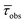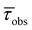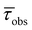Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A stable and true-blue emissive hexacoordinate Si(IV) N-heterocyclic carbene complex and its use in organic light-emitting diodes†
Thibault
Thierry‡
a,
Valerio
Giuso‡
 a,
Federico
Polo
a,
Federico
Polo
 bc,
Pierluigi
Mercandelli
bc,
Pierluigi
Mercandelli
 d,
Yi-Ting
Chen
e,
Chih-Hao
Chang
d,
Yi-Ting
Chen
e,
Chih-Hao
Chang
 e,
Matteo
Mauro
e,
Matteo
Mauro
 *a and
Stéphane
Bellemin-Laponnaz
*a and
Stéphane
Bellemin-Laponnaz
 *a
*a
aInstitut de Physique et Chimie des Matériaux de Strasbourg UMR 7504 - Université de Strasbourg & CNRS, 23 rue du Loess, 67034 Strasbourg, France. E-mail: bellemin@unistra.fr; mauro@unistra.fr
bDepartment of Molecular Sciences and Nanosystems, Ca’ Foscari University of Venice, Via Torino 155, 30172 Venice, Italy
cEuropean Centre for Living Technology (ECLT), Ca’ Foscari University of Venice, Ca’ Bottacin, 30124, Venice, Italy
dUniversità degli Studi di Milano, Dipartimento di Chimica, 20133 Milan, Italy
eDepartment of Electrical Engineering, Yuan Ze University, 32003 Taoyuan, Taiwan
First published on 8th March 2024
Abstract
A neutral hexacoordinate Si(IV) complex containing two tridentate N-heterocyclic carbene ligands is synthesised and characterized by X-ray crystallography, optical spectroscopy, electrochemistry and computational methods. The stable compound exhibits remarkable deep-blue photoluminescence particularly in the solid state, which enables its use as an electroluminescent material in organic light-emitting diodes.
Introduction
Organosilicon compounds are an interesting class of photo-functional species with appealing application as electroluminescent and electron transport materials as well as photodynamic therapy agents.1 Selected examples are displayed in Chart 1. The majority of organosilicon derivatives are conjugated molecules that contain Si–C bond(s), and their unique optoelectronic properties have been reported to date.2–5 Hypercoordinated Si derivatives for such applications are much less explored. An interesting example is based on Si(IV) phthalocyanines where the tetracoordinated macrocycle helps stabilize the otherwise labile Si–N bonds.6 Little efforts have been devoted to the stabilization of silicon complexes using chelating bi- and/or tridentate architectures with C–Si, O–Si and N–Si bonds. Tetracoordinate N–Si complexes containing 1,2-bis(indol-2-yl)benzene have shown blue photoluminescence with high efficiency.7 Dipyrromethene moieties have been used to form tetracoordinated Si complexes8,9 and adjacent hydroxy anchors gave pentacoordinate O^N^N^O complexes with efficient red and near-IR luminescence.10,11 More recently, hexacoordinate N–Si complexes featuring two 2,6-bis(benzimidazol-2-yl)pyridine pincer-type ligands have been prepared and their excellent optoelectronic properties and thermal stability have enabled their use in Organic Light-Emitting Diodes (OLEDs) and photovoltaic devices.12,13 Silicon remains very attractive as a tetravalent, earth-abundant, non-toxic lightweight atom and it often has significant influence on the optical and electronic properties. On the other hand, there is currently great interest in developing stable deep-blue emitters for light-emitting devices. Despite these promising recent examples, the coordination chemistry of Si(IV) chelates is still overlooked most likely due to their supposed instability. | ||
| Chart 1 Selected examples of tetra-, penta- and hexacoordinate Si complexes with remarkable photophysical properties and the molecular structure of the Si(OCO)2 complex. | ||
N-Heterocyclic carbenes (NHCs) have been recognised as privileged ligands in organometallic chemistry of transition metals15–17 and to provide robust (electro)luminescent materials for optoelectronics.18,19 Interestingly, the use of NHCs to stabilize Si(IV) is gaining increasing attention.14 Although NHCs can stabilize low valence states and induce new reactivities,20–24 they have been mostly used with halogenated Si(IV) reagents to exploit hypervalent pentacoordinate Si(IV) intermediates and, more recently, to stabilize less common Si(IV) alkoxysilanes with highly electronegative bis-trifluoroalkoxy ligands. Pentacoordinate NHC–Si intermediates have been postulated to account for the stability of free NHC carbenes in silicones as well.25
A few years ago we introduced a new family of LX2pincer-type NHC ligands with the O^C^O motif, namely (OCO)H3, characterised by a central imidazolylidene NHC moiety with two lateral chelating phenolic groups (see Scheme 1).26,27 This ligand has proven to be very versatile and robust for coordinating oxophilic metals with high oxidation states such as Ti(IV), Zr(IV) and Hf(IV).28–32 Herein, we report the synthesis and comprehensive characterization of a homoleptic, neutral, hexacoordinate luminescent complex, namely Si(OCO)2, which displays high thermal and photo-stability in solution and in the solid state under air and moisture. The optical properties are investigated and elucidated also with the help of computational approaches. Finally, proof-of-concept OLED devices are successfully fabricated, and the electroluminescence performances are described.
Results and discussion
The complex Si(OCO)2 was straightforwardly synthesized from SiCl4 and the corresponding benzimidazolium precursor (OCO)H3 resulting in a robust and air stable compound that can be easily purified by column chromatography (Scheme 1 and the ESI for experimental details†).33 NMR data are reported in Fig. S1–S3 of the ESI.† The 1H NMR spectrum displays a highly symmetric resonance pattern consistent with an average D2d symmetry of the molecule, expectedly, as shown in Fig. S1 in the ESI.†29Si NMR spectroscopy confirms the hexacoordinate nature of the silicon with a δ = −197.4 ppm (Fig. S3†), a chemical shift in the same range as the hexacoordinated complex reported by Schmedake and coworkers which displays a δ = –186 ppm (see Chart 1).12Single crystals suitable for X-ray investigation were obtained and the structure is displayed in Fig. 1 and S4.† Crystallographic refinement parameters are listed in Table S1 of the ESI.† In the crystal structure, the silicon center adopts an octahedral geometry with CNHC atoms located mutually in the trans geometry. The Si–O distances are long with an average bond length d(Si–O) = 1.78 Å, typical for a hypervalent silicon, and the d(Si–CNHC) = 1.90 Å. The phenolates are outside the plane formed by the carbene heterocycle with an average angle of ca. 41° between the two phenolate planes of the ligand, with an O–Si–O angle of 174.7°. Interestingly, the Si(OCO)2 complex displays a very high thermal stability as demonstrated by thermogravimetric analysis where degradation starts above 200 °C with the 5% weight loss temperature T5% as high as 352 °C (Fig. S5†). Cyclic voltammetry was employed to assess the electrochemical behavior of Si(OCO)2 in CH2Cl2 solution. At negative potentials it presents an irreversible reduction process R1 at −2.30 V vs. Fc|Fc+, which is assigned to the reduction of the ligand (see Fig. S6 and Table S2 in the ESI†).
 | ||
| Fig. 1 Molecular structure of Si(OCO)2 (CCDC 2284375). The tBu groups and H atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: Si(1)–O(1), 1.7707(15); Si(1)–O(4), 1.7709(15); Si(1)–O(3), 1.7849(15); Si(1)–O(2), 1.7865(15); Si(1)–C(56), 1.904(2); Si(1)–C(21) 1.909(2); O(1)–Si(1)–O(4), 89.80(7); O(1)–Si(1)–O(3), 89.67(7); O(4)–Si(1)–O(3), 174.50(7); O(1)–Si(1)–O(2), 174.84(7). | ||
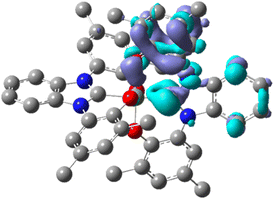
Density Functional Theory (DFT) computations were performed on a species analogous to Si(OCO)2 in which the tert-butyl substituents on the phenyl rings have been replaced with methyl groups, namely Si(OMeCOMe)2. The optimized geometry of the latter complex in its ground state shows an exact S4 symmetry. Molecular orbitals (MOs) closer to the frontier region are displayed in Fig. 2, whereas a larger set of MO density surfaces is reported in Fig. S9,† together with their symmetry and energy.
The eight higher-energy filled orbitals are located on the four phenolate moieties. They are various combinations (of a, b and e symmetry) of the p orbitals of the oxygen atoms with two of the π orbitals of the phenyl groups (corresponding to the e1g degenerate bonding orbitals of benzene). The first four lower unoccupied molecular orbitals are located on the two NHC moieties. The degenerate LUMOs of e symmetry have contribution mainly from the five-membered rings while the next two orbitals (of a and b symmetry) have contribution mainly from the six-membered rings. The following two degenerate orbitals are somehow delocalized all over the molecule. No significant contribution from the silicon atom can be found in any of the orbitals described.
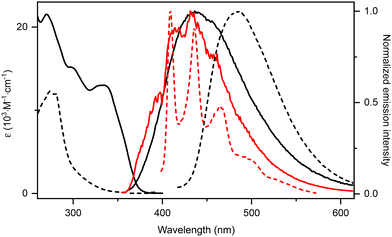
The experimental electronic absorption and emission spectra recorded for Si(OCO)2 and the comparison with those of the pro-ligand (OCO)H3 are displayed in Fig. 3 and the data are summarized in Table 1. For dilute CH2Cl2 samples (2 × 10−5 M), the UV-Vis spectrum of Si(OCO)2 displays three main regions of absorption with a broad profile. The two bands at lower energy, λabs = 332 and 299 nm, display lower intensity, with ε = 1.3 × 104 and 1.5 × 104 M−1 cm−1, respectively. A more intense band is present with a maximum at λabs = 271 nm (ε = 2.2 × 104 M−1 cm−1).
| λ max (ε) [nm, (103 M−1 cm−1)] | λ em [nm] | PLQY (%) | τ obs [ns] | [ns] | k r [107 s−1] | k nr [108 s−1] | λ em [nm] | PLQY (%) | τ obs [ns] | [ns] | k r [107 s−1] | k nr [107 s−1] | λ em [nm] | τ obs [ns] | [ns] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH2Cl2, 2 × 10−5 M, air-equilibrated | Crystalline solid | 77 K, 2-MeTHF | ||||||||||||||
| (OCO)H3 | 274 (12.25), 280 (12.01) | 485 | 1 | 1.87 | — | 0.5 | 5.3 | 434 | 1 | 5.7 (38%), 1.81 (62%) | 4.38 | 0.23 | 22.6 | 410, 436, 467, 500sh | 1.75 (34%), 1.11 (64%) | 1.58 |
| Si(OCO)2 | 271 (21.55), 299sh (15.02), 332 (12.88) | 437, 456sh | 2 | 0.21 | — | 9.6 | 46.9 | 418 | 32 | 2.98 | — | 10.7 | 22.8 | 414, 433, 462sh | 1.35 (25%), 5.60 (75%) | 4.31 |
Time-dependent DFT (TD-DFT) supported the photophysical characterization. Given the composition of the HOMOs and LUMOs, all the transitions up to ca. 4.95 eV (corresponding to ca. 250 nm) are mainly intraligand charge transfer (1ILCT) transitions, characterized by a large electron density displacement (ca. 0.25–0.45 e−) from the phenolate moieties to the NHC rings. In particular, the transitions computed at 348 nm (S1 and S2, of E symmetry), 309 nm (S8), and 280 nm (S12) involve both the NCN atoms and the annulated ring of the NHC moiety, while the transitions computed at 283 nm (S9 and S10, of E symmetry) and 274 nm (S13 and S14, of E symmetry) involve the six-membered ring only. All the degenerate transitions correspond to transfer of electrons within a single ligand with πOPh → π*NHC nature. In addition, transitions S9, S10 and S12 involve excitations in orbitals delocalized also on the OPh groups, assuming a partial πOPh → π*OPh character. Data for all the described transitions are reported in Table S3.†
In the experimental absorption of pro-ligand (OCO)H3 only the band at λabs = ca. 270 nm is present with about half of the intensity compared to that observed for Si(OCO)2, supporting these attributions. Additionally, a more intense (ε = 5.5 × 104 M−1 cm−1) band is clearly visible at λabs = 245 nm in solvents of larger optical transparency window (e.g. THF) which can be ascribed with confidence to singlet-manifold 1π → π* processes mainly involving the NHC scaffold (Fig. S7†). A minor solvent effect is observed for the lower energy band at λabs = ca. 340 nm which shifts bathochromically by ca. 440 cm−1 upon decreasing solvent polarity in the order MeOH → CH2Cl2 → THF → toluene. Despite the overall CT character of the transitions, small solvatochromism is observed since these excitation processes involve both the OPhO^CNHC^OPhO chromophoric scaffolds with an overall spherically symmetric redistribution of the electron–hole pair density at Franck–Condon.
Upon photoexcitation in the lower-energy band, samples of Si(OCO)2 in CH2Cl2 display deep-blue emission with a broad and structureless profile peaking at λem = 437 nm with a photoluminescence quantum yield (PLQY) of 2% (Fig. 3 and Table 1). This emission arises from a short-lived excited state τ = 209 ps, insensitive to oxygen quenching. The high radiative rate constant (kr) for this process (kr = 9.6 × 107 s−1) is characteristic of a strongly allowed transition originating from a singlet manifold. On the other hand, the non-radiative rate constant (knr) was as high as 4.9.6 × 109 s−1, indicative of the presence of efficient quenching channels coupling with the emitting excited state. The quenching is attributable to the rotational motion of the tert-butyl substituents, the fluxional twisting motion of the two PhO–Si–OPh moieties and the large geometrical distortion occurring in the S1 (Table S3†). The emission S1 → S0 is computed at 481/394 nm (vertical/adiabatic). The large difference between the S0 and S1 geometries is responsible for the significant difference between the two computed values. The corresponding electron density-difference map shows the expected electron transfer from the NHC moiety to the OPh groups (ca. 0.40 e−, see Fig. 4).
Emission spectra appear to be slightly influenced by the solvent with an overall shift by about 926 cm−1 from MeOH to CH2Cl2 (Fig. S7†). An increase of the PLQY up to 4% is also observed in MeOH and toluene samples along with a prolongation of the excited state lifetime (Table S3†). Solvent polarity is likely not the only parameter affecting the emission solvatochromism of this compound: specific solvent–solute interactions are expected to be at play as well. As a consequence, being based on a polarizable continuum model, TD-DFT results only qualitatively agree with the observed shift due to solvent effects (Table S6†).
Photophysical measurements of Si(OCO)2 carried out in a 2-MeTHF glassy matrix at 77 K yield a slightly structured emission profile with a minor hypsochromic shift compared to the emission in fluid THF and a few ns-scale lifetime, indicative of a partial 1CT character. On the other hand, for (OCO)H3 the spectrum at 77 K becomes highly structured and shows a larger hypsochromic shift, supporting the sizeable 1CT character of the excited state in the cationic pro-ligand (see Fig. 3).
Remarkably, spin-coated thin film samples of Si(OCO)2 at 10 wt% doping level in polymer matrices such as poly-methylmethacrylate (PMMA) and polystyrene (PS) switch-on the emission properties of the samples as a consequence of the rigidification of the environment and restriction of the roto-vibrational freedom of the emitters. Indeed, doped thin films display intense, structureless, deep-blue photoluminescence with maxima at λem = 419 and 422 nm, and PLQYs as high as 28% and 32%, respectively (Fig. S8† and Table 2), with the CIEy coordinate as low as 0.11, making Si(OCO)2 of great potential interest for the fabrication of deep-blue OLED devices. Interestingly, crystalline samples of Si(OCO)2 show a narrower emission profile at λem = 418 nm due to the even more rigid environment provided by the dense crystal packing with a PLQY of 32%. The (OCO)H3 derivative, on the other hand, shows a low PLQY of 1% and red-shifted emission at λem = 434 nm in the solid state. For both species, this emission is ascribed to an excited state with 1ILCT (1πPhO → π*NHC, S1 → S0) character.
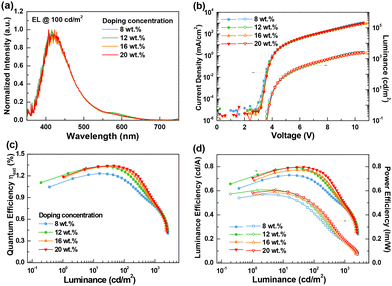
Owing to its interesting photophysical properties, the Si(OCO)2 complex was employed as an emitter in vacuum-processed OLED devices to explore its potential as an electroluminescent material under various doping concentrations between 8 and 20 wt%. The device performances in terms of electroluminescence (EL) spectra, current density–voltage–luminescence (J–V–L), external quantum efficiency (EQE) vs. luminance and luminance efficiency vs. luminance are displayed in Fig. 5. The corresponding details and additional characteristics of the fabricated OLEDs are provided in Fig. S10 and Table S7 of the ESI.† The optimized architecture for the tested devices was as follows: ITO (120 nm)/TAPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MoO3 20 wt% (5 nm)/TAPC (25 nm)/TCTA (10 nm)/mCP
MoO3 20 wt% (5 nm)/TAPC (25 nm)/TCTA (10 nm)/mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si(OCO)2 (20 nm)/TmPyPB (50 nm)/LiF (0.8 nm)/Al (120 nm), where TAPC is 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane, TCTA is 4,4′,4′′-tris(carbazol-9-yl)-triphenylamine, mCP is N,N′-dicarbazolyl-3,5-benzene and TmPyPB is 1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene.
Si(OCO)2 (20 nm)/TmPyPB (50 nm)/LiF (0.8 nm)/Al (120 nm), where TAPC is 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane, TCTA is 4,4′,4′′-tris(carbazol-9-yl)-triphenylamine, mCP is N,N′-dicarbazolyl-3,5-benzene and TmPyPB is 1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene.
The OLED devices fabricated by embedding the complex Si(OCO)2 in the electroluminescent layer showed featureless and relatively narrow EL spectra peaking at λEL,max = 408–423 nm depending on the doping concentration in the investigated range. The EL spectra largely resembled the profile observed in the photoluminescence measurements of doped thin films at 10 wt% doping level in either the PMMA or PS polymer matrix (see above). This is indicative of the fact that the same excited state is involved in both photo- and electro-generated luminescence processes. Optimized devices displayed adequate EL performance with a peak EQE of 1.3% (0.8 cd A−1), maximum luminance value of 2566 cd m−2 and saturated true-blue emission. Notably, the tested devices with varying doping concentrations showed similar EL performances, including the current density, luminance, efficiency, and spectrum, highlighting that Si(OCO)2 is insensitive to the doping level.
Conclusions
In conclusion, we have synthesized and characterized a neutral hexacoordinate Si(IV) complex containing two tridentate N-heterocyclic carbene ligands. Our studies included X-ray crystallography, optical spectroscopy, electrochemistry and computational methods. The homoleptic complex exhibits remarkable dark-blue photoluminescence, particularly in the solid state, enabling it to be used as an electroluminescent material in organic light-emitting diodes. This study demonstrates the benefits of combining silicon and carbenes for achieving a novel class of stable emitters based on the Si(IV)–NHC core and suitable as true-blue EL materials in OLED devices for the first time.Experimental details
General experimental remarks and details including the synthesis, X-ray crystallographic analysis, NMR spectroscopy, photophysical, electrochemical, and computational characterisation as well as OLED device fabrication can be found in the ESI† section along with the additional data.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Université de Strasbourg and the CNRS. This research was financed in part by the French National Research Agency (Agence Nationale de la Recherche – ANR) under the project ANR-22-CE07-0049-01, the ITI-CSC via the IdEx Unistra (ANR-10-IDEX-0002), and the National Science and Technology Council (Taiwan) under the project NSTC 112-2923-E-155-002-MY4. N. Kyritsakas of the Service de Radiocristallographie, Fédération de chimie Le Bel – FR2010, Université de Strasbourg & CNRS, is kindly acknowledged for the help in solving the X-ray structure.References
-
N. Auner and J. Weis, Organosilicon Chemistry III From Molecules to Materials, Wiley-VCH, Weinheim, 2008 Search PubMed
.
- Y. Tokoro, H. Yeo, K. Tanaka and Y. Chujo, Chem. Commun., 2012, 48, 8541 RSC
.
- S. Yamaguchi and K. Tamao, J. Chem. Soc., Dalton Trans., 1998, 3693–3702 RSC
.
- K. L. Chan, M. J. McKiernan, C. R. Towns and A. B. Holmes, J. Am. Chem. Soc., 2005, 127, 7662–7663 CrossRef CAS PubMed
.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740–1741 RSC
.
- A. J. Pearson, T. Plint, S. T. E. Jones, B. H. Lessard, D. Credgington, T. P. Bender and N. C. Greenham, J. Mater. Chem. C, 2017, 5, 12688–12698 RSC
.
- T. Tanaka and A. Osuka, Chem. Commun., 2015, 51, 8123–8125 RSC
.
- A. Kämpfe, E. Kroke and J. Wagler, Organometallics, 2014, 33, 112–120 CrossRef
.
- A. Kämpfe, E. Brendler, E. Kroke and J. Wagler, Chem. – Eur. J., 2014, 20, 9409–9418 CrossRef PubMed
.
- N. Sakamoto, C. Ikeda, M. Yamamura and T. Nabeshima, J. Am. Chem. Soc., 2011, 133, 4726–4729 CrossRef CAS PubMed
.
- M. Yamamura, M. Albrecht, M. Albrecht, Y. Nishimura, T. Arai and T. Nabeshima, Inorg. Chem., 2014, 53, 1355–1360 CrossRef CAS PubMed
.
- M. Kocherga, J. Castaneda, M. G. Walter, Y. Zhang, N.-A. Saleh, L. Wang, D. S. Jones, J. Merkert, B. Donovan-Merkert, Y. Li, T. Hofmann and T. A. Schmedake, Chem. Commun., 2018, 54, 14073–14076 RSC
.
- M. Kocherga, K. M. Boyle, J. Merkert, T. A. Schmedake and M. G. Walter, Mater. Adv., 2022, 3, 2373–2379 RSC
.
- V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt and S. Inoue, Chem. Rev., 2018, 118, 9678–9842 CrossRef CAS PubMed
.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed
.
- C. Romain, S. Bellemin-Laponnaz and S. Dagorne, Coord. Chem. Rev., 2020, 422, 213411 CrossRef CAS
.
- S. Bellemin-Laponnaz and S. Dagorne, Chem. Rev., 2014, 114, 8747–8774 CrossRef CAS PubMed
.
- Y. Chi, T.-K. Chang, P. Ganesan and P. Rajakannu, Coord. Chem. Rev., 2017, 346, 91–100 CrossRef CAS
.
- A. Bonfiglio and M. Mauro, Eur. J. Inorg. Chem., 2020, 3427–3442 CrossRef CAS
.
- D. Lutters, C. Severin, M. Schmidtmann and T. Müller, J. Am. Chem. Soc., 2016, 138, 6061–6067 CrossRef CAS PubMed
.
- Y. Wang, M. Chen, Y. Xie, P. Wei, H. F. Schaefer, P. von R. Schleyer and G. H. Robinson, Nat. Chem., 2015, 7, 509–513 CrossRef CAS PubMed
.
- Y. Xiong, S. Yao, R. Müller, M. Kaupp and M. Driess, Nat. Chem., 2010, 2, 577–580 CrossRef CAS PubMed
.
- Y. Wang, M. Chen, Y. Xie, P. Wei, H. F. Schaefer and G. H. Robinson, J. Am. Chem. Soc., 2015, 137, 8396–8399 CrossRef CAS PubMed
.
- A. Burchert, S. Yao, R. Müller, C. Schattenberg, Y. Xiong, M. Kaupp and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 1894 CrossRef CAS PubMed
.
- F. Bonnette, T. Kato, M. Destarac, G. Mignani, F. P. Cossío and A. Baceiredo, Angew. Chem., Int. Ed., 2007, 46, 8632–8635 CrossRef CAS PubMed
.
- S. Bellemin-Laponnaz, R. Welter, L. Brelot and S. Dagorne, J. Organomet. Chem., 2009, 694, 604–606 CrossRef CAS
.
- S. Bellemin-Laponnaz, S. Dagorne, R. Dümpelmann and P. Steffanut, Chimia, 2014, 68, 500 CrossRef CAS PubMed
.
- C. Romain, B. Heinrich, S. Bellemin-Laponnaz and S. Dagorne, Chem. Commun., 2012, 48, 2213 RSC
.
- S. Dagorne, S. Bellemin-Laponnaz and C. Romain, Organometallics, 2013, 32, 2736–2743 CrossRef CAS
.
- C. Romain, C. Fliedel, S. Bellemin-Laponnaz and S. Dagorne, Organometallics, 2014, 33, 5730–5739 CrossRef CAS
.
- E. Borré, G. Dahm, A. Aliprandi, M. Mauro, S. Dagorne and S. Bellemin-Laponnaz, Organometallics, 2014, 33, 4374–4384 CrossRef
.
- C. Romain, S. Choua, J.-P. Collin, M. Heinrich, C. Bailly, L. Karmazin-Brelot, S. Bellemin-Laponnaz and S. Dagorne, Inorg. Chem., 2014, 53, 7371–7376 CrossRef CAS PubMed
.
- L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin-Laponnaz and V. César, Chem. Rev., 2011, 111, 2705–2733 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental remarks, details including the synthesis, X-ray crystallographic data, 1H, 13C{1H} and 19F{1H} NMR spectra, additional photophysical, electrochemical, and computational characterisation as well as OLED device fabrication. CCDC 2284375 for Si(OCO)2. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00420e |
| ‡ These authors have equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |