 Open Access Article
Open Access ArticleAntimony centre in three different roles: does donor strength or acceptor ability determine the bonding pattern?†
Richard
Chlebík
a,
Csilla
Fekete
 b,
Roman
Jambor
b,
Roman
Jambor
 a,
Aleš
Růžička
a,
Aleš
Růžička
 a,
Zoltán
Benkő
a,
Zoltán
Benkő
 *bc and
Libor
Dostál
*bc and
Libor
Dostál
 *a
*a
aDepartment of General and Inorganic Chemistry, University of Pardubice, Studentská 573, CZ 532 10 Pardubice, Czech Republic. E-mail: libor.dostal@upce.cz
bDepartment of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Műegyetem rkp 3, H-1111 Budapest, Hungary. E-mail: benko.zoltan@vbk.bme.hu
cHUN-REN-BME Computation Driven Chemistry Research Group, Műegyetem rkp 3, H-1111 Budapest, Hungary
First published on 8th October 2024
Abstract
A set of antimony(III) compounds containing a ligand (Ar) with a pendant guanidine function (where Ar = 2-[(Me2N)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N]C6H4) was prepared and characterized. This includes triorgano-Ar3Sb, diorgano-Ar2SbCl and monoorgano-ArSbCl2 compounds and they were characterized by 1H and 13C NMR spectroscopy and by single-crystal X-ray diffraction analysis (sc-XRD). The coordination capability of Ar3Sb and Ar2SbCl was examined in the reactions with either cis-[PdCl2(CH3CN)2] or PtCl2 and complexes cis-[(κ2-Sb,N-Ar3Sb)MCl2] (M = Pd 1, Pt 2) and [(κ3-N,Sb,N-Ar2SbCl)MCl2] (M = Pd 3, Pt 4) were isolated, while their structures were determined by sc-XRD. Notably, the ligands Ar3Sb and Ar2SbCl exhibit different coordination modes – bidentate and tridentate, respectively – and the antimony exhibits three distinct bonding modes in complexes 1–4, which were also subjected to theoretical studies.
N]C6H4) was prepared and characterized. This includes triorgano-Ar3Sb, diorgano-Ar2SbCl and monoorgano-ArSbCl2 compounds and they were characterized by 1H and 13C NMR spectroscopy and by single-crystal X-ray diffraction analysis (sc-XRD). The coordination capability of Ar3Sb and Ar2SbCl was examined in the reactions with either cis-[PdCl2(CH3CN)2] or PtCl2 and complexes cis-[(κ2-Sb,N-Ar3Sb)MCl2] (M = Pd 1, Pt 2) and [(κ3-N,Sb,N-Ar2SbCl)MCl2] (M = Pd 3, Pt 4) were isolated, while their structures were determined by sc-XRD. Notably, the ligands Ar3Sb and Ar2SbCl exhibit different coordination modes – bidentate and tridentate, respectively – and the antimony exhibits three distinct bonding modes in complexes 1–4, which were also subjected to theoretical studies.
Introduction
For a long time, triorganostibines have been considered heavier counterparts of ubiquitous phosphines regarding their coordination chemistry1 and they usually behave as classical 2e L-type ligands. However, they also exhibit other interesting coordination properties, e.g. serving as bridging ligands, as demonstrated by Werner et al.2 The family of Z-type ligands,3 that function as σ-acceptors for transition metals, has recently gained considerable attention. Although the first examples were reported as chemical curiosities in the 1970s as complexes with SO2 or Ph3Al,4 the main breakthrough started in the early 2000s.5 Examples of unsupported M → Z interactions are also known in the literature,5a,6 but a majority of such interactions are mediated by pendant L donors that coordinate to the same central metal and simultaneously support the Z interaction.5 This concept often utilizes inherently Lewis acidic group 13 elements,5,7 but systems based on other p-block elements8 including antimony9 have also emerged. The cornerstone in the field of antimony was laid by the Gabbaï group using the triphosphanylstibine Ar′3Sb (Ar′ = [2-(Ph2P)C6H4]3Sb) that after oxidation of the antimony atom revealed a Z-type Au → Sb interaction (Fig. 1A).10 Since that time, various Au,11 Ni,12 Cu or Ag13 complexes profiting from this well-designed backbone have been reported. Ar′3Sb or its congeners Ar′2SbX (X = Ph or Cl) were also coordinated with PdCl214 and PtCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 yielding Z-type complexes (e.g.Fig. 1B), while a new Sb–Cl bond formed due to the coordination non-innocent behaviour of the ligands. However, all the examples mentioned above rely on the utilization of phosphines as necessary supporting donors. A system bearing guanidine pendant functions, i.e.Ar3Sb (Ar = 2-[(Me2N)2C
15 yielding Z-type complexes (e.g.Fig. 1B), while a new Sb–Cl bond formed due to the coordination non-innocent behaviour of the ligands. However, all the examples mentioned above rely on the utilization of phosphines as necessary supporting donors. A system bearing guanidine pendant functions, i.e.Ar3Sb (Ar = 2-[(Me2N)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N]C6H4) has only recently been introduced into Cu or Ag chemistry, including remarkable coordination of the single Sb donor toward the M3X3 (M = Cu or Ag; X = halide) fragment using its 5s lone pair.16 As one may expect different donor properties of the pendant imino-group in Ar compared to a phosphino-one in Ar′, we herein examine this point and report on the coordination behaviour of Ar3Sb and Ar2SbCl in Pd(II) and Pt(II) complexes. Antimony is found in three distinct bonding modes, including a Z-interaction in five-coordinate complexes (Fig. 1C). However, no coordination non-innocence was observed for these ligands, thus differing from the phosphorus-based systems known so far (cf.Fig. 1B).
N]C6H4) has only recently been introduced into Cu or Ag chemistry, including remarkable coordination of the single Sb donor toward the M3X3 (M = Cu or Ag; X = halide) fragment using its 5s lone pair.16 As one may expect different donor properties of the pendant imino-group in Ar compared to a phosphino-one in Ar′, we herein examine this point and report on the coordination behaviour of Ar3Sb and Ar2SbCl in Pd(II) and Pt(II) complexes. Antimony is found in three distinct bonding modes, including a Z-interaction in five-coordinate complexes (Fig. 1C). However, no coordination non-innocence was observed for these ligands, thus differing from the phosphorus-based systems known so far (cf.Fig. 1B).
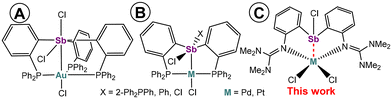 | ||
| Fig. 1 Examples of Z-type ligands based on the antimony σ-acceptor (A and B) and complexes from the current study (C). | ||
Results and discussion
Treatment of the lithium precursor ArLi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 with SbCl3 in an appropriate stoichiometric ratio furnished a set of organoantimony compounds Ar3Sb
17 with SbCl3 in an appropriate stoichiometric ratio furnished a set of organoantimony compounds Ar3Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (67%), Ar2SbCl (58%) and ArSbCl2 (49%). The 1H and 13C NMR spectra revealed one set of signals for all the studied compounds (see the ESI†), including the typical signal for the guanidine N3C carbon atom (δ(13C) in the range 157.6–159.2 ppm). This fact proves the magnetic equivalence of all Ar groups, indicating that the fluxional behavior of these compounds is rapid on the NMR time scale. Molecular structures were also determined by sc-XRD analysis (Fig. 2).
16 (67%), Ar2SbCl (58%) and ArSbCl2 (49%). The 1H and 13C NMR spectra revealed one set of signals for all the studied compounds (see the ESI†), including the typical signal for the guanidine N3C carbon atom (δ(13C) in the range 157.6–159.2 ppm). This fact proves the magnetic equivalence of all Ar groups, indicating that the fluxional behavior of these compounds is rapid on the NMR time scale. Molecular structures were also determined by sc-XRD analysis (Fig. 2).
 | ||
| Fig. 2 Molecular structure of starting antimony compounds showing 30% probability ellipsoids and the crystallographic numbering scheme. Hydrogen atoms are omitted for clarity. | ||
In the case of Ar3Sb, the central atom adopts the expected trigonal pyramidal geometry. All the Sb–N distances (in the range 2.983(2)–3.059(2) Å) are similar to each other and are far longer than expected for a Sb–N covalent bond (Σcov(Sb, N) = 2.11 Å),18 thereby ruling out any significant intramolecular N → Sb interaction. In contrast, there is an obvious difference between the Sb1–N1/2 distances in Ar2SbCl, i.e. 2.5440(17) vs. 3.0906(18) Å. These values are comparable to those of the related compound [2-(Me2N)C6H4]Sb(Mes)Cl (2.619(3) Å),19 Finally, the Sb1–N1 distance of 2.4382(15) Å is the shortest in this series being consistent with the highest Lewis acidity of the central atom, but slightly longer than in, e.g., [2-(Me2NCH2)C6H4]SbCl2 (2.407(5) Å)20 and [2-(DippN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)C6H4]SbCl2 (2.416(2) Å).21 The enhanced Lewis acidity of the antimony atom also leads to the formation of intermolecular Sb(1)–Cl(1)⋯Sb(1a) contacts (3.225 Å; cf. Σcov(Sb, Cl) = 2.39 Å).18
CH)C6H4]SbCl2 (2.416(2) Å).21 The enhanced Lewis acidity of the antimony atom also leads to the formation of intermolecular Sb(1)–Cl(1)⋯Sb(1a) contacts (3.225 Å; cf. Σcov(Sb, Cl) = 2.39 Å).18
Their coordination toward either cis-[PdCl2(CH3CN)2] or PtCl2 was examined. Unfortunately, no stable complexes could be isolated from the respective reactions with ArSbCl2. Nevertheless, Ar3Sb and Ar2SbCl provided the compounds cis-[(κ2-Sb,N-Ar3Sb)MCl2] (M = Pd 1, Pt 2) and [(κ3-N,Sb,N-Ar2SbCl)MCl2] (M = Pd 3, Pt 4, Scheme 1), respectively.
Ar3Sb behaves as an Sb,N-chelate in 1 and 2 containing tetracoordinated Pd and Pt centers in a square planar coordination environment (Fig. 3). To our knowledge, these complexes represent rare structurally characterized examples containing five-membered C2SbNM metallacycles (M = Pd or Pt) as only a few related six-membered metallacycles have been reported: i.e. cis-[κ2-Sb,N-(2-(Me2NCH2)C6H4SbMes2)PdCl2],22cis-[κ2-Sb,N-(2,6-(Me2NCH2)2C6H3SbPh2)PtCl2],23cis-[κ2-Sb,N-(2-(Me2NCH2)C6H3)3SbPtCl2]24 and cis-[κ2-Sb,N-(2-(Me2NCH2)Fc)3SbPtCl2].25 The Sb1–Pd/Pt bond lengths of 2.4573(7)/2.4661(7) Å in 1/2 are a little shorter than those found in the abovementioned six-membered cycles (cf. Sb–Pd 2.4831(5) and Sb–Pt 2.4935(5)–2.5162(4) Å) and the same applies to the N1–Pd/Pt bonds of 2.082(3)/2.072(5) Å (cf. literature data 2.096(5)–2.133(4) Å).22–25
 | ||
| Fig. 3 Molecular structures of 1 and 2 showing 30% probability ellipsoids and the crystallographic numbering scheme. Hydrogen atoms are omitted for clarity. | ||
The M–Cl bond located trans to the antimony is elongated to 2.4004(12) vs. 2.2988(13) Å in 1 and 2.3863(14) vs. 2.3024(15) Å in 2. In line with the solid-state structures, the 1H and 13C NMR spectra of 1 and 2 revealed two sets of signals for the aryl ligands in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 integral ratio, indicating that only one of the guanidines forms a chelate with the metal. The methyl groups within this set of signals are magnetically non-equivalent (δ(13C) = 40.4/41.7 and 40.1/42.0 ppm, for 1 and 2, respectively) suggesting hindered rotation around the phenyl–N bond due to the closure of this metallacycle. The formation of the chelate is also reflected in a pronounced down-field shift of one of the guanidine carbons N3C 159.4/168.9 and 159.9/169.6 ppm in 1 and 2, respectively, and this is also consistent with the value found in the non-coordinated parent Ar2SbCl (158.9 ppm). Although both compounds were shown to be unstable at elevated temperatures by high-temperature 1H NMR experiments (Fig. S17 and S18†), the 1H, 1H-EXSY experiment (with a mixing time of 1 s) in the case of palladium complex 1 clearly showed chemical exchange between all accessible guanidine donors in solution (Fig. S10†), proving its dynamic behavior. In contrast, similar experiments with 2 (with a mixing time of up to 3 s) revealed chemical exchange only in the region of the methyl groups of the coordinated guanidine function, while solely NOESY cross-peaks were obtained in the aromatic region of the spectra (Fig. S14–S16†). This suggests that the metallacycle in complex 2 is more rigid in solution.
2 integral ratio, indicating that only one of the guanidines forms a chelate with the metal. The methyl groups within this set of signals are magnetically non-equivalent (δ(13C) = 40.4/41.7 and 40.1/42.0 ppm, for 1 and 2, respectively) suggesting hindered rotation around the phenyl–N bond due to the closure of this metallacycle. The formation of the chelate is also reflected in a pronounced down-field shift of one of the guanidine carbons N3C 159.4/168.9 and 159.9/169.6 ppm in 1 and 2, respectively, and this is also consistent with the value found in the non-coordinated parent Ar2SbCl (158.9 ppm). Although both compounds were shown to be unstable at elevated temperatures by high-temperature 1H NMR experiments (Fig. S17 and S18†), the 1H, 1H-EXSY experiment (with a mixing time of 1 s) in the case of palladium complex 1 clearly showed chemical exchange between all accessible guanidine donors in solution (Fig. S10†), proving its dynamic behavior. In contrast, similar experiments with 2 (with a mixing time of up to 3 s) revealed chemical exchange only in the region of the methyl groups of the coordinated guanidine function, while solely NOESY cross-peaks were obtained in the aromatic region of the spectra (Fig. S14–S16†). This suggests that the metallacycle in complex 2 is more rigid in solution.
In marked contrast to the conventional coordination mode in 1 and 2, Ar2SbCl in 3 and 4 behaves as an N,Sb,N-pincer ligand (Scheme 1 and Fig. 4) pointing to a Z-type coordination of the antimony atom (vide infra). The central metals are coordinated by two chlorine and two nitrogen atoms in a cis fashion. The M–N (2.055(3) and 2.060(3) Å for 3; 2.049(5) and 2.049(7) Å for 4) and M–Cl bond lengths (2.3004(10) and 2.3138(10) Å for 3; 2.3085(18) and 2.319(2) Å for 4) are in the expected range for single bonds (cf. Σcov(Pd/Pt, N) = 1.91/1.94 Å; Σcov(Pd/Pt, Cl) = 2.19/2.22 Å).18 Importantly, the coordination number of the metals is increased to five by the coordination of the antimony atom, giving rise to a distorted square-pyramidal geometry for the Pt and Pd atoms. The atoms-in-molecules (AIM) study (see the ESI†) also located five bond critical points around the Pd/Pt centers (ωB97X-D/def2-TZVP level). This observation contrasts with the coordination non-innocent behaviour of the related systems containing pendant P-donors, where the coordination is accompanied by the formation of a new Sb–Cl bond, thereby preserving the square planar environment at the metal centers (Fig. 1B).14b,15b,i The bond lengths Pd–Sb 2.8829(5) Å in 3 and Pt–Sb 2.8163(6) in 4 are elongated compared to those in 1/2 and with the closest counterparts from Fig. 1B (R = Cl), cf. Sb–Pd 2.4230(3)14b and Sb–Pt 2.4407(5) Å.15b Nevertheless both values are still close to the respective Σcov(Pd/Pt, Sb) = 2.60/2.63 Å![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 indicating significant bonding interactions (vide infra). The coordination sphere of the antimony atom in 3 and 4 is completed by a chlorine atom (Sb–Cl 2.4605(12)/2.515(2) Å for 3/4) that is coordinated trans to the metal (Cl–Sb–M 171.35(3)/170.91(5)° for 3/4) and is best described as having a see-saw geometry bearing a stereochemically active lone pair. Although complex 4 after isolation remains insoluble, the solubility of 3 was at an acceptable level that allowed recording of 1H and 13C NMR spectra, which revealed two sets of signals for both ligands in a mutual 1
18 indicating significant bonding interactions (vide infra). The coordination sphere of the antimony atom in 3 and 4 is completed by a chlorine atom (Sb–Cl 2.4605(12)/2.515(2) Å for 3/4) that is coordinated trans to the metal (Cl–Sb–M 171.35(3)/170.91(5)° for 3/4) and is best described as having a see-saw geometry bearing a stereochemically active lone pair. Although complex 4 after isolation remains insoluble, the solubility of 3 was at an acceptable level that allowed recording of 1H and 13C NMR spectra, which revealed two sets of signals for both ligands in a mutual 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 integral ratio. The chemical shifts of both N3C guanidine carbons at 165.9/169.9 are consistent with coordination toward the palladium atom, when compared with the values found in 1 and 2 (vide supra).
1 integral ratio. The chemical shifts of both N3C guanidine carbons at 165.9/169.9 are consistent with coordination toward the palladium atom, when compared with the values found in 1 and 2 (vide supra).
 | ||
| Fig. 4 Molecular structure of 3 and 4 showing 30% probability ellipsoids and the crystallographic numbering scheme. Hydrogen atoms are omitted for clarity. | ||
To explore the bonding situations in the experimentally obtained complexes, quantum chemical calculations were performed using various DFT functionals (ωB97X-D, BP86, BP86-D3, B3LYP-D3, and M06-2X) in combination with the def2-TZVP basis set and the more accurate LNO-CCSD(T)/aug-cc-pVTZ level. Based on comparing the computed geometrical parameters with those obtained from sc-XRD experiments, the ωB97X-D (and BP86-D3) functional shows the best performance and, in the following, we only discuss the findings obtained at the ωB97X-D/def2-TZVP level. To scrutinize the nature of possible metal–antimony interactions that may stabilize these complexes, we performed NBO (Natural Bonding Orbital) calculations. In the case of complexes 1 and 2, two important stabilizing donor–acceptor interactions were found: one from the N atom and another from the Sb center into the σ*(M–Cl) antibonding orbital (Fig. 5A). In contrast, for the Pd–Sb interaction in complex 3, the NBO analysis identifies two different types of interactions that are similar to those in a previous Au complex reported by the Gabbaï group:11a (i) a donation from the d-orbital at the palladium center into the σ* antibonding orbital of the Sb–Cl bond, lp(Pd) → σ*(Sb–Cl) (Fig. 5B), with a stabilization energy of only E(2) = 10.6 kcal mol−1 (according to the second order perturbation theory in the Fock matrix); and (ii) additional interactions arising from a donation of the s-type Sb lone pair toward the σ*(Pd–Cl) orbitals with a summarized stabilization energy of 8.1 kcal mol−1 (Fig. 5C). Although complex 4 shows similar characteristics, (i) the donation from the d-type Pt “lone pair” into the σ*(Sb–Cl) orbital exhibits a significantly larger E(2) of 25.7 kcal mol−1 (compared to 3), while (ii) the two lp(Sb) → σ*(Pt–Cl) donations have a lower cumulative stabilization energy of 7.5 kcal mol−1. The deletion procedures on the Fock matrix elements (for the interactions between the Sb–metal and Sb–Cl) in both 3 and 4 also delivered similar results for the E(2) values.
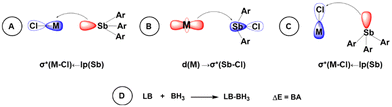 | ||
| Fig. 5 Various M–Sb bonding interactions obtained in 1–4 (A–C) and the definition of borane affinity used in the study (D). | ||
These donor–acceptor interactions also affect the geometrical parameters. On one hand, the backdonation from the M center into the σ*(Sb–Cl) antibonding orbital is manifested in the slight elongation of the Sb–Cl bond in both complexes (2.461/2.515 Å, for 3/4) compared to the free ligand Ar2SbCl (2.442 Å). On the other hand, we focused on gaining evidence for the presence of the stabilizing interaction from the Sb lone pair toward the M center. Therefore, the lone pair of Sb was blocked by a BH3 molecule in complexes 3 and 4 to hamper the lp(Sb) → σ*(M–Cl) donation. As a result, the Sb–M bond distance lengthens significantly upon the BH3 addition from 2.88 to 3.17 Å for complex 3, and a smaller but still notable change from 2.81 to 3.04 Å is observed for complex 4. These Sb–M bond elongations are consistent with the lack of stabilizing interaction from the Sb center toward the metal in the borane adducts.
Finally, we aimed to identify the reasons why the Ar3Sb and Ar2SbCl ligands prefer different kinds of coordination modes. A simple and straightforward explanation seems to be the higher Lewis acidity of the Ar2SbCl ligand compared to that of the Ar3Sb counterpart. However, the backdonation in the Pd complex 3 offers stabilization similar to that of the Sb → Pd donation, and this does not justify the formation of a pentacoordinate Pd center. To clarify this situation, we studied the borane affinities (BA, Fig. 5D) of the ligands at both Sb and N centers defined as the complexation energy of the donor center (LB, Lewis base) utilizing BH3 as the Lewis acid (which cannot offer backdonation). The BA of the Sb center in the Ar3Sb ligand is much lower (−32.4 kcal mol−1) than that in Ar2SbCl (−14.4 kcal mol−1), highlighting that the Sb in the Ar2SbCl ligand is a weaker donor than that in Ar3Sb. Interestingly, the BA of the nitrogen in a guanidine arm (−24.4 kcal mol−1) has a value intermediate between the BAs of the two types of Sb centers. Comparing the BAs of the various centers in these ligands reveals that the best stabilization using the triaryl ligand Ar3Sb can be achieved via bidentate coordination utilizing the Sb and N donors.
In contrast to the Ar3Sb ligand, the Sb center of the Ar2SbCl ligand cannot compete with another N donor due to its highest (least negative) BA among the studied ones, and thus coordination is established by two imino-N centers rather than by an Sb and N donor. Altogether, the reason for the coordination fashion observed for complexes 3 and 4 is the lower donor strength of the Sb center compared to that of an imino-group rather than its higher Lewis acidity.
Conclusions
In conclusion, herein, we presented the synthesis and characterization of triaryl and monochloro-diaryl stibine Pd(II) and Pt(II) complexes displaying remarkably different molecular structures. Importantly, the Sb center in these complexes exhibits three different kinds of bonding: in the triaryl ligand Ar3Sb, it behaves as a conventional Lewis base, and its donor ability clearly exceeds that of an imino-N center. The situation with the ligand Ar2SbCl is more complicated: in its Pd complex, two different kinds of weak donor–acceptor interactions are established between the Pd and Sb centers. In one of these interactions, the Sb center acts as a donor (and the acceptor is the σ*(Pd–Cl) orbital), while in the other, backbonding interaction, the σ*(Sb–Cl) orbital accepts the electron density from the filled d-orbital at Pd. Moreover, the magnitudes of these stabilization effects (in reverse directions) are quite similar. In contrast to the Pd complex, in the Pt complex of Ar2SbCl the donation of the Sb lone pair is clearly suppressed by backdonation, showing that the Pt center is a better donor than Pd. Our results indicate that the primary driving force for these different coordination modes lies in the lower donor strength of the Sb center in Ar2SbCl than its higher Lewis acidity compared to Ar3Sb. It is also noteworthy that the utilization of this guanidine-based ligand may allow for different coordination modes in comparison with well-established Gabbaï systems. We note that during proceeding of this paper, two related works dealing with nitrogen donors containing antimony ligands in Z-type coordination appeared in the literature.26,27Experimental
All experimental details including synthesis, NMR spectra, X-ray crystallography, and theoretical studies can be found in the ESI.†Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for Ar3Sb, Ar2SbCl, ArSbCl2 and complexes 1–4 have been deposited at the CCDC under 2313659, 2313660, 2313661, 2313662, 2313664, 2313665, and 2313666.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. D. and A. R. acknowledge the Czech Science Foundation (project no. 21-02964S). The support from the National Research, Development and Innovation Found for project K-147095 (for Z.B.) and the EKÖP-24-3-BME-358 project (for Cs.F.) is gratefully acknowledged. We also thank the generous computational resources offered by the Ministry of Education, Youth and Sports of the Czech Republic through the e-INFRA CZ (project: OPEN-31-21).Notes and references
- For relevant reviews see: (a) V. K. Greenacre, W. Levason and G. Reid, Coord. Chem. Rev., 2021, 432, 213698 CrossRef; (b) S. L. Benjamin and G. Reid, Coord. Chem. Rev., 2015, 297–298, 168 CrossRef; (c) W. Levason and G. Reid, Coord. Chem. Rev., 2006, 250, 2565 CrossRef; (d) N. R. Champnes and W. Levason, Coord. Chem. Rev., 1994, 133, 115 CrossRef.
- (a) P. Schwab, N. Mahr, J. Wolf and H. Werner, Angew. Chem., Int. Ed. Engl., 1994, 33, 97 CrossRef; (b) U. Herber, B. Weberndörfer and H. Werner, Angew. Chem., Int. Ed., 1999, 38, 1609 CrossRef; (c) P. Schwab, J. Wolf, N. Mahr, P. Steinert, U. Herber and H. Werner, Chem. – Eur. J., 2000, 6, 4471 CrossRef CAS PubMed; (d) T. Pechmann, C. D. Brandt and H. Werner, Dalton Trans., 2003, 1495 RSC; (e) M. Pietsch, F. P. Gabbaï and M. Scheer, Z. Anorg. Allg. Chem., 2021, 647, 266 CrossRef.
- M. L. H. A. Green, J. Organomet. Chem., 1995, 500, 127 CrossRef CAS.
- (a) K. W. Muir and J. A. Ibers, Inorg. Chem., 1969, 8, 1921 CrossRef CAS; (b) J. M. Burlitch, M. E. Leonowicz, R. B. Petersen and R. E. Hughes, Inorg. Chem., 1979, 18, 1097 CrossRef CAS.
- (a) A. Amgoune and D. Bourissou, Chem. Commun., 2011, 47, 859 RSC; (b) G. Bouhadir, A. Amgoune and D. Bourissou, Adv. Organomet. Chem., 2010, 58, 1 CrossRef; (c) G. Bouhadi and D. Bourissou, Chem. Soc. Rev., 2016, 45, 1065 RSC.
- For several examples see: (a) C. A. Miller, T. S. Janik, M. R. Churchill and J. D. Atwood, Inorg. Chem., 1996, 35, 3683 CrossRef; (b) M. Albrecht, R. A. Gossage, M. Lutz, A. L. Spek and G. van Koten, Chem. – Eur. J., 2000, 6, 1431 CrossRef; (c) H. Braunschweig, K. Gruss and K. Radacki, Angew. Chem., Int. Ed., 2007, 46, 7782 CrossRef PubMed; (d) H. Braunschweig, K. Gruss and K. Radacki, Inorg. Chem., 2008, 47, 8595 CrossRef PubMed; (e) B. R. Barnett, C. E. Moore, R. Chandrasekaran, S. Sproules, A. L. Rheingold, S. DeBeer and J. S. Figueroa, Chem. Sci., 2015, 6, 7169 RSC; (f) M. Cokoja, C. Gemel, T. Steinke, F. Schroder and R. A. Fischer, Dalton Trans., 2005, 44 RSC.
- For several examples see: (a) A. F. Hill, G. R. Owen, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 1999, 38, 2759 CrossRef; (b) M. J. Lopez-Gomez, N. G. Connelly, M. F. Haddow, A. Hamilton and A. G. Orpen, Dalton Trans., 2010, 39, 5221 RSC; (c) K. Pang, J. M. Tanski and G. Parkin, Chem. Commun., 2008, 1008 RSC; (d) S. Senda, Y. Ohki, H. Yasuhiro, T. Tomoko, D. Toda, J. L. Chen, T. Matsumoto, H. Kawaguchi and K. Tatsumi, Inorg. Chem., 2006, 45, 9914 CrossRef PubMed; (e) S. Bontemps, H. Gornitzka, G. Bouhadir, K. Miqueu and D. Bourissou, Angew. Chem., Int. Ed., 2006, 45, 1611 CrossRef; (f) M. Sircoglou, S. Bontemps, M. Mercy, N. Saffon, M. Takahashi, G. Bouhadir, L. Maron and D. Bourissou, Angew. Chem., Int. Ed., 2007, 46, 8583 CrossRef; (g) M. Sircoglou, S. Bontemps, G. Bouhadir, N. Saffon, K. Miqueu, W. Gu, M. Mercy, C. H. Chen, B. M. Foxman, L. Maron, O. V. Ozerov and D. Bourissou, J. Am. Chem. Soc., 2008, 130, 16729 CrossRef PubMed; (h) M. Sircoglou, M. Mercy, N. Saffon, Y. Coppel, G. Bouhadir, L. Maron and D. Bourissou, Angew. Chem., Int. Ed., 2009, 48, 3454 CrossRef PubMed; (i) P. A. Rudd, S. Liu, L. Gagliardi, V. G. Young, Jr. and C. C. Lu, J. Am. Chem. Soc., 2011, 133, 20724 CrossRef PubMed; (j) R. C. Cammarota and C. C. Lu, J. Am. Chem. Soc., 2015, 137, 12486 CrossRef.
- For examples see: (a) J. Grobe, N. Krummen, R. Wehmschulte, B. Krebs and M. Laege, Z. Anorg. Allg. Chem., 1994, 620, 1645 CrossRef; (b) J. Wagler and E. Brendler, Angew. Chem., Int. Ed., 2010, 49, 624 CrossRef PubMed; (c) P. Gualco, T. P. Lin, M. Sircoglou, M. Mercy, S. Ladeira, G. Bouhadir, L. M. Perez, A. Amgoune, L. Maron, F. P. Gabbaï and D. Bourissou, Angew. Chem., Int. Ed., 2009, 48, 9892 CrossRef; (d) P. Gualco, M. Mercy, S. Ladeira, L. Maron, A. Amgoune and D. Bourissou, Chem. – Eur. J., 2010, 16, 10808 CrossRef PubMed; (e) M. Karimi, E. S. Tabei, R. Fayad, M. R. Saber, E. O. Danilov, C. Jones, F. N. Castellano and F. P. Gabbaï, Angew. Chem., Int. Ed., 2021, 60, 22352 CrossRef PubMed; (f) M. Karimi and F. P. Gabbaï, Organometallics, 2022, 41, 642 CrossRef CAS; (g) T. P. Lin and F. P. Gabbaï, Polyhedron, 2017, 125, 18 CrossRef CAS; (h) H. Kameo, K. Ikeda, S. Sakaki, S. Takemoto, H. Nakazawa and H. Matsuzaka, Dalton Trans., 2016, 45, 7570 RSC; (i) T. P. Lin and F. P. Gabbaï, J. Am. Chem. Soc., 2012, 134, 12230 CrossRef; N. Ansmann, J. Munch, M. Schorpp and L. Greb, Angew. Chem., Int. Ed., 2023, 62, e202313636 Search PubMed.
- For reviews see: (a) J. S. Jones and F. P. Gabbaï, Acc. Chem. Res., 2013, 8, 1720 Search PubMed; (b) D. You and F. P. Gabbaï, Trends Chem., 2019, 1, 485 CrossRef.
- C. R. Wad and F. P. Gabbaï, Angew. Chem., Int. Ed., 2011, 50, 7369 CrossRef.
- (a) I. S. Ke and F. P. Gabbaï, Inorg. Chem., 2013, 52, 7145 CrossRef PubMed; (b) H. Yan and F. P. Gabbaï, J. Am. Chem. Soc., 2015, 137, 13425 CrossRef; (c) S. Sen, I. S. Ke and F. P. Gabbaï, Inorg. Chem., 2016, 55, 9162 CrossRef; (d) J. S. Jones and F. P. Gabbaï, Chem. – Eur. J., 2017, 23, 1136 CrossRef CAS PubMed; (e) S. Sen, I. S. Ke and F. P. Gabbaï, Organometallics, 2017, 36, 4224 CrossRef CAS; (f) Y. H. Lo and F. P. Gabbaï, Angew. Chem., Int. Ed., 2019, 58, 10194 CrossRef CAS PubMed.
- (a) J. S. Jones, C. R. Wade and F. P. Gabbaï, Angew. Chem., Int. Ed., 2014, 53, 8876 CrossRef; (b) J. S. Jones, C. R. Wade, M. Yang and F. P. Gabbaï, Dalton Trans., 2017, 46, 5598 RSC.
- I. S. Ke and F. P. Gabbaï, Aust. J. Chem., 2013, 66, 1281 CrossRef.
- (a) C. R. Wade, I. S. Ke and F. P. Gabbaï, Angew. Chem., Int. Ed., 2012, 51, 478 CrossRef; (b) S. Sahu and F. P. Gabbaï, J. Am. Chem. Soc., 2017, 139, 5035 CrossRef PubMed.
- (a) I. S. Ke, J. S. Jones and F. P. Gabbaï, Angew. Chem., Int. Ed., 2014, 53, 2633 CrossRef; (b) I. H. Yang and F. P. Gabbaï, J. Am. Chem. Soc., 2014, 136, 10866 CrossRef; (c) J. S. Jones, C. R. Wade and F. P. Gabbaï, Organometallics, 2015, 34, 2647 CrossRef; (d) D. You and F. P. Gabbaï, J. Am. Chem. Soc., 2017, 139, 6843 CrossRef; (e) D. You, H. Yang, S. Sen and F. P. Gabbaï, J. Am. Chem. Soc., 2018, 140, 9644 CrossRef; (f) D. You, J. E. Smith, S. Sen and F. P. Gabbaï, Organometallics, 2020, 39, 4169 CrossRef; (g) R. R. Rodriguez and F. P. Gabbaï, Molecules, 2021, 26, 1985 CrossRef; (h) J. E. Smith, H. Yang and F. P. Gabbaï, Organometallics, 2021, 23, 3886 CrossRef; (i) S. Furan, E. Hupf, J. Boidol, J. Brünig, E. Lork, S. Mebs and J. Beckmann, Dalton Trans., 2019, 48, 4504 RSC.
- M. Sharma, R. M. Fritz, J. O. Adebanjo, Z. Lu, T. R. Cundari, M. A. Omary, A. Choudhury and P. Stavropoulos, Organometallics, 2024, 43, 634 CrossRef.
- C. Manankandayalage, D. K. Unmruh and C. Krempner, Chem. – Eur. J., 2021, 27, 6263 CrossRef.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 186 CrossRef.
- D. Sharma, A. Benny, R. Gupta, E. D. Jemmins and A. Venugopal, Chem. Commun., 2022, 58, 11009 RSC.
- L. M. Opris, A. Silvestru, C. Silvestru, H. J. Breunig and E. Lork, Dalton Trans., 2003, 4367 RSC.
- L. Dostál, R. Jambor, A. Růžička and P. Šimon, Eur. J. Inorg. Chem., 2011, 2380 CrossRef.
- D. Copolovici, F. Isaia, H. J. Breunig, C. I. Rat and C. Silvestru, RSC Adv., 2014, 4, 26569 RSC.
- T. Řezníček, L. Dostál, A. Růžička, J. Vinklárek, M. Řezáčová and R. Jambor, Appl. Organomet. Chem., 2012, 26, 237 CrossRef.
- P. Sharma, D. Castillo, N. Rosas, A. Cabrera, E. Gomez, A. Toscano, F. Lara, S. Hernández and G. Espinosa, J. Organomet. Chem., 2004, 689, 2593 CrossRef.
- D. Pérez, P. Sharma, N. Rosas, A. Cabrera, J. L. Arias, F. del Rio-Portilla, J. Vazquez, R. Gutierrez and A. Toscano, J. Organomet. Chem., 2008, 693, 3357 CrossRef.
- C. R. Wade, B. L. Murphy, S. Bedajna and F. P. Gabbaï, Organometallics, 2024, 43, 1785–1788 CrossRef PubMed.
- C. K. Webber, F. Kong, J. Kumawat, J. Joy, E. K. Richardson, P. Siano, D. A. Dickie, D. H. Ess and T. B. Gunnoe, Organometallics, 2024, 43, 1789–1802 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental data including NMR spectra and theoretical studies. CCDC 2313661 (Ar2SbCl); 2313662 (Ar2SbCl); 2313664 (Ar3Sb); 2313665 (1); 2313666 (2); 2313660 (3); 2313659 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02787f |
| This journal is © The Royal Society of Chemistry 2024 |

