Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The formation, reactivity and transformation pathways of formaldehyde in the methanol-to-hydrocarbon conversion†
Vladimir
Paunović
 *a,
Xiangkun
Wu
b,
Luca
Maggiulli
ab,
Davide
Ferri
*a,
Xiangkun
Wu
b,
Luca
Maggiulli
ab,
Davide
Ferri
 b,
Patrick
Hemberger
b,
Patrick
Hemberger
 b,
Andras
Bodi
b,
Andras
Bodi
 b and
Jeroen A.
van Bokhoven
b and
Jeroen A.
van Bokhoven
 *ab
*ab
aInstitute for Chemical and Bioengineering, ETH Zurich, Vladimir-Prelog-Weg 1, 8093 Zurich, Switzerland. E-mail: vladimir.paunovic@chem.ethz.ch; jeroen.vanbokhoven@chem.ethz.ch
bPaul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen, Switzerland
First published on 1st February 2024
Abstract
Coke-induced catalyst deactivation is one of the major challenges in commercial methanol-to-hydrocarbon (MTH) conversion processes. It is suspected to be critically dependent on formaldehyde-mediated reactions. Using photoelectron photoion coincidence and operando diffuse reflectance UV-vis spectroscopy techniques, we reveal details of formaldehyde formation and reactivity over industrially relevant ZSM-5 catalysts, and its role in the coke formation. We show that in the early stage of the MTH reaction, formaldehyde is mainly obtained via methanol disproportionation, with a moderate apparent activation energy of 70 kJ mol−1. In the fully developed reaction phase, it readily converts alkenes into dienes and aliphatic polyenes, which are then promptly converted to methylbenzenes, naphthalenes, and polycyclic arenes that compose coke. We evidence increased reactivity of formaldehyde and dienes over catalysts exhibiting higher productivity of arenes and high propensity to coking. The results suggest that the consumption of this intermediate is not only dependent on the concentration of Brønsted acid sites, but also on their nature, the presence of Lewis acid sites, and framework defects. A high reactivity of cyclopentadienes towards formaldehyde and detection of fulvenes indicate their involvement as key intermediates in the transformation of alkenes to akylbenzenes. The identification of styrene and indene derivatives suggests a pathway to polycyclic arenes by condensation of formaldehyde with aliphatic substituents on arene rings, followed by cyclization. The results demonstrate the pivotal role of formaldehyde in the MTH reaction.
Introduction
Methanol-to-hydrocarbon (MTH) conversion over molecular sieve catalysts is an important technology for the production of light alkenes, aromatics, and fuels from a range of fossil and renewable alternatives to petroleum.1–3 After fast methanol–dimethyl ether (DME) equilibration and evolution of the first C2+ species, C–C bonds are assembled via methylation and cracking reactions of the alkene- and arene-based chain carriers, which can be interconverted via hydrogen transfer (HT) and cyclization reactions.1–5 This unique array of reactions is referred to as the dual-cycle hydrocarbon pool (DCHP) mechanism. It proceeds via carbenium ion intermediates formed by the interaction of hydrocarbon chain carriers with Brønsted acid sites (BAS) in the confined micropore space.1,3–5 The cracking of alkene chain carriers results in the formation of propene and higher alkenes, whereas arene-mediated reactions produce ethene, propene, and mono- and bicyclic methylated aromatics. However, MTH reaction sequences also yield higher molecular weight by-products, predominantly alkylated polycyclic aromatic hydrocarbons (PAHs).1,6–12 The impaired diffusion and strong adsorption of PAHs on BAS lead to their accumulation in the form of internal (micropore) and external coke deposits, causing MTH catalyst deactivation.7–9,11,12 Catalyst coking is the central challenge in the MTH conversion, because coke limits the catalyst turnover capacity, dissipates a substantial part of up to 8% of the methanol feed, and introduces the need for high-temperature catalyst regeneration that causes permanent structural degradation of the catalysts.6,11,12The formation of coke species is primarily associated with HT reactions, which convert alkenes into conjugated aliphatics, i.e., dienes and polyenes, arenes, and finally PAHs, in order of increasing thermodynamic stability.12,13 Methanol and DME, i.e., surface methoxy-groups, are highly potent hydrogen donors in addition to unsaturated hydrocarbons, such as alkenes. In methanol-induced HT (MIHT), hydride is abstracted by another methoxy or carbenium ion, which is thus converted into methane or C2+ alkanes, respectively.14–20 An important outcome of MIHT is the dehydrogenation of methanol to formaldehyde. Previous reports showed that the yield of formaldehyde decreases with the progress of the MTH reaction over a ZSM-5 catalyst, almost vanishing at high conversions.17 Moreover, a prominent increase in arene formation and catalyst coking propensity was reported in the presence of a formaldehyde co-feed.13,21 The alkene cycle and catalyst stability are enhanced under the conditions that either minimize MIHT,13,21 promote the decomposition of formaldehyde,22 or reduce its reactivity.23,24 It has been proposed that formaldehyde promotes the formation of dienes, polyenes, and aromatics via Prins condensation and/or hydroacylation reaction with alkenes,13,17,21 as well as diphenylmethane formation via condensation with arene chain carriers.25 All these steps are catalyzed by BAS.18 These findings imply that the product distribution and the coking propensity of the MTH catalysts critically depend on their activity for formaldehyde formation and subsequently, formaldehyde-mediated reactions (FMRs). In particular, zeolites exhibiting high acid site density are expected to be more reactive in formaldehyde formation and FMRs than their low-acidity counterparts,13,17 which may explain the higher arene selectivity and decreased stability of the former compared to the latter.26–28 However, the relationships between formaldehyde formation and reactivity, product distribution, and catalyst stability have not been systematically explored so far, and the FMR reaction intermediates remain elusive.
In this study, formaldehyde evolution and reactivity were assessed over several ZSM-5 catalysts with varying acidity and MTH catalytic performance by photoelectron photoion coincidence (PEPICO) spectroscopy, which enables fragmentation-free and isotope- and isomer-specific detection of molecules at high sampling frequency.20,29–34 Formaldehyde co-feeding and isotope labeling PEPICO spectroscopy experiments were complemented with operando diffuse reflectance UV-vis (DRUV-vis) spectroscopic analysis of the zeolite-confined intermediates, and by the assessment of deactivating potential of different species via co-feeding experiments. Thus, we gained a thorough picture of formaldehyde formation and FMRs by identifying the reactive intermediates and uncovering the relationships between the formaldehyde reactivity and catalytic performance. The results verify the prominent role of formaldehyde in the evolution of aromatics and coke-forming reactions.
Results and discussion
Kinetics of formaldehyde formation in the MTH conversion
The kinetics of formaldehyde formation in the MTH process was investigated over Z40 and CaZ40 zeolites by using a PEPICO spectroscopy set-up (Fig. S1†). Z40 is a prototypical MTH catalyst, which is composed of ca. 0.4–1 μm particles and exhibits moderate acidity (Fig. S2, S3 and Table S1†). The incorporation of alkaline-earth counterions such as calcium enables the concentration and strength of BAS to be substantially reduced (Fig. S3, Table S1†), without modifying the particle size and significantly altering the distribution of defects in the material.28,34 In addition, CaZ40 serves as a pertinent model material for analyzing the potential influence of extra-framework calcium cations on the formaldehyde formation in the MTH reaction. This is particularly noteworthy because the incorporation of calcium significantly diminishes the production of arenes,28,34 which may arise from reduced generation of this intermediate compound. Owing to their medium and low BAS densities, respectively, these Z40 and CaZ40 catalysts display reduced methanol conversion activities (Fig. 1a and S4†). This, along with their operation at increased WHSV, permits the study of the MTH reaction in the low conversion regime that limits the consumption of formaldehyde in subsequent reactions with hydrocarbons.17,19 The evolution of formaldehyde and other species in the reactor outlet stream was assessed by PEPICO spectroscopy (Fig. S1†). The formaldehyde ion (m/z 30) was detected at a photon energy of hν = 10.9 eV, slightly above its ionization potential (IP = 10.88 eV, Fig. 1a and S1†).20,35 Its identity was unequivocally confirmed by recording photoionization (PI) and photoion mass-selected threshold photoelectron (ms-TPE) spectra (Fig. S1†). The onset of formaldehyde formation over the Z40 and CaZ40 catalysts was observed at ca. 625 K (Fig. 1a and b). This was paralleled by the appearance of methane (m/z 16, hν = 13.6 eV), which was the only observable hydrocarbon at low temperatures (Fig. 1a). With increasing temperature, the production of formaldehyde and methane increased, and signals associated with ethene (m/z 28), propene (m/z 42), and butene (m/z 56) started to evolve, implying the formation of the HP (Fig. 1a and c). Nonetheless, the carbon-based yields of detected light alkenes (ca. 0.8% in total) were lower than those of methane and formaldehyde (ca. 2.2%, Fig. 1c), indicating that MIHT still prevailed over the reactions of the hydrocarbon pool. Notably, alkanes, such as propane (m/z 44) and butane (m/z 58), methylated benzenes (MBs), such as methylbenzene (1 MB, m/z 92) and dimethylbenzene (2 MB, m/z 106), and dienes, such as butadiene (m/z 54), were not detected under these reaction conditions (Fig. 1a). The lack of alkanes in the outlet reactor feed indicates the absence of MIHT to alkenes and points that methanol disproportionation being the main pathway of the formaldehyde formation.17 This is further corroborated by the equivalent yields of formaldehyde and methane, which is in good agreement with the reaction stoichiometry (Fig. 1c). The absence of dienes and arenes, which are the expected products of the formaldehyde-mediated reaction sequences such as Prins condensation, suggests that subsequent formaldehyde conversion is greatly suppressed (Fig. 1c).15,17 Formaldehyde may be also consumed through the reactions leading to the formation of HP intermediates, which are expected to propagate the formation of the C2+ hydrocarbons. However, the measurement of the catalytic performance of the CaZ40 catalyst showed that its activity and product distribution remained unaltered over longer reaction time, which indicates that the concentration of HP species does not change significantly (Fig. S5†). In addition, as shown in the example of the CaZ40 catalyst, the yields of detectable products are invariant to the inlet methanol concentration, indicating that the formation of formaldehyde and methane, as well as the synthesis of light alkenes, exhibits a first-order dependence with respect to this reactant (Fig. 1c and d). The apparent order of formaldehyde formation of ca. 1 is in agreement with the mechanism in which the hydride transfer occurs between the surface-bound methyl species and weakly bound methanol molecule (please see ESI† Discussion S2.1).18 The methanol-to-DME ratio at different inlet methanol concentrations remained constant and was close to the equilibrium ratio (ca. 0.5). These conditions allow us to determine the apparent activation energy of formaldehyde evolution to be ca. 70 kJ mol−1 over both the Z40 and CaZ40 catalysts (Fig. 1e). This value is significantly lower than the value estimated by theoretical calculations (ca. 130–220 kJ mol−1)18,19 and is comparable to the typical activation barriers for the alkylation of alkene and arene chain carriers (40–80 kJ mol−1).36–39 Therefore, the kinetics of formaldehyde formation is competitive with that of alkene evolution in the low conversion regime. It is interesting to note that the apparent rates of formaldehyde evolution over the Z40 catalyst are ca. 7.2× higher than those over the CaZ40 catalyst. This activity difference is significantly higher than the ratio of the total BAS, 4.6, in these two catalysts and is comparable to the ratio of their strong BAS concentrations, 8.3 (Table S1†). This suggests that strong BAS are primarily responsible for methanol disproportionation, and that extra-framework calcium ions do not affect this reaction.Relationship between the MTH performance and formaldehyde reactivity
After analyzing the formaldehyde evolution in the initial stages of the MTH reaction, its yield and the relationship with the MTH catalytic performance were investigated in a broader range of conversions. Commercial Z15 and Z40 zeolites were selected as representative model catalysts because of their distinct MTH performance (Fig. 2, S4 and S6†). Scanning electron microscopy (SEM) shows that both Z15 and Z40 catalysts contain particles of similar size (0.4–1 μm, Fig. S1 and S2†). Besides, they also exhibit very similar textural properties (Table S1†). The most prominent difference between the Z15 and Z40 samples is in their acid properties, wherein the former sample contains much higher concentration of BAS and LAS. The highly acidic Z15 zeolite displays higher MTH activity (STY, Fig. 2a), lower cumulative turnover capacity (CT0, Fig. 2b), and higher propensity to form ethene and aromatics with respect to C3+ alkenes than its less acidic Z40 counterpart (Fig. 2c, S3 and S4†). Such a performance profile implies that the arene chain carriers are more abundant over Z15 than over Z40, which is reflected by the higher ethene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (2-methylbutane + 2-methylbutene) ratio (DC2H4/mC5H10,12, Fig. 2d).40 Consistently, Z15 displayed a higher propensity for catalyzing HT reactions than Z40, inferred from the higher selectivities to methane and the higher fraction of C2–4 alkanes relative to the total amount of C2–4 hydrocarbon products and as expressed by the hydrogen transfer index (HTI, Fig. 2e).41
(2-methylbutane + 2-methylbutene) ratio (DC2H4/mC5H10,12, Fig. 2d).40 Consistently, Z15 displayed a higher propensity for catalyzing HT reactions than Z40, inferred from the higher selectivities to methane and the higher fraction of C2–4 alkanes relative to the total amount of C2–4 hydrocarbon products and as expressed by the hydrogen transfer index (HTI, Fig. 2e).41
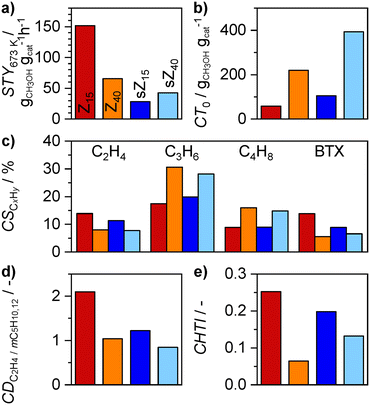 | ||
Fig. 2 (a) Space–time yields and (b) cumulative turnover capacities, (c) cumulative selectivities to selected hydrocarbon products, (d) cumulative ethene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (2-methylbutane + 2-methylbutene) ratios and (e) cumulative hydrogen transfer indices of the Z15 and Z40, and steamed sZ15, and sZ40 catalysts in the MTH reaction. The space–time yield was assessed at 673 K, and other parameters were determined at 773 K. The cumulative product distribution parameters were calculated in the 20–95% conversion range. The complete selectivity–conversion profiles of the catalysts are presented in Fig. S6.† All reactants and products were detected and quantified by the GC-FID method. The color code in (a) applies to all plots. Other conditions: CH3OH (2-methylbutane + 2-methylbutene) ratios and (e) cumulative hydrogen transfer indices of the Z15 and Z40, and steamed sZ15, and sZ40 catalysts in the MTH reaction. The space–time yield was assessed at 673 K, and other parameters were determined at 773 K. The cumulative product distribution parameters were calculated in the 20–95% conversion range. The complete selectivity–conversion profiles of the catalysts are presented in Fig. S6.† All reactants and products were detected and quantified by the GC-FID method. The color code in (a) applies to all plots. Other conditions: CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 19 Ar = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 mol%, WHSV = 76 or 253 (Z15 at 673 K) gCH3OH gcat−1 h−1, and P = 1.6 bar. 81 mol%, WHSV = 76 or 253 (Z15 at 673 K) gCH3OH gcat−1 h−1, and P = 1.6 bar. | ||
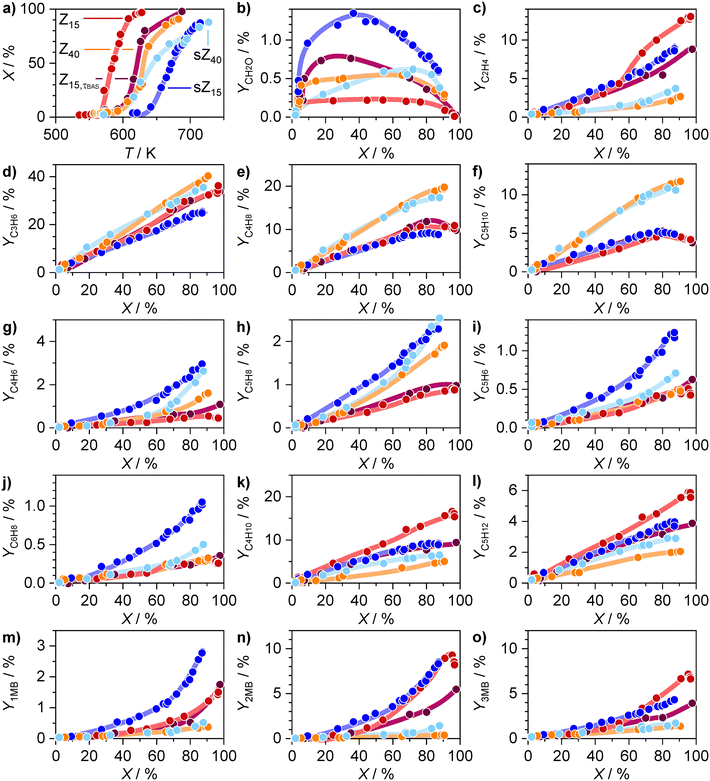
In view of previous findings indicating that MIHT is the prevailing HT mechanism at sub-complete conversion and suggesting that formaldehyde strongly affects the MTH reaction mechanism,12,15,17,20,22 it is relevant to compare the formaldehyde concentration profiles over these two materials as a function of methanol conversion, which was adjusted by gradually increasing the reactor temperature. Although PEPICO catalytic experiments were performed at lower pressures than laboratory tests because of the high sensitivity and arrangement of the detection system, all the catalysts were active in a characteristic temperature window of the MTH reaction of ca. 580–730 K, wherein their light-off curves follow the order of activities assessed in a conventional lab-based fixed-bed reactor (Fig. S4†). The performance of Z15 and Z40 was compared at a constant contact time per total BAS (τBAS) concentration and at a constant WHSV. In line with the established catalytic role of BAS in MTH conversion, the Z15 and Z40 catalysts displayed almost identical sigmoidal light-off profiles at a constant τBAS in the operando PEPICO experiment (Fig. 3a). Consistent with the fixed-bed catalytic tests (Fig. 2 and S6†), the Z40 catalyst displayed a higher selectivity to C3+ alkenes (Fig. 3d–f) and a lower selectivity to ethene (Fig. 3c), alkanes (Fig. 3k), and MBs (Fig. 3m–o), demonstrating that the product distribution trends are preserved in the PEPICO experiments.
PEPICO spectroscopy enables quantitative monitoring of the changes in the yield of formaldehyde along with other characteristic MTH products (Fig. 3b–o) and analysis of the relationships in their evolution. Herein, different structural isomers of C4+ products could be identified based on PI and photoion ms-TPE spectra (Fig. S7†). In agreement with the observation that formaldehyde is the primary reaction intermediate that readily reacts with different MTH species, the yield of formaldehyde increases in the lower and decreases in the higher conversion range, attaining an almost zero value at near complete conversion levels (Fig. 3b). After a similar increase up to conversion levels of ca. 6%, the formaldehyde yield over the Z15, τBAS catalyst is ca.1.5× higher than the one over Z40 in the conversion range up to ca. 40%. This is accompanied by the higher yield of C4 and C5 alkanes over Z15, τBAS with respect to the Z40 catalyst in this conversion range, suggesting that the higher yield of formaldehyde over the Z15, τBAS catalyst arises from the more prominent MIHT between methanol and alkenes. In the case of the Z40 catalyst, a decrease of the formaldehyde yield is observable at conversion levels exceeding ca. 60%. At this turning point, the yields of acyclic dienes display a pronounced upward trend of the change of the yield (C4H6, C5H8, Fig. 3g and h), while 1MB and 3MB also display only a small positive inflection. C4 and C5 alkenes show a downward curve of the change of the yield at conversions above ca. 60% (Fig. 3e and f). These trends in changes in the yield indicate that the conversion of formaldehyde over the Z40 catalyst is associated with the formation of acyclic dienes, likely via the Prins reaction.17 Consistent with this observation, the Z40 catalyst displays ca. 2× higher yield of acyclic dienes than Z15, τBAS (C4H6, C5H8, Fig. 3g and h). This parallels the ca. 2× higher yields of C3–5 alkenes over the latter system, which indicates that the higher average concentration of alkenes in the reactor comprising the Z40 material favors the reaction of formaldehyde with these products. The yield of formaldehyde over Z15, τBAS decreases at conversions above ca. 40%, displaying a steep decay at conversions above ca. 80%. Both inflection points in the formaldehyde yield versus conversion curve coincide with the upward changes of the MB yield (Fig. 3m–o). This provides a strong hint that formaldehyde is consumed in the reaction sequences leading to the formation of MBs and/or in reactions with these arenes. Additional insights are obtained from the experiment over the Z15 catalyst performed at identical WHSV as the experiment over the Z40 material, which corresponds to ca. 2.4 higher τBAS. Because of longer residence time, the light-off curve of the methanol conversion shifted to ca. 45 K lower temperature (Z15, Fig. 3a). Notably, the yield of formaldehyde remains significantly lower than those measured over the same catalyst at higher WHSV (i.e., Z15, τBAS) and over the Z40 catalyst under the same WHSV conditions (Fig. 3b). Since a lower WHSV (i.e., higher τBAS) favors the production of formaldehyde, as also supported by the higher yields of MIHT products (C4,5 alkanes, Fig. 3k and l), the lower integral yield of formaldehyde over Z15 with respect to Z15, τBAS evidences enhanced consumption of this intermediate over the longer catalyst bed. Among the products, 2MB and 3MB display the most pronounced relative gain in yield (Fig. 3n and o), which further supports the hypothesis that the enhanced consumption of formaldehyde is linked with the formation of these arenes and/or reactions with them.
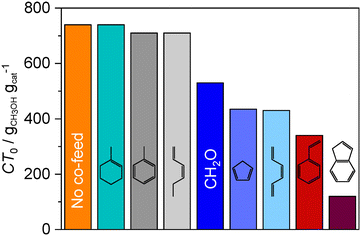
The experiments performed over the Z15 and Z40 catalysts indicate that the average concentration of formaldehyde across the catalyst bed increases with decreasing τBAS. In realistic industrial MTH operation, the residence time increases as a result of progressive coke deposition and because of steam-induced hydrolysis of the framework aluminum atoms.6,26,42 Regarding the latter source of deactivation, we assess the formaldehyde evolution profiles over severely steamed sZ15 and sZ40 zeolites that can be viewed as aged Z15 and Z40 catalysts that have been subjected to multiple regeneration cycles. In comparison with their parent materials, both steamed sZ15 and sZ40 catalysts display lower catalytic activity, higher CT0 values, and similar selectivity to C3–4 alkenes (Fig. 2a–c). While sZ40 and Z40 catalysts show similar selectivities to ethene and BTX, sZ15 exhibits significantly lower selectivity to these products than its parent counterpart. This indicates that the arene cycle is less prevalent in sZ15 than in parent Z15, which is also reflected by the lower value of the CDC2H4/mC5H10,12 parameter (Fig. 2d). In comparison with Z40 and sZ40 catalysts, sZ15 displays higher selectivities to ethene and BTX and lower selectivities to propene and butene, as well as lower CT0 values, although its BAS concentration is lower than those measured in the former two zeolites (Table S1†). Since all three catalysts exhibit similar particle-size distribution and porous properties (Fig. S2, Table S1†), this result indicates that the higher formation of HT products such as alkanes and arenes is induced by other factors including the higher concentration of LAS (Table S1†),15 which can be linked to the higher fraction of extra-framework aluminum species (Fig. S3†). Besides, the FTIR spectra of sZ15 indicate a higher relative fraction of internal silanols (Fig. S3†), which are proposed to promote coking.43
In view of these structural and performance differences between the steamed and parent zeolites, it was relevant to assess if these variances are also reflected in the formaldehyde reactivity profiles over these catalysts. The PEPICO data show that the formaldehyde yield over sZ15 exhibits a prominent maximum at moderate conversions of ca. 45%, which is higher than that of the parent Z15 material (Fig. 3b). However, at conversions exceeding ca. 45%, the formaldehyde yield exhibits a very steep decline, indicating that the reactivity of formaldehyde remains high over the steamed catalyst sample. In contrast, the formaldehyde yield over sZ40 increases more steadily and reaches the maximum value at slightly higher conversions than for the parent Z40 catalyst (75% vs. 65%). Another peculiar feature of steamed zeolites is the higher yield of dienes with respect to their parent counterparts (Fig. 3g–j), indicating that the decrease in BAS concentration impedes the transformations of these conjugated aliphatics. This result, along with the observed longer catalyst lifetime of the steamed samples, is in good agreement with the previous hypothesis that reactions of dienes and formaldehyde have a profound contribution to the formation of heavier aromatics and catalyst coking.13,21,25
Impact of formaldehyde on the MTH reactions
The FMRs are additionally evaluated by step-introduction of formaldehyde to the methanol feed (CH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH = 0.55
CH3OH = 0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1) over the representative Z40 catalyst. This perturbation caused a substantial change in the product distribution (Fig. 4a). In particular, a rapid increase in the formaldehyde peak (m/z 30) was accompanied by an increase in methanol (m/z 32) and DME (m/z 46) signals (Fig. 4b). The total carbon-based conversion of ca. 90% was essentially unaltered after the addition of formaldehyde, indicating an approximately first-order reaction response to the added formaldehyde. The reactivity of formaldehyde is additionally studied by introducing 12C formaldehyde (12CH2O) into a 13C methanol (13CH3OH) feed (Fig. 5). The 13C content in formaldehyde, methanol, and DME, as well as in representative hydrocarbon products, such as propene, 2MB, and 3MB, follows a virtually identical trend and eventually reaches a constant value of ca. 0.64, which matches that anticipated from the relative ratio between 13C methanol and 12C formaldehyde (12CH2O
1 mol mol−1) over the representative Z40 catalyst. This perturbation caused a substantial change in the product distribution (Fig. 4a). In particular, a rapid increase in the formaldehyde peak (m/z 30) was accompanied by an increase in methanol (m/z 32) and DME (m/z 46) signals (Fig. 4b). The total carbon-based conversion of ca. 90% was essentially unaltered after the addition of formaldehyde, indicating an approximately first-order reaction response to the added formaldehyde. The reactivity of formaldehyde is additionally studied by introducing 12C formaldehyde (12CH2O) into a 13C methanol (13CH3OH) feed (Fig. 5). The 13C content in formaldehyde, methanol, and DME, as well as in representative hydrocarbon products, such as propene, 2MB, and 3MB, follows a virtually identical trend and eventually reaches a constant value of ca. 0.64, which matches that anticipated from the relative ratio between 13C methanol and 12C formaldehyde (12CH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13CH3OH = 0.55
13CH3OH = 0.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1, eqn (S11) in the ESI†). These results demonstrate prompt interconversion between DME/methanol and formaldehyde, which is faster than the conversion of methanol and formaldehyde to hydrocarbons. DME/methanol–formaldehyde interconversion likely proceeds via hydride scrambling between adsorbed methanol and formaldehyde species (eqn (1)).17
1 mol mol−1, eqn (S11) in the ESI†). These results demonstrate prompt interconversion between DME/methanol and formaldehyde, which is faster than the conversion of methanol and formaldehyde to hydrocarbons. DME/methanol–formaldehyde interconversion likely proceeds via hydride scrambling between adsorbed methanol and formaldehyde species (eqn (1)).17| 12CH3OH + 13CH2O ⇌ 12CH2O + 13CH3OH | (1) |
 | ||
| Fig. 5 The fraction of 13C carbon in the representative reaction species after the introduction of formaldehyde to a 13C-labeled methanol feed as detected by PEPICO spectroscopy. The inset shows the 13C fraction after attaining steady state. Reaction conditions are equivalent to those reported in the caption of Fig. 4. | ||
In addition to MBs, the formaldehyde co-feed induced a substantial increase in signals at m/z 116 (IP = 8.2 eV), 118 (IP = 8.30 eV), 130 (IP = 8.16 eV), 132 (IP = 8.16 eV), and 146 (IP = 7.98 eV, calculated IPs for likely spectral carriers, see Fig. 4b and S9†). Their ionization profiles indicate that these species likely represent the structural isomers of indene (m/z 116, 130, 144), indane (m/z 146), styrene (m/z 118, 132, 146), and divinylbenzene (m/z 130), exhibiting different degrees of methylation. Moreover, the species at the high m/z values of 156, 170, and 184 also increased substantially. Although these species cannot be unequivocally identified, their photoionization onsets (7.8–7.9 eV) and photoionization spectra suggest that they represent alkylated and likely methylated naphthalenes (MNs, Fig. S7†). Notably, the relative increase of the outlet concentrations of styrene (e.g., m/z 118, ca. 6×), indene (e.g., m/z 116, ca. 19×), and indane/styrene (e.g., m/z 146, ca. 34×) species, as well as for MNs (e.g., 3MN ca. 20×), is even higher than those observed for most of the MBs (Fig. 4b). This reactivity pattern is consistent with the proposed FMRs in which formaldehyde promotes the transformation of alkenes into MBs, MNs, and PAHs via series of sequential steps (Fig. 4b, top scheme). Herein, the alkenes as the starting reactants are expected to display the most prominent decrease, while dienes as the intermediate species that are both formed and consumed are expected to exhibit a lower relative decrease. MBs, indene and styrene species, and MNs, which are positioned in the latter steps of the reaction sequence, are expected to show the most prominent increase in the relative concentration, since their formation is in multiple steps promoted by the addition of the formaldehyde co-feed.
It is further interesting to note that the outlet concentration changes of specific hydrocarbon groups induced by formaldehyde addition follow similar transient profiles (Fig. 6). Herein, the outlet concentrations of alkenes and dienes decrease almost instantaneously with the addition of formaldehyde (phase I, Fig. 6). The onset of MBs and ethene (phase II), indene and styrene species (phase III), and MNs occurs after a certain lag-time (phase IV), which progressively increases with the increases from MBs to MNs. Considering the integral nature of the MTH reactor under study, the successive onset of various species may also arise from the differences in the micropore diffusivities among various reactants. However, the absence of lag-times among the hydrocarbons of the same group (e.g., between C3H6 and C6H12, 1MB and 4MB) in spite of their different diffusivities (which monotonically increase with molecular weight), and different responses of hydrocarbons displaying comparable diffusivities (e.g., C2H6 and C3H6, 1MB–3MB and C5+ aliphatics) suggest that the transients likely reflect the induction phases of various arene species. Notably, a fast response of alkenes and diene signals and the sequential onset of arene formation are consistent with the proposed reaction sequential reaction scheme (Fig. 4b, top scheme).
 | ||
| Fig. 6 Temporal evolution of relative concentration changes of various reaction species induced by introducing the formaldehyde co-feed to the MTH reaction as detected by PEPICO spectroscopy. Phases I through IV represent the time periods in which the changes of the outlet concentrations for particular hydrocarbon products commence. Reaction conditions are equivalent to those reported in the caption of Fig. 4. | ||
To complement the above findings, DR-UV/vis spectroscopy was applied to monitor the evolution of the reaction intermediates in the zeolite pores induced by the addition of formaldehyde to the MTH reaction over the Z40 catalyst (Fig. 7 and S10†). In agreement with previous reports,45–47 the bands centered at ca. 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 32
500 and 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1, attributed to monoenyl carbenium ions (cyclic and acyclic), the band centered at ca. 28
500 cm−1, attributed to monoenyl carbenium ions (cyclic and acyclic), the band centered at ca. 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1 associated with lower MB and/or dienyl (cyclic and acyclic) carbenium ions, and the band centered at ca. 26
500 cm−1 associated with lower MB and/or dienyl (cyclic and acyclic) carbenium ions, and the band centered at ca. 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, associated with higher MB carbenium ions, represent the prevailing spectral features in the early stage of the MTH reaction (Fig. 7a, top). Higher-wavelength spectral components associated with MNs (ca. 23
000 cm−1, associated with higher MB carbenium ions, represent the prevailing spectral features in the early stage of the MTH reaction (Fig. 7a, top). Higher-wavelength spectral components associated with MNs (ca. 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1), and lower (ca. 20
000 cm−1), and lower (ca. 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) and higher (ca. 18
000 cm−1) and higher (ca. 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) polycyclic aromatic hydrocarbons (PAHs), i.e., coke precursors and species, increase as the reaction progresses (Fig. 7b).45–47 A significantly different behavior is obtained if only a small amount of formaldehyde is co-fed with methanol (CH3OH
000 cm−1) polycyclic aromatic hydrocarbons (PAHs), i.e., coke precursors and species, increase as the reaction progresses (Fig. 7b).45–47 A significantly different behavior is obtained if only a small amount of formaldehyde is co-fed with methanol (CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2O = 10.6
CH2O = 10.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 mol%, Fig. 7a, bottom). The relative fraction of the bands associated with the monoenyl carbenium ion components was very low at the start of the reaction, whereas the signals associated with MBs, and especially with MNs and PAHs, intensified immediately after the reaction started. These spectral profiles indicate that formaldehyde greatly enhances the conversion of monoenyl and dienyl species into MBs and further into MNs and coke species, which is consistent with the product evolution profiles observed in the PEPICO experiments.
0.2 mol%, Fig. 7a, bottom). The relative fraction of the bands associated with the monoenyl carbenium ion components was very low at the start of the reaction, whereas the signals associated with MBs, and especially with MNs and PAHs, intensified immediately after the reaction started. These spectral profiles indicate that formaldehyde greatly enhances the conversion of monoenyl and dienyl species into MBs and further into MNs and coke species, which is consistent with the product evolution profiles observed in the PEPICO experiments.
 | ||
Fig. 7 (a) DR-UV/vis spectra of Z40 during the MTH reaction in the absence (top) and in the presence (bottom) of formaldehyde in the feed. (b) Temporal evolution of the relative fractions of specific spectral components obtained by deconvolution into the Gaussian peaks as indicated in Fig. S10.† Reaction conditions: CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2O CH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 10.6 Ar = 10.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0(0.2) 0(0.2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89.4(89.2) mol%, WHSV = 41 gCH3OH gcat−1 h−1, T = 673 K, and P = 1.2 bar. 89.4(89.2) mol%, WHSV = 41 gCH3OH gcat−1 h−1, T = 673 K, and P = 1.2 bar. | ||
Formaldehyde-mediated reactions
PEPICO analysis of formaldehyde formation in the early stage of MTH conversion revealed that the formation of this primary reaction product proceeds with an apparent activation barrier of 70 kJ mol−1. This value is comparable to the apparent activation barriers of the methylation and cracking steps of the DCHP mechanism (40–80 kJ mol−1),36–39 indicating that the formaldehyde formation is competitive with the formation of the hydrocarbon products during the MTH reaction.The formaldehyde concentration profiles recorded over Z15 and Z40 and their steamed sZ15 and sZ40 counterparts demonstrate the higher reactivity of formaldehyde over catalysts with higher propensity to coking. Therefore, a faster decrease in the formaldehyde yield over Z15 with respect to Z40 at constant τBAS, as well as over sZ15 with respect to the Z40 material, despite the lower BAS concentration in the former (Fig. 3b), indicates that the nature of BAS and, potentially, of the LAS and framework defects such as silanols also affects the reactivity of formaldehyde in addition to the concentration. Moreover, the correlations between a decrease in formaldehyde and alkene yield and an increase in dienes corroborate the proposal that FMRs are initiated by the formation of the latter products from alkenes via Prins condensation. In this respect, higher yields of dienes over more stable catalysts that are also less reactive towards formaldehyde (e.g., Z40vs. Z15, sZ15 and sZ40vs. Z15 and sZ40) suggest the importance of the diene–formaldehyde reactions in the formation of aromatics and coke (Fig. 4b, top scheme). Both PEPICO and DRUV-vis analyses indicate high dienyl and, especially, cyclopentadienyl intermediate reactivity with formaldehyde, which correlates with the enhancement in the production rates of higher MNs and PAHs. The critical role of more conjugated triene products and their cyclic derivatives, cyclopentadienyl species, in promoting catalyst deactivation is also supported by the much higher deactivation potential of hexatriene and cyclopentadiene co-feeds with respect to analogues hexadiene and methylcyclohexene feeds at similar concentration levels (Fig. 8 and S11†). Notably, the PEPICO spectra indicate the formation of fulvene derivatives, which can be obtained via Prins condensation from cyclopentadiene and can readily isomerize into benzene rings (Fig. 4b, top scheme). This enables the prompt buildup of arene chain carriers. These observations indicate that the critical role of formaldehyde in the MTH mechanism originates primarily from its ability to enhance the formation of longer conjugated hydrocarbons and arene rings via condensation reactions that bypass the HT steps.
In addition to the enhancement of the alkene–polyene–MBs reaction cascade, PEPICO experiments indicate that other higher molecular weight species are formed in FMRs. In particular, the observation of signals attributed to indene and styrene derivatives indicates that formaldehyde enhances the stepwise growth of polycyclic aromatics, potentially via side-chain condensation reactions that generate the unsaturated alkenyl substituents that can undergo cyclization reactions (Fig. 4b, top scheme). Styrene and especially indene species exhibit strong catalyst deactivation potential, as demonstrated by co-feeding of small amounts of these hydrocarbons over the Z40 catalyst (Fig. 8 and S10†). Finally, the formation of MNs may proceed via cyclization of the vinyl substituents of divinylbenzene species (Fig. 4b, top scheme).
Conclusions
This study provides an in-depth assessment of formaldehyde formation and transformation in the MTH reaction over practically relevant ZSM-5 catalysts and their contributions to catalyst deactivation. In the early stage of the MTH reaction, formaldehyde formation mainly proceeds via methanol disproportionation, with an apparent activation energy of ca. 70 kJ mol−1. This value is comparable to the activation barriers of the methylation steps in the DCHP reaction sequences, indicating that formaldehyde formation is competitive with the evolution of hydrocarbon products. The formaldehyde yield profiles indicate that it primarily reacts with alkenes, transforming them into (cyclo)dienes with increasing reactivity as a function of alkene size. This is followed by the prompt conversion of dienes into aromatics, wherein cyclopentadienes are particularly reactive towards formaldehyde. The detection of fulvenes, which can be produced via Prins condensation of cyclopentadienes with formaldehyde and may isomerize into MBs, suggests that they can be the intermediates between dienes and arenes. The prominent build-up of styrene, indene, and naphthalene derivatives in the presence of formaldehyde co-feeds implies that condensation of the side chain-substitutes with formaldehyde, coupled with ring closure reactions of the obtained alkenyl, constitutes a possible reaction pathway leading to the formation of PAHs, i.e., coke. The measured product distribution profiles over the ZSM-5 catalysts demonstrate a positive correlation between formaldehyde reactivity and catalyst propensity to coking. Herein, the formaldehyde-mediated reactions are not only dependent on the concentration of the BAS but also on their speciation and are likely affected by the LAS and framework-associated defects such as silanols. The results evidence an important role of formaldehyde in the reaction sequences causing deactivation of the MTH catalysts.Experimental
Catalysts and characterization
ZSM-5 catalysts with nominal Si/Al ratios of 15 (Zeolyst, CBV 3024E) and 40 (Zeolyst, CBV 8014), labeled Z15 and Z40, respectively, were received in the ammonium form and transformed into their protonic form by calcination. Calcium-modified ZSM-5 with a nominal loading of 1 wt%, denoted as CaZ40, was prepared by dry impregnation of the ammonium form of Z40 using an aqueous solution of Ca(NO3)2·4H2O (Sigma-Aldrich, >99%), followed by drying in a vacuum (30 mbar) at 353 K for 12 h. Severely steamed ZSM-5 catalysts, labeled sZ15 and sZ40, were prepared by steaming the Z15 and Z40 parent materials, respectively, in a quartz fixed-bed reactor at 773 K for 5 h using a feed of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 18
Ar = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 mol% and a weight-hourly space velocity (WHSV) of 10.2 gH2O gcat−1 h−1. All catalysts were calcined under an oxygen flow (PanGas 5.0, FO2 = 100 cm3STP min−1) at 823 K for 5 h at a heating rate of 2 K min−1. The materials were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy with energy dispersive X-ray analysis (SEM–EDX), 27Al magic angle spinning nuclear magnetic resonance (27Al MAS NMR), and Fourier transform infrared spectroscopy (FTIR), as detailed in the ESI.†
72 mol% and a weight-hourly space velocity (WHSV) of 10.2 gH2O gcat−1 h−1. All catalysts were calcined under an oxygen flow (PanGas 5.0, FO2 = 100 cm3STP min−1) at 823 K for 5 h at a heating rate of 2 K min−1. The materials were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy with energy dispersive X-ray analysis (SEM–EDX), 27Al magic angle spinning nuclear magnetic resonance (27Al MAS NMR), and Fourier transform infrared spectroscopy (FTIR), as detailed in the ESI.†
Catalyst testing in the MTH conversion
MTH catalytic tests were performed in a continuous-flow fixed-bed reactor setup using a methanol (CH3OH, Sigma-Aldrich, ≥99.9%) in argon (PanGas, 5.0) feed at a molar ratio of CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 19
Ar = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 mol%, WHSV = 25, 76, or 253 gCH3OH gcat−1 h−1 at T = 673 or 773 K, and P = 1.6 bar. Details of the reactor setup, catalytic tests, and analysis protocols are provided in the ESI.†
81 mol%, WHSV = 25, 76, or 253 gCH3OH gcat−1 h−1 at T = 673 or 773 K, and P = 1.6 bar. Details of the reactor setup, catalytic tests, and analysis protocols are provided in the ESI.†
PEPICO spectroscopy experiments
Species exiting the MTH reactor were analyzed using PEPICO spectroscopy at the vacuum ultraviolet (VUV) beamline of the Swiss Light Source (Fig. S1†).31,48–50 Argon (used as a diluent) and xenon (Xe, 2% in Ar, Messer, 5.0, used as an internal calibrant) were fed using digital mass flow controllers (MKS). Diluted methanol vapor and 13C-labeled methanol (13CH3OH, Sigma-Aldrich, 99 atom% 13C) were introduced by passing the argon flow through a methanol bubbler, placed in a water thermostat maintained at a constant temperature of 278 K. The diluted formaldehyde feed was prepared by passing the argon flow through a small vessel loaded with 1,3,5-trioxane flakes (Sigma-Aldrich, ≥99%), which acted as an anhydrous source of this aldehyde. Four-way valves were used to direct the reactive feed to an exhaust vacuum line for stabilization or to the MTH reactor, as well as to introduce formaldehyde into the methanol feed. All catalytic tests were performed in a quartz reactor (di = 4 mm) with an outlet pinhole. The zeolite catalysts (Wcat = 6–24 mg, dp = 0.18–0.25 mm) were well mixed with quartz particles (Thommen-Furler, washed in HNO3 and calcined, dp = 0.355–0.420 mm) and loaded in the form of a fixed bed between two thin layers of quartz wool. The tip of a type K thermocouple was placed in the center of the catalyst bed and used to monitor the reaction temperature. The reactor temperature was controlled using a home-made, resistively heated electric oven, which was connected to two type K thermocouples and a PID controller. The reaction pressure was monitored using a pressure transducer above the reaction zone. Prior to the experiment, the reactors were thermally treated under an argon flow at 773 K for 0.5 h to remove any potential organic impurities. Unless otherwise stated, the MTH reaction was conducted using a reaction mixture of the composition CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2O
CH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xe
Xe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 1.95
Ar = 1.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0(1.1)
0(1.1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15
0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97.9(96.8) mol%, WHSV of 1.4, 3.4, or 5.4 gCH3OH gcat−1 h−1, T = 537–727 K, and P = 0.4 bar.
97.9(96.8) mol%, WHSV of 1.4, 3.4, or 5.4 gCH3OH gcat−1 h−1, T = 537–727 K, and P = 0.4 bar.
The molecular beam leaving the reactor was skimmed as it entered the ionization chamber, operating at 2 × 10−9 bar. There, it was ionized by VUV synchrotron radiation. The radiation was dispersed by a 150 mm−1 grating working in grazing incidence and focused at the 200 μm exit slit in a rare gas filter to monochromatize it. Higher order radiation of the grating was suppressed in the 9–14 eV photon energy range by using a Ne![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar
Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kr mixture in the gas filter (Messer, Ne
Kr mixture in the gas filter (Messer, Ne![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar
Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kr = 60
Kr = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mol%), operating at 8 × 10−3 bar over an optical length of 10 cm. The photoions and photoelectrons generated by photoionization were accelerated vertically in opposite directions by a constant electric field (213 V cm−1) towards two delay-line anode detectors (Roentdek, DLD40), where they were velocity map imaged and detected in delayed coincidence. Further details of the methanol and product analyses are provided in the ESI.†
10 mol%), operating at 8 × 10−3 bar over an optical length of 10 cm. The photoions and photoelectrons generated by photoionization were accelerated vertically in opposite directions by a constant electric field (213 V cm−1) towards two delay-line anode detectors (Roentdek, DLD40), where they were velocity map imaged and detected in delayed coincidence. Further details of the methanol and product analyses are provided in the ESI.†
DRUV-vis spectroscopy experiments
Surface-confined MTH reaction intermediates were studied by operando diffuse reflectance UV-visible (DRUV-vis) spectroscopy using a home-built plug-flow reactor cell closed with a calcium fluoride window and connected to a GC-FID analyzer (Fig. S1†).51 Spectra were recorded with a fiber optic spectrometer (AvaSpec ULS2048CL, Avantes) combined with a deuterium halogen light source (Avalight-DHS, Avantes) and a reaction probe (FCR-7UVIR600, Avantes) placed in front of the calcium fluoride window perpendicular to the reactor. Formaldehyde (CH2O, 23.5% in water, methanol-free, Roth) was dissolved in methanol. MTH catalytic tests were performed using a feed of CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2O
CH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 10.6
Ar = 10.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0(0.2)
0(0.2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89.4(89.2) mol%, WHSV = 41 gCH3OH gcat−1 h−1 at T = 673 K, and P = 1.2 bar. Further details of the DRUV-vis setup and data analysis are provided in the ESI.†
89.4(89.2) mol%, WHSV = 41 gCH3OH gcat−1 h−1 at T = 673 K, and P = 1.2 bar. Further details of the DRUV-vis setup and data analysis are provided in the ESI.†
Author contributions
V. P. conceived the idea, performed the i2PEPICO and UV-vis experiments and MTH catalytic tests, analyzed the data, and coordinated this project. X. W., P. H. and A. B. supported the planning and execution of the i2PEPICO experiments and the analysis of the obtained spectra, and discussed the i2PEPICO data. L. M. and D. F. supported the execution of the UV-vis experiments and the spectra analysis. J. A. v. B. acquired funding and discussed the data. V. P. wrote the manuscript with comments and corrections from all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the VUV beamline of the Swiss Light Source at the Paul Scherrer Institute (PSI), Villigen, Switzerland for granting a beamtime, Dr. Zeyou Pan and Mr. Patrick Ascher from the PSI for technical assistance during the i2PEPICO measurements, Dr. Przemyslaw Rzepka from ETH Zurich and the Paul Scherrer Institute for help with SEM–EDX analysis, ScopeM at ETH Zurich for the access to the SEM facility, and the Energy System Integration platform of the PSI for financial support. L. M. and D. F. would like to thank the ETH Board (Switzerland) for financial support through the program ‘SynFuels – Synthetic Fuels from Renewable Resources’.References
- U. Olsbye, S. Svelle, M. Bjrgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS PubMed.
- P. Tian, Y. Wei, M. Ye and Z. Liu, ACS Catal., 2015, 5, 1922–1938 CrossRef CAS.
- I. Yarulina, A. D. Chowdhury, F. Meirer, B. M. Weckhuysen and J. Gascon, Nat. Catal., 2018, 1, 398–411 CrossRef CAS.
- S. Ilias and A. Bhan, ACS Catal., 2013, 3, 18–31 CrossRef CAS.
- V. Van Speybroeck, K. De Wispelaere, J. Van Der Mynsbrugge, M. Vandichel, K. Hemelsoet and M. Waroquier, Chem. Soc. Rev., 2014, 43, 7326–7357 RSC.
- V. Paunović, V. Sushkevich, P. Rzepka, L. Artiglia, R. Hauert, S. Sik Lee and J. A. van Bokhoven, J. Catal., 2022, 407, 54–64 CrossRef.
- J. Valecillos, H. Vicente, A. G. Gayubo, A. T. Aguayo and P. Castaño, J. Catal., 2022, 408, 115–127 CrossRef CAS.
- K. Barbera, S. Sørensen, S. Bordiga, J. Skibsted, H. Fordsmand, P. Beato and T. V. W. Janssens, Catal. Sci. Technol., 2012, 2, 1196 RSC.
- Y. Wei, C. Yuan, J. Li, S. Xu, Y. Zhou, J. Chen, Q. Wang, L. Xu, Y. Qi, Q. Zhang and Z. Liu, ChemSusChem, 2012, 5, 906–912 CrossRef CAS PubMed.
- J. S. Martinez-Espin, M. Mortén, T. V. W. Janssens, S. Svelle, P. Beato and U. Olsbye, Catal. Sci. Technol., 2017, 7, 2700–2716 RSC.
- S. Lee and M. Choi, J. Catal., 2019, 375, 183–192 CrossRef CAS.
- S. Müller, Y. Liu, M. Vishnuvarthan, X. Sun, A. C. van Veen, G. L. Haller, M. Sanchez-Sanchez and J. A. Lercher, J. Catal., 2015, 325, 48–59 CrossRef.
- B. L. Foley, B. A. Johnson and A. Bhan, ACS Catal., 2021, 11, 3628–3637 CrossRef CAS.
- Y. Liu, S. Müller, D. Berger, J. Jelic, K. Reuter, M. Tonigold, M. Sanchez-Sanchez and J. A. Lercher, Angew. Chem., Int. Ed., 2016, 55, 5723–5726 CrossRef CAS PubMed.
- S. Müller, Y. Liu, F. M. Kirchberger, M. Tonigold, M. Sanchez-Sanchez and J. A. Lercher, J. Am. Chem. Soc., 2016, 138, 15994–16003 CrossRef PubMed.
- J. S. Martínez-Espín, K. De Wispelaere, T. V. W. Janssens, S. Svelle, K. P. Lillerud, P. Beato, V. Van Speybroeck and U. Olsbye, ACS Catal., 2017, 7, 5773–5780 CrossRef.
- Y. Liu, F. M. Kirchberger, S. Müller, M. Eder, M. Tonigold, M. Sanchez-Sanchez and J. A. Lercher, Nat. Commun., 2019, 10, 1462 CrossRef PubMed.
- L. Kilburn, M. DeLuca, A. J. Hoffman, S. Patel and D. Hibbitts, J. Catal., 2021, 400, 124–139 CrossRef CAS.
- F. M. Kirchberger, Y. Liu, P. N. Plessow, M. Tonigold, F. Studt, M. Sanchez-Sanchez and J. A. Lercher, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, 1–10 CrossRef PubMed.
- V. Paunović, P. Hemberger, A. Bodi, R. Hauert and J. A. van Bokhoven, ACS Catal., 2022, 12, 13426–13434 CrossRef.
- Y. Ni, W. Zhu and Z. Liu, ACS Catal., 2019, 9, 11398–11403 CrossRef CAS.
- A. Hwang and A. Bhan, ACS Catal., 2017, 7, 4417–4422 CrossRef CAS.
- S. S. Arora, D. L. S. Nieskens, A. Malek and A. Bhan, Nat. Catal., 2018, 1, 666–672 CrossRef CAS.
- S. S. Arora, Z. Shi and A. Bhan, ACS Catal., 2019, 9, 6407–6414 CrossRef CAS.
- J. S. Martinez-Espin, K. De Wispelaere, M. Westgård Erichsen, S. Svelle, T. V. W. Janssens, V. Van Speybroeck, P. Beato and U. Olsbye, J. Catal., 2017, 349, 136–148 CrossRef CAS.
- S. M. T. Almutairi, B. Mezari, E. A. Pidko, P. C. M. M. Magusin and E. J. M. Hensen, J. Catal., 2013, 307, 194–203 CrossRef CAS.
- T. Liang, J. Chen, Z. Qin, J. Li, P. Wang, S. Wang, G. Wang, M. Dong, W. Fan and J. Wang, ACS Catal., 2016, 6, 7311–7325 CrossRef CAS.
- I. Yarulina, K. De Wispelaere, S. Bailleul, J. Goetze, M. Radersma, E. Abou-Hamad, I. Vollmer, M. Goesten, B. Mezari, E. J. M. Hensen, J. S. Martínez-Espín, M. Morten, S. Mitchell, J. Perez-Ramirez, U. Olsbye, B. M. Weckhuysen, V. Van Speybroeck, F. Kapteijn and J. Gascon, Nat. Chem., 2018, 10, 804–812 CrossRef CAS PubMed.
- J. Krüger, G. A. Garcia, D. Felsmann, K. Moshammer, A. Lackner, A. Brockhinke, L. Nahon and K. Kohse-Höinghaus, Phys. Chem. Chem. Phys., 2014, 16, 22791–22804 RSC.
- P. Hemberger, V. B. F. Custodis, A. Bodi, T. Gerber and J. A. van Bokhoven, Nat. Commun., 2017, 8, 15946–15955 CrossRef PubMed.
- B. Sztáray, K. Voronova, K. G. Torma, K. J. Covert, A. Bodi, P. Hemberger, T. Gerber and D. L. Osborn, J. Chem. Phys., 2017, 147, 13944–13954 CrossRef PubMed.
- P. Hemberger, J. A. Van Bokhoven, J. Pérez-Ramírez and A. Bodi, Catal. Sci. Technol., 2020, 10, 1975–1990 RSC.
- A. Cesarini, S. Mitchell, G. Zichittella, M. Agrachev, S. P. Schmid, G. Jeschke, Z. Pan, A. Bodi, P. Hemberger and J. Pérez-Ramírez, Nat. Catal., 2022, 5, 605–614 CrossRef CAS PubMed.
- A. Liutkova, H. Zhang, J. F. M. Simons, B. Mezari, M. Mirolo, G. A. Garcia, E. J. M. Hensen and N. Kosinov, ACS Catal., 2023, 13, 3471–3484 CrossRef CAS PubMed.
- S. G. Lias, J. E. Bartmess, J. F. Liebman, J. L. Holmes, R. Levin and W. G. Mallard, in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, 2023 Search PubMed.
- I. M. Hill, Y. S. Ng and A. Bhan, ACS Catal., 2012, 2, 1742–1748 CrossRef CAS.
- I. Hill, A. Malek and A. Bhan, ACS Catal., 2013, 3, 1992–2001 CrossRef CAS.
- V. Van Speybroeck, J. Van Der Mynsbrugge, M. Vandichel, K. Hemelsoet, D. Lesthaeghe, A. Ghysels, G. B. Marin and M. Waroquier, J. Am. Chem. Soc., 2011, 133, 888–899 CrossRef CAS PubMed.
- L. Qi, Y. Wei, L. Xu and Z. Liu, ACS Catal., 2015, 5, 3973–3982 CrossRef CAS.
- S. Ilias, R. Khare, A. Malek and A. Bhan, J. Catal., 2013, 303, 135–140 CrossRef CAS.
- M. Bjørgen, F. Joensen, M. Spangsberg Holm, U. Olsbye, K. P. Lillerud and S. Svelle, Appl. Catal., A, 2008, 345, 43–50 CrossRef.
- S. M. Campbell, D. M. Bibby, J. M. Coddington and R. F. Howe, J. Catal., 1996, 161, 350–358 CrossRef CAS.
- K. Barbera, F. Bonino, S. Bordiga, T. V. W. Janssens and P. Beato, J. Catal., 2011, 280, 196–205 CrossRef CAS.
- R. I. Kaiser, L. Zhao, W. Lu, M. Ahmed, M. V Zagidullin, V. N. Azyazov and A. M. Mebel, J. Phys. Chem. Lett., 2022, 13, 208–213 CrossRef CAS PubMed.
- D. Mores, E. Stavitski, M. H. F. Kox, J. Kornatowski, U. Olsbye and B. M. Weckhuysen, Chem. – Eur. J., 2008, 14, 11320–11327 CrossRef CAS PubMed.
- J. Goetze, F. Meirer, I. Yarulina, J. Gascon, F. Kapteijn, J. Ruiz-Martínez and B. M. Weckhuysen, ACS Catal., 2017, 7, 4033–4046 CrossRef CAS PubMed.
- D. Fu, O. Heijden, K. Stanciakova, J. E. Schmidt and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15502–15506 CrossRef CAS PubMed.
- A. Bodi, B. Sztáray, T. Baer, M. Johnson and T. Gerber, Rev. Sci. Instrum., 2007, 78, 084102 CrossRef PubMed.
- D. L. Osborn, C. C. Hayden, P. Hemberger, A. Bodi, K. Voronova and B. Sztáray, J. Chem. Phys., 2016, 145, 164202 CrossRef PubMed.
- A. Bodi, M. Johnson, T. Gerber, Z. Gengeliczki, B. Sztáray and T. Baer, Rev. Sci. Instrum., 2009, 80, 034101 CrossRef PubMed.
- G. L. Chiarello, M. Nachtegaal, V. Marchionni, L. Quaroni and D. Ferri, Rev. Sci. Instrum., 2014, 85, 074102 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cy01786a |
| This journal is © The Royal Society of Chemistry 2024 |