Open Access Article
Open Access ArticleFlow chemistry enhances catalytic alcohol-to-alkene dehydration†
D. J.
Ward
 a,
D. J.
Saccomando
b,
F.
Vilela
a,
D. J.
Saccomando
b,
F.
Vilela
 a,
G.
Walker
b and
S. M.
Mansell
a,
G.
Walker
b and
S. M.
Mansell
 *a
*a
aInstitute of Chemical Sciences, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK. E-mail: s.mansell@hw.ac.uk; Web: https://www.mansellresearch.org.uk
bLubrizol Limited, The Knowle, Nether Lane Hazelwood, Derby, Derbyshire DE56 4AN, UK
First published on 3rd October 2024
Abstract
Hf(OTf)4 was identified as an excellent catalyst for the low temperature (180 °C) dehydration of 1-hexanol to hexenes and 2-methyl-1-butanol to 2-methylbutenes. Batch conditions limited yields of alkene to 50% despite >90% conversions of 1-hexanol, 16% yield and 55% conversion for 2-methyl-1-butanol, but dramatically better yields were achieved using flow chemistry. For 2-methyl-1-butanol, steady-state conditions were achieved at 180 °C at flow rates of 0.1–0.2 mL min−1 that gave excellent mass balance and allowing selectivities and activities to be meaningfully compared. Hf(OTf)4 was the most active (51 h−1) with a selectivity of 50% at 50% conversion. Optimising for the production of purer alkene was achieved by raising the pressure producing 2.1 g h−1 of 2-methylbutenes (up to 98% pure by mass).
Introduction
Alkenes are essential raw materials for the chemical industry, traditionally sourced from fossil fuels through cracking processes.1–3 New routes to sustainable alkenes are therefore necessary to move away from the exploitation of fossil fuels.4–7 One route is the simple dehydration of alcohols to alkenes. This is a well-studied reaction that has the potential to provide an alternative pathway to alkenes using renewable resources,4 however, it may be hindered by competing side reactions (Scheme 1).8 | ||
| Scheme 1 Alcohol dehydration and competing side reactions.8 | ||
The dehydration of alcohols under simple hydrothermal conditions is possible at elevated temperatures where the entropic effects help drive the equilibrium towards the alkene and water. For 1-propanol, this is temperatures above 155 °C.9 Many different catalysts have been identified for alcohol dehydration including simple Brønsted10–13 and Lewis acids,14–16 as well as metal-based homogeneous17–19 and heterogeneous catalysts.5,20,21 Some of the most active catalysts for the low temperature dehydration of alcohols to alkenes are transition metal triflates, specifically Ti(OTf)4, Hf(OTf)4 and Fe(OTf)3 (OTf = OSO2CF3).18 The correlation of high oxophilicity and Lewis acidity with high conversion and yield was reflected in the performance of Ti(OTf)4 and Hf(OTf)4, both of which are highly oxophilic and Lewis acidic, which give >99% conversions of 2-octanol (a secondary alcohol) with octene yields of 71% and 93% respectively at 150 °C.18 Fe(OTf)3 is able to achieve alkene yields of 80–85% once the temperature is increased to 165 °C.18 Other key homogeneous catalysts used for alcohol dehydration include the rhenium complexes ReMeO3 (MTO), Re2(CO)10 and Re2O7 which have been shown to dehydrate secondary benzylic alcohols in good yields, but are poor catalysts for primary alcohols.17,22,23 The identification of iron catalysts for dehydration reactions is potentially important for producing a cost-effective and sustainable process.24 Selecting the best metals for use in catalysis based on low toxicity and environmental friendliness can be a complicated procedure,25 but it is clear that some transition metals – including iron – are better than others.26 Abundance and low cost are also important factors for delivering more sustainable catalytic processes.27 The promise of titanium complexes has also been identified, offering further options for developing efficient and sustainable catalysts.18,28 Classic organometallic chemistry can be applied to alcohol dehydration, via oxidative addition and β-hydride elimination, by using an acid to form the alkyl iodide in situ in combination with ammonium halide additives.19 With a wide variety of processes possible, it is important that comparisons between catalysts are carried out to give definitive information on relative rates of reactions, lifetimes of catalysts and other important properties under similar conditions. Homogeneous and heterogeneous approaches can be compared and perhaps combined through supported or immobilised catalyst designs.29–32 For instance, the dehydration of ethanol to ethene is already a commercially successful process,33–35 but expanding the substrate scope to different alcohols36 would require screening of many catalysts to determine those with the highest yields and selectivities for each substrate. Additionally, these catalyst systems would need to be implemented as low energy-intensive processes, such as using low reaction temperatures, to target more sustainable catalytic procedures.37,38
The focus of this work is to investigate a variety of known catalysts using two processes (batch and flow) for the dehydration of branched primary alcohols. Primary alcohols are the focus as they are known to be more difficult to dehydrate than secondary or tertiary alcohols.4 A very recent paper has investigated metal triflates for the dehydration of 1-hexanol, and identified Ti(OTf)4 and Hf(OTf)4 as the best catalysts.39 The simplest branched primary alcohol is isobutanol that would be dehydrated to give isobutylene (2-methylpropene). Isobutylene is a significant fraction of the C4 products formed in naphtha cracking (ca. 23% of the C4 fraction),5 and is an important industrial raw material.40,41 There are important future commercial opportunities in the production of more sustainable isobutylene as the amount of naphtha cracking is decreasing, reducing the future availability of fossil-fuel-derived isobutylene.41 As isobutylene is a gas at room temperature and atmospheric pressure, collection and analysis can be challenging, especially as it forms explosive mixtures with air.40 For our investigations, 2-methyl-1-butanol was selected as a model substrate because both alcohol and the products (2-methylbutenes) are liquid at room temperature. In addition, dehydration comparisons to linear 1-hexanol were also carried out (Scheme 2). 2-Methyl-1-butene is also of interest as it can be reacted with methanol to make tert-amyl methyl ether (TAME, 2-methoxy-2-methylbutane), an oxygenate similar to methyl tert-butyl ether (MTBE) both of which are used in gasoline.42 Homogeneous catalysts were targeted because they typically function at lower temperatures and can be more selective compared to heterogeneous catalysts,4 so an investigation into catalyst activity and selectivity was conducted, with the benefits of performing the reaction in flow highlighted.
Results and discussion
Preliminary screening of catalysts for n-hexanol dehydration in batch
After the batch reactor was validated in a series of control reactions (see ESI†),43 a preliminary screen of catalysts for 1-hexanol dehydration was performed to assess the activity of a series of known catalysts measuring the yield of alkene under fixed reaction conditions of 0.5 mol% catalyst in relation to the alcohol substrate (unless otherwise stated) and 1-hexanol (5 mL) at 180 °C for 4 h with a 500 r min−1 stir rate (magnetic stirrer bar) in a 100 mL Hastelloy autoclave. The results determined by GC-FID (Fig. 1) showed Hf(OTf)4 to be the standout performer with the highest yield (33%) of hexenes (all three isomers combined) after 4 h at 0.5 mol% loading. Fe(OTf)3 at a higher loading of 2.5 mol% also performed well (49% yield), better than triflic acid (5 mol%: 3.9% yield), the typical Brønsted acid H2SO4 (5 mol%: 7% yield) or the typical Lewis acid BF3OEt2 (5 mol%: 7% yield). The other catalysts did not show any meaningful activity, even once the catalyst loading was increased by a factor of ten to 5 mol%. The conversion of 1-hexanol using triflic acid was high despite giving a low yield of hexenes, and mass spectrometry and GC identified di-n-hexyl ether as the major product. Brønsted acid catalysts often promote the formation of ethers in alcohol dehydration reactions (Scheme 1).44 The identification of Hf(OTf)4 as a suitable catalyst, and the formation of ethers using Brønsted acids, has been independently confirmed.39 | ||
| Fig. 1 Yield of hexenes from 1-hexanol dehydration after 4 h at 180 °C as a function of catalyst. Average of two runs with yields determined by calibrated GC-FID. | ||
Further investigations of the time profile of the dehydration reaction of 1-hexanol with hafnium triflate found that dihexyl ether was formed in large quantities very quickly (77% yield of ether in 1 hour; Fig. 2). This type of etherification reaction has been seen previously using aryl and alkyl alcohols by Gunanathan and co-workers with iron triflate as the catalyst at temperatures between 0 °C and 25 °C, giving an explanation as to why such high ether yields were achieved in an hour at our elevated temperature.45 The yield of ether then drops over time as the yield of hexene increases to 50% where it plateaus. The yield of ether continues to drop to 2% and the conversion of alcohol remains above 90% at 16 hours showing that the ether is the first product formed and is then decomposed to the alkene. The proportions of alcohol, ether and alkene are linked as the reactions that form and consume them are reversible (Scheme 1). Alkenes are reactive, and the hexenes that are produced can polymerise to form oligomeric poly(hexene) species, affecting the position of any equilibria leading to ether decomposition and additional alkene formation according to Le Chatelier's principle. Triflic acid reactions with 1-hexanol show a different time profile to that of hafnium triflate (Fig. 2, right). The alcohol is not consumed as quickly demonstrating slower formation of dihexyl ether with an ether yield of 33% (47% conversion of alcohol) in 1 hour, compared to 77% ether yield (85% alcohol conversion) in 1 hour using hafnium triflate. Using triflic acid in batch conditions, dihexyl ether does not appear to decompose to produce the alkene even after 16 hours (Fig. 2, right). Both catalysts were also tested under the same conditions (4 h, 180 °C) using dihexyl ether as the substrate but with no alcohol or water present. Hafnium triflate showed decomposition of the ether to produce 20% hexenes with very little alcohol produced. Triflic acid was comparatively worse at decomposing the ether starting material only forming 5% alkenes under the same conditions.
Using 2-methyl-1-butanol as the substrate in a batch reaction at 180 °C with Hf(OTf)4 as the catalyst, after 1 h the conversion of 2-methyl-1-butanol was 55% and the yield of 2-methylbutenes was 16%, but with a higher proportion of oligomeric products observed than was the case with hexanol. This indicates that the branched alkene reacts faster than the linear alkene to give oligomers. The oligomeric reactions indicate a major drawback of batch reactions whereby the increasing concentration of product alkenes leads to side reactions at higher conversions. Gröger and co-workers circumvented this problem through the use of reactive distillation, which allowed the alkene to escape the reaction vessel that contained the metal triflate catalyst.39 Vorholt and co-workers used phosphoric acid as the catalyst and a reactive distillation setup to achieve high yields of linear alkenes; interestingly, dioctyl ether was left untouched under the same conditions.46 We chose to use flow chemistry to avoid these unwanted oligomeric products (vide infra).
Characterisation of products formed in batch reactions
The products of the 16-hour batch dehydration reaction of n-hexanol were found to be oligomeric in nature with the mass spectrum (analysed using an atmospheric solids analysis probe, ASAP) showing two main trends (Fig. 3). A series of peaks at 84m/z difference (mass of hexene monomer unit) suggested a linear oligomer of polyhexene. A second series was found with highly unsaturated products (high double bond equivalents, DBEs) in a series with 14m/z difference (equating to a CH2 unit). The lack of molecular ions and high degree of unsaturation suggests the formation of a highly branched oligomer which has fragmented at a quaternary carbon centre. This process can happen at any branching point on the oligomer and the varying size in oligomer results in highly overlapping series with 14m/z difference. | ||
| Fig. 3 ASAP mass spectrum of oligomers formed in a 16 hour batch dehydration reaction of n-hexanol with 0.5 mol% Hf(OTf)4 as the catalyst. | ||
Using isobutanol as a substrate with 0.5 mol% Hf(OTf)4 as a catalyst at 180 °C, under short reaction times (1 hour) linear polyisobutylene can be formed with low molecular weights (Mn up to 405 g mol−1). This is evidenced by 1H NMR spectra matching literature data47 (Fig. 4a) and a mass spectrum containing a series of peaks with 56m/z differences equating to the monomer (Fig. S14†). However, once the reaction is left for longer the 1H NMR spectrum changes dramatically with the methylene signal at 1.25 ppm broadened and the formation of other broad peaks further down-field in the alkyl region (Fig. 4b). Mass spectra showed highly unsaturated products with high DBEs as several overlapping series of 14m/z difference. The lack of molecular ions and degree of unsaturation again suggests a highly branched oligomer which has fragmented (Fig. S13†). Products similar to this, formed in consecutive alcohol dehydration, oligomerisation and hydrogenation reactions as described in a patent by Chevron, have been identified to have potential uses as jet fuel.48 The same oligomerisation processes were also seen for 2-methyl-1-butanol, however, the initial alkene product seems to be more reactive leading to only the complicated mixture within a one hour time period as shown by mass spectra which displayed no molecular ions and highly unsaturated compounds again suggesting fragmentation due to a high degree of branching. Further evidence is seen in the 13C{1H} NMR spectrum where many peaks were observed in the alkyl region.
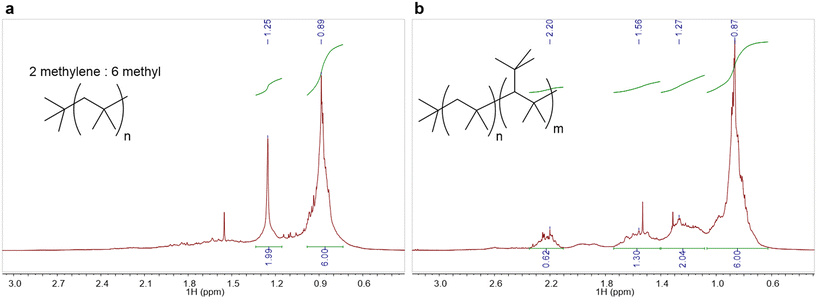 | ||
| Fig. 4 a: 1H NMR spectrum of linear oligomers of isobutene; b: 1H NMR spectrum of highly branched oligomers of isobutene. | ||
Utilising flow chemistry for improved 2-methyl-1-butanol dehydration
To obtain continuous steady-state conditions allowing for true catalyst performance to be evaluated, a flow reaction setup was devised using a continuous stirred tack reactor (CSTR) allowing for steady-state conditions to be achieved with respect to concentration, temperature and pressure. The reactor design was inspired by Guironnet and coworkers' design for a flow reactor applied to homogeneously catalysed Guerbet alcohol upgrading.49 The schematic in Fig. 5 illustrates the process of pumping alcohol into the CSTR using peristaltic pumps. Additionally, a stream of nitrogen is introduced at the inlet, serving as a carrier gas to facilitate the flow of volatile products through the system. The reactor contains the catalyst in paraffinic oil. As the reactor was heated above the boiling point of the reagent and products, the gaseous reactant and products will flow out of the reactor with the stream of nitrogen and into a collection flask. The back pressure regulator allowed for control over the pressure in the CSTR. The conversion and yield were determined by GC-FID.The best four catalysts from the batch reactions (Hf(OTf)4, Fe(OTf)3, HOTf and H2SO4) were used for the screening under steady-state flow conditions. After the reactor was validated in a series of control reactions (see ESI†),43 standard conditions were developed for screening catalysts to determine catalyst activity and selectivity (Table 1). For these two sets of conditions, particular attention was paid to achieving good mass balance, which required modification of the reaction setup to heat the outlet pipe from the CSTR to avoid condensation of the products in this pipe and remove any complications arising from ‘slugs’ of reaction mixture interspersed with the nitrogen. These optimised catalyst testing conditions led to excellent mass balances with conversions of 70–80% and yields up to 60% (Fig. 6).
| Optimised catalytic conditions | For screening activity | For screening selectivity | For producing pure alkene |
|---|---|---|---|
| Paraffin oil | 5 mL | 5 mL | 5 mL |
| Catalyst | 0.705 mmol | 0.705 mmol | 0.705 mmol |
| Reactor volume | 100 mL | 100 mL | 100 mL |
| Stirring rate | 500 rpm | 500 rpm | 500 rpm |
| N2 | 100 mL min−1 (gas) | 100 mL min−1 (gas) | 2 mL min−1 (gas) |
| 2-Methyl-1-butanol | 0.1 mL min−1 (liquid) | 0.1–0.2 mL min−1 (liquid) | 0.1 mL min−1 (liquid) |
| Pressure | 1.4 barg | 1.4 barg | 3.1 barg |
| Reactor temperature | 180 °C | 180 °C | 180 °C |
| Outlet temperature | 160 °C | 160 °C | Room temperature |
 | ||
| Fig. 6 Reaction conditions: 180 °C, Hf(OTf)4 (0.705 mmol), paraffin oil (5 mL), 500 rpm, 2-Me-BuOH flow rate 0.1 mL min−1, flow rate N2 10 mL min−1, back-pressure 2.1 barg. | ||
Catalyst activity and lifetime
One measure of catalyst activity can be defined as a turnover rate or frequency, which is the number of molecules that react per active site per unit of time. For a CSTR reactor this can be easily calculated by a simple mass balance equation (eqn (1)).43 For meaningful comparisons of activity, it is important to test all the catalysts under the same conditions when the turnover frequency is measured for each. | (1) |
x: fractional conversion of reactant 
F 0: molar flowrate of reactant into the reactor
F 1: molar flow rate of unconverted reactant leaving the reactor
n c: moles of catalyst.
Eqn (1). Mass balance equation for calculating turnover frequency from a CSTR.43
We evaluated catalyst activity by measuring the conversion of 2-methyl-1-butanol under the same conditions for each catalyst (Table 1). From these conversions the turnover frequencies were calculated (eqn (1)). The results were plotted (Fig. 7) and showed that Hf(OTf)4 was the most active catalyst (51 h−1) followed by HOTf (42 h−1) and Fe(OTf)3 (24 h−1) then H2SO4 (2 h−1). This showed a change from the preliminary screen performed in batch for n-hexanol where HOTf was active but not selective and produced very little hexene. This change is ascribed to the continuous nature of the CSTR setup meaning that the alkene generated did not undergo further reactions as it was removed from the catalyst before this could happen. This activity data was verified by changing flow rates and measuring activity, and comparisons of activity at different conversions can then be extrapolated (see ESI†). This data shows that Hf(OTf)4 was the most active followed by HOTf then Fe(OTf)3.
 | ||
| Fig. 7 Activity of the catalysts for the dehydration of 2-methyl-1-butanol under optimised conditions (see Table 1). | ||
The CSTR reaction setup allowed us to monitor the reaction progress over 28 hours in order to get an assessment of the catalyst lifetime for Hf(OTf)4. The first 8 hours showed no great reduction in turnover frequency with the activity staying around 50 h−1 over this time, however, after 24 hours the activity had dropped to 31 h−1 then to 18 h−1 after 26 hours. A best-fit of this activity data shows that roughly 1 h−1 is lost per hour and the catalyst therefore has 50 hours activity before it will no longer convert 2-methyl-1-butanol to 2-methylbutenes (Fig. 8).
 | ||
| Fig. 8 Catalyst lifetime tests: Hf(OTf)4 (0.7 mmol), 180 °C reaction temperature, 5 mL paraffin oil, 0.1 mL min−1 2-Me-butan-1-ol. 100 min−1 N2, outlet temperature 160 °C. | ||
Flow rate and selectivity
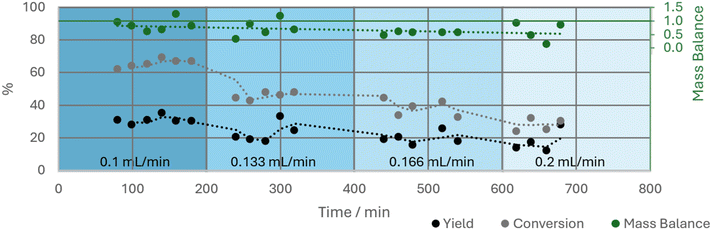
Selectivity is a measurement of the efficiency of a catalyst to produce the desired product over other products and, therefore, is a function of relative rates of reaction.43,50 This means that any comparison made must be performed under the same temperature, pressure, reactor geometry and degree of conversion. So, to obtain a fair comparison of catalyst selectivity, the selectivity must be measured at the same conversion. This can be challenging due to every catalyst having different activities and therefore will have different levels of conversion under the same conditions. The flow rate of 2-methyl-1-butanol through the system was therefore used to alter the residence time in the CSTR to decrease and increase the conversion of the catalysts to allow selectivity to be measured at a range of conversions. To achieve a steady state, the flow CSTR setup was first run for 1 h at 0.1 mL L−1 alcohol. Then, a series of different flow rates of the alcohol were then tested: 0.1, 0.133, 0.166 and 0.2 mL min−1 for Hf(OTf)4 (Fig. 9) and HOTf; for the less active Fe(OTf)3 lower flow rates of 0.05, 0.1, 0.133 and 0.166 mL min−1 were used. Each flow rate was kept constant for three hours and samples taken at 20-minute intervals to probe the change in selectivity with flow rate (Fig. 10). As expected, conversion to alkene increased with lower flow rates because the residence time of the alcohol in the CSTR had been increased (Fig. 10). This allowed us to correlate conversion of alcohol with selectivity to the alkene, showing that selectivity increased with decreasing conversion for the metal triflate catalysts that were tested, but that selectivity remained constant for triflic acid (at ca. 50%), highlighting the benefit of a high flow rate for this catalyst. Overall, Hf(OTf)4 was the most selective catalyst at conversions of 25–45%, but at higher conversions triflic acid was more selective. Most importantly, these results show much better catalytic performance than in the batch setup and in a CSTR all three catalysts are viable for the dehydration of 2-methyl-1-butanol.Increasing back pressure to optimise alkene yield
In order to increase conversion and reduce the amount of starting material in the collection flask, it was found that the system can be tuned to intentionally trap the higher boiling alcohol starting material in the CSTR whilst allowing for the lower boiling alkene to be volatilised and flow out of the reactor with the stream of nitrogen. To obtain these conditions, the back pressure applied to the system was increased from 1.4 barg to 3.1 barg increasing the boiling point of the alcohol, and the heating tape on the outlet pipe was also removed ensuring that any alcohol that boiled would be condensed at the outlet forcing it back into the CSTR, effectively trapping it until it had reacted. This resulted in an increase in the percentage of alkene in the product up to 98% from ca. 50% under the previous steady-state conditions over a 3-hour time period thereby providing a cleaner stream of product (Fig. 11). The optimised set up allows for an average of 2.1 g of 2-methylbutenes to be produced per hour, with the alkene product separated from water by freezing and decanting. Over the total 3-hour reaction time, the yield was 54% as 3.9 g of alkene would be expected. Under the same conditions in batch, 55% conversion was achieved but with a selectivity of 29% showing the optimised flow system has enhanced selectivity to alkene, improved the reaction work-up and allowed the production of a continuous stream of product.Comparisons to literature
The catalytic dehydration of secondary alcohols has been discussed extensively in the literature with many active and selective catalysts shown to work at relatively low temperatures.4 The more challenging primary alcohol substrates have been covered to a lesser extent with varying success. In a reactive distillation system, Hf(OTf)4 was shown to dehydrate 1-octanol at 180 °C at 0.5 mol% loading in 12 hours to give a 65% yield of octenes.18 Fe(OTf)3 could only generate 2% yield under the same conditions for 6 hours.18 Other homogeneous catalysts such as a molybdenum acac complex converted 1-octanol to octene at 250 °C for 20 hours with 60% conversion and 2% yield.51 Our work therefore shows the advantage of flow chemistry applied to homogeneous catalysis to enhance dehydration reactions of primary alcohols by the slow addition of primary alcohol. To compare with heterogeneous systems, the dehydration of isobutanol using rhodium-doped alumina catalysts at temperatures of 700 °C achieved 80% conversion with 90% selectivity, but at a much higher temperature.52 A similar reactor to this work was used in 1994 by Air Products and Chemicals Inc. for the dehydration of isobutanol to isobutylene using heterogeneous alumina catalysts at 300 °C and showed high conversion and selectivity towards isobutylene (75 to 98% conversion with 92 to 94% selectivity).53 Heterogeneous zeolite catalysts for the conversion of isobutanol have also been investigated and showed further reactions from oligomerisation, cracking, hydrogen transfer and dehydrogenation resulting in various olefins (C2–C8) and aromatics.54 Overall, we have demonstrated that homogeneous catalysis works at a lower temperature for dehydration reactions without sacrificing conversion and maintaining a high selectivity.Conclusion
Screening of homogeneous catalysts for alcohol dehydration in batch reactions was completed using 1-hexanol as a substrate and showed that the top three most active catalysts were all triflates: Hf(OTf)4, Fe(OTf)3 and HOTf. For the branched primary alcohol 2-methyl-1-butanol, batch reactions showed higher conversions but poorer selectivities than for the linear alcohol requiring an adapted reaction setup. A fully validated CSTR reactor was chosen to complete this study allowing the dehydration of 2-methyl-1-butanol to be monitored under steady state conditions. The activity screen showed Hf(OTf)4 to be the most active, with HOTf also performing well. Selectivity for all three catalysts was also determined as a function of conversion showing Hf(OTf)4 and HOTf to have similar selectivities at 50% conversion (50% and 51% respectively). Optimum conditions to produce alkene were developed using Hf(OTf)4 as the most active catalyst, producing 2.1 g of 2-methylbutenes per hour with up to 98% purity. Overall, key advantages to using flow chemistry over batch reactions setup for both catalyst testing and generating pure alkene products have been demonstrated.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
DJW thanks Lubrizol and EaSI-cat for funding a PhD studentship. DJW and SMM thank the Royal Society of Chemistry for an RSC Research Enablement Grant (E21-8488203428) and DJW thanks the RSC for a travel grant. FV thanks Vapourtec for their invaluable technical support.References
- C. W. Fernelius, H. Wittcoff and R. E. Varnerin, J. Chem. Educ., 1979, 56, 385 CrossRef
.
- P. Wiseman, J. Chem. Educ., 1977, 54, 154 CrossRef CAS
.
-
R. Sadeghbeigi, in Fluid Catalytic Cracking Handbook, ed. R. Sadeghbeigi, Butterworth-Heinemann, 4th edn, 2020, pp. 1–22 Search PubMed
.
- D. J. Ward, D. J. Saccomando, G. Walker and S. M. Mansell, Catal. Sci. Technol., 2023, 13, 2638–2647 RSC
.
- Y. Nakagawa, M. Yabushita and K. Tomishige, RSC Sustainability, 2023, 1, 814–837 RSC
.
- B. M. Stadler, C. Wulf, T. Werner, S. Tin and J. G. de Vries, ACS Catal., 2019, 9, 8012–8067 CrossRef CAS
.
- E. T. C. Vogt and B. M. Weckhuysen, Nature, 2024, 629, 295–306 CrossRef CAS PubMed
.
-
A. de Klerk, Dehydration, Etherification, and Hydration, in Fischer-Tropsch Refining, John Wiley & Sons, Incorporated, 2011, ch. 17, pp. 335–352 Search PubMed
.
- C. Bockisch, E. D. Lorance, H. E. Hartnett, E. L. Shock and I. R. Gould, ACS Earth Space Chem., 2018, 2, 821–832 CrossRef CAS
.
- M. Zhang and Y. Yu, Ind. Eng. Chem. Res., 2013, 52, 9505–9514 CrossRef CAS
.
-
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Pergamon Press Ltd., Oxford, 1984, p. 601 Search PubMed
.
- V. Siracusa and I. Blanco, Polymers, 2020, 12, 1641 CrossRef CAS PubMed
.
- J. B. Friesen and R. Schretzman, J. Chem. Educ., 2011, 88, 1141–1147 CrossRef CAS
.
- G. L. O'Connor and H. R. Nace, J. Am. Chem. Soc., 1955, 77, 1578–1581 CrossRef
.
- C. Liu, B. Pan and Y. Gu, Chin. J. Catal., 2016, 37, 979–986 CrossRef CAS
.
- H. Firouzabadi, N. Iranpoor, H. Hazarkhani and B. Karimi, Synth. Commun., 2003, 33, 3653–3660 CrossRef CAS
.
- S. Raju, M.-E. Moret and R. J. M. Klein Gebbink, ACS Catal., 2015, 5, 281–300 CrossRef CAS
.
- J. Keskiväli, A. Parviainen, K. Lagerblom and T. Repo, RSC Adv., 2018, 8, 15111–15118 RSC
.
- G. R. M. Dowson, I. V. Shishkov and D. F. Wass, Organometallics, 2010, 29, 4001–4003 CrossRef CAS
.
- J. M. Müller, G. C. Mesquita, S. M. Franco, L. D. Borges, J. L. de Macedo, J. A. Dias and S. C. L. Dias, Microporous Mesoporous Mater., 2015, 204, 50–57 CrossRef
.
- Y. Tavan, S. H. Hosseini, M. Ghavipour, M. R. Khosravi Nikou and A. Shariati, Chem. Eng. Process., 2013, 73, 144–150 CrossRef CAS
.
- Z. Zhu and J. H. Espenson, J. Org. Chem., 1996, 61, 324–328 CrossRef CAS
.
- T. J. Korstanje, J. T. B. H. Jastrzebski and R. J. M. Klein Gebbink, ChemSusChem, 2010, 3, 695–697 CrossRef CAS PubMed
.
- O. Nachtigall, A. I. VanderWeide, W. W. Brennessel and W. D. Jones, ACS Catal., 2021, 11, 10885–10891 CrossRef CAS
.
- K. S. Egorova and V. P. Ananikov, Angew. Chem., Int. Ed., 2016, 55, 12150–12162 CrossRef CAS PubMed
.
- M. Bystrzanowska, P. Petkov and M. Tobiszewski, ACS Sustainable Chem. Eng., 2019, 7, 18434–18443 CrossRef CAS
.
- K. M. P. Wheelhouse, R. L. Webster and G. L. Beutner, Org. Process Res. Dev., 2023, 27, 1157–1159 CrossRef CAS
.
- R. Schobert, Simple syntheses and reactions of (di-η5-cyclopentadienyl)methyl(alkoxy)- and -(acyloxy)titanium(IV) complexes, J. Organomet. Chem., 1991, 405, 201–205 CrossRef CAS
.
- C. Godard, C. Claver and A. C. Albéniz, Eur. J. Inorg. Chem., 2022, 2022, e202101024 CrossRef CAS
.
- Á. Molnár and A. Papp, Coord. Chem. Rev., 2017, 349, 1–65 CrossRef
.
- R. Munirathinam, J. Huskens and W. Verboom, Adv. Synth. Catal., 2015, 357, 1093–1123 CrossRef CAS
.
- D. Cantillo and C. O. Kappe, ChemCatChem, 2014, 6, 3286–3305 CrossRef CAS
.
- V. A. Chumachenko and E. V. Ovchinnikova, Katal. Prom-sti., 2016, 8, 134–138 Search PubMed
.
- D. Fan, D.-J. Dai and H.-S. Wu, Materials, 2013, 6, 101–115 CrossRef CAS PubMed
.
- H. Xiang, R. Xin, N. Prasongthum, P. Natewong, T. Sooknoi, J. Wang, P. Reubroycharoen and X. Fan, Resour. Chem. Mater., 2022, 1, 47–68 CAS
.
- Y. Shi, A. S. Weller, A. J. Blacker and P. W. Dyer, Catal. Commun., 2022, 164, 106421 CrossRef CAS
.
- M. E. H. Tijani, H. Zondag and Y. Van Delft, ACS Sustainable Chem. Eng., 2022, 10, 16070–16089 CrossRef CAS
.
- R. Chauhan, R. Sartape, N. Minocha, I. Goyal and M. R. Singh, Energy Fuels, 2023, 37, 12589–12622 CrossRef CAS
.
- A. Allahverdiyev, J. Yang and H. Gröger, Green Chem., 2024, 26, 7869–7878 RSC
.
- W. E. Luttrell, J. Chem. Health Saf., 2013, 20, 35–37 Search PubMed
.
- P. C. Zonetti, V. L. Bridi, G. G. Gonzalez, C. R. Moreira, O. C. Alves, R. R. de Avillez and L. G. Appel, ChemCatChem, 2019, 11, 4011–4020 CrossRef CAS
.
- J. A. Linnekoski, A. O. I. Krause and L. K. Struckmann, Appl. Catal., A, 1998, 170, 117–126 CrossRef CAS
.
-
Catalytica, A Practical Guide to Catalyst Testing: Basic Guide, Catalytica, 1987 Search PubMed
.
- A. de Klerk and A. DeKlerk, Dehydration, Etherification, and Hydration, 2011 Search PubMed.
- P. K. Sahoo, S. S. Gawali and C. Gunanathan, ACS Omega, 2018, 3, 124–136 CrossRef CAS PubMed
.
- J. T. Vossen, A. J. Vorholt and W. Leitner, ACS Sustainable Chem. Eng., 2022, 10, 5922–5931 CrossRef CAS
.
- Q. Liu, Y. Wu, P. Yan, Y. Zhang and R. Xu, Macromolecules, 2011, 44, 1866–1875 CrossRef CAS
.
-
B. K. Chang, H.-K. C. Timken and M. K. Young, Process for the production of renewable distillate-range hydrocarbons, U.S. Pat., 2023, 11639320 Search PubMed
.
- N. M. Wang, S. Dillon and D. Guironnet, React. Chem. Eng., 2022, 7, 711–718 RSC
.
- G. A. Somorjai and J. Y. Park, Angew. Chem., Int. Ed., 2008, 47, 9212–9228 CrossRef CAS PubMed
.
- T. J. Korstanje, E. Folkertsma, M. Lutz, J. T. B. H. Jastrzebski and R. J. M. Klein Gebbink, Eur. J. Inorg. Chem., 2013, 2013, 2195–2204 CrossRef CAS
.
- I. C. Lee, J. G. St. Clair and A. S. Gamson, Catalytic Oxidative Dehydration of Butanol Isomers: 1-Butanol, 2-Butanol, and Isobutanol, Army Research LAB Adelphi MD Sensors and Electron Devices Directorate, 2011, Accession Number: ADA550017 Search PubMed.
- B. E. Latshaw, Dehydration of isobutanol to isobutene in a slurry reactor, Air Products and Chemicals, Inc., Allentown, PA (United States), 1994 Search PubMed.
- Z.-Y. Du, B.-B. Zhang, T.-S. Chen, Y. Betancur and W.-Y. Li, Energy Fuels, 2019, 33, 10176–10184 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy00913d |
| This journal is © The Royal Society of Chemistry 2024 |