Solvating lithium and tethering aluminium using di-coordination-strength anions for low-temperature lithium metal batteries†
Jin-Xiu
Chen
a,
Jin-Hao
Zhang
a,
Xiao-Zhong
Fan
a,
Fang-Fang
Wang
a,
Wen
Tang
b,
Wei
Xia
*b,
Yusheng
Zhao
*bc and
Long
Kong
 *a
*a
aInstitute of Flexible Electronics (IFE), Northwestern Polytechnical University, Xi'an 710129, China. E-mail: iamlkong@nwpu.edu.cn
bEastern Institute for Advanced Study, Eastern Institute of Technology, Ningbo 315201, China. E-mail: wxia@eitech.edu.cn; yzhao@eitech.edu.cn
cDepartment of Physics, Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen 518055, China
First published on 13th March 2024
Abstract
Lithium bis(fluorosulfonyl)imide (LiFSI) corrodes aluminium (Al) current collector, but this corrosion can be impeded using di-coordination-strength anions. This concept adopts the weakly coordinated anion (FSI−) to exert high ionic transport kinetics, and the strongly coordinated anion (nitride, NO3−) to stabilize the Al foil surface. Such simultaneous Li solvation and Al tethering, highlight the importance of solvation chemistry to mediating Li transport and Al corrosion.
Broader contextLow-temperature batteries need high-disassociation lithium (Li) salts to increase the charge carriers for favorable transport kinetics under harsh operation conditions. Lithium bis(fluorosulfonyl)imide (LiFSI) is being developed for low-temperature electrolytes due to its weakly coordinated anion that enables a high dissociation constant. However, LiFSI corrodes aluminium (Al) foil and causes electron pathways to disconnect between the active material and Al current collector, endangering battery stability at high voltage. Here, a new concept of di-coordination-strength anions is proposed to circumvent this dilemma. The concept is based on solvation chemistry where Li is solvated and Al is simultaneously tethered through weakly and strongly coordinated anions: the weakly coordinated anion (FSI−) exerts high ionic transport kinetics while the strongly coordinated anion (nitride, NO3−) stabilizes the Al surface. The electrolyte achieves impressive low-temperature performance even at a high areal loading of 4.5 mA h cm−2. The cell equipped with this electrolyte can be operated for 120 and 200 cycles at −20 °C and 25 °C with a capacity retention of 80% and 85%, respectively. This work is expected to highlight the use of solvation chemistry in mediating Li transport and Al corrosion to promote low-temperature Li metal battery performance. |
Introduction
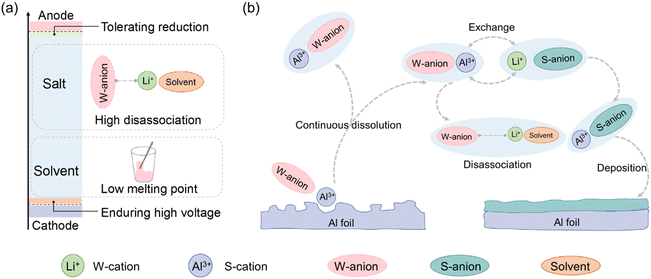
Emerging electrochemical energy applications demand high power output at low temperatures, such as aerospace, polar exploration, and deep-sea operations, and therefore have pressing requirements for lithium–ion (Li–ion) batteries.1–7 The main challenges restricting battery performance at subzero conditions are sluggish redox and transport kinetics across the bulk and electrode/electrolyte interface that arise from a large desolvation energy barrier, low species mobility and inherent interfacial impedance.1,8–12 These challenges are critically linked to low-temperature solvation chemistry through the coordination competition among cations, anions and solvents (Fig. 1a),9,13–16 leading to the formation of charged or uncharged species that are generally grouped into solvent-separated ion pair (SSIP), contact ion pair (CIP) and aggregate (AGG).17–20 SSIP is more weighted in the electrolyte design, since excessive CIP or AGG in electrolytes contribute to a decrease in charged species and slow down ion mobility, both of which impact internal charge migration and reduce external current delivery during battery operation at low temperature.9,20–23Selecting Li salts with anions that have weak coordinating strength toward Li+ benefits the quantity of SSIP in the electrolyte and promotes rapid Li+ transport11,24 (Fig. 1a). However, such Li salts generally engender corrosion for aluminium (Al) current collectors, leading to the rapid capacity degradation in a relatively high voltage. For example, lithium bis(fluorosulfonyl)imide (LiFSI), an amide-based Li salt to be widely used in low-temperature batteries,25–27 has a high-valence N atom in the center, and strong electron-withdrawing F atom at the terminal sites, which make the electrons of the FSI− anion delocalize and thus reduce coordination strength toward Li+.28 The high dissociation in bulk electrolyte and beneficial fluorination of the solid electrolyte interphase (SEI) of LiFSI have been widely corroborated to boost low-temperature battery performance.21,29–31 Nonetheless, extensive adoption of LiFSI to replace lithium hexafluorophosphate (LiPF6) would corrode Al foil, which restricts the broad application of LiFSI-based electrolytes at low temperature.32–35 There have been proposed corrosion mechanisms for Al foil with weak-coordination amide-based Li salts.32,36–38 A classic one is considered as the formation and dissolution of [Al–imide anion] complexes,32,39,40 which accelerates Al corrosion and results in detachment of active electrode materials from the Al current collector.41 Therefore, the interface stability of the Al current collector in the presence of a low coordination strength anion is of great importance and interest in low-temperature battery fields.
Two sets of approaches are ubiquitously employed to circumvent the above dilemma. The straightforward one is based on physical separation of the electrolyte from Al foil by coating with an inert functional layer, such as previously reported Al-doped ZnO,42 CrOx,43 graphene oxide (GO),44,45 graphene46,47 and conductive polymers.48 Another approach can be categorized to trap solvents and de-activate their solvation capability through recently proposed concepts of high concentrated electrolytes and locally high concentrated electrolytes, which can be attributed to the quantity reduction of free solvents and consequently mitigate the formation of soluble [Al–imide anion] complexes.49–54
A more general consideration for stabilizing the Al foil with the presence of a weak coordination anion is the significant reduction of Al species solubility using an in situ high-voltage enduring layer on the Al side. Once Al3+ is generated (Al2O3 → 2Al3+ + 3/2O2 + 6e−, Al → Al3+ + 3e−) under relatively high voltage, strong anions are needed to bind with Al3+ and locally deposit on the Al surface to avoid the continuous exposure of the fresh Al surface. For example, Al foil does not corrode with LiPF6-based electrolytes below 4.3 V, due to the relatively strong coordination strength that promotes Al(PF6)n precipitation and finally develops into a protective layer to locally protect the Al surface from further corrosion, as evidenced by the presence of Al–P–F–O containing species.36 Extraordinarily strong anions inherently facilitate salt precipitation due to easy formation of AGG and clusters, like lithium fluoride (LiF) that is rarely soluble in aprotic electrolytes.
The salt disassociation and precipitation mechanism based on anion chemistry encouraged us to adopt electrolytes with di-coordination strength anions to bifunctionally solvate Li and tether the Al in Li metal batteries. The Li salt with weak coordinating strength anion (FSI−), guarantees sufficient SSIP to promote excellent dynamics of Li+ transport, while the Li salt with a strong coordinating anion (e.g., NO3−) necessarily binds with Al3+ to prevent dissolution of Al species (Fig. 1b). Thanks to the above two aspects, the cell with dominant LiFSI exhibits stable operation in both ambient and low temperature conditions at relative high voltage (∼4.4 V), a value that was previously considered unstable in battery cycling with LiFSI.37,55 The concept of di-coordination strength anions to protect the Al surface and promote Li transport are experimentally and computationally verified through potentiostatic etching and molecular dynamics simulations. These benefits endow the cell with stable operation over 120 and 200 cycles at −20 °C and room temperature, respectively, even with the high areal capacity of NCM622 electrode (~4.5 mA h cm−2). Although a body of literature has reported the protection of the Al surface using coating technology, inhibition of Al corrosion based on electrolyte solvation chemistry has rarely been considered, and such a di-coordination strength anion concept based on electrolyte solvation chemistry provides an alternative approach to balance Li+ transport kinetics and Al protection at low temperatures in Li metal batteries.
Results and discussion
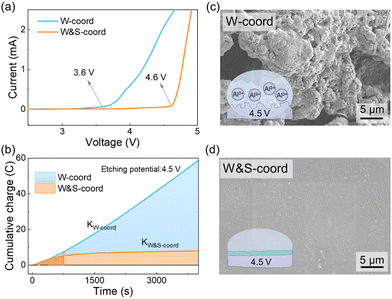
The concept of di-coordination strength anions is proven by deliberately selecting an electrolyte formulation for a low-temperature Li metal battery system. The LiFSI with weakly coordinated anion is used to facilitate SSIP species for better transport kinetics,22,56 while ethyl methyl carbonate (EMC) and fluoroethylene carbonate (FEC) exhibit desired low-temperature performance and excellent film forming capability, respectively.57 LiNO3 with a strong coordination anion is dissolved in 1,2-dimethoxyethane (DME), which is then mixed with the above ester mixture to obtain an electrolyte with di-coordination strength anions. The detailed description of electrolyte preparation are available in the ESI.†Linear sweep voltammetry (LSV) was performed to acquire the Al anodic stability against different electrolytes in Li‖Al cells. The Li‖Al cell with 1 M LiFSI in DME/FEC/EMC (denoted as W-coord) delivers a significant anodic current at 3.6 V vs. Li/Li+ (Fig. 2a), which is attributed to the corrosion of the Al surface by LiFSI instead of DME decomposition. The detailed causes that exclude DME decomposition in the current system are available in the ESI.† Incorporation of LiNO3 in the W-coord electrolyte, forming the W&S-coord electrolyte, markedly extends the threshold of the upper voltage to 4.6 V. As a strong Lewis acid, Al3+ coordinated by solvents results in dissolution of Al(solvent)n3+ and corrosion of the Al foil. However, if strongly coordinated anions are introduced to regulate the solvation structure of Al3+, they tend to bind with Al3+ and aggregate into a cluster, which subsequently precipitate locally to prevent further corrosion until higher voltage is required (>4.6 V).
The stability of the Al surface is reflected by the leakage current that serves as an indicator to quantify the rate of parasitic reactions, using chronoamperometry of a Li‖Al cell at 4.5 V (Fig. S3, ESI†). The cumulative charge as a function of time is obtained by integrating time and current, with the slope representing the corrosion rate. The cell with W&S-coord electrolyte exhibits a similar rate to the cell with W-coord electrolyte in the initial stage, while its slope close to 0 from the marked point shown in Fig. 2b, indicating the negligible corrosion of Al foil. The initial corrosion current in the W&S-coord cell originates from surface passivation reactions that involve redox impurities and Al-species dissolution and precipitation during NO3− and FSI− coordination competition to Al3+. On the contrary, the cell with W-coord electrolyte exhibits a stable yet high Al corrosion rate throughout the entire potentiostatic process, reflecting that the passivation layer fails to inhibit Al corrosion. The weakly coordinated anion (FSI−) promotes the Al–FSI–solvent complex that can be dissolved in the bulk electrolyte, endowing the FSI−-based cell with a profound corrosive charge. The anti-corrosive effect of the Al surface imparted by NO3− is visually corroborated using scanning electron microscopy (SEM) images obtained after potentiostatic etching measurement. The Al surface with FSI− is characterized by plenty of pits and holes, indicating the aggressive corrosion on the Al surface (Fig. 2c). This is in sharp contrast to the Al surface with NO3− anion, which is nearly intact, fully proving the importance of strongly coordinated anions in the inhibition of Al corrosion (Fig. 2d). The schematic illustration of Al corrosion in the presence of the different coordinated electrolytes is inserted in Fig. 2c and d. To further illustrate the anti-corrosive effect of NO3− for Al foil, the Li‖Al cells were also assembled to etch at −20 °C. The Al surface with W-anion electrolyte is pitted with deep holes, implying that some Al3+ has been solvated and diffused into the bulk electrolyte. On the contrary, the smooth Al surface is observed for the W&S-anion cell (Fig. S4, ESI†). The SEM images of fresh Al foil have also been provided in Fig. S5 (ESI†).
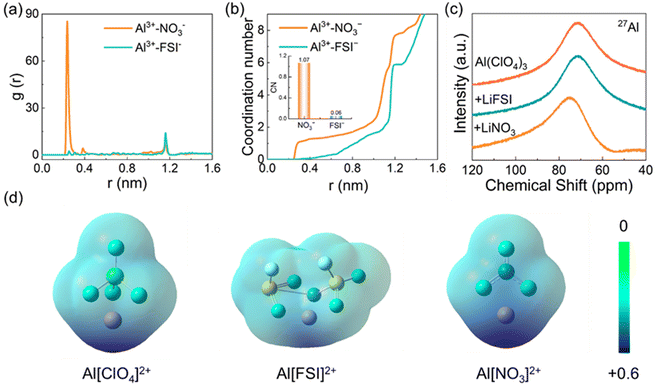
Ab initio molecular dynamics (AIMD) simulations were performed to probe the ion solvation environment, which affords a molecule-level clue to reveal an Al-protective mechanism enabled by di-coordination-strength anions. The detailed electrolyte formulations to be modelled are presented in the ESI.† The radial distribution function (RDF) shows a pronounced peak for the Al3+–NO3− interaction at 0.36 nm (Fig. 3a), significantly dwarfing the Al3+–FSI−, which implies that NO3− is more likely to exist in the first solvation sheath of Al3+ due to the stronger coordination capability. The FSI− anions are forced to the second sheath at ∼1.1 nm. The RDF derived coordination number around Al3+ is quantified at 0.36 nm (the first sheath). The coordination number of NO3− is ∼1.07, while that of FSI− is almost 0, unambiguously hinting that NO3− preferentially coordinates with Al3+ (Fig. 3b). This cation–anion interaction implies that NO3− readily binds with Al and prevents the Al complex dissolution and diffusion. It is worth noting that the high-voltage performance is not due to oxidation-intolerant solvents being squeezed out of the first solvated layer after the addition of NO3−, since the sufficient solvents are presented in the Al current collector surface, and these solvents have high statistical probability to contact with the Al surface. If these solvents exhibit low anodic decomposition voltage, the leakage current can be recorded in the potentiostatic etching measurement. Meanwhile, the limited amount of DME in the W&S-coord electrolyte does not statistically interact with Al3+ (Fig. S6, ESI†).58 On the contrary, the coordination number of FSI− is about 1.5 at a distance of 0.4 nm from Al3+ in the W-coord electrolyte (Fig. S7a, ESI†). The detailed information of Al3+-DME, Al3+-EMC and Al3+-FEC in W-coord electrolyte also shows a huge difference from the W&S-coord electrolyte (Fig. S7b and c, ESI†). According to the RDF and coordination number, the possible solvation structure of Al3+ is depicted in Fig. S8 (ESI†). And the simulation snapshots are shown in Fig. S9 (ESI†).
To experimentally explore anion coordination strength derived ion species, selected salts are dissolved in N,N-dimethylformamide (DMF) for nuclear magnetic resonance (NMR). The Al–imide salts [Al(FSI)3] are not commercially available, and their previously reported preparation processes are complex59,60 and beyond our ability to synthesize. Therefore, the perchlorate (ClO4−) with a moderate coordination strength for Al3+ is selected as a benchmark to evaluate anion coordination strength. According to the literature, the sequence of anion coordination strength follows FSI− < ClO4− < NO3−,61,62 and anion coordination competition results show the variation of electron cloud around Al3+. To this end, a DMF solution containing Al(ClO4)3 is prepared and then added in equal molar LiNO3 and LiFSI to monitor the 27Al and 17O signals.
A down-field shift can be observed in the 27Al NMR spectra with the addition of LiNO3, suggesting that the NO3− is involved in the solvation structure of Al3+ (Fig. 3c). The stronger electron absorbing capability of NO3− over DMF takes responsibility for the down-field shift of 27Al and weakens its coordination to DMF molecules. The atomic charge distribution in various molecules also supports the conclusion of NMR (Fig. 3d). The electron cloud density of Al in Al[NO3]2+ is lower than that in Al[FSI]2+ and Al[ClO4]2+. On the contrary, no significant shift was detected when an equal molar quantity of LiFSI was added, since the coordination strength of FSI− is weaker than that of DMF and ClO4−. The weaker coordination strength of FSI− renders it to locate outside the sheath of Al3+ while DMF molecules dominated the first solvation sheath of Al3+, leading to the negligible resonance in 27Al spectra. The 27Al NMR spectra imply that Al3+ has a higher binding tendency with NO3− than FSI−, indicating the stronger ion–ion interaction between Al3+ and NO3−. Meanwhile, the binding energy of Al3+ and NO3− is much higher than that of Li+ and NO3−, unambiguously indicating that Al3+ has a greater tendency to interact with NO3− and prevent further Al corrosion (Fig. S10, ESI†).
Furthermore, although the strong binding energy between NO3− and Li+ results in prevalent LiNO3 with the CIP and AGG structure in DME, the limited amount of LiNO3/DME in the entire electrolyte (<90 vol%) does not affect Li ion conduction in the bulk electrolyte and Li anode interface. The ionic conductivities of the W-anion and W&S-anion electrolytes are 3.3 and 3.1 mS cm−1 at −20 °C. The Li ion conduction across the interface is corroborated by the Li–Cu cell at −20 °C (Fig. S11, ESI†), showing the lower Li plating overpotential. The detailed discussion can be found in the ESI.†
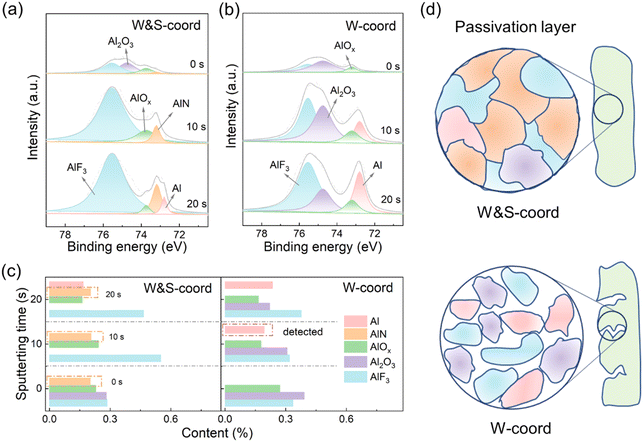
X-ray photoelectron spectroscopy (XPS) is conducted to explore the Al interface evolution in the presence of NO3− after potentiostatic etching. The Al 2p spectra of XPS can be deconvoluted in Al (72.8 eV), AlN (73.74 eV), Al2O3 (74.75 eV) and AlF3 (75.54 eV), as shown in Fig. 4a and b.37,63,64 The Al–N compounds are generated from the decomposition of Al3+–NO3− species on the Al surface at relatively high voltage, and such Al–N compounds are considered to protect Al foil.65 In the case of the electrolyte without NO3−, the corroded Al3+ cannot be tethered on the Al surface and suffers from continuous migration toward the electrolyte, so a tiny amount of Al–N compound can be found in the surface composition. Another worthy observation is the Al metal signal in the XPS measurement. Since the surface of the Al foil is corroded to generate cracks (Fig. 2c), the Al metal signal can be detected earlier (Fig. 4b). These chemical evolutions are statistically plotted as a function of the different etching depths (Fig. 4c). A clear implication is the relatively stable content of the Al–N compound, indicating that NO3− could readily coordinate with Al3+ to form a uniform passivation layer in the W&S-coord electrolyte, while the Al metal signal can be noticeably detected in the W-coord electrolyte due to the formation of pits and holes. And the distribution of various elements at different depths is also given with the N 1s, F 1s, Li 1s spectra of XPS in Fig. S12–S14 (ESI†). Based on the SEM observations (Fig. 2c and d) and chemical evolution (Fig. 4a–c), a schematic diagram of the Al foil surface is shown in Fig. 4d. The passivation layer containing the Al–N compound is more compact and uniform due to tethering Al3+ with the help of NO3−.
Meanwhile, the obvious cracks and pores are finally developed in the W-coord electrolyte, which is incurred by the dissolution of Al3+.
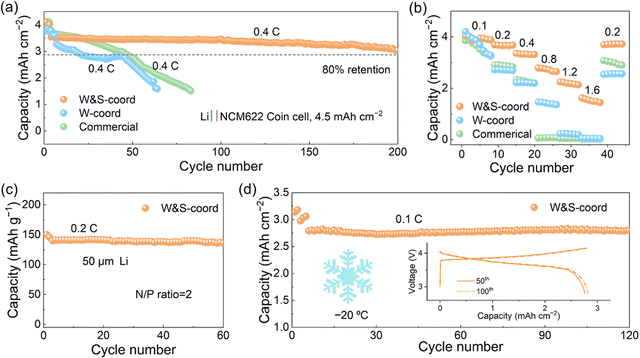
In order to evaluate the effect of di-coordination strength anions on battery performance, the Li‖NCM622 cells with the proposed electrolytes were tested at 25 °C (Fig. 5a), with a high areal capacity of 4.5 mA h cm−2 corresponding to an areal mass loading of 27 mg cm−2. The cell with the W&S-coord electrolyte can be cycled for more than 200 cycles at a current density of 1.8 mA cm−2, maintaining a capacity retention of 85%. However, the cell with W-coord electrolyte exhibits a sharp capacity decay attributed to the corrosion of the Al current collector that causes the disconnection of the electron pathway between the active material and Al current collector. In contrast, such corrosion can be inhibited in the W&S-coord electrolyte by virtue of the strongly coordinated anion. For the cell with commercial electrolyte (1 M LiPF6 in EC/DEC), it survives only 50 cycles due to the unstable SEI and the uncontrolled Li dendrite growth (Fig. 5a).66 In addition, remarkable rate capability is achieved with the support of the W&S-coord electrolyte (Fig. 5b), delivering an area capacity of 1.5 mA h cm−2 at 1.6 C. The cell with W-coord and commercial electrolyte cannot work even at 1.2 C and 0.8 C, respectively. It may originate from the formation of “dead” Li, since mossy and dendritic Li morphology are observed on Cu foils in the Li‖Cu cells with a capacity of 1 mA h cm−2 at 0.5 mA cm−2 (Fig. S15, ESI†). The improved rate capability of cells with the W&S-coord electrolyte can be ascribed to the highly disassociated LiFSI that assists greater ionic conductivity,67 as well as protecting the Li anode surface (Fig. S16, ESI†) and stable cathode structure (Fig. S17, ESI†).68 Based on the excellent performance of proposed electrolyte, Li‖NMC622 coin cells were evaluated by pairing with 50 μm ultra-thin Li with a negative/positive (N/P) capacity ratio of 2 to tap its potential under practical conditions. The cell can work for more than 60 cycles at 25 °C without distinct capacity decline (Fig. 5c).
As shown in Fig. 2a, the electrolyte also has a high voltage tolerance because of the strongly coordinated anion NO3−, which sustains the cell for 50 cycles even at a cut-off voltage of 4.3 V (Fig. S18a, ESI†). The charge/discharge profiles display the negligible increase of polarization after 45 cycles (Fig. S18b, ESI†). More importantly, the cell containing electrolyte based on di-coordination strength anions survives more than 120 cycles at −20 °C with a capacity retention of 85%. The identical charge and discharge curves (inset in Fig. 5d) at the 50th and 100th cycles indicate the highly reversible capacity under low temperature. To more clearly identify the high voltage tolerance of the electrolyte, Li‖NCM811 cells were conducted with the upper cut-off voltage of 4.4 V at −20 °C (Fig. S19a, ESI†). The cells matched the various NCM811 loading yield with a capacity retention above 98% after 60 cycles. The charge/discharge curves are shown in Fig. S19b–d (ESI†). In summary, the electrochemical performance of the cells equipped with di-coordination strength electrolyte are markedly improved compared with the cells with commercial and W-coord electrolyte, unambiguously stressing the importance of beneficial ionic transport and favorable Al surface stabilization.
The low temperature favors the ion–ion interaction, which leads to decreased SSIP and charge transport. To this end, highly dissociated Li salts with a weakly coordinating anion (like FSI−) are advocated. However, the Li salts with weakly coordinating anion corrode the Al current collector, which requires a strong anion (like NO3−) to compete with the weak anion and form Al–NO3 deposits to prevent further Al corrosion. Based on this rational consideration, the concept of di-coordination-strength anions is unambiguously proposed to simultaneously address the kinetic issues related to low temperature performance and corrosion issues related to rapid cell failure. This concept is basically different from previous ones that use LiNO3 to alter the SEI component or Helmholtz layer.69–71 In addition, the currently proposed concept can be placed in the broad context of electrolyte design for low temperature Li metal batteries. There are several Li salts having a high donor number.72 This concept provides a general principle to rationally select appropriate anion pairs for advanced electrolytes to further improve low-temperature battery performance, which is another point that this work would like to highlight.
Conclusions
In summary, the concept of di-coordination-strength anions is proposed to concurrently solvate Li and tether Al, simultaneously achieving beneficial ion transport and favorable Al stability. The weakly coordinated FSI− anion facilitates charge transport, but it is unable to coordinate with Al3+ to form a stable complex, which would be dissociated into electrolytes and results in continuous Al corrosion. The strongly coordinated anion NO3− was introduced into the electrolyte to interact with Al3+ and such a complex is in situ gathered to form a stable layer, as evidenced by developing a uniform passivation layer containing the AlN compound on the Al foil surface. The electrolyte based on di-coordination-strength anions balanced ionic transport and Al stability, endowing NCM622‖Li and NCM811‖Li cells with superior low-temperature electrochemical performance. This work reveals the effect of ion–ion interaction on inhibiting Al3+ dissolution based on solvation chemistry, and provides a new point for inhibiting Al corrosion while retaining rapid ionic transport in low-temperature Li metal batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22379121), Shenzhen Research Foundation (JCYJ20210324104412034), Fundamental Research Funds for the Central Universities (G2022KY0606), and the Zhejiang Province Key Laboratory of Flexible Electronics (2023FE005). We thank Ms Jia-Yue Duan, Mr Xiong Xiao and Ms Zhen-Zhen Dong for helpful discussion.Notes and references
- Y. Tian, C. Lin, X. Chen, X. Yu, R. Xiong and Q. Zhang, Energy Storage Mater., 2023, 56, 412–423 CrossRef.
- Z. Li, Y. Yao, S. Sun, C. Jin, N. Yao, C. Yan and Q. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202303888 CrossRef CAS PubMed.
- Y. Qin, P. Zuo, X. Chen, W. Yuan, R. Huang, X. Yang, J. Du, L. Lu, X. Han and M. Ouyang, J. Energy Chem., 2022, 72, 442–452 CrossRef CAS.
- M. Qin, Z. Zeng, Q. Wu, X. Liu, Q. Liu, S. Cheng and J. Xie, J. Energy Chem., 2023, 85, 49–57 CrossRef CAS.
- S. Zhang, N. S. Andreas, R. Li, N. Zhang, C. Sun, D. Lu, T. Gao, L. Chen and X. Fan, Energy Storage Mater., 2022, 48, 44–73 CrossRef.
- J. Chen, Y. Peng, Y. Yin, M. Liu, Z. Fang, Y. Xie, B. Chen, Y. Cao, L. Xing, J. Huang, Y. Wang, X. Dong and Y. Xia, Energy Environ. Sci., 2022, 15, 3360–3368 RSC.
- S. Kim, J.-A. Lee, T. K. Lee, K. Baek, J. Kim, B. Kim, J. H. Byun, H.-W. Lee, S. J. Kang, J.-A. Choi, S.-Y. Lee, M.-H. Choi, J.-H. Lee and N.-S. Choi, Energy Environ. Sci., 2023, 16, 5108 RSC.
- S. G. Yoon, K. A. Cavallaro, B. J. Park, H. Yook, J. W. Han and M. T. McDowell, Adv. Funct. Mater., 2023, 33, 2302778 CrossRef CAS.
- R. L. Hou, S. H. Guo and H. S. Zhou, Adv. Energy Mater., 2023, 13, 2300053 CrossRef CAS.
- D. Hu, G. Chen, J. Tian, N. Li, L. Chen, Y. Su, T. Song, Y. Lu, D. Cao, S. Chen and F. Wu, J. Energy Chem., 2021, 60, 104–110 CrossRef CAS.
- Z. Li, N. Fu and Z. Yang, Particuology, 2023, 83, 129–141 CrossRef CAS.
- Z. Liu, L. Li, L. Qin, S. Guo, G. Fang, Z. Luo and S. Liang, Adv. Mater., 2022, 34, 2204681 CrossRef CAS PubMed.
- H. Hu, J. Li, Q. Zhang, G. Ding, J. Liu, Y. Dong, K. Zhao, M. Yu, H. Wang and F. Cheng, Chem. Eng. J., 2023, 457, 141273 CrossRef CAS.
- G. Leverick and Y. Shao-Horn, Adv. Energy Mater., 2023, 13, 2204094 CrossRef CAS.
- I. S. Buyuker, B. Pei, H. Zhou, X. Cao, Z. Yu, S. Liu, W. Zhang, W. Xu, J.-G. Zhang, Z. Bao, Y. Cui, C. Wang and M. S. Whittingham, ACS Energy Lett., 2023, 8, 1735–1743 CrossRef CAS.
- Q. Zhang, Y. Ma, Y. Lu, L. Li, F. Wan, K. Zhang and J. Chen, Nat. Commun., 2020, 11, 4463 CrossRef CAS PubMed.
- Z. Yu, N. P. Balsara, O. Borodin, A. A. Gewirth, N. T. Hahn, E. J. Maginn, K. A. Persson, V. Srinivasan, M. F. Toney, K. Xu, K. R. Zavadil, L. A. Curtiss and L. Cheng, ACS Energy Lett., 2022, 7, 461–470 CrossRef CAS.
- B. Nan, L. Chen, N. D. Rodrigo, O. Borodin, N. Piao, J. Xia, T. Pollard, S. Hou, J. Zhang, X. Ji, J. Xu, X. Zhang, L. Ma, X. He, S. Liu, H. Wan, E. Hu, W. Zhang, K. Xu, X.-Q. Yang, B. Lucht and C. Wang, Angew. Chem., Int. Ed., 2022, 61, e202205967 CrossRef CAS PubMed.
- N. Sun, R. Li, Y. Zhao, H. Zhang, J. Chen, J. Xu, Z. Li, X. Fan, X. Yao and Z. Peng, Adv. Energy Mater., 2022, 12, 2200621 CrossRef CAS.
- J. Holoubek, K. Kim, Y. J. Yin, Z. H. Wu, H. D. Liu, M. Q. Li, A. Chen, H. P. Gao, G. R. Cai, T. A. Pascal, P. Liu and Z. Chen, Energy Environ. Sci., 2022, 15, 1647–1658 RSC.
- H. V. Ramasamy, S. Kim, E. J. Adams, H. Rao and V. G. Pol, Chem. Commun., 2022, 58, 5124–5127 RSC.
- H. Zhang, Z. Zeng, F. Ma, Q. Wu, X. Wang, S. Cheng and J. Xie, Angew. Chem., Int. Ed., 2023, 62, e202300771 CrossRef CAS PubMed.
- J. Shi, C. Xu, J. Lai, Z. Li, Y. Zhang, Y. Liu, K. Ding, Y.-P. Cai, R. Shang and Q. Zheng, Angew. Chem., Int. Ed., 2023, 62, e202218151 CrossRef CAS PubMed.
- W. Lin, J. D. Li, J. S. Wang, K. C. Gu, H. Li, Z. Xu, K. X. Wang, F. Wang, M. Y. Zhu, Y. Fan, H. B. Wang, G. J. Tao, N. Liu, M. F. Ding, S. Chen, J. Wu and Y. X. Tang, Small, 2023, 19, 2207093 CrossRef CAS PubMed.
- W. Zhang, Y. Lu, L. Wan, P. Zhou, Y. Xia, S. Yan, X. Chen, H. Zhou, H. Dong and K. Liu, Nat. Commun., 2022, 13, 2029 CrossRef CAS PubMed.
- H. Q. Pham, G. J. Chung, J. Han, E.-H. Hwang, Y.-G. Kwon and S.-W. Song, J. Chem. Phys., 2020, 152, 094709 CrossRef CAS PubMed.
- L. Li, S. Zhou, H. Han, H. Li, J. Nie, M. Armand, Z. Zhou and X. Huang, J. Electrochem. Soc., 2011, 158, A74 CrossRef CAS.
- W. Linert, A. Camard, M. Armand and C. Michot, Coord. Chem. Rev., 2002, 226, 137–141 CrossRef CAS.
- Z. Li, Y.-X. Yao, S. Sun, C.-B. Jin, N. Yao, C. Yan and Q. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202303888 CrossRef CAS PubMed.
- T. D. Pham, A. Bin Faheem and K.-K. Lee, Small, 2021, 17, 2103375 CrossRef CAS PubMed.
- G. Yang, Y. Li, S. Liu, S. Zhang, Z. Wang and L. Chen, Energy Storage Mater., 2019, 23, 350–357 CrossRef.
- C. L. Li, S. W. Zeng, P. Wang, Z. J. Li, L. Yang, D. N. Zhao, J. Wang, H. N. Liu and S. Y. Li, Trans. Nonferrous Met. Soc. China, 2021, 31, 1439–1451 CrossRef CAS.
- L. J. Krause, W. Lamanna, J. Summerfield, M. Engle, G. Korba, R. Loch and R. Atanasoski, J. Power Sources, 1997, 68, 320–325 CrossRef CAS.
- A. Abouimrane, J. Ding and I. J. Davidson, J. Power Sources, 2009, 189, 693–696 CrossRef CAS.
- H. Wu, Z. Song, X. Wang, W. Feng, Z. Zhou and H. Zhang, Nano Res., 2023, 16, 8269–8280 CrossRef CAS.
- P. Meister, X. Qi, R. Kloepsch, E. Kramer, B. Streipert, M. Winter and T. Placke, ChemSusChem, 2017, 10, 804–814 CrossRef CAS PubMed.
- E. Cho, J. Mun, O. B. Chae, O. M. Kwon, H. T. Kim, J. H. Ryu, Y. G. Kim and S. M. Oh, Electrochem. Commun., 2012, 22, 1–3 CrossRef CAS.
- C. Luo, Y. Li, W. Sun, P. Xiao, S. Liu, D. Wang and C. Zheng, Electrochim. Acta, 2022, 419, 140353 CrossRef CAS.
- L. PÉTer and J. Arai, J. Appl. Electrochem., 1999, 29, 1053–1061 CrossRef.
- X. Wang, E. Yasukawa and S. Mori, Electrochim. Acta, 2000, 45, 2677–2684 CrossRef CAS.
- P. Du, D. Liu, X. Chen, H. Xie, X. Qu, D. Wang and H. Yin, Energy Storage Mater., 2023, 57, 371–399 CrossRef.
- G. Teucher, T. Van Gestel, M. Krott, H.-G. Gehrke, R.-A. Eichel and S. Uhlenbruck, Thin Solid Films, 2016, 619, 302–307 CrossRef CAS.
- N. Piao, L. Wang, T. Anwar, X. Feng, S. E. Sheng, G. Tian, J. Wang, Y. Tang and X. He, Corros. Sci., 2019, 158, 108100 CrossRef CAS.
- E. J. Yoon, J. H. Lee, S. M. Byun, D. Y. Kim and T. H. Yoon, Adv. Funct. Mater., 2022, 32, 2200026 CrossRef CAS.
- S. J. Richard Prabakar, Y.-H. Hwang, E. G. Bae, D. K. Lee and M. Pyo, Carbon, 2013, 52, 128–136 CrossRef CAS.
- X. Li, S. Deng, M. N. Banis, K. Doyle-Davis, D. Zhang, T. Zhang, J. Yang, R. Divigalpitiya, F. Brandys, R. Li and X. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 32826–32832 CrossRef CAS PubMed.
- S. Y. Kim, Y. I. Song, J.-H. Wee, C. H. Kim, B. W. Ahn, J. W. Lee, S. J. Shu, M. Terrones, Y. A. Kim and C.-M. Yang, Carbon, 2019, 153, 495–503 CrossRef CAS.
- D. Lepage, L. Savignac, M. Saulnier, S. Gervais and S. B. Schougaard, Electrochem. Commun., 2019, 102, 1–4 CrossRef CAS.
- F. Ren, Z. Li, J. Chen, P. Huguet, Z. Peng and S. Deabate, ACS Appl. Mater. Interfaces, 2022, 14, 4211–4219 CrossRef CAS PubMed.
- J.-D. Xie, J. Patra, P. Chandra Rath, W.-J. Liu, C.-Y. Su, S.-W. Lee, C.-J. Tseng, Y. A. Gandomi and J.-K. Chang, J. Power Sources, 2020, 450, 227657 CrossRef CAS.
- C. Zhang, S. Gu, D. Zhang, J. Ma, H. Zheng, M. Zheng, R. Lv, K. Yu, J. Wu, X. Wang, Q.-H. Yang, F. Kang and W. Lv, Energy Storage Mater., 2022, 52, 355–364 CrossRef.
- J. Wang, Y. Yamada, K. Sodeyama, C. H. Chiang, Y. Tateyama and A. Yamada, Nat. Commun., 2016, 7, 12032 CrossRef CAS PubMed.
- Z. Wang, L.-P. Hou, Q.-K. Zhang, N. Yao, A. Chen, J.-Q. Huang and X.-Q. Zhang, Chin. Chem. Lett., 2023, 108570 Search PubMed.
- D. W. McOwen, D. M. Seo, O. Borodin, J. Vatamanu, P. D. Boyle and W. A. Henderson, Energy Environ. Sci., 2014, 7, 416–426 RSC.
- K. Hirata, T. Kawase and Y. Sumida, J. Electrochem. Soc., 2020, 167, 140534 CrossRef CAS.
- J. Holoubek, H. Liu, Z. Wu, Y. Yin, X. Xing, G. Cai, S. Yu, H. Zhou, T. A. Pascal, Z. Chen and P. Liu, Nat. Energy, 2021, 6, 303–313 CrossRef CAS PubMed.
- R. Deng, F. Chu, F. Kwofie, Z. Guan, J. Chen and F. Wu, Angew. Chem., Int. Ed., 2022, 61, e202215866 CrossRef CAS PubMed.
- C. Park, M. Kanduč, R. Chudoba, A. Ronneburg, S. Risse, M. Ballauff and J. Dzubiella, J. Power Sources, 2018, 373, 70–78 CrossRef CAS.
- J. Krummacher and A. Balducci, Chem. Mater., 2018, 30, 4857–4863 CrossRef CAS.
- M. Chiku, S. Matsumura, H. Takeda, E. Higuchi and H. Inoue, J. Electrochem. Soc., 2017, 164, A1841–A1844 CrossRef CAS.
- D. Y. Kim, N. J. Singh and K. S. Kim, J. Chem. Theory Comput., 2008, 4, 1401–1407 CrossRef CAS PubMed.
- C. W. Kim, J. Ahn, S. M. Kim, T. H. Noh and O.-S. Jung, Transition Met. Chem., 2011, 36, 545–551 CrossRef CAS.
- R. Hauert, J. Patscheider, M. Tobler and R. Zehringer, Surf. Sci., 1993, 292, 121–129 CrossRef CAS.
- C. J. Powell, J. Electron Spectrosc. Relat. Phenom., 2012, 185, 1–3 CrossRef CAS.
- C.-H. Yen, A. R. Neale, J. Lim, D. Bresser, L. J. Hardwick and C.-C. Hu, Electrochim. Acta, 2022, 431, 141105 CrossRef CAS.
- X. Hu, J. Liu, Y. Yang, Y. Liu, Q. Wu and J. Ma, Chin. Chem. Lett., 2023, 34, 108456 CrossRef CAS.
- Y. Cai, H. Zhang, Y. Cao, Q. Wang, B. Cao, Z. Zhou, F. Lv, W. Song, D. Duo and L. Yu, J. Power Sources, 2022, 535, 231481 CrossRef CAS.
- Z. Cui, Z. Guo and A. Manthiram, Angew. Chem., Int. Ed., 2023, 62, e202313437 CrossRef CAS PubMed.
- Y. Mao, T. Li, S. Abuelgasim, X. Hao, Y. Xiao, C. Li, W. Wang, Y. Li and E. Bao, J. Energy Storage, 2024, 79, 110215 CrossRef.
- L. Fu, X. Wang, L. Wang, M. Wan, Y. Li, Z. Cai, Y. Tan, G. Li, R. Zhan, Z. W. Seh and Y. Sun, Adv. Funct. Mater., 2021, 31, 2010602 CrossRef CAS.
- C. Yan, H.-R. Li, X. Chen, X.-Q. Zhang, X.-B. Cheng, R. Xu, J.-Q. Huang and Q. Zhang, J. Am. Chem. Soc., 2019, 141, 9422–9429 CrossRef CAS PubMed.
- M. Schmeisser, P. Illner, R. Puchta, A. Zahl and R. van Eldik, Chem. - Eur. J., 2012, 18, 10969–10982 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee03809b |
| This journal is © The Royal Society of Chemistry 2024 |