Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An investigation of the structural and electronic origins of enhanced chemical looping air separation performance of B-site substituted SrFe1−xCoxO3−δ perovskites†
Qianwenhao
Fan
 ab,
Haiyan
Li
c,
Syed
Saqline
ab,
Haiyan
Li
c,
Syed
Saqline
 abd,
Felix
Donat
abd,
Felix
Donat
 e,
Mingwu
Tan
f,
Longgang
Tao
f,
Christoph R.
Müller
e,
Mingwu
Tan
f,
Longgang
Tao
f,
Christoph R.
Müller
 e,
Zhichuan J.
Xu
e,
Zhichuan J.
Xu
 g and
Wen
Liu
g and
Wen
Liu
 *abd
*abd
aSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 62 Nanyang Drive, Singapore 637459, Singapore. E-mail: wenliu@ntu.edu.sg
bCambridge Centre for Advanced Research and Education in Singapore, 1 Create Way, Singapore 138602, Singapore
cCollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou, 310027, China
dNanyang Environmental and Water Research Institute, Nanyang Technological University, 1 Cleantech Loop, Singapore 637141, Singapore
eDepartment of Mechanical and Process Engineering, ETH Zurich, Leonhardstrasse 21, Zürich 8092, Switzerland
fInstitute of Sustainability for Chemicals, Energy and Environment, Agency for Science, Technology and Research (A*STAR), 1 Pesek Road, Jurong Island, 627833, Singapore
gSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore
First published on 16th July 2024
Abstract
Chemical looping air separation (CLAS) is a promising process intensification technology for extracting oxygen from air for oxygen enrichment in process streams. Co-doped strontium ferrites (SrFe1−xCoxO3−δ) have been found to have outstanding activities for CLAS processes. In this study, we explore the underlying factors driving the enhancement in oxygen uptake and release performance of perovskite structured SrFe1−xCoxO3−δ oxygen carriers for CLAS. Phase-pure perovskites, with B site substituted by up to 75 mol% Co, were prepared by a sol–gel method and systematically investigated through a wide range of well controlled experimental and computational approaches. While all SrFe1−xCoxO3−δ oxygen carriers showed excellent cyclic stability and structural reversibility over CLAS cycles, increased B site occupancy by Co resulted in monotonic decrease in onset temperature for oxygen release and increase in oxygen carrying capacity. These experimental trends can be fundamentally explained by an increase in the structural tolerance factor, an elevation in transition metal d-band, as well as an increased degree of hybridization between the metal d-band and the O p band. Therefore, these ab initio structural and electronic descriptors are useful design rationales for the hypothesis-driven synthesis of high-performing oxygen carriers for CLAS.
1 Introduction
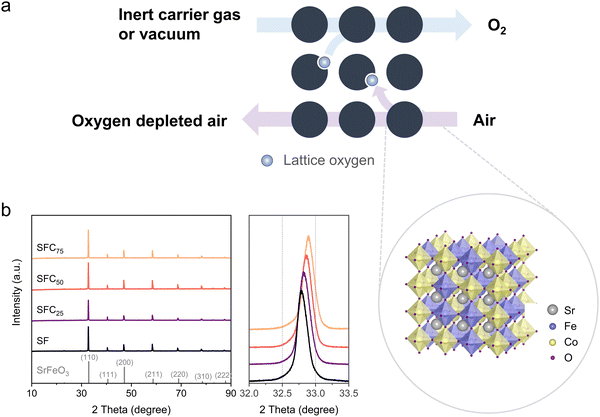
Air separation is a common process in many industrial applications. It plays a crucial role in the production of essential processing gases such as oxygen, nitrogen, and argon, which are extensively used in steel production, oil and gas refining, chemical manufacturing, healthcare, and semiconductors manufacturing.1,2 Traditional methods of air separation, including cryogenic distillation and pressure swing adsorption, have been the standard practices in industry for many years.3 Cryogenic distillation, which separates air components based on their different boiling points, is particularly prevalent owing to its ability to produce high-purity gases at large scales. However, cryogenic air separation is a highly energy-intensive process and requires significant capital investment.4 Pressure swing adsorption (PSA), on the other hand, separates gases based on their molecular characteristics such as size and polarity. While less energy-intensive than cryogenic distillation, PSA is generally less efficient and produces gases of lower purity.2 In addition, membrane-based methods have also been developed to achieve air separation.5 By using semi-permeable membranes to separate gases based on their interaction with the membrane materials, air separation may be energy-efficient and simple to operate. However, reliable and high-performance membranes are rare while the energy penalties for operating membrane units are still high.6 Given the limitations of existing air separation technologies, there is an interest to develop novel air separation technologies that are energy efficient, cost-effective, and environmentally friendly.Chemical looping air separation (CLAS) has emerged as a promising alternative to existing air separation approaches.7 CLAS relies on the redox reactions of oxide-based materials, also known as oxygen carriers, which can reversibly take up and release gaseous oxygen in response to variations in oxygen partial pressure and, or temperature. As illustrated by Fig. 1a, when the oxygen partial pressure is low, the lattice oxygen within the oxide is liberated in the form of gaseous oxygen, accompanied by the reduction of the oxide. The lattice oxygen in the reduced oxide is subsequently regenerated by put in contact with air. Repeating this cyclic process facilitates continuous air separation and oxygen production. The advantages of CLAS include high separation efficiency, low energy consumption, and the potential for integration with thermal power generation cycles, making it an attractive technology for potential industrial applications.8–11
Perovskite structured oxide materials have been extensively studied as potential oxygen carriers for CLAS owing to their unique structural and redox properties.12–15 Perovskite structured oxides, with a general formula of ABO3, are a class of materials in which A and B are cations of different ionic radii, while O represent oxygen anions. When the B-site cation undergoes redox reactions, the perovskites would take up or release oxygen based on the principle of charge balance, thereby facilitating CLAS.16 Among the various perovskites structured oxide materials, strontium ferrite (SrFeO3−δ) has shown promising performance owing to its high oxygen capacity and excellent cyclability.17 Indeed, the perovskite structured SrFeO3−δ, with δ ranging between 0 and 0.5, has drawn considerable scholarly interests in recent years for its application in CLAS and other chemical looping processes.18–20 In addition, the abundant oxygen vacancies in SrFeO3−δ based oxides afford excellent oxygen ion conductivity, rendering them excellent materials for oxygen permeation membranes.21
In SrFeO3−δ, the Fe ions exist in mixed valence states between +4 and +3 as the oxygen non-stoichiometry, δ varies between 0 and 0.5, where δ = 0.5 signifies a substantial number of oxygen vacancies and a potential to undergo phase transformation to a brownmillerite structure (i.e. Sr2Fe2O5). To further promote the oxygen release behavior of SrFeO3−δ, B-site substitution, e.g. by Co and Mn, has been explored.22,23 Notably, B-site substitution by cobalt has been reported to modify the electronic structure of SrFeO3−δ, increase the oxygen desorption rate and lower the operating temperature of the CLAS process.24 Fujishiro et al. conducted in situ X-ray absorption spectroscopy studies and have resolved the structural evolution of the SrFe1−xCoxO3−δ perovskites when they undergo oxygen release at elevated temperatures. They found that the reduction of the Fe centers is primarily responsible for oxygen release.22 Li and coworkers have conducted extensive investigation of A-site and B-site doped SrFeO3 oxygen carriers and have identified (i) the net electronic charge of the oxygen anion,25 (ii) the Gibbs free energy of formation of an oxygen vacancy12 and (iii) the activation energy for oxygen diffusion and surface oxygen exchange20 as energetic descriptors capable of predicting the CLAS activities of the perovskite material in terms of (a) the onset temperature for oxygen release and (b) the oxygen carrying capacities.
Building on the prior knowledge discussed above, we set out to further probe the origin of the variations in the oxygen release properties of B-site doped SrFeO3 oxygen carriers, viz. SrFe1−xCoxO3−δ (SFC), when they are subjected to CLAS cycles under industrially relevant operating conditions (in the presence of high partial pressure of CO2). Accordingly, a series of phase-pure perovskite samples with B site substitution by Co up to 75 mol%, viz. SrFeO3−δ (SF), SrFe0.75Co0.25O3−δ (SFC25), SrFe0.5Co0.5O3−δ (SFC50), and SrFe0.25Co0.75O3−δ (SFC75) were synthesized via a sol–gel method. Temperature and oxygen partial pressure swing experiments, coupled with in situ structural, electronic characterizations, and ab initio simulations revealed the structural and electronic descriptors that gave rise to the improved CLAS performance of the SFC-based oxygen carriers.
2 Experimental section
2.1 Material preparation
The SrFe1−xCoxO3−δ samples were prepared by sol–gel synthesis, which is widely employed for preparing perovskite-structured oxide powders and thin films.26–29 Sr(NO3)2 (Sigma-Aldrich, 99.9%), Fe(NO3)3·9H2O (Sigma-Aldrich, ≥98%), and Co(NO3)2·6H2O (Sigma-Aldrich, 99%) were used as the metal precursors without any further purification. In a typical synthesis, stoichiometric amounts of metal nitrates (exact quantities given in Table S1, ESI†) were dissolved in 60 mL deionized water together with 5.845 g (20 mmol) of ethylenediaminetetraacetic acid (EDTA) and 7.685 g (40 mmol) of citric acid at room temperature. 100 mL of NH4OH (30.0%) was used to adjust the pH to allow a clear solution to re-appear after mixing. Next, the solution was heated to 95 °C and held at 95 °C until a gel formed (i.e., when the mixture is not visibly fluid). The gel was dried at 250 °C for 3 h in an oven and subsequently crushed to a fine powder. The powders were calcined in air at 1000 °C with a heating rate of 5 °C min−1 and a holding time of 5 h. The calcined powders were sieved to 100–200 μm for characterization and chemical looping air separation experiments.2.2 Thermogravimetric analysis
A series of thermogravimetric analyses (TGA) were performed using a TGA/DSC2 (Mettler Toledo) thermogravimetric analyzer to investigate the relationship between the temperature (T), oxygen partial pressure (pO2), oxygen stoichiometry (3 − δ), and oxygen carrier compositions (x). The absolute δ value of the as-synthesized perovskite oxygen carriers was first determined by temperature programed desorption (TPD) experiment, in which 50 mg of the sample was heated from 150 °C to 1000 °C at a rate of 5 °C min−1 in a N2 flow (200 mL min−1). This program allowed the perovskite phase to be fully reduced to brownmillerite (SrFe1−xCoxO2.5, δ = 0.5).30 The mass change (Δm) between 150 °C in air and 1000 °C in N2 was measured. This mass change corresponds to the loss of lattice oxygen in the perovskite structure (i.e., a change in δ) prior to phase transition to brownmillerite. Subsequently, the value of δ0 at 150 °C in air was calculated using the following equation, assuming δ = 0.5 at 1000 °C in N2:| Δm/m = (0.5 − δ0)MO/MS | (1) |
| Δm/m = (δ − δ0)MO/MS | (2) |
In a typical TGA experiment, 50 mg of sample was placed in an alumina crucible (150 μL), heated to 150 °C at a rate of 5 °C min−1 and held for 30 min in air. After the isothermal period, pO2 was changed to 0.2 bar, 0.1 bar, 0.05 bar, or 0.02 bar by using either air or a N2/air mixture of. Then, the sample was heated from 250 °C to 650 °C in increments of 50 °C, and held at each incremental temperature for 30 min to investigate the relationship between T, pO2, δ, and x for each sample when it is in equilibrium with the gas. The total gas flow over the microbalance was maintained at 200 mL min−1 (measured at room temperature and pressure) throughout the experiment.
2.3 Material characterization
The oxygen contents of the as-synthesized oxygen carriers were quantified by iodometric titration. In a typical titration experiment, a perovskite sample weighing 250 mg was dissolved in 50 mL of 6 M HCl. Subsequently, 10 mL of this solution was transferred into an Erlenmeyer flask, to which 1.00 g of potassium iodide (KI) was introduced. A few minutes later, the solution was diluted with deionized (DI) water and the released iodine was titrated using 0.05 M sodium thiosulfate (Na2S2O3). Prior to each titration, sodium thiosulfate was standardized against potassium iodate (KIO3). The titration endpoint was identified using a starch indicator, which was added into the solution prior to the titration.The morphologies of the samples were examined by a field emission scanning electron microscope with equipped with energy dispersive spectroscopy (JEOL JSM-7200F). The crystal structures of the as-prepared oxygen carriers were studied by powder X-ray diffraction (XRD) in a Bruker D8 Advance diffractometer with filtered Cu Kα radiation (λ = 1.5418 Å) at 40 kV and 40 mA under ambient condition. The XRD patterns were collected with 2θ range from 10° to 90°, a step size of 0.02°, and a collection time of 1 s per step. In addition, in situ XRD experiments were conducted to monitor the structural changes of the perovskite oxygen carriers over a CLAS cycle. In the first in situ XRD experiment, the sample was heated up in air at 10 °C min−1 to 100, 200, 300, 400, and 500 °C, consecutively, dwelling for 2 h at each temperature, followed by a 2 h scan for 2θ = 10–90° at that temperature before heating up to the next set point temperature. After the scan at 500 °C, the sample was cooled down consecutively to 400, 300, 200, and 100 °C, while following the same temperature dwelling and XRD scanning program. In the second in situ XRD experiment, a fresh sample was first heated up in air stepwise to 100, 200, 300, 400, 500 °C, consecutively, with a pattern collected over the 2θ range of 30–35° at each temperature point before cooling down. Then, the same temperature-XRD program was repeated in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of air and CO2. The XRD data collected were analyzed by Rietveld refinement using GSAS software through EXPGUI.31
1 mixture of air and CO2. The XRD data collected were analyzed by Rietveld refinement using GSAS software through EXPGUI.31
2.4 Density functional theory (DFT) calculation
Density of states (DOS) of the oxygen carriers were obtained by DFT computation using the Vienna ab initio simulation package (VASP) software32,33 with the projector-augmented wave (PAW) approach34 and the Perdew–Burk–Ernzerhof (PBE) exchange–correlation functional.32,33,35 To account for the strongly localized d-electrons, effective Hubbard U parameters for Fe and Co are set to be 4.0 and 3.3 eV, respectively, taken from the literature.36 Structural models used for the calculation were 2 × 2 × 2 cubic perovskite supercells with various extent of B-site substitution. The Brillouin zone was sampled in a 6 × 6 × 6 mesh, and the plane-wave cut-off energy was set to be 520 eV.3 Results and discussion
3.1 Characterization of oxygen carriers
The crystal structures of the as-prepared perovskite oxygen carriers were studied by powder X-ray diffraction (XRD). The undoped SrFeO3−δ (SF) and Co-doped SFC all showed cubic-phase perovskite structures (with Pm3m space group), with major peaks at 32.8, 40.4, 47.0, 58.5, 68.7, and 78.2°, indexed to (110), (111), (200), (211), (220), and (310) reflections, respectively (Fig. 1b). Accordingly, the Pm3m space group was used as a reference structure for analyzing the data collected by the in situ XRD experiments following Rietveld refinement. The results of the Rietveld refinement, including lattice constant, crystallite size, dislocation density,37 and microstrain, are summarized in Table S4 (ESI†). These structural parameters are known to influence the properties of perovskite-structured materials.15,38–40 From the XRD results, no impurity phase was detected, verifying the successful synthesis of phase-pure, perovskite structured, Co-doped SrFeO3−δ, even at a Co occupancy as high as 75% (i.e., 25% Fe occupancy). Little or no sign of lattice distortion could be observed from the refinement results, whereas the microstrain of the perovskite structure appears slightly increased upon Co doping. A clear shift of diffraction peaks to higher 2θ is observed with increasing Co content. This indicates Co cations are well-confined in the B sites, substituting Fe, leading to lattice contraction as a result of the relatively small radii of Co4+ and Co3+ ions cf. the Fe4+ and Fe3+ ions. This trend is also corroborated by the quantitative analysis of the refined XRD pattern shown in Table S4 (ESI†). Therefore, despite the different extent of Co doping, the lattice structure of the SrFe1−xCoxO3−δ closely resembles that of the undoped SrFeO3−δ.The sample morphology and elemental homogeneity at the microscopic level were analyzed using SEM and EDS mapping. As shown in Fig. 2, a uniform dispersion of all constituent elements could be confirmed, without any observation of agglomeration, locally high concentration, or impurity phases. The SF and SFC oxygen carrier particles were observed to have porous surfaces, which are beneficial for rapid oxygen uptake and release during CLAS.
 | ||
| Fig. 2 SEM and EDX mappings of the as-prepared (a) SF, (b) SFC25, (c) SFC50, and (d) SFC75 perovskite oxygen carriers. | ||
Even in ambient air, SrFeO3 and its Co-doped variants can have oxygen non-stoichiometry. Therefore, the oxygen non-stoichiometries (i.e., δ) of the oxygen carriers were first determined prior to any further evaluation, by both iodometric titration and temperature programed desorption (TPD). The results of the latter analysis are discussed in the next section. As shown in Table S2 (ESI†), the (3 − δ) value of the as-prepared oxygen carriers in air was found to decrease systematically from 2.83 to 2.68 as Co ratio (x) increase from 0 to 0.75. The values obtained here are similar to those determined in the literature for SrFe1−xCoxO3−δ prepared by sol–gel methods.41,42
3.2 Oxygen uptake and release through temperature swings
The temperature programed oxygen release experiments were first conducted in nitrogen to validate the oxygen content estimated by iodometric titration, as well as to reveal the change in the activity of oxygen carriers induced by Co-substitution. The complete decomposition of perovskite resulted in a phase transformation to SrFe1−xCoxO2.5 brownmillerite.43 The weight loss curves (see Fig. S1, ESI†) during this process were collected to determine the initial oxygen content, 3 − δ0. In the N2-TPD profiles, SFC was found to lose less weight than undoped SF, suggesting its initial oxygen content is closer to that of brownmillerite (2.5). As summarized in Table S2 (ESI†), the results derived from N2-TPD are in close agreement with those obtained by iodometric titration, with higher Co occupancy (x) corresponding to greater oxygen non-stoichiometry.The effect of Co doping could be clearly observed from the N2-TPD profiles. As shown in Fig. 3a, the SFC perovskites tend to lose weight at lower temperatures than the undoped SF. For SF, the onset temperature for O2 release was around 400 °C. After Co doping, the onset temperature as well as the peak temperature for oxygen release shift to increasingly lower temperatures with increasing Co occupancy, indicating higher activity for oxygen release. Here, the onset temperature for oxygen release was defined as the temperature at which the oxygen carriers started to lose weight. In general, the enhancement in oxygen release activity is more profound for samples with more Co doping. For SFC75, oxygen release started at a temperature as low as 300 °C. Air-TPD have also been conducted, as summarized in Fig. 3b. Although the heating rate appears to influence the onset temperature, especially when the heating rate is high, the promotional effect of Co-doping is ubiquitous at all heating rates (1, 3, 5 and 10 °C min−1). SFC75 exhibited an onset temperature ∼100 °C lower than that of SF, indicating that the promotional effect of Co doping is applicable across a wide range of oxygen partial pressure (i.e., any mixture of N2 and air).
 | ||
| Fig. 3 Temperature-programmed oxygen desorption profiles of the perovskite oxygen carriers in (a) N2 and (b) air. There N2-TPD profiles (derivative weight) were derived from the weight loss curves shown in Fig. S1 (ESI†). | ||
TGA experiments across a wide range of temperatures (T) and oxygen partial pressures (pO2) were conducted to systematically investigate the oxygen uptake-release behavior of the SF and SFC oxygen carriers. In the isothermal experiments (detailed program shown in Fig. S2a, ESI†), oxygen carrier samples were subjected to a specified pO2 (between 0.02 and 0.2 bar) under elevated temperature (150–650 °C). As shown in Fig. S2b (ESI†), under each pO2, SFC75 started losing weight at the lowest temperature amongst all samples. All perovskite oxygen carriers exhibited fast oxygen release behavior, as indicated by the observation that the sample mass quickly equilibrated to a steady value upon reaching a new temperature set point, as shown Fig. S2b (ESI†). At each time point (i.e., under the same T and pO2), the mass change of the oxygen carriers followed the trend of SFC75 > SFC50 > SFC25 > SF, indicating that the SFC75 possesses the highest oxygen carrying capacity for any given temperature swing.
The oxygen stoichiometry (3 − δ) and oxygen carrying capacity (with reference to T = 150 °C and pO2 = 0.2 bar) were determined according to eqn (2). In general, a lower oxygen partial pressure corresponds to a lower (3 − δ) value. At pO2 = 0.2 bar, the 3 − δ values approach 2.5 (δ = 0.5) when the temperature is above 500 °C, indicating the tendency of phase change from perovskite to brownmillerite. While the value of (3 − δ) could be reduced below 2.5 under highly reducing conditions (e.g. T > 800 °C, log(pO2) < −3), such conditions are not applicable to CLAS processes.38 The capacity of these perovskite oxygen carriers for CLAS applications could be further evaluated by analyzing the oxygen carrying capacities. As expected, all perovskite-structured oxygen carriers release more gaseous oxygen when exposed to higher temperatures. As shown in Fig. 4, SFC75 was found to possess the highest oxygen carrying capacity amongst all oxygen carriers, especially in the temperature range above 400 °C. Under the condition of T = 650 °C and pO2 = 0.02 bar, SFC75 shows an oxygen carrying capacity of ∼1.5 wt%, i.e. a ∼20% improvement compared to undoped SF. In summary, the results from isothermal experiments under various oxygen partial pressure have confirmed the enhanced CLAS performance of the Co-doped SF perovskites.
The stability of the crystal structure of the perovskite oxygen carriers is essential to ensuring cyclic durability, since it will undergo cyclic phase changes upon oxygen release and uptake. Accordingly, the structural changes of the oxygen carriers were investigated by in situ XRD. During the temperature excursion from 100 °C to 500 °C and back, the refined in situ XRD data indicate that all SF and SFC oxygen carriers were able to fully restore their original lattice structures and lattice parameters (Fig. 5a), suggesting excellent structural reversibility and cyclic stability. Then, the perovskite samples were subjected to temperature cycles in air and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 air/CO2 mixture (temperature program shown in Fig. 5b). As shown by the in situ diffractograms in Fig. 5c, as the O2 release progressed with temperature increase (i.e., as the scan number increased), the characteristic (110) diffraction peak shifted towards smaller angles, indicative of an expanding perovskite unit cell due to thermal expansion accompanied by the depletion of lattice oxygen. Upon cooling back to room temperature, the diffraction peak restored its initial position, demonstrating reversibility and thermal stability over the redox process. Moreover, the same experiment conducted in a 1
1 air/CO2 mixture (temperature program shown in Fig. 5b). As shown by the in situ diffractograms in Fig. 5c, as the O2 release progressed with temperature increase (i.e., as the scan number increased), the characteristic (110) diffraction peak shifted towards smaller angles, indicative of an expanding perovskite unit cell due to thermal expansion accompanied by the depletion of lattice oxygen. Upon cooling back to room temperature, the diffraction peak restored its initial position, demonstrating reversibility and thermal stability over the redox process. Moreover, the same experiment conducted in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 air/CO2 mixture revealed a shift in the main perovskite peak (110) to lower angles, signifying further O2 release. This is expected as the degree of oxygen release increases with decreasing oxygen partial pressure. At the same time, the in situ XRD patterns in air/CO2 have shown no evidence of carbonate phase formation, which would be detrimental to the performance of the perovskite materials, especially because Sr-containing perovskites are known to undergo carbonation in CO2 to form SrCO3.44 The resistance to carbonation assumes critical importance, especially in processes such as CLAS or oxyfuel combustion, the oxygen release could take place in highly concentrated CO2. Consequently, materials suffering severely from carbonation are unsuitable for CLAS.45 In summary, the in situ XRD experiments corroborates the feasibility of employing the SF and SFCs for CLAS applications using CO2 as a carrier gas, which is relevant to processes such as oxy-fuel combustion and Allam cycle.
1 air/CO2 mixture revealed a shift in the main perovskite peak (110) to lower angles, signifying further O2 release. This is expected as the degree of oxygen release increases with decreasing oxygen partial pressure. At the same time, the in situ XRD patterns in air/CO2 have shown no evidence of carbonate phase formation, which would be detrimental to the performance of the perovskite materials, especially because Sr-containing perovskites are known to undergo carbonation in CO2 to form SrCO3.44 The resistance to carbonation assumes critical importance, especially in processes such as CLAS or oxyfuel combustion, the oxygen release could take place in highly concentrated CO2. Consequently, materials suffering severely from carbonation are unsuitable for CLAS.45 In summary, the in situ XRD experiments corroborates the feasibility of employing the SF and SFCs for CLAS applications using CO2 as a carrier gas, which is relevant to processes such as oxy-fuel combustion and Allam cycle.
The cyclic durability of the perovskite oxygen carriers was examined by isothermal redox swing cycles (i.e., switching between air and N2) at 500 °C. Each cycle consists of a 10 min oxidation stage in air, followed by a 30 min reduction stage in N2. For all samples, the cyclic mass changes have stabilized after the fifth cycle and became highly reproducible for the remaining 45 cycles (Fig. 6). In other words, all the SF and SFC perovskite oxygen carriers have shown excellent cyclic stability. Amongst all the samples, SFC75 has shown the highest oxygen carrying capacity of ∼1.3 wt%, whereas the undoped SF only have a capacity of ∼0.5 wt%, signifying the apparent benefit of Co doping. It could also be observed from the detailed weight loss curve during cycles 2–3 (Fig. 6e) and cycles 49–50 (Fig. 6f) that the SFC75 exhibited superior redox kinetics, with almost identical apparent rate of oxygen uptake and significantly faster apparent rate of oxygen release than all other samples; both rates remain unchanged over 50 cycles. Upon completion of the extended cycling experiment, the morphology of the spent oxygen carriers was characterized by SEM, as shown in Fig. 7. Remarkably, the morphologies of all the perovskite oxygen carrier particles remained nearly unchanged, with the porous surface morphology well preserved after 50 redox cycles. Therefore, the results above showed the suitability of SF and SFC oxygen carriers for CLAS up to 500 °C, without inducing any irreversible or undesirable (i.e., transformation from perovskite to brownmillerite) change in phase composition or morphology.
 | ||
| Fig. 7 SEM images of the (a) SF, (b) SFC25, (c) SFC50, and (d) SFC75 perovskites after 50 TGA cycles. | ||
3.3 Ab initio rationalization of oxygen carrier performance
Several structural descriptors, derived from ab initio calculations, were investigated in order to probe the composition-structure–function relationship of the B-site substituted SFC perovskites for CLAS applications. For perovskite materials, the Goldschmidt tolerance factor may be used as a simple descriptor of the perovskite's stability and is often used to describe the perceived activity of doped perovskite phases:46 | (3) |
| Oxygen carrier | Tolerance factor | O 2p band center (eV) | Hybridization intensity (%) |
|---|---|---|---|
| SF | 1.019 | −1.951 | 32.98 |
| SFC25 | 1.027 | −1.948 | 37.29 |
| SFC50 | 1.034 | −1.902 | 44.32 |
| SFC75 | 1.042 | −1.887 | 51.29 |
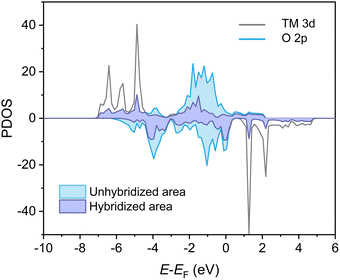
Besides crystal structure parameters, B-site substitution can alter the electronic structures of the perovskites and changes the chemical activities of the perovskites, both in terms of thermodynamics and kinetics. Here, density functional theory (DFT) calculations were carried out to analyze the electronic structures of SF, SFC25, SFC50, and SFC75 perovskite oxygen carriers. Fig. S3 (ESI†) shows the calculated partial density of state (DOS) plots of the 3d orbitals of the transition metals (viz. Fe and Co) and the O 2p orbitals. For all perovskite samples, the DOS at the Fermi energy (EF) is, with the band gap being less than 2 eV, i.e., these Fe-based perovskite are semiconductors. It could be noticed that the Fe 3d band become less intense while the Co 3d band intensifies with increasing the Co content in the SFC. Besides, the valence band near the Fermi level is primarily occupied by O 2p states, while the conduction band (unoccupied states above EF) is mainly dominated by Fe 3d states. The above observations are in agreement with previous studies.25,49 The relationship between the O 2p-band center and transition metal–oxygen interaction in perovskite structures is often considered important to the redox activities of transition metal oxide catalysts.50 Here, the O 2p-band center (ε2p) is defined as:
 | (4) |
The degree of hybridization between O 2p and TM 3d states is also proposed to represent the nature of metal–oxygen interaction, and subsequently the redox activity of metal oxides.51,52Fig. 8 illustrates the hybridization between O 2p and TM 3d states of SFC75. Here, the degree of hybridization is estimated by quantifying the overlap between the TM 3d and O 2p bands (annotated in purple in Fig. 8), calculated according:
| Hybridization intensity = PDOS (O 2p)overlapped/PDOS (O 2p) | (5) |
The results of hybridization of the various perovskite structured oxygen carriers are summarized in Table 1. As depicted in Fig. 9, there is a strongly negative correlation between the degree of hybridization and the onset temperature for oxygen release. In fact, the onset temperature for oxygen release shows apparently greater correlation with the degree of hybridization than the O 2p center as descriptors. In prior studies, the relationships between the CLAS performance of substituted strontium ferrite perovskites oxygen carriers and other DFT calculated descriptors, such as energy for oxygen vacancy formation and net electronic charge have also been reported.12,25 Accordingly, the findings in the present study offer further insights into the electronic origins of the activity perturbation by B-site substitution.
Besides B-site substitution, further studies on A-site substitution as well as other substitutional B-site dopants are also worthwhile, particularly for validating the applicability of the descriptors discussed in the present study, including tolerance factor, O 2p-band center, and the degree of TM–O hybridization. In addition, future simulation work should also investigate the release of gaseous oxygen by perovskite structured oxides with existing oxygen vacancies.
4 Conclusions
In this study, the consequence of Co substitution at the B sites of SrFeO3 is systematically investigated for the application of CLAS. Phase-pure perovskites with B-site Co substitution up to 75% was prepared by a sol–gel method. Thermogravimetric analysis revealed that the oxygen uptake and release performance of the strontium ferrite-based perovskite materials, characterized by onset temperature for oxygen release and oxygen carrying capacity, can be effectively altered through B-site substitutional doping. All the perovskite structured oxygen carriers, including SF and SFC, showed excellent cyclic stability over pressure swing (air-N2) cycles at 500 °C, suggesting their suitability for long-term CLAS operation. Deactivation due to carbonation of Sr is generally absent in the presence of CO2 in the process stream. The experimental observations can be rationalized by the enhanced activity of the TM–O bonds, which can originate from structural perturbation as characterized by the Goldschmidt tolerance factor, as well as changes in the electronic structure as characterized by the O 2p center and the degree of TM–O hybridization. These correlations offer ab initio rationales for designing high-performance perovskite-structured oxygen carriers for CLAS and other chemical looping applications through hypothesis-driven synthesis.Data availability
Data for this article, including empirical data (e.g. tabular files, text files, images, etc.) are available at NTU Research Data Repository at https://doi.org/10.21979/N9/OGIWAS.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the financial support by Ministry of Education Singapore's Academic Research Fund Tier 1 (RT03/19 and RG112/18) and the National Research Foundation (NRF), Prime Minister's Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.References
- K. Shah, B. Moghtaderi and T. Wall, Energy Fuels, 2012, 26, 2038–2045 CrossRef CAS.
- F. Wu, M. D. Argyle, P. A. Dellenback and M. Fan, Prog. Energy Combust. Sci., 2018, 67, 188–205 CrossRef.
- A. R. Smith and J. Klosek, Fuel Process. Technol., 2001, 70, 115–134 CrossRef CAS.
- W. Castle, Int. J. Refrig., 2002, 25, 158–172 CrossRef CAS.
- R. S. Murali, T. Sankarshana and S. Sridhar, Sep. Purif. Rev., 2013, 42, 130–186 CrossRef CAS.
- N. F. Himma, A. K. Wardani, N. Prasetya, P. T. Aryanti and I. G. Wenten, Rev. Chem. Eng., 2019, 35, 591–625 CrossRef CAS.
- Q. Imtiaz, D. Hosseini and C. R. Müller, Energy Technol., 2013, 1, 633–647 CrossRef CAS.
- Z. Deng, B. Jin, Y. Zhao, H. Gao, Y. Huang, X. Luo and Z. Liang, Energy Convers. Manage., 2018, 160, 289–301 CrossRef CAS.
- C. Zhou, K. Shah, H. Song, J. Zanganeh, E. Doroodchi and B. Moghtaderi, Energy Fuels, 2016, 30, 1741–1755 CrossRef CAS.
- S. Saqline, H. Wang, Q. Fan, F. Donat, C. Müller and W. Liu, Appl. Energy Combust. Sci., 2024, 17, 100238 Search PubMed.
- S. Saqline, L. Yang, A. Romagnoli and W. Liu, J. Cleaner Prod., 2023, 418, 138097 CrossRef CAS.
- E. Krzystowczyk, X. Wang, J. Dou, V. Haribal and F. Li, Phys. Chem. Chem. Phys., 2020, 22, 8924–8932 RSC.
- J. Dou, E. Krzystowczyk, X. Wang, A. R. Richard, T. Robbins and F. Li, J. Phys.: Energy, 2020, 2, 025007 CAS.
- E. Krzystowczyk, V. Haribal, J. Dou and F. Li, ACS Sustainable Chem. Eng., 2021, 9, 12185–12195 CrossRef CAS.
- H. Kusaba, G. Sakai, K. Shimanoe, N. Miura and N. Yamazoe, Solid State Ionics, 2002, 152–153, 689–694 CrossRef CAS.
- J. Vieten, B. Bulfin, F. Call, M. Lange, M. Schmucker, A. Francke, M. Roeb and C. Sattler, J. Mater. Chem. A, 2016, 4, 13652–13659 RSC.
- B. Bulfin, J. Lapp, S. Richter, D. Gubàn, J. Vieten, S. Brendelberger, M. Roeb and C. Sattler, Chem. Eng. Sci., 2019, 203, 68–75 CrossRef CAS.
- E. Marek, W. T. Hu, M. Gaultois, C. P. Grey and S. A. Scott, Appl. Energy, 2018, 223, 369–382 CrossRef CAS.
- R. H. Görke, E. J. Marek, F. Donat and S. A. Scott, Int. J. Greenhouse Gas Control, 2020, 94, 102891 CrossRef.
- J. Dou, E. Krzystowczyk, X. Wang, T. Robbins, L. Ma, X. Liu and F. Li, ChemSusChem, 2020, 13, 385–393 CrossRef CAS.
- Y. Hayamizu, M. Kato and H. Takamura, J. Membr. Sci., 2014, 462, 147–152 CrossRef CAS.
- F. Fujishiro, N. Oshima, T. Sakuragi and M. Oishi, J. Solid State Chem., 2022, 312, 123254 CrossRef CAS.
- F. Fujishiro, N. Oshima, N. Kamioka, T. Sakuragi and M. Oishi, J. Solid State Chem., 2020, 283, 121152 CrossRef CAS.
- G. Luongo, F. Donat and C. R. Müller, Phys. Chem. Chem. Phys., 2020, 22, 9272–9282 RSC.
- X. Wang, E. Krzystowczyk, J. Dou and F. Li, Chem. Mater., 2021, 33, 2446–2456 CrossRef CAS.
- F. Mikailzade, F. Önal, M. Maksutoglu, M. Zarbali and A. Göktaş, J. Supercond. Novel Magn., 2018, 31, 4141–4145 CrossRef CAS.
- A. Goktas, I. H. Mutlu and A. Kawashi, Thin Solid Films, 2012, 520, 6138–6144 CrossRef CAS.
- H. Gencer, A. Goktas, M. Gunes, H. I. Mutlu and S. Atalay, Int. J. Mod. Phys. B, 2008, 22, 497–506 CrossRef CAS.
- H. Gencer, M. Gunes, A. Goktas, Y. Babur, H. I. Mutlu and S. Atalay, J. Alloys Compd., 2008, 465, 20–23 CrossRef CAS.
- M. Schmidt, J. Phys. Chem. Solids, 2000, 61, 1363–1365 CrossRef CAS.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- Y.-L. Lee, J. Kleis, J. Rossmeisl, Y. Shao-Horn and D. Morgan, Energy Environ. Sci., 2011, 4, 3966–3970 RSC.
- L. K. Parmar, R. Joshi, P. Yadav, T. Garg, A. Kumar and A. Yadav, Chem. Phys. Impact, 2024, 8, 100603 CrossRef.
- A. Holt, T. Norby and R. Glenne, Ionics, 1999, 5, 434–443 CrossRef CAS.
- J. P. Hodges, S. Short, J. D. Jorgensen, X. Xiong, B. Dabrowski, S. M. Mini and C. W. Kimball, J. Solid State Chem., 2000, 151, 190–209 CrossRef CAS.
- A. Göktaş, A. Tumbul and F. Aslan, J. Sol-Gel Sci. Technol., 2016, 78, 262–269 CrossRef.
- L. Kokhanovskii, V. Vashuk, O. Ol'shevskaya and O. Kirilenko, Inorg. Mater., 2001, 37, 730–736 CrossRef CAS.
- I. Starkov, S. Bychkov, A. Matvienko and A. Nemudry, Phys. Chem. Chem. Phys., 2014, 16, 5527–5535 RSC.
- V. L. Kozhevnikov, I. A. Leonidov, M. V. Patrakeev, E. B. Mitberg and K. R. Poeppelmeier, J. Solid State Chem., 2001, 158, 320–326 CrossRef CAS.
- V. Kharton, A. Yaremchenko, A. Kovalevsky, A. Viskup, E. Naumovich and P. Kerko, J. Membr. Sci., 1999, 163, 307–317 CrossRef CAS.
- C. Y. Lau, M. T. Dunstan, W. Hu, C. P. Grey and S. A. Scott, Energy Environ. Sci., 2017, 10, 818–831 RSC.
- J. Vieten, B. Bulfin, P. Huck, M. Horton, D. Guban, L. Zhu, Y. Lu, K. A. Persson, M. Roeb and C. Sattler, Energy Environ. Sci., 2019, 12, 1369–1384 RSC.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Energy Environ. Sci., 2015, 8, 1404–1427 RSC.
- K. J. May, C. E. Carlton, K. A. Stoerzinger, M. Risch, J. Suntivich, Y.-L. Lee, A. Grimaud and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 3264–3270 CrossRef CAS.
- R. Jacobs, J. Hwang, Y. Shao-Horn and D. Morgan, Chem. Mater., 2019, 31, 785–797 CrossRef CAS.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS.
- J. Suntivich, W. T. Hong, Y.-L. Lee, J. M. Rondinelli, W. Yang, J. B. Goodenough, B. Dabrowski, J. W. Freeland and Y. Shao-Horn, J. Phys. Chem. C, 2014, 118, 1856–1863 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Determination of the oxygen non-stoichiometry, oxygen carrying capacity, weight loss curves during the N2-TPD and isothermal TGA measurements under different pO2. See DOI: https://doi.org/10.1039/d4cp02152e |
| This journal is © the Owner Societies 2024 |