 Open Access Article
Open Access ArticleGradual spin crossover behavior encompasing room temperature in an iron(II) complex based on a heteroscorpionate ligand†
Oleksandr Ye.
Horniichuk
 ,
Laure
Vendier
,
Lionel
Salmon
,
Laure
Vendier
,
Lionel
Salmon
 * and
Azzedine
Bousseksou
*
* and
Azzedine
Bousseksou
*
LCC, CNRS and Université de Toulouse (UPS, INP), 205 Route de Narbonne, F-31077 Toulouse, France. E-mail: lionel.salmon@lcc-toulouse.fr
First published on 27th September 2024
Abstract
In this paper, we report the synthesis of six novel triazole-based heteroscorpionate ligands based on heterocycle metathesis reactions and their iron(II) complexes. Single crystal structure analyses were performed, the spectroscopic and magnetic properties of the obtained complexes were studied and their spin crossover–structural relationships were compared to those obtained for their pyrazole-based analogues reported in the literature. In particular, the amino derivative complex bis[hydrobis(pyrazol-1-yl)(3-amino-1,2,4-triazol-1-yl)]iron(II) obtained by post-synthetic catalytic nitro-group reduction under pressure of hydrogen in an autoclave presents a scarce gradual spin crossover behavior at room temperature. The profile of the SCO curve can be explained by the presence of only relatively weak H bonds, spreading only in one dimension. Among the interesting spin transition behaviors observed for the different complexes, such stable, complete and gradual spin crossover at room temperature makes this neutral complex a good candidate for sublimation and future investigation as an active element notably for thermoreflectance-based surface microthermometry applications.
Introduction
The spin crossover (SCO) phenomenon refers to a change in the spin state of first-row transition metal complexes with central ion electronic configurations of d4–d7 induced by external stimuli that include temperature, pressure, light or magnetic field.1–3 The phenomenon typically occurs in complexes with an octahedral ligand field and is most abundant for the electronic configuration d6 where iron(II) complexes are prevalent examples. One of the objectives to better integrate these materials into devices for real applications is to develop (thin) films.4,5 Over decades of research, various techniques to obtain such films have been developed, which include the Langmuir–Blodgett technique,6,7 spin coating,8,9 drop casting,10 surface layer-by-layer assembly,11,12 vacuum deposition,13,14etc.4 The vast majority of applications involving thin films require high quality, purity and homogeneity of the film. To fulfil these criteria, thermal vacuum deposition serves as a powerful means.13,15 Currently, only a limited number of SCO complexes are known to be capable of being deposited by this technique.13–29 Such a limited range of spin crossover compounds suitable for vacuum deposition can be explained by the fact that the compound candidates for thermal vacuum evaporation should be prone to sublimation and thus meet certain criteria, such as relatively low molecular weight, charge neutrality, a discrete structure and the absence of solvent or other guest molecules in their crystal lattice. However, the cationic iron(III) complex [Fe(pap)2]ClO4·H2O (pap = N-2-pyridylmethylidene-2-hydroxyphenylaminato) is the only exception to this rule.20 Heteroleptic FeII complexes [Fe(H2B(pz)2)2phen], [Fe(H2B(pz)2)2bpy] (pz = pyrazol-1-yl; phen = 1,10-phenanthroline; and bpy = 2,2′-bipyridine) and some of their derivatives substituted at the phen or bpy cores and [Fe(pypyr(CF3)2)2phen] (pypyr = 2-(2′-pyridyl)pyrrolide) can undergo surface-induced fragmentation to [FeL2] or [FeL]+ (L = H2B(pz)2 or pypyr respectively) and phen (bpy) on certain metallic substrates at monolayer and sub-monolayer coverages.30–32 In contrast, homoleptic iron(II) complexes with scorpionate-type tripodal hydrotris(azol-1-yl)borate ligands33 do not dissociate on surfaces and can represent a stable alternative to their heteroleptic counterparts.34The majority of applications require materials that switch around or above room temperature with or without a hysteresis loop.35 Among the sublimable SCO complexes, only a few are known to undergo spin crossover above room temperature in both bulk and thin-film states; those include [Fe(pypyr(CF3)2)2phen],36 [Fe(HB(pz)3)2],23,37 [Fe(HB(1,2,3-tz)3)2]25,38 and [Fe(HB(1,2,4-tz)3)2].16,39 It is noteworthy that the latter three belong to the scorpionate family of complexes. The temperature of spin transition T1/2 for Fe-pypyr thin films (∼330 K) is nearly 60 K lower compared to the bulk substance (390 K), while the other three compounds show comparable SCO properties in bulk and thin film forms (T1/2 ≈ 393 K, 373 K and 335 K for the pyrazolyl, 1,2,3-triazolyl and 1,2,4-triazolyl derivatives, respectively). The properties of [Fe(HB(1,2,4-tz)3)2] thin films were investigated in the 20–200 nm thickness range. It was found that the considered films are of high quality and show unprecedented endurance towards thermal cycling withstanding over 10 million heating–cooling cycles while remaining intact.24
Such an outstanding stability of the films makes them appropriate materials to elaborate miscellaneous functional devices for photonics, molecular electronics, actuators, sensors and temperature damping.24,40–44 In particular, thermometry based on thermoreflectance allows high thermal and temporal resolution and provides the possibility to perform both qualitative and quantitative measurements. Despite the advantages that an abrupt spin transition can offer for thermometry, it does not allow for continuous real-time temperature monitoring enabled by a gradual transition. The issue was addressed by the use of a coating of the SCO [Fe(HB(1,2,3-tz)3)2] complex.25
Recent work of several research groups has reported a potential extension to the family of sublimable spin crossover compounds switching their spin states above room temperature.45–47 The reported compounds belong to the class of heteroscorpionates. Unlike common (homo)scorpionates, in heteroscorpionates the N-donor azol-1-yl rings are not identical.33 Flötotto et al. have developed a synthetic path to access new iron(II) heteroscorpionates, which involves the exchange of the pyrazole ring for a 4-nitropyrazole in the initial [HB(pz)3]− ligand driven by the difference in the pKa of the heterocycles.45 They have successfully demonstrated that by controlling the temperature and stoichiometry, it is possible to selectively substitute one to all three pyrazol-1-yl moieties in the anionic ligand with their nitro counterpart. Moreover, the susceptibility of the NO2 group to reduction offers a route to post-modification of the complexes. Using the above-discussed reaction of pyrazole exchange with 3(5)-nitro- and 4-nitropyrazoles, Ma et al. have complemented the library of nitro- and amino-decorated iron(II) hydrotris(pyrazol-1-yl)borates.46 They have shown that the occurrence of SCO behavior in the resulting complexes depends on steric and electronic factors determined by the substituent position of the pyrazole ring, its steric demands and electronic properties. Desrochers et al. used the reaction of heterocycle metathesis to replace one 3,5-dimethylpyrazole ring in the [HB(3,5-Me2pz)3]− ligand with benzotriazole (btz) to obtain the first ferrous heteroazolyl heteroscorpionate [Fe(HB(3,5-Me2pz)2(btz)2)2].47 As synthesized, the substance constitutes a mixture of cis- and trans-isomers separable by column chromatography. Both isomers display spin state changes in the high temperature region (T1/2 ≈ 385 K for the cis-isomer and T1/2 ≈ 372 K for the trans-isomer).
Thus, a range of evaporable spin crossover complexes have been synthesized up to date; however, this domain of research is still on the rise and requires the development of new SCO coordination compounds and their thin-film materials. The present work concerns the synthesis of novel triazole-based heteroazolyl heteroscorpionate ligands and their iron(II) complexes. Their structure, physico-chemical properties and magnetic behavior were analyzed and compared to those obtained for their pyrazole-based analogues reported in the literature.
Experimental section
Techniques and methods
IR spectra were recorded on a PerkinElmer FT-IR Frontier MIR/FIR spectrometer, using a diamond ATR (Attenuated Total Reflection). NMR spectra were recorded with a Bruker Avance III 400 (400 MHz) at 298 K using deuterated solvents CD3OD and CD2Cl2. The chemical shifts, δ, for 1H, 13C and 11B NMR are expressed in ppm. 11B NMR spectra were recorded using BF3·OEt2 as an external reference. Elemental analyses (for C, H and N) were performed using a PerkinElmer 2400 Series II instrument. Single-crystal X-ray diffraction analysis for nBu4NL1 was performed on a Rigaku Gemini Ultra diffractometer using graphite-monochromated Cu-Kα radiation (λ = 1.54184 Å). The single crystal of the trans-isomer complex 3 was analyzed on a Bruker Kappa Apex II diffractometer equipped with a 30 W air-cooled microfocus source using MoKα radiation (λ = 0.71073 Å). Suitable single crystals of the cis-isomer complexes 3 and 5 were analyzed using a Rigaku XtaLAB Synergy Dualflex diffractometer equipped with a PhotonJet X-ray source (Cu, λ = 1.54184 Å). Data were collected between 100 and 393 K; all diffractometers are equipped with an Oxford Instrument Cooler Device. The structures have been solved using the new dual-space algorithm program SHELXT48 and refined by means of least-squares procedures either using the SHELXL-2018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 program included in the software package WinGX49 version 1.639 or with the aid of the program CRYSTALS.50 The atomic scattering factors were taken from the International Tables for X-Ray Crystallography.51 Hydrogen atoms were placed geometrically and refined using a riding model. All non-hydrogen atoms were anisotropically refined. Ellipsoid plots in the figures of the Crystallography section were generated using ORTEP-3 software.52 The crystal structures have been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition numbers CCDC 2370860–2370867.† X-ray powder diffractograms for complexes 4 and 6 were recorded on capillaries, on a Rigaku Smartlab Powder Diffractometer using a Cu source (λKα1 = 1.54056 Å) and a high energy linear detector (D Tex Ultra 250 from Rigaku). CCDC 2370860–2370867.† The diffractometer was used in a theta/theta configuration with multilayer mirror optics (CBO series for Smartlab). Data were recorded with 2θ between 5 and 70° or 5 and 50° (step: 0.01° or 0.02°). Variable-temperature magnetic susceptibility data for complexes 2 and 3 were obtained in the 4–300 K range, at cooling and heating rates of 2 K min−1 under a field of 1 kOe using a Quantum Design MPMS magnetometer. For complexes 4–7, the data were acquired in the 4–400 K range on a Quantum Design MPMS3 SQUID magnetometer using the same parameters. 57Fe Mössbauer spectra were recorded using a conventional constant-acceleration-type spectrometer equipped with a 50 mCi 57Co source and a liquid nitrogen cryostat. Least-squares fitting of the Mössbauer spectra was carried out with the assumption of Lorentzian line shapes using Recoil software.53
48 program included in the software package WinGX49 version 1.639 or with the aid of the program CRYSTALS.50 The atomic scattering factors were taken from the International Tables for X-Ray Crystallography.51 Hydrogen atoms were placed geometrically and refined using a riding model. All non-hydrogen atoms were anisotropically refined. Ellipsoid plots in the figures of the Crystallography section were generated using ORTEP-3 software.52 The crystal structures have been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition numbers CCDC 2370860–2370867.† X-ray powder diffractograms for complexes 4 and 6 were recorded on capillaries, on a Rigaku Smartlab Powder Diffractometer using a Cu source (λKα1 = 1.54056 Å) and a high energy linear detector (D Tex Ultra 250 from Rigaku). CCDC 2370860–2370867.† The diffractometer was used in a theta/theta configuration with multilayer mirror optics (CBO series for Smartlab). Data were recorded with 2θ between 5 and 70° or 5 and 50° (step: 0.01° or 0.02°). Variable-temperature magnetic susceptibility data for complexes 2 and 3 were obtained in the 4–300 K range, at cooling and heating rates of 2 K min−1 under a field of 1 kOe using a Quantum Design MPMS magnetometer. For complexes 4–7, the data were acquired in the 4–400 K range on a Quantum Design MPMS3 SQUID magnetometer using the same parameters. 57Fe Mössbauer spectra were recorded using a conventional constant-acceleration-type spectrometer equipped with a 50 mCi 57Co source and a liquid nitrogen cryostat. Least-squares fitting of the Mössbauer spectra was carried out with the assumption of Lorentzian line shapes using Recoil software.53
Syntheses of ligands and complexes
If not specified otherwise, all reactants and reagents used in this study were procured from commercial suppliers and employed as received. A solvent purification system PS-MD-5 (Inert Corporation, USA) was used to provide dry air-free dichloromethane for the synthesis of iron(II) complexes 1–4. The synthesis of KTp was performed according to the procedure developed by Trofimenko,54 with modifications specified in the respective protocol below. nBu4NTp was synthesized according to the literature protocol.55 All the ligands are poorly soluble in water, but soluble in alcohols, DCM and aprotic polar organic solvents, in particular, tetrahydrofuran (THF), acetonitrile and acetone.The CN-bearing derivatives of L3 and L4 were obtained following the same protocols as for their nitro-featured counterparts using 3-cyano-1,2,4-triazole instead of 3-nitro-1,2,4-triazole.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)), 1500w, 1470s, 1413w, 1384s, 1291s, 1161vs, 1107vs, 1096vs, 1034vs; 1H NMR (400 MHz, CD2Cl2) δ 7.99 (s, 1H), 7.52 (d, J = 2.1 Hz, 2H), 7.50 (d, J = 2.1 Hz, 2H), 6.16 (t, J = 2.1, 2H), 4.78 (q, JHB = 138 Hz, 1H), 3.10–2.93 (m, 8H), 1.61–1.49 (m, 8H), 1.44–1.30 (m, 8H), 0.99 (t, J = 7.3 Hz, 12H); 13C NMR (101 MHz, CD2Cl2) δ 149.35, 140.28, 139.29, 134.12, 114.61, 104.20, 59.10, 24.19, 20.03, 13.72. 11B NMR (128 MHz, CD2Cl2) δ −1.66 (d); C25H44BN9 (M = 481.50). Anal. calc.: C, 62.36; H, 9.21; N, 26.18%. Found: C, 62.70; H, 9.52; N, 26.09%.
N)), 1500w, 1470s, 1413w, 1384s, 1291s, 1161vs, 1107vs, 1096vs, 1034vs; 1H NMR (400 MHz, CD2Cl2) δ 7.99 (s, 1H), 7.52 (d, J = 2.1 Hz, 2H), 7.50 (d, J = 2.1 Hz, 2H), 6.16 (t, J = 2.1, 2H), 4.78 (q, JHB = 138 Hz, 1H), 3.10–2.93 (m, 8H), 1.61–1.49 (m, 8H), 1.44–1.30 (m, 8H), 0.99 (t, J = 7.3 Hz, 12H); 13C NMR (101 MHz, CD2Cl2) δ 149.35, 140.28, 139.29, 134.12, 114.61, 104.20, 59.10, 24.19, 20.03, 13.72. 11B NMR (128 MHz, CD2Cl2) δ −1.66 (d); C25H44BN9 (M = 481.50). Anal. calc.: C, 62.36; H, 9.21; N, 26.18%. Found: C, 62.70; H, 9.52; N, 26.09%.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)), 1506w, 1470s, 1415w, 1386m, 1315s, 1166vs, 1104s, 1030vs; 1H NMR (400 MHz, CD2Cl2) δ 8.19 (s, 2H), 7.65 (d, J = 2.2 Hz, 1H), 7.60 (d, J = 2.2 Hz, 1H), 6.23 (t, J = 2.2 Hz, 1H), 4.77 (q, JHB = 142 Hz, 1H), 3.15–3.03 (m, 8H), 1.67–1.52 (m, 8H), 1.45–1.31 (m, 8H), 0.98 (t, J = 7.3 Hz, 12H); 13C NMR (101 MHz, CD2Cl2) δ 149.58, 141.27, 139.81, 134.96, 114.22, 104.91, 59.32, 24.17, 20.02, 13.66; 11B NMR (128 MHz, CD2Cl2) δ −2.59 (d); C25H42BN11 (M = 507.50). Anal. calc.: C, 59.17; H, 8.34; N, 30.36%. Found: C, 59.40; H, 8.33; N, 30.40%.
N)), 1506w, 1470s, 1415w, 1386m, 1315s, 1166vs, 1104s, 1030vs; 1H NMR (400 MHz, CD2Cl2) δ 8.19 (s, 2H), 7.65 (d, J = 2.2 Hz, 1H), 7.60 (d, J = 2.2 Hz, 1H), 6.23 (t, J = 2.2 Hz, 1H), 4.77 (q, JHB = 142 Hz, 1H), 3.15–3.03 (m, 8H), 1.67–1.52 (m, 8H), 1.45–1.31 (m, 8H), 0.98 (t, J = 7.3 Hz, 12H); 13C NMR (101 MHz, CD2Cl2) δ 149.58, 141.27, 139.81, 134.96, 114.22, 104.91, 59.32, 24.17, 20.02, 13.66; 11B NMR (128 MHz, CD2Cl2) δ −2.59 (d); C25H42BN11 (M = 507.50). Anal. calc.: C, 59.17; H, 8.34; N, 30.36%. Found: C, 59.40; H, 8.33; N, 30.40%.
The synthesis of the iron(II) complexes 2–5 was carried out under an argon atmosphere using common anaerobic Schlenk techniques. The reduction of the nitro-derived complexes 2 and 3 was conducted in a 50 ml autoclave (Autoclave-Maxitech, France) pressurized with hydrogen gas. Synthetic procedures to access iron(II) complexes with ligands L1–L4 were conducted as follows.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)), 1501w, 1469w, 1406m, 1315m, 1220m, 1171m, 1112m, 1049m, 755s, 713vs; C18H16B2FeN16 (M = 533.90). Anal. calc.: C, 40.49; H, 3.02; N, 41.98%. Found: C, 40.53; H, 2.95; N, 41.82%.
N)), 1501w, 1469w, 1406m, 1315m, 1220m, 1171m, 1112m, 1049m, 755s, 713vs; C18H16B2FeN16 (M = 533.90). Anal. calc.: C, 40.49; H, 3.02; N, 41.98%. Found: C, 40.53; H, 2.95; N, 41.82%.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)), 1505w, 1464w, 1405w, 1337m, 1317w, 1167m, 1109w, 1020m, 761s, 712vs; C18H12B2FeN20 (M = 585.90). Anal. calc.: C, 36.90; H, 2.06; N, 47.81%. Found: C, 36.40; H, 2.33; N, 47.49%.
N)), 1505w, 1464w, 1405w, 1337m, 1317w, 1167m, 1109w, 1020m, 761s, 712vs; C18H12B2FeN20 (M = 585.90). Anal. calc.: C, 36.90; H, 2.06; N, 47.81%. Found: C, 36.40; H, 2.33; N, 47.49%.
Results and discussion
Inspiration to design new scorpionate ligands and their FeII complexes was based on the heterocycle metathesis reaction described in the literature,45,46 where the authors obtained novel spin-crossover complexes by replacing pyrazole units in tris(pyrazol-1-yl)borate derivatives with nitropyrazoles, with the possibility of post-functionalization of the resulting complexes. Instead of nitropyrazoles, 1,2,4-triazoles substituted with an electron withdrawing group (–NO2 and –CN) at position 3 were used in this work. Nitro-functionalized derivatives were successfully converted to amines. At first, potassium hydrotris(pyrazol-1-yl)borate (hereafter KTp) was synthesized following the protocol developed by Trofimenko,54 with a slightly modified work-up procedure (see the Experimental part). Then, potassium cation in the product was exchanged for tetrabutylammonium (nBu4N+ or TBA) according to the procedure reported in ref. 55. Then, the resulting TBA derivative nBu4NTp was heated with 1 (2) equivalent(s) of an EWG-bearing 1,2,4-triazole to obtain mono(di)substituted products, as shown in Fig. 1. As electron-withdrawing groups, nitro-group and cyano-group were selected. Thus, commercially available 3-nitro-1,2,4-triazole and 3-cyano-1,2,4-triazole were used for the reaction of heterocycle exchange to afford the new heteroscorpionate ligands. The reactions between nBu4NTp and the triazole reactants were conducted without any solvent under static vacuum conditions to promote the pyrazole release. The degree of substitution of pyrazole moieties with triazole moieties was controlled through stoichiometry and temperature alike. Thus, to replace one pyrazole unit (n = 1, Fig. 1), heating the reaction mixture at 90 °C was sufficient for the reaction to proceed. The optimal temperature range for monosubstitution was set between 90 and 105 °C. The primary evidence of the reaction is the condensation of the eliminated pyrazole crystals on the walls of the reaction vessel. The latter can be distilled under dynamic vacuum and collected in a receiving flask. This allowed us to conveniently monitor the reaction completion by measuring the weight of the released pyrazole. To this purpose, the latter was distilled off from the reaction vessel every one hour, and the collected distillate was weighed. The majority of pyrazole was released in the first hour of the reaction. Heating the reaction mixture for an additional hour yielded the rest of pyrazole; the pyrazole release was quantitative. The formation of the disubstituted derivatives was possible via heating the stoichiometric mixture of reactants to 130 °C. After around 95% of pyrazole was eliminated, its release ceased, reaching a maximum after 3 h of heating. As in the case of analogous reactions with nitropyrazoles, heterocycle metathesis involving the above-mentioned triazole reactants leads to selective formation of the desired mono- and disubstituted products, which can be controlled through temperature and stoichiometry.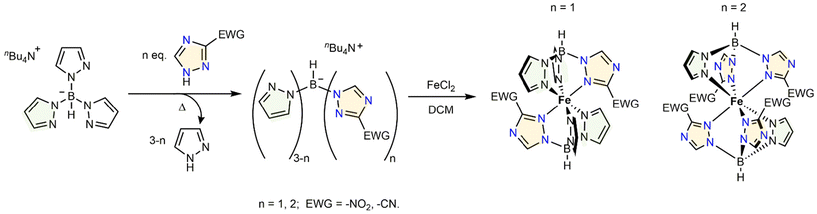 | ||
| Fig. 1 Synthetic pathway to obtain triazole-based heteroazolyl heteroscorpionate ligands and their respective iron complexes. | ||
However, attempts at trisubstitution, requiring 3 equivalents of a triazole reactant and higher temperatures (about 150 °C), led to the formation of a mixture of products with different degrees of substitution and by-products that were formed, most probably, as a result of side reactions taking place at high temperatures. The separation of products in this mixture appeared to be challenging. The scope of the synthesized ligands is depicted in Chart 1.
In the literature,45,46 the authors propose a heterocycle metathesis mechanism based on the considerations of HSAB theory, according to which more acidic nitropyrazoles (pKa = 9.50–9.75), attacking the boron core, readily give their pyrrole-type protons to the more basic pyrazolyl moiety, which eliminates as a free pyrazole (pKa = 14.21).56 Given that the acidity of 1,2,4-triazole (pKa = 9.89)57 is close to that of nitropyrazoles, it was interesting to carry out this reaction with unsubstituted triazole. Though the synthetic conditions for mono- and disubstitution were the same as those in the case of the substituted triazoles, the reactions with pristine triazole did not provide the same selectivity and resulted in a mixture of products that are challenging to separate. Similar results were achieved, when a triazole bearing an electron-donating amino group at position 3 was used as the reactant in an attempt to synthesize directly an amino-functionalized heteroscorpionate ligand. Considering the electron density donation from the amino group to the aromatic core, the pKa of 3-amino-1,2,4-triazole is expected to be higher than that of pristine triazole; consequently, such a result was expected. Consequently, these results point to the conclusion that the presence of EWG in the triazole precursor is crucial for heterocycle metathesis to proceed as it provides a sufficient difference in pKa between a triazole reactant and pyrazole, to shift the equilibrium towards the formation of the desired heteroscorpionates.
As the nitro group can be reduced to a primary amine, it was interesting to try to convert a nitro-derived ligand to an NH2-bearing ligand that can be either directly coordinated to FeII or undergo functionalization of the amino group with the subsequent coordination of a modified ligand to the metal ion. For this purpose, the monosubstituted nitro-derived ligand nBu4NL1 was selected for reduction. The reduction reaction conditions were similar to those in the case of complexes reported in ref. 46, which involve the use of an organic reducing agent, a palladium catalyst, a protic solvent and microwave heating. In the present case, 1,4-cyclohexadiene was used as the reducing agent and palladium (5 wt%) on carbon was selected as the catalyst, while alcohols such as methanol or ethanol were used as solvents either pure or in an alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (3
toluene (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture similar to those reported by Ma et al.46 The first approach involved microwave heating (closed-vessel mode) of nBu4NL1 stirred methanolic solution at 120 °C for 5 minutes with an excess (6 equivalents) of 1,4-cyclohexadiene and in the presence (8 mol%) of Pd/C. Colorless crystals isolated after concentration of the obtained solution were analyzed by means of single crystal X-ray diffraction. Instead of the expected amino-derived hydroborate anion, the resulting crystal structure revealed an associate composed of a 3-amino-1,2,4-triazole anion binding three neutral pyrazole molecules via hydrogen bonding (see Fig. S1†). The negatively charged associate is compensated for by a TBA cation. Such a result indicates the degradation of the hydroborate anion, possibly due to solvolysis under the given conditions. The degradation is supported by NMR spectroscopy data. Solution 1H NMR of an aliquot of the reaction mixture taken after 5 minutes of the reaction revealed two signals of free-pyrazole aromatic protons, while in the spectra of the scorpionate ligands, three signals are typically observed for pyrazole bound to boron (see the NMR spectrum in Fig. S2†). Changing the reaction conditions, e.g., reducing the temperature together with increasing the time only retarded the degradation, but did not prevent it. Moreover, even after leaving the mixture of the reactants and the reagents overnight at room temperature with no stirring, degradation signs, such as the presence of the liberated pyrazole, could be detected. Varying the solvents did not have any notable impact on the reaction outcome. Such a chemical behavior is probably related to the presence of the palladium catalyst that in the protic solvent medium can promote the solvolysis of the hydroborate as is known in the case of borohydride.58 Considering the fragility of the hydroborate anion, direct reduction of the nitro group in L1 and L2 to obtain L5 and L6 is probably not the most appropriate strategy. The reduction of the NO2 group of the ligands in their iron(II) complexes (post-functionalization) is another approach, which is discussed further together with the synthesis of coordination compounds based on heteroscorpionate ligands.
1) mixture similar to those reported by Ma et al.46 The first approach involved microwave heating (closed-vessel mode) of nBu4NL1 stirred methanolic solution at 120 °C for 5 minutes with an excess (6 equivalents) of 1,4-cyclohexadiene and in the presence (8 mol%) of Pd/C. Colorless crystals isolated after concentration of the obtained solution were analyzed by means of single crystal X-ray diffraction. Instead of the expected amino-derived hydroborate anion, the resulting crystal structure revealed an associate composed of a 3-amino-1,2,4-triazole anion binding three neutral pyrazole molecules via hydrogen bonding (see Fig. S1†). The negatively charged associate is compensated for by a TBA cation. Such a result indicates the degradation of the hydroborate anion, possibly due to solvolysis under the given conditions. The degradation is supported by NMR spectroscopy data. Solution 1H NMR of an aliquot of the reaction mixture taken after 5 minutes of the reaction revealed two signals of free-pyrazole aromatic protons, while in the spectra of the scorpionate ligands, three signals are typically observed for pyrazole bound to boron (see the NMR spectrum in Fig. S2†). Changing the reaction conditions, e.g., reducing the temperature together with increasing the time only retarded the degradation, but did not prevent it. Moreover, even after leaving the mixture of the reactants and the reagents overnight at room temperature with no stirring, degradation signs, such as the presence of the liberated pyrazole, could be detected. Varying the solvents did not have any notable impact on the reaction outcome. Such a chemical behavior is probably related to the presence of the palladium catalyst that in the protic solvent medium can promote the solvolysis of the hydroborate as is known in the case of borohydride.58 Considering the fragility of the hydroborate anion, direct reduction of the nitro group in L1 and L2 to obtain L5 and L6 is probably not the most appropriate strategy. The reduction of the NO2 group of the ligands in their iron(II) complexes (post-functionalization) is another approach, which is discussed further together with the synthesis of coordination compounds based on heteroscorpionate ligands.
The syntheses of iron(II) complexes with ligands L1–L4 were performed under an argon atmosphere in a degassed dichloromethane (DCM) solution starting from 2 equivalents of the respective ligand and 1 equivalent of FeCl2·4H2O (Fig. 1). Attempts to reduce complexes [Fe(L1)2] under microwave conditions suggested by Ma et al.46 and tried for uncoordinated L1 faced issues to obtain any reproducible results, which are related to the absence of possibility of control over the reaction outcome to obtain the desired product, resulting in fairly low yields (2–33%) of the reduction reaction product. Conversely, the classical technique of catalytic nitro-group reduction under pressure of hydrogen in an autoclave served as a reliable tool to convert NO2-decorated substrates [Fe(L1)2] and [Fe(L2)2] into di- and tetraamines [Fe(L5)2] and [Fe(L6)2], respectively (Fig. 2). The reaction conditions were similar to those proposed by Flötotto et al. for the pyrazole-based counterpart.45 The synthetic procedures to access the ligands and their iron(II) complexes are provided in the Experimental part.
Spectroscopic characterization
The NMR spectra are presented in Fig. S3–S14.† In the proton NMR spectra of the ligands, the pyrazolyl moiety is characterized by three signals: two doublets at about 7.61 and 7.58 ppm (average chemical shift values) assigned to protons at positions 5 and 3 of the pyrazole ring, and a triplet at around 6.20 ppm corresponding to the proton at position 4. The triazolyl units bear only a single proton, the singlet signal of which appears at 7.93 (7.99) ppm for L1 (L3) and at 8.20 ppm in the case of L2 and L4. The integral intensity ratio of the triazole singlet to the pyrazole multiplets (s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t) is 1
t) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for L1 and L3 and 2
2 for L1 and L3 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for L2 and L4, which is in line with the expected structures of the respective heteroscorpionate anions. The 13C NMR spectra of the ligands featuring nitro groups are characterized by the presence of four signals in the aromatic region of the spectra: one weak-field signal (∼149 ppm) corresponding to the carbon nucleus at position 5 of the triazolyl moiety and three signals in the 140–105 ppm region from the pyrazole carbon nuclei. A large number of scans (>3000) in the NMR experiment may allow us to observe a weak signal of the carbon nucleus at position 3 of the triazole ring. For example, the signal can be observed for L1 at 152.39 ppm. In the spectra of the cyano-functionalized ligands, an extra signal of low intensity observed at 114.61 ppm for L3 and at 114.22 for L4 is assigned to the carbon nucleus of the CN group. Similar to the nitro derivatives, large scan numbers allow the observation of the position 3 aromatic carbon signal at 139.29 and 139.81 ppm for L3 and L4, respectively. The boron NMR spectra of all the ligands contain one doublet lying in the region from −2.59 to −1.57 ppm. The presence of the sole signal in the spectra as well as the multiplicity of the signal correlates well with the fact that the compounds contain only one type of boron nucleus directly linked to the proton nuclei of identical nuclear spin. The NMR spectra of the heteroscorpionate ligands recorded weeks or months after their synthesis reveal signs of stability issues in the ligands upon their storage under ambient conditions. Thus, an extra signal appears in the 11B NMR spectra as a singlet at around 18 ppm, which is characteristic of boric acid and its esters.59 Meanwhile in the proton NMR, an extra weak-field singlet appears in the 8.3–8.0 ppm region, most probably corresponding to the free triazole species. It should be noted that these impurity signals are also observed in the spectra of the crude products. Similar to the observations of L1 degradation under reduction conditions, the considered observations highlight the fact that heteroscorpionates have an inclination to degrade over time under ambient conditions, probably due to slow hydrolysis of the hydroborate species caused by moisture in the ambient air. This fact should be taken into account during the planning of ligand synthesis as well as the synthesis of complexes. Thus, practices such as using the ligands shortly after their synthesis or employing anaerobic techniques for preparation, handling and storage of the compounds can be applied to ensure substance purity and to minimize or avoid undesired degradation processes.
1 for L2 and L4, which is in line with the expected structures of the respective heteroscorpionate anions. The 13C NMR spectra of the ligands featuring nitro groups are characterized by the presence of four signals in the aromatic region of the spectra: one weak-field signal (∼149 ppm) corresponding to the carbon nucleus at position 5 of the triazolyl moiety and three signals in the 140–105 ppm region from the pyrazole carbon nuclei. A large number of scans (>3000) in the NMR experiment may allow us to observe a weak signal of the carbon nucleus at position 3 of the triazole ring. For example, the signal can be observed for L1 at 152.39 ppm. In the spectra of the cyano-functionalized ligands, an extra signal of low intensity observed at 114.61 ppm for L3 and at 114.22 for L4 is assigned to the carbon nucleus of the CN group. Similar to the nitro derivatives, large scan numbers allow the observation of the position 3 aromatic carbon signal at 139.29 and 139.81 ppm for L3 and L4, respectively. The boron NMR spectra of all the ligands contain one doublet lying in the region from −2.59 to −1.57 ppm. The presence of the sole signal in the spectra as well as the multiplicity of the signal correlates well with the fact that the compounds contain only one type of boron nucleus directly linked to the proton nuclei of identical nuclear spin. The NMR spectra of the heteroscorpionate ligands recorded weeks or months after their synthesis reveal signs of stability issues in the ligands upon their storage under ambient conditions. Thus, an extra signal appears in the 11B NMR spectra as a singlet at around 18 ppm, which is characteristic of boric acid and its esters.59 Meanwhile in the proton NMR, an extra weak-field singlet appears in the 8.3–8.0 ppm region, most probably corresponding to the free triazole species. It should be noted that these impurity signals are also observed in the spectra of the crude products. Similar to the observations of L1 degradation under reduction conditions, the considered observations highlight the fact that heteroscorpionates have an inclination to degrade over time under ambient conditions, probably due to slow hydrolysis of the hydroborate species caused by moisture in the ambient air. This fact should be taken into account during the planning of ligand synthesis as well as the synthesis of complexes. Thus, practices such as using the ligands shortly after their synthesis or employing anaerobic techniques for preparation, handling and storage of the compounds can be applied to ensure substance purity and to minimize or avoid undesired degradation processes.
The IR spectra are presented in Fig. S15–S24.† A characteristic vibration band for boron-based scorpionate ligands is the B–H stretch appearing well apart from other bands within the 2460–2526 cm−1 range in the case of the considered ligands and complexes. As observed for pyrazole-based scorpionates in ref. 46, the B–H stretching vibration appeared to be sensitive to the coordination of the scorpionate ligands to the metal ion. Following the coordination, the B–H band position experienced a shift towards higher wavenumbers by 27–45 cm−1 for NO2-bearing ligands and by 50–56 cm−1 in the case of CN derivatives. The high-frequency shift of the B–H stretch is related to the withdrawal of the electron density from the ligand and, consequently, from the boron, upon the coordination. In the IR spectra of the amino-functionalized complexes, the B–H band is located at lower wavenumbers than in the rest of the complexes, e.g., 2474 cm−1 for [Fe(L5)2] vs. 2487 cm−1 for [Fe(L1)2] and 2470 cm−1 for [Fe(L6)2] vs. 2512 cm−1 for [Fe(L2)2], which is expected given the electron-donating effect of the NH2 group increasing the electron density at boron. An additional characteristic band, positioned away from the rest of the bands, is caused by the stretching vibration of the nitryl group's triple bond inherent in L3, L4, 3 and 4. In these compounds, the band position is situated in a narrow 2245–2251 cm−1 range. Unlike the B–H stretching, the position of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibration band did not experience any significant shift upon the ligand coordination, though the band is useful for the identification of the respective compounds.
N stretching vibration band did not experience any significant shift upon the ligand coordination, though the band is useful for the identification of the respective compounds.
The IR spectra of nitro compounds typically feature the characteristic asymmetric and symmetric stretching vibrations of the NO2 group. However, they are usually located in the fingerprint region containing a set of other bands that are positioned tightly, which might complicate the assignment of the nitro-group vibrations. Thus, in the spectra of the nitro ligands and their complexes, no bands were found that can be clearly assigned to the NO2 stretches.
Single crystal and powder X-ray diffraction studies
Single crystals of nBu4NL1 suitable for X-ray diffraction grow in the crude product oily substance in one day. The crystal structure of the heteroscorpionate ligand is shown in Fig. 3. The compound crystallizes in the monoclinic P21/n space group, with the desired structure of the heteroscorpionate tetrahedral anion with hydrogen, two pyrazolyl substituents and one 3-nitro-1,2,4-triazolyl function attached to boron as well as tetrabutylammonium as a countercation. Efforts to obtain crystals for the rest of the ligands were not successful.The crystallization of complexes 1 and 2 by slow evaporation of their saturated solutions or vapor diffusion techniques yielded only polycrystalline aggregates or very small crystals, the quality of which did not meet the requirements for the single crystal X-ray diffraction experiment. Similarly, the endeavor to crystallize complexes 4 and 6 proved unsuccessful. Single crystals of bis[hydrobis(pyrazol-1-yl)(3-amino-1,2,4-triazol-1-yl)]iron(II) (5) were grown from a saturated methanolic solution by means of slow evaporation of the solvent. The complex crystallizes in the P21/n space group (see Table S1† for crystal data and structural refinement). The coordination environment of FeII is formed by two coordinated tridentate ligands, the donor atoms of which are resting on the facets of the octahedron. The triazolyl units are located on opposite sides of the axial vertices of the octahedron. Thus, the obtained complex appears in the trans-form. Despite the probability of the existence of both cis- and trans-isomers, only the trans-isomer of 5 was observed. The crystal structure of 5 is shown in Fig. 3.
The crystal structure of 5 was acquired at four different temperatures: 100 K, 293 K, 320 K and 393 K. The average distance Fe–N (![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) Fe–N) in the complex at 100 K equals 1.986(11) Å, while the octahedron volume (Vp) at this temperature is around 10.46 Å3, which is inherent in low spin iron(II) complexes. The
Fe–N) in the complex at 100 K equals 1.986(11) Å, while the octahedron volume (Vp) at this temperature is around 10.46 Å3, which is inherent in low spin iron(II) complexes. The ![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) Fe–N value, at room temperature, reaches 2.107(2) Å, which already falls within the range of Fe–N distances found in the HS complexes. At higher temperatures of 320 K and 393 K, the mean iron–nitrogen distances are 2.130(3) Å and 2.159(15) Å, while the polyhedral volume values are 12.80 Å3 and 13.32 Å3, respectively. The octahedron distortion parameter Σ at the termini of the low and high temperature values equals 16.77° and 47.53°, respectively. The unit cell volume (Vc) increases with temperature from 2142.1(10) Å3 (at 100 K) to 2314.06(10) Å3 (at 393 K). Thus, Fe–N distances increase by 8.7% in the full range of temperatures, while the cell volume increases by 8.0%. However, the percentage changes in the thermal evolution parameters should be considered with care, as the thermal expansion in the given temperature range may be non-negligible. The evolution of the structural parameters with temperature for complex 5 is summarized in Table 1 (see detailed crystallographic data in Tables S3 and S4†).
Fe–N value, at room temperature, reaches 2.107(2) Å, which already falls within the range of Fe–N distances found in the HS complexes. At higher temperatures of 320 K and 393 K, the mean iron–nitrogen distances are 2.130(3) Å and 2.159(15) Å, while the polyhedral volume values are 12.80 Å3 and 13.32 Å3, respectively. The octahedron distortion parameter Σ at the termini of the low and high temperature values equals 16.77° and 47.53°, respectively. The unit cell volume (Vc) increases with temperature from 2142.1(10) Å3 (at 100 K) to 2314.06(10) Å3 (at 393 K). Thus, Fe–N distances increase by 8.7% in the full range of temperatures, while the cell volume increases by 8.0%. However, the percentage changes in the thermal evolution parameters should be considered with care, as the thermal expansion in the given temperature range may be non-negligible. The evolution of the structural parameters with temperature for complex 5 is summarized in Table 1 (see detailed crystallographic data in Tables S3 and S4†).
| T, K |
![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) Fe–N, Å
Fe–N, Å |
Σ, ° | V p, Å3 | V c, Å3 |
|---|---|---|---|---|
| 100 | 1.986(11) | 16.77 | 10.46 | 2142.1(10) |
| 293 | 2.107(2) | 38.59 | 12.41 | 2256.1(11) |
| 320 | 2.130(3) | 42.81 | 12.80 | 2269.4(2) |
| 393 | 2.159(15) | 47.53 | 13.32 | 2314.06(10) |
Besides the metal octahedron distortions accompanying the spin crossover, there are also distortions in the ligands. In scorpionate ligands, two main types of distortions in response to iron–nitrogen distance increase were established: twisting of a heterocycle ring defined by the B–N–N–Fe torsion angle, and pyramidalization of the boron tetrahedron defined by the distance from boron to the centroid of the B–N-bonded N3 plane or B–Ct(N3).60 These SCO-responsive parameters were measured in coordination compound 5. It was found that temperature had no considerable effect on the twisting of heterocycle rings. Thus, at 100 K, the triazole rings are twisted to a quite small extent: torsion angles B1–N5–N6–Fe1 and B2–N13–N14–Fe1 are −0.05° and 1.32°, respectively. Meanwhile, at 393 K, these values change to −2.21° and −0.08° respectively. Similar to those for the triazole rings, the B–N–N–Fe torsion angles of the pyrazole changed only by 2.38° maximum. In contrast, the elongation of iron–nitrogen bonds induces pyramidalization of the boron geometry, i.e., a decrease in the distance between boron and the centroid of the mean plane of the boron-bound nitrogen atoms B–Ct(N3) from 0.57(2) Å at 100 K to 0.522(2) Å at 393 K, which leads to an increase in the ligand bite angle.
Among the interactions determining the crystal packing of [Fe(L5)2] molecules, one can highlight the intermolecular H-bonding between the triazole units formed by a hydrogen atom of the amino group (H-bond donor or D) and the nitrogen atom at position 4 (H-bond acceptor or A) of the heterocycle ring (Fig. 4a). Hydrogen bonding connects the complex molecules in a 1D cascade growing along the a crystallographic axis. The distance between hydrogen-bond donors and acceptors d(D⋯A) is 3.016(3) Å for N8⋯N15 and 3.012(3) Å for N7⋯N16 at 100 K. The mean donor–acceptor distance increases by ca. 2% at high temperature. Alternatively, C–H⋯N interactions are present between the triazole and pyrazole units of adjacent [Fe(L6)2] molecules (Fig. 4b). The X-ray diffractogram acquired on the powder of 5 at room temperature matches that simulated from the single crystal diffraction data (Fig. S25†).
Single crystals of complex 3 were obtained by slow evaporation of its saturated DCM solution. The elongated prismatic crystals correspond to the compound that crystallizes in the monoclinic C2/c space group as a trans-isomer (Table S1†). Together with the prismatic crystals, seldom small orthorhombic crystals can be found in the same crystallization batch. These crystals show distinct unit cell parameters and correspond to the cis-isomer of the complex that crystallizes as a dichloromethane solvate [cis-Fe(L4)2]·CH2Cl2 (Fig. 5) in the P21/n space group (see detailed crystallographic data in Table S2†). Thus, crystallization of both isomers was observed for complex 3. However, unlike the case described in ref. 47, no separation of the isomers was achieved by means of column chromatography. Moreover, the PXRD pattern of the same crystallization batch does not contain reflection peaks that might correspond to the cis-isomer (Fig. S26†). These observations suggest that the latter may form during the crystallization; however, it is likely a very minor product.
At 100 K, the average Fe–N distance value is 1.976(14) Å in the trans- and 1.968(4) Å in the cis-isomer. The octahedron volume at the given temperature equals 10.27 Å3 and 10.16 Å3 in the trans- and cis-isomers, respectively. These values indicate that both isomers of the complex are low spin at this temperature. The octahedron distortion of the low-spin trans-isomer appears to be higher (19.80°) than that of the LS cis-isomer (16.10°). The crystal packing of the isomers is dominated by C–H⋯N and C–H⋯π interactions between the complex molecules. The mean D⋯A distances characterizing the C–H⋯N interactions in both isomers are 3.424(3) Å in the trans- and 3.307(6) Å in the cis-isomer. In the case of the C–H⋯π interactions in the cis-isomer, the CH/π-plane distance equals 2.762(4) Å, while the C–H⋯π access angle is 146.0(4)°, which is in line with typical reported values for such a type of interaction.61
Spin crossover properties
The magnetic behavior of the complexes under temperature variation was studied by means of variable-temperature SQUID magnetometry on bulk powders of the complexes. Molar magnetic susceptibility (χMT) was measured in 4–300 K (for 1 and 2) and 4–400 K (for 3–6) temperature ranges (Fig. 6). Nitro-functionalized complexes 1 and 2 are high spin at room temperature and retain their HS state at low temperatures until their χMT starts to decrease brusquely below 50 K due mainly to the zero-field splitting. This behavior was also observed for their heteroanalogues containing 3-nitropyrazolyl moieties instead of the nitrotriazole moieties.46 Referring to the crystallographic data, Ma et al. suggested that the effect of the sterically demanding NO2-substituent situated closely to the metal center allows the ligands to adopt exclusively HS FeII. | ||
| Fig. 6 Molar magnetic susceptibility of 1–6 as a function of temperature in both cooling and heating modes. | ||
Unlike the nitro group, the CN function has no considerable steric effect. Thus, complex 3 bearing two cyano-substituents is low spin at low temperatures, which agrees with the X-ray structural data, and it remains LS at room temperature, whereafter its χMT firmly increases. The transition is not over at 400 K and probably occurs completely at higher temperatures out of the range attainable by the SQUID magnetometry setup used. A similar trend is observed in 4 (tetracyano derivative); however, the threshold of χMT is above 350 K. The amino group is much less bulky compared to the nitro group, and thus the NH2-substituted ligands are expected to adopt the metal ion in both LS and HS configurations. Indeed, the magnetic measurement data reveal a complete spin transition in diamino-featured complex 5 and incomplete SCO behavior in tetraamino-derived complex 6. The SCO behavior in the complexes is reproducible over three consecutive thermal cycles. Complex 5 undergoes a gradual spin crossover in the 150–350 K range with T1/2 centered around 276 K. The SCO curves of 5 acquired over three consecutive heating/cooling cycles together with the hysteresis loop are shown in Fig. S27.† Despite the presence of hydrogen bonding between [Fe(L5)2] molecules in the bulk of the substance, the SCO occurs gradually. The magnetometry data and the crystallography data correlate well with each other. By comparing the SCO behavior in complex 5 to that of bis[hydrotris(1,2,3-triazol-1-yl)borate]iron(II), one may note that the spin transition in bis[hydrobis(pyrazol-1-yl)(3-amino-1,2,4-triazol-1-yl)]iron(II) is more gradual. The hydrogen bonding present in the crystal is supposed to favor a more abrupt spin transition. It should be noted however that the H-bonding in the structure is spread only in one dimension, while in previously reported [Fe(HB(1,2,3-tz)3)2] and [Fe(HB(1,2,4-tz)3)2] similar intermolecular C–H⋯N interactions form 2D and 3D supramolecular networks, respectively, which may promote the cooperativity. Thus, such a gradual SCO behavior in 5 may be explained by the absence of any multidimensional supramolecular network and the lack of other strong intermolecular interactions apart from hydrogen bonding. Besides the structural and magnetochemical characterization, Mössbauer spectra were acquired for the bulk powder of bis[hydrobis(pyrazol-1-yl)(3-amino-1,2,4-triazol-1-yl)] (Fig. S28†). The spectrum acquired at 80 K allows us to conclude that the substance is virtually fully low spin (γHS = 4%) at this temperature, while at 320 K the HS fraction reaches 94%. Expectedly, γHS is at equilibrium at 270 K (γHS = 50%), while at room temperature the compound is 74% high spin. The isomeric shifts, quadrupole splittings, half widths at half maximum (HWHM), acquisition time and high-spin fraction for each temperature are given in Table S5.† The full correlation between the temperature-dependent magnetic susceptibility and Mössbauer spectral data is depicted in Fig. S29.†
The collected structural, magnetochemical and spectroscopic data on the properties of the complexes, when compared to those reported for their 3-nitro- and 3-aminopyrazolyl analogues, can yield some clues on the structure–property relationship. Indeed, iron(II) complexes with heteroscorpionate ligands substituted with bulky NO2 groups at the 3 position of a heterocycle do not undergo spin crossover as only the HS configuration is sterically favorable for them. Changing the nitro group to the non-bulky CN group affords low spin complexes, which may undergo SCO at high temperatures. Concerning the amino derivatives, one may conclude that replacing the 3-aminopyrazole unit with 3-amino-1,2,4-triazole units makes the HS state more favorable at lower temperatures, thus lowering T1/2 or even favoring the incomplete SCO behavior.
Conclusions
Six novel iron(II) coordination complexes incorporating six new heteroscorpionate ligands were synthesized and characterized. SCO behavior (or its absence) in the compounds depended on the steric effect of the substituent (–NO2, –CN or –NH2) of the triazole ring at the position proximal to the metal ion. The SCO-active new complexes potentially may provide an extension to the family of thin film thermo-responsive materials. The gradual spin transition in the diamine complex [Fe(L5)2] represents particular interest as it covers cryogenic to above-room-temperature ranges (150–350 K), which is interesting in particular for thermoreflectance-based surface microthermometry applications. Indeed, among the scarce compounds presenting gradual behavior at room temperature (300 K) and without a significant hysteresis loop,46,62,63 the present compound may represent a unique candidate for sublimation. Thus, future research will concern the assessment of their evaporability and delving into their spin crossover behavior within thin films. This dual focus on SCO phenomena and thermometry highlights the intention to develop advanced materials with tailored characteristics for precise temperature measurement techniques. Moreover, the versatile reactivity of the NH2 group may allow using amino derivatives as building blocks to extend the family of (evaporable) SCO compounds.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
No conflict of interest.References
- Spin-Crossover Materials: Properties and Applications, ed. M. A. Halcrow, John Wiley & Sons Ltd, Oxford, UK, 2013 Search PubMed.
- P. Gütlich and H. A. Goodwin, Spin Crossover—An Overall Perspective, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Springer-Verlag Berlin Heidelberg, Berlin, Heidelberg, 2004, vol. 233, pp. 1–47 Search PubMed.
- A. Bousseksou, Spin Crossover Phenomenon, C. R. Chim., 2018, 21(12), 1055–1300 CrossRef CAS.
- M. Cavallini, Status and Perspectives in Thin Films and Patterning of Spin Crossover Compounds, Phys. Chem. Chem. Phys., 2012, 14(34), 11867 RSC.
- T. Mallah and M. Cavallini, Surfaces, Thin Films and Patterning of Spin Crossover Compounds, C. R. Chim., 2018, 21(12), 1270–1286, DOI:10.1016/j.crci.2018.02.007.
- H. Soyer, E. Dupart, C. J. Gómez-García, C. Mingotaud and P. Delhaès, First Magnetic Observation of a Spin Crossover in a Langmuir-Blodgett Film, Adv. Mater., 1999, 11(5), 382–384, DOI:10.1002/(SICI)1521-4095(199903)11:5<382::AID-ADMA382>3.0.CO;2-U.
- O. Roubeau, B. Agricole, R. Clérac and S. Ravaine, Triazole-Based Magnetic Langmuir–Blodgett Films: Paramagnetic to Spin-Crossover Behavior, J. Phys. Chem. B, 2004, 108(39), 15110–15116, DOI:10.1021/jp048194i.
- A. Tissot, J.-F. Bardeau, E. Rivière, F. Brisset and M.-L. Boillot, Thermo- and Photoswitchable Spin-Crossover Nanoparticles of an Iron(II) Complex Trapped in Transparent Silica Thin Films, Dalton Trans., 2010, 39(33), 7806, 10.1039/c0dt00321b.
- A. Akou, I. A. Gural'skiy, L. Salmon, C. Bartual-Murgui, C. Thibault, C. Vieu, G. Molnár and A. Bousseksou, Soft Lithographic Patterning of Spin Crossover Complexes. Part 2: Stimuli-Responsive Diffraction Grating Properties, J. Mater. Chem., 2012, 22(9), 3752–3757, 10.1039/c2jm15663f.
- K. Kuroiwa, T. Shibata, S. Sasaki, M. Ohba, A. Takahara, T. Kunitake and N. Kimizuka, Supramolecular Control of Spin-Crossover Phenomena in Lipophilic Fe(II)-1,2,4-Triazole Complexes, J. Polym. Sci., Part A: Polym. Chem., 2006, 44(17), 5192–5202 CrossRef CAS.
- C. Bartual-Murgui, L. Salmon, A. Akou, C. Thibault, G. Molnár, T. Mahfoud, Z. Sekkat, J. A. Real and A. Bousseksou, High Quality Nano-Patterned Thin Films of the Coordination Compound {Fe(Pyrazine)[Pt(CN)4]} Deposited Layer-by-Layer, New J. Chem., 2011, 35(10), 2089–2094, 10.1039/c1nj20212j.
- S. Cobo, G. Molnár, J. A. Real and A. Bousseksou, Multilayer Sequential Assembly of Thin Films That Display Room-Temperature Spin Crossover with Hysteresis, Angew. Chem., Int. Ed., 2006, 45(35), 5786–5789 CrossRef CAS PubMed.
- K. S. Kumar and M. Ruben, Sublimable Spin-Crossover Complexes: From Spin-State Switching to Molecular Devices, Angew. Chem., Int. Ed., 2021, 60(14), 7502–7521 CrossRef CAS PubMed.
- M. Gavara-Edo, F. J. Valverde-Muñoz, R. Córdoba, M. C. Muñoz, J. Herrero-Martín, J. A. Real and E. Coronado, Sublimable Complexes with Spin Switching: Chemical Design, Processing as Thin Films and Integration in Graphene-Based Devices, J. Mater. Chem. C, 2023, 11, 8107–8120 RSC.
- L. Kipgen, M. Bernien, F. Tuczek and W. Kuch, Spin-Crossover Molecules on Surfaces: From Isolated Molecules to Ultrathin Films, Adv. Mater., 2021, 33(24), 2008141, DOI:10.1002/adma.202008141.
- V. Shalabaeva, S. Rat, M. D. Manrique-Juarez, A.-C. Bas, L. Vendier, L. Salmon, G. Molnár and A. Bousseksou, Vacuum Deposition of High-Quality Thin Films Displaying Spin Transition near Room Temperature, J. Mater. Chem. C, 2017, 5(18), 4419–4425 RSC.
- V. Davesne, M. Gruber, M. Studniarek, W. H. Doh, S. Zafeiratos, L. Joly, F. Sirotti, M. G. Silly, A. B. Gaspar, J. A. Real, G. Schmerber, M. Bowen, W. Weber, S. Boukari, V. Da Costa, J. Arabski, W. Wulfhekel and E. Beaurepaire, Hysteresis and Change of Transition Temperature in Thin Films of Fe{[Me2Pyrz]3BH}2, a New Sublimable Spin-Crossover Molecule, J. Chem. Phys., 2015, 142(19), 194702, DOI:10.1063/1.4921309.
- M. Gruber, T. Miyamachi, V. Davesne, M. Bowen, S. Boukari, W. Wulfhekel, M. Alouani and E. Beaurepaire, Spin Crossover in Fe(Phen)2(NCS)2 Complexes on Metallic Surfaces, J. Chem. Phys., 2017, 146(9), 092312, DOI:10.1063/1.4973511.
- O. Iasco, M.-L. Boillot, A. Bellec, R. Guillot, E. Rivière, S. Mazerat, S. Nowak, D. Morineau, A. Brosseau, F. Miserque, V. Repain and T. Mallah, The Disentangling of Hysteretic Spin Transition, Polymorphism and Metastability in Bistable Thin Films Formed by Sublimation of Bis(Scorpionate) Fe(II) Molecules, J. Mater. Chem. C, 2017, 5(42), 11067–11075, 10.1039/C7TC03276E.
- T. Jasper-Tönnies, M. Gruber, S. Karan, H. Jacob, F. Tuczek and R. Berndt, Deposition of a Cationic FeIII Spin-Crossover Complex on Au(111): Impact of the Counter Ion, J. Phys. Chem. Lett., 2017, 8(7), 1569–1573, DOI:10.1021/acs.jpclett.7b00457.
- A. Mosey, A. S. Dale, G. Hao, A. N'Diaye, P. A. Dowben and R. Cheng, Quantitative Study of the Energy Changes in Voltage-Controlled Spin Crossover Molecular Thin Films, J. Phys. Chem. Lett., 2020, 11(19), 8231–8237, DOI:10.1021/acs.jpclett.0c02209.
- S. Beniwal, S. Sarkar, F. Baier, B. Weber, P. A. Dowben and A. Enders, Site Selective Adsorption of the Spin Crossover Complex Fe(Phen)2(NCS) on Au(111), J. Phys.: Condens. Matter, 2020, 32(32), 324003, DOI:10.1088/1361-648X/ab808d.
- T. Mahfoud, G. Molnár, S. Cobo, L. Salmon, C. Thibault, C. Vieu, P. Demont and A. Bousseksou, Electrical Properties and Non-Volatile Memory Effect of the [Fe(HB(Pz)3)2] Spin Crossover Complex Integrated in a Microelectrode Device, Appl. Phys. Lett., 2011, 99(5), 9–11 CrossRef.
- K. Ridier, A. C. Bas, Y. Zhang, L. Routaboul, L. Salmon, G. Molnár, C. Bergaud and A. Bousseksou, Unprecedented Switching Endurance Affords for High-Resolution Surface Temperature Mapping Using a Spin-Crossover Film, Nat. Commun., 2020, 11(1), 1–9 CrossRef PubMed.
- O. Y. Horniichuk, K. Ridier, L. Zhang, Y. Zhang, G. Molnar, L. Salmon and A. Bousseksou, ACS Appl. Mater. Interfaces, 2022, 14, 52140–52148 CrossRef CAS PubMed.
- T. Palamarciuc, J. C. Oberg, F. El Hallak, C. F. Hirjibehedin, M. Serri, S. Heutz, J.-F. Létard and P. Rosa, Spin Crossover Materials Evaporated under Clean High Vacuum and Ultra-High Vacuum Conditions: From Thin Films to Single Molecules, J. Mater. Chem., 2012, 22(19), 9690, 10.1039/c2jm15094h.
- M. Bernien, D. Wiedemann, C. F. Hermanns, A. Krüger, D. Rolf, W. Kroener, P. Müller, A. Grohmann and W. Kuch, Spin Crossover in a Vacuum-Deposited Submonolayer of a Molecular Iron(II) Complex, J. Phys. Chem. Lett., 2012, 3(23), 3431–3434, DOI:10.1021/jz3011805.
- A. Pronschinske, R. C. Bruce, G. Lewis, Y. Chen, A. Calzolari, M. Buongiorno-Nardelli, D. A. Shultz, W. You and D. B. Dougherty, Iron(II) Spin Crossover Films on Au(111): Scanning Probe Microscopy and Photoelectron Spectroscopy, Chem. Commun., 2013, 49(89), 10446, 10.1039/c3cc44904a.
- H. Naggert, J. Rudnik, L. Kipgen, M. Bernien, F. Nickel, L. M. Arruda, W. Kuch, C. Näther and F. Tuczek, Vacuum-Evaporable Spin-Crossover Complexes: Physicochemical Properties in the Crystalline Bulk and in Thin Films Deposited from the Gas Phase, J. Mater. Chem. C, 2015, 3(30), 7870–7877 RSC.
- T. G. Gopakumar, M. Bernien, H. Naggert, F. Matino, C. F. Hermanns, A. Bannwarth, S. Mühlenberend, A. Krüger, D. Krüger, F. Nickel, W. Walter, R. Berndt, W. Kuch and F. Tuczek, Spin-Crossover Complex on Au(111): Structural and Electronic Differences Between Mono- and Multilayers, Chem. – Eur. J., 2013, 19(46), 15702–15709 CrossRef CAS PubMed.
- S. Ossinger, H. Naggert, L. Kipgen, T. Jasper-Toennies, A. Rai, J. Rudnik, F. Nickel, L. M. Arruda, M. Bernien, W. Kuch, R. Berndt and F. Tuczek, Vacuum-Evaporable Spin-Crossover Complexes in Direct Contact with a Solid Surface: Bismuth versus Gold, J. Phys. Chem. C, 2017, 121(2), 1210–1219 CrossRef CAS.
- S. Rohlf, J. Grunwald, T. Jasper-Toennies, S. Johannsen, F. Diekmann, M. Studniarek, R. Berndt, F. Tuczek, K. Rossnagel and M. Gruber, Influence of Substrate Electronic Properties on the Integrity and Functionality of an Adsorbed Fe(II) Spin-Crossover Compound, J. Phys. Chem. C, 2019, 123(29), 17774–17780 CrossRef CAS.
- S. Trofimenko, in Scorpionates: The Coordination Chemistry of Polypyrazolylborate Ligands, Imperial College Press, London, United Kingdom, 1st edn, 1999 Search PubMed.
- K. Bairagi, A. Bellec, C. Fourmental, O. Iasco, J. Lagoute, C. Chacon, Y. Girard, S. Rousset, F. Choueikani, E. Otero, P. Ohresser, P. Sainctavit, M.-L. Boillot, T. Mallah and V. Repain, Temperature-, Light-, and Soft X-Ray-Induced Spin Crossover in a Single Layer of FeII-Pyrazolylborate Molecules in Direct Contact with Gold, J. Phys. Chem. C, 2018, 122(1), 727–731, DOI:10.1021/acs.jpcc.7b11874.
- A. Bousseksou, G. Molnár, L. Salmon and W. Nicolazzi, Molecular Spin Crossover Phenomenon: Recent Achievements and Prospects, Chem. Soc. Rev., 2011, 40(6), 3313 RSC.
- S. Rohlf, M. Gruber, B. M. Flöser, J. Grunwald, S. Jarausch, F. Diekmann, M. Kalläne, T. Jasper-Toennies, A. Buchholz, W. Plass, R. Berndt, F. Tuczek and K. Rossnagel, Light-Induced Spin Crossover in an Fe(II) Low-Spin Complex Enabled by Surface Adsorption, J. Phys. Chem. Lett., 2018, 9(7), 1491–1496 CrossRef CAS PubMed.
- L. Salmon, G. Molnár, S. Cobo, P. Oulié, M. Etienne, T. Mahfoud, P. Demont, A. Eguchi, H. Watanabe, K. Tanaka and A. Bousseksou, Re-Investigation of the Spin Crossover Phenomenon in the Ferrous Complex [Fe(HB(Pz)3)2], New J. Chem., 2009, 33(6), 1283 RSC.
- O. Y. Horniichuk, K. Ridier, G. Molnar, V. O. Kotsyubynsky, S. Shova, V. M. Amirkhanov, I. A. Gural'skiy, L. Salmon and A. Bousseksou, New J. Chem., 2022, 46, 11734 RSC.
- S. Rat, K. Ridier, L. Vendier, G. Molnár, L. Salmon and A. Bousseksou, Solvatomorphism and Structural-Spin Crossover Property Relationship in Bis[Hydrotris(1,2,4-Triazol-1-Yl)Borate]Iron(II), CrystEngComm, 2017, 19(24), 3271–3280 RSC.
- M. D. Manrique-Juarez, F. Mathieu, V. Shalabaeva, J. Cacheux, S. Rat, L. Nicu, T. Leïchlé, L. Salmon, G. Molnár and A. Bousseksou, A Bistable Microelectromechanical System Actuated by Spin-Crossover Molecules, Angew. Chem., Int. Ed., 2017, 56(28), 8074–8078 CrossRef CAS PubMed.
- V. Shalabaeva, K. Ridier, S. Rat, M. D. Manrique-Juarez, L. Salmon, I. Séguy, A. Rotaru, G. Molnár and A. Bousseksou, Room Temperature Current Modulation in Large Area Electronic Junctions of Spin Crossover Thin Films, Appl. Phys. Lett., 2018, 112(1), 013301 CrossRef.
- Y. Zhang, K. Ridier, V. Shalabaeva, I. Séguy, S. Pelloquin, H. Camon, S. Calvez, L. Routaboul, L. Salmon, G. Molnár and A. Bousseksou, A Molecular Spin-Crossover Film Allows for Wavelength Tuning of the Resonance of a Fabry-Perot Cavity, J. Mater. Chem. C, 2020, 8(24), 8007–8011 RSC.
- K. Ridier, Y. Zhang, M. Piedrahita-Bello, C. M. Quintero, L. Salmon, G. Molnár, C. Bergaud and A. Bousseksou, Heat Capacity and Thermal Damping Properties of Spin-Crossover Molecules: A New Look at an Old Topic, Adv. Mater., 2020, 32(21), 2000987 CrossRef CAS PubMed.
- Y. Zhang, I. Séguy, K. Ridier, V. Shalabaeva, M. Piedrahita-Bello, A. Rotaru, L. Salmon, G. Molnár and A. Bousseksou, Resistance Switching in Large-Area Vertical Junctions of the Molecular Spin Crossover Complex [Fe(HB(Tz)3)2]: ON/OFF Ratios and Device Stability, J. Phys.: Condens. Matter, 2020, 32(21), 214010, DOI:10.1088/1361-648X/ab741e.
- H. Flötotto, T. Secker, P. Kögerler and C. Besson, Amine-Functionalized Spin Crossover Building Blocks, Eur. J. Inorg. Chem., 2019, 4621–4624 CrossRef PubMed.
- C. Ma and C. Besson, Precise Control of the Degree and Regioselectivity of Functionalization in Nitro- and Amino-Functionalized Di(Trispyrazolylborato)Iron(II) Spin Crossover Complexes, Dalton Trans., 2021, 50(48), 18077–18088 RSC.
- P. J. Desrochers, A. Abdulrahim, K. R. Demaree, J. A. Fortner, J. D. Freeman, M. Provorse Long, M. E. Martin, C. J. Gómez-García and N. Gerasimchuk, Rational Design of Iron Spin-Crossover Complexes Using Heteroscorpionate Chelates, Inorg. Chem., 2022, 61(47), 18907–18922 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837–838 CrossRef CAS.
- P. W. Betteridge, J. R. Carruthers, K. Prout and D. J. Watkin, CRYSTALS version 12: software for guided crystal structure analysis, J. Appl. Crystallogr., 2003, 36, 1487 CrossRef CAS.
- International tables for X-ray crystallography, ed. A. Ibers and W. C. Hamilton, Kynoch Press, Birmingham, England, 1974, vol. IV Search PubMed.
- L. J. Farrugia, ORTEP3 for Windows, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
- K. Lagarec and D. G. Rancourt, Extended Voigt-Based Analytic Lineshape Method for Determining N-Dimensional Correlated Hyperfine Parameter Distributions in Mössbauer Spectroscopy, Nucl. Instrum. Methods Phys. Res., Sect. B, 1997, 129(2), 266–280 CrossRef CAS.
- S. Trofimenko, Poly(1-pyrazolyl)Borates, Their Transition-Metal Complexes, and Pyrazaboles, Inorganic Syntheses, 1970, vol. 12, pp. 99–109 Search PubMed.
- J. R. Bour, N. M. Camasso and M. S. Sanford, Oxidation of Ni(II) to Ni(IV) with Aryl Electrophiles Enables Ni-Mediated Aryl–CF3 Coupling, J. Am. Chem. Soc., 2015, 137(25), 8034–8037 CrossRef CAS PubMed.
- G. Yagil, The Proton Dissociation Constant of Pyrrole, Indole and Related Compounds, Tetrahedron, 1967, 23(6), 2855–2861, DOI:10.1016/0040-4020(67)85151-2.
- E. Radzyminska-Lenarcik, I. Pyszka and W. Urbaniak, New Polymer Inclusion Membranes in the Separation of Palladium, Zinc and Nickel Ions from Aqueous Solutions, Polymers, 2021, 13(9), 1424 CrossRef CAS PubMed.
- J. Sun, Y. Fu, G. He, X. Sun and X. Wang, Catalytic Hydrogenation of Nitrophenols and Nitrotoluenes over a Palladium/Graphene Nanocomposite, Catal. Sci. Technol., 2014, 4(6), 1742–1748 RSC.
- B. Wrackmeyer and O. L. Tok, Indirect Nuclear Spin-Spin Coupling Constants 1J(17O, 11B). First Observation And Calculation Using Density Functional Theory (Dft), Z. Naturforsch., B: J. Chem. Sci., 2006, 61(8), 949–955, DOI:10.1515/znb-2006-0804.
- D. L. Reger, J. R. Gardinier, J. D. Elgin, M. D. Smith, D. Hautot, G. J. Long and F. Grandjean, Structure-Function Correlations in Iron(II) Tris(Pyrazolyl)Borate Spin-State Crossover Complexes, Inorg. Chem., 2006, 45(22), 8862–8875, DOI:10.1021/ic0607437.
- M. Nishio, The CH/π Hydrogen Bond in Chemistry. Conformation, Supramolecules, Optical Resolution and Interactions Involving Carbohydrates, Phys. Chem. Chem. Phys., 2011, 13(31), 13873 RSC.
- C. Rajadurai, F. Schramm, S. Brink, O. Fuhr, M. Ghafari, R. Kruk and M. Ruben, Inorg. Chem., 2006, 45, 10019–10021 CrossRef CAS PubMed.
- J.-H. Liu, X.-Q. Guo, Z.-C. Wang, L.-X. Cai, L. P. Zhou, C. B. Tian and Q.-F. Sun, Dalton Trans., 2022, 51, 3894 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and IR spectra, X-ray diffraction data and magnetism and Mössbauer measurements. CCDC 2370860–2370867. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02244k |
| This journal is © The Royal Society of Chemistry 2024 |





