A multi-stimuli–response metal–organic framework nanopesticide for smart weed control in agriculture†
Dingyang
Zhang
a,
Xueping
Guo
a,
Wenhua
Rao
c,
Danmei
Pan
d,
Fang
Cao
a,
Tianyun
Zhai
a,
Wenhui
Zheng
a,
Yakubu Saddeeq
Abubakar
ae,
Xiong
Guan
a,
Zhi
Chen
*b and
Xiaohong
Pan
 *a
*a
aState Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops College of Plant Protection & Key Lab of Biopesticide and Chemical Biology, Ministry of Education, College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, P. R. China. E-mail: panxiaohong@163.com
bCollege of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, P. R. China. E-mail: chenzhi0529@163.com
cInstitute of Plant protection, Fujian Academy of Agriculture Sciences, Fuzhou 350003, P. R. China
dFujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China
eDepartment of Biochemistry, Faculty of Life Sciences, Ahmadu Bello University, Zaria 810281, Nigeria
First published on 27th September 2024
Abstract
Herbicides play an important role in weed control when it comes to ensuring a high and consistent yield in agriculture, but their effectiveness is often compromised by climatic variables. Therefore, improving the climatic adaptability of pesticides is crucial to ensure sustainable agricultural development. In this study, a novel bispyribac-sodium (BIS)–zeolitic imidazolate framework-8 (ZIF-8) nanopesticide (BIS@ZIF-8) with excellent multi-stimuli-responsive properties was synthesized. The nanopesticide BIS@ZIF-8 showed a multi-stimuli response and efficient weed control. In addition, the BIS@ZIF-8 nanocomposite showed strong resistance to rainwater erosion on leaf surfaces with a BIS retention rate of 76.26% under simulated rainwater, which was 41.54% higher than the BIS retention rate of the pure herbicide. Under UV light and acidic conditions, a high concentration of BIS was released from the BIS@ZIF-8 nanocomposite, resulting in an improved weed control effect. Further analyses showed that the BIS@ZIF-8 nanocomposite retained its structural stability and adhered to the weed under rainy conditions through electrostatic interaction. Conversely, the BIS@ZIF-8 nanocomposite was depolymerized under UV light irradiation and released BIS to kill weeds. In addition, BIS@ZIF-8 showed excellent herbicidal activity under field conditions with good biosafety. This work provides a new strategy to avoid environmental and climate-induced pesticide losses and paves the way for smart weed control in agriculture.
Environmental significanceGiven the urgent need to reduce the environmental impact of agrochemicals while preserving crop yields, the BIS@ZIF-8 nanopesticide study represents a breakthrough in sustainable agriculture by improving the climatic adaptability of herbicides. This nanoformulation has a high retention rate on leaf surfaces, thus reducing runoff and pollution. Its intelligent release mechanism ensures efficient weed control under UV light and acidic conditions, thereby optimizing chemical use and minimizing ecological impact. The structural stability of BIS@ZIF-8 under rain and its depolymerization under UV light contribute to targeted herbicide action, which reduces the need for reapplication. This research supports smart agriculture to achieve sustainable development goals by preserving the environment and ensuring efficient farming practices with good biosafety and improved weed control. |
1. Introduction
Weed invasion of crops is one of the significant challenges in agricultural production.1 Weed invasion is responsible for 34% of agricultural losses.2 Chemical herbicides are generally used to control weeds. The global proportion of herbicide use is steadily increasing annually and accounts for up to 49% of agricultural pesticides.3 However, the efficacy of most herbicides is closely linked to environmental and climatic conditions.4 Bispyribac-sodium (BIS) is a post-emergent herbicide commonly used to control rice grass and sedges, belongs to the pyrimidinyloxybenzoic acid ester family and primarily controls susceptible weeds by getting absorbed through stems and leaves, inhibiting the acetolactate synthase (ALS) enzyme, which is involved in the synthesis of branched-chain amino acids.5 The global market for herbicides used in rice cultivation has reached $2.45 billion, with the market sales of BIS ranking sixth in position.6 Owing to its water solubility, BIS cannot effectively adhere to the hydrophobic or superhydrophobic leaves of weeds during seasonal rains, which severely limits its effectiveness in weed control.7 Reports indicate that only about 0.1% of herbicides and other pesticides in the natural environment can effectively affect target organisms.8–10 The development of new, environmentally friendly, and highly efficient pesticide systems is therefore crucial and urgently needed.Due to the excellent properties of nanomaterials, they are widely used to improve the efficiency of pesticides and reduce residual pollution.11–15 Wang et al. found that UV photolysis and migration losses of photosensitive agrochemicals can be effectively reduced by lignin/surfactant coacervates.16 Zhang et al. developed a series of coacervates composed of bile salt and cationic surfactants, which can effectively encapsulate various solutes, improve adhesion and deposition efficiency on hydrophobic leaves, and thereby reduce pesticide use.17 Recent studies have also explored the use of environmental factors, such as temperature, pH, enzymes, and UV light, to improve the release and efficacy of the pesticide on the target organisms.18 Liang et al. developed a novel redox and α-amylase responsive pesticide delivery system using functionalized mesoporous silica nanoparticles to load abamectin. The system showed excellent sustained release ability and UV protection.19 However, these studies primarily focus on improving the efficiency of pesticides in a single aspect, but the efficiency of the practical application of pesticides can be affected by various environmental factors. Therefore, selecting suitable nanomaterials can effectively improve the climatic adaptability of pesticides.
The zeolitic imidazolate framework-8 (ZIF-8) is a subclass of metal–organic frameworks (MOFs) that contain 2-methylimidazole and Zn2+ ions with a high specific surface area and can be easily synthesized.18 Compared to MOFs containing heavy metals, ZIF-8 has better biosafety for non-target organisms. ZIF-8 is widely used for gas storage, catalysis, drug release, and antimicrobial protection.20–22 Several studies have also demonstrated the use of ZIF-8 as a carrier for pesticides, such as alkaloids, β-cypermethrin, and prochloraz, which enables the targeting of pesticides and decreases side effects of the pesticides due to its pH-responsive properties.8,20,23 Xu et al. reported the efficacy of copper-doped ZIF-8 nanoparticles when loaded with fludioxonil, and the acid-dependent release properties of ZIF-8 enabled the controlled release of pesticides.24 Recent studies have also shown that irradiation with UVA, UVB, and near UV-visible light can accelerate the hydrolysis-based degradation of ZIF-8 in water.25 However, as far as we know, no study has addressed the enhancement of the targeted release of pesticides using the UV sensitivity of ZIF-8. In this study, a multi-stimuli–response metal–organic framework nanopesticide was synthesized using a simple one-pot method to encapsulate BIS in ZIF-8 nanoparticles for smart control of barnyard grass (Echinochloa crus-galli) (Scheme 1). The aim of this study was to investigate the herbicidal activity of the BIS@ZIF-8 nanocomposite. We also evaluated the resistance to rain erosion and the environmental behavior of BIS release under different pH conditions and UV light exposure. Importantly, the study demonstrated that ZIF-8 exhibits a UV light-dependent release mechanism, which modulates the rate at which BIS is released upon UV irradiation. This property is crucial for optimizing the effectiveness of the nanocomposite in targeted applications. In addition, the safety of using ZIF-8 was assessed against non-targeted organisms to ensure its environmental compatibility. This study provides new strategies for pesticide release in response to climate change and improves pesticide utilization efficiency.
 | ||
| Scheme 1 Schematic showing the synthesis process for BIS@ZIF-8 and its control of barnyard grass in response to multiple-stimuli factors. | ||
2. Experimental section
2.1 Materials and chemicals
BIS (purity: 96.2%) was purchased from Ruiping Chemical Co., Ltd. (Jiangsu, China). 2-Methylimidazole and zinc nitrate hexahydrate (Zn(NO3)2·6H2O) (purity: 98%) was purchased from Macklin Company (Shanghai, China). Weed seeds (barnyard grass) were collected from vegetable fields in Fairy Town (Yangzhou, Jiangsu, China). Other chemicals were provided by Sinopharm Chemical Reagent Company (Shanghai, China) and used without further purification.2.2 Synthesis of ZIF-8 and BIS@ZIF-8 nanocomposite
ZIF-8 and BIS@ZIF-8 were prepared following previously reported procedures with minor modifications.26–28 Briefly, 8 mL of BIS stock solution (0.04 g mL−1) was added to 32 mL of 2-methylimidazole solution (0.25 g mL−1) and stirred at room temperature for 5 minutes at 1200 rpm. Subsequently, 3.2 mL of Zn(NO3)2·6H2O solution (0.25 g mL−1) was added dropwise to the mixture and stirred at 1200 rpm for 2 hours at 25 °C. The mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to obtain the precipitates, which were further washed three times with deionized water to remove residual reactants. For comparison, ZIF-8 without BIS was similarly synthesized according to the procedure above.
000 rpm for 10 min to obtain the precipitates, which were further washed three times with deionized water to remove residual reactants. For comparison, ZIF-8 without BIS was similarly synthesized according to the procedure above.
Analysis of BIS loading revealed that BIS@ZIF-8 was destroyed by an aqueous hydrochloric acid solution, and drug loading was determined by UV-vis spectrophotometer (UV1800). BIS@ZIF-8 (20 mg) was dissolved in 20 mL aqueous HCl solution (pH 1) and sonicated (250 W, 40 kHz) for 20 min. The absorbance of the filtrate was determined by UV-vis absorption spectroscopy at 247 nm according to the measured standard curve (Fig. S1A–D†). In addition, we used HPLC to check the accuracy of the UV-vis results (Fig. S2†).
2.3 Characterization
Characterization of morphology and microstructure was performed using a field emission scanning electron microscope (SEM, Hitachi, SU8010, Japan) and a transmission electron microscope (TEM, JEOL, JEM-2100F, Japan), respectively. X-ray diffraction patterns were recorded in the range of 5–35° using an X-ray diffractometer (XRD, Bruker, D8 Focus, Germany) with CuKα at 40 kV and 40 mA. The Brunauer–Emmett–Teller surface was measured at 77 K on a Quantachrome autosorb automated gas sorption system (BET, Micromeritics, ASAP 2460, USA). Infrared spectra were recorded with an FT-IR spectrometer (FT-IR, Nicolet IS10, Nicolet USA) using KBr pellets, scanning the range from 4000 to 400 cm−1 at a scan time of 32 with a resolution of 4 cm−1. The zeta potentials of BIS, ZIF-8 and BIS@ZIF-8 nanocomposites were measured using dynamic light scattering on a Zeta Sizer Nano Series Nano-ZS (Zetasizer Nano ZS90, Brookhaven, USA), with each solution or dispersion diluted to the correct concentration and measured in triplicate. The thermal stability of the samples was measured using a thermogravimetric analyzer from 28 to 800 °C under a N2 atmosphere at a heating rate of 10 °C min−1 (TGA, SDT Q600, TA Instruments, USA). Energy dispersive spectroscopy (EDS) was performed using energy dispersive X-ray spectroscopy (FEI Talos F200X), and the BIS concentration was analyzed using a UV-vis spectrophotometer (UV-vis, UV1800, AUCY Scientific Instruments, China). XPS was carried out using Escalab 250Xi (ThermoFisher, USA) to analyze the mechanism of interaction between BIS and ZIF-8.2.4 Herbicidal activity analysis of BIS@ZIF-8 nanocomposite
To investigate the control efficiency of the BIS@ZIF-8 nanocomposite on barnyard grass, aqueous ZIF-8 solution (positive control), deionized water (negative control), BIS (0.1 mg mL−1) and BIS@ZIF-8 (1.0 mg mL−1) were separately sprayed onto the leaves of 4-week-old barnyard grass at room temperature. The concentration of BIS@ZIF-8 was set at 1.0 mg mL−1 to ensure that the actual BIS content was consistent between the BIS@ZIF-8 and pure BIS treatments. The treated barnyard grasses were maintained in an incubator (T = 26 ± 3 °C, RH = 60 ± 5%, L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = 16
D = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) for 10 days, after which the plant heights, fresh weights, and root lengths were measured. The weeding effects of BIS@ZIF-8 after scouring simulated rainwater and UV irradiation were further investigated (see ESI†).
8) for 10 days, after which the plant heights, fresh weights, and root lengths were measured. The weeding effects of BIS@ZIF-8 after scouring simulated rainwater and UV irradiation were further investigated (see ESI†).
2.5 Foliar retention rate of BIS@ZIF-8 nanocomposite
To study the leaf surface resistance to BIS@ZIF-8 during rain erosion, the weeds were placed at an angle of 30° on a glass petri dish,29 and 5 mL of aqueous BIS (0.1 mg mL−1) and BIS@ZIF-8 (1.0 mg mL−1) solutions were separately and uniformly sprayed on barnyard grass leaves. Then, 5 mL of PBS (pH 5.0) solution was evenly sprayed on the weed leaves for 5 min (the simulated rainfall was moderate), and the leaves were further air-dried and immersed in 5 mL PBS solution and agitated at 150 rpm for 10 min to remove excess BIS from the leaf surfaces. Finally, the concentration of BIS was detected by UV-vis spectroscopy. The concentration of BIS on the leaves before (C0) and after (C1) washing was measured, and the retention rate was calculated using C1/C0 as the retention rate of pesticide on the leaves. All experiments were repeated three times.2.6 Contact angle determination
Similar sizes of fresh and intact barnyard grass leaves were selected and carefully washed with distilled water to remove dust. The leaf surfaces were air-dried at room temperature, cut into smaller pieces (0.5 cm × 3 cm), and placed flat on a contact angle (CA) measuring platform (SL2008, Solon Tech. Co., Ltd., Shanghai, China). Precisely, 100 μL each of aqueous BIS@ZIF-8 (1.0 mg mL−1), BIS aqueous solution (0.1 mg mL−1), and ZIF-8 solutions (1.0 mg mL−1) were sprayed on the leaves prior to CA measurements. Distilled water was sprayed for the control.2.7 Distribution and translocation of BIS@ZIF-8 nanocomposite on the leaf surface
To examine the distribution of nanomaterials on the leaf surface of barnyard grass, 5 mL of BIS@ZIF-8 and ZIF-8 suspensions were separately sprayed onto the leaves of 4-week-old barnyard grass at room temperature. After the leaves were naturally air-dried at room temperature, they were cut into small slices of uniform size, and SEM was used to examine the leaf surface morphology and the distribution of nanomaterials on the leaf surface of barnyard grass.To investigate the potential absorption and subsequent distribution of both ZIF-8 and BIS@ZIF-8 by barnyard grass, the leaves and leaf sheath of barnyard grass were exposed to FITC-labeled nanoparticles. For foliar distribution, 5 mL of FITC-labeled nanoparticle suspension (1 mg mL−1) was separately sprayed onto the leaves of 4-week-old barnyard grass at room temperature. Following natural air-drying of the leaves at room temperature, a wash-off test was conducted by spraying 5 mL of deionized water onto the leaves, and this process was repeated in triplicate. Changes before and after spraying were observed using CLSM (Nikon, CSU-W1, Japan) with a laser excitation wavelength of 488 nm.
In the leaf sheath translocation experiment, 0.5 mL of the FITC-labeled nanoparticle suspension (1 mg mL−1) was pipetted onto the leaf sheath of 4-week-old barnyard grass. Subsequently, the barnyard grass leaf sheath was placed in a dark humid box (25 °C, 90% humidity) for continuous cultivation over 24 hours. After rinsing the leaf sheath with 0.5 mL of deionized water, the barnyard grass leaf sheath membrane was carefully excised. The distribution of nanoparticles in the leaf sheath cells was observed using CLSM with a laser excitation wavelength of 488 nm.
Furthermore, the bioaccumulation of zinc (Zn) in barnyard grass subjected to cultivation and treatment with BIS@ZIF-8 NPs was systematically monitored. The Zn concentration in different plant tissues, including leaves, stems, and roots, was meticulously determined. Upon harvest, the plants underwent thorough washing with tap water, followed by rinsing with distilled water. Subsequently, the leaves, stems, and roots were meticulously separated and subjected to drying at 65 °C for 48 hours. The Zn concentration in each tissue was quantitatively analyzed using an inductively coupled plasma mass spectrometer (ICPMS, Agilent 7800, US).
2.8 Controlled release of BIS@ZIF-8 at different pH and UV conditions
To study the release of BIS@ZIF-8 under complex conditions, BIS@ZIF-8 was first dissolved in PBS solutions at pH 5.0 and 7.4 and then separately stored in dark and UV light conditions. The solution was taken at the corresponding time point to measure the concentration of BIS. Specifically, 20.0 mg of BIS@ZIF-8 samples were added to 20 mL of 0.05 mol L−1 PBS buffer of different pH (pH 5.0 and pH 7.4) and separately stored under dark and UV light irradiation conditions (UVA lamps with a wavelength of 352 nm and UVB lamps with a wavelength of 306 nm). Then, 0.2 mL of the suspension was collected from the system at certain time intervals (0, 1, 2, 3, 4, 5, 10, 12, and 24 h), followed by 0.2 mL of the same pH PBS buffer. The absorbance value of the filtrate at 247 nm was measured with UV-vis after filtration using a 0.22 μm filter membrane. The concentration of BIS in the filtrate was calculated, and the release ratio of BIS@ZIF-8 was calculated using eqn (1). | (1) |
2.9 Field weeding effect of BIS@ZIF-8
To study the weeding effect of BIS@ZIF-8 in the actual field (experimental field of the Fujian Agriculture and Forestry University), 30 mL of BIS (0.1 mg mL−1) and BIS@ZIF-8 (1.0 mg mL−1) were uniformly sprayed onto the leaves of 4-week-old barnyard grass at room temperature, separately. Then, under natural conditions (specific climatic conditions can be found in Table S2†), the treated barnyard grass was continuously cultured for 10 days, and the plant height, fresh weight, and chlorophyll content of barnyard grass were measured to evaluate the weeding effect. Deionized water was used as the control group in the experiment.2.10 Biosafety evaluation
To investigate the biosafety of ZIF-8 and BIS@ZIF-8, the effects of nanoparticles on the growth and enzyme activity of rice and the growth of goldfish, silkworms and earthworms were studied. Moreover, the high-throughput sequencing of soil bacterial 16S rRNA genes was conducted to evaluate the effect of BIS@ZIF-8 and ZIF-8 on the taxonomic abundance of soil community diversity using an Illumina MiSeq platform (Majorbio. Inc., Shanghai, China). More details can be found in the ESI† (Methods).2.11 Statistical analysis
All data were expressed as mean ± standard deviation. The results were analysed by SPSS 18.0 software (IBM) using a t-test. In the figures for each assay, values followed by different capital P < 0.01 and lowercase were significantly different at P ≤ 0.05; no statistical significance is indicated by ns.3. Result and discussion
3.1 Synthesis and characterization of BIS@ZIF-8 nanocomposite
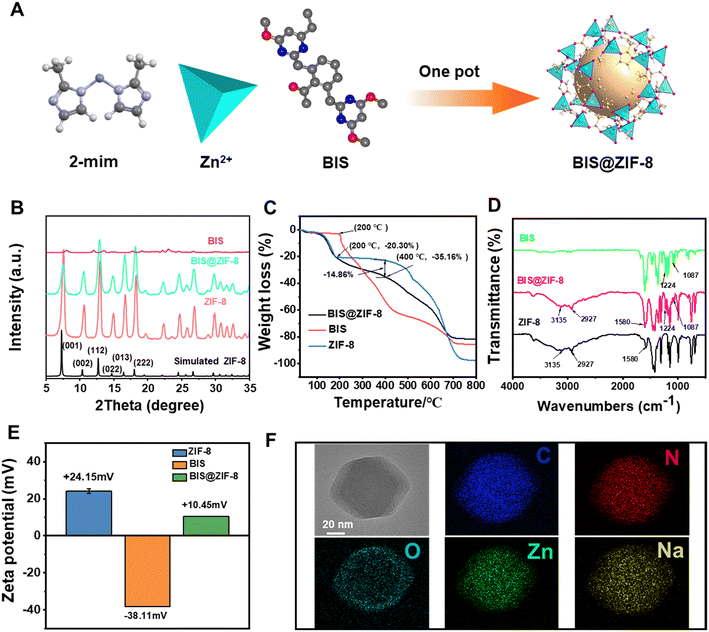
The BIS@ZIF-8 nanocomposite was synthesized using a one-pot method (Fig. 1A). As shown in Fig. 1B, the typical XRD diffraction peaks and the positions of the peaks were consistent with previous reports,30 indicating that the sample was ZIF-8 crystals with a high degree of crystallization and that the simultaneous addition of drugs during the synthesis of ZIF-8 did not affect the nanocrystal structure. In addition, analysis using Nano Measurer 1.2 software revealed that the mean particle size of BIS@ZIF-8 (118.46 ± 1.08 nm) was larger than that of ZIF-8 (90.79 ± 0.87 nm) (Fig. S3†). EDS mappings showed a uniform distribution of C, N, O, Zn, and Na in the nanoparticles, proving the presence of BIS in the ZIF-8 nanoparticles (Fig. 1F). In addition, the UV-vis spectra showed that BIS@ZIF-8 had the characteristic absorption peak of BIS at 247 nm. BIS@ZIF-8 also had an absorption peak at about 210 nm, similar to the absorption peak of ZIF-8 (Fig. S1A†). The FT-IR spectra showed that BIS@ZIF-8 and ZIF-8 exhibited the same characteristic peaks (C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the imidazole ring)20,23 and the characteristic peaks of BIS (C–O stretching at 1224 cm−1) were observed in BIS@ZIF-8, confirming the successful loading of BIS onto ZIF-8 (Fig. 1D).
N stretching vibrations of the imidazole ring)20,23 and the characteristic peaks of BIS (C–O stretching at 1224 cm−1) were observed in BIS@ZIF-8, confirming the successful loading of BIS onto ZIF-8 (Fig. 1D).
The thermal stability of the material and the drug loading capacity were investigated using TGA. As shown in Fig. 1C, the mass loss of ZIF-8 (20.30%) from 27 to 200 °C is related to the evaporation loss of water in the sample and the presence of other impurities.31 When the temperature increased to about 400 °C, the organic ligand of ZIF-8 started to thermally decompose rapidly. The decomposition rate continued until about 700 °C and reached the equilibrium state, meaning that ZIF-8 was completely decomposed while the remaining carbon and zinc oxide did not decompose.28 However, the mass of BIS decreased significantly when the temperature was about 200 °C, indicating that the thermal decomposition of BIS started at 200 °C.32 Due to the presence of carrier materials in BIS@ZIF-8, the difference in weight loss between ZIF-8 and BIS@ZIF-8 at a temperature range of 200–400 °C can be used for drug loading. The drug loading of BIS was calculated to be about 14.86%. The TGA results were consistent with the loading content of BIS analysis (15.45 ± 0.61%).
Surface charge is a basic parameter for studying the nature and predicting the long-term stability of nanoparticles.33 The zeta potential results (Fig. 1E) show that BIS exhibited a strong electronegativity of −38.11 ± 0.20 mV due to the presence of –COOH.34 Due to the strong positive charge of ZIF-8 (24.15 ± 1.27 mV), the charge value of BIS@ZIF-8 increased to 10.45 ± 0.09 mV after a successful ZIF-8 loading of the BIS. As shown in Fig. S4 and Table S1,† the specific surface areas of ZIF-8 and BIS@ZIF-8 were 1451.08 m2 g−1 and 936.97 m2 g−1, while their pore volumes were 0.67 cm3 g−1 and 0.41 cm3 g−1, respectively. The decrease in the BET-specific surface area and pore volume of BIS@ZIF-8 could be attributed to BIS entering and occupying some parts of the pore area of ZIF-8 during the synthesis process. In addition, the average particle sizes of BIS@ZIF-8 and ZIF-8 were 6.40 nm and 4.13 nm, respectively, indicating that the addition of BIS increased the size of the nanoparticles.
Moreover, the XPS survey spectra (Fig. S5†) revealed that there was no difference between ZIF-8 and BIS@ZIF-8, except for the presence of a peak corresponding to Na 1s at 1072 eV, which was observed only in the spectra of BIS@ZIF-8, indicating that BIS was successfully loaded onto ZIF-8. To study the interaction mechanism of BIS and ZIF-8 nanoparticles, the samples were analyzed using Zn 2p, O 1s, and N 1s high resolution. All binding energies were referenced to the C 1s peak at 284.80 eV to compensate for any possible charging-up effect (Fig. S6†). As shown in Fig. 2A–C, the Zn 2p3/2 and 2p1/2 peaks shifted from 1021.52 and 1044.52 eV to 1022.02 and 1045.02 eV, respectively, after loading the BIS, indicating the existence of interaction forces, likely through coordinate bonding between Zn and the functional groups (carboxy groups or nitrogen atoms) in the BIS. The O 1s XPS spectrum in BIS (Fig. 2A, D and E) yielded two peaks at 530.80 and 533.56 eV, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, respectively. However, the peaks of C
O and C–O, respectively. However, the peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O in BIS@ZIF-8 shifted to 532.49 eV and 533.99 eV, respectively, and a peak of Zn–O was observed at 531.65 eV. This could be attributed to the formation of coordinate bonds between some C
O and C–O in BIS@ZIF-8 shifted to 532.49 eV and 533.99 eV, respectively, and a peak of Zn–O was observed at 531.65 eV. This could be attributed to the formation of coordinate bonds between some C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the BIS and Zn in the ZIF-8 nanoparticles, which reduced the electron density of O atoms, thereby increasing the binding energy (Fig. 2A). Furthermore, for N 1s spectra in ZIF-8 (Fig. 2F–H), peaks were observed at 398.14 and 398.68 eV, which correspond to the binding of pyridinic and pyrrolic N, respectively. For N 1s in BIS, the peaks observed at 399.07 eV could be due to –N
O in the BIS and Zn in the ZIF-8 nanoparticles, which reduced the electron density of O atoms, thereby increasing the binding energy (Fig. 2A). Furthermore, for N 1s spectra in ZIF-8 (Fig. 2F–H), peaks were observed at 398.14 and 398.68 eV, which correspond to the binding of pyridinic and pyrrolic N, respectively. For N 1s in BIS, the peaks observed at 399.07 eV could be due to –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. Compared to ZIF-8, the peaks of BIS@ZIF-8 shifted to 398.71 eV and 400.31 eV after BIS loading (Fig. 2H). The results indicate that N might play a role in the process of BIS loading (Fig. 2A), and coordinate bonds, hydrogen bonds, or electronic effects between the N of the pyrimidine rings and the N in ZIF-8 are likely involved.
C. Compared to ZIF-8, the peaks of BIS@ZIF-8 shifted to 398.71 eV and 400.31 eV after BIS loading (Fig. 2H). The results indicate that N might play a role in the process of BIS loading (Fig. 2A), and coordinate bonds, hydrogen bonds, or electronic effects between the N of the pyrimidine rings and the N in ZIF-8 are likely involved.
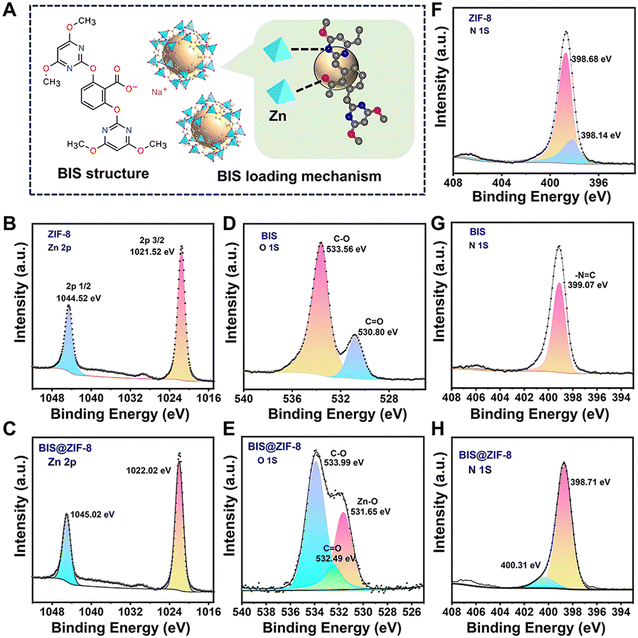 | ||
| Fig. 2 (A) Schematic representation of the BIS-loading mechanism, (B and C) Zn 2p XPS spectra, (D and E) O 1s XPS spectra, and (F–H) N 1s XPS spectra of ZIF-8, BIS and BIS@ZIF-8. | ||
3.2 Control effects, adhesion, and distribution of BIS@ZIF-8 on weed
For the preliminary determination of the herbicidal activity of BIS@ZIF-8, the growth of barnyard grass was analyzed in a pot experiment by spraying the leaves with BIS@ZIF-8, BIS, and ZIF-8. As shown in Fig. S7,† ZIF-8 had no discernible effect on the development of barnyard grass, proving that ZIF-8 had no herbicidal effect (P > 0.05). However, the plant height, fresh weight, and root length of barnyard grass decreased significantly when treated with BIS@ZIF-8 and BIS compared to the control group (Fig. S7†) (P < 0.05), proving that BIS@ZIF-8 has an equivalent herbicidal effect to BIS at the same dose.Studies have shown that BIS can inhibit acetolactate synthase and can be absorbed through the leaf surface and transferred to the whole plant, affecting the synthesis of branched-chain amino acids and inhibiting the growth of weeds.35,36 Therefore, the efficient deposition and strong adhesion of pesticides on the leaf surface of plants are crucial for improving their utilization efficiency.37,38 We found that both the BIS solution and water had high CA values on the surface of the weed leaves (130.58 ± 5.35° and 105.40 ± 2.71°, respectively), indicating that the leaf had strong hydrophobicity and BIS did not readily adhere to the leaf (Fig. 3B). However, the ZIF-8 solution had a better affinity with the leaf surface (CA value 91.84 ± 1.66°), and BIS@ZIF-8 had a lower CA value with the leaf surface 92.36 ± 1.65° compared to BIS. Further investigation revealed that the retention rate of BIS on barnyard grass leaves was 53.88%, while the retention rate of BIS in the form of BIS@ZIF-8 was 76.26% (Fig. 3A), indicating that BIS@ZIF-8 significantly increased the retention of BIS on barnyard grass leaves. These results show that the addition of ZIF-8 reduces the CA value between the pesticide and the leaf surface, thus improving the wettability and distribution area of the pesticide on the leaf surface.39–41
To further compare the herbicidal activities of BIS and BIS@ZIF-8 after the simulated rainfall, the height and fresh weight of barnyard grass and chlorophyll content in the plants were examined after the plants were sprayed with different chemicals and then flushed with the simulated rainwater. The results showed that the heights of both BIS- and BIS@ZIF-8-treated barnyard grass plants were significantly different from those of the control (P < 0.05). However, the BIS@ZIF-8-treated barnyard grass had lower heights and chlorophyll contents than the BIS-treated barnyard grass plants (Fig. 3C–F). These results indicate that BIS@ZIF-8 had better rainwater scouring resistance with greater herbicidal activity on the barnyard grass than the independent BIS.
In addition, the distribution of nanoparticles on the barnyard grass leaves sprayed with BIS@ZIF-8 and ZIF-8 was investigated by SEM to explore the improved mechanism behind the BIS retention rate in BIS@ZIF-8. As shown in Fig. 3G1–G3, barnyard grass leaves without nanomaterial spraying exhibited many papillae and waxy layers (indicated by red arrows). These structures increased the CA between the pesticide and the leaf surface, which was not conducive to the distribution and adhesion of the pesticide on the leaf surface.42 Moreover, numerous nanoparticles adhered to the leaves of barnyard grass sprayed with ZIF-8 and BIS@ZIF-8, forming a film consisting of nanoparticles (Fig. 3G4–G9). The leaf surface is generally negatively charged because the micron-sized wax layer contains several types of higher fatty acids, alcohols, and aldehydes,8,43–45 whereas BIS@ZIF-8 has a positive charge (Fig. 1E). Therefore, BIS@ZIF-8 adhered to the weed leaves by electrostatic interaction. The CA value between BIS@ZIF-8 and leaves was significantly reduced. This result is consistent with the previous CA data (Fig. 3B). The increased BIS retention rate of BIS@ZIF-8 might be due to the polar groups on the leaf surface forming stronger electrostatic or H-bond interactions with the ZIF-8 nanoparticles or the topological structure formed between the wax layer and the ZIF-8 nanoparticles.8,39,40,44
The absorption and distribution of ZIF-8 and BIS@ZIF-8 nanoparticles (NPs) in barnyard grass were investigated by inductively coupled plasma mass spectrometry (ICP-MS) and confocal fluorescence microscopy. As shown in Fig. 4A, the ZIF-8 NPs deposited on the leaves to a considerable extent when FITC-labeled NPs were applied. This phenomenon is consistent with similar studies conducted on other plant species, such as rice and wheat, where nanoparticle deposition was observed on the leaf surfaces.24,46 When FITC-labeled nanoparticles were applied to the leaf surface of barnyard grass, the nanomaterials showed uniform distribution across the leaf surface, which is consistent with the SEM results (Fig. 3G). This uniform distribution pattern is consistent with observations in studies on maize and barley, suggesting a common mechanism of nanoparticle adhesion and spread across various plant species.47,48 Following washing with water, the nanoparticles remained adhered to the leaf surface, indicating that BIS@ZIF-8 had a strong affinity for the barnyard grass leaves. Similar results were reported in studies on Arabidopsis thaliana, where engineered nanoparticles showed prolong adhesion even after rinsing, emphasizing the robust interaction between the nanoparticles and plant surfaces.49 Hu et al. have shown that negatively charged nanoparticles can pass through the cell membrane and enter the cell.50 By observing the nanoparticles in the leaf sheath cells of barnyard grass, we found that some nanoparticles still exist in the leaf sheath cells of the barnyard grass after washing. Therefore, we hypothesized that the negatively charged ZIF-8 nanoparticles could have been transported into the leaf sheath cells. Quantitative evidence from ICP-MS results (Fig. 4B) indicated the distribution of ZIF-8 NPs sprayed on the plants. Zinc (Zn) was predominantly concentrated in the leaves of the barnyard grass (Zn content: 280.93 mg kg−1, constituting 72.96% of the total plant), consistent with confocal laser scanning microscopy (CLSM) findings. Over a 5-day period, a discernible translocation of Zn occurred to stems (Zn content: 70.69 mg kg−1, comprising 18.36% of the total plant) and roots (Zn content: 33.44 mg kg−1, comprising 8.68% of the total plant). Our previous research has shown that NPs can be transported along with nutrients and have a good control effect against bacterial wilt.51 Therefore, we speculate that the translocation of ZIF-8 nanoparticles in weeds will enhance their efficacy as carriers. These findings indicate the presence and stability of the ZIF-8 nanoparticles within the leaves and leaf sheaths of barnyard grass, ensuring their consistent transport in the plant tissues.
3.3 Release behavior of BIS@ZIF-8
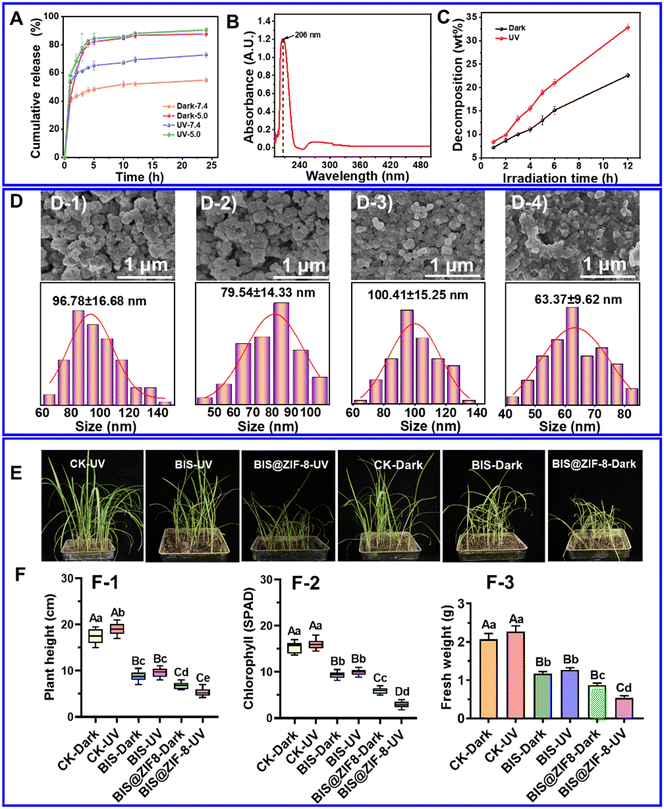
A previous study has shown that ZIF-8 is self-assembled from the Zn2+ ions and the organic ligand 2-methylimidazole.20 The coordination bond between the metal ion and the organic ligand can easily dissociate under acidic conditions and release the encapsulated agent, indicating the excellent pH-response property of ZIF-8.20,24 In addition, UVA and UVB are the UV lights that naturally travel to the earth's surface. Studies have shown that ZIF-8 is hydrolyzed in water in the presence of UVA and UVB irradiation, implying that ZIF-8 release is UV-dependent.25,52 Therefore, in this study, the pH and UV responses of BIS@ZIF-8 under different pH and UV conditions were investigated.As shown in Fig. 5A, the cumulative release rate of BIS from BIS@ZIF-8 at pH 5.0 was markedly higher than that at pH 7.4 after a 24-hour release period under both dark and UV light conditions. Specifically, under dark conditions, the cumulative release rates of BIS from BIS@ZIF-8 were 87.61 ± 2.11% at pH 5.0 and 54.93 ± 1.68% at pH 7.4. The impact of light on the cumulative release rate of BIS@ZIF-8 at the same pH was further evaluated, revealing a 32.86% increase in the cumulative release rate after UV irradiation (72.98 ± 2.35%) compared to dark conditions (54.93 ± 1.68%) at pH 7.4. Notably, at pH 5.0, the cumulative release rate of BIS@ZIF-8 after UV irradiation only surpassed that in the dark within the initial 5 hours. Subsequently, the cumulative release rates under UV irradiation and dark conditions became nearly identical after 5 hours. This observation gave us the impression that at pH 5.0, BIS@ZIF-8 undergoes rapid decomposition to release BIS at the early stages, and this is primarily influenced by the combined effects of UV irradiation and pH. However, under single-action conditions, the impact was less pronounced, likely due to the acidic pH value. Hence, the cumulative release rate of BIS@ZIF-8 under specific pH conditions was observed to be lower than that under UV irradiation conditions. Over time, a gradual breakdown of BIS@ZIF-8 occurred, resulting in nearly equal cumulative release profiles under both conditions (Fig. 5C). However, it is noteworthy that BIS@ZIF-8 exhibited a higher release of agents when exposed to UV light stimulation, indicating a more pronounced response to UV irradiation compared to the influence of pH alone.
To reveal the UV-response release mechanism of BIS@ZIF-8, the decomposition behavior of ZIF-8 under UV irradiation was investigated by determining the 2-methylimidazole intermediate as previously reported.25 Based on the strongest absorption peak of 2-methylimidazole at 206 nm and the standard curve (Fig. 5B and S9†), the ZIF-8 aqueous solution was continuously irradiated at different times using a UV irradiation device, and its decomposition rate was calculated. The results showed that the decomposition rate of ZIF-8 under the dark condition was consistently lower than that in the UV irradiation group, which indicated that UV light facilitates the decomposition of ZIF-8, and the results are consistent with a previous study.25 Meanwhile, the morphology of BIS@ZIF-8 under different conditions (dark, UV irradiation, pH 7.4 and 5.0) was examined using SEM, and the results showed BIS@ZIF-8 breakdown and dissolution after UV irradiation compared to the morphology of BIS@ZIF-8 after exposure to the dark. The average particle size of the nanoparticles in the dark (96.78 ± 16.68 nm) was significantly larger than that after UV irradiation (79.54 ± 14.33 nm) (Fig. 5D-1 and D-2). Compared to pH 7.4, BIS@ZIF-8 showed severe morphological breakage and agglomeration of nanoparticles after pH 5.0 treatment, and the average particle size decreased from 100.41 ± 15.25 nm to 63.37 ± 9.62 nm (Fig. 5D-3 and D-4). The above results demonstrate that BIS@ZIF-8 was continuously decomposed under the UV and pH conditions, thereby releasing the loaded drug, consistent with the in vitro release results (Fig. 5A).
Further studies confirmed the weeding effect under UV irradiation. The heights, fresh weights, and chlorophyll contents of the barnyard grasses were analyzed under the different treatments. The results showed that BIS@ZIF-8 had a better control effect on barnyard grass than BIS alone, both in the dark and under UV light (Fig. 5E and F). Under dark and UV conditions, chlorophyll contents in the barnyard grass subjected to BIS@ZIF-8 treatment decreased by 36.4 and 34.1%, plant height decreased by 22.6 and 44.6% while fresh weight decreased by 25.7 and 57.9%, respectively. In summary, under UV light irradiation, BIS@ZIF-8 showed an improved control effect on barnyard grass.
3.4 Weeding effect and biosafety evaluation of BIS@ZIF-8 under natural conditions
Preliminary experimental results showed that BIS@ZIF-8 responded to pH and UV light under indoor conditions by releasing more pesticides. However, in the practical application process, various environmental factors could affect the pesticide efficacy. Therefore, barnyard grass was separately treated with BIS and BIS@ZIF-8 and cultured under natural conditions for 10 days (Fig. 6A). Most of the pesticides in the BIS treatment group were washed away by rainwater after application, which significantly impacted their efficacy. The growth of treated barnyard grass was slightly lower than that of the control group, indicating that BIS@ZIF-8 was effectively bound to the barnyard grass (Fig. S10†). Additionally, BIS@ZIF-8 responded to UV light in natural conditions to release pesticides, leading to the death of all the barnyard grass. The fresh weight, plant height, and chlorophyll content of the barnyard grass were further evaluated (Fig. 6B). The result revealed that these parameters were significantly lower in the BIS@ZIF-8 treatment group than those in the BIS treatment group, indicating that BIS@ZIF-8 had greater herbicidal activities under natural conditions.The safety of ZIF-8 should not be ignored when used as pesticide carriers because ZIF-8 nanoparticles can slowly release metal ions and organic ligands.21,53,54 The original microbial sequence data of soil samples under different treatments were quality-controlled, resulting in a total of 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 high-quality 16S rRNA gene sequences obtained through high-throughput amplification sequencing. The result showed that Proteobacteria represent the dominant bacteria, accounting for 25.30 to 34.36% in all the samples, followed by Acidobacteriota, Actinobacteriota, Chloroflexi, and Firmicutes, with relative abundances of 15.77–32.70%, 12.90–18.63%, 8.55–10.87% and 4.36–5.32%, respectively (Fig. 6I and J). ZIF-8 significantly increased the abundance of Acidobacteriota and decreased that of Actinobacteriota compared to the control group. This is consistent with a previous work.55 In addition to taxonomic composition, we conducted a detailed analysis of microbial community structure, focusing on community complexity and stability. Alpha diversity indices (ACE, Chao1, Shannon, and Simpson) indicated that the overall complexity of the microbial communities remained stable, with only minor fluctuations between treatments (Fig. S13†). This suggests that ZIF-8 did not induce significant changes in microbial diversity. These findings indicate that ZIF-8 affects the relative abundances of specific bacterial taxa without dramatically altering the overall microbial community composition or its structural complexity and stability.
663 high-quality 16S rRNA gene sequences obtained through high-throughput amplification sequencing. The result showed that Proteobacteria represent the dominant bacteria, accounting for 25.30 to 34.36% in all the samples, followed by Acidobacteriota, Actinobacteriota, Chloroflexi, and Firmicutes, with relative abundances of 15.77–32.70%, 12.90–18.63%, 8.55–10.87% and 4.36–5.32%, respectively (Fig. 6I and J). ZIF-8 significantly increased the abundance of Acidobacteriota and decreased that of Actinobacteriota compared to the control group. This is consistent with a previous work.55 In addition to taxonomic composition, we conducted a detailed analysis of microbial community structure, focusing on community complexity and stability. Alpha diversity indices (ACE, Chao1, Shannon, and Simpson) indicated that the overall complexity of the microbial communities remained stable, with only minor fluctuations between treatments (Fig. S13†). This suggests that ZIF-8 did not induce significant changes in microbial diversity. These findings indicate that ZIF-8 affects the relative abundances of specific bacterial taxa without dramatically altering the overall microbial community composition or its structural complexity and stability.
Moreover, the different concentrations of ZIF-8 had no significant effects on the fresh weight, plant height, and chlorophyll content of the rice plants (Fig. S11A–C and S12A†). Antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), play an important role in protecting plants from oxidative stress.56 Compared to the control group, the activities of SOD, POD, and CAT significantly increased under ZIF-8, BIS, and BIS@ZIF-8 treatments in the rice samples (Fig. 6C–E). Malondialdehyde (MDA) is one of the most important products of membrane lipid peroxidation, and its production can trigger membrane damage.57 MDA production in the rice plants significantly decreased under ZIF-8 treatment (33.72%) compared to the non-treated controls (Fig. 6F). The decrease in MDA indicates reduced oxidative stress in the rice plants under ZIF-8, BIS, and BIS@ZIF-8 treatments, which in turn lowered the need for high antioxidant enzyme activities (SOD, POD, CAT). This reduced stress likely prevented any damage that would affect plant height, fresh weight, or chlorophyll content, explaining why these growth parameters remained unchanged. Furthermore, insects (silkworms), aquatic organisms (goldfish), and earthworms (Aporrectodea caliginosa) were treated with higher concentrations of ZIF-8 than the actual concentration to test its potential toxicity to the animal subjects. The results showed that the different concentrations of ZIF-8 did not affect the growth and survival rate of the silkworms, goldfish, or earthworms (Fig. 6G, H, S12B, C and S14†). Studies have shown that ZIF-8 has no significant toxic effect on cells during short-term exposure.8,31,58 Our results also show that ZIF-8 is harmless to non-targeted organisms such as rice, insects, earthworms, aquatic organisms and soil microorganisms. It rather improved antioxidant enzymatic activities and reduced ROS-induced damage in the rice. Therefore, ZIF-8 exhibited excellent biological safety and broad application prospects as a pesticide carrier.
4. Conclusions
In summary, the effectiveness, safety, and smart weed control properties of BIS@ZIF-8 composites were investigated. The BIS@ZIF-8 composites had a mean diameter of 118.46 ± 1.08 nm and a drug loading of approximately 14.86%. The nanocomposites had a smaller CA with weed leaves than simple herbicide BIS. BIS@ZIF-8 nanocomposites adhere to weed leaves via electrostatic forces, maintaining structural stability under rainy conditions and resisting water erosion. Simultaneously, upon attachment to the leaf surface, the nanoparticles demonstrate excellent downward transport capability. Some nanoparticles are capable of entering the sheath cells of the weeds, facilitating targeted delivery of pesticide molecules. In addition, the nanocomposites were easily depolymerized under UV light and acidic conditions, resulting in the substantial release of BIS and a significant improvement in weed control efficiency. Moreover, BIS@ZIF-8 exhibited excellent herbicidal activity and biosafety under field conditions. This method is a simple and effective release system for responding to environmental and climate factors, which may provide a sustainable strategy for improving pesticide utilization and smart weed control.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Dingyang Zhang: responsible for writing the whole paper, data curation, and implementing most of the experiments; Xueping Guo: responsible for drawing a model picture of the article; Wenhua Rao, Danmei Pan, Fang Cao: responsible for conducting the biosafety test and revising the first draft of the article; Tianyun Zhai: handle the synthesis of BIS@ZIF-8; Wenhui Zheng and Yakubu Saddeeq Abubakar: responsible for editing the grammar of the article; Xiong Guan and Zhi Chen: responsible for obtaining funding for the project, checking the research ideas of this study. Xiaohong Pan: responsible for sourcing funds for the project, checking the whole experimental ideas, revising the language and logical relationships of the paper, and communicating with the editor.Conflicts of interest
The authors declare that they do not have any identifiable financial conflicts of interest or personal relationships that might have influenced the findings presented in this paper.Acknowledgements
This work was supported by the National Key R&D Program of China (grant no. 2022YFD1400700), the Scientific Research Foundation of the Graduate School of FAFU to Excellent Master's Thesis (1122YS01006), the Open Funds of the Key Laboratory of Biopesticide and Chemical Biology, and Ministry of Education, FAFU (Keylab2020-04). Dr. Baitong Liu (Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong SAR 999077, P. R. China) is gratefully acknowledged for supporting nanomaterial synthesis and analysis.References
- M. Kostina-Bednarz, J. Płonka and H. Barchanska, Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture, Rev. Environ. Sci. Bio/Technol., 2023, 22, 471–504 CrossRef CAS
.
- E. V. R. Campos, J. Ratko, N. Bidyarani, V. Takeshita and L. F. Fraceto, Nature-based herbicides and micro−/nanotechnology fostering sustainable agriculture, ACS Sustainable Chem. Eng., 2023, 11, 9900–9917 CrossRef CAS
.
- FAO, Pesticides Use., FAOSTAT, 2023, https://www.fao.org/faostat/en/#data/RP.
- D. Wang, N. B. Saleh, A. Byro, R. Zepp, E. Sahle-Demessie, T. P. Luxton, K. T. Ho, R. M. Burgess, M. Flury, J. C. White and C. Su, Nano-enabled pesticides for sustainable agriculture and global food security, Nat. Nanotechnol., 2022, 17, 347–360 CrossRef CAS PubMed
.
- N. Sharma, P. Kaur, D. Jain and M. S. Bhullar, In-vitro evaluation of rice straw biochars' effect on bispyribac-sodium dissipation and microbial activity in soil, Ecotoxicol. Environ. Saf., 2020, 191, 1102204–1110304 Search PubMed
.
- P. McDougall, Proceedings The Global Agrochemical Market Trends by Crop, Shanghai, 2017 Search PubMed.
- N. K. Kalsi and P. Kaur, Dissipation of bispyribac-sodium in aridisols: impact of soil type, moisture and temperature, Ecotoxicol. Environ. Saf., 2019, 170, 375–382 CrossRef CAS PubMed
.
- W. L. Liang, Z. G. Xie, J. L. Cheng, D. Xiao, Q. Xiong, Q. Wang, J. Zhao and W. Gui, A light-triggered pH-responsive metal-organic framework for smart delivery of fungicide to control Sclerotinia diseases of oilseed rape, ACS Nano, 2021, 15, 6987–6997 CrossRef CAS PubMed
.
- X. Cao, X. Chen, Y. Liu, C. Wang, L. Yue, W. H. Elmer, J. C. White, Z. Wang and B. Xing, Lanthanum silicate nanomaterials enhance sheath blight resistance in rice: Mechanisms of action and soil health evaluation, ACS Nano, 2023, 17, 15821–15835 CrossRef CAS PubMed
.
- X. Zhao, H. Cui, Y. Wang, C. Sun, B. Cui and Z. Zeng, Development strategies and prospects of nano-based smart pesticide formulation, J. Agric. Food Chem., 2018, 66, 6504–6512 CrossRef CAS
.
- D. X. Zhang, J. Du, R. Wang, J. Luo, T. F. Jing, B. X. Li, W. Mu, F. Liu and Y. M. Hou, Core/Shell dual-responsive nanocarriers via iron-mineralized electrostatic self-assembly for precise pesticide delivery, Adv. Funct. Mater., 2021, 31, 2102027 CrossRef CAS
.
- X. L. Deng, P. Y. Zhao, Y. Xie and L. Y. Bai, Self-assembled sphere covalent organic framework with enhanced herbicidal activity by loading cyhalofop-butyl, J. Agric. Food Chem., 2023, 71, 1417–1425 CrossRef CAS
.
- W. Rao, Q. Yue, S. Gao, M. Lei, T. Lin, X. Pan, J. Hu and G. Fan, Visible-light-driven water-soluble zinc oxide quantum dots for efficient control of citrus canker, Pest Manage. Sci., 2024, 6, 3022–3034 CrossRef
.
- X. Pan, X. Guo, T. Zhai, D. Zhang, W. Rao, F. Cao and X. Guan, Nanobiopesticides in sustainable agriculture: developments, challenges, and perspectives, Environ. Sci.: Nano, 2023, 10, 41–61 RSC
.
- X. Pan, F. Cao, X. Guo, Y. Wang, Z. Cui, T. Huang, Y. Hou and X. Guan, Development of a safe and effective Bacillus thuringiensis-Based nanobiopesticide for controlling tea pests, J. Agric. Food Chem., 2024, 72, 7807–7817 CrossRef CAS PubMed
.
- J. Wang, Y. Fan, H. Wang, J. Yin, W. Tan, X. Li, Y. Shen and Y. Wang, Promoting efficacy and environmental safety of photosensitive agrochemical stabilizer via lignin/surfactant coacervates, Chem. Eng. J., 2022, 430, 132920 CrossRef CAS
.
- L. Zhang, J. Wang, Y. Fan and Y. Wang, Coacervate-enhanced deposition of sprayed pesticide on hydrophobic/superhydrophobic abaxial leaf surfaces, Adv. Sci., 2023, 10, 2300270 CrossRef CAS
.
- M. Hoop, C. F. Walde, R. Riccò, F. Mushtaq, A. Terzopoulou, X. Chen, A. J. deMello, C. J. Doonan, P. Falcaro, B. J. Nelson, J. Puigmartí-Luis and S. Pané, Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications, Appl. Mater. Today, 2018, 11, 13–21 CrossRef
.
- Y. Liang, Y. Gao, W. Wang, H. Dong, R. Tang, J. Yang, J. Niu, Z. Zhou, N. Jiang and Y. Cao, Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery, J. Hazard. Mater., 2020, 389, 122075 CrossRef CAS PubMed
.
- W. Liang, J. Cheng, J. Zhang, Q. Xiong, M. Jin and J. Zhao, pH-Responsive on-demand alkaloids release from core-shell ZnO@ZIF-8 nanosphere for synergistic control of bacterial wilt disease, ACS Nano, 2022, 16, 2762–2773 CrossRef CAS PubMed
.
- C. Pettinari, R. Pettinari, C. Di Nicola, A. Tombesi, S. Scuri and F. Marchetti, Antimicrobial MOFs, Coord. Chem. Rev., 2021, 446, 214121–214189 CrossRef CAS
.
- R. Yu and Z. Wu, High adsorption for ofloxacin and reusability by the use of ZIF-8 for wastewater treatment, Microporous Mesoporous Mater., 2020, 308, 110494–110502 CrossRef CAS
.
- Y. Ma, R. Zhao, H. Shang, S. Zhen, L. Li, X. Guo, M. Yu, Y. Xu, J. Feng and X. Wu, pH-Responsive ZIF-8-Based metal–organic-framework nanoparticles for termite control, ACS Appl. Nano Mater., 2022, 5, 11864–11875 CrossRef CAS
.
- C. Xu, L. Cao, T. Liu, H. Chen and Y. Li, pH-responsive copper-doped ZIF-8 MOF nanoparticles for enhancing the delivery and translocation of pesticides in wheat plants, Environ. Sci.: Nano, 2023, 10, 2578–2590 RSC
.
- M. Taheri and T. Tsuzuki, Photo-accelerated hydrolysis of metal organic framework ZIF-8, ACS Mater. Lett., 2021, 3, 255–260 CrossRef CAS
.
- H. Q. Zheng, Y. N. Zhang, L. F. Liu, W. Wan, P. Guo, A. M. Nystrom and X. Zou, One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery, J. Am. Chem. Soc., 2016, 138, 962–968 CrossRef CAS
.
- C. Yang, J. Xu, D. D. Yang, X. X. Wang, B. Liu, N. Y. He and Z. F. Wang, ICG@ZIF-8: One-step encapsulation of indocyanine green in ZIF-8 and use as a therapeutic nanoplatform, Chin. Chem. Lett., 2018, 29, 1421–1424 CrossRef CAS
.
- N. Liédana, A. Galve, C. Rubio, C. Téllez and J. Coronas, CAF@ZIF-8: One-step encapsulation of caffeine in MOF, ACS Appl. Mater. Interfaces, 2012, 4, 5016–5022 CrossRef PubMed
.
- W. H. Rao, Y. T. Zhan, S. L. Chen, Z. Y. Xu, T. Z. Huang, X. X. Hong, Y. L. Zheng, X. H. Pan and X. Guan, Flowerlike Mg(OH)2 cross-nanosheets for controlling Cry1AC protein loss: Evaluation of insecticidal activity and biosecurity, J. Agric. Food Chem., 2018, 66, 3651–3657 CrossRef CAS
.
- S. R. Venna, J. B. Jasinski and M. A. Carreon, Structural evolution of zeolitic imidazolate framework-8, J. Am. Chem. Soc., 2010, 132, 18030–18033 CrossRef CAS PubMed
.
- D. Luo, C. J. Wang, Y. Tong, C. Liu, Y. M. Xiao, Z. X. Zhu, D. N. Liu and Y. Y. Wang, An NIF-doped ZIF-8 hybrid membrane for continuous antimicrobial treatment, RSC Adv., 2020, 10, 7360–7367 RSC
.
- H. Ma, M. Li, T. Yu, H. Zhang, M. Xiong and F. Li, Magnetic ZIF-8-Based mimic multi-enzyme system as a colorimetric biosensor for detection of aryloxyphenoxypropionate herbicides, ACS Appl. Mater. Interfaces, 2021, 13, 44329–44338 CrossRef CAS
.
- N. Abdollahi, S. A. Akbar Razavi, A. Morsali and M.-L. Hu, High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation, J. Hazard. Mater., 2020, 387, 121667 CrossRef CAS PubMed
.
- M. Guo, J. Zhou, Y. Tian, X. Du, X. Tang, H. Lu, Y. Li, Y. Xu, Z. Yuan and Z. Qin, Synthesis, herbicidal activity against barnyard grass, and photolytic behavior of aryl 2,6-dipyrimidinoxybenzoates, J. Agric. Food Chem., 2024, 72, 300–312 CrossRef CAS PubMed
.
- Q. Zhang, Y. Zhao, S. Fan, A. Bai, X. Li and C. Pan, Dissipation and residues of bispyribac-sodium in rice and environment, Environ. Monit. Assess., 2013, 185, 9743–9749 CrossRef CAS
.
- G. Ding, X. Li, F. Zhang, J. Chen, L. Huang and X. Qiao, Mechanism-based quantitative structure-activity relationships on toxicity of selected herbicides to Chlorella vulgaris and Raphidocelis subcapitata, Bull. Environ. Contam. Toxicol., 2009, 83, 520–524 CrossRef CAS PubMed
.
- L. Zheng, C. Cao, L. Cao, Z. Chen, Q. Huang and B. Song, Bounce behavior and regulation of pesticide solution droplets on rice leaf surfaces, J. Agric. Food Chem., 2018, 66, 11560–11568 CrossRef CAS
.
- H. Qu, S. Wu and J. Gong, A sustainable and smart fungicide release platform through cocrystal nanocapsules for improved utilization rate and environmental safety, Chem. Eng. J., 2023, 473, 145284 CrossRef CAS
.
- K. Koch, K. D. Hartmann, L. Schreiber, W. Barthlott and C. Neinhuis, Influences of air humidity during the cultivation of plants on wax chemical composition, morphology and leaf surface wettability, Environ. Exp. Bot., 2006, 56, 1–9 CrossRef CAS
.
- J. J. Nairn, W. A. Forster and R. M. V. Leeuwen, Effect of solution and leaf surface polarity on droplet spread area and contact angle, Pest Manage. Sci., 2016, 72, 551–557 CrossRef CAS
.
- M. Hunsche, K. Bringe, M. Schmitz-Eiberger and G. Noga, Leaf surface characteristics of apple seedlings, bean seedlings and kohlrabi plants and their impact on the retention and rainfastness of mancozeb, Pest Manage. Sci., 2006, 62, 839–847 CrossRef CAS
.
- D. Wu, J. N. Wang, S. Z. Wu, Q. D. Chen, S. Zhao, H. Zhang, H. Sun and L. Jiang, Three-level biomimetic rice-leaf surfaces with controllable anisotropic sliding, Adv. Funct. Mater., 2011, 21, 2927–2932 CrossRef CAS
.
- M. Yu, J. Yao, J. Liang, Z. Zeng, B. Cui, X. Zhao, C. Sun, Y. Wang, G. Liu and H. Cui, Development of functionalized abamectin poly (lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention, RSC Adv., 2017, 7, 11271–11280 RSC
.
- K. Zhao, J. Hu, Y. Ma, T. Wu, Y. Gao and F. Du, Topology-regulated pesticide retention on plant leaves through concave janus carriers, ACS Sustainable Chem. Eng., 2019, 7, 13148–13156 CrossRef CAS
.
- H. Zhu and Z. Guo, Wetting characterizations of oilseed rapes, J. Bionic Eng., 2016, 13, 213–219 CrossRef
.
- L. Yang, H. Chen, S. Zhu, S. Zhao, S. Huang, D. Cheng, H. Xu and Z. Zhang, Pectin-coated iron-based metal–organic framework nanoparticles for enhanced foliar adhesion and targeted delivery of fungicides, ACS Nano, 2024, 18, 6533–6549 CrossRef CAS PubMed
.
- S. Rodrigues, A. Avellan, G. D. Bland, M. C. R. Miranda, C. Larue, M. Wagner, D. A. Moreno-Bayona, H. Castillo-Michel, G. V. Lowry and S. M. Rodrigues, Effect of a zinc phosphate shell on the uptake and translocation of foliarly applied zno nanoparticles in pepper plants (Capsicum annuum), Environ. Sci. Technol., 2024, 58, 3213–3223 CAS
.
- H. Ullah, X. Li, L. Peng, Y. Cai and H. W. Mielke, In vivo phytotoxicity, uptake, and translocation of PbS nanoparticles in maize (Zea mays L.) plants, Sci. Total Environ., 2020, 737, 139558 CrossRef CAS PubMed
.
- H. Jia, Y. Wei, H. An, Q. Wang, J. Yang and C. Li, Copper oxide nanoparticles alter the uptake and distribution of cadmium through disturbing the ordered structure of the cell wall in Arabidopsis root, Plant Physiol. Biochem., 2024, 207, 108430 CrossRef CAS PubMed
.
- P. Hu, J. An, M. M. Faulkner, H. Wu, Z. Li, X. Tian and J. P. Giraldo, Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles, ACS Nano, 2020, 14, 7970–7986 CrossRef CAS
.
- X. Pan, D. Nie, X. Guo, S. Xu, D. Zhang, F. Cao and X. Guan, Effective control of the tomato wilt pathogen using TiO2 nanoparticles as a green nanopesticide, Environ. Sci.: Nano, 2023, 10, 1441–1452 RSC
.
- W. H. Rao, D. Y. Zhang, X. Guan and X. H. Pan, Recycling of spent mushroom substrate biowaste as an anti-UV agent for Bacillus thuringiensis, Sustainable Chem. Pharm., 2022, 30, 100811 CrossRef CAS
.
- G. Huang, C. Huang, Y. Tao and H. Li, Localized heating driven selective growth of metal-organic frameworks (MOFs) in wood: A novel synthetic strategy for significantly enhancing MOF loadings in wood, Appl. Surf. Sci., 2021, 564, 150325–150333 CrossRef CAS
.
- H. Zhang, M. Zhao, Y. Yang and Y. S. Lin, Hydrolysis and condensation of ZIF-8 in water, Microporous Mesoporous Mater., 2019, 288, 109568–109575 CrossRef CAS
.
- N. Shi, T. Bai, X. Wang, Y. Tang, C. Wang and L. Zhao, Toxicological effects of WS2 nanomaterials on rice plants and associated soil microbes, Sci. Total Environ., 2022, 832, 154987 CrossRef CAS
.
- T. Ahmed, M. Noman, H. Jiang, M. Shahid, C. Ma, Z. Wu, M. M. Nazir, M. A. Ali, J. C. White, J. Chen and B. Li, Bioengineered chitosan-iron nanocomposite controls bacterial leaf blight disease by modulating plant defense response and nutritional status of rice (Oryza sativa L.), Nano Today, 2022, 45, 101547 CrossRef CAS
.
- D. Bonnes-Taourel, M.-C. Guérin and J. Torreilles, Is malonaldehyde a valuable indicator of lipid peroxidation?, Biochem. Pharmacol., 1992, 44, 985–988 CrossRef CAS PubMed
.
- L. X. Shi, J. Wu, X. R. Qiao, Y. Ha, Y. P. Li, C. Peng and R. B. Wu, In situ biomimetic mineralization on ZIF-8 for smart drug delivery, ACS Biomater. Sci. Eng., 2020, 6, 4595–4603 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: The method of the ultraviolet scanning spectra of ZIF-8, BIS, and BIS@ZIF-8, the BIS standard curve preparation, synthesis of FITC-labelled ZIF-8 and FITC-labelled BIS@ZIF-8, weeding effect after simulated rainwater scouring, weeding effect after UV irradiation, UV degradation of ZIF-8, biosafety evaluation of BIS@ZIF-8, impact of BIS@ZIF-8 on the taxonomic abundance of microbial community. Fig. S1 to S14, Tables S1 and S2. See DOI: https://doi.org/10.1039/d4en00695j |
| This journal is © The Royal Society of Chemistry 2025 |