Cu2O facet controlled reactivity for peroxidase-like activity†
Shivanand
Chettri‡
 a,
Liang-Ting
Wu‡
a,
Liang-Ting
Wu‡
 b,
Sagarmani
Rasaily
b,
Sagarmani
Rasaily
 a,
Debesh
Sharma
a,
Debesh
Sharma
 a,
Bikram
Gurung
a,
Bikram
Gurung
 a,
Rajani
Dewan
a,
Rajani
Dewan
 c,
Sudarsan
Tamang
c,
Sudarsan
Tamang
 a,
Jyh-Chiang
Jiang
a,
Jyh-Chiang
Jiang
 *b and
Anand
Pariyar
*b and
Anand
Pariyar
 *a
*a
aDepartment of Chemistry, Sikkim University, Tadong 737102, Gangtok, East Sikkim, India. E-mail: anandpariyar@gmail.com
bDepartment of Chemical Engineering, National Taiwan University of Science and Technology, Taipei 106335, Taiwan. E-mail: jcjiang@mail.ntust.edu.tw
cDepartment of Chemistry, St. Joseph's College, Darjeeling 734101, West Bengal, India
First published on 28th November 2023
Abstract
Replicating the enzymatic surface microenvironment in vitro is challenging, but constructing an analogous model could facilitate our understanding of surface effects and aid in developing an efficient bioinspired catalytic system. In this study, we generate five unique Cu2O morphologies: cubic (c-Cu2O), octahedral (o-Cu2O), rhombododecahedral (r-Cu2O), cuboctahedral (co-Cu2O), and spherical (s-Cu2O) with an average edge length between 0.59 and 0.61 μm. By precisely controlling crystal growth on distinct surface planes, with surface energies in the order γ{100} < γ{111} < γ{110}, we achieve a wide spectrum of shape variations during the evolution of these structures. The variations in surface morphology are a consequence of differences in the exposure of low-index facets, such as {100}, {111}, and {110}, leading to varying numbers of unsaturated copper sites at the surface. The peroxidase-like activity was investigated by altering the Cu2O surface structures in the reduction of H2O2 using chromogenic substrates like TMB/ABTS. The activity catalyzed by various Cu2O surfaces (r-Cu2O, o-Cu2O, and c-Cu2O) followed the typical Michaelis–Menten kinetics, with Km values ranging from 0.096 to 0.120 mM for TMB and 1.57 to 2.90 mM for H2O2 as substrates, respectively. Compared to native peroxidase enzymes (with Km values of 0.434 mM for TMB and 3.70 mM for H2O2 under identical conditions), these Cu2O catalysts exhibited a higher affinity. In general, the reactivity order observed was as follows: co-Cu2O ≈ r-Cu2O > o-Cu2O > c-Cu2O > s-Cu2O. Mechanistically, the H2O2 reduction on the surface produces hydroxyl radicals that undergo H-abstraction from TMB, where the latter was found to be the rate-determining step. Both kinetic and DFT studies have unveiled that the heightened reactivity of r-Cu2O can be attributed to its higher proportion of {110} planes, which contain a higher number of dangling (unsaturated) Cu atoms that facilitate H2O2 decomposition. Additionally, they exhibit sufficiently low energy barriers (TS2: 0.70 eV) that enable OH radicals to efficiently oxidize TMB molecules compared to other morphologies.
Introduction
PODs, which stand for peroxidases, are a crucial group of enzymes found in virtually all living organisms. These efficient metalloenzymes catalyse the reduction of peroxides which are harmful to the tissues, while also serving as crucial radical scavengers for toxin elimination in biological systems. However, due to their intrinsic limitations, such as high cost, difficulty in purification/recyclability, inadequate storage, easy inactivation, and sensitivity to solution, pH, and temperature, their catalytic activities are limited, hindering their broader usage. Peroxidase-assisted H2O2 reduction involves the active participation of the protein chains that provide a facile surface for the active site interaction with the substrate for enzymatic transformation. The structure of the native horse radish peroxidase (HRP) enzyme is depicted in (Fig. 1a). Specifically, the active site in HRP comprises an Fe(III)-protoporphyrin IX coordinated to a proximal His-170 and two distal His-42 and Arg-38 amino acids.1 These amino acids have a crucial role in activating H2O2 towards the cleavage of the O–O bond. To approach the active site of the heme prosthetic group, H2O2 must diffuse from the protein surface towards the heme pocket through a fluctuating entry point located between Phe-68 and Phe-142 (Fig. 1b), subsequently traversing a channel resembling a bottle to ultimately reach the heme iron.2 The reduction of H2O2 on the active site is only then favoured where Fe(III) (resting state) first oxidises to an Fe(IV) oxo π cation radical complex (compound I). Consequently, in the presence of two electron donor substrates (like TMB), it reduces to an Fe(IV) oxo complex (compound II) and finally completes the cycle through the generation of the resting Fe(III) enzyme (Fig. 1c).3–6 The resplendent surface structures displayed by protein molecules provide an opulence of invaluable information for understanding the biological roles of proteins.7,8 Proteins/enzymes and dynamic functional materials exhibit intricate interactions, and biological cells can modify the surfaces, ranging from mechanical disruption to protein excretion and enzymatic degradation.9 Thus, by treating proteins and material surfaces as chemical systems, it becomes possible to comprehend these complex biological reactions. Accordingly, developing chemically defined surfaces can enhance our understanding of biological systems and enable the design of biomimetic surfaces with targeted catalytic activity. | ||
| Fig. 1 (a) Native horse radish peroxidase (HRP) enzyme (PDB: 1HCH). (b) Schematic representation of the active site of HRP. (c) Peroxidase cycle for two electron donor substrates as catalyzed by HRP. | ||
In this context, we screened chemical analogues to study peroxidase-like activity. Metal oxides emerge as an obvious choice, as they offer a range of surfaces that can be systematically tuned for facile adsorption and reduction of H2O2 on their surface that can generate reactive oxygen species.10,11 Although a plethora of metal oxides such as TiO2,12,13 V2O5,14 MnO2,15 Fe2O3,16,17 Fe3O4,18 Co3O4,19,20 NiO,21 Cu2O,22 CuO,23–25 ZnO,26,27 RuO2,28 ZrO2 (ref. 29) and CeO2 (ref. 30–32) are known to exhibit peroxidase-like activity, a comprehensive study to understand the relative reactivity differences over various morphologies is still illusive. Interestingly, Cu2O is an important p-type semiconductor with a direct band gap of 2.17 eV, which displays diverse facet-dependent electrochemical33–38 and photocatalytic39–43 properties. Varying the shape of ionic solids, like metal oxides with a cubic crystal structure, can regulate the exposed facets on their surface. Cu2O can be tuned easily to generate surfaces with various exposed facets and surface energies that control their reactivity. For instance, rhombic dodecahedral Cu2O is known to exhibit excellent photocatalytic activity towards the degradation of methyl orange.39 In another study, Cu2O with different exposed facets was investigated for electrocatalytically sensing H2O2, wherein the {111}-facet bound hexapod, octahedral and {110} bound rhombododecahedral Cu2O catalysts were found to have higher reactivity than {100} bound cubic Cu2O.44
In one relevant computational study, Yuan et al. examined transition metal oxides such as Cu2O and Fe3O4 as peroxidase mimics by analyzing their H2O2 adsorption energies. However, their kinetic properties still need further understanding.22 In another study, Shen et al. systematically studied POD-like reactions catalyzed by iron oxides by calculating the energy potential surface along the reaction coordinate.45 They proposed a three-step reaction mechanism, which includes H2O2 dissociative adsorption on the catalyst and consecutive oxidation of TMB by two adsorbed OH radicals. However, the steps involving the H2O2 and substrate (TMB) adsorption were not considered, which might lead to an underestimation of the energy barriers for H2O2 dissociation and TMB oxidation. Additionally, solvents also influence the energy of the adsorbed species and solvated molecules within the solution. The absence of solvent effects and van der Waals (vdW) interaction corrections in both computational works could result in a significant deviation from the actual experimental conditions. Therefore, a more detailed potential energy surface is necessary for exploring the kinetics of POD-like reactions catalyzed by Cu2O. In this work, we have synthesized Cu2O with different morphologies such as cubic (c-Cu2O), octahedral (o-Cu2O), rhombododecahedral (r-Cu2O), cuboctahedral (co-Cu2O), and spherical (s-Cu2O) as a surface mimic towards peroxidase activity. We employed UV-vis spectroscopy to investigate the TMBox production rate and utilized the Michaelis–Menten model to study steady-state kinetics. Furthermore, we conducted DFT calculations to examine the reaction mechanism on different exposed facets under more rigorous modelling conditions, including the consideration of the implicit solvent model, DFT-D3 van der Waals correction, and H2O2/TMB molecular adsorption steps.
Results and discussion
Synthesis and characterization
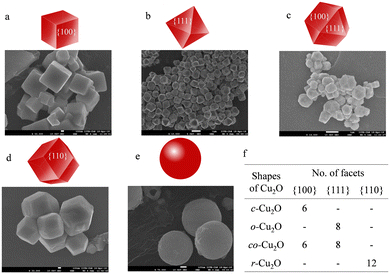
The synthesis of various shaped Cu2O catalysts was achieved by controlling the growth of the {100}, {111}, {110} planes.39,46,47 The facet growth of a metal oxide is driven by the increased growth of the high surface area facet, which plays a crucial role in elucidating the final morphological outcome of the metal oxides. The shape evolution of these metal oxides is predicted by the Gibbs–Curie–Wulff theorem,48,49 which suggests that the surfaces or facets with higher surface energy have rapid perpendicular growth and are eventually eliminated from the final morphology, whereas the facets having low surface energy grow slowly and are retained in the final shape of the crystals. Therefore, to preserve the facets associated with a higher surface energy in the final morphology of a metal oxide, their growth rate is substantially reduced with the introduction of surface capping agents like polyvinylpyrrolidone (PVP) and sodium dodecyl sulphate (SDS) in the case of Cu2O. The syntheses of five different morphologies of Cu2O, viz., cubic (c-Cu2O), octahedral (o-Cu2O), rhombododecahedral (r-Cu2O), cuboctahedral (co-Cu2O), and spherical (s-Cu2O) were achieved by controlling the concentration of the capping agents and reaction time.39,47 FT-IR spectra show a characteristic Cu–O bond stretching band at around 626 cm−1 for Cu2O crystals (Fig. 2a).50 The phase purity of the as-synthesized Cu2O crystals was confirmed using PXRD and indexed to the cubic phase (JCPDS: 05-0667) (Fig. 2b).Here, the relative difference in the intensity of {111} and {200} peaks is indicative of the evolution of different morphologies.51 For example, due to the presence of a higher number of {111} planes on o-Cu2O, the relative ratio of the peak intensity between {111} and {200} is very high. This was furthermore confirmed by studying the surface morphologies with SEM.
Fig. 3 and S1† show the SEM images of different as-synthesized Cu2O crystals along with the geometric representation of the evolved facets and vertices with the change in morphology. These different shapes of Cu2O display distinct edges and relatively uniform sizes. Each cube has six {100} facets, an octahedron has eight {111} facets, a rhombododecahedron consists of twelve {110} facets, and a cuboctahedron has 14 facets comprising six {100} and eight {111} facets (Fig. 3f). As evident from the SEM images, the shape of the Cu2O crystal on increasing the PVP concentration evolves from cubic to octahedral. This is due to the controlled/slow growth of the high energy {111} plane in o-Cu2O with higher PVP concentration. The average edge lengths (Fig. S2†) in c-Cu2O and o-Cu2O were found to be 616.68 (±78.88) nm and 595.85 (±65.40) nm, respectively. Furthermore, excessive use of capping agents resulted in the formation of s-Cu2O with an average diameter of 603.02 (±71.62) nm. Generally, excessive use of capping agents results in indiscriminate binding around planes, resulting in the lowest energy morphology. Similarly, with the use of SDS as a capping agent and the stoichiometric concentration of NH2OH·HCl, other morphologies, viz., r-Cu2O and co-Cu2O, were obtained with an average edge length of 600.75 (±73.26) nm and 615.67 (±69.32) nm, respectively (see the ESI† for details). XPS analysis show typical Cu(I) signature peaks (2P3/2 at 931 eV and 2P1/2 at 950.9 eV). An additional Cu 2P peak at higher binding energy centered around 933 eV and 953 eV is attributed to the partial surface oxidation to Cu (II) (Fig. S3†).52
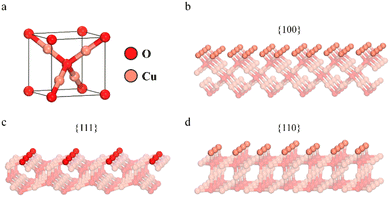
In a typical unit cell of Cu2O in a cubic crystal system (Fig. 4a), each O atom is tetrahedrally coordinated to four Cu atoms, and each Cu atom is bonded to two neighbouring O atoms. It is well established that the surface energy of the low-indexed facets {100}, {111}, and {110} is closely associated with the density of the coordinatively unsaturated Cu atoms.39Fig. 4b–d show the atomic arrangements along these three low-indexed facets. Generally, the {100} facets are terminated by O atoms, only making them relatively neutral, while the {111} facets have dangling coordinatively unsaturated Cu sites (CuCUS) perpendicular to them, making them positively charged.53 Again, the {110} facets are terminated by Cu atoms in association with the dangling Cu atom perpendicular to them. As a result, the number of Cu sites in the {110} facet is found to be approximately 1.5 times higher than that found in the {111} facet.53 Relatively, this makes the {110} facet more positively charged than the {111} facet. The density of these unsaturated dangling copper sites affects the surface energies of the low-indexed facets {100}, {111}, and {110}.39 Likewise, the surface energies (γ) of Cu2O follow the order: γ{100} < γ{111} < γ{110}.48 However, Su et al. reported that the presence of capping agents, such as SDS, significantly affects the surface termination of Cu2O nanoparticles.54 This is because the –OSO3− group in SDS tends to adsorb onto Cu sites rather than O sites. As a result, the Cu terminated surface is more favorably exposed than the O terminated surface, leading to different surface properties than initially anticipated. In terms of surface termination, the {100} and {110} surfaces have considerable dangling Cu atoms, while the {111} surface is terminated by O atoms in addition to a few dangling Cu atoms (Fig. 4).
Cu2O as a peroxidase mimic
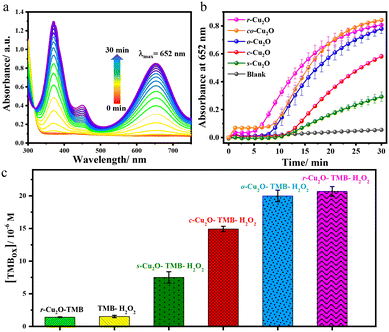
The peroxidase-like activity of the as-prepared Cu2O with different exposed facets was first investigated by monitoring the evolution of absorption variation of the chromogenic peroxidase substrate, 3,3′,5,5′-tetramethylbenzidine (TMB), in a time scale mode at 652 nm. TMB is a two-electron donor assay used to test peroxidase-like activity for the reduction of H2O2. The absorption band at 652 nm may be assigned to a charge transfer complex dimer TMBox (ε652nm = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1)55 formed between one electron oxidised radical cation and an unoxidized TMB, which is blue (Scheme 1).
000 M−1 cm−1)55 formed between one electron oxidised radical cation and an unoxidized TMB, which is blue (Scheme 1).
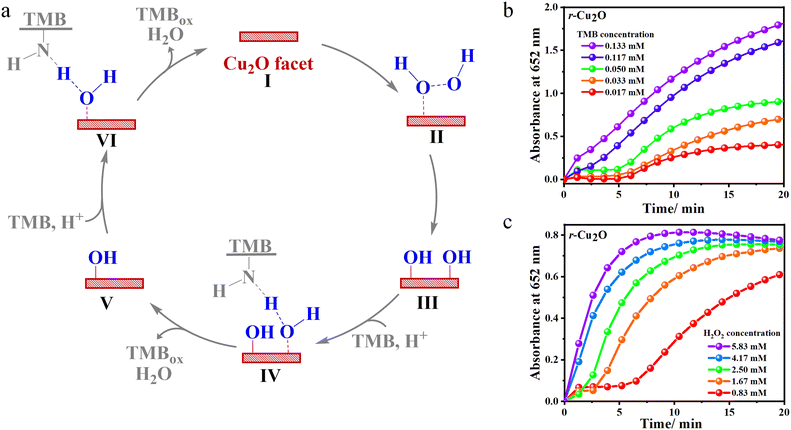
Further, one electron oxidation of TMBox resulted in the saturation of the peak at 652 nm and evolution of the peak for the diimine complex at λmax of 450 nm, which is yellow. As a representative case, r-Cu2O was taken for the optimisation of pH, temperature and catalytic loading, which may affect the reactivity. Buffer solutions having a wide range of pH values (2–10) was used and the reaction was found be progressive under acidic conditions. When the reaction was initiated in a buffer solution at pH 2, the rate of reaction towards the formation of a yellow coloured 2e− oxidised diimine complex (λmax = 450 nm) was drastically fast (Fig. S4†).56,57 Consequently, the rate of reaction for the formation of the 1e− oxidised product, viz., TMBox (λmax = 652 nm) was found to be optimal with the buffer solution of pH 4. The reaction was also performed in a wide range of temperatures (0–60 °C) (Fig. S5†). At 0 °C, there was no evident formation of TMBox and the rate of formation of the charge transfer complex (λmax = 652 nm) was found to increase with increasing temperature. However, the formation of TMBox was found to be maximum at 25 °C making it an ideal temperature for the reaction. Concisely, at higher temperature the increasing reaction rate for the formation of TMBox and its rapid conversion to the 2e− oxidised diimine complex (λmax = 450 nm) was observed.57 Lastly, the reaction was further optimized at various catalytic loadings ranging from 8–33 μg mL−1 (Fig. S6†). A steady increase in the formation of TMBox was observed on increasing the Cu2O catalytic loading from 8 to 33 μg mL−1. As a result, 33 μg mL−1 of Cu2O was taken as the optimised catalytic loading for the reaction. Furthermore, under the optimised conditions, a set of commercially available metal oxides were screened towards TMB oxidation, where Cu2O and CuO were found to have a comparatively better reactivity (Fig. S7†). Additionally, Cu2O had a much greater reactivity than CuO as anticipated which can be attributed to the lower oxidation state of the Cu centre.22 Thus, we proceeded with 33 μg mL−1 catalytic loading of various Cu2O catalysts, H2O2, and TMB as a peroxidase assay at room temperature in pH = 4 acetate buffer solution as the optimised conditions. The initial reaction using Cu2O gave the instant formation of blue coloured species along with the evolution of a peak at 652 nm showing peroxidase-like activity (Fig. 5a).18
The relative temporal evolution of the 652 nm peak on different Cu2O catalysts shows that the catalytic oxidation of TMB by H2O2 goes through a sedate initial phase (up to 5–10 min) due to the adsorption of the substrate on the surface of the catalyst, the phase is also referred to as the induction time.58 After the induction time, Cu2O with various morphologies showed different initial rate trajectories (Fig. 5b). For instance, c-Cu2O with exposed {100} facets exhibited slow oxidation of TMB, whereas the evolution is accelerated on r-Cu2O with exposed {110} facets. The efficiency of the different as-synthesized Cu2O catalysts under the optimised conditions was found to follow the order: cuboctahedral (co-Cu2O) ≈ rhombododecahedral (r-Cu2O) > octahedral (o-Cu2O) > cubic (c-Cu2O) > spherical (s-Cu2O) (Fig. 5b). Interestingly, the conversion of TMB using co-Cu2O and r-Cu2O was found to be comparable at 30 min. However, their induction time differed. The high reactivity of r-Cu2O may be attributed to the presence of a higher number of dangling copper sites on the {110} facet that gives rise to the high metal density and, thereby, high surface energy.59,60 Similar observation was found by Zhao and coworkers where the rhombododecahedral morphology of Fe3O4 with exposed high surface energy {110} facets showed a better catalytic activity towards TMB oxidation than the spherical morphology.61 Notably, the atomic constitutions of the facets may vary from compound to compound, but the surface reactivity depends exclusively on exposed planes and the corresponding surface energy. For example, the high energy surface is the {100} facet in the case of CeO2 nanocubes. Therefore, CeO2 nanocubes with exposed high surface energy {100} facets displayed higher peroxidase activity towards TMB oxidation than CeO2 nanorods with exposed {110} facets.31 Furthermore, the higher reactivity of co-Cu2O vs. c-Cu2O may be attributed by comparing the relative reactivity of {111} and {100} facets in Cu2O. Although both co-Cu2O and c-Cu2O contain 6 {100} planes, co-Cu2O has additional 8 {111} planes. Likewise, the surface energy of the {111} facet being greater than that of {100} facet, the reactivity of o-Cu2O was found to be greater than that of c-Cu2O or other morphologies. The overall conversion measured at t = 30 min shows that the relative conversion with respect to a blank (without catalyst) increased up to 5, 10, 13, and 14 fold for s-Cu2O, c-Cu2O, o-Cu2O, and r-Cu2O. These results also depict the role and difference of various Cu2O facets in peroxidase-like activity.
Furthermore, the peroxidase-like activity of Cu2O surfaces was also investigated using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) as a chromogenic peroxidase assay to test the substrate versatility of the catalyst. Catalytic oxidation of the ABTS substrate in the presence of H2O2 generates a green coloured radical cation complex (ABTSox) at λmax of 420 nm (ε420nm = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, Scheme 2). Indeed, Cu2O crystals were also found to catalyse the oxidation of ABTS, evident from the evolution of the 420 nm band (Fig. 6a).
000 M−1 cm−1, Scheme 2). Indeed, Cu2O crystals were also found to catalyse the oxidation of ABTS, evident from the evolution of the 420 nm band (Fig. 6a).
 | ||
| Fig. 6 (a) Evolution of the absorbance of ABTSox at 420 nm catalysed by Cu2O crystals in the presence of H2O2. (b) Different reaction systems viz., r-Cu2O–ABTS, ABTS–H2O2 and r-Cu2O–ABTS–H2O2. | ||
In the case of ABTS as well, the conversion of the reaction for the r-Cu2O–ABTS–H2O2 system was found to be much higher than ABTS–H2O2 (blank) and r-Cu2O–ABTS systems with the latter showing no significant absorbance at 420 nm even after 30 min (Fig. 6b). In fact, the relative conversion with respect to a blank (ABTS–H2O2) was found to be increased up to 10 fold for the r-Cu2O–TMB–H2O2 system.
Steady state kinetic studies
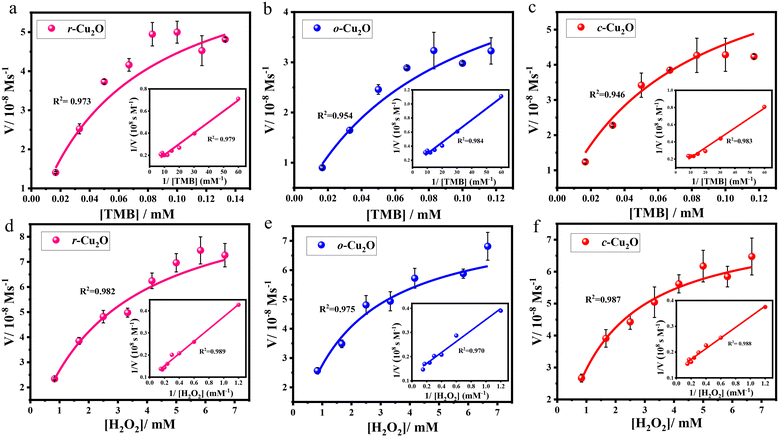
To test the reactivity of different surfaces of Cu2O towards H2O2 reduction, specifically r-Cu2O, o-Cu2O, and c-Cu2O, which contain unique {110}, {111}, and {100} facets, respectively, TMB as a chromogenic substrate was selected (Fig. S8–S11†). The measured initial rates vs. TMB/H2O2 concentrations are displayed in Fig. 7a–f; both followed a typical Michaelis–Menten behaviour in a suitable range of substrate concentrations. The kinetic parameters were calculated using the Lineweaver–Burk plots, which is a double reciprocal plot of the Michaelis–Menten equation: , where V is the initial velocity, Vmax is the maximal reaction velocity, [S] is the substrate concentration, and Km is the Michaelis constant.62 These parameters obtained for the different Cu2O surfaces are highlighted in Table 1.
, where V is the initial velocity, Vmax is the maximal reaction velocity, [S] is the substrate concentration, and Km is the Michaelis constant.62 These parameters obtained for the different Cu2O surfaces are highlighted in Table 1.
| Sl. no. | Catalyst | K m/mM | V max/10−8 M s−1 | Ref. | ||
|---|---|---|---|---|---|---|
| vs. TMB | vs. H2O2 | vs. TMB | vs. H2O2 | |||
| 1 | r-Cu2O {110} | 0.096 ± 0.004 | 2.898 ± 0.060 | 9.64 ± 0.93 | 10.45 ± 0.64 | This work |
| 2 | o-Cu2O {111} | 0.120 ± 0.015 | 1.800 ± 0.001 | 7.51 ± 1.36 | 7.93 ± 0.57 | This work |
| 3 | c-Cu2O {100} | 0.115 ± 0.012 | 1.570 ± 0.012 | 9.95 ± 1.75 | 7.63 ± 0.26 | This work |
| 4 | HRP | 0.434 | 3.70 | 10.00 | 8.71 | 18 |
| 5 | Commercial-Cu2O | — | 44.4 | — | 4.83 | 22 |
The Km values for r-Cu2O (0.096 mM), o-Cu2O (0.120 mM), and c-Cu2O (0.115 mM) with TMB as a substrate were found to be 4.5, 3.6, and 3.8 times, respectively, lower than that for the natural enzyme HRP (0.434 mM) (Table 1, entries 1–3 vs. TMB). This clearly indicates that the affinity of TMB for all Cu2O surfaces is far greater than that for native HRP. Further, r-Cu2O exhibited the highest affinity towards TMB, as reflected in a substantially low Km of 0.096 mM. This implies that a lower concentration of TMB is required to obtain the maximum reaction velocity for these Cu2O catalysts over HRP.62 Under various H2O2 concentrations, the calculated Km values for r-Cu2O (2.898 mM), o-Cu2O (1.800 mM), and c-Cu2O (1.570 mM) were also found to be lower than that for the natural enzyme HRP (3.70 mM) which indicates the greater affinity of H2O2 for Cu2O surfaces over the latter (Table 1, entries1–3 vs. H2O2).18 The role of the exposed facets may be highlighted here, wherein a recent study reported a high Km value of 44.4 mM for commercially available Cu2O nanoparticles with H2O2 as a substrate.22 Interestingly, c-Cu2O was found to possess the highest affinity towards H2O2, followed by o-Cu2O and then r-Cu2O. This clearly reveals that for r-Cu2O, a higher concentration of H2O2 is required to attain the maximum reaction velocity compared to c-Cu2O. Precisely, the Vmax values for r-Cu2O were found to be 1.4 and 1.3 times greater than those for c-Cu2O and o-Cu2O, respectively. Incidentally, a higher substrate-to-product conversion was observed for r-Cu2O (10.45 ± 0.64 × 10−8 M s−1), which is higher than that for the native enzyme HRP (8.71 × 10−8 M s−1).18
Mechanistic & DFT studies
Here, we conducted DFT calculations to investigate the potential energy diagram of TMB oxidation by H2O2 on various Cu2O surfaces. All the optimized minima and transition states are shown in Fig. S14† (see the ESI† for details). The optimized {100}, {110}, and {111} exposed surfaces (IS), as displayed in Fig. 4, were used to calculate the potential energy diagram, as illustrated in Fig. 8. Here, the unsaturated dangling Cu sites play a crucial role in the O–O bond cleavage on the surface. These sites attract, adsorb, and dissociate H2O2, which distinguishes them from saturated Cu in the bulk with an oxidation number of +1. According to Bader charge analysis, these dangling Cu sites carry a positive charge of approximately +0.3 |e|. The total charge brought by dangling Cu per unit area is calculated as +2.85, +2.30, and +1.03 |e| per nm2 for {100}, {110}, and {111} facets, respectively, with detailed surface properties in Table S1.† Consequently, the interaction strength of the long-range H2O2–Cu interaction can be estimated by comparing the electrostatic interaction between oxygen atoms from H2O2 and dangling Cu on the surface, following this order: {100} > {110} > {111}. This suggests that the {100} facet is the most attractive to H2O2 molecules near the surface, followed by {110} and then {111} facets. For the surface reaction, initially, the H2O2 molecule is adsorbed on the Cu2O surface (IM1) and forms bonds with CuCUS (CUS: coordinatively unsaturated sites). The calculated adsorption energies are −0.43, −1.45, and −0.91 eV on {100}, {110}, and {111} surfaces, respectively. This is consistent with the observed variation in the induction time (Fig. 5b). The activation of the O–O bond in leads to subsequent dissociative adsorption. The energy barriers of the O–O bond cleavage (TS1) are 0.06, 0.15, and 0.25 eV on {100}, {110}, and {111} surfaces, respectively. The vibrational mode of imaginary frequency related to O–O bond breaking is shown in Fig. S15.† O–O bond dissociation occurs almost barrierless on the {100} surface, making it the most favorable facet for forming OH˙ radicals after H2O2 adsorption, followed by the {110} surface, and then the {111} surface. The formation of OH˙ radicals was established by performing the reaction with terephthalic acid (TA) in place of TMB. TA is generally used as a fluorescent probe for the detection of free hydroxyl radicals, which can generate highly fluorescent 2-hydroxyterephthalic acid upon reacting with OH radicals.22,25,63 Indeed, when the reaction was performed in the presence of TA, H2O2, and Cu2O, a strong fluorescence peak at 420 nm for 2-hydroxyterephthalic acid was observed, while a very weak fluorescence emission was detected in the absence of either H2O2 or Cu2O (Fig. S13a†). Further, on increasing the concentration of H2O2 (Fig. S13b†), there was a significant increase in the intensity of the fluorescence peak, clearly indicating the formation of hydroxyl radical intermediates during the peroxidase-like catalytic reaction.22,25,63
leads to subsequent dissociative adsorption. The energy barriers of the O–O bond cleavage (TS1) are 0.06, 0.15, and 0.25 eV on {100}, {110}, and {111} surfaces, respectively. The vibrational mode of imaginary frequency related to O–O bond breaking is shown in Fig. S15.† O–O bond dissociation occurs almost barrierless on the {100} surface, making it the most favorable facet for forming OH˙ radicals after H2O2 adsorption, followed by the {110} surface, and then the {111} surface. The formation of OH˙ radicals was established by performing the reaction with terephthalic acid (TA) in place of TMB. TA is generally used as a fluorescent probe for the detection of free hydroxyl radicals, which can generate highly fluorescent 2-hydroxyterephthalic acid upon reacting with OH radicals.22,25,63 Indeed, when the reaction was performed in the presence of TA, H2O2, and Cu2O, a strong fluorescence peak at 420 nm for 2-hydroxyterephthalic acid was observed, while a very weak fluorescence emission was detected in the absence of either H2O2 or Cu2O (Fig. S13a†). Further, on increasing the concentration of H2O2 (Fig. S13b†), there was a significant increase in the intensity of the fluorescence peak, clearly indicating the formation of hydroxyl radical intermediates during the peroxidase-like catalytic reaction.22,25,63
After H2O2 dissociation, each OH˙ radical is adsorbed on the CuCUS top side on {110} and {111} surfaces, while on the {100} surface, each OH˙ is adsorbed on the bridge side between two CuCUS (IM2). The H2O2 dissociation is highly exothermic, especially on the {100} surface, due to the strong O–Cu interactions resulting from the bridge site adsorption configuration. The TMB molecule was adsorbed on the Cu2O surface in the presence of two OH˙ radicals, and a TMB molecule interacted with two OH˙ radicals via N–H⋯O hydrogen bonds between the amine group (–NH2) of TMB and the oxygen of OH (IM3). The calculated adsorption energies are −0.66, −0.73, and −0.77 eV on {100}, {110}, and {111} surfaces, respectively. The TMB adsorption energies on {110} and {111} are comparable since the interactions between TMB and two OH˙ radicals are similar on both surfaces. However, the TMB adsorption energy on {100} is slightly smaller than that on other facets, attributed to the strongest O–Cu interactions between OH˙ and the surface, thereby weakening the hydrogen bonds between OH˙ and TMB. In the redox reactions, one OH˙ radical attracts a hydrogen radical from the amine group of TMB (TS2), forming an adsorbed water, H2O*, and an oxidized TMB radical (FS), TMBox. The vibrational mode of imaginary frequency associated with H radical transfer is shown in Fig. S15.† The energy barrier of OH˙ attack is 1.51, 0.70, and 0.42 eV on {100}, {110}, and {111} surfaces, respectively. The extremely high energy barrier of 1.51 eV when OH˙ attracted one H atom from the TMB molecule on the {100} facet renders it less active in oxidizing the TMB molecule. This result is also attributed to the strongest O–Cu interactions. On the other hand, {110} and {111} surfaces exhibit moderate energy barriers in this step, which can be overcome by thermal energy at room temperature.
Discussion
The above experimental and theoretical data reveal that the catalytic oxidation of TMB in the presence of H2O2 proceeds via the initial adsorption of H2O2 over the Cu2O facet (intermediate II in Fig. 9a).45 At a lower substrate concentrations, the initial phase of the reaction displays a sluggish evolution of the absorbance peak at 652 nm for TMBox (Fig. 9b–c). This can be attributed to the adsorption of the substrate on the surface of the catalyst. With the increase in the concentration of H2O2, the active surface on the exposed facets of Cu2O becomes increasingly saturated with H2O2. After complete saturation of the surface, the catalytic conversion of TMB to TMBox is highly favoured, as evident from the steeper slope of the initial phase of the reaction at a higher substrate concentration. Hence, at a higher substrate concentration, there is a drastic increase in the rate of the reaction.The dissociative adsorption of H2O2 over the Cu2O facet occurs through the formation of hydroxyl radical (intermediate III, Fig. 9a).22,64 DFT calculations add that the transition state involved in homolytic O–O cleavage over all the Cu2O surfaces is kinetically favourable (<0.25 eV) and thermodynamically exothermic. Furthermore, relatively barrierless TS1 and the lower reactivity of the {100} surface clearly indicate the non-involvement of this process in the rate-determining step. The reduction of H2O2 then proceeds via the protonation-coupled hydrogen transfer from the amino group of TMB to the adsorbed hydroxyl radical, thereby facilitating the liberation of one equivalent of TMBox and H2O (intermediate IV, Fig. 9a).45 This proton-coupled H-transfer step invokes a transition state TS2 that reflects an endothermic IM3 to FS pathway in favour of r-Cu2O ({110} facet) with a relatively lower energy barrier (0.70 eV) than that of {100} containing c-Cu2O (1.51 eV). The lower reactivity of c-Cu2O, lower Km values (vs. TMB), and the observation of TMB-dependent reactivity (under limiting H2O2 conditions) indicates TS2 to be the rate-determining step. The reduction of the second adsorbed hydroxyl radical consequently liberates another equivalent of TMBox and H2O with the recovery of the Cu2O facet at the end of the catalytic cycle (intermediates V and VI, Fig. 9a).
Overall, the entire reactions on different Cu2O facets are thermodynamically favorable (driving force: FS–IS = −6.18 eV, −4.37 eV and −5.36 eV for c-Cu2O, o-Cu2O and r-Cu2O, respectively, Table S2†), driven largely by H2O2 adsorption and dissociation. Among these facets, the {100} facet exhibits the highest ability to activate the O–O bond and stabilize OH˙ radicals, while the {111} facet is the least effective. The strong O–Cu interaction between surface Cu and OH˙ leads to a high barrier for hydrogen radical transfer from TMB to OH˙, effectively suppressing the oxidation of TMB by OH˙ radicals. Furthermore, comparing the computational results from DFT calculations with measurements from the evolution of the absorbance peak, both support the conclusion that the {100} facet exhibits the lowest oxidative activity among the three facets. However, experimental observations show that {110} shows higher oxidative activity than {111}, despite the computational study indicating that {110} has a higher energy barrier in the rate-determining step (0.70 eV) compared to {111} (0.42 eV). To clarify this seemingly contradictory observation, we calculate the amount of active CuCUS atoms on each facet. The concentration of CuCUS is 10.95, 7.74, and 3.16 atoms per nm2 on {100}, {110}, and {111} facets, respectively. The {110} surface has ∼2.5 times more CuCUS atoms than the {111} surface, which is the crucial factor that causes the {110} facet to exhibit the highest oxidative activity in dissociating H2O2 and producing active OH˙ radicals. Interestingly, we observed that co-Cu2O exhibits a higher TMB conversion rate than r-Cu2O after 20 min in absorbance evolution measurement. This strongly suggests the coexistence of {100} and {111} facets with a synergistic effect in oxidizing TMB. While modeling polycrystalline surfaces and investigating reactions between two facets remain a challenge, the experimental results suggest a cooperative effect between the two facets in co-Cu2O crystals. In Fig. 5b, there is a rapid increase in absorbance intensity on co-Cu2O around 9 minutes, indicating an accelerated production of TMBox. Since the energy barrier for TMB oxidation on {100} is significantly higher than that on {111} (1.51 eV vs. 0.42 eV), the initial overall production rate of TMBox on co-Cu2O is primarily attributed to the ongoing reactions on the {111} surface. Additionally, {100} exhibits a lower activation energy (0.06 eV) for  dissociation compared to {111} (0.25 eV). Consequently, the initial OH˙ generation rate on the {100} facet is higher than that on the {111} facets, leading to a more rapid increase in OH˙ concentration on the {100} facets compared to {111}. Moreover, due to the high energy barrier, the initial consumption rate of OH˙ on the {100} facet is slower than that on {111}, resulting in an accumulation of OH˙ on the {100} facet. To make an estimate of the amount of accumulated OH˙ radicals, we calculated the migration barriers of OH˙ on {100} and {111} facets, respectively, and consistently found both to be 1.19 eV (Fig. 10).
dissociation compared to {111} (0.25 eV). Consequently, the initial OH˙ generation rate on the {100} facet is higher than that on the {111} facets, leading to a more rapid increase in OH˙ concentration on the {100} facets compared to {111}. Moreover, due to the high energy barrier, the initial consumption rate of OH˙ on the {100} facet is slower than that on {111}, resulting in an accumulation of OH˙ on the {100} facet. To make an estimate of the amount of accumulated OH˙ radicals, we calculated the migration barriers of OH˙ on {100} and {111} facets, respectively, and consistently found both to be 1.19 eV (Fig. 10).
It is noted that the OH˙ migration rate on each facet is dependent on the concentration of OH˙ and the concentration of active sites on the other facet. Notably, this energy barrier is lower than the energy barrier for the rate-determining step of TMB oxidation on the {100} surface, which stands at 1.51 eV. This result strongly suggests that OH˙ radicals can migrate between any two unsaturated dangling Cu sites on both {100} and {111} facets, enabling them to move towards the edge of the facet. Since the empty active sites on {100} are significantly occupied by OH˙ compared to {111}, migration from {100} to {111} is more favorable than from {111} to {100}. Ultimately, OH˙ migration from {100} to {111} increases the OH˙ concentration on {111} and converts TMB to TMBox efficiently. This OH˙ migration mechanism explains the accelerated production of TMBox around 9 minutes and the “absorbance crossover” with co-Cu2O and r-Cu2O around 20 minutes (Fig. S12†).
Conclusions
In this study, we examined different Cu2O morphologies, namely r-Cu2O, co-Cu2O, o-Cu2O, c-Cu2O, and s-Cu2O, which exhibit variations in exposed facets, for their peroxidase-like activity using TMB as a substrate. The reactivity of different Cu2O morphologies as peroxidase mimics can be ranked as follows: co-Cu2O ≈ r-Cu2O > o-Cu2O > s-Cu2O > c-Cu2O. This trend aligns with DFT calculations, where r-Cu2O, o-Cu2O, and c-Cu2O primarily consist of {110}, {111}, and {100} facets, respectively, and the variations in reactivity are due to differences in exposed facet reactivity, with {110} > {111} > {100}.These Cu2O surfaces exhibit enzyme-like properties within a suitable range of substrate concentrations, with Km values (for r-Cu2O, o-Cu2O, and c-Cu2O) with both TMB and H2O2 as substrates being lower than those for the native enzyme (HRP). In fact, the conversion rate from the substrate to product for r-Cu2O (10.45 ± 0.64 × 10−8 M s−1) exceeded that for HRP (8.71 × 10−8 M s−1). The mechanism of TMB oxidation in the presence of H2O2 involves the generation of hydroxyl radicals. Cu2O plays a crucial role in activating H2O2 through adsorption on exposed facets, followed by the homolytic cleavage of H2O2. This leads to hydroxyl radical generation, a critical step in the TMB oxidation, followed by the rate-determining H-abstraction pathway. Our computational results revealed that the energy barrier for the rate-determining step of TMB oxidation on {100} facets is exceptionally high at 1.51 eV, resulting in the lowest TMBox production rate on the c-Cu2O surface. In contrast, the {111} facet exhibits the smallest energy barrier of 0.42 eV for the rate-determining step, followed by the {110} facet (0.70 eV). However, the {110} facet has a higher surface CuCUS concentration of 7.74 atoms per nm2 compared to the {111} facet (3.16 atoms per nm2), resulting in more active sites for H2O2 adsorption and dissociation. This leads to a higher TMBox production rate on the {110} facet than on the {111} facet.
Additionally, Cu2O catalysts with specifically exposed facets outperformed commercially available Cu2O, with significantly lower Km values for H2O2 as the substrate (2.9 vs. 44.4 mM). This underscores the excellent substrate affinity of catalysts with singularly exposed facets. These findings offer insights into the facet-dependent enzymatic transformations, potentially paving the way for the future development of artificial enzymes.
Author contributions
AP and ST designed the project. SC, SR, BG and DS performed all experimental work and analyzed the data. LTW and JCJ performed the DFT calculations and analyzed the computational data. AP, JCJ, ST, RD, LTW and SC compiled, reviewed and completed the final manuscript. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AP and ST would like to thank SERB-DST (EEQ/2016/000685), SERB-DST (EEQ/2016/000751), DST-INSPIRE (DST/INSPIRE/04/2015/002674) and SERB-DST, (EMR/2016/002505), Govt. of India for financial support. The computational work has been financially supported by the National Science and Technology Council, Taiwan (NSTC 110-2113-M-011-002-MY3). LTW and JCJ thank the Taiwan National Center of High-Performance Computing (NCHC) for computing resources. SC would like to acknowledge Sikkim University for the fellowship. We would like to thank Dr M. U. Sreekuttan, CECRI Madras Unit, CSIR-Chennai for the XPS.Notes and references
- M. Khajehpour, I. Rietveld, S. Vinogradov, N. V. Prabhu, K. A. Sharp and J. M. Vanderkooi, Proteins: Struct., Funct., Genet., 2003, 53, 656–666 CrossRef CAS PubMed.
- N. Mogharrab, H. Ghourchian and M. Amininasab, Biophys. J., 2007, 92, 1192–1203 CrossRef CAS PubMed.
- G. L. Kedderis and P. F. Hollenberg, J. Biol. Chem., 1983, 258, 8129–8138 CrossRef CAS PubMed.
- H. Kwon, J. Basran, C. M. Casadei, A. J. Fielding, T. E. Schrader, A. Ostermann, J. M. Devos, P. Aller, M. P. Blakeley, P. C. E. Moody and E. L. Raven, Nat. Commun., 2016, 7, 1–6 Search PubMed.
- M. Kathiresan and A. M. English, Chem. Sci., 2017, 8, 1152–1162 RSC.
- G. I. Berglund, G. H. Carlsson, A. T. Smith, H. Szöke, A. Henriksen and J. Hajdu, Nature, 2002, 417, 463–468 CrossRef CAS PubMed.
- M. Ozboyaci, D. B. Kokh, S. Corni and R. C. Wade, Q. Rev. Biophys., 2016, 49, 1–45 CrossRef PubMed.
- A. V. Gribenko, M. M. Patel, J. Liu, S. A. McCallum, C. Wang and G. I. Makhatadze, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 2601–2606 CrossRef CAS PubMed.
- M. D. Mager, V. Lapointe and M. M. Stevens, Nat. Chem., 2011, 3, 582–589 CrossRef CAS PubMed.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- A. Kessler, J. Hedberg, E. Blomberg and I. Odnevall, Nanomaterials, 2022, 12, 1–24 CrossRef PubMed.
- S. Lin, W. Tan, P. Han, X. Li, J. Li, Z. Nie and K. Li, Nano Res., 2022, 15, 9073–9081 CrossRef CAS.
- H. Zhao, Y. Dong, P. Jiang, G. Wang and J. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 6451–6461 CrossRef CAS PubMed.
- R. André, F. Natálio, M. Humanes, J. Leppin, K. Heinze, R. Wever, H. C. Schröder, W. E. G. Müller and W. Tremel, Adv. Funct. Mater., 2011, 21, 501–509 CrossRef.
- L. Han, J. Shi, A. Liu and A. Liu, Sens. Actuators, B, 2017, 252, 919–926 CrossRef CAS.
- Y. S. Mingyun Zhu, Y. Dai, Y. Wu, K. Liu and X. Qi, Nanotechnology, 2018, 29, 0–31 Search PubMed.
- M. K. Masud, J. Kim, M. M. Billah, K. Wood, M. J. A. Shiddiky, N. T. Nguyen, R. K. Parsapur, Y. V. Kaneti, A. A. Alshehri, Y. G. Alghamidi, K. A. Alzahrani, M. Adharvanachari, P. Selvam, M. S. A. Hossain and Y. Yamauchi, J. Mater. Chem. B, 2019, 7, 5412–5422 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- J. Mu, Y. Wang, M. Zhao and L. Zhang, Chem. Commun., 2012, 48, 2540–2542 RSC.
- J. Mu, L. Zhang, G. Zhao and Y. Wang, Phys. Chem. Chem. Phys., 2014, 16, 15709–15716 RSC.
- C. Ray, S. Dutta, S. Sarkar, R. Sahoo, A. Roy and T. Pal, J. Mater. Chem. B, 2014, 2, 6097–6105 RSC.
- B. Yuan, H. L. Chou and Y. K. Peng, ACS Appl. Mater. Interfaces, 2022, 14, 22728–22736 CrossRef CAS PubMed.
- W. Chen, J. Chen, A. L. Liu, L. M. Wang, G. W. Li and X. H. Lin, ChemCatChem, 2011, 3, 1151–1154 CrossRef CAS.
- X. Wang, Q. Han, S. Cai, T. Wang, C. Qi, R. Yang and C. Wang, Analyst, 2017, 142, 2500–2506 RSC.
- A. P. Nagvenkar and A. Gedanken, ACS Appl. Mater. Interfaces, 2016, 8, 22301–22308 CrossRef CAS PubMed.
- A. Chakraborty, D. A. Islam and H. Acharya, Mater. Res. Bull., 2019, 120, 110592 CrossRef CAS.
- A. Hayat, W. Haider, Y. Raza and J. L. Marty, Talanta, 2015, 143, 157–161 CrossRef CAS PubMed.
- H. Li, H. Sun, J. Ding, T. Wu, S. Cai, C. Wang and R. Yang, Microchim. Acta, 2022, 189, 1–10 CrossRef PubMed.
- K. Sobańska, P. Pietrzyk and Z. Sojka, ACS Catal., 2017, 7, 2935–2947 CrossRef.
- X. Xiao, Q. Luan, X. Yao and K. Zhou, Biosens. Bioelectron., 2009, 24, 2447–2451 CrossRef CAS PubMed.
- Y. Yang, Z. Mao, W. Huang, L. Liu, J. Li, J. Li and Q. Wu, Sci. Rep., 2016, 6, 1–7 CrossRef CAS PubMed.
- Z. Tian, J. Li, Z. Zhang, W. Gao, X. Zhou and Y. Qu, Biomaterials, 2015, 59, 116–124 CrossRef CAS PubMed.
- Y. H. Won and L. A. Stanciu, Sensors, 2012, 12, 13019–13033 CrossRef CAS PubMed.
- Z. G. Liu, Y. F. Sun, W. K. Chen, Y. Kong, Z. Jin, X. Chen, X. Zheng, J. H. Liu, X. J. Huang and S. H. Yu, Small, 2015, 11, 2493–2498 CrossRef CAS PubMed.
- Y. Jiang, T. Xia, L. Shen, J. Ma, H. Ma, T. Sun, F. Lv and N. Zhu, ACS Catal., 2021, 11, 2949–2955 CrossRef CAS.
- Q. Ma, J. Young, S. Basuray, G. Cheng, J. Gao, N. Yao and W. Zhang, Nano Today, 2022, 101538, 1–9 Search PubMed.
- C. S. Tan, S. C. Hsu, W. H. Ke, L. J. Chen and M. H. Huang, Nano Lett., 2015, 15, 2155–2160 CrossRef CAS PubMed.
- B. Liu, X. Yao, Z. Zhang, C. Li, J. Zhang, P. Wang, J. Zhao, Y. Guo, J. Sun and C. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 39165–39177 CrossRef CAS PubMed.
- W. C. Huang, L. M. Lyu, Y. C. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261–1267 CrossRef CAS PubMed.
- G. Z. Yuan, C. F. Hsia, Z. W. Lin, C. Chiang, Y. W. Chiang and M. H. Huang, Chem. – Eur. J., 2016, 22, 12548–12556 CrossRef CAS PubMed.
- C. Y. Chu and M. H. Huang, J. Mater. Chem. A, 2017, 5, 15116–15123 RSC.
- Y. A. Wu, I. McNulty, C. Liu, K. C. Lau, Q. Liu, A. P. Paulikas, C. J. Sun, Z. Cai, J. R. Guest, Y. Ren, V. Stamenkovic, L. A. Curtiss, Y. Liu and T. Rajh, Nat. Energy, 2019, 4, 957–968 CrossRef CAS.
- H. E. Rudel, M. K. M. Lane, C. L. Muhich and J. B. Zimmerman, ACS Nano, 2020, 14, 16472–16501 CrossRef CAS PubMed.
- Y. Zhong, Y. Li, S. Li, S. Feng and Y. Zhang, RSC Adv., 2014, 4, 40638–40642 RSC.
- X. Shen, Z. Wang, X. Gao and Y. Zhao, ACS Catal., 2020, 10, 12657–12665 CrossRef CAS.
- X. Wang, M. Chen, Y. He and J. Zhu, J. Alloys Compd., 2014, 628, 50–56 CrossRef.
- D. F. Zhang, H. Zhang, L. Guo, K. Zheng, X. D. Han and Z. Zhang, J. Mater. Chem., 2009, 19, 5220–5225 RSC.
- Y. Shang and L. Guo, Adv. Sci., 2015, 2, 1500140 CrossRef PubMed.
- R. Li, X. Zhang, H. Dong, Q. Li, Z. Shuai and W. Hu, Adv. Mater., 2016, 28, 1697–1702 CrossRef CAS PubMed.
- D. Guo, L. Wang, Y. Du, Z. Ma and L. Shen, Mater. Lett., 2015, 160, 541–543 CrossRef CAS.
- Y. Sui, W. Fu, H. Yang, Y. Zeng, Y. Zhang, Q. Zhao, Y. Li, X. Zhou, Y. Leng, M. Li and G. Zou, Cryst. Growth Des., 2010, 10, 99–108 CrossRef CAS.
- M. C. Biesinger, Surf. Interface Anal., 2017, 49, 1325–1334 CrossRef CAS; C. Wang, H. Tissot, C. Escudero, V. P. Dieste, D. J. Stacchiola and J. Weissenrieder, J. Phys. Chem. C, 2018, 122, 28684–28691 CrossRef.
- J. Y. Ho and M. H. Huang, J. Phys. Chem. C, 2009, 113, 14159–14164 CrossRef CAS.
- Y. Su, H. Li, H. Ma, J. Robertson and A. Nathan, ACS Appl. Mater. Interfaces, 2017, 9, 8100–8106 CrossRef CAS PubMed.
- P. D. Josephy, T. Eling and R. P. Mason, J. Biol. Chem., 1982, 257, 3669–3675 CrossRef CAS PubMed.
- M. Drozd, M. Pietrzak, P. G. Parzuchowski and E. Malinowska, Anal. Bioanal. Chem., 2016, 408, 8505–8513 CrossRef CAS PubMed.
- A. Dalui, B. Pradhan, U. Thupakula, A. H. Khan, G. S. Kumar, T. Ghosh, B. Satpati and S. Acharya, Nanoscale, 2015, 7, 9062–9074 RSC.
- A. Pariyar, S. Gopalakrishnan, J. Stansbery, R. L. Patel, X. Liang, N. Gerasimchuk and A. Choudhury, RSC Adv., 2016, 6, 38533–38540 RSC.
- W. Huang, Acc. Chem. Res., 2016, 49, 520–527 CrossRef CAS PubMed.
- J. Paier, C. Penschke and J. Sauer, Chem. Rev., 2013, 113, 3949–3985 CrossRef CAS PubMed.
- X. L. Cheng, J. Sen Jiang, D. M. Jiang and Z. J. Zhao, J. Phys. Chem. C, 2014, 118, 12588–12598 CrossRef CAS.
- W. Chen, S. Li, J. Wang, K. Sun and Y. Si, Nanoscale, 2019, 11, 15783–15793 RSC.
- S. Kulandaivel, C. H. Lin and Y. C. Yeh, Chem. Commun., 2022, 58, 569–572 RSC.
- H. Wang, X. Ren, Z. Liu and B. Lv, Chem. Commun., 2022, 58, 908–924 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: All experimental details, synthesis of different Cu2O crystals, spectral data, SEM images and computational details are included. See DOI: https://doi.org/10.1039/d3cy01399e |
| ‡ First authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |