Sustainable DMSNs nano-biopesticide platform built by a “one-pot” method focusing on injury-free drug demonstration of pine wood nematodes
Long
Chen
a,
Jiamin
Hu
a,
Haiwei
Pang
a,
Shuyan
Yin
ab,
Huixiang
Liu
ab,
Yehan
Tian
ab,
Shangkun
Gao
ab,
Chenggang
Zhou
ab,
Puxia
Wu
c,
Shuo
Miao
d,
Yingchao
Ji
 *ab,
Chaoqiong
Liang
*c and
Yanxue
Liu
*ab
*ab,
Chaoqiong
Liang
*c and
Yanxue
Liu
*ab
aCollege of Plant Protection, Shandong Agricultural University, Tai'an 271018, China. E-mail: 2535269325@qq.com; jiyc2018@sdau.edu.cn; liuyouyou2018@163.com
bShandong Forestry Pest Prevention and Control Engineering Technology Research Center, Shandong Agricultural University, Tai'an 271018, China
cShaanxi Academy of Forestry, Xi'an 710082, China. E-mail: lcq19880305@126.com
dExperimental Centre of Forestry in North China, Chinese Academy of Forestry, China
First published on 19th December 2023
Abstract
Pine wilt disease (PWD) is an infestation caused by pine wood nematodes (PWN, Bursaphelenchus xylophilus), and has caused significant disruption to forest ecosystems worldwide. Trunk injection is effective in controlling PWD, but the long-term use of abamectin (AVM) and other drugs in large quantities has caused resistance problems, and the annual trunk injections have caused some damage to the trunks themselves. In order to reduce drug resistance and damage to the tree trunks, in this study, dendritic mesoporous silica nanoparticles (DMSNs) were prepared by using a “one-pot” method, which is the easiest to industrialise, and AVM@DMSNs nano-pesticides with a uniform particle size, high loading efficiency (80.2%) and sustained release were prepared by physical adsorption. And cellular uptake and toxicity experiments were carried out on sf9 cells, and the results showed that the nano DMSNs could be enriched on sf9 cells and had good inhibition of cellular activity. The lethality of the nano-pesticides AVM@DMSNs and AVM on PWN was investigated using the insect dip method. The results showed that the corrected mortality rate of AVM@DMSNs was significantly higher than that of free AVM within 72 h. In addition, AVM@DMSNs compounded with a plant essential oil bark penetrant could be applied locally outside the bark and penetrate the drug into the tree to achieve the purpose of injury-free drug delivery, which could effectively reduce the damage of perforation injection on the tree and provide a new way for the control of forest pests.
Environmental significanceNano-pesticides are conducive to the promotion of green agricultural development, improvement of pesticide efficacy, reduction of pesticide dosage, extension of the effective period of the agent, and production and processing of environmentally friendly water instead of organic solvents, without the use of highly toxic benzene solvents and additives, which can be a fundamental solution to the problem of agricultural and forestry environmental pollution. Highly efficient, safe, low-residue “nano-pesticides” have become the mainstream of green pesticide innovation and development. Dendritic mesoporous silica nanoparticles (DMSNs) are prepared by the simplest industrial “one-pot” method, physically adsorbed and loaded with the biogenic pesticide abamectin, and using nanocarriers with a small-size effect, interfacial effect, tunneling effect, and controlled-release function, the drug can be delivered to the target point of action at regular intervals, quantitatively, or dynamically to maximise the degree of utilisation (improvement of utilisation). This provides a new platform for green and sustainable control of agricultural and forestry pests and diseases. |
1. Introduction
Pine wilt disease (PWD) is causing significant disruption to forest ecosystems globally.1 PWD is an invasive disease caused by pine wood nematodes (PWN), which are mainly transmitted by a vector of the genus Monochamus, such as M. alternatus, M. saltuarius and so on.2,3 PWN infest healthy trees through wounds when the vector insects feed on healthy trees with supplemental nutrients, destroying the thinning system of the pine tree and causing it to die out.4,5 Known as the cancer of pine trees, PWD was first discovered in 1982 at the Zhongshan Mausoleum in Nanjing, China, and as of 2023, it has spread to 19 provinces,6 causing hundreds of billions of dollars in economic losses,7 and has become a major forest disaster in China.8,9 In Japan, the total economic losses over a 10-year period from 2004 to 2014 were estimated to be $3.7 billion.10 In South Korea, the number of infected pine trees has increased dramatically since 2011, with the total losses over the past decade estimated at KRW 8.4 billion.11,12 Despite quarantine measures to prevent PWN from entering European countries, the disease has spread to Portugal and northern Spain.13 It is predicted that by 2030, PWNs could spread to 8–34% of Europe and the cumulative value of losses is expected to reach EUR 22 billion.1,14,15 As a result, PWD has become one of the most serious forest diseases in the world.16,17At present, control of PWD is mainly based on clearing dead trees, trunk injection and insect vector fly control.18 Manual clearing of dead trees requires a lot of manpower and material resources, and the economic cost is high.19 Chemical control has been widely adopted to control PWNs and vector insects because of its immediate effects.2 Trunk injection has become one of the important methods to control PWD at home and abroad because of its simple operation and good control effect.20,21 Currently, the main agents used for trunk injection to control PWN are abamectin (AVM) and emamectin benzoate,22 but trunk injection may cause blue fungal infection, and pest resistance may occur in the long term.23 Moreover, annual trunk injections can also cause some damage to the tree, which in turn can lead to other problems such as pests and diseases. With the great promotion of the zero-growth pesticide action plan, it is the need of the world development to actively explore efficient, safe and green nano pesticide technologies.24
In recent years, nanotechnology has been greatly developed and applied in agriculture and forestry.25 The combination of nanotechnology and pesticide active ingredients forms nano-pesticides, which have certain slow-release, controllability and targeting, and can greatly improve the efficiency of pesticide utilization.26,27 Polshettiwar's research group prepared dendritic mesoporous silica nanoparticles (DMSNs) in 2010, which are widely used because of their high specific surface area, large pores and other characteristics.28 Lv Haixiang et al. (2023) constructed rough surface hollow mesoporous silica (RHMS) and loaded dsRNA and imidacloprid by electrostatic action, and the toxicity of the complex was increased by 1.95-fold compared to imidacloprid. The efficacy against Aphis gossypii was increased by 19.95% within 5 days.29 Wang et al. (2023) utilized DMSNs loaded with the pesticides chlorantraniliprole and spinosad with a loading rate of 52.03%, which improved the insecticidal toxicity against Spodoptera frugiperda.30 The biopesticide AVM is currently the most widely used and fastest developing biopesticide due to destroy insects a table wide, low toxicity, low residue, and high biosafety,31 and has been used for trunk injection to control pine wood nematodes. However, due to its easy degradation and short persistence period, it needs to be used in large quantities for a long period of time for the control of PWNs, which produces a series of problems of drug resistance.32,33
In our previous study, we have reported that DMSNs loaded with abamectin have a good preventive and control effect on M. alternatus.34 In this study, we took up the previous report and continued to explore its control effect on PWN in depth. It is difficult for aqueous nanomaterials alone to directly penetrate the bark to reach the xylem. On this basis, we innovatively use the compounding of a plant essential oil bark penetrant and nano-pesticide, and adopt the new technology of localised application, which avoids the secondary damage caused to the trees and the weakening of the trees by the traditional way of injecting drugs to prevent and control the stem-boring pests. The nano compound bark penetrant will avoid to a great extent that nano in the process of direct use, due to the dispersion and aggregation of nano itself, resulting in insufficient dosage and unsatisfactory effect of dosage. Nano-pesticides still need to be developed in the control of forest and fruit pests to improve the performance of nano-drugs and the efficiency and value of drug application. Therefore, in this study, DMSNs-loaded biopesticide AVM was selected to synthesize the nano-pesticide AVM@DMSNs for the control of PWN, aiming to reduce the emergence of pest resistance through the use of new nano-pesticides, and to reduce the damage of perforation and injection on tree trunks through the spraying method of application in conjunction with the bark penetrant of essential oils, which can be used as a reference to increase the efficiency of the control and to reduce the cost of the control. DMSNs-loaded biopesticides will provide new green control strategies for pine wilt disease (an incurable disease) worldwide. The new technology will significantly reduce the economic losses caused by pine wood nematodes, revitalize the forest ecosystem and assist in building a green and sustainable ecological environment (Fig. 1).
 | ||
| Fig. 1 Schematic diagrams for the preparation of DMSNs and loading of pesticides (AVM@DMSNs), as well as mortality assessment and drug penetration and transmission through the bark. | ||
2. Materials and Methods
2.1. The materials
The reagents used for the preparation of DMSNs included: triethylamine (purchased from Tianjin Kaitong Chemical Reagent Co., Ltd.), sodium salicylate (purchased from Sinopharm Chemical Reagent Co., Ltd.), and tetraethyl silicate (purchased from Shanghai McLean Biochemistry & Science Co). The reagent used for biodistribution and bark penetration was fluorescein isothiocyanate (FITC) (supplied by Beijing Coordinator Science and Technology Co.). Toucui, a plant essential oil penetrant, was purchased from Novozymes Ltd. (Shenzhen, China). The reagent used for mortality determination was AVM supplied by Aladdin Industrial Co.The scanning electron microscope (JSM-7800F) and transmission electron microscope (HT7800) were provided by Hitachi Manufacturing Co., Ltd. The ultraviolet visible spectrophotometer (UV-2600) was purchased from Shimadzu Instruments (Suzhou) Co., Ltd. The nano laser particle size analyzer (90 Plus PALS) was provided by Brookhaven Instruments in the United States, and the BET specific surface area analyzer (ASAP2460) was provided by McMurdoch Instruments (Shanghai) Co., Ltd. Insect cell culture medium sf9 was provided by Fudan University (Shanghai, China).
2.2. Preparation of dendritic mesoporous organic silicon nanoparticles (DMSNs)
This experiment used the “one pot” method to synthesize DMSNs. 0.068 g of triethanolamine was dispersed in 25 mL of deionized water and stirred in an oil bath, then 380 mg of CTAB and 168 mg of NaSal were added and stirred for 1 h, and then 4 mL of TEOS was added to the solution. The solution was stirred continuously for 2 h, centrifuged, and washed repeatedly with anhydrous ethanol. The precipitate was collected, and 120 mL of methanol was added to the precipitate. Ultrasound dispersion was performed, followed by addition of 1.54 mL of concentrated hydrochloric acid. The above solution was stirred and heated in a water-bath for several times to wash the precipitate, and then freeze-dried. The DMSNs were obtained.2.3. Preparation of AVM@DMSNs and FTIC@DMSNs
80 mg of prepared DMSNs carriers were dissolved in 25 mL of AVM saturated solution, uniformly dispersed under ultrasonication, stirred in the dark for 48 h, and washed by centrifugation to obtain the precipitates of AVM@DMSNs. Gradient dilution solutions of AVM (48 μg mL−1, 32 μg mL−1, 16 μg mL−1, 8 μg mL−1, 4 μg mL−1) were prepared, and the UV absorption spectra of AVM methanolic solution were measured using a UV-vis spectrophotometer. The standard curve of AVM was established with the concentration of AVM solution as the X-axis and the absorbance as the Y-axis. The absorbance at different concentrations was obtained, the data were recorded, the standard curve of AVM was plotted, and the equation of the standard curve was obtained.5 mg of the prepared DMSNs carriers were dissolved in 20 mL of methanol, uniformly dispersed under ultrasonication, stirred in the dark for 4 h, and washed by centrifugation to obtain the precipitate of FITC@DMSNs.
2.4. Release performance of AVM@DMSNs
In order to explore the AVM@DMSNs slow-release performance by simulating the external environment, AVM@DMSNs was taken and dispersed into PBS buffer solutions with different pH values (pH 6.5, pH 7.4, pH 8.2), fully sonicated and homogenized, and then placed in a constant temperature oscillator for processing. The sample was tested at different times, and a certain mixture was centrifuged and washed repeatedly with methanol solution, and the absorbance of the supernatant was measured using a UV spectrophotometer, and then the absorbance was substituted into the standard curve to calculate the release concentration of AVM, and thus the release rate was calculated and the release curve was plotted.2.5. Biodistribution, cellular uptake and toxicity
FITC labeled DMSNs (FITC@DMSNs) were used to resolve the attachment of DMSNs to mycelium and nematode uptake in more detail. In this experiment, FITC-labelled DMSNs were sprayed onto the surface of mycelium by the grey grapevine spray method. First, FITC@DMSNs was sprayed on the mycelium to attach FITC@DMSNs on the surface of Botrytis cinerea mycelium. The mycelium of Botrytis cinerea was picked to observe the fluorescence distribution on the surface of the mycelium under the fluorescence microscope. The nematodes were then inoculated to observe the distribution of fluorescence on the mycelium and the distribution of DMSNs inside the body of the nematode through fungal feeding. PWN were separated from the culture medium by the Baermann funnel method,35 centrifuged for 3 min at 2000 rpm. PWN were sucked (10 μL) and placed on a glass slide, then placed under a stereoscopic optical microscope (Nikon, SMZ 25) to observe the fluorescence distribution on PWN.The distribution of FITC@DMSNs on the surface of sf9 cells was investigated using sf9 cells as a model cell line. First, treated sf9 cells were inoculated into 24-well plates at 5 × 104 cell wells and cultured in medium at 28 °C for 24 h. After that sf9 cells were incubated in fresh culture medium with FITC@DMSNs (10 μg mL−1) for 4 h. Then, the cells were gently rinsed with DPBS, resuspended, and fixed in 4% paraformaldehyde for 15 min. Then, the DAPI solution was added to the cells after treatment for 5 min, washed three times with DPBS, and stored at 4 °C. The fluorescence distribution on the surface of sf9 cells at 488 nm was observed by confocal microscopy to analyze the ability of nanoparticle DMSNs to capture cells.
In this study, the toxicity of DMSNs, AVM and AVM@DMSNs on cells was detected by CCK8 method. sf9 cells were prepared into cell suspensions and placed in 96-well plates and incubated at 28 °C. sf9 cell suspensions were added into DMSNs, AVM, and AVM@DMSNs solutions with different concentrations (6.67, 8, 10, 13.33, 20, 40, 100, and 200 μg mL−1), and were incubated for 24 h and 48 h, respectively. Each treatment was repeated three times. 20 μL of CCK-8 solution was added into 96-well plates and incubated at 28 °C protected from light for 1 h. Cell viability was measured and calculated.
2.6. Absorption transmission performance evaluation
The practical value of AVM@DMSNs was used to control PWN. Therefore, their uptake and diffusion within the tree were also investigated. The field transmission experiment site is located in Mount Taishan Forest Area, Tai'an, Shandong Province (117.06° E, 36.23° N). All healthy Pinus thunbergii with a diameter of approximately 8 cm and good growth were used.FITC labeled DMSNs (FITC@DMSNs) were used to observe their diffusion in pine trees. Two application methods, hole punching injection and trunk spraying, were used for the experiments. Holes (0.5 cm in diameter) were drilled at an angle of 45° to the base of the pine trunk and 30 cm from the ground. FITC@DMSNs were injected into the trunks. After different treatment times, pine trees were cut into disks. The diffusion distribution of FITC@DMSNs on the tree was observed with a small animal imager. Plant essential oil bark penetrant was diluted with deionised water and mixed with prepared nanocarriers, which were sprayed on the surface of the trunk to penetrate into the inner trunk for transmission. The original solution of plant essential oil penetrant was diluted 30 times and mixed with FITC@DMSNs. The mixed solution was sprayed on the surface of the bark of pine tree segments. After being kept in the dark for a period of time, the fluorescence distribution was observed with a small animal imager.
2.7. Determination of the mortality rate of nano pesticides
The test sample Bursaphelenchus xylophilus was provided by the Laboratory of Forest Pathogens and Host Molecular Interaction at Shandong Agricultural University.Under laboratory conditions, Botrytis cinerea was inoculated into a culture dish containing potato glucose agar (PDA) medium and inoculated at 25 °C for 7 days, under 8 hours of light per day. After B. cinerea fills the culture dish, B. xylophilus was inoculated onto B. cinerea and placed it in the dark at 25 °C.
After B. cinerea was eaten in the culture dish, the PWN growing on the mold was separated by the Baermann method, centrifuged at 2000 rpm for 3 minutes, and repeatedly washed with sterile water to remove impurities, and the PWN concentration has been adjusted to 1000 nematodes per mL for future use. The mortality of nano pesticides and AVM to pine wood nematodes was studied using an impregnation method.
On the basis of preliminary experiments, the nematicidal activity of AVM and AVM@DMSNs was determined by an impregnation method. AVM, DMSNs and AVM@DMSNs were dissolved in water and the same concentration of test solution (6.25 μg mL−1) was configured. Each time, 100 μL of pine wood nematode suspension (about 100 individuals) was added to the 96-well plate, and then the agent to be tested was added. The 96-well plates were placed in the incubator for continuous dark treatment and the mortality rate was calculated at 12 h, 24 h and 48 h of treatment, respectively. Nematodes that continuously swing or have bodies in an “S”, curly, wavy, or spiral shape are classified as live nematodes; nematodes that do not move have a “J” or “C” shaped body, or if the body is stiff, it is considered a dead nematode.36 After centrifuging and shaking the eluting solution of pine wood nematodes, insect needles were used to detect nematodes under a microscope to avoid false death.
2.8. Statistical Analysis
The data in the table are mean ± SD of the three replicate; different letters indicate significant differences (ANOVA, p < 0.05).This study used IBM SPSS Statistical 25 software for analysis of variance to calculate the mortality rate and correct mortality rate.37 Origin 2021 software was used for graphic rendering.
3. Results and Discussion
3.1. Characterization of DMSNs and AVM @ DMSNs
In this study, DMSNs were prepared by the “one-pot” method and AVM@ DMSNs nano-pesticides were prepared by a physical adsorption method. From the TEM images (A and B) and SEM images (C and D) in Fig. 2, it can be seen that the prepared DMSNs have a particle size of 200–300 nm and are spheres with an internal dendritic structure. | ||
| Fig. 2 TEM image of DMSNs (A) and the enlarged image (B); SEM image of DMSNs (C) and the enlarged image (D). | ||
The XRD images of DMSNs and AVM@DMSNs showed (Fig. 3A) that the 2θ of DMSNs and AVM@DMSNs were between 20° and 30°, and the central feature was around 22.5°, indicating that the synthesized DMSNs were amorphous SiO2, and the morphology and structure did not change after loading AVM, indicating good stability. The adsorption–desorption isotherms of DMSNs on N2 (Fig. 3B), showing the N2 adsorption–desorption isotherms and pore size distribution curves of DMSNs, indicated that the adsorption type of DMSNs was type IV and the hysteresis loop was type H3. Meanwhile, the presence of the hysteresis loop indicated that the prepared DMSNs had a mesoporous structure. When the isotherm curve was close to 1, there was no plateau phenomenon, indicating the presence of microporous structure in addition to mesoporous structure in the DMSNs. The internal plot of BJH showed that the pore size of the material was concentrated between 20 and 30. After the calculation of BET and BJH methods, the average mesopore size of DMSNs was 18.05 nm, the specific surface area was 648.078 m2 g−1, and the pore volume was 0.195 cm3 g−1. Based on the above results, it indicated that DMSNs have larger specific surface area and pore volume and can be loaded with more AVM.
The FTIR images of DMSNs, AVM, and AVM@DMSNs are shown in curves a, b, and c in Fig. 3C. From curve a in the figure, it can be seen that the asymmetric stretching vibration peak of Si–O–Si appeared at 1099.71 cm−1. On the other hand, curve b, showed the FTIR spectrum of AVM at the absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the stretching vibration peak of C–H at 2977.55. By comparing the three curves a, b, and c, it can be found that the FTIR spectra of DMSN loaded with AVM not only have the characteristic peaks of DMSN, but also have the characteristic absorption peaks of AVM. The infrared spectra of other bands were basically the same as those of DMSNs without drug loading, indicated that the loading process of AVM on DMSNs was a physical adsorption process without any chemical reactions or new substances. This result showed that DMSNs had good stability and does not react when loaded with AVM.
O and the stretching vibration peak of C–H at 2977.55. By comparing the three curves a, b, and c, it can be found that the FTIR spectra of DMSN loaded with AVM not only have the characteristic peaks of DMSN, but also have the characteristic absorption peaks of AVM. The infrared spectra of other bands were basically the same as those of DMSNs without drug loading, indicated that the loading process of AVM on DMSNs was a physical adsorption process without any chemical reactions or new substances. This result showed that DMSNs had good stability and does not react when loaded with AVM.
3.2. Drug loading rate of DMSNs
The UV absorption curve (Fig. 3D) was measured by preparing a methanolic solution of AVM in gradient concentration and a standard curve of AVM was established (Fig. 3E). The fitted equation was y = 0.0194x −0.0049, R2 = 0.9997, and the mass of the DMSNs being loaded with AVM was calculated using the above equation, and the drug loading was calculated to be 80.2%.3.3. Release performance of AVM@DMSNs-NPs at different pH levels
In this experiment, phosphate buffers of different pH values (pH 6.5, 7.4, 8.2) were selected to simulate the slow and controlled release of drugs in common agroforestry environments. AVM@DMSNs were dissolved in the buffer solutions, and the slow-release solutions were removed at different time points and the release rates were calculated using UV spectrophotometer. The experiment was stopped when the cumulative release rate reached 50% or more. The release was stable at different pH values, and the release trends were similar at all three pH values (Fig. 3F). The cumulative release increased with time and the release rate was relatively slow. At day 14, more than half of the drug was released.3.4. Trunk transmission distribution
Fluorescein isothiocyanate (FITC) shows green fluorescence in the blue spectrum. Fluorescence intensity represented in red. FITC is used to label DMSNs that can be used to simulate transport within trees. The cross-sectional fluorescence images of the trunk at 1 h, 3 h, 5 h and 7 h after injection (Fig. 4A) and the original images (Fig. 4B) are shown. As the time of drug on the tree increased, the fluorescence in the pith began to accumulate, and the fluorescence at the perforations also accumulated significantly. This suggests that FITC@DMSNs can be transported within the black pine tree and that DMSNs contribute to AVM transport within the tree. Fig. 5A and B show the fluorescence images and the original images after using the essential oil penetrant of the plant. And after using the bark penetrant, FITC@DMSNs could reach the xylem through the bark and be transported within the xylem. In addition, it can be shown that the combined use of AVM@DMSNs and bark penetrant can achieve the effect of applying the drug on the surface of bark and transporting it within the tree, providing a new method to solve the problem of damage to trees caused by traditional perforation injection.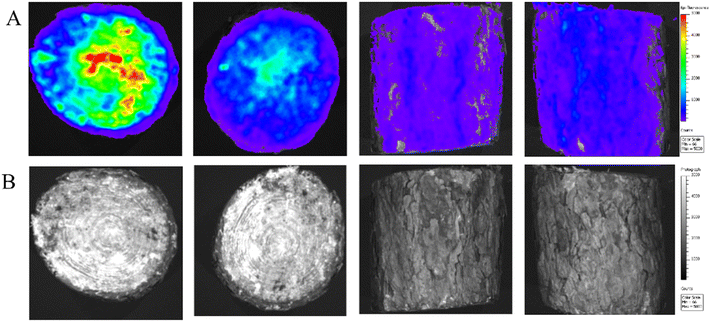 | ||
| Fig. 5 Fluorescence images (A) and original images (B) of the tree bark sprayed with FITC@DMSNs and the plant essential oil penetrating agent. | ||
3.5. Biodistribution, cellular uptake and mortality rate
Distribution of nano-pesticides in PWN was observed using FITC labeled DMSNs (FITC@DMSNs). The observed distribution of fluorescence into the pine wood nematode is shown in Fig. 6A and B. Fig. 6C shows the fluorescence image attached to the Botrytis cinerea mycelium of the culture medium. Fig. 6D and E show the original images of Botrytis cinerea mycelium and PWN under the microscope, respectively. The fluorescence distribution graph showed that the mycelium was labeled with fluorescence and the nematodes were also fluorescent. It is possible that the DMSNs are carried to the interior of the nematode through feeding by the nematode, and it is certainly possible that they enter the interior of the worm through contact with the nematode by the mycelium. In conclusion, we investigated the infiltration of FITC@DMSNs into DMSNs by tracking mycelia and nematodes by the reported methods to provide experimental support for AVM@DMSNs efficacy experiments. | ||
| Fig. 6 The fluorescence images (A and B) and original images (D and E) of a pine wood nematode, respectively. Fluorescence image (C) and original image (F) of grey mould. | ||
To evaluate the ability of the nano-bio-pesticide AVM@DMSNs to internalize in the cells, sf9 cells were incubated with FITC@DMSNs for 4 hours. The fluorescence distribution of FITC (green, Fig. 7B) and DAPI (blue, Fig. 7A) was observed by confocal microscopy after application. Based on the intensity of the fluorescence distribution in Fig. 7C, it can be seen that a large amount of FITC@DMSNs was adsorbed on the surface of the cell membranes of the DAPI-labeled cells, and the fluorescence uptake was enhanced, which demonstrated that the DMSNs could be efficiently delivered to the surface of sf9 cells.
The cytotoxicity characteristics of AVM@DMSNs on sf9 cells were evaluated by CCK-8 and compared with AVM prodrugs and DMSNs (Fig. 7D and E). The cell survival rate of sf9 cells in the AVM@DMSNs treated group was significantly lower than that of the AVM prodrugs group at the same concentration for the same treatment duration. This indicated that AVM@DMSNs were more toxic to the cells than the AVM prodrugs, which provided a basis for pesticide nanosizing to achieve reduction and efficiency.sf9 cells at the same concentration of AVM@DMSNs solution showed a decrease in the cell survival rate with the increase in the treatment time. This indicates that there is a continuous toxicity of AVM@DMSNs to the cells. Based on these data, it was also revealed that DMSNs are almost non-toxic to sf9 cells at lower concentrations, which provides an opportunity for safe diffusion of DMSNs into the cells.
3.6. Pharmacodynamic evaluation
In this experiment, the in vitro toxicity of AVM@DMSNs against pine nematodes was determined. The prepared AVM@DMSNs were compared with AVM raw materials and DMSNs was used as a blank control. The results were shown in Table 1. The corrected mortality of B. xylophilus by DMSNs at 12 h, 24 h and 48 h was 7.23%, 11.9% and 14.1%, respectively. Based on data analysis and comparison with the toxicity of AVM feedstock (99%), the corrected mortality rates of pine wood nematodes treated with the two pesticides and DMSNs (experimental control) at a specific 48 h were analyzed for significant differences and the statistics are presented in Table 1. The nematicidal activity of AVM@DMSNs was superior to that of AVM feedstock. In the initial stage, the release rate was slow, and with the extension of time, more AVM were gradually released from the AVM@DMSNs nanoparticles, which further enhanced the nematicidal effect and improved the efficiency of pine wood nematode control.| Time | 12 h | 24 h | 48 h |
|---|---|---|---|
| Note: lowercase letters indicate significant differences in corrected mortality rates among different formulations at the same time and concentration (P < 0.05). | |||
| AVM@DMSNs | 0.5670 ± 0.0374a | 0.8211 ± 0.0164a | 0.8824 ± 0.0197a |
| AVM | 0.3758 ± 0.0701b | 0.6383 ± 0.0726b | 0.6416 ± 0.0381b |
| DMSNs | 0.0723 ± 0.00324c | 0.119 ± 0.003c | 0.141 ± 0.0119c |
4. Conclusion
In this study, DMSNs were synthesised by a one-pot method with uniform size of about 200 nm, and up to 80.2% of AVM@DMSNs nano-pesticides were prepared by physical adsorption using AVM as a model drug with high loading efficiency and simple operation. Moreover, it also has a certain slow-release performance, which can achieve the effect of long-lasting medication. According to the comparison of the mortality of AVM and AVM@DMSNs against PWN, DMSNs increased the utilisation rate of the active ingredients of AVM, improved the control effect and reduced the dosage. This provides an improved technology for the application of AVM to better utilise its broad-spectrum and efficient effects.In addition, the combination of AVM@DMSNs and a plant essential oil bark penetrant provides an innovative non-invasive drug delivery pathway for the control of pine wood nematodes and related wood-boring pests. In traditional control methods, drugs are often directly injected into the interior of trees through perforation injection, which carries certain risks and causes damage. However, the combination of AVM@DMSNs and the plant essential oil bark penetrant enhances the penetration capability of AVM@DMSNs, allowing for localized drug application. Through the penetrating effect, the drug is delivered to the interior of the trees, achieving the desired control effect. Compared to traditional perforation injection methods, this non-invasive drug delivery approach is safer and more reliable, minimizing damage to the trees. This innovative drug delivery method provides new ideas and approaches for research and application in the field of plant protection.
This study shows that with this approach we can progressively advance the research on the control of stem-boring pests, and we will continue to explore green and sustainable auxiliary agents to achieve the reduction and efficiency of nano-pesticides.
Author contributions
YJ, CL, and YL contributed to study design. SY, HL, YT, SG and CZ supervised the experiments. LC, JH, HP, PW and SM contributed to data acquisition. LC contributed to original writing. All authors read and approved the final manuscript.Conflicts of interest
All authors declare that they have no competing interests.Acknowledgements
This work was supported by the Natural Science Basic Research Program of Shaanxi (2022JQ-212), the Philosophy and Forestry Science and Technology Innovation Project of Shaanxi Province (SXLK2023-0302), the Social Science Research Project of Shaanxi Province (2022HZ1758), and the Project of Mount Taishan Scenic Area (2022TSGS001).References
- W. Gan, X. B. Kong, J. X. Fang, X. Shi, S. F. Zhang, Y. X. Li, L. J. Qu, F. Liu, Z. Zhang, F. B. Zhang and X. Y. Zhang, A pH-responsive fluorescent nanopesticide for selective delivery and visualization in pine wood nematode control, Chem. Eng. J., 2023, 463, 142353 CrossRef CAS
.
- B. H. Lee, J. O. Yang, S. Beckett and Y. L. Ren, Preliminary trials of the ethanedinitrile fumigation of logs for eradication of Bursaphelenchus xylophilus and its vector insect Monochamus alternatus, Pest Manage. Sci., 2017, 73, 1446–1452 CrossRef CAS
.
- F. M. Shi, S. X. Ge, Z. H. Hou, Y. B. Xu, J. Tao, H. Wu and S. X. Zong, Species-specific primers for rapid detection of Monochamus saltuarius, an effective vector of Bursaphelenchus xylophilus in China, J. Appl. Entomol., 2022, 146, 636–647 CrossRef CAS
.
- J. L. Feng, R. G. Li, C. Wang, H. Yang, W. J. Deng, G. C. Du and Q. Q. Guo, Nematicidal phytochemicals against pine wood nematode, Bursaphelenchus xylophilus (nematoda: aphelenchoididae), J. Plant Dis. Prot., 2022, 130, 215–223 CrossRef
.
- K. Togashi, S. Akbulut, K. Matsunaga, H. Sugimoto and K. Yanagisawa, Transmission of Bursaphelenchus xylophilus between Monochamus alternatus and Monochamus saltuarius through interspecific mating behaviour, J. Appl. Entomol., 2019, 143, 483–486 CrossRef
.
- National Forestry and Grassland Administration of the People's Republic of China, Announcement of National Forestry and Grassland Administration of the People's Republic of China. (No. 7 of 2023).
- X. Hao, J. Chen, Y. X. Li, X. F. Liu, Y. Li, B. W. Wang, J. X. Cao, Y. R. Gu, W. Ma and L. Ma, Molecular Defense Response of Bursaphelenchus xylophilus to the Nematophagous Fungus Arthrobotrys robusta, Cell, 2023, 12, 543 CrossRef CAS
.
- F. Wang, Q. L. Chen, R. Z. Zhang, D. L. Li, Y. M. Ling and R. Q. Song, The anti-phytoalexin gene Bx-cathepsin W supports the survival of Bursaphelenchus xylophilus under Pinus massoniana phytoalexin stress, BMC Genomics, 2019, 20, 779 CrossRef PubMed
.
- Z. X. Li, J. Tao and S. X. Zong, Cold Tolerance in Pinewood Nematode Bursaphelenchus xylophilus Promoted Multiple Invasion Events in Mid-Temperate Zone of China, Forests, 2022, 13, 1100 CrossRef
.
- A. Hirata, K. Nakamura, K. Nakao, Y. Kominami, N. Tanaka, H. Ohashi, K. T. Takano, W. Takeuchi and T. Matsui, Potential distribution of pine wilt disease under future climate change scenarios, PLoS One, 2017, 12, e0182837 CrossRef
.
- B.-N. Kim, J. H. Kim, J.-Y. Ahn, S. Kim, B.-K. Cho, Y.-H. Kim and J. Min, A short review of the pinewood nematode, Bursaphelenchus xylophilus, J. Toxicol. Environ. Health Sci., 2020, 12, 297–304 CrossRef
.
- T.-S. Kwon, J. H. Shin, J.-H. Lim, Y.-K. Kim and E. J. Lee, Management of pine wilt disease in Korea through preventative silvicultural control, For. Ecol. Manage., 2011, 261, 562–569 CrossRef
.
- C. Robinet, A. Roques, H. Pan, G. Fang, J. Ye, Y. Zhang and J. Sun, Role of Human-Mediated Dispersal in the Spread of the Pinewood Nematode in China, PLoS One, 2009, 4, e4646 CrossRef PubMed
.
- S. Tarek, M. C. M. Mourits, V. D. W. Wopke, G. M. Hengeveld, R. Christelle and A. G. J. M. Lansink, Framework for Modelling Economic Impacts of Invasive Species, Applied to Pine Wood Nematode in Europe, PLoS One, 2012, 7, e45505 CrossRef
.
- S. Matsuhashi, A. Hirata, M. Akiba, K. Nakamura and T. Matsui, Developing a point process model for ecological risk assessment of pine wilt disease at multiple scales, For. Ecol. Manage., 2020, 463, 118010 CrossRef
.
-
C. Vicente, M. Espada, P. Vieira and M. Mota, Pine Wilt Disease: a threat to European forestry, Springer Netherlands, 2012, vol. 133, pp. 89–99 Search PubMed
.
-
A. Roques, L. Zhao, J. Sun and C. Robinet, Pine wood nematode, pine wilt disease, vector beetle and pine tree: How a multiplayer system could reply to climate change, Climate change and insect pests, 2015, vol. 12, pp. 220–234 Search PubMed
.
- O. E. Douda, M. Zouhar, M. Maňasová, M. Dlouhy, J. Lišková and P. Ryšánek, Hydrogen cyanide for treating wood against pine wood nematode (Bursaphelenchus xylophilus): results of a model study, J. Wood Sci., 2015, 61, 204–210 CrossRef CAS
.
- G. Y. Liu, X. Lin, S. Y. Xu, G. Liu, Z. Y. Liu, F. Liu and W. Mu, Efficacy of fluopyram as a candidate trunk-injection agent against Bursaphelenchus xylophilus, Eur. J. Plant Pathol., 2020, 157, 403–411 CrossRef CAS
.
- K. Takai, T. Soejima, T. Suzuki and K. Kawazu, Development of a water-soluble preparation of emamectin benzoate and its preventative effect against the wilting of pot-grown pine trees inoculated with the pine wood nematode, Bursaphelenchus xylophilus, Pest Manage. Sci., 2001, 57, 463–466 CrossRef CAS
.
- J. F. Byrne, I. R. Krieger, G. J. Morse and J. Doccola, Seasonal timing of neonicotinoid and organophosphate trunk injections to optimize the management of avocado thrips in California avocado groves, Crop Prot., 2014, 57, 20–26 CrossRef
.
- H. Y. Cui, H. Jin, Q. Liu, Z. Q. Yan, L. Ding and B. Qin, Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus, Pest Manage. Sci., 2014, 70, 827–835 CrossRef CAS
.
- S. M. Lee, D. S. Kim, S. G. Lee, N. C. Park and D. W. Lee, Selection of Trunk Injection Pesticides for Preventive of Pine Wilt Disease, Bursaphelenchus xylophilus on Japanese Black Pine (Pinus thunbergii). Korean, J. Pestic. Sci., 2009, 13 CAS
.
- D. J. Chitwood, Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service, Pest Manage. Sci., 2010, 59, 748–753 CrossRef
.
- Y. Wang, C. J. Sun, X. Zhao, B. Cui, Z. H. Zeng, A. Q. Wang, G. Q. Liu and H. X. Cui, The Application of Nano-TiO2 Photo Semiconductors in Agriculture, Nanoscale Res. Lett., 2016, 11, 529 CrossRef PubMed
.
- L. Cao, H. Zhang, C. Cao, J. Zhang and Q. Huang, Quaternized Chitosan-Capped Mesoporous Silica Nanoparticles as Nanocarriers for Controlled Pesticide Release, Nanomaterials, 2016, 6, 126 CrossRef PubMed
.
- Y. Sun, J. Liang, L. Tang, H. Li, Y. Zhu, D. N. Jiang, B. Song, M. Chen and G. M. Zeng, Nano-pesticides: A great challenge for biodiversity?, Nano Today, 2019, 28, (100757)1-4 CrossRef
.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology, Angew. Chem., Int. Ed., 2010, 49, 9652–9656 CrossRef CAS
.
- H. X. Lv, X. C. Li, J. Q. Li, C. Yu, Q. H. Zeng, G. G. Ning, H. Wan, J. H. Li, K. S. Ma and S. He, Overcoming resistance in insect pest with a nanoparticle-mediated dsRNA and insecticide co-delivery system, Chem. Eng. J., 2023, 475, 146239 CrossRef CAS
.
- K. Wang, Y. Wang, Y. Y. Wu, J. J. Jiang, Y. X. Zhang, N. Yu and Z. W. Liu, A novel dual stimuli-responsive and double-loaded insecticidal nanoformulation for efficient control of insect pest, Chem. Eng. J., 2023, 474, 146012 CrossRef CAS
.
- K. Hanif, M. Zubair, D. Hussain, S. Ali, M. Saleem, H. A. A. Khan, T. Nazir and M. W. ul Hassan, Biopesticides and insect pest management. International Journal of Tropical Insect, Science, 2022, 42, 3631–3637 Search PubMed
.
- H. Zhang, H. Qin, L. X. Li, X. T. Zhou, W. Wang and C. Y. Kan, Preparation and Characterization of Controlled-Release Avermectin/Castor Oil-Based Polyurethane Nanoemulsions, J. Agric. Food Chem., 2018, 66, 6552–6560 CrossRef CAS
.
- S. Wyche and C. Steinfield, Why Don't Farmers Use Cell Phones to Access Market Prices? Technology Affordances and Barriers to Market Information Services Adoption in Rural Kenya, Inf. Technol. Dev., 2016, 22, 320–333 CrossRef
.
- G. H. Wang, X. Y. Xu, Q. Q. Cheng, J. M. Hu, X. Y. Xu, Y. W. Zhang, S. Guo, Y. C. Ji, C. G. Zhou, F. Gao, L. Yang, Y. X. Liu, S. Y. Yin and C. Y. Su, Preparation of sustainable release mesoporous silica nano-pesticide for control of Monochamus alternatus, Sustainable Mater. Technol., 2023, 35, e00538 CrossRef CAS
.
- J. R. Christie and V. G. Perry, Removing nematodes from soil, Proc. Helminthol. Soc. Wash., 1951, 18, 106–108 Search PubMed
.
- W. J. Zhang, X. Q. Wu, J. R. Ye, C. Q. Li, L. J. Hu, L. Rui, Y. Zhang, X. F. Shi and L. Wang, Toxicity of an Emamectin Benzoate Microemulsion against Bursaphelenchus xylophilus and Its Effect on the Prevention of Pine Wilt Disease, Forests, 2023, 14, 1476 CrossRef
.
- Q. Q. Guo, G. C. Du, H. T. Qi, Y. A. Zhang, T. Q. Yue, J. C. Wang and R. G. Li, A nematicidal tannin from Punica granatum L. rind and its physiological effect on pine wood nematode (Bursaphelenchus xylophilus), Pestic. Biochem. Physiol., 2017, 135, 64–68 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |





