Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
[Ag(IPr)(bpy)][PF6]: brightness and darkness playing with aggregation induced phosphorescence for light-emitting electrochemical cells†
Ginevra
Giobbio
 ab,
Pedro B.
Coto
ab,
Pedro B.
Coto
 *c,
Jean-François
Lohier
a,
Jean-Luc
Renaud
*c,
Jean-François
Lohier
a,
Jean-Luc
Renaud
 ad,
Sylvain
Gaillard
ad,
Sylvain
Gaillard
 *a and
Rubén D.
Costa
*a and
Rubén D.
Costa
 *b
*b
aNormandy University, ENSICAEN, UNICAEN, CNRS, LCMT, 1400 Caen, France. E-mail: sylvain.gaillard@ensicaen.fr
bTechnical University of Munich, Campus Straubing for Biotechnology and Sustainability, Chair of Biogenic Functional Materials, Schulgasse 22, 94315 Straubing, Germany. E-mail: ruben.costa@tum.de
cSpanish National Research Council (CSIC) and Donostia International Physics Center (DIPC), Material Physics Center (CFM), 20018 Donostia – San Sebastián, Spain. E-mail: pedro.brana@csic.es
dSorbonne Université, CNRS, Institut Parisien de Chimie Moléculaire, UMR 8232, 75005 Paris, France
First published on 19th June 2024
Abstract
Heteroleptic silver(I) complexes have recently started to attract attention in thin-film lighting technologies as an alternative to copper(I) analogues due to the lack of flattening distortion upon excitation. However, the interpretation of their photophysical behavior is challenging going from traditional fluorescence/phosphorescence to a temperature-dependent dual emission mechanism and ligand-lock assisted thermally activated delayed fluorescence. Herein, we unveil the photoluminescence behavior of a three-coordinated Ag(I) complex with the N-heterocyclic carbene (NHC) ligand and 2,2′-bipyridine (bpy) as the N^N ligand. In contrast to its low-emissive Cu(I) complex structural analogues, a strong greenish emission was attributed to the presence of aggregates formed by π–π intermolecular interactions as revealed by the X-ray structure and aggregation induced emission (AIE) studies in solution. In addition, the temperature-dependent time-resolved spectroscopic and computational studies demonstrated that the emission mechanism is related to a phosphorescence emission mechanism of two very close lying (ΔE = 0.08 eV) excited triplet states, exhibiting a similar delocalized nature over the bipyridine ligands. Unfortunately, this favourable AIE is lost upon forming homogeneous thin films suitable for lighting devices. Though the films showed very poor emission, the electrochemical stability under device operation conditions is remarkable compared to the prior-art, highlighting the potential of [Ag(NHC)(N^N)][X] complexes in thin-film lighting.
Introduction
Known since ancient times, coinage metals have always played an important role in everyday life. Recently, modern chemistry fueled more sophisticated uses of d10-metal derivatives. They have been widely exploited as catalysts,1–8 while they have become more and more attractive for the replacement of rare and expensive metal-based active components, such as Ir(III), Ru(II) and Pt(II)-based compounds, in optoelectronics.9–14In this context, the primacy of heteroleptic Cu(I)-complexes combining diimine (N^N) and diphosphine (P^P) ligands ([Cu(N^N)(P^P)]+) for thin-films, solid-state lighting (i.e., light-emitting electrochemical cells or LECs and organic light-emitting diodes or OLEDs)15–17 and photovoltaics (i.e., zombie dye-sensitized solar cells or DSSCs)18,19 has been heralded owing to (i) their well-established chemistry to tune the photophysical and electrochemical properties and (ii) their highly efficient emission mechanism via thermally activated delayed fluorescence (TADF); the ability to harvest both singlet and triplet excitons leads to virtual lighting device efficiencies of 100%.15,17,20,21 Conversely, the chemistry and the photophysical and electrochemical behaviors of their homologous Ag(I) complexes ([Ag(N^N)(P^P)]+) have attracted much less attention.12,22–33 However, research interest in silver(I) complexes is back with the promise to overcome the limitations of their copper(I) homologues arising from their labile coordination sphere.24 In short, their emitting excited state features a strong metal-to-ligand charge transfer (MLCT) character, in which the metal ion center is formally oxidized (d9 configuration), leading to a square-planar coordination geometry.34 This flattening distortion causes (i) unforeseen changes in the emission features going from powder to solution and to thin films applied to devices,35,36 (ii) a reduction of the photoluminescence quantum yield (ϕ),17,37–39 and (iii) better accessibility of nucleophiles, like solvent molecules, forming non-emissive species during device fabrication.40 In stark contrast, the 4d orbitals of the silver metal center are lower in energy compared to those related to the ligands. Thus, Ag(I) complexes are usually characterized by ligand-centered (LC) emitting excited states with a small MLCT contribution.41 While this usually results in higher stabilities due to the lack of flattening distortion upon excitation,24 the emission mechanism is difficult to predict even for similar heteroleptic [Ag(N^N)(P^P)]+. Indeed, more and more contributions are disclosing complexes with standard emission mechanisms like fluorescence/phosphorescence42,43 and others with, for example, more sophisticated temperature-dependent dual phosphorescence44 and ligand-lock assisted TADF.31,33 Finally, their application in OLEDs and LECs is also starting to flourish, highlighting limitations, such as formation of Ag(0) nanoclusters upon electrochemical stress.22,28
Herein, we expand the above prior-art designing of a three-coordinated Ag(I) complex bearing both an N-heterocyclic carbene (NHC) ligand and 2,2′-bipyridine (bpy) as the N^N ligand – Fig. 1. Previously, our copper(I) complex design proved to be effective for the stabilization of the metal center due to the strong σ-donation provided by the NHC ligand.45–47 However, the rigidity of the bipyridyl ligand and the intermolecular π–π interactions led to low-emissive aggregates in powder form (λem = 639 nm, ϕ ≥ 1% – Fig. S1†).45,48,49 In stark contrast, the same complex design for silver(I) complexes results in a highly emissive powder with a unique emission mechanism. In short, the intermolecular π–π interaction-induced aggregation phenomena activate a phosphorescence mechanism, leading to a remarkable ϕ value of 20% and a long excited state lifetime of around 571 μs. Joint temperature-dependent steady-state and time-resolved spectroscopy and theoretical studies confirmed the presence of two temperature equilibrated triplet excited states with experimental data rendering an energy splitting of 0.08 eV. Finally, this complex also exhibits a stable electrochemical behavior without showing the formation of Ag(0)-nanoclusters upon electrochemical stress. Thus, they were implemented into LECs that showed the expected ion-assisted electrical behavior with remarkable electrochemical stability, but no light response, since the lack of aggregation in the desired smooth and homogeneous active layers leads to the formation of non-emissive excitons. A trigonal Ag(I) complex with NHC and bipyridine ligands shows strong phosphorescence when aggregated into a powder. This aggregation-induced emission is lost in thin films, but the LEC devices show remarkable electrochemical stability.
 | ||
| Fig. 1 The chemical structure, picture of the powder under 305 nm UV lamp irradiation, and synthesis scheme of [Ag(IPr)(bpy)][PF6]. | ||
Results and discussion
Synthesis and structural characterization
At first, IPr (IPr = 1,3-bis(2,6-di-iso-propylphenyl)imidazol-2-ylidene) was selected as the NHC ligand for its well-known steric and σ-donation properties50,51 and its wide use in copper congeners.45–47 Then, following the procedure reported by Nolan and co-workers,51 the [AgCl(IPr)] precursor was prepared in 88% isolated yield. The archetypal [Ag(IPr)(bpy)][PF6] was synthesized using our previously reported procedure for analogous Cu(I) complexes.45 In detail, a mixture of 1 eq. of [AgCl(IPr)] with 1.05 eq. of 2,2′-bipyridine (bpy) was heated up to 78 °C in EtOH for 1 h. Precipitation with a saturated aqueous solution of KPF6 (10 eq.) furnished the expected complex. 1H NMR analysis of the crude reaction showed the presence of 10% of the homoleptic complex [Ag(IPr)2][PF6]. However, two consecutive slow gas-diffusion recrystallizations, from a dichloromethane solution of the crude silver complex using diethyl ether as a light solvent, furnished the pure complex [Ag(IPr)(bpy)][PF6] in 52% isolated yield as suggested by NMR spectroscopy. The coordination of the NHC ligand was confirmed by 13C NMR spectroscopy in the presence of the typical two doublets centered at 186.2 ppm, resulting from the coupling between the carbenic carbon and the two magnetically active isotopes of silver (107Ag and 109Ag).51Next, suitable single crystals were grown from a concentrated CH2Cl2 solution using a slow gas-diffusion technique with diethyl ether as a light solvent. X-ray diffraction analysis was conducted to unambiguously prove the structure. The most relevant values of bond lengths and angles are reported in Table 1 and Fig. 2, while all the structural values and experimental details are provided in the experimental part and in the ESI – Table S1.† In short, the 2,2′-bipyridine ligand and the Ag(I) metal centre formed the expected five-membered chelating ring. With the additional IPr ligand, the expected trigonal geometry for such a complex was also proven – Fig. 2.
| Ag–CNHC [Å] | Ag–N [Å] | N–Ag–N [°] | CNHC–Ag–N [°] | CH⋯Cg [Å] | Cg⋯Cg distance [Å] | Displacement [°] |
|---|---|---|---|---|---|---|
| 2.0723(12) | 2.2928(11) | 71.86(4) | 142.31(4) | 3.09 | 3.82 | 21.26 |
| 2.2944(11) | 145.78(4) |
Then, the Ag–C bond length was measured at 2.0723(12) Å and the two Ag–N bonds at 2.2928(11) and 2.2944(11) Å, in line with data from the literature.51–53 The bite angle of the N^N ligands toward the metal center was found at 71.86(4)° which was smaller by around 10° than in the copper analogue reported previously (80.04(7)°).45 The distances between the hydrogen atoms in the α position of the nitrogen atoms in 2,2′-bipyridine and the centroid of the N-substituted aromatic rings of NHC (CH⋯Cg) was estimated over 3 Å, resulting in the loss of the CH–π interaction that was observed in the copper(I) complexes.45 Nevertheless, long-range intermolecular π–π interactions occurred between the pyridines of the bpy ligand with a centroid–centroid (Cg⋯Cg) distance of 3.82 Å and displacement, defined as the angle between the centroid-ring vector and the ring normal, of 21.3° – Fig. 2.54
Photophysical and computational studies
The most relevant photophysical figures are summarized in Table 2. The UV-Vis absorption features in diluted solution (10−5 M in THF) show two typical structured bands centred at ca. 250 and 290 nm that are assigned to π–π* ligand-centred transitions – Fig. 3A, while a very weak (ϕ < 1%) and structured emission centred at 365 nm is associated with very short excited state lifetimes (τ) in the ns regime. Thus, this emission mechanism is attributed to a ligand-centered fluorescence. In stark contrast, the crystalline powder features a broad emission band centred at 500 nm – Fig. 3B, representing a significant wavelength shift of around 150 nm that is associated with ϕ and τ values of 18% and 571 μs, respectively. This could be attributed to a change of the nature of the excited state going from solution to crystalline powder form caused by the intermolecular π–π interactions described above. To investigate the presence of aggregation induced emission (AIE) phenomena,55–57 we simply force the aggregation phenomena in THF solution (10−5 M) by adding different volume ratios of distilled water (fw) – Fig. 3C. Upon increasing the fw to 70% (v/v), a new low-energy emission band centred at 500 nm starts evolving, while a broad and intense emission similar to that of the crystalline powder was finally noted at fw = 90%. Interestingly, the emission band shape as well as ϕ and τ values nicely match with those noted for powder – Table 2.| Conditions a | Parameters | Data |
|---|---|---|
| a λ ex = 290 nm for emission (λem), lifetime (τ) and quantum yield (ϕ). | ||
| THF solution | λ abs (ε)/nm (104 L mol−1 cm−1) | 258 (1.45), 296 (1.17), 318 (1.32) |
| λ em 298 K nm−1 | 321, 365 | |
| τ 298 K ns−1 | 11.3 | |
| ϕ/% | <1 | |
| Powder | λ em 298 K nm−1 | 498 |
| λ em 77 K nm−1 | 428 | |
| τ 298 K μs−1 | 571 | |
| τ 77 K ms−1 | 13.8 | |
| ϕ/% | 20 | |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v% H2O/THF solution 10 v% H2O/THF solution |
λ em 298 K nm−1 | 502 |
| τ 298 K ms−1 | 464 | |
| ϕ/% | 18 | |
| Spin coated thin film | λ em 298 K nm−1 | 540 |
| τ 298 K μs−1 | 133 | |
| ϕ/% | 2 | |
| Dropcast thin film | λ em 298 K nm−1 | 497 |
| τ 298 K ms−1 | 449 | |
| ϕ/% | 18 | |
The τ values of [Ag(IPr)(bpy)][PF6] are around 500 μs, suggesting that the AIE phenomenon induces a change in the emission mechanism from fluorescence (THF solution) to phosphorescence (aggregated state in solution and powder).
To further study the emission mechanism, temperature-dependent emission was carried for the powder samples going from 77 K to 400 K – Fig. 4. Here, the emission band is red-shifted from 458 nm at 77 K to 498 nm at 300 K, indicating that this complex does not feature the typical TADF mechanism (i.e. a blue-shifted emission at 298 K related to the lowest emitting triplet excited state in thermal equilibrium with its singlet excited state), but a temperature-dependent dual-phosphorescence mechanism – Fig. 4A. This is confirmed by the τ vs. T plot that follows a Boltzmann-type distribution described using eqn (1) – Fig. 4B:44
 | (1) |
 | ||
| Fig. 4 (A) Emission spectra of [Ag(IPr)(bpy)][PF6] in powder at different temperatures – see the legend. (B) τ vs. T plot along the fitted eqn (1) (red). | ||
Finally, we also studied the photoluminescence features in thin films similar to those applied in devices – vide infra. The films were prepared via spin-coating deposition onto a quartz slide from 15 mg ml−1 acetonitrile solutions, forming homogeneous films with no visual aggregates and a roughness below 1 nm – Fig. 5A. In contrast to the photoluminescence in solution and powder, the emission of the films consists of a broad band centered at 540 nm (i.e., 40 nm red-shifted) and exhibits a dramatic reduction of the ϕ and τ values (i.e., <2.0% and 133 μs; Table 2 and Fig. 5A). This is typically ascribed to aggregation induced quenching phenomena occurring upon film formation.36 Here, the formation of aggregates induced by the quick drying of the solvent upon spinning as well as the presence of solvent molecules leads to a non-emissive species, in which short- and long-range intermolecular interactions are different from those noted in their X-ray structures. Indeed, drop-cast films formed by slow evaporation under ambient conditions led to non-homogeneous films with the same photoluminescence features similar to those in powder – Table 2 and Fig. 5B. Though aggregation is key to unlocking the photoluminescence features of [Ag(IPr)(bpy)][PF6], our efforts changing thin-film forming conditions (spinning/blading, solvents, and additives, such as ionic liquids and polymers) did not lead to simultaneously meeting good film quality for devices and photoluminescence features similar to those in powder.
Preparation and characterization of LECs
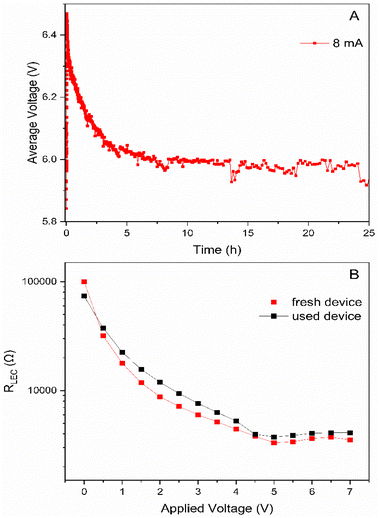
Despite the poor photoluminescence features in thin films, we decided to study the electroluminescence behavior in LECs. In short, a layer (70 nm) of poly(3,4-ethylene dioxythiophene):polystyrene sulfonate (PEDOT:PSS) was spin-coated onto a clean indium-tin-oxide (ITO) electrode-coated glass to increase reproducibility. Then, the active layer (ca. 90 nm) was deposited via spin-coating as explained above. Finally, 100 nm of the aluminum cathode was deposited via physical vapour deposition to finalize the devices’ architecture. The devices were driven at a pulsed current of 8 mA, using a 1 kHz block-wave and 50% duty cycle on 10 mm2 pixels. Unfortunately, all the devices did not light up as expected by the poor photophysical features in thin films, but showed a stable electrical behavior compared to that of the prior-art – Fig. 6A.22,28,44 In detail, the average voltage reduces exponentially until a stable plateau is reached. This is related to the formation of electrical double layers (EDLs) at the electrode interface by the drifting of ions upon applying an external electric field. The second stage is achieved once the ohmic contacts are formed. Here, charge injection is stable and the doped regions are slowly growing until a stable p-i-n junction is formed. This is typically affected by the electrochemical degradation of the oxidized/reduced species that leads to unforeseen changes in the average voltage profile over time that either increase caused by the formation of carrier trappers, (e.g., Cu(II) species in the case of Cu(I)-based devices)58 or decrease caused by the formation of highly conductive degradative compounds, such as Ag(0) nanoclusters.28 Since the voltage profile is stable, we can infer that electrochemical degradation of [Ag(IPr)(bpy)][PF6] in thin-films is not happening. Electrochemical impedance spectroscopy (EIS) assays were performed to further investigate the electrical behavior using a single resistor/capacitor equivalent circuit to analyse the dominant processes upon increasing the applied voltage – Fig. S7.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 Thus, the device resistance profile is characterized by (i) an exponential decrease during the formation of the EDLs at low applied voltages before carrier injection occurs and (ii) a linear regime related to a balanced growth of the p- and n-regions at voltages above the electrochemical band-gap of the complex.60–62 The representative values calculated at 0 V are dielectric constant (εr) and ionic conductivity (σ) that are 13.95 and 8.08 × 10−8 S m−1, respectively. While the εr value is high compared to that of other thin-films with similar d10-complexes,60–62 suggesting that the formation of EDLs is highly favourable in [Ag(IPr)(bpy)][PF6], and the σ values are two-order of magnitude lower, indicating that the growing of the doped regions must be slower than in analogues complexes. However, the most relevant feature is the lack of meaningful changes in the resistance profile of the device upon comparing both fresh and used devices – Fig. 6. This nicely confirms that the electrochemical stability of [Ag(IPr)(bpy)][PF6] thin-films is remarkable.
59 Thus, the device resistance profile is characterized by (i) an exponential decrease during the formation of the EDLs at low applied voltages before carrier injection occurs and (ii) a linear regime related to a balanced growth of the p- and n-regions at voltages above the electrochemical band-gap of the complex.60–62 The representative values calculated at 0 V are dielectric constant (εr) and ionic conductivity (σ) that are 13.95 and 8.08 × 10−8 S m−1, respectively. While the εr value is high compared to that of other thin-films with similar d10-complexes,60–62 suggesting that the formation of EDLs is highly favourable in [Ag(IPr)(bpy)][PF6], and the σ values are two-order of magnitude lower, indicating that the growing of the doped regions must be slower than in analogues complexes. However, the most relevant feature is the lack of meaningful changes in the resistance profile of the device upon comparing both fresh and used devices – Fig. 6. This nicely confirms that the electrochemical stability of [Ag(IPr)(bpy)][PF6] thin-films is remarkable.
Conclusions
This work highlights the bright and dark sides of unique aggregation induced emission (AIE) in archetypal tri-coordinate [Ag(IPr)(bpy)][PF6] complexes. Intermolecular π–π interactions determined in the X-ray structures play a pivotal role in the photophysical behavior of the complex that shows a remarkable ϕ value of 20%. What is more, temperature-dependent lifetime measurement revealed the presence of a phosphorescence behavior related to two thermally interconnected triplet excited states (ΔE of 0.08 eV). This was further supported by computational studies that determined the presence of two very close lying excited triplet states (adiabatic ΔE of 0.008 eV) with a similar delocalized nature over the bipyridine ligands. Unfortunately, this favorable AIE is lost upon forming homogeneous thin-films suitable for lighting devices. Here, films feature very poor emission with ϕ < 2% which leads to dark LECs. However, the electrical behavior of the device is outstanding compared to that of devices with tetragonal heteroleptic Ag(I) complexes, since the formation of Ag(0) nanoclusters is not observed by electrochemical impedance spectroscopy. Finally, the photophysical characteristics and the stability of [Ag(IPr)(bpy)][PF6] open up new possibilities for the exploration of new [Ag(NHC)(N^N)][X] complexes, seeking the optimization of the AIE behavior upon device fabrication.Experimental part
General considerations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), distilled water, ethanol and propan-2-ol as solvents in a warm ultrasonic bath (60 °C, 37–70 Hz) for 15 min at each step. Then, the substrates were dried in N2 flow and treated in a UV-ozone cleaner for 8 min. The aqueous PEDOT:PSS (Clevios P VP.Al4083) was diluted with propan-2-ol (ratio 3
1), distilled water, ethanol and propan-2-ol as solvents in a warm ultrasonic bath (60 °C, 37–70 Hz) for 15 min at each step. Then, the substrates were dried in N2 flow and treated in a UV-ozone cleaner for 8 min. The aqueous PEDOT:PSS (Clevios P VP.Al4083) was diluted with propan-2-ol (ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The resulting solution was sonicated at room temperature for 15 min and then filtered (0.45 μm pore diameter) before the spin-coating onto the clean ITO substrates. The resulting layers were dried on a heating plate at 120 °C and stored in a glovebox (N2 atmosphere, <0.1 ppm O2 and H2O, Ångstrom Engineering). The complex was dissolved in acetonitrile, reaching a concentration of 15 mg ml−1. The solution was sonicated for 10 min and filtered before the deposition. The films were prepared by spin-coating of 60 μl of solution with a three-step deposition program (800 rpm, 30 s; 1500 rpm, 30 s; and 3000 rpm, 10 s) reaching thick layers (95 nm). Then, the active layers were dried in a vacuum overnight. Finally, the aluminum cathode was deposited onto the active layer via physical vapor deposition (Ångstrom Covap evaporator integrated with the glovebox, <1 × 10−6 mbar). A shadow mask was used to define pixels with an area of 10 mm2. Voltage and current performances of the devices were evaluated with a Botest OLT OLED Lifetime-Test System operating in the pulsed mode. A Metrohm μAutolab III potentiostat/galvanostat equipped with a frequency analyzer module (FRA2) was used to carry out electrochemical spectroscopy (ESI) assays. The range of the applied voltage was set between 0 V and 7 V and fitted (Nova 2.1) with the equivalent circuit model reported in the ESI – S18.†
1). The resulting solution was sonicated at room temperature for 15 min and then filtered (0.45 μm pore diameter) before the spin-coating onto the clean ITO substrates. The resulting layers were dried on a heating plate at 120 °C and stored in a glovebox (N2 atmosphere, <0.1 ppm O2 and H2O, Ångstrom Engineering). The complex was dissolved in acetonitrile, reaching a concentration of 15 mg ml−1. The solution was sonicated for 10 min and filtered before the deposition. The films were prepared by spin-coating of 60 μl of solution with a three-step deposition program (800 rpm, 30 s; 1500 rpm, 30 s; and 3000 rpm, 10 s) reaching thick layers (95 nm). Then, the active layers were dried in a vacuum overnight. Finally, the aluminum cathode was deposited onto the active layer via physical vapor deposition (Ångstrom Covap evaporator integrated with the glovebox, <1 × 10−6 mbar). A shadow mask was used to define pixels with an area of 10 mm2. Voltage and current performances of the devices were evaluated with a Botest OLT OLED Lifetime-Test System operating in the pulsed mode. A Metrohm μAutolab III potentiostat/galvanostat equipped with a frequency analyzer module (FRA2) was used to carry out electrochemical spectroscopy (ESI) assays. The range of the applied voltage was set between 0 V and 7 V and fitted (Nova 2.1) with the equivalent circuit model reported in the ESI – S18.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the “Ministère de l'Enseignement Supérieur et de la Recherche”, CNRS (Centre National de la Recherche Scientifique) and the LABEX SynOrg (ANR-11-LABX-0029). S. G. acknowledges the “Région Normandie” (G. G.), the Graduate School of Research XL-Chem (ANR-18-EURE-0020 XL-Chem) for funding.References
- S. Saranya and G. Anilkumar, Copper Catalysis in Organic Synthesis, John Wiley & Sons, Ltd, 2020, pp. 1–5 Search PubMed.
- A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed.
- A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed.
- S. A. Shahzad, M. A. Sajid, Z. A. Khan and D. Canseco-Gonzalez, Synth. Commun., 2017, 47, 735 CrossRef CAS.
- S. Witzel, A. S. K. Hashmi and J. Xie, Chem. Rev., 2021, 121, 8868 CrossRef CAS PubMed.
- A. M. Echavarren, N. Jiao and V. Gevorgyan, Chem. Soc. Rev., 2016, 45, 4445 RSC.
- Q.-Z. Zheng and N. Jiao, Chem. Soc. Rev., 2016, 45, 4590 RSC.
- K. Sekine and T. Yamada, Chem. Soc. Rev., 2016, 45, 4524 RSC.
- A. Barbieri, G. Accorsi and N. Armaroli, Chem. Commun., 2008, 2185 RSC.
- J. Feng, A.-P. M. Reponen, A. S. Romanov, M. Linnolahti, M. Bochmann, N. C. Greenham, T. Penfold and D. Credgington, Adv. Funct. Mater., 2021, 31, 2005438 CrossRef CAS.
- J. Hossain, R. Akhtar and S. Khan, Polyhedron, 2021, 201, 115151 CrossRef CAS.
- C.-W. Hsu, C.-C. Lin, M.-W. Chung, Y. Chi, G.-H. Lee, P.-T. Chou, C.-H. Chang and P.-Y. Chen, J. Am. Chem. Soc., 2011, 133, 12085 CrossRef CAS PubMed.
- M. Osawa, I. Kawata, R. Ishii, S. Igawa, M. Hashimoto and M. Hoshino, J. Mater. Chem. C, 2013, 1, 4375 RSC.
- T. S. Teets, D. V. Partyka, A. J. Esswein, J. B. Updegraff, M. Zeller, A. D. Hunter and T. G. Gray, Inorg. Chem., 2007, 46, 6218 CrossRef CAS PubMed.
- G. U. Mahoro, J. Fernandez-Cestau, J.-L. Renaud, P. B. Coto, R. D. Costa and S. Gaillard, Adv. Opt. Mater., 2020, 8, 2000260 CrossRef CAS.
- E. Fresta and R. D. Costa, J. Mater. Chem. C, 2017, 5, 5643 RSC.
- R. Czerwieniec, M. J. Leits, H. H. H. Homeier and H. Yersin, Coord. Chem. Rev., 2016, 325, 2 CrossRef CAS.
- M. Freitag, Q. Daniel, M. Pazoki, K. Sveinbjörnsson, J. Zhang, L. Sun, A. Hagfeldt and G. Boschloo, Energy Environ. Sci., 2015, 8, 2634 RSC.
- Y. Saygili, M. Stojanovic, H.-S. Kim, J. Teuscher, R. Scopelliti, M. Freitag, S. M. Zakeeruddin, J.-E. Moser, M. Grätzel and A. Hagfeldt, J. Phys. Chem. C, 2020, 124, 7071 CrossRef CAS.
- C. E. Housecroft and E. C. Constable, J. Mater. Chem. C, 2022, 10, 4456–4482 RSC.
- N. Armaroli and H. J. Bolink, Top. Curr. Chem., 2016, 374, 44 CrossRef PubMed.
- O. Moudam, A. C. Tsipis, S. Kommanaboyina, P. N. Horton and S. J. Coles, RSC Adv., 2015, 5, 95047 RSC.
- A. Kaeser, O. Moudam, G. Accorsi, I. Séguy, J. Navarro, A. Bel-bakra, C. Duhayon, N. Armaroli, B. Delavaux-Nicot and J.-F. Nieren-garten, Eur. J. Inorg. Chem., 2014, 8, 1345 CrossRef.
- M. Z. Shafikov, A. F. Suleymanova, R. Czerwieniec and H. Yersin, Chem. Mater., 2017, 29, 1708 CrossRef CAS.
- M. Z. Shafikov, A. F. Suleymanova, A. Schinabeck and H. Yersin, J. Phys. Chem., 2018, 9, 702 CAS.
- D. Zare, C. Piguet, A. Prescimone, C. E. Housecroft and E. C. Constable, Chem. – Eur. J., 2022, 28, e202200912 CrossRef CAS PubMed.
- J. M. Carbonell-Vilar, E. Fresta, D. Armentano, R. D. Costa, M. Viciano-Chumillas and J. Cano, Dalton Trans., 2019, 48, 9765 RSC.
- E. Fresta, J. M. Carbonell-Vilar, J. Yu, D. Armentano, J. Cano, M. Viciano-Chumillas and R. D. Costa, Adv. Funct. Mater., 2019, 29, 1901797 CrossRef.
- A. V. Artem’ev, M. Z. Shafikov, A. Schinabeck, O. V. Antonova, A. S. Berezin, I. Y. Bagryanskaya, P. E. Plusnin and H. Yersin, Inorg. Chem. Front., 2019, 6, 3168 RSC.
- M. Z. Shafikov, R. Czerwieniec and H. Yersin, Dalton Trans., 2019, 48, 2802 RSC.
- T. Teng, K. Li, G. Cheng, Y. Wang, J. Wang, J. Li, C. Zhou, H. Liu, T. Zou, J. Xiong, C. Wu, H.-X. Zhang, C.-M. Che and C. Yang, Inorg. Chem., 2020, 59, 12122 CrossRef CAS PubMed.
- M. Calvo, O. Crespo, M. C. Gimeno, A. Laguna, M. T. Oliván, V. Polo, D. Rodríguez and J.-M. Sáez-Rocher, Inorg. Chem., 2020, 59, 14447 CrossRef CAS PubMed.
- J.-H. Jia, D. Liang, R. Yu, X.-L. Chen, L. Meng, J.-F. Chang, J.-Z. Liao, M. Yang, X.-N. Li and C.-Z. Lu, Chem. Mater., 2020, 32, 620 CrossRef CAS.
- C. Förster and K. Heinze, Chem. Soc. Rev., 2020, 49, 1057 RSC.
- E. Fresta, G. U. Mahoro, L. M. Cavinato, J.-F. Lohier, J.-L. Renaud, S. Gaillard and R. D. Costa, Adv. Opt. Mater., 2022, 10, 2101999 CrossRef CAS.
- E. Fresta, G. Volpi, C. Garino, C. Barolo and R. D. Costa, Polyhedron, 2018, 140, 129 CrossRef.
- M. Iwamura, S. Takeuchi and T. Tahara, Acc. Chem. Res., 2015, 48, 782 CrossRef CAS PubMed.
- M. W. Mara, K. A. Fransted and L. X. Chen, Coord. Chem. Rev., 2015, 282–283, 2 CrossRef CAS.
- H. Yersin, R. Czerwieniec, M. Z. Shafikov and A. F. Suleymanova, ChemPhysChem, 2017, 18, 3508 CrossRef CAS PubMed.
- C. T. Cunningham, J. J. Moore, K. L. H. Cunningham, P. E. Fanwick and D. R. McMillin, Inorg. Chem., 2000, 39, 3638 CrossRef CAS PubMed.
- M. Z. Shafikov, A. F. Suleymanova, R. Czerwieniec and H. Yersin, Inorg. Chem., 2017, 56, 13274 CrossRef CAS PubMed.
- M. Barwiolek, A. Wojtczak, A. Kozakiewicz, R. Szczesny, M. Babinska, L. Skowronski and E. Szlyk, New J. Chem., 2018, 42, 18559 RSC.
- A. Castiñeiras and R. Pedrido, Inorg. Chem., 2009, 48, 4847 CrossRef PubMed.
- S. Lipinski, L. M. Cavinato, T. Pickl, G. Biffi, A. Pöthig, P. B. Coto, J. Fernández-Cestau and R. D. Costa, Adv. Opt. Mater., 2023, 11, 2203145 CrossRef CAS.
- R. Marion, F. Sguerra, F. Di Meo, E. Sauvageot, J.-F. Lohier, R. Daniellou, J.-L. Renaud, M. Linares, M. Hamel and S. Gaillard, Inorg. Chem., 2014, 53, 9181 CrossRef CAS PubMed.
- M. Elie, F. Sguerra, F. Di Meo, M. D. Weber, R. Marion, A. Grimault, J.-F. Lohier, A. Stallivieri, A. Brosseau, R. B. Pansu, J.-L. Renaud, M. Linares, M. Hamel, R. D. Costa and S. Gaillard, ACS Appl. Mater. Interfaces, 2016, 8, 14678 CrossRef CAS PubMed.
- M. Elie, M. D. Weber, F. Di Meo, F. Sguerra, J.-F. Lohier, R. B. Pansu, J.-L. Renaud, M. Hamel, M. Linares, R. D. Costa and S. Gaillard, Chem. – Eur. J., 2017, 23, 16328 CrossRef CAS PubMed.
- V. A. Krylova, P. I. Djurovich, M. T. Whited and M. E. Thompson, Chem. Commun., 2010, 46, 6696 RSC.
- K. A. Barakat, T. R. Cundari and M. A. Omary, J. Am. Chem. Soc., 2003, 125, 14228 CrossRef CAS PubMed.
- D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 2000, 100, 39–92 CrossRef CAS PubMed.
- P. de Frémont, N. M. Scott, E. D. Stevens, T. Ramnial, O. C. Lightbody, C. L. B. Macdonald, J. A. C. Clyburne, C. D. Abernethy and S. P. Nolan, Organometallics, 2005, 24, 6301 CrossRef.
- S. D. Adhikary, A. Mondal, H. K. Kisan, C. W. Bielawski and J. Dinda, Appl. Organomet. Chem., 2020, 34, e5335 CrossRef.
- L. Jhiulki, R. Purkait, H. K. Kisan, V. Bertolasi, A. A. Isab, C. Sinha and J. Dinda, Appl. Organomet. Chem., 2020, 34, e5663 CrossRef.
- C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740–1741 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- M. D. Weber, E. Fresta, M. Elie, M. E. Miehlich, J.-L. Renaud, K. Meyer, S. Gaillard and R. D. Costa, Adv. Funct. Mater., 2018, 28, 1707423 CrossRef.
- S. B. Meier, D. Hartmann, A. Winnacker and W. Sarfert, J. Appl. Phys., 2014, 116, 104504 CrossRef.
- B. M. D. Puscher, M. F. Aygüler, P. Docampo and R. D. Costa, Adv. Energy Mater., 2017, 7, 1602283 CrossRef.
- M. D. Weber, M. Viciano-Chumillas, D. Armentano, J. Cano and R. D. Costa, Dalton Trans., 2017, 46, 6312 RSC.
- E. Fresta, M. D. Weber, J. Fernandez-Cestau and R. D. Costa, Adv. Opt. Mater., 2019, 7, 1900830 CrossRef CAS.
- A. Sillen and Y. Engelborghs, Photochem. Photobiol., 1998, 67, 475 CrossRef CAS.
- C. Ullrich, Time-Dependent Density-Functional Theory: Concepts and Applications, Oxford University Press, 2012 Search PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- D. Andrae, U. Häußermann, M. Dolg, H. Stoll and H. Preuß, Theor. Chim. Acta, 1990, 77, 123 CrossRef CAS.
- S. G. Balasubramani, P. G. Chen, S. Coriani, M. Diedenhofen, M. S. Frank, Y. J. Franzke, F. Furche, R. Grotjahn, M. E. Harding, C. Hättig, A. Hellweg, B. Helmich-Paris, C. Holzer, U. Huniar, M. Kaupp, A. Marefat Khah, S. Karbalaei Khani, T. Müller, F. Mack, B. D. Nguyen, S. M. Parker, E. Perlt, D. Rappoport, K. Reiter, S. Roy, M. Rückert, G. Schmitz, M. Sierka, E. Tapavicza, D. P. Tew, C. van Wüllen, V. K. Voora, F. Weigend, A. Wodyński and J. M. Yu, TURBOMOLE: Modular program suite for ab initio quantum-chemical and condensed-matter simulations, J. Chem. Phys., 2020, 152, 184107 CrossRef CAS PubMed.
- TURBOMOLE V7.6 2022, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007; available from https://www.turbomole.com.
- E. van Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1993, 99, 4597 CrossRef CAS.
- E. van Lenthe, E. J. Baerends and J. G. Snijders, Chem. Phys., 1994, 101, 9783 CAS.
- J. D. Rolfes, F. Neese and D. A. Pantazis, J. Comput. Chem., 2020, 41, 1842 CrossRef CAS PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73 CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057 RSC.
- F. Neese, J. Chem. Phys., 2005, 122, 034107 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2347259. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt01056f |
| This journal is © The Royal Society of Chemistry 2024 |