Open Access Article
Open Access ArticleSynergistically enhanced photoelectrocatalytic degradation of ciprofloxacin via oxygen vacancies and internal electric field on a NiSe2/WO3 photoanode†
Tunde L.
Yusuf
 *ab,
Babatope O.
Ojo
c,
Talifhani
Mushiana
c,
Nonhlangabezo
Mabuba
*ab,
Babatope O.
Ojo
c,
Talifhani
Mushiana
c,
Nonhlangabezo
Mabuba
 c,
Omotayo A.
Arotiba
c,
Omotayo A.
Arotiba
 c and
Seshibe
Makgato
c and
Seshibe
Makgato
 a
a
aDepartment of Chemical & Materials Engineering, University of South Africa, South Africa
bDepartment of Chemistry, Faculty of Natural and Agricultural Sciences, University of Pretoria, Private Bag X20, Hatfield 0028, Pretoria, South Africa. E-mail: yusuf.tl@up.ac.za
cDepartment of Chemical Science, University of Johannesburg, South Africa
First published on 19th August 2024
Abstract
This study presents the in situ deposition of nickel selenide (NiSe2) on tungsten trioxide (WO3) nanorods to enhance the photoelectrocatalytic degradation of organic pollutants in water. The synthesis involves integrating nickel selenide (NiSe2) and tungsten trioxide (WO3) nanorod to form a heterojunction, utilizing a facile in situ growth method. The resulting NiSe2/WO3 heterojunction exhibits enhanced photocatalytic properties attributed to efficient charge separation, improved charge transfer dynamics, and synergistic catalytic activity created by an internal electric field and oxygen vacancy. The heterojunction demonstrates remarkable performance in the degradation of ciprofloxacin under visible light irradiation. Under optimum conditions, the photodegradation of ciprofloxacin reached 89% (0.0179 min−1) compared to pristine WO3, which only achieved 48% (0.0069 min−1) under the same conditions. The study systematically investigates the structural and morphological characteristics of the NiSe2/WO3 heterojunction and elucidates its superior photocatalytic efficacy through comprehensive experimental analyses. The primary reactive species responsible for CIP degradation were identified as photogenerated h+ and ˙OH. The successful development of the NiSe2/WO3 heterojunction holds significant promise for advancing environmentally sustainable technologies in water treatment and pollution remediation.
Introduction
The dependence of humans and livestock on pharmaceuticals is increasing as the world population continues to expand. In as much as pharmaceuticals have been very beneficial, their ever-rising production and abuse of various types have raised concerns about the environment.1,2 Numerous kinds of pharmaceutical residues have been detected in worrisome concentrations in wastewater, surface water and drinking water. Due to their adverse effects on the human population and the environment, many efforts have been made to find ways to eliminate these harmful substances from water.3 Various techniques have been suggested, including adsorption, coagulation, flocculation, membrane filtration, chemical precipitation, ion exchange, etc., to eliminate these toxic organic compounds from wastewater.4 However, these approaches have proven ineffective and are deemed unsatisfactory due to the generation of substantial sludge requiring appropriate disposal, high operational costs, incomplete removal, and the potential production of secondary pollutants.5,6Interestingly, advanced oxidation processes (AOPs), such as photocatalysis, electrochemical oxidation, photoelectrocatalysis, sonolysis, hydrodynamic cavitation and microwave oxidation, have been identified to be particularly effective for treating recalcitrant and persistent organic compounds, including pharmaceutical residues.7–9 AOPs are innovative water treatment methods that involve the generation of highly reactive hydroxyl radicals (·OH) to break down and eliminate organic pollutants in water.10,11 Photoelectrocatalysis is attractive because it is an AOP that synergistically combines photocatalysis and electrochemical oxidation to degrade and eliminate organic pollutants in water.12,13 This process utilizes a semiconductor material as a photocatalyst illuminated by light to generate electron–hole pairs.14,15 The generated charge carriers participate in electrochemical reactions, producing reactive species that can oxidize and degrade organic contaminants. Photoelectrocatalysis offers several advantages, including improved efficiency in pollutant degradation, enhanced control over the reaction conditions, catalyst recovery, and the potential for direct utilization of solar energy.8,16 The efficiency of the photoelectrocatalysis process largely depends on the photoanode materials, usually semiconductors. It has been observed that photoanodes consisting of semiconductor–semiconductor heterojunctions offer higher removal efficiencies of organic pollutants in water.17,18 Hence, there is a constant effort to develop photoanodes with appropriately matched semiconductors for photoelectrocatalytic degradation of organic pollutants.
In the pool of visible light-responsive metal oxide semiconductors, tungsten trioxide (WO3) has garnered considerable attention as a photocatalyst in recent years, offering a promising solution for environmental remediation and sustainable energy applications.19–21 Tungsten trioxide possesses unique properties that make it well-suited for photocatalytic applications.22 Its semiconductor nature, high stability, and excellent light-absorbing capabilities contribute to its effectiveness in harnessing solar energy for catalytic reactions.23,24 The various crystalline forms of WO3, including monoclinic, hexagonal, and orthorhombic structures, add versatility to its photocatalytic performance.25 However, while WO3 exhibits significant promise as a photocatalyst, challenges, such as the recombination of electron–hole pairs and the need for optimal band gap engineering, persist.25 In particular, oxygen defect modulation in WO3 arising from heterojunction construction with suitable semiconductors is highly desired in catalytic reactions.26 This confers a visible light response and superior charge separation advantages on the heterojunction photoanode, thus improving the overall catalytic process. Hence, to improve the photocatalytic efficiency of WO3, research endeavours have focused on refining WO3 band engineering by doping a heteroatom, heterojunction, or surface defects to enhance their stability for prolonged use in real-world applications.
Another promising photoelectrocatalytic material is nickel selenide (NiSe2).27 This unique material, with its distinctive properties, plays a crucial role in enhancing the efficiency and performance of photoelectrocatalytic processes.27 As a co-catalyst, NiSe2 facilitates efficient charge transfer at the semiconductor–electrolyte interface, minimizing electron–hole recombination. The material's unique electronic structure promotes rapid electron transfer, improving overall photocatalytic performance.28 The presence of NiSe2 in photoelectrocatalytic systems results in increased photocurrent and photovoltage, indicative of improved charge carrier dynamics.27 This enhancement translates to higher efficiency in converting solar energy into chemical energy during photoelectrocatalysis. NiSe2 demonstrates notable efficacy as a water-splitting co-catalyst, particularly in the hydrogen evolution reaction (HER).29 Its electrocatalytic activity for the HER complements the photocatalytic oxidation of water, contributing to the overall efficiency of the water-splitting process.29–31 NiSe2's versatility extends to various photocatalytic reactions beyond water splitting, including the degradation of organic pollutants and the reduction of carbon dioxide.32,33 Its catalytic properties make it suitable for various photoelectrocatalytic applications, addressing environmental and energy-related challenges. As an emerging co-catalyst in photocatalytic applications, nickel selenide has been utilized as a co-catalyst for improving other semiconductors such as TiO2,34 BiVO4,34 Cd0.5Zn0.5S,35,36 Mn0.5Cd0.5S,37 Mn0.05Cd0.95S,38 and CdS.28 NiSe2 exhibits good stability and durability under photoelectrocatalytic conditions, ensuring prolonged and effective performance in diverse applications. Numerous studies reported in heterojunction engineering for effective acceleration of photogenerated charge separation during photoelectrocatalytic applications need consolidation. Hence, exploring the benefits of the oxygen vacancy and internal electric field in heterojunction photocatalysts to achieve charge separation and catalytic efficiency enhancement is desirable for wastewater remediation applications.
To our knowledge, NiSe2 has yet to be employed as a co-catalyst to enhance the catalytic efficiency of WO3 in degrading pharmaceuticals in water. Consequently, we present a facile synthesis method for fabricating a NiSe2/WO3 heterojunction through the in situ deposition of NiSe2 onto WO3. The heterojunction was sufficiently characterized by XRD, SEM, TEM, XPS, and photoelectrochemical characterization (EIS, PL, photocurrent response). The catalytic performance of the heterojunction was evaluated for the degradation of ciprofloxacin. The total organic carbon removal, degradation pathway, and detection of the reaction species are also reported.
Results and discussion
Structure, morphology, and composition
The NiSe2/WO3 heterojunction was synthesized using a solvothermal strategy, as illustrated in Scheme 1. Powder XRD was used to determine the phase purity and crystallinity of the synthesized compounds, as shown in Fig. 1a. When compared to the corresponding crystallographic planes (100), (002), (110), (102), (200), (202), and (220), the occurrence of diffraction peaks at 2θ = 14.01, 22.92, 24.33, 27.07, 28.18, 33.57, and 50.0 was well matched.39 The peaks fall within the typical hexagonal WO3 range (JCPDS card #33-1387), with a and c being the lattice constants. The peaks for NiSe2 are almost unnoticed in the NiSe2/WO3 diffractogram due to the low concentration of NiSe2 in the composite.The morphology of the catalysts was examined using scanning electron microscopy (SEM). In the pristine WO3, the micrographs revealed a distinct nanorod rhombohedral shape. Fig. 1b illustrates the SEM image of pristine WO3, where cylindrical nanorods were observed in an aggregated state. However, in the SEM images of NiSe2/WO3 (Fig. 1c), the introduction of NiSe2 led to a dispersed configuration of WO3 nanorods, resulting in an increased surface area. This enhanced surface area is particularly advantageous for photocatalytic applications, increasing the active sites and overall activity. The TEM micrographs provide additional support to the findings derived from SEM analysis. In the TEM images of WO3, an assortment of nanorods with an average diameter of 23 nm was observed. However, with the introduction of NiSe2, a noticeable change occurred in the nanorod structure, characterized by breakage and a slightly increased dispersion. This suggests that the deposition of NiSe2 onto the WO3 surface enhanced the surface area by reducing the particle size. Consequently, this modification rendered the nanorods more suitable for catalytic applications.
BET analysis was also conducted to investigate the surface area enhancement emanating from the successful formation of the NiSe2/WO3 heterojunction photocatalyst. The isotherms showing the N2 gas adsorption–desorption behaviour for WO3 and NiSe2/WO3 photocatalysts in Fig. 1f revealed that both photocatalysts exhibited a type IV pattern. Adsorption–desorption hysteresis observed between p/po of 0.5–1.0 is characteristic of mesoporous materials by IUPAC classification. In particular, the surface area of 12.29 m2 g−1 obtained for NiSe2/WO3 further reinforces the surface area enhancement exhibited by the NiSe2/WO3 heterojunction photocatalyst in comparison to 8.43 m2 g−1 obtained for pristine WO3. This further establishes the dispersed configuration of the synthesized NiSe2/WO3 photocatalyst, as illustrated by the SEM image. This surface enhancement underscores the successful formation of the NiSe2/WO3 heterojunction photocatalyst, bestowing tremendous benefit to the catalytic process.
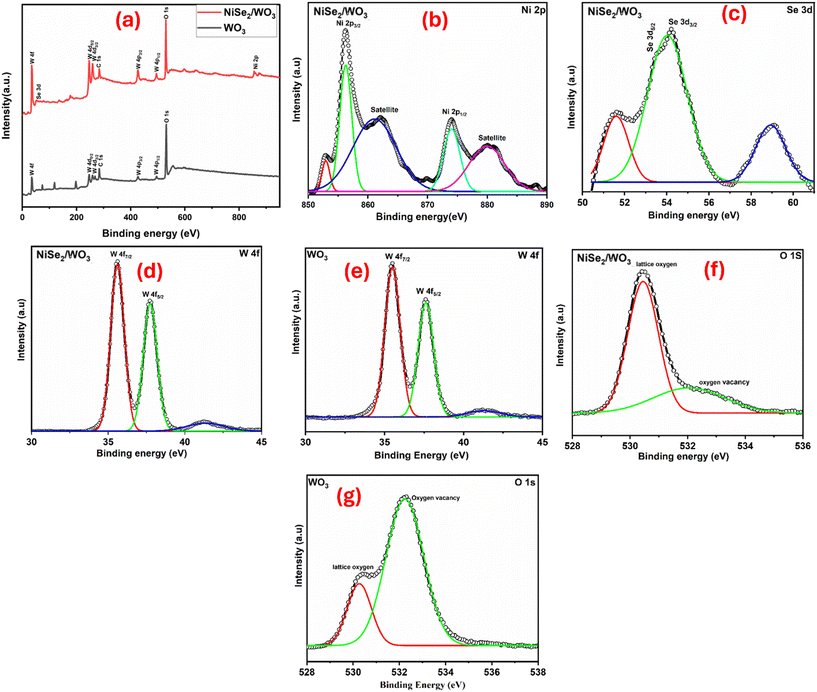
The surface chemistry comprising the chemical composition and valence state of the WO3 and NiSe2/WO3 was investigated with X-ray photoelectron spectroscopy (XPS). The total survey spectrum in Fig. 2a shows peak signals associated with the elements Ni, Se, W and O and XPS deconvoluted peaks of Se 3d, W 4d, O 1s and Ni 2p in high-resolution spectra of WO3 and NiSe2/WO3 are presented in Fig. 2b–h. The Ni 2p characteristic peaks at approximately 856.4 and 873.8 eV are illustrative of the respective Ni 2p3/2 and Ni 2p1/2 states of Ni2+ in NiSe2/WO3 (Fig. 2b). Characteristics satellite peaks attributed to the Ni oxidation states were also observed at approximately 862.8 and 879.7 eV.40 In Fig. 2c, peaks observed at approximately 53.6 and 54.3 eV correspond to Se 3d5/2 and Se 3d3/2 of the Se2− anion in NiSe2/WO3, confirming the presence of the Ni–Se bond. In addition, the peak at approximately 59.1 eV can be attributed to SeOx species, whose presence has been credited to NiSe2 exposure to air.41 Comparing the W 4f peaks in the high-resolution spectra of WO3 and NiSe2/WO3 (Fig. 2d and e), characteristic peaks of W 4f7/2 and W 4f5/2 observed at approximate binding energies of 35.6 and 37.8 eV in the WO3 spectra corresponds to the W6+ state.42,43 The presence of these peaks in the high-resolution spectra of NiSe2/WO3 (Fig. 2d) further confirms the complete formation of WO3 in NiSe2/WO3 after thermal treatment. The high-resolution O 1s spectra of NiSe2/WO3 and WO3 showing two distinct peaks are presented in Fig. 2f and g. The intense peak at the binding energy of 530.3 eV observed for NiSe2/WO3 (Fig. 2f) can be attributed to the lattice oxygen (O2−) in WO3 of the NiSe2/WO3 heterojunction. Whereas a pronounced peak at 532.2 eV, corresponding to the oxygen vacancy, is observed in pristine WO3 (Fig. 2g), and a significant reduction was observed in NiSe2/WO3. This suggests the occurrence of electron flow from WO3 to NiSe2 as the NiSe2/WO3 heterojunction was achieved, indicative of charge separation improvement via oxygen vacancy density in NiSe2/WO3.
Optical properties
Ultraviolet-visible diffuse reflectance spectroscopy (UV-DRS) is a valuable technique for determining the band gap energies of semiconductor materials and their composites. Understanding the band gap energies is crucial for optimizing their photocatalytic properties and designing efficient photocatalysts for environmental remediation and other applications. The absorption edge for WO3 was extrapolated at 449 nm (Fig. 3a). NiSe2/WO3 exhibited a stronger absorption in the visible region, indicative of the influence of NiSe2 on WO3. The band gap energy of NiSe2 typically falls in the range of 1.0 to 2.0 eV, as we have previously reported.27 The band gaps of NiSe2/WO3 and WO3 were calculated using the Kubelka–Munk function.| ahv = A(hv − Eg)n/2 | (1) |
| Evb = X − Ee + 0.5Eg | (2) |
| Ecb = Evb − Eg | (3) |
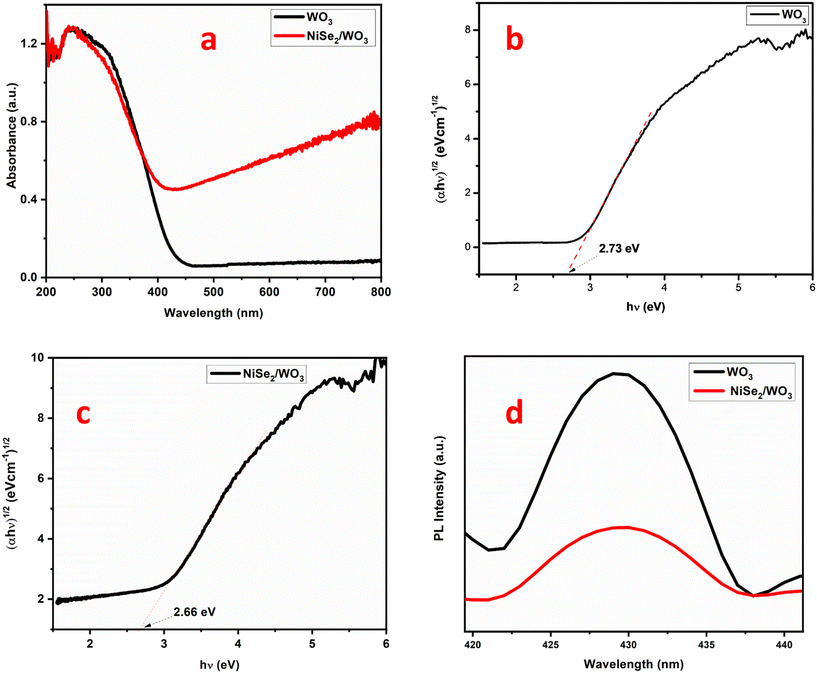 | ||
| Fig. 3 a) UV-DRS spectra for WO3 and NiSe2/WO3, b) Tauc plot for WO3, c) Tauc plot for NiSe2/WO3, and d) photoluminescence for WO3 and NiSe2/WO3. | ||
Electrochemical and photoelectrochemical properties
Electrochemical impedance spectroscopy (EIS), a valuable technique, was employed to analyze the electrical properties of the NiSe2/WO3 semiconductor-based photocatalysts. The charge transfer resistance (Rct) at the photocatalyst–electrolyte interface is proportional to the semicircle diameter in the Nyquist plot. Whereas a larger diameter often indicates higher charge transfer resistance, a small diameter can propel the photoelectrocatalytic reaction to achieve significant efficiency. As observed in Fig. 4a, the Rct of WO3 and NiSe2/WO3 are 398.5 and 48.5 ohm, respectively, which implies that the ease of charge transfer is greater in NiSe2/WO3. The transient photocurrent density response in Fig. 4b indicates that NiSe2/WO3 possesses a significantly enhanced photocurrent response over the pristine WO3. This further indicates that the NiSe2/WO3 heterojunction promoted a sustained charge carrier separation due to its superior visible light response edge. This superior charge-separation edge possessed by NiSe2/WO3 can promote the photoelectrocatalytic activity by prompting a significant formation of reactive species often responsible for the degradation of target organics. | ||
| Fig. 4 (a) Nyquist plot of WO3 and NiSe2/WO3, (b) photocurrent density of WO3 and NiSe2/WO3, and (c) Mott–Schottky plot for WO3. | ||
Furthermore, the valence and conduction band positions of WO3 were evaluated using the Mott–Schottky (MS) plot provided in Fig. 4c. The E–C−2 plot for both samples displays a positive plot, indicating n-type semiconductivity. The flat band potential obtained from the Mott–Schottky plot for WO3 was +0.42 eV (Fig. 4c), and by employing the Nernst equation: ENHE = EAg/AgCl + 0.197, and the Efb of WO3 is calculated to be +0.62 eV vs. NHE, respectively. Generally, n-type semiconductors have conduction bands of about 0.1–0.2 eV, which is more negative than the Efb.44 Therefore, the Ecb values for WO3 were evaluated to be +0.72 eV vs. NHE. In addition, by applying the Ecb = Evb − Eg relation, the valence band (Evb) for WO3 was estimated to be 3.45 eV vs. NHE.
Photoelectrocatalytic performance
| Catalysts | Process | Catalyst dosage | Pollutant | Degradation efficiency | Rate constant | Ref |
|---|---|---|---|---|---|---|
| FTO/Fe2O3/BiOI@BiOBr | Photoelectrocatalysis | Electrodeposition | Ciprofloxacin, 3.0 × 10−5 mol L−1, 120 min | 90% | 0.07 min−1 | 45 |
| NiSe2/MoS2 | Photoelectrocatalysis | 50 mg | Ciprofloxacin, 5 mg L−1, 120 min | 78% | 0.0111 min−1 | 47 |
| TiO2/AgBiS2 | Photoelectrocatalysis | 40 mg | Ciprofloxacin, 10 ppm, 100 min | 62% | — | 48 |
| g-C3N4/MIL-101 (Fe) | Photoelectrocatalysis | 50 mg | Ciprofloxacin, 10 mg L−1, 240 min | 87.6% | 0.009 min−1 | 49 |
| WO3/NiSe2 | Photoelectrocatalysis | 10 mg | Ciprofloxacin, 5 mg L−1, 120 min | 89% | 0.012 min−1 | This study |
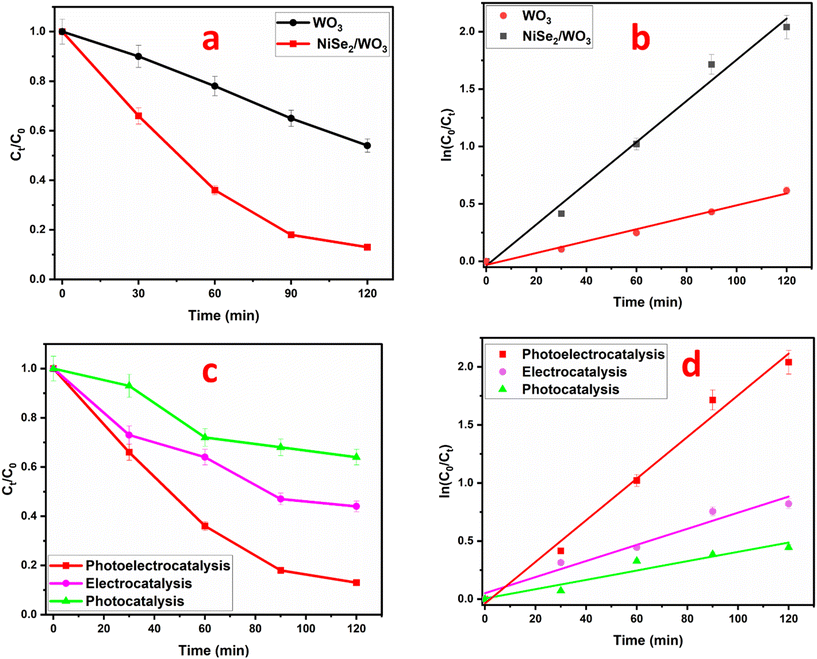
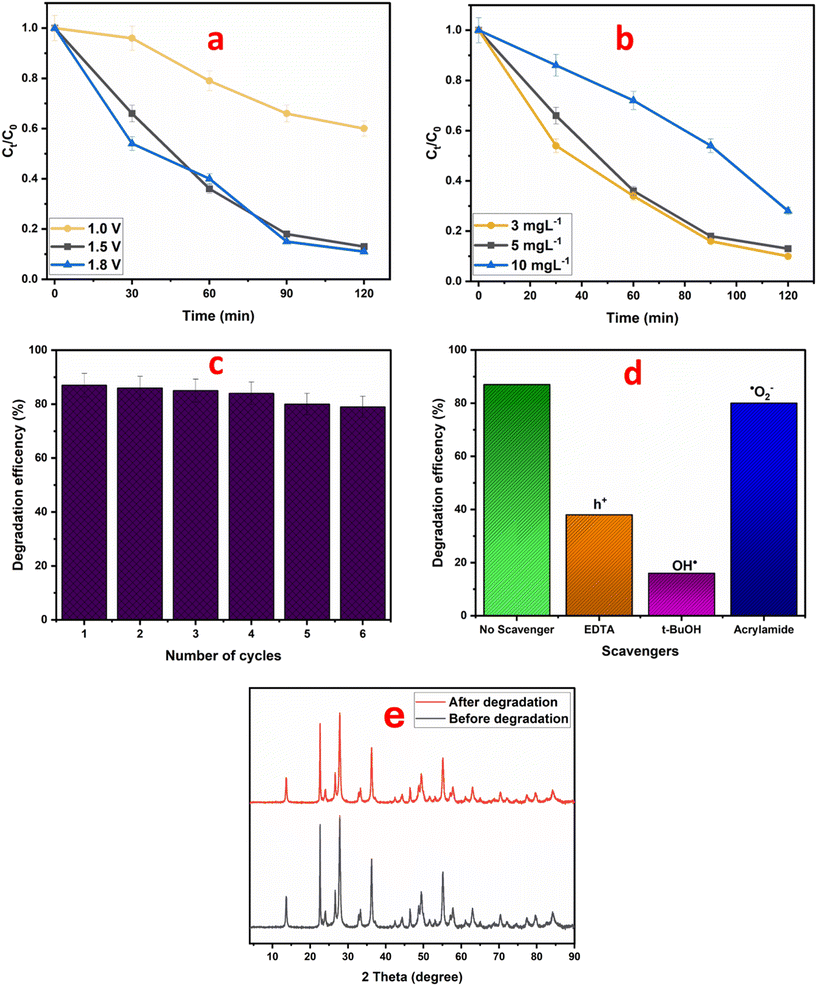
To assess the superiority of photoelectrocatalytic degradation over photocatalytic and electrocatalytic degradation, the NiSe2/WO3 electrode was further employed to degrade CIP under electrocatalytic and photocatalytic conditions. As shown in Fig. 5c and d, the percentage removal of the pharmaceutical was significantly reduced to 37% (0.0049 min−1) and 56% (0.0073 min−1) degradation in the photocatalytic and electrocatalytic processes, respectively. Evidently, the enhanced removal of CIP through the photoelectrocatalytic process arose from a synergy between the applied potential and light energy. This advantage arose from a pronounced effect exerted by the photocatalytic process upon illumination of the photoanode, thereby initiating the generation of charge carriers that migrate to its surface. The resulting holes oxidize CIP and interact with water molecules, yielding highly oxidizing hydroxyl radicals, further aiding CIP degradation. However, the occurrence of charge carrier recombination hindered this process, resulting in reduced degradation efficiency. Nonetheless, the presence of the bias potential aided charge carrier separation as electrons travel through the circuit to the cathode, reducing recombination rates. The observed variation in the apparent first-order rate constants (NiSe2/WO3 > WO3) further highlights the superior efficiency of the NiSe2/WO3 heterojunction photoanode.
Moreover, the internal electric field driven by the bias potential in the heterojunction further led to electron flow from the conduction band of WO3 to the valence band of NiSe2. Thus, establishing the availability of photogenerated charge carriers (holes and electrons) in the PEC process produced hydroxyl, which is primarily responsible for the degradation of CIP. This charge-redistribution mechanism agrees with the XPS high-resolution O 1s spectra (Fig. 2f and g) and the scavenger study (Fig. 6d). Eqn (4)–(6) represent reactions establishing the photoelectrocatalytic activity via the NiSe2/WO3 heterojunction photoanode.
| NiSe2/WO3 + Vis → h+ + e− | (4) |
| H2O + h+ → ˙OH + H+ | (5) |
| ˙OH + h+ + CIP → Byproducts + H2O + CO2 | (6) |
 | ||
| Fig. 8 Proposed degradation pathway showing byproducts I–VII for the CIP PEC process at the NiSE2/WO3 photoanode. | ||
Moreover, the opened piperayl ring was oxidized to form an amide attachment on the quinolone moiety (V–VII). These intermediates can undergo further oxidation to form CO2, H2O, and NH3. The formation of these identified by-products further substantiates the superior suppression of charge carrier recombination during the process, thus leading to the sustained generation of species responsible for the decomposition of CIP and improved PEC activity.
Conclusions
In summary, we present a systematic strategy for removing CIP via an improved photoelectrocatalytic approach using a nickel selenide–tungsten trioxide heterojunction. The heterostructured NiSe2/WO3 band gap was effectively narrowed through a precise composite formation. Consequently, the recombination of photogenerated charge carriers was significantly suppressed while achieving a significant improvement in charge-transfer efficiency as established by the optical and electrochemical properties of the photoactive composite. Moreover, these advantages improved the kinetics of the reaction and degradation efficiency of ciprofloxacin during the photoelectrocatalytic process. Furthermore, the heterostructured photoanode demonstrated a markedly substantial degradation efficiency at a low bias potential against higher initial concentrations of CIP. The scavenger analysis of the study revealed that hydroxyl and superoxide radicals generated from the interaction of photogenerated charge carriers with hydroxyl ions and oxygen were primarily responsible for the degradation of CIP. Our investigation provides a robust framework for optimising photoelectrochemical energy conversion, capitalizing on exploiting solar illumination and low bias potential for water remediation.Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Additionally, supplementary data and materials can be found in the ESI† files associated with this publication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the University of Pretoria, the University of Johannesburg and the University of South Africa for providing the facility for this research. BO is grateful to URC, University of Johannesburg, for the award of a postdoctoral fellowship.References
- J.-L. Liu and M.-H. Wong, Environ. Int., 2013, 59, 208–224 CrossRef CAS PubMed.
- P. Chaturvedi, P. Shukla, B. S. Giri, P. Chowdhary, R. Chandra, P. Gupta and A. Pandey, Environ. Res., 2021, 194, 110664 CrossRef CAS PubMed.
- K. Kümmerer, J. Environ. Manage., 2009, 90, 2354–2366 CrossRef.
- A. Saravanan, P. S. Kumar, S. Jeevanantham, S. Karishma, B. Tajsabreen, P. Yaashikaa and B. Reshma, Chemosphere, 2021, 280, 130595 CrossRef CAS.
- T. A. Saleh, M. Mustaqeem and M. Khaled, Environ. Nanotechnol., Monit. Manage., 2022, 17, 100617 CAS.
- P. Rajasulochana and V. Preethy, Resour.-Effic. Technol., 2016, 2, 175–184 Search PubMed.
- E. Brillas, I. Sirés and M. A. Oturan, Chem. Rev., 2009, 109, 6570–6631 CrossRef CAS PubMed.
- T. L. Yusuf, B. O. Orimolade, D. Masekela, B. Mamba and N. Mabuba, RSC Adv., 2022, 12, 26176–26191 RSC.
- O. C. Olatunde, T. L. Yusuf, N. Mabuba, D. C. Onwudiwe and S. Makgato, J. Water Process Eng., 2024, 59, 105074 CrossRef.
- M. Cheng, G. Zeng, D. Huang, C. Lai, P. Xu, C. Zhang and Y. Liu, Chem. Eng. J., 2016, 284, 582–598 CrossRef CAS.
- Q. Yang, Y. Ma, F. Chen, F. Yao, J. Sun, S. Wang, K. Yi, L. Hou, X. Li and D. Wang, Chem. Eng. J., 2019, 378, 122149 CrossRef CAS.
- P. Alulema-Pullupaxi, P. J. Espinoza-Montero, C. Sigcha-Pallo, R. Vargas, L. Fernandez, J. M. Peralta-Hernandez and J. L. Paz, Chemosphere, 2021, 281, 130821 CrossRef CAS.
- S. Garcia-Segura and E. Brillas, J. Photochem. Photobiol., C, 2017, 31, 1–35 CrossRef CAS.
- J. D. García-Espinoza, I. Robles, A. Durán-Moreno and L. A. Godínez, Chemosphere, 2021, 274, 129957 CrossRef.
- E. Mousset and D. D. Dionysiou, Environ. Chem. Lett., 2020, 18, 1301–1318 CrossRef CAS.
- T. Mohlala, T. L. Yusuf and N. Mabuba, J. Electroanal. Chem., 2023, 947, 117806 CrossRef CAS.
- T. L. Yusuf, S. A. Ogundare, F. Opoku and N. Mabuba, Surf. Interfaces, 2023, 36, 102534 CrossRef CAS.
- K. D. Jayeola, D. S. Sipuka, T. I. Sebokolodi, O. V. Nkwachukwu, C. Muzenda, B. A. Koiki, J. O. Babalola, M. Zhou and O. A. Arotiba, Chem. Eng. J., 2024, 479, 147482 CrossRef CAS.
- J. Murillo-Sierra, A. Hernández-Ramírez, L. Hinojosa-Reyes and J. Guzmán-Mar, Chem. Eng. J. Adv., 2021, 5, 100070 CrossRef CAS.
- V. Dutta, S. Sharma, P. Raizada, V. K. Thakur, A. A. P. Khan, V. Saini, A. M. Asiri and P. Singh, J. Environ. Chem. Eng., 2021, 9, 105018 CrossRef CAS.
- M. G. Peleyeju and E. L. Viljoen, J. Water Process Eng., 2021, 40, 101930 CrossRef.
- S. Adhikari, K. S. Chandra, D.-H. Kim, G. Madras and D. Sarkar, Adv. Powder Technol., 2018, 29, 1591–1600 CrossRef CAS.
- O. Samuel, M. H. D. Othman, R. Kamaludin, O. Sinsamphanh, H. Abdullah, M. H. Puteh and T. A. Kurniawan, Ceram. Int., 2022, 48, 5845–5875 CrossRef CAS.
- S. Adhikari, M. Murmu and D. H. Kim, Small, 2022, 18, 2202654 CrossRef CAS PubMed.
- P. Shandilya, S. Sambyal, R. Sharma, P. Mandyal and B. Fang, J. Hazard. Mater., 2022, 428, 128218 CrossRef CAS PubMed.
- M. Zhang, D. Wang, H. Ma, H. Wei and G. Wang, Sci. Total Environ., 2024, 924, 171383 CrossRef CAS PubMed.
- T. L. Yusuf, S. A. Ogundare, F. Opoku, O. A. Arotiba and N. Mabuba, J. Environ. Chem. Eng., 2023, 11, 110711 CrossRef CAS.
- S. Shen, L. Yan, K. Song, Z. Lin, Z. Wang, D. Du and H. Zhang, RSC Adv., 2020, 10, 42008–42013 RSC.
- I. H. Kwak, H. S. Im, D. M. Jang, Y. W. Kim, K. Park, Y. R. Lim, E. H. Cha and J. Park, ACS Appl. Mater. Interfaces, 2016, 8, 5327–5334 CrossRef CAS PubMed.
- L. Yang, L. Huang, Y. Yao and L. Jiao, Appl. Catal., B, 2021, 282, 119584 CrossRef CAS.
- C. Liu, T. Gong, J. Zhang, X. Zheng, J. Mao, H. Liu, Y. Li and Q. Hao, Appl. Catal., B, 2020, 262, 118245 CrossRef CAS.
- C. Yang, Y. Lu, W. Duan, Z. Kong, Z. Huang, T. Yang, Y. Zou, R. Chen and S. Wang, Energy Fuels, 2021, 35, 14283–14303 CrossRef CAS.
- X. Xia, L. Wang, N. Sui, V. L. Colvin and W. Y. William, Nanoscale, 2020, 12, 12249–12262 RSC.
- S. Shen, H. Zhang, A. Xu, Y. Zhao, Z. Lin, Z. Wang, W. Zhong and S. Feng, J. Alloys Compd., 2021, 875, 160071 CrossRef CAS.
- X. Zhang, Z. Cheng, P. Deng, L. Zhang and Y. Hou, Int. J. Hydrogen Energy, 2021, 46, 15389–15397 CrossRef CAS.
- H. Gong, Z. Li, Z. Chen, Q. Liu, M. Song and C. Huang, ACS Appl. Nano Mater., 2020, 3, 3665–3674 CrossRef CAS.
- X. Jiang, H. Gong, Q. Liu, M. Song and C. Huang, Appl. Catal., B, 2020, 268, 118439 CrossRef CAS.
- H. Liu, T. Yan, Z. Jin and Q. Ma, New J. Chem., 2020, 44, 14879–14889 RSC.
- V. Ramar and K. Balasubramanian, ACS Appl. Nano Mater., 2021, 4, 5512–5521 CrossRef CAS.
- K. Lu, J. Sun, H. Xu, C. Jiang, W. Jiang, F. Dai, H. Wang and H. Hao, Mater. Adv., 2022, 3, 2139–2145 RSC.
- Z. Li, X. Ma, L. Wu, H. Ye, L. Li, S. Lin, X. Zhang, Z. Shao, Y. Yang and H. Gao, RSC Adv., 2021, 11, 6842–6849 RSC.
- M. Tong, J. Yang, Q. Jin, X. Zhang, J. Gao and G. Li, J. Mater. Sci., 2019, 54, 10656–10669 CrossRef CAS.
- A. K. Mohamedkhair, Q. A. Drmosh, M. Qamar and Z. H. Yamani, Catalysts, 2021, 11, 381 CrossRef CAS.
- X. Yue, S. Yi, R. Wang, Z. Zhang and S. Qiu, Nano Energy, 2018, 47, 463–473 CrossRef CAS.
- M. H. A. Feitosa, A. M. Santos, A. Wong, C. A. F. Moraes, G. M. Grosseli, O. R. Nascimento, P. S. Fadini and F. C. Moraes, Chem. Eng. J., 2024, 493, 152291 CrossRef CAS.
- X. Li, Q. Liu, F. Deng, J. Huang, L. Han, C. He, Z. Chen, Y. Luo and Y. Zhu, Appl. Catal., B, 2022, 314, 121502 CrossRef CAS.
- T. L. Yusuf, O. C. Olatunde, D. Masekela, K. D. Modibane, D. C. Onwudiwe and S. Makgato, ChemElectroChem, 2024, e202400309 CrossRef CAS.
- Laxmiputra, D. B. Nityashree, Udayabhanu, S. M. Anush, K. Pramoda, K. Prashantha, B. U. M. B N, Y. R. Girish and H. Nagarajaiah, Mater. Res. Bull., 2024, 169, 112489 CrossRef CAS.
- H. Liu, C. Du, M. Li, S. Zhang, H. Bai, L. Yang and S. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 28686–28694 CrossRef CAS.
- Z. Chen, W. Lai, Y. Xu, G. Xie, W. Hou, P. Zhanchang, C. Kuang and Y. Li, J. Hazard. Mater., 2021, 405, 124262 CrossRef CAS PubMed.
- Z. Jia, R. Lv, L. Guo, J. Zhang, R. Li, J. Liu and C. Fan, Sep. Purif. Technol., 2021, 257, 117872 CrossRef CAS.
- C. Wang, T. Zhang, J. Luo, M. Wu, J. Niu, E. Shang, C. Ni and J. Ni, Sep. Purif. Technol., 2022, 297, 121528 CrossRef CAS.
- M. Sayed, M. Ismail, S. Khan, S. Tabassum and H. M. Khan, Environ. Technol., 2016, 37, 590–602 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cy00729h |
| This journal is © The Royal Society of Chemistry 2024 |