Open Access Article
Open Access ArticleRecent advances and perspectives for intercalation layered compounds. Part 2: applications in the field of catalysis, environment and health
Chiara
Bisio
 *ab,
Jocelyne
Brendlé
*ab,
Jocelyne
Brendlé
 *c,
Sébastien
Cahen
*c,
Sébastien
Cahen
 d,
Yongjun
Feng
d,
Yongjun
Feng
 e,
Seong-Ju
Hwang
e,
Seong-Ju
Hwang
 f,
Morena
Nocchetti
f,
Morena
Nocchetti
 *g,
Dermot
O'Hare
*g,
Dermot
O'Hare
 h,
Pierre
Rabu
h,
Pierre
Rabu
 i,
Klara
Melanova
i,
Klara
Melanova
 j and
Fabrice
Leroux
j and
Fabrice
Leroux
 *k
*k
aDipartimento di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale, Viale Teresa Michel 11, 15121 Alessandria, AL, Italy. E-mail: chiara.bisio@uniupo.it
bCNR-SCITEC Istituto di Scienze e Tecnologie Chimiche “Giulio Natta”, Via C. Golgi 19, 20133 Milano, MI, Italy
cInstitut de Science des Matériaux de Mulhouse CNRS UMR 7361, Université de Haute-Alsace, Université de Strasbourg, 3b rue Alfred Werner, 68093 Mulhouse CEDEX, France. E-mail: jocelyne.brendle@uha.fr
dInstitut Jean Lamour – UMR 7198 CNRS-Université de Lorraine, Groupe Matériaux Carbonés, Campus ARTEM – 2 Allée André Guinier, B.P. 50840, F54011, NancyCedex, France
eState Key Laboratory of Chemical Resource Engineering, Beijing Engineering Center for Hierarchical Catalysts, Beijing University of Chemical Technology, No. 15 Beisanhuan East Road, Beijing, 100029, China
fDepartment of Materials Science and Engineering, College of Engineering, Yonsei University, Seoul 03722, Republic of Korea
gDepartment of Pharmaceutical Sciences, University of Perugia, Via del Liceo 1, 06123 Perugia, Italy. E-mail: morena.nocchetti@unipg.it
hChemistry Research Laboratory, University of Oxford Department of Chemistry, 12 Mansfield Road, Oxford, OX1 3TA, UK
iInstitut de Physique et Chimie des Matériaux de Strasbourg, CNRS – Université de Strasbourg, UMR7504, 23 rue du Loess, BP43, 67034 Strasbourg cedex 2, France
jCenter of Materials and Nanotechnologies, Faculty of Chemical Technology, University of Pardubice, Studentská 95, 532 10 Pardubice, Czech Republic. E-mail: klara.melanova@upce.cz
kInstitut de Chimie de Clermont-Ferrand, Université Clermont Auvergne, UMR CNRS 6296, Clermont Auvergne INP, 24 av Blaise Pascal, BP 80026, 63171 Aubière cedex, France. E-mail: fabrice.leroux@uca.fr
First published on 18th June 2024
Abstract
Intercalation compounds represent a unique class of materials that can be anisotropic (1D and 2D-based topology) or isotropic (3D) through their guest/host superlattice repetitive organisation. Intercalation refers to the reversible introduction of guest species with variable natures into a crystalline host lattice. Different host lattice structures have been used for the preparation of intercalation compounds, and many examples are produced by exploiting the flexibility and the ability of 2D-based hosts to accommodate different guest species, ranging from ions to complex molecules. This reaction is then carried out to allow systematic control and fine tuning of the final properties of the derived compounds, thus allowing them to be used for various applications. This review mainly focuses on the recent applications of intercalation layered compounds (ILCs) based on layered clays, zirconium phosphates, layered double hydroxides and graphene as heterogeneous catalysts, for environmental and health purposes, aiming at collecting and discussing how intercalation processes can be exploited for the selected applications.
1. Introduction
Intercalation is a way to specifically tune the structure, composition, and physico-chemical properties of materials by exploiting interactions between a host matrix (generally a 2D-layered material) and guest species of various chemical natures. Intercalation materials thus represent a broad class of compounds generated by the reversible insertion of guest species, which can range from ions to organic species, into a layered host matrix that can have either an amorphous or crystalline structure. As a result of the intercalation procedure, the structure of the host normally remains unmodified, or it is only slightly modified in the guest–host complex. The intercalation reaction can be chemically or thermally reversible, and it can be carried out using different approaches including insertion and introduction or topotactic exchange of atomic or molecular species inside the host matrix, thus allowing a controlled and systematic tuning of the physico-chemical properties.As a general feature, intercalation layered compounds (ILCs) prepared by the different methods described in the first part of this perspective paper are a class of very flexible compounds because the appropriate choice of host and guest species results in the possibility of tuning their final properties, thus spreading their potential application to numerous technological fields.
These materials can be suitable for many applications, for instance as catalysts, sorbents, electrochromic displays, electrodes for secondary batteries (Li-ion batteries), components for fuel cells and drug delivery systems. The different intercalation approaches can be indeed used for: (i) modifying the catalytic, optic, electronic and magnetic performances of different host structures through the introduction of specific functionalities; (ii) thermally stabilising and protecting the guest species from light and oxygen; (iii) developing multifunctional carriers for specific applications.
In this review, representative examples from the state of the art of the last 8 years (since 2015) of the application of intercalation materials in the fields of catalysis, environment, health and polymeric composites are reported.
This perspective is especially focused on selected cationic and anionic layered materials as host matrices for the preparation of intercalation compounds.
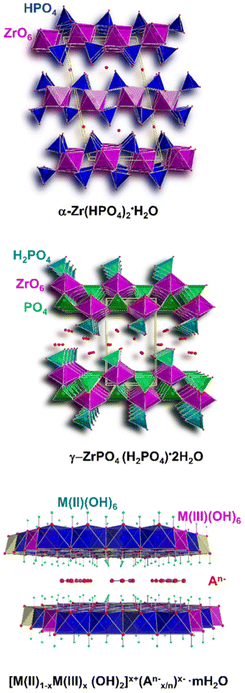
Emphasis is placed on the use cationic layered materials such as clays, of both natural and synthetic origin (Fig. 1), and zirconium phosphates (ZrP). Layered double hydroxides (LDHs) are considered for the class of anionic layered materials (Fig. 2). Some recent examples of ILCs derived from graphene are also reported.
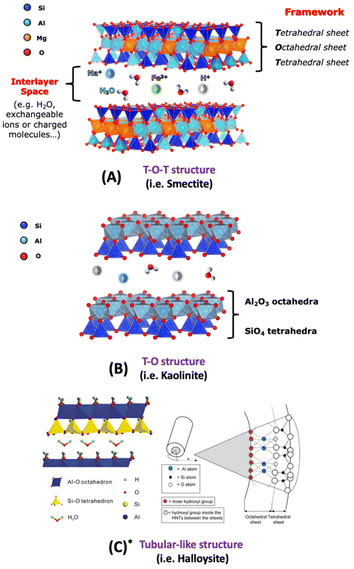 | ||
| Fig. 1 Schematic view of different clay structures: T-O-T (A), T-O (B) structures and tubular-like structure (C* is adapted with permission from ref. 1, Copyright 2020 MDPI). | ||
The number of scientific manuscripts reporting solids based on clays, ZrP, LDH for the applications of interest (e.g. heterogeneous catalysis, environment and health) in the last decade deserves great attention. The amount of papers dealing with the use of selected matrices for the different applications is reported in the histograms of Fig. 3.
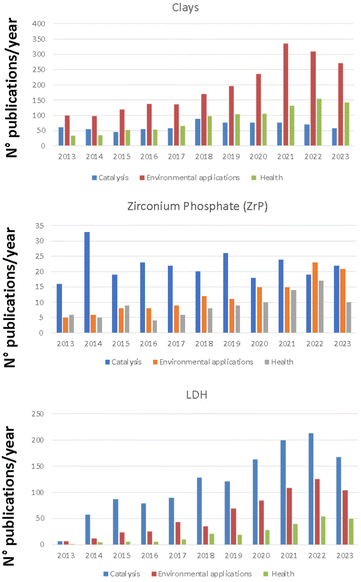 | ||
| Fig. 3 Histograms reporting the amount of publications/year for clays, LDHs and ZrP for the selected applications. Data are obtained from SCOPUS database. | ||
In the following sections, the contributions of the selected solids in the different applications are reported, with the focus of attention on ILCs prepared by using clays, ZrP, LDHs and graphene.
1.1 Catalytic applications of intercalation compounds
In this section particular emphasis will be placed on the description of recent applications of intercalation compounds based on clays, ZrP and LDHs for heterogeneous catalysis.Clay-based catalysts have been used in the conversion of crude oil to biodiesel, and they are employed as catalysts for several reactions such as esterification, addition, transesterification, oxidation, hydrogenation, epoxidation, alkylation, polymerisation, allylation, diazotisation, acylation, rearrangement, isomerisation, cyclisation condensation, organic synthesis etc.3,4 It was pointed out that catalysts derived from layered silicate are regenerable, low cost, and active not only for producing biodiesel but also for other chemical processes such as oxidation reactions, water treatment and photovoltaic applications. These solids showed efficient catalytic potential, allowing a high yield of FAMEs (fatty acid methyl esters) at less harsh reaction conditions, and they were proved to be easily recoverable, highly reusable and thus highly sustainable.2
Among clays, halloysite nanotube (HNT)-based heterogeneous catalysts deserve additional comments. Composite and hybrid materials obtained by the surface modification of halloysite, a natural clay with tubular morphology, are much proposed in the literature. It was observed that the synergy between halloysite and different modifying species (i.e. polymers, dendrimers, porphyrin, metal organic frameworks (MOFs), ionic liquids) allows an increase in the performance of the final hybrid/composite. However, the use of halloysite-based hybrids/composites also has some disadvantages due to the time-consuming multistep production processes, often involving (toxic) solvents/reagents for the large-scale production of these materials.5 Modified halloysites have been also proposed for environmental and catalytic applications. For this latter application, the presence of metal nanoparticles (NPs) is essential to impart catalytic performance. Besides methods allowing the introduction of NPs on the outer surface of halloysite, the methods to embed metallic NPs of small size in the inner part of HNTs have been recently reviewed.6 These methods have the advantage of increasing the resistance of NPs to cleavage and agglomeration of the embedded metallic NPs, thus improving the catalytic activity of the obtained solid.
Jiang et al. prepared Pd/HNTs by exploiting a modification of the hydrophobicity of halloysite by impregnating the solid with CTAB species (see Fig. 4). The proposed method allowed the authors to obtain uniformly dispersed palladium particles (ca. 2 nm), with a suitable ratio of Pd2+/Pd4+ species and appropriate surface acidity: this solid showed improved catalytic activity with respect to the catalyst prepared without adding CTAB.7
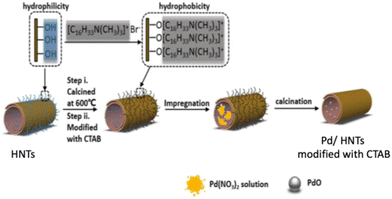 | ||
| Fig. 4 Schematic diagram of the preparation method of Pd/HNTs catalyst. Adapted with permission from ref. 7, Copyright 2020, American Chemical Society. | ||
For methane combustion, high-performance halloysite-supported palladium catalysts have been also proposed through appropriate pre-treatments of the support.8
Cu–Ni-containing halloysite solids prepared by ligand-assisted reduction of Cu2+ and Ni2+ cations followed by annealing have been also tested for the purification of vehicle exhaust gases through catalysed processes.9 For this process, catalysts based on confinement of Cu–Ni species, Pd, and Ag (particle size below 3 nm) have been proposed. It was shown that the nano-confinement allows an increase in the stability of embedded NPs, reducing detachment and sintering, and improving the catalytic reaction kinetics.6
Modification of the clay's chemical composition was also proposed to introduce catalytic active sites into the clay framework, thus leading to active materials for the catalytic decomposition and/or simultaneous detection of toxic molecules, e.g. chemical warfare agents.10–12 In this case, the synergistic presence of proton sites (normally introduced by ion exchange in acidic aqueous solutions) and metal centres (either present naturally in clays or introduced by modifying the preparation steps of the support) are beneficial for the catalytic performances of the final material.
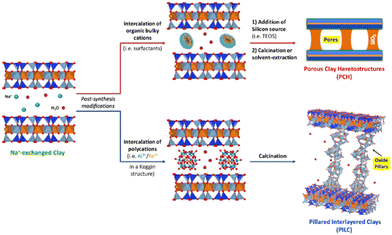
Thermally stable pillared clays (or pillared interlayered clays, PILCs) derive from the intercalation of inorganic polycations in the clay interlayer space. The clay pillaring procedure allowed the preparation of solids mainly based on transition metal species, with great interest in the catalytic purposes particularly required in advanced oxidation processes and chemical transformations. The pillaring procedure allows the introduction of specific catalytic species on the clay's surface and this, together with the improvement in specific surface area and thermal stability, makes these solids suitable for different catalytic reactions (e.g. nitration of chlorobenzene, catalytic combustion of acetone, methyl-ethyl-ketone), oxidation and isomerisation reactions and as Fenton-like systems for the degradation of organic pollutants in wastewaters.13 Clays pillared with TiO2 (Ti-PILC) are characterised by surface acidity higher than other pillared clays, such as Al or Zn-PILC.13 The origins of Brønsted and Lewis acid sites in Ti-PILC have been studied.14 The photocatalytic degradation of organic species (i.e. organic dyes, herbicides and aromatic amines, phenolic compounds, emerging contaminants) over PILCs with TiO2 species has been recently reviewed, with particular emphasis on photocatalysts based on kaolinite, palygorskite, montmorillonite and halloysite.15
Very recently, the use of PILCs for the catalytic reduction of NOx with hydrocarbons at low temperature was reviewed.16 Special interest was paid to metal-supported PILCs, and the catalytic performances of different samples together with the possible reaction mechanisms in relation to the different active sites, metal loading and reaction conditions were reported. It was underlined that Ag- and Cu-based PILC displayed good performances for NOx conversion in the presence of short hydrocarbons (HC) at low temperature. Nevertheless, additional studies are needed to better clarify the HC-SCR reaction mechanisms in the presence of metal-modified PILC catalysts. Major challenges are represented by the preparation of stable metal-modified PILC catalysts for NOx reduction in the presence of H2O and SO2.16
Moreover, TiO2/clay heterostructures for the remediation of water polluted by organic species have been recently proposed.17 In this respect, particular emphasis was placed on the use of phyllosilicates as supports (e.g. montmorillonite, sepiolite, palygorskite, vermiculite, saponite). The introduction of pillars in the clays’ galleries results in a stable porosity leading to heterostructured materials with high specific surface area (SSA) characterised by the presence of micro- and mesopores. The pore architecture and the high SSA have a positive effect on the photocatalytic activity of TiO2/clay materials. Moreover, long-chain hydrophobic cationic surfactants can be exploited to tune the porosity of TiO2-based clays and to produce porous clay heterostructures (PCHs) and delaminated porous clay heterostructures (DCPHs).18–20
These composite materials have been proposed for overcoming the problems related to practical and environmental issues linked to the use of nanosized TiO2 powders. Different clays such as kaolinite, hectorite, saponite, montmorillonite, palygorskite, halloysite and imogolite have been proposed as support materials for TiO2 nanoparticles, thus obtaining solids with high photocatalytic efficiency and easy separation from treated water. The different examples reported in the literature suggested that the combination of clay support characterised by high specific surface areas and adsorption capacity improves the photocatalytic activity of TiO2 nanoparticles and thus the decomposition capacity for organic pollutants. It was pointed out that the anchoring of TiO2 on the clay's surface is useful to reduce the TiO2 agglomeration, thus leading to more active surface sites.15
A schematic representation of the formation of PILC and PCH starting from intercalation processes is reported in Fig. 5.
1.2 Catalytic applications of zirconium phosphate (ZrP)
ZrP can be also considered as a promising material for catalytic applications. ZrP shows very interesting physical and chemical properties, such as high ion-exchange capacity, good chemical and thermal stability, and easy functionalisation. The presence of acidic P-OH groups on the layers makes this solid an efficient catalyst in acid-catalyzed reactions. The acidity and the hydrophobicity of the ZrP surface can be modulated by totally or partially replacing the phosphate groups with other phosphonate groups. The modification of the ZrP surface has also been achieved by exploiting the reactivity of the surface hydroxyl groups with organofunctional silanes that allowed the immobilisation of catalytically active nanoparticles.21,22 Moreover, several catalysts have been prepared considering the ion-exchange capacity of ZrP. Intercalation compounds containing metal ions or metal complexes have shown good performance as catalysts for converting biomass-derived molecules into added-value products such as oxygenated fuels and fine chemicals. In particular, ZrP-Ru(III) and Ni/ZrP (Fig. 6A) showed high catalytic activity towards the oxidation of 5-hydroxymethylfurfural (HMF) under mild conditions.23,24 ZrP intercalated with Ru(II) complex ([(p-cymene)Ru(phen)Cl]Cl) showed higher catalytic performance in selective hydrogenation, with molecular H2, of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of furfural and of other carbonyl compounds into the corresponding alcohols. Experimental study and DFT calculation gave evidence that the catalytic performance can be attributed to the interaction of –POH groups of ZrP and Ru complex, leading to Ru–O–P active species.25 Cu/ZrP catalyst enabled the direct oxidation of phenol, also derived from lignin depolymerisation, to cis,cis-muconic acid that can be further converted into high-added-value chemicals.26
O group of furfural and of other carbonyl compounds into the corresponding alcohols. Experimental study and DFT calculation gave evidence that the catalytic performance can be attributed to the interaction of –POH groups of ZrP and Ru complex, leading to Ru–O–P active species.25 Cu/ZrP catalyst enabled the direct oxidation of phenol, also derived from lignin depolymerisation, to cis,cis-muconic acid that can be further converted into high-added-value chemicals.26
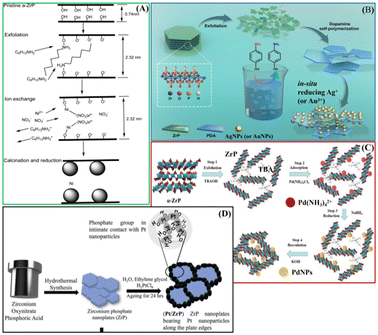 | ||
| Fig. 6 Schematic illustration of preparation steps of the catalysts: (A) Ni/ZrP adapted with permission from ref. 24, Copyright 2018 American Chemical Society; (B) ZrP@PDA/AuNP or ZrP@PDA/AuNP, adapted with permission from ref. 30, Copyright 2020 MDPI; (C) ZrP-PdNPs, adapted with permission from ref. 31, Copyright 2022 Taiwan Institute of Chemical Engineers; (D) Pt/ZrP. Adapted with permission from ref. 36, Copyright 2023 John Wiley and sons. | ||
Composite ZrP/silver-based materials are obtained by exploiting the intercalation properties of ZrP and then used as catalysts for the degradation of organic pollutants. Silver-exchanged ZrP treated with halogenidric acids gave rise to ZrP/AgX (X = Cl and Br) composites able to catalyze the photodegradation of rhodamine B (RhB). The action of ZrP as a source of silver ions led to a reduction of AgCl particle growth and aggregation, thus increasing the number of catalytic active sites. In addition, the –POH groups of ZrP promote the chemisorption of the RhB and then its degradation.27,28 A composite ZrP-polydopamine (ZrP@PDA) was prepared by polymerization of dopamine in the aqueous dispersion of exfoliated ZrP. The catechol groups of PDA reduced the noble metal ions, such as silver or gold, generating metallic NPs on the ZrP@PDA surface. These composites showed very interesting catalytic activity towards the reduction of 4-nitrophenol to 4-aminophenol (Fig. 6B).29,30 The exfoliation/restacking method was exploited to immobilise Pd precursor (Pd(NH3)4Cl2) on α-ZrP nanosheets. The treatment with a reducing agent gave rise to Pd NPs, with an average diameter of 5.17 nm, immobilised on ZrP nanosheets (Fig. 6C). The composite showed good performance as a heterogeneous catalyst for the Heck reaction.31
Due to ZrP's electrochemical inertness it has been studied as a support for transition metals for the electrocatalysis of the oxygen evolution reaction (OER) in alkaline media.32 Ions Fe2+, Fe3+, Co2+, and Ni2+ were intercalated or adsorbed on α-ZrP material. The systems display interesting activity for the OER, requiring between 0.5–0.7 V of overpotential to reach 10 mA cm−2 (the benchmark for OER catalyst activity). It was found that the OER occurs especially on the external surfaces of the nanoparticles, thus the catalytic activity increases upon the exfoliation of ZrP materials.33 Moreover, the OER activity depends on the loading and coverage of cobalt and nickel on ZrPs with different morphologies.34 More recently, mixtures of nickel–iron were confined in ZrP; the hydrated confined environment of the layered structure stabilised Fe-rich compositions, yielding highly active OER catalysts.35
Carbon-free electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells were prepared by growing Pt nanoparticles on ZrP, which is a solid-state proton conductor (Fig. 6D). The Pt/ZrP composites showed high durability and superior performance with respect to commercially available platinum-supported carbon catalysts.36
1.3 Catalytic applications of LDHs
A recent review underlines the versatility of LDHs as anion-exchange frameworks as well as considering their ability to face today's societal issues, such as their high biocompatibility combined with their relatively low impact in terms of biodegradation.37 LDHs are used in photocatalysis, electrocatalysis, CO2 handling, biomass conversion, lowering the anthropogenic effects on the environment as scavenger for pollutants for water and soil treatments, and as additives for polymer and for polymer coatings.The importance of the use of catalysts with reduced size (nano-catalysts) is covered in an interesting review focused on biomass conversion including reduction, oxidation or reforming reactions.38 The tunability of the chemical composition of LDHs, associated with the flexibility to accommodate interleaved guests and a developed surface that can provide exposed and well-dispersed active species (for example metals or nanoparticles in an atomic-level uniform distribution) make LDH-based materials suitable and efficient nano-catalysts.
This is exemplified by de(hydrogenative) transformations and catalytic hydrogenations using transition metals in LDHs,39 and more specifically Ni-based LDH to catalytically convert bio-ethanol to butanol.40 In the latter case, the aldol-condensation to use biomass and produce butanol was optimised from a supported Cu/NiAlOx material in a time-on-stream of 110 h at 280 °C reaching appropriate performance in terms of ethanol conversion, butanol selectivity and catalyst stability. Adapted cation composition as well as particle size can stabilise LDHs even in an acidic medium. For instance, LiAl2 LDH-based sheets prepared by a urea method were covered with NHC-heterocyclic carbene gold anionic complexes for a reaction of hydration of alkynes.41 Another study reports LDH of Mg4Al composition covered this time by silver NP for the alkylation of nitriles, oxindoles and other carboxylic acid derivatives with alcohols.42 In this study NP and the LDH support are co-acting in the cyclisation of N[2-(hydroxymethyl)phenyl]-2-phenylacetamides to yield 3-arylquinolin-2(1H): NP in the dehydrogenation and hydrogenation steps, while the LDH support catalyses the condensation step.
LDHs are also studied as photocatalysts; a recent review covers the latest advances in that domain43 as well as electrocatalysts, mostly for the water-splitting to produce green hydrogen as well as other compound families such as transition metal chalcogenides.44 As far as water electrolysis is concerned, they should possess electrocatalytic activity associated with long-term stability. LDHs rank well for developing such sustainable and clean energy, as discussed in critical reviews45,46 where the relationships between structural defects and electrocatalytic performances are scrutinised, as for Ce-doped NiFe LDH-based material dispersed onto carbon paper47 or NiFe heterostructure nanorods48 for the OER. Other interesting articles mainly focus on LDHs and the possibility for seawater electrolysis, since they are stable in alkaline electrolyte suitable for seawater oxidation at the anode through the OER and not that of chloride ions.49 However, NiFe LDHs-based electrocatalysts for the OER suffer from instability upon cycling, mostly due to the leaching of Fe active sites migrating out of the structure to form ferrihydrite by-product after repetitive potential cycling.50
Among 2D carbon-based materials, iron- and nitrogen-doped graphene appear as promising platinum-free materials for the oxygen reduction reaction (ORR),51 and g-C3N4 and other doped graphenic materials (i.e. single or double doping with N, B, S and P) for ORR, hydrogen evolution (HER), and reports of OER rivalling that of noble metals and transition metal-based catalysts have been reviewed by Chen et al.52
The examples described above are summarised in Table 1. Wherever possible, the catalytic performances of ILCs are compared with those of reference materials (e.g. non-hybridised systems, non-intercalated heterogeneous catalysts).
| Catalyst | Active species | Catalyst preparation | Reaction | Substrate | Catalytic conditions | Products | Ref. |
|---|---|---|---|---|---|---|---|
| Pd/HTNs | Pd | CTAB surface modification, impregnation and calcination | Oxidation | Methane | 0.2 g catalysts with 1 wt% Pd nominal loading; gas feed: 1 vol% CH4, 10 vol% O2 in N2 | Methane conversion: 99% at 425 °C, ca. 195 °C lower than of Pd/HNTs prepared without any surface modification | 7 |
| Flow rate: 100 mL min−1 | |||||||
| T: 425–620 °C | |||||||
| Pd/HNTs | Pt | Surface modification using acid, basic media and surfactants, impregnation, calcination | Oxidation | Methane | 50 mg catalysts CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar (1% Ar (1%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% 20%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79%) 79%) |
Methane conversion depends on the surface treatment. Best performances with Pd/HNTs-NaOH that shows 90% conversion at 373 °C. | 8 |
| Flow rate: 60 mL min−1 | |||||||
| T: 250–450 °C | |||||||
| Cu-Ni/HTNs | Metallic Cu and Ni particles | Wet chemical synthesis | Rreduction | Simulated exhaust gas (equimolar mixture of nitrogen monoxide and carbon monoxide) | 10 mg catalysts, NO (5.00 kPa) and CO (5.00 kPa) T: 275–400 °C | NO conversion to N2: over 90% at 325 °C after 30 min. | 9 |
| Nb/saponite | Nb in framework position | Nb introduction in framework position | Selective oxidation | (2-Chloroethyl)ethylsulfide (CEES) | 20 mg catalyst | CEES conversion: 98% (8 h) | 10 |
| Synthetic saponite clay | Intercalated H+ species | 14 mM CEES; 70 mm 30% aq. H2O2; n-heptane; T: 25 °C | Selectivity to CEESO: 73% | ||||
| Synthetic saponite clay (without Nb and protons): CEES conversion ca. 20% (8 h) | |||||||
| Fe/montmorillonite | Fe3+ and H+ | Ion exchange of montmorillonite natural clay | Selective oxidation | (2-Chloroethyl)ethylsulfide (CEES) | 20 mg catalyst | CEES conversion: >99% over acid sample after 48 h | 11 |
| H/montmorillonite | 14 mM CEES; 70 mm 30% aq. H2O2; n-heptane; T: 25 °C | The parent sample (without any modification): CEES conversion of 7.5% after 48 h | |||||
| Commercial (non modified) sample | |||||||
| TiO2-MMT PLC | TiO2 | Intercalation of Ti alkoxide precursor with supercritical CO2 | Photocatalytic degradation | Toluene and acetaldehyde | Toluene (100 vol ppm) and acetaldehyde (500 vol ppm) in humidified air, 250 W high-pressure mercury lamp | VOCs decomposition rate between 2 and 6 mol min−1 mol−1 TiO2 | 18 |
| TiO2-MMT PLC | TiO2 | Acidic solutions of hydrolyzed Ti alkoxides in the presence of high-molecular-weight polymer | Photocatalytic degradation | Methylene blue (MB) | Aqueous suspensions of MB (250 ml, initial concentration 30 mg l−1) and photocatalyst powder (50 mg), 250 W high-pressure mercury lamp | Maximum removal efficiency of methylene blue is for PIL up to 83% within 90 min | 19 |
| Compared with TiO2 P25 | For P25: degradation efficiency ca. 46% | ||||||
| Multicomponent PCHs | SiO2/Al2O3, SiO2/TiO2 and Si/ZrO2 pillars | Pillaring by surfactant directed methods and template ion exchange method. | Dehydration reactions | Methanol and ethanol | 100 mg catalysts | Methanol conversion to dimethyl ether (DME): between 40 and 77% at 300 °C (best results obtained in the presence of SiO2 pillars) | 20 |
| Gas mixture containing 3.9 vol% of methanol diluted in helium (total flow rate of 20 ml min−1, isothermal saturator at 0 °C) or 5.7 vol% of ethanol diluted in pure helium (total flow rate of 20 ml min−1, isothermal saturator at 20 °C) | Ethanol conversion to diethyl ether (DEE) and ethylene: between 80 and 100% at 300 °C (best results achieved in the presence of Al2O3 pillars) | ||||||
| T: between 100 and 325 °C | |||||||
| ZrP-Ru | Ru(III) | Ion-exchange | Oxidation | 5-Hydroxymethylfurfural | T = 110 °C | 2,5-Furandicarboxylic acid (selectivity: 28%) | 23 |
| P = atmospheric O2 pressure, flow rate of 20 ml min−1 | 2,5-Diformylfuran (selectivity: 62%) | ||||||
| t = 12 h | |||||||
| Ni/ZrP | Ni, Lewis acidic sites of Zr(IV) | Exfoliation, ion-exchange, calcination and reduction | Hydrodeoxygenation | 5-Hydroxymethylfurfural | T = 240 °C | 2,5-Dimethylfuran (yield: 68.1%) | 24 |
| P = 5 MPa H2 | |||||||
| t = 20 h | |||||||
| ZrP-[(p-cymene)Ru(phen)Cl] | Ru–O–P species formed by interaction of cationic Ru(II) complex and P-OH group of ZrP | Exfoliation, ion-exchange | Hydrogenation | Furfural | T = 100 °C | Furfuryl alcohol (selectivity: 99%) | 25 |
| Compared with: Ru/C | P = 1.5 MPa H2 | For Ru/C: furfuryl alcohol (selectivity: 34%) | |||||
| T = 8 h | |||||||
| Cu/ZrP | Cu(II) bound with phosphate groups, Cu(II)–O–P species | Wet impregnation, calcination at 200 °C | Oxidation | Phenol | HCOOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 5 H2O2 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 12 mmol H2O2, 0.2 mmol H3PO4 1, 12 mmol H2O2, 0.2 mmol H3PO4 |
cis,cis-Muconic acid (selectivity: 60%; yield: 40%) | 26 |
| Compared with: Cu(OAc)2 | T = 30 °C | For Cu(OAc)2: cis,cis-muconic acid (selectivity: 55%; yield: 35%) | |||||
| T = 2 h | |||||||
| ZrP/AgCl | Ag@AgCl | Ion-exchange, treatment with HCl | Photodegradation | Rhodamine B | mg AgCl per mL RhB = 0.74 | Chromophore cleavage: 92% in 10 min | 27 |
| Compared with: AgCl | [RhB] = 10−5 M | For AgCl: chromophore cleavage: 57% in 30 min | |||||
| ZrP/AgBr | Ag@AgBr | Ion-exchange, treatment with HBr | Photodegradation | Rhodamine B | mg AgBr per mL RhB = 1.1 | Chromophore cleavage: 90% in 3 min | 28 |
| Compared with: AgCl | Ag@AgBr | [RhB] = 10−5 M | Chromophore cleavage: 91% in 30 min | ||||
| ZrP@PDA/Ag | AgNPs | Reduction and deposition of silver on ZrP-polydopamine | Reduction | 4-Nitrophenol (4NP) | [4NP] = 0.12 mM, [NaBH4] = 38 mM [Ag] = 0.125 μg mL−1 | 4-Aminophenol (apparent rate constant = 34.76 × 10−3 s−1) | 29 |
| Compared with: AgNPs/SNTs | T = 25 °C | For AgNPs/SNTs: 4-aminophenol (apparent rate constant = 38.41 × 10−3 s−1) | |||||
| ZrP@PDA/Au | AuNPs | Reduction and deposition of gold on ZrP-polydopamine | Reduction | 4-Nitrophenol (4-NP) | [4NP] = 0.24 mM, [NaBH4] = 76 mM [Ag] = 0.125 μg mL−1 | 4-Aminophenol (apparent rate constant = 7.4 × 10−3 s−1) | 30 |
| Compared with: Au@MOF-3 | T = 25 °C | For Au@MOF-3: 4-aminophenol (apparent rate constant = 68.8 × 10−3 s−1) | |||||
| Pd@ZrP | PdNPs | Exfoliation, restacking, in situ reduction | Carbon–carbon bond formation | Iodobenzene (IB) and methyl acrylate (MA) | IB = 1.0 mmol, MA = 1.5 mmol NEt3 = 1.5 mmol DMF = 2 mL, T = 100 °C, t = 0.5 h, Pd@ZrP = 15 mg, 0.204 mol% of Pd, NEt3, GVL, 130 °C, 2 h | Methyl cinnamate (conversion: 95.8%) | 31 |
| Compared with: PS-TEA | For PS-TEA: methyl cinnamate (conversion: 91%) | ||||||
| Ni0.1Fe0.9-ZrP | Ni2+/Fe3+ | Ion-exchange reaction | OER | Water | Catalyst loading = 0.125 mg cm−2 | Overpotential = 350 mV at 10 mA cm−2 | 35 |
| Compared with: Ni1Fe2-250 | KOH = 0.1 M | For Ni1Fe2-250: overpotential = 310 mV at 10 mA cm−2 | |||||
| Catalyst loading = 0.17 mg cm−2 | |||||||
| Pt/ZrP | PtNPs | Ageing ZrP with H2PtCl6 | Oxygen reduction reaction (ORR) in polymer electrolyte membrane fuel cells (PEMFC) | Oxygen | PEMFC at an operating potential of 0.60 V | Maximum power densities = 0.95 W cm−2 | 36 |
| Compared with: Pt/C | For Pt/C: maximum power densities = 0.83 W cm−2 | ||||||
| Cu/NiAl-LDH | Cu/NiAlOx | Hydrothermal deposition–precipitation method | Guerbet coupling reaction | Biomass-derived ethanol | T = 280 °C, P = 2 MPa N2 | n-Butanol (conversion = 35%, selectivity = 25%) | 40 |
| T = 110 h | |||||||
| LiAl2-LDH-NHC-Au(I) | Gold/carben complex NHC, Au(I)(NHC-Au(I)) | Direct coprecipitation | Hydration | Alkynes alcohol (i.e. pent-4-ynol) | Catalyst = 0.75 mol% | 5-Hydroxypentan2-one (conversion = 100%) | 41 |
| T = 60 °C | |||||||
| t = 2 h | |||||||
| pH = 3 | |||||||
| Ag/Mg4Al-LDH | AgNPs and LDH | Formation of new C–C bonds from alcohols and C nucleophiles | Nitriles, oxindoles | Nitrile or oxindole = 0.125 mmol alcohol = 0.375 mmol | Isolated products (yield = from 70% to 97%) | 42 | |
| Ag/Mg4Al-LDH = 134 mg, 5 mol% Ag | |||||||
| Mesitylene = 1.5 mL | |||||||
| T = 140 °C under N2 | |||||||
| T = 24 h | |||||||
| NiFeCe-LDH on carbon paper (CP) | Ni2+/Fe3+ | Precipitation with urea | OER | Water | KOH = 1 M | Overpotential = 232 mV at 10 mA cm−2 | 47 |
| Compared with: commercial IrO2 benchmark | 50 μL of the ink (IrO2 = 3 mg, nafion = 15 μL, ethanol = 1 mL) was dropped on CP | For IrO2: overpotential = 451 mV at 10 mA cm−2 | |||||
| KOH = 1 M | |||||||
| NiFe-LDH/Fe1–N–C heterostructure hollow nanorods | NiFe-LDH and Fe1–N–C | Preparation of Fe1–N–C hollow nanorods through a ZIF-phase-transition principle, decoration with NiFe-LDH nanodots | Bifunctional ORR/OER in rechargeable Zn–air batteries | O2/water | Catalyst (carbon cloth) as the air cathode, a zinc plate as the anode, 6 M KOH solution containing 0.2 M Zn(Ac)2 filled between the electrodes as electrolyte. | Power density = 205 mW cm−2 ultralong cyclability up to 400 h | 48 |
| Compared with: Pt/C||IrO2 | For Pt/C||IrO2: power density = 111.6 mW cm−2 |
2. Environmental applications of intercalation materials
This section is devoted to the description of the most representative examples of intercalation compounds based on clays, ZrP and LDHs for the removal of environmental contaminants. An overview of the most significant examples of these materials for environmental applications is also reported in Table 2.| Material | Preparation | Mechanism of pollutant removal | Pollutants removed | Maximum adsorption capacity | Ref. |
|---|---|---|---|---|---|
| Synthetic saponite clays | Hydrothermal synthesis followed by Na+ ion exchange | Ion-exchange | La3+, Gd3+ and Lu3+ | Gd3+: 46.94 ± 1.71 mg g−1 | 56 |
| La3+: 45.21 ± 1.12 mg g−1 | |||||
| Lu3+: 54.89 ± 1.31 mg g−1 | |||||
| From simulated freshwaters: Gd3+: 49.48 ± 1.98 mg g−1 | |||||
| HNTs modified with Fe2O3 | HTNs modified by sol gel method | Electrostatic attraction, ligand exchange, and Lewis acid–base interactions | Phosphate anions | Maximum ads capacity: 5.46 mg g−1 | 62 |
| Fe2O3/attapulgite | Ultrasonic co-precipitation method | Adsorption, electrostatic interactions | Arsenite (AsIII) | ca. 150 mg As(III) per g Fe | 63 |
| Zero valent iron/kaolin | Fe(III) reduction method | Adsorption, reduction | Pb(II) | 440 mg g−1 | 64 |
| Zero valent iron/PILC | Chemical reduction procedure on PILC | Adsorption, reduction | Nitrates | — | 65 |
| Zero valent iron/MMT | Impregnation | Adsorption, reduction | Pb(II) | — | 66 |
| Organo-modified MMT clays | Ion exchange | Adsorption, π–π interaction and hydrophobic affinity | Bisphenol A | Maximum adsorption: 222.2 mg g−1 | 71 |
| Organo-modified MMT clays | Ion exchange with gemini surfactants | Surface adsorption and intraparticle diffusion | p-Nitrophenol | 81.30 mg g−1 (for sample modified with 1,3-bis(dodecyldimethylammonio)-2-hydroxypropane dichloride (BDHP)) | 72 |
| Fe3O4-MWCNT-bentonite | In situ growth process | Physical adsorption | Methylene blue (MB) | MB adsorption: 48.2 mg g−1 | 73 |
| Organo-modified paligorskite and sepiolite | Grafting process | Adsorption mechanism via hydrophobic interaction and/or hydrogen bonds | Methylene blue (MB) and metanil yellow (MY) | 60.00 and 59.78 mg g−1 for grafted sepiolite, for MB and MY, respectively | 76 |
| Organo-bentonite (AOBent)/sodium (SA) composite | Intercalation of sodium alginate in activated organo-bentonite | Hydrophobic interactions | Methylene blue (MB) and methyl orange (MO) | 414 mg g−1 for MB and 116 mg g−1 for MO | 79 |
| Organo-modified acid-treated bentonite | Acid treatment and ion exchange with surfactants | Surface adsorption and intraparticle diffusion | 2,4,5-Trichlorophenol (TC) | TC: 200.6 mg g−1 | 80 |
| Organo-modified montmorillonite PILCs | Grafting process | Ion exchange and coordination interactions | Co2+ ions | Co2+ ions: 60 mg g−1 | 86 |
| AlCr-pillared clays | One pot hydrothermal methods and ion exchange | Physical adsorption | Benzene | Benzene: 48.3 μmol g−1 | 87 |
| ZrP nanoparticles | ZrOCl2, H3PO4 and aqueous Tritron X-100 surfactant | Column separation, ion-exchange | Radioanalytical separation of the no-carrier-added 137mBa from 137Cs | Cs+ = 2.593 meq g−1 | 91 |
| α-ZrP flower-like | ZrOCl2, 8H2O, Na3PO4, at 80 °C for 96 h | Electrostatic interaction and ion-exchange | 90Sr from high-level liquid waste | Sr2+ = 293.43 mg g−1 | 92 |
| ZrP-SO3H | ZrP exfoliation, grafting of (3-mercaptopropyl)trimethoxysilane, oxidation | Column separation, surface chemical adsorption | 90Sr from high-level liquid waste | Sr2+ = 183.21 mg g−1 | 94 |
| γ-ZrP | — | Ion-exchange | Rare earth metal cations | 0.06–0.10 mol per mol of γ-ZrP | 95 |
| γ-ZrP | ZrOCl2·8H2O, NaH2PO4·H2O and NaF ground in an agate mortar, 120 °C for different times | Ion-exchange | Selective adsorption of Cs+ | Cs+ removal efficiency >98% | 96 |
| K2Zr(PO4)2 | Solid-state reaction using ZrO2 and KPO3 (750 °C for 24 h) | Ion-exchange | Separation of 90Sr from nuclear waste | Sr2+ = 603 μmol g−1 | 97 |
| Zr(NaPO4)2·H2O | ZrOCl2·8H2O, Na2HPO4 and NaF were ground in an agate mortar. Heated to 120 °C for 24 h. | Ion-exchange | Heavy metals such as Pb2+, Cu2+, Cd2+, and Tl+ | Highly toxic Tl+ = 1036 mg g−1 | 98 |
| α-Zr-(NH4PO4)2·H2O | ZrOCl2·8H2O, (NH4)2HPO4 and NaF were ground in an agate mortar. Heated to 120 °C for 24 h. | Ion-exchange | Pb2+ and Cu2+ | Pb2+ = 398 mg g−1 | 99 |
| Cu2+ = 144 mg g−1 | |||||
| AM-ZrP, AM = 4-amino-benzo-18 crown 6 | Intercalation of AM in α-ZrP preintercalated with butylamine | Complexation by AM | 90Sr generated in the nuclear fuel cycle | Sr2+ = 320.16 mg g−1 | 100 |
| M-ZrP, M = melamine | Preparation of melamine phosphate (MP). Precipitation reaction between MP and ZrCl4 to obtain M-ZrP | Chelation by M | Pb2+ | Pb2+ = 1000 mg g−1 | 101 |
| γ-ZrP-p-aminoazobenzene | Intercalation of the azo compounds into γ-ZrP | Ion-exchange and complexation by p-aminoazobenzene | Lanthanoid elements | Lanthanoid elements = 370 mg L−1 on average | 102 |
| Zirconium phenylenediphosphonate-phosphate | Hydrothermal synthesis (120 °C for 4 days) of ZrOCl2·8H2O, C6H4(PO3H2)2 and phosphoric acid solution | Ion-exchange | Nd3+, Tb3+ | Nd3+ = 99% of uptake | 103 and 104 |
| Tb3+ = 98% of uptake | |||||
| GO-Zr-P | Addition of zirconium chloride to the GO suspension under sonication | Chemisorption, mainly through surface complexation | Pb2+, Cd2+, Cu2+, Zn2+ | Pb2+ = 363.42 mg g−1 | 105 |
| Cd2+ = 232.36 mg g−1 | |||||
| Cu2+ = 328.56 mg g−1 | |||||
| Zn2+ = 251.58 mg g−1 | |||||
| Fe3O4@ZrP | ZrOCl2, (NH4)2HPO4, sodium lauryl sulphate and Fe3O4 nanoparticles were stirred for 3 h at room temperature | Chemical adsorption | Hg2+ | Hg2+ = 181.8 mg g−1 | 106 |
| ZrP@MPS, MPS = mesoporous polystyrene | MPS was immersed into ethanol solutions containing ZrOCl2, followed by evaporation. The beads were incubated with H3PO4 for 24 | Highly specific inner-sphere coordination of nanoconfined γ-ZrP | Pb2+ | Pb2+ = 180 mg g−1 | 107 |
| PVA/ZrP PVDF membrane | PVA and zirconium ions could be coated onto the PVDF membrane through crosslinking reactions with glutaradehyde. The Zr/PVA modified membrane was immersed into the phosphate solution | Ion exchange | Pb2+ | Pb2+ = 121.2 mg g−1 | 108 |
| Amorphous-ZrP | Solutions of ZrCl4 and H3PO4 were mixed, the precipitated solid was allowed to stand over night | Ion exchange | Nd3+, Dy3+ | Nd3+ = 0.610 meq g−1 | 109 |
| Dy3+ = 0.628 meq g−1 | |||||
| Hydrophilic cellulose-α-ZrP | Dispersion of nano-sized a-ZrP was sprayed onto the surface of the pure cellulose membranes under vacuum filtration | Electrostatic attraction between the heavy metal ions and the high-negatively charged membrane's surface, ion exchange | Pb2+, Ni2+, Cu2+, Zn2+ | Cu2+, Zn2+, Ni2+, Pb2+ = removal efficiency of 97.0, 98.0, 99.5, and 91.5%, respectively | 110 |
| Chitosan-ZrP | Chitosan and ZrP solution was mixed and kept in stirring condition for 24 h | Electrostatic interactions | Cr6+, reactive blue-21 (RB-21), reactive red 141 (RR-141), rhodamine-6G (Rh-6G) | Cr6+ = 311.53 mg g−1 | 111 |
| RB-21 = 457 mg g−1 | |||||
| RR-141 = 435.1 mg g−1 | |||||
| Rh-6G = 438.6 mg g−1 | |||||
| Gelatin-ZrP | Sol–gel method | Ion exchange | Selective adsorption of Cd2+ | Cd2+ removal efficiency >96% | 112 |
| ZrP monolith | ZrOCl2·6H2O, HCl, glycerol, poly(ethylene glycol), polyacrylamide, H3PO4 | Electrostatic interactions | Adsorption of Ag+, Cs+, Sr2+, Zn2+, Cu2+, Pb2+, Cd2+, Fe3+ | Percentage of all cations adsorption% >92% | 113 |
| ZrP nanoflake | ZrOCl2 in ethanol, HCl, H3PO4, stirred thoroughly for 24 h at 60 °C | Strong inner-sphere complexation achieved by Zr–F bonds | Fluoride scavenging | F− = 55.7 mg g−1 | 114 |
| C3N4-ZrP | α-ZrP nanoparticles and C3N4 colloid was dispersed in deionised water and ultrasonicated. The mixture was stirred at 80 °C for 3 h | Photodegradation | RhB | RhB degradation efficiency = 99.95% | 115 |
| ZrP/BMIMCl, BMIMCl = 1-n-butyl-3-methylimidazolium chloride hybrids | Exfoliated ZrP was acid treated mixed with BMIMCl under ultrasonication | Physical and chemical absorption | CO2 | CO2 capture capacities = 0.73 mmol g−1 at 60 °C | 116 |
| MgAl-MoS4-LDH | (MoS4)2− was intercalated in MgAl-NO3via ion exchange reaction | Chemisorption with the adsorption mechanism via M–S bonding | Selective removal of Cu2+, Pb2+, Ag+, and Hg2+ | Hg2+ = 500 mg g−1 | 117 |
| Ag+ = 450 mg g−1 | |||||
| MgAl-CMC, CMC = carboxymethylcellulose | Coprecipitation at low supersaturation of LDH in the presence of CMC | Chemisorption | 4-Methyl-, 4-propyl- and 4-benzylparaben | 4-Methylparaben = adsorption capacity >70% | 118 |
| Cu–Zn oxide/Cu–Zn aluminate | CuZnAl-LDH decomposed to CuZn oxide/CuZn aluminate pre-catalyst. Electrochemical reduction to generates CuZn alloy | Electrochemical CO2 reduction reaction | CO2 | Faradaic efficiency of 88.5% for the conversion of CO2 to C2+ products | 123 |
| (LDH/FAS)n-PDMS, FAS = formamidine sulfinic acid, PDMS = poly(dimethylsiloxane) | Self-assembly of LDH, FAS, followed by spray-coating with PDMS layer | Synergy of enhanced solubility, diffusivity and chemical affinity for CO2 in the sub-nanometre channels | Separation of CO2 | Maximum CO2 selectivity factor (CO2/CH4): 62 | 124 |
| Vertically aligned graphene sheets membran | Antifreeze-assisted freezing technique | Solar thermal purification | Cr3+, Pb2+, Zn2+, Ni2+, Cu2+ | Removal efficiency 99.5% | 125 |
| Au/Pt/g-C3N4 | Calcination-photodeposition technique | Plasmonic photocatalys | Tetracycline hydrochloride (TC) | 93.0% of TC degraded in 3 h | 126 |
2.1 Environmental applications of clays
Clay minerals have been largely studied in the last few decades as solid adsorbents to remove heavy metals with high toxicity (i.e. cadmium, copper, lead, zinc, chromium(VI) but also mercury, cobalt, manganese, chromium(III)), organic dyes and other pollutants (i.e. antibiotics, per- and polyfluoroalkyl substances, organic species…) from aqueous solutions and industrial wastewaters.53–57 Natural clays are used because of their non-toxic nature, low cost, wide availability, and high adsorption capabilities. Examples of clay modifications to allow the detection of specific anions are also given.58Natural clays are very often modified by using thermal treatments, acid washing, pillaring strategies, or through the intercalation of organic species in the galleries, aiming at improving adsorption properties. Materials such as clay nanosheets, nanotubes, nanorods and clay-supported nanoparticles are reported.59 In this respect, interesting recent approaches are linked to clay exfoliation processes to obtain low-dimensional clays (with 1D or 2D morphology). This method is used to fully expose adsorption sites and to increase the specific surface area of the adsorbents.60,61
Moreover, coupling agents and/or polymers are used to create nano-clay-based composites with a porous structure allowing exploitation of the exposed adsorption sites and/or introduction of functional groups aiming at increasing the affinity to heavy metals and toxic dyes.59
Clays have been also proposed as supports for nanoparticles of metals and metal oxides (i.e. iron and copper oxides, TiO2, ZnO, Al2O3), thus obtaining more effective adsorbing agents. As an example, the presence of Fe3O4 on halloysite nanotubes has been proposed for treating the excess of phosphate in water media: it was reported that the supported metal species improves the clay surface charge density thus leading better phosphate control.62 The presence of the clay seems to improve the adsorption capacity of the pristine Fe2O3 species towards arsenite species.63
Supported metal oxide species are also useful for the degradation of toxic species. The case of nanoparticle-sized zero-valent iron (normally named nZVI or nFe0) deserves special attention in wastewater and groundwater remediation because of its ability in the degradation and removal of different metals (i.e. As, Cr(VI)), Pb and other toxic pollutants.64–66
The combination of nZVI and clay support is a positive strategy to avoid the aggregation of metal species, thus allowing the development of effective systems for water remediation. The use of these composite systems was systematically reviewed in 2017.67
The preparation and use of several types of clay-based composite materials for contaminant remediation (with emphasis on heavy metals, nitrate, selenate, dyes, nitroaromatic compounds, polybrominated diphenyl ethers and chlorinated compounds) are also reported. Data related to an extended use of the type of nanocomposites for the removal of chlorinated volatile organic compounds from soils are also discussed, and it was shown that concentration of pollutant species is strongly reduced.68 Nevertheless, further studies to clarify the mechanism of degradation of various contaminants on nZVI/clay composites (i.e. adsorption, oxidation, reduction and precipitation processes) are still needed to optimise the preparation and application conditions of these materials.
Intercalation of natural clays with surfactants (i.e. quaternary ammonium cations (QACs)) aiming at improving the adsorption capacity for water pollutants has been also reviewed.69 Introduction of QACs allows modification of the hydrophobicity of the clay surface and expansion of the interlayer space of 2D materials. Different types of surfactants have been already proposed for this use (i.e. organic surfactants with benzyl, phenyl, tetramethylammonium (TMA), trimethylphenylammonium (TMPA), hexadecyltrimethylammonium (HDTMA), cetyltrimethylammonium (CTMA)).70 The use of Gemini surfactants, characterised by the presence of both aromatic rings and alkyl chain, was also reported for the remediation of water containing pollutants such as bisphenol, p-nitrophenol and triclosan.71,72
Very recently, the removal of methylene blue from aqueous solutions has been carried out by using Fe3O4-multiwalled carbon nanotubes–bentonite composite material.73
The anion exchange capacity of clays can be exploited by using physical (i.e. thermo-activation processes) and chemical modifications devoted to modifying the interlayer properties of these materials. Chemical activation includes metal or surfactant impregnation and amine grafting. All these methods have, as a general scope, the enrichment of the clay's surface with positive charges that can be exploitable to interact with anions. The results of these modification methods on nitrate removal have been recently reviewed.74 Clay modification for the removal of anionic dyes was also recently discussed.75,76
Magnetic and mechano-chemical modifications of clays have also been proposed for improving the recovery of clays after toxic dye adsorption and/or to improve their adsorption capacity.77 In the same review, the influence of different physico-chemical properties of modified clays (i.e. surface area, particle dimensions, morphology, electrical charges, zeta potential, charge density and swelling properties) on the adsorption of synthetic dyes while varying several parameters such as pH, ionic strength, dye concentration, contact time, and temperature, were reported.
Clays treated in acid media and then modified with polymers, surfactants (both anionic and cationic) or materials derived from the combination of clays with magnetic species and polymers and intercalated surfactants and polymers have been proposed for the recovery of heavy metals and inorganic ions, organic dyes, antibiotics, phenolic compounds.78–80
Polymer-amended bentonites for application as barriers to limit the contaminants’ diffusion in solids have been recently reviewed, with particular emphasis on the different types of modification and application fields.81
These composite materials have generally excellent impermeability properties that are related to the formation of specific bentonite/polymer intercalated and exfoliated structures. Their applicability as a barrier can be exploited in the presence of inorganic salts, heavy metal species and organic contaminants.
Biochar (BC)/clay composites have been also proposed for the adsorption of both inorganic (i.e. heavy metals such as Cs, Cr(VI), Zn, nitrates, NH4+…) and organic contaminants (i.e. dyes, antibiotics, herbicides…) in soil and water.82
BCs are very often produced through low-cost methods and have the potential capability for the removal of environmental contaminants. The combination of BC with clay mineral has been therefore proposed in the literature to improve the SSA and thus the adsorption efficiency of BC. These composites can be obtained essentially by using pre-treatment83 or post-treatment methods.84 From reports in the literature it emerges that the synergic combination of clays and BC allows low-cost materials with better capacities for water and soil remediation with respect to the parent constituents to be obtained, because of improved specific surface area and high porosity, chemical, thermal and mechanical stability.
The synthesis steps together with physico-chemical properties, data related to toxicity, and the regeneration ability of various types of modified clay for environmental purposes have been recently reviewed.78
The use of pillared clays for environmental applications also deserves some comment.
Among the different pillaring agents, inorganic ligands such as hydroxo and chloro ligands, metal complex ions with organic ligands and polyhydroxy cations (such as AlCl3·6H2O, ZrOCl2·8H2O) metal ions, metal complexes or neutral particles (such as silica sol particles) are reported. The presence of metal oxide pillars (formed upon the calcination step) in the interlamellar space of clays results in a modification of the thermal and hydrothermal stability of the clays. The use of pillared clays for environmental applications has been recently reviewed.85 Applications include the removal of heavy metals from wastewaters,86 the adsorption of VOCs and gases such as methane, ethane, benzene, CO2 coming from industrial activities,87 and catalytic applications.
Aerogels derived from clays, characterised by a porous microstructure, have been proposed in the last decade for their interesting thermal retardant and adsorption properties that are associated with low toxicity, low cost, and unique structural properties. In particular, montmorillonite (MMT)-based aerogels have been studied to improve their adsorption properties by modifying their preparation method (Fig. 7). A concise review on the possible use of MMT-aerogels for environmental remediation recently appeared in the literature.88
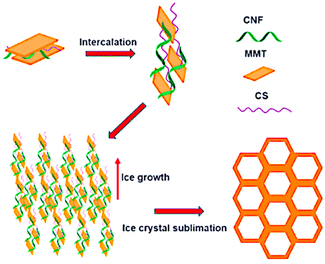 | ||
| Fig. 7 Schematic view of the mechanism of the pore structure formation of the MMT–chitosan aerogels. Reproduced with permission from ref. 88, Copyright 2023 Elsevier. | ||
2.2 Environmental applications of ZrP
Due to the presence of hydroxyl groups with acid properties, ZrP shows a high ion exchange capacity, metal ion selectivity and fast kinetics. Its ability to withstand heat, ionizing radiation, acidic media and an oxidizing environment makes it a very promising material for sorption of radioactive and heavy metal cations.89,90 Special attention has been paid to the removal of Cs+ and Sr2+ cations (because 90Sr and 137Cs are present in high-level liquid nuclear waste) and highly toxic Pb2+, Hg2+ and Cd2+ ions. With the increasing use of rare earth elements in industry in recent times, the need to remove them from contaminated water is also increasing.The ion exchange capacity of ZrP nanoparticles was found to increase with decreasing particle size.91 Recently, there were some attempts to affect Sr2+ adsorption by changing particle morphology (nanoflowers92 or spheres93) or by surface modification with SO3H groups.94 Another approach is based on the use of ZrP derivatives with greater gallery height, such as γ-ZrP (for Cs+ and rare earths),95,96 α-K2Zr(PO4)2 (for Sr2+),97 α-Na2Zr(PO4)2·H2O (for heavy metal cations)98 and α-(NH4)2Zr(PO4)2·H2O (for Pb2+ and Cu2+).99 The sorption capacity of ZrP can be enhanced by its combination with organic molecules with high metal affinity. For example, intercalation of 4-amino-benzo-18-crown-6, which has a strong complexing ability for Sr2+, into α-ZrP leads to a composite with excellent stability under acid and radioactive conditions and an adsorption capacity exceeding other similar zirconium adsorbents.100 Melamine zirconium phosphate,101 synthesized by the addition of zirconium tetrachloride to melamine phosphate solution, was tested for adsorption of Pb2+, Hg2+ and Cu2+. The sorption ability of γ-ZrP towards rare earth metals can be significantly enhanced by intercalation of p-aminoazobenzene.102 Clearfield's group reported the enhanced ability of mixed zirconium phenylenediphosphonate-phosphate to take up Nd and Tb cations, where the selectivity for Nd3+ depends on the amount of phosphate.103,104
Graphene oxide-zirconium phosphate nanocomposite, prepared by dropwise addition of zirconium chloride to a sonicated graphene oxide suspension, with the subsequent addition of NaH2PO4, was tested for the removal of Pb2+, Cd2+ and Cu2+ cations.105 Fe3O4@ZrP nanocomposite, prepared in a similar way, was efficiently used for Hg2+ removal from solution.106 An important benefit of this adsorbent is its simple separation by magnetic power.
The abovementioned adsorbents based on ZrP were tested for metal exchange in batch experiments at a laboratory scale, but these materials are not suitable for column operation on a large scale. To overcome this problem, composites with various polymers were tested. α-ZrP and γ-ZrP encapsulated into mesoporous polystyrene107 or zirconium phosphate-modified polyvinyl alcohol-poly(vinylenedifluoride) membrane108 demonstrate promising sorption performance for Pb2+. Amorphous ZrP nanoparticles exhibit promising adsorption capacity and selectivity for Dy and Nd cations.109 Recently, biocomposites of ZrP with cellulose,110 chitosan,111 and gelatin112 were used for the sorption of heavy metals. Another elegant way to make ZrP suitable for large-scale application is the preparation of ZrP monoliths combining micro- and mesopores.113
All the abovementioned examples describe ZrP as a cation adsorbent, but α-ZrP nanoflakes were also used as a fluoride scavenger. Preferable fluoride adsorption was ascribed to the formation of Zr–F complexes.114
Another relevant source of environmental pollution is organic dyes. Organic dyes may have a positive charge or a group that can be protonated and intercalated by zirconium phosphate, but ZrP can also act as a support for species with photocatalytic properties such as titania, silver nanoparticles or graphene oxide and carbon nitrides.90 Rhodamine B was successfully decomposed using zirconium phosphate/silver/silver halide27 or an α-ZrP carbon nitride composite115 under ultraviolet light. A ZrP-gelatin nanocomposite was able to decompose methylene blue and Fast green under solar light.112 A ZrP-chitosan nanocomposite was used for the sorption of several types of organic dye (copper phthalocynanine dye – reactive blue-21, azo dye reactive red 141, and xanthene dye-rhodamine-6G), and can also serve as a catalyst for the hydrogen peroxide degradation of these dyes.94
Co-assembled ZrP nanosheet/1-n-butyl-3-methylimidazolium chloride hybrids could serve as highly efficient CO2 absorbents.116
2.3 Environmental applications of LDHs
As has been known for decades, ion exchange compounds present properties to trap or release species according to their affinity. Such chemical speciation is often imposed by the charge of the species present. However, the uptake of ions incompatible with the exchanger is possible, as shown by an elegant approach which circumvents the problem of charge compatibility by first intercalating a complexing guest species MoS42− into LDH with respect to ions of opposite charge such as Co2+, Ni2+, Zn2+ < Cd2+ ≪ Pb2+ < Cu2+ < Hg2+ < Ag+, ranked by their selectivity of removal by the hybrid LDH.117 Due to its high adsorption capacity, LDH/MoS42− is very well positioned for the remediation of water polluted by heavy metals. The interlayer species of LDHs may be useful for the adsorption of contaminants. This is the case of carboxymethylcellulose (CMC) as an interlayer anion to sorb parabens.118 Interestingly, the hybrid CMC LDH can be regenerated at least five times without any pronounced loss of efficiency. LDHs can photocatalytically degrade pollutants such as dyes and nitro-compounds, as recently reviewed.119 Specifically Fenton, photoFenton, and electro-Fenton reactions are of great interest in advanced oxidation processes to decontaminate wastewater, and LDH-based frameworks including FeMn as cation in their composition compare well with other materials such as spinel-type, perovskite-type or MOF.120In addition to wastewater treatment and the role played by LDH as a scavenger, some new trends are appearing concerning CO2 photocatalytic reduction121 and capture for limiting anthropogenic pollution.122
To face the new challenge of carbon neutrality, one possibility is to decompose CO2 electrocatalytically, as this also addresses the energy domain by producing a renewable fuel.123 A template based on Cu–Zn–Al LDH is used to form Cu–Zn oxide/Cu–Zn aluminate, uniformly dispersed thanks to the cation dispersion within the LDH pristine precursor. The optimised composition is found to reach a faradaic efficiency of 88.5% for the conversion of CO2 to C2+ products. Membranes composed of LDH nanosheets self-assembled with formamidine sulfinic acid and formed with poly(dimethylsiloxane) are reported to perform effectively for selective CO2 separation.124
The shaping of graphene and the complementary plasma-assisted oxidation of material open the way to novel devices as efficient membranes for water treatment, thanks to the optimised diffusion pathway and hydrophilic feature improving the capillary effect for effective water infiltration. This can clearly be considered more as water insertion rather than a true intercalation reaction.125
Concerning water cleaning, metal/g-C3N4 can display enhanced photocatalytic activity thanks to plasmonic effects in the heterostructure with noble metals such as gold and/or platinum. These catalysts can be used for the degradation of antibiotic residues and other pharmaceutical contaminants that induce negative environmental effects,126 but this once again consists more of surface reactions than intercalation.
3. Applications of intercalation materials for health purposes
Intercalation compounds are being increasingly studied for their possible application in the health domain. Once their biocompatibility has been verified, it is indeed possible to consider inorganic structures that can provide solutions for the release of active ingredients, for antimicrobial or bone reconstruction activity, or even more generally for theranostic applications, as reviewed recently.127,128Representative applications of intercalation compounds based on clays, ZrP and LDHs for health purposes are described below, and then summarised in Table 3.
| Material/substrate | Active species | Procedure to introduce the active species | Application | Biological system | Ref. |
|---|---|---|---|---|---|
| Palygorskite and HNTs clays | Eu(DBM)3(H2O)2 (HDBM dibenzoylmethane) | PEI grafting and further modification with terpyridine derivatives to introduce an antenna species. | Bioimaging applications | Tested for bioimaging with HeLa cell line | 134 |
| The Eu complex is introduced via a ligand exchange reaction. | Promising in applications as targeting cancer cells and in drug delivery systems | ||||
| Amino-modified clays in liposomes | Budesomide | Polyelectrolytes, aminoclay and Eudragit® S100, were assembled directly on the liposomal surface via a layer by layer deposition | Drug-releasing carriers | Colon-targeted delivery system | 138 |
| Porous clay heterostructures based on MMT clay | Methotrexate (MTX) | MTX encapsulation | MTX delivery systems, chemotherapy | Potential use of PCHs/MTX in pharmaceutical field for cancer treament | 139 |
| Organo-clay (alkylammonium on MMT) | Methylene blue (MB) (as a model drug) | Intercalation process | Drug delivery system | Thermo-sensitive hydrogel based on Pluronic and nanoclay potentially exploitable as injectable systems and as a long-term delivery system | 141 |
| MMT clay | Tetracycline hydrochloride | Ion exchange process | Antibacterial effect | Gram-positive and Gram-negative bacteria | 142 |
| MMT clay in polylactide matrix | Gentamicin and neomycin | Intercalation process | Antibacterial activity | Escherichia coli (E. coli) | 143 |
| Smectite clays | Ciprofloxacin | Ion exchange | Antibacterial Effect | E. coli and Staphylococcus aureus (S. aureus) | 144 |
| MMT modified with PEGylated chitosan (PEG-CS) | Doxorubicin (DOX) | Impregnation | Drug delivery | TRAMP-C1 cells epithelial cell line | 145 |
| Fe-MMT clay | Photosensitizer TPCI | Ion exchange, intercalation | Photodynamic therapy, antibacterial effect, wound healing | E. coli, Pseudomonas aeruginosa (P. aeruginosa) and S. aureus | 146 |
| In vivo antibacterial and wound healing assay | |||||
| HNTs | Vancomycin | Physical dispersion, sonication, vacuuming | Local drug delivery system | HL-60 cell lines (in vitro cell cytotoxicity) | 151 |
| Antibacterial activity on S. aureus and B. streptococcus | |||||
| HNTs | Ciprofloxacin and polymyxin B sulfate | Physical dispersion, sonication, vacuuming | Wound dressing, antimicrobial activity | S. aureus and P. aeruginosa | 153 |
| HNTs | Thymol | Cryogel synthesis | Wound healing | — | 154 |
| HNTs | DOX | Impregnation | Drug delivery | MFC mouse gastric cancer cells | 156 |
| HNTs | Icariin | Vacuum loading method | Bone tissue engineering | In vitro osteogenic differentiation | 161 |
| HNTs-poly[bis(carboxyphenoxy)phosphazene] | Amoxicillin and marine sponge | Inclusion and adsorption | Antibacterial and antibiofilm coating for the Ti–6Al–4 V screw for dental implantation | S. aureus, E. coli, Porphyromonas gingivalis | 164 |
| HNTs | Deferoxamine | Inclusion | 3D-printed scaffold to realise the coupling regeneration of blood vessels and bones | Bone marrow mesenchymal stem cells (BMSCs), human umbilical endothelial cells (HUVECs) | 165 |
| Saponites | Gd-complexes | Cation exchange | Diagnostic MRI and theranostics | — | 166 |
| Saponites | Gd-(or Y3+)TETA-monoamide chelates | Cation exchange | Diagnostic MRI and theranostics | — | 167 |
| Fe3O4@HNTs | Lanthanide complex, iron oxide | One-step hydrothermal process combined with the coupling grafting method | Bioimaging and biological applications in human hepatic adenocarcinoma cells and diagnostic MRI | Human hepatic (LO2) and human hepatic adenocarcinoma (HepG2) cell line, MRI was performed in vivo in tumour-bearing rabbits | 168 |
| Fe3O4 – LAPONITE® | Fe3O4 | Schikorr reaction | Localised magnetic hyperthermia (MH) and diagnostic MRI | Human glioblastoma cells (U87EGFRvIII) and human foreskin fibroblasts (HFF), MRI was performed in vivo in rodent brain | 169 |
| γ-ZP | Alendronate | Exfoliation, topotactic exchange | Drug delivery capable of promoting bone mineralisation and inhibiting bone resorption | Human bone osteosarcoma (MG-63) | 171 |
| ZrP | DOX | Intercalation | Drug delivery of anticancer drugs | MCF-7 and MDA-MB 231 cancer cell lines, MCF-10A cells, human prostate cancer PC3 | 173 and 174 |
| ZrP | Curcumin | Adsorption | Drug delivery of anticancer drugs | MDA-MB-231 breast cancer cell lines | 175 and 176 |
| Fe3O4/α-ZrP | 5-Fluorouracil folate acid-chitosan-rhodamine6G complex | Exfoliation, intercalation | Deliver anticancer drug for tumour optical imaging and therapy | A549, HeLa and HEK293 cells | 177 |
| α-ZrP | Methylene blue | Cation exchange | Photodynamic therapy | MDA-MB-231 breast cancer cell | 178 |
| α-ZrP/carboxymethylcellulose | Chlorhexidine | Intercalation, solvent casting | Antimicrobial and antibiofilm wound dressings | HDF human dermal fibroblast cells (HuDe) and human keratinocyte (NCTC2544) cell lines | 179 |
| α-ZrP | Ag+ | Cation exchange | Antimicrobial | E. coli | 180 |
| Saos-2 (Sarcoma osteogenic) cell line | |||||
| α-ZrP/poly(lactide-co-glycolic acid) | NorA efflux pump inhibitors | Intercalation, solvent casting | Antibiofilm composites | S. aureus | 181 |
| Staphylococcus epidermidis | |||||
| α-ZrP | Bromfenac | Exfoliation, adsorption | Drug delivery of anti-inflammatory drug | — | 182 |
| α-ZrP | Caffeine | Exfoliation, intercalation | Topical application | — | 183 |
| α-ZrP or LDH/poly(ethyleneoxideterephthalate)/poly(butyleneterephthalate) | Gentamicin, ciprofloxacin | Intercalation, melt-blending, melt extrusion | 3D scaffolds for bone infection prevention and tissue regeneration | S. epidermidis, P. aeruginosa cytotoxicity on human mesenchymal stromal cells (hMSCs) osteogenic differentiation capacity of hMSCs | 184 |
| Alg-Cu MOF-LDH beads | DOX | Co-precipitation and in situ growth method. | Drug delivery system for cancer therapy | L929 non-cancerous cells, MCF-7 human breast cancer cells | 188 |
| MgAl-LDH/poly(ε-caprolactone) | LDH | Electrospinning technique | Nanocomposite scaffolds | Mouse adipose derived stem cells (mADSC) | 194 |
| Graphene oxide | Graphene oxide nanosheets | Intercalation/exfoliation (Hummers’ method) | Antimicrobial surface coatings | E. coli | 195 |
3.1 Applications of clays in the health domain
Nanoclays are attracting increasing interest for their enhanced therapeutic effects. Indeed, their biocompatibility, the bacteriostatic or bactericidal activity exhibited by some clays,129 and their high surface area, adsorption capacity, ability to serve as carrier for therapeutics and to release drugs in a controlled manner make them ideal candidates for applications in biomedical engineering,130 tissue engineering and regenerative medicine,131 wound healing,132 drug delivery systems,133 bioimaging134 and 3D bio inks for use in medical purposes.135 It is worthy to note that attention has also been paid to commercially available clays such as LAPONITE® in the field of health, as reviewed by Tomás et al.,136 owing to their purity but above all due to their high aspect ratio derived from their 25 nm disk-shaped particles. The most promising use of clays and clay-based nanocomposites is in the field of drug delivery systems,137–141 including antimicrobial,142,143 antibiotic,143,144 and anti-cancer145 as well as theranostic146,147 and antihistamine treatments.148Montmorillonite (MMT) clay has been frequently utilised as a drug vehicle due to its high specific surface area, excellent cation exchange capacity and biocompatibility. To avoid flocculation of MMT under physiological conditions the surface has to be modified. For example, PEGylated chitosan (PEG-CS) adducts can be used to modify the clay surface, thus leading to a good dispersion in a serum-containing environment (Fig. 8) as well as resulting in interesting drug carriers.145
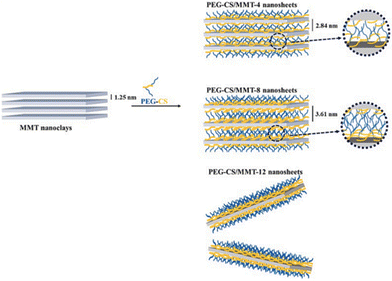 | ||
| Fig. 8 Microstructures of PEG-CS/MMT nanosheets with various PEG-CS/MMT. Reproduced with permission from ref. 145, Copyright 2022 Elsevier. | ||
Examples of the uses of PCHs derived from MMT clays for drug delivery applications are also reported. PCHs prepared using montmorillonite as starting material are interesting supports for methotrexate (MTX) encapsulation due to the superior drug encapsulation efficiency (EE) with respect to MMT parent clay (EE values higher than 97% for PHCs compared with EE = 45% for MMT clay).134 It was also observed that PCH hosts can influence drug release in relation to their pore architecture. The drug is released in a proportion higher than 70%, whereas from MMT parent clay only 9.7% of MTX is released.134
Halloysite is one of clay minerals showing maximum efficiency as a nano drug carrier, and demonstrates exceptional biocompatibility and low cytotoxicity when tested for use in cell cultures and tissues.149,150 Moreover, thanks to its size, the inner lumen can accommodate molecules of interest, while small drug molecules can be filled into the wall interlayer spaces and molecules can be either adsorbed or covalently bonded on the outer surface. As different interactions can occur, an initial drug burst can start from the outer surface and be followed by a sustained release of the drug from the inner surface (lumen) owing to a slower leakage.
Drugs can be loaded into HNTs from saturated drug solutions or a melt of low water-soluble drugs. Pan et al.151 loaded vancomycin, a drug used for serious Gram-positive bacterial infections, into the internal cavity of halloysite by using different mass ratios of vancomycin and halloysite via sonication and vacuuming, obtaining a solid with high antibacterial activity against S. aureus and B. streptococcus. The use of HNTs allowed the preparation of a local antibiotic delivery system without the need for a particularly energy-consuming process, thus allowing the controlled release of molecules beneficial for targeted applications.
Norfloxacin, an anti-microbial agent, was loaded within HNT and then embedded with chitosan to prepare bio-nanocomposite films.152
Antimicrobial gelatin-based elastomer nanocomposite membranes loaded with ciprofloxacin and polymyxin B sulfate-loaded halloysite nanotubes were formed for wound dressing applications.153 A combination of ciprofloxacin (characterised by a broad-spectrum antimicrobial activity inhibiting both Gram-negative and Gram-positive bacteria) and polymyxin B-sulfate, with a narrow spectrum of activity mainly against Gram-negative bacteria, aimed at having a co-operative anti-infection effect. It was reported that HTNs play a dual role of enhancing the matrix tensile strength and slowing down the release rate of highly dissoluble drugs.
Recently, the formation of a hybrid system based on biocompatible poly 2-hydroxyethyl methacrylate (pHEMA) and thymol-loaded halloysite was reported for application as portable wound dressing to protect injured skin;154 the antibacterial, antifungal and anti-inflammatory properties of thymol make this compound interesting for the development of devices for tissue repair. Also in this case, HTNs were exploited to entrap the thymol in the lumen to ensure a sustained release of the drug.
Doxorubicin (DOX) is widely used as a chemotherapeutic drug, owing to its effectiveness in combating numerous types of cancers. However, as some serious side effects have been witnessed, DOX delivery vectors based on different hosts were developed, as recently reported.155 In this regard DOX was, for example, loaded into HNTs and then encapsulated by soybean phospholipid (LIP) to form HNTs/DOX/LIP.156 The optimal DOX loading efficiency was higher than 22%. A pH-responsive release property with fast drug release under acidic conditions (pH = 5.4) was evidenced by in vitro drug release testing, and in vivo experimental results revealed that such compounds exhibit a significantly higher inhibitory efficacy on the growth of mouse gastric cancer cells than free DOX at the same drug concentration.
The development of chitosan/halloysite/graphitic-carbon nitride compounds for quercetin targeted delivery was performed to overcome the weak hydrophilicity, chemical instability and low bioavailability of quercetin which, however, presents anti-inflammatory, antiviral and mainly anticancer effects. The combination of carbon nitride (g-C3N4), which is similar in structure to graphene, has a low biological toxicity and can be metabolised in biological systems,157 with HNT and chitosan to form hydrogels, followed by a loading of quercetin using a water-in-oil-in-water emulsification process to attain quercetin sustained-release, leads to a remarkable encapsulation loading and loading efficiency (up to 86%) and the formation of a pH-responsive behaviour that minimizes the side effects of quercetin by controlling its burst release at neutral conditions. These new nanocomposites also revealed a significantly higher cytotoxicity against breast cancer cells in comparison with quercetin as a free drug.
The reported examples suggested that clays can be exploited as nano containers for applications in drug delivery, antimicrobial materials, self-healing polymeric composites, and regenerative medicine that entail time-extended functions.
In the field of tissue engineering, which aims to develop biological substitutes that replace and restore structural and functional properties of tissues, HNTs are also arguably the most promising candidates, as besides all their already-mentioned properties, their unique structure can significantly increase the mechanical and chemical stability of tissue engineering scaffolds.158–160 Applications for bone tissues,161 cartilage repair,162 implants,163 dental fillings,164 and tissue scaffolds165 underline all the advantages of HNTs. As interesting results, in the field of orthopedic implants, Dharmaraj et al.163 mentioned the antibacterial, anti-cancer and osteosarcoma cell growth-impediment properties of composites prepared by electrodeposition of strontium-halloysite nanotubes (Sr-HNT)/lanthanum cerium-substituted hydroxyapatite composite coatings on titanium used as implant materials. In addition, an anti-corrosion effect of this new type of coating was also revealed. A 3D-printed scaffold with a sequential delivery platform was also formed starting from deferoxamine-loaded HTNs, bone morphogenetic protein-2 and poly(L-lactide-co-glycolide)/b-tricalcium phosphate solution with the aim of realizing the coupling regeneration of blood vessels and bones.165 These new composites promote cell–scaffold interactions by enabling cell adhesion, spreading, and differentiation in vitro, and also enhance subcutaneous ectopic bone formation in vivo via the synergistic effect of deferoxamine and bone morphogenetic protein-2, therefore paving the way for new treatments for bone defects.
The inherent properties of clay minerals, such as fluorescence and paramagnetism, enable their utilisation in biomedical imaging techniques. Functionalised clay nanoparticles have been developed as contrast agents for magnetic resonance imaging (MRI), providing improved imaging resolution and targeting capabilities. Paramagnetic clays have been obtained by introducing, in the interlayer space of saponite clays, Gd3+-complexes by a classical ion exchange reaction. It was pointed out that the interactions of the complexes with the saponite layers have a certain influence on both the local rotational dynamics and exchange process of water molecules between the internal Gd3+ coordination sphere and the bulk.166,167 Clay-based lanthanide polymer nanocomposites were for example formed,135 starting from polyethyleneimine and a lanthanide complex attached onto the surface of halloysite or palygorskite, leading to nanocomposites exhibiting both intense red emission under visible light excitation, long luminescent lifetime as well as high quantum yields, and improved photoluminescence stability. The photoluminescent and magnetic properties of multifunctional halloysite nanotube Fe3O4@HNT-polyethyleneimine-Tip-Eu(dibenzoylmethane)3 were also reported,168 one interesting feature being that these composites displayed superparamagnetic behaviour and also worked as a magnetic resonance imaging (MRI) contrast agent in vitro and in vivo. The Schikorr reaction, which involves the oxidation of layered Fe(OH)2, was used to prepare superparamagnetic Fe3O4 – LAPONITE®.169
3.2 ZrP-based compounds for health applications
The discovery that ZrP is harmless to the body, since it is highly hemocompatible, not toxic, and not associated with any metabolic function, has paved the way for using these compounds in the nanomedicine field.170 The insertion into ZrP of molecules of biological interest can occur via ion-exchange reactions between the acid protons of the solid and the molecular cations, intercalation of basic molecules and topotactic exchange of phosphate by phosphonate groups. This last strategy is mainly applied in γ-ZrP. In this context, the alendronate, a gem-bisphosphonate used to treat bone diseases, was immobilised in γ-ZrP by topotactic exchange to obtain a drug delivery system.171 When the biological molecules are particularly bulky a preintercalation step in alpha type is mandatory to enlarge the interlayer region; a strategy to avoid this step is the use of θ-ZrP, a hydrated form of α-ZrP with six water molecules per formula unit. Otherwise, one-pot intercalation can be achievable by starting from a gel of nanometric α-ZrP in ethanol or propanol.172 The alcohol present in the interlayer region keeps the layers far enough apart to promote the intercalation of bulky species.Particular attention has been paid to delivering chemotherapeutic drugs loaded in nanocarriers, to target cancer cells and minimize drug side-effects. DOX, one of the most used anticancer drugs, is highly toxic to cancer cells and to normal tissue, causing several adverse effects. To overcome these drawbacks, DOX was intercalated into θ-ZrP to obtain a compound (DOX@ZrP) with a drug loading of 34.9 wt%. Two-dimensional 31P NMR studies showed that DOX is intercalated as a neutral molecule and interacts with the ZrP layers by hydrogen bonds among protonated phosphate groups and the amine and –OH groups of DOX, involving also hydration water molecules. The release of DOX from DOX@ZrP in simulated body fluid at pH 7.42 is prolonged, extending to 11–14 days. Cellular studies showed that DOX@ZrP samples were favoured for cellular uptake and cytotoxicity in MCF-7 and MDA-MB 231 cancer cell lines compared with free DOX. In vitro studies in MCF-7 and MCF-10A cells showed that ZrP nanoparticles are able to promote the uptake of DOX@ZrP by endocytosis, especially in cancerous cells.173 The biocompatibility of DOX@ZrP was improved by surface functionalisation with monomethyl-poly(ethylene glycol)-monophosphate, and cytotoxicity towards human prostate cancer PC3 cells was about 20% higher than free DOX.174
Kalita et al. prepared nanometric zirconium pyrophosphate, having the dimension of about 48 nm, by a sonochemical method in the presence of cetyltrimethylammonium bromide as surfactant followed by calcination at 700 °C. The nanoparticles were used to immobilise curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), a model drug with anti-inflammatory, antioxidant and anti-tumour properties. The loading of the drug in the composite (ZP-CUR) was 11.5 wt%. The in vitro drug release from ZP-CUR was pH sensitive and was higher at pH 5 than pH 7.4, making the composite suitable for delivering and releasing the drug in the acidic intracellular environment of tumour cells. The cytotoxicity studies of free curcumin and of ZP-CUR, performed on MDA-MB-231 breast cancer cell lines, showed an enhancement of the cytotoxic effect of the formulate curcumin as compared with the native form.175
Antitumour drugs lack specificity for tumour cells, thus exhibiting high cytotoxicity also for normal cells. The use of magnetic nanoparticles (Fe3O4), anchored to the nanocarrier, allows, through an external magnetic field, the delivery and anchoring of the drug in a specific site, enhancing the drug activity. In this context, Kalita et al. extended their work with curcumin-loaded amorphous zirconium phosphate as the shell for magnetite core nanocarriers.176 The release profile and cytotoxicity in MDA-MB-231 cell lines was comparable to those of ZP-CUR previously described.
To improve targeted drug delivery to tumour cells, nanocarriers conjugated with folate seem to be a promising strategy since the cancer cells over-express folate receptors. Magnetite nanoparticles and 5-fluorouracil (5-FU) were intercalated in α-ZrP preintercalated with butylamine. The compound obtained was covered with a folate acid-chitosan-rhodamine6G (FA-CHI-R6G) complex to obtain the system 5-FU/Fe3O4/α-ZrP@FA-CHI-R6G (Fig. 9). The presence of R6G, which acts as a fluorescent probe, suggests the nanocomposite as a promising material for bioimaging. Moreover, the nanocomposite has no cytotoxic effects on A549 cells, interacts with FA-positive HeLa cells while the FA-negative HEK293 cells show little uptake of the nanomaterials, and shows a sustainable 5-FU release.177
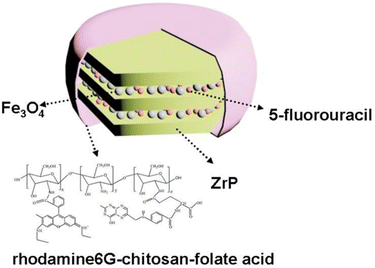 | ||
| Fig. 9 Schematic representation of the 5-FU/Fe3O4/α-ZrP@CHI-FA-R6G nanocomposites. Reproduced with permission from ref. 177, Copyright 2015 American Chemical Society. | ||
An alternative to the use of chemotherapeutic drugs for cancer treatment is photodynamic therapy (PDT), which is based on photoactivation of a photosensitizer able to form cytotoxic reactive oxygen species. The photosensitizers, as the antitumour drugs, have nonspecific interactions that also cause damage to normal cells.
The photosensitizer methylene blue (MB) was intercalated in α-ZrP by cation exchange with the acid protons. The ZrP-MB nanoparticles were able to deliver MB to MDA-MB-231 breast cancer cells and to reduce the toxicity of pure MB in a dark experiment. PDT efficacy on ZrP-MB-treated MDA-MB-231 cells was enhanced upon MB intercalation. The nanoparticles protect the MB from the reduction to “leuco-methylene blue” in biological systems that generally limits the clinical use of MB, and release it preferentially in cancer cells due to their acidic microenvironment.178
Several active molecules were intercalated starting from the gel of nanometric α-ZrP in ethanol. Chlorhexidine (CLX), a broad spectrum antimicrobial agent, was intercalated starting from the gel of nanometric α-ZrP, reaching a drug loading 50 wt%. ZrP(CLX) was used as a filler of sodium carboxymethylcellulose to prepare films as potential wound dressings. The films showed antimicrobial and antibiofilm activity. Moreover, in vitro cytotoxicity tests of the films in HDF human dermal fibroblast cells (HuDe) and human keratinocyte cell lines (NCTC2544) showed reduced cytotoxicity of CLX due to its prolonged release, which maintains a low CLX concentration.179 Silver NPs were immobilised on α-ZrP via the H+/Ag+ ion-exchange process followed by thermal treatment. The Ag-enriched α-ZrP showed antimicrobial activity against Escherichia coli.180
The intercalation properties of the gel of nanometric α-ZrP in ethanol were exploited to intercalate potent in-house S. aureus NorA efflux pump inhibitors. The intercalation compounds were used as fillers of poly(lactide-co-glycolic acid). The composites showed an antibiofilm activity comparable to that of film containing ZrP intercalated with thioridazine, a NorA and biofilm inhibitor.181
An alternative way to promote the interactions between the α-ZrP surface and biomolecules is the use of a gel of zirconium phosphate obtained by exfoliation with propylamine and regenerated with HCl. The gel was able to immobilise the anti-inflammatory drug bromfenac (BFS)182 and caffeine,183 a central nervous system stimulant. The compound ZrP-BFS showed a BFS release profile that was pH dependent; a complete release of BFS was obtained at pH 7.
Finally, a ZrP-gentamicin intercalation compound was used as a filler of a thermoplastic polymer and the composite was processed via high-temperature melt extrusion into 3D scaffolds. The antibiotic, released in a sustained manner, maintained its activity against Gram+ and Gram− bacteria, and the scaffolds were not cytotoxic towards hMSCs cells and did not prevent their osteogenic differentiation.184
3.3 Applications of LDHs for health purposes
LDHs are also studied today for their possible biomedical applications.185 Their trapping and release ability is important regarding the delivery of drugs or bioactive molecules as well as their ease of handling and scale-up.186 Accommodation of bioactive molecules within the LDH host structure leads to bioactive hybrid materials that can be efficient in administration and associated with low cytotoxicity, this triggered by the pH of human fluids to precisely target a site. They may be of use for delivering cosmeceutical, natural or synthetic biomolecules. When considering the formed bio-hybrid composite, it may be considered as a win–win partnership, the host LDH protecting the bioactive molecule from aggressive media while the guest maintains the layered structure until its ingress out of the LDH cargo. Due to their biocompatibility, LDHs are also studied in terms of clinical translation of their bio-activity and bio-reactivity concerning orthopaedic diseases and other bone tumours.187LDH-supported MOFs loaded in alginate beads, a pH-sensitive biopolymer extracted from seaweed,188 have been prepared to control the release of DOX for cancer therapy. In vitro cytotoxicity in MCF-7 cells (human breast cancer cells) showed that the loaded beads have a higher cytotoxic effect than the free DOX. The higher DOX activity may be due to the presence of the Alg coating and the controlled DOX release into cancer cells. Other reviews report the combination of organic polymer with LDH as well.189 From in vivo assays it is demonstrated that such bio-hybrid composites containing bioactive guests are of interest in pharmaceutical and medical applications, and that review more specifically focused on drug delivery and tissue engineering regarding skin and bone therapies. For the latter, it is observed that LDH composites can be safely integrated into living matter as well as being stimuli-responsive when exposed to collagen. Of course, other naturally occurring clay minerals such as montmorillonite and halloysite may also be combined with biocompatible polymers to carry the active ingredient of interest in drug delivery or in tissue engineering.190 In the latter case, the bio-hybrid composites are found to induce cell adhesion and proliferation, helping in regenerating skin damage. It is indeed a set of 2D materials which can be considered as bio-platforms in theranostics (therapeutic and diagnostic) when endowed with bio-functionalities,191 as found in the critical and cutting-edge review discussing the current challenges and their future research directions regarding such applications in human health.
Chemotherapeutic and gene-therapeutic functions for hetero-structured layered nanohybrids using clays (in general) have been recently reviewed, underlining the possibility of drug–clay nanohybrids being relevant for drug administration and release.192
Similarly, another review reports such carriers for gene-LDH focusing on intercellular gene uptake mechanisms and their intracellular ingress, but also with imaging and targeting functions.193
Other health domains may be relevant, such as tissue and bone engineering, where LDH dispersed into fibers of poly(ε-caprolactone) (PCL) by the electrospinning technique are studied to mimic tissue-engineering scaffolds.194 The study reports the viability, proliferation, and adipogenic differentiation of mouse adipose-derived stem cells and shows that LDH-enriched electrospun PCL scaffolds with high porosity increase cell adhesion and proliferation, suitable for application in soft tissue regeneration.
More specifically for bone healing, 3D additive-manufactured scaffolds made with biodegradable polymers and LDH intercalated with ciprofloxacin have been designed to present possible antibiotic release for antimicrobial activity to prevent infection in the case of an open bone fracture or in implant surgery. Incorporation of LDH-ciprofloxacin into the scaffolds did not affect the viability of human mesenchymal stromal cells (hMSCs) or prevent their osteogenic differentiation.184 An intercalation/exfoliation procedure following Hummers’ method led to the synthesis of graphene oxide (GO). Consecutively to this intercalation process, the so-obtained materials exhibited a size-dependency of GO antimicrobial activity. The defect quantity is correlated to the size/specific surface area of the GO, giving guidelines for future development of graphene-based antimicrobial surface coatings.195
Tunable surface properties, transparency, electronic capabilities, biocompatibility and outstanding flexible mechanical properties make graphene a promising material for biomedical applications such as biomedical electronic devices or implants. However, the material generally called “graphene” is, in fact, a large variety of carbon-based materials. Following these considerations, graphene-based materials used for biomedical applications must be regarded while paying particular attention to the characterisations defining them. Consequently, some “rules” must be established concerning the synthesis considerations and the possible biomedical applications depending on the characteristics of graphene. If graphene can be obtained by the CVD procedure, intercalation using Hummers’ method can lead to GO and then reduced graphene oxide (RGO) interesting for health purposes.196
4. Applications of intercalation materials in polymer science
Clays and graphene have been extensively studied as fillers of polymeric composite, and the research has been summarised in several reviews and thematic books. Therefore, to avoid redundancy, discussion of the main aspects related to polymeric composites based on these layered materials is entrusted to the most recent reviews and books.197–204Here, the latest examples of the use of ZrP and LDHs as a filler of polymeric composites are reported. Table 4 summarises the recent applications of intercalation compounds based on ZrP and LDHs for polymer science.
| Filler | Polymeric matrix | Preparation method | Purpose | Performances | Ref. |
|---|---|---|---|---|---|
| α-ZrP | Polydopamine (PDA) | In situ polymerisation | Composite epoxy coating | Corrosion protection (improved anticorrosion performance with Icorr of 6.60 × 10−9 A cm−2, which are one order of magnitude lower than that of blank epoxy coating) | 206 |
| α-ZrP | Polyurethane | In situ polymerisation | Advanced coatings | Reduction of ca. 40% in H2O permeability, anti-corrosion protection | 207 |
| α-ZrP | Polybenzoxazine | Thermal curing process | Flame retardance | Improved thermal stability and flame retardancy (49.3% peak of heat release rate reduction (PHRR) with respect to pure matrix) | 209 |
| α-ZrP | Polyamide6 | Melt blending | Flame retardance | Improved flame retardancy (ca. 83% PHRR with respect to pure matrix) | 210 |
| α-ZrP | Polyacrylate | In situ emulsion polymerisation | Flame retardance | Improved flame retardancy. LOI (limited oxygen index) value of 25% against 23% of pure PPA | 211 |
| α-ZrP | Poly(vinyl alcohol) | Solution blending | Flame retardance | Improved graphitization of char residues and thermal stability | 212 |
| α-ZrP | Polypropylene | Melt blending | Flame retardance | Improved formation of char residues and higher thermal stability with respect to pure matrix. LOI value ca. 26% vs. 17% in pure PP matrix | 213 |
| Organo-modified ZrP LDH (m-ZrP) | Ethylene vinyl acetate | Melt blending | Flame retardance | Incorporation of mZrP/LDH mixture into EVA/ATH composite resulted in a reduction in thermal stability but improved the char yield at 750 °C. EVA composites exhibited improvement in fire retardancy (decreased PHRR, THR, TSP and the enhanced char yield): LDH was more effective than mZrP in char formation; on the other hand, mZrP showed a 73% reduction in PHRR, which was more efficient than LDH (58% reduction in PHRR) | 214 |
| Organophilic α-ZrP | Low-density polyethylene and ethylene–vinyl acetate (LDPE/EVA) hybrid | Bradender extrusion | Flame retardance | Improved flame retardancy. LOI value of the composite ca. 32 against 20.3 of the pure polymer | 215 |
| Hierarchical graphene-confined zirconium phosphate nanosheets (ZrP/RGO) | Cellulose-based aerogels | Unidirectional freeze-casing technique | Thermal insulation, flame retardance, mechanical properties | Thermal conductivity (18 mW m−1 K−1), and high LOI (33.5) as well as very low PHRR (14.1 kW m−2) | 216 |
| α-ZrP | PET film | Layer-by-layer (LbL) assembly | Oxygen barrier properties | Oxygen transmission rate (OTR) reduced from 57 to 0.87 cc per m2 per day for the best composite material. | 218 |
| Organo-modified MgAl LDHs | Polybutylene succinate (PBS) | Melt extrusion | Viscoelastic properties, rate of hydrolysis, photodegradation, antibacterial activity | Small rate of hydrolysis, photostability, biocide activity, chain extension is obtained using a combination of LDHs-L-ascorbate and LDH-3-(4-hydroxyphenyl)propionate | 222 |
| LDH-RA (RA = rosmarinic acid), LDH-SA (SA = salicylic acid) | Low-density polyethylene(LDPE) | Two-step melt compounding procedure | Antibacterial activity | LDPE/MgAl-RA was selective and strongly inhibitory toward S. aureus, LDPE/ZnAl-SA inhibited the growth of E. coli and S. aureus | 223 |
| LDH MgAl-D (D = 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid) | Polypropylene | Solvent mixing method | Anti-aging | Good stability against thermal aging, low migration of molecules out of the PP films | 224 |
| LDH MgAl-AO (AO = Irganox 1010) | Polypropylene | Solvent mixing method | Anti-aging | LDH-AO significantly enhances long-term performance of LDH/PP composites against thermal/thermo-oxidative degradation | 227 |
| LDH ZnAl-HxDy (H = 4-oxo-4-((2,2,6,6-tetramethylpiperidin-4-yl)amino)butanoic acid; D = 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic) | Polypropylene | Solvent or extrusion mixing | Anti-aging | The co-intercalated hybrid materials are found to decelerate the oxidative degradation for PP | 228 |
| LDH CaAl-HnMn′ (M = Irganox 1425; H = hindered amine light stabilizer) | Polypropylene | Solvent casting method | Anti-aging | Higher overall resistance against thermal degradation and photooxidation | 229 |
4.1 ZrP compounds for polymer science applications
Zirconium phosphate nanosheets, the size and thickness of which can be precisely controlled, are suitable nanofillers for improving the anti-corrosion properties of polymeric coatings.205 It was shown that improved barrier properties, caused by self-assembly of ZrP nanosheets, are mainly responsible for the increased corrosion resistance of ZrP/epoxy resin (Fig. 10),206 ZrP/polyurethane207 or ZrP/polybenzoxazine coatings.208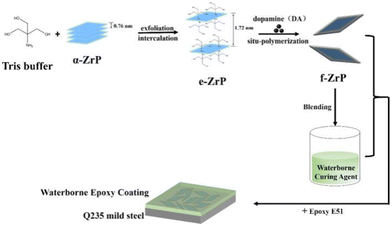 | ||
| Fig. 10 Exfoliation and functionalisation process of α-ZrP (f-ZrP) and the preparation of f-ZrP/WEP nanocomposite coatings. Reproduced with permission from ref. 206, Copyright 2020 Elsevier. | ||
Zirconium phosphate is also a possible candidate for flame retardancy applications, due to its high thermal stability and phosphorus content. In addition, the aspect ratio and size of the nanoparticles can be controlled by the choice of synthesis and exfoliation method. Exfoliated ZrP significantly enhanced the thermal stability and flame retardation behaviour of polybenzoxazine by improving the stability of the char layer and reducing the amount of flammable gases (Fig. 11).209
 | ||
| Fig. 11 (a) and (b) SEM of original α-ZrP, (c) original α-ZrP in acetone, (d) exfoliated α-ZrP in acetone, (e) Ba/α-ZrP-8.4% in acetone, (f) and (g) TEM of PBa/α-ZrP-8.4% nanocomposites, (h) photographic images of PBa/α-ZrP-8.4% nanocomposites and (i) light transmission spectra of samples. Adapted with permission from ref. 209, Copyright 2016 Royal Society of Chemistry. | ||
Hybrid platelet nanostructures formed by the self-assembly of ZrP, melamine and cyanuric acid significantly improved the flame retardancy and crystallinity of polyamide6 (Fig. 12).210 Acrylamide-modified ZrP/polyacrylate nanocomposite was prepared via in situ polymerization and the effect of ZrP (dimension of ca. 120–160 nm) amount and the quality of its dispersion on flame retardancy was described.211 When ca. 0.5 wt% of modified ZrP was added, a decrease of nanoplatelets dimensions was visible because of the exfoliation process (Fig. 12B-B′). An increase in ZrP content to 3 wt% leads to the formation of large aggregates (Fig. 12C-C′) that might have a negative effect on the mechanical properties and flame retardancy of the composite.
 | ||
| Fig. 12 TEM images of ZrP/polyacrylate nanocomposite. A-A′: α-ZrP, B-B′ and C-C′: PPA/AM-ZrP-1 composites with 0.5 and 3 wt%, respectively. Reproduced with permission from ref. 211, Copyright 2017 Royal Society of Chemistry. | ||
ZrP nanoplates modified with hexachlorocyclotriphosphane and melamine in polyvinyl alcohol composite improve the yield and graphitization of char residues, and make the char more stable, compact and continuous, inhibiting the underlying polymer from coming into contact with heat and oxygen.212 The synergistic effect between polyphosphazene and α-ZrP in polypropylene composites improved the thermal stability of the matrix and increased the amount of char yield in comparison with polypropylene containing either only polyphosphazene or only ZrP.213 Organo-modified ZrP combined with another traditional flame retardant agent aluminium hydroxide leads to improved flame retardancy of ethylene vinyl acetate, low-density polyethylene/ethylene-vinyl acetate copolymer, and polystyrene.214,215 Hierarchical graphene-confined zirconium phosphate nanosheets were used to improve the thermal conductivity, mechanical properties and flame resistance of cellulose nanofiber aerogels.216
The use of α-ZrP nanosheets as a nano-filler in polymers for food packaging applications is very limited; some examples are given in Ghanbarzadeh's et al. review.217 A combination of graphene and ZrP nanosheets with different two-dimensional scales results in a synergistic ordering effect that increased the tortuosity of the permeation path for oxygen molecules to travel through the PVA nanocomposite films and significantly decreased their oxygen permeability. A similar nano-bricks wall architecture was obtained through layer-by-layer deposition of polyacrylic acid, poly(dimethyl diallyl ammonium chloride), and α-ZrP nanosheets onto polyethylene terephthalate film (Fig. 13).218 The oxygen transmission decreased exponentially with the number of the deposited quadlayers (consisting of three repeat units of polyacrylic acid, poly (dimethyl diallyl ammonium chloride) and α-ZrP), and the transparency of the film was not significantly reduced; therefore, this composite film shows great potential for application in packaging.
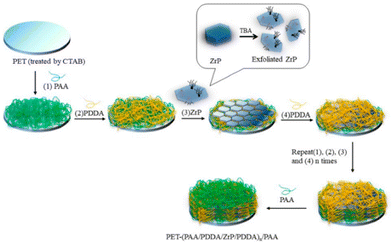 | ||
| Fig. 13 Schematic of the layer-by-layer (LbL) assembly for polyacrylic acid (PAA)/poly(dimethyl diallyl ammonium chloride) (PDDA)/zirconium phosphate (ZrP)/PDDA quadlayers. PET: polyethylene terephthalate; TBA: tetrabutylammonium; CTAB: hexadecyl trimethyl ammonium bromide. Reproduced with permission from ref. 218, Copyright 2018 MDPI. | ||
4.2 LDHs-based compounds for polymer science
LDHs are often used as a polymer filler as they can be customised to accommodate functionalities such as fire retardancy, UV-stabilization, biocide, water or moisture permeability or repellant (increase of the hydrophobicity by the presence of platelets organo-modified by surfactants) while providing the expected barrier-effect by the enhancement of tortuosity coming from the high mechanical modulus of the anisotropic platelets.219 More specifically, they may be of interest in food packaging by increasing the shelf life of food products and adding organoleptic and nutritional value.220 LDHs contribute to stabilizing or enhancing certain properties of PVC221 or PBS.222 In the latter case, with organic anions interleaved into LDH fillers (L-tyrosine (TYR), L-tryptophan (TRP), L-ascorbate (ASA) and 3-(4-hydroxyphenyl)propionate (HPP)), it was possible to demonstrate antibacterial properties for the films in combination with a high chain extension effect suitable for polymer processability. When LDH intercalated with salicylate and rosmarinate anions is dispersed into LDPE, the formed polymer nanocomposites present strong antibacterial activity against Staphylococcus aureus and antioxidant properties altogether suitable for packaging materials.223Furthermore, LDH has been used as a barrier to protect antioxidants from migrating out of the polymer.224 Hindered phenolic antioxidants have gained widespread recognition and application in enhancing the heat resistance and oxidative aging performance of polypropylene (PP) composites.225 However, the use of low molecular weight antioxidants poses certain challenges. These antioxidants have a greater tendency to volatilise, migrate, and be extracted from PP or PP-based products. Such behaviour directly diminishes the anti-thermal oxidative aging efficacy of PP and its composites, and additionally presents a risk of contaminating food or drugs when used in packaging.226 LDHs are highly desirable as antioxidant protection materials due to their adjustable compositions (M2+ and M3+) within the host sheet, replaceable interlayer anions (An−) in the interlayer region, and adaptable charge density (M2+/M3+ ratio). Consequently, it is plausible to inhibit the migration of antioxidants utilizing host–guest interactions and supramolecular forces by incorporating low molecular weight antioxidant species into the interlayer gaps of transparent LDHs. The 3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate anions (AO, one-fourth of the most used antioxidant in industry, Irganox 1010) were intercalated into Mg2Al-LDH, thus leading to AO-LDH samples by a one-step synthesis. Moreover, AO-LDH/PP composites were obtained through a solvent washing method. It was proved that the intercalation of AO anions between LDH led to an improvement of the antioxidative performance of PP composites. A barrier effect was offered by the host sheet of MgAl-AO-LDH thus allowing greater migration resistance than that of AO/PP composite.227 Based on theory, the composition of the host layer and the interlayer guests in the intercalation structure antioxidants was controlled to achieve homogeneity of the resulting compounds, leading to the construction of a class of supramolecular intercalated structure antioxidants with anti-migration properties, such as the single-intercalated MgAl-HALS-LDH photostabiliser228 and the co-intercalated CaAl-HxMy-LDH anti-aging agent.229
As far as corrosion inhibition is concerned, LDHs are also found to be of interest in polymer coatings for metal substrates such as magnesium alloys (AZ91D, an alloy of interest but lacking stability when exposed to the marine environment) using the so-called self-healing behaviour of LDH cargo, since the release is triggered by corrosion.230 LDHs can be mixed with other frameworks as exemplified in one article,231 where the idea is to build a 3D fir tree-like hierarchical architecture by utilizing pulsed plasma electrolysis with LDHs and a zeolitic imidazolate framework to protect the magnesium alloy substrate.
5. Remarks and outlook
This perspective has focused on the role played by intercalation chemistry as an evaluable tool for the design and synthesis of materials to meet some of the challenges of our century. Particular attention has been paid to developing catalysts for producing clean energy, converting molecules derived from biomass into value-added products, degrading pollutants, materials for environmental remediation, systems for use in medicinal chemistry and for the preparation of polymeric composites.As far as heterogeneous catalysts are concerned, the layered materials here reviewed provide sustainable catalyst sources with several advantages: easily recoverable and highly reusable, economical, and efficient catalytic potential under mild conditions. The intercalation chemistry allows us to obtain solids with transition metal species, with great interest for catalytic purposes. Metals can be intercalated as inorganic polycations within the interlayer space of clay (PILCs) or as cations or cationic complexes in ZrP. Transition metals can be inserted as constituents of the LDH layers. All these possibilities allow to obtain catalysts with improvements in specific surface area, thermal stability, dispersion of the active phase and sometimes catalytic performance thanks to specific interactions of the active species with the functional groups of the layer or their confinement in the interlayer region. Layered materials have been used as systems capable of favouring the growth of metallic nanoparticles upon intercalation of metallic ions followed by reduction in a confined environment. Otherwise, preformed metallic NPs can be immobilized on material surface. The confinement of NPs improves their resistance to cleavage and agglomeration as well as their catalytic activity. In addition, the immobilisation of the NPs favours their recovery and reuse. Recently, superior catalytic performance has been achieved by combining different materials to obtain heterostructures (i.e. TiO2/clays, HNT/MOFs, etc.).
The ability of layered materials to intercalate ionic or molecular species has made them very good candidates as solid adsorbents for the removal of pollutants. The literature survey indicated that clays, ZrP and LDH can be successfully modified to improve the adsorption ability towards environmental contaminants, thus making them potential candidates for wastewater treatments. The presence of intercalated functional groups (classically leading to an increase of the basal spacing, modification of textural properties and surface hydrophobicity) and, in some case, the increase in swelling ability are key factors affecting the adsorption properties of intercalated compounds and leading to an improvement of adsorption properties with respect to raw materials.
ILCs can be also active as photocatalysts for pollutant degradation. This property is very often achieved in the case of compounds containing metal oxide particles, that can be thus stabilised from the layered matrices.
In this sense, efforts must be made in searching for sustainable synthetic methods (especially in terms of time and production costs) for layered materials-based intercalation compounds. Few methods are, for instance, related to ultrasound-accelerated synthesis. As an example, environmentally safe low-cost adsorbents can indeed be obtained also starting from natural clays by using microwave-assisted heating methods.232 Another emerging green and powerful synthesis is mechanochemistry, due its scalability and tunability. This technique has great potential for the environmentally friendly production of layered materials for applications in the sustainable energy and environmental sectors.233
Most of the papers in the recent literature are related to the use of ILCs for the removal of pollutants from simulated matrices. Since the solution pH and the presence of interfering ions and/or molecules can interfere with the main adsorption mechanism, the performances of materials must be tested in different conditions (i.e. distilled water, groundwater, industrial wastewater, sea water) to assess the real applicability of materials.234
Moreover, there are still problems to be solved in the use of materials as catalysts, especially when used to remove pollutants from water. For practical applications, correct monitoring of the species formed during the catalytic reaction, together with the detection of possible toxic intermediates, must be carried out.
For health purposes, the interlayer region of layered materials can be considered as a nanocontainer capable of protecting the bioactive species from light and oxygen. Moreover, the intercalation compounds, suitably modified, can provide targeted delivery of therapeutic species by releasing them upon a specific stimulus (variation of pH, temperature).
Among clays, halloysite is a promising local delivery system that does not require exfoliation or any other complicated energy-consuming process, thus permitting the storage and controlled release of molecules.
The application of zirconium phosphate for drug delivery has not been extensively studied. However, the ability of ZrP to exfoliate in aqueous solution makes this material a good candidate for hosting bulky cations and basic species with therapeutic activity. Attention should be focused on scalable and green syntheses of ZrP, taking into account mechanochemistry. As for LDHs, ILCs have long been considered for the preparation of drug delivery systems. Future opportunities for these materials are related to the modulation of the layer composition, which can be constituted by several metal cations. The correct choice of these intralayer metals (i.e. Zn, Ga, Cu, Mg…), which can be released in appropriate media, can produce materials with therapeutic properties. In addition, a new frontier is the possibility of creating defect sites in the LDH layer that can be tailored to the needs of cancer immunotherapy. For photothermal/photodynamic therapy, sonodynamic therapy, and pH-sensitive magnetic resonance imaging, the modulation of defect sites in the crystal structure of LDH enables it to respond sensitively to external stimuli.235
Finally, layered materials have been used as nanofillers to improve the thermal stability, mechanical properties of polymers and anti-corrosion properties of polymeric coatings. The inorganic filler, intercalated with active species, absolves to another task that is to impart to the polymer additional properties such as antioxidant and antimicrobial properties.
However, in general, one of the major issues in materials development in many areas is scaling up from the laboratory level to large-scale implementation as an available and affordable commercial solution. The production of large quantities of intercalation compounds means the simplification of preparation steps, the elimination of toxic solvents/reagents, and reduced production costs. The last of these could be achieved by using waste materials where possible, in accordance with the principles of the circular economy.
The recovery and reuse processes of these adsorbents are not always studied in the literature. Moreover, the fate of recovered pollutants merits additional consideration to develop sustainable and circular economy processes.234 An interesting example of Cr-based PILCs for the recovery of anionic and cationic species was recently reported in the literature.236 The study is especially focusing on the possibility of reducing the synthesis time and cost, identifying the optimal conditions for effective adsorption of individual dyes using Cr-pillared clay after statistical analysis through the design of experiments. Moreover, in many cases, we must continue efforts to understand the structure–property relationships. As an example, for PILCs different characterisation techniques are needed to reveal their still unknown properties, thus paving the way for intelligent material design.
Data availability
Manuscript Ms. ref. no.: DT-PER-03-2024-000757 entitled “Recent advances and perspectives for intercalation compounds. Part 2: applications in the field of catalysis, environment and health”, authored by Chiara Bisio, Jocelyne Brendlé, Sébastien Cahen, Yongjun Feng, Seong-Ju Hwang, Morena Nocchetti, Dermot O'Hare, Pierre Rabu, Klara Melanova, Fabrice Leroux. No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All co-authors as well as all colleagues from the board of the International Symposium on Intercalation Compounds (ISIC) are warmly acknowledged.References
- M. Massaro, R. Noto and S. Riela, Molecules, 2020, 25, 4863 CrossRef CAS PubMed.
- S. Nawaz, M. Ahmad, S. Asif, J. J. Klemes, M. Mubashir, M. Munir, M. Zafar, A. Bokhari, A. Mukhtar, S. Saqib, K. S. Khoo and P. L. Show, Bioresour. Technol., 2022, 343, 126068 CrossRef CAS PubMed.
- A. Ramli, M. Farooq, A. Naeem, S. Khan, M. Hummayun, A. Iqbal, S. Ahmed and L. A. Shah, in Frontiers in Bioenergy and Biofuels, ed. E. Jacob-Lopes and L. Queiroz Zepka, IntechOpen, 2017, vol. 14, pp. 285–308 Search PubMed.
- T. Chellapandi and G. Madhumitha, Mol. Divers., 2022, 26, 2311–2339 CrossRef CAS PubMed.
- S. Sadjadi, Appl. Clay Sci., 2020, 189, 105537 CrossRef CAS.
- Y. Li, X. Yuan, L. Jiang, H. Dai, Y. Zhao, X. Guan, J. Bai and H. Wang, Environ. Sci.: Nano, 2022, 9, 841–866 RSC.
- Y. Zheng, L. Wang, F. Zhong, G. Cai, Y. Xiao and L. Jiang, Ind. Eng. Chem. Res., 2020, 59, 5636–5647 CrossRef CAS.
- Y. H. Ahmad, A. T. Mohamed and S. Y. Al-Qaradawi, Appl. Clay Sci., 2021, 201, 105956 CrossRef CAS.
- N. M. Sanchez-Ballester, G. V. Ramesh, T. Tanabe, E. Koudelkova, J. Liu, L. K. Shrestha, Y. Lvov, J. P. Hill, K. Ariga and H. Abe, J. Mater. Chem. A, 2015, 3, 6614–6619 RSC.
- F. Carniato, C. Bisio, R. Psaro, L. Marchese and M. Guidotti, Angew. Chem., Int. Ed., 2014, 53, 10095–10098 CrossRef CAS PubMed.
- F. Carniato, C. Bisio, C. Evangelisti, R. Psaro, L. Marchese and M. Guidotti, Dalton Trans., 2018, 47, 2939–2948 RSC.
- S. Marchesi, M. Guidotti, L. Marchese, F. Carniato and C. Bisio, Chem. – Eur. J., 2021, 27(14), 4723–4730 CrossRef CAS PubMed.
- E. M. Serwicka, Catalysts, 2021, 11, 1087 CrossRef CAS.
- K. Bahranowski, W. Włodarczyk, E. Wisła-Walsh, A. Gaweł, J. Matusik, A. Klimek, B. Gil, A. Michalik-Zym, R. Dula and R. P. Socha, Microporous Mesoporous Mater., 2015, 202, 155–164 CrossRef CAS.
- B. Szczepanik, Appl. Clay Sci., 2017, 141, 227–239 CrossRef CAS.
- M. Kashif, M. Yuan, Y. Su, P. M. Heynderickx and A. Memon, Appl. Clay Sci., 2023, 233, 106847 CrossRef CAS.
- M. C. Dlamini, M. S. Maubane-Nkadimeng and J. A. Moma, J. Environ. Chem. Eng., 2021, 9, 106546 CrossRef CAS.
- S. Yoda, Y. Sakurai, A. Endo and T. Miyata, J. Mater. Chem., 2004, 14, 2763–2767 RSC.
- D. Chen, Q. Zhu, F. Zhou, X. Deng and F. Li, J. Hazard. Mater., 2012, 235–236, 186–193 CrossRef CAS PubMed.
- L. Chmielarza, A. Kowalczyk, M. Skoczek, M. Rutkowska, B. Gil, P. Natkański, M. Radko, M. Motak, R. Dębek and J. Ryczkowski, Appl. Clay Sci., 2018, 160, 116–125 CrossRef.
- M. Pica, Catalysts, 2017, 7, 190 CrossRef.
- H. Ding, A. Ahmed, K. Shen and L. Sun, Aggregate, 2022, 3, e174 CrossRef CAS.
- F. Wang, Z. Yuan, B. Liu, S. Chen and Z. Zhang, J. Ind. Eng. Chem., 2016, 38, 181–185 CrossRef CAS.
- C. Zhu, Q. Liu, D. Li, H. Wang, C. Zhang, C. Cui, L. Chen, C. Cai and L. Ma, ACS Omega, 2018, 3, 7407–7417 CrossRef CAS PubMed.
- M. Chen, J. Xia, H. Li, X. Zhao, Q. Peng, J. Wang, H. Gong, S. Dai, P. An, H. Wang and Z. Hou, ChemCatChem, 2021, 13, 3801–3814 CrossRef CAS.
- J. He, Y. Jiang, B. Ding, Y. Wang, H. Qiu, S. Dai, X. Zhao and Z. Hou, Appl. Catal., A, 2023, 664, 119351 CrossRef CAS.
- M. Pica, M. Nocchetti, B. Ridolfi, A. Donnadio, F. Costantino, P. L. Gentili and M. Casciola, J. Mater. Chem. A, 2015, 3, 5525–5534 RSC.
- M. Pica, S. Calzuola, A. Donnadio, P. L. Gentili, M. Nocchetti and M. Casciola, Catalysts, 2019, 9, 3 CrossRef.
- Y. Xu, F. Zhou, M. Chen, H. Hu, L. Lin, J. Wua and M. Zhanga, New J. Chem., 2020, 44, 9793–9801 RSC.
- L. Lin, Y. Wen, L. Li, Y. Tan, P. Yang, Y. Liang, Y. Xu, H. Hu and Y. Xu, Nanomaterials, 2022, 12, 3339 CrossRef CAS PubMed.
- J. Zhou, H. Sun, C. Xu, Z. Wang, H. Zhang, D. Guo, J. Zhang, X. Ji, L. Liu, J. Ma and Z. Tong, J. Taiwan Inst. Chem. Eng., 2022, 138, 104478 CrossRef CAS.
- M. V. Ramos-Garcés and J. L. Colón, Nanomaterials, 2020, 10, 822 CrossRef PubMed.
- M. V. Ramos-Garcés, J. Sanchez, D. E. Del Toro-Pedrosa, I. B. Alvarez, Y. Wu, E. Valle, D. Villagrán, T. F. Jaramillo and J. L. Colón, ACS Appl. Energy Mater., 2019, 2, 3561–3567 CrossRef.
- M. V. Ramos-Garcés, J. Sanchez, K. La Luz-Rivera, D. E. Del Toro-Pedrosa, T. F. Jaramillo and J. L. Colón, Dalton Trans., 2020, 49, 3892–3900 RSC.
- J. Sanchez, M. B. Stevens, A. R. Young, A. Gallo, M. Zhao, Y. Liu, M. V. Ramos-Garcés, M. Ben-Naim, J. L. Colón, R. Sinclair, L. A. King, M. Bajdich and T. F. Jaramillo, Adv. Energy Mater., 2021, 11, 2003545 CrossRef CAS.
- S. Kumar, A. Yoyakki, A. Pandikassala, R. Soni and S. Kurungot, Adv. Sustainable Syst., 2023, 7, 2200330 CrossRef CAS.
- A. Sharma, S. Kumari, S. Sharma, T. Singh, S. Kumar, A. Thakur, S. K. Bhatia and A. K. Sharma, Mater. Today Sustainability, 2023, 22, 100399 CrossRef.
- J. Wu, Y. Xie, Y. Li, M. Jin, L. Liu, G. Pan, C. Wang and F. Li, Coord. Chem. Rev., 2023, 497, 215437 CrossRef CAS.
- B. Sardar and D. Srimani, Tetrahedron, 2023, 138, 133414 CrossRef CAS.
- Y. Xiao, J. Li, Y. Tan, X. Chen, F. Bai, W. Luo and Y. Ding, Int. J. Mol. Sci., 2023, 24(19), 14859 CrossRef CAS PubMed.
- C. Gastaldi, C. Taviot-Gueho, C. Guerard-Helaine and C. Forano, Appl. Clay Sci., 2023, 238, 106931 CrossRef CAS.
- L. Izquierdo-Aranda, R. Adam and J. R. Cabrero-Antonino, ChemSusChem, 2023, 16, e2023008 CrossRef PubMed.
- U. A. Mohanty, D. P. Sahoo, L. Paramanik and K. Parida, Sustainable Energy Fuels, 2023, 7, 1145–1186 RSC.
- Y. Liu, Y. Guo, Y. Liu, Z. Wei, K. Wang and Z. Shi, Energy Fuels, 2023, 37, 2608–2630 CrossRef CAS.
- P. R. Chowdhury, H. Medhi, K. G. Bhattacharyya and C. M. Hussain, Coord. Chem. Rev., 2024, 501, 215547 CrossRef.
- D. K. Perivoliotis, J. Ekspong, X. Zhao, G. Hu, T. Wagberg and E. Gracia-Espino, Nano Today, 2023, 50, 101883 CrossRef CAS.
- Y. Liao, R. He, W. Pan, Y. Li, Y. Wang, J. Li and Y. Li, Chem. Eng. J., 2023, 464, 142669 CrossRef CAS.
- Z.-Q. Liu, X. Liang, F.-X. Ma, Y.-X. Xiong, G. Zhang, G. Chen, L. Zhen and C.-Y. Xu, Adv. Energy Mater., 2023, 13, 2203609 CrossRef CAS.
- R. Dai, C. Dai, S. Hou, Q. He, B. Liu, M. Huang, H. Jiang, M. Li, L. Pan and Z. Guo, J. Mater. Chem. A, 2023, 11, 20383–20407 RSC.
- D. Tyndall, M. J. Craig, L. Gannon, C. McGuinness, N. McEvoy, A. Roy, M. Garcia-Melchor, M. P. Browne and V. Nicolosi, J. Mater. Chem. A, 2023, 11, 4067–4077 RSC.
- A. Zitolo, V. Goellner, V. Armel, M.-T. Sougrati, T. Mineva, L. Stievano, E. Fonda and F. Jaouen, Nat. Mater., 2015, 14, 937–942 CrossRef CAS PubMed.
- J. Duan, S. Chen, M. Jaroniec and S. Z. Qiao, ACS Catal., 2015, 5, 5207–5234 CrossRef CAS.
- M. K. Uddin, Chem. Eng. J., 2017, 308, 438–462 CrossRef CAS.
- V. B. Yadav, R. Gadi and S. Kalr, J. Environ. Manage., 2019, 232, 803–817 CrossRef CAS PubMed.
- S. Manna, P. Das, P. Basak, A. K. Sharma, V. K. Singh, R. K. Patel, J. K. Pandey, V. Ashokkumar and A. Pugazhendhi, Chemosphere, 2021, 280, 130961 CrossRef CAS PubMed.
- S. Marchesi, F. Carniato, M. Guidotti, M. Botta, L. Marchese and C. Bisio, New J. Chem., 2020, 44, 10033–10041 RSC.
- S. Marchesi, S. Nascimbene, M. Guidotti, C. Bisio and F. Carniato, Dalton Trans., 2022, 51, 4502–4509 RSC.
- S. Marchesi, C. Bisio and F. Carniato, RSC Adv., 2020, 10, 29765–29771 RSC.
- T. Zhang, W. Wang, Y. Zhao, H. Bai, T. Wen, S. Kang, G. Song, S. Song and S. Komarneni, Chem. Eng. J., 2021, 420, 127574 CrossRef CAS.
- H. Bai, Y. Zhao, X. Zhang, W. Wang, T. Zhang, C. Liu, H. Yi and S. Song, J. Am. Ceram. Soc., 2019, 3908–3922 CrossRef CAS.
- H. Bai, Y. Zhao, W. Wang, T. Zhang, H. Yi and S. Song, Ceram. Int., 2019, 45, 17054–17063 CrossRef CAS.
- D. A. Almasri, N. B. Saleh, M. A. Atieh, G. McKay and S. Ahzi, Sci. Rep., 2019, 9, 1–13 CrossRef CAS PubMed.
- D. Yao, Y. Shi, H. Pan, D. Zhong, H. Hou, X. Wu, J. Chen, L. Wang, Y. Hu and J. C. Crittenden, Chem. Eng. J., 2020, 392, 123637 CrossRef CAS.
- X. Zhang, S. Lin, X. Q. Lu and Z. L. Chen, Chem. Eng. J., 2010, 163, 243–248 CrossRef CAS.
- Y. Zhang, Y. Li, J. Li, L. Hu and X. Zheng, Chem. Eng. J., 2011, 171, 526–531 CrossRef CAS.
- N. Arancibia-Miranda, S. E. Baltazar, A. García, D. Munoz-Lira, P. Sepúlveda, M. A. Rubio and D. Altbir, J. Hazard. Mater., 2016, 301, 371–380 CrossRef CAS PubMed.
- N. Ezzatahmadi, G. A. Ayoko, G. J. Millar, R. Speight, C. Yan, J. Li, S. Li, J. Zhu and Y. Xi, Chem. Eng. J., 2017, 312, 336–350 CrossRef CAS.
- M. R. Olson, T. C. Sale, C. D. Shackelford, C. Bozzini and J. Skeean, Groundwater Monit. Rem, 2012, 32, 63–74 CrossRef CAS.
- A. M. Awad, S. M. R. Shaikh, R. Jalab, M. H. Gulied, M. S. Nasser, A. Benamor and S. Adham, Sep. Purif. Technol., 2019, 228, 115719 CrossRef CAS.
- Y. Park, G. A. Ayoko and R. L. Frost, J. Colloid Interface Sci., 2011, 354, 292–305 CrossRef CAS PubMed.
- Q. Yang, M. Gao, Z. Luo and S. Yang, Chem. Eng. J., 2016, 285, 27–38 CrossRef CAS.
- G. Xue, M. Gao, Z. Gu, Z. Luo and Z. Hu, Chem. Eng. J., 2013, 218, 223–231 CrossRef CAS.
- A. Abutaleb, M. Imran, N. Zouli, A. H. Khan, S. Hussain, M. A. Ali, O. Bakather, M. A. Gondal, N. A. Khan, H. Panchal and S. Zahmatkesh, Chemosphere, 2023, 316, 137824 CrossRef CAS PubMed.
- C. V. Lazaratou, D. V. Vayenas and D. Papoulis, Appl. Clay Sci., 2020, 185, 105377 CrossRef CAS.
- A. Kausar, M. Iqbal, A. Javed, K. Aftab, Z. Nazli, H. N. Bhatti and S. Nouren, J. Mol. Liq., 2018, 256, 395–407 CrossRef CAS.
- M. A. Moreira, K. J. Ciuffi, V. Rives, M. A. Vicente, R. Trujillano, A. Gil, S. A. Korili and E. H. de Faria, Appl. Clay Sci., 2017, 135, 394–404 CrossRef CAS.
- T. Ngulube, J. R. Gumbo, V. Masindi and A. Maity, J. Environ. Manage., 2017, 191, 35–57 CrossRef CAS PubMed.
- H. Han, M. K. Rafiq, T. Zhou, R. Xu, O. Mašek and X. Li, J. Hazard. Mater., 2019, 369, 780–796 CrossRef CAS PubMed.
- N. Belhouchat, H. Zaghouane-Boudiaf and C. Viseras, Appl. Clay Sci., 2017, 135, 9–15 CrossRef CAS.
- H. Zaghouane-Boudiaf, M. Boutahala, S. Sahnoun, C. Tiar and F. Gomri, Appl. Clay Sci., 2014, 90, 81–87 CrossRef CAS.
- Q. Cui and B. Chen, Appl. Clay Sci., 2023, 235, 106869 CrossRef CAS.
- M. Arif, G. Liu, B. Yousaf, R. Ahmed, S. Irshad, A. Ashraf, M. Zia-ur-Rehman and M. S. Rashid, J. Cleaner Prod., 2021, 310, 127548 CrossRef CAS.
- M. M. Mian and G. Liu, Chem. Eng. J., 2020, 392, 123681 CrossRef CAS.
- K. S. D. Premarathna, A. Upamali, B. Sarkar and E. E. Kwon, Chem. Eng. J., 2019, 372, 536–550 CrossRef CAS.
- P. Pandey and V. K. Saini, Environ. Chem. Lett., 2019, 17, 721–727 CrossRef CAS.
- Z. Huang, P. Wu, B. Gong, Y. Dai, P. C. Chiang, X. Lai and G. Yu, PLoS One, 2016, 11, e0159802 CrossRef PubMed.
- M. Ding, S. Zuo and C. Qi, Appl. Clay Sci., 2015, 115, 9–16 CrossRef CAS.
- C. Liu, Z. Li, B. Li, H. Zhang and J. Han, Appl. Clay Sci., 2023, 236, 106887 CrossRef CAS.
- A. Bashir, S. Ahad, L. A. Malik, A. Qureashi, T. Manzoor, G. N. Dar and A. H. Pandith, Ind. Eng. Chem. Res., 2020, 59, 22353–22397 CrossRef CAS.
- M. Pica, Molecules, 2021, 26, 2392 CrossRef CAS PubMed.
- R. Chakraborty, K. Bhattacharaya and P. Chattopadhyay, Appl. Radiat. Isot., 2014, 85, 34–38 CrossRef CAS PubMed.
- W. J. Mu, Q. H. Yu, R. Zhang, X. L. Li, R. Hu, Y. He, H. Y. Wei, Y. Jian and Y. C. Yang, J. Mater. Chem. A, 2017, 5, 24388–24395 RSC.
- A. Bashir, L. A. Malik, G. N. Dar and A. H. Pandith, in Application of Ion Exchange Materials in the Environment, ed. A. M. Inamuddin and A. Assiri, Springer, Cham, 2019, pp. 95–108 Search PubMed.
- W. J. Mu, S. Z. Du, Q. H. Yu, X. L. Li, H. Y. Wei, Y. C. Yang and S. M. Peng, Chem. Eng. J., 2019, 361, 538–546 CrossRef CAS.
- T. Takei, K. Iidzuka, A. Miura, S. Yanagida, N. Kumada, E. Magome, C. Moriyoshi and Y. Kuroiwa, Langmuir, 2016, 32, 9993–9999 CrossRef CAS PubMed.
- Y. Cheng, X. D. Wang, S. Jaenicke and G. K. Chuah, Inorg. Chem., 2018, 57, 4370–4378 CrossRef CAS PubMed.
- S. Bevara, P. Giri, S. J. Patwe, S. N. Achary, R. K. Mishra, A. Kumar, A. K. Sinha, C. P. Kaushik and A. K. Tyagi, J. Environ. Chem. Eng., 2018, 6, 2248–2261 CrossRef CAS.
- Y. Cheng, S. S. Y. Chui, X. D. T. Wang, S. Jaenicke and G. K. Chuah, Inorg. Chem., 2019, 58, 13020–13029 CrossRef CAS PubMed.
- Y. Cheng, H. W. Zhang, J. A. Jaenicke, E. C. P. Tan and G. K. Chuah, ACS Sustainable Chem. Eng., 2019, 7, 895–904 CrossRef CAS.
- W. J. Mu, Q. H. Yu, J. Y. Gu, X. L. Li, Y. C. Yang, H. Y. Wei and S. M. Peng, Sep. Purif. Technol., 2020, 240, 116658 CrossRef CAS.
- A. M. Bakry, F. S. Awad, J. A. Bobb, A. A. Ibrahim and M. S. El-Shall, RSC Adv., 2020, 10, 37883–37897 RSC.
- S. Fujimoto, T. Takei, S. Yanagida and N. Kumada, J. Ceram. Soc. Jpn., 2019, 127, 830–836 CrossRef CAS.
- R. Silbernagel, C. H. Martin and A. Clearfield, Inorg. Chem., 2016, 55, 1651–1656 CrossRef CAS PubMed.
- M. W. Terban, C. Shi, R. Silbemagel, A. Clearfield and S. J. L. Billinge, Inorg. Chem., 2017, 56, 8837–8846 CrossRef CAS PubMed.
- S. Pourbeyram, Ind. Eng. Chem. Res., 2016, 55, 5608–5617 CrossRef CAS.
- T. Ahamad, M. Naushad, B. M. Al-Maswari, J. Ahmed, Z. A. Alothman, S. M. Alshehri and A. A. Alqadami, J. Ind. Eng. Chem., 2017, 53, 268–275 CrossRef CAS.
- X. L. Zhang, J. L. Shen, S. Y. Pan, J. S. Qian and B. C. Pan, Adv. Funct. Mater., 2020, 30, 1909014 CrossRef CAS.
- D. D. Zhao, Y. Yu and J. P. Chen, Water Res., 2016, 101, 564–573 CrossRef CAS PubMed.
- J. H. Xu, R. Koivula, W. Z. Zhang, E. Wiikinkoski, S. Hietala and R. Harjula, Hydrometallurgy, 2018, 175, 170–178 CrossRef CAS.
- Y. Ibrahim, E. Abdulkarem, V. Naddeo, F. Banat and S. W. Hasan, Sci. Total Environ., 2019, 690, 167–180 CrossRef CAS PubMed.
- R. Bhatt, V. Ageetha, S. B. Rathod and P. Padmaja, Carbohydr. Polym., 2019, 208, 441–450 CrossRef CAS PubMed.
- M. Thakur, D. Pathania, G. Sharma, M. Naushad, A. Bhatnagar and M. R. Khan, J. Polym. Environ., 2018, 26, 1415–1424 CrossRef CAS.
- Y. Zhu, T. Shimizu, T. Kitajima, K. Morisato, N. Moitra, N. Brun, T. Kiyomura, K. Kanamori, K. Takeda, H. Kurata, M. Tafu and K. Nakanishi, New J. Chem., 2015, 39, 2444–2450 RSC.
- Q. R. Zhang, Y. X. Li, P. Phanlavong, Z. K. Wang, T. F. Jiao, H. Qiu and Q. M. Peng, J. Cleaner Prod., 2017, 161, 317–326 CrossRef CAS.
- S. Qing, H. Chen, L. J. Han, Z. B. Ye, L. T. Shi, Z. Shu, L. Chen, L. Xu and Q. Q. Xu, ACS Omega, 2020, 5, 27873–27879 CrossRef CAS PubMed.
- Y. J. Zhou, J. J. Liu, M. Xiao, Y. Z. Meng and L. Y. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 5547–5555 CrossRef CAS PubMed.
- L. Ma, Q. Wang, S. M. Islam, Y. Liu, S. Ma and M. G. Kanatzidis, J. Am. Chem. Soc., 2016, 138(8), 2858–2866 CrossRef CAS PubMed.
- D. Cosano, D. Esquivel, F. J. Romero-Salguero, C. Jimenez-Sanchidrian and J. R. Ruiz, Langmuir, 2023, 39, 5294–5305 CrossRef CAS PubMed.
- A. Haleem, A. Shafiq, S.-Q. Chen and M. Nazar, Molecules, 2023, 28, 1081 CrossRef CAS PubMed.
- Y.-D. Dong, Y. Shi, Y.-L. He, S.-R. Yang, S.-Y. Yu, Z. Xiong, H. Zhang, G. Yao, C.-S. He and B. Lai, Ind. Eng. Chem. Res., 2023, 62, 10828–10848 CrossRef CAS.
- Z. Bi, R.-T. Guo, X. Hu, J. Wang, X. Chen and W.-G. Pan, Nanoscale, 2022, 14(9), 3367–3386 RSC.
- C. Zhao, L. Wang, L. Huang, N. M. Musyoka, T. Xue, J. Rabeah and Q. Wang, J. Energy Chem., 2024, 90, 435–452 CrossRef CAS.
- Z.-Y. Zhang, H. Tian, L. Bian, S.-Z. Liu, Y. Liu and Z.-L. Wang, J. Energy Chem., 2023, 83, 90–97 CrossRef CAS.
- X. Xu, J. Wang, A. Zhou, S. Dong, K. Shi, B. Li, J. Han and D. O'Hare, Nat. Commun., 2021, 12, 3069 CrossRef CAS PubMed.
- P. Zhang, J. Li, L. Lv, Y. Zhao and L. Qu, ACS Nano, 2017, 11, 5087–5093 CrossRef CAS PubMed.
- J. Xue, S. Ma, Y. Zhou, Z. Zhang and M. He, ACS Appl. Mater. Interfaces, 2015, 7(18), 9630–9637 CrossRef CAS PubMed.
- G. Mishra, B. Dash and S. Pandey, Appl. Clay Sci., 2018, 153, 172–186 CrossRef CAS.
- T. Hu, X. Mei, Y. Wang, X. Weng, R. Liang and M. Wei, Sci. Bull., 2019, 64, 1707–1727 CrossRef CAS PubMed.
- C. F. Gomes, J. H. Gomes and E. F. da Silva, Environ. Geochem. Health, 2020, 42, 3507–3527 CrossRef CAS PubMed.
- P. Das, S. Manna, A. K. Behera, M. Shee, P. Basak and A. K. Sharma, Environ. Res., 2022, 212, 113534–123546 CrossRef CAS PubMed.
- A. Sharma, G. R. Kokil, Y. He, B. Lowe, A. Salam, T. A. Altalhi, Q. Ye and T. Kumeria, Bioact. Mater., 2023, 24, 535–550 CAS.
- C. de Sousa Figueiredo Gomes, Environ. Geochem. Health, 2018, 40, 1739–1765 CrossRef PubMed.
- N. Khatoon, M. Q. Chu and C. H. Zhou, J. Mater. Chem. B, 2020, 8, 7335–7351 RSC.
- L. Jia, T. Zhou, J. Xu, Z. Xu, M. Zhang, Y. Wang, Z. Li and T. Zhu, J. Mater. Sci., 2016, 5, 1324–1332 CrossRef.
- S. Murugesan and T. Scheibel, J. Polym. Sci., 2021, 59, 1610–1642 CrossRef CAS.
- H. Tomás, C. S. Alves and J. Rodrigues, Nanomedicine, 2018, 14, 2407–2420 CrossRef PubMed.
- Y. M. Lvov, Y. M. M. DeVilliers and R. F. Fakhrullin, Expert Opin. Drug Delivery, 2016, 13, 977–986 CrossRef CAS PubMed.
- H. Y. Kim, J. H. Jae, H. Cheon, S. H. Lee, J. Y. Min, S.-Y. Back, J. G. Song, D. H. Kim, J.-J. Lim and H.-K. Han, J. Nanobiotechnol., 2020, 18, 17–31 CrossRef CAS PubMed.
- A. I. Voicu, S. A. Gârea, A. Ghebaur, C. L. Nistor, A. Sârbu, E. Vasile, R. Mitran and H. Iovu, Microporous Mesoporous Mater., 2021, 328, 111434 CrossRef CAS.
- F. García-Villén and C. Viseras, Pharmaceutics, 2020, 12, 1142–1150 CrossRef PubMed.
- C. Tipa, M. T. Cidade, T. Vieira, J. C. Silva, P. I. P. Soares and J. P. Borges, Nanomaterials, 2021, 11(1), 25–47 CrossRef CAS PubMed.
- K. Dutta, K. Saha, P. Sarkar and D. Chattopadhyay, Bull. Mater. Sci., 2020, 43(1), 248–257 CrossRef CAS.
- A. Rapacz-Kmita, B. Szaraniec, M. Mikołajczyk, E. Stodolak-Zych, E. Dzierzkowska, M. Gajek and P. Dudek, Acta Bioeng. Biomech., 2020, 22(2), 83–93 Search PubMed.
- C. F. Memenfo, N. Tabary, J. A. Mbey, S. Degoutin, F. Cazaux, M. Bacquet, M. Maton, B. Martel and D. Njopwouo, J. Mater. Sci. Chem. Eng., 2021, 9, 21–40 CAS.
- H.-J. Huang, S.-Y. Huang, T.-H. Wang, T.-Y. Lin, N.-C. Huang, O. Shih, U.-S. Jeng, C.-Y. Chu and W.-H. Chiang, Carbohydr. Polym., 2023, 302, 120390 CrossRef CAS PubMed.
- J. J. Zhang, F. Zhou, Z. He, Y. Pan, S. Zhou, C. Yan, L. Luo and Y. Gao, ACS Appl. Mater. Interfaces, 2022, 14(27), 30533–30545 CrossRef CAS PubMed.
- D. Peixoto, I. Pereira, M. Pereira-Silva, F. Veiga, M. R. Hamblin, Y. Lvov, M. Liu and A. C. Paiva-Santos, Coord. Chem. Rev., 2021, 440, 213956 CrossRef CAS.
- M. Massaro, C. G. Colletti, G. Lazzara and S. Riela, J. Funct. Biomater., 2018, 9, 58–70 CrossRef CAS PubMed.
- D. Sawicka, L. Zapor, L. Chojnacka-Puchta and K. Miranowicz-Dzierzawska, Toxicol. Res., 2021, 37, 301–331 CrossRef CAS PubMed.
- G. Biddeci, G. Spinelli, P. Colomba and F. Di Blasi, Int. J. Mol. Sci., 2023, 24(5), 4801–4822 CrossRef CAS PubMed.
- Q. Pan, N. Li, Y. Hong, H. Tang, Z. Zheng, S. Weng, Y. Zheng and L. Huang, RSC Adv., 2017, 7(34), 21352–21359 RSC.
- M. Barman, S. Mahmood, R. Augustine, A. Hasan, S. Thomas and K. Ghosal, Int. J. Biol. Macromol., 2020, 162, 1849–1861 CrossRef CAS PubMed.
- R. Shi, Y. Niu, M. Gong, J. Ye, W. Tian and L. Zhang, Mater. Sci. Eng., C, 2018, 87, 128–138 CrossRef CAS PubMed.
- C. Zagni, A. A. Scamporrino, P. M. Riccobene, G. Floresta, V. Patamia, A. Rescifina and S. C. Carroccio, Nanomaterials, 2023, 13, 741 CrossRef CAS PubMed.
- R. Arshad, M. S. Arshad, A. Rahdar, D. Hassan, R. Behzadmehr, S. Ghotekar, D. I. Medina and S. Pandey, J. Drug Delivery Sci. Technol., 2023, 83, 104432–104444 CrossRef CAS.
- K. Li, Y. Zhang, M. Chen, Y. Hu, W. Jiang, L. Zhou, S. Li, M. Xu, Q. Zhao and R. Wan, Int. J. Nanomed., 2018, 13, 19–30 CrossRef CAS PubMed.
- M. Perveen, S. Nazir, A. W. Arshad, M. I. Khan, M. Shamim, K. Ayub, M. A. Khan and J. Iqbal, Biophys. Chem., 2020, 267, 106461–106473 CrossRef CAS PubMed.
- E. Naumenko and R. Fakhrullin, Biotechnol. J., 2019, e1900055 CrossRef PubMed.
- E. Gkouma, E. Gianni, K. Avgoustaki and D. Papoulis, Appl. Clay Sci., 2021, 214, 106291 CrossRef CAS.
- L. W. Wong, C. B. S. Goh, P. Pasbakhsh and J. B. L. Tan, J. Sci.-Adv. Mater. Dev., 2022, 7, 100431–100445 CAS.
- F. Kazemi-Aghdam, V. Jahed, M. Dehghan-Niri, F. Ganji and E. Vasheghani-Farahani, Carbohydr. Polym., 2021, 269, 118311–118320 CrossRef CAS PubMed.
- M. A. Bonifacio, A. Cochis, S. Cometa, A. Scalzone, P. Gentile, G. Procino, S. Milano, A. C. Scalia, L. Rimondini and E. De Giglio, Mater. Sci. Eng., C, 2020, 108, 110444 CrossRef CAS PubMed.
- B. M. Dharmaraj, R. Subramani, G. Dhanaraj and K. Louis, Compos., Part C: Open Access, 2020, 3, 10007–10019 Search PubMed.
- S. Mehnath and M. Jeyaraj, Mater. Chem. Phys., 2023, 295, 127061–127074 CrossRef CAS.
- J. Ji, C. Wang, Z. Xiong, Y. Pang and W. Sun, Mater. Today Adv., 2022, 15, 100259–100271 CrossRef CAS.
- S. Marchesi, F. Carniato, C. Bisio, L. Tei, M. Botta and L. Marchese, Dalton Trans., 2018, 47, 7896–7904 RSC.
- S. Marchesi, D. Lalli, F. Carniato, C. Bisio, L. Tei, L. Marchese and M. Botta, Dalton Trans., 2020, 49, 6566–6571 RSC.
- T. Zhou, L. Jia, Y. F. Luo, J. Xu, R. H. Chen, Z. J. Ge, T. L. Ma, H. Chen and T. F. Zhu, J. Nanomed., 2016, 11, 4765–4776 CrossRef CAS PubMed.
- G. Basina, G. Diamantopoulos, E. Devlin, V. Psycharis, S. M. Alhassan, M. Pissas, G. Hadjipanayis, A. Tomou, A. Bouras, C. Hadjipanayis and V. Tzitzios, J. Mater. Chem. B, 2022, 10, 4935–4945 RSC.
- M. V. Ramos-Garcés, J. González-Villegas, A. López-Cubero and J. L. Colón, Acc. Mater. Res., 2021, 2, 793–803 CrossRef.
- A. Donnadio, G. Paul, M. Barbalinardo, V. Ambrogi, G. Pettinacci, T. Posati, C. Bisio, R. Vivani and M. Nocchetti, Nanomaterials, 2023, 13, 742 CrossRef CAS PubMed.
- M. Pica, A. Donnadio and M. Casciola, Coord. Chem. Rev., 2018, 374, 218–235 CrossRef CAS.
- M. L. González, M. Ortíz, C. Hernández, J. Cabán, A. Rodríguez, J. L. Colón and A. Báez, J. Nanosci. Nanotechnol., 2016, 16, 117–129 CrossRef PubMed.
- J. González-Villegas, Y. Kan, V. I. Bakhmutov, A. García-Vargas, M. Martínez, A. Clearfield and J. L. Colón, Inorg. Chim. Acta, 2017, 468, 270–279 CrossRef.
- H. Kalita, B. N. P. Kumar, S. Konar, S. Tantubay, M. K. Mahto, M. Mandal and A. Pathak, Mater. Sci. Eng., C, 2016, 60, 84–91 CrossRef CAS PubMed.
- H. Kalita, S. Rajput, B. N. P. Kumar, M. Mandal and A. Pathak, RSC Adv., 2016, 6, 21285 RSC.
- S. Yu, X. Gao, H. Baigude, X. Hai, R. Zhang, X. Gao, B. Shen, Z. Li, Z. Tan and H. Su, ACS Appl. Mater. Interfaces, 2015, 7, 5089–5096 CrossRef CAS PubMed.
- R. Hosseinzadeh and K. Khorsandi, Sci. Rep., 2019, 9, 14899 CrossRef PubMed.
- A. Donnadio, V. Ambrogi, D. Pietrella, M. Pica, G. Sorrentino and M. Casciola, RSC Adv., 2016, 6, 46249–46257 RSC.
- I. García, C. Trobajo, Z. Amghouz and A. Adawy, Materials, 2021, 14, 1481 CrossRef PubMed.
- M. Pica, N. Messere, T. Felicetti, S. Sabatini, D. Pietrella and M. Nocchetti, Materials, 2021, 14, 670 CrossRef CAS PubMed.
- H. Ueoka, O. Shimomura, M. Pica, A. Donnadio and R. Nomura, Colloids Interface Sci. Commun., 2019, 28, 29–33 CrossRef CAS.
- M. Bastianini, M. Sisani, A. Petracci, I. Di Guida, C. Faffa and F. Cardellini, Mater. Adv., 2021, 2, 1313–1319 RSC.
- M. Camara-Torres, S. Duarte, R. Sinha, A. Egizabal, N. Alvarez, M. Bastianini, M. Sisani, P. Scopece, M. Scatto, A. Bonetto, A. Marcomini, A. Sanchez, A. Patelli, C. Mota and L. Moroni, Bioact. Mater., 2021, 6, 1073–1082 CAS.
- H. Du, D. Zhang, F. Peng, K. W. K. Yeung and X. Liu, Prog. Mater. Sci., 2024, 142, 101220 CrossRef CAS.
- S. Kumari, V. Sharma, S. Soni, A. Sharma, A. Thakur, S. Kumar, K. Dhama, A. K. Sharma and S. K. Bhatia, Environ. Res., 2023, 238, 117171 CrossRef CAS PubMed.
- Y. Bian, X. Cai, Z. Lv, Y. Xu, H. Wang, C. Tan, R. Liang and X. Weng, Adv. Sci., 2023, 10, 2301806 CrossRef CAS PubMed.
- S. Karimi, H. Rasuli and R. Mohammadi, Int. J. Biol. Macromol., 2023, 234, 123538 CrossRef CAS PubMed.
- V. R. L. Constantino, M. P. Figueiredo, V. R. Magri, D. Eulalio, V. R. R. Cunha, A. C. S. Alcantara and G. F. Perotti, Pharmaceutics, 2023, 15, 413 CrossRef CAS PubMed.
- C. Nomicisio, M. Ruggeri, E. Bianchi, B. Vigani, C. Valentino, C. Aguzzi, C. Viseras, S. Rossi and G. Sandri, Pharmaceutics, 2023, 15, 1368 CrossRef CAS PubMed.
- Q. Li, X. Wu, S. Mu, C. He, X. Ren, X. Luo, M. Adeli, X. Han, L. Ma and C. Cheng, Adv. Sci., 2023, 10, 2207759 CrossRef CAS PubMed.
- J.-H. Yang, J.-H. Lee, H.-J. Ryu, A. A. Elzatahry, Z. A. Alothman and J.-H. Choy, Appl. Clay Sci., 2016, 130, 20–32 CrossRef CAS.
- G. Choi, S. Eom, A. Vinu and J.-H. Choy, Chem. Rec., 2018, 18, 1033–1053 CrossRef CAS PubMed.
- S. S. Shafiei, M. Shavandi, G. Ahangari and F. Shokrolahi, Appl. Clay Sci., 2016, 127, 52–63 CrossRef.
- F. Perreault, A. Fonseca de Faria, S. Nejati and M. Elimelech, ACS Nano, 2015, 9(7), 7226–7236 CrossRef CAS PubMed.
- G. Reina, J.-M. Gonzalez-Dominguez, A. Criado, E. Vazquez, A. Bianco and M. Prato, Chem. Soc. Rev., 2017, 46(15), 4400–4416 RSC.
- P. Das, S. Manna, A. K. Behera, M. Shee, P. Basak and A. K. Sharma, Environ. Res., 2022, 212, 113534–123546 CrossRef CAS PubMed.
- R. Mukhopadhyay, D. Bhaduri, B. Sarkar, R. Rusmin, D. Hou, R. Khanam, S. Sarkar, J. K. Biswas, M. Vithanage, A. Bhatnagar and Y. S. Ok, J. Hazard. Mater., 2020, 383, 121125 CrossRef CAS PubMed.
- X. Fu, J. Lin, Z. Liang, R. Yao, W. Wu, Z. Fang, W. Zou, Z. Wu, H. Ning and J. Peng, Surf. Interfaces, 2023, 37, 102747 CrossRef CAS.
- S. Gungordu Er, M. Edirisinghe and T. A. Tabish, Adv. Healthcare Mater., 2023, 12, 2201523 CrossRef CAS PubMed.
- X. You, Q. Zhang, J. Yang and S. Dong, Composites, Part A, 2023, 167, 107420 CrossRef CAS.
- S. Ren, M. Cui, C. Liu and L. Wang, Corros. Sci., 2023, 212, 110939 CrossRef CAS.
- Polymer Nanocomposites Containing Graphene: Preparation, Properties, and Applications, ed. M. Rahaman, L. Nayak, I. A. Hussein and N. C. Das, Woodhead Publishing, 2022 Search PubMed.
- C. Cheng, W. Song, Q. Zhao and H. Zhang, Nanotechnol. Rev., 2020, 9, 323–344 CrossRef.
- H. W. Huang, X. X. Sheng, Y. Q. Tian, L. Zhang, Y. Chen and X. Y. Zhang, Ind. Eng. Chem. Res., 2020, 59(35), 15424–15446 CrossRef CAS.
- H. Huang, M. Li, Y. Tian, Y. Xie, X. Sheng, X. Jiang and X. Zhang, Prog. Org. Coat., 2020, 138, 105390 CrossRef CAS.
- T. C. Huang, G. H. Lai, C. E. Li, M. W. Tsai, P. Y. Wang, Y. H. Chung and M. H. Lin, RSC Adv., 2017, 7(36), 22540–22540 RSC.
- S. L. Li, C. X. Zhao, H. L. Gou, Y. T. Li, X. J. He and L. Zhao, Int. J. Electrochem. Sci., 2018, 13(3), 2661–2675 CrossRef CAS.
- C. X. Zhao, P. Li, D. He, Y. T. Li, F. Lei and H. J. Sue, RSC Adv., 2016, 6, 73485–73495 RSC.
- Y. F. Xiao, J. Y. Xu, S. W. Huang and H. M. Deng, Int. J. Polym. Sci., 2017, 6034741 Search PubMed.
- K. Q. Li, H. M. Lei, X. R. Zeng, H. Q. Li, X. J. Lai and S. Y. Chai, RSC Adv., 2017, 7, 49290–49298 RSC.
- L. F. Xu, C. H. Lei, R. J. Xu, X. Q. Zhang and F. Zhang, Polym. Degrad. Stab., 2016, 133, 378–388 CrossRef.
- L. F. Xu, C. H. Lei, R. J. Xu, X. Q. Zhang and F. Zhang, RSC Adv., 2016, 6, 77545–77552 RSC.
- E. N. Kalali, S. De Juan, X. Wang, S. Nie, R. Wang and D.-Y. Wang, J. Therm. Anal., 2015, 121, 619–626 CrossRef CAS.
- D. D. Yang, Y. Hu, H. T. Li, L. Song, H. P. Xu and B. Li, J. Therm. Anal., 2015, 119, 619–624 CrossRef CAS.
- D. Wang, H. Y. Peng, B. Yu, K. Q. Zhou, H. F. Pan, L. P. Zhang, M. Li, M. Liu, A. L. Tian and S. H. Fu, Chem. Eng. J., 2020, 389, 124449 CrossRef.
- B. Ghanbarzadeh, S. A. Oleyaei and H. Almasi, Crit. Rev. Food Sci. Nutr., 2015, 55, 1699–1723 CrossRef CAS PubMed.
- D. Han, Y. Luo, Q. Ju, X. Xiao, M. Xiao, N. Xiao, S. Chen, X. Peng, S. Wang and Y. Meng, Polymers, 2018, 10, 1082 CrossRef PubMed.
- H. Oosthuizen, L. Jones, S. Naseem, F. J. W. J. Labuschagne and A. Leuteritz, J. Polym. Sci., 2023, 61, 1749–1777 CrossRef CAS.
- S. Kumari, S. Soni, Aj. Sharma, S. Kumar, V. Sharma, V. S. Jaswal, S. K. Bhatia and A. K. Sharma, Appl. Clay Sci., 2024, 247, 107216 CrossRef CAS.
- Y. Guo, F. Leroux, W. Tian, D. Li, P. Tang and Y. Feng, Appl. Clay Sci., 2021, 211, 106198 CrossRef CAS.
- A. A. Marek, V. Verney, G. Totaro, L. Sisti, A. Celli, N. Bozzi Cionci, D. Di Gioia, L. Massacrier and F. Leroux, Appl. Clay Sci., 2020, 188, 105502 CrossRef CAS.
- S. Coiai, F. Cicogna, S. Pinna, R. Spiniello, M. Onor, W. Oberhauser, M.-B. Coltelli and E. Passaglia, Appl. Clay Sci., 2021, 214, 106276 CrossRef CAS.
- Q. Zhang, Y. Guo, F. Leroux, P. Tang, D. Li, L. Wang and Y. Feng, New J. Chem., 2020, 44(24), 10119–10126 RSC.
- J. Zhang, Q. Ke, J. Bai and M. Yang, Polym. Degrad. Stab., 2023, 218, 110550 CrossRef CAS.
- L. Han, J. Geng, Z. Wang and J. Hua, Polym. Adv. Technol., 2022, 33, 3619–3627 CrossRef CAS.
- Y. Feng, Y. Jiang, Q. Huang, S. Chen, F. Zhang, P. Tang and D. Li, Ind. Eng. Chem. Res., 2014, 53(6), 2287–2292 CrossRef CAS.
- Q. Zhang, Y. Guo, A. A. Marek, V. Verney, F. Leroux, P. Tang, D. Li and Y. Feng, Inorg. Chem. Front., 2019, 6, 2539–2549 RSC.
- Q. Zhang, Q. Gu, F. Leroux, P. Tang, D. Li and Y. Feng, Beilstein J. Nanotechnol., 2018, 9, 2980–2988 CrossRef CAS PubMed.
- N. K. Kumbhar, M. D. Joshi, V. Kumar and S. S. Hosmani, Adv. Eng. Mater., 2023, 25, 2201680 CrossRef CAS.
- A. Chaouiki, M. Chafiq, T. Suhartono and Y. G. Ko, Chem. Eng. J., 2023, 470, 144355 CrossRef CAS.
- F. G. Nunes Filho, E. C. Silva Filho, J. A. Osajima, A. P. de Melo Alves and M. G. Fonseca, Appl. Clay Sci., 2023, 239, 106952 CrossRef CAS.
- Z. Chen, G.-F. Han, A. Mahmood, J. Hou, W. Wei, H. K. Shon, G. Wang, T. D. Waite, J.-B. Baek and B.-J. Ni, Prog. Mater. Sci., 2024, 145, 101299 CrossRef CAS.
- D. Evis, M. M. Ba-Abbad, A. Benamor and M. H. El-naas, Appl. Clay Sci., 2022, 229, 106686 CrossRef.
- C. Zhu, J. Jiang, Y. Jia, Z. P. Xu and L. Zhang, Acc. Mater. Res., 2023, 4, 758–771 CrossRef CAS.
- H. Desai, A. Kannan and G. S. K. Reddy, Microporous Mesoporous Mater., 2023, 352, 112488 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |