retention and related enzyme activities during storage of strawberry juices processed by high-intensity pulsed electric fields or heat, Food Chem., 2009, 116(1), 59–65 CrossRef.
- M. S. Aday and C. Caner, The shelf life extension of fresh strawberries using an oxygen absorber in the bio based package, LWT--Food Sci. Technol., 2013, 52(2), 102–109 CrossRef CAS.
- M. Hasanuzzaman, M. R. Raihan, A. A. Masud, K. Rahman, F. Nowroz, M. Rahman, K. Nahar and M. Fujita, Regulation of reactive oxygen species and antioxidant defense in plants under salinity, Int. J. Mol. Sci., 2021, 22(17), 9326 CrossRef.
- S. Singh, A. K. Singh and H. K. Joshi, Prolonging storability of Indian gooseberry (Emblica officinalis) under semi-arid ecosystem of Gujarat, Indian J. Agric. Sci., 2005, 75(10), 647–650 Search PubMed.
- M. C. Wu, Effect of vacuum packaging on the color of frozen sugar apple (Annona squamosa L.), J. Food Qual., 2000, 23(3), 305–315 CrossRef.
- R. K. Pal, M. S. Ahmad, S. K. Roy and M. Singh, Influence of storage environment, surface coating, and individual shrink wrapping on quality assurance of guava (Psidium guajava) fruits, Plant Foods Hum. Nutr., 2004, 59, 67–72 CrossRef PubMed.
- B. Tirkey, U. S. Pal, L. M. Bal, N. R. Sahoo, C. K. Bakhara and M. K. Panda, Evaluation of physico-chemical changes of fresh-cut unripe papaya during storage, Food Packag. Shelf Life, 2014, 1(2), 190–197 CrossRef.
- M. Hailu, T. Seyoum Workneh and D. Belew, Effect of packaging materials on shelf life and quality of banana cultivars (Musa spp.), J. Food Sci. Technol., 2014, 51, 2947–2963 CrossRef PubMed.
- V. Rodov, R. Porat, A. Sabag, B. Kochanek and H. Friedman, Microperforated compostable packaging extends shelf life of ethylene-treated banana fruit, Foods, 2022, 11(8), 1086 CrossRef.
- K. Ncama, L. S. Magwaza, A. Mditshwa and S. Z. Tesfay, Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review, Food Packag. Shelf Life, 2018, 16, 157–167 CrossRef.
- E. D. Mullins, T. G. McCollum and R. E. McDonald, Consequences on ethylene metabolism of inactivating the ethylene receptor sites in diseased non-climacteric fruit, Postharvest Biol. Technol., 2000, 19(2), 155–164 CrossRef.
- M. Farcuh, R. M. Rivero, A. Sadka and E. Blumwald, Ethylene regulation of sugar metabolism in climacteric and non-climacteric plums, Postharvest Biol. Technol., 2018, 139, 20–30 CrossRef.
- A. Islam, R. Acıkalın, B. Ozturk, E. Aglar and C. Kaiser, Combined effects of Aloe vera gel and modified atmosphere packaging treatments on fruit quality traits and bioactive compounds of jujube (Ziziphus jujuba Mill.) fruit during cold storage and shelf life, Postharvest Biol. Technol., 2022, 187, 111855 CrossRef.
- A. Torres, G. Aguilar-Osorio, M. Camacho, F. Basurto and A. Navarro-Ocana, Characterization of polyphenol oxidase from purple sweet potato (Ipomoea batatas L. Lam) and its affinity towards acylated anthocyanins and caffeoylquinic acid derivatives, Food Chem., 2021, 356, 129709 CrossRef PubMed.
- Z. Zhang, D. J. Huber, H. Qu, Z. E. Yun, H. Wang, Z. Huang, H. Huang and Y. Jiang, Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols, Food Chem., 2015, 171, 191–199 CrossRef.
- X. Wang, H. Liu, F. Yu, B. Hu, Y. Jia, H. Sha and H. Zhao, Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering, Sci. Rep., 2019, 9(1), 8543 CrossRef PubMed.
- S. Ali, A. S. Khan, A. U. Malik, M. A. Anjum, A. Nawaz and H. M. Shah, Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage, Sci. Hortic., 2019, 254, 14–20 CrossRef.
- Y. Sun, Y. Huang, X. Y. Wang, Z. Y. Wu and Y. X. Weng, Kinetic analysis of PGA/PBAT plastic films for strawberry fruit preservation quality and enzyme activity, J. Food Compos. Anal., 2022, 108, 104439 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |