 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Safety concerns in solid-state lithium batteries: from materials to devices
Yang
Luo†
ab,
Zhonghao
Rao†
a,
Xiaofei
Yang
 *bd,
Changhong
Wang
c,
Xueliang
Sun
*bd,
Changhong
Wang
c,
Xueliang
Sun
 *c and
Xianfeng
Li
*c and
Xianfeng
Li
 *bd
*bd
aSchool of Energy and Environmental Engineering, Hebei University of Technology, Tianjin, 300401, China
bDalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: yangxf@dicp.ac.cn; lixianfeng@dicp.ac.cn
cDepartment of Mechanical and Materials Engineering, University of Western Ontario, London, Ontario N6A 5B9, Canada. E-mail: xsun9@uwo.ca
dLaboratory of Advanced Spectro-Electrochemistry and Lithium-Ion Batteries, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 22nd August 2024
Abstract
Solid-state lithium-metal batteries (SSLMBs) with high energy density and improved safety have been widely considered as ideal next-generation energy storage devices for long-range electric vehicles. Nevertheless, the potential safety issues in SSLMBs during solid-state electrolyte synthesis, battery operation and battery post-processing have been often ignored, which presents difficulties for their practical application. This review primarily evaluates the safety concerns in SSLMBs, especially thermal runaway and hazardous product release induced by the undesirable chemical/thermal/interfacial dynamic stability of the electrode and electrolyte materials. Subsequently, the recent advancements in addressing safety concerns by relying on electrolyte innovation and interface regulation as well as engineering SSLMB design are summarized, and future directions are speculated upon. The purpose of this review is to encourage researchers to devote more efforts to this field and pave the way for practical applications of SSLMBs.
Broader contextEnsuring both safety and high energy density is crucial for advancing energy storage technologies. Notably, the energy density of existing lithium-ion batteries is approaching its theoretical limit, and hence there is an urgent need to develop novel battery systems. In addition, flammable organic liquid electrolytes and their gaseous derivatives pose serious safety risks for batteries. Among various battery systems, solid-state Li metal batteries (SSLMBs) have emerged as promising candidates owing to their safety. Despite extensive research focused on enhancing ionic conductivity and optimizing electrode/electrolyte interfaces, recent studies have revealed unexpected safety issues, including thermal runaway events even in seemingly stable oxide-based SSLMB systems. Addressing these concerns is imperative for the continued progress of SSLMB technology. Herein, this review will comprehensively summarize the safety challenges of SSLMBs including the intrinsic stability of SSEs and the interfacial stability, as well as gas release. Corresponding strategies will be proposed via material design, battery operation, post-processing, etc., to guide the future development and application of high-safety SSLMBs. |
1. Introduction
As electric vehicles (EVs) rapidly develop, frequent safety incidents caused by thermal runaway and the demand for long ranges drive the development of the next generation batteries with high safety and high energy density. To date, conventional lithium-ion batteries (LIBs) hardly satisfy the above requirements due to their tricky safety concerns and limited energy density (<300 W h kg−1).1,2 Li metal batteries (LMBs) using the Li metal anode with high theoretical capacity (3860 mA h g−1) and the lowest electrochemical potential (−3.04 V vs. standard hydrogen electrode) have attracted growing interest due to their higher energy density compared to state-of-the-art LIBs.3 Nevertheless, the safety issues of LMBs including short circuits and thermal runaway still should be addressed.Generally, the dendritic Li piercing the separator is the primary cause of short circuits. Furthermore, flammable organic liquid electrolytes and their gaseous derivatives are regarded as being responsible for violent burning or jet combustion.4 Compared with liquid electrolytes, solid-state electrolytes (SSEs) possess obvious superiorities in many aspects, including (i) non-volatile nature and higher thermal stability, (ii) excellent mechanical strength to inhibit dendrite growth, and (iii) wider electrochemical stability windows and low reactivity, which allows for superior compatibility with electrodes. Given the above virtues, SSEs offer a promising route for the implementation of Li anodes to achieve higher energy density and safer SSLMBs, making SSLMBs one of the most promising types of next-generation batteries.5–7
In the last few years, most of the efforts have been devoted to exploring high ionic conductors and solving the mismatched electrode/electrolyte interfaces, and eye-catching progress has been made. For instance, both sulfide and halide SSEs have achieved high ionic conductivities of over 10−2 S cm−1 that are comparable to those of liquid electrolytes.8–11 More importantly, relying on structural innovation and interface decoration, the assembled SSLMBs have demonstrated an ultralong lifespan of over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.12,13 It seems that the practically accessible SSLMBs are near at hand. Nevertheless, very recently, some noteworthy exceptions contradicting the conventional “safe SSLMBs” concept have been reported. The thermal runaway was detected, even in highly stable oxide-based SSLMB systems.14 Moreover, recent reports showed that solid polymer electrolytes (SPEs) and sulfide SSEs are also likely to react exothermically.15–17 On grinding the sulfide SSEs (e.g. Li3PS4, Li7P3S11) with Li or lithiated Si anodes, even in an inert atmosphere, an obvious combustion phenomenon was observed. When oxidation-state cathode materials were involved, the combustion reaction was more severe.18 In other words, the risks of fire and explosion potentially exist in SSLMBs. These findings provoked us to think about whether SSLMBs are intrinsically safe.14 Systematically pointing out the safety concerns and providing potential solutions are of significance to pave the way for practical application of SSLMBs.
000 cycles.12,13 It seems that the practically accessible SSLMBs are near at hand. Nevertheless, very recently, some noteworthy exceptions contradicting the conventional “safe SSLMBs” concept have been reported. The thermal runaway was detected, even in highly stable oxide-based SSLMB systems.14 Moreover, recent reports showed that solid polymer electrolytes (SPEs) and sulfide SSEs are also likely to react exothermically.15–17 On grinding the sulfide SSEs (e.g. Li3PS4, Li7P3S11) with Li or lithiated Si anodes, even in an inert atmosphere, an obvious combustion phenomenon was observed. When oxidation-state cathode materials were involved, the combustion reaction was more severe.18 In other words, the risks of fire and explosion potentially exist in SSLMBs. These findings provoked us to think about whether SSLMBs are intrinsically safe.14 Systematically pointing out the safety concerns and providing potential solutions are of significance to pave the way for practical application of SSLMBs.
In this review, as shown in Fig. 1, the safety concerns involved in material synthesis, battery operation and battery failure in SSLMBs will be first summarized. Furthermore, the unsafe factors including thermal runaway and hazardous product release induced by unstable SSEs, undesirable interfacial reactions, mismatched interface contact as well as uncontrollable Li dendrite growth will be deeply analyzed. Following that, potential solutions, mainly focused on electrolyte innovation and interfacial engineering design, will be discussed to improve the safety of SSLMBs. Then, advanced characterization techniques and theoretical calculations are systematically reviewed to create a toolbox to understand battery failure mechanisms in terms of their heat evolution, dendrite growth, and gas emission. Finally, the rational design of practically accessible SSLMBs from the viewpoint of engineering (e.g., building battery safety management systems and safe battery post-processing routes) will be discussed and the future directions will be speculated upon.
2. Safety challenges of SSLMBs
Conventional liquid electrolytes show poor safety performance owing to their flammability and reactivity. To address the safety concerns, SSLMBs using SSEs, especially inorganic solid electrolytes, are developed due to the theoretical nonflammability of SSEs. Nevertheless, recent studies have found that even solid-state lithium batteries suffer from severe exothermic reactions, which seriously affect battery safety.Generally speaking, attention should be paid to the following processes, which seriously affect the safety of SSLMBs. (1) Intrinsic thermal/chemical stability of materials during preparation. (2) Interfacial dynamics processes during battery operation, including interfacial reactions and their products, as well as dendrite growth issues. Special attention should be paid to the heat accumulation and hazard release caused by the ohmic heat and internal reactions of cells during cycling. With this in mind, the intrinsic stability of SSEs and their compatibility with electrodes are particularly important. (3) The overlooked potential safety hazard of failed SSLMBs, i.e. the handling of harmful gases and the subsequent reaction of ruptured batteries should also be emphasized. In the following sections, we will evaluate the safety of SSLMBs by taking the intrinsic stability of SSEs and the interfacial stability as well as gas release into consideration.
2.1. Intrinsic instability of SSEs
The electrochemical performance and safety of SSLMBs are highly dependent on the intrinsic stability of SSEs. Notably, inorganic solid electrolytes such as sulfides and halides are reported to be sensitive to humidity, while SPEs exhibit poorer thermal stability compared with inorganic solid electrolytes. The intrinsic instability of SSEs would cause decomposition of SSEs as well as hazard release, limiting the safety and electrochemical performance improvement. Thus, the chemical and thermal stability of SSEs will be evaluated below. | ||
| Fig. 2 (a)–(c) Factors affecting the thermodynamic stability of SSEs. (d) Chemical reaction mechanisms of several typical SSEs.19,26,27 (e) The upper limit temperature of thermal stability of different SSEs. Reproduced with permission from ref. 28. (f) Thermal conductivity of different SSEs. Reproduced with permission from ref. 29. | ||
In summary, almost all inorganic solid electrolytes are unstable in moist air. In terms of SSE reactivity and their decomposition products, the chemical stability of SSEs may be in the following order: oxides > halides > sulfides > hydrides. By-products such as H2S, H2, NH3 and HX (X = F, Cl, Br, I) when inorganic solid electrolytes are exposed to humid air will induce unsafe factors during large-scale production. Thus, appropriate synthesis approaches and stabilization strategies for SSEs as well as specific handling conditions should be considered during the synthesis process.
For SPEs, the thermal stability of the polymer matrix is mainly affected by the chemical structure of the polymer monomer, degree of polymerization, length of the polymerization segment, and degree of crystallization. Taking polyethylene oxide (PEO) as an example, polymer chain segments may break and form small molecular compounds or gaseous products when reacted with the Li anode or under elevated temperature. Therefore, designing stable chain segment structures is beneficial for improving the chemical/thermal stability of SPEs. Apart from the polymer matrix, Li salts and additives are the main components of SPEs, which also directly affect the overall thermal stability of SPEs from the perspectives of their decomposition temperature and interactions with polymers.31 Abels et al. investigated the effect of several commonly used Li salts on the thermal stability of PEO.31 The results showed that LiClO4 accelerated PEO decomposition and showed strong exothermic combustion around 347 °C. In contrast, lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(pentafluoroethanesulfonyl)imide (LiBETI) had a negligible effect on PEO decomposition, where PEO decomposed at roughly 400 °C. Therefore, LiTFSI and LiBETI are much more appropriate in terms of safety than LiClO4. Apart from Li salts, introducing flame retardants (e.g. halogen, phosphorus and nitrogen-based flame retardants) as additives can effectively inhibit SPE combustion. Generally, flame retardants play the following two roles in improving the safety of SPE-based SSLMBs: (1) promoting the dehydration and carbonization of polymer materials, and preventing flammable gases from releasing and (2) forming a protective film to isolate the outside air and heat. Among them, organophosphorus-based flame retardants (phosphates, phosphites, phosphonates, phosphazenes, etc.) are widely adopted due to their low toxicity and high flame retarding efficiency.32
Besides the intrinsic thermal stability, the thermal conductivity of SSEs also affects the temperature distribution inside SSLMBs. The electrochemical reaction accompanied by heat release affects the internal temperature distribution of SSLMBs, and vice versa. More seriously, massive heat accumulation may cause thermal runaway of SSLMBs; however it has received little attention. Recently, the thermal conductivity (k) of SSEs was systematically studied.29 As described in Fig. 2f, oxide SSEs possess higher k (1–2.2 W m−1 K−1) than SPEs (0.2–0.7 W m−1 K−1), which facilitates the uniform distribution and conduction of heat and prevents SSLMBs from heating up drastically due to heat accumulation. Nevertheless, the high thermal conductivity of SSEs can’t totally ensure superior internal heat transfer. Recent research studies have shown that the main thermal resistance of batteries is derived from the interfacial thermal resistance; thus reducing the interfacial thermal resistance is of great significance for enhancing the internal heat transport performance of batteries.33
2.2. Interfacial dynamic instability
As mentioned above, the intrinsic stability of SSEs can’t represent the thermal stability of SSLMBs, and in actual situations, the electrode/SSE interface usually exhibits different chemical/dynamic/thermal behavior compared with either electrodes or SSEs. The Li plating/stripping process is accompanied by interface dynamics variations including the interfacial reaction products, physical contact, Li deposition morphology and temperature distribution, which also affect the safety of SSLMBs. | ||
| Fig. 3 Interfacial dynamic instability of SSLMBs. (a) Interfacial reaction mechanisms of SSEs. (b) The issues caused by mismatched interfacial physical contact. (c) Scheme of multi-step thermal runaway reaction between oxide SSEs and Li metal. Reproduced with permission from ref. 14. (d) Schematic diagram of the two distinct failure routes of different sulfide SSEs with NCM. Reproduced with permission from ref. 17. | ||
Despite the tremendous efforts in studying electrode/SSE interfaces, reports on interface security hazards are rare to date. Several factors related to interfacial stability, including material compatibility, heat accumulation, and gas release, should be considered when designing high-safety SSLMBs.
(1) Li/SSE interfacial reactions. Recent reports showed that the exothermic reaction of SSEs and Li metal will affect Li deposition behavior and the stability of SSEs. On one side, high-valence-metal-element-containing SSEs such as Li10GeP2S12 (LGPS), Li1+xAlxGe2−x(PO4)3 (LAGP), and Li1+xAlxTi2−x(PO4)3 (LATP) will be reduced by Li metal to form an unstable MCI. The MCI enables the continuous decomposition of SSEs and increased interfacial resistance. The electrical properties of the interphase strongly affect the mechanical integrity of LAGP. The MCI forces Li+ reduction at the LAGP side rather than the Li side, causing a local volume expansion, crack formation and the pulverization of LAGP. Furthermore, interfacial reactions are usually accompanied by heat generation. When MCI reacts dramatically with melted Li when the temperature is over 200 °C, the thermal runaway occurs.36 To further reveal the failure mechanism of oxide SSEs, multiscale characterization was employed to study the thermal reaction of LATP and Li.37 It was found that LATP would react with Li to form Li3PO4, LiP, Li0.5TiO2, etc., at elevated temperatures and release a large amount of heat. Besides, molten Li diffuses along the microcracks of LATP and its products, intensifying the thermal reaction with LATP, and causing thermal runaway for LATP. In another case, it was found that O2 is another by-product of the interfacial reactions of Li and LATP, where O2 participated in the subsequent reaction with molten Li to induce thermal runaway (Fig. 3c).14 These results suggested that even high-stability SSEs are threatened by interfacial decomposition products, which reminds one to reconsider the fundamental interfacial safety issues of SSLMBs.
(2) Cathode/SSE interfacial reactions. In addition to the SSE/Li interface, safety concerns regarding the SSE/cathode interface should be considered. The electrochemical and thermodynamic instability is a hidden safety concern. For SPEs, their poor anti-oxidant properties cause incompatibility with high-voltage cathode materials, even releasing O2, CO, C2H2, etc.38 Additionally, some hydrocarbon polymers also face the risk of burning.39,40 Taking PEO as an example, when matching to high voltage cathode materials, such as LiCoO2 or LiNixCoyMn1−x−yO2 (NCM), the LiCoO2 surface will catalyze the PEO electrolyte to release unexpected H2 gas,38 and NCM may react with thermally degrading PEO or fluorine radicals (from lithium salts) to release O2 and subsequently polymer combustion may occur, threatening battery safety.31 Similarly, inorganic solid electrolytes also face the risk of gas production/heat accumulation from the reaction with cathode materials. Typically, sulfides are a class of SSEs with high ionic conductivity, while showing relatively poor electrochemical stability. Recently, Wang and co-authors explored the thermal stability of sulfide SSEs and the Li1−xCoO2 cathode at 400–500 °C.18 It was found that Li3PS4 would decompose into reducible and flammable Li2S and S. Such products may be in direct contact with Li1−xCoO2 and lead to an exothermic reaction or even combustion at a certain temperature. The interfacial stability of Li3PS4 and NCM was further estimated via differential thermal analysis (DTA) measurements, and the results showed exothermic peaks appearing at 340–420 °C, suggesting their lower thermal stability. Recent research found that the interfacial stability of cathode/SSE materials is highly dependent on the structure of SSE and cathode materials. For instance, when the P in Li3PS4 is totally substituted by Sn, the interfacial reactions are suppressed and no distinct exothermic peaks are observed in NCM–Li4SnS4. It can be attributed to the stronger Sn–S bond, where S is more difficult to be substituted by O (from NMC) compared with the P–S bond.41
Notably, the thermal safety of SSLMBs is threatened by lattice oxygen (O2) release from cathodes. Recently, a new view reported that Li presented relatively high thermal stability against sulfide SSEs and O2 under practical working conditions. It is confirmed that no thermal runaway was detected in an Ah-level Li|NCM pouch cell using the Li6PS5Cl electrolyte before charging. Nevertheless, when charging to 100% state-of-charge (SOC), there is intense exothermic heat generation due to the reaction of O2 and Li6PS5Cl. After the temperature reached 275.5 °C, a short circuit occurred. Thus, inhibiting the release of lattice oxygen is important for enhancing the thermal stability of SSLMBs.42 Furthermore, differential scanning calorimetry-mass spectrometry (DSC-MS) characterization is used to understand the thermal failure of the sulfide SSE and the NCM cathode. Here two distinct thermal runaway mechanisms of sulfide-based SSLMBs using glass-ceramic (Li3PS4 and Li7P3S11) and crystalline (Li6PS5Cl and Li10GeP12S2) sulfide SSEs were revealed, named the gas–solid and the solid–solid reactions (Fig. 3d).17 The glassy-ceramic SSEs experienced a gas–solid reaction, which was oxidized by O2 (released from NCM cathodes) at approximately 200 °C, leading to toxic SO2 generation and tremendous heat. In contrast, the crystalline sulfide SSEs remained stable against lattice O2 at 200 °C without SO2 generation, while solid–solid reactions occurred with the decomposition products of NCM cathodes (transition-metal oxides, etc.) at 300 °C. The mechanism suggests that strategies such as inhibiting O2 release from cathode materials and optimizing the crystal structure of SSEs facilitate achieving safer SSLMBs. In addition, cathode materials also play an important role in determining interfacial stability. Compared to layered metal oxide cathode materials, sulfide SSEs show better thermal stability towards LiFePO4. Taking Li6PS5Cl as an example, it is thermally stable towards LiFePO4 even at 350 °C. In contrast, Li6PS5Cl showed vigorous exothermic chemical reactions with delithiated Li1−xNi0.8Co0.1Mn0.1O2 (NCM811).43 In short, the proposed mechanisms established the relationship among the sulfide SSE structures, gas-driven crosstalk reactions, and interfacial reactions during the thermal runaway, guiding the rational design of interfaces.
Compared with sulfide SSEs, halide SSEs possess better air stability and anti-oxidation, especially fluoride-based SSEs. However, their interfacial-level electrochemical/thermal stability is unclear. Recently, the interfacial thermal stability of halide SSEs and electrodes was explored, and it was confirmed that anti-perovskite-type Li2OHCl was compatible with the cathode and anode at a relatively low temperature, while exhibiting high chemical reactivity after melting over 320 °C. In contrast, rock-salt-type Li3InCl6 showed higher thermal stability and interfacial compatibility.44 Nevertheless, the oxidation by-products may contain toxic Cl2 based on Mo's simulation for most chloride-based SSEs. In practical pouch cells, the sealing packages are easily blasted and result in the leakage of Cl2, which increases safety risks.45 As another important SSE with high stability against Li anode, hydride SSEs have also attracted great attention.46 However, according to Lu's simulation, the hydride SSEs present poor oxidation stability. LiBH4 and Li2B12H12 would be oxidized into flammable H2 under a voltage of ∼2 V and ∼3.3 V (vs. Li+/Li), respectively, which poses potential hazards.47
As aforementioned, the structure of SSEs and their compatibility with anodes/cathodes have a significant effect on interfacial stability. More considerable efforts are required to study the interfacial stability mechanisms of SSEs and electrodes to guide the design of high-performance SSEs. More efforts are recommended to focus on exploring SSEs with wide electrochemical stability windows to match both cathode and anode materials.
2.3. Gas release
In addition to short circuits and heat accumulation, gas accumulation is another factor leading to battery failure. As mentioned in Sections 2.1 and 2.2, gases (such as O2, H2, HX, NH3 and H2S) are mainly derived from material preparation and battery operation processes (Fig. 4a). Firstly, these hazardous gases would cause environmental pollution and health hazards. Moreover, the gases derived from interfacial reactions during battery operation may accelerate side reactions and deteriorate interfacial contact, resulting in interfacial impedance increase and fast battery capacity decay (Fig. 4b). What's worse, the gases (such as O2) may continuously react with Li or other components and cause significant heat release and internal temperature rise, eventually leading to the thermal runaway of SSLMBs. Notably, battery recycling and post-treatment processes may be accompanied by the release of massive amounts of harmful gases, leading to the destruction and corrosion of battery packs, and even combustion and explosion (Fig. 4c). Furthermore, gas accumulation, pulverized Li metal and unstable SSEs in the spent batteries pose a huge safety hazard to their recycling management.51 Thus, it is a critical path to enhance the stability of SSLMBs via rational design of electrode materials, SSEs, and electrode/SSE interfaces as well as building an appropriate safety management/recycling system. | ||
| Fig. 4 (a)–(c) Gas sources and corresponding safety hazards for SSLMBs. (d) Safety landscape for SSLMBs. Reproduced with permission from ref. 52. | ||
In brief summary, SSLMBs are not completely safe, as heat accumulation, gas release, and short circuits will cause degradation and thermal runaway of SSLMBs52 (Fig. 4d). More comprehensive correlative characterization is advised to explore the gas release mechanism and the thermal behavior of SSLMBs, thus, guiding the exploration of targeted improvement strategies.
3. Recent advances in improving the safety of SSLMBs
To address the aforementioned issues, this section focuses on enhancing the safety of SSLMBs by improving the thermal/chemical/electrochemical stability of SSEs and the corresponding interfacial stability of electrode/SSE materials.3.1. SSE stability improvement
As aforementioned, the air/moisture instability of inorganic solid electrolytes and poor thermal stability of SPEs are critical issues that hinder the development of high-safety SSLMBs. In the following section, we will summarize the recent progress in enhancing the chemical stability of inorganic solid electrolytes and the thermal stability of SPEs. | ||
| Fig. 5 Electrolyte design strategies. (a) Crystal structures of LASI and LASI-80Si. Reproduced with permission from ref. 20. (b) Inorganic solid electrolyte's surface coating. (c) Liquid-state brush-like polymer PPZ. Reproduced with permission from ref. 58. (d) Inorganic–polymer composite electrolytes. (e) Chemical structures of flame retardants. (f) Schematic illustration of thermal polymerization of VC with AIBN as its initiator. Reproduced with permission from ref. 59. | ||
Besides the sulfide SSEs, the doping strategy has also proved to enhance the humidity stability of halide SSEs.60 Among the available halide SSEs, Li3InCl6 is considered relatively stable in ambient air in the chloride system, and can even be synthesized by water-mediated synthesis.61 However, recent results suggested that Li3InCl6 degraded upon exposure to humid air: Li3InCl6 reacts with H2O to form In2O3, LiCl, and corrosive HCl.25 When Cl was partially replaced by F in Li3InCl6 (labeled as Li3InCl5.6F0.4), the water absorption rate was reduced by 3 times from 0.607 g h−1 to 0.198 g h−1, while the ionic conductivity decreased from 4.5 times to 1.7 times in the 20 ± 3 °C dew-point dry room, indicating the improved moisture stability.62
The element doping strategy can regulate the inorganic solid electrolyte crystal structure, yet are not useful on the crystal surface where the hydrolysis reaction first occurs. The surface coating or core–shell nanostructure strategy is a valid approach to resist chemical attack by O2, H2O and even electrodes for inorganic solid electrolytes. To date, polymers, metal oxides, air-stable inorganic solid electrolytes, and fluorides are commonly used surface layer materials that are expected to inhibit the decomposition and rupture of SSEs (Fig. 5b). For instance, to enhance the moisture stability of Li3InCl6, a thin Al2O3 coating layer was created by atomic layer deposition (ALD, labeled as Li3InCl6@Al2O3), which reduced the water absorption rate to 1/4 and prolonged the liquefaction time to 7 times compared with the coating-free Li3InCl6.63 In another case, a sulfide SSE with an oxysulfide shell was designed to prevent deterioration of the bulk SSE by moisture.64 When exposing the SSE to 35% humid air for 5 min, the ionic conductivity of the SSE with the oxysulfide shell slightly decreased by 4.53%, which is only 21% of the coating-free SSE (21.7%).
(1) Thermal stability improvement. Polymer matrices typically offer superior chemical stability and air tolerance, whereas their thermal stability is not satisfactory. Actually, the stability of SPEs primarily depends on the chemical structure of the polymer monomer, the degree of crystallinity and other components. Taking PEO as an example, it possesses a relatively low melting point (65 °C). The crystallinity would be reduced and enable the PEO to exhibit a high ionic conductivity of over 10−4 S cm−1 when the temperature is over 60 °C, while an ultralow room-temperature ionic conductivity of roughly 10−8 S cm−1 is presented due to the low dissociation degree of Li salt in highly crystalline PEO.65 However, PEO will thermally decompose and release gas at 330 °C.66 To enhance the thermal stability of PEO-based SPEs, some thermostable polymers (such as poly(m-phenylene isophthalamide) (PMIA), poly(tetra-fluoroethylene) (PTFE), poly(vinylidene fluoride) (PVDF)) are introduced as reinforcement matrices.16,67,68 In particular, fluorinated polymers possess high Tm (>155 °C) and high Tdec (>350 °C) owing to the strong C–F bond, which improves the thermal stability of as-prepared SPEs.69 A fly in the ointment is that the ionic conductivity of such fluorinated polymer-based SPEs is still unsatisfactory.70 Polyimide (PI), possessing excellent thermal stability up to 500 °C and chemical inertness, is another promising electrolyte host.71 Cui's group designed safe SPEs via filling PEO/LiTFSI into an 8.6-μm-thick nanoporous polyimide (PI) film to improve the thermal stability of PEO-based SPEs.72 Then they further introduced decabromodiphenyl ethane (DBDPE) as a flame retardant to improve the safety of SSLMBs.73 Such a PI/DBDPE SPE ensures stable running of Li|Li symmetric batteries for 300 h without short circuits owing to its thermal stability, non-flammability, and high mechanical strength. More eye-catching evidence of the improved safety is provided by the pouch cells, which work properly even when tested under flame.
Alternatively, dispersing inorganic fillers74 and fast Li-ion conductors75,76 into SPEs to integrate composite electrolytes has been adopted to improve the ionic conductivity, mechanical properties, and thermal stability (Fig. 5d). Firstly, inorganic fillers improve ionic conductivity by inhibiting polymer matrix crystallization and promoting the dissociation of Li salts, as well as providing rich migration sites of Li+ drawing on their Lewis acid–base groups and oxygen vacancy. Secondly, inorganic fillers possess excellent chemical stability and non-flammability, improving the thermal stability of SPEs. For example, the introduction of 10% Zn2(OH)BO3 and 30% Li6.4La3Zr1.4Ta0.6O12 (LLZTO) improved the Tdec of the PEO SPE from 350 °C to 428–458 °C and 400 °C, respectively.77,78
Besides decomposition at high temperatures, PEO-based SPEs will undergo exothermic reactions and may release large amounts of heat/gas during combustion, and introducing flame retardant functional groups is of significance to prevent the thermal runaway in SSLMBs. In this regard, a nonflammable liquid polymer electrolyte (LPE) was designed by using a liquid-state brush-like polymer (consisting of flame-retardant polyphosphazene as the backbone and methoxytriethoxy substituents as the side chains) as the sole solvent (Fig. 5c).58 Significantly, such a non-flammable LPE enables the full cells to stably run for over 1000 cycles in a wide operating temperature range of 60–90 °C. Besides, the maximum heating rate was only 0.49 °C min−1, and no thermal runaway occurred throughout the accelerating rate calorimeter test.
(2) Flame retardants/thermoresponsive additives. In addition to the polymer structural design, the introduction of flame retardants is another effective strategy to suppress the combustion of SPEs. Generally, flame retardants can be divided into two types: (i) additive-type (i.e., non-reactive inorganic particles or nonflammable polymers) and (ii) reactive-type flame retardants (e.g., phosphate, fluoride and phosphonitrile) (Fig. 5e).79 The former additives are inert and difficult to react exothermically, thus preventing the combustion of SPEs to a certain extent. In the case of the latter reactive-type flame retardants, their reaction mechanism is as follows: (i) halogen flame retardants firstly produce free radicals X˙ (X = F, Cl, Br) after decomposition, and then X˙ will capture the highly active free radicals (H˙ and OH˙) that are produced by the pyrolysis of SPEs. Their decomposition products including HX, H2O and X2 dilute the concentrations of combustible gases and O2, thus delaying the combustion of SPEs. (ii) Phosphorus-based flame retardants will break down and produce phosphate and phosphate oxygen radicals, which diffuse into the gas phase to combine with the combustible radical to delay SPE burning. Meanwhile, phosphoric acid and its derivatives dehydrate the polymer to form a carbon layer that slows down heat transfer and traps O2, inhibiting the decomposition and combustion of SPEs.71 For example, triethyl phosphate (TEP) was introduced into an in situ polymerized electrolyte to inhibit electrolyte combustion and enhance thermal stability, which enabled the Li metal anode with high temperature (>60 °C) survivability.80
Apart from flame retardant additives, thermoresponsive additives have been developed to improve the safety of SSLMBs. The thermoresponsive additives are a class of thermally sensitive materials. They are capable of inhibiting the occurrence of thermal runaway by increasing battery resistance, blocking ion transportation channels, or releasing blocking mediators. Introducing thermoresponsive additives into polymer electrolytes gives LMBs the ability to recognize overheating temperatures in the early stages, which is an effective thermal safety prevention technology. For instance, certain small molecular solvents (vinyl carbonate (VC) and azodiisobutyronitrile) were reported as thermoresponsive electrolytes, which can be polymerized into poly(VC) as the battery temperature abnormally increases (Fig. 5f).59 The in situ formed poly(VC) can act as a barrier to prevent direct contact between electrodes and immobilize the free liquid solvent at high temperatures, thereby reducing the exothermic reactions between electrodes and electrolytes. Consequently, the internal-short-circuit temperature and “ignition point” temperature (the starting temperature of thermal runaway) of LMBs are largely increased from 126.3 and 100.3 °C to 176.5 and 203.6 °C, respectively.
3.2. Interface modifications
In Section 3.1, we have summarized the recent advances in developing SSEs with chemical/thermal stability to meet the requirements of safer SSLMBs. Apart from being intrinsically stable, an ideal SSE should be chemically and physically robust with cathodes/anodes, or capable of forming a stable interface as mentioned in Section 2.2, to achieve safer SSLMBs. However, experimental evidence indicates that the safety of SSLMBs is still limited by the undesirable side reactions and dendrite growth due to the mismatched electrode/SSE interface. Furthermore, interfacial exothermic reactions bring about heat accumulation and gas production issues, which tend to cause SSLMBs to heat up and inflate, and more seriously, trigger thermal runaway. In this section, recent advances in terms of electrochemical/thermally stable electrode/electrolyte interlayers and grain boundaries are summarized from an interface engineering perspective to design stable and safe SSLMBs.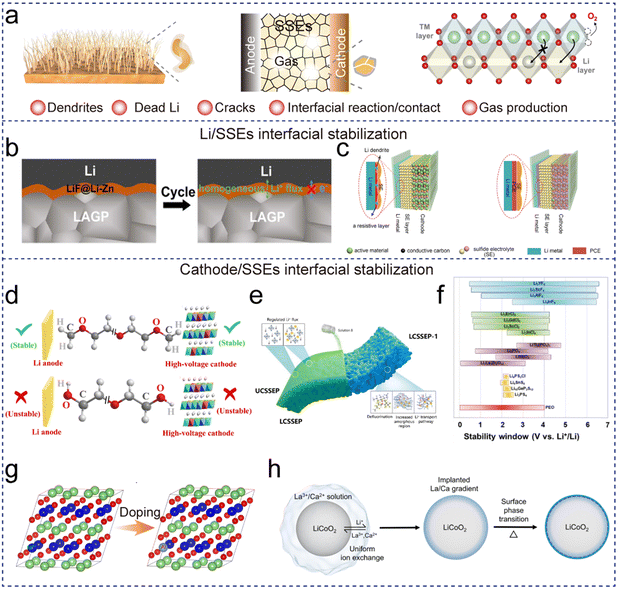 | ||
| Fig. 6 Interface engineering. (a) Interfacial issues of SSLMBs. (b) Interface evolution between metallic Li anode and ZnF2 layer-coated LAGP. Reproduced with permission from ref. 85. (c) Schematic diagram of SSLMBs without or with the PCE interlayer. (d) The stability of PEGDME and PEG in contact with the anode and high-voltage cathode. Reproduced with permission from ref. 86. (e) Structure of the Janus composite electrolyte and schematic description of the fabrication of the JCSSE. Reproduced with permission from ref. 87. (f) The electrochemical stability windows of various SSEs. Reproduced with permission from ref. 88. (g) Cathode material crystal structure doping. (h) A protective coating on the LiCoO2 surface based on ion exchange reactions. Reproduced with permission from ref. 89. | ||
Compared with SPEs, the interfacial stability of inorganic solid electrolytes is primarily influenced by the interfacial reaction and interfacial contact resistance. As described in Section 2.2, some SSEs such as NASICON and LGPS may react with the Li metal anode, resulting in electrolyte decomposition and heat emission. Furthermore, a “point-to-point” solid–solid contact interface due to rigid inorganic solid electrolytes (especially crystalline oxide SSEs) also leads to large interface resistance, uneven current distribution as well as dendrite growth. To solve the issues, functional interlayers such as lithiophilic alloy layers (Ag, In, etc.)90,91 are developed to regulate Li+ deposition.92 For instance, a LiF@Li–Zn alloy layer was constructed at the LAGP|Li interface (Z-LAGP) via in situ conversion reactions, which exhibited a strong wetting interaction with Li metal and low interfacial resistance, and provided a homogeneous Li-ion flux (Fig. 6b).85 As a consequence, the Li|Z-LAGP|Li cells presented a critical current density (CCD) of 2.0 mA cm−2 and a long lifespan of 1000 h at 0.1 mA cm−2. Notably, although facing similar challenges as alloy anodes, lithiophilic alloy layers help regulate Li-ion flux and suppress Li dendrite growth, but the side reaction still exists due to the mixed conductive layer. Thus, flexible polymer buffer layers (PEO, Kevlar aramid nanofiber)93,94 have been developed to facilitate intimate interfacial contact. For example, the solid-state plastic crystal electrolyte (PCE) acting as a flexible interlayer can effectively suppress the reduction of Ge4+ into metallic Ge, thus inhibiting the decomposition of LGPS at the Li/LGPS interface (Fig. 6c).95 As a consequence, the assembled Li|S full cells showed stable cycling performance for over 100 cycles at 0.13 mA cm−2.
(1) High anti-oxidation SSEs. In high-safety SSLMBs, an ideal SSE should possess a wide electrochemical stability window, being stable against both anode and cathode materials. Although PEO-based SPEs have demonstrated high stability against the Li anode, their oxidation potential is below 4.0 V, which can hardly match with the high-voltage cathode materials.88 On the cathode side, the electrochemical oxidation of SSEs on the surface of the cathode results in the formation of a passivating layer and subsequently increases interfacial resistance, and even gas release. Based on the simulation and experiments, –OH in the PEO-based SPEs will be oxidized into –COOH(Li), which is the limiting factor of electrochemical stability windows for PEO-based SPEs (Fig. 6d).86 End-group substitution (–OCH3)86 or the introduction of inorganic particles (e.g., Al2O3, SiO2)96,97 interacting with –OH had a positive effect on improving the anti-oxidation of PEO-based SPEs. For example, the PEO-based SPE with mono-dispersed ultrafine SiO2 delivered a high oxidation stability potential of 5.5 V.96 Alternatively, creating a high-voltage-resistant interlayer for SPEs towards the cathode has also demonstrated a positive effect on inhibiting SPE decomposition.98,99 For instance, designing conductive coatings of anionic molecules at the interface enabled the Li|NCM cell with a diglyme electrolyte to work stably at 4.2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V.98 Although the introduction of fillers and interfacial modification can improve the anti-oxidation performance of PEO-based SPEs to some extent, the intrinsic oxidative stability of PEO requires further enhancement.
V.98 Although the introduction of fillers and interfacial modification can improve the anti-oxidation performance of PEO-based SPEs to some extent, the intrinsic oxidative stability of PEO requires further enhancement.
Alternatively, designing composite electrolytes with both anti-oxidation and anti-reduction is a promising direction. Janus electrolytes (or bilayer or multi-layer composite SSEs) are regarded as promising candidates to achieve high-stability SSLMBs due to their exceptional compatibility with both cathode and anode. Generally, the SSE facing the cathode possesses high antioxidant properties, while the SSE towards the anode presents high anti-reduction properties. As shown in Fig. 6e, a Janus electrolyte with mortise and tenon joints (JCSSE), composed of a poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP)/Li6.4La3Zr1.4Ta0.6O12 layer toward the cathode and a poly(diallyldimethylammonium) bis(trifluoro-methanesulfonyl)imide (PDADMATFSI)/UiO-66-SO3Li layer toward the Li anode, is proposed with broadened electrochemical stability windows.87 The assembled SSLMBs demonstrated good cycling stability for 100 cycles under a charging cutoff voltage of 4.3 V, as well as superior cycling stability in the wide temperature range of 25–100 °C. In another case, a heterogeneous multilayered solid electrolyte (HMSE) was developed with a reduction tolerant polyethylene glycol diacrylate layer facing the Li anode, while an oxidation-resistant poly(acrylonitrile) (PAN) layer contacts with the cathode. The HMSE possesses a wide electrochemical stability window of 0–5 V, enabling Li|NMC811 SSLMBs stably run for over 175 cycles with a high capacity retention of 94.4% at 0.2C (charging cutoff voltage of 4.3 V).100
Compared with SPEs, sulfide SSEs possess higher ionic conductivity, even beyond 10−2 S cm−1 at room temperature. Nevertheless, the narrow electrochemical stability windows in the range of 1.5–2.5 V are considered to be a major obstacle to matching high-voltage cathodes.101 It is difficult to broaden the electrochemical stability window of sulfide SSEs via elemental doping, where the coating technique102 and composite electrolytes103 are adopted as alternative strategies to suppress interfacial reactions in sulfide SSE-based batteries.
Generally speaking, the oxidation potentials of oxide and halide electrolytes are satisfactory compared to sulfide electrolytes as shown in Fig. 6f.88 For oxide-SSEs, garnet-type LLZO shows a wide electrochemical stability window of 0–6 V, and is regarded as a promising SSE to match the high-voltage cathode materials. However, the large interfacial resistance because of rigid oxide SSEs is a limitation. To solve the issue, the following strategies are adopted: (1) cathode/electrolyte co-sintering to enable the amorphous oxide SSE to crystallize on the surfaces of electrode particles and the electrode materials embedded within the lattice of the oxide SSE, guaranteeing intimate interface contact;104 (2) introducing high-voltage resistant polymers as a buffer layer; and (3) introducing materials with low melting points (e.g. Li3PO4) as an intermediate phase to bridge the active materials and SSEs together, ensuring good contact. For instance, the low-melting-point Li3PO4 helps build a compatible and Li+-conductive self-integrated layer to provide continuous Li+ transport pathways at the NMC811/LLZTO interface. The conclusion is confirmed by the density functional theory (DFT) calculation. The surface energy differences of NCM811/Li3PO4 and Li3PO4/LLZTO are 0.87 and 0.98 J m−2, which are lower than that of NCM811/LLZTO (1.85 J m−2), indicating the faster Li+ transport across the NCM811/Li3PO4 and Li3PO4/LLZTO interfaces. Generally speaking, high-safety and high-voltage SSLMBs are still one of the most interesting routes and more efforts should be devoted to reducing interfacial impedance.105
Halide SSEs have recently emerged with relatively high oxidation potentials over 4.2 V (especially for the Cl- and F-based halide SSEs).60,106 Altering halogen anions could adjust the oxidation stability of halide SSEs (F− > Cl− > Br− > I−); e.g., Li3YCl6 shows a wider electrochemical stability window of 0.62–4.21 V than Li3YBr6 (0.59–3.15 V).106 Nevertheless, chloride electrolytes still struggle to satisfy high-voltage demands. Thus, an F-substitution strategy is proposed to achieve the above targets. For instance, 20% Cl in Li3InCl6 was substituted with F to obtain the Li3InCl4.8F1.2 SSE, which extended the oxidation stability potential from 4.3 V to over 6 V.107 DFT was employed to clarify the underlying mechanism, and it was found that an F-containing CEI was formed on the cathode materials, thus suppressing the further decomposition of SSEs under high voltages. It should be mentioned that the SSLMBs using the high voltage LCO cathode still present fast capacity decay, indicating the existence of interfacial side reactions. Thus, developing high-ionic-conductivity fluoride-based SSEs that are compatible with high-voltage cathode materials is highly recommended in the next step.
In brief summary, designing SSEs with high voltage resistance is of significance in achieving high-voltage and high-safety SSLMBs. Considering the intrinsic poor anti-oxidation ability of sulfide SSEs and SPEs, multi-layered SSEs or coating strategies are advised for stabilizing the interface. The rigid oxide and halide SSEs possess high voltage stability, whereas the interfacial contact with cathode materials should be improved.
(2) Cathode oxygen release inhibition. As mentioned in Section 2.2.1, the structural stability of layered oxide LiTMO2 cathodes against O2 release depends predominantly on their chemical composition (e.g. lithium content and TM species) and SSE/cathode interfacial reaction.108 Firstly, higher Ni content in LiTMO2 cathodes triggers lower onset temperatures for phase decomposition and more severe oxygen loss attributed to the instability of Ni–O bonding in the charged cathode. Computational results indicate that the TM–O bond strength increases as Ni2+–O < Ni3+–O < Co3+–O < Mn4+–O.109 Meanwhile, lower Li content (i.e., higher state of charge, SOC) in LiTMO2 cathodes causes structural instability and more O2 release at elevated temperatures.110 Secondly, SSE/cathode interfacial reactions will consume surface-activated oxygen and promote bulk-to-surface oxygen migration, aggravating the reaction and releasing massive heat, in turn causing an explosion.111 Doping with strong covalent elements (Mg, Al, etc.)112,113 has been demonstrated to be an effective approach to thermodynamically stabilize the lattice oxygen (Fig. 6g). The bonding energy is closely related to the covalency of the chemical bond: the more the covalent bonds, the more the delocalized electrons, and the easier for the bond to break. For example, Al and Mg doping has been proven effective in postponing O2 evolution and reducing heat release as the bonding energy follows the order Al–O/Mg–O > Ni–O > Co–O. To characterize gas evolution from cells, online electrochemical mass spectrometry (OEMS) is employed at high voltages of 4.4 V. The amount of gas released is as follows: LiNi0.90Mn0.05Al0.05O2 (207 μmol m−2) < LiNi0.90Mn0.10O2 (239 μmol m−2) < LiNi0.90Co0.05Al0.05O2 (290 μmol m−2) < LiNi0.90Mn0.05Co0.05O2 (336 μmol m−2) < LiNi0.90Co0.10O2 (513 μmol m−2).114 The above experimental characterization demonstrates the gas release of cathodes is dependent on the identity of the dopant in the lattice. Apparently, Mn and Al are effective dopants to suppress gas release from overcharged cathodes. Surface coating89,115–117 is another strategy to hinder interfacial reactions, thus stabilizing the structure of LiTMO2 and against oxygen release. Recently, a Li-poor perovskite structure protection shell and a La/Ca gradient-doped layered-structure buffer layer were constructed to stabilize LiCoO2 (La-LCO) based on ion exchange reactions (Fig. 6h). In situ differential electrochemical mass spectrometry (DEMS) was performed to detect gas evolution at first charging. It was found that pristine-LCO exhibits severe O2 (onset at ∼4.33
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) and CO2 (onset at ∼4.17
V) and CO2 (onset at ∼4.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V) release, while the release is delayed for La-LCO (no O2 release up to 4.7
V) release, while the release is delayed for La-LCO (no O2 release up to 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V and low content CO2 emission onset at ∼4.57
V and low content CO2 emission onset at ∼4.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V). The constructed surface architecture helped suppress surface oxygen and gas release, enabling the assembly pouch cells to stably cycle at 4.8
V). The constructed surface architecture helped suppress surface oxygen and gas release, enabling the assembly pouch cells to stably cycle at 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V.89 Based on the above results, crystal structure optimization, surface engineering and coating by high-oxygen-activity passivation are promising directions.
V.89 Based on the above results, crystal structure optimization, surface engineering and coating by high-oxygen-activity passivation are promising directions.
(1) Modifying grain boundaries. Introducing ionic-conducting polymers or inorganic compounds at the grain boundaries is promising to alleviate Li dendrite growth. Recently, Yang et al. proposed a grain-boundary electronic insulation (GBEI) strategy, aiming to block electron transport via introducing an insulating polymer layer (Fig. 7a).118 Benefitting from the GBEI strategy, Li|Li symmetric cells with 30 times longer lifespan and Li|LiCoO2 full cells with 3 times lower self-discharging rate than pristine sulfide SSEs were developed. Furthermore, LiPO2F2 was introduced into LATP during ceramic sintering, to suppress the reactions between Li via modifying the defect sites inside the LATP pellet (Fig. 7b).50 Also, the delayed thermal runaway of the LATP pellet with LiPO2F2 confirmed the as-proposed feasibility of the grain boundary modification strategies.
 | ||
| Fig. 7 Grain boundary decoration. (a) Schematic illustrations of SSLMBs with and without PEGDME modified LPSCl electrolytes. Reproduced with permission from ref. 118. (b) ARC test results of the Li/LATP pellet and the Li/LATP@LPF-pellet sample. Reproduced with permission from ref. 50. (c) A schematic diagram of the VIGLAS-based electrode fabrication process. Reproduced with permission from ref. 119. | ||
(2) Reducing electronic conductivity of inorganic solid electrolytes. Notably, the non-negligible electronic conductivity of inorganic solid electrolytes will induce Li deposits inside inorganic solid electrolytes and self-discharge. Recent experimental research showed that this “bulk” dendrite growth is more prevalent in SSEs with high electronic conductivity. To resist dendrite growth, the empirical upper limit thresholds of electronic conductivity should be controlled at 10−10 and 10−12 S cm−1 under current densities of 1 and 10 mA cm−2, respectively.120 Nevertheless, the electronic conductivity of most reported inorganic solid electrolytes is in the range of 10−9 to 10−7 S cm−1.121 To reduce the electronic conductivity, a low-electronic-conductivity sulfide SSE was synthesized by microwave-induced thermal shock to enhance crystallization and suppress carbonization. The obtained microwave-derived Li6PS5Cl exhibits an electronic conductivity of 1.2 × 10−9 S cm−1, which is 4 orders of magnitude lower than that of furnace-derived Li6PS5Cl (1.7 × 10−5 S cm−1).122 However, recent research found that the reported electronic conductivity of inorganic solid electrolytes is inconsistent with the large bandgaps (e.g., 5.79 eV for c-LLZO, 3.7 eV for β-Li3PS4) computed from DFT.123 Theoretically, inorganic solid electrolytes should possess an ultralow electronic conductivity of lower than 10−10 S cm−1. The huge deviation between experimental and theoretical results is attributed to the cracks and defects that affect electronic transport.121 Thus, the cracks and defects are suggested to be eliminated to suppress Li dendrite growth.
(3) Eliminating cracks and defects. The formation of cracks and defects in the SSE during the Li plating process is another reason leading to the short circuit and thermal runaway. To reduce the cracks and defects that arise in the SSEs, designing an integrated electrolyte structure124 or increasing operating pressure125 can be considered. Apparently, external stack pressure helps reduce the porosity of the SSE pallet and interfacial impedance.126,127 Nevertheless, higher stack pressure may short-circuit the cell during battery preparation immediately or after a few cycles. This stems from the inherent ductility of the Li metal, allowing Li to creep through the pores of the SSEs. Hence, an appropriate stack pressure should be adopted to enable the stable cycling of SSLMBs.128,129 Alternatively, compared with crystalline SSEs, amorphous SSEs present the primary advantages of softness, easy fabrication, low grain boundaries, wider compositional variations, and isotropic ionic conductivity.83,119,130 Recently, a viscoelastic inorganic glass (VIGLAS) was reported to serve as a SSE by simply replacing the chlorine of tetrachloroaluminates with oxygen.119 The VIGLAS with polymer-like mechanical properties holds great promise, which improves the mechanical stability of inorganic solid electrolytes and enables the successful operation of pressure-less SSLMBs (<0.1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MPa, Fig. 7c).
MPa, Fig. 7c).
3.3. Advanced analytical technique
Previously, we summarized failure mechanisms of SSLMBs and strategies for manipulating SSEs and electrode/SSE interfaces to enhance their safety. This section focuses on advanced characterization techniques and theoretical analysis for mechanistic studies of SSLMBs, including heat evolution, dendrite growth, gas emission, etc. (Fig. 8). | ||
| Fig. 8 Advanced analytical techniques to reveal the internal reaction mechanisms of SSLMBs. Reproduced with permission from ref. 14, 38 and 131–133. | ||
In brief summary, the advanced characterization techniques are helpful to comprehensively understand the underlying mechanisms from different angles, and the non-destructive and in situ characterization techniques are powerful tools to understand the actual situation and internal evolution of SSLMBs during cycling.
Besides the advanced characterization, theoretical analysis is another powerful strategy to understand the mechanism at an atomic level. COMSOL has been developed to simulate the internal evolution, Li deposition behavior, thermal management and thermal runaway of SSLMBs. For instance, COMSOL multi-physics field simulation was adopted to build a 3D electrochemical model with the coupling of electric and temperature fields, which helps understand the temperature changes133 and Li dendrite growth.138,139 Additionally, machine learning (ML) has been widely used to predict properties and learn the rules underlying datasets, thus efficiently simplifying the material-discovery process and performance predictions. Recently, Ahmad and his coworkers screened over 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inorganic materials based on their mechanical properties, aiming to predict their ability to inhibit Li dendrite formation and stabilize the interface as SSEs.132 Based on ML, they predicted over 20 mechanically anisotropic interfaces with the Li metal anode, and four of them enable dendrite growth suppression. Besides, the screened candidates (e.g., LiOH and Li2WS4) are generally soft and highly anisotropic, which show great potential to be high-ionic-conductivity SSEs with strong Li dendrite suppression capability. Although great progress has been achieved, the application of ML still faces two critical challenges: massive data and reliable models. Apparently, the acquisition and screening of reliable data is a priority for the future. More approaches should be explored for material discovery to address the data scarcity challenge. Apparently, advanced analysis techniques reveal the dynamic deposition behavior of the Li anode, as well as the reaction mechanism, gas release and thermal conduction at the interface, which provide a guideline to optimize electrodes, SSEs and their interfaces. In future, the combination of more experimental and computational techniques is suggested to enable deep understanding of the underlying mechanism buried in SSLMBs, guiding the development of safer SSLMBs.
000 inorganic materials based on their mechanical properties, aiming to predict their ability to inhibit Li dendrite formation and stabilize the interface as SSEs.132 Based on ML, they predicted over 20 mechanically anisotropic interfaces with the Li metal anode, and four of them enable dendrite growth suppression. Besides, the screened candidates (e.g., LiOH and Li2WS4) are generally soft and highly anisotropic, which show great potential to be high-ionic-conductivity SSEs with strong Li dendrite suppression capability. Although great progress has been achieved, the application of ML still faces two critical challenges: massive data and reliable models. Apparently, the acquisition and screening of reliable data is a priority for the future. More approaches should be explored for material discovery to address the data scarcity challenge. Apparently, advanced analysis techniques reveal the dynamic deposition behavior of the Li anode, as well as the reaction mechanism, gas release and thermal conduction at the interface, which provide a guideline to optimize electrodes, SSEs and their interfaces. In future, the combination of more experimental and computational techniques is suggested to enable deep understanding of the underlying mechanism buried in SSLMBs, guiding the development of safer SSLMBs.
4. Engineering SSLMB design
Apart from developing inert and thermally stable SSEs as well as stabilizing the interface, the operation and post-failure state of SSLMBs need to be considered. In this section, the factors affecting the safety of SSLMBs under practical application and post-processing are discussed to guide and design safer SSLMBs.4.1. Battery safety management system
As aforementioned, the factors including the material and SSLMB assembly process, the working environment and operating conditions, etc., affect the lifespan and safety of SSLMBs. Besides, given the unpredictable operation environment for grid energy storage and EVs, more reliable state monitoring and safety management of SSLMBs is also required to maintain the optimal working temperature (typically between 25 and 40 °C) and against thermal runaway.140Fig. 9a shows potential safety hazards and corresponding monitoring means for SSLMBs. Among the numerous monitoring techniques, electrochemical testing has been widely adopted. Voltage and current are the most important parameters to initially judge whether the SSLMBs work properly. However, it is difficult to diagnose the internal state and predict sudden thermal runaway inside the SSLMBs. | ||
| Fig. 9 (a) Potential safety hazards and corresponding monitoring means for SSLMBs. Reproduced with permission from ref. 136 and 141–143. (b) Overview of feasibility and challenges of different recycling technologies for SSLMBs. Reproduced with permission from ref. 144. | ||
Alternatively, temperature and gas are important signals reflecting thermal runaway, since the internal reactions of SSLMBs are usually accompanied by temperature change and gas release. In the following sections, advanced safety management systems will be discussed according to the above two parameters.
Detecting the internal temperature changes in SSLMBs not only predicts thermal runaway, but ensures that battery packs work in a safe and mild temperature range by regulating temperature.148 According to the transferring medium, three thermal management approaches including air cooling, liquid cooling and phase change material (PCM) cooling have been widely used to regulate temperature.149,150 Notably, the former two approaches generally require additional cooling equipment, decreasing the energy density of the battery systems. Alternatively, PCMs, via absorbing/releasing latent heat at the phase change temperature to achieve thermal management, have attracted much attention due to the non-requirement of auxiliary cooling equipment. Nevertheless, PCMs such as paraffin are limited by low thermal conductivity and flammability. More efforts are recommended to explore non-flammable PCMs with higher thermal conductivity.151
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cells to operando monitor internal pressure and temperature during cycling.153 The two internal pressure peaks of sensor's signal correspond to safety venting and the onset of thermal runaway. The sensor provides a scalable solution to determine the safe warning range of thermal runaway based on the change of the temperature and pressure differential curves. By raising an alert before safe venting, the sensor provides critical abilities for battery safety assessment and thermal runaway warning.
650 cells to operando monitor internal pressure and temperature during cycling.153 The two internal pressure peaks of sensor's signal correspond to safety venting and the onset of thermal runaway. The sensor provides a scalable solution to determine the safe warning range of thermal runaway based on the change of the temperature and pressure differential curves. By raising an alert before safe venting, the sensor provides critical abilities for battery safety assessment and thermal runaway warning.
It is worth noting that most gases accumulated in the SSLMBs are toxic, flammable and corrosive, posing a great safety hazard.154 When thermal runaway occurs, these gases and internal dust particles will be ejected, which will be harmful to human health or even lead to spontaneous explosions. Therefore, it is essential to develop appropriate fire-extinguishing technology. Referring to the experience of LIBs, the current fire extinguishing agents are mainly divided into water-based fire extinguishing agents, air-based fire extinguishing agents, dry powder fire extinguishing agents and aerosol fire extinguishing agents.155 Here these agents were compared, and water-based fire-extinguishing agents showed the best performance since they possess high cooling capacity and excellent anti-reflash performance for the fire.156 However, they are not applicable to SSLMBs due to the high activity of Li metal and the low humidity tolerance of SSEs. With this in mind, more efforts should be devoted to developing environmentally friendly and efficient fire extinguishing agents, aiming to build the last safety barrier for SSLMBs when thermal runaway occurs.
4.2. Battery post-processing
Rapid growth in the market for EVs presents post-treatment/recycling challenge for the spent SSLMBs: the safety of spent SSLMBs and recycling of valuable secondary key materials. Here the potential approaches for SSLMB post-treatment/recycling are summarized and future directions are highlighted.Fig. 9b summarizes and compares the feasibility and challenges of the three recycling technologies. Pyrometallurgy and hydrometallurgy primarily involve the destruction of the crystal structure of the materials at the atomic level and the extraction of valuable metallic elements, while they may be not suitable for all SSEs. Firstly, the pyrometallurgical technique is operated under high temperatures, which accelerates the reaction between gases and dead Li, leading to intense exothermic phenomena. Besides, SPEs and sulfide SSEs would be burned off and could not be recovered using this method. Different from pyrometallurgy, hydrometallurgy is an aqueous chemical treatment but has certain limitations. For example, Li metal can react violently with water and generate flammable H2 if not pretreated beforehand. In addition, sulfide SSEs cannot be dispersed in water due to their instability and production of toxic H2S gas.144,165 Therefore, it is still urgently required to explore alternative recycling strategies to improve the recycling safety of SSEs. Of the three recycling technologies, direct regeneration is superior in terms of maximizing the economic value and minimizing environmental impact. For instance, polar solvents such as ethanol and acetonitrile are used to dissolve the PS43− thiophosphate unit-containing sulfide SSEs, where the sulfide SSEs can be regenerated through heat treatment and relithiation without structural damage and safety hazard.166,167 However, the recycling of SSEs is still at its infancy, where opportunities and challenges coexist. To accelerate the development of SSLMB recycling technologies, both recycling efficiency and economic benefits should be taken into consideration.
5. Perspectives and outlooks
At present, SSLMBs have proven the most promising next-generation rechargeable batteries for portable electronic devices and stationary energy storage systems in terms of their improved energy density and safety. Nevertheless, challenges such as undesirable safety and lifespan should be addressed to accelerate their path to commercialization.(1) Advanced SSE development and interface engineering
To the best of our knowledge, heat accumulation and gas production mainly caused by SSE decomposition and interfacial reactions seriously affect the safety of SSLMBs. On one side, more efforts are recommended to be devoted to developing SSEs with high thermal stability, moisture-stability and wide electrochemical stability windows. For example, composite SSEs have demonstrated their merits in extending the electrochemical stability windows and improving the thermal stability while their preparation process and cost need further improvement and refinement.168 It is proposed to construct multilayer composite SSEs to enhance the antioxidant to cathode side and the anti-reduction to anode side, thus enhancing the overall stabilization of SSEs.169 Moreover, high-ionic-conductivity fluoride-based SSEs with wide electrochemical stability windows and high thermal stability are also highly recommended. Apart from SSEs, future works would focus on interfacial engineering, e.g. constructing flexible insulation layers at the interfaces and grain boundaries. It is recommended to design an ideal interphase with high ionic conductivity and low electronic conductivity, as well as good densification to inhibit interfacial side reactions, thus improving the safety of SSLMBs.170(2) High-throughput screening and advanced theoretical calculations/characterization
Theoretical chemistry facilitates rapid screening of materials and significantly enhances synthesis efficiency. Li's group discovered 12 promising super Li-ion conductors from 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 garnets for SSEs using artificial intelligence (AI) technologies. This approach directly cuts across at least 95 years of computational cycles to screen SSEs, accelerating the innovation of SSEs.171 Advanced theoretical calculation has a huge influence in terms of structural properties, ion transport mechanisms, and material optimization, thus guiding the design of safer materials and SSLMB systems.172,173 Meanwhile, more in situ and coupling techniques are suggested, and theoretical predictions are used to provide more comprehensive evidence. Photoacoustic microscopy techniques174,175 combining the merits of non-destructive testing, high temporal resolution and three-dimensional imaging are powerful tools to analyze the material structure and the physical properties (e.g., temperature).
008 garnets for SSEs using artificial intelligence (AI) technologies. This approach directly cuts across at least 95 years of computational cycles to screen SSEs, accelerating the innovation of SSEs.171 Advanced theoretical calculation has a huge influence in terms of structural properties, ion transport mechanisms, and material optimization, thus guiding the design of safer materials and SSLMB systems.172,173 Meanwhile, more in situ and coupling techniques are suggested, and theoretical predictions are used to provide more comprehensive evidence. Photoacoustic microscopy techniques174,175 combining the merits of non-destructive testing, high temporal resolution and three-dimensional imaging are powerful tools to analyze the material structure and the physical properties (e.g., temperature).
(3) Application
To realize the commercialization of SSLMBs, energy density, safety and cycling performance should be balanced. Compared with the high reactivity of Li metal, high-capacity alloying anodes are also a good choice by balancing energy density and safety. To reduce the generation of gas, the internal water content of SSLMBs should be minimized during material preparation and SSLMB assembly. Furthermore, AI-management systems are proposed for real-time battery health monitoring and aging state control, as well as for early warning of abnormal battery operation.176,177 In addition, advanced sensing technologies are advised during battery operation to obtain valuable data signals. Relying on the collected data, AI-management helps improve the health, efficiency and safety of SSLMBs. Notably, most research has focused on thermal runaway prevention and battery management systems, paying little attention to fire suppression after cell thermal runaway. Delayed combustion systems are suggested in conjunction with early warning systems and fire suppression under extreme conditions (e.g. nail penetrations or high temperatures) to promise high safety.178Data availability
This is a review paper. Most of the figures and results used in this review are collected from the publications listed in the references. The remaining data are available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 22279135, U1808209), CAS Project of Young Scientists in Basic Research (No. YSBR-058), CAS Strategic Leading Science & Technology Program (A) (XDA21070100), CAS Engineering Laboratory for Electrochemical Energy Storage (KFJ-PTXM-027), Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Research Chair Program (CRC), Canada Foundation for Innovation (CFI), Hebei Natural Science Foundation (B2023202027), and Science and Technology Project of Hebei Education Department (No. JZX2024003, QN2024080).References
- J. Xiao, F. Shi, T. Glossmann, C. Burnett and Z. Liu, Nat. Energy, 2023, 8, 329–339 CrossRef.
- J. T. Frith, M. J. Lacey and U. Ulissi, Nat. Commun., 2023, 14, 420 CrossRef CAS PubMed.
- Q. Li, C. Geng, L. Wang, Q.-H. Yang and W. Lv, Renewables, 2023, 1, 374–396 CrossRef.
- A. Yang, C. Yang, K. Xie, S. Xin, Z. Xiong, K. Li, Y.-G. Guo and Y. You, ACS Energy Lett., 2023, 8, 836–843 CrossRef CAS.
- S. Kim, G. Park, S. J. Lee, S. Seo, K. Ryu, C. H. Kim and J. W. Choi, Adv. Mater., 2023, 35, 2206625 CrossRef CAS PubMed.
- Y.-C. Yin, J.-T. Yang, J.-D. Luo, G.-X. Lu, Z. Huang, J.-P. Wang, P. Li, F. Li, Y.-C. Wu, T. Tian, Y.-F. Meng, H.-S. Mo, Y.-H. Song, J.-N. Yang, L.-Z. Feng, T. Ma, W. Wen, K. Gong, L.-J. Wang, H.-X. Ju, Y. Xiao, Z. Li, X. Tao and H.-B. Yao, Nature, 2023, 616, 77–83 CrossRef CAS PubMed.
- S. Zhou, M. Li, P. Wang, L. Cheng, L. Chen, Y. Huang, S. Yu, F. Mo and J. Wei, Electrochem. Energy Rev., 2023, 6, 34 CrossRef.
- Y. Li, S. Song, H. Kim, K. Nomoto, H. Kim, X. Sun, S. Hori, K. Suzuki, N. Matsui, M. Hirayama, T. Mizoguchi, T. Saito, T. Kamiyama and R. Kanno, Science, 2023, 381, 50–53 CrossRef CAS PubMed.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- Y. Tanaka, K. Ueno, K. Mizuno, K. Takeuchi, T. Asano and A. Sakai, Angew. Chem., Int. Ed., 2023, 62, e202217581 CrossRef CAS PubMed.
- L. Ye and X. Li, Nature, 2021, 593, 218–222 CrossRef CAS PubMed.
- W. Yan, Z. Mu, Z. Wang, Y. Huang, D. Wu, P. Lu, J. Lu, J. Xu, Y. Wu, T. Ma, M. Yang, X. Zhu, Y. Xia, S. Shi, L. Chen, H. Li and F. Wu, Nat. Energy, 2023, 8, 800–813 CrossRef CAS.
- R. Chen, A. M. Nolan, J. Lu, J. Wang, X. Yu, Y. Mo, L. Chen, X. Huang and H. Li, Joule, 2020, 4, 812–821 CrossRef CAS.
- L. Han, Y. Liu, C. Liao, Y. Zhao, Y. Cao, Y. Kan, J. Zhu and Y. Hu, Nano Energy, 2023, 112, 108448 CrossRef CAS.
- S. Yang, X. He, T. Hu, Y. He, S. Lv, Z. Ji, Z. Zhu, X. Fu, W. Yang and Y. Wang, Adv. Funct. Mater., 2023, 33, 2304727 CrossRef CAS.
- X. Rui, D. Ren, X. Liu, X. Wang, K. Wang, Y. Lu, L. Li, P. Wang, G. Zhu, Y. Mao, X. Feng, L. Lu, H. Wang and M. Ouyang, Energy Environ. Sci., 2023, 16, 3552–3563 RSC.
- S. Wang, Y. Wu, T. Ma, L. Chen, H. Li and F. Wu, ACS Nano, 2022, 16, 16158–16176 CrossRef CAS PubMed.
- Z. Gao, H. Sun, L. Fu, F. Ye, Y. Zhang, W. Luo and Y. Huang, Adv. Mater., 2018, 30, 1705702 CrossRef PubMed.
- P. Lu, Y. Xia, G. Sun, D. Wu, S. Wu, W. Yan, X. Zhu, J. Lu, Q. Niu, S. Shi, Z. Sha, L. Chen, H. Li and F. Wu, Nat. Commun., 2023, 14, 4077 CrossRef CAS PubMed.
- X. Yang, X. Gao, M. Jiang, J. Luo, J. Yan, J. Fu, H. Duan, S. Zhao, Y. Tang, R. Yang, R. Li, J. Wang, H. Huang, C. Veer Singh and X. Sun, Angew. Chem., Int. Ed., 2023, 135, e202215680 CrossRef.
- R. Koerver, I. Aygün, T. Leichtweiß, C. Dietrich, W. Zhang, J. O. Binder, P. Hartmann, W. G. Zeier and J. Janek, Chem. Mater., 2017, 29, 5574–5582 CrossRef CAS.
- J. P. Goudon, F. Bernard, J. Renouard and P. Yvart, Int. J. Hydrogen Energy, 2010, 35, 11071–11076 CrossRef CAS.
- S. Wang, X. Xu, C. Cui, C. Zeng, J. Liang, J. Fu, R. Zhang, T. Zhai and H. Li, Adv. Funct. Mater., 2022, 32, 2108805 CrossRef CAS.
- W. Li, J. Liang, M. Li, K. R. Adair, X. Li, Y. Hu, Q. Xiao, R. Feng, R. Li, L. Zhang, S. Lu, H. Huang, S. Zhao, T.-K. Sham and X. Sun, Chem. Mater., 2020, 32, 7019–7027 CrossRef CAS.
- W. Li, M. Li, P.-H. Chien, S. Wang, C. Yu, G. King, Y. Hu, Q. Xiao, M. Shakouri, R. Feng, B. Fu, H. Abdolvand, A. Fraser, R. Li, Y. Huang, J. Liu, Y. Mo, T.-K. Sham and X. Sun, Sci. Adv., 2023, 9, eadh4626 CrossRef CAS PubMed.
- F. Takeiri, A. Watanabe, K. Okamoto, D. Bresser, S. Lyonnard, B. Frick, A. Ali, Y. Imai, M. Nishikawa, M. Yonemura, T. Saito, K. Ikeda, T. Otomo, T. Kamiyama, R. Kanno and G. Kobayashi, Nat. Mater., 2022, 21, 325–330 CrossRef CAS PubMed.
- Y. Wu, S. Wang, H. Li, L. Chen and F. Wu, InfoMat, 2021, 3, 827–853 CrossRef CAS.
- C.-W. Wu, X. Ren, W.-X. Zhou, G. Xie and G. Zhang, APL Mater., 2022, 10, 040902 CrossRef CAS.
- Z. Gao, S. Rao, T. Zhang, F. Gao, Y. Xiao, L. Shali, X. Wang, Y. Zheng, Y. Chen, Y. Zong, W. Li and Y. Chen, Adv. Sci., 2022, 9, e2103796 CrossRef PubMed.
- G. Abels, I. Bardenhagen, J. Schwenzel and F. Langer, J. Electrochem. Soc., 2022, 169, 020560 CrossRef CAS.
- X. L. Yao, S. Xie, C. H. Chen, Q. S. Wang, J. H. Sun, Y. L. Li and S. X. Lu, J. Power Sources, 2005, 144, 170–175 CrossRef CAS.
- R. Kantharaj and A. M. Marconnet, Nanoscale Microscale Thermophys. Eng., 2019, 23, 128–156 CrossRef CAS.
- S. S. Shinde, N. K. Wagh, S.-H. Kim and J.-H. Lee, Adv. Sci., 2023, 10, 2304235 CrossRef CAS PubMed.
- Y. Xiao, Y. Wang, S.-H. Bo, J. C. Kim, L. J. Miara and G. Ceder, Nat. Rev. Mater., 2020, 5, 105–126 CrossRef CAS.
- H. Chung and B. Kang, Chem. Mater., 2017, 29, 8611–8619 CrossRef CAS.
- J. Yan, D. Zhu, H. Ye, H. Sun, X. Zhang, J. Yao, J. Chen, L. Geng, Y. Su, P. Zhang, Q. Dai, Z. Wang, J. Wang, J. Zhao, Z. Rong, H. Li, B. Guo, S. Ichikawa, D. Gao, L. Zhang, J. Huang and Y. Tang, ACS Energy Lett., 2022, 7, 3855–3863 CrossRef CAS.
- K. Nie, X. Wang, J. Qiu, Y. Wang, Q. Yang, J. Xu, X. Yu, H. Li, X. Huang and L. Chen, ACS Energy Lett., 2020, 5, 826–832 CrossRef CAS.
- L. Wang, Z. Chen, Y. Liu, Y. Li, H. Zhang and X. He, eTransportation, 2023, 16, 100239 CrossRef.
- P. Lv, S. Xie, Q. Sun, X. Chen and Y. He, ACS Appl. Energy Mater., 2022, 5, 7199–7209 CrossRef CAS.
- M. Otoyama, A. Sakuda, M. Tatsumisago and A. Hayashi, ACS Appl. Mater. Interfaces, 2020, 12, 29228–29234 CAS.
- S.-J. Yang, J.-K. Hu, F.-N. Jiang, X.-B. Cheng, S. Sun, H.-J. Hsu, D. Ren, C.-Z. Zhao, H. Yuan, M. Ouyang, L.-Z. Fan, J.-Q. Huang and Q. Zhang, eTransportation, 2023, 18, 100279 CrossRef.
- T. Kim, K. Kim, S. Lee, G. Song, M. S. Jung and K. T. Lee, Chem. Mater., 2022, 34, 9159–9171 CrossRef CAS.
- G. Jia, Z. Deng, D. Ni, Z. Ji, D. Chen, X. Zhang, T. Wang, S. Li and Y. Zhao, Front. Chem., 2022, 10, 952875 CrossRef CAS PubMed.
- Y. Xiao, Y. Wang, S.-H. Bo, J. C. Kim, L. J. Miara and G. Ceder, Nat. Rev. Mater., 2019, 5, 105–126 CrossRef.
- S. Kim, H. Oguchi, N. Toyama, T. Sato, S. Takagi, T. Otomo, D. Arunkumar, N. Kuwata, J. Kawamura and S. I. Orimo, Nat. Commun., 2019, 10, 1081 CrossRef PubMed.
- Z. Lu and F. Ciucci, Chem. Mater., 2017, 29, 9308–9319 CrossRef CAS.
- T. Famprikis, P. Canepa, J. A. Dawson, M. S. Islam and C. Masquelier, Nat. Mater., 2019, 18, 1278–1291 CrossRef CAS PubMed.
- X. Liu, R. Garcia-Mendez, A. R. Lupini, Y. Cheng, Z. D. Hood, F. Han, A. Sharafi, J. C. Idrobo, N. J. Dudney, C. Wang, C. Ma, J. Sakamoto and M. Chi, Nat. Mater., 2021, 20, 1485–1490 CrossRef CAS PubMed.
- R. Chen, C. Yao, Q. Yang, H. Pan, X. Yu, K. Zhang and H. Li, ACS Appl. Mater. Interfaces, 2021, 13, 18743–18749 CrossRef CAS PubMed.
- J. Mao, C. Ye, S. Zhang, F. Xie, R. Zeng, K. Davey, Z. Guo and S. Qiao, Energy Environ. Sci., 2022, 15, 2732–2752 RSC.
- B. S. Vishnugopi, M. T. Hasan, H. Zhou and P. P. Mukherjee, ACS Energy Lett., 2022, 8, 398–407 CrossRef.
- Y. Zhu and Y. Mo, Angew. Chem., Int. Ed., 2020, 59, 17472–17476 CrossRef CAS PubMed.
- Y. Li, J. Cheng, J. Li, Z. Zeng, Y. Guo, H. Zhang, H. Liu, X. Xu, Y. Rao, D. Li and L. Ci, J. Power Sources, 2022, 542, 231794 CrossRef CAS.
- H. Liu, Q. Zhu, Y. Liang, C. Wang, D. Li, X. Zhao, L. Gao and L.-Z. Fan, Chem. Eng. J., 2023, 462, 142183 CrossRef CAS.
- F. Zhao, S. H. Alahakoon, K. Adair, S. Zhang, W. Xia, W. Li, C. Yu, R. Feng, Y. Hu, J. Liang, X. Lin, Y. Zhao, X. Yang, T. K. Sham, H. Huang, L. Zhang, S. Zhao, S. Lu, Y. Huang and X. Sun, Adv. Mater., 2021, 33, e2006577 CrossRef PubMed.
- J. Liang, N. Chen, X. Li, X. Li, K. R. Adair, J. Li, C. Wang, C. Yu, M. Norouzi Banis, L. Zhang, S. Zhao, S. Lu, H. Huang, R. Li, Y. Huang and X. Sun, Chem. Mater., 2020, 32, 2664–2672 CrossRef CAS.
- G.-R. Zhu, Q. Zhang, Q.-S. Liu, Q.-Y. Bai, Y.-Z. Quan, Y. Gao, G. Wu and Y.-Z. Wang, Nat. Commun., 2023, 14, 4617 CrossRef CAS PubMed.
- F. N. Jiang, X. B. Cheng, S. J. Yang, J. Xie, H. Yuan, L. Liu, J. Q. Huang and Q. Zhang, Adv. Mater., 2023, 35, 2209114 CrossRef CAS PubMed.
- C. Wang, J. Liang, J. T. Kim and X. Sun, Sci. Adv., 2022, 8, eadc9516 CrossRef CAS PubMed.
- X. Li, J. Liang, N. Chen, J. Luo, K. R. Adair, C. Wang, M. N. Banis, T.-K. Sham, L. Zhang, S. Zhao, S. Lu, H. Huang, R. Li and X. Sun, Angew. Chem., Int. Ed., 2019, 58, 16427–16432 CrossRef CAS PubMed.
- X. Chen, Z. Jia, H. Lv, C. Wang, N. Zhao and X. Guo, J. Power Sources, 2022, 545, 231939 CrossRef CAS.
- S. Wang, X. Xu, C. Cui, C. Zeng, J. Liang, J. Fu, R. Zhang, T. Zhai and H. Li, Adv. Funct. Mater., 2021, 32, 2108805 CrossRef.
- W. D. Jung, M. Jeon, S. S. Shin, J.-S. Kim, H.-G. Jung, B.-K. Kim, J.-H. Lee, Y.-C. Chung and H. Kim, ACS Omega, 2020, 5, 26015–26022 CrossRef CAS PubMed.
- X. Lu, Y. Wang, X. Xu, B. Yan, T. Wu and L. Lu, Adv. Energy Mater., 2023, 13, 2301746 CrossRef CAS.
- L.-Z. Fan, H. He and C.-W. Nan, Nat. Rev. Mater., 2021, 6, 1003–1019 CrossRef CAS.
- Y.-N. Liu, Z. Xiao, W.-K. Zhang, J. Zhang, H. Huang, Y.-P. Gan, X.-P. He, G. G. Kumar and Y. Xia, Rare Met., 2022, 41, 3762–3773 CrossRef CAS.
- Q. Liang, L. Chen, J. Tang, X. Liu, J. Liu, M. Tang and Z. Wang, Energy Storage Mater., 2023, 55, 847–856 CrossRef.
- Z. Cui, E. Drioli and Y. M. Lee, Prog. Polym. Sci., 2014, 39, 164–198 CrossRef CAS.
- S. Zhang, Z. Li, Y. Guo, L. Cai, P. Manikandan, K. Zhao, Y. Li and V. G. Pol, Chem. Eng. J., 2020, 400, 125996 CrossRef CAS.
- L. Han, L. Wang, Z. Chen, Y. Kan, Y. Hu, H. Zhang and X. He, Adv. Funct. Mater., 2023, 33, 2300892 CrossRef CAS.
- J. Wan, J. Xie, X. Kong, Z. Liu, K. Liu, F. Shi, A. Pei, H. Chen, W. Chen, J. Chen, X. Zhang, L. Zong, J. Wang, L.-Q. Chen, J. Qin and Y. Cui, Nat. Nanotechnol., 2019, 14, 705–711 CrossRef CAS PubMed.
- Y. Cui, J. Wan, Y. Ye, K. Liu, L. Y. Chou and Y. Cui, Nano Lett., 2020, 20, 1686–1692 CrossRef CAS PubMed.
- W. Tang, S. Tang, C. Zhang, Q. Ma, Q. Xiang, Y.-W. Yang and J. Luo, Adv. Energy Mater., 2018, 8, 1800866 CrossRef.
- D. Cai, D. Wang, Y. Chen, S. Zhang, X. Wang, X. Xia and J. Tu, Chem. Eng. J., 2020, 394, 124993 CrossRef CAS.
- P. Shi, J. Ma, M. Liu, S. Guo, Y. Huang, S. Wang, L. Zhang, L. Chen, K. Yang, X. Liu, Y. Li, X. An, D. Zhang, X. Cheng, Q. Li, W. Lv, G. Zhong, Y.-B. He and F. Kang, Nat. Nanotechnol., 2023, 18, 602–610 CrossRef CAS PubMed.
- Z. Wu, Z. Xie, J. Wang, T. Yu, X. Du, Z. Wang, X. Hao, A. Abudula and G. Guan, Int. J. Hydrogen Energy, 2020, 45, 19601–19610 CrossRef CAS.
- S. H.-S. Cheng, K.-Q. He, Y. Liu, J.-W. Zha, M. Kamruzzaman, R. L.-W. Ma, Z.-M. Dang, R. K. Y. Li and C. Y. Chung, Electrochim. Acta, 2017, 253, 430–438 CrossRef CAS.
- K. Deng, Q. Zeng, D. Wang, Z. Liu, G. Wang, Z. Qiu, Y. Zhang, M. Xiao and Y. Meng, Energy Storage Mater., 2020, 32, 425–447 CrossRef.
- J. Zhao, M. Li, H. Su, Y. Liu, P. Bai, H. Liu, L. Ma, W. Li, J. Sun and Y. Xu, Small Methods, 2023, 7, 2300228 CrossRef CAS PubMed.
- H. Duan, C. Wang, R. Yu, W. Li, J. Fu, X. Yang, X. Lin, M. Zheng, X. Li, S. Deng, X. Hao, R. Li, J. Wang, H. Huang and X. Sun, Adv. Energy Mater., 2023, 13, 2300815 CrossRef CAS.
- X. Fu, H. Duan, L. Zhang, Y. Hu and Y. Deng, Adv. Funct. Mater., 2023, 33, 2308022 CrossRef CAS.
- L. Hu, J. Wang, K. Wang, Z. Gu, Z. Xi, H. Li, F. Chen, Y. Wang, Z. Li and C. Ma, Nat. Commun., 2023, 14, 3807 CrossRef CAS PubMed.
- H. Pan, M. Zhang, Z. Cheng, H. Jiang, J. Yang, P. Wang, P. He and H. Zhou, Sci. Adv., 2022, 8, eabn4372 CrossRef CAS PubMed.
- J. Yu, Q. Liu, X. Hu, S. Wang, J. Wu, B. Liang, C. Han, F. Kang and B. Li, Energy Storage Mater., 2022, 46, 68–75 CrossRef.
- X. Yang, M. Jiang, X. Gao, D. Bao, Q. Sun, N. Holmes, H. Duan, S. Mukherjee, K. Adair, C. Zhao, J. Liang, W. Li, J. Li, Y. Liu, H. Huang, L. Zhang, S. Lu, Q. Lu, R. Li, C. V. Singh and X. Sun, Energy Environ. Sci., 2020, 13, 1318–1325 RSC.
- Q. Ruan, M. Yao, J. Lu, Y. Wang, J. Kong, H. Zhang and S. Zhang, Energy Storage Mater., 2023, 54, 294–303 CrossRef.
- X. Yang, Q. Yin, C. Wang, K. Doyle-Davis, X. Sun and X. Li, Prog. Mater. Sci., 2023, 140, 101193 CrossRef CAS.
- M. Cai, Y. Dong, M. Xie, W. Dong, C. Dong, P. Dai, H. Zhang, X. Wang, X. Sun, S. Zhang, M. Yoon, H. Xu, Y. Ge, J. Li and F. Huang, Nat. Energy, 2023, 8, 159–168 CrossRef CAS.
- S. Lee, K.-S. Lee, S. Kim, K. Yoon, S. Han, M. H. Lee, Y. Ko, J. H. Noh, W. Kim and K. Kang, Sci. Adv., 2022, 8, eabq0153 CrossRef CAS PubMed.
- Y. Chen, J. Qian, X. Hu, Y. Ma, Y. Li, T. Xue, T. Yu, L. Li, F. Wu and R. Chen, Adv. Mater., 2023, 35, 2212096 CrossRef CAS PubMed.
- H. Wan, Z. Wang, W. Zhang, X. He and C. Wang, Nature, 2023, 623, 739–744 CrossRef CAS PubMed.
- W. Zhou, S. Wang, Y. Li, S. Xin, A. Manthiram and J. B. Goodenough, J. Am. Chem. Soc., 2016, 138, 9385–9388 CrossRef CAS PubMed.
- W. Kong, Z. Jiang, Y. Liu, Q. Han, L.-X. Ding, S. Wang and H. Wang, Adv. Funct. Mater., 2023, 33, 2306748 CrossRef CAS.
- C. Wang, K. Adair, J. Liang, X. Li, Y. Sun, X. Li, J. Wang, Q. Sun, F. Zhao, X. Lin, R. Li, H. Huang, L. Zhang, R. Yang, S.-G. Lu and X. Sun, Adv. Funct. Mater., 2019, 29, 1900392 CrossRef.
- D. Lin, W. Liu, Y. Liu, H. R. Lee, P.-C. Hsu, K. Liu and Y. Cui, Nano Lett., 2016, 16, 459–465 CrossRef CAS PubMed.
- Q. Zhou, J. Ma, S. Dong, X. Li and G. Cui, Adv. Mater., 2019, 31, 1902029 CrossRef CAS PubMed.
- S. Choudhury, Z. Tu, A. Nijamudheen, M. J. Zachman, S. Stalin, Y. Deng, Q. Zhao, D. Vu, L. F. Kourkoutis, J. L. Mendoza-Cortes and L. A. Archer, Nat. Commun., 2019, 10, 3091 CrossRef PubMed.
- S.-J. Yang, N. Yao, F.-N. Jiang, J. Xie, S.-Y. Sun, X. Chen, H. Yuan, X.-B. Cheng, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202214545 CrossRef CAS PubMed.
- H. Duan, M. Fan, W.-P. Chen, J.-Y. Li, P.-F. Wang, W.-P. Wang, J.-L. Shi, Y.-X. Yin, L.-J. Wan and Y.-G. Guo, Adv. Mater., 2019, 31, 1807789 CrossRef PubMed.
- H. Xu, G. Cao, Y. Shen, Y. Yu, J. Hu, Z. Wang and G. Shao, Energy Environ. Mater., 2022, 5, 852–864 CrossRef CAS.
- K. Wang, Z. Liang, S. Weng, Y. Ding, Y. Su, Y. Wu, H. Zhong, A. Fu, Y. Sun, M. Luo, J. Yan, X. Wang and Y. Yang, ACS Energy Lett., 2023, 8, 3450–3459 CrossRef CAS.
- C. Liao, C. Yu, S. Chen, C. Wei, Z. Wu, S. Chen, Z. Jiang, S. Cheng and J. Xie, Renewables, 2023, 1, 266–276 CrossRef.
- F. Li, J. Li, F. Zhu, T. Liu, B. Xu, T.-H. Kim, M. J. Kramer, C. Ma, L. Zhou and C.-W. Nan, Matter, 2019, 1, 1001–1016 CrossRef.
- Z. Zhao, Z. Wen, X. Liu, H. Yang, S. Chen, C. Li, H. Lv, F. Wu, B. Wu and D. Mu, Chem. Eng. J., 2021, 405, 127031 CrossRef CAS.
- T. Yu, J. Liang, L. Luo, L. Wang, F. Zhao, G. Xu, X. Bai, R. Yang, S. Zhao, J. Wang, J. Yu and X. Sun, Adv. Energy Mater., 2021, 11, 2101915 CrossRef CAS.
- S. Zhang, F. Zhao, S. Wang, J. Liang, J. Wang, C. Wang, H. Zhang, K. Adair, W. Li, M. Li, H. Duan, Y. Zhao, R. Yu, R. Li, H. Huang, L. Zhang, S. Zhao, S. Lu, T.-K. Sham, Y. Mo and X. Sun, Adv. Energy Mater., 2021, 11, 2100836 CrossRef CAS.
- J. Wang, R. Chen, L. Yang, M. Zan, P. Chen, Y. Li, W. Li, H. Yu, X. Yu, X. Huang, L. Chen and H. Li, Adv. Mater., 2022, 34, 2200655 CrossRef CAS PubMed.
- J. Jiao, G. Lai, S. Qin, C. Fang, X. Xu, Y. Jiang, C. Ouyang and J. Zheng, Acta Mater., 2022, 238, 118229 CrossRef CAS.
- H.-J. Noh, S. Youn, C. S. Yoon and Y.-K. Sun, J. Power Sources, 2013, 233, 121–130 CrossRef CAS.
- L. Geng, J. Liu, D. L. Wood, Y. F. Qin, W. Lu, C. J. Jafta, Y. Bai and I. Belharouak, ACS Appl. Energy Mater., 2020, 3, 7058–7065 CrossRef CAS.
- J.-N. Zhang, Q. Li, C. Ouyang, X. Yu, M. Ge, X. Huang, E. Hu, C. Ma, S. Li, R. Xiao, W. Yang, Y. Chu, Y. Liu, H. Yu, X.-Q. Yang, X. Huang, L. Chen and H. Li, Nat. Energy, 2019, 4, 594–603 CrossRef CAS.
- L. Zou, J. Li, Z. Liu, G. Wang, A. Manthiram and C. Wang, Nat. Commun., 2019, 10, 3447 CrossRef PubMed.
- R. Sim, Z. Cui and A. Manthiram, ACS Energy Lett., 2023, 8, 5143–5148 CrossRef CAS.
- H.-H. Ryu, H.-W. Lim, S. G. Lee and Y.-K. Sun, Nat. Energy, 2024, 9, 47–56 CrossRef CAS.
- Y. Wang, Z. Wang, D. Wu, Q. Niu, P. Lu, T. Ma, Y. Su, L. Chen, H. Li and F. Wu, eScience, 2022, 2, 537–545 CrossRef.
- S. Sun, C.-Z. Zhao, H. Yuan, Z.-H. Fu, X. Chen, Y. Lu, Y.-F. Li, J.-K. Hu, J. Dong, J.-Q. Huang, M. Ouyang and Q. Zhang, Sci. Adv., 2022, 8, eadd5189 CrossRef CAS PubMed.
- X. Yang, X. Gao, M. Jiang, J. Luo, J. Yan, J. Fu, H. Duan, S. Zhao, Y. Tang, R. Yang, R. Li, J. Wang, H. Huang, X. Sun and C. V. Singh, Angew. Chem., Int. Ed., 2023, 62, e202215680 CrossRef CAS PubMed.
- T. Dai, S. Wu, Y. Lu, Y. Yang, Y. Liu, C. Chang, X. Rong, R. Xiao, J. Zhao, Y. Liu, W. Wang, L. Chen and Y.-S. Hu, Nat. Energy, 2023, 8, 1221–1228 CrossRef CAS.
- F. Han, A. S. Westover, J. Yue, X. Fan, F. Wang, M. Chi, D. N. Leonard, N. J. Dudney, H. Wang and C. Wang, Nat. Energy, 2019, 4, 187–196 CrossRef CAS.
- B. Shao, Y. Huang and F. Han, Adv. Energy Mater., 2023, 13, 2204098 CrossRef CAS.
- B. Jang, J. Woo, Y. B. Song, H. Kwak, J. Park, J. S. Kim, H. Lim and Y. S. Jung, Energy Storage Mater., 2024, 65, 103154 CrossRef.
- H.-K. Tian, Z. Liu, Y. Ji, L.-Q. Chen and Y. Qi, Chem. Mater., 2019, 31, 7351–7359 CrossRef CAS.
- X. W. Chi, M. L. Li, J. C. Di, P. Bai, L. N. Song, X. X. Wang, F. Li, S. Liang, J. J. Xu and J. H. Yu, Nature, 2021, 592, 551–557 CrossRef CAS PubMed.
- J.-M. Doux, H. Nguyen, D. H. S. Tan, A. Banerjee, X. Wang, E. A. Wu, C. Jo, H. Yang and Y. S. Meng, Adv. Energy Mater., 2020, 10, 1903253 CrossRef CAS.
- J. Sang, B. Tang, Y. Qiu, Y. Fang, K. Pan and Z. Zhou, Energy Environ. Mater., 2024, e12670 CrossRef CAS.
- L. Qian, T. Or, Y. Zheng, M. Li, D. Karim, A. Cui, M. Ahmed, W. Park Hey, Z. Zhang, Y. Deng, A. Yu, Z. Chen and K. Amine, Renewables, 2023, 1, 114–141 CrossRef.
- S.-Y. Ham, H. Yang, O. Nunez-cuacuas, D. H. S. Tan, Y.-T. Chen, G. Deysher, A. Cronk, P. Ridley, J.-M. Doux, E. A. Wu, J. Jang and Y. S. Meng, Energy Storage Mater., 2023, 55, 455–462 CrossRef.
- B. Lu, W. Bao, W. Yao, J.-M. Doux, C. Fang and Y. S. Meng, J. Electrochem. Soc., 2022, 169, 070537 CrossRef CAS.
- S. Zhang, F. Zhao, J. Chen, J. Fu, J. Luo, S. H. Alahakoon, L.-Y. Chang, R. Feng, M. Shakouri, J. Liang, Y. Zhao, X. Li, L. He, Y. Huang, T.-K. Sham and X. Sun, Nat. Commun., 2023, 14, 3780 CrossRef PubMed.
- C. Zhu, T. Fuchs, S. A. L. Weber, F. H. Richter, G. Glasser, F. Weber, H.-J. Butt, J. Janek and R. Berger, Nat. Commun., 2023, 14, 1300 CrossRef CAS PubMed.
- Z. Ahmad, T. Xie, C. Maheshwari, J. C. Grossman and V. Viswanathan, ACS Cent. Sci., 2018, 4, 996–1006 CrossRef CAS PubMed.
- J. H. Cho, C. N. Im, C. H. Choi, S. H. Ha, H. K. Yoon, Y. Choi and J. Bae, Electrochim. Acta, 2020, 35, 136612 CrossRef.
- T. Inoue and K. Mukai, ACS Appl. Mater. Interfaces, 2017, 9, 1507–1515 CrossRef CAS PubMed.
- X. Feng, M. Fang, X. He, M. Ouyang, L. Lu, H. Wang and M. Zhang, J. Power Sources, 2014, 255, 294–301 CrossRef CAS.
- C. Wang, T. Deng, X. Fan, M. Zheng, R. Yu, Q. Lu, H. Duan, H. Huang, C. Wang and X. Sun, Joule, 2022, 6, 1770–1781 CrossRef CAS.
- D. Cao, K. Zhang, W. Li, Y. Zhang, T. Ji, X. Zhao, E. Cakmak, J. Zhu, Y. Cao and H. Zhu, Adv. Funct. Mater., 2023, 33, 2307998 CrossRef CAS.
- C. Yuan, B. W. Sheldon and J. Xu, Adv. Energy Mater., 2022, 12, 2201804 CrossRef CAS.
- G. Lu, W. Liu, Z. Yang, Y. Wang, W. Zheng, R. Deng, R. Wang, L. Lu and C. Xu, Adv. Funct. Mater., 2023, 33, 2304407 CrossRef CAS.
- O. Furat, D. P. Finegan, Z. Yang, M. Neumann, S. Kim, T. R. Tanim, P. Weddle, K. Smith and V. Schmidt, Energy Storage Mater., 2024, 64, 103036 CrossRef.
- T. M. M. Heenan, I. Mombrini, A. Llewellyn, S. Checchia, C. Tan, M. J. Johnson, A. Jnawali, G. Garbarino, R. Jervis, D. J. L. Brett, M. Di Michiel and P. R. Shearing, Nature, 2023, 617, 507–512 CrossRef CAS PubMed.
- J.-P. Schmiegel, M. Leißing, F. Weddeling, F. Horsthemke, J. Reiter, Q. Fan, S. Nowak, M. Winter and T. Placke, J. Electrochem. Soc., 2020, 167, 060516 CrossRef CAS.
- J. Gu, X. Chen, R. Ma, Z. He, Z. Liang, H. Zhong, Y. Su, J. Shi and Y. Yang, Energy Storage Mater., 2023, 63, 103052 CrossRef.
- X. Wu, G. Ji, J. Wang, G. Zhou and Z. Liang, Adv. Mater., 2023, 35, 2301540 CrossRef CAS PubMed.
- J. Fleming, T. Amietszajew, J. Charmet, A. J. Roberts, D. Greenwood and R. Bhagat, J. Energy Storage, 2019, 22, 36–43 CrossRef.
- D. P. Finegan, M. Scheel, J. B. Robinson, B. Tjaden, I. Hunt, T. J. Mason, J. Millichamp, M. Di Michiel, G. J. Offer, G. Hinds, D. J. L. Brett and P. R. Shearing, Nat. Commun., 2015, 6, 6924 CrossRef CAS PubMed.
- Y. Zhang, J. Feng, J. Qin, Y. L. Zhong, S. Zhang, H. Wang, J. Bell, Z. Guo and P. Song, Adv. Sci., 2023, 10, 2301056 CrossRef CAS PubMed.
- R. S. Longchamps, X.-G. Yang and C.-Y. Wang, ACS Energy Lett., 2022, 7, 1103–1111 CrossRef CAS.
- J. Deng, X. Li, C. Li, T. Wang, R. Liang, S. Li, Q. Huang and G. Zhang, J. Energy Storage, 2023, 72, 108313 CrossRef.
- X. Li, Q. Huang, J. Deng, G. Zhang, Z. Zhong and F. He, J. Power Sources, 2020, 451, 227820 CrossRef CAS.
- Z. Rao, P. Lyu, P. Du, D. He, Y. Huo and C. Liu, Battery Energy, 2022, 1, 20210019 CrossRef CAS.
- J. Gu, Z. Liang, J. Shi and Y. Yang, Adv. Energy Mater., 2023, 13, 2203153 CrossRef CAS.
- W. Mei, Z. Liu, C. Wang, C. Wu, Y. Liu, P. Liu, X. Xia, X. Xue, X. Han, J. Sun, G. Xiao, H.-Y. Tam, J. Albert, Q. Wang and T. Guo, Nat. Commun., 2023, 14, 5251 CrossRef CAS.
- P. Liu, L. Yang, B. Xiao, H. Wang, L. Li, S. Ye, Y. Li, X. Ren, X. Ouyang, J. Hu, F. Pan, Q. Zhang and J. Liu, Adv. Funct. Mater., 2022, 32, 2208586 CrossRef CAS.
- Y. Lv, X. Geng, W. Luo, T. Chu, H. Li, D. Liu, H. Cheng, J. Chen, X. He and C. Li, J. Energy Storage, 2023, 72, 108389 CrossRef.
- S. Yuan, C. Chang, S. Yan, P. Zhou, X. Qian, M. Yuan and K. Liu, J. Energy Chem., 2021, 62, 262–280 CrossRef CAS.
- Z. Jia, Y. Min, P. Qin, W. Mei, X. Meng, K. Jin, J. Sun and Q. Wang, J. Energy Chem., 2024, 89, 195–207 CrossRef CAS.
- X.-Q. Xu, X.-B. Cheng, F.-N. Jiang, S.-J. Yang, D. Ren, P. Shi, H. Hsu, H. Yuan, J.-Q. Huang, M. Ouyang and Q. Zhang, SusMat, 2022, 2, 435–444 CrossRef CAS.
- M. Hu, Z. Tong, C. Cui, T. Zhai and H. Li, Nano Lett., 2022, 22, 3047–3053 CrossRef CAS.
- S.-J. Yang, N. Yao, X.-Q. Xu, F.-N. Jiang, X. Chen, H. Liu, H. Yuan, J.-Q. Huang and X.-B. Cheng, J. Mater. Chem. A, 2021, 9, 19664–19668 RSC.
- S.-J. Yang, F.-N. Jiang, J.-K. Hu, H. Yuan, X.-B. Cheng, S. Kaskel, Q. Zhang and J.-Q. Huang, Electron, 2023, 1, e8 CrossRef.
- Z. J. Baum, R. E. Bird, X. Yu and J. Ma, ACS Energy Lett., 2022, 7, 712–719 CrossRef CAS.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS PubMed.
- X. Yu, W. Li, V. Gupta, H. Gao, D. Tran, S. Sarwar and Z. Chen, Global Challenges, 2022, 6, 2200099 CrossRef.
- L. Azhari, S. Bong, X. Ma and Y. Wang, Matter, 2020, 3, 1845–1861 CrossRef.
- D. H. Kim, D. Y. Oh, K. H. Park, Y. E. Choi, Y. J. Nam, H. A. Lee, S.-M. Lee and Y. S. Jung, Nano Lett., 2017, 17, 3013–3020 CrossRef CAS PubMed.
- A. Miura, N. C. Rosero-Navarro, A. Sakuda, K. Tadanaga, N. H. H. Phuc, A. Matsuda, N. Machida, A. Hayashi and M. Tatsumisago, Nat. Rev. Chem., 2019, 3, 189–198 CrossRef CAS.
- T. Yang, C. Wang, W. Zhang, Y. Xia, H. Huang, Y. Gan, X. He, X. Xia, X. Tao and J. Zhang, J. Energy Chem., 2023, 84, 189–209 CrossRef CAS.
- Y. Ishiguro, K. Ueno, S. Nishimura, G. Iida and Y. Igarashib, Chem. Lett., 2023, 52, 237–241 CrossRef CAS.
- H. Wan, B. Zhang, S. Liu, Z. Wang, J. Xu and C. Wang, Adv. Energy Mater., 2024, 14, 2303046 CrossRef CAS.
- Z. Wang, X. Lin, Y. Han, J. Cai, S. Wu, X. Yu and J. Li, Nano Energy, 2021, 89, 106337 CrossRef CAS.
- Y. Xiao, Y. Wang, S.-H. Bo, J. C. Kim, L. Miara and G. Ceder, Nat. Rev. Mater., 2020, 5, 105–126 CrossRef CAS.
- S. Zhang, J. Ma, S. Dong and G. Cui, Electrochem. Energy Rev., 2023, 6, 4 CrossRef.
- H. Liu, Y. Zhao, J. Zhou, P. Li, S.-H. Bo and S.-L. Chen, ACS Appl. Energy Mater., 2020, 3, 1260–1264 CrossRef CAS.
- J. Zhou, Y. Zhao, H. Liu, X. Tang, S.-L. Chen and S.-H. Bo, J. Mater. Res., 2022, 37, 3283–3296 CrossRef CAS.
- K. Liu, Z. Wei, C. Zhang, Y. Shang, R. Teodorescu and Q. L. Han, IEEE/CAA J. Autom. Sin., 2022, 9, 1139–1165 Search PubMed.
- J. Tian, R. Xiong, W. Shen, J. Lu and X.-G. Yang, Joule, 2021, 5, 1521–1534 CrossRef.
- L. Bravo Diaz, X. He, Z. Hu, F. Restuccia, M. Marinescu, J. V. Barreras, Y. Patel, G. Offer and G. Rein, J. Electrochem. Soc., 2020, 167, 090559 CrossRef.
Footnote |
| † Y. L. and Z. R. contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2024 |







