Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon flows and biochar stability during co-pyrolysis of human faeces with wood biomass†
M. E.
Koulouri
 *,
M.
Qiu
,
M. R.
Templeton
and
G. D.
Fowler
*,
M.
Qiu
,
M. R.
Templeton
and
G. D.
Fowler
Department of Civil and Environmental Engineering, Imperial College London, SW7 2AZ, UK. E-mail: maria.koulouri17@imperial.ac.uk
First published on 28th August 2024
Abstract
As non-sewered toilets are now the most commonly used sanitation facilities, the faecal sludge management (FSM) sector is starting to be recognised as an important actor for global carbon management. The development of systematic strategies to calculate avoided emissions and carbon storage opportunities is currently constrained by a lack of understanding of carbon flows during faecal sludge treatment. This study investigated carbon sequestration potential for faecal sludge treatment systems that involve co-pyrolysis of human faeces (HF) and wood biomass (WB) at different blending ratios HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB (100
WB (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 75
0, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 25
50, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, 0
75, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and temperatures (450, 550, 650 °C). The systematic investigation of analytical biochar stability parameters and the quantification of carbon flows among pyrolysis products were carried out for the first time in the context of faecal sludge. The stability of the produced biochars was assessed based on their remaining volatility, carbon structure (H/C and O/C ratios, SEM and FTIR analyses) and oxidation resistance (chemical oxidation by H2O2 and thermal degradation by thermogravimetric analysis [R50 index]). Overall, co-pyrolysis of HF and WB improved carbon fixation and biochar stability, enhancing carbon sequestration potential compared to pyrolysis of pure faecal feedstocks. Biochars produced from 50
100) and temperatures (450, 550, 650 °C). The systematic investigation of analytical biochar stability parameters and the quantification of carbon flows among pyrolysis products were carried out for the first time in the context of faecal sludge. The stability of the produced biochars was assessed based on their remaining volatility, carbon structure (H/C and O/C ratios, SEM and FTIR analyses) and oxidation resistance (chemical oxidation by H2O2 and thermal degradation by thermogravimetric analysis [R50 index]). Overall, co-pyrolysis of HF and WB improved carbon fixation and biochar stability, enhancing carbon sequestration potential compared to pyrolysis of pure faecal feedstocks. Biochars produced from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 HF
50 HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blends at 550 °C had the highest carbon retention (41.1%); this feedstock blending ratio corresponds to ∼30 g dry wood added in toilets as a cover material (per user per day), based on the expected daily excretion quantities. For these conditions, the H/C, O/C ratios, H2O2 oxidation and R50 index values suggest that the produced biochars have developed an aromatic structure and are suitable for long-term carbon storage. The biochar characteristics were found to be more dependent on feedstock composition than pyrolysis temperature – provided that the temperature reached was sufficient to ensure completion of the main pyrolytic reactions (≥500 °C) – while carbon flows to the bio-oil and non-condensable gas fractions were significantly influenced by pyrolysis operational parameters (retention time and inert gas flow rate). The formation of CaCO3 was observed via SEM/EDX and can be further investigated as a potential additional carbon storage mechanism in FSM. The findings of this research can be used to create a methodological dataset to inform carbon assessments and future modelling applications, paving the way towards the establishment of carbon-negative FSM.
WB blends at 550 °C had the highest carbon retention (41.1%); this feedstock blending ratio corresponds to ∼30 g dry wood added in toilets as a cover material (per user per day), based on the expected daily excretion quantities. For these conditions, the H/C, O/C ratios, H2O2 oxidation and R50 index values suggest that the produced biochars have developed an aromatic structure and are suitable for long-term carbon storage. The biochar characteristics were found to be more dependent on feedstock composition than pyrolysis temperature – provided that the temperature reached was sufficient to ensure completion of the main pyrolytic reactions (≥500 °C) – while carbon flows to the bio-oil and non-condensable gas fractions were significantly influenced by pyrolysis operational parameters (retention time and inert gas flow rate). The formation of CaCO3 was observed via SEM/EDX and can be further investigated as a potential additional carbon storage mechanism in FSM. The findings of this research can be used to create a methodological dataset to inform carbon assessments and future modelling applications, paving the way towards the establishment of carbon-negative FSM.
Water impactGlobally, more people use on-site sanitation than sewered connections. This sector emits ∼6% of global CH4, but knowledge on carbon flows along the sanitation chain is very limited. This article introduces a systematic methodology to calculate carbon flows and storage potential when co-pyrolysing human excreta with wood cover materials. Results can be used directly by WASH practitioners and for future research. |
1. Introduction
On-site non-sewered toilets have become the most commonly used sanitation systems globally and the amount of faecal sludge (FS) generated from these facilities is expected to increase over the coming decades.1 The management of FS is not only essential to maintain environmental and public health safety, but also creates opportunities for resource recovery and climate change mitigation, via avoided carbon emissions and carbon storage, during containment and treatment of human waste.2,3 Although there are currently very limited studies on the quantification and systematic assessment of carbon flows along the sanitation chain,4 a new paradigm is starting to be established whereby on-site sanitation is being recognised as an important sector for carbon management, estimated to contribute to up to 6% of global CH4 emissions.5 Container-based sanitation (CBS) in particular is garnering interest as an emerging technology with significant opportunities for carbon benefits.6 Container-based systems provide a service-based approach to sanitation provision and offer regular collection services to households that contribute to avoided greenhouse gas emissions from traditional long-term containment facilities like pit latrines and septic tanks.7 Efforts to quantify these benefits are ongoing and carbon credit schemes are starting to be established as a viable revenue stream for CBS business models.8Less is known about the flow of carbon after collection of human waste (i.e., during treatment and end-uses of recovered products), as well as on the effect of FS composition on carbon dynamics.9 Notably, dry toilets such as CBS that do not use water for flushing are usually urine diverting and involve the addition of a cover material to the faecal stream, to prevent odour and insects and to facilitate on-site treatment.10,11 Often this cover material is some form of wood biomass, like sawdust, while agricultural waste (incl. coconut shells, rice husk, coffee husk, etc.) are also used, depending on local availability.12 The addition of these biomass feedstocks alters the material balance in FS treatment, but there is currently limited understanding and control of the relative quantities of materials in sanitation facilities.13 The organic composition of human faeces is different to that of lignocellulosic biomass sources such as wood, and therefore, the systematic addition of these materials can be expected to alter the behaviour of carbon within FS management (FSM) systems.14
This is particularly true for pyrolysis technologies, as their process operation and end-product characteristics are highly dependent on feedstock decomposition behaviour and system carbon dynamics.14 Pyrolysis is a mature technology for the treatment of several carbonaceous materials, such as wood or agricultural waste. Although it has not been widely applied for excreta-based sludges, it is emerging as a technology of interest among researchers and practitioners, as it ensures pathogen sterilisation and offers several resource recovery opportunities.15 Pyrolysis encompasses the thermal decomposition of carbonaceous materials in the absence of oxygen, producing a solid output known as biochar, as well as bio-oil and non-condensable gases (NCG).16 Recent studies on FS pyrolysis have focused on the uses of the produced biochar, particularly as a soil amendment or as a solid fuel.17,18
Agricultural applications of biochar can be used as a soil-based carbon sequestration strategy because biochar is an aromatic form of carbon that can potentially remain stable for hundreds or thousands of years.19 In that context, FS pyrolysis can be deployed when aiming to establish carbon-neutral (or carbon-negative) FS treatment systems, especially for developing countries that have largely agriculture-centred economies and abundant FS and biomass waste sources.20 Traditional FS pyrolysis applications that do not require electricity to operate (e.g., using biomass-based fuels)16 are currently being used in several developing countries (Uganda, Rwanda, etc.). Still, very little is known about carbon flows during FS pyrolysis and the impact that different blending ratios of cover materials have on carbon stability and sequestration potential, for different operational conditions.
This study aims to assess carbon sequestration potential for FS pyrolysis systems that involve co-pyrolysis of human faeces with a wood-based cover material at different blending ratios and treatment temperatures by: 1) investigating thermal behaviour and end-product characteristics; 2) quantifying carbon retention and stability in the biochar; and 3) for the first time, mapping the distribution of carbon flows among pyrolysis products. This research can be used to assess the carbon footprint of FS pyrolysis systems during their life cycle and to inform sustainability decision-making in sanitation and FSM chains. The research methods presented in this paper can be used for the development of protocols to comprehensively assess FS stability and carbon sequestration potential. Supporting sustainability claims with transparent and systematically documented technical knowledge is crucial to understand and maximise carbon benefits, as well as overcome scepticism around climate finance schemes.21
2. Materials & methods
2.1 Sample collection
Samples of source-separated human faeces (HF) were collected from 12 volunteers in the UK according to the protocol described by Koulouri et al.22 The HF samples were dried at 105 °C to constant weight, subsequently dry-sterilised at 150 °C for 150 min as suggested by Krueger et al.,23 and were mechanically homogenised and ground into particle sizes <10 mm before pyrolysis experiments. Wood biomass (WB) (willow wood) was also locally collected, dried, and ground homogeneously to the same particle size. HF and WB were blended at ratios of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, 50
75, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 75
50 and 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (dry w/w%), corresponding to sample names HF25, HF50 and HF75 respectively.
25 (dry w/w%), corresponding to sample names HF25, HF50 and HF75 respectively.
2.2 Pyrolysis experiments
Pyrolysis experiments were conducted using a Carbolite HTR 11/75 rotary furnace fitted with a quartz reactor connected to an oil trap and condenser (ESI† Fig. S1). A 20 g sample aliquot was placed in the reactor and N2 was purged through the vessel to ensure that an inert atmosphere was maintained. The biochar characterisation experiments were performed for three prescribed temperatures (450, 550, 650 °C) with a heating rate of 10 °C min−1 and a 2 h retention time at the highest heating temperature (HHT). For the investigation of carbon flows among pyrolysis products, several retention times (0.5, 1, 2 h) and inert gas flow rates (0.5, 1, 1.5 L min−1) were tested. Pyrolysis conditions were chosen based on preliminary findings and previously published studies.22,24,25 Biochar samples are named B-HF, B-HF25, B-HF50, B-HF75 and B-WB corresponding to the feedstock types defined in section 2.1. For the carbon flow assessment, a “FS pyrolysis system” is defined based on the boundaries of the pyrolysis treatment unit; i.e., from the (dry) feedstock entering the pyrolysis furnace, to the final products (biochar, bio-oil, NCG) collected after treatment.2.3 Biochar stability assessment
There is currently very little knowledge on the stability and carbon sequestration potential of biochars produced from human excreta and FS, as well as on the effect of co-pyrolysis with other types of waste biomass. Existing research on biochars produced from other biomass sources, such as wood or various agricultural wastes, indicates that both feedstock composition and operational conditions can have a significant effect on the stability and carbon sequestration potential of biomass pyrolysis applications.26–28 Methods to evaluate biochar stability have been categorised as: a) carbon structure analysis (qualitative and quantitative); b) oxidation resistance analysis (thermal and chemical degradation); and c) biochar persistence analysis (incubation and modelling).29 Although interest in carbon sequestration via FS pyrolysis is increasing rapidly, these technical parameters of biochar stability have not been systematically investigated for FS.In this study, the stability of biochars produced from co-pyrolysis of HF and WB was assessed for the first time, using previously established methods for carbon structure and oxidation resistance analysis, and for the different HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB ratios and pyrolysis temperatures tested. Proximate analysis and extractable dissolved organic carbon (DOC) content measurements were carried out as preliminary screenings to test the volatile carbon content of biochars produced at different temperatures and assess their suitability for carbon storage applications (section 3.2.1). For the carbon structure analysis, results include the H/C and O/C ratios, scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) analyses (section 3.2.2). For the oxidation resistance of the biochars, H2O2 was used to assess chemical oxidation potential, and the recalcitrance index (R50) method by thermogravimetric analysis (TGA) was implemented to study thermal degradation (section 3.2.3).30,31
WB ratios and pyrolysis temperatures tested. Proximate analysis and extractable dissolved organic carbon (DOC) content measurements were carried out as preliminary screenings to test the volatile carbon content of biochars produced at different temperatures and assess their suitability for carbon storage applications (section 3.2.1). For the carbon structure analysis, results include the H/C and O/C ratios, scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) analyses (section 3.2.2). For the oxidation resistance of the biochars, H2O2 was used to assess chemical oxidation potential, and the recalcitrance index (R50) method by thermogravimetric analysis (TGA) was implemented to study thermal degradation (section 3.2.3).30,31
2.4 Analytical methods
Thermal analysis was conducted using a PL Thermal Sciences Simultaneous Thermal Analyser (PL-STA 1500) to obtain the TGA and derivative thermogravimetry (DTG) curves (under N2 flow, 50 ml min−1). Proximate analysis was also conducted by TGA to determine the volatile matter, fixed carbon and ash content, based on ASTM D7582-1532 as adapted by Krueger et al.23 The surface morphology of the biochars was analysed under field-emission SEM with energy dispersive X-ray spectroscopy (EDX) on a TM4000 Tabletop Microscope (Hitachi, Japan). FTIR spectra were collected based on ASTM E1252-98,33 on a Nicolet 6700 FTIR Spectrometer (Thermo Fisher Scientific USA) over the wavenumber range 4000–500 cm−1, using a diamond ATR-crystal. CHNS analysis was performed on a ThermoScientific Flash Smart Elemental Analyzer according to BS EN ISO 16948:2015.34 Carbon flows among pyrolysis products (biochar, bio-oil, NCG) were calculated based on eqn (1)–(3): | (1) |
 | (2) |
| Carbon_NCG [%] = 100 − Carbon_bio_oil [%] − Carbon_biochar [%] | (3) |
For the DOC analysis, biochar samples were mixed with deionised water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 g
50 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL ratio) and shaken on a reciprocal shaker at 150 rpm for 16 h at room temperature (method modified from Tang et al.35 and Wang et al.36). Samples were then centrifuged and the supernatant was analysed in a total organic carbon (TOC) analyser (Shimadzu, TOC-V WS). The carbon chemical stability of the biochars was determined by H2O2 oxidation, indicating the oxidation resistance in natural soils.30,37 Briefly, 40 mL 5% H2O2 was added to 0.1 g biochar and left in a thermostatic water bath at 60 °C for 24 h as suggested by Han et al.38 The C stability was then determined based on eqn (4). The recalcitrance index method proposed by Harvey et al.31 (R50) was applied to illustrate the relative thermal stability of the biochar compared to that of graphite. TGA was performed under an air atmosphere, heating from room temperature to 900 °C at 20 °C min−1. The R50 value was calculated using eqn (5) after correcting for the moisture and ash content of the biochar.31
mL ratio) and shaken on a reciprocal shaker at 150 rpm for 16 h at room temperature (method modified from Tang et al.35 and Wang et al.36). Samples were then centrifuged and the supernatant was analysed in a total organic carbon (TOC) analyser (Shimadzu, TOC-V WS). The carbon chemical stability of the biochars was determined by H2O2 oxidation, indicating the oxidation resistance in natural soils.30,37 Briefly, 40 mL 5% H2O2 was added to 0.1 g biochar and left in a thermostatic water bath at 60 °C for 24 h as suggested by Han et al.38 The C stability was then determined based on eqn (4). The recalcitrance index method proposed by Harvey et al.31 (R50) was applied to illustrate the relative thermal stability of the biochar compared to that of graphite. TGA was performed under an air atmosphere, heating from room temperature to 900 °C at 20 °C min−1. The R50 value was calculated using eqn (5) after correcting for the moisture and ash content of the biochar.31
 | (4) |
 | (5) |
Samples were ground to <500 μm before all analytical procedures. All analyses were performed in triplicate and results are presented as mean values. Standard deviation values are presented in brackets. Results were statistically analysed by ANOVA tests to investigate the influence of the different HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB ratios and pyrolysis operational parameters on output variables, at the 0.05 significance level. Multiple comparison tests were used to localise the impact among factor levels, where a significant influence was detected.
WB ratios and pyrolysis operational parameters on output variables, at the 0.05 significance level. Multiple comparison tests were used to localise the impact among factor levels, where a significant influence was detected.
3. Results & discussion
3.1 Feedstock characteristics
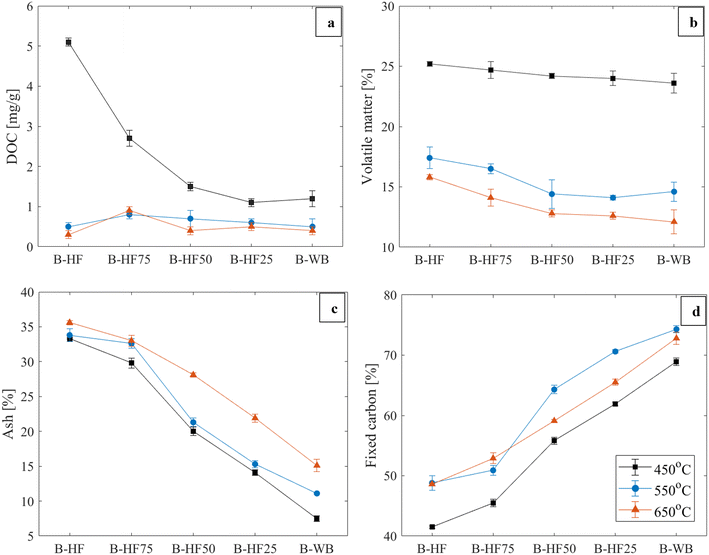
The chemical bonds and organic constituents present in HF, HF50 and WB samples, as qualitatively investigated via FTIR analysis, are shown in Fig. 1. The carbon content and proximate analysis for all feedstock combinations are shown in Fig. 2 (ANOVA results in Table S1†).Differences in the temperature and intensity of peak decomposition rates during pyrolysis can be expected due to differences in the organic composition of HF compared to the purely lignocellulosic composition of WB.39,40 Typical peaks of aliphatic C–H bonds (2920, 2850 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (∼1750 cm−1) were more intense for feedstock blends containing more HF, due to the presence of fat and lipids (Fig. 1).41 All the samples exhibited strong peaks in the 1750–1450 cm−1 range but several differences in the vibrational frequency are observed, as both protein (more prevalent in HF) and lignin (more prevalent in WB) have characteristic peaks in that range. These different organic constituents are expected to have an effect on the yield distribution of pyrolysis products (biochar, bio-oil, NCG) and the composition of evolved gases.42,43 For example, fatty constituents contribute to the bio-oil yield during pyrolysis, while protein and lignin contribute to biochar formation.44,45
O stretching (∼1750 cm−1) were more intense for feedstock blends containing more HF, due to the presence of fat and lipids (Fig. 1).41 All the samples exhibited strong peaks in the 1750–1450 cm−1 range but several differences in the vibrational frequency are observed, as both protein (more prevalent in HF) and lignin (more prevalent in WB) have characteristic peaks in that range. These different organic constituents are expected to have an effect on the yield distribution of pyrolysis products (biochar, bio-oil, NCG) and the composition of evolved gases.42,43 For example, fatty constituents contribute to the bio-oil yield during pyrolysis, while protein and lignin contribute to biochar formation.44,45
Proximate analysis results (Fig. 2) confirm that adding WB in higher ratios increased fixed carbon content (from 14.6% to 20.5% for HF and HF25, respectively) and decreased ash content (from 12.9% to 7.9% for HF and HF25, respectively) in HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blends. These trends indicate the different carbon retention and stability potential of the materials, suggesting that carbon flows between process end-products are expected to be different due to WB addition, despite the feedstocks having almost identical initial total C contents. Therefore, total C is not an appropriate indicator of the feedstock's carbon retention potential and does not provide sufficient information to optimise feedstock composition from a carbon management perspective.
WB blends. These trends indicate the different carbon retention and stability potential of the materials, suggesting that carbon flows between process end-products are expected to be different due to WB addition, despite the feedstocks having almost identical initial total C contents. Therefore, total C is not an appropriate indicator of the feedstock's carbon retention potential and does not provide sufficient information to optimise feedstock composition from a carbon management perspective.
The nature of the carbon in the feedstock material(s), which is dependent on the organic composition and has an influence on decomposition behaviour and biochar characteristics, needs to be understood – both quantitatively and qualitatively – to optimise carbon flows in pyrolysis. Previous research by Somorin et al.43 on the thermogravimetric analysis of these materials provided baseline information on their expected behaviour during pyrolysis. The authors found that co-pyrolysis of HF and WB had synergistic effects and enhanced the degradation of HF regardless of the mixing ratio.43 For the present study, the thermal decomposition behaviour of samples HF, HF50 and WB is depicted in the TGA and DTG curves shown in the ESI† (Fig. S2).
3.2 Biochar stability and carbon storage
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB ratio.46 Biochars produced at 450 °C also had consistently high remaining volatile matter (VM) content (>20%) (Fig. 3b), indicating potentially incomplete carbonisation at temperatures below 500 °C.22,47 Therefore, pyrolysis of HF, WB and HF
WB ratio.46 Biochars produced at 450 °C also had consistently high remaining volatile matter (VM) content (>20%) (Fig. 3b), indicating potentially incomplete carbonisation at temperatures below 500 °C.22,47 Therefore, pyrolysis of HF, WB and HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blends at 450 °C was considered unsuitable for carbon storage applications, as it cannot be safely assumed that carbon would remain stable in the long-term, while the high VM content may also have negative effects on soil properties and environmental safety.48 At 550 °C and 650 °C, the remaining DOC dropped to <1 mg g−1 for all feedstock blending ratios and no statistically significant differences were observed between these temperatures. The low DOC and VM values are indicative of the completion of main pyrolysis reactions after 500 °C, similar to previously reported results for biomass- and manure-derived biochars.49,50
WB blends at 450 °C was considered unsuitable for carbon storage applications, as it cannot be safely assumed that carbon would remain stable in the long-term, while the high VM content may also have negative effects on soil properties and environmental safety.48 At 550 °C and 650 °C, the remaining DOC dropped to <1 mg g−1 for all feedstock blending ratios and no statistically significant differences were observed between these temperatures. The low DOC and VM values are indicative of the completion of main pyrolysis reactions after 500 °C, similar to previously reported results for biomass- and manure-derived biochars.49,50
The WB fraction had a dual beneficial influence upon the ash and fixed carbon content (Fig. 3c and d). Ash generally increased with temperature, due to the concentration of minerals in the biochar fraction, and decreased with higher WB content, in line with the relative ash content of the feedstock materials (Fig. 2). The fixed carbon content, which is positively correlated to the presence of aromatic, stable forms of carbon,51 increased with WB addition and was maximised at a pyrolysis temperature of 550 °C. Based on these results, wood addition in sanitation facilities can realistically be investigated as a method to increase the carbon-fixing capacity of FS pyrolysis systems, as long as the minimum temperature for complete carbonisation is reached, in this case >500 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB biochars, regardless of the blending ratio, but not for pure HF biochars. These findings confirm the added-value of biomass addition in sanitation systems. Nevertheless, these values are based on the total carbon content that does not necessarily reflect the aromatic structure associated with carbon stability, and therefore, should be complemented with further structural and oxidative assessments.29,55
WB biochars, regardless of the blending ratio, but not for pure HF biochars. These findings confirm the added-value of biomass addition in sanitation systems. Nevertheless, these values are based on the total carbon content that does not necessarily reflect the aromatic structure associated with carbon stability, and therefore, should be complemented with further structural and oxidative assessments.29,55
The change in the nature of carbon present for different HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blending ratios was also observed via SEM analysis (Fig. 5). Biochars containing more HF than WB have a sheet-like texture that is characteristic of the HF-based carbon matrix (B-HF; Fig. 5a), with distinctive mineral constituents that appear as brighter areas, due to compositional contrast (B-HF75; Fig. 5b).22 Biochars made from HF50 and HF25 (Fig. 5c and d) exhibited an ordered honeycomb structure with longitudinal cracking that is characteristic of lignocellulosic WB-derived biochars.36,56 For HF
WB blending ratios was also observed via SEM analysis (Fig. 5). Biochars containing more HF than WB have a sheet-like texture that is characteristic of the HF-based carbon matrix (B-HF; Fig. 5a), with distinctive mineral constituents that appear as brighter areas, due to compositional contrast (B-HF75; Fig. 5b).22 Biochars made from HF50 and HF25 (Fig. 5c and d) exhibited an ordered honeycomb structure with longitudinal cracking that is characteristic of lignocellulosic WB-derived biochars.36,56 For HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB mixtures, the presence of CaCO3 on the carbon structure was further observed (B-HF50; Fig. 5c) and confirmed by simultaneous EDX analysis. The formation of CaCO3 in faecal biochars can occur spontaneously for Ca-rich biochars during cooling (i.e., when the biochar enters an oxygen-containing atmosphere) based on the reactions shown in eqn (6) and (7) or following an intermediate hydration step, as shown in eqn (8) and (9).22,57 These findings suggest that the high Ca content in HF can enable a supplementary carbon storage mechanism in FS pyrolysis, while the porous structure of WB provides a suitable matrix for the combination of organic and inorganic forms of carbon.22,58
WB mixtures, the presence of CaCO3 on the carbon structure was further observed (B-HF50; Fig. 5c) and confirmed by simultaneous EDX analysis. The formation of CaCO3 in faecal biochars can occur spontaneously for Ca-rich biochars during cooling (i.e., when the biochar enters an oxygen-containing atmosphere) based on the reactions shown in eqn (6) and (7) or following an intermediate hydration step, as shown in eqn (8) and (9).22,57 These findings suggest that the high Ca content in HF can enable a supplementary carbon storage mechanism in FS pyrolysis, while the porous structure of WB provides a suitable matrix for the combination of organic and inorganic forms of carbon.22,58
| 2Ca + O2 → 2CaO | (6) |
| CaO + CO2 → CaCO3 | (7) |
| CaO + H2O → Ca(OH)2 | (8) |
| Ca(OH)2 + CO2 → CaCO3 + H2O | (9) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (1600–1500 cm−1) and C–H bending vibrations (∼900–700 cm−1), which are indicative of lignin and aromatic carbon, were stronger for samples containing more WB.59 On the contrary, strong peaks at 1040–1020 cm−1 and 550 cm−1, which have been associated with carbohydrates and phosphates, were more intense for the biochars containing more HF and almost eliminated for B-HF25.42,60 The presence of carbonates, which was previously validated via SEM analysis, was also confirmed by identified FTIR peaks (∼1030 cm−1, 870 cm−1 and vibrations at 1500–1300 cm−1).61
C stretching (1600–1500 cm−1) and C–H bending vibrations (∼900–700 cm−1), which are indicative of lignin and aromatic carbon, were stronger for samples containing more WB.59 On the contrary, strong peaks at 1040–1020 cm−1 and 550 cm−1, which have been associated with carbohydrates and phosphates, were more intense for the biochars containing more HF and almost eliminated for B-HF25.42,60 The presence of carbonates, which was previously validated via SEM analysis, was also confirmed by identified FTIR peaks (∼1030 cm−1, 870 cm−1 and vibrations at 1500–1300 cm−1).61
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB co-pyrolysis, in terms of carbon stability. Therefore, 50% WB content is deemed sufficient to alter carbon dynamics and enhance carbon stability, while maximising the quantity of faecal matter safely treated and minimising the required treatment temperature to reach stability.
WB co-pyrolysis, in terms of carbon stability. Therefore, 50% WB content is deemed sufficient to alter carbon dynamics and enhance carbon stability, while maximising the quantity of faecal matter safely treated and minimising the required treatment temperature to reach stability.
Another established method to assess biochar stability is by investigating its thermal degradation potential in comparison to that of graphite, after applying corrections for moisture and ash content. The recalcitrance index (R50) method to assess the stability of plant-derived biochars was proposed by Harvey et al.,31 and has since been used and modified by other studies.28,62 According to Harvey et al.31 biochars with R50 ≥ 0.7 (class A) have carbon sequestration potential comparable to graphite, biochars with R50 < 0.5 are comparable to uncharred plant biomass and those with 0.7 > R50 ≥ 0.5 are considered to have intermediate carbon sequestration potential.
Results (Fig. 6b) suggest that biochars produced from HF50, HF25 and WB had R50 ≥ 0.5 for both treatment temperatures (550, 650 °C), while all biochars with <50% WB content did not meet the R50 = 0.5 threshold for adequate stability. Contrary to previous research by Somorin et al.43 reporting that the observed HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB synergy is independent of the blending ratio, these findings suggest that significant stability synergy takes place when a minimum of 50% WB is included in the feedstock mix.
WB synergy is independent of the blending ratio, these findings suggest that significant stability synergy takes place when a minimum of 50% WB is included in the feedstock mix.
A strong linear correlation was observed between R50 and H2O2 oxidation results (eqn (10)) (R2 = 0.86) and all the samples with R50 ≥ 0.5 had H2O2 stability ≥90%, indicating that the threshold suggested by Harvey et al.31 can be potentially translated into an oxidation stability threshold value that may be more useful for excreta-derived biochars. The chemical oxidation method showed a significantly wider range of values for different temperatures and feedstock types, with clear statistical significance, while the R50 method had a small range of values, potentially due to the applied correction factors to account for high ash and moisture content in faecal biochars, that may hinder the observation of statistically significant differences (ESI† Table S3). These findings can be useful for at-scale stability assessments or for comparisons among studies, although the correlation relationship should be verified by research with larger sample sizes.
| R50 = 0.0017 × CS [%] + 0.3483 (R2 = 0.86) | (10) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB biochar samples, and showed that both carbon and fixed carbon values are well predicted based on the ash content of (fully carbonised) biochars, within the 95% confidence interval (R2 > 0.95) (Fig. 7a and eqn (11)–(13)). Therefore, the high-level estimation of carbon storage benefits when using FS biochars can be achievable even for low-cost applications, for example to screen between different feedstock types or operational parameters.
WB biochar samples, and showed that both carbon and fixed carbon values are well predicted based on the ash content of (fully carbonised) biochars, within the 95% confidence interval (R2 > 0.95) (Fig. 7a and eqn (11)–(13)). Therefore, the high-level estimation of carbon storage benefits when using FS biochars can be achievable even for low-cost applications, for example to screen between different feedstock types or operational parameters.
 | ||
| Fig. 7 Correlation between carbon and ash content (%) (a); and carbon retention (%) results (b) for biochars B-HF, B-HF75, B-HF50 and B-HF25. Carbon retention results calculated based on eqn (1). Error bars note the standard deviation of measured values (n = 3). | ||
A more meaningful metric than total C content (Fig. 4) is the C retention% based on eqn (1) (Fig. 7b), that also takes the biochar yield (ESI† Fig. S4) and feedstock C content (Fig. 2) into consideration, and therefore represents the carbon retention in the whole FS pyrolysis system. The biochar yield during pyrolysis decreased with increasing WB content and temperature, from 32% for B-HF (450 °C) to 24.3% for B-HF25 (650 °C), due to the change in ash content and organic composition and the release of volatile compounds.14 Based on these data, the B-HF50 biochar produced at 550 °C had the highest carbon retention (41.1%) compared to all the HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blends (Fig. 7b), despite the higher absolute C concentration in B-HF25 (Fig. 4). In practice, for HF50 more carbon is retained in the system per mass of feedstock treated, while for HF25 more carbon can be stored per mass of biochar applied by the end-user, assuming that the biochar is used for agricultural applications or other non-volatilising uses (i.e., not as a fuel). From a systematic perspective, HF50 blends had the best performance in terms of carbon retention benefits.
WB blends (Fig. 7b), despite the higher absolute C concentration in B-HF25 (Fig. 4). In practice, for HF50 more carbon is retained in the system per mass of feedstock treated, while for HF25 more carbon can be stored per mass of biochar applied by the end-user, assuming that the biochar is used for agricultural applications or other non-volatilising uses (i.e., not as a fuel). From a systematic perspective, HF50 blends had the best performance in terms of carbon retention benefits.
For all the blends, more C is retained in the biochar at 550 °C compared to 650 °C, indicating that prolonged carbonisation past the temperature where the initial char stability is reached (in this case between 450–550 °C) does not offer further carbon retention benefits and leads to further carbon loss due to secondary decomposition (carbonisation) reactions.63 These findings highlight the importance of presenting comprehensive carbon management data that also consider operational parameters, biochar yield and feedstock characteristics. The biochar C content can be a misleading figure if used out-of-context, as biochars with higher C concentrations may be produced by systems that have emitted more C per mass of feedstock treated.
Overall, the biochar produced at 550 °C by co-pyrolysing HF and WB at a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio had the best performance in terms of both retention and stability of carbon. From a practical perspective, this corresponds to approximately 30 g dry wood being added daily to a toilet as a cover material by each user, according to the expected daily faecal excretion mass per person.40
50 ratio had the best performance in terms of both retention and stability of carbon. From a practical perspective, this corresponds to approximately 30 g dry wood being added daily to a toilet as a cover material by each user, according to the expected daily faecal excretion mass per person.40
| C [%] = −0.9946 × ASH [%] + 85.857 (R2 = 0.96) | (11) |
| FC [%] = −1.1075 × ASH [%] + 88.224 (R2 = 0.98) | (12) |
| C [%] = 0.9035 × FC [%] + 6.298 (R2 = 0.99) | (13) |
3.3 Carbon flow distribution during pyrolysis
The distribution of carbon flows among pyrolysis products (biochar, bio-oil, NCG) is shown in Fig. 8 for HF, HF50 and WB samples, and for different pyrolysis retention times (0.5, 2 h) and N2 gas flow rates used (0.5, 1.5 L min−1). Preliminary tests showed no significant differences between 1–2 h retention time and 1–1.5 L min−1 N2 gas flow rate, hence results for intermediate factor levels are not shown. The chosen HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blend ratio (50
WB blend ratio (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) and temperature (550 °C) for this analysis were based on previous findings (section 3.2), in order to study the change in carbon flow distribution when aiming to maximise carbon stability and retention in the biochar. Further statistical analysis data are included in the ESI† (Tables S4–S9).
50) and temperature (550 °C) for this analysis were based on previous findings (section 3.2), in order to study the change in carbon flow distribution when aiming to maximise carbon stability and retention in the biochar. Further statistical analysis data are included in the ESI† (Tables S4–S9).
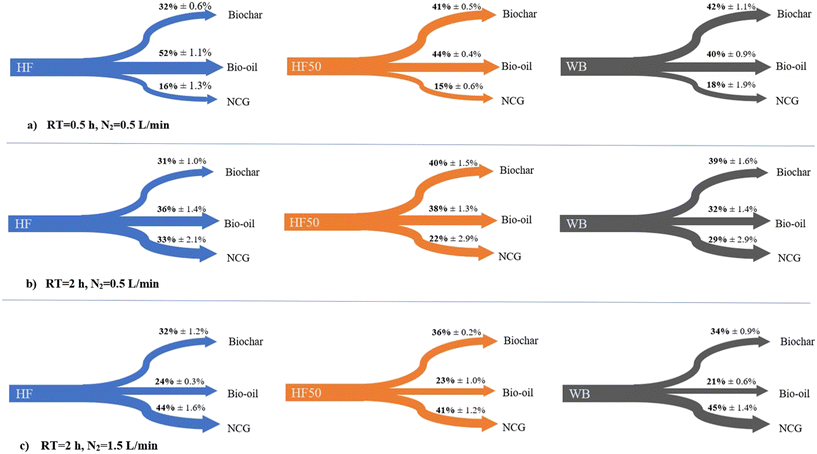 | ||
| Fig. 8 Distribution of carbon flows to biochar, bio-oil and non-condensable gas (NCG) streams expressed as the % of the total carbon content in the feedstock (for HF, HF50 and WB) (pyrolysis experiments at 550 °C). Results for different combinations of retention time (RT) and N2 gas flow rate: (a) RT = 0.5 h, N2 = 0.5 L min−1; (b) RT = 2 h, N2 = 0.5 L min−1; and (c) RT = 2 h, N2 = 1.5 L min−1. The reported values are calculated based on eqn (1)–(3), following total C analysis on a CHNS elemental analyser (see section 2.4). | ||
The addition of WB to faecal samples increased C retention in the biochar fraction regardless of the process operational parameters, due to the increase of lignin content in the feedstock mix and the dilution of the fat and protein content of HF which suppressed bio-oil and NCG formation.42,44,64 In terms of operational parameters, the biochar carbon yield reached its maximum values (∼40%) when using a low inert gas flow rate (0.5 L min−1) that encouraged the recondensation of volatiles on the char fraction, while the retention time did not have a significant influence on solid carbon retention.
Synergy during HF and WB co-pyrolysis was evident in terms of solid carbon retention effects, as there were no significant differences between HF50 and WB for any of the operational parameters tested (ESI† Tables S4 and S5) (i.e., the relationship between the HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB ratio and the carbon retention % is not linear). In a theoretical system where no synergistic reactions take place, considering the scenario shown in Fig. 8a where HF and WB biochars contain 32% and 42% of the total feedstock C respectively, HF50 would contain 37% of the total C. In reality, 41% of the feedstocks' C was retained in the B-HF50 fraction, confirming the synergistic effect when co-pyrolysing HF and WB.
WB ratio and the carbon retention % is not linear). In a theoretical system where no synergistic reactions take place, considering the scenario shown in Fig. 8a where HF and WB biochars contain 32% and 42% of the total feedstock C respectively, HF50 would contain 37% of the total C. In reality, 41% of the feedstocks' C was retained in the B-HF50 fraction, confirming the synergistic effect when co-pyrolysing HF and WB.
In terms of oil and gas products, the carbon in the bio-oil fraction increased with decreasing retention time and inert gas flow rate, while the carbon in NCG followed the opposite trend. Therefore, for traditional pyrolysis applications that may not recover gases for energy/heat recovery, the minimum retention time and inert gas flow to achieve full carbonisation and efficient volatile release are preferable to minimise C gas emissions. This is achieved by minimising the solid retention time in the reactor (i.e., short hold time preventing prolonged carbonisation of the formed char) and maximising volatile retention time in the bio-oil condensation system (i.e., slow inert gas flow rate encouraging bio-oil formation from condensable volatiles).27,65 The bio-oil can then be exploited for its energy value as it contains the majority of volatile compounds with higher calorific values.66 Interestingly, the bio-oil produced from HF50 contained more C compared to pure WB bio-oil, due to the increased bio-oil yield when co-pyrolysing with a feedstock high in fat (i.e., HF). There was therefore a dual benefit of HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB co-pyrolysis, as the WB and HF fractions increase the relative C flows to the biochar and bio-oil products respectively.
WB co-pyrolysis, as the WB and HF fractions increase the relative C flows to the biochar and bio-oil products respectively.
For an alternative scenario where the produced gases are exploited for energy or heat recovery, maximising the calorific value of the NCG fraction may be the preferable objective. Ultimately, the optimum choice of operational parameters needs to be considered on a case-specific basis, also depending on the site location and type of kiln used. ANOVA results (ESI† Tables S4–S6) suggest that the C in the biochar fraction is mainly influenced by feedstock composition, while the bio-oil and NCG fractions are greatly influenced by operational parameters. Findings confirm the influence of chosen operational parameters upon the carbon distribution among FS pyrolysis products (biochar, bio-oil, NCG); this information should be considered when optimising process operation or when comparing the performance of different treatment systems with varying process controls.
For the context of developing countries where traditional kilns are used with minimal operational control, the critical aspects to achieve carbon stability and storage benefits are pyrolysis temperature and feedstock composition respectively.67,68 In terms of stability, operators seeking to achieve carbon credit accreditation need to ensure that a minimum temperature of ∼500 °C is reached to produce stable biochars. For carbon retention, additional biomass sources, such as wood or agricultural waste, can be used for co-pyrolysis (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio recommended) to maximise the amount of carbon contributing to biochar formation. Ultimately, findings suggest that having a condensation system in place to separate bio-oils (to be used for energy generation or heat recovery – subject to further research) and/or exploiting NCG for energy/heat generation and controlling emissions are also critical aspects towards achieving carbon-negative FS pyrolysis systems.69,70 Efforts to incorporate condensation systems and emission controls into low-cost pyrolysis solutions, such as drum kilns, should be investigated by further research, while also considering suitable methods for handling corrosive bio-oils and combustible gases within low-income settings.71
50 ratio recommended) to maximise the amount of carbon contributing to biochar formation. Ultimately, findings suggest that having a condensation system in place to separate bio-oils (to be used for energy generation or heat recovery – subject to further research) and/or exploiting NCG for energy/heat generation and controlling emissions are also critical aspects towards achieving carbon-negative FS pyrolysis systems.69,70 Efforts to incorporate condensation systems and emission controls into low-cost pyrolysis solutions, such as drum kilns, should be investigated by further research, while also considering suitable methods for handling corrosive bio-oils and combustible gases within low-income settings.71
4. Conclusions
This study assessed different blending ratios of HF and WB during pyrolysis at three prescribed temperatures (450, 550, 650 °C) for their carbon sequestration potential. Key findings are:• Co-pyrolysis of HF and WB improved carbon fixation and stability in produced biochars, enhancing carbon sequestration potential compared to pyrolysis of pure faecal feedstocks. Findings suggest that synergistic effects take place during HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB co-pyrolysis, both in terms of carbon stability and carbon storage benefits.
WB co-pyrolysis, both in terms of carbon stability and carbon storage benefits.
• Biochars produced from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 HF
50 HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB blends at 550 °C had the highest carbon retention (41.1%). Pyrolysing at a higher temperature (650 °C) did not show any benefits in terms of carbon retention, while biochars produced at 450 °C showed potential to release labile organic carbon.
WB blends at 550 °C had the highest carbon retention (41.1%). Pyrolysing at a higher temperature (650 °C) did not show any benefits in terms of carbon retention, while biochars produced at 450 °C showed potential to release labile organic carbon.
• A 50% WB content was found to be sufficient to alter carbon dynamics and enhance carbon stability. From a practical perspective, this corresponds to each user adding approximately 30 g dry wood to a toilet as a cover material daily.
• The H/C, O/C ratios, R50 index and H2O2 oxidation values at 550 °C and 650 °C suggest a high aromatic structure of the HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WB biochars containing ≥50% WB.
WB biochars containing ≥50% WB.
• The formation of CaCO3 during pyrolysis of HF (pure or mixed with other biomass) should be further investigated as a potential additional carbon storage mechanism in FSM.
• Biochar characteristics overall were more dependent on the feedstock type and blending ratios rather than pyrolysis temperature, provided that the temperature reached was sufficient to ensure completion of the main pyrolytic reactions (≥500 °C according to thermal analysis results).
• The C flows to the biochar fraction were mainly dependent on the feedstock composition, while the bio-oil and NCG fractions were influenced by pyrolysis operational parameters (retention time and inert gas flow rate). Absolute values of C flows should be treated as system-specific but the identified statistically significant trends related to specific operational parameters and wood addition ratio can be used for technical and high-level decision making related to carbon management in sanitation systems.
5. Practical recommendations
The findings of this research set the tone for sustainable FSM concepts and further techno-economic assessments of carbon management in sanitation businesses, that could introduce a new sustainability paradigm in non-sewered sanitation. While climate change impact assessments for FSM are still mostly based on the use of global databases that include several assumptions and simplifications,72 simple carbon mass flow calculations and carbon stability tests provide a methodology for more robust, transparent and context-specific assessments. When incorporated into wider methodologies for sanitation carbon assessments during containment, collection and transport,8 this approach can provide a systematic way to measure and analyse carbon offsetting opportunities for sanitation business models.This study focused on the carbon stability and mass balance for a pyrolysis FS system where wood is used as a cover material, but the concepts and methods applied can be used for other biomass materials or alternative treatment systems where biochar is used in conjunction with other technologies (e.g., biochar used in composting or as a urine treatment medium).73,74 Additional methods for carbon sequestration investigation, including emerging technologies such as hydro-pyrolysis, can be investigated by further research for wider contexts. Ultimately, high-level carbon mass balance calculations can be a good starting point to minimise the – currently unmanaged – carbon footprint of a diverse global sanitation sector. While the retention and recalcitrance of the biochar are important indicators to determine its long-term sequestration potential, soil interactions and the influence of environmental conditions, such as temperature, also need to be considered in a case-specific context.46,75
The proposed methods for carbon mass balance calculations can therefore be used to create a library of data based on real measurements for human excreta and faecal sludge to inform and support carbon assessments and future modelling applications. These can be supplemented with data for other parts of the FSM chain, for example avoided emissions when using the produced biochars as fertilisers or meeting energy requirements for drying, hence enabling comprehensive climate change assessments. For the calculation of the overall energy balance of a pyrolysis system, the heating source of the process also needs to be considered. For example, if electricity is used, the energy mix (i.e., renewable vs. non-renewable sources) needs to be known, while for the case of developing countries, solid fuels are often used as the heating source for traditional kilns (e.g., wood, charcoal, biochar or briquettes).
For detailed emission calculations, the composition of emitted gases should be accounted for, as varied C forms will have significantly different climate change potential. For human faeces, CO2 and CH4 have been identified as the main gaseous emissions at mid-scale pyrolysis temperatures.76 While CO2 emissions from biomass are considered as biogenic,3,77 several studies suggest that at least part of the emissions from FS should be included in assessments as non-biogenic due to potential contamination and additional waste discarded in toilets.2 Nitrogen-containing evolved gases also need to be considered for wider climate change assessments, as both urine diversion and biomass addition are expected to reduce nitrous oxide emissions during FS containment and treatment.
Overall, the results of this research demonstrate that both feedstock composition and treatment conditions can significantly influence the overall carbon balance of FS pyrolysis, providing useful information towards the establishment of carbon-negative sanitation systems. Container-based sanitation business models are particularly relevant to the context of this research, as they provide a service-based approach with frequent maintenance, collection and treatment services, allowing interventions along the entire FSM chain. Nevertheless, several institutional, operational and financial challenges need to be overcome before rapid upscaling is feasible.78 Quantifying the added value of resource recovery and climate change mitigation can provide meaningful incentives for operators and governments to maintain proper use and collection services for on-site sanitation systems.
Ethical approvals
Approvals and permissions for the collection of human excreta samples used in this research project were obtained via the Imperial College Research Governance and Integrity Team (ref. no. 21IC6817) and the Imperial College Healthcare Tissue Bank (licence: 12275) [supported by the National Institute for Health Research Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London]. Informed consents were obtained from all human participants of this study.Data availability
The data supporting this article have been included as part of the ESI.† Further data can be provided upon request. Data collected from human participants are not available for confidentiality reasons.Author contributions
M. E. Koulouri: conceptualisation, methodology, formal analysis, visualisation, writing – original draft; M. Qiu: methodology, formal analysis, writing – review & editing; M. R. Templeton, G. D. Fowler: supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to thank Imperial College London for the award of a Skempton Scholarship and the Society of Chemical Industry (SCI) for supporting this work via a Sydney Andrew Scholarship. This project was also supported by the Royal Academy of Engineering under the Research Chairs and Senior Research Fellowships programme and by the EPSRC [EP/R010161/1, EP/R017727/1 – UKCRIC Coordination Node].References
- WHO/UNICEF, Progress on household drinking-water, sanitation and hygiene 2000-2022, https://www.who.int/publications/m/item/progress-on-household-drinking-water--sanitation-and-hygiene-2000-2022---special-focus-on-gender, (accessed 22 November 2023).
- A. Gallego-Schmid and R. R. Z. Tarpani, Life cycle assessment of wastewater treatment in developing countries: A review, Water Res., 2019, 153, 63–79 CrossRef CAS PubMed.
- L. S. Rowles, V. L. Morgan, Y. Li, X. Zhang, S. Watabe, T. Stephen, H. A. C. Lohman, D. DeSouza, J. Hallowell, R. D. Cusick and J. S. Guest, Financial Viability and Environmental Sustainability of Fecal Sludge Treatment with Pyrolysis Omni Processors, ACS Environ. Au, 2022, 2, 455–466 CrossRef CAS PubMed.
- M. Manga, B. E. Evans, T. M. Ngasala and M. A. Camargo-Valero, Recycling of Faecal Sludge: Nitrogen, Carbon and Organic Matter Transformation during Co-Composting of Faecal Sludge with Different Bulking Agents, Int. J. Environ. Res. Public Health, 2022, 19, 10592 CrossRef CAS PubMed.
- G. McNicol, J. Jeliazovski, J. J. François, S. Kramer and R. Ryals, Climate change mitigation potential in sanitation via off-site composting of human waste, Nat. Clim. Change, 2020, 10, 545–549 CrossRef CAS.
- R. Ryals, G. McNicol, S. Porder and S. Kramer, Greenhouse gas fluxes from human waste management pathways in Haiti, J. Cleaner Prod., 2019, 226, 106–113 CrossRef CAS.
- M. C. Reid, K. Guan, F. Wagner and D. L. Mauzerall, Global Methane Emissions from Pit Latrines, Environ. Sci. Technol., 2014, 48, 8727–8734 CrossRef CAS.
- CBSA, Unlocking carbon credits for sanitation – Container Based Sanitation Alliance Briefing paper, 2023.
- J. Johnson, F. Zakaria, A. G. Nkurunziza, C. Way, M. A. Camargo-Valero and B. Evans, Whole-system analysis reveals high greenhouse-gas emissions from citywide sanitation in Kampala, Uganda, Commun. Earth Environ., 2022, 3, 1–10 CrossRef.
- T. Hübner, A. Herrmann, J. Kretzschmar and F. Harnisch, Suitability of fecal sludge from composting toilets as feedstock for carbonization, J. Water, Sanit. Hyg. Dev., 2019, 9, 616–626 CrossRef.
- F. VanRiper, K. C. Russel, L. A. Cramer, D. Tillias, J. Laporte, E. Lloyd and S. Kramer, Container-Based Sanitation Services and Attrition: An Examination of Drivers and Implications, Front. Environ. Sci., 2022, 9, 817142 CrossRef.
- World Bank, Evaluating the Potential of Container-Based Sanitation, World Bank, Washington DC, 2019 Search PubMed.
- S. Hashemi, S. Boudaghpour and M. Han, Evaluation of different natural additives effects on the composting process of source separated feces in resource-oriented sanitation systems, Ecotoxicol. Environ. Saf., 2019, 185, 109667 CrossRef CAS PubMed.
- J. A. Ippolito, L. Cui, C. Kammann, N. Wrage-Mönnig, J. M. Estavillo, T. Fuertes-Mendizabal, M. L. Cayuela, G. Sigua, J. Novak, K. Spokas and N. Borchard, Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review, Biochar, 2020, 2, 421–438 CrossRef.
- B. C. Krueger, G. D. Fowler, M. R. Templeton and B. Moya, Resource recovery and biochar characteristics from full-scale faecal sludge treatment and co-treatment with agricultural waste, Water Res., 2020, 169, 115253 CrossRef CAS PubMed.
- J. Lehmann and S. Joseph, Biochar for Environmental Management: Science, Technology and Implementation, Taylor & Francis Group, 2015 Search PubMed.
- M. Bleuler, M. Gold, L. Strande and A. Schönborn, Pyrolysis of Dry Toilet Substrate as a Means of Nutrient Recycling in Agricultural Systems: Potential Risks and Benefits, Waste Biomass Valorization, 2021, 12, 4171–4183 CrossRef CAS.
- M. Gold, M. Cunningham, M. Bleuler, R. Arnheiter, A. Schönborn, C. Niwagaba and L. Strande, Operating parameters for three resource recovery options from slow-pyrolysis of faecal sludge, J. Water, Sanit. Hyg. Dev., 2018, 8, 707–717 CrossRef.
- S. Mandlebaum and J. Nriagu, in Encyclopedia of Environmental Health, ed. J. O. Nriagu, Elsevier, Burlington, 2011, pp. 498–504 Search PubMed.
- R. R. Tan, Data challenges in optimizing biochar-based carbon sequestration, Renewable Sustainable Energy Rev., 2019, 104, 174–177 CrossRef.
- R. Clark, J. Reed and T. Sunderland, Bridging funding gaps for climate and sustainable development: Pitfalls, progress and potential of private finance, Land Use Policy, 2018, 71, 335–346 CrossRef.
- M. E. Koulouri, M. R. Templeton and G. D. Fowler, Source separation of human excreta: Effect on resource recovery via pyrolysis, J. Environ. Manage., 2023, 338, 117782 CrossRef CAS PubMed.
- B. C. Krueger, G. D. Fowler and M. R. Templeton, Critical analytical parameters for faecal sludge characterisation informing the application of thermal treatment processes, J. Environ. Manage., 2021, 111658 CrossRef PubMed.
- X. Liu, Z. Li, Y. Zhang, R. Feng and I. B. Mahmood, Characterization of human manure-derived biochar and energy-balance analysis of slow pyrolysis process, Waste Manage., 2014, 34, 1619–1626 CrossRef CAS PubMed.
- B. J. Ward, T. W. Yacob and L. D. Montoya, Evaluation of Solid Fuel Char Briquettes from Human Waste, Environ. Sci. Technol., 2014, 48, 9852–9858 CrossRef CAS PubMed.
- K. Crombie and O. Mašek, Pyrolysis biochar systems, balance between bioenergy and carbon sequestration, GCB Bioenergy, 2015, 7, 349–361 CrossRef CAS.
- M. Somerville and A. Deev, The effect of heating rate, particle size and gas flow on the yield of charcoal during the pyrolysis of radiata pine wood, Renewable Energy, 2020, 151, 419–425 CrossRef CAS.
- J. H. Windeatt, A. B. Ross, P. T. Williams, P. M. Forster, M. A. Nahil and S. Singh, Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment, J. Environ. Manage., 2014, 146, 189–197 CrossRef CAS.
- L. Leng, H. Huang, H. Li, J. Li and W. Zhou, Biochar stability assessment methods: A review, Sci. Total Environ., 2019, 647, 210–222 CrossRef CAS.
- A. Cross and S. P. Sohi, A method for screening the relative long-term stability of biochar, GCB Bioenergy, 2013, 5, 215–220 CrossRef CAS.
- O. R. Harvey, L.-J. Kuo, A. R. Zimmerman, P. Louchouarn, J. E. Amonette and B. E. Herbert, An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars), Environ. Sci. Technol., 2012, 46, 1415–1421 CrossRef CAS PubMed.
- ASTM, ASTM D7582-15 Standard: Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis, ASTM International, 2015 Search PubMed.
- ASTM, ASTM E1252-98 (2021) Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis, ASTM International, 2021 Search PubMed.
- ISO, BS EN ISO 16948:2015: Solid biofuels, Determination of total content of carbon, hydrogen and nitrogen.
- J. Tang, C. Cao, F. Gao and W. Wang, Effects of biochar amendment on the availability of trace elements and the properties of dissolved organic matter in contaminated soils, Environ. Technol. Innovation, 2019, 16, 100492 CrossRef.
- F. Wang, R. Zhang, S. W. Donne, Y. Beyad, X. Liu, X. Duan, T. Yang, P. Su and H. Sun, Co-pyrolysis of wood chips and bentonite/kaolin: Influence of temperatures and minerals on characteristics and carbon sequestration potential of biochar, Sci. Total Environ., 2022, 838, 156081 CrossRef CAS PubMed.
- S. Mia, F. A. Dijkstra and B. Singh, in Adv. Agron., ed. D. L. Sparks, Academic Press, 2017, vol. 141, pp. 1–51 Search PubMed.
- L. Han, K. S. Ro, Y. Wang, K. Sun, H. Sun, J. A. Libra and B. Xing, Oxidation resistance of biochars as a function of feedstock and pyrolysis condition, Sci. Total Environ., 2018, 616–617, 335–344 CrossRef CAS PubMed.
- B. Fidalgo, M. Chilmeran, T. Somorin, A. Sowale, A. Kolios, A. Parker, L. Williams, M. Collins, E. J. McAdam and S. Tyrrel, Non-isothermal thermogravimetric kinetic analysis of the thermochemical conversion of human faeces, Renewable Energy, 2019, 132, 1177–1184 CrossRef CAS PubMed.
- C. Rose, A. Parker, B. Jefferson and E. Cartmell, The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology, Crit. Rev. Environ. Sci. Technol., 2015, 45, 1827–1879 CrossRef CAS PubMed.
- A.-S. De Koninck, K. Nys, B. Vandenheede, S. Van Biervliet, M. M. Speeckaert and J. R. Delanghe, Detailed faecal fat analysis using Fourier transform infrared spectroscopy: Exploring the possibilities, Clin. Biochem., 2016, 49, 1283–1287 CrossRef CAS PubMed.
- B. C. Krueger, G. D. Fowler, M. R. Templeton and S. Septien, Faecal sludge pyrolysis: Understanding the relationships between organic composition and thermal decomposition, J. Environ. Manage., 2021, 298, 113456 CrossRef CAS.
- T. Somorin, A. Parker, E. McAdam, L. Williams, S. Tyrrel, A. Kolios and Y. Jiang, Pyrolysis characteristics and kinetics of human faeces, simulant faeces and wood biomass by thermogravimetry–gas chromatography–mass spectrometry methods, Energy Rep., 2020, 6, 3230–3239 CrossRef.
- F. Abnisa and W. M. A. Wan Daud, A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil, Energy Convers. Manage., 2014, 87, 71–85 CrossRef CAS.
- A. Hassen, T. Kraiem, S. Naoui and H. Belayouni, Pyrolysis of waste animal fats in a fixed-bed reactor: Production and characterization of bio-oil and bio-char, Waste Manage., 2013, 34, 210–218 Search PubMed.
- Y. Fang, B. Singh, B. P. Singh and E. Krull, Biochar carbon stability in four contrasting soils, Eur. J. Soil Sci., 2014, 65, 60–71 CrossRef CAS.
- N. Ren, Y. Tang and M. Li, Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar, Process Saf. Environ. Prot., 2018, 115, 70–78 CrossRef CAS.
- A. Tomczyk, Z. Sokołowska and P. Boguta, Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects, Rev. Environ. Sci. Biotechnol., 2020, 19, 191–215 CrossRef CAS.
- X. Gui, C. Liu, F. Li and J. Wang, Effect of pyrolysis temperature on the composition of DOM in manure-derived biochar, Ecotoxicol. Environ. Saf., 2020, 197, 110597 CrossRef CAS PubMed.
- H. Zhang, W. Qian, L. Wu, S. Yu, R. Wei, W. Chen and J. Ni, Spectral characteristics of dissolved organic carbon (DOC) derived from biomass pyrolysis: Biochar-derived DOC versus smoke-derived DOC, and their differences from natural DOC, Chemosphere, 2022, 302, 134869 CrossRef CAS PubMed.
- K. Crombie, O. Mašek, S. P. Sohi, P. Brownsort and A. Cross, The effect of pyrolysis conditions on biochar stability as determined by three methods, GCB Bioenergy, 2013, 5, 122–131 CrossRef CAS.
- EBC, European Biochar Certificate (EBC) - guidelines version 6.1, 2012, DOI:10.13140/RG.2.1.4658.7043.
- IBI, International Biochar Initiative - Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil, 2015 Search PubMed.
- K. A. Spokas, Review of the stability of biochar in soils: predictability of O:C molar ratios, Carbon Manage., 2010, 1, 289–303 CrossRef CAS.
- A. Enders, K. Hanley, T. Whitman, S. Joseph and J. Lehmann, Characterization of biochars to evaluate recalcitrance and agronomic performance, Bioresour. Technol., 2012, 114, 644–653 CrossRef CAS PubMed.
- J. B. Zicherman and R. B. Williamson, Microstructure of wood char, Wood Sci. Technol., 1981, 15, 237–249 CrossRef.
- M. Galván-Ruiz, J. Hernández, L. Baños, J. Noriega-Montes and M. E. Rodríguez-García, Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction, J. Mater. Civ. Eng., 2009, 21, 694–698 CrossRef.
- R. Chang, S. Kim, S. Lee, S. Choi, M. Kim and Y. Park, Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism, Front. Energy Res., 2017, 5, 17 CrossRef.
- K. Sahoo, A. Kumar and J. Chakraborty, A comparative study on valuable products: bio-oil, biochar, non-condensable gases from pyrolysis of agricultural residues, J. Mater. Cycles Waste Manage., 2021, 23, 186–204 CrossRef CAS.
- W. Jastrzębski, M. Sitarz, M. Rokita and K. Bułat, Infrared spectroscopy of different phosphates structures, Spectrochim. Acta, Part A, 2011, 79, 722–727 CrossRef PubMed.
- F. B. Reig, J. V. G. Adelantado and M. C. M. Moya Moreno, FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples, Talanta, 2002, 58, 811–821 CrossRef CAS.
- N. Gómez, J. G. Rosas, S. Singh, A. B. Ross, M. E. Sánchez and J. Cara, Development of a gained stability index for describing biochar stability: Relation of high recalcitrance index (R50) with accelerated ageing tests, J. Anal. Appl. Pyrolysis, 2016, 120, 37–44 CrossRef.
- P. Basu, Biomass Gasification and Pyrolysis: Practical Design and Theory, Elsevier Science & Technology, Academic Press, Burlington, 2010 Search PubMed.
- A. H. Hubble and J. L. Goldfarb, Synergistic effects of biomass building blocks on pyrolysis gas and bio-oil formation, J. Anal. Appl. Pyrolysis, 2021, 156, 105100 CrossRef CAS.
- A. J. Bowles, Á. Nievas and G. D. Fowler, Consecutive recovery of recovered carbon black and limonene from waste tyres by thermal pyrolysis in a rotary kiln, Sustainable Chem. Pharm., 2023, 32, 100972 CrossRef CAS.
- T. Bond, Q. Tse, C. L. Chambon, P. Fennell, G. D. Fowler, B. C. Krueger and M. R. Templeton, The feasibility of char and bio-oil production from pyrolysis of pit latrine sludge, Environ. Sci.: Water Res. Technol., 2018, 4, 253–264 RSC.
- S. Sangsuk, C. Buathong and S. Suebsiri, High-energy conversion efficiency of drum kiln with heat distribution pipe for charcoal and biochar production, Energy Sustainable Dev., 2020, 59, 1–7 CrossRef.
- A. B. Smebye, M. Sparrevik, H. P. Schmidt and G. Cornelissen, Life-cycle assessment of biochar production systems in tropical rural areas: Comparing flame curtain kilns to other production methods, Biomass Bioenergy, 2017, 101, 35–43 CrossRef CAS.
- V. Karlsson and L. Nilsson, Co-production of pyrolysis oil and district cooling in biomass-based CHP plants: Utilizing sequential vapour condensation heat as driving force in an absorption cooling machine, Appl. Therm. Eng., 2015, 79, 9–16 CrossRef.
- A. Inayat, A. Ahmed, R. Tariq, A. Waris, F. Jamil, S. F. Ahmed, C. Ghenai and Y.-K. Park, Techno-Economical Evaluation of Bio-Oil Production via Biomass Fast Pyrolysis Process: A Review, Front. Energy Res., 2022, 9, 770355 CrossRef.
- T. Namaswa, D. F. R. P. Burslem and J. Smith, Emerging trends in appropriate kiln designs for small-scale biochar production in low to middle income countries, Bioresour. Technol. Rep., 2023, 24, 101641 CrossRef CAS.
- K. L. Lam, L. Zlatanović and J. P. van der Hoek, Life cycle assessment of nutrient recycling from wastewater: A critical review, Water Res., 2020, 173, 115519 CrossRef CAS.
- D. Castro-Herrera, K. Prost, D.-G. Kim, F. Yimer, M. Tadesse, M. Gebrehiwot and N. Brüggemann, Biochar addition reduces non-CO2 greenhouse gas emissions during composting of human excreta and cattle manure, J. Environ. Qual., 2023, 52, 814–828 CrossRef CAS.
- M. E. Koulouri, M. R. Templeton and G. D. Fowler, Enhancing the nitrogen and phosphorus content of faecal-derived biochar via adsorption and precipitation from human urine, J. Environ. Manage., 2024, 352, 119981 CrossRef CAS PubMed.
- K. Lorenz and R. Lal, Biochar application to soil for climate change mitigation by soil organic carbon sequestration, J. Plant Nutr. Soil Sci., 2014, 177, 651–670 CrossRef CAS.
- T. W. Yacob, R. Fisher, K. G. Linden and A. W. Weimer, Pyrolysis of human feces: Gas yield analysis and kinetic modeling, Waste Manage., 2018, 79, 214–222 CrossRef CAS PubMed.
- M. F. Agunyo, J. Born, E. Wozei and B. Moeller, Exploring the Environmental Feasibility of Integrated Sanitation Systems for Uganda, J. Sustain. Dev. Energy Water Environ. Syst., 2019, 7, 28–43 CrossRef.
- A. Mallory, A. Mdee, D. Agol, L. Hyde-Smith, D. Kiogora, J. Riungu and A. Parker, The potential for scaling up container-based sanitation in informal settlements in Kenya, J. Int. Dev., 2022, 34, 1347–1361 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ew00513a |
| This journal is © The Royal Society of Chemistry 2024 |