Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
2,3-Diamino-4,5-diarylcyclopentadienone iron carbonyl complexes as catalysts for reductive amination reactions†
Lukas
Körner
 ,
Dirk
Bockfeld
,
Thomas
Bannenberg
and
Matthias
Tamm
,
Dirk
Bockfeld
,
Thomas
Bannenberg
and
Matthias
Tamm
 *
*
Institut für Anorganische und Analytische Chemie, Technische Universtiät Braunschweig, Hagenring 30, 38106 Braunschweig, Germany. E-mail: m.tamm@tu-bs.de
First published on 8th July 2024
Abstract
The reaction of dipiperidinoacetylene, (CH2)5NC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CN(CH2)5, with a series of 2,3-diarylcyclopropenones (Ar)2C3O (Ar = phenyl, 4-tert-butylphenyl, 2,5-dimethylphenyl, 4-trifluoromethylphenyl) afforded 2,3-dipiperidino-4,5-diarylcyclopentadienones, which were used to prepare cyclopentadienone (CPD) iron tricarbonyl complexes [(CPD)Fe(CO)3] by the reaction with Fe2(CO)9. Subsequent treatment with trimethylamine-N-oxide in the presence of acetonitrile afforded the corresponding acetonitrile complexes [(CPD)Fe(CO)2(NCCH3)], which were used as catalysts for the reductive amination of citronellal with various secondary amines under 5 bar of dihydrogen pressure. The first iron-catalysed reductive amination for the preparation of the pharmaceutically important antidepressant sertraline is also reported.
CN(CH2)5, with a series of 2,3-diarylcyclopropenones (Ar)2C3O (Ar = phenyl, 4-tert-butylphenyl, 2,5-dimethylphenyl, 4-trifluoromethylphenyl) afforded 2,3-dipiperidino-4,5-diarylcyclopentadienones, which were used to prepare cyclopentadienone (CPD) iron tricarbonyl complexes [(CPD)Fe(CO)3] by the reaction with Fe2(CO)9. Subsequent treatment with trimethylamine-N-oxide in the presence of acetonitrile afforded the corresponding acetonitrile complexes [(CPD)Fe(CO)2(NCCH3)], which were used as catalysts for the reductive amination of citronellal with various secondary amines under 5 bar of dihydrogen pressure. The first iron-catalysed reductive amination for the preparation of the pharmaceutically important antidepressant sertraline is also reported.
Introduction
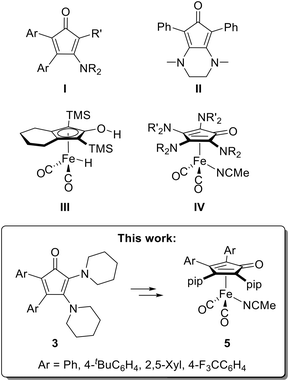
In recent years, the chemistry of iron cyclopentadienone (CPD) carbonyl complexes and their application in homogeneous catalysis has developed into an extensive and diverse field of research.1–3 Although such complexes have been known since the 1950s,4–8 the beginnings of comprehensive research can be traced back to Knölker, who first demonstrated the cyclisation of alkynes in the presence of iron pentacarbonyl to form CPD iron tricarbonyl complexes in 1992.9 Remarkably, the original intention of the synthesis was to obtain the free CPDs by oxidative decomposition of the iron complexes, mainly with trimethylamine-N-oxide, and to study their Diels–Alder follow-up chemistry.9–12 Other methods for obtaining free CPDs are widespread and mostly involve condensation reactions of 1,3-substituted propan-2-ones with benzil derivatives.13,14 Other protocols use alkynes and diarylated cyclopropenones in the presence of rhodium catalysts.15–20In contrast, more electron-rich amino-substituted congeners are rare, with only two classes known to date (Fig. 1, I and II). As early as 1966, the uncatalysed (3 + 2)-cycloaddition of 1-diethylamino-2-phenylacetylene with diphenylcyclopropenone was shown to yield 3-diethylamino-2,4,5-triphenylcyclopentadienone.21 In 1980, this reaction type was used to obtain a larger series of 3-amino-2,4,5-triaryl-CPD derivatives I.22 The proposed mechanism involves a nucleophilic attack by the aminoalkyne on the carbonyl group of the cyclopropenone, which is then followed by an electrocyclic rearrangement to form the CPD. In 2007, Haak introduced the first and only known synthesis of diamino-CPDs. Among these, the bicyclic compound II is preferably used for the preparation of catalysts and can be obtained in two steps from 1,3-diphenylpropan-2-one, diethyl oxalate, and N,N-dimethylethylenediamine.23 The CPD can then be transferred to ruthenium or iron by reacting it with suitable precursors, such as Ru3(CO)12 or Fe2(CO)9.24,25 The use of CPD complexes as catalysts originated from the discovery of the Shvo catalyst; this bimetallic hydride-bridged CPD ruthenium dimer was discovered as the reaction product of diphenylacetylene and Ru3(CO)12. Shvo and coworkers demonstrated that the catalyst dissociates in solution, providing both a hydrogen acceptor and a hydrogen donor.24 This property enables broad applicability in catalytic oxidation and reduction reactions through dehydrogenation and hydrogenation, respectively.26,27
Related CPD iron complexes, despite their first appearance as early as the 1950s (vide supra),4–7 only became the target of catalytic applications after 1999, when Knölker published the preparation of the hydroxy–hydride complex III (Fig. 1) through a Hieber-base type transformation of the corresponding iron CPD tricarbonyl complex with sodium hydroxide, followed by an acidic workup with H3PO4.28 Casey and Guan were the first to use III for catalytic purposes. They investigated the (transfer-)hydrogenation of various carbonyl compounds and one imine and also provided mechanistic insights by substantiating the widely accepted “outer-sphere” mechanism for the dihydrogen transfer, which involves the hydroxy–hydride complex as the catalytically active species.29,30 For most CPD-iron catalyst systems, however, the active catalyst is typically generated from a stable CPD iron tricarbonyl complex, which is achieved by dissociating one CO ligand, either chemically with trimethylamine-N-oxide or photochemically by UV irradiation. The resulting unsaturated species then forms the corresponding hydroxy–hydride complex upon reacting with the hydrogen donor.31–33 Alternatively, direct activation with the hydrogen donor can be achieved by using the corresponding dicarbonyl complexes that contain a labile ligand, such as acetonitrile, which can be readily dissociated in situ.34
As part of our research on the chemistry of diaminoacetylenes (DAAs),35–40 we have recently introduced various symmetric and asymmetric tetraamino-CPD iron complexes of type IV (Fig. 1) with cyclic amino substituents such as piperidine. These complexes were obtained by reacting two DAA equivalents with Fe(CO)5, followed by CO substitution with the acetonitrile ligand.41 As previously shown by Filippou,42 these reactions proceed via a ferracyclobutenone intermediate, which allows the subsequent incorporation of two different DAA units (NR2 ≠  , Fig. 1). Investigation of the catalytic activity of these complexes in the (transfer-)hydrogenation of carbonyl compounds revealed a significantly enhanced catalytic activity compared to the established Knölker-type congeners. In particular, the hydrogenation of aldehydes and ketones with dihydrogen proceeded under remarkably mild reactions conditions (3 bar dihydrogen pressure, room temperature). Attempts to isolate a hydroxy–hydride intermediate similar to III proved unsuccessful, and its relative thermodynamic instability compared to its dehydrogenated form was confirmed by DFT calculations.41 It should also be noted that our recent attempts to decompose complexes IV or their Fe(CO)3 precursors to release the as yet inaccessible free 2,3,4,5-tetraamino-CPDs were also unsuccessful.
, Fig. 1). Investigation of the catalytic activity of these complexes in the (transfer-)hydrogenation of carbonyl compounds revealed a significantly enhanced catalytic activity compared to the established Knölker-type congeners. In particular, the hydrogenation of aldehydes and ketones with dihydrogen proceeded under remarkably mild reactions conditions (3 bar dihydrogen pressure, room temperature). Attempts to isolate a hydroxy–hydride intermediate similar to III proved unsuccessful, and its relative thermodynamic instability compared to its dehydrogenated form was confirmed by DFT calculations.41 It should also be noted that our recent attempts to decompose complexes IV or their Fe(CO)3 precursors to release the as yet inaccessible free 2,3,4,5-tetraamino-CPDs were also unsuccessful.
Since enhanced catalytic activity was also observed by Renaud and coworkers for 3,4-diamino-CPD iron complexes derived from II,25,43–50 we reasoned that 2,3-diamino-CPDs 3 should also give rise to promising CPD iron catalysts and that they should be readily accessible from diarylcyclopropenones and diaminoacetylenes (DAAs), in analogy to similar reactions with monoaminoalkynes to give CPDs I.21,22 Accordingly, we report here the preparation of various 2,3-diamino-4,5-diarylcyclopentadienones 3 and their corresponding CPD-iron complexes 5, which were used as catalysts in hydrogenation and reductive amination reactions (see box in Fig. 1). It should be noted that the metal coordination of CPDs 3via their enantiotopic faces affords planar chiral metal complexes which, upon resolution, could serve as stereoselective hydrogenation catalysts, an area that has received comparatively little attention in the case of CPD iron complexes.51–59 The best results so far, however, have not been obtained with chiral CPD complexes, but with Knölker's catalyst III in combination with chiral BINOL-derived phosphoric acids, and these systems have been used by Beller and coworkers in the asymmetric hydrogenation and reductive amination reactions with high enantiomeric excesses.60–63
Results and discussion
Synthesis of 2,3-dipiperidino-4,5-diarylcyclopentadienones
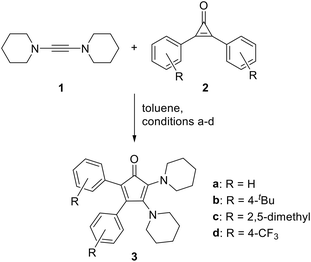
Treatment of dipiperidinoacetylene (1) with diphenylcyclopropenone (2a) in toluene at ambient temperature resulted in a rapid colour change from light yellow to dark purple, and the reaction was complete within 30 minutes (Scheme 1). Thin-layer chromatography revealed a purple spot under visible light, and the corresponding fraction could be isolated by flash column chromatography on neutral alumina. 1H NMR spectroscopy of the resulting purple solid revealed three multiplets in the aromatic region attributable to ten phenyl protons, two multiplets at 3.20 and 2.97 ppm each corresponding to four protons of the α-CH2 groups of two chemically distinct piperidino moieties, and a multiplet at 1.52–1.27 ppm corresponding to twelve protons of the β- and γ-CH2 groups, respectively. In the 13C{1H} NMR spectrum, the signal for the carbonyl carbon atom is found at 197.7 ppm, which is in good agreement with the reported value for the bicyclic CPD II (195.2 ppm).23 These observations led to the conclusion that the cycloaddition was successful and cyclopentadienone 3a was obtained. Initially also aiming at the preparation of tetraaminocyclopentadienones, we treated dipiperidinocyclopropenone with dipiperidinoacetylene, but no reaction was observed, indicating that this electron-rich cyclopropenone was too unreactive. Similarly, no reactions were observed for dimesityl-, dianisyl- and dinaphtylcyclopropenone, suggesting that these substituents are too electron-donating and/or sterically demanding.In contrast, di(4-tert-butylphenyl)cyclopropenone (2b) and bis(2,5-dimethylphenyl)cyclopropenone (2c) underwent a colour change when treated with dipiperidinoacetylene (1) at ambient temperature over a few hours. The resulting purple substances were isolated by column chromatography and characterised by NMR spectroscopy. The NMR spectra of cyclopentadienone 3b exhibited the expected signals, whereas 3c produced more signals than anticipated. This observation can be attributed to hindered rotation around the C4/5–Caryl bonds and the presence of diastereomeric atropisomers in solution at ambient temperature (ESI,† Fig. S6). Variable-temperature 1H NMR spectra were recorded in tetrachloroethene up to 100 °C, however, coalescence and fast interconversion could not be observed (ESI,† Fig. S8). The structural integrity of 3c is supported by the recorded IR and UV/Vis spectra as well as from the ESI high-resolution mass spectrum. Another CPD, 3d, was synthesized in a similar fashion from bis(4-trifluoromethylphenyl)cyclopropenone (2d), but at lower temperature due to the higher reactivity of the CF3-substituted, less electron-rich cyclopropenone. 3d was characterized, inter alia, by NMR spectroscopy, including the 19F NMR spectrum, which showed two singlets at −63.0 and −63.1 ppm.
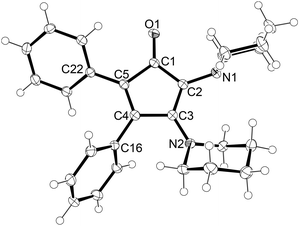
Furthermore, the molecular structures of 3a and 3d could be determined by X-ray diffraction analysis after growing suitable single crystals from diethylether/n-hexane and n-hexane solutions, respectively, both at −40 °C. The molecular structure of 3a is shown in Fig. 2, while that of 3d is presented in the ESI† section (Table S2). In both structures, the C3–N2 bond lengths are significantly shorter than the corresponding C2–N1 bonds, i.e. 1.3604(17) vs. 1.4277(13) Å in 3a, indicating a significant double bond character of the C3–N2 bonds. Furthermore, the C1–C2 bonds are shortened compared to the C1–C5 bonds and the angle sums of the nitrogen atoms show planarised N2 atoms (356.6 in 3a). This suggests a significant π-interaction with the N2 lone pair and consequently a marked polarisation towards an imininium-enolate mesomeric form.
Theoretical study of the mechanism of 2,3-diamino-4,5-diarylcyclopentadienone formation
It has been proposed that the formation of monoaminocyclopentadienones of type I (Fig. 1) may proceed via a nucleophilic attack of an aminoacetylene (yneamine) at the 1- or 2-position of the diarylcyclopropenone.22 To establish a similar mechanism for the formation of diaminocyclopentadienones 3, we investigated the formation of 3a from dipiperidinoacetylene (1) and diphenylcyclopropenone (2a). For both reaction pathways, the energy profile begins with the formation of van der Waals (vdW) complexes between 1 and 2a, in which the two reactants are suitably oriented to attack either at the C1 (CO black profile a) or C2 (CPh, red profile b) carbon atom of the cyclopropenone (Fig. 3). The nucleophilic attack at C2, which corresponds to a Michael-type addition, is clearly kinetically and thermodynamically favoured, with a barrier of ΔG° = +19.2 kcal mol−1 relative to 1/2a. The resulting intermediate IN1b is formed exergonically (ΔG° = −13.6 kcal mol−1); it can be described as a ketene derivative, and its transformation to CPD 3a proceeds with a very small barrier (ΔG° = +3.4 kcal mol−1) by intramolecular attack of the remaining aminocarbene moiety at the carbonyl carbon atom. The overall reaction and formation of 3a is highly exergonic (ΔG° = −60.6 kcal mol−1), and the activation energies are consistent with the reaction occurring at room temperature. In contrast, pathway a, involving nucleophilic attack at C1 via the cyclopropenolate intermediate INa, is strongly disfavoured with a maximum barrier of +31.2 kcal mol−1 (relative to 1/2a). However, since the reaction of 1 does not lead to different regioisomers, unlike in the case of monoaminoacetylenes,22 it is not possible to distinguish experimentally between the two pathways.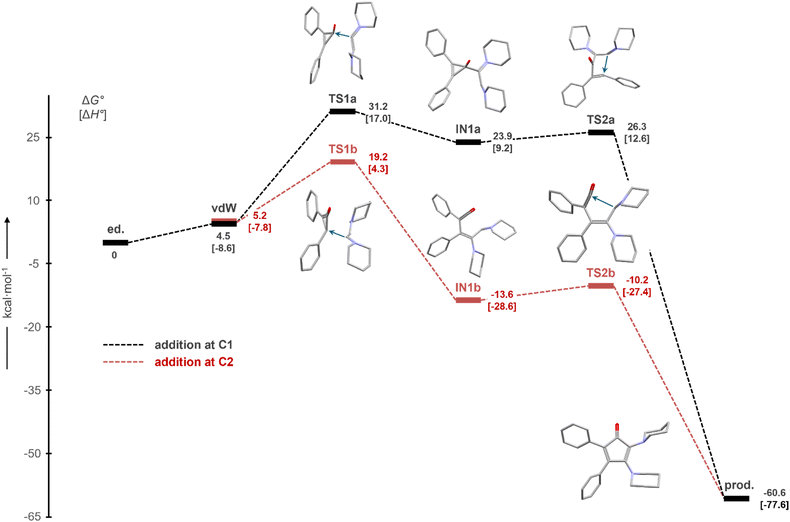 | ||
| Fig. 3 Calculated energy profiles (a in black, b in red) for the formation of 2,3-dipiperidinyl-4,5-diphenylcyclopentadienone (3a) from dipiperidinoacetylene (1) and diphenylcyclopropenone (2a), scaled to standard Gibbs free energies (ΔG°); standard enthalpies are given in square brackets. The densitiy functional theory (DFT) method ωB97XD/6-311G(d,p) together with a universal solvent model (SMD) for toluene was used (ESI†). | ||
Synthesis of iron cyclopentadienone complexes
The cyclopentadienones 3a–3d were reacted with Fe2(CO)9 in toluene at 110 °C, following the synthesis of known CPD iron tricarbonyl complexes such as [(II)Fe(CO)3] (Scheme 2).25 The desired complexes 4a–4d were obtained as air-stable yellow solids in moderate to good yields (66–84%) after work-up by flash column chromatography on alumina. The carbonyl ligands in 4a–4c give rise to characteristic low-field signals at 211.0–211.3 ppm in the 13C{1H} NMR spectra, which is the same range as found for related tetraamino-CPD complexes.41 In 4d, which carries electron-withdrawing CF3 groups on the CPD ligand, this resonance appears at a slightly higher field (209.9 ppm), and its 19F NMR spectrum shows two singlets at −63.1 and −63.3 ppm for the two chemically distinct CF3 groups. It should be noted that, in contrast to CPD 3c, the 1H and 13C{1H} NMR spectra of the corresponding CPD complex 4c show four sharp signals for the o-CH3 and m-CH3 groups of the two different xylyl substituents, revealing a fast rotation around the C4,5–Caryl bonds with rapid interconversion between the four possible atropisomers at room temperature on the NMR time scale. The IR spectra of 4a–4d exhibit two absorption bands at 2037–2031 cm−1 and 1975–1960 cm−1, respectively, which can be assigned to the symmetric and asymmetric CO stretching frequencies.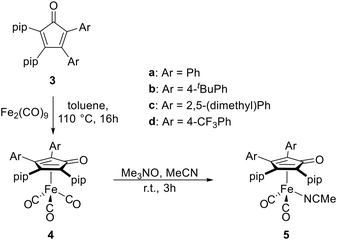 | ||
| Scheme 2 Synthesis of 2,3-dipiperidino-4,5-diarylcyclopentadienone iron complexes 4 from free CPDs 3 and transformation to corresponding acetonitrile complexes 5. | ||
The CPD complexes exhibit markedly different solubilities; the phenyl-substituted complex 4a is almost insoluble in unpolar media such as n-hexane, whereas the addition of substituents to the phenyl moieties increases the solubility. Accordingly, single crystals suitable for X-ray diffraction analysis could be obtained from a THF/n-hexane solution for 4a, chloroform/n-hexane for 4b·CHCl3, hot n-hexane by cooling to room temperature for 4c, and a saturated cyclohexane solution of 4d·cyclohexane at ambient temperature. The molecular structure of 4a is shown in Fig. 4, while those of 4b–4d are presented in the ESI† section (Tables S4–S6). Relevant bond lengths of the four tricarbonyl complexes are summarised in Table 1 for comparison. All complexes exhibit the expected three-legged piano-stool geometry around the iron atoms with η4-coordinated CPD ligands that have slightly longer Fe–Cpip (2.18–2.22 Å) than Fe–Caryl (2.05–2.11 Å) bond lengths. The Fe–C1 distances are significantly longer (2.40–2.46 Å) with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units bending away from the iron atoms, precluding any significant binding interaction. Accordingly, short C1–O bond lengths of about 1.23 Å are found. It is noteworthy that the C2–N1 and C3–N2 bond lengths are uniform (∼1.39 Å) and both nitrogen atoms are clearly pyramidalised in each case, in contrast to the markedly different nitrogen environments found in the free CPD ligands (vide supra). Overall, however, the structural parameters are in very good agreement with those determined for other CPD complexes, especially those with amino substituents.25,41,42
O units bending away from the iron atoms, precluding any significant binding interaction. Accordingly, short C1–O bond lengths of about 1.23 Å are found. It is noteworthy that the C2–N1 and C3–N2 bond lengths are uniform (∼1.39 Å) and both nitrogen atoms are clearly pyramidalised in each case, in contrast to the markedly different nitrogen environments found in the free CPD ligands (vide supra). Overall, however, the structural parameters are in very good agreement with those determined for other CPD complexes, especially those with amino substituents.25,41,42
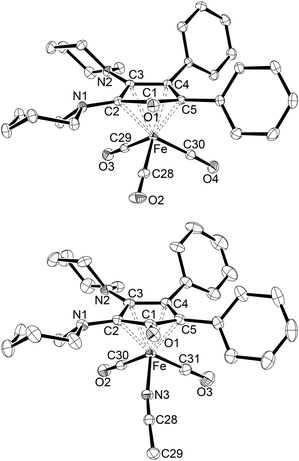 | ||
| Fig. 4 Molecular structures of 4a (top) and 5a (bottom) with thermal displacement parameters drawn at 50% probability. Hydrogen atoms as well as a toluene molecule in the asymmetric unit of 5a·toluene are omitted for clarity. Selected bond lengths are listed in Table 1. | ||
| 4a | 4b | 4c | 4d | 5a | 5b | 5c | 5d | 6 | |
|---|---|---|---|---|---|---|---|---|---|
a The bond lengths of the two independent molecules in the asymmetric unit are shown.
b Distance between Fe1 and the centroid of C2–C3–C4–C5 is shown.
c L = C28![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O for 4a–d; L = NCMe for 5a–d and 6.
d Due to different connectivity here, C–N and C–Ci are meant as follows: C2–N1 = C3–N1; C3–N2 = C4–N2; C4–Ci = C2–Ci. O for 4a–d; L = NCMe for 5a–d and 6.
d Due to different connectivity here, C–N and C–Ci are meant as follows: C2–N1 = C3–N1; C3–N2 = C4–N2; C4–Ci = C2–Ci.
|
|||||||||
| Fe–CPDb | 1.7760(9) | 1.7807(9) | 1.7783(4) | 1.8228(10)/1.7789(11) | 1.7526(7) | 1.7692(13) | 1.7812(3) | 1.7653(14) | 1.7706(5) |
| Fe–Lc | 1.805(2) | 1.8094(13) | 1.7998(7) | 1.803(2)/1.803(2) | 1.9543(13) | 1.951(3) | 1.9458(3) | 1.951(2) | 1.9752(10) |
| Fe–C29O | 1.7994(19) | 1.7975(18) | 1.8036(6) | 1.795(2)/1.798(3) | 1.7843(10) | 1.774(3) | 1.784(2) | 1.781(4) | 1.7860(11) |
| Fe–C30O | 1.7962(16) | 1.8025(18) | 1.7930(7) | 1.8038(18)/1.8049(17) | 1.7826(17) | 1.785(3) | 1.783(2) | 1.795(3) | 1.7737(10) |
| Fe–C2 | 2.2210(15) | 2.2185(17) | 2.2072(6) | 2.225(2)/2.220(2) | 2.1994(14) | 2.202(3) | 2.238(2) | 2.200(2) | 2.1244(9) |
| Fe–C3 | 2.1842(14) | 2.1891(16) | 2.1874(5) | 2.204(2)/2.205(2) | 2.1265(14) | 2.150(3) | 2.1678(18) | 2.166(2) | 2.121(1) |
| Fe–C4 | 2.0707(16) | 2.0660(14) | 2.0634(6) | 2.0503(19)/2.059(2) | 2.0557(14) | 2.046(3) | 2.0605(18) | 2.028(3) | 2.1700(9) |
| Fe–C5 | 2.0724(16) | 2.0986(16) | 2.1112(6) | 2.0846(17)/2.0819(17) | 2.090(1) | 2.134(3) | 2.098(2) | 2.118(4) | 2.1180(9) |
| C1–C2 | 1.505(2) | 1.495(2) | 1.5042(5) | 1.503(2)/1.502(2) | 1.4955(13) | 1.487(5) | 1.485(3) | 1.488(5) | 1.4751(12) |
| C2–C3 | 1.451(3) | 1.4494(17) | 1.4520(7) | 1.452(3)/1.453(3) | 1.4469(16) | 1.441(4) | 1.450(2) | 1.442(4) | 1.4428(2) |
| C3–C4 | 1.4426(19) | 1.445(2) | 1.4478(7) | 1.445(2)/1.449(3) | 1.4407(16) | 1.445(3) | 1.443(3) | 1.446(4) | 1.4431(12) |
| C4–C5 | 1.450(3) | 1.449(2) | 1.4573(5) | 1.452(3)/1.453(3) | 1.4465(15) | 1.453(5) | 1.446(3) | 1.449(4) | 1.4418(12) |
| C5–C1 | 1.469(3) | 1.4719(18) | 1.4736(8) | 1.478(3)/1.477(3) | 1.4688(16) | 1.473(4) | 1.462(3) | 1.467(4) | 1.4775(12) |
| C1–O1 | 1.227(3) | 1.2375(17) | 1.2316(6) | 1.229(3)/1.235(3) | 1.2382(13) | 1.240(4) | 1.240(2) | 1.238(4) | 1.2373(11) |
| C2–N1 | 1.385(2) | 1.3900(17) | 1.3892(8) | 1.389(2)/1.392(3) | 1.3956(14) | 1.398(3) | 1.385(3) | 1.397(4) | 1.3850(11) |
| C3–N2 | 1.386(2) | 1.386(2) | 1.3852(5) | 1.385(2)/1.380(2) | 1.4010(14) | 1.395(4) | 1.387(2) | 1.385(4) | 1.3727(12) |
| C4–Ci | 1.488(3) | 1.4878(16) | 1.4951(7) | 1.487(3)/1.490(3) | 1.4879(16) | 1.495(4) | 1.495(3) | 1.493(4) | 1.4738(12) |
|
1.486(2) | 1.484(2) | 1.4888(6) | 1.479(2)/1.477(3) | 1.4780(15) | 1.482(4) | 1.494(3) | 1.480(5) | 1.4861(12) |
For the application of iron CPD complexes in homogeneous catalysis, a coordinatively unsaturated species must be provided by dissociation of one carbonyl ligand. For this purpose, in situ activation by treatment with trimethylamine-N-oxide (Me3NO) is widely used in the literature, but according to our studies on tetraamino-cyclopentadienone iron complexes, the preparation of the corresponding dicarbonyl-acetonitrile complexes is a more favourable method to obtain sufficiently active precatalysts.41 The tricarbonyl complexes 4 were therefore treated with Me3NO in a toluene/acetonitrile mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at ambient temperature to effect the oxidation of a carbonyl ligand to carbon dioxide and its substitution by the acetonitrile ligand (Scheme 2). The complexes 5 could be isolated as analytically pure orange solids in moderate yields (50–65%) after recrystallization from toluene/n-hexane mixtures (5a–5c) or acetonitrile (5d). NMR spectroscopic characterisation in CD2Cl2 revealed characteristic additional signals for the coordinated acetonitrile ligands at ca. 2.2 ppm (CH3) in the 1H NMR spectra and at ca. 127 ppm (CN) and 4.8 ppm (CH3) in the 13C{1H} NMR spectra, in good agreement with the chemical shifts reported for the corresponding tetraamino-CPD complexes IV.41 In contrast to symmetric complexes of the latter type, complexes 5 are asymmetric, resulting in two distinct signals for the two remaining diastereotopic carbonyl ligands, found at 216.0/214.1 (5a), 216.1/214.3 (5b), 217.4/214.1 (5c), and 215.2/213.4 ppm (5d), respectively. Again, the 1H and 13C{1H} NMR spectra of the xylyl-substituted CPD complex 5c show four sharp signals for the o-CH3 and m-CH3 groups of the two different xylyl substituents, indicating rapid interconversion between the four possible atropisomers at room temperature on the NMR time scale. The IR spectra of complexes 5 exhibit two equally strong CO absorption bands with averaged CO stretching frequencies (
1) at ambient temperature to effect the oxidation of a carbonyl ligand to carbon dioxide and its substitution by the acetonitrile ligand (Scheme 2). The complexes 5 could be isolated as analytically pure orange solids in moderate yields (50–65%) after recrystallization from toluene/n-hexane mixtures (5a–5c) or acetonitrile (5d). NMR spectroscopic characterisation in CD2Cl2 revealed characteristic additional signals for the coordinated acetonitrile ligands at ca. 2.2 ppm (CH3) in the 1H NMR spectra and at ca. 127 ppm (CN) and 4.8 ppm (CH3) in the 13C{1H} NMR spectra, in good agreement with the chemical shifts reported for the corresponding tetraamino-CPD complexes IV.41 In contrast to symmetric complexes of the latter type, complexes 5 are asymmetric, resulting in two distinct signals for the two remaining diastereotopic carbonyl ligands, found at 216.0/214.1 (5a), 216.1/214.3 (5b), 217.4/214.1 (5c), and 215.2/213.4 ppm (5d), respectively. Again, the 1H and 13C{1H} NMR spectra of the xylyl-substituted CPD complex 5c show four sharp signals for the o-CH3 and m-CH3 groups of the two different xylyl substituents, indicating rapid interconversion between the four possible atropisomers at room temperature on the NMR time scale. The IR spectra of complexes 5 exhibit two equally strong CO absorption bands with averaged CO stretching frequencies (![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) av) of 1954 (5a), 1952 (5b), 1949 (5c), and 1959 cm−1 (5d). These values are slightly higher than those determined for the tetraamino congeners IV (
av) of 1954 (5a), 1952 (5b), 1949 (5c), and 1959 cm−1 (5d). These values are slightly higher than those determined for the tetraamino congeners IV (![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) av ≈ 1944 cm−1),41 which is consistent with reduced metal-carbonyl π-backbonding in the presence of diamino- rather than tetraamino-CPD ligands.
av ≈ 1944 cm−1),41 which is consistent with reduced metal-carbonyl π-backbonding in the presence of diamino- rather than tetraamino-CPD ligands.
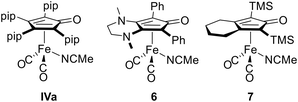
For comparison, the diamino-CPD iron dicarbonyl acetonitrile complex [(II)Fe(CO)2(NCMe)] (6, Fig. 5, vide infra) was synthesised from the corresponding tricarbonyl complex [(II)Fe(CO)3], as only the latter has previously been used as a precatalyst by in situ activation with Me3NO.25,43,506 was isolated as an orange solid in 75% yield following the same procedure described for complexes 5. Here, the 1H and 13C{1H} NMR signals of the acetonitrile ligand are found at 2.14 ppm (CH3) and at 125.8 ppm/4.6 ppm (CN, CH3). The IR spectrum of 6 allows to establish an averaged CO stretching frequency of ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) av(CO) = 1940 cm−1, indicating a stronger π-electron releasing ability of the bicyclic 3,4-diamino-CPD ligand II compared to the 2,3-diamino-CPD ligands 3 and consequently an enhanced metal-carbonyl π-backbonding interaction.
av(CO) = 1940 cm−1, indicating a stronger π-electron releasing ability of the bicyclic 3,4-diamino-CPD ligand II compared to the 2,3-diamino-CPD ligands 3 and consequently an enhanced metal-carbonyl π-backbonding interaction.
The molecular structures of all five acetonitrile complexes were determined by X-ray diffraction analysis of suitable single crystals isolated from toluene/n-hexane solutions (5a·toluene, 5c·0.7 toluene·0.3 hexane), from an n-hexane solution (5b·0.5 hexane) or from an acetonitrile solution (5d), each at −40 °C. The molecular structure of 5a is shown in Fig. 4, all other structures are presented in the ESI† section (Tables S8–S11). Selected bond lengths are given in Table 1. All complexes exhibit the expected three-legged piano-stool geometry, with an ecliptic orientation of the acetonitrile ligand towards the CPD carbonyl group, as found in all other previously structurally characterised complexes of the type [(CPD)Fe(CO)2(NCMe)],64,65 including the tetraamino-CPD congeners IV.41 As observed for 4a–4d (vide supra, Table 1), the η4-coordinated CPD ligands in 5a–5d again have slightly longer Fe–Cpip (2.20–2.24 Å) than Fe–Caryl (2.05–2.13 Å) bond lengths. Complex 6, on the other hand, shows a more symmetrical binding with Fe–C bond lengths ranging from 2.1180(9) to 2.1700(9) Å. The Fe–N bond lengths in 5a–5d are ca. 1.95 Å, while a slightly longer bond length of 1.9752(10) Å is found for 6.
Catalytic reductive amination
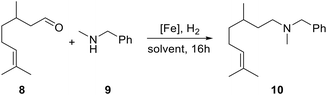
Transition metal catalysed reductive amination with dihydrogen is an industrially important reaction to produce alkyl amines from readily available aldehydes or ketones. The reaction generally proceeds by condensation of the carbonyl compound with ammonia or primary and secondary amines, followed by hydrogenation of the intermediate imine or enamine derivatives.66 Homogeneous iron catalysts have also become increasingly important in promoting such reductive amination reactions,67,68 including CPD-iron tricarbonyl complexes.1–3 In particular, Renaud and coworkers have shown that the latter serve as efficient precatalysts for the model reaction between rac-citronellal (8) and N-methylbenzylamine (9) under dihydrogen pressure (5 bar) in ethanol,69 with enhanced catalytic activity observed for diamino-CPD complexes such as [(II)Fe(CO)3] compared to non-nitrogen substituted species.25,43,50 For comparison with the established systems, we have studied the same reaction using our new precatalysts 5 together with the tetraamino-CPD congener IVa and the Renaud- and Knölker-type acetonitrile complexes 6 and 7 (Fig. 5).Varying the reaction conditions showed that complex 5a is able to promote the reaction at elevated temperature (85 °C) under 5 bar dihydrogen pressure to achieve full conversion to amine 10 at 1 mol% catalyst loading (Table 2, entries 1–4). Further reduction of the catalyst loading to 0.5 mol% resulted in a slight decrease in conversion to 88% (entry 5). Attempts to perform the reaction at atmospheric dihydrogen pressure or in toluene at ambient temperature resulted only in the formation of the intermediate enamine 9, with no further conversion to the desired amine 10 (entries 6 and 7). The former observation, however, rules out the ethanol solvent as a possible transfer hydrogenation reagent to any significant extent. The diamino-CPD derivatives 5b, 5c and 5d were also successfully applied and performed just as well as 5a (entries 4 and 8–10). In contrast, the tetraamino-CPD complex IVa gave full conversion at a lower temperature (45 °C), but no reactivity was observed at ambient temperature (entries 11 and 12). This performance is similar to that of precatalyst 6, which promoted the reaction efficiently at 85 °C and 45 °C with catalyst loadings of 1 and 2.5 mol%, respectively (entries 13 and 14). The latter reactivity agrees with the results reported by Renaud for the corresponding tricarbonyl complex, albeit activated in situ with trimethylamine-N-oxide.43 Finally, the non-nitrogen substituted Knölker-type complex 7 proved to be less reactive even at elevated temperature (entry 15). It can therefore be concluded that amino substituents on the CPD ring are clearly favourable for catalytic activity, with both tetraamino and 3,4-diamino substitution leading to improved catalytic activity compared to 2,3-diamino substitution.
| Entry | [Fe] | Mol% | H2 | Solvent | Temp. | Conv.a |
|---|---|---|---|---|---|---|
| Reaction conditions: 0.5 mmol citronellal, 0.6 mmol N-methylbenzylamine, 1 mL solvent, 16 h.a Conversion determined by GC/MS, isolated yield in brackets. | ||||||
| 1 | 5a | 2 | 5 bar | EtOH | rt | 1% |
| 2 | 5a | 2 | 5 bar | EtOH | 45 °C | 2% |
| 3 | 5a | 2 | 5 bar | EtOH | 85 °C | >99% |
| 4 | 5a | 1 | 5 bar | EtOH | 85 °C | >99% (89%) |
| 5 | 5a | 0.5 | 5 bar | EtOH | 85 °C | 88% |
| 6 | 5a | 1 | 1 atm | EtOH | 85 °C | 0% |
| 7 | 5a | 2 | 5 bar | Toluene | rt | 0% |
| 8 | 5b | 2 | 5 bar | EtOH | 85 °C | >99% |
| 9 | 5c | 1 | 5 bar | EtOH | 85 °C | >99% |
| 10 | 5d | 1 | 5 bar | EtOH | 85 °C | >99% |
| 11 | IVa | 2 | 5 bar | EtOH | 45 °C | >99% (93%) |
| 12 | IVa | 2 | 5 bar | EtOH | rt | 0% |
| 13 | 6 | 1 | 5 bar | EtOH | 85 °C | >99% |
| 14 | 6 | 2.5 | 5 bar | EtOH | 45 °C | >99% |
| 15 | 7 | 2 | 5 bar | EtOH | 85 °C | 92% |
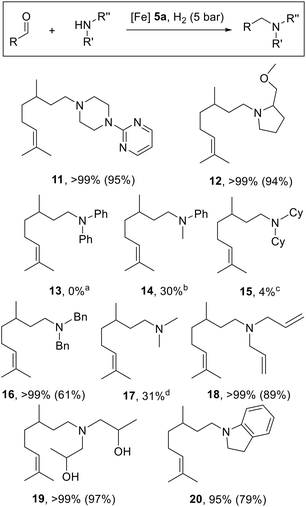
To expand the substrate range for the new catalysts, various analogous reductive amination reactions were carried out using different secondary amines, 5 bar dihydrogen pressure, 2 mol% 5a and 85 °C in EtOH for 16 h (Scheme 3). Fortunately, the piperazyl pyrimidine derivative 11 was obtained in a high isolated yield of 95%. An equally excellent yield was achieved using (S)-prolinol methyl ether and 12 was isolated in 94% yield, but complete racemisation was observed due to the formation of an intermediate enamine containing the asymmetrically substituted carbon atom. Accordingly, the NMR signals for the two diastereomers are found (ESI†). In contrast, diphenylamine showed no conversion to the intermediate enamine, but a complete hydrogenation of citronellal to citronellol, tentatively attributed to the steric demand of the amine and the resulting slow enamine formation. When N-methylaniline was used instead, 30% conversion to the desired amine 14 was observed, but 70% citronellol was still obtained. The same problem was encountered with dicyclohexylamine targeting amine 15, whereas dibenzylamine was suitable to produce amine 16 with complete conversion. Using an ethanolic dimethylamine solution, 31% of the desired amine 17 was obtained, which could potentially be improved by increasing the excess of the gaseous amine. Of note, amine 18 was successfully synthesised with full chemoselectivity and no reduction of the allyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in the diallylamino group was observed. The presence of additional hydroxy groups using diisopropanolamine did not appear to be a problem either and amine 19 could be obtained in high yield (97%). Finally, the reductive amination of citronellal with indoline was tested, with the expectation that an aromatic indole derivative might be formed as an intermediate. Fortunately, the desired amine 20 was obtained in 79% yield and according to the GC/MS analysis the aromatic enamine was present in only 4%.
C double bonds in the diallylamino group was observed. The presence of additional hydroxy groups using diisopropanolamine did not appear to be a problem either and amine 19 could be obtained in high yield (97%). Finally, the reductive amination of citronellal with indoline was tested, with the expectation that an aromatic indole derivative might be formed as an intermediate. Fortunately, the desired amine 20 was obtained in 79% yield and according to the GC/MS analysis the aromatic enamine was present in only 4%.
Reductive amination is one of the most important classes of reactions in the production of substances in demand for pharmaceutical and medicinal applications.70 At the same time, sustainable strategies that replace precious metals, harsh reaction conditions and environmentally unfriendly solvents are becoming increasingly important.71 Furthermore, the search for chiral catalysts that allow asymmetric transformations to yield enantiomerically pure substances has become an increasingly demanded field in order to reduce waste in terms of stereoisomeric by-products and also the need for enantioselective separation.72 With the asymmetric CPD iron hydrogenation catalysts 5 in hand, the opportunity to contribute to this important field seems possible after the separation of the racemic mixtures obtained in the reaction of the CPDs 3 with Fe2(CO)9. As a first attempt to assess the general suitability of the planar chiral complexes 5 for the preparation of a chiral drug, we chose sertraline, the (S,S)-enantiomer of 22 (Scheme 4), which is one of the most demanded drugs for the treatment of mood and anxiety disorders.73 The key transformation is the reductive amination of the racemic tetralone derivative 21 with H2 over Pd/C, patented by Pfizer Inc.,74,75 which is used industrially. The desired enantiomer is then separated by crystallisation with (R)-mandelic acid.73,76 In order to apply our new iron catalysts to this transformation, we reacted the racemic tetralone derivative 21 with methylamine over molecular sieves in ethanol for 48 h and observed a complete conversion to the corresponding imine. Subsequently, reduction under 5 bar H2 in ethanol for 16 h led to a complete hydrogenation to the corresponding amine 22 with a diastereomeric ratio of 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 (cis
37 (cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans), which is comparable to those achieved in the industrial process (70
trans), which is comparable to those achieved in the industrial process (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30).74 Thus, this application demonstrates a promising and potentially green alternative by replacing the solvent tetrahydrofuran with ethanol and a palladium catalyst with an iron catalyst. The potential for asymmetric hydrogenation using enantiomerically pure derivatives of our catalyst after chiral resolution is currently being investigated.
30).74 Thus, this application demonstrates a promising and potentially green alternative by replacing the solvent tetrahydrofuran with ethanol and a palladium catalyst with an iron catalyst. The potential for asymmetric hydrogenation using enantiomerically pure derivatives of our catalyst after chiral resolution is currently being investigated.
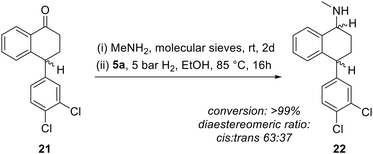 | ||
| Scheme 4 Reductive amination to afford sertraline; reaction conditions: 6 eq. MeNH2, 1 g mmol−1 molecular sieves (3–5 Å), 2 mol% 5a. | ||
Conclusion
We have shown that 2,3-diamino-4,5-diarylcyclopentadienones (3) can be readily prepared by an uncatalysed (3 + 2)-cycloaddition reaction between diarylcyclopropenones and diaminoacetylenes, i.e. dipiperidinoacetylene (1). Their reaction with Fe2(CO)6 provided the corresponding cyclopentadienone (CPD) iron tricarbonyl complexes 4. Carbonyl substitution in acetonitrile solution in the presence of trimethylamine-N-oxide afforded the acetonitrile complexes 5, which were successfully employed as catalysts for the reductive amination of citronellal with secondary amines under dihydrogen pressure (5 bar). Similar to previous studies, the reaction proceeds with the formation of intermediate enamines, followed by chemoselective hydrogenation, leaving unpolarised carbon–carbon double bonds as well as hydroxy and ether groups unaffected. The successful synthesis of a diastereomeric mixture of the antidepressant sertraline through reductive amination of a tetralone precursor reveals the principal suitability of the novel CPD complexes for use in stereoselective catalysis if methods for the resolution of the planar chiral CPD complexes can be established. Investigations in this direction are currently underway in our laboratories.Conflicts of interest
There are no conflicts to declare.Acknowledgements
LK would like to thank Ekaterina Bergmann, née Korotenko, and Philipp Meinhold for preparative assistance and Alexandra Dierks (Prof. Dr. Thomas Lindel) for providing amines.References
- A. Quintard and J. Rodriguez, Iron cyclopentadienone complexes: discovery, properties, and catalytic reactivity, Angew. Chem., Int. Ed., 2014, 53, 4044–4055 CrossRef CAS PubMed.
- L. Pignataro and C. Gennari, Recent Catalytic Applications of (Cyclopentadienone)iron Complexes, Eur. J. Org. Chem., 2020, 2020, 3192–3205 CrossRef CAS.
- M. Akter and P. Anbarasan, (Cyclopentadienone)iron Complexes: Synthesis, Mechanism and Applications in Organic Synthesis, Chem. – Asian J., 2021, 16, 1703–1724 CrossRef CAS PubMed.
- W. Reppe and H. Vetter, Carbonylierung VI. Synthesen mit Metallcarbonylwasserstoffen, Justus Liebigs Ann. Chem., 1953, 582, 133–161 CrossRef CAS.
- V. R. Stimson, E. R. H. Jones, P. C. Wailes, M. C. Whiting, J. E. Baines, C. Eaborn, A. Mackie, A. L. Misra, H. Suschitzky, W. Bradley and H. E. Nursten, Tetracarbonyliron Bisphenyhcet ylide, J. Chem. Soc., 1955, 4020 RSC.
- E. Weiss and W. Hübel, Organometallic complexes—V(1) π-komplexe aus cyclischen dienverbindungen und metallcarbonylen, J. Inorg. Nucl. Chem., 1959, 11, 42–55 CrossRef CAS.
- W. Hübel, E. H. Braye, A. Clauss, E. Weiss, U. Krüerke, D. A. Brown, G. S. D. King and C. Hoogzand, Organometallic complexes—I the reaction of metal carbonyls with acetylenic compounds, J. Inorg. Nucl. Chem., 1959, 9, 204–210 CrossRef.
- K. Hoffmann and E. Weiss, Kristall- und Molekülstruktur von Tricarbonyl(η4-cyclopentadienon)eisen, J. Organomet. Chem., 1977, 128, 237–246 CrossRef CAS.
- H.-J. Knölker, J. Heber and C. H. Mahler, Transition Metal-Diene Complexes in Organic Synthesis, Part 14. 1 Regioselective Iron-Mediated [2+2+1] Cycloadditions of Alkynes and Carbon Monoxide: Synthesis of Substituted Cyclopentadienones, Synlett, 1992, 1992, 1002–1004 CrossRef.
- H.-J. Knölker and J. Heber, Transition Metal-Diene Complexes in Organic Synthesis, Part 18. 1 Iron-Mediated [2+2+1] Cycloadditions of Diynes and Carbon Monoxide: Selective Demetalation Reactions, Synlett, 1993, 1993, 924–926 CrossRef.
- A. J. Pearson and A. Perosa, Selectivity in the pentacarbonyliron-Promoted Cyclocarbonylation of Enediynes, Organometallics, 1995, 14, 5178–5183 CrossRef CAS.
- H.-J. Knölker, E. Baum and J. Heber, Transition Metal-Diene Complexes in Organic Synthesis, Part 25.1 Cycloadditions of Annulated 2,5-Bis(trimethylsilyl)cyclopentadienones, Tetrahedron Lett., 1995, 36, 7647–7650 CrossRef.
- C. F. H. Allen and J. A. VanAllan, Dimerization of Cyclopentadienones, J. Am. Chem. Soc., 1950, 72, 5165–5167 CrossRef CAS.
- T. W. Funk, A. R. Mahoney, R. A. Sponenburg, K. P. Zimmerman, D. K. Kim and E. E. Harrison, Synthesis and Catalytic Activity of (3,4-Diphenylcyclopentadienone)Iron Tricarbonyl Compounds in Transfer Hydrogenations and Dehydrogenations, Organometallics, 2018, 37, 1133–1140 CrossRef CAS.
- P. A. Wender, T. J. Paxton and T. J. Williams, Cyclopentadienone synthesis by rhodium(I)-catalyzed 3 + 2 cycloaddition reactions of cyclopropenones and alkynes, J. Am. Chem. Soc., 2006, 128, 14814–14815 CrossRef CAS PubMed.
- H. M. Peng, R. D. Webster and X. Li, Quinoline-Tethered N-Heterocyclic Carbene Complexes of Rhodium and Iridium: Synthesis, Catalysis, and Electrochemical Properties, Organometallics, 2008, 27, 4484–4493 CrossRef CAS.
- M. K. Thorson, K. L. Klinkel, J. Wang and T. J. Williams, Mechanism of Hydride Abstraction by Cyclopentadienone-Ligated Carbonylmetal Complexes (M = Ru, Fe), Eur. J. Inorg. Chem., 2009, 2009, 295–302 CrossRef.
- B. Rao, H. Tang, X. Zeng, L. L. Liu, M. Melaimi and G. Bertrand, Cyclic (Amino)(aryl)carbenes (CAArCs) as Strong σ-Donating and π-Accepting Ligands for Transition Metals, Angew. Chem., Int. Ed., 2015, 54, 14915–14919 CrossRef CAS PubMed.
- T. Kondo, R. Taniguchi and Y. Kimura, Ruthenium- and Rhodium-Catalyzed Ring-Opening Coupling Reactions of Cyclopropenones with Alkenes or Alkynes, Synlett, 2018, 29, 717–722 CrossRef CAS.
- A. Gutiérrez-Blanco, E. Peris and M. Poyatos, Pyrene-Connected Tetraimidazolylidene Complexes of Iridium and Rhodium. Structural Features and Catalytic Applications, Organometallics, 2018, 37, 4070–4076 CrossRef.
- M. Franck-Neumann, Reaction d'une ynamine avec la diphenylcyclopropenone, Tetrahedron Lett., 1966, 7, 341–345 CrossRef.
- T. Eicher and M. Urban, Zur Reaktion von Diarylcyclopropenonen mit Inaminen, Chem. Ber., 1980, 113, 408–423 CrossRef CAS.
- E. Haak, Ruthenium Complexes of Electronically Coupled Cyclopentadienone Ligands – Catalysts for Transformations of Propargyl Alcohols, Eur. J. Org. Chem., 2007, 2007, 2815–2824 CrossRef.
- Y. Blum and Y. Shvo, Catalytically Reactive Ruthenium Intermediates in the Homogeneous Oxidation of Alcohols to Esters, Isr. J. Chem., 1984, 24, 144–148 CrossRef CAS.
- T.-T. Thai, D. S. Mérel, A. Poater, S. Gaillard and J.-L. Renaud, Highly active phosphine-free bifunctional iron complex for hydrogenation of bicarbonate and reductive amination, Chem. – Eur. J., 2015, 21, 7066–7070 CrossRef CAS PubMed.
- Y. Blum, D. Czarkie, Y. Rahamim and Y. Shvo, (Cyclopentadienone)ruthenium carbonyl complexes - a new class of homogeneous hydrogenation catalysts, Organometallics, 1985, 4, 1459–1461 CrossRef CAS.
- Y. Shvo, D. Czarkie, Y. Rahamim and D. F. Chodosh, A new group of ruthenium complexes: structure and catalysis, J. Am. Chem. Soc., 1986, 108, 7400–7402 CrossRef CAS.
- H.-J. Knölker, E. Baum, H. Goesmann and R. Klauss, Demetalation of Tricarbonyl(cyclopentadienone)iron Complexes Initiated by a Ligand Exchange Reaction with NaOH—X-Ray Analysis of a Complex with Nearly Square-Planar Coordinated Sodium, Angew. Chem., Int. Ed., 1999, 38, 2064–2066 CrossRef.
- C. P. Casey and H. Guan, An efficient and chemoselective iron catalyst for the hydrogenation of ketones, J. Am. Chem. Soc., 2007, 129, 5816–5817 CrossRef CAS PubMed.
- C. P. Casey and H. Guan, Cyclopentadienone iron alcohol complexes: synthesis, reactivity, and implications for the mechanism of iron-catalyzed hydrogenation of aldehydes, J. Am. Chem. Soc., 2009, 131, 2499–2507 CrossRef CAS PubMed.
- S. A. Moyer and T. W. Funk, Air-stable iron catalyst for the Oppenauer-type oxidation of alcohols, Tetrahedron Lett., 2010, 51, 5430–5433 CrossRef CAS.
- T. C. Johnson, G. J. Clarkson and M. Wills, (Cyclopentadienone)iron Shvo Complexes: Synthesis and Applications to Hydrogen Transfer Reactions, Organometallics, 2011, 30, 1859–1868 CrossRef CAS.
- A. Berkessel, S. Reichau, A. von der Höh, N. Leconte and J.-M. Neudörfl, Light-Induced Enantioselective Hydrogenation Using Chiral Derivatives of Casey's Iron–Cyclopentadienone Catalyst, Organometallics, 2011, 30, 3880–3887 CrossRef CAS.
- T. N. Plank, J. L. Drake, D. K. Kim and T. W. Funk, Air-Stable, Nitrile-Ligated (Cyclopentadienone)iron Dicarbonyl Compounds as Transfer Reduction and Oxidation Catalysts, Adv. Synth. Catal., 2012, 354, 597–601 CrossRef CAS.
- L. Hackl, L. Körner and M. Tamm, Diaminoacetylenes: Synthesis and Coordination Chemistry, Chem. Lett., 2021, 50, 1428–1440 CrossRef CAS.
- A. R. Petrov, C. G. Daniliuc, P. G. Jones and M. Tamm, A novel synthetic approach to diaminoacetylenes: structural characterization and reactivity of aromatic and aliphatic ynediamines, Chem. – Eur. J., 2010, 16, 11804–11808 CrossRef CAS PubMed.
- L. Hackl, A. R. Petrov, T. Bannenberg, M. Freytag, P. G. Jones and M. Tamm, Dimerisation of Dipiperidinoacetylene: Convenient Access to Tetraamino-1,3-Cyclobutadiene and Tetraamino-1,2-Cyclobutadiene Metal Complexes, Chem. – Eur. J., 2019, 16148–16155 CrossRef CAS PubMed.
- L. Körner, L. P. Ho, R. Puchta, A. Stanger and M. Tamm, Dimorpholinoacetylene and Its Use for the Synthesis of Tetraaminocyclobutadiene Species, Chem. – Eur. J., 2022, e202202737 CrossRef PubMed.
- L. Körner, L. Yang, D. Bockfeld and M. Tamm, 1,1-Enediamines as highly polarized olefin ligands, J. Organomet. Chem., 2023, 984, 122572 CrossRef.
- L. Hackl, L. Körner, L. P. Ho, T. Bannenberg and M. Tamm, Iridium(I) 1,3,4,4-Tetraamino-1,2-cyclobutadiene Complexes, Eur. J. Inorg. Chem., 2023, e202300680 Search PubMed.
- L. Hackl, L. P. Ho, D. Bockhardt, T. Bannenberg and M. Tamm, Tetraaminocyclopentadienone Iron Complexes as Hydrogenation Catalysts, Organometallics, 2022, 41, 836–851 CrossRef CAS.
- A. C. Filippou and T. Rosenauer, A Reaction Pathway of [Fe(CO)5] with Alkynes via Ferrabicyclobutenones, Angew. Chem., Int. Ed., 2002, 41, 2393–2396 CrossRef CAS PubMed.
- A. Lator, Q. G. Gaillard, D. S. Mérel, J.-F. Lohier, S. Gaillard, A. Poater and J.-L. Renaud, Room-Temperature Chemoselective Reductive Alkylation of Amines Catalyzed by a Well-Defined Iron(II) Complex Using Hydrogen, J. Org. Chem., 2019, 84, 6813–6829 CrossRef CAS PubMed.
- L. Bettoni, C. Seck, M. D. Mbaye, S. Gaillard and J.-L. Renaud, Iron-Catalyzed Tandem Three-Component Alkylation: Access to α-Methylated Substituted Ketones, Org. Lett., 2019, 21, 3057–3061 CrossRef CAS PubMed.
- L. Bettoni, S. Gaillard and J.-L. Renaud, Iron-Catalyzed β-Alkylation of Alcohols, Org. Lett., 2019, 21, 8404–8408 CrossRef CAS PubMed.
- L. Bettoni, S. Gaillard and J.-L. Renaud, A phosphine-free iron complex-catalyzed synthesis of cycloalkanes via the borrowing hydrogen strategy, Chem. Commun., 2020, 56, 12909–12912 RSC.
- S. Coufourier, D. Ndiaye, Q. G. Gaillard, L. Bettoni, N. Joly, M. D. Mbaye, A. Poater, S. Gaillard and J.-L. Renaud, Iron-catalyzed chemoselective hydride transfer reactions, Tetrahedron, 2021, 90, 132187 CrossRef CAS.
- M. Gimferrer, N. Joly, S. Escayola, E. Viñas, S. Gaillard, M. Solà, J.-L. Renaud, P. Salvador and A. Poater, Knölker Iron Catalysts for Hydrogenation Revisited: A Nonspectator Solvent and Fine-Tuning, Organometallics, 2022, 41, 1204–1215 CrossRef CAS.
- M.-S. Abdallah, N. Joly, S. Gaillard, A. Poater and J.-L. Renaud, Blue-Light-Induced Iron-Catalyzed α-Alkylation of Ketones, Org. Lett., 2022, 24, 5584–5589 CrossRef CAS PubMed.
- N. Joly, M. Gimferrer, S. Escayola, M. Cendra, S. Coufourier, J.-F. Lohier, Q. G. Gaillard, S. Gaillard, M. Solà, J.-L. Renaud and A. Poater, Enhancement of Knölker Iron Catalysts for Imine Hydrogenation by Predictive Catalysis: From Calculations to Selective Experiments, Organometallics, 2023, 42, 1784–1792 CrossRef CAS.
- U. Piarulli, S. V. Fachini and L. Pignataro, Enantioselective Reductions Promoted by (Cyclopentadienone)iron Complexes, Chimia, 2017, 71, 580–585 CrossRef CAS PubMed.
- P. Gajewski, M. Renom-Carrasco, S. V. Facchini, L. Pignataro, L. Lefort, J. G. de Vries, R. Ferraccioli, U. Piarulli and C. Gennari, Synthesis of (R)-BINOL-Derived (Cyclopentadienone)iron Complexes and Their Application in the Catalytic Asymmetric Hydrogenation of Ketones, Eur. J. Org. Chem., 2015, 2015, 5526–5536 CrossRef CAS.
- D. S. Mérel, S. Gaillard, T. R. Ward and J.-L. Renaud, Achiral Cyclopentadienone Iron Tricarbonyl Complexes Embedded in Streptavidin: An Access to Artificial Iron Hydrogenases and Application in Asymmetric Hydrogenation, Catal. Lett., 2016, 146, 564–569 CrossRef.
- D. Brenna, S. Rossi, F. Cozzi and M. Benaglia, Iron catalyzed diastereoselective hydrogenation of chiral imines, Org. Biomol. Chem., 2017, 15, 5685–5688 RSC.
- A. Del Grosso, A. E. Chamberlain, G. J. Clarkson and M. Wills, Synthesis and applications to catalysis of novel cyclopentadienone iron tricarbonyl complexes, Dalton Trans., 2018, 47, 1451–1470 RSC.
- C. A. M. R. van Slagmaat, K. C. Chou, L. Morick, D. Hadavi, B. Blom and S. M. A. de Wildeman, Synthesis and Catalytic Application of Knölker-Type Iron Complexes with a Novel Asymmetric Cyclopentadienone Ligand Design, Catalysts, 2019, 9, 790 CrossRef CAS.
- X. Bai, M. Cettolin, G. Mazzoccanti, M. Pierini, U. Piarulli, V. Colombo, A. Dal Corso, L. Pignataro and C. Gennari, Chiral (cyclopentadienone)iron complexes with a stereogenic plane as pre-catalysts for the asymmetric hydrogenation of polar double bonds, Tetrahedron, 2019, 75, 1415–1424 CrossRef CAS.
- R. Hodgkinson, A. Del Grosso, G. Clarkson and M. Wills, Iron cyclopentadienone complexes derived from C2-symmetric bis-propargylic alcohols; preparation and applications to catalysis, Dalton Trans., 2016, 45, 3992–4005 RSC.
- G. M. Fusi, S. Gazzola and U. Piarulli, Chiral Iron Complexes in Asymmetric Organic Transformations, Adv. Synth. Catal., 2022, 364, 696–714 CrossRef CAS.
- S. Zhou, S. Fleischer, K. Junge and M. Beller, Cooperative transition-metal and chiral Brønsted acid catalysis: enantioselective hydrogenation of imines to form amines, Angew. Chem., Int. Ed., 2011, 50, 5120–5124 CrossRef CAS PubMed.
- S. Fleischer, S. Zhou, S. Werkmeister, K. Junge and M. Beller, Cooperative iron-Brønsted acid catalysis: enantioselective hydrogenation of quinoxalines and 2H-1,4-benzoxazines, Chem. – Eur. J., 2013, 19, 4997–5003 CrossRef CAS PubMed.
- S. Zhou, S. Fleischer, H. Jiao, K. Junge and M. Beller, Cooperative Catalysis with Iron and a Chiral Brønsted Acid for Asymmetric Reductive Amination of Ketones, Adv. Synth. Catal., 2014, 356, 3451–3455 CrossRef CAS.
- K. H. Hopmann, Iron/Brønsted Acid Catalyzed Asymmetric Hydrogenation: Mechanism and Selectivity-Determining Interactions, Chem. – Eur. J., 2015, 21, 10020–10030 CrossRef CAS PubMed.
- M. Kamitani, Y. Nishiguchi, R. Tada, M. Itazaki and H. Nakazawa, Synthesis of Fe–H/Si–H and Fe–H/Ge–H Bifunctional Complexes and Their Catalytic Hydrogenation Reactions toward Nonpolar Unsaturated Organic Molecules, Organometallics, 2014, 33, 1532–1535 CrossRef CAS.
- A. Bütikofer and P. Chen, Cyclopentadienone Iron Complex-Catalyzed Hydrogenation of Ketones: An Operando Spectrometric Study Using Pressurized Sample Infusion-Electrospray Ionization-Mass Spectrometry, Organometallics, 2022, 41, 2349–2364 CrossRef.
- T. Irrgang and R. Kempe, Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen, Chem. Rev., 2020, 120, 9583–9674 CrossRef CAS PubMed.
- S. Gaillard and J.-L. Renaud, Iron-catalyzed hydrogenation, hydride transfer, and hydrosilylation: an alternative to precious-metal complexes?, ChemSusChem, 2008, 1, 505–509 CrossRef CAS PubMed.
- D. Wei and C. Darcel, Iron Catalysis in Reduction and Hydrometalation Reactions, Chem. Rev., 2019, 119, 2550–2610 CrossRef CAS PubMed.
- S. Moulin, H. Dentel, A. Pagnoux-Ozherelyeva, S. Gaillard, A. Poater, L. Cavallo, J.-F. Lohier and J.-L. Renaud, Bifunctional (cyclopentadienone)iron-tricarbonyl complexes: synthesis, computational studies and application in reductive amination, Chem. – Eur. J., 2013, 19, 17881–17890 CrossRef CAS PubMed.
- O. I. Afanasyev, E. Kuchuk, D. L. Usanov and D. Chusov, Reductive Amination in the Synthesis of Pharmaceuticals, Chem. Rev., 2019, 119, 11857–11911 CrossRef CAS PubMed.
- P. Gupta and A. Mahajan, Green chemistry approaches as sustainable alternatives to conventional strategies in the pharmaceutical industry, RSC Adv., 2015, 5, 26686–26705 RSC.
- N. U. D. Reshi, V. B. Saptal, M. Beller and J. K. Bera, Recent Progress in Transition-Metal-Catalyzed Asymmetric Reductive Amination, ACS Catal., 2021, 11, 13809–13837 CrossRef CAS.
- G. MacQueen, L. Born and M. Steiner, The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders, CNS Drug Rev., 2001, 7, 1–24 CrossRef CAS PubMed.
- W. M. Welch, C. A. Harbert, B. K. Koe and A. R. Kraska, Antidepressant derivatives of cis-4-phenyl-1,2,3,4-tetrahydro-1-naphthalenamine, US4536518, 1985.
- J. C. Spavins, Process for Preparing a Ketimine, US4855500, 1988.
- W. M. Welch, A. R. Kraska, R. Sarges and B. K. Koe, Nontricyclic antidepressant agents derived from cis- and trans-1-amino-4-aryltetralins, J. Med. Chem., 1984, 27, 1508–1515 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2338289–2338299. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cy00372a |
| This journal is © The Royal Society of Chemistry 2024 |