Open Access Article
Open Access ArticleHow the spin state tunes the slow magnetic relaxation field dependence in spin crossover cobalt(II) complexes†
Renato
Rabelo
 ab,
Luminita
Toma
a,
Miguel
Julve
ab,
Luminita
Toma
a,
Miguel
Julve
 a,
Francesc
Lloret
a,
Francesc
Lloret
 a,
Jorge
Pasán
a,
Jorge
Pasán
 c,
Danielle
Cangussu
c,
Danielle
Cangussu
 b,
Rafael
Ruiz-García
b,
Rafael
Ruiz-García
 a and
Joan
Cano
a and
Joan
Cano
 *a
*a
aInstituto de Ciencia Molecular (ICMol), Universitat de València, 46980 Paterna, València, Spain. E-mail: joan.cano@uv.es
bInstituto de Química, Universidade Federal de Goiás, Av. Esperança Campus Samambaia, Goiânia, GO, Brazil
cLaboratorio de Materiales para Análisis Químico (MAT4LL), Departamento de Química, Facultad de Ciencias, Universidad de La Laguna, 38200 Tenerife, Spain
First published on 20th February 2024
Abstract
A novel family of cobalt(II) compounds with tridentate pyridine-2,6-diiminephenyl type ligands featuring electron-withdrawing substituents of general formula [Co(n-XPhPDI)2](ClO4)2·S [n-XPhPDI = 2,6-bis(N-n-halophenylformimidoyl)pyridine with n = 4 (1–3) and 3 (4); X = I (1), Br (2 and 4) and Cl (3); S = MeCN (1 and 2) and EtOAc (3)] has been synthesised and characterised by single-crystal X-ray diffraction, electron paramagnetic resonance, and static (dc) and dynamic (ac) magnetic measurements combined with theoretical calculations. The structures of 1–4 consist of mononuclear bis(chelating) cobalt(II) complex cations, [CoII(n-XPhPDI)2]2+, perchlorate anions, and acetonitrile (1 and 2) or ethyl acetate (3) molecules of crystallisation. This unique series of mononuclear six-coordinate octahedral cobalt(II) complexes displays both thermally-induced low-spin (LS)/high-spin (HS) transition and field-induced slow magnetic relaxation in both LS and HS states. A complete LS ↔ HS transition occurs for 1 and 2, while it is incomplete for 4, one-third of the complexes being HS at low temperatures. In contrast, 3 remains HS in all the temperature range. 1 and 2 show dual spin relaxation dynamics under the presence of an applied dc magnetic field (Hdc), with the occurrence of faster- (FR) and slower-relaxing (SR) processes at lower (Hdc = 1.0 kOe) and higher fields (Hdc = 2.5 kOe), respectively. On the contrary, 3 and 4 exhibit only SR and FR relaxations, regardless of Hdc. Overall, the distinct field-dependence of the single-molecule magnet (SMM) behaviour along with this family of spin-crossover (SCO) cobalt(II)-n-XPhPDI complexes is dominated by Raman mechanisms and, occasionally, with additional temperature-independent Intra-Kramer [LS or HS (D > 0)] or Quantum Tunneling of Magnetisation mechanisms [HS (D < 0)] also contributing.
Introduction
Mononuclear transition metal complexes constitute the most miniature molecular magnetic units for quantum data storage and processing applications in the emerging fields of molecular spintronics and quantum computing.1 Spin crossover (SCO) compounds2–13 and mononuclear single-molecule magnets (SMMs),14–27 in their excellent paradigms of addressable and stimuli-responsive magnetic materials, are bistable magnetic molecules with potential applications in molecular spintronic devices and quantum computers.28–52From a historical viewpoint, in the molecular magnetism field, great attention was devoted to mononuclear cobalt(II) complexes as illustrative examples of SCO compounds53–56 or SMMs.57–60 Now, many cobalt(II)-based SCO compounds and SMMs are known, where both high- (HS, SCo = 3/2) and low-spin (LS, SCo = 1/2) states are available for a 3d7 cobalt(II) ion depending on the metal coordination environment. To date, a few examples are known where both properties coexist in the same system, leading to a new class of multiresponsive and multifunctional SCO/SMM materials as potential candidates for multistable molecular quantum bits (qubits) for quantum information processing (QIP).61–63 Known cobalt(II)-based SCO/SMM systems include a series of double salts whereby the SCO and SMM behaviour have been separately identified on the two components of the ion pair.64,65 In such cases, the SCO feature occurs in cationic six-coordinate octahedral complexes, and the SMM response arises from anionic four-coordinate tetrahedral ones. More interestingly, a series of mononuclear five-coordinate cobalt(II) SCO complexes with a square-pyramidal coordination geometry exhibits a field-induced SMM behaviour in the LS state;66,67 one of them was recently tested as a molecular qubit prototype.68 In truth, the LS cobalt(II) ion is a genuine two-level magnetic quantum system represented by the mS = +1/2 and −1/2 states, able to play the role of a qubit for quantum computing applications. By comparison, the HS cobalt(II) ion possesses an effective doublet or quartet ground spin state (Seff = 1/2 or 3/2) coming from the well-isolated ground Kramers doublet resulting from a large first-order spin–orbit coupling (SOC) with either easy-plane xy- (D > 0) or easy-axis Ising-type (D < 0) magnetic anisotropy, respectively. This feature makes this effective spin state an alternative candidate for a “single” qubit. Likewise, related mononuclear octahedral cobalt(II) complexes exhibit a chemo-, electro-, or photo-switching of the SCO and SMM behaviour, being thus postulated as promising candidates for chemical sensors and switches.69–73
Cobalt(II) complexes with bidentate 2,2′-bipyridine (bipy) or tridentate 2,2′:6′,2′′-terpyridine (terpy) ligands and related imine derivatives constitute a major class of SCO systems. In this respect, Figgins and Busch reported in 1960 the first examples of the SCO phenomenon in mononuclear octahedral cobalt(II) complexes with N-methyl substituted, pyridine-2-imine (PI) and pyridine-2,6-diimine type (PDI) ligands.74,75 Herein, we report the synthesis, structural, and spectroscopic characterisation, as well as the static (dc) and dynamic (ac) magnetic properties for a related series of mononuclear octahedral cobalt(II) compounds of formula [Co(n-XPhPDI)2](ClO4)2·S [1: X = I (n = 4), S = MeCN; 2: X = Br (n = 4), S = MeCN; 3: X = Cl (n = 4), S = MeCO2Et; and 4: X = Br (n = 3)] (Scheme 1). This novel family of cobalt(II) complexes displays thermally-induced LS–HS transition and field-induced slow magnetic relaxation in the LS and HS states. The distinct SCO and SMM behaviours depend on the halogen substituent, the para or meta substitution, or occasionally the presence of additional crystallisation solvent molecules (acetonitrile or ethyl acetate). This study allows us to obtain appropriate magneto-structural correlations that, with the aid of ab initio and density functional theory (DFT) calculations, could be useful in designing new SCO/SMM materials as prototypes of molecular spintronic devices for QIP.
Experimental
Materials
All chemicals were of reagent-grade quality, purchased from commercial sources, and used as received.Preparation of the ligands
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 4-IPhPDI ligand]. 1H NMR (CDCl3; 300 MHz, ppm): δ = 8.64 (s, 2H, Him), 8.28 (d, 2H, m-Hpy, J = 7.8 Hz), 7.95 (t, 1H, p-Hpy, J = 7.8 Hz), 7.75 (d, 4H, m-HPh, J = 8.6 Hz), 7.06 (d, 4H, o-HPh, J = 8.6 Hz). Anal. calc. for C19H13N3Br2 (4-BrPhPDI): C, 51.50; H, 2.96; N, 9.48. Found: C, 51.25; H, 3.12; N, 9.66%. IR (KBr, cm−1): 1625(m) [ν(C
N) from 4-IPhPDI ligand]. 1H NMR (CDCl3; 300 MHz, ppm): δ = 8.64 (s, 2H, Him), 8.28 (d, 2H, m-Hpy, J = 7.8 Hz), 7.95 (t, 1H, p-Hpy, J = 7.8 Hz), 7.75 (d, 4H, m-HPh, J = 8.6 Hz), 7.06 (d, 4H, o-HPh, J = 8.6 Hz). Anal. calc. for C19H13N3Br2 (4-BrPhPDI): C, 51.50; H, 2.96; N, 9.48. Found: C, 51.25; H, 3.12; N, 9.66%. IR (KBr, cm−1): 1625(m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 4-BrPhPDI ligand]. 1H NMR (CDCl3; 300 MHz, ppm): δ = 8.64 (s, 2H, Him), 8.28 (d, 2H, m-Hpy, J = 7.8 Hz), 7.95 (t, 1H, p-Hpy, J = 7.8 Hz), 7.55 (d, 4H, m-HPh, J = 8.6 Hz), 7.19 (d, 4H, o-HPh, J = 8.6 Hz). Anal. calc. for C19H13N3Cl2 (4-ClPhPDI): C, 64.42; H, 3.70; N, 11.86. Found: C, 63.25; H, 3.65; N, 11.99%. IR (KBr, cm−1): 1626(m) [ν(C
N) from 4-BrPhPDI ligand]. 1H NMR (CDCl3; 300 MHz, ppm): δ = 8.64 (s, 2H, Him), 8.28 (d, 2H, m-Hpy, J = 7.8 Hz), 7.95 (t, 1H, p-Hpy, J = 7.8 Hz), 7.55 (d, 4H, m-HPh, J = 8.6 Hz), 7.19 (d, 4H, o-HPh, J = 8.6 Hz). Anal. calc. for C19H13N3Cl2 (4-ClPhPDI): C, 64.42; H, 3.70; N, 11.86. Found: C, 63.25; H, 3.65; N, 11.99%. IR (KBr, cm−1): 1626(m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 4-ClPhPDI ligand]. 1H NMR (CDCl3; 300 MHz, ppm): δ = 8.65 (s, 2H, Him), 8.28 (d, 2H, m-Hpy, J = 7.8 Hz), 7.95 (t, 1H, p-Hpy, J = 7.8 Hz), 7.40 (d, 4H, m-HPh, J = 8.6 Hz), 7.26 (d, 4H, o-HPh, J = 8.6 Hz).
N) from 4-ClPhPDI ligand]. 1H NMR (CDCl3; 300 MHz, ppm): δ = 8.65 (s, 2H, Him), 8.28 (d, 2H, m-Hpy, J = 7.8 Hz), 7.95 (t, 1H, p-Hpy, J = 7.8 Hz), 7.40 (d, 4H, m-HPh, J = 8.6 Hz), 7.26 (d, 4H, o-HPh, J = 8.6 Hz).
Preparation of the complexes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solutions in glass tubes. Yield: 66 (1), 73 (2) and 62% (3). Anal. calc. for C40H29N7O8Cl2I4Co (1): C, 34.99; H, 2.13; N, 7.14. Found: C, 35.21; H, 2.08; N, 7.42%. IR (KBr, cm−1): 2244(w) [ν(C
1 v/v) solutions in glass tubes. Yield: 66 (1), 73 (2) and 62% (3). Anal. calc. for C40H29N7O8Cl2I4Co (1): C, 34.99; H, 2.13; N, 7.14. Found: C, 35.21; H, 2.08; N, 7.42%. IR (KBr, cm−1): 2244(w) [ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) from acetonitrile], 1580(m) [ν(C
N) from acetonitrile], 1580(m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 4-IPhPDI], 1086(vs) [ν(Cl–O) from perchlorate]. UV-Vis (MeCN): νmax (cm−1) [ε (M−1 cm−1)] = 50
N) from 4-IPhPDI], 1086(vs) [ν(Cl–O) from perchlorate]. UV-Vis (MeCN): νmax (cm−1) [ε (M−1 cm−1)] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762 [114
762 [114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883], 43
883], 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 479 [71
479 [71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 901] and 28
901] and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 [34
654 [34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774]. Anal. calc. for C40H29N7O8Cl2Br4Co (2): C, 40.54; H, 2.47; N, 8.27. Found: C, 40.66; H, 2.45; N, 8.45%. IR (KBr, cm−1): 2244(w) [ν(C
774]. Anal. calc. for C40H29N7O8Cl2Br4Co (2): C, 40.54; H, 2.47; N, 8.27. Found: C, 40.66; H, 2.45; N, 8.45%. IR (KBr, cm−1): 2244(w) [ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) from acetonitrile], 1580(m) [ν(C
N) from acetonitrile], 1580(m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 4-BrPhPDI], 1086(vs) [ν(Cl–O) from perchlorate]. νmax (cm−1) [ε (M−1 cm−1)] = 51
N) from 4-BrPhPDI], 1086(vs) [ν(Cl–O) from perchlorate]. νmax (cm−1) [ε (M−1 cm−1)] = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282 [105
282 [105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132], 43
132], 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 [51
860 [51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854] and 29
854] and 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326 [26
326 [26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960]. Anal. calc. for C42H34N6O10Cl6Co (3): C, 47.84; H, 3.25; N, 7.97. Found: C, 47.66; H, 3.35; N, 7.92%. IR (KBr, cm−1): 1730 (m) [ν(C
960]. Anal. calc. for C42H34N6O10Cl6Co (3): C, 47.84; H, 3.25; N, 7.97. Found: C, 47.66; H, 3.35; N, 7.92%. IR (KBr, cm−1): 1730 (m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) from ethyl acetate], 1580(m) [ν(C
O) from ethyl acetate], 1580(m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 4-ClPhPDI], 1086(vs) [ν(Cl–O) from perchlorate]. νmax (cm−1) [ε (M−1 cm−1)] = 51
N) from 4-ClPhPDI], 1086(vs) [ν(Cl–O) from perchlorate]. νmax (cm−1) [ε (M−1 cm−1)] = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282 [10
282 [10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 351], 44
351], 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 053 [55
053 [55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620] and 29
620] and 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 586 [28
586 [28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428].
428].
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution in a glass tube. Yield 83%. Anal. calc. for C38H26N6O8Cl2Br4Co (4): C, 39.89; H, 2.29; N, 7.35. Found: C, 40.02; H, 2.36; N, 7.65%. IR (KBr, cm−1): 1572(m) [ν(C
1 v/v) solution in a glass tube. Yield 83%. Anal. calc. for C38H26N6O8Cl2Br4Co (4): C, 39.89; H, 2.29; N, 7.35. Found: C, 40.02; H, 2.36; N, 7.65%. IR (KBr, cm−1): 1572(m) [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) from 3-BrPhPDI], 1088(vs) [ν(Cl–O) from perchlorate]. νmax (cm−1) [ε (M−1 cm−1)] = 50
N) from 3-BrPhPDI], 1088(vs) [ν(Cl–O) from perchlorate]. νmax (cm−1) [ε (M−1 cm−1)] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 [68
505 [68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630], 45
630], 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872 [61
872 [61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 543], and 30
543], and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 395 [19
395 [19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985].
985].
Physical techniques
Elemental analyses (C, H, N) were performed at the Servicio Central de Soporte a la Investigación (SCSIE) at the Universitat de València (Spain). FTIR spectra were recorded on a Nicolet-5700 spectrophotometer as KBr pellets. Electronic absorption spectra of the ligands and complexes 1–4 (cL = cM = 5 × 10−3 mM) were carried out in acetonitrile solutions at room temperature with a Jasco UV/Vis/NIR V-670 spectrophotometer. 1H NMR spectra were recorded at room temperature on a Bruker AC 300 (300 MHz) spectrometer. Deuterated chloroform was used as solvent and internal standard (δ = 7.26 ppm). Q-band EPR spectra of powdered samples of 1–4 were recorded at 4.0 K under non-saturating conditions with a Bruker ER 200 D spectrometer equipped with a helium-flow cryostat. PXRD data were obtained on a powder X-ray diffractometer (model Ultima IV, Rigaku, Japan) using Cu Kα radiation (λ = 1.5418 Å) at a voltage of 40 kV and a current of 30 mA with θ–2θ geometry. Data were collected using a 2θ step of 0.02° and a counting time of 0.25° 2θ min−1 in the angular range from 5 to 40° (2θ).Magnetic measurements
Variable-temperature (2.0–300 K) direct current (dc) magnetic susceptibility measurements under applied fields of 0.25 (T < 20 K) and 5.0 kOe (T > 20 K) and variable-field (0–50 kOe) magnetisation measurements at 2.0 K were carried out on crushed crystals of 1–4 with a Quantum Design SQUID magnetometer. Alternating current (ac) magnetic susceptibility measurements of 1–4 in the temperature range 0–12.0 K under ±5.0 Oe oscillating field at frequencies in the range of 0.1–10.0 kHz were performed under different applied static dc fields (0–2.5 kOe) with a Quantum Design Physical Property Measurement System (PPMS). The magnetic susceptibility data were corrected for the diamagnetism of the constituent atoms and the sample holder (a plastic bag).Crystal structure data collection and refinement
X-ray data on single crystals of 1–4 were collected with Mo-Kα radiation (λ = 0.71073 Å) at 150 K on a Bruker APEX-II CCD (1 and 3) and an Agilent Supernova diffractometers (2 and 4), the last one equipped with an EosS2 detector. Collection, scalation, and integration of data were carried out with Bruker SAINT76 (1 and 3) and CrysAlisPro77 (2 and 4) software. The crystal structures were solved by intrinsic phasing methods integrated into the SHELXTL software478 with the Olex2 platform.79 The models were refined by full-matrix least-squares on F2 using the SHELXL-2018/3 program. Non-hydrogen atoms were anisotropically refined. Hydrogen atoms were set on geometrical positions and refined with a riding model. Two positions were found for the I2 and I7 iodine and perchlorate (Cl4) oxygen atoms of 1. The site occupancy factors (s.o.f., 0.454 and 0.546 for I2 and I2A, and 0.817 and 0.183 for I7 and I7A) of iodine atoms were refined, and constraints were applied to the bond lengths and anisotropic factors of the perchlorate oxygen atoms. Since the perchlorate Cl6 in 3 is disordered in two positions, their s.o.f. were refined considering soft restraints on the distances between oxygen atoms. Two bromophenyl groups in 4 occupy two positions connected by a rotation (ca. 180°) around the Nim–CPh bond; therefore, they were modelled in these positions, and their s.o.f. refined (0.484 and 0.516 for Br8 and Br8B, and 0.831 and 0.169 for Br4 and Br4B). Some constraints were also applied to the anisotropic displacements of the carbon atoms. Three perchlorate groups in 4 are also disordered into two positions; their s.o.f. were refined, and some restraints on Cl–O bond lengths and anisotropic displacements of the chlorine and oxygen atoms were applied to make more accessible the convergence of the refinement. The graphical manipulations and calculations were performed with the CRYSTALMAKER and MERCURY programs.80,81Tables 1, S1 and S2† summarise crystallographic data, refinement conditions, and relevant structural parameters for 1–4. Crystallographic data (excluding structure factors) of 1–4 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 2040602 (1), 2040600 (2), 2040601 (3) and 2040599 (4).†| R 1(Co–Npy)b (Å) | R 2(Co–Nim)c (Å) | R 3(Co–N′im)c (Å) |
R
eq![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (Å) d (Å) |
R (Å) | ||
|---|---|---|---|---|---|---|
| a Structural data for each crystallographically independent cobalt atom. b Average axial cobalt to pyridyl–nitrogen bond distance from the two ligands. c Average equatorial cobalt to imine–nitrogen bond lengths from each ligand. d Average equatorial cobalt–nitrogen bond distance defined as Req = (R2 + R3)/2. e Average cobalt–nitrogen bond distance defined as R = (R1 + R2 + R3)/3. | ||||||
| 1 | Co1 | 1.907(2) | 2.113(3) | 2.126(3) | 2.117(3) | 2.047(3) |
| Co2 | 1.902(2) | 2.026(2) | 2.209(2) | 2.117(2) | 2.046(2) | |
| 2 | Co1 | 1.892(3) | 2.035(3) | 2.192(3) | 2.114(3) | 2.039(3) |
| Co2 | 1.884(3) | 2.094(3) | 2.126(3) | 2.110(3) | 2.035(3) | |
| 3 | Co1 | 2.0255(19) | 2.185(2) | 2.228(2) | 2.207(2) | 2.146(2) |
| 4 | Co1 | 1.904(5) | 2.018(5) | 2.245(5) | 2.132(5) | 2.055(5) |
| Co2 | 1.940(5) | 2.081(5) | 2.194(5) | 2.138(5) | 2.071(5) | |
| Co3 | 2.024(5) | 2.205(5) | 2.214(5) | 2.210(5) | 2.148(5) | |
Computational details
Results and discussion
Synthesis and general characterisation
The n-XPhPDI ligands (with n-X = 4-I, 4-Br, 3-Br, 4-Cl) were synthesised from the straightforward condensation of pyridine-2,6-diformaldehyde and the corresponding para- or meta-monosubstituted aniline (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar stoichiometry) in acid media, as reported earlier for the parent unsubstituted PhPDI ligand.70 All these 4-XPhPDI ligands were isolated as crystalline powders in good yields, and they were characterised by elemental analyses (C, H, N), Fourier-transform infrared (FT-IR) and proton nuclear magnetic resonance (1H NMR) spectroscopies. In contrast, the 3-BrPhPDI ligand could not be isolated as a solid and was used as an oily crude product.
2 molar stoichiometry) in acid media, as reported earlier for the parent unsubstituted PhPDI ligand.70 All these 4-XPhPDI ligands were isolated as crystalline powders in good yields, and they were characterised by elemental analyses (C, H, N), Fourier-transform infrared (FT-IR) and proton nuclear magnetic resonance (1H NMR) spectroscopies. In contrast, the 3-BrPhPDI ligand could not be isolated as a solid and was used as an oily crude product.
The reaction between cobalt(II) perchlorate hexahydrate and the corresponding 4-XPhPDI ligand (X = I, Br, and Cl) in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in methanol led to crystalline powders of 1–3. Compound 4 was synthesised by an in situ reaction of pyridine-2,6-diformaldehyde and 3-bromoaniline with cobalt(II) perchlorate hexahydrate. X-ray suitable single crystals of 1–4 were grown in a test tube by layering ethyl acetate on saturated acetonitrile/chloroform solutions of the crystalline powder. The experimental and calculated (from the CIF files) PXRD patterns of 1–4 (Fig. S1†) showed a remarkable coincidence of the position of all peaks, confirming the purity of the bulk materials for each sample.
2 molar ratio in methanol led to crystalline powders of 1–3. Compound 4 was synthesised by an in situ reaction of pyridine-2,6-diformaldehyde and 3-bromoaniline with cobalt(II) perchlorate hexahydrate. X-ray suitable single crystals of 1–4 were grown in a test tube by layering ethyl acetate on saturated acetonitrile/chloroform solutions of the crystalline powder. The experimental and calculated (from the CIF files) PXRD patterns of 1–4 (Fig. S1†) showed a remarkable coincidence of the position of all peaks, confirming the purity of the bulk materials for each sample.
Description of the structures
Compounds 1 and 2 crystallise in the P21/c space group of the monoclinic system; 3 and 4 do so in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and P21/n space groups of triclinic and monoclinic systems (Table S1†). All structures consist of mononuclear cobalt(II) complex cations, [CoII(n-XPhPDI)2]2+ [n = 4 (1–3) and 3 (4); X = I (1), Br (2 and 4) and Cl (3)], perchlorate anions, and acetonitrile (1 and 2) or ethyl acetate (3) crystallisation solvent molecules. However, while 3 embodies a single crystallographically independent cationic complex, two coexist in 1 and 2 and even three in 4 (Fig. 1 and 2).
and P21/n space groups of triclinic and monoclinic systems (Table S1†). All structures consist of mononuclear cobalt(II) complex cations, [CoII(n-XPhPDI)2]2+ [n = 4 (1–3) and 3 (4); X = I (1), Br (2 and 4) and Cl (3)], perchlorate anions, and acetonitrile (1 and 2) or ethyl acetate (3) crystallisation solvent molecules. However, while 3 embodies a single crystallographically independent cationic complex, two coexist in 1 and 2 and even three in 4 (Fig. 1 and 2).
Due to some asymmetry observed in these compounds, these bond distances are difficult to assign to a specific spin state when viewed individually. Nevertheless, the mean values [R = (2Req + Rax)/3 = 2.047 (1), 2.037 (2), and 2.147 Å (3)] are typical for LS CoII complexes in 1 and 2, but HS in 3. However, both LS and HS CoII complexes coexist in 4 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio [R = 2.057 (Co1/Co2) and 2.148 Å (Co3)]. The stronger electron-withdrawing character of the p-chloro substituent (3) compared to the p-iodo- and p-bromo ones (1 and 2) should provide a weaker ligand field, explained by the HS t2g5eg2 electronic configuration in 3. Since the d orbitals mainly participate in antibonding molecular orbitals and the eg ones have their electron density better directed to the bond regions than the t2g ones, the greater occupation of the former in the HS state (3) is responsible for its Co–N bond lengthening. However, the electronic effects induced in 4 by the weaker inductive character of the bromine substituent are partially compensated by its meta placement in the phenyl ring. Nevertheless, it is well known that the network, including the crystal packing, plays a relevant role in stabilising a particular electronic configuration of a SCO system, which is probably the case here where there are Br⋯Br strong intermolecular contacts. This circumstance is probably why the HS and LS configurations occur together in 4.
1 ratio [R = 2.057 (Co1/Co2) and 2.148 Å (Co3)]. The stronger electron-withdrawing character of the p-chloro substituent (3) compared to the p-iodo- and p-bromo ones (1 and 2) should provide a weaker ligand field, explained by the HS t2g5eg2 electronic configuration in 3. Since the d orbitals mainly participate in antibonding molecular orbitals and the eg ones have their electron density better directed to the bond regions than the t2g ones, the greater occupation of the former in the HS state (3) is responsible for its Co–N bond lengthening. However, the electronic effects induced in 4 by the weaker inductive character of the bromine substituent are partially compensated by its meta placement in the phenyl ring. Nevertheless, it is well known that the network, including the crystal packing, plays a relevant role in stabilising a particular electronic configuration of a SCO system, which is probably the case here where there are Br⋯Br strong intermolecular contacts. This circumstance is probably why the HS and LS configurations occur together in 4.
The springing up of intermolecular halogen–halogen interactions between neighbouring [CoII(n-XPhPDI)2]2+ units also contribute to stabilising the crystal packing of 1, 2, and 4 (Fig. 2 and S3†), but they are not present in 3 or in the HS cobalt(II) units of 4. The interhalogen distances [I⋯I = 3.858–3.964 Å (1), Br⋯Br = 3.624–3.680 Å (2), and Br⋯Br = 3.072–3.492 Å (4)] are slightly shorter than the sum of the van der Waals radii (I⋯I < 4.0 Å and Br⋯Br < 3.7 Å). Both intermolecular interactions in 1 and 2 lead to a supramolecular three-dimensional array along the crystallographic a axis (Fig. S3 and S4†), the holes being filled by perchlorate anions and acetonitrile molecules. In contrast, this situation in 4 promotes π-linked supramolecular chains of cationic mononuclear cobalt(II) complexes rather well-isolated from each by perchlorate anions placed into the interchain space (Fig. S6†). Mononuclear cobalt(II) complex cations and perchlorate anions in 3 are arranged to build a segregated layer array (Fig. S5†), where ethyl acetate molecules occupy the interlayer space establishing weak intermolecular van der Waals contacts with complex cations.
EPR spectra and theoretical calculations
Q-Band EPR spectra of crushed crystals of 1–4 at 4.0 K are in Fig. 3. All spectra show a strong predominant signal around g = 2.0 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 G), typical for LS cobalt(II) complexes, split in two by the high distortion (axial and rhombic) imposed by n-XPhPDI ligands. However, two low-field signals, whose patterns are characteristic of HS cobalt(II) octahedral complexes, are also recorded for 3 and 4.57 The presence of additional LS signatures in 3 indicates a small residual fraction of the LS phase, not inferred from crystallography and the less sensitive magnetometry techniques (see discussion below).
000 G), typical for LS cobalt(II) complexes, split in two by the high distortion (axial and rhombic) imposed by n-XPhPDI ligands. However, two low-field signals, whose patterns are characteristic of HS cobalt(II) octahedral complexes, are also recorded for 3 and 4.57 The presence of additional LS signatures in 3 indicates a small residual fraction of the LS phase, not inferred from crystallography and the less sensitive magnetometry techniques (see discussion below).
EPR spectra of 1–4 were simulated jointly for the LS and HS fractions. LS contributions were satisfactorily simulated considering a highly axial (gx = gy ≠ gz) for 3 and a rhombic octahedron (gx ≠ gy ≠ gz) for the rest. Table S3† summarises the g values used in these simulations.
Based on the crystal structure, 4 is the only compound in which HS and LS configurations patently coexist. Its low-field EPR signals (geff = [2.39, 3.47, 6.42]), marked with an asterisk in Fig. 3d, can only correspond to the ground Kramers doublet of the HS fraction. These values are typical of an easy-plane zfs (D > 0), the most common situation in octahedral cobalt(II) complexes, and agree with those found by ab initio CASSCF/NEVPT2 calculations (gx = 3.25, gy = 7.21 and gz = 2.06). A subsequent analysis considering a zfs on the quartet ground state (gx = 2.47, gy = 2.77, gz = 2.13, D > 0, and E/D = 0.11) and ab initio calculations (D = +92.6 cm−1 and E/D = 0.207) confirm the easy-plane zfs and a moderate rhombicity. While there is reasonable agreement between theoretical and experimental results, it is important to note that the theoretical predictions should be considered semiquantitative at best, as indicated by an abnormal g1 value (1.91) and a large mixing of the spin components into the ground Kramers doublet, suggesting that the three spin states comprising the 4T1 term in an ideal octahedral symmetry are nearly degenerate (0, 512, and 623 cm−1). As a result, second-order perturbation theory may encounter challenges in its applicability.98
However, among the four compounds, the only one exhibiting a HS configuration down to 2.0 K is 3, which shows a split signal at low fields (H < 4000 G), also occurring in the X-band spectrum. The gi values for the two components of this signal are strongly dependent on the microwave frequency band but not their centre of gravity, which is indicative of an effect of the field-modulation amplitude, being this midpoint that provides the real g value. This signal at gz = 7.8 is conclusive evidence of an easy-axis zfs (D < 0).99,100 In such a situation, the two remaining geff components must take low values, enough so that they cannot be observed by Q-band EPR spectroscopy, but it does in X-band. Thus, the X-band spectrum shows an asymmetric signal at fields higher than those for the residual LS fraction, a consequence of the proximity between the signals at gx = 1.43 and gy = 1.54. According to that, the analysis of the X-band spectrum considering a zfs on a quartet spin state provided the following results: gx = 2.25, gy = 2.05, gz = 2.78, D < 0, and E/D = 0.25, which are in agreement with the previous discussion and those found from ab initio calculations (geff = [1.19, 1.39, 8.56] or g3/2 = [1.95, 2.15, 3.04], D = −92.1 cm−1, and E/D = 0.196).
In conclusion, EPR spectroscopy allows determining the spin state and also the presence of the minor molecules undergoing a spin transition or the opposite. Supported by theoretical calculations, this spectroscopic technique has shown that this family of compounds, with unusual molecular geometries, can show both easy-axis and easy-plane anisotropies on the HS configurations. These electronic changes in a highly distorted geometry must occur by subtle geometric modifications imposed by the packing in the crystal network arising from the observation or not of a spin transition phenomenon.
Magnetic properties and theoretical calculations
The spin crossover and spin dynamics for this unique family of mononuclear cobalt(II)-n-XPhPDI complexes with electron-withdrawing substituents at the terminal phenyl rings were investigated through static (dc) and dynamic (ac) magnetic measurements in the temperature ranges 2.0–300 and 2.0–10.0 K, respectively.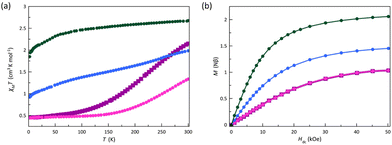 | ||
| Fig. 4 (a) Temperature dependence of χMT and (b) field dependence of M at 2.0 K for 1 (■), 2 (●), 3 (●), and 4 (●). The solid lines are only eye guides. | ||
The χMT versus T plots for 1–4 reveal a behaviour dependence on the electron-withdrawing character of the substituents and their meta or para location (Fig. 4a). At 300 K, χMT for 3 is 2.68 cm3 mol−1 K, a value expected for a HS cobalt(II) ion with an unquenched orbital momentum contribution (2.70 cm3 mol−1 K with gCo = 2.4). However, the χMT values at room temperature for 1, 2, and 4 (2.15, 1.33, and 1.98 cm3 mol−1 K, respectively) are much smaller. For 3, the smooth but gradual decrease in χMT on cooling to 1.85 cm3 mol−1 K at 2.0 K reveals the occurrence of a significant zfs coming from the first-order SOC typical of an octahedral HS cobalt(II) complex (4T1g term with SCo = 3/2 and LCo = 1). Upon cooling, a complete spin transition (with no hysteresis) occurs for 1 and 2, as reported earlier for the related complex with the electron-donating p-methoxy-substituted PDI ligand.70 This fact is revealed by the gradual decrease of χMT to reach a sort of plateau [ca. 50 (1) and 150 K (2)]. The χMT value of 0.45 cm3 mol−1 K at 2.0 K for 1 and 2 is expected for an LS cobalt(II) ion [χMT = (Nβ2gCo2/3kB)SCo(SCo + 1) = 0.45 cm3 mol−1 K with SCo = 1/2 and gCo = 2.2]. In contrast, compound 4 exhibits a gradual and incomplete spin transition in agreement with the coexistence of the LS and HS signature signals observed in the EPR spectra, as reported earlier for the parent unsubstituted complex.75 Even though the spin assignment for 4 is challenging, the χMT value of 0.92 cm3 mol−1 K at 2.0 K agrees with that estimated for one HS and two LS cobalt(II) ions inferred from its single-crystal X-ray structure.
The M versus H plots at 2.0 K for 1–4 further conform to the aforementioned interpretation (Fig. 4b). So, the value of M at 50 kOe for 1 and 2 is 1.00Nβ, being then close to the calculated one for the saturation magnetisation of a doublet spin state of an LS cobalt(II) ion (Ms = gCoSCoNβ = 1.10Nβ with SCo = 1/2 and gCo = 2.2). Besides, for an LS octahedral cobalt(II) complex with SCo = 1/2 on which a magnetic anisotropy does not cause any splitting, the isothermal magnetisation curves should superimpose if they are magnetically isolated, as practically occurs for 1 and 2 in the temperature range from 2.0 to 10.0 K (Fig. S7a and b†). In contrast, M at 50 kOe for 3 is 2.06Nβ, a value which agrees with the calculated one for the saturation magnetisation for an effective doublet spin state of an HS cobalt(II) ion (Ms = gCoSCoNβ = 2.10Nβ with SCo = Seff = 1/2 and gCo = 4.2). In this case, the ground ±3/2 and excited ±1/2 Kramers doublets for D < 0, or vice versa for D > 0, are well-separated due to the sizeable zfs effects operating on the quartet ground state, recording only the magnetisation for the ground Kramers doublet. On the other hand, the value of M equal to 1.45Nβ for 4 at 50 kOe is close to that estimated for the saturation magnetisation corresponding to one HS and two LS cobalt(II) ions [Ms = (2.10 + 2 × 1.10)/3 = 1.43Nβ]. In contrast, isothermal magnetisation curves of 3 and 4 do not superimpose below 10.0 K (Fig. S7c and d†), supporting the occurrence of substantial zfs effects resulting from the significant SOC acting on their HS cobalt(II) ions. Unfortunately, all the attempts to fit the χMT vs. T and M vs. H/T data of 3 taking into account either a first-order spin–orbit coupling or a dominant zfs were unsuccessful, a consequence of the presence of a residual LS phase, evidenced in the EPR study (see below).
Direct and IK processes may seem to be the same, but they are not. Direct relaxation can be applied to easy-axis non-Kramer or any Kramer ions, while the IK mechanism only works on easy-plane Kramer or S = 1/2 ions. Therefore, the IK contribution in our systems is related to a passage between the Zeeman-split components mS = ±1/2 of the LS CoII ion (S = 1/2) or the easy-plane HS CoII ion (S = 3/2). Interestingly, both mechanisms are temperature-independent only without an external magnetic field. However, under a Hdc, the thermal dependence differs since an IK process should follow an Arrhenius law with a minimal energy barrier corresponding to the Zeeman-splitting of the ground Kramer doublet, which makes this relaxation appear temperature-independent with most common working magnetic fields.
This series of compounds reveals that the HS and LS configurations behave differently, the blocking temperature being higher for the former and intermediate for 4 (Fig. S11†), which contains both in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LS/HS ratio below 150 K (see above). In this case, the presence of HS CoII ions would affect the magnetic relaxation of the adjacent LS CoII ions, and vice versa.
1 LS/HS ratio below 150 K (see above). In this case, the presence of HS CoII ions would affect the magnetic relaxation of the adjacent LS CoII ions, and vice versa.
Similarly, while the blocking temperature strongly depends on Hdc for the LS configuration, it is entirely invariant for the HS one (Fig. S10†). These characteristics suggest that the mechanisms of magnetic relaxation or how they operate will be different in the two spin configurations, and they are probably strongly linked to electronic aspects such as the mixing of Ms functions by the rhombic parameter of zfs, nature of the occupied 3d orbitals, spin delocalisation on the ligands, or low-energy molecular vibrational modes. Additionally, new signals displayed as incipient or a shoulder appear in the χ′′Mvs. T curves, mainly in 3 and under the Hdc of 2.5 kOe, allowing to discern higher (HT) and lower (LT) temperature magnetic relaxation processes.
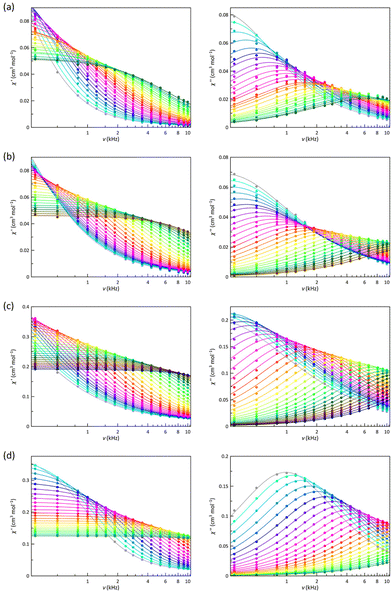
The best way to calculate the magnetic relaxation time (τ) is the joint analysis of the χ′M and χ′′Mvs. ν data (Fig. 5 and 6) through the generalised Debye model, which also takes into account the static and infinite frequency magnetic susceptibility (χS and χT), and the exponential factor that describes the spectral breadth (α), as additional fitting parameters.101
Two types of graphic representations are recommended according to the nature of the relaxation mechanisms involved. For thermally activated two-phonon Orbach-type relaxations [τORB−1 = τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/T)], an Arrhenius plot is preferable since they show a linear dependence where the slope directly estimates the energy barrier (Ueff) controlling the spin reversal. However, a ln
exp(−Ueff/T)], an Arrhenius plot is preferable since they show a linear dependence where the slope directly estimates the energy barrier (Ueff) controlling the spin reversal. However, a ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. ln
τ vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T plot showing linear dependencies of slope n is more appropriate for one-phonon direct or two-phonons Raman-like mechanisms (τ−1 ∝ Tn). When n takes values close to 2 or 8, optical or acoustic phonon-assisted Raman mechanisms dispense the fastest spin reversal.102 Instead, a direct mechanism plays the main role for n values close to unity. Nevertheless, both representations are helpful for temperature-independent relaxations, such as QTM or IK.
T plot showing linear dependencies of slope n is more appropriate for one-phonon direct or two-phonons Raman-like mechanisms (τ−1 ∝ Tn). When n takes values close to 2 or 8, optical or acoustic phonon-assisted Raman mechanisms dispense the fastest spin reversal.102 Instead, a direct mechanism plays the main role for n values close to unity. Nevertheless, both representations are helpful for temperature-independent relaxations, such as QTM or IK.
That being so, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. ln
τ vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T (Fig. 7) and ln
T (Fig. 7) and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. 1/T Arrhenius plots (Fig. S14†) were drawn for 1–4 at Hdc = 0.5–5.0 kOe. In all cases, there is a deviation from a linear dependence, suggesting that several relaxations coexist and compete. In the Arrhenius plots, it is not easy to distinguish straight lines, indicating that one of the mechanisms utterly prevails, which is not surprising for LS 1 and 2 since a S = 1/2 spin state cannot present an energy barrier for the spin reversal. However, the energy barrier so evaluated has sometimes been related to the energy required to reach low-lying vibrational modes that make the spin reversal easier and faster. A similar case would be that for an easy-plane HS cobalt(II) complex (D > 0), as in the HS site of 4, where the |±1/2〉 ground Kramers doublet allows fast relaxation, at least in the absence of a Hdc, being required a Hdc to observe slow magnetic relaxation effects.103 However, 3, exhibiting a easy-axis zfs (D < 0), should be a different case where an energy barrier exists. Therefore, both representations were analysed for the four compounds. In general, a relatively high energy barrier acts at high temperatures and a significantly lower one at low temperatures. The values of this second energy barrier are too low to be related to vibrational modes and are probably a consequence of the magnetic effect on a fast IK relaxation or even competing direct or Raman mechanisms. Similarly, the energy barriers found for 3 are quite lower than that provided by the zfs. So, the fastest relaxation mechanisms must be of a different nature and probably, once again, Raman or direct type.
τ vs. 1/T Arrhenius plots (Fig. S14†) were drawn for 1–4 at Hdc = 0.5–5.0 kOe. In all cases, there is a deviation from a linear dependence, suggesting that several relaxations coexist and compete. In the Arrhenius plots, it is not easy to distinguish straight lines, indicating that one of the mechanisms utterly prevails, which is not surprising for LS 1 and 2 since a S = 1/2 spin state cannot present an energy barrier for the spin reversal. However, the energy barrier so evaluated has sometimes been related to the energy required to reach low-lying vibrational modes that make the spin reversal easier and faster. A similar case would be that for an easy-plane HS cobalt(II) complex (D > 0), as in the HS site of 4, where the |±1/2〉 ground Kramers doublet allows fast relaxation, at least in the absence of a Hdc, being required a Hdc to observe slow magnetic relaxation effects.103 However, 3, exhibiting a easy-axis zfs (D < 0), should be a different case where an energy barrier exists. Therefore, both representations were analysed for the four compounds. In general, a relatively high energy barrier acts at high temperatures and a significantly lower one at low temperatures. The values of this second energy barrier are too low to be related to vibrational modes and are probably a consequence of the magnetic effect on a fast IK relaxation or even competing direct or Raman mechanisms. Similarly, the energy barriers found for 3 are quite lower than that provided by the zfs. So, the fastest relaxation mechanisms must be of a different nature and probably, once again, Raman or direct type.
As with the Arrhenius plots, the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. ln
τ vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T plots for 1–4 do not usually show regions with linear dependencies. However, for 2 under a 2.5 kOe magnetic field, these features are observed at high and low temperatures. The derived n values show that Raman mechanisms assisted by optical and acoustic phonons govern the relaxation of the magnetisation at low and high temperatures, respectively. Although linear dependencies are not observed clearly under other magnetic fields or compounds, there is no reason to think that this does not occur in all of them, particularly in those that also exhibit a LS configuration. In this sense, these cases are usually described with n values intermediate between those corresponding to optical and acoustic phonons. However, good simulations are also found with values closer to what might be expected. Therefore, obtaining accurate values for n requires an extensive data range, which is not always possible, and a meticulous analysis of the experimental data to get the relaxation times. Besides, it is also essential to avoid, without physical evidence, using an oversised set of relaxation mechanisms, which will lead to additional overparameterisation problems. Considering these conclusions, we tried to carry out a similar analysis in all cases. Furthermore, a dominant temperature-independent relaxation becomes apparent under specific magnetic fields. For a LS electronic configuration, this new process can only be assigned to an IK relaxation, a direct and enabled interchange between the two Ms components of ground doublet (|+1/2〉 ⇔ |−1/2〉). The difficulties in achieving accurate relaxation times and subsequent analysis can make it challenging to establish general conclusions, but qualitative scrutiny of the ln
T plots for 1–4 do not usually show regions with linear dependencies. However, for 2 under a 2.5 kOe magnetic field, these features are observed at high and low temperatures. The derived n values show that Raman mechanisms assisted by optical and acoustic phonons govern the relaxation of the magnetisation at low and high temperatures, respectively. Although linear dependencies are not observed clearly under other magnetic fields or compounds, there is no reason to think that this does not occur in all of them, particularly in those that also exhibit a LS configuration. In this sense, these cases are usually described with n values intermediate between those corresponding to optical and acoustic phonons. However, good simulations are also found with values closer to what might be expected. Therefore, obtaining accurate values for n requires an extensive data range, which is not always possible, and a meticulous analysis of the experimental data to get the relaxation times. Besides, it is also essential to avoid, without physical evidence, using an oversised set of relaxation mechanisms, which will lead to additional overparameterisation problems. Considering these conclusions, we tried to carry out a similar analysis in all cases. Furthermore, a dominant temperature-independent relaxation becomes apparent under specific magnetic fields. For a LS electronic configuration, this new process can only be assigned to an IK relaxation, a direct and enabled interchange between the two Ms components of ground doublet (|+1/2〉 ⇔ |−1/2〉). The difficulties in achieving accurate relaxation times and subsequent analysis can make it challenging to establish general conclusions, but qualitative scrutiny of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ vs. ln
τ vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T and Arrhenius plots is often more informative.
T and Arrhenius plots is often more informative.
It is worth noting that the temperature-independent relaxation in 3, due to an energy barrier arising from a negative D, is instead governed by a QTM mechanism. Thus, the relaxation times corresponding to this process prevailing at lower temperatures are greater for the QTM mechanism (3) than for the IK one (1, 2, and 4), consistent with its lower probability and wavefunctions mixing between the components of the ground Kramers doublet (Table 2). As the rhombicity of the zfs tensor (E/D) increases, so does this probability, and the QTM process becomes faster. Similarly, as expected, the relaxation rate for IK and QTM processes decreases as the magnetic field increases and, therefore, the splitting of the Kramers doublet.
| H dc (kOe) |
τ
IK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (×10−3 s) b (×10−3 s) |
C
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (s−1 K−n) c (s−1 K−n) |
n
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
C
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (s−1 K−n) c (s−1 K−n) |
n
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|
|---|---|---|---|---|---|---|
| a The fits correspond to double or triple relaxation models. b Coefficient factor for the temperature-independent IK process (τ−1 = IK). c Coefficient and polynomial factor for the Raman process (τ−1 = CTn). | ||||||
| 1 | 1.0 | — | 1480 ± 50 | 1.98 ± 0.03 | 1.13 ± 0.09 | 5.6 ± 0.4 |
| 2.5 | 1.1 ± 0.4 | 23 ± 2 | 2.4 ± 1.0 | 3.73 ± 0.03 | 4.69 ± 0.07 | |
| 2 | 1.0 | 0.32 ± 0.23 | 900 ± 600 | 2.5 ± 0.4 | 1.3 ± 0.3 | 5.7 ± 0.8 |
| 2.5 | 1.10 ± 0.07 | 58 ± 9 | 1.92 ± 0.10 | 1.15 ± 0.03 | 5.26 ± 0.09 | |
| 3 | 1.0 | 1.39 ± 0.06 | 25 ± 5 | 2.5 ± 0.6 | 1.43 ± 0.19 | 5.16 ± 0.06 |
| 2.5 | 2.4 ± 0.4 | 100 ± 20 | 2.20 ± 0.15 | 1.000 ± 0.016 | 5.30 ± 0.05 | |
| 4 | 1.0 | — | 2340 ± 80 | 1.91 ± 0.03 | 0.10 ± 0.03 | 7.33 ± 0.13 |
| 2.5 | 0.59 ± 0.09 | 1050 ± 60 | 1.95 ± 0.15 | 8.1 ± 1.3 | 5.04 ± 0.07 | |
However, the two spin configurations coexist in 4, the majority being the LS form (2/3). Thus, the relaxation time for the temperature-independent process is expected to be closer to those observed for 1 and 2. A slower IK is still presumed for the HS portion even though it exhibits a |±1/2〉 ground Kramers doublet, as does the LS form. However, this Kramers doublet arises from an S = 3/2 and not an S = 1/2 state, or what is the same from an HS and not an LS form; so, this conclusion is not surprising, being also confirmed by the way it depends on Hdc, which is more like 3. However, what is the cause behind it?
An isolated electron, i.e., a single |±1/2〉 Kramers doublet, in the absence of an external magnetic field or even being moderate, must show a swift spin reversal with an oscillating field. This same doublet arising from an unpaired electron in a metal complex cannot be described as that of an isolated electron since its spin density is delocalised on the ligands. Moreover, the local spin densities caused by a spin delocalisation mechanism polarise spin densities into neighbouring atoms and even, together with the participation of magnetic orbitals in the metal centre, into some internal orbitals of the own metal ion.104 In such a situation, reversing the spin density laying on the paramagnetic centre entails opposing spin densities in inner layers and also on neighbouring atoms becomes a more difficult task that requires a reversal of the overall density on the complex at once, leading to a significant slowdown in the spin reversal. On the other hand, the spin delocalisation in the HS configuration on cobalt(II) complexes is larger than in the LS form since the former one shows a larger occupation of the eg orbitals and therefore, a less localised spin density,104 as confirmed by DFT calculations (Fig. 8 and Table S4†). Hence, the relaxation of the magnetisation in a HS configuration is likely slower, as it is observed. Besides, a distinct σ- or π-type polarisation contribution in the pyridinediimine fragment is observed for the LS and HS configuration, which agrees with the different nature of the magnetic orbitals involved in each case.
How the magnetic field affects the particular dynamic magnetic behaviour of HS and LS forms is not apparent and requires a more detailed study. However, it can be argued that the most significant influence at lower temperatures observed in the LS form may be due solely to the fact that such an effect has already “saturated” under smaller magnetic fields since the Zeeman splitting is larger on a S = 3/2 than on a S = 1/2 state. In contrast, the dynamic behaviour tends to become invariant with Hdc for both HS and LS forms in the high-temperature region since a Raman mechanism governs the spin reversal relaxation,66,67,70 which involves virtual states associated with a spin–phonon coupling or low-energy vibrations for the complex or network and they are therefore not affected by Hdc.
Conclusions
A novel series of mononuclear spin crossover cobalt(II) compounds with a field-induced single-molecule magnet behaviour has been prepared through ligand design from a series of N,N′-diphenyl-substituted pyridine-2,6-diimine (n-XPhPDI) tridentate ligands bearing electron-withdrawing iodo, bromo, or chloro substituents at the para or meta positions of the terminal N-substituted phenyl rings.This series of compounds exhibits from complete and gradual, thermally-induced spin transition to its absence, including partial transitions. No direct correlation of the SCO phenomenology with the electron acceptor nature of the ligand substituents is observed along this series. Otherwise, the role played by the crystal lattice through its organisation assisted by covalent bonds or intermolecular interactions in the SCO phenomenon must be highlighted. Then, the effects of the intermolecular halogen⋯halogen contacts seem to be responsible for the distinct SCO behaviour. Thus, weak X⋯X interactions only concerns the LS cobalt(II) units.
These cobalt(II)-n-XPhPDI complexes show different spin relaxation dynamics under a dc magnetic field with slower- (SR) and faster-relaxing (FR) characters depending on the magnitude of the applied dc magnetic field or their LS and HS nature. This disparate field-induced SMM behaviour is dominated by IK or QTM plus Raman processes at low- and high-temperature regions. The observed Raman mechanisms are assisted by both optical and acoustic phonons, involving virtual states that can be ascribed to low-energy vibrational modes. The presence of two-phonon Raman processes at higher temperatures was confirmed by its weak or null dependence on the dc magnetic field; however, at least for the LS configuration, the strong magnetic field influence suggests that IK, QTM, or even direct mechanisms govern the spin reversal at lower temperatures. Our results and theoretical studies support that the larger spin delocalisation in the HS configuration slows down the magnetic relaxation.
Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish MINECO (Projects PID2019-109735GB-I00 and Unidad de Excelencia María de Maeztu CEX2019-000919-M) and the Generalitat Valenciana (AICO/2020/183 and AICO/2021/295). This work has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 748921-SIMOF. R. R. thanks the Generalitat Valenciana and CAPES for the doctoral (GRISOLIAP/2017/057) and postdoctoral (88887.798611/2022-00) grants. J. P. also thanks the Cabildo de Tenerife for the Agustín de Betancourt research contract, respectively. We are indebted to Dr Gloria Agustí and José María Martínez (Universitat de València) for their assistance with the EPR measurements.References
- J. Ferrando-Soria, J. Vallejo, M. Castellano, J. Martínez-Lillo, E. Pardo, J. Cano, I. Castro, F. Lloret, R. Ruiz-García and M. Julve, Coord. Chem. Rev., 2017, 339, 17–103 CrossRef CAS.
- M. A. Halcrow, Chem. Soc. Rev., 2011, 40, 4119–4142 RSC.
- M. A. Halcrow, Chem. Lett., 2014, 43, 1178–1188 CrossRef CAS.
- M. A. Halcrow, Crystals, 2016, 6, 58 CrossRef.
- T. Mallah and M. Cavallini, C. R. Chim., 2018, 21, 1270–1286 CrossRef CAS.
- G. Molnár, S. Rat, L. Salmon, W. Nicolazzi and A. Bousseksou, Adv. Mater., 2018, 30, 17003862 CrossRef PubMed.
- G. Molnár, M. Mikolasek, K. Ridier, A. Fahs, W. Nicolazzi and A. Bousseksou, Ann. Phys., 2019, 531, 1900076 CrossRef.
- A. Bousseksou, G. Molnár, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313–3335 RSC.
- C. M. Quintero, G. Félix, I. Suleimanov, J. S. Costa, G. Molnár, L. Salmon, W. Nicolazzi and A. Bousseksou, Beilstein J. Nanotechnol., 2014, 5, 2230–2239 CrossRef PubMed.
- E. Ruiz, Phys. Chem. Chem. Phys., 2014, 16, 14–22 RSC.
- R. Bertoni, M. Cammarata, M. Lorenc, S. F. Matar, J.-F. Matar, J. F. Létard, E. Lemke and H. T. Collet, Acc. Chem. Res., 2015, 48, 774–781 CrossRef CAS PubMed.
- D. J. Harding, P. Harding and W. Phonsri, Coord. Chem. Rev., 2016, 313, 38–61 CrossRef CAS.
- M. M. Khusniyarov, Chem. – Eur. J., 2016, 22, 15178–15191 CrossRef CAS PubMed.
- J. Cirera, E. Ruiz, S. Alvarez, F. Neese and J. Kortus, Chem. – Eur. J., 2009, 15, 4078–4087 CrossRef CAS PubMed.
- F. Neese and D. A. Pantazis, Faraday Discuss., 2011, 148, 229–238 RSC.
- M. Atanasov, D. Aravena, E. Suturina, E. Bill, D. Maganas and F. Neese, Coord. Chem. Rev., 2015, 289–290, 177–214 CrossRef CAS.
- S. Gomez-Coca, D. Aravena, R. Morales and E. Ruiz, Coord. Chem. Rev., 2015, 289–290, 379–392 CrossRef CAS.
- L. F. Chibotaru, Struct. Bonding, 2015, 164, 185–229 CrossRef CAS.
- G. A. Craig and M. Murrie, Chem. Soc. Rev., 2015, 44, 2135–2147 RSC.
- J. M. Frost, K. L. M. Harriman and M. Murugesu, Chem. Sci., 2016, 7, 2470–2491 RSC.
- A. K. Bar, C. Pichon and J.-P. Sutter, Coord. Chem. Rev., 2016, 308, 346–380 CrossRef CAS.
- Y.-S. Meng, S.-D. Jiang, B.-W. Wang and S. Gao, Acc. Chem. Res., 2016, 49, 2381–2389 CrossRef CAS PubMed.
- J. M. Frost, K. L. M. Harriman and M. Murugesu, Chem. Sci., 2016, 7, 2470–2491 RSC.
- E. Moreno-Pineda, C. Godfrin, F. Balestro, W. Wernsdorfer and M. Ruben, Chem. Soc. Rev., 2018, 47, 501–513 RSC.
- E. Moreno-Pineda and W. Wernsdorfer, Nat. Rev. Phys., 2021, 3, 645–659 CrossRef CAS.
- A. Zabala-Lekuona, J. M. Seco and E. Colacio, Coord. Chem. Rev., 2021, 441, 213984 CrossRef CAS.
- N. F. Chilton, Annu. Rev. Mater. Res., 2022, 52, 79–101 CrossRef CAS.
- J. J. Parks, A. R. Champagne, T. A. Costi, W. W. Shum, A. N. Pasupathy, E. Neuscamman, S. Flores-Torres, P. S. Cornaglia, A. A. Aligia, C. A. Balseiro, G.-K.-L. Chan, H. D. Abruña and D. C. Ralph, Science, 2010, 328, 1370–1373 CrossRef CAS PubMed.
- R. Sessoli, ACS Cent. Sci., 2015, 1, 473–474 CrossRef CAS PubMed.
- B. Doistau, L. Benda, B. Hasenknopf, V. Marvaud and G. Vives, Magnetochemistry, 2018, 4, 5 CrossRef.
- Y.-S. Ding, Y.-F. Deng and Y.-Z. Zheng, Magnetochemistry, 2016, 2, 40 CrossRef.
- M. Warner, S. Din, I. S. Tupitsyn, G. W. Morley, A. M. Stoneham, J. A. Gardener, Z. Wu, A. J. Fisher, S. Heutz, C. W. M. Kay and G. Aeppli, Nature, 2013, 503, 504–508 CrossRef CAS PubMed.
- M. Atzori, L. Tesi, E. Morra, M. Chiesa, L. Sorace and R. Sessoli, J. Am. Chem. Soc., 2016, 138, 2154–2157 CrossRef CAS PubMed.
- K. Bader, D. Dengler, S. Lenz, B. Endeward, S.-D. Jiang, P. Neugebauer and J. van Slageren, Nat. Commun., 2014, 5, 5304 CrossRef CAS PubMed.
- G. D. Harzmann, R. Frisenda, H. S. J. van der Zant and M. Mayor, Angew. Chem., Int. Ed., 2015, 54, 13425–13430 CrossRef CAS PubMed.
- A. C. Aragonès, D. Aravena, J. I. Cerdá, Z. Acís-Castillo, H. Li, J. A. Real, F. Sanz, J. Hihath, E. Ruiz and I. Díez-Pérez, Nano Lett., 2016, 16, 218–226 CrossRef PubMed.
- E. J. Devid, P. N. Martinho, M. V. Kamalakar, I. Salitros, U. Prendergast, J.-F. Dayen, V. Meded, T. Lemma, R. González-Prieto, F. Evers, T. E. Keyes, M. Ruben, B. Doudin and S. J. van der Molen, ACS Nano, 2015, 9, 4496–4507 CrossRef CAS PubMed.
- A. Bousseksou, G. Molnár, P. Demont and J. Menegotto, J. Mater. Chem., 2003, 13, 2069–2071 RSC.
- Y. Hasegawa, K. Takahashi, S. Kume and H. Nishihara, Chem. Commun., 2011, 47, 6846–6848 RSC.
- K. Takahashi, Y. Hasegawa, R. Sakamoto, M. Nishikawa, S. Kume, E. Nishibori and H. Nishihara, Inorg. Chem., 2012, 51, 5188–5198 CrossRef CAS PubMed.
- M. Milek, F. W. Heinemann and M. M. Khusniyarov, Inorg. Chem., 2013, 52, 11585–11592 CrossRef CAS PubMed.
- M. G. Cowan, J. Olguín, S. Narayanaswamy, J. L. Tallon and S. Brooker, J. Am. Chem. Soc., 2012, 134, 2892–2894 CrossRef CAS PubMed.
- D. Aravena and E. Ruiz, J. Am. Chem. Soc., 2012, 134, 777–779 CrossRef CAS PubMed.
- B. Warner, J. C. Oberg, T. G. Gill, F. El Hallak, C. F. Hirjibehedin, M. Serri, S. Heutz, M.-A. Arrio, P. Sainctavit, M. Mannini, G. Poneti, R. Sessoli and P. Rosa, J. Phys. Chem. Lett., 2013, 4, 1546–1552 CrossRef CAS PubMed.
- V. Meded, A. Bagrets, K. Fink, R. Chandrasekar, M. Ruben, F. Evers, A. Bernand-Mantel, J. S. Seldenthuis, A. Beukman and H. S. J. van der Zant, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 245415 CrossRef.
- B. Rösner, M. Milek, A. Witt, B. Gobaut, P. Torelli, R. H. Fink and M. M. Khusniyarov, Angew. Chem., Int. Ed., 2015, 54, 12976–12980 CrossRef PubMed.
- T. Mahfoud, G. Molnár, S. Cobo, L. Salmon, C. Thibault, C. Vieu, P. Demont and A. Bouseksou, Appl. Phys. Lett., 2011, 99, 053307 CrossRef.
- K. Bairagi, O. Iasco, A. Bellec, A. Kartsev, D. Li, J. Lagoute, C. Chacon, Y. Girard, S. Rousset, F. Miserque, Y. J. Dappe, A. Smogunov, C. Barreteau, M.-L. Boillot, T. Mallah and V. Repain, Nat. Commun., 2016, 7, 12212 CrossRef CAS PubMed.
- M. D. Jenkins, D. Zueco, O. Roubeau, G. Aromí, J. Majer and F. Luis, Dalton Trans., 2016, 45, 16682–16693 RSC.
- A. C. Aragonès, D. Aravena, F. J. Valverde-Muñoz, J. A. Real, F. Sanz, J. Hihath, I. Díez-Pérez and E. Ruiz, J. Am. Chem. Soc., 2017, 139, 5768–5778 CrossRef PubMed.
- T. Jasper-Toennies, M. Gruber, S. Karan, H. Jacob, F. Tuczek and R. Berndt, Nano Lett., 2017, 17, 6613–6619 CrossRef CAS PubMed.
- K. Kaushik, S. Mehta, M. Das, S. Ghosh, S. Kamilya and A. Mondal, Chem. Commun., 2023, 59, 13107–13124 RSC.
- U. Beckmann and S. Brooker, Coord. Chem. Rev., 2003, 245, 17–29 CrossRef CAS.
- H. A. Goodwin, Top. Curr. Chem., 2004, 234, 23–47 CrossRef CAS.
- I. Krivokapic, M. Zerara, M. L. Daku, A. Vargas, C. Enachescu, C. Ambrus, P. Tregenna-Piggott, N. Amstutz, E. Krausz and A. Hauser, Coord. Chem. Rev., 2007, 251, 364–378 CrossRef CAS.
- S. Hayami, Y. Komatsu, T. Shimizu, H. Kamihata and Y. H. Lee, Coord. Chem. Rev., 2011, 255, 1981–1990 CrossRef CAS.
- M. Murrie, Chem. Soc. Rev., 2010, 39, 1986–1995 RSC.
- X.-N. Yao, J.-Z. Du, Y.-Q. Zhang, X.-B. Leng, M.-W. Yang, S.-D. Jiang, Z.-X. Wang, Z.-W. Ouyang, L. Deng, B.-W. Wang and S. Gao, J. Am. Chem. Soc., 2017, 139, 373–380 CrossRef CAS PubMed.
- P. C. Bunting, M. Atanasov, E. Damgaard-Møller, M. Perfetti, I. Crassee, M. Orlita, J. Overgaard, J. van Slageren, F. Neese and J. R. Long, Science, 2018, 362, 7319 CrossRef PubMed.
- A. Landart-Gereka, M. M. Quesada-Moreno, M. A. Palacios, I. F. Díaz-Ortega, H. Nojiri, M. Ozerov, J. Krzystek and E. Colacio, Chem. Commun., 2023, 59, 952–955 RSC.
- X. Feng, C. Mathonière, I.-R. Jeon, M. Rouzières, A. Ozarowski, M. L. Aubrey, M. I. Gonzalez, R. Clérac and J. R. Long, J. Am. Chem. Soc., 2013, 135, 15880–15884 CrossRef CAS PubMed.
- C. Mathonière, H.-J. Lin, D. Siretanu, R. Clérac and J. M. Smith, J. Am. Chem. Soc., 2013, 135, 19083–19086 CrossRef PubMed.
- A. Urtizberea and O. Roubeau, Chem. Sci., 2017, 8, 2290–2295 RSC.
- J. Palion-Gazda, B. Machura, R. Kruszynski, T. Grancha, N. Moliner, F. Lloret and M. Julve, Inorg. Chem., 2017, 56, 6281–6296 CrossRef CAS PubMed.
- D. Shao, L.-D. Deng, L. Shi, D.-Q. Wu, X.-Q. Wei, S.-R. Yang and X.-Y. Wang, Eur. J. Inorg. Chem., 2017, 3862–3867 CrossRef CAS.
- H.-H. Cui, J. Wang, X.-T. Chen and Z.-L. Xue, Chem. Commun., 2017, 53, 9304–9307 RSC.
- L. Chen, J. Song, W. Zhao, G. Yi, Z. Zhou, A. Yuan, Y. Song, Z. Wang and Z.-W. Ouyang, Dalton Trans., 2018, 47, 16596–16602 RSC.
- M.-X. Xu, Z. Liu, B.-W. Dong, H.-H. Cui, Y.-X. Wang, J. Su, Z. Wang, Y. Song, X.-T. Chen, S.-D. Jiang and S. Gao, Inorg. Chem., 2019, 58, 2330–2335 CrossRef CAS PubMed.
- D. Shao, L. Shi, J. Yin, B.-L. Wang, Z.-X. Wang, Y.-Q. Zhang and X.-Y. Wang, Chem. Sci., 2018, 9, 7986–7991 RSC.
- R. Rabelo, L. Toma, N. Moliner, M. Julve, F. Lloret, J. Pasán, C. Ruiz-Pérez, R. Ruiz-García and J. Cano, Chem. Commun., 2020, 56, 12242–12245 RSC.
- R. Rabelo, L. Toma, N. Moliner, M. Julve, F. Lloret, M. Inclán, E. García-España, J. Pasán, R. Ruiz-García and J. Cano, Chem. Sci., 2023, 14, 8850–8859 RSC.
- F. Kobayashi, Y. Komatsumaru, R. Akiyoshi, M. Nakamura, Y. Zhang, L. F. Lindoy and S. Hayami, Inorg. Chem., 2020, 59, 16843–16852 CrossRef CAS PubMed.
- S. E. Canton, M. Biednov, M. Pápai, F. A. Lima, T.-K. Choi, F. Otte, Y. Jiang, P. Frankenberger, M. Knoll, P. Zalden, W. Gawelda, A. Rahaman, K. B. Møller, C. Milne, D. J. Gosztola, K. Zheng, M. Retegan and D. Khakhulin, Adv. Sci., 2023, 10, 2206880 CrossRef CAS PubMed.
- P. E. Figgins and D. H. Busch, J. Am. Chem. Soc., 1960, 82, 820–824 CrossRef CAS.
- G. Juhász, S. Hayami, K. Inoue and Y. Maeda, Chem. Lett., 2003, 32, 882–883 CrossRef.
- Bruker, SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2012 Search PubMed.
- Agilent, CrysAlis PRO, Agilent Technologies Ltd, Yarnton, Oxfordshire, England, 2014 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. J. Puschmann, Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- CrystalMaker, CrystalMaker Software, Bicester, England, 2015 Search PubMed.
- Mercury, The Cambridge Crystallographic Data Centre, Cambridge, UK.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- A. Schäfer, H. Horn and R. Ahlrichs, J. Chem. Phys., 1992, 97, 2571–2577 CrossRef.
- A. Schäfer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- C. Angeli, R. Cimiraglia and J.-P. Malrieu, Chem. Phys. Lett., 2001, 350, 297–305 CrossRef CAS.
- C. Angeli, R. Cimiraglia and J.-P. Malrieu, J. Chem. Phys., 2002, 117, 9138–9153 CrossRef CAS.
- C. Angeli, R. Cimiraglia, S. Evangelisti, T. Leininger and J.-P. Malrieu, J. Chem. Phys., 2001, 114, 10252–10264 CrossRef CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- K. Eichkorn, O. Treutler, H. Ohm, M. Haser and R. Ahlrichs, Chem. Phys. Lett., 1995, 242, 652–660 CrossRef CAS.
- K. Eichkorn, F. Weigend, O. Treutler, H. Ohm and R. Ahlrichs, Theor. Chem. Acc., 1997, 97, 119–124 Search PubMed.
- F. Neese, F. Wennmohs, A. Hansen and U. Becker, Chem. Phys., 2009, 356, 98–109 CrossRef CAS.
- R. Izsák and F. Neese, J. Chem. Phys., 2011, 135, 144105 CrossRef PubMed.
- R. Izsák, A. Hansen and F. Neese, Mol. Phys., 2012, 110, 2413–2417 CrossRef.
- R. Boca and J. Titis, Magnetochemistry, 2023, 9, 100 CrossRef CAS.
- A. Landart-Gereka, M. M. Quesada-Moreno, I. F. Díaz-Ortega, H. Nojiri, M. Ozerov, J. Krzystek, M. A. Palacios and E. Colacio, Inorg. Chem. Front., 2022, 9, 2810–2831 RSC.
- S. Sottini, G. Poneti, S. Ciattini, N. Levesanos, E. Ferentinos, J. Krzystek, L. Sorace and P. Kyritsis, Inorg. Chem., 2016, 55, 9537–9548 CrossRef CAS PubMed.
- K. S. Cole and R. H. Cole, J. Chem. Phys., 1941, 9, 341–351 CrossRef CAS.
- K. J. Stanley and R. A. Vaughan, Electronic Spin Relaxation Phenomena in Solids, Springer, 1969.
- S. Gómez-Coca, A. Urtizberea, E. Cremades, P. J. Alonso, A. Camón, E. Ruiz and F. Luis, Nat. Commun., 2014, 5, 4300 CrossRef PubMed.
- J. Cano, E. Ruiz, S. Alvarez and M. Verdaguer, Comments Inorg. Chem., 1998, 20, 27–56 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of powder X-ray diffraction (Fig. S1), crystallographic drawings (Fig. S2–S6 and Tables S1 and S2), EPR spectroscopic (Table S3) and magnetic data of 1–4 (Fig. S7–S14), and theoretical calculations (Scheme S1 and Table S4). CCDC 2040599–2040602. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00059e |
| This journal is © The Royal Society of Chemistry 2024 |