Unraveling the temperature-responsive solvation structure and interfacial chemistry for graphite anodes†
Yanbing
Mo
,
Gaopan
Liu
 ,
Jiawei
Chen
,
Xiao
Zhu
,
Yu
Peng
,
Yonggang
Wang
,
Jiawei
Chen
,
Xiao
Zhu
,
Yu
Peng
,
Yonggang
Wang
 ,
Congxiao
Wang
,
Xiaoli
Dong
,
Congxiao
Wang
,
Xiaoli
Dong
 * and
Yongyao
Xia
* and
Yongyao
Xia
 *
*
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), Fudan University, Shanghai 200433, China. E-mail: xldong@fudan.edu.cn; yyxia@fudan.edu.cn
First published on 14th November 2023
Abstract
The variation of temperature induces a corresponding transformation of the primary solvation structure of Li+ due to the competition coordination of solvents and anions with Li+. However, the specific variations and their effect on the interfacial chemistry are less-studied and ambiguous. Herein, the correlation of the temperature-responsive solvation structure, interfacial chemistry and electrochemical performance of graphite anodes is systematically investigated to figure out the structure–property relationships. Spectra analysis and molecular dynamics simulations reveal that increasing the temperature causes enhanced Li+–anion interaction and weakened Li+–solvent interaction in the primary solvation structure of Li+. This easily generates the anion-dominated solvation sheath and the corresponding inorganic-rich solid electrolyte interphase (SEI) with increasing temperature. However, the projected density-of-states calculations and thermal analysis witness that more solvents tend to be reduced at high temperatures, which results in an obvious increase of organic species in the interphase. Given the synergistic effect of the temperature-responsive solvation structure and thermal reduction, the SEI formed at 25 °C has been equipped as a stable LiF-rich inorganic film with moderate thickness and low energy barrier for smooth Li+ diffusion. These features enable graphite anodes with a super-fast rate capability of 256 mA h g−1 at 5C under 25 °C and high-capacity retention of 50.4% even at −45 °C compared to that at 25 °C. This study reveals the correlation between the temperature-responsive solvation structure and interfacial chemistry, providing a viewpoint on designing temperature-adaptative batteries.
Broader contextGraphite-based lithium-ion batteries have been extensively employed in portable devices and electric vehicles. However, their all-weather application is still limited due to the performance degradation when operated under harsh temperatures. This is largely affected by the ion movement kinetics in electrolytes and solid electrolyte interphase (SEI) on graphite. The formation of SEI is closely linked to the solvation structure of Li+ and reduction stability of coordinated components. The varied temperatures induce the solvation sheath of Li+, which influences the properties of the as-formed SEI and the performance of the graphite electrode. Nevertheless, the intricate relationship between temperature and these critical aspects remains ambiguous. In this work, the temperature effects on the solvation structure, interfacial chemistry, and performance of graphite anodes are uncovered. The solvation sheath of Li+ is demonstrated to spontaneously transform with more anions and less solvents participating in the first solvation sheath of Li+ as the temperature increases, which facilitates the generation of the inorganic-rich interphase. Meanwhile, the reduced reduction stability and slight thermal reduction of solvents lead to the increase of interfacial organic species at elevated temperature. The unique solvation structure and moderate reduction stability of the coordinated solvents at 25 °C ensure the generation of LiF-rich inorganic SEI with a low energy barrier for Li+ migration. These features enable excellent performance for graphite anodes with SEI formation at 25 °C, providing insights into the development of temperature-adaptative batteries. |
Introduction
Lithium-ion batteries (LIBs) are extensively employed to power portable devices and electric vehicles, whereas they still encounter great challenges when subjected to harsh temperatures.1–4 Operation under low temperatures usually leads to the undesirable capacity/energy deterioration of LIBs, limiting their applications in cold scenarios, which include military missions, polar expeditions and ocean exploration. It is generally considered that the degradation under subzero temperature should be attributed to the increased resistance, mainly arising from the slow de-solvation process and sluggish ion transport across the solid electrolyte interphase (SEI) on the low-potential graphite (Gr) side.5–8 This would thus cause huge polarization and severe capacity loss, which even propels the intercalation potential of the Gr anodes below 0 V (vs. Li+/Li) and makes it difficult to charge the batteries. A more critical concern is the occurrence of disastrous Li plating on the Gr anodes, leading to the irreversible depletion of active Li+ and rapid capacity deterioration.9–12 Different from the low-temperature conditions, elevating the operation temperature can accelerate the Li+ movement kinetics and charge transfer process. Nevertheless, despite the benefits of the accelerated rate performance and output power density, the enhanced side reactions at high temperatures cause the irreversible electrolyte consumption and more severe SEI reconstruction. As a consequence, this leads to the reduced interfacial stability and the thickening of the SEI film.13–16 Obviously, the operation temperature would impact the ion movements and thermal stability of the electrolyte components, which leaves the rocking-chair LIBs with the temperature-related electrochemical performance.During the operation of LIBs, ion movements in both the liquid electrolytes and the SEI film at the electrode/electrolyte interface exert significant influence on the polarization of the Gr anode side.17–20 Meanwhile, the properties of SEI are related to the electrolyte components, where the primary solvation sheath of Li+ in the electrolyte is considered as the origin of the SEI film. For example, more anions involved in the first solvation sheath of Li+ are believed to generate anion-derived inorganic-rich SEI, which has been universally acknowledged as guidance for facilitating the rapid migration of Li+ across the SEI films.7,21–24 It is worth noting that various types of interactions coexist in the liquid electrolytes in the microscopic view, among which the cation–anion and cation–solvent interactions are two prominent factors competitively affecting the primary solvation chemistry in the electrolytes. Specifically, the competing coordination of Li+ with the solvents and anions will induce the formation of diverse primary solvation structures, including solvent-separated ion pairs (SSIPs), contact ion pairs (CIPs), and aggregates (AGGs).25–28 Moreover, the variation of the temperatures can induce the corresponding changes in the coordination interaction of Li+ with solvents and anions, leading to the transformation of the primary solvation structure of Li+.29–32 The interaction between the electrolyte and the SEI becomes even more complex because the SEI formation process is closely linked to the reduction stability of the solvated components, especially when thermal reduction interferes at elevated temperature.13,33 This highlights the significant effect of temperature on the solvation sheath and SEI film. Nevertheless, despite these considerations, it still remains ambiguous how temperature impacts the solvation structure, interfacial chemistry and battery performance in the microscopic view. Therefore, a comprehensive and systematic investigation is imperative to elucidate the intricate relationship between the temperature and these critical aspects.
In this work, the temperature-responsive solvation structure and reduction stability of the solvated components are explored at the microscopic level to unravel the temperature-related interfacial chemistry and corresponding electrochemical performance of the Gr anodes. The temperature-variable nuclear magnetic resonance (NMR) and spectra analysis, together with molecular dynamics (MD) simulations, reveal the reduced ion–dipole interaction and the enhanced Li+–anion interaction with the increase of temperatures. This induces the generation of inorganic-rich SEI derived from the anion-dominated solvation sheath at elevated temperature. However, the projected density of states (PDOS) calculations and thermal analysis confirm that more solvents are prone to be reduced at high temperature, leading to the increase of the organic species. Moreover, the strengthened solvent coordination with Li+ at low temperature causes the high ratio of organic content in the inner SEI formed at −20 °C, resulting in the high energy barrier for Li+ diffusion and thus poor electrochemical performance. The unique solvation structure at 25 °C generates a LiF-rich inorganic SEI with medium thickness and minimal activation energy for smooth Li+ diffusion. Such SEI endows Gr anodes with a super-fast rate response of 256 mA h g−1 at 5C and long cycling stability with 93.3% capacity retention after 300 cycles. Furthermore, Gr anodes exhibit a high reversible capacity of 175 mA h g−1 at a lower temperature of −45 °C. This work reveals the temperature-responsive solvation structures and interfacial chemistry at the microscopic level, providing a guideline to understand the temperature-related performance of Gr-based LIBs.
Results and discussion
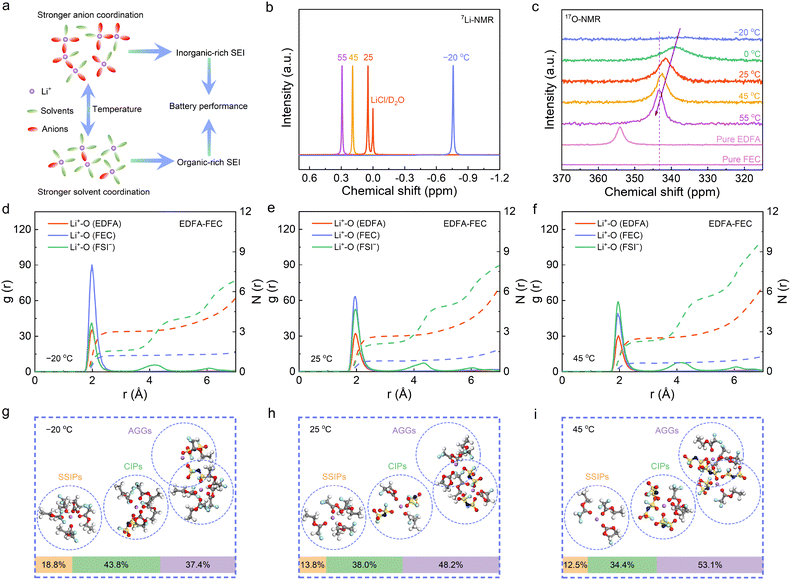
Temperature-responsive solvation structure
In a typical liquid electrolyte, Li+ ions exist in the form of solvated Li+ surrounded by polar solvents and anions. With the variable temperatures, the solvation sheath of Li+ usually undergoes a corresponding transformation due to the competition coordination of the solvent molecules and anions with the Li+ ions.29 Such a feature can significantly affect the interfacial chemistry and the electrochemical performance of batteries, especially at low temperature. The relationship between the temperature variety and solvation sheath of Li+, as well as the resultant SEI, is illustrated in Fig. 1(a). More specifically, the enhanced ion–dipole interaction leads to the formation of organic-rich SEI films on Gr anodes, and the increased Li+–anion interaction can instead generate inorganic-rich SEI films. Therefore, it is crucial to firstly comprehend how the solvation sheath changes with temperature, which will further provide the optimum formation temperature of the SEI film and insights into the understanding of the electrochemical behaviors of batteries. Noteworthily, most LIBs used in mobile devices and electric vehicles are generally recommended to operate within the temperature range from −20 °C to 45 °C.34 Accordingly, three representative temperatures including 45 °C, 25 °C and −20 °C are selected in order to track the variations under practical temperatures. Meanwhile, an optimized electrolyte, 1 mol L−1 lithium bis(fluorosulfonyl)imide (LiFSI) in ethyl difluoroacetate (EDFA)/fluoroethylene carbonate (FEC) (9/1 by volume, marked as EDFA-FEC), was utilized as a model electrolyte to ensure facile Li+ intercalation/de-intercalation in the Gr anodes due to its superior low-temperature ionic conductivity and weak solvation energy.35 Fourier transform infrared (FTIR) spectroscopy was performed to gain better insight into the solvation structure of the electrolyte (Fig. S1, ESI†). A redshift for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak can be detected after adding LiFSI compared to free EDFA, indicating the coordination of EDFA to Li+. Noteworthily, the shift in solvent mixtures (EDFA + FEC, Δ = 3.4 cm−1) is smaller than that in pure EDFA solvents (Δ = 5.7 cm−1) upon adding LiFSI, which implies a slight decrease of the coordinated EDFA. This can be attributed to the participation of FEC into the solvation sheath of Li+, as proved with the appearance of a new peak of solvated FEC. Afterwards, the 7Li-NMR spectra were recorded to unveil the solvation chemistry of the electrolyte with varied temperatures. A downfield shift of the 7Li signal is observed as the temperature increases (Fig. 1(b)), indicating the dispersion of the electron cloud around Li+ and the deshielding effects. This reflects the variation of the adjacent chemical environment of the Li+ ions, indicating the weakening of the ion solvation or a decrease of ion pairs. Unfortunately, the 7Li-NMR spectra alone are inadequate to distinguish the relative strength of the two interactions. Therefore, the 17O-NMR spectra were obtained to reveal the variation of the coordination strength of solvents to Li+ with temperatures. The carbonyl 17O of EDFA and FEC solvents exhibits upfield chemical shifts with the addition of LiFSI (Fig. S2a, ESI†). This is due to the shielding effect of Li+ on the lone pair electron density, suggesting the coordination interaction of solvents with Li+.36 With the increasing temperature, the carbonyl 17O peak on solvated EDFA displays a downfield shift, an indication for the weakened coordination interaction between the carbonyl O of EDFA with Li+ (Fig. 1(c)). Moreover, the peak broadening at lower temperature is likely owing to the quadrupolar relaxation. This can be expected due to the slowed molecular reorientation and the weak anisotropic quadrupolar interaction of 17O at lower temperatures.37 A similar but lower downfield shift with increasing temperature can be observed for the carbonyl 17O peak on solvated FEC, showing the slightly weakened coordination of FEC with Li+ (Fig. S2b, ESI†). The varied solvation environments around Li+ with temperatures are consistent with the Raman spectra. The band around 739.9 cm−1 indicates that FSI− anions participate in the Li+ solvation sheath with the formation of ion pairs (Fig. S3, ESI†). As the temperature increases, the enhanced interaction between the FSI− anions and Li+ can be viewed from the blueshifted band of FSI− (Fig. S4a and b, ESI†). The intensive Li+–FSI− interaction with temperatures can be further confirmed by the blueshift of the band of the S–N–S stretching vibration with temperature for the 1 M LiFSI/EDFA electrolyte (Fig. S5, ESI†), which excludes the interference of the peaks of FEC. Moreover, the blueshift of the carbonyl O on EDFA further confirms a decrease of the coordinated EDFA in the Li+ solvation sheath with the increasing temperature (Fig. S4c, ESI†).
O stretching vibration peak can be detected after adding LiFSI compared to free EDFA, indicating the coordination of EDFA to Li+. Noteworthily, the shift in solvent mixtures (EDFA + FEC, Δ = 3.4 cm−1) is smaller than that in pure EDFA solvents (Δ = 5.7 cm−1) upon adding LiFSI, which implies a slight decrease of the coordinated EDFA. This can be attributed to the participation of FEC into the solvation sheath of Li+, as proved with the appearance of a new peak of solvated FEC. Afterwards, the 7Li-NMR spectra were recorded to unveil the solvation chemistry of the electrolyte with varied temperatures. A downfield shift of the 7Li signal is observed as the temperature increases (Fig. 1(b)), indicating the dispersion of the electron cloud around Li+ and the deshielding effects. This reflects the variation of the adjacent chemical environment of the Li+ ions, indicating the weakening of the ion solvation or a decrease of ion pairs. Unfortunately, the 7Li-NMR spectra alone are inadequate to distinguish the relative strength of the two interactions. Therefore, the 17O-NMR spectra were obtained to reveal the variation of the coordination strength of solvents to Li+ with temperatures. The carbonyl 17O of EDFA and FEC solvents exhibits upfield chemical shifts with the addition of LiFSI (Fig. S2a, ESI†). This is due to the shielding effect of Li+ on the lone pair electron density, suggesting the coordination interaction of solvents with Li+.36 With the increasing temperature, the carbonyl 17O peak on solvated EDFA displays a downfield shift, an indication for the weakened coordination interaction between the carbonyl O of EDFA with Li+ (Fig. 1(c)). Moreover, the peak broadening at lower temperature is likely owing to the quadrupolar relaxation. This can be expected due to the slowed molecular reorientation and the weak anisotropic quadrupolar interaction of 17O at lower temperatures.37 A similar but lower downfield shift with increasing temperature can be observed for the carbonyl 17O peak on solvated FEC, showing the slightly weakened coordination of FEC with Li+ (Fig. S2b, ESI†). The varied solvation environments around Li+ with temperatures are consistent with the Raman spectra. The band around 739.9 cm−1 indicates that FSI− anions participate in the Li+ solvation sheath with the formation of ion pairs (Fig. S3, ESI†). As the temperature increases, the enhanced interaction between the FSI− anions and Li+ can be viewed from the blueshifted band of FSI− (Fig. S4a and b, ESI†). The intensive Li+–FSI− interaction with temperatures can be further confirmed by the blueshift of the band of the S–N–S stretching vibration with temperature for the 1 M LiFSI/EDFA electrolyte (Fig. S5, ESI†), which excludes the interference of the peaks of FEC. Moreover, the blueshift of the carbonyl O on EDFA further confirms a decrease of the coordinated EDFA in the Li+ solvation sheath with the increasing temperature (Fig. S4c, ESI†).
To further achieve an explicit deciphering of the temperature effect on the solvation sheath of Li+, classical MD simulations were conducted (Fig. 1(d)–(f)). According to the radial distribution functions (RDFs) and coordination numbers, one Li+ is solvated by 2.63 EDFA solvents, 2.09 FSI− anions, and 0.79 FEC additives on average at 25 °C, verifying the weak solvation capability of the EDFA solvents. When the temperature decreases to −20 °C, the coordination number of EDFA increases to 2.99, while that of FSI− decreases to 1.58 in the primary solvation sheath of Li+. This suggests that the EDFA solvents tend to preferentially occupy the first solvation sheath of Li+ at a low temperature of −20 °C. Moreover, with the temperature rising from 25 °C to 45 °C, the average coordination number of the EDFA solvent mildly decreases from 2.63 to 2.49, indicating a reduced coordination interaction between the EDFA solvent and Li+ ions. Meanwhile, the average coordination number of the FSI− anions slightly increases (Table S1, ESI†), showing that more FSI− anions appear in the primary solvation sheath of Li+ under a high temperature of 45 °C. The weakened ion–dipole interaction and strengthened Li+–FSI− interaction with the increase of temperatures are further confirmed according to the MD simulations. The existent form of the FSI− anions can provide insights into the solvation structure of the electrolyte (that is, the SSIPs, CIPs and AGGs). The statistics are illustrated and compared in Fig. 1(g)–(i). At the low temperature of −20 °C, 18.8% of Li+ ions are coordinated in the form of SSIPs with a representative [Li-EDFA4FEC1]+ structure, while 43.8% of Li+ ions are bound to form CIPs with a representative [Li-EDFA3FEC1]+[FSI]− structure and the remaining Li+ coordinates to form the AGGs structure (37.4%, Fig. 1(g)). As the temperature increases to 25 °C, the solvation structure spontaneously transforms with more FSI− anions participating in the primary solvation sheath of Li+, contributing to the higher AGGs proportion of 48.2% and lower SSIPs proportion of 13.8% (Fig. 1(h)). The solvation structure further changes at 45 °C with the formation of a new CIPs structure and the proportion of AGGs boosting to 53.1% (Fig. 1(i)), indicating the enhanced interaction between the FSI− anions and Li+ ions. In addition, the proportion of SSIPs is decreased to 12.5% at 45 °C, verifying the lessened solvent coordination with Li+ and the reduced ion–dipole interaction with increasing temperature. The abovementioned MD simulations are in agreement with the temperature-variable NMR and Raman results, jointly corroborating the temperature-responsive solvation structure with weakened ion–solvent interaction and strengthened Li+–FSI− interaction as the temperature increases. It is worth mentioning that the weakened Li+–solvent interaction with elevated temperatures would facilitate the Li+ desolvation process at the electrode/electrolyte interface (Fig. S6, ESI†).
Temperature-dependent interphase chemistry
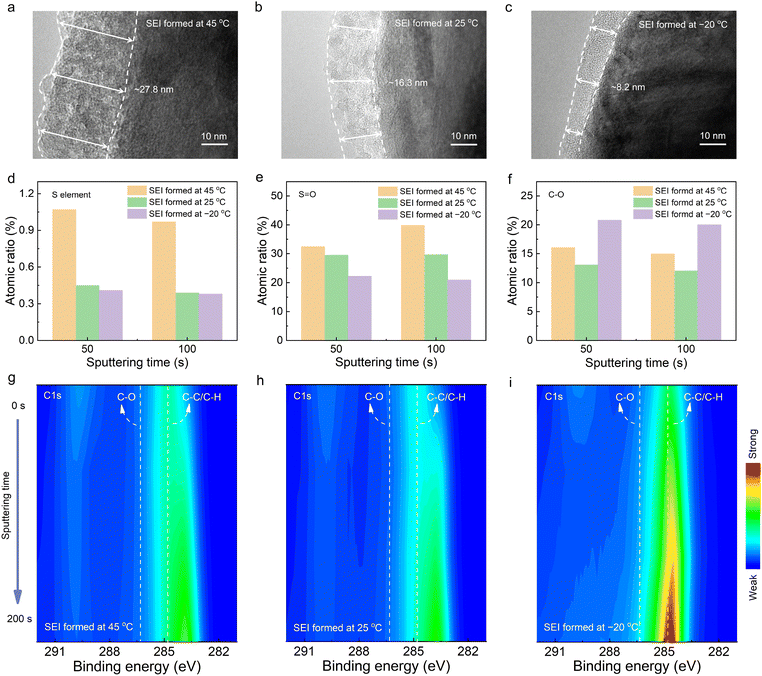
The primary solvation sheath of Li+ is generally considered as the precursor of the SEI, where its temperature-responsive variety would inevitably affect the interfacial behaviors, as well as the components and microstructures of the as-formed SEI film. Therefore, the interfacial film was further explored to establish a bridge between the temperature-responsive solvation sheath and interphase chemistry. Transmission electron microscope (TEM) characterization was performed on Gr anodes pre-cycled for 10 cycles at different temperatures (−20, 25 and 45 °C). It can be seen from Fig. 2(a) that an inhomogeneous and thick SEI film of about 27.8 nm is constructed at the elevated temperature of 45 °C, which is consistent with most previous reported SEI formed at high temperature.38,39 With temperatures decreasing to 25 °C, a homogeneous and relatively thin SEI film around 16.3 nm is fabricated on the Gr anodes (Fig. 2(b)). As illustrated in Fig. 2(c), a thinner SEI film with an average thickness of 8.2 nm is generated at the temperature of −20 °C, indicating the decrease of the electrolyte decomposition. This illustrates that the thickness of the SEI films gradually increases with the increasing temperature.Apart from the thickness, the migration of Li+ across SEI is highly relevant to the chemical components and microstructures of the SEI, which was investigated via X-ray photoelectron spectroscopy (XPS) technique with an Ar+ sputtering depth profiling. The atomic ratio of the S element in the inner layer of SEI was firstly summarized in Fig. 2(d), which can reflect the decomposition of the solvation sheath. When the sputtering time is 50 s, the SEI formed at 45 °C delivers the highest S element proportion of 1.07%, followed by that formed at 25 °C (0.45%) and −20 °C (0.41%). It can be detected that the trend of the S element content in the SEI formed at different temperatures remains the same with the extended sputtering time. The distinct differences of the electrolyte decomposition can be further verified by the S 2p spectra (Fig. S7, ESI†) and the ratio of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the O 1s spectra (Fig. 2(e) and Fig. S8, ESI†). The relatively high content of the anion-derived decomposition products can be found in the inner layer of SEI formed at 45 °C, followed by that formed at 25 °C and −20 °C, affirming the solvation structure transformation by involving greater anion participation in the solvation shell of Li+ at high temperature. The C–C/C–H species and the C–O species in the C 1s spectra represent the decomposition of solvents. Moreover, the ratio of C–O in the inner layer of SEI is also summarized in Fig. 2(f). The SEI film formed at 25 °C exhibits the lowest ratio of C–O species, indicating that the room-temperature environment promotes the low organic content of the SEI film. With the temperature decreasing, the SEI formed at −20 °C displays a significantly higher C–O ratio compared to that formed at 25 °C and 45 °C, suggesting the formation of the organic-rich SEI on the Gr anodes and correlating to more solvent participation into the first solvation sheath of Li+ at −20 °C. This corresponds to the C–C/C–H species in the C 1s spectra, where higher C–C/C–H content can be observed from the SEI film formed at −20 °C (Fig. 2(g)–(i) and Fig. S9, ESI†). In addition, relatively high contents of the C–O species are observed in the SEI formed at 45 °C compared to that formed at 25 °C. This is likely due to an intensified decomposition reaction of the organic solvents and slight thermal reduction induced by high temperature (Fig. S10 and S11, ESI†). The above XPS results confirm the temperature-dependent interphase chemistry, where the content of the anion-derived products corresponds to the temperature-responsive cation–anion coordination interaction with elevated temperature. What is more complex is that the thermal reduction of organic solvents at high temperature would also affect the interfacial components and film thickness.
O in the O 1s spectra (Fig. 2(e) and Fig. S8, ESI†). The relatively high content of the anion-derived decomposition products can be found in the inner layer of SEI formed at 45 °C, followed by that formed at 25 °C and −20 °C, affirming the solvation structure transformation by involving greater anion participation in the solvation shell of Li+ at high temperature. The C–C/C–H species and the C–O species in the C 1s spectra represent the decomposition of solvents. Moreover, the ratio of C–O in the inner layer of SEI is also summarized in Fig. 2(f). The SEI film formed at 25 °C exhibits the lowest ratio of C–O species, indicating that the room-temperature environment promotes the low organic content of the SEI film. With the temperature decreasing, the SEI formed at −20 °C displays a significantly higher C–O ratio compared to that formed at 25 °C and 45 °C, suggesting the formation of the organic-rich SEI on the Gr anodes and correlating to more solvent participation into the first solvation sheath of Li+ at −20 °C. This corresponds to the C–C/C–H species in the C 1s spectra, where higher C–C/C–H content can be observed from the SEI film formed at −20 °C (Fig. 2(g)–(i) and Fig. S9, ESI†). In addition, relatively high contents of the C–O species are observed in the SEI formed at 45 °C compared to that formed at 25 °C. This is likely due to an intensified decomposition reaction of the organic solvents and slight thermal reduction induced by high temperature (Fig. S10 and S11, ESI†). The above XPS results confirm the temperature-dependent interphase chemistry, where the content of the anion-derived products corresponds to the temperature-responsive cation–anion coordination interaction with elevated temperature. What is more complex is that the thermal reduction of organic solvents at high temperature would also affect the interfacial components and film thickness.
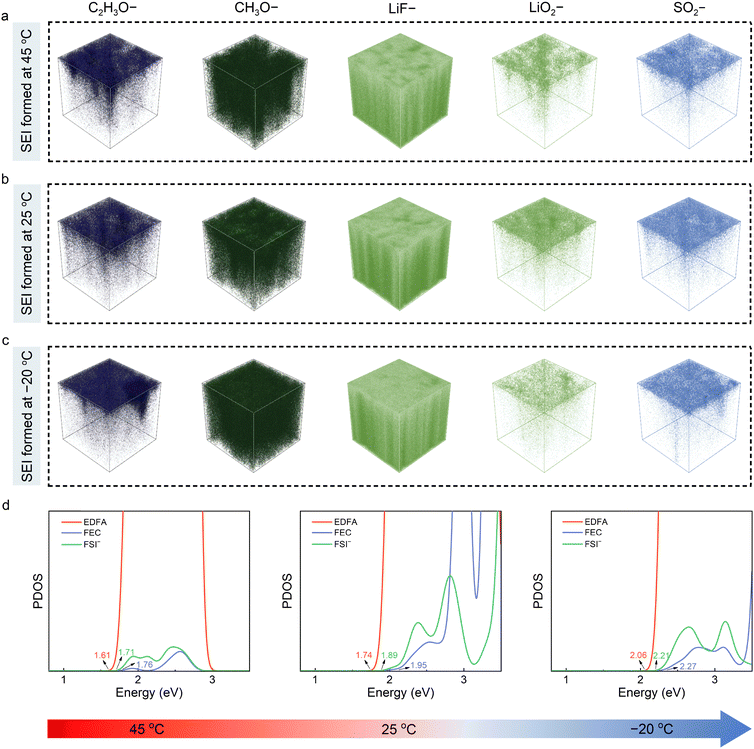
To visualize the spatial distribution and content of interfacial components, the SEI films formed at different temperatures were further meticulously characterized by time-of-flight secondary ion mass spectrometry (ToF-SIMS) with spatial resolution property. As shown in Fig. 3(a)–(c), the chemical components of the SEI films are parallel under different formation temperatures, consisting of organic species (represented by C2H3O–, CH3O– fragments) and inorganic compounds (including LiF–, LiO2–, and SO2– fragments). However, the content and distribution of the species in the SEI prominently vary, as presented in the 3D ToF-SIMS mapping images. It can be detected that the SEI formed at 25 °C exhibits lower contents of C2H3O– and CH3O– fragments with sputtering and higher content of the LiF– fragment in the inner SEI film, proving that the LiF-rich inorganic species dominates the SEI generated at 25 °C. This is consistent with the F 1s spectra of XPS, in which the strong and uniformly-distributed LiF signal throughout the depth profiling can be observed in the SEI formed at 25 °C (Fig. S12, ESI†). It is worth mentioning that the content of the LiF species can not reflect the decomposition tendency of the FSI− anions at different temperatures. This is because the LiF species are derived from the joint reduction decomposition of the fluorinated solvents (including EDFA and FEC solvents) and FSI− anions. Moreover, the LiO2– fragment is more uniformly distributed in the surface and its content visibly increases for the SEI formed at 25 °C than those formed at −20 °C and 45 °C. With the temperature increasing to 45 °C, the content of the organic species is distinctly increased, which can be ascribed to the severe electrolyte decomposition reaction under high temperature. Meanwhile, the greater amount of SO2− fragments recorded under ToF-SIMS suggest a higher decomposition tendency of the FSI− anions, also correlating to the strong coordination interaction between the Li+ and FSI− anions at high temperature. In addition, the SEI formed at −20 °C displays a higher content of organic species and lower content of inorganic species compared to that formed at 25 °C. This result indicates the generation of the solvent-dominated organic-rich SEI, which conforms the solvation sheath with more solvent participation at the low-temperature environment, as demonstrated by previous characterizations. Moreover, the normalized depth profiles of the representative fragments were also provided to identify the SEI structure, where a high content of LiF– fragments can be detected for the SEI formed at 25 °C (Fig. S13, ESI†). The abovementioned ToF-SIMS and XPS results jointly confirm the SEI variations with formation temperatures and highlight the superiority of the SEI formed at 25 °C.
It should be mentioned that the interfacial chemistry is not only related to the solvation structure, but also to the reduction behaviors of the EDFA-FEC electrolyte under different temperatures. To unravel the temperature effects on the reduction behaviors of the electrolyte, the PDOS calculation was performed to figure out the reduction stability of the solvated components at different temperatures. As shown in Fig. 3(d), the electrolyte components, including EDFA solvents, FSI− anions and FEC additives, are involved in the formation of SEI films at the surface of the Gr anodes. Under the higher temperature of 45 °C, the lowest unoccupied molecular orbital (LUMO) levels of EDFA, FSI− and FEC are located at 1.61 eV, 1.71 eV and 1.76 eV, respectively. However, the transformation of the solvation structure caused by the transition of the temperatures generates the different reduction stabilities of the solvated components. The LUMO level of the solvated components decreases with the increase of temperatures, indicating the weakening of the reduction stability of solvated components. This explains the observed relatively higher organic species at 45 °C compared to that at room temperature and the thinner SEI film under low temperature owing to the slow reduction kinetics.
Temperature-related electrochemical performance
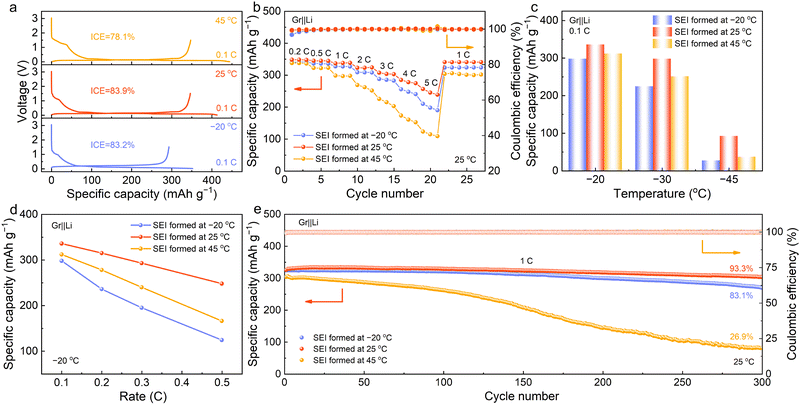
The Gr electrode is a widely-used anode material in the LIBs, whose Li+-intercalation potential is low and sensitive to the polarization change caused by the solvation structure of Li+ and the interfacial film.40 Therefore, it was selected as a model to investigate the effect of temperature-dependent SEI on the electrochemical performance. Fig. 4(a) presents the 1st discharge–charge curves of the Gr||Li half cells equipped with the EDFA-FEC electrolyte at various temperatures (−20, 25 and 45 °C). The reaction kinetics decelerate and the polarization of the batteries increases as the temperature drops, leading to increased hindrance for Li+-intercalation and reduced reversible capacity at −20 °C. Meanwhile, the cell exhibits a low irreversible capacity (59 mA h g−1) owing to the lesser decomposition of the electrolyte, resulting in the initial coulombic efficiency (ICE) with 83.2% at −20 °C. Under high temperature, however, severe decomposition reactions of the electrolyte lead to the dramatic increase of the irreversible discharge capacity (97 mA h g−1). Compared to the relatively high ICE of 83.9% under 25 °C, the ICE at 45 °C is pronouncedly deteriorated to 78.1%, which corresponds to the thickest SEI shown in Fig. 2(a). The temperature-related electrochemical behavior was then systematically examined in Gr||Li cells with as-formed SEI at different temperatures. It can be detected that the Gr electrode with SEI formation at 25 °C exhibits outstanding rate performance with a reversible capacity of 256 mA h g−1 at 5C and the smallest polarization (Fig. 4(b) and Fig. S14, ESI†). Moreover, it shows the smallest overpotential compared to that at −20 °C and 45 °C when charging under −20 °C (corresponding to the de-intercalation of Li+, Fig. S15, ESI†), making it feasible under low-temperature scenarios. Therefore, the Gr||Li cells after SEI formation at different temperatures were characterized via galvanostatic discharge–charge process within the low-temperature range. As shown in Fig. 4(c), the Gr||Li cell with SEI formation at 25 °C delivers a high reversible capacity of up to 336 mA h g−1 at 0.1C under −20 °C. When lowering the temperature to −30 °C, it still exhibits low polarization and retains a high reversible capacity of 298 mA h g−1, which is superior to the cells with SEI formation at −20 °C and 45 °C under the same conditions (Fig. S16, ESI†). With the temperature further decreasing to −45 °C, the cell with SEI formation at 25 °C can achieve a reversible capacity of 175 mA h g−1 at 0.05C, corresponding to a capacity retention of 50.4% compared to that at 25 °C (Fig. S17, ESI†). When the rate increases to 0.1C, it can exhibit a reversible capacity of 92 mA h g−1 at −45 °C, which is far superior to that with SEI formation at −20 °C and 45 °C (Fig. S16, ESI†). Furthermore, the rate capabilities of the Gr||Li cells with different SEI formation temperatures were evaluated under low temperature. At a low temperature of −20 °C, the Gr||Li cell with SEI formation at 25 °C exhibits a capacity of 315 mA h g−1 at 0.2C and the best rate capability with reversible capacity of 248 mA h g−1 at 0.5C, corresponding to a capacity retention of 71.5% compared to that at room temperature (Fig. 4(d)). In comparison, the cells with SEI formation at −20 °C and 45 °C show large polarization at higher rates and poor rate capabilities (Fig. S18, ESI†). Specifically, the SEI formed at −20 °C exhibits the worst rate performance, which may be chiefly attributed to its poor ion migration across the interphase. The low-temperature performance of Gr anodes with high mass loading was also investigated to validate the effects of the SEI formation temperature on the electrochemical performance of the Gr anodes. The results show that the Gr anode with SEI forming at 25 °C can achieve a reversible capacity of 303 mA h g−1 at 0.1C under −20 °C, which is higher than that with SEI forming at −20 °C and 45 °C (Fig. S19, ESI†).To further investigate the effects of the formation temperatures on SEI, the de-intercalation of Li+ from the Gr electrodes was explored under low temperatures, which can eliminate the effect of the sluggish de-solvation process during the intercalation of Li+ into the Gr electrodes. The result reveals that the SEI formed at 25 °C experiences relatively low voltage polarization during de-intercalation, contributing to the superior capacity retention (Fig. S20 and S21, ESI†). Even at lower temperatures down to −70 °C, a slightly higher capacity retention of 98.5% is obtained with an excellent de-intercalation capacity of 339 mA h g−1 (Fig. S21, ESI†). However, the SEI formed at 45 °C delivers a rapidly increasing polarization and obvious capacity decay (5.6%) at −70 °C. The huge polarization can be attributed to the enormous migration resistance of Li+ through the SEI film (Fig. S22, ESI†), which was acquired by imposing the electrochemical impedance spectroscopy (EIS) technology on the symmetric Gr||Gr cells. The Nyquist plots display one semi-circle at high frequency and one upward-sloping line at low frequency, which are assigned to the impedance of the Li+ diffusion through SEI and inside the bulk Gr electrode, respectively.41,42 The results exhibit much lower and slow-growing SEI resistance under such low temperature, confirming that the superior interfacial film is formed at 25 °C. Moreover, the SEI film formed at 45 °C exhibits larger resistance than that formed at −20 °C, which corresponds to higher polarization and lower charge capacity retention of the Gr anodes at low temperature. Afterwards, the activation energy was further investigated by fitting the SEI impedance under different temperatures (Fig. S23, ESI†). The activation energy of the Li+ migration across SEI formed at 25 °C experiences a decrease (14.9 kJ mol−1) compared with that formed at −20 °C (21.9 kJ mol−1) and 45 °C (17.2 kJ mol−1). Such a result confirms the rapid transport kinetics of Li+ diffusion through the inorganic SEI formed at 25 °C, which endows the Gr electrodes with excellent low-temperature tolerance. The cycling performance of the Gr electrode was also examined to uncover the effect of the SEI formation temperatures on its electrochemical stability (Fig. 4(e)). The Gr electrode with SEI formation at 25 °C affords decent cycling stability with 93.3% capacity retention after 300 cycles, in contrast to the rapid capacity fading for the SEI formed at 45 °C. Of note is that such a poor cycling performance should be attributed to the unstable interfacial film formed at high temperature and the fast increase in voltage polarization (Fig. S24, ESI†). The above results imply that the temperature can notably affect the SEI and thus the electrochemical performance of Gr anodes, highlighting the superiority of the SEI formed at room temperature.
Bridging the temperature-responsive solvation sheath and electrochemical behavior
Based on the above discussions, the formation of different SEI layers stems from the temperature-responsive solvation sheath and the reduction stability of the solvated components, which further influences the electrochemical performance of the Gr anodes, particularly under low temperature. At a low temperature of −20 °C, more solvent molecules participate in the primary solvation sheath of Li+, suggesting the stronger ion–dipole interaction. Such solvation structure with more coordinating solvents leads to the formation of the organic-dominated SEI, which exhibits higher activation energy for Li+ migration and causes worse electrochemical performance of the Gr anodes at high rate or low temperature. Nevertheless, the improved reduction stability of the solvents at low temperature effectively reduces the electrolyte decomposition, contributing to a thin SEI film and higher ICE of the Gr anodes (Fig. 5(a)). With the temperature increasing to 25 °C, the solvation sheath spontaneously transforms with more FSI− anions participating in the primary solvation sheath of Li+, contributing to the formation of abundant ion pairs. Owing to the enhanced reduction ability of solvents at 25 °C compared to −20 °C, the resulting synergistic reduction of the FSI− anions and fluorinated solvents is expected to occur and generates a stable LiF-rich inorganic SEI. Such SEI possesses great advantages in inhibiting the further parasitic reactions with the electrolyte, as confirmed from the higher ICE and moderate SEI thickness. The reduced activation energy for the smooth Li+ transport across SEI endows the Gr anodes with superior rate capabilities and low-temperature performance (Fig. 5(b)). When the temperature further increases to 45 °C, more FSI− anions coordinate with Li+ to form an anion-dominated solvation structure with more AGGs being observed, resulting in the generation of higher-content anion-derived decomposition products in the SEI (Fig. 5(c)). However, the significantly enhanced reduction activation of solvents at high temperature leads to the more organic contents formed in the SEI, which is responsible for the greatly increased thickness and larger energy barrier of the Li+ diffusion across the SEI. Such thick SEI raises the difficulty for ion migration and causes the higher polarization of the Gr anodes operated under sub-zero temperature. Therefore, it can be concluded that SEI can be optimized under the synergy of the temperature-responsive solvation structure and reduction behavior of the coordinated components, which elucidates the temperature factor on the SEI formation process and the electrochemical performance of the low-potential Gr anodes, and provides some insights into the formation process in practical application.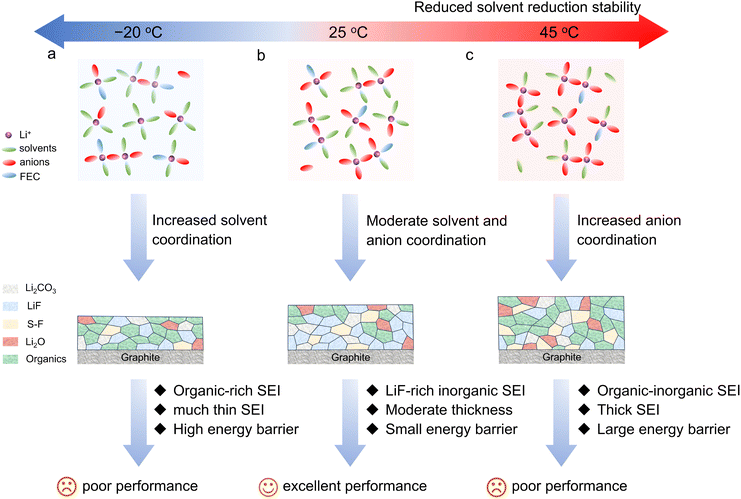 | ||
| Fig. 5 Schematic illustration of the effects of the temperatures on the solvation structures, the as-formed SEIs and the electrochemical performance of the Gr anodes. | ||
Conclusions
In summary, our thorough theoretical and experimental analysis uncover the structure–performance relationship, which depicts the correlations of the temperature-responsive solvation sheath, interfacial chemistry and electrochemical performance of the Gr anodes. The temperature-variable NMR and Raman studies confirm that the first solvation sheath of Li+ in the EDFA-FEC electrolyte experiences the spontaneous transformation process in response to the variable temperatures. More specifically, more FSI− anions and less solvents participate in the primary solvation sheath of Li+ as the temperature increases, contributing to the strengthened Li+–FSI− anion interaction and the weakened ion–dipole interaction. This induces the generation of anion-dominated solvation structure and inorganic-rich interphase with increasing temperature. Meanwhile, the PDOS calculations prove that the reduction stability of the EDFA solvent decreases with the increasing temperature, contributing to the relatively high organic species of SEI formed at high temperature. The unique solvation structure and moderate reduction stability of solvents at 25 °C yield a stable LiF-rich inorganic SEI, which can effectively prevent parasitic reactions with electrolytes. Such SEI possesses great advantages in favoring Li+ migration, which exhibits moderate thickness and minimal activation energy barrier for smooth Li+ migration. These features endow the Gr anodes with ultrafast rate capabilities with 256 mA h g−1 at 5C and long cycling stability with 93.3% capacity retention after 300 cycles under 25 °C. Moreover, the Gr anode with SEI formation at 25 °C achieves high reversible capacity with 175 mA h g−1 at temperatures under −45 °C, corresponding to a capacity retention of 50.4% compared to that at 25 °C. This work emphasizes the pivotal yet less-studied direction, the temperature-responsive solvation sheath and interfacial chemistry, providing insight for understanding the behavior of electrolytes at the molecular level, including the bulk solution and electrode/electrolyte interface.Author contributions
Yanbing Mo: formal analysis, data curation, investigation, and writing – original draft. Gaopan Liu: data curation and investigation. Jiawei Chen, Xiao Zhu and Yu Peng: formal analysis and investigation. Yonggang Wang and Congxiao Wang: data curation and writing – review & editing. Xiaoli Dong: conceptualization, methodology, formal analysis, data curation, and writing – review & editing. Yongyao Xia: writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2022YFB3803400), the National Natural Science Foundation of China (21935003, 22379028 and 22109028), the Natural Science Foundation of Shanghai (22ZR1404400), and the Chenguang Program sponsored by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (19CG01). We appreciate the Shiyanjia Lab (www.shiyanjia.com) for the assistance of the XPS test.Notes and references
- J.-M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- G. G. Eshetu, H. Zhang, X. Judez, H. Adenusi, M. Armand, S. Passerini and E. Figgemeier, Nat. Commun., 2021, 12, 5459 CrossRef CAS PubMed.
- Y. Feng, L. Zhou, H. Ma, Z. Wu, Q. Zhao, H. Li, K. Zhang and J. Chen, Energy Environ. Sci., 2022, 15, 1711–1759 RSC.
- N. Zhang, T. Deng, S. Zhang, C. Wang, L. Chen, C. Wang and X. Fan, Adv. Mater., 2022, 34, 2107899 CrossRef CAS PubMed.
- J. Holoubek, K. Kim, Y. Yin, Z. Wu, H. Liu, M. Li, A. Chen, H. Gao, G. Cai, T. A. Pascal, P. Liu and Z. Chen, Energy Environ. Sci., 2022, 15, 1647–1658 RSC.
- B. Nan, L. Chen, N. D. Rodrigo, O. Borodin, N. Piao, J. Xia, T. Pollard, S. Hou, J. Zhang, X. Ji, J. Xu, X. Zhang, L. Ma, X. He, S. Liu, H. Wan, E. Hu, W. Zhang, K. Xu, X. Q. Yang, B. Lucht and C. Wang, Angew. Chem., Int. Ed., 2022, 61, e202205967 CrossRef CAS.
- S. Weng, X. Zhang, G. Yang, S. Zhang, B. Ma, Q. Liu, Y. Liu, C. Peng, H. Chen, H. Yu, X. Fan, T. Cheng, L. Chen, Y. Li, Z. Wang and X. Wang, Nat. Commun., 2023, 14, 4474 CrossRef.
- D. Hubble, D. E. Brown, Y. Zhao, C. Fang, J. Lau, B. D. McCloskey and G. Liu, Energy Environ. Sci., 2022, 15, 550–578 RSC.
- S. Tan, U. N. D. Rodrigo, Z. Shadike, B. Lucht, K. Xu, C. Wang, X.-Q. Yang and E. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 24995–25001 CrossRef CAS.
- X. Zheng, Z. Cao, W. Luo, S. Weng, X. Zhang, D. Wang, Z. Zhu, H. Du, X. Wang, L. Qie, H. Zheng and Y. Huang, Adv. Mater., 2022, 35, 2210115 CrossRef.
- X. Ding, Q. Huang and X. Xiong, Acta Phys.-Chim. Sin., 2022, 38, 2204057 Search PubMed.
- Y. Zhu, W. Li, L. Zhang, W. Fang, Q. Ruan, J. Li, F. Zhang, H. Zhang, T. Quan and S. Zhang, Energy Environ. Sci., 2023, 16, 2825–2855 RSC.
- T. Chen, Z. Jin, Y. Liu, X. Zhang, H. Wu, M. Li, W. Feng, Q. Zhang and C. Wang, Angew. Chem., Int. Ed., 2022, 61, 202207645 CrossRef.
- Y. Du, S. Shironita, E. Hosono, D. Asakura, Y. Sone and M. Umeda, J. Power Sources, 2023, 556, 232513 CrossRef CAS.
- L. Chen, J. Lu, Y. Wang, P. He, S. Huang, Y. Liu, Y. Wu, G. Cao, L. Wang, X. He, J. Qiu and H. Zhang, Energy Storage Mater., 2022, 49, 493–501 CrossRef.
- D. J. Yoo, Q. Liu, O. Cohen, M. Kim, K. A. Persson and Z. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 11910–11918 CrossRef CAS.
- J. Tan, J. Matz, P. Dong, J. Shen and M. Ye, Adv. Energy Mater., 2021, 11, 2100046 CrossRef CAS.
- S. Y. Sun, N. Yao, C. B. Jin, J. Xie, X. Y. Li, M. Y. Zhou, X. Chen, B. Q. Li, X. Q. Zhang and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, 202208743 CrossRef.
- D. Zhang, L. Li, W. Zhang, M. Cao, H. Qiu and X. Ji, Chin. Chem. Lett., 2023, 34, 107122 CrossRef CAS.
- J. Xu, J. Zhang, T. P. Pollard, Q. Li, S. Tan, S. Hou, H. Wan, F. Chen, H. He, E. Hu, K. Xu, X.-Q. Yang, O. Borodin and C. Wang, Nature, 2023, 614, 694–700 CrossRef CAS.
- H. Zhang, Z. Zeng, F. Ma, Q. Wu, X. Wang, S. Cheng and J. Xie, Angew. Chem., Int. Ed., 2023, 62, 202300771 CrossRef PubMed.
- Y. X. Yao, X. Chen, C. Yan, X. Q. Zhang, W. L. Cai, J. Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 4090–4097 CrossRef CAS PubMed.
- J. Chen, Y. Zhang, H. Lu, J. Ding, X. Wang, Y. Huang, H. Ma and J. Wang, eScience, 2023, 3, 100135 CrossRef.
- Y. Liu, Y. Huang, X. Xu, Y. Liu, J. Yang, J. Lai, J. Shi, S. Wang, W. Fan, Y. P. Cai, Y. Q. Lan and Q. Zheng, Adv. Funct. Mater., 2023, 33, 2303667 CrossRef CAS.
- D. Guo, J. Wang, T. Lai, G. Henkelman and A. Manthiram, Adv. Mater., 2023, 35, 2300841 CrossRef CAS.
- M. Mao, X. Ji, Q. Wang, Z. Lin, M. Li, T. Liu, C. Wang, Y. S. Hu, H. Li, X. Huang, L. Chen and L. Suo, Nat. Commun., 2023, 14, 1082 CrossRef CAS.
- Y. Zhao, T. Zhou, T. Ashirov, M. E. Kazzi, C. Cancellieri, L. P. H. Jeurgens, J. W. Choi and A. Coskun, Nat. Commun., 2022, 13, 2575 CrossRef CAS.
- C. Yang, X. Liu, Y. Lin, L. Yin, J. Lu and Y. You, Adv. Mater., 2023, 35, 2301817 CrossRef CAS.
- Q. Hou, P. Li, Y. Qi, Y. Wang, M. Huang, C. Shen, H. Xiang, N. Li and K. Xie, ACS Energy Lett., 2023, 8, 3649–3657 CrossRef CAS.
- S. Wu, T. Wada, H. Shionoya, J. Hwang, K. Matsumoto and R. Hagiwara, Energy Storage Mater., 2023, 61, 102897 CrossRef.
- S. Ardhra, P. Prakash, R. Siva Dev and A. Venkatnathan, J. Phys. Chem. B, 2022, 126, 2119–2129 CrossRef CAS PubMed.
- C. Yan, Y.-X. Yao, W.-L. Cai, L. Xu, S. Kaskel, H. S. Park and J.-Q. Huang, J. Energy Chem., 2020, 49, 335–338 CrossRef.
- J. Wang, Q. Zheng, M. Fang, S. Ko, Y. Yamada and A. Yamada, Adv. Sci., 2021, 8, 2101646 CrossRef CAS.
- Y. Mo, G. Liu, Y. Yin, M. Tao, J. Chen, Y. Peng, Y. Wang, Y. Yang, C. Wang, X. Dong and Y. Xia, Adv. Energy Mater., 2023, 13, 2301285 CrossRef CAS.
- X. Bogle, R. Vazquez, S. Greenbaum, A. Cresce and K. Xu, J. Phys. Chem. Lett., 2013, 4, 1664–1668 CrossRef CAS PubMed.
- A. C. Thenuwara, P. P. Shetty, N. Kondekar, S. E. Sandoval, K. Cavallaro, R. May, C.-T. Yang, L. E. Marbella, Y. Qi and M. T. McDowell, ACS Energy Lett., 2020, 5, 2411–2420 CrossRef CAS.
- M.-T. F. Rodrigues, F. N. Sayed, H. Gullapalli and P. M. Ajayan, J. Power Sources, 2018, 381, 107–115 CrossRef CAS.
- S. J. An, J. Li, C. Daniel, D. Mohanty, S. Nagpure and D. L. Wood, Carbon, 2016, 105, 52–76 CrossRef CAS.
- H. Cheng, Q. Sun, L. Li, Y. Zou, Y. Wang, T. Cai, F. Zhao, G. Liu, Z. Ma, W. Wahyudi, Q. Li and J. Ming, ACS Energy Lett., 2022, 7, 490–513 CrossRef CAS.
- Y. Yang, Z. Fang, Y. Yin, Y. Cao, Y. Wang, X. Dong and Y. Xia, Angew. Chem., Int. Ed., 2022, 61, 202208345 CrossRef.
- Y. Yin, T. Zheng, J. Chen, Y. Peng, Z. Fang, Y. Mo, C. Wang, Y. Wang, Y. Xia and X. Dong, Adv. Funct. Mater., 2023, 33, 2215151 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee03176d |
| This journal is © The Royal Society of Chemistry 2024 |