Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Temperature-dependent excited states for detecting reversible phase transitions in 2D lead(II) iodide perovskites†
Mahboubeh
Jamshidi
 * and
James M.
Gardner
* and
James M.
Gardner
 *
*
Department of Chemistry, Division of Applied Physical Chemistry, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden. E-mail: mahjam@kth.se; jgardner@kth.se
First published on 24th May 2024
Abstract
Significant interest exists in water-tolerant 2D lead iodide perovskites owing to their stability and proven potential in photovoltaic and photonic applications. These materials have solid-state phase transitions that are accessible below 100 °C. Here, the study witnesses the multiple phase transitions of the last members of a series of organic–inorganic hybrid materials, [(CnH2n+1NH3)2PbI4], with even n as n = 14, 16, and 18, once again. By employing temperature-dependent steady-state photoluminescence (PL) and temperature-dependent time-resolved photoluminescence (TRPL) spectroscopy in the temperature range of −18 to +90 °C and at −196 °C, we explore the thermal responses of these materials. The investigation reveals reversible phase transitions occurring between room temperature (RT) and elevated temperatures, impacting the optical properties and emitting colors of the perovskite compounds. The longer the alkyl chain, the higher the phase transition temperature, attributed to increased conformational disorder and enhanced perovskite symmetry. The decay constants for all compounds are very close in value, which confirms the underlying excited-state dynamics, pointing to contributions primarily from inorganic components across different phases. We anticipate that our results on the detection of phase transitions in 2D perovskites will not only motivate the use of these techniques for detecting phase transitions but also would help to understand their excited states in more details to selectively use them for solar cell and next-generation display technologies.
1. Introduction
Two-dimensional (2D) lead halide perovskite semiconductors are a class of materials that have recently found applications in solar cells, light-emitting diodes, and photodetector technologies.1 Although 3D perovskites, such as CH3NH3PbI3, could be transformative for optoelectronic applications, they are presently limited by their chemical and thermal stability. Alternatively, 2D perovskites may be more promising for their incorporation in practical devices due to their long, hydrophobic alkyl chain cations.2–4 The general formula of these organic–inorganic hybrids is (R-NH3)2PbX4 where R is a long-chain alkylammonium cation, and X is a halide. These perovskites consist of two-dimensional sheets of the corner-sharing octahedral anions [PbX6]4− capped with alternating double layers of cations (CnH2n+1NH3)+, which are stabilized by hydrogen bonding between ammonium cations and halides in the inorganic layers.5The layered structure creates a quantum well structure with strong electronic confinement effects that separates the inorganic lead(II) halide sheets from the organic cation double layers.6 The dielectric and excitonic characteristics of 2D perovskites are associated with their quantum well arrangement and can be adjusted through the incorporation of different halide anions and by modifying the thickness of the inorganic layer.7–9 Various alkylammonium ligand-based cations have been investigated for use within the context of 2D perovskites.10–13 Typically, short-chain cations are employed in 2D perovskite structures to minimize the separation between quantum wells, thereby facilitating charge transfer.14,15 Conversely, larger organic cations increase this separation, impeding charge transport and consequently diminishing device efficiency.16,17
Two-dimensional perovskite compounds can undergo solid-state phase transitions. The temperature for the phase transitions increases as the alkylammonium cation carbon chain length increases, as confirmed by differential scanning calorimetry (DSC), differential thermal analysis (DTA), Raman spectroscopy, and single-crystal X-ray diffraction.18–22 According to single-crystal X-ray diffraction, there are three changes involved in the phase transitions, which may occur independently or cooperatively in each phase transition.23,24 As the temperature increases, the Pb–X–Pb angle increases and results in lead(II) halide layers becoming more co-planar and the planes shifting from a staggered geometry to an eclipsed geometry. Also, the shift of the organic cation layer relative to the inorganic layer causes the angle of the ammonium cations to change from obtuse to acute. Lastly, there are rotations around C–C bonds in the alkylammonium cations, which have less impact on the inorganic layer.18,25,26 The number of unique phases depends on the length of the organic chain, for instance, the number of unique phases for a 2D lead(II) iodide perovskite with an alkyl chain of n = 11 and 15 is three, while there are four phases for n = 13.3
Recently, we investigated the longest, even-numbered members of the series of organic–inorganic hybrid materials, [(CnH2n+1NH3)2PbI4], with n = 14, 16, and 18 for their use as water-tolerant perovskites in solar cells.2 From the crystallographic data and DSC, these materials undergo two first-order phase transitions in the range from +45 to +100 °C from fluctuations in the thermal motion of the alkylammonium chains positioned between the layers (Fig. 1).27,28 At room temperature, the organic chains are interdigitated in a planar conformation and nearly perpendicular to the inorganic layers. By increasing the temperature, the disorder in the alkyl chains and interlayer spacing increases.18,29
 | ||
| Fig. 1 The crystal structures of the (C14H29NH3)2PbI4 perovskite (a) are orthorhombic at room temperature (RT) and (b) monoclinic at an elevated temperature (HT). The interdigitated arrangement of alkylammonium chains is shown as lines. The yellow spheres inside the polyhedra are Pb2+ cations and the black spheres are I− anions. (c) DSC curves of the (CnH2n+1NH3)2PbI4 (n = 14, 16 and 18) 2D perovskite powder samples measured in both heating and cooling cycles. Reprinted by permission from ref. 28. | ||
For optoelectronic devices utilizing perovskites, it is necessary to have a detailed photophysical understanding of the compounds. There are several reports on excited states, photoluminescence quantum yields, and responsiveness to external stimuli. For example, for APbX3 perovskites such as A = Cs+, CH3NH3+, or NH2CHNH2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30–33 and 2D perovskites such as (C4H9NH3)2PbI4 and (BDA)PbBr4.34–36 However, the current study aims to show how photophysical properties change as a function of temperature across phase transitions which have not been thoroughly investigated and warrant further study.
30–33 and 2D perovskites such as (C4H9NH3)2PbI4 and (BDA)PbBr4.34–36 However, the current study aims to show how photophysical properties change as a function of temperature across phase transitions which have not been thoroughly investigated and warrant further study.
Herein, we studied the excited states of two-dimensional perovskites in the powder form by temperature-dependent time-resolved photoluminescence spectroscopy (at −196 °C and from −18 to 95 °C) to investigate their phase transitions and develop an in-depth understanding of the excited state behavior across phase transitions. Above −18 °C, the PL spectra of all the perovskites were fitted with multipeak Gaussian functions to determine the contributions of individual phases.
2. Experimental methods
The synthesis protocol of 2D perovskites given in ref. 2 and 27 is followed for the preparation of (C14H29NH3)2PbI4, (C16H33NH3)2PbI4, and (C18H37NH3)2PbI4 2D perovskites. For the diffuse reflectance spectroscopic measurements, an Avantes AvaSpec-2048 dual UV-vis spectrophotometer, equipped with an integrating sphere and an integrated light source, was used to record the absorption of powders. Photoluminescence measurements were carried out on a Fluorolog FL 3–22 spectrometer (Horiba Jobin Yvon, Longjumeau, France), equipped with a double excitation monochromator, a single emission monochromator (HR320) and an R928P PMT detector. A continuous xenon lamp (450 W) was used for steady state measurements. A Delta Diode (λex = 360 nm) was employed as the pulsed source for time correlated single photon counting (TCSPC) lifetime acquisition. The absolute quantum yield (QY) was measured with a G8 integrating sphere (GMP), using BaSO4 and pure solvents as blanks at 25 °C. Integrated emission intensity as a function of temperature was compared with the measured absolute QY at room temperature to calculate the relative quantum yield based on the fixed parameters and conditions. Measurements at −196 °C were performed in liquid N2. A Luma 40 heat exchanger connected to a TC 1 temperature controller filled with 50% methanol was used for temperature adjustment from −18 to 95 °C. A multi-exponential function was selected to fit PL decay curves:I = ∑Ai![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp−t/τi, exp−t/τi, |
3. Results and discussion
The photoluminescence time constants, quantum yields, and emission maxima for (CnH2n+1NH3)2PbI4 (n = 14, 16 and 18) powder samples are summarized in Table 1 and Table S1.† The tables reflect the heating and cooling of the samples up to +85 °C and cooling to −18 and −196 °C.| Compound | Temperature | λ em (nm) | τ em (ns) ± standard error | A i | Φ PL [%] |
|---|---|---|---|---|---|
| (C18H37NH3)2PbI4 | 85 °C | 584 | λ det = 578 nm | 0.08 | |
| τ 1 = 53.3 ± 0.9 | 0.56 | ||||
| τ 2 = 261 ± 4 | 0.33 | ||||
| τ 3 = 1043 ± 12 | 0.12 | ||||
| 25 °C | 493, 523 | λ det = 493 nm | 2.75 | ||
| τ 1 = 57 ± 1 | 0.60 | ||||
| τ 2 = 272 ± 5 | 0.30 | ||||
| τ 3 = 1095 ± 17 | 0.10 | ||||
| λ det = 523 nm | |||||
| τ 1 = 59 ± 3 | 0.60 | ||||
| τ 2 = 297 ± 19 | 0.30 | ||||
| τ 3 = 1253 ± 85 | 0.10 | ||||
| −10 °C | 490, 507 | λ det = 490 nm | 3.06 | ||
| τ 1 = 50 ± 2 | 0.72 | ||||
| τ 2 = 270 ± 17 | 0.22 | ||||
| τ 3 = 1200 ± 70 | 0.06 | ||||
| −196 °C | 484 | λ det = 484 nm | |||
| τ 1 = 42 ± 4 | 0.90 | ||||
| τ 2 = 394 ± 28 | 0.10 | ||||
| (C16H33NH3)2PbI4 | 25 °C | 498, 536 | λ det = 498 nm | 0.27 | |
| τ 1 = 42 ± 1 | 0.67 | ||||
| τ 2 = 241 ± 6 | 0.25 | ||||
| τ 3 = 1081 ± 25 | 0.08 | ||||
| λ det = 536 nm | |||||
| τ 1 = 54 ± 1 | 0.58 | ||||
| τ 2 = 260 ± 5 | 0.31 | ||||
| τ 3 = 1041 ± 15 | 0.11 | ||||
| −196 °C | 483, 645 | λ det = 498 nm | |||
| τ 1 = 39 ± 1 | 0.85 | ||||
| τ 2 = 385 ± 8 | 0.15 | ||||
| (C14H29NH3)2PbI4 | 25 °C | 495, 525 | λ det = 495 nm | 1.45 | |
| τ 1 = 28 ± 2 | 0.80 | ||||
| τ 2 = 184 ± 9 | 0.15 | ||||
| τ 3 = 931 ± 36 | 0.05 | ||||
| λ det = 525 nm | 0.05 | ||||
| τ 1 = 53 ± 2 | 0.60 | ||||
| τ 2 = 265 ± 8 | 0.30 | ||||
| τ 3 = 1093 ± 29 | 0.10 | ||||
| −196 °C | 485 | λ det = 485 nm | |||
| τ 1 = 37 ± 3 | 0.79 | ||||
| τ 2 = 234 ± 12 | 0.16 | ||||
| τ 3 = 1125 ± 57 | 0.05 |
The emission spectra of samples from room temperature to high temperature are similar to those in our previous report on the same samples but on thin film. The PL spectra reveal the presence of two resolved peaks in all three powder samples, which is complementary to the absorption results (Fig. S1†). The presence of two resolved peaks in the absorption and PL emission spectra could originate from the dual excitonic emission, vibronic coupling, simultaneous coexistence of the two crystallographic phases, or temperature-induced exciton switching in these compounds.37–42 The robust absorbance aligned with both PL peaks implies that neither of the two PL peaks stems from defect states. Typically, emissions linked to defects or traps lack the corresponding absorption, as localized defect states exhibit a notably low absorption cross-section.34,43
Regarding the dual excitonic emission, there are some reports which could be seen in the single crystals of some 2D perovskites such as (C4H9NH3)2PbI4,40 (C8H17NH3)2PbI4,44 and A2SnI4 (with A = phenylethylammonium, butylammonium, hexylammonium, and octylammonium monovalent cations).37 However, herein we believe that the dual peaks are coming from two different phases. To further confirm the origin of the peaks and confirm the existence of two phases, we have measured the PL emission spectra of the freshly prepared powder samples that were not annealed (Fig. S2†). The PL emission peak at a shorter wavelength exactly overlaps with the PL emission spectra recorded in the fresh powder samples, indicating that the peak at longer wavelengths results from the presence of the HT phase. It suggests that the RT phase is the product which takes time to be converted partially to the HT phase at room temperature without annealing. This is further demonstrated by PXRD, wherein the initial peak exhibits a shift over time (Fig. S3†).
In order to distinguish the influences originating from the emission states of different phases in the photoluminescence spectra, we utilized a Gaussian fitting function with multiple peaks to compare the photoluminescence spectra for temperatures between −18 and 85 °C. Starting the steady state measurements at −18 °C, a sharp emission peak (Peak 1) with the maximum intensity at 489 nm and a broad peak at 503 nm (Peak 2) were observed with a quantum yield of 6.3% (Fig. 2a and S8†). The population of the peaks at −18 °C was fitted using gaussian distributions as shown in Fig. 2b. The population of Peak 1 is lower than that of Peak 2 in this temperature range (Fig. 2d). Increasing the temperature leads to a bathochromic shift in Peak 1 and a pronounced bathochromic shift in Peak 2 (Fig. 2a) with a relatively broad emission bandwidth. At 0 °C, the integrated PL intensity of both peaks significantly decreased (Fig. 2c). The intensity remained relatively constant between 0 and 20 °C. The intensities gradually decreased with the increasing temperature and were quenched at 70 °C and 85 °C for Peak 1 and Peak 2, respectively.
The absolute quantum yield was measured as 2.75% at 20 °C (Fig. 2c). At temperatures above 20 °C, the intensities diminished and at 85 °C, only Peak 2 (584 nm) was observed and quantum yield reduced significantly to 0.06% compared to room temperature (Fig. 2e and S8†). Finally, the emission is quenched below the detection limit at 90 °C, which is consistent with the reported phase transition temperature for (C18H37NH3)2PbI4 (Fig. 1c).18,28
It is noteworthy to mention that the quenching of the emission is accompanied by the emergence of a higher temperature phase due to annealing the sample to 100 °C.28 When viewed under ambient light, the color of the sample is yellow below the phase transition temperature, but it is orange at 80 °C (Fig. 3a and b). Additionally, the bright green emission from UV excitation at ambient temperature is quenched at 85 °C (Fig. 3c) and above as non-emissive phase dominates at higher temperatures.
The inverse temperature response is observed upon cooling the same sample from 85 to −18 °C, which confirms the reversibility of the phase transition (Fig. S4†).
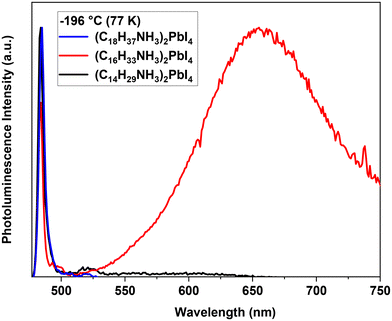
At −196 °C (77 K) there is a sharp, narrow photoluminescence peak (Peak 1) at 483 nm, which is hypsochromically shifted at ∼420 cm−1 compared to room temperature (493 nm), as shown in Fig. 2e. We speculate that dual emission comes from the co-existence of the room temperature phase and the high temperature phase (Peak 2, orthorhombic structure). If the peaks were originated from the same phase, then the vibronic structure of the emission would have sharp, distinct peaks, which was not observed. The stability of the compounds was confirmed by comparing the PL of a sample that was thermally cycled with that of a sample that was not. The spectra of the two samples are the same, which confirms the reversibility.
To gain further insights into the optical and physical transitions, variable temperature time-resolved photoluminescence (TRPL) measurements were performed. Herein, decay traces were fitted to multiexponential functions with short (<100 ns), medium (100–400 ns), and long (>400 ns) time constants.
As shown in Fig. 4a, the decay traces of the sample were recorded at two detection wavelengths that correspond to the two maxima in the PL spectra. At room temperature, the PL decay traces are fitted as a triple exponential decay process. The time constants for the traces at 493 nm and 523 nm are similar (τ1 = 57 vs. 59 ns, τ2 = 272 vs. 297 ns, and τ3 = 1.09 vs. 1.25 μs for λdet = 493 nm and 523 nm, respectively) and suggesting that they are produced by the same process or that there is mixing between the two excited states. The pre-exponential factors show consistent contributions at both PL maxima (A1 = 0.6, A2 = 0.3, and A3 = 0.10). In addition, the same decay dynamics in the emission wavelengths suggest that these two bands are not caused by defect states, which could present different decay dynamics.40
Based on the time constants, we posit that the emission of Peak 2 originates from the inorganic ordered sublattice singlet state and triplet states in the orthorhombic structure (RT phase). Excitation principally occurs within the inorganic quantum well singlet state; however, the perovskites have heavy ions (Pb2+ and I−), which facilitate spin–orbit coupling between singlet and triplet states of the inorganic well.45,46 At 85 °C, all detected emissions come from Peak 2, which refers to the HT phase (Fig. 4a). Above 85 °C, the emission is quenched (Fig. 3a and b).
Measuring the time-resolved PL from the room temperature phase (λdet = 490 nm) at −10 °C indicates that the time constants are similar to those at 25 °C (τ1 = 50 ns, τ2 = 270 ns, and τ3 = 1.2 μs), but the contribution of the shortest time constant increased, and the overall quantum yield increased to 3.06%. At −196 °C, only the narrow PL peak is observed (Peak 1), and the PL decay curve (λdet = 484 nm) no longer includes the longest time component, and the data are best fit by a bi-exponential decay process (τ1 = 39 ns and τ2 = 394 ns) that is heavily weighted towards the shortest time constant (A1 = 0.85 vs. A2 = 0.15) as seen in Fig. 4b. We have associated the single PL peak at −196 °C with the RT phase (484 nm emission). At this temperature, we did not observe the HT phases. By decreasing the temperature, shorter lifetimes are observed, which corresponds with more excitonic character and less of a trap-state or triplet character.
For (C16H33NH3)2PbI4, the integrated intensities of Peak 1 and Peak 2 are identical for temperatures between −18 °C and −5 °C (Fig. 5b and S5c†). Upon warming the sample to 0 °C, the population of Peak 2 (λmax = 514 nm) begins to dominate and a bathochromic shift to 525 nm is present until 30 °C. Unlike (C18H37NH3)2PbI4, for (C16H33NH3)2PbI4, the intensity of both peaks increased from −15 °C and reached to its maxima at +25 °C for Peak 1 and at +30 °C for Peak 2. After that, the intensity of both bands decreases until they were below the detection limit at 70 °C for Peak 1 and 80 °C for Peak 2 and the third phase (HT, monoclinic) which is poorly emissive appeared higher than 80 °C. According to XRD, an increase in the temperature results in a more symmetrical geometry of the inorganic planes and the average bond length decreasing slightly in the new monoclinic system compared to that in the orthorhombic system (RT phase).18
At −196 °C, there is one narrow peak positioned at 483 nm and a broad and intense emission band at 625 nm. The sharp, higher energy peak corresponds to the RT phase, which appears as another phase transition below −20 °C. The lower energy band was not observed for (C18H37NH3)2PbI4 or (C14H29NH3)2PbI4 and may indicate that internal energy transfer is more important for (C16H33NH3)2PbI4 than in the other compounds (Fig. 6). The results are consistent with the observed lower quantum yield for (C16H33NH3)2PbI4 when compared with those of (C18H37NH3)2PbI4 and (C14H29NH3)2PbI4 (Table 1 and Fig. S8†).
The behavior of (C14H29NH3)2PbI4 is similar to that of (C18H37NH3)2PbI4 and in both compounds, the integrated PL intensity dropped precipitously at −10 °C and continued for both PL peaks until they were below the detection limit at 65 and 75 for Peak 1 and Peak 2, respectively (Fig. S6c†). At −196 °C, only the first narrow PL band is observed for (C14H29NH3)2PbI4, which is associated with the LT phase (Fig. 6). The decay constants and pre-exponential factors for (C14H29NH3)2PbI4 were similar at room temperature and low temperature, which may suggest that there is greater similarity between the two phases (LT and RT) as compared to (C18H37NH3)2PbI4 (Fig. S7†).
4. Conclusion
In summary, temperature-dependent steady-state photoluminescence (PL) and temperature-dependent time-resolved photoluminescence (TRPL) spectroscopy have been successfully applied to study the multiple phase transitions and excited-state decay in the temperature range of −18 to 90 °C and at −196 °C. The observed reversible temperature response confirms the reversibility of the phase transition in the studied compounds. Our observations show that the color of the sample transitions from yellow to orange at the phase transition temperature from the RT to HT phase and the emission changes from blue to green from low to room temperature (RT phase) due to different excited states. Furthermore, it seems that the longer the alkyl chain in the organic chain is, the higher the temperature of the phase transition. This involves enhanced conformational and dynamic disorder (or melting) of the organic chain, and an increase in the perovskite symmetry. Furthermore, the decay constants for all compounds are very close in value and showed multi-component constants confirming the contribution of the same fragments in all of them (inorganic part). However, a longer PL decay is not observed at a low temperature (−196 °C) for (C18H37NH3)2PbI4 and (C16H33NH3)2PbI4 which is evidence for more changes in the inorganic part of the compounds between LT and RT phases. Further investigations in the temperature range from −196 to −18 °C by powder X-ray diffraction could provide more information on the structures and conformational alteration, especially for the LT phase and the exact temperature of phase transition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Swedish Energy Agency for financial support (Grant number: 49278-1). Further support for this research was possible due to the generous contributions of the Swedish government through the strategic research area "STandUP for ENERGY". We would like to thank Dr Veronica Paterlini for helping us with the time-resolved measurements at Stockholm University and KTH's Department of Chemistry for supplying the caffeine required for completing this work. JG would like to thank Brian David Gardner and Rachael Elizabeth Gardner for their insightful conversations and vigorous debates.References
- E. Elahi, G. Dastgeer, A. S. Siddiqui, S. A. Patil, M. W. Iqbal and P. R. Sharma, A review on two-dimensional (2D) perovskite material-based solar cells to enhance the power conversion efficiency, Dalton Trans., 2022, 51, 797–816 RSC.
- B. P. Kore, W. Zhang, B. W. Hoogendoorn, M. Safdari and J. M. Gardner, Moisture tolerant solar cells by encapsulating 3D perovskite with long-chain alkylammonium cation-based 2D perovskite, Commun. Mater., 2021, 2, 100 CrossRef CAS.
- A. Lemmerer, Thermochromic Phase Transitions of Long Odd-Chained Inorganic–Organic Layered Perovskite-Type Hybrids [(CnH2n+1NH3)2PbI4], n = 11, 13, and 15, Inorg. Chem., 2022, 61, 6353–6366 CrossRef CAS PubMed.
- B. P. Kore, M. Jamshidi and J. M. Gardner, The impact of moisture on the stability and degradation of perovskites in solar cells, Mater. Adv., 2024, 5, 2200–2217 RSC.
- M. M. Abdelkader and W. M. Gamal, Characterization and coordination of intercalation - Non-intercalation phase transition in a new hybrid halide perovskite (n-C16H33NH3)2 ZnCl4, Solid State Sci., 2016, 61, 173–184 CrossRef CAS.
- B. Traore, L. Pedesseau, L. Assam, X. Che, J.-C. Blancon, H. Tsai, W. Nie, C. C. Stoumpos, M. G. Kanatzidis, S. Tretiak, A. D. Mohite, J. Even, M. Kepenekian and C. Katan, Composite Nature of Layered Hybrid Perovskites: Assessment on Quantum and Dielectric Confinements and Band Alignment, ACS Nano, 2018, 12, 3321–3332 CrossRef CAS PubMed.
- C. M. Mauck and W. A. Tisdale, Excitons in 2D Organic–Inorganic Halide Perovskites, Trends Chem., 2019, 1, 380–393 CrossRef CAS.
- J. C. Blancon, A. V. Stier, H. Tsai, W. Nie, C. C. Stoumpos, B. Traoré, L. Pedesseau, M. Kepenekian, F. Katsutani, G. T. Noe, J. Kono, S. Tretiak, S. A. Crooker, C. Katan, M. G. Kanatzidis, J. J. Crochet, J. Even and A. D. Mohite, Scaling law for excitons in 2D perovskite quantum wells, Nat. Commun., 2018, 9, 2254 CrossRef PubMed.
- D. B. Straus and C. R. Kagan, Electrons, Excitons, and Phonons in Two-Dimensional Hybrid Perovskites: Connecting Structural, Optical, and Electronic Properties, J. Phys. Chem. Lett., 2018, 9, 1434–1447 CrossRef CAS PubMed.
- L. Mao, C. C. Stoumpos and M. G. Kanatzidis, Two-Dimensional Hybrid Halide Perovskites: Principles and Promises, J. Am. Chem. Soc., 2019, 141, 1171–1190 CrossRef CAS PubMed.
- F. Zhang, H. Lu, J. Tong, J. J. Berry, M. C. Beard and K. Zhu, Advances in two-dimensional organic–inorganic hybrid perovskites, Energy Environ. Sci., 2020, 13, 1154–1186 RSC.
- A. H. Proppe, M. Wei, B. Chen, R. Quintero-Bermudez, S. O. Kelley and E. H. Sargent, Photochemically Cross-Linked Quantum Well Ligands for 2D/3D Perovskite Photovoltaics with Improved Photovoltage and Stability, J. Am. Chem. Soc., 2019, 141, 14180–14189 CrossRef CAS PubMed.
- D. H. Kim, C. P. Muzzillo, J. Tong, A. F. Palmstrom, B. W. Larson, C. Choi, S. P. Harvey, S. Glynn, J. B. Whitaker, F. Zhang, Z. Li, H. Lu, M. F. A. M. van Hest, J. J. Berry, L. M. Mansfield, Y. Huang, Y. Yan and K. Zhu, Bimolecular Additives Improve Wide-Band-Gap Perovskites for Efficient Tandem Solar Cells with CIGS, Joule, 2019, 3, 1734–1745 CrossRef CAS.
- K. Zheng, Y. Chen, Y. Sun, J. Chen, P. Chábera, R. Schaller, M. J. Al-Marri, S. E. Canton, Z. Liang and T. Pullerits, Inter-phase charge and energy transfer in Ruddlesden–Popper 2D perovskites: critical role of the spacing cations, J. Mater. Chem. A, 2018, 6, 6244–6250 RSC.
- A. H. Proppe, M. H. Elkins, O. Voznyy, R. D. Pensack, F. Zapata, L. V. Besteiro, L. N. Quan, R. Quintero-Bermudez, P. Todorovic, S. O. Kelley, A. O. Govorov, S. K. Gray, I. Infante, E. H. Sargent and G. D. Scholes, Spectrally Resolved Ultrafast Exciton Transfer in Mixed Perovskite Quantum Wells, J. Phys. Chem. Lett., 2019, 10, 419–426 CrossRef CAS PubMed.
- M. Safdari, P. H. Svensson, M. T. Hoang, I. Oh, L. Kloo and J. M. Gardner, Layered 2D alkyldiammonium lead iodide perovskites: synthesis, characterization, and use in solar cells, J. Mater. Chem. A, 2016, 4, 15638–15646 RSC.
- F. Li, Y. Xie, Y. Hu, M. Long, Y. Zhang, J. Xu, M. Qin, X. Lu and M. Liu, Effects of Alkyl Chain Length on Crystal Growth and Oxidation Process of Two-Dimensional Tin Halide Perovskites, ACS Energy Lett., 2020, 5, 1422–1429 CrossRef CAS.
- D. G. Billing and A. Lemmerer, Synthesis, characterization and phase transitions of the inorganic–organic layered perovskite-type hybrids [(CnH2n+1NH3)2PbI4] (n = 12, 14, 16 and 18), New J. Chem., 2008, 32, 1736–1746 RSC.
- D. Spirito, Y. Asensio, L. E. Hueso and B. Martín-García, Raman spectroscopy in layered hybrid organic-inorganic metal halide perovskites, Journal of Physics: Materials, 2022, 5, 034004 CAS.
- J. M. Hoffman, C. D. Malliakas, S. Sidhik, I. Hadar, R. McClain, A. D. Mohite and M. G. Kanatzidis, Long periodic ripple in a 2D hybrid halide perovskite structure using branched organic spacers, Chem. Sci., 2020, 11, 12139–12148 RSC.
- K. Fedoruk, D. Drozdowski, M. Maczka, J. K. Zareba, D. Stefańska, A. Gagor and A. Sieradzki, [Methylhydrazinium]2PbCl4, a Two-Dimensional Perovskite with Polar and Modulated Phases, Inorg. Chem., 2022, 61, 15520–15531 CrossRef CAS PubMed.
- M. Zhang, M. Li, X. You, Z. Wei, W. Rao, L. Wang and H. Cai, A fluorine and chlorine substituted 2D lead bromide perovskite with high phase transition temperature, J. Solid State Chem., 2021, 302, 122409 CrossRef CAS.
- Q. Gao, J. Qi, K. Chen, M. Xia, Y. Hu, A. Mei and H. Han, Halide Perovskite Crystallization Processes and Methods in Nanocrystals, Single Crystals, and Thin Films, Adv. Mater., 2022, 34, 2200720 CrossRef CAS PubMed.
- W. Paritmongkol, N. S. Dahod, A. Stollmann, N. Mao, C. Settens, S.-L. Zheng and W. A. Tisdale, Synthetic Variation and Structural Trends in Layered Two-Dimensional Alkylammonium Lead Halide Perovskites, Chem. Mater., 2019, 31, 5592–5607 CrossRef CAS.
- A. Lemmerer and D. G. Billing, Synthesis, characterization and phase transitions of the inorganic–organic layered perovskite-type hybrids [(CnH2n+1NH3)2PbI4], n = 7, 8, 9 and 10, Dalton Trans., 2012, 41, 1146–1157 RSC.
- C. C. Stoumpos, D. H. Cao, D. J. Clark, J. Young, J. M. Rondinelli, J. I. Jang, J. T. Hupp and M. G. Kanatzidis, Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors, Chem. Mater., 2016, 28, 2852–2867 CrossRef CAS.
- N. V. Venkataraman, S. Bhagyalakshmi, S. Vasudevan and R. Seshadri, Conformation and orientation of alkyl chains in the layered organic–inorganic hybrids: (CnH2n+1NH3)2PbI4 (n = 12,16,18), Phys. Chem. Chem. Phys., 2002, 4, 4533–4538 RSC.
- B. P. Kore and J. M. Gardner, Water-resistant 2D lead(II) iodide perovskites: correlation between optical properties and phase transitions, Mater. Adv., 2020, 1, 2395–2400 RSC.
- N. V. Venkataraman, S. Barman, S. Vasudevan and R. Seshadri, Structural analysis of alkyl chain conformation in the layered organic–inorganic hybrids (CnH2n+1NH3)2PbI4 (n=12,16,18) by IR spectroscopy, Chem. Phys. Lett., 2002, 358, 139–143 CrossRef CAS.
- K.-H. Wang, L.-C. Li, M. Shellaiah and K. W. Sun, Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals, Sci. Rep., 2017, 7, 13643 CrossRef PubMed.
- D. W. deQuilettes, W. Zhang, V. M. Burlakov, D. J. Graham, T. Leijtens, A. Osherov, V. Bulović, H. J. Snaith, D. S. Ginger and S. D. Stranks, Photo-induced halide redistribution in organic–inorganic perovskite films, Nat. Commun., 2016, 7, 11683 CrossRef CAS PubMed.
- A. Osherov, E. M. Hutter, K. Galkowski, R. Brenes, D. K. Maude, R. J. Nicholas, P. Plochocka, V. Bulović, T. J. Savenije and S. D. Stranks, The Impact of Phase Retention on the Structural and Optoelectronic Properties of Metal Halide Perovskites, Adv. Mater., 2016, 28, 10757–10763 CrossRef CAS PubMed.
- O. Vukovic, G. Folpini, E. L. Wong, L. Leoncino, G. Terraneo, M. D. Albaqami, A. Petrozza and D. Cortecchia, Structural effects on the luminescence properties of CsPbI3 nanocrystals, Nanoscale, 2023, 15, 5712–5719 RSC.
- T. Sheikh, A. Shinde, S. Mahamuni and A. Nag, Possible Dual Bandgap in (C4H9NH3)2PbI4 2D Layered Perovskite: Single-Crystal and Exfoliated Few-Layer, ACS Energy Lett., 2018, 3, 2940–2946 CrossRef CAS.
- Y. Han, Y. Li, Y. Wang, G. Cao, S. Yue, L. Zhang, B.-B. Cui and Q. Chen, From Distortion to Disconnection: Linear Alkyl Diammonium Cations Tune Structure and Photoluminescence of Lead Bromide Perovskites, Adv. Opt. Mater., 2020, 8, 1902051 CrossRef CAS.
- C.-W. Lin, F. Liu, T.-Y. Chen, K.-H. Lee, C.-K. Chang, Y. He, T. L. Leung, A. M. C. Ng, C.-H. Hsu, J. Popović, A. Djurišić and H. Ahn, Structure-Dependent Photoluminescence in Low-Dimensional Ethylammonium, Propylammonium, and Butylammonium Lead Iodide Perovskites, ACS Appl. Mater. Interfaces, 2020, 12, 5008–5016 CrossRef CAS PubMed.
- V. V. Nawale, T. Sheikh and A. Nag, Dual Excitonic Emission in Hybrid 2D Layered Tin Iodide Perovskites, J. Phys. Chem. C, 2020, 124, 21129–21136 CrossRef CAS.
- D. Cortecchia, S. Neutzner, A. R. S. Kandada, E. Mosconi, D. Meggiolaro, F. De Angelis, C. Soci and A. Petrozza, Broadband Emission in Two-Dimensional Hybrid Perovskites: The Role of Structural Deformation, J. Am. Chem. Soc., 2017, 139, 39–42 CrossRef CAS PubMed.
- M. Jamshidi, S. R. Barzegar-Kiadehi, M. G. Haghighi and B. Notash, Dual-Emissive Bis(diphenylphosphino)amine Platinum Complexes: Structural, Reactivity, Photophysical, and Theoretical Investigations, Organometallics, 2020, 39, 3099–3111 CrossRef CAS.
- R. F. Moral, J. C. Germino, L. G. Bonato, D. B. Almeida, E. M. Therézio, T. D. Z. Atvars, S. D. Stranks, R. A. Nome and A. F. Nogueira, Influence of the Vibrational Modes from the Organic Moieties in 2D Lead Halides on Excitonic Recombination and Phase Transition, Adv. Opt. Mater., 2020, 8, 2001431 CrossRef CAS.
- B. Dryzhakov, B. J. Lawrie, J. Z. Celio, M. Wang, M. Koehler and B. Hu, Dual Emission Bands of a 2D Perovskite Single Crystal with Charge Transfer State Characteristics, ACS Nano, 2023, 17, 12200–12207 CrossRef CAS PubMed.
- K. Pradeesh, J. J. Baumberg and G. V. Prakash, Temperature-induced exciton switching in long alkyl chain based inorganic-organic hybrids, J. Appl. Phys., 2012, 111, 013511 CrossRef.
- M. Bruzzi, F. Gabelloni, N. Calisi, S. Caporali and A. Vinattieri, Defective States in Micro-Crystalline CsPbBr3 and Their Role on Photoconductivity, Nanomaterials, 2019, 9, 177 CrossRef CAS PubMed.
- T. Sheikh, A. Shinde, S. Mahamuni and A. Nag, Dual excitonic emissions and structural phase transition of octylammonium lead iodide 2D layered perovskite single crystal, Mater. Res. Express, 2019, 6, 124002 CrossRef CAS.
- Laxmi and D. Kabra, Origin of Contrasting Emission Spectrum of Bromide versus Iodide Layered Perovskite Semiconductors, J. Phys. Chem. Lett., 2022, 13, 2737–2743 CrossRef CAS PubMed.
- B. B. Chen, S. Wang, S. W. Jiang, Z. G. Yu, X. G. Wan, H. F. Ding and D. Wu, The role of heavy metal ions on spin transport in organic semiconductors, New J. Phys., 2015, 17, 013004 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, global fitting parameters, relative quantum yields, and additional results of PL and decay constant measurements. See DOI: https://doi.org/10.1039/d4dt01210k |
| This journal is © The Royal Society of Chemistry 2024 |