Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of the structural, electrochemical, and spectroscopic properties of two cryptates of trivalent uranium†
D. Nuwangi
Kulasekara
a,
Matthew D.
Bailey
 a,
Cassandra L.
Ward
b and
Matthew J.
Allen
a,
Cassandra L.
Ward
b and
Matthew J.
Allen
 *a
*a
aDepartment of Chemistry, Wayne State University, 5101 Cass Avenue, Detroit, MI 48202, USA. E-mail: mallen@chem.wayne.edu
bLumigen Instrument Center, Wayne State University, 5101 Cass Avenue, Detroit, MI 48202, USA
First published on 2nd May 2024
Abstract
We describe a study of the influence of cryptand denticity on the structural, electronic, and electrochemical properties of UIII-containing cryptates. Two cryptands (2.2.2 and 2.2.1) are reported. The cryptand with the smaller denticity leads to negative electrochemical potentials and shorter bond lengths that are consistent with a better fit for UIII than the larger cryptand. These studies provide insight into the rational design of cryptand-based ligands for trivalent uranium.
Introduction
The accumulation of depleted uranium as a waste product from uranium enrichment encourages the development of research focused on uranium coordination chemistry.1–3 Reported studies in this area provide insight into potential uses of depleted uranium and fundamental knowledge of uranium coordination chemistry involved in applications such as actinide separations in nuclear waste.4–8 Within this context, there have been widespread reports of redox-active and redox-inactive ligands used to form complexes of UIII,9–16 and one of those ligands, 2.2.2-cryptand, has been widely used to encapsulate metal ions, including UIII.17–19 Further, the coordination chemistry of UIII, NpIII, and PuIII with 2.2.2-cryptand has been reported recently,20 expanding cryptand chemistry into the actinides. The thermodynamic and kinetic stability of a cryptate is governed by the cavity size and denticity of the coordinated cryptand as well as the ionic radii and oxidation state of the given metal ion.21–24 For example, 2.2.1-cryptand fits better with EuIII, and 2.2.2-cryptand fits better with EuII; moreover, the Gibbs free energy of EuIII(2.2.1-cryptand) is 1.8 times greater than that of EuIII(2.2.2-cryptand), and the dissociation constant of EuIII(2.2.1-cryptand) is 2.7 × 103 times smaller than that of EuIII(2.2.2-cryptand).24 Because the size of UIII (1.025 Å) is closer to the size of EuIII (0.947 Å) than EuII (1.17 Å)25 and the charge density of UIII is closer to EuIII than EuII, we suspected that 2.2.1-cryptand would be a good ligand for UIII. Additional support for this suspicion is in our recent report that the flexible counterpart of 2.2.2-cryptand, tris[2-(2-methoxyethoxy)ethyl]amine (TDA-1), forms UIII-containing complexes with smaller coordination numbers (nine) compared to all reported 2.2.2-cryptates (with coordination numbers of ten).26 This report of an acyclic ligand implies that trivalent uranium can be encapsulated by cryptands with smaller denticities than that of 2.2.2-cryptand. Therefore, based on the studies of EuIII cryptand chemistry and UIII chemistry with acyclic TDA-1, we hypothesized that 2.2.1-cryptand is a better match for UIII than 2.2.2-cryptand. Here, we report UIII-containing cryptates of 2.2.2- and 2.2.1-cryptand (Fig. 1) to investigate how ligand denticity affects the structural, spectroscopic, and electrochemical properties of UIII.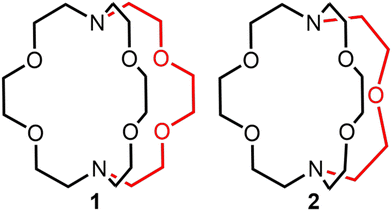 | ||
| Fig. 1 2.2.2-Cryptand, 1, and 2.2.1-cryptand, 2, that were studied with UIII. The red color highlights the difference between the two ligands. | ||
Results and discussion
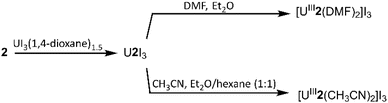
To evaluate the structural properties of UIII-containing cryptates, crystals were grown from N,N-dimethylformamide (DMF) or acetonitrile (CH3CN) (Schemes 1 and 2), and the structures of [UIII1(DMF)2]I3, [UIII2(CH3CN)2]I3, and [UIII2(DMF)2]I3 were solved from the crystals (Fig. 2). All three complexes contained coordinated solvent molecules. The structure with 1 is like reported structures of 2.2.2-cryptates of trivalent uranium that share ten-coordinate structures with bound solvent molecules, iodide,18,20 triflate,22 or water molecules.18,20 The geometry of [UIII1(DMF)2]I3, analyzed by SHAPE (v.2.1),27 is sphenocorona. We also performed SHAPE analysis for the reported structure of [UIII1I(CH3CN)]I2 with one inner-sphere iodide and one inner-sphere molecule of CH3CN (CCDC number 2020050),19 and we found that it also has the sphenocorona geometry. The crystal structure that we report here contains two coordinated molecules of DMF instead of iodide, water, or triflate, but the change in monodentate donors does not change the geometry about UIII. In contrast to [UIII1(DMF)2]I, UIII with 2 adopts a nine-coordinate structure with two molecules of CH3CN {[UIII2(CH3CN)2]I3} or two molecules of DMF {[UIII2(DMF)2]I3} coordinated to the opposite face of uranium relative to the single-oxygen-bearing arm of 2. Both [UIII2(CH3CN)2]I3 and [UIII2(DMF)2]I3 possess spherical-relaxed capped cube geometries as determined by SHAPE (v.2.1). UIII-containing complexes of 1 and 2 exhibit poor solubility in ethereal and nonpolar solvents, and for this reason, DMF and CH3CN were used for crystallization. Comparison of bond lengths between [UIII2(CH3CN)2]I3 and [UIII2(DMF)2]I3 reveals no noticeable variation in bond lengths between uranium and the donor atoms in 2; however, the U–ODMF bond in the complex with coordinated DMF is shorter than the U–NCH3CN bond in the complex with coordinated CH3CN, as expected based on the difference in size between O and N (Table 1). Similarly, U–Oether bonds in [UIII2(CH3CN)2]I3, [UIII2(DMF)2]I3, and [UIII1(DMF)2]I3 are shorter than U–Namine bonds. The complexes [UIII2(CH3CN)2]I3 and [UIII2(DMF)2]I3 each contain U–Oether and U–Namine bonds that are 0.1–0.2 Å shorter than similar bonds in [UIII1(DMF)2]I3. However, U–ODMF bond lengths in [UIII1(DMF)2]I3 and [UIII2(DMF)2]I3 do not exhibit large differences relative to each other. The U–Oether and U–Namine bonds in [UIII2(CH3CN)2]I3 and [UIII2(DMF)2]I3 are shorter than analogous bonds in reported UIII-containing complexes with TDA-1.26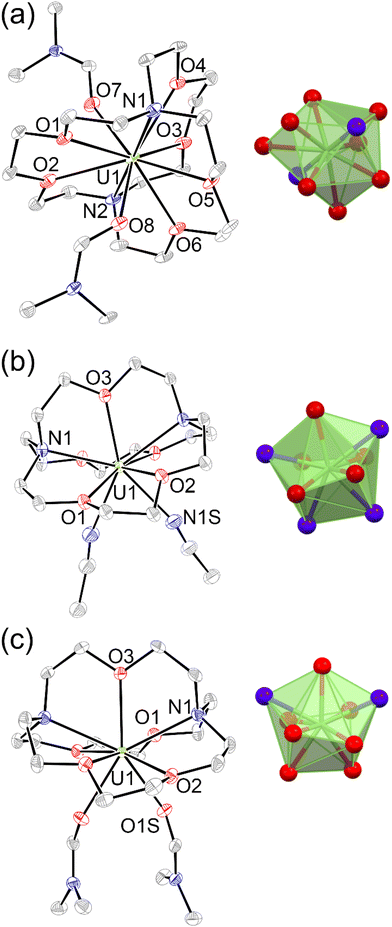 | ||
| Fig. 2 Molecular structures in crystals of (a) [UIII1(DMF)2]I3, (b) [UIII2(CH3CN)2]I3, and (c) [UIII2(DMF)2]I3. Thermal ellipsoids are drawn at 50% probability. Hydrogen atoms and iodide counter ions are omitted for clarity. Blue = nitrogen; red = oxygen; gray = carbon; and green = uranium. Crystallographic data for these structures have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC (2248985–2248987†). | ||
| Average U–donor distances (Å) | ||||
|---|---|---|---|---|
| Complex | U–O(ether) | U–N(amine) | U–I | U–S(solvent) |
| NA = not applicable. | ||||
| [UIII1(DMF)2]I3 | 2.656(5) | 2.790(6) | NA | 2.434(6) |
| [UIII2(DMF)2]I3 | 2.547(3) | 2.683(3) | NA | 2.471(3) |
| [UIII2(CH3CN)2]I3 | 2.507(3) | 2.661(3) | NA | 2.644(4) |
[UIII1(I)(CH3CN)]I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
2.640(2) | 2.818(3) | 3.2594(7) | 2.647(3) |
[UIII(TDA-1)(I)2]I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
2.605(6) | 2.767(7) | 3.2045(7) | NA |
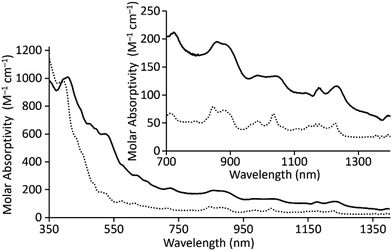
In addition to solid-state characterization, electronic spectroscopic characterization was performed for U1I3 and U2I3 to analyze how ligand denticity influences solution properties of the complexes. We performed UV-visible and near-IR experiments using elementally pure UIII-containing complexes of 1 and 2 that does not include coordinated solvent molecules. Those complexes are referred to as U1I3 and U2I3. UV-visible and near-IR electronic absorption data were collected from 350 to 1400 nm in CH3CN (Fig. 3). In the visible region, color-producing bands in U1I3 appeared for the green-yellow solutions in acetonitrile with maximum absorbances at 395 nm (ε = 978 M−1 cm−1). U2I3 forms reddish-pink solutions in CH3CN with color-producing bands having maxima at 407 nm (ε = 1008 M−1 cm−1) with shoulders at 521 nm (ε = 603 M−1 cm−1). Weak bands appear in the near-IR region for U1I3 and U2I3 like the other complexes of trivalent uranium with 5f3 electronic configurations with Laporte-forbidden f–f transitions.18,28,29 These bands in near IR-region are broader than the UI3 bands in acetonitrile (Fig. S3†) and the reported acyclic UIIITDA-1.26 The differences in the optical properties of UIII1I3 and UIII2I3 in solution, specifically the large differences in shifts of color producing bands, indicate an influence of the change of ligand denticity from octadentate to heptadentate on 5f–6d orbital energy gaps.
To study the electrochemical behavior of UIII-containing cryptates, cyclic voltammetry was performed for U1I3 and U2I3 in CH3CN (Fig. 4). Oxidation peaks corresponding to the UIII/IV couple appear at −0.26 V versus ferrocene/ferrocenium (Fc/Fc+) for U1I3. The oxidation potential of the UIII/IV couple of U1I3 in CH3CN is like the reported oxidation potential (−0.31 V versus Fc/Fc+) of UIII2.2.2-cryptate.19 The cyclic voltammogram of U2I3 contains an oxidation peak corresponding to the UIII/IV couple at −0.46 V versus Fc/Fc+. Both U1I3 and U2I3 contain an oxidation peak corresponding to a UIII-to-UIV oxidation that is not observed in the voltammogram of UI3 (Fig. S2†). Cyclic voltammograms of U1I3 and U2I3 were performed using elementally pure powdered compounds that do not contain coordinated solvent molecules. Both U1I3 and U2I3 can coordinate at least one acetonitrile molecules in that solid state as evidenced by reported crystal structures19 and this study. Similarly, in solution, acetonitrile can coordinate to U1I3, U2I3, and UI3. However, cyclic voltammetry of U1I3, U2I3, and UI3 in this study were performed in the same solvent, acetonitrile; consequently, the variability in shifts of oxidation potentials that arises from the coordination of solvent is minimized. Therefore, the observed 0.2 V difference in oxidation potentials between U1I3 and U2I3 in acetonitrile most likely arises from changes in the ionization energies resulting from changes in the ligand structure. These results indicate that oxidation potentials of U2I3 shift to more negative potentials with the decrease of coordination number compared to the octadentate cryptand in U1I3. The negative shift in oxidation potential is likely due to the smaller denticity of 2 compared to 1 and consequent size match between UIII and 2. A similar relationship is observed in the reported study between EuIII-containing complexes of 1 and 2.30 The formal potential of EuIII2 (−425 mV versus saturated calomel electrode) is more negative than that of EuIII1 (−225 mV versus saturated calomel electrode). Therefore, the negative shift in cyclic voltammetry of U2I3 is consistent with the electrochemistry of 4f systems. Interestingly, U1I3 and U2I3 have electrochemical potentials among the most positive of reported UIII/IV couples of monometallic complexes of uranium.31 The reported UIII complexes that coordinated to negatively charged donor atoms increase the electron density of uranium and consequently result in more negative electrochemical potentials.31 Therefore, the observed positive shift in electrochemical potential is not surprising for UIII-containing cryptates of 1 and 2 when compared to complexes of cyclopentadienyls, bis(trimethylsilyl)amides, tris(aryloxides), and β-diketiminates.31 However, to the best of our knowledge, the relationship of cryptand denticity on electrochemical studies of actinide series has not been reported; therefore, the insight gained from the electrochemical studies described here provides information regarding the influence of cryptand denticity on the tuning of the electrochemical potential of UIII.
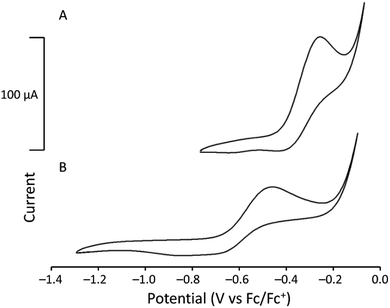 | ||
| Fig. 4 Cyclic voltammograms of (A) [UIII1]I3 (3.36 mM) and (B) [UIII2]I3 (3.42 mM) in CH3CN (scan rate = 100 mV s−1). | ||
Experimental
General methods
All air- and moisture-sensitive reactions were performed using standard Schlenk technique with Ar or using an inert atmosphere dry glove box under an atmosphere of N2. Commercially available anhydrous solvents were used for air- and moisture-sensitive reactions after degassing under reduced pressure and drying over activated molecular sieves (CH3CN with 3 Å sieves, diethyl ether and DMF with 4 Å sieves, and tetrahydrofuran and 1,4-dioxane with 5 Å sieves).Depleted uranium was purchased from Manufacturing Science Cooperation (Oak Ridge, TN) and purified following a reported procedure.32 UI3(1,4-dioxane)1.5 was synthesized following a reported procedure.32 4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane (1) and 4,7,13,16,21-pentaoxa-1,10-diazabicyclo[8.8.5]tricosane (2) were purchased from commercial sources.
Elemental analyses were performed by the Microanalytical Facility at the University of California, Berkeley. Electronic absorption spectra were collected using a Jasco UV-Vis-NIR spectrophotometer, and metal concentrations were determined using energy-dispersive X-ray fluorescence spectroscopy with a Shimadzu EDX-7000 spectrometer at the Lumigen Instrument Center in the Department of Chemistry at Wayne State University.
Cyclic voltammetry was performed using a three-electrode setup with a Pine Wavenow USB potentiostat under an atmosphere of Ar with a glassy carbon working electrode, a freshly prepared Ag/AgCl wire pseudo reference electrode (Ag wire with AgCl coating was prepared by dipping a polished Ag wire in bleach for 5–10 min), and a Pt-wire auxiliary electrode. Acquisition parameters of [UIII1]I3 and [UIII2]I3 were eight segments, an initial potential of −0.5 or 0.0 V (rising), an upper potential of 0.7 V, a lower potential of −0.5 V or 0.0 V against Ag/AgCl pseudo reference electrode, and a scan rate of 100 mV s−1. Tetrabutylammonium trifluoromethanesulfonate (0.1 M) was used as the electrolyte, and analyte concentrations were 3.0–3.5 mM. All cyclic voltammograms were recorded in CH3CN referenced to an internal standard of Fc/Fc+.
A crystal of [UIII1(DMF)2]I3 was mounted on a Bruker X8 APEX-II diffractometer with Mo radiation and a graphite monochromator. The X-ray diffraction intensities were measured using a Bruker APEX-II charge-couple device detector. Crystals of [UIII2(DMF)2]I3 and [UIII2(CH3CN)2]I3 were mounted on a Bruker D8 Venture diffractometer with kappa geometry, an Incoatec IμS micro-focus source X-ray tube (Mo Kα radiation), and a multilayer mirror for monochromatization. The X-ray diffraction intensities were measured using a Photon III CPAD area detector. Data for [UIII1(DMF)2]I3, [UIII2(DMF)2]I3, and [UIII2(CH3CN)2]I3 were acquired at 100 K with an Oxford 800 Cryostream low-temperature apparatus. The intensities were integrated using SAINT V8.38a software. A multiscan absorption correction was applied with SADABS v2016/2 using APEX4 v2021.10-0. Crystal structures were solved using a dual-space approach as implemented in SHELXT program33 and difference Fourier maps during least-squares refinement, as embedded in SHELXL-2018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 running under Olex2.35
34 running under Olex2.35
Synthesis of UIII-containing cryptates
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) into a solution of UIII2I3 (∼20 mg) in CH3CN (∼1.5 mL).
1) into a solution of UIII2I3 (∼20 mg) in CH3CN (∼1.5 mL).
Conclusions
This study reports differences in the structural, spectroscopic, and electrochemical properties of UIII encapsulated into neutral, redox-inactive cryptands. The smaller denticity and cavity size of heptadentate 2 enabled greater bonding interactions with UIII compared to octadentate 1. This evidence of a favorable size match between 2.2.1-cryptand and UIII is supported by shorter bond lengths and negative shifts of the electrochemical potentials of UIII2 compared to UIII1. These findings provide valuable insight into the encapsulation of trivalent uranium that has potential use areas such as actinide separations relevant to the management of radioactive waste.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the ACS-PRF (59577-ND3) for funding. NMR spectroscopy and X-ray crystallography were performed in the Lumigen Instrument Center at Wayne State University that is partially supported by the National Institutes of Health (3R01EB027103-02S1 and S10OD028488). We thank Professor Long Luo for helpful discussions.Notes and references
- Q. Y. Zhang, L. J. Zhang, J. Q. Zhu, L. L. Gong, Z. C. Huang, F. Gao, J. Q. Wang, X. Q. Xie and F. Luo, Nat. Commun., 2024, 15, 453 CrossRef CAS PubMed.
- M. A. Boreen and J. Arnold, Dalton Trans., 2020, 49, 15124–15138 RSC.
- B. M. T. C. Peluzo and E. Kraka, Int. J. Mol. Sci., 2022, 23, 4655 CrossRef PubMed.
- D. R. Hartline and K. Meyer, JACS Au, 2021, 1, 698–709 CrossRef CAS PubMed.
- M. G. Ferrier, B. C. Childs, C. M. Silva, M. M. Greenough, E. E. Moore, K. A. Erickson, M. J. Monreal, C. A. Colla, M. A. T. Marple, L. D. Winston, J. N. Burks, A. Martin, J. R. Jeffries and K. S. Holliday, Inorg. Chem., 2024, 63, 1938–1946 CrossRef CAS PubMed.
- J. C. Wedal, J. Murillo, J. W. Ziller, B. L. Scott, A. J. Gaunt and W. J. Evans, Inorg. Chem., 2023, 62, 5897–5905 CrossRef CAS PubMed.
- J.-B. Lu, X.-L. Ma, J.-Q. Wang, Y.-F. Jiang, Y. Li, H.-S. Hu, H. Xiao and J. Li, Inorg. Chem., 2019, 58, 7433–7439 CrossRef CAS PubMed.
- N. Kaltsoyannis, Inorg. Chem., 2013, 52, 3407–3413 CrossRef CAS PubMed.
- M. Mazzanti, R. Wietzke, J. Pécaut, J.-M. Latour, P. Maldivi and M. Remy, Inorg. Chem., 2002, 41, 2389–2399 CrossRef CAS PubMed.
- I. Castro-Rodriguez, K. Olsen, P. Gantzel and K. Meyer, J. Am. Chem. Soc., 2003, 125, 4565–4571 CrossRef CAS PubMed.
- S. J. Kraft, P. E. Fanwick and S. C. Bart, Inorg. Chem., 2010, 49, 1103–1110 CrossRef CAS PubMed.
- P. L. Arnold, C. J. Stevens, J. H. Farnaby, M. G. Gardiner, G. S. Nichol and J. B. Love, J. Am. Chem. Soc., 2014, 136, 10218–10221 CrossRef CAS PubMed.
- D. K. Modder, R. Scopelliti and M. Mazzanti, Inorg. Chem., 2024 DOI:10.1021/acs.inorgchem.3c03668 , online ahead of print.
- T. Cantat, B. L. Scott, D. E. Morris and J. L. Kiplinger, Inorg. Chem., 2009, 48, 2114–2127 CrossRef CAS PubMed.
- J. Hochholzer, P. Waldschmidt, F. W. Heinemann, H. Grützmacher and K. Meyer, Eur. J. Inorg. Chem., 2024, e202300592 CrossRef CAS.
- D. Pividori, M. E. Miehlich, B. Kestel, F. W. Heinemann, A. Scheurer, M. Patzschke and K. Meyer, Inorg. Chem., 2021, 60, 16455–16465 CrossRef CAS PubMed.
- J. I. Bullock and A. E. Storey, Inorg. Chim. Acta, 1979, 36, L399–L400 CrossRef CAS.
- D. N. Huh, C. J. Windorff, J. W. Ziller and W. J. Evans, Chem. Commun., 2018, 54, 10272–10275 RSC.
- D. N. Huh, J. M. Barlow, S. R. Ciccone, J. W. Ziller, J. Y. Yang and W. J. Evans, Inorg. Chem., 2020, 59, 17077–17083 CrossRef CAS PubMed.
- C. A. P. Goodwin, S. R. Ciccone, S. Bekoe, S. Majumdar, B. L. Scott, J. W. Ziller, A. J. Gaunt, F. Furche and W. J. Evans, Chem. Commun., 2022, 58, 997–1000 RSC.
- N.-D. H. Gamage, Y. Mei, J. Garcia and M. J. Allen, Angew. Chem., Int. Ed., 2010, 49, 8923–8925 CrossRef CAS PubMed.
- B. G. Cox, N. Van Truong, J. Garcia-Rosas and H. Schneider, J. Phys. Chem., 1984, 88, 996–1001 CrossRef CAS.
- X. X. Zhang, R. M. Izatt, J. S. Bradshaw and K. E. Krakowiak, Coord. Chem. Rev., 1998, 174, 179–189 CrossRef CAS.
- E. L. Yee, O. A. Gansow and M. J. Weaver, J. Am. Chem. Soc., 1980, 102, 2278–2285 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, A32, 751–767 CrossRef CAS.
- S. S. Bokouende, D. N. Kulasekara, S. A. Worku, C. L. Ward, A. B. Kajjam, J. C. Lutter and M. J. Allen, Inorg. Chem., 2023 DOI:10.1021/acs.inorgchem.3c02752 , online ahead of print.
- M. Llunell, D. Casanova, J. Cirera, P. Alemany and S. Alvarez, SHAPE, version 2.1, Barcelona, Spain, 2013 Search PubMed.
- C. L. Clark, J. J. Lockhart, P. E. Fanwick and S. C. Bart, Chem. Commun., 2015, 51, 14084–14087 RSC.
- L. R. Avens, S. G. Bott, D. L. Clark, A. P. Sattelberger, J. G. Watkin and B. D. Zwick, Inorg. Chem., 1994, 33, 2248–2256 CrossRef CAS.
- O. A. Gansow, A. R. Kausar, K. M. Triplett, M. J. Weaver and E. L. Yee, J. Am. Chem. Soc., 1977, 99, 7087–7089 CrossRef CAS.
- J. Riedhammer, D. P. Halter and K. Meyer, Chem. Rev., 2023, 123, 7761–7781 CrossRef CAS PubMed.
- M. J. Monreal, R. K. Thomson, T. Cantat, N. E. Travia, B. L. Scott and J. L. Kiplinger, Organometallics, 2011, 30, 2031–2038 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, A71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, C71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, SHAPE analysis, cyclic voltammetry, and electronic absorption spectroscopy. CCDC 2248985–2248987. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00521j |
| This journal is © The Royal Society of Chemistry 2024 |