Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular gels: a versatile crystallization toolbox
Rafael
Contreras-Montoya
 *a,
Luis
Álvarez de Cienfuegos
*a,
Luis
Álvarez de Cienfuegos
 b,
José A.
Gavira
b,
José A.
Gavira
 c and
Jonathan W.
Steed
c and
Jonathan W.
Steed
 d
d
aNanoscopy-UGR Laboratory, Faculty of Pharmacy, University of Granada, E-18071, Granada, Spain. E-mail: rcm@ugr.es
bDepartamento de Química Orgánica, Unidad de Excelencia Química Aplicada a Biomedicina y Medioambiente (UEQ), Universidad de Granada, E-18071, Granada, Spain
cLaboratorio de Estudios Cristalográficos, Instituto Andaluz de Ciencias de la Tierra (IACT, CSIC), E-18100, Granada, Spain
dDepartment of Chemistry, Durham University, Durham, DH1 3LE, UK
First published on 11th September 2024
Abstract
Supramolecular gels are unique materials formed through the self-assembly of molecular building blocks, typically low molecular weight gelators (LMWGs), driven by non-covalent interactions. The process of crystallization within supramolecular gels has broadened the scope of the traditional gel-phase crystallization technique offering the possibility of obtaining crystals of higher quality and size. The broad structural diversity of LMWGs allows crystallization in multiple organic and aqueous solvents, favouring screening and optimization processes and the possibility to search for novel polymorphic forms. These supramolecular gels have been used for the crystallization of inorganic, small organic compounds of pharmaceutical interest, and proteins. Results have shown that these gels are not only able to produce crystals of high quality but also to influence polymorphism and physicochemical properties of the crystals, giving rise to crystals with potential new bio- and technological applications. Thus, understanding the principles of crystallization in supramolecular gels is essential for tailoring their properties and applications, ranging from drug delivery systems to composite crystals with tunable stability properties. In this review, we summarize the use of LMWG-based supramolecular gels as media to grow single crystals of a broad range of compounds.
Introduction
A major aim of the crystallization of small molecules and macromolecules is structure elucidation through single crystal X-ray diffraction. In the case of biomacromolecules, obtaining crystals has been crucial to unravel the secrets of their biological function, although the recent development of deep learning-based methods, i.e. AlphaFold,1 as well as access to high-resolution structures from cryo-EM has reduced the pressure to obtain high quality crystals.2 For example, knowing the structure of active and allosteric centres of enzymes, allows us to understand how they work in nature and also identifies their malfunctioning by a genetic alteration. The same information can be employed to design drugs to target specific proteins. In the pharmaceutical industry, crystallization is used as a powerful tool to modify the properties of active pharmaceutical ingredients (APIs). Crystal habit and polymorphism of APIs can have a huge impact on important pharmaceutical properties such as stability, dissolution rate, solubility, bioavailability, processing characteristics, compressibility, flowability and surface characteristics, so having control over solid state properties is a key economic and formulation problem.3 Having tools to increase the scope of API polymorph screening, would greatly help the pharmaceutical industry.Obtaining crystals, especially high-quality samples (better optical quality, larger size, and fewer defects), is the result of an optimization process that often proceeds by an iterative trial-and-error process. Due to the great relevance of crystallization, new and improved methodologies have been developed throughout history, with the aim of having a broader and better toolset of techniques. It is already well accepted that reducing convection and gravity effects are, in general, beneficial for obtaining crystals of better quality and larger size.4 It has also been demonstrated that this can be achieved from the use of gelled media without the need to perform experiments under microgravity conditions. Gels are useful environments because inside them, dynamic processes can happen in solution, but thanks to their solid-like nature, crystallization occurs under static conditions. Gels avoid mass transport convection, buoyancy-driven convection flow due to the depletion of solute molecules around growing-crystals, crystal sedimentation and the associated convective mass flow, temperature, or concentration gradients (Marangoni effect), etc.5 Therefore, in gel media, both nucleation and growth, proceed smoothly as they are governed by the mass transport of the reactants providing an ideal environment for growing crystals of high quality for structure determination.
Since the gel surface is an additional component in the crystallization media, its chemical composition may influence crystal nucleation, growth or both processes. The effect of silica gel as a nucleation inhibitor6 or the promoter action of agarose7 has already been described and characterized in the case of macromolecular crystallization.6 Those studies are less common in the case of inorganic or small molecule crystallization.8
Gels can be found in day-to-day life in different forms such as gelatin jelly, pen ink, shampoo, etc. Although solid-like in appearance, they have useful properties of both liquids and solids. Gels can be defined as composite materials resulting from the combination of a solid (gelator) capable of generating a three-dimensional network that retains the flow of a much larger quantity of fluid due to surface tension. They are solid-like materials from a mechanical point of view, consisting almost exclusively of a fluid. While most gels used industrially are made with polymeric gelators (e.g., polyethylene glycol, silica, polyacrylamide), there is another increasingly important group of gels composed of low molecular weight gelators (LMWGs).9,10 These are a special class of molecules defined by their ability to self-assemble through non-covalent or supramolecular interactions (e.g., hydrogen bonds, solvophobic interactions, π–π stacking), generating three-dimensional lattices yielding gels. LMWGs based gels are also known as supramolecular gels and are relatively new materials. They have shown numerous potential applications due to, among other qualities, their tremendous versatility.11 Unlike polymers, LMWGs can be designed to gel different organic or aqueous solvents under the application of multiple stimuli,12,13 can be tailored to present catalytic,14 optical and chiroptical activities,15–17 to obtain composite or hybrid materials,18–23 and to be biocompatible or have therapeutic activity.24–28
Recently, supramolecular gels have been introduced in the field of crystallization. Their small molecule-based composition has allowed highly sought-after properties such as easy crystal harvesting from gel-based crystallization media, observation of the influence of matrix's chirality over protein crystallization or have control over polymorphism of APIs among others. Because of their virtually unlimited versatility, they have the potential to be a crystallization toolbox that will allow the selection of gel properties and specific functionalities to match the compound and crystallization conditions of interest. Thanks to the broad structural diversity of gelator molecules, the ability to gel different solvents increases the physicochemical environments available for crystallization.
In this review, we summarize the progress in the use of supramolecular gels based on LMWGs as media to promote crystallization, emphasizing the importance of the supramolecular nature of the gels. We focus particularly on single crystal growth, excluding nanoparticles or nanostructured materials grown in supramolecular gels, for which several review articles have been published.29–31 Unlike previous reviews,26,32,33 herein, we have tried to describe all types of molecules that have been crystallized in these types of supramolecular gels: inorganic solids, small organic molecules, and proteins. This broader overview makes it easier for the reader to find particular characteristics, similarities and advantages of using LMWG for crystallization. All gelators successfully employed in crystallization featured in this review article, have been compiled into tables as a guide for the reader (Tables 1–3). A last critical section has been included, as a way to frame the current trend of crystallization in supramolecular gels and the role it may play in the future.
Crystallization of inorganic solids
| Gelator | Solvents | Crystallized substances | Main observations over crystallization | Ref. |
|---|---|---|---|---|
|
Water | Calcite (CaCO3) | Reinforcement of crystals | 38 |
|
Water | NaCl, KCl, KI | High aspect ratio wires of NaCl, KCl and KI | 41 |
|
Acetonitrile | Fullerene (C60) | Super-long 1D fullerene crystals with tunable lengths and diameters. Easy crystal harvesting by dissolving gel matrix. | 42 |
| Large area 2D fullerene crystals. Easy crystal harvesting by dissolving gel matrix. | 43 | |||
|
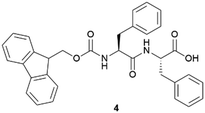
Water | ZIF-8, MOF-808 (MOFs) | Fibres exert strong control over the size of ZIF-8 crystals. MOF-808 grown on these hydrogels showed enhanced catalytic activity. | 44 |
| Gelators | Solvents | Crystallized substances | Main observations over crystallization | Ref. |
|---|---|---|---|---|
|
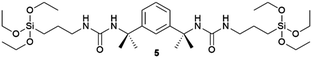
Toluene | Carbamazepine | Larger crystal size and well-defined faces. Easy crystal harvesting by dissolving gel matrix. | 48 |
|
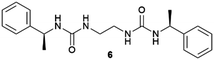
Acetonitrile | Aspirin | Crystals in gel-phase and not in solution. Easy crystal harvesting by dissolving gel matrix. | 48 |
|
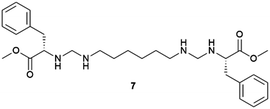
Toluene | 2-Hydroxybenzyl alcohol | Larger crystal size. Easy crystal harvesting by dissolving gel matrix. | 48 |
Acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene chloroform toluene chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene DMSO toluene DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water water |
Piroxicam | Polymorph selection. Easy crystal harvesting by dissolving gel matrix. | 48 | |
|
Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water water |
Carbamazepine | Control over crystal habit. Easy crystal harvesting by dissolving gel matrix. | 48 |
|
Toluene | Aspirin, indomethacin, caffeine, carbamazepine | Easy crystal harvesting by dissolving gel matrix. | 53 |
|
Toluene | 6,13-Pentacenequinone, 9,10-bis-phenylethyl-anthracene, N,N,N′,N′-tetraphenylbenzidine, triphenylamine | Easy crystal harvesting by dissolving gel matrix. | 54 |
|
o-Xylene | Cis-platinum (Cis-Pt) | New interfacial crystallization technique. New crystal habit obtained. New dimethylacetamide solvate of Cis-Pt | 56 |
|
Hexylamine and decylamine | Caffeine, carbamazepine | Non-acid substrates (caffeine and carbamazepine) crystallized and acid ones (aspirin and indomethacin) inhibit gelation and crystallization. | 57 |
|
1,2,4-Trichlorobenzene | Paracetamol, fenbufen, monobenzone, chlorphenesin | Proof-of-principle: calyxarene-based gels to obtain crystals of drugs containing hydrophobic residues. New polymorph of chlorphenesin. Easy recovery of crystals by collapsing gel through addition of KPF6. | 58 |
|
Toluene | 5-Methyl-2-[(2-nitrophenyl)-amino]-thiophene-3-carbonitrile (ROY) | Heteronucleation induced by specific interactions between gelator and crystallization substrate allowing a selection of polymorphs obtained. | 59 |
|
1,2-Dibromoethane | Mexiletine hydrochloride | Only known route to access high-temperature-stable form 2 at room temperature. | 63 |
|
DMF | Mexiletine hydrochloride | Type A solvate not found in DMF solution | 63 |
|
8 organic solvents | Mexiletine hydrochloride | Same polymorphs than in solution | 63 |
|
10 organic solvents | Isoniazid | New tool developed for isoniazid polymorphism screening. | 64 |
|
Multitude of organic solvents | Theophylline, sulfathiazole, sulfamerazine, ninfumic acid | Kinetic form I of sulfathiazole obtained in 1-propanol gel and thermodynamic form II obtained in solution | 66 |
|
Dichloromethane | Theophylline, sulfamerazine, sulfathiazole, flufenamic acid | Selection of thermodynamic form III of flufenamic acid over the form IV obtained in solution. | 67 |
|
Nitrobenzene | Metronidazole | Different crystal habit found in non-mimetic gel (plate-like shape) than in mimetic-gel phase (needle shape) | 68 |
|
Methanol | Vitamin C, isonicotinamide | Control over the stoichiometry of vitamin C and isonicotinamide co-crystals. Easy crystals harvesting by adding triethylamine | 71 |
|
DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water water |
Caffeine, carbamazepine, piroxicam | Different size, habit and/or polymorphism than in solution. Strong influence over crystalline parameters in function of gel composition | 73 |
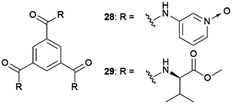
|
Water | Cu(II) isonicotinate-N-oxide | Block-shaped crystals in solution and agarose gel while plate-like in supramolecular gel. Selection of form-I crystals only within non-tailored gel | 74 |
|
Ethanol, 1-butanol, 1,4-butane-diol, 1-pentanol, nitrobenzene, nitromethane, cyclohexanone | Barbital, thalidomide | Concomitant crystallization of both barbital and thalidomide avoided within drug-mimetic gels | 75 |
| Gelators | Crystallized proteins | Main observations over crystallization | Ref. |
|---|---|---|---|
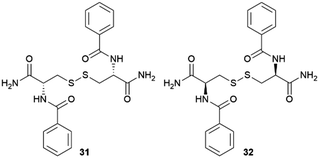
|
Lysozyme | Firsts model protein crystallized in supramolecular hydrogels | 77 |
| Compatibility with batch crystallization producing the best diffracting crystals. | 78 | ||
| Glucose isomerase | New orthorhombic polymorph selectively obtained only in the D enantiomer (32) | 77 | |
| Compatibility with batch crystallization producing the best diffracting crystals. | 78 | ||
| Fomamidase (B. cereus) | The highest quality crystals in the D enantiomer (32) | 77 | |
| Two different polymorphs in each enantiomer, P622 (31) and C121, respectively (32). | 78 | ||
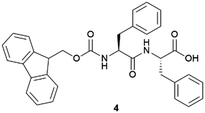
|
Lysozyme | Firstly crystallized in commercially available 4 | 78 |
| First reported inclusion of a hydrophobic material, SWCNTs, in protein crystal. First conversion of protein crystals in conductor. Enzymatically active crystal over 85 °C. | 19 | ||
| Glucose isomerase | Firstly crystallized in commercially available 4. Batch crystallization | 78 | |
| Fomamidase (B. cereus) | Firstly crystallized in commercially available 4 | 78 | |
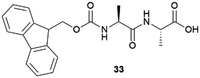
|
Lysozyme | Firstly crystallized in commercially available 33. Slow dissolution rate | 78 |
| Glucose isomerase, formamidase (B. cereus), thaumatin | Firstly crystallized in commercially available 33 | 78 | |
| Insulin | Firstly crystallized in commercially available 33. Dissolution rate and thermal stability enhanced tested in vivo | 87 | |
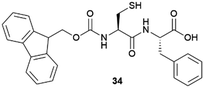
|
Lysozyme | Demonstration of the free-radical scavenger effect. Protection against local radiation damage | 81 |
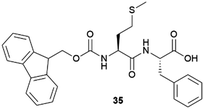
|
Lysozyme, glucose isomerase, thaumatin | Induction nucleation effect as a function of concentration. Remarkable effect in the case of thaumatin | 79 |
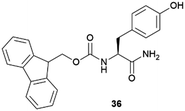
|
Lysozyme | Firstly crystallized using a single Fmoc-Y (36). Top resolution limit, 1.0 Å | 79 |
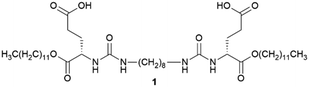
The first known example of a supramolecular gel appeared in the mid-19th century,34 but it was relatively recently that interest in this kind of material has exploded.35 In 2000, Weiss and Abdallah,36 and Estroff and Hamilton,37 predicted the use of supramolecular organogels and hydrogels (respectively) as crystallization media. This latter group, reported in 2004 the first crystallization performed in a supramolecular gel.38 The authors grew calcite (CaCO3) crystals within a supramolecular hydrogel made with 1 (Fig. 3a). They observed that the gel matrix actively participates in the crystallization process, influencing nucleation and crystal growth. This influence is related to the previously known ability of 1 to bind Ca2+.39 Occlusion of the gel fibres within the crystals40 yielded unique surface features (surface etching) and altered their dissolution behaviour making them more soluble. The authors also pointed out that this kind of material could be used as a model to gain insights into how mineralization happens in Nature. Notably, in this study the gelator was chosen to be an active participant in the crystallization process, not only an inert spectator. This was possible thanks to its low molecular weight nature which allowed the selection of the precise chemical functionalities needed, something that is difficult to achieve using traditional macromolecular gels.
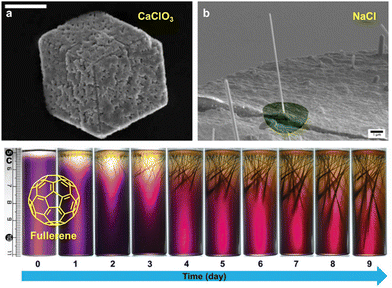 | ||
| Fig. 3 (a) SEM image of a calcite crystal obtained within hydrogels of 1 after 24 h with etched surface provoked by the occlusion of the gel matrix, scale bar 20 μm (Adapted with permission from ref. 38. Copyright 2004 Royal Society of Chemistry). (b) SEM image high aspect NaCl nanowires grown on the surface of Eu3+ cross-linked gel 2 (Adapted with permission from ref. 41. Copyright 2013 American Chemical Society). (c) Photographs of the two-layer counter-diffusion crystallization of C60 within m-xylene gels of 3 over 9 days triggered by the addition of acetonitrile (Adapted with permission from ref. 42. Copyright 2019 John Wiley and Sons). | ||
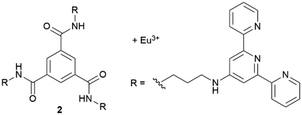
Gunnlaugsson's group have reported the growth of NaCl, KCl and KI nanowires from supramolecular gels (Fig. 3b).41 The gels they employed were made of fibres of self-assembled 2 cross-linked with europium(III). They show that the supramolecular gel stabilizes the growth of these wires by facilitating a diffusion-driven base growth mechanism. Such high aspect-ratio wires were only obtained using the supramolecular hydrogel and were not detected in non-supramolecular standard gels (LAPONITE® and Carbopol).
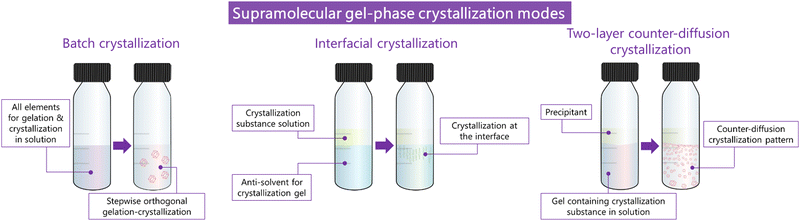
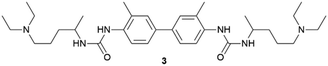
Supramolecular gels have also proven to be useful as media to crystallize carbon materials as Liu and co-workers demonstrated producing super-long crystalline fibres of fullerene C60.42 The authors obtained m-xylene-based supramolecular organogels containing fullerene in solution, using the bis(urea)-based supramolecular gelator 3 specifically designed to not interact with C60. A two-layer counter-diffusion crystallization was employed (Fig. 2) where the crystallization was triggered by the gradual diffusion of acetonitrile which act as antisolvent provoking the supersaturation of fullerene. The authors were able to obtain 1D long fullerene crystals with tunable lengths and diameters (Fig. 3c). In a more recent work, the same group followed a similar strategy to obtain large-area 2D crystals of C60 for the fabrication of high-performance photodetectors.43 To achieve this, the antisolvent was slowly diffused through the gel via vapor diffusion. Tunning the temperature and the concentration of fullerene and gelator, allowed that the immobilized solvent molecules drove the crystal growing through the most favourable crystallographic plane {220}. It is worth highlighting that in both works, the obtained crystals were easily collected through just the dissolution of the gel matrix by adding methanol in which the fullerene is completely insoluble. These beautiful crystallization strategies are possible thanks to the supramolecular nature of the gel: (i) the structure of the gelator was designed ad hoc thanks to its LWMG nature, (ii) the gelator is able to gel the two organic solvents needed to act as solvent and antisolvent, and (iii) the gel becomes soluble just by adding a solvent in which the crystal is insoluble, allowing simple and clean crystal harvesting (see below).
Recently, the Álvarez de Cienfuegos group has used short-peptide supramolecular hydrogels for the growth of ZIF-8 (Zn(mIm)2, mIm = 2-methylimidazolate) metal–organic frameworks (MOFs).44 Similar to the strategy used for the growth of calcite crystals mentioned above, these authors used a short-peptide, Fmoc–PhePhe (fluorenylmethoxicarbonyl–diphenylalanine) (4), able to interact and self-assemble with Zn2+ cations needed to form the ZIF-8. Different crystallization protocols were assayed but the best in this case was a diffusion protocol, in which the HmIm (2-methylimidazole) was allowed to diffuse on top of the hydrogel containing the Zn2+ salt, and an in situ MOF growth by simultaneous gel formation.45 Both processes promoted the formation of ZIF-8 particles that required a lower ratio of HmIm versus Zn, making these protocols more efficient. The same hydrogel was also used for the in situ growth of MOF-808 using Zr6 oxoclusters as seeds. MOF-808 obtained in this hydrogel showed improved catalytic activity toward the degradation of methyl paraoxon.
Crystallization of small organic molecules
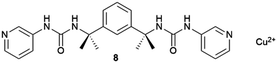
Supramolecular gels are often thought of as metastable forms. Actually, it has been recently observed that a supramolecular gel-to-crystal transition, consistent with Ostwald's rule of stages, occurs during the organogelation of a mono-iodinated 2,4,5-triphenylimidazole (lophine) derivative (I-TPI), in which the gel emerges as a kinetically trapped intermediate, prior to the crystallization of a series of increasingly stable methanol solvates (Fig. 1).46 The gel-to-crystal transition of supramolecular organogels made from a dipeptide functionalized with azide and alkyne at its termini allowed the formation of different polymorphs, depending on whether crystallization occurred in solution or from the gel phase and also on the solvent. Polymorphs of a polymer yielded from topochemical polymerization of the different polymorphic crystals.47 However, the first deliberate attempt to crystallize molecular crystals within supramolecular gels was reported by Steed's group in 2010.48 This work was presented as a proof-of-concept to explore the potential of supramolecular gels as crystallization media and how they can influence crystal growth. They employed bis(urea) based organogels 5, 6, 7 chosen because of their ability to gel several organic solvents, and bis(urea) metallogel 8 which gels when complexed with Cu2+, as media for crystallizing non-drugs and drugs. Prior to this work, gel phase crystallization of organic molecules was only achieved in macromolecular-based or clay-like hydrogels.49 Supramolecular gels opened the door to the use organic solvents, significantly expanding the range of gel-based polymorph screening tools. The use of organic solvents in the gel-phase allowed the preparation of carbamazepine (CBZ) crystals grown inside toluene gels of 5 (Fig. 4a) exhibiting more well-defined crystal faces and significantly larger sizes than those in non-gel crystallization control experiments, as well as the isolation of the high temperature Form I. A similar phenomenon occurred with 2-hydroxybenzyl alcohol (HBA) crystals within toluene gels of 7. The possibility of tuning the gel's chemical composition gives an inert matrix that does not interact with the solute triggering homogenous nucleation in a confined environment yielding larger crystals. Alternatively, the gelator can be chosen to interact with the substrate to promote heterogeneous nucleation. In this manner, aspirin (ASP) crystals were produced in acetonitrile gels of 6 (Fig. 4b), not observed in acetonitrile solution. Something very interesting derived from this work was the observation of a selection of polymorphs of the same substance depending on the composition of the supramolecular gel, as was the case of piroxicam (PIR) crystallized in 7-based gels in different solvents. The same happened with crystal habit, with different morphologies of CBZ arising from methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) copper metallogels of 8 depending on the copper content of the gel. The non-covalent nature of supramolecular gels implies that they are inherently reversible, thus the application of a stimulus to provoke the gel-to-sol transition is feasible, which facilitates harvesting of the crystals. In the case of urea-based organogels, the transition can be triggered by the addition of acetate anions,50 allowing the recovery of clean crystals (Fig. 4c). In this work, both self-assembly processes, gelation, and crystallization, are orthogonal,51,52 so an effective phase separation between gel and crystal occurred, which made crystallization possible (Fig. 1).
1) copper metallogels of 8 depending on the copper content of the gel. The non-covalent nature of supramolecular gels implies that they are inherently reversible, thus the application of a stimulus to provoke the gel-to-sol transition is feasible, which facilitates harvesting of the crystals. In the case of urea-based organogels, the transition can be triggered by the addition of acetate anions,50 allowing the recovery of clean crystals (Fig. 4c). In this work, both self-assembly processes, gelation, and crystallization, are orthogonal,51,52 so an effective phase separation between gel and crystal occurred, which made crystallization possible (Fig. 1).
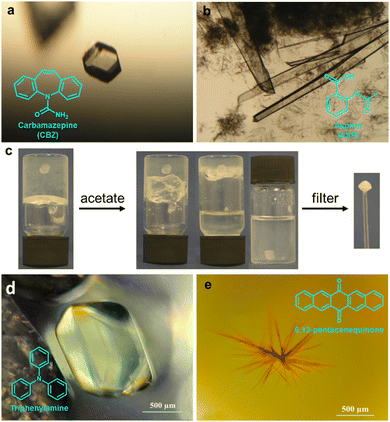 | ||
Fig. 4 (a) Crystal form III of CBZ obtained in 6 wt% of 5 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 chloroform 9 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (×25 magnification). (b) ASP crystals grown from 0.3 wt% acetonitrile gel of 6. (c) Recovery of a single crystal of CBZ form III obtained within 1 toluene (×25 magnification). (b) ASP crystals grown from 0.3 wt% acetonitrile gel of 6. (c) Recovery of a single crystal of CBZ form III obtained within 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 chloroform 9 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene gel of 7. (Adapted with permission from ref. 48. Copyright 2010, Springer Nature). (d) and (e) Organic semiconductor triphenylamine and 6,13-pentacenequinone grown in an 11-toluene gel (Adapted with permission from ref. 54. Copyright 2016 American Chemical Society). toluene gel of 7. (Adapted with permission from ref. 48. Copyright 2010, Springer Nature). (d) and (e) Organic semiconductor triphenylamine and 6,13-pentacenequinone grown in an 11-toluene gel (Adapted with permission from ref. 54. Copyright 2016 American Chemical Society). | ||
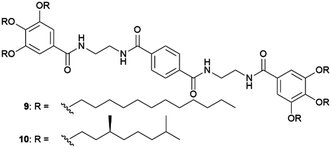
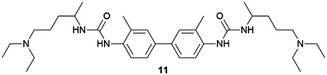
The possibility of harvesting crystals in gel-phase crystallization by dissolving the gel matrix without the use of heating is potentially a great advantage. Sánchez and co-workers used this idea to collect crystals of different APIs (ASP, indomethacin (IND), caffeine (CAF), and CBZ) obtained within tetraamide 9 and 10 toluene-based organogels, just by adding more toluene.53 In another example, this strategy was employed to recover crystals of organic semiconductors (6,13-pentacenequinone, 9,10-bis-phenylethyl-anthracene, N,N,N′,N′-tetraphenylbenzidine and triphenylamine) crystallized in toluene-base gels of the bis-urea derivative 11via dissolving the gel by adding a liquid organic acid (Fig. 4d and e).54
Supramolecular gels for polymorph screening.
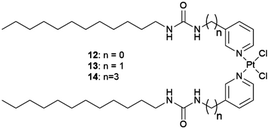
Supramolecular gels offer the possibility of designing gelators that match or mimic the structure of the crystallized substance favouring, matrix–substrate interactions which drives the crystallization process. In 2015, Steed's group suggested this possibility55 and developed a targeted supramolecular gel as a media to crystallize the anticancer drug Cisplatin (Cis-Pt).56 Crystallization was performed by diffusing a DMF solution of Cis-Pt into o-xylene gels of the targeted gelators 12–14 and in parallel in a generic supramolecular gelator. This gel–sol interfacial crystallization technique (Fig. 2) allowed the preparation of crystals by placing a solution of the molecule on a supramolecular gel made of a different solvent in which the substrate is insoluble. As the gel is already formed when the substrate is added, this strategy avoids heating the substance to be crystallized and opens the window to adjusting the solubility in a gel-phase crystallization. In all of gels studied, yellow plate-like triclinic DMF solvate crystals of Cis-Pt were obtained, but only yellow hexagonal plate-like crystals were observed using gelator 14. This new habit for Cis-Pt suggests selective adsorption of the gelator on the fastest-growing face of the crystals. Using dimethylacetamide (DMA) instead of DMF, high-quality yellow rectangular plate-like single crystals were observed only using gelator 14 (Fig. 5a). In contrast, in the other o-xylene-based gels studied, a mass of small microcrystals was obtained suggesting nucleation-control exerted by the targeted gel 14. Single crystal X-ray diffraction revealed that the crystals obtained with 14 diffusing DMA were a new 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
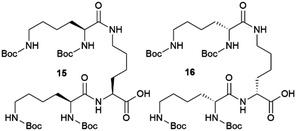
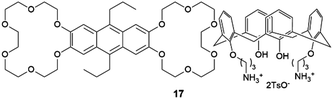
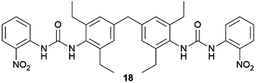
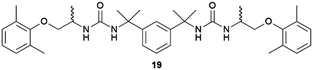
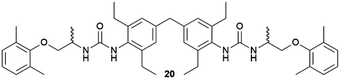
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 solvate form of Cis-Pt incorporating one DMA molecule per two Cis-Pt molecules. Also, in 2015 Sánchez and coworkers reported the crystallization of a range of drugs using multi-component gels based on lysine-based dendrons (15, 16) and alkyl amines.57 This work established the active role of all the components of the mixture with observation of both enhancement and antagonistic effects. The following year, Steed reported a more general approach to small-molecule pharmaceutical crystallization involving both one- and two-component gels based on calix[4]arenes (17). The concept was the inclusion of molecules of a hydrophobic crystallization target within the calixarene cavity exposed on the surface of the gel fibre that will be exposed to the second component. This proof of principle study was limited by the range of solvents gelled but did result in the crystallization of the agrochemical chlorphenesin. In the case of the two-component gel, gelation could be reversed by complexation of the crown ether-based linker with K+ (Fig. 5b).58 The idea of using specifically targeted gelators to influence crystallization for polymorphism screening was further explored by Steed's group by preparing gelators mimicking the structure of the molecule 5-methyl-2-[(2-nitrophenyl)-amino]-thiophene-3-carbonitrile, also known as ROY (red-orange-yellow).59 ROY is the precursor of the antipsychotic drug olanzapine and has claimed the record as the molecule with the most polymorphs known (currently 12 single crystal structures and two additional less well characterised phases).60,61 The targeted bis(urea) gelator 18, which includes the o-nitroaniline-derived functional group found in ROY, was designed (Fig. 5c), intended to self-assemble into fibres which expose a locally ordered array of molecular features mimicking a part of ROY. This distribution was designed to act as a template to drive the epitaxial overgrowth of metastable or hard-to-nucleate solid forms specifically. Crystallization experiments in toluene solution or within other non-targeted supramolecular toluene-based gels, yielded large yellow blocks identified as the thermodynamically most stable monoclinic Y form (Fig. 5d). However, when the experiment was performed at the same ROY concentration within 18-based toluene gels produced red crystals corresponding to the metastable, triclinic form R (Fig. 5e). The heteronucleation induced by the specific interaction of the solute with matching groups periodically distributed through the fibres, allowed the selection of the polymorph obtained. These results prove the potential of designed supramolecular gels to mimic the crystallization substrate's structure as a way to influence polymorphism. This gelator is now commercially available as a polymorph screening aid. The crystallization of ROY has also been studied using polymer microgel particles.62 The microconfinement provided by the polymer gel environment was found to significantly improve crystallization selectivity in a range of systems, including ROY. The targeted approach has also been applied to the very complex polymorphism of the heart antiarrhythmic drug mexiletine hydrochloride.63 This is a particularly complex system both because of the wide range of polymorphs formed and because it exists as a salt with the potential of the chloride anion to bind to the gelator. The use of mexiletine-derived bis(urea) gelators (19–21) resulted in a range of metastable forms produced under conditions that differ from solution and most strikingly, the gels gave rise to the high-temperature-stable form 2 (gelator 19 in 1,2-dibromoethane): the only known route to access this form at room temperature.
2 solvate form of Cis-Pt incorporating one DMA molecule per two Cis-Pt molecules. Also, in 2015 Sánchez and coworkers reported the crystallization of a range of drugs using multi-component gels based on lysine-based dendrons (15, 16) and alkyl amines.57 This work established the active role of all the components of the mixture with observation of both enhancement and antagonistic effects. The following year, Steed reported a more general approach to small-molecule pharmaceutical crystallization involving both one- and two-component gels based on calix[4]arenes (17). The concept was the inclusion of molecules of a hydrophobic crystallization target within the calixarene cavity exposed on the surface of the gel fibre that will be exposed to the second component. This proof of principle study was limited by the range of solvents gelled but did result in the crystallization of the agrochemical chlorphenesin. In the case of the two-component gel, gelation could be reversed by complexation of the crown ether-based linker with K+ (Fig. 5b).58 The idea of using specifically targeted gelators to influence crystallization for polymorphism screening was further explored by Steed's group by preparing gelators mimicking the structure of the molecule 5-methyl-2-[(2-nitrophenyl)-amino]-thiophene-3-carbonitrile, also known as ROY (red-orange-yellow).59 ROY is the precursor of the antipsychotic drug olanzapine and has claimed the record as the molecule with the most polymorphs known (currently 12 single crystal structures and two additional less well characterised phases).60,61 The targeted bis(urea) gelator 18, which includes the o-nitroaniline-derived functional group found in ROY, was designed (Fig. 5c), intended to self-assemble into fibres which expose a locally ordered array of molecular features mimicking a part of ROY. This distribution was designed to act as a template to drive the epitaxial overgrowth of metastable or hard-to-nucleate solid forms specifically. Crystallization experiments in toluene solution or within other non-targeted supramolecular toluene-based gels, yielded large yellow blocks identified as the thermodynamically most stable monoclinic Y form (Fig. 5d). However, when the experiment was performed at the same ROY concentration within 18-based toluene gels produced red crystals corresponding to the metastable, triclinic form R (Fig. 5e). The heteronucleation induced by the specific interaction of the solute with matching groups periodically distributed through the fibres, allowed the selection of the polymorph obtained. These results prove the potential of designed supramolecular gels to mimic the crystallization substrate's structure as a way to influence polymorphism. This gelator is now commercially available as a polymorph screening aid. The crystallization of ROY has also been studied using polymer microgel particles.62 The microconfinement provided by the polymer gel environment was found to significantly improve crystallization selectivity in a range of systems, including ROY. The targeted approach has also been applied to the very complex polymorphism of the heart antiarrhythmic drug mexiletine hydrochloride.63 This is a particularly complex system both because of the wide range of polymorphs formed and because it exists as a salt with the potential of the chloride anion to bind to the gelator. The use of mexiletine-derived bis(urea) gelators (19–21) resulted in a range of metastable forms produced under conditions that differ from solution and most strikingly, the gels gave rise to the high-temperature-stable form 2 (gelator 19 in 1,2-dibromoethane): the only known route to access this form at room temperature.
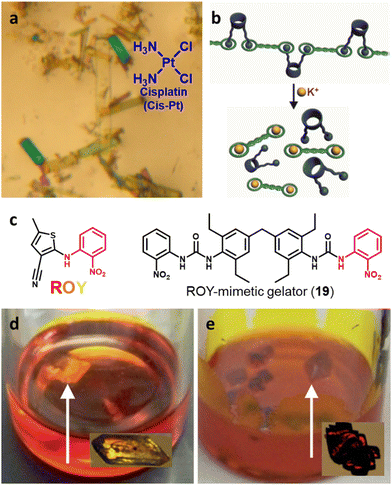 | ||
| Fig. 5 (a) Rectangular plate-like DMA solvate crystals of Cis-Pt obtained adding Cis-Pt dissolved in DMA over gels made of 14 in o-xylene (90× magnification) (Adapted with permission from ref. 56. Copyright 2015 American Chemical Society). (b) Schematic representation of the K+-mediated disassembly of 1,2,4-trichlorobenzene-17 gel (Adapted with permission from ref. 58. Copyright 2016 Royal Society of Chemistry). (c) Chemical structures of ROY and a ROY-mimetic bis(urea)-based gelator 18 synthetized to influence ROY's crystallization. (d) Y (yellow) form of ROY obtained within toluene non-tailored gel. (e) R (red) form of ROY obtained inside a toluene gel of ROY-mimetic gelator 18 (Adapted with permission from ref. 59. Copyright 2017 Royal Society of Chemistry). | ||
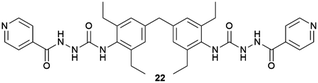
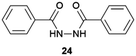
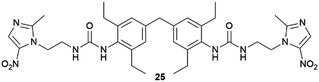
Supramolecular gels have also been employed as a versatile polymorphism-screening toolbox to test the supposed monomorphism of the drug used in the treatment of tuberculosis, isoniazid.64 Crystallization experiments were performed with targeted gels made of 22 prepared in several solvents, in parallel with microemulsion. As a result of this study, some significant differences in terms of crystal habit and crystal size were observed between experiments. However, the one known polymorph of Isoniazid was observed in all cases, and it was the alternative technique of melt crystallization that revealed two new forms.65 Another example of an effect of the supramolecular gel over polymorphism was observed when the drug sulfathiazole was crystallized in pH-reversible 23 gels obtaining kinetic form I, in comparison with solution experiments where thermodynamically stable form II was obtained.66 Closely related results were recently observed for the crystallization of flufenamic acid.67 Its crystallization in dichloromethane gels of 24 inhibited nucleation the thermodynamic form IV obtained in solution, allowing the selection of the thermodynamic form III. Tailored gels have also been used68 to modify drug crystal habit as in the use of gels with functional groups similar to the antibiotic metronidazole (25), which gave rise to habit changes implying surface adsorption of the gem on the drug while control compounds did not influence the crystal shape (Fig. 6a).
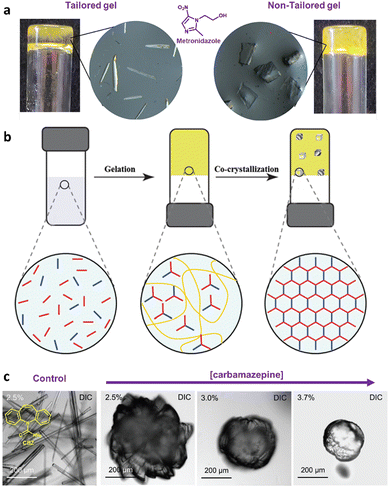 | ||
Fig. 6 (a) Different crystal habits of metronidazole obtained in tailored (needles) or non-tailored gels (plate-shape) (Adapted with permission from ref. 68. Copyright 2021 American Chemical Society). (b) Schematic representation of the orthogonal self-assembly of vitamin B9 gelation and vitamin C and isonicotinamide crystallization (Adapted with permission from ref. 71. Copyright 2016 Royal Society of Chemistry). (c) Differential interference contrast (DIC) microscopy images of needle-like crystals of CBZ crystals grown in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution and near-spherical shape crystals within TA–TiIV gels. Gel-phase grown crystals are more spherical as drug concentration increases (Adapted with permission from ref. 73. Copyright 2018 John Wiley and Sons). water solution and near-spherical shape crystals within TA–TiIV gels. Gel-phase grown crystals are more spherical as drug concentration increases (Adapted with permission from ref. 73. Copyright 2018 John Wiley and Sons). | ||
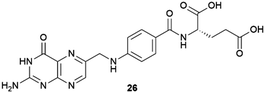
The co-crystallization of APIs is employed in the pharmaceutical industry for the development of new drug formulations with more suitable physical and pharmacological properties.69,70 In co-crystals, one of the most relevant parameters is their stoichiometry, and having control over this is still a tremendously difficult task. There is a need for new methodologies to exert such control. As seen in previous sections, the versatility of supramolecular gels makes them excellent candidates for this purpose. Xuenfeng Mei and co-workers were the first to explore this.71 They prepared pH-switchable vitamin B9 (26) gels in methanol and used them as media to crystallize and control the stoichiometry of vitamin C and isonicotinamide co-crystals. This success is attributed to the gel phase crystallization (Fig. 6b), which slows down diffusion providing a homogeneous growth medium, and to the local ordering of the fibres which potentially drives the nucleation of co-crystals. An additional advantage of the supramolecular nature of the vitamin B9 gel is that crystals obtained within, can be easily harvested just adding triethylamine to dissolve the gel and filtering. The gel can also be easily recycled to repeat the process just adding the solutes and acetic acid to regenerate the gel.
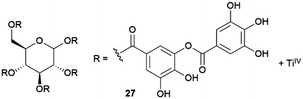
Caruso and co-workers described the possibilities of their versatile supramolecular metallogels made by complexation of naturally occurring tannic acid with titanium(IV) (TA–TiIV gels, 27)72 as API crystallization media.73 TA–TiIV are robust gels which can be obtained in several solvents including water. They can be prepared including additives and their mechanical properties can be easily tuned changing the concentration and stoichiometry. These gels can also be disassembled in mild conditions just adding a competing ligand such as pyrocatechol. The authors studied the crystallization of CAF, CBZ and PIR, and observed that the size, habit and/or polymorphism of crystals grown within gels were different from those grown in solution, and that parameters could be modulated by varying the gel composition. For CAF they observed a far more uniform distribution of needle-like crystals than in solution at room temperature. However, when the crystallization was performed at 4 °C, crystals of completely different morphology than in solution, and in function of the proportion of TiIV in the gel-phase, were observed. While thin needle-like crystals were observed in solution, in the gel-phase, hair-like structures on rod cores, leaf-like and blade-like crystals were obtained as the proportion of TiIV was increased. In the case of CBZ, the matrix exerted a stronger influence on the crystal morphology (Fig. 6c). While needle-like crystals were obtained in solution, near-spherical aggregates of densely stacked plate-like crystals were observed in gel-phase. In both cases, dihydrate and form III polymorphs were detected, but form III was the dominant phase in control and the dihydrate one in TA–TiIV gel matrix. A significant influence over crystal habit and polymorphism was also observed for PIR. Globular aggregates of thin needle and block type crystals were obtained in solution and gel-phase respectively. In this case, bigger crystals were obtained as the PIR concentration was increased. This behaviour can be explained by a different interaction with the gel matrix. Regarding polymorphism, only form I of PIR was detected in the control experiment, and a mixture of form I and form B, being form B the dominant one, were obtained in gel-phase.
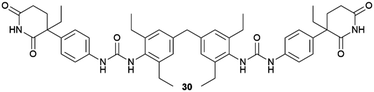
The crystallization of the coordination complex Cu(II) isonicotinate-N-oxide was studied in a tailored (28) and a non-tailored (29) supramolecular gel, and in comparison with solution and in agarose gel.74 When the crystallization was performed in aqueous media, only the blue form I of this complex was obtained. Crystals obtained in aqueous solution and in agarose gel were block-shaped, whereas in both supramolecular gels, plate-like crystals were obtained. The experiments performed in ethanol/water mixtures, only allowed in solution and in the non-tailored gel, resulted in a selection of crystal forms: in solution, a mixture of green form II and blue form I was observed, and only form I was detected in gel 29. The possibility of tailoring supramolecular gels has also been explored to exert control over the polymorphism of drug barbital crystals to avoid undesired concomitant crystallization.75 Simultaneous crystallization of different polymorphs (concomitant crystallization) is a major inconvenient in pharma industry, where obtaining a pure single form of a drug is crucial. Barbital (BAR) is known to crystallize concomitantly in three polymorphs (i.e., forms I, III and V).76 Its gel-phase crystallization within a supramolecular gel bearing the drug-mimetic imide group that occurs in barbiturates 30 in a multitude of solvents, allowed to obtain the kinetic crystal form III exclusively avoiding concomitant crystallization of the other forms. No selection was observed in sol-phase crystallization or within another non-mimetic supramolecular gel, indicating that the interaction of the drug with the imide moiety at the gel fibre surface might increase the nucleation rate of the kinetic form. Similar results were obtained for the crystallization of thalidomide in nitromethane solution, where concomitant crystallization of polymorphs α and β were obtained and a selective crystallization of form α within nitromethane-30 organogel.
Protein crystallization
In 2015 Álvarez de Cienfuegos and Gavira together with their teams, presented the first protein crystallization within supramolecular gels.77 Two short peptide-based supramolecular hydrogels 31 and 32 were tested as media for crystallization of two model proteins, hen egg-white lysozyme (HEWL) and glucose isomerase, and one target protein, a formamidase extracted from B. cereus. Following the same logic as that for small molecules discussed above, they wondered if the crystallization of protein within these kind of materials was feasible, and what advantages supramolecular gels would bring compared to other methodologies. They studied the influence of matrix chirality in protein crystallogenesis, which was unexplored at that time owing to the difficulty in obtaining a pair of enantiomeric crystallization environments, easily overcome using supramolecular gels. Hydrogels made of 31 and 32 were chosen because (i) they are stable and transparent, (ii) are 100% water-based, which is needed for proteins, (iii) are peptide-based, so they are native media for protein, facilitating the interaction to mediate nucleation and molecular influence, and (iv) are chiral and both enantiomers are easily synthetized. Following a two-layer counter-diffusion methodology (Fig. 2), lysozyme crystals were obtained in both enantiomeric hydrogels showing a typical counter-diffusion pattern (Fig. 7a). Crystals obtained in 32 were of higher quality than those in 31, as shown by the synchrotron-radiation diffraction data. In the case of glucose isomerase crystals, those obtained in 31 were of higher quality than those obtained in 32; nevertheless, a second polymorph (P21212) was identified only in the experiments run with hydrogel 32 (Fig. 7d) that co-exist with the other typical polymorph (I222) (Fig. 7c). In addition, the highest quality crystals of formamidase grew exclusively in hydrogel 32. The usefulness of peptide-based supramolecular hydrogels as media for obtaining excellent quality protein crystals was established. The different crystallization outcomes in both enantiomer hydrogels suggests that there are diastereomeric interactions between the fibre matrix and proteins at the nucleation stage. In this work, the incorporation of hydrogel fibres within the crystal structure was also demonstrated, opening the door to the development of novel composite crystals.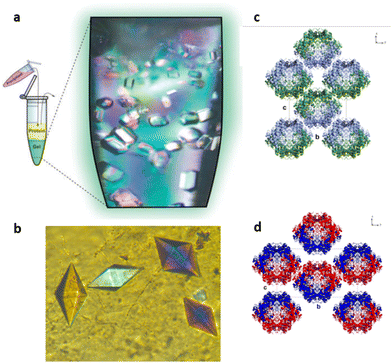 | ||
| Fig. 7 (a) Crystallization of lysozyme in hydrogel 32 using the two-layers configuration of the counter-diffusion set-up in Eppendorf tubes schematically represented (Adapted with permission from ref. 77. Copyright 2015 Royal Society of Chemistry). (b) Thaumatin crystals grown within hydrogel 33 (Adapted with permission from ref. 78. Copyright 2015 Royal Society of Chemistry). (c) and (d) Glucose isomerase crystal packing in the (c) already reported (PDB ID. 1OAD) obtained in both enantiomeric hydrogels 31 and 32, and (d) the new P21212 polymorphs (PDB ID. 4US6) obtained exclusively within 32 hydrogels (Adapted with permission from ref. 77. Copyright 2015 Royal Society of Chemistry). | ||
After the good results obtained in the first attempt crystallizing proteins within short peptide-based supramolecular hydrogels, the same group continued expanding this methodology to evaluate its scope.78 New proteins (thaumatin and insulin) and together with the previously employed 31 and 32 hydrogels, the well-known Fmoc-derivative peptide-based hydrogels Fmoc–PhePhe (4) and Fmoc–AlaAla (33) were also tested. These kinds of hydrogels have been extensively studied and applied for several purposes.26 Most of them are also commercially available, so this crystallization methodology can be more accessible and applicable. Thanks to the orthogonal self-assembly of gels and crystals under mild conditions (Fig. 1),51 it what possible to test a ‘batch’ crystallization methodology together with the previously employed two-layer counter diffusion technique. In this work, the different influence of hydrogel chirality over nucleation and growth was corroborated. Although, dramatic effects in crystallogenesis were also observed based on the gelator's chemical composition. Peptide-based supramolecular hydrogels have therefore demonstrated to be useful and versatile materials for protein crystallization.79 The fibre–protein composite crystals were obtained again. Composite lysozyme crystals obtained within 33 hydrogels showed remarkable slower dissolution rate than in the other gels. This synergistic interaction was observed thanks to the interaction of compounds through crystallization.
Protein-gel composite crystals
A peculiar aspect of protein crystals grown in hydrogels is the molecular level influence that the hydrogel fibres exert over the crystals. This is due to the direct interaction fibre–protein during crystallization which yields composite crystals. In several cases, a synergistic interaction happens generating composite crystals with new or improved properties. This phenomenon has been observed before for macromolecular hydrogels6,80 but also for supramolecular hydrogels, as has been discussed above. The great advantage of the latter is, as in the case of small molecules crystallization, their enormous versatility, and the possibility of being designed ad hoc with specific features. Gel promotors can be chemically designed to provide properties to the composite crystal. With this idea in mind, the groups of Álvarez de Cienfuegos and Gavira hypothesized that reductant hydrogels’ fibres occluded within protein crystals, would be able to capture free radicals during the exposition to synchrotron radiation source and therefore reduce the indirect radiation damage.81 Briefly, to achieve the structural resolution of a protein crystal, synchrotron radiation source is normally needed. As a counterpart, this radiation causes a collateral damage also called ‘radiation damage’ which reduce the quality of the diffraction pattern. This can be over the structure itself (direct damage) or generating reactive species from the media contained in the structure which afterwards reacts with sensitive groups of the proteins (indirect damage). The direct damage can only be reduced by decreasing the exposure time and intensity, but the indirect damage is greatly attenuated by lowering the temperature to 100 K which slows the diffusion of generated free radicals. The counterpart of this methodology is the need to add cryoprotectant agents and unexpected differences between the structure found at 100 K and at room temperature. To overcome these issues authors crystallized lysozyme within Fmoc–CysPhe hydrogels (34) to capitalize the well-known ability of thiol group from cysteine to capture free-radicals and thus reduce indirect damage. It was proven that composite lysozyme crystals containing fibres made of 34, unlike those grown in agarose, have an enhanced resistance against degradation caused by an intense exposure to synchrotron X-ray irradiation at room temperature. The gel fibres contained in the crystal structure show a clear local protection of the most sensitive groups (disulphide bonds and methionines) of the protein. The fact that cysteines are locked within the rigid hydrogel fibres minimize cross-reactions with the protein and also immobilize the radicals after their scavenging unlike what is observed with cysteine in solution.82 The fibrillar nature of the hydrogel and the possibility of being designed on demand due to its supramolecular nature, have been key for the success on the protection of lysozyme against radiation damage (Fig. 8a).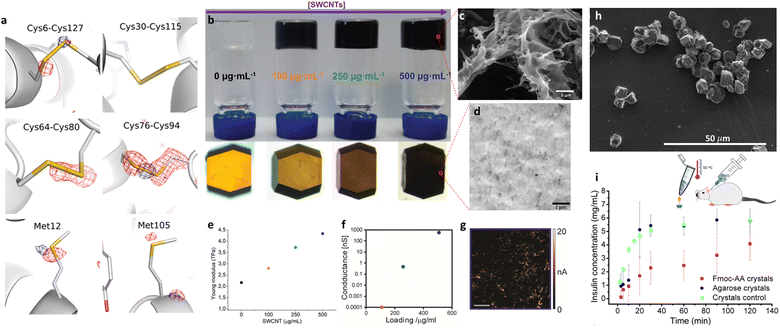 | ||
| Fig. 8 (a) Detailed ribbon representation of the lysozyme structure showing the most sensitive groups to radiation damage. The D9–D1 maps (contoured at −0.15 e Å−3) showing the specific protection of the disulfide bonds 64–80 and 76–94 only in crystals grown in hydrogel 34 (Adapted with permission from ref. 81. Copyright 2019 American Chemical Society). (b)–(g) Preparation and characterization of electron conducting reinforced lysozyme crystals doped with single walled carbon nanotubes (0 to 500 μg mL−1) while maintaining its intrinsic enzymatic activity; (c) environmental scanning electron microscopy (ESEM) picture of 4-SWCNTs composite hydrogels; (d) transmission electron microscopy (TEM) picture of inner section of SWCNTs lysozyme crystal where homogeneously distribute bundles of SWCNTs can be distinguished as black dots; plotting of Young's modulus of increasing (e) and electrical conductivity (f) as [SWCNTs] increases in doped lysozyme crystals; (g) conductive-atomic force microscopy (c-AFM) picture of the electrical conductivity through bundles of nanotubes distributed through the whole volume of the loaded lysozyme crystals (Adapted with permission from ref. 19. Copyright 2019 John Wiley and Sons). (h) Scanning electron microscopy (SEM) picture of injectable insulin crystals grown in Fmoc–AlaAla (33) hydrogels with enhanced dissolution rate (i) and thermal stability (Adapted with permission from ref. 87. Copyright 2021 American Chemical Society). | ||
The fact that supramolecular gels are macroporous materials allow them to host nanomaterials which can give the composite gel new properties such as: increased mechanical performance by combination with iron nanoparticles,84 making them fluorescent and with antimicrobial activity by adding silver nanoparticles,83 or making them electricity conductors using carbon nanotubes.85 The combination of carbon nanomaterials such as carbon nanotubes or graphene86 with supramolecular hydrogels is especially interesting because these materials are remarkably hydrophobic. The amphiphilic nature of supramolecular hydrogelators is exploited in this case to act as a bridge between the nanomaterial and water. This strategy was employed by Álvarez de Cienfuegos and Gavira to generate pristine single-walled carbon nanotubes (SWCNTs) and 4 composite hydrogels (Fig. 8b). The crystallization of lysozyme within this novel composite hydrogels allowed, for the first time, to introduce carbon nanotubes as bundles of SWCNTs homogeneously distributed throughout the whole volume of a protein crystal (Fig. 8d).19 The homogeneous dispersion of SWCNTs through a hydrogel matrix was crucial to achieve this successfully because unaltered carbon nanotubes are not water soluble and due to their huge aspect ratio, cannot be introduced inside the crystals once formed. The inclusion of SWCNTs within the protein crystal lattice results in a synergistic interaction and the transference of properties from carbon nanotubes to the resulting composite crystals. These materials maintained the enzymatic activity when cross-linked, being catalytically active at 90 °C (the highest temperature ever tested for lysozyme's enzymatic activity) thanks to the thermal stabilization provided by the SWCNTs. This stabilization was also manifested by the improvement of their mechanical behaviour, being stronger as SWCNTs’ concentration was increased (Fig. 8e). The cross-linked composite crystals also showed isotropic electron conduction, presenting the highest conductivity (600 nS) ever registered for a protein crystal (Fig. 8f and g). This work exemplifies the potential of supramolecular gels as a vehicle to introduce different kind of materials within protein crystals, thus conferring them new properties.
The interaction between 33 supramolecular gel fibrils and lysozyme crystals results in a reduction of the rate of dissolution of the composite crystals. The ability of supramolecular gels of altering physicochemical properties of protein crystals, was thought to be extremely useful for therapeutic proteins. This idea was exemplified with insulin as therapeutically essential protein.87 In this work, a batch crystallization methodology (see Fig. 2) was developed to produce tiny insulin crystals (4 ± 1 μm) ideal for in vivo subcutaneous administration, within agarose and 33 gels. Both gel-phase crystallization yielded stabilized crystals which remained pharmacologically unaltered after being maintained at 50 °C for 7 days (equivalent to more than two years at room temperature88). Specifically, insulin crystals grown in 33 hydrogels remained active even after one day at 60 °C and showed slower dissolution rate which was then translated into slow-release profile in vivo (Fig. 8h and i). This example highlights the enormous potential of supramolecular gels to improve pharmaceutical formulations based on protein crystals.
Conclusions and perspectives
Supramolecular gels, which are formed through self-assembly processes driven by non-covalent interactions, provide a controlled environment for crystalline domain formation. The broad diversity of chemical structures that can behave as LMWGs offers the possibility to explore crystallization in a great variety of organic and aqueous solvents, favouring the study of crystallization as never before. Moreover, the chemical simplicity of most LMWGs allows an easy access and facile customization to include in their structures similarities with molecules to crystallize. This has shown to promote crystallization and polymorph selection, which is of fundamental importance in the case of APIs. In addition, the gelator molecules and self-assembled derivatives can also be designed to interact with the grown crystals, influencing the process of crystallization and altering the physicochemical properties of the resulting crystals. This has been notorious in the case of protein crystals, in which their porous structure allows the incorporation of gel fibres in their interior giving rise to composite protein crystals. Additionally, LMWGs can trigger gel formation under the application of different stimuli, such as, change in pH, temperature, solvent, addition of salts, etc. These parameters have a direct impact on the macro- and microscopic properties of the resulting gels. The ability to easily manipulate gel parameters offers an extra degree of control for the fine-tuning of crystal size, polymorphism, and morphology. Finally, the possibility to obtain composite or hybrid gels by the homogeneous incorporation of different additives within the three-dimensional gel matrix, allows the formation of composite or hybrid crystals that hold great promise for advancing research in nanotechnology, materials science, and biomedicine.One of the drawbacks supramolecular gels have, is that they are unknown materials for non-chemists who also can benefit from their use for crystallization. These materials can be seen as difficult to handle for specialists in other disciplines. This problem is being progressively overcome thanks to companies (e.g., BiogelX™) which are producing ready-to-use gelation kits based on supramolecular gels. There is still much room for improvement in this sense and this review tries to stimulate the interest of the scientific community for the impressive ‘crystallization toolbox’ supramolecular gels are.
Using nucleobase-based supramolecular gels to crystallize nucleic acids or carbohydrate-based gels for carbohydrate crystallization could be an interesting strategy to explore to what extent structural similarities can enhance the crystallization process, a question that is still under debate.
Easy collection of protein crystals is essential to prepare crystals for X-ray diffraction characterization and use them for additional studies, such as dissolution studies or to measure biological activities in vitro or in vivo.
Supramolecular hydrogels as a vehicle to include other types of nanomaterials within protein crystals have paved the way to develop new composite or hybrid protein crystals that may present novel properties, as discussed in this review. This strategy can be of great importance in the field of protein engineering, the ultimate goal of which is to create new functional materials based on protein crystals.
These last paragraphs are just a few glimpses of the exciting possibilities that the use of supramolecular gels can have in the field of crystallization and in obtaining new advanced materials. In this regard, we believe that this review can be of great help to all those scientists who feel attracted by obtaining crystals, their study and the possibility of creating new materials with them.
Author contributions
R. C.-M.: conceptualization, formal analysis, writing – original draft, writing – review and editing; L. A. C.: conceptualization, supervision, funding acquisition, writing – review and editing; J. A. G.: conceptualization, funding acquisition, writing – review and editing; J. W. S.: conceptualization, funding acquisition, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for funding to the European Commission (HORIZON-MSCA-PF-2022, project 101110492), to the Ministerio de Ciencia, Innovación y Universidades (Spain) MCIN/AEI/10.13039/501100011033 (PID2020-118498GB-I00 and PID2020-116261GB-I00), to the FEDER/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento y Universidades (Spain) (P18-FR-3533), and to the Engineering and Physical Research Council (EP/S035877/1). Funding for open access charge: Universidad de Granada/CBUA.References
- J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov, O. Ronneberger, K. Tunyasuvunakool, R. Bates, A. Žídek, A. Potapenko, A. Bridgland, C. Meyer, S. A. A. Kohl, A. J. Ballard, A. Cowie, B. Romera-Paredes, S. Nikolov, R. Jain, J. Adler, T. Back, S. Petersen, D. Reiman, E. Clancy, M. Zielinski, M. Steinegger, M. Pacholska, T. Berghammer, S. Bodenstein, D. Silver, O. Vinyals, A. W. Senior, K. Kavukcuoglu, P. Kohli and D. Hassabis, Nature, 2021, 596, 583–589 CrossRef CAS PubMed.
- K. Haubrich, V. A. Spiteri, W. Farnaby, F. Sobott and A. Ciulli, Curr. Opin. Struct. Biol., 2023, 79, 102534 CrossRef CAS PubMed.
- G. P. Stahly, Cryst. Growth Des., 2007, 7, 1007–1026 CrossRef CAS.
- W. E. Lutz, J. Azadmanesh, J. J. Lovelace, C. Kolar, L. Coates, K. L. Weiss and G. E. O. Borgstahl, NPJ Microgravity, 2023, 9, 39 CrossRef CAS PubMed.
- A. Moreno and M. E. Mendoza, in Handbook of Crystal Growth: Bulk Crystal Growth, Elsevier, 2015, pp. 1277–1315 Search PubMed.
- J. A. Gavira, A. E. S. Van Driessche and J. M. Garcia-Ruiz, Cryst. Growth Des., 2013, 13, 2522–2529 CrossRef CAS.
- F. Artusio, A. Castellví, R. Pisano and J. A. Gavira, Crystals, 2021, 11, 466 CrossRef CAS.
- H. Li, Y. Fujiki, K. Sada and L. A. Estroff, CrystEngComm, 2011, 13, 1060–1062 RSC.
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3160 CrossRef CAS PubMed.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- D. K. Smith, in Molecular Gels, ed. R. G. Weiss, Royal Society of Chemistry, Cambridge, Cambridge, United Kingdom, 2018, pp. 300–371 Search PubMed.
- S. Panja and D. J. Adams, Chem. Soc. Rev., 2021, 50, 5165–5200 RSC.
- R. Contreras-Montoya, J. P. Smith, S. C. Boothroyd, J. A. Aguilar, M. Mirzamani, M. A. Screen, D. S. Yufit, M. Robertson, L. He, S. Qian, H. Kumari and J. W. Steed, Chem. Sci., 2023, 14, 11389–11401 RSC.
- W. Fang, Y. Zhang, J. Wu, C. Liu, H. Zhu and T. Tu, Chem. – Asian J., 2018, 13, 712–729 CrossRef CAS.
- A. Vidyasagar, K. Handore and K. M. Sureshan, Angew. Chem., Int. Ed., 2011, 50, 8021–8024 CrossRef CAS PubMed.
- L. Zhang, H. X. Wang, S. Li and M. Liu, Chem. Soc. Rev., 2020, 49, 9095–9120 RSC.
- P. Duan, H. Cao, L. Zhang and M. Liu, Soft Matter, 2014, 10, 5428–5448 RSC.
- R. Contreras-Montoya, A. B. Bonhome-Espinosa, A. Orte, D. Miguel, J. M. Delgado-López, J. D. G. Duran, J. M. Cuerva, M. T. Lopez-Lopez and L. Álvarez de Cienfuegos, Mater. Chem. Front., 2018, 2, 686–699 RSC.
- R. Contreras-Montoya, G. Escolano, S. Roy, M. T. Lopez-Lopez, J. M. Delgado-López, J. M. Cuerva, J. J. Díaz-Mochón, N. Ashkenasy, J. A. Gavira and L. Álvarez de Cienfuegos, Adv. Funct. Mater., 2019, 29, 1807351 CrossRef.
- S. Firouzeh, S. Illescas-Lopez, M. A. Hossain, J. M. Cuerva, L. Álvarez de Cienfuegos and S. Pramanik, ACS Nano, 2023, 17, 20424–20433 CrossRef CAS PubMed.
- M. Cametti and Z. Dolic, ChemComm, 2014, 50, 8273–8286 RSC.
- S. Bhattacharya and S. K. Samanta, Chem. Rev., 2016, 116, 11967–12028 CrossRef CAS.
- A. Prathap and K. M. Sureshan, Angew. Chem., Int. Ed., 2017, 56, 9405–9409 CrossRef CAS PubMed.
- M. C. Mañas-Torres, C. Gila-Vilchez, F. J. Vazquez-Perez, P. Kuzhir, D. Momier, J. C. Scimeca, A. Borderie, M. Goracci, F. Burel-Vandenbos, C. Blanco-Elices, I. A. Rodriguez, M. Alaminos, L. Álvarez de Cienfuegos and M. T. Lopez-Lopez, ACS Appl. Mater. Interfaces, 2021, 13, 49692–49704 CrossRef.
- C. Gila-Vilchez, M. C. Mañas-Torres, Ó. D. García-García, A. Escribano-Huesca, L. Rodríguez-Arco, V. Carriel, I. Rodriguez, M. Alaminos, M. T. Lopez-Lopez and L. Álvarez de Cienfuegos, ACS Appl. Polym. Mater., 2023, 5, 2154–2165 CrossRef CAS PubMed.
- M. C. Mañas-Torres, S. Illescas-Lopez, J. A. Gavira, L. Álvarez de Cienfuegos and S. Marchesan, Isr. J. Chem., 2022, e202200018 CrossRef.
- J. Y. C. Lim, Q. Lin, K. Xue and X. J. Loh, Mater. Today Adv., 2019, 3, 100021 CrossRef.
- R. Dong, Y. Pang, Y. Su and X. Zhu, Biomater. Sci., 2015, 3, 937–954 RSC.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- M. Llusar and C. Sanchez, Chem. Mater., 2008, 20, 782–820 CrossRef CAS.
- T. Kar and P. K. Das, in Functional Molecular Gels, ed. B. Escuder and J. F. Miravet, The Royal Society of Chemistry, Cambridge, United Kingdom, 1st edn, 2014, pp. 255–303 Search PubMed.
- A. Dawn, Int. J. Mol. Sci., 2019, 20, 781 CrossRef CAS.
- H. Sharma, B. K. Kalita, D. Pathak and B. Sarma, Cryst. Growth Des., 2023, 24, 17–37 CrossRef.
- T. Graham, Philos. Trans. R. Soc. London, 1861, 151, 197–224 Search PubMed.
- R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed.
- D. J. Abdallah and R. G. Weiss, Adv. Mater., 2000, 12, 1237–1247 CrossRef CAS.
- L. A. Estroff and A. D. Hamilton, Angew. Chem., Int. Ed., 2000, 39, 3447–3450 CrossRef CAS.
- L. A. Estroff, L. Addadi, S. Weiner and A. D. Hamilton, Org. Biomol. Chem., 2004, 2, 137–141 RSC.
- L. A. Estroff, L. Leiserowitz, L. Addadi, S. Weiner and A. D. Hamilton, Adv. Mater., 2003, 15, 38–42 CrossRef CAS.
- H. Li, H. L. Xin, D. A. Muller and L. A. Estroff, Science, 2009, 326, 1244–1247 CrossRef CAS PubMed.
- R. Daly, O. Kotova, M. Boese, T. Gunnlaugsson and J. J. Boland, ACS Nano, 2013, 7, 4838–4845 CrossRef CAS.
- K. Liu, S. Gao, Z. Zheng, X. Deng, S. Mukherjee, S. Wang, H. Xu, J. Wang, J. Liu, T. Zhai and Y. Fang, Adv. Mater., 2019, 31, 1808254 CrossRef PubMed.
- C. Shen, P. Han, Z. Zheng, W. Jiang, S. Gao, C. Hua, C. L. Chen, F. Xia, T. Zhai, K. Liu and Y. Fang, Adv. Sci., 2022, 9, 2203662 CrossRef CAS PubMed.
- S. Illescas-Lopez, J. D. Martin-Romera, M. C. Mañas-Torres, M. T. Lopez-Lopez, J. M. Cuerva, J. A. Gavira, F. J. Carmona and L. Álvarez de Cienfuegos, ACS Appl. Mater. Interfaces, 2023, 15, 32597–32609 CrossRef CAS PubMed.
- M. C. Mañas-Torres, G. B. Ramírez-Rodríguez, J. I. García-Peiro, B. Parra-Torrejón, J. M. Cuerva, M. T. Lopez-Lopez, L. Álvarez de Cienfuegos and J. M. Delgado-López, Inorg. Chem. Front., 2022, 9, 743–752 RSC.
- J. L. Andrews, E. Pearson, D. S. Yufit, J. W. Steed and K. Edkins, Cryst. Growth Des., 2018, 18, 7690–7700 CrossRef CAS.
- R. Mohanrao, K. Hema and K. M. Sureshan, Nat. Commun., 2020, 11, 865 CrossRef CAS.
- J. A. Foster, M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke, J. A. K. Howard and J. W. Steed, Nat. Chem., 2010, 2, 1037–1043 CrossRef CAS PubMed.
- M. Pauchet, T. Morelli, S. Coste, J.-J. Malandain and G. Coquerel, Cryst. Growth Des., 2006, 6, 1881–1889 CrossRef CAS.
- J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686–3699 RSC.
- D. K. Kumar and J. W. Steed, Chem. Soc. Rev., 2014, 43, 2080–2088 RSC.
- A. Dawn, M. Mirzamani, C. D. Jones, D. S. Yufit, S. Qian, J. W. Steed and H. Kumari, Soft Matter, 2018, 14, 9489–9497 RSC.
- F. Aparicio, E. Matesanz and L. Sánchez, ChemComm, 2012, 48, 5757–5759 RSC.
- S. Gao, S. Wang, J. Ma, Y. Wu, X. Fu, R. K. Marella, K. Liu and Y. Fang, Langmuir, 2016, 32, 12805–12813 CrossRef CAS.
- H. Kumari, S. E. Armitage, S. R. Kline, K. K. Damodaran, S. R. Kennedy, J. L. Atwood and J. W. Steed, Soft Matter, 2015, 11, 8471–8478 RSC.
- A. Dawn, K. S. Andrew, D. S. Yufit, Y. Hong, J. P. Reddy, C. D. Jones, J. A. Aguilar and J. W. Steed, Cryst. Growth Des., 2015, 15, 4591–4599 CrossRef CAS.
- J. Buendía, E. Matesanz, D. K. Smith and L. Sánchez, CrystEngComm, 2015, 17, 8146–8152 RSC.
- L. Kaufmann, S. R. Kennedy, C. D. Jones and J. W. Steed, ChemComm, 2016, 52, 10113–10116 RSC.
- J. A. Foster, K. K. Damodaran, A. Maurin, G. M. Day, H. P. G. Thompson, G. J. Cameron, J. Cuesta Bernal and J. W. Steed, Chem. Sci., 2017, 8, 78–84 RSC.
- L. Yu, Acc. Chem. Res., 2010, 43, 1257–1266 CrossRef CAS PubMed.
- G. J. O. Beran, I. J. Sugden, C. Greenwell, D. H. Bowskill, C. C. Pantelides and C. S. Adjiman, Chem. Sci., 2022, 13, 1288–1297 RSC.
- Y. Diao, K. E. Whaley, M. E. Helgeson, M. A. Woldeyes, P. S. Doyle, A. S. Myerson, T. A. Hatton and B. L. Trout, J. Am. Chem. Soc., 2012, 134, 673–684 CrossRef CAS PubMed.
- J. L. Andrews, S. R. Kennedy, D. S. Yufit, J. F. Mccabe and J. W. Steed, Cryst. Growth Des., 2022, 22, 6785 CrossRef PubMed.
- S. R. Kennedy, C. D. Jones, D. S. Yufit, C. E. Nicholson, S. J. Cooper and J. W. Steed, CrystEngComm, 2018, 20, 1390–1398 RSC.
- K. Zhang, N. Fellah, A. G. Shtukenberg, X. Fu, C. Hu and M. D. Ward, CrystEngComm, 2020, 22, 2705–2708 RSC.
- I. Torres-Moya, B. Saikia, P. Prieto, J. R. Carrillo and J. W. Steed, CrystEngComm, 2019, 21, 2135–2143 RSC.
- I. Torres-Moya, A. Sánchez, B. Saikia, D. S. Yufit, P. Prieto, J. R. Carrillo and J. W. Steed, Gels, 2023, 9, 26 CrossRef CAS PubMed.
- S. S. Jayabhavan, J. W. Steed and K. K. Damodaran, Cryst. Growth Des., 2021, 21, 5383–5393 CrossRef CAS.
- S. A. Ross, D. A. Lamprou and D. Douroumis, ChemComm, 2016, 52, 8772–8786 RSC.
- R. Thipparaboina, D. Kumar, R. B. Chavan and N. R. Shastri, Drug Discovery Today, 2016, 21, 481–490 CrossRef CAS PubMed.
- J.-R. Wang, J. Bao, X. Fan, W. Dai and X. Mei, ChemComm, 2016, 52, 13452–13455 RSC.
- M. A. Rahim, M. Björnmalm, T. Suma, M. Faria, Y. Ju, K. Kempe, M. Müllner, H. Ejima, A. D. Stickland and F. Caruso, Angew. Chem., Int. Ed., 2016, 55, 13803–13807 CrossRef CAS PubMed.
- M. A. Rahim, Y. Hata, M. Björnmalm, Y. Ju and F. Caruso, Small, 2018, 14, 1801202 CrossRef PubMed.
- D. Ghosh, K. Ferfolja, Z. Drabavicius, J. W. Steed and K. K. Damodaran, New J. Chem., 2018, 42, 19963–19970 RSC.
- B. Saikia, M. T. Mulvee, I. Torres-Moya, B. Sarma and J. W. Steed, Cryst. Growth Des., 2020, 20, 7989–7996 CrossRef CAS.
- N. Zencirci, U. J. Griesser, T. Gelbrich, D. C. Apperley and R. K. Harris, Mol. Pharmaceutics, 2014, 11, 338–350 CrossRef CAS PubMed.
- M. Conejero-Muriel, J. A. Gavira, E. Pineda-Molina, A. Belsom, M. Bradley, M. Moral, J. de, D. G. López-Durán, A. Luque González, J. J. Díaz-Mochón, R. Contreras-Montoya, Á. Martínez-Peragón, J. M. Cuerva and L. Álvarez de Cienfuegos, ChemComm, 2015, 51, 3862–3865 RSC.
- M. Conejero-Muriel, R. Contreras-Montoya, J. J. Díaz-Mochón, L. Álvarez de Cienfuegos and J. A. Gavira, CrystEngComm, 2015, 17, 8072–8078 RSC.
- G. Escolano-casado, R. Contreras-montoya, M. Conejero-muriel, A. Castellv, J. Juanhuix, M. T. Lopez-lopez, L. Álvarez de Cienfuegos and J. A. Gavira, Crystals, 2019, 9, 244 CrossRef CAS.
- J. A. Gavira and J. M. García-Ruiz, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2002, 58, 1653–1656 CrossRef PubMed.
- R. Contreras-Montoya, A. Castellví, G. Escolano-Casado, J. Juanhuix, M. Conejero-Muriel, M. T. Lopez-Lopez, J. M. Cuerva, L. Álvarez de Cienfuegos and J. A. Gavira, Cryst. Growth Des., 2019, 19, 4229–4233 CrossRef CAS.
- J. Kmetko, M. Warkentin, U. Englich and R. E. Thorne, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2011, 67, 881–893 CrossRef CAS PubMed.
- J. Nanda, B. Adhikari, S. Basak and A. Banerjee, J. Phys. Chem. B, 2012, 116, 12235–12244 CrossRef CAS PubMed.
- R. Contreras-Montoya, A. B. Bonhome-Espinosa, A. Orte, D. Miguel, J. M. Delgado-López, J. D. G. Duran, J. M. Cuerva, M. T. Lopez-Lopez and L. Álvarez de Cienfuegos, Mater. Chem. Front., 2018, 2, 686–699 RSC.
- S. Roy and A. Banerjee, RSC Adv., 2012, 2, 2105–2111 RSC.
- B. Adhikari and A. Banerjee, Soft Matter, 2011, 7, 9259–9266 RSC.
- R. Contreras-Montoya, M. Arredondo-Amador, G. Escolano-Casado, M. C. Mañas-Torres, M. González, M. Conejero-Muriel, V. Bhatia, J. J. Díaz-Mochón, O. Martínez-Augustin, F. Sánchez de Medina, M. T. Lopez-Lopez, F. Conejero-Lara, J. A. Gavira and L. Álvarez de Cienfuegos, ACS Appl. Mater. Interfaces, 2021, 13, 11672–11682 CrossRef CAS PubMed.
- B. Shenoy, Y. Wang, W. Shan and A. L. Margolin, Biotechnol. Bioeng., 2001, 73, 358–369 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |