Open Access Article
Open Access ArticleChoosing between Ti(II) and Ti(III): selective reduction of titanocene dichloride by elemental lanthanides†
Guillaume
Bousrez
 ab,
Dominique
Harakat
ab,
Dominique
Harakat
 a,
Sylviane
Chevreux
ac,
Isabelle
Déchamps-Olivier
a and
Florian
Jaroschik
a,
Sylviane
Chevreux
ac,
Isabelle
Déchamps-Olivier
a and
Florian
Jaroschik
 *ad
*ad
aUniversité de Reims Champagne Ardenne, CNRS UMR 7312, ICMR, URCATech, 51100 Reims, France
bDepartment of Biological and Chemical Engineering, Aarhus University, 8000 Aarhus C, Denmark
cInstitut de Recherche de Chimie Paris, UMR CNRS 8247, Chimie ParisTech, PSL University, 75005 Paris, France
dICGM, Univ. Montpellier, CNRS, ENSCM, 34090 Montpellier, France. E-mail: florian.jaroschik@enscm.fr
First published on 27th August 2024
Abstract
The reduction of titanocene dichloride Cp2TiCl2 with lanthanide metals has led to the discovery of a surprising lanthanide effect: while with most lanthanides, a divalent [Cp2Ti] equivalent was obtained, the use of samarium or ytterbium only led to the reduction to trivalent [Cp2TiCl]-type complexes, including the structurally characterized heterobimetallic complex [Cp2Ti(μ-Cl)2SmCl2(THF)3]. These results were corroborated by reactivity studies (alkyne coupling and radical reactions), EPR spectroscopy and electrospray mass spectrometry, providing new insights into the reduction chemistry of lanthanide metals.
Introduction
Low-valent titanocene complexes have found widespread applications in organic and organometallic transformations.1 Trivalent [Cp2TiCl] is a reliable source of radicals, which can be applied in stoichiometric or catalytic amounts for the synthesis of complex organic molecules, involving, for example, epoxide openings, free radical additions, cycloadditions or reductive umpolung reactions.2 The synthesis of [Cp2TiCl] is mainly achieved by reduction of the tetravalent precursor Cp2TiCl2 employing Grignard reagents, metal hydrides, dihydropyrazines or mild metallic reducing agents, such as Zn or Mn metal. Electrochemical preparation,3 and more recently, photocatalytic pathways have also been described (Scheme 1a).4 | ||
| Scheme 1 (a) Reduction of Cp2TiCl2 leading to low-valent titanocene species and (b) Takeda carbonyl olefination using Cp2TiCl2/Ln systems. | ||
On the other hand, divalent [Cp2Ti] equivalents, such as [Cp2Ti(CO)2], [Cp2Ti(alkyne)], and [Cp2Ti(P(OEt)3)2], have been investigated in dinitrogen activation and cleavage, water splitting, diene transformations or carbonyl olefination.5 For accessing these divalent [Cp2Ti] equivalents from Cp2TiCl2, strong reducing agents, such as Mg or Na, and the presence of stabilising ligands or Lewis bases (phosphines, alkynes, and CO) are required (Scheme 1a).6 Base-free titanocenes were only obtained with highly bulky Cp ligands.7
Lanthanide metals are strong reducing agents (E°(Ln3+/Ln0) = −2.3 V), and have been employed in organic chemistry, e.g. for radical generation, for the formation of Grignard-type reagents, in C–F bond activation and in bimetallic systems combined with transition metals.8,9 They are also excellent starting materials for the synthesis of organometallic complexes using redox transmetallation approaches.10 Despite the similar redox potential of these metals along the series from La to Lu, their reactivity and efficiency are not necessarily the same, which may be related to their different size, density or activation methods.8,11
The reduction of Cp2TiCl2 with lanthanides, especially Sm, has been investigated since 1995, and this bimetallic system has been employed successfully in radical reactions.12 The formation of a bimetallic complex comprising Ti(III) and Sm(II) moieties was suggested,12a but to date, no structurally characterized complex has been reported. Another rare example of the combination of TiCl4 with Yb metal has shown its utility in the McMurry carbonyl olefination reaction, in which the involvement of a Ti(III) complex was proposed.13
At the same time, in 2015, we reported on the reduction of Cp2TiCl2 with different lanthanide metals in the absence of any Lewis base to access a new [Cp2Ti] equivalent, solely stabilised by the in situ formed LnCl3 salt.14 Dysprosium was shown to be most efficient among a selection of lanthanides, as evidenced by the application of the Cp2TiCl2/Dy system in the Takeda carbonyl olefination reaction (Scheme 1b). In this reaction, initially, Ti(IV) was reduced to Ti(II), which then reacted with dithioacetals to form an intermediate Ti-carbene species, which reacted with carbonyl groups to form the corresponding alkene. Under classical Takeda conditions,15 Ti(II) was stabilised by triethyl phosphite; however, in our case, no phosphite was necessary. In addition, even a one-pot reaction with all reagents present from the start was successful. During the initial metal screening, we observed a surprising lanthanide effect: while La, Nd and Dy enabled the olefination reaction in moderate to good yields, no product was obtained with Yb and Sm, suggesting that the latter cannot afford Ti(II) species (Scheme 1b).14
We have now investigated this outcome in detail, conducting further reactivity studies and employing EPR spectroscopy, electrospray mass spectrometry (ESI-MS) and X-ray diffraction analysis on the in situ formed low-valent titanocene complexes, unveiling new insights into the reduction chemistry of lanthanide metals. This study provides, in fine, the possibility to choose between the formation of Ti(III) or Ti(II) complexes by selecting the right lanthanide metal.
Results and discussion
Reactivity studies
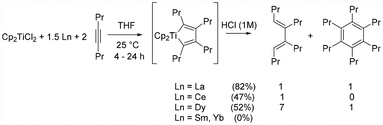
Reductive dimerization of alkynes is a well-known reaction with low-valent group 4 metallocenes (Ti and Zr).1b,5 While this reaction is mostly restricted to internal alkynes, the previously reported bimetallic Cp2ZrCl2/La system also allowed the efficient coupling of terminal alkynes, which we have successfully applied for the synthesis of 2,4-disubstituted phospholes.16 We therefore set out to investigate the reaction of 4-octyne with the Cp2TiCl2/Ln system. To our surprise, when using La metal as the reducing agent, we observed the formation of not only the expected butadiene but also a hexa-substituted benzene derivative in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Scheme 2). On the other hand, when using Ce or Dy, the major product was by far the butadiene with only trace or small amounts of benzene byproduct. Importantly, the use of Sm or Yb did not provide any coupling product, in agreement with the ineffective reduction to Ti(II) complexes, even upon heating at 50 °C for several hours. In the case of the previously reported Cp2ZrCl2/La system, trimerization was only observed for terminal alkynes.17 A possible explanation for this surprising reaction behaviour of the Cp2TiCl2/La system could be derived from the results of the ESI-MS studies (see below).
1 ratio (Scheme 2). On the other hand, when using Ce or Dy, the major product was by far the butadiene with only trace or small amounts of benzene byproduct. Importantly, the use of Sm or Yb did not provide any coupling product, in agreement with the ineffective reduction to Ti(II) complexes, even upon heating at 50 °C for several hours. In the case of the previously reported Cp2ZrCl2/La system, trimerization was only observed for terminal alkynes.17 A possible explanation for this surprising reaction behaviour of the Cp2TiCl2/La system could be derived from the results of the ESI-MS studies (see below).
As mentioned above, titanocene(III) species are often involved in radical reactions. We therefore screened Cp2TiCl2/Ln systems for the homocoupling of cinnamyl bromide, a reaction known to occur with Mn as the reducing agent.18 In the case of Sm and Yb, the reaction always provided the expected radical–radical homocoupling products in similar ratios, comparable to Mn, even when a large excess of Ln metal was employed (Scheme 3). In the case of Dy, two different results were observed. When equimolar amounts of Cp2TiCl2 and Dy were employed, the same homocoupling reaction was observed as with Yb and Sm. A similar result was obtained with equimolar amounts of Nd. However, with an excess of Dy no coupling reaction occurred, which is in agreement with the formation of a Ti(II) species, which does not undergo radical reactions. We previously showed by EPR studies that the amount of Dy was important to obtain selectively Ti(III) or Ti(II) species.14 It should be noted that when the reaction was carried out with SmI2 instead of the Cp2TiCl2/Ln system, the coupling products were obtained in a different ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), pointing to the likely absence of a divalent Sm species in the Cp2TiCl2/Sm system.
1), pointing to the likely absence of a divalent Sm species in the Cp2TiCl2/Sm system.
Electrospray-MS studies
ESI-MS has become an important tool for the elucidation of reaction mechanisms and the characterization of reactive organometallic complexes.19 This is due to the rather mild experimental conditions and the compatibility with air-sensitive compounds via introduction under an inert atmosphere. We have studied four reduction processes of Cp2TiCl2 with Sm, Yb, Dy and La metals in THF at room temperature for 3 hours. Samples of these solutions were taken directly from the Schlenk tube under argon and injected without further dilution. The ESI-MS spectra were recorded in positive and negative modes. As expected, in the case of Sm and Yb, the major signal could be attributed in the negative mode to the [LnCl4]− anionic species. However, upon closer inspection, signals corresponding to bimetallic {[Cp2TiCl][LnCl3]Cl}− type complexes were also detected in both cases (m/z = 506.8 for Sm and 528.8 for Yb), albeit in low intensities. The isotope distribution is in perfect agreement between calculated and observed values (Fig. 1 and ESI†). This is a first indication of the formation of a [Ti(III)/Ln(III)] type bimetallic complex, which will be further corroborated by XRD studies (see below).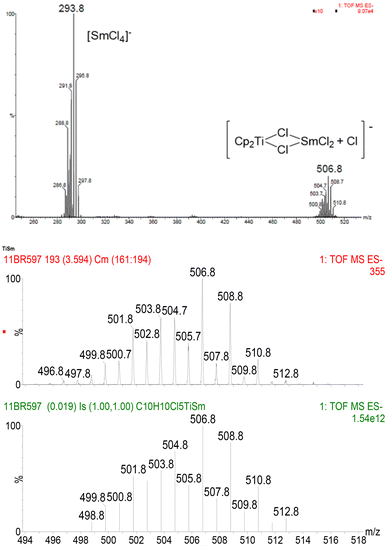 | ||
| Fig. 1 ESI-MS spectrum (negative mode) for the reduction of Cp2TiCl2 with Sm in THF (above); observed (middle) vs. calculated (bottom) isotope distribution of the bimetallic complex. | ||
In the case of Dy, [DyCl4]− was detected as the major species in the negative mode, whereas in the positive mode titanocene-derivatives could be observed, including [Cp2Ti]+ at m/z = 178.0 (from the oxidation of divalent [Cp2Ti] in the spectrometer), and [Cp2TiO2]+ at m/z = 209.9 (from trace amounts of oxygen) (see the ESI†). The former was previously observed from [Cp2TiMe2] under EI-MS conditions.20 On the other hand, for other supposedly [Cp2Ti] species in the literature, the corresponding bimetallic fulvalene titanium hydride complex was observed by mass spectrometry,21 which is not present in our case (no peak at m/z = 354 or 356). From these experiments, the formation of stable divalent [Cp2Ti][DyCl3] type complexes in solution can be deduced.
When La metal was used for the reduction of titanocene dichloride, a very intriguing observation was made in the ESI-MS spectrum in the negative mode. In addition to the major signal corresponding to [LaCl4]−, several other products corresponding to [CpLaCl3]−, [Cp2LaCl2]− and [Cp3LaCl]− were observed (Fig. 2). Such byproducts resulting from Cp abstraction from the Ti centre were not observed for the other lanthanides or observed only in trace amounts. This phenomenon, which has been sporadically reported in the literature,22 may explain why in the case of La the formation of trimerization occurs (Scheme 2). It is well established now that [TiX2] species can trigger the trimerization of alkynes,23 whereas [Cp2Ti] does not.
EPR studies
The paramagnetism of Ti(III) precludes further characterisation by NMR spectroscopy studies; however, EPR spectroscopy has been extensively employed for such complexes.24 The reduction of Cp2TiCl2 was conducted in THF with an excess of Yb or Sm at room temperature and the resulting solutions were measured, after dilution, by EPR with the X-band at 150 K. In both cases, a signal centred at 3400 G, which can be attributed to a trivalent [Cp2TiCl]-type complex, was observed (see the ESI†). The giso value of 1.976 derived from the spectra is identical to the data from the literature for [Cp2TiCl(THF)].24b We previously reported a similar signal for the reduction with 0.33 equivalent of Dy metal. However, when an excess of Dy metal was employed, the EPR signal disappeared, in agreement with the reduction to titanocene(II).14 In the case of Yb and Sm, the signal did not disappear in the presence of excess metal. It should be noted that most trivalent lanthanides (except Gd) cannot be detected in EPR spectroscopy above 15 K due to line-broadening.25XRD structure of [Cp2Ti(μ-Cl)2SmCl2(THF)3]·THF
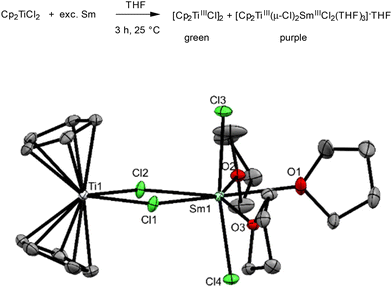
As described above, the quest for identifying unambiguously the reaction product of the reduction of Cp2TiCl2 with Sm in THF has been going on for 30 years.12a Our ESI-MS experiments have already pointed towards a bimetallic Ti(III)–Sm(III) species. This has now been confirmed by XRD studies on an isolated complex. From a concentrated THF solution, a mixture of two types of crystals was obtained after standing for several weeks, which were separated manually under a microscope for XRD determination: a green complex identified as the known titanocene dimer [Cp2TiCl]2, confirmed by the determination of its unit cell26 and a purple bimetallic complex identified as [Cp2Ti(μ-Cl)2SmCl2(THF)3]·THF (Scheme 4).In this bimetallic structure, the Sm ion is seven-coordinate in a distorted pentagonal bipyramidal coordination environment with the terminal chloride ligands in the apical positions. The structure has many similarities to a previously described di-samarium(III) complex [CpSmCl(μ-Cl)2SmCl2(THF)3] with respect to bond distances and coordination,27 and it also compares well with the heterotrimetallic complexes [(Cp2TiCl)2MCl2] (with M = Zn, Mn, and Ni).28
Discussion
The reduction of titanocene dichloride has intrigued chemists for over fifty years. In this contribution, we have shown by various experimental and analytical techniques that depending on the choice of lanthanide metal as the reducing agent, different outcomes are possible (Scheme 5). The two elements Sm and Yb lead to trivalent [Cp2TiCl] species, according to radical coupling experiments, and ESI-MS, EPR and XRD analyses. Why these two metals are not capable of reducing the intermediate [Cp2TiCl] species further to the divalent complex [Cp2Ti] has not been elucidated so far. The standard redox potentials of these metals (E°(Sm3+/Sm0) = −2.30 V and E°(Yb3+/Yb0) = −2.19 V) were reported to be in the same range as those of other lanthanide metals, e.g. E°(Dy3+/Dy0) = −2.29 V.29 Furthermore, if direct oxidation of the lanthanide metal to divalent Ln intermediates occurred, the potentials would be even more negative (E°(Sm2+/Sm0) = −2.68 V and E°(Yb2+/Yb0) = −2.76 V)29 and more favorable to access [Cp2TiII]. Consequently, at this stage, we cannot rule out that other factors or other reaction pathways influence the further reduction of [Cp2TiCl] to divalent titanocene species.In contrast to Sm and Yb, other lanthanide metals, such as Dy, Ce, and Nd, which also proceed via the [Cp2TiCl] species as shown by the radical coupling experiment and EPR analysis, provide a relatively stable divalent titanocene complex, with a suggested composition of [Cp2Ti][LnCl3], based on ESI-MS and reactivity studies (alkyne coupling and carbonyl olefination). Intriguingly, La metal, the largest and often the most reactive lanthanide metal, reduces titanocene dichloride to divalent species but also exchanges not only chloride but also Cp ligands with the titanium ion. The resulting low-valent titanium species have been shown to facilitate the trimerization of internal alkynes.
Experimental
General
All reactions were conducted under an atmosphere of argon using standard Schlenk techniques and an argon-filled Jacomex BS531-type dry box. Tetrahydrofuran was collected under argon from a PURSOLV MD-3 (Innovative Technologies Inc.) solvent purification unit. Titanocene dichloride, iodine and manganese were purchased from Strem Chemicals. 4-Octyne, cinnamylbromide and diethylether were purchased from Aldrich and used as received. Neodymium, samarium, europium, dysprosium and ytterbium ingots were purchased from Aldrich or Strem and, prior to use, were freshly filed in a drybox under argon. SmI2 was prepared in situ by reacting elemental samarium with iodine in THF.ESI-MS
All experiments (MS and HRMS) were performed on a hybrid tandem quadrupole/time-of-flight (Q-TOF) instrument, equipped with a pneumatically assisted electrospray (Z-spray) ion source (Micromass, Manchester, UK) operated in positive mode. The electrospray potential was set to 3 kV in positive ion mode and the extraction cone voltage was usually varied between 30 and 60 V, with an injection flow rate of 5 μL min−1.EPR
EPR spectra were recorded using a Bruker ESP300e spectrometer (X-band) equipped with a Bruker E035M gaussmeter and an HP 5350B microwave frequency counter. Samples were prepared at a concentration of 2 mmol L−1 in THF frozen solutions (150 K, Bruker ER4111VY variable-temperature unit). The best resolution was obtained at T = 150 K by using a modulation amplitude of 16.789 G, a time constant of 81.92 ms, a conventional time of 20.48 ms and a sweep time of 20.972 s.XRD
A single crystal coated with viscous hydrocarbon oil was mounted on a cryoloop. Single crystal X-ray diffraction was performed on a Bruker D8 Venture diffractometer. The D8 Venture was equipped with a Cu microsource (Kα1 radiation λ = 1.54056 Å) and a PHOTON100 CMOS detector. Data were collected at −173.15 °C (100 K). Data collection and integration were performed using Bruker Apex2 software.30 The structures were solved using SHELXS-97 and refined by full-matrix least-squares on all F2 data using SHELXL-97.31 All hydrogen atoms were placed in calculated positions using the riding model.Alkyne coupling
A Schlenk tube was loaded with dichlorotitanocene (1.0 mmol), lanthanide (1.5 mmol (excess)) and THF (5 mL) under an argon atmosphere. The resulting mixture was stirred vigorously at room temperature until a deep green color appeared. 4-Octyne (2.0 mmol) was added to the mixture and the solution was vigorously stirred for 4 hours at room temperature. The reaction was then quenched by the addition of a hydrochloric acid solution (1 M). An extraction with diethylether (3 × 25 mL) was performed and the organic phases were collected, washed with brine, dried over magnesium sulfate, and filtered over cotton. The solvent was concentrated under vacuum and the crude mixture was purified by column chromatography with petroleum ether as the eluent, leading to 1,2,3,4-tetrapropylbutadiene (colorless oil) and 1,2,3,4,5,6-hexapropylbenzene (white solid). The NMR data were in agreement with the literature.16a,32Radical coupling
A Schlenk tube was loaded with dichlorotitanocene (1.0 mmol), lanthanide (0.33 mmol (equimolar) or 1.0 mmol (excess)) and THF (5 mL) under an argon atmosphere. The resulting mixture was stirred vigorously at room temperature for 2 h, leading to a deep green solution. Cinnamylbromide (1.0 mmol) was solubilized in THF (2 mL) and then introduced into the mixture. The solution was vigorously stirred for 30 minutes at room temperature and the reaction was quenched by the addition of a hydrochloric acid solution (1 M). The aqueous phase was extracted with diethylether (3 × 25 mL). The organic phases were collected, washed with brine, dried over magnesium sulfate, and filtered over cotton. The solvent was concentrated under vacuum and the crude was purified by column chromatography with petroleum ether as the eluent, leading to a mixture of three dienes as a colorless oil. The NMR data were in agreement with the literature.18Conclusions
The reduction of titanocene dichloride by different lanthanide metals has been investigated revealing a substantial lanthanide effect. While with Sm and Yb, the reduction stopped at trivalent titanocene species, with Dy, Ce and Nd, the formation of divalent titanocenes was evidenced. In the case of La, an additional feature is the abstraction of the Cp ligand from the titanium centre during the reduction process. Further studies on selective synthetic applications are currently underway.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for [Cp2Ti(μ-Cl)2SmCl2(THF)3] have been deposited at the CCDC under 2369904 and can be obtained from https://www.ccdc.cam.ac.uk/.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the CNRS, the Université de Reims Champagne-Ardenne and the Université de Montpellier is acknowledged. G. B. thanks the Region Grand Est for a PhD scholarship. We thank Dr G. Dantelle for helpful discussions on EPR spectroscopy.References
- (a) F. Sato, H. Urabe and S. Okamoto, Chem. Rev., 2000, 100, 2835 CrossRef CAS PubMed; (b) Titanium and Zirconium in Organic Synthesis, ed. I. Marek, Wiley-VCH, Weinheim, 2002 Search PubMed; (c) P. J. Chirik, Organometallics, 2010, 29, 1500 CrossRef CAS; (d) M. Manßen and L. L. Schafer, Chem. Soc. Rev., 2020, 49, 6947 RSC; (e) S. Fortier and A. Gomez-Torres, Chem. Commun., 2021, 57, 10292 RSC; (f) X. Wu, Y. Chang and S. Lin, Chem, 2022, 8, 1805 CrossRef CAS PubMed; (g) U. N. Oloyede and R. A. Flowers, Dalton Trans., 2024, 53, 2413 RSC.
- Reviews: (a) A. R. Martínez, L. P. Morales, E. D. Ojeda, M. C. Rodríguez and I. Rodríguez-García, J. Org. Chem., 2021, 86, 1311 CrossRef PubMed; (b) T. Hilche, S. L. Younas, A. Gansäuer and J. Streuff, ChemCatChem, 2022, 14, e202200530 CrossRef CAS Original research papers: (c) P. K. Mandal, G. Maiti and S. C. Roy, J. Org. Chem., 1998, 63, 2829 CrossRef CAS; (d) H. R. Diéguez, A. López, V. Domingo, J. F. Arteaga, J. A. Dobado, M. M. Herrador, J. F. Quílez del Moral and A. F. Barrero, J. Am. Chem. Soc., 2010, 132, 254 CrossRef PubMed; (e) I. Sancho-Sanz, D. Miguel, A. Millán, R. E. Estévez, J. L. Oller-López, E. Álvarez-Manzaneda, R. Robles, J. M. Cuerva and J. Justicia, J. Org. Chem., 2011, 76, 732 CrossRef CAS PubMed; (f) A. Gansäuer, M. Behlendorf, D. von Laufenberg, A. Fleckhaus, C. Kube, D. V. Sadasivam and R. A. Flowers II, Angew. Chem., Int. Ed., 2012, 51, 4739 CrossRef PubMed; (g) S. Begum and T. K. Chakraborty, J. Org. Chem., 2021, 86, 11812 CrossRef CAS PubMed; (h) J. Ni, X. Xia, W.-F. Zheng and Z. Wang, J. Am. Chem. Soc., 2022, 144, 7889 CrossRef CAS PubMed; (i) T. Saito, H. Nishiyama, H. Tanahashi, K. Kawakita, H. Tsurugi and K. Mashima, J. Am. Chem. Soc., 2014, 136, 5161 CrossRef CAS PubMed; (j) G. Frey, J. N. Hausmann and J. Streuff, Chem. – Eur. J., 2015, 21, 5693 CrossRef CAS PubMed; (k) D. S. G. Henriques, K. Zimmer, S. Klare, A. Meyer, E. Rojo-Wiechel, M. Bauer, R. Sure, S. Grimme, O. Schiemann, R. A. Flowers and A. Gansäuer, Angew. Chem., Int. Ed., 2016, 55, 7671 CrossRef CAS PubMed.
- (a) I. M. M. Fussing, D. Pletcher and R. J. Whitby, J. Organomet. Chem., 1994, 470, 119–125 CrossRef CAS; (b) R. J. Enemærke, J. Larsen, T. Skrydstrup and K. Daasbjerg, J. Am. Chem. Soc., 2004, 126, 7853 CrossRef PubMed; (c) A. Gansäuer, C. Kube, K. Daasbjerg, R. Sure, S. Grimme, G. D. Fianu, D. V. Sadasivam and R. A. Flowers II, J. Am. Chem. Soc., 2014, 136, 1663 CrossRef PubMed.
- (a) Z. Zhang, R. B. Richrath and A. Gansäuer, ACS Catal., 2019, 9, 3208 CrossRef CAS; (b) A. Gualandi, F. Calogero, M. Mazzarini, S. Guazzi, A. Fermi, G. Bergamini and P. G. Cozzi, ACS Catal., 2020, 10, 3857 CrossRef CAS; (c) Z. Zhang, D. Slak, T. Krebs, M. Leuschner, N. Schmickler, E. Kuchuk, J. Schmidt, L. I. Domenianni, J. B. Kleine Büning, S. Grimme, P. Vöhringer and A. Gansäuer, J. Am. Chem. Soc., 2023, 145, 26667 CrossRef CAS PubMed; (d) Z. Zhang, T. Hilche, D. Slak, N. R. Rietdijk, U. N. Oloyede, R. A. Flowers and A. Gansäuer, Angew. Chem., Int. Ed., 2020, 59, 9355 CrossRef CAS PubMed.
- Reviews: (a) T. Takeda, Chem. Rec., 2007, 7, 24 CrossRef CAS PubMed; (b) U. Rosenthal, Organometallics, 2020, 39, 4403 CrossRef CAS Selected original papers: (c) S. A. Cohen and J. E. Bercaw, Organometallics, 1985, 4, 1006 CrossRef CAS; (d) H. G. Alt, K.-H. Schwind and M. D. Rausch, J. Organomet. Chem., 1987, 321, C9 CrossRef CAS; (e) S. C. Berk, R. B. Grossman and S. L. Buchwald, J. Am. Chem. Soc., 1994, 116, 8593 CrossRef CAS; (f) K. Sato, Y. Nishihara, S. Huo, Z. Xi and T. Takahashi, J. Organomet. Chem., 2001, 633, 18 CrossRef CAS; (g) K. Kaleta, M. Ruhmann, O. Theilmann, T. Beweries, S. Roy, P. Arndt, A. Villinger, E. D. Jemmis, A. Schulz and U. Rosenthal, J. Am. Chem. Soc., 2011, 133, 5463 CrossRef CAS PubMed; (h) M. Kessler, S. Hansen, C. Godemann, A. Spannenberg and T. Beweries, Chem. – Eur. J., 2013, 19, 6350 CrossRef CAS PubMed; (i) A. A. Oluyadi, S. Ma and C. N. Muhoro, Organometallics, 2013, 32, 70 CrossRef CAS; (j) L. O. Khafizova, M. G. Shaibakova, N. A. Rikhter, T. V. Tyumkina and U. M. Dzhemilev, Tetrahedron, 2019, 75, 906 CrossRef CAS; (k) M. Reiß, F. Reiß, A. Spannenberg, P. Arndt and T. Beweries, Organometallics, 2018, 37, 4415 CrossRef; (l) G. R. Kiel, A. E. Samkian, A. Nicolay, R. J. Witzke and T. D. Tilley, J. Am. Chem. Soc., 2018, 140, 2450 CrossRef CAS PubMed; (m) J. Kim, D. T. Egger, C. W. Frye, E. P. Beaumier and I. A. Tonks, Organometallics, 2023, 42, 1331 CrossRef CAS PubMed.
- (a) N. El Murr and A. Chaloyard, J. Organomet. Chem., 1982, 231, 1 CrossRef CAS; (b) B. H. Edwards, R. D. Rogers, D. J. Sikora, J. L. Atwood and M. D. Rausch, J. Am. Chem. Soc., 1983, 105, 416 CrossRef CAS; (c) G. S. Girolami, G. Wilkinson, M. Thornton-Pett and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 1984, 2347 RSC; (d) D. W. Stephan, Organometallics, 1992, 11, 996 CrossRef CAS.
- (a) P. B. Hitchcock, F. M. Kerton and G. A. Lawless, J. Am. Chem. Soc., 1998, 120, 10264 CrossRef CAS; (b) M. D. Walter, C. D. Sofield and R. A. Andersen, Organometallics, 2008, 27, 2959 CrossRef CAS.
- Reviews: (a) G. Bousrez and F. Jaroschik, Eur. J. Org. Chem., 2022, e202200202 CrossRef CAS; (b) M. A. Halim, Z. Guo, V. L. Blair, G. B. Deacon and P. C. Junk, J. Organomet. Chem., 2024, 1010, 123094 CrossRef CAS; (c) A. Mortis and R. Anwander, Eur. J. Inorg. Chem., 2024, 27, e202400127 CrossRef CAS.
- (a) T. Kumar, F. Massicot, D. Harakat, S. Chevreux, A. Martinez, K. Bordolinska, P. Preethalayam, R. Kokkuvayil Vasu, J.-B. Behr, J.-L. Vasse and F. Jaroschik, Chem. – Eur. J., 2017, 23, 16460 CrossRef CAS PubMed; (b) X. Y. Cao, F. Huang and S. Zhang, Synlett, 2019, 1437 CAS; (c) S. Xiao, C. Liu, B. Song, L. Wang, Y. Qi and Y. Liu, Chem. Commun., 2021, 57, 6169 RSC.
- Z. Guo, R. Huo, Y. Q. Tan, V. Blair, G. B. Deacon and P. C. Junk, Coord. Chem. Rev., 2020, 415, 213232 CrossRef CAS.
- B. K. Banik, Eur. J. Org. Chem., 2002, 2431 CrossRef CAS.
- (a) Y. Zhang, Y. Yu and W. Bao, Synth. Commun., 1995, 25, 1825 CrossRef CAS; (b) Y. Huang and Y. Zhang, Synth. Commun., 1996, 26, 2911 CrossRef CAS.
- L. Zhang, X. Yu, L. Zhang, X. Zhou and Y. Lin, Org. Chem. Front., 2014, 1, 929 RSC.
- G. Bousrez, I. Déchamps, J. L. Vasse and F. Jaroschik, Dalton Trans., 2015, 44, 9359 RSC.
- Y. Horikawa, M. Watanabe, T. Fujiwara and T. Takeda, J. Am. Chem. Soc., 1997, 119, 1127 CrossRef CAS.
- (a) C. Denhez, S. Medegan, F. Helion, J.-L. Namy, J.-L. Vasse and J. Szymoniak, Org. Lett., 2006, 8, 2945 CrossRef CAS PubMed; (b) G. Bousrez, F. Jaroschik, A. Martinez, D. Harakat, E. Nicolas, X. F. Le Goff and J. Szymoniak, Dalton Trans., 2013, 42, 10997 RSC.
- A. Joosten, M. Soueidan, C. Denhez, D. Harakat, F. Hélion, J.-L. Namy, J.-L. Vasse and J. Szymoniak, Organometallics, 2008, 27, 4152 CrossRef CAS.
- (a) D. L. J. Clive, P. C. Anderson, N. Moss and A. Singh, J. Org. Chem., 1982, 47, 1641 CrossRef CAS; (b) A. F. Barrero, M. M. Herrador, J. F. Quílez del Moral, P. Arteaga, J. F. Arteaga, H. R. Dieguez and E. M. Sanchez, J. Org. Chem., 2007, 72, 2988 CrossRef CAS PubMed; (c) T. Strašák, F. Jaroschik, M. Malý, J. Čermák, J. Sýkora, R. Fajgar, J. Karban and D. Harakat, Inorg. Chim. Acta, 2014, 409, 137 CrossRef.
- Reviews: (a) Reactive Intermediates: MS Investigations in Solution, ed. L. S. Santos, Wiley-VCH, Weinheim, 2010 Search PubMed; (b) J. Mehara and J. Roithová, Chem. Sci., 2020, 11, 11960 RSC Original research papers: (c) W. J. Evans, M. A. Johnston, C. H. Fujimoto and J. Greaves, Organometallics, 2000, 19, 4258 CrossRef CAS; (d) D. P. Daniels, G. B. Deacon, D. Harakat, F. Jaroschik and P. C. Junk, Dalton Trans., 2012, 41, 267 RSC; (e) H. Zhang, K. Yu, N. Li, J. He, H. You and J. Jiang, J. Mass Spectrom., 2018, 53, 511 CrossRef CAS PubMed; (f) L. P. E. Yunker, Z. Ahmadi, J. R. Logan, W. Wu, T. Li, A. Martindale, A. G. Oliver and J. S. McIndoe, Organometallics, 2018, 37, 4297 CrossRef CAS; (g) M. Regnacq, D. Lesage, M. S. M. Holmsen, K. Miqueu, D. Bourissou and Y. Gimbert, Dalton Trans., 2023, 52, 13528 RSC.
- M. Polášek and J. Kubišta, J. Organomet. Chem., 2007, 692, 4073 CrossRef.
- H. Brintzinger and J. E. Bercaw, J. Am. Chem. Soc., 1970, 92, 6182 CrossRef CAS.
- (a) J. E. Bercaw, R. H. Marvich, L. G. Bell and H. H. Brintzinger, J. Am. Chem. Soc., 1972, 94, 1219 CrossRef CAS; (b) V. Varga, K. Mach, J. Heyrovský, G. Schmid and U. Thewalt, J. Organomet. Chem., 1994, 475, 127 CrossRef CAS.
- (a) M. G. Shaibakova, L. O. Khafizova, N. M. Chobanov, R. R. Gubaidullin, N. Y. R. Popod'ko and U. M. Dzhemilev, Tetrahedron Lett., 2014, 55, 1326 CrossRef CAS; (b) B. R. Reiner and I. A. Tonks, Inorg. Chem., 2019, 58, 10508 CrossRef CAS PubMed; (c) S. Okamoto, T. Yamada, Y.-K. Tanabe and M. Sakai, Organometallics, 2018, 37, 4431 CrossRef CAS; (d) G. Siemiaszko and Y. Six, New J. Chem., 2018, 42, 20219 RSC; (e) S. Ohta, N. Miura, K. Saitoh, K. Itoh, S. Satoh, R. Miyamoto and M. Okazaki, Organometallics, 2021, 40, 2826 CrossRef CAS.
- (a) J. G. Kenworthy, J. Myatt and M. C. R. Symons, J. Chem. Soc. A, 1971, 3428 RSC; (b) E. Samuel and J. Vedel, Organometallics, 1989, 8, 237 CrossRef CAS; (c) E. Samuel and J. Hénique, J. Organomet. Chem., 1996, 512, 183 CrossRef CAS; (d) S. Van Doorslaer, J. J. Shane, S. Stoll, A. Schweiger, M. Kranenburg and R. J. Meier, J. Organomet. Chem., 2001, 634, 185 CrossRef CAS.
- A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions, Clarendon Press, London, 1970 Search PubMed.
- F. Lacroix, C. E. Plecnik, S. Liu, F.-C. Liu, E. A. Meyers and S. G. Shore, J. Organomet. Chem., 2003, 687, 69 CrossRef CAS.
- G. Depaoli, P. Zanonato and G. Valle, Inorg. Chim. Acta, 1990, 170, 109 CrossRef CAS.
- (a) D. G. Sekutowski and G. D. Stucky, Inorg. Chem., 1975, 14, 2192 CrossRef CAS; (b) D. G. Sekutowski, R. Jungst and G. D. Stucky, Inorg. Chem., 1978, 17, 1848 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, FL, 87th edn, 2006 Search PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- K. Yoshida, I. Morimoto, K. Mitsudo and H. Tanaka, Tetrahedron, 2008, 64, 5800 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of X-ray diffraction, EPR spectroscopy, and ESI-MS analysis. CCDC 2369904. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02004a |
| This journal is © The Royal Society of Chemistry 2024 |